Abstract
Experimental, epidemiological and clinical evidence implicates insulin resistance and its accompanying hyperinsulinaemia in the development of cancer, but the relative importance of these disturbances in cancer remains unclear. There are, however, theoretical mechanisms by which hyperinsulinaemia could amplify such growth-promoting effects as insulin may have, as well as the growth-promoting effects of other, more potent, growth factors. Hyperinsulinaemia may also induce other changes, particularly in the IGF (insulin-like growth factor) system, that could promote cell proliferation and survival. Several factors can independently modify both cancer risk and insulin resistance, including subclinical inflammation and obesity. The possibility that some of the effects of hyperinsulinaemia might then augment pro-carcinogenic changes associated with disturbances in these factors emphasizes how, rather than being a single causative factor, insulin resistance may be most usefully viewed as one strand in a network of interacting disturbances that promote the development and progression of cancer.
Keywords: cancer, inflammation, insulin, insulin-like growth factor-1 (IGF-1), mitogen-activated protein kinase (MAPK), obesity, p21Ras
Abbreviations: AMPK, AMP-activated protein kinase; BMI, body mass index; Fox, forkhead box; GH, growth hormone; Grb2, growth-factor-receptor-bound protein 2; IGF, insulin-like growth factor; IGFBP, IGF-binding protein; IL-6, interleukin-6; IRS-1, insulin receptor substrate-1; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; mSos, mammalian Son of sevenless; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor κB; IκB, inhibitor of NF-κB; IKKβ, IκB kinase; OGTT, oral glucose tolerance test; p70S6K, p70 S6 kinase; PI3K, phosphoinositide 3-kinase; PPAR, peroxisome-proliferator-activated receptor; Ptpn1, protein tyrosine phosphatase, non-receptor type 1; ROS, reactive oxygen species; SHBG, sex hormone-binding globulin; SIRT1, sirtuin 1; TNF, tumour necrosis factor
INTRODUCTION
Insulin resistance is a state of reduced sensitivity of insulin-responsive tissues to insulin and its key consequences include an impaired ability of insulin to suppress hepatic glucose production and stimulate peripheral glucose elimination. Circulating glucose levels would, therefore, be expected to rise with insulin resistance, but glucose is insulin's principal secretagogue and, providing β-cell function is adequate, insulin secretion increases to overcome insulin resistance and glucose levels are normalized. The resulting ‘compensatory hyperinsulinaemia’ is a hallmark of insulin resistance.
Insulin resistance coincides with an increased risk of cancer in a variety of conditions, including aging, physical inactivity [1], obesity [2], central body fat distribution [3], Type 2 diabetes [4], hyperglycaemia [5], the metabolic syndrome [6], liver disease [7], subclinical inflammation [8], acromegaly [9], a high-glycaemic-index diet [10] and a high-saturated-fat diet [11], and there is extensively reviewed evidence linking measures associated with insulin resistance with the subsequent development of cancer or pre-cancerous changes [12–21]. These reviews have, however, tended to focus on specific conditions, e.g. diabetes, obesity or colorectal cancer, or on epidemiological rather than experimental evidence. The present review begins by highlighting key aspects of the experimental, epidemiological and clinical evidence that justify an in-depth consideration of insulin resistance and hyperinsulinaemia as factors in carcinogenesis. It then examines the various mechanisms by which insulin resistance and its accompanying hyperinsulinaemia could have an impact on cancer risk. The review also makes a distinction between promotion of cancer by insulin resistance and hyperinsulinaemia and promotion of cancer by conditions that coincidentally cause insulin resistance and hyperinsulinaemia.
EXPERIMENTAL, EPIDEMIOLOGICAL AND CLINICAL EVIDENCE FOR LINKS BETWEEN INSULIN RESISTANCE, HYPERINSULINAEMIA AND CANCER
Experimental studies
Azoxymethane induction of colon cancer or pre-cancerous aberrant crypt foci in the rat has provided the most widely used experimental model with which links between insulin resistance and cancer have been evaluated. Tumorigenesis in this model is enhanced by injection of medium-acting insulin [22,23], and calorie restriction, perhaps the most familiar means of reducing insulin resistance, diminishes the development of aberrant crypt foci [24]. Calorie restriction is also effective in other animal models [25,26]. Low fat in the diet provides additional protection [27] and this emphasizes further the possible importance of insulin resistance, given that low-fat diets are associated with reduced insulin resistance independent of calorie content [28]. Conversely, high calorie intake correlates significantly with pre-cancerous changes [29], and this effect is again modulated by dietary composition, the most tumorigenic being a high-fat diet [30]. It is worth noting that, in an animal model of breast cancer, the presence of obesity had no apparent effect on mammary tumour development, but the carcinogen employed was more able to promote colon cancer [31]. Insulin resistance and hyperinsulinaemia are typical of obesity, so this observation draws attention to the possibility that insulin resistance may be particularly important in colon cancer.
Bruce and co-workers [32] have extended the dietary studies to include an evaluation of accompanying changes in insulin resistance and insulinaemia. The development of colonic aberrant crypt foci was preceded by insulin resistance and hyperinsulinaemia, and there was a significant positive relationship between the size of aberrant crypt foci and non-fasting insulin concentrations [29]. A stronger relationship was observed with increasing OGTT (oral glucose tolerance test) glucose concentrations which, when resulting from dietary modification, would be expected to reflect increasing insulin resistance. In subsequent experiments, a positive relationship was observed between the number of aberrant crypt foci and insulin concentrations 3 h following an oral glucose load, but a stronger relationship was apparent with a direct measure of insulin sensitivity obtained using the euglycaemic–hyperinsulinaemic clamp [33]. The significant correlates of precursors of colon cancer were, therefore, those that might be expected to express most accurately net insulin exposure. Key findings from these experiments are summarized in Table 1.
Table 1. Correlation coefficients for the relationships between aberrant crypt foci and indices and measures of insulin resistance and insulinaemia in Fischer 344 rats initiated by azoxymethane and given diets differing in calorie and saturated fat content (Koohestani et al. [29]) or saturated fat content (Tran et al. [33]).
ACF, aberrant crypt foci; AUC, area under the curve.
Epidemiological studies
A complicating factor in evaluating whether experimental associations between insulin resistance and cell proliferation and survival translate into increased risk in humans has been the difficulty in assessing insulin resistance, requiring as it does quantification of the rate of elimination of glucose from the blood relative to the accompanying plasma insulin concentration. Plasma insulin concentrations can, nevertheless, provide a surrogate index since, in the presence of adequate β-cell function, the degree of compensatory hyperinsulinaemia is directly related to the degree of insulin resistance. C-peptide may provide a more effective index of insulinaemia as it is secreted simultaneously and in equimolar quantities with insulin but has a longer plasma half-life, which may render it less subject to individual variation. Associations between insulin or C-peptide and cancer have been evaluated in a number of studies. Studies of colorectal cancer are divided between those showing no association [34–38] or a significant positive association [3,39–44], although three studies have found significantly higher insulin or C-peptide concentrations among patients in whom colorectal adenoma has been detected [45–47], particularly in those with advanced lesions [45]. As with colorectal cancer, studies are divided for breast cancer (no association [48–56]; positive association [57–64]), endometrial cancer (no association [65,66]; positive association [67–69]) and prostate cancer (no association [70–72]; positive association [73,74]). Two studies of pancreatic cancer both found a positive association [75,76].
Studies of colorectal, breast, endometrial and pancreatic cancer were the subject of a meta-analysis by Pisani [19] published in 2008. The majority of studies were population-based and, for colorectal and endometrial cancers, there was little difference between prospective and cross-sectional studies. Pisani did, however, note that, for breast cancer, prospective studies all showed no association with insulin or C-peptide, with all of the evidence for a positive association coming from cross-sectional studies. Positive associations could, therefore, reflect secondary effects of existing cancer in the cases. However, more recently, two prospective analyses from the U.S. Women's Health Initiative Observational Study have reported a positive association between insulin concentrations and incident breast cancer [60,61]. Importantly, one of these evaluated serial insulin measurements [61], which would be expected to provide a more accurate assessment of net insulin exposure than a single baseline measure. The serially derived insulin measures showed an appreciably stronger relationship with incident breast cancer than the single baseline measurements.
Epidemiological studies of the relationships between insulin, C-peptide and cancer vary considerably in study design and the extent to which confounding factors have been taken into account. However, the majority standardized for the principal confounder, adiposity. The extent to which there is a continuous or threshold relationship between insulinaemia and cancer is difficult to assess, the majority of studies simply comparing risk in the top and bottom percentiles. There is, however, some evidence for a continuous relationship [43]. The weight of epidemiological evidence, therefore, suggests that hyperinsulinaemia (and, by implication, insulin resistance) is a risk factor for cancer and it should be noted that, with studies divided between those showing no association or a positive association, the weight of evidence favours a positive association. Only with prostate cancer has there been any evidence for a lower risk of cancer with elevated insulin levels [77,78]. Studies of prostate cancer will be confounded by the low androgen concentrations, and therefore lower risk of prostate cancer, that may be found in association with insulin resistance. Nevertheless, as mentioned above, there are also studies showing no association [70–72] or a positive association [73,74] between prostate cancer and insulinaemia. Relative risks for all studies of colorectal, breast, endometrial and pancreatic cancer in the meta-analysis of Pisani [19] are summarized in Table 2, with estimates for prostate cancer from the meta-analysis of Hsing et al. [18].
Table 2. Summary relative risk estimates for plasma insulin or C-peptide as predictors of colorectal, breast, endometrial and pancreatic cancer from the meta-analysis of Pisani [19] and for insulin as a predictor of prostate cancer from the meta-analysis of Hsing et al. [18].
CI, confidence interval.
| Type of cancer | Relative risk (95% CI) | Number of cases |
|---|---|---|
| Colorectal | 1.35 (1.13–1.61) | 1257 |
| Breast | 1.26 (1.06–1.48) | 1164 |
| Endometrial | 1.11 (0.74–1.65) | 388 |
| Pancreatic | 1.70 (1.10–2.63) | 209 |
| Prostate | 1.53 (1.01–2.32) | 220 |
Other indices of insulin resistance or hyperinsulinaemia include plasma leptin concentrations, the coincident insulin-resistance-related cardiovascular risk factors of the metabolic syndrome and polymorphisms of the insulin signalling system. As with measures of insulinaemia, studies are divided according to whether they show no significant association or a significant positive association between these indices and cancer risk [12–18,79–81]. It is noteworthy, however, that for the metabolic syndrome, which would be expected to reflect long-term insulin resistance and hyperinsulinaemia, the weight of evidence appears to favour a significant positive association [6,17,18,38,82–87]. It should also be noted that insulin-resistance-related abnormalities may be associated, not only with cancer itself, but also with more aggressive cancer or with mortality from cancer in those with existing cancer [88–92]. Equivocal evidence regarding diabetes mellitus may reflect a lack of discrimination between hypo- and hyper-insulinaemic forms of diabetes mellitus [17,93] and possible differences according to diabetes therapy [21] (see the next section).
Clinical evidence
Restoration of insulin action by a reduction in insulin resistance and replacement of plasma insulin is central to the treatment of diabetes mellitus, and monitoring of the long-term effects of the different treatments available has provided suggestive evidence regarding links between insulin resistance, hyperinsulinaemia and cancer. A number of insulin-sensitization agents have recently become available, but only metformin has been in use long enough for reliable conclusions to be drawn regarding its effects on cancer in vivo. Metformin augments signalling via AMPK (AMP-activated protein kinase), resulting in inhibition of hepatic glucose and lipid synthesis and increased muscle glucose uptake [94]. Consequently less insulin is needed to achieve these effects and a net insulin sensitization is apparent. Additionally, AMPK activation can inhibit cell growth and proliferation, possibly via inhibition of the mTOR (mammalian target of rapamycin) complex [95], and there are now several studies showing a reduced risk of cancer in patients treated with metformin [96–99]. It could be argued, however, that this reflects metformin's intracellular actions rather than any effect it may have on insulin resistance and hyperinsulinaemia. Moreover, evidence from in vitro studies indicates that metformin also has effects on cell proliferation via intracellular targets other than AMPK [100,101].
Injection of insulin by patients with diabetes to control their blood glucose levels has been a mainstay of Type 1 diabetes therapy for over 80 years and is also an important option in the treatment of Type 2 diabetes. There is some evidence that insulin therapy can augment colorectal cancer risk in patients with Type 2 diabetes [102,103]. Importantly, one of these studies corrected for metformin use, this being likely to be more frequent in the non-insulin-using controls and to be associated with lower colorectal cancer risk [102]. Moreover, that study found that risk increased significantly with increasing duration of chronic insulin use. Further suggestive evidence comes from a study published recently based on a German insurance fund cohort in which a significant increase in the risk of malignant neoplasm or cancer mortality was observed with each S.D. increase in dose of human insulin [104].
Recombinant DNA technology has enabled the construction of insulin analogues with enhanced absorption properties and a more sustained biological effect on account of a higher affinity of binding to the insulin receptor. Such properties might also be expected to enhance any effects insulin might have on cell proliferation and survival. A dose-dependent increase in malignant tumour formation was observed in rats injected with one of the earliest of these analogues to be investigated, B10-Asp (AspB10 insulin), which has an aspartate residue substituted at the B10 position of the insulin molecule [105]. A 7-fold increase in the rate of proliferation of cultured human cancer cells was observed with this analogue [106], and similar effects have been reported in vitro with other analogues [107]. Insulin analogues can undergo modification at the site of injection, so the relevance of proliferative effects identified in vitro to in vivo risks is questionable. There are, nevertheless, several observational epidemiological studies that link use of some insulin analogues with increased risk of cancer [98,104,108,109]. Although controversial [110,111], the possibility has been raised that the pro-proliferative effects of insulin analogues result primarily from enhanced activation of the IGF-1 (insulin-like growth factor-1) receptor [21]. Their relevance to any effects native human insulin may have are, therefore, questionable.
It should be noted that the other major therapy option for increasing circulating native insulin levels is use of insulin secretagogues, of which the sulfonylurea drugs have been available the longest. There is some evidence suggesting an increased risk of cancer in patients using these agents [97–99,112], but firm conclusions are difficult because of the inclusion of reduced risk metformin users in the comparison groups. Sulfonylurea users may be at a somewhat lower risk than insulin users [97], but information in this area is very limited and, even if an increased risk was confirmed, a pharmacological effect of these drugs cannot be excluded.
MITOGENIC AND ANTI-APOPTOTIC EFFECTS OF INSULIN
Growth promotion via classical insulin signalling
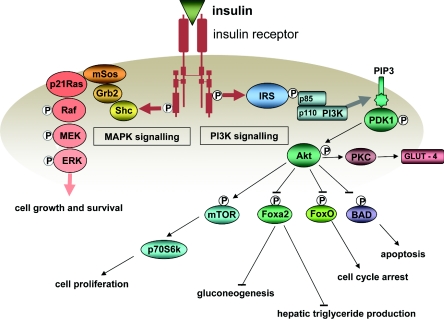
The possibility that insulin might increase cell proliferation in vivo was highlighted decades ago by Stout and Vallance-Owen [113] in relation to the development of atherosclerosis and, at the same time, in vitro and in vivo studies were indicating that many tumours might have an absolute dependence on insulin for continuing growth [114,115]. Since then, there has been considerable progress in elucidating how insulin could promote cell proliferation and survival. Insulin action begins with binding to its specific receptor, a transmembrane receptor tyrosine kinase. Signalling by the activated receptor involves autophosphorylation of tyrosine residues in the intracellular domain, which then promotes the phosphorylation of IRS-1 (insulin receptor substrate-1) and transmission of the insulin signal via two major phosphorylation cascades. These are distinguished by their principal mediators: PI3K (phosphoinositide 3-kinase) and MAPK (mitogen-activated protein kinase), as illustrated in Figure 1.
Figure 1. Principal signalling pathways activated by the insulin receptor.
Positive influences are shown by lines ending in arrows and inhibitory influences by lines ending in bars. MEK, MAPK/extracellular-signal-regulated kinase kinase; PIP3, PtdIns(3,4,5)P3; PKC, protein kinase C; triglyceride, triacylgycerol.
Signalling via the PI3K pathway involves the interaction of phosphorylated IRS-1 with the p85 regulatory subunit of PI3K and, as a consequence, activation of the PI3K p110 catalytic subunit. Activated PI3K locates to the cell membrane where it catalyses the production of PtdIns(3,4,5)P3. PtdIns(3,4,5)P3 stimulates the translation of the serine/threonine protein kinase Akt to the cell membrane, where it is phosphorylated by the PtdIns(3,4,5)P3-dependent protein kinase PDK1 (phosphoinositide-dependent kinase 1). Akt is capable of stimulating the phosphorylation and consequent inhibition or activation of a broad range of proteins that affect cell growth, division and survival as well as lipid and carbohydrate metabolism, for example the pro-apoptotic Bcl-2 family member BAD, the metabolism and growth-related Fox (forkhead box) transcription factors such as Foxa2 and FOXO1a, and the growth-related mTOR and its downstream effector kinase p70S6K (p70 S6 kinase). Signalling via the PI3K pathway is also a key factor in the translocation of GLUT-4 glucose transporters to the cell membrane. The importance of signalling via the PI3K pathway for cell proliferation and survival is evidenct from the high prevalence in a variety of cancers of a loss-of-function of the PtdIns(3,4,5)P3-phosphatase PTEN (phosphatase and tensin homologue deleted on chromosome 10), which results in elevated levels of PtdIns(3,4,5)P3 and enhanced PI3K signalling [116].
Phosphorylated IRS-1 can also mediate the formation of a complex between the adaptor protein Grb2 (growth-factor-receptor-bound protein 2) and the guanine nucleotide-exchange factor mSos (mammalian Son of sevenless). The Grb2–mSos complex can then promote GTP loading and consequent activation of the small-molecular-mass GTPase p21Ras, which lies at the head of the MAPK signalling cascade and which provides a point of convergence for signalling by a number of growth factors. Via a series of intermediate kinases, activated p21Ras promotes the activation of MAPKs, which then activate transcription factors involved in cell proliferation. The importance of signalling via the Ras/MAPK pathway for cell proliferation and survival is shown by the high prevalence of p21Ras overexpression in a range of different cancers [117].
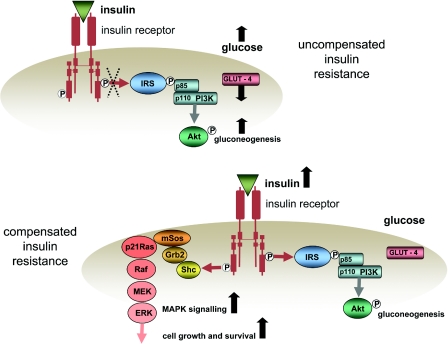
In vitro studies, including studies of cancer cells [118], provide ample evidence for the promotion of cell division by insulin. However, supra-physiological insulin concentrations have generally been used in these studies and it has been questioned whether, on its own, insulin binding to the insulin receptor has any growth-promoting effects [119]. Nevertheless, the in vivo milieu in which insulin operates may be very different from in vitro conditions and, as will be shown, insulin can induce other changes that could amplify any growth-promoting properties it may have. It could be objected that the compensatory hyperinsulinaemia of insulin resistance merely restores insulin signalling to non-resistant levels so, in principle, no additional drive to cell division and survival might be expected. This is likely to be true of signalling via the PI3K pathway, which also mediates the glucoregulatory effects of insulin. There is, nevertheless, some evidence from an in vitro study in breast cancer cells that insulin signalling via the MAPK pathway is preserved despite inhibition of IRS-1/PI3K signalling [120]. The possibility that compensatory hyperinsulinaemia might provide additional drive to cell proliferation via the MAPK pathway is illustrated in Figure 2.
Figure 2. Hypothetical scheme for enhanced cell growth and survival in insulin resistance.
In uncompensated insulin resistance (upper panel), signalling via the PI3K pathway is diminished, glucose uptake by the cell via GLUT-4 transporters is reduced, suppression of gluconeogenesis is reduced and glucose levels rise. In compensated insulin resistance (lower panel), the pancreas responds to the rise in glucose levels with increased insulin secretion, insulin levels rise, signalling through the PI3K pathway is restored and cell glucose uptake and gluconeogenesis are normalized. However, the increased insulin concentrations increase signalling via the kinases of the MAPK pathway {p21Ras, Raf, MEK [MAPK/ERK (extracellular-signal-regulated kinase) kinase] and ERK} to increase cell proliferation and survival.
Other growth-promoting effects of insulin
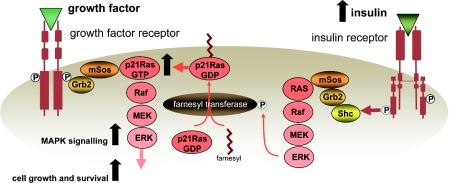
Small-molecular-mass GTPases play a key role in the signalling pathways involved in cell proliferation and there is evidence, largely from the work of Goalstone, Draznin and co-workers [121–123], for specific activation of these proteins and amplification of their effects by insulin. p21Ras is among the most prominent of these enzymes and prenylation of p21Ras with a farnesyl moiety by the enzyme farnesyl transferase is an essential step in anchoring p21Ras to the plasma membrane in readiness for GTP loading and signalling via the MAPK pathway. Insulin can activate farnesyl transferase by phosphorylation of its regulatory α subunit [121], and insulin-induced increases in p21Ras farnesylation have indeed been shown to be associated with increased GTP loading of membrane-bound p21Ras [122]. Activation of farnesyl transferase appears to be mediated via the insulin receptor, as shown by increased levels of farnesylated p21Ras in cells overexpressing insulin receptors and reduced levels in cells expressing a mutant insulin receptor with impaired function [123]. These studies also suggest that farnesyl transferase activation by insulin is not mediated by the IRS-1/PI3K pathway, but via another target of the activated insulin receptor, Shc, which then promotes Grb2/mSos recruitment, p21Ras activation and phosphorylation of farnesyl transferase via MAPK pathway activation. Different isoforms of Shc that can be phosphorylated by the activated insulin receptor [124] include the p52 isoform which, upon tyrosine phosphorylation, activates p21Ras and the p66 isoform which has an additional domain with a phosphorylatable serine residue and acts as a competitive inhibitor of p21Ras activation by the other isoforms [125].
This stimulation of farnesyl transferase activity appears to be specific to insulin [126], and prolonged hyperinsulinaemia has been shown to increase the cellular pool of farnesylated p21Ras and loading of p21Ras with GTP [127,128]. This effect is in addition to the general up-regulation of p21Ras-GTP associated with growth factor signalling and it can, therefore, synergize with stimulation of the guanine nucleotide-exchange activity of mSos by insulin or other growth factors to amplify growth factor signalling [129,130]. These interactions are illustrated in Figure 3.
Figure 3. Farnesylation of p21Ras causes it to locate to the plasma membrane where it can undergo GTP loading, which is essential for signalling via the MAPK pathway.
Insulin increases activation of farnesyl transferase, which increases farnesylation of p21Ras. As a consequence, the pool of p21Ras-GTP is increased. This has the effect of amplifying growth factor signalling via the MAPK pathway. MEK, MAPK/ERK kinase.
Up-regulation of insulin receptors and insulin-regulated intracellular signalling molecules
Carcinogenesis is a multifactorial multistage process and one of these stages can include overexpression of insulin receptors. The compensatory hyperinsulinaemia of insulin resistance could, therefore, provide an additional stimulus to cell proliferation and survival in situations where insulin signalling has already been rendered hypersensitive by increased expression of insulin receptors. In non-malignantly transformed cells overexpressing insulin receptors, exposure to insulin can result in a malignantly transformed phenotype [131,132]. Insulin receptors may be overexpressed 2–6-fold in cancer cells [133–135]; moreover, although the up-regulated receptors can appear structurally normal, the sensitivity of their receptor tyrosine kinase activity is increased [136]. Two isoforms of the insulin receptor exist, the A isoform predominating in fetal tissues and the B isoform in adult tissues. In addition to binding insulin, the A form of the receptor binds IGF-2 and, in cancer cells overexpressing insulin receptors, the A form appears to predominate [137]. Overexpression of insulin receptors therefore exposes the cell to the growth-promoting effects of IGF-2.
Cancer cells appear to loose their ability to down-regulate insulin receptors in response to hyperinsulinaemia [138] and in cells with up-regulated insulin receptors, a given concentration of insulin can increase production of p21Ras-GTP 7-fold compared with normal cells [139]. The observation that p21Ras-GTP, farnesylated p21 Ras and phosphorylated Shc are all increased in cells overexpressing insulin receptors or overexpressing a mitogenically active mutant insulin receptor emphasizes the functional consequences of receptor overexpression [135,140]. As well as interacting with up-regulated insulin receptors, the compensatory hyperinsulinaemia of insulin resistance could also interact with the up-regulation of intracellular mediators involved in growth factor signalling frequently observed in carcinogenesis. An example is provided by p21Ras itself, which, when overexpressed, can greatly enhance the effect of insulin on cell growth [141].
INSULIN RESISTANCE, HYPERINSULINAEMIA AND SIGNALLING VIA THE IGF-1 RECEPTOR
IGF-1, insulin resistance and cell proliferation and survival
IGF-1 signalling via the IGF-1 receptor has effects on cell proliferation and survival that are appreciably stronger than those of insulin [142]. IGF-1 can act as a potent growth factor for cancer cells both in vivo [143] and in vitro [144], and in vivo overexpression of IGF-1 can promote tumour formation [145], whereas its down-regulation can inhibit tumorigenesis [146]. IGF-1 can overcome the beneficial effect of calorie restriction in animal models of cancer [147] and can increase expression of angiogenic factors in cancer cells [148]. Epidemiological evidence supports a role for elevated circulating IGF-1 levels in the development of a variety of cancers, including colorectal, prostate and breast cancers [149] (although an inverse relationship has been reported for free IGF-1 levels [67]). It should also be noted that stimulation of PI3K or MAPK signalling via the IGF-1 receptor can promote phosphorylation of TAF-1 (transcriptional activation function-1) of the oestrogen receptor, thus promoting oestrogen-independent cell growth and division in oestrogen-sensitive tissues [150].
There is considerable homology between the insulin receptor and the IGF-1 receptor, and insulin can bind to and activate the IGF-1 receptor. Insulin could, therefore, enhance IGF signalling by direct stimulation of the IGF-1 receptor [151], although the relevance of this is uncertain [152]. Nevertheless, given that signalling via the IGF-1 receptor is more tightly linked to growth promotion, any augmentation of IGF-1-receptor-mediated signalling could markedly affect cell proliferation and survival. In accordance with this, relatively minor increases in serum IGF-1 concentrations are associated with an increased risk of prostate [153,154], breast [155], colon [156] and lung [157] cancers.
Indirect effects of insulin on IGF signalling
Insulin can up-regulate human hepatic GH (growth hormone) receptors [158], and receptor-mediated GH signalling in the liver is the principal stimulus for IGF-1 release [159]. Hyperinsulinaemia could, therefore, specifically augment IGF-1 levels. The potential importance of such an effect is suggested by the observations that, in contrast with IGF-1, IGF-2 levels appear to be unaffected by GH levels [160] and do not predict incident cancer [156]. As IGF-1 signalling involves similar pathways to those utilized by insulin, another mechanism by which insulin could amplify IGF-1 signalling could involve insulin's ability to stimulate p21Ras farnesylation. Moreover, as with the insulin receptor, the IGF-1 receptor is frequently overexpressed in cancer cells [161,162]. In addition to amplifying any signalling by insulin via the IGF-1 receptor, overexpression of the IGF-1 receptor in cancer cells will augment the formation of insulin receptor/IGF-1 receptor hybrids. This could increase the possibility of cross-talk, whereby insulin binding to the insulin receptor moiety of the heterodimer activates the IGF-1 receptor moiety [163], and there is evidence for this in relation with hybrids with the A isoform of the insulin receptor [164].
IGFBPs (IGF-binding proteins)
Insulin could affect IGF-1 signalling further by modulating the availability of IGFBPs. Six IGFBPs have been identified and the most abundant, IGFBP-3, binds approx. 80% of circulating IGF-1 and has intrinsic anti-tumorigenic properties [165], possibly mediated by inhibition of farnesyl transferase activity [166]. There is also evidence that IGFBP-3 may promote apoptosis [167,168]; however, hepatic production of IGFBP-3 and formation of the IGF-1–IGFBP-3 complex appears to be positively controlled by GH, which, given the up-regulation of GH receptors by insulin described above, would predict higher IGFBP-3 levels in association with compensatory hyperinsulinaemia [169]. These interactions would then suggest an anti-tumorigenic action of insulin.
In contrast, hyperinsulinaemia suppresses IGFBP-1 levels [170,171] and, although IGFBP-2 is not acutely regulated by insulin, there is some evidence that chronically low levels of insulin are associated with an increase in IGFBP-2 [172]. How important these effects are with regard to the control of IGF availability might be questioned given that less than 25% of IGF appears to be bound by these proteins [173]. However, although IGFBP-3 is responsible for the bulk of IGF binding, it is the smaller, capillary permeable, rapidly turning over pool of IGFBPs, which includes IGFBP-1 and IGFBP-2, that may be important in modulating IGF bioactivity [173,174]. Suppression of the levels of these proteins by insulin might, therefore, increase the extravascular availability of bioactive IGF-1, although much more evidence is needed to clarify the complex dynamic relationships that are likely to be involved.
INSULIN-RESISTANCE-RELATED PRO-CARCINOGENIC CHARACTERISTICS
Insulin resistance and its compensatory hyperinsulinaemia have effects that can promote cancer and these effects can interact and synergize with other growth-promoting changes, as illustrated in Figure 4. Nevertheless, insulin resistance and hyperinsulinaemia may be a consequence of other processes that can themselves promote cancer by other pathways. Two key areas in this respect are increased activation of the inflammatory system and increased adiposity, both of which can induce insulin resistance and both of which may promote the development and progression of cancer independently of their effects on insulin resistance.
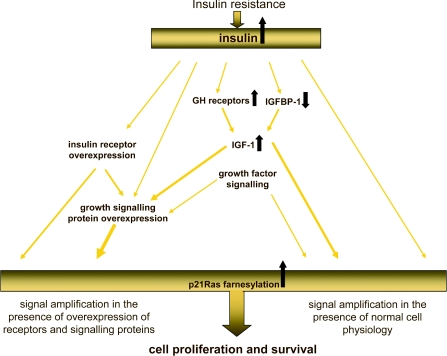
Figure 4. Interaction between insulin resistance and cell proliferation and survival.
Theoretically, interactions between the effects of insulin resistance and hyperinsulinaemia could lead to amplification of the cell proliferation and survival signals induced by insulin. This amplification will be even greater against a background of insulin or IGF-1 receptor overexpression or growth signalling protein overexpression. The ability of insulin to increase the pool of p21Ras-GTP by enhancing p21Ras farnesylation has the potential to amplify further these effects.
Activation of inflammatory mediators
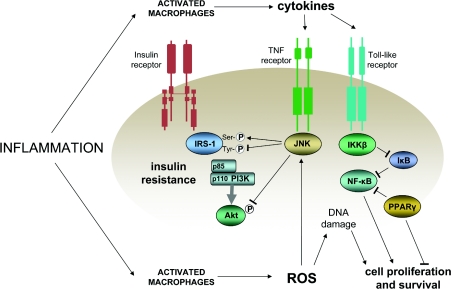
Inflammation, chronic and subclinical, has come to be recognized as a key factor in tumorigenesis and cancer progression [175–177]. One means by which activated cells of the immune system exert their protection against pathogens is by production of ROS (reactive oxygen species), and ROS can disrupt insulin signalling by activating JNK (c-Jun N-terminal kinase). JNK, in turn, increases the serine phosphorylation of IRS-1, decreases the tyrosine phosphorylation of IRS-1 and inhibits serine phosphorylation of Akt, each of which contribute to reduced insulin signalling [178]. ROS can also cause oxidative damage to DNA, a key factor in the somatic mutations that underlie tumorigenesis [179]. It should be noted that, as well as inducing insulin resistance, production of ROS can also be increased by some of the consequences of insulin resistance, including elevations in glucose levels [180] and increased β-oxidation of fatty acids [181].
Activation of inflammatory pathways can induce insulin resistance by a variety of other mechanisms [182]. For example, signalling by TNF-α (tumour necrosis factor-α) via the TNF receptor can activate JNK. Another possibility involves signalling via NF-κB (nuclear factor κB), which is promoted by an inhibitor of NF-κB inactivation, IKKβ [IκB (inhibitor of NF-κB) kinase β]. The importance of NF-κB in the induction of insulin resistance is apparent in the improvements in insulin sensitivity resulting from inhibition of IKKβ by high dose salicylate [183]. Equally, the importance of NF-κB in the promotion of carcinogenesis is apparent in the reduction in risk of cancer associated with the use of aspirin and other NSAIDs (non-steroidal anti-inflammatory agents) [184], which is mediated by the inhibition of signalling via NF-κB [185]. A further convergence between inflammation, insulin resistance and cancer is observed in relation to the nuclear receptor PPAR-γ (peroxisome-proliferator-activated receptor-γ). As a well-established mediator of insulin action, PPAR-γ can also antagonize NF-κB [186] and can inhibit pro-carcinogenic changes in human colon cancer cells [187]. Interactions between inflammation, insulin resistance and cell proliferation and survival are illustrated in Figure 5.
Figure 5. Coincident promotion of insulin resistance and cell proliferation and survival in inflammation.
Activated cells of the immune system release cytokines and ROS, which induce insulin resistance via JNK and cell proliferation and survival via NF-κB. Positive influences are shown by lines ending in arrows and inhibitory influences by lines ending in bars.
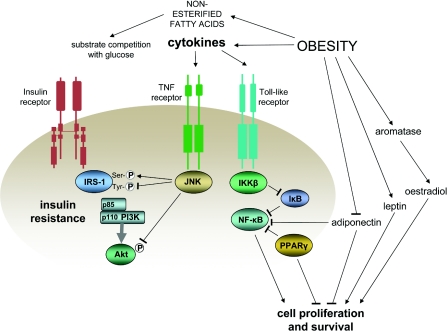
Obesity
Increased adiposity is a major determinant of insulin resistance, as well as being a risk factor for cancer. It is associated with increased NEFAs (non-esterified fatty acid levels) which, by competing with glucose as a metabolic fuel, can induce insulin resistance and can also increase fatty acid oxidation and production of ROS. Moreover, adipose tissue, with its resident macrophages, can release a variety of so-called ‘adipokines’ that include both proteins with signalling properties and cytokines. The cytokines include TNF-α and IL-6 (interleukin-6), both of which have pro-oncogenic effects and can induce insulin resistance [188]. Visceral adipose tissue appears to be a particularly rich source of adipokine cytokines [189], and insulin resistance has a stronger relationship with visceral fat than with overall adiposity. Visceral fat may particularly predispose to the development of cancer [3,190], especially in post-menopausal women [191–193].
Among the adipokine proteins with signalling properties, leptin acts as an ‘adipostat’ by suppressing centrally generated appetite signalling. Obesity is, however, associated with leptin resistance and hyperleptinaemia. Leptin concentrations are strongly correlated with insulin resistance [194], but leptin appears to have pro-oncogenic effects of its own since, in vitro, it can enhance proliferation of both normal [195] and cancer [196] cell lines and can stimulate angiogenesis [197]. Moreover, at levels observed in obesity, leptin may be able to induce the adipose tissue aromatase enzyme complex [198], which would be expected to increase concentrations of the mitogenic sex hormone oestradiol (see below).
Obesity is also associated with reduced levels of the anti-inflammatory pro-apoptotic adipokine adiponectin. Adiponectin is inversely associated with insulin resistance and this may reflect more than mere co-variation with adiposity, since adiponectin has a number of effects at the cellular level that could increase the sensitivity of glucose and lipid metabolism to insulin via its activation of the cell energy sensor AMPK and the nuclear receptor PPAR-α [199]. As mentioned previously with regard to metformin, activation of AMPK has anti-proliferative effects, and there is evidence for this in relation to the activation of AMPK signalling by adiponectin [200]. It should be noted that adiponectin can inhibit TNF-α-induced phosphorylation of IKKβ and, consequently, the activation of NF-κB [201].
Beyond the pro-inflammatory effects of increased adiposity, adipose tissue can mediate potentially pro-oncogenic transformations in gonadal or adrenal steroids: specifically, increased synthesis of oestradiol from testosterone and oestrone from Δ4-androstenedione by the aromatase enzyme complex. This increases oestradiol concentrations, and oestradiol is a potent mitogen for breast and endometrial tissue. The potential importance of these interconversions is apparent in post-menopausal women, in whom oestradiol levels are significantly correlated with BMI (body mass index), and the risk of breast cancer associated with BMI is substantially reduced by including endogenous oestrogen concentrations as a covariate [202]. Interactions between obesity, insulin resistance and cell proliferation and survival are illustrated in Figure 6.
Figure 6. Coincident promotion of insulin resistance and cell proliferation and survival in obesity.
Positive influences are shown by lines ending in arrows and inhibitory influences by lines ending in bars.
Other factors related to insulin resistance and cancer
Specifically with regard to the sex-hormone-dependent cancers, i.e. cancers of the breast, prostate and endometrium, SHBG (sex hormone-binding globulin) levels are reduced in states of insulin resistance, apparently due to a direct suppressive effect of hyperinsulinaemia on SHBG production by the liver [203]. This could act to increase the availability of free sex hormones [204] and, therefore, favour the development of sex-hormone-dependent cancers. Amplification of oestradiol levels in obesity could also result from induction of aromatase activity by the pro-inflammatory adipokines TNF-α and IL-6 [205], which, as mentioned above, will be present in adipose tissue in relatively high concentrations.
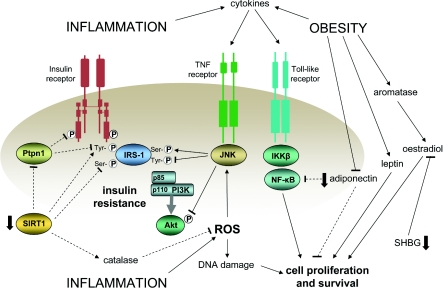
Increased inflammation and obesity and decreased SHBG provide extracellular points of focus that relate to insulin resistance and have pro-carcinogenic effects of their own. Conversely, there are several intracellular points of focus that can both mediate insulin sensitization and diminish cell proliferation and survival. PPAR-γ has already been mentioned in relation to inflammation. Another, more recently recognized point of focus is provided by the deacetylase SIRT1 (sirtuin 1). Sirtuins are key mediators in the longevity associated with calorie restriction [206], and sirtuin action can modulate the activities of several key transcription factors (reviewed in [207,208]). For example, SIRT1 can diminish inflammatory pathway signalling by deacetylating the p65 transcription factor subunit of NF-κB and can deacetylate Foxo1, thus promoting transcription of the adiponectin gene. It can also promote deacetylation of Foxo3a, leading to enhanced expression of catalase, the enzyme responsible for metabolizing H2O2 and thus reducing ROS levels. SIRT1 can also enhance post-receptor insulin signalling by increasing serine phosphorylation of Akt and tyrosine phosphorylation of the insulin receptor and IRS-1, primarily through transcriptional repression of the phosphorylase Ptpn1 (protein tyrosine phosphatase, non-receptor type 1). The actions of SIRT1 appear to depend on the nutritional context in which they are studied, but their net effects generally point to enhancement of insulin sensitivity. Their effect on cancer risk is complicated by the fact that, under normal conditions, there is a mutually reinforced inverse relationship between SIRT1 and p53 [209,210]. Given the critical role of p53 in eliminating DNA damage via apoptosis, its down-regulation would, conventionally, be regarded as oncogenic. However, SIRT1 can also inhibit cell cycle progression [211], so it may be that it acts to provide a check on both cell cycle progression and apoptosis, thus allowing for more effective DNA repair [207]. In accord with this, in vivo studies show a tumour-suppressive effect of experimentally enhanced SIRT1 levels [212].
DISCUSSION
There are plausible mechanisms by which insulin resistance and hyperinsulinaemia could promote cancer development and progression, but it remains to be established how important these are. A number of conditions that lead to insulin resistance, particularly subclinical inflammation and obesity, have effects of their own that are pro-carcinogenic. Therefore, in epidemiological studies, detailed baseline and long-term evaluation will be needed not only of insulin resistance and hyperinsulinaemia, but also subclinical inflammation and adiposity. To date, very few studies meet these requirements.
The inter-relatedness of the various factors that connect with insulin resistance and cancer risk is illustrated in Figure 7, and it should be apparent from this Figure that it may be unrealistic to consider insulin resistance as a standalone cancer risk factor. Both inflammation and obesity have effects of their own that could promote cancer, but the hyperinsulinaemia they induce may itself feedback and augment further their effects on cell proliferation and survival. One possibility for gaining a better understanding of the relative importance of the role of insulin resistance in cancer development may be to evaluate the co-associated metabolic disturbances that insulin resistance is characterized by, rather than insulin resistance itself. Conventionally, insulin resistance is quantified according to its glucoregulatory effects, but it also has effects on lipid metabolism and renal function. A measure of the insulin resistance syndrome could, therefore, provide a potentially useful index of whole-body insulin resistance. Given the close relationship between insulin resistance and hyperinsulinaemia, such a measure could also provide a better index of net insulin exposure than a single fasting insulin measurement or even insulin concentrations in response to glucose challenge. It is worth noting, as described above, that the evidence for the metabolic syndrome (an approximate index of insulin-resistance-related metabolic disturbance) as a risk factor for cancer is more consistent than for insulin alone.
Figure 7. Selected key inter-relationships in an insulin-resistant pro-carcinogenic state associated with increased inflammation, obesity and reduced levels of SIRT1.
Potentially beneficial effects, with regard to reducing both insulin resistance and cell proliferation and survival, that are diminished in this state are shown by the broken lines. Positive influences are shown by lines ending in arrows and inhibitory influences by lines ending in bars. Variables independently reduced in insulin-resistant states are shown by the solid arrows.
An important question that has yet to be resolved is, to what extent is insulin resistance a risk factor for cancer in general or for specific types of cancer? As mentioned, there is some evidence for insulin resistance being particularly related to colorectal cancer, and studies specifically concerned with colorectal cancer predominate. However, the majority of mechanisms summarized in the present review could affect the risk of any kind of cancer, with some additional mechanisms applying to sex-hormone-dependent cancers. Colorectal cancer is, nevertheless, a major cancer and insulin-resistance-related factors could provide useful points of intervention by analogy with cigarette smoking and lung cancer, and sex hormone action and breast and prostate cancer.
Recent reports of an increased incidence of cancer in users of insulin analogues [98,104,108,109] highlight the potential importance of insulin as a cancer risk factor. However, from the clinical point of view, these are still preliminary observations, with relatively short follow-up times and inconsistencies in the types of cancer for which an increased risk was apparent. Future studies will need to focus on whether these are chance findings, whether they reflect the influence of a particular at-risk subgroup and, supposing there is increased risk, whether this places incidence rates among those using long-acting insulins above those in the population in general. This latter point reflects the possibility mentioned previously that some patients with diabetes may be relatively hypoinsulinaemic and, therefore, at relatively low risk of cancer. If such patients form the comparison group in studies of long-acting insulins, an exaggerated impression of risk may be given.
In conclusion, it bears repeating that cancer is a multifactorial process. Insulin itself does not induce somatic cell mutations and cannot, therefore, be considered a carcinogen. However, pre-malignant lesions may be present in a high proportion of healthy individuals, and whether these progress to an invasive metastatic fatal cancer can depend on an extraordinarily complex interacting network of errors, imbalances and disturbances. It should be apparent from the present review that insulin resistance and its associated hyperinsulinaemia could form a significant strand in such a network.
FUNDING
The author's work is funded by the Heart Disease and Diabetes Research Trust.
References
- 1.Giovannucci E., Colditz G. A., Stampfer M. J., Willett W. C. Physical activity, obesity, and risk of colorectal adenoma in women (United States) Cancer Causes Control. 1996;7:253–263. doi: 10.1007/BF00051301. [DOI] [PubMed] [Google Scholar]
- 2.Calle E. E., Thun M. J. Obesity and cancer. Oncogene. 2004;23:6365–6378. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- 3.Schoen R. E., Tangen C. M., Kuller L. H., Burke G. L., Cushman M., Tracy R. P., Dobs A., Savage P. J. Increased blood glucose and insulin, body size, and incident colorectal cancer. J. Natl. Cancer Inst. 1999;91:1147–1154. doi: 10.1093/jnci/91.13.1147. [DOI] [PubMed] [Google Scholar]
- 4.Coughlin S. S., Calle E. E., Teras L. R., Petrelli J., Thun M. J. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am. J. Epidemiol. 2004;159:1160–1167. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 5.Rapp K., Schroeder J., Klenk J., Ulmer H., Concin H., Diem G., Oberaigner W., Weiland S. K. Fasting blood glucose and cancer risk in a cohort of more than 140,000 adults in Austria. Diabetologia. 2006;49:945–952. doi: 10.1007/s00125-006-0207-6. [DOI] [PubMed] [Google Scholar]
- 6.Trevisan M., Liu J., Muti P., Misciagna G., Menotti A., Fucci F. Markers of insulin resistance and colorectal cancer mortality. Cancer Epidemiol. Biomarkers. Prev. 2001;10:937–941. [PubMed] [Google Scholar]
- 7.Bugianesi E. Non-alcoholic steatohepatitis and cancer. Clin. Liver Dis. 2007;11:191–207. doi: 10.1016/j.cld.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Coussens L. M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenkins P. J. Acromegaly and cancer. Horm. Res. 2004;62(Suppl. 1):108–115. doi: 10.1159/000080768. [DOI] [PubMed] [Google Scholar]
- 10.Augustin L. S., Galeone C., Dal Maso L., Pelucchi C., Ramazzotti V., Jenkins D. J., Montella M., Talamini R., Negri E., Franceschi S., La Vecchia C. Glycemic index, glycemic load and risk of prostate cancer. Int. J. Cancer. 2004;112:446–450. doi: 10.1002/ijc.20416. [DOI] [PubMed] [Google Scholar]
- 11.Bruce W. R., Wolever T. M., Giacca A. Mechanisms linking diet and colorectal cancer: the possible role of insulin resistance. Nutr. Cancer. 2000;37:19–26. doi: 10.1207/S15327914NC3701_2. [DOI] [PubMed] [Google Scholar]
- 12.McKeown-Eyssen G. Epidemiology of colorectal cancer revisited: are serum triglycerides and/or plasma glucose associated with risk? Cancer Epidemiol. Biomarkers Prev. 1994;3:687–695. [PubMed] [Google Scholar]
- 13.Komninou D., Ayonote A., Richie J. P., Jr, Rigas B. Insulin resistance and its contribution to colon carcinogenesis. Exp. Biol. Med. 2003;228:396–405. doi: 10.1177/153537020322800410. [DOI] [PubMed] [Google Scholar]
- 14.Giovannucci E. Nutrition, insulin, insulin-like growth factors and cancer. Horm. Metab. Res. 2003;35:694–704. doi: 10.1055/s-2004-814147. [DOI] [PubMed] [Google Scholar]
- 15.Kaaks R. Nutrition, insulin, IGF-1 metabolism and cancer risk: a summary of epidemiological evidence. Novartis Found. Symp. 2004;262:247–260. [PubMed] [Google Scholar]
- 16.Cowey S., Hardy R. W. The metabolic syndrome: A high-risk state for cancer? Am. J. Pathol. 2006;169:1505–1522. doi: 10.2353/ajpath.2006.051090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue F., Michels K. B. Diabetes, metabolic syndrome, and breast cancer: a review of the current evidence. Am. J. Clin. Nutr. 2007;86(Suppl):823S–835S. doi: 10.1093/ajcn/86.3.823S. [DOI] [PubMed] [Google Scholar]
- 18.Hsing A. W., Sakoda L. C., Chua S. C. J. Obesity, metabolic syndrome and prostate cancer. Am. J. Clin. Nutr. 2007;86(Suppl):843S–857S. doi: 10.1093/ajcn/86.3.843S. [DOI] [PubMed] [Google Scholar]
- 19.Pisani P. Hyper-insulinaemia and cancer, meta-analyses of epidemiological studies. Arch. Physiol. Biochem. 2008;114:63–70. doi: 10.1080/13813450801954451. [DOI] [PubMed] [Google Scholar]
- 20.Renehan A. G., Roberts D. L., Dive C. Obesity and cancer: pathophysiological and biological mechanisms. Arch. Physiol. Biochem. 2008;114:71–83. doi: 10.1080/13813450801954303. [DOI] [PubMed] [Google Scholar]
- 21.Smith U., Gale E. A. Does diabetes therapy influence the risk of cancer? Diabetologia. 2009;52:1699–1708. doi: 10.1007/s00125-009-1441-5. [DOI] [PubMed] [Google Scholar]
- 22.Tran T. T., Medline A., Bruce W. R. Insulin promotion of colon tumors in rats. Cancer Epidemiol. Biomarkers Prev. 1996;5:1013–1015. [PubMed] [Google Scholar]
- 23.Corpet D. E., Jacquinet C., Peiffer G., Taché S. Insulin injections promote the growth of aberrant crypt foci in the colon of rats. Nutr. Cancer. 1997;27:316–320. doi: 10.1080/01635589709514543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinbach G., Kumar S. P., Reddy B. S., Lipkin M., Holt P. R. Effects of caloric restriction and dietary fat on epithelial cell proliferation in rat colon. Cancer Res. 1993;53:2745–2749. [PubMed] [Google Scholar]
- 25.Hursting S. D., Perkins S. N., Phang J. M., Barrett J. C. Diet and cancer prevention studies in p53-deficient mice. J. Nutr. 2001;131(Suppl):3092S–3094S. doi: 10.1093/jn/131.11.3092S. [DOI] [PubMed] [Google Scholar]
- 26.Bonorden M. J., Rogozina O. P., Kluczny C. M., Grossmann M. E., Grambsch P. L., Grande J. P., Perkins S., Lokshin A., Cleary M. P. Intermittent calorie restriction delays prostate tumor detection and increases survival time in TRAMP mice. Nutr. Cancer. 2009;61:265–275. doi: 10.1080/01635580802419798. [DOI] [PubMed] [Google Scholar]
- 27.Lasko C. M., Good C. K., Adam J., Bird R. P. Energy restriction modulates the development of advanced preneoplastic lesions depending on the level of fat in the diet. Nutr. Cancer. 1999;33:69–75. doi: 10.1080/01635589909514750. [DOI] [PubMed] [Google Scholar]
- 28.Lovejoy J. C., Windhauser M. M., Rood J. C., de la Bretonne J. A. Effect of a controlled high-fat versus low-fat diet on insulin sensitivity and leptin levels in African-American and Caucasian women. Metab. Clin. Exp. 1998;47:1520–1524. doi: 10.1016/s0026-0495(98)90080-4. [DOI] [PubMed] [Google Scholar]
- 29.Koohestani N., Chia M. C., Pham N. A., Tran T. T., Minkin S., Wolever T. M., Bruce W. R. Aberrant crypt focus promotion and glucose intolerance: correlation in the rat across diets differing in fat, n-3 fatty acids and energy. Carcinogenesis. 1998;19:1679–1684. doi: 10.1093/carcin/19.9.1679. [DOI] [PubMed] [Google Scholar]
- 30.Rao C. V., Hirose Y., Indranie C., Reddy B. S. Modulation of experimental colon tumorigenesis by types and amounts of dietary fatty acids. Cancer Res. 2001;61:1927–1933. [PubMed] [Google Scholar]
- 31.Lee W. M., Lu S., Medline A., Archer M. C. Susceptibility of lean and obese Zucker rats to tumorigenesis induced by N-methyl-N-nitrosourea. Cancer Lett. 2001;162:155–160. doi: 10.1016/s0304-3835(00)00635-2. [DOI] [PubMed] [Google Scholar]
- 32.Koohestani N., Tran T. T., Lee W., Wolever T. M., Bruce W. R. Insulin resistance and promotion of aberrant crypt foci in the colons of rats on a high-fat diet. Nutr. Cancer. 1997;29:69–76. doi: 10.1080/01635589709514604. [DOI] [PubMed] [Google Scholar]
- 33.Tran T. T., Gupta N., Goh T., Naigamwalla D., Chia M. C., Koohestani N., Mehrotra S., McKeown-Eyssen G., Giacca A., Bruce W. R. Direct measure of insulin sensitivity with the hyperinsulinemic-euglycemic clamp and surrogate measures of insulin sensitivity with the oral glucose tolerance test: correlations with aberrant crypt foci promotion in rats. Cancer Epidemiol. Biomarkers Prev. 2003;12:47–56. [PubMed] [Google Scholar]
- 34.Saydah S. H., Platz E. A., Rifai N., Pollak M. N., Brancati F. L., Helzlsouer K. J. Association of markers of insulin and glucose control with subsequent colorectal cancer risk. Cancer Epidemiol. Biomarkers Prev. 2003;12:412–418. [PubMed] [Google Scholar]
- 35.Palmqvist R., Stattin P., Rinaldi S., Biessy C., Stenling R., Riboli E., Hallmans G., Kaaks R. Plasma insulin, IGF-binding proteins-1 and -2 and risk of colorectal cancer: a prospective study in northern Sweden. Int. J. Cancer. 2003;107:89–93. doi: 10.1002/ijc.11362. [DOI] [PubMed] [Google Scholar]
- 36.Stattin P., Lukanova A., Biessy C., Söderberg S., Palmqvist R., Kaaks R., Olsson T., Jellum E. Obesity and colon cancer: does leptin provide a link? Int. J. Cancer. 2004;109:149–152. doi: 10.1002/ijc.11668. [DOI] [PubMed] [Google Scholar]
- 37.Wei E. K., Ma J., Pollak M. N., Rifai N., Fuchs C. S., Hankinson S. E., Giovannucci E. A prospective study of C-peptide, insulin-like growth factor-I, insulin-like growth factor binding protein-1, and the risk of colorectal cancer in women. Cancer Epidemiol. Biomarkers Prev. 2005;14:850–855. doi: 10.1158/1055-9965.EPI-04-0661. [DOI] [PubMed] [Google Scholar]
- 38.Stocks T., Lukanova A., Johansson M., Rinaldi S., Palmqvist R., Hallmans G., Kaaks R., P S. Components of the metabolic syndrome and colorectal cancer risk; a prospective study. Int. J. Obes. 2008;32:304–314. doi: 10.1038/sj.ijo.0803713. [DOI] [PubMed] [Google Scholar]
- 39.Limburg P. J., Stolzenberg-Solomon R. Z., Vierkant R. A., Roberts K., Sellers T. A., Taylor P. R., Sellers T. A., Taylor P. R., Virtamo J., Cerhan J. R., Albanes D. Insulin, glucose, insulin resistance, and incident colorectal cancer in male smokers. Clin. Gastroenterol. Hepatol. 2006;4:1514–1521. doi: 10.1016/j.cgh.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaaks R., Toniolo P., Akhmedkhanov A., Lukanova A., Biessy C., Dechaud H., Rinaldi S., Zeleniuch-Jacquotte A., Shore R. E., Riboli E. Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J. Natl. Cancer Inst. 2000;92:1592–1600. doi: 10.1093/jnci/92.19.1592. [DOI] [PubMed] [Google Scholar]
- 41.Ma J., Giovannucci E., Pollak M., Leavitt A., Tao Y., Gaziano J. M., Stampfer M. J. A prospective study of plasma C-peptide and colorectal cancer risk in men. J. Natl. Cancer Inst. 2004;96:546–553. doi: 10.1093/jnci/djh082. [DOI] [PubMed] [Google Scholar]
- 42.Tripkovic I., Tripkovic A., Strnad M., Capkun V., Zekan L. Role of insulin-like growth factor-1 in colon cancerogenesis: a case-control study. Arch. Med. Res. 2007;38:519–525. doi: 10.1016/j.arcmed.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 43.Otani T., Iwasaki M., Sasazuki S., Inoue M., Tsugane S. Plasma C-peptide, insulin-like growth factor-I, insulin-like growth factor binding proteins and risk of colorectal cancer in a nested case-control study: the Japan public health center-based prospective study. Int. J. Cancer. 2007;120:2007–2012. doi: 10.1002/ijc.22556. [DOI] [PubMed] [Google Scholar]
- 44.Jenab M., Riboli E., Cleveland R. J., Norat T., Rinaldi S., Nieters A., Biessy C., Tjønneland A., Olsen A., Overvad K., et al. Serum C-peptide, IGFBP-1 and IGFBP-2 and risk of colon and rectal cancers in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer. 2007;121:368–376. doi: 10.1002/ijc.22697. [DOI] [PubMed] [Google Scholar]
- 45.Schoen R. E., Weissfeld J. L., Kuller L. H., Thaete F. L., Evans R. W., Hayes R. B., Rosen C. J. Insulin-like growth factor-I and insulin are associated with the presence and advancement of adenomatous polyps. Gastroenterology. 2005;129:464–475. doi: 10.1016/j.gastro.2005.05.051. [DOI] [PubMed] [Google Scholar]
- 46.Keku T. O., Lund P. K., Galanko J., Simmons J. G., Woosley J. T., Sandler R. S. Insulin resistance, apoptosis, and colorectal adenoma risk. Cancer Epidemiol. Biomarkers Prev. 2005;14:2076–2081. doi: 10.1158/1055-9965.EPI-05-0239. [DOI] [PubMed] [Google Scholar]
- 47.Wei E. K., Ma J., Pollak M. N., Rifai N., Fuchs C. S., Hankinson S. E., Giovannucci E. C-peptide, insulin-like growth factor binding protein-1, glycosylated hemoglobin, and the risk of distal colorectal adenoma in women. Cancer Epidemiol. Biomarkers Prev. 2006;15:750–755. doi: 10.1158/1055-9965.EPI-05-0820. [DOI] [PubMed] [Google Scholar]
- 48.Jernström H., Barrett-Connor E. Obesity, weight change, fasting insulin, proinsulin, C-peptide, and insulin-like growth factor-1 levels in women with and without breast cancer: the Rancho Bernardo Study. J. Womens Health Gender-Based Med. 1999;8:1265–1272. doi: 10.1089/jwh.1.1999.8.1265. [DOI] [PubMed] [Google Scholar]
- 49.Muti P., Quattrin T., Grant B. J., Krogh V., Micheli A., Schünemann H. J., Ram M., Freudenheim J. L., Sieri S., Trevisan M., Berrino F. Fasting glucose is a risk factor for breast cancer: a prospective study. Cancer Epidemiol. Biomarkers Prev. 2002;11:1361–1368. [PubMed] [Google Scholar]
- 50.Mink P. J., Shahar E., Rosamond W. D., Alberg A. J., Folsom A. R. Serum insulin and glucose levels and breast cancer incidence: the Atherosclerosis Risk in Communities Study. Am. J. Epidemiol. 2002;156:349–352. doi: 10.1093/aje/kwf050. [DOI] [PubMed] [Google Scholar]
- 51.Eliassen A. H., Tworoger S. S., Mantzoros C. S., Pollak M. N., Hankinson S. E. Circulating insulin and c-peptide levels and risk of breast cancer among predominately premenopausal women. Cancer Epidemiol. Biomarkers Prev. 2007;16:161–164. doi: 10.1158/1055-9965.EPI-06-0693. [DOI] [PubMed] [Google Scholar]
- 52.Toniolo P., Bruning P. F., Akhmedkhanov A., Bonfrèr J. M., Koenig K. L., Lukanova A., Shore R. E., Zeleniuch-Jacquotte A. Serum insulin-like growth factor-I and breast cancer. Int. J. Cancer. 2000;88:828–832. doi: 10.1002/1097-0215(20001201)88:5<828::aid-ijc22>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 53.Keinan-Boker L., Bueno De Mesquita H. B., Kaaks R., Van Gils C. H., Van Noord P. A., Rinaldi S., Riboli E., Seidell J. C., Grobbee D. E., Peeters P. H. Circulating levels of insulin-like growth factor I, its binding proteins -1,-2, -3, C-peptide and risk of postmenopausal breast cancer. Int. J. Cancer. 2003;106:90–95. doi: 10.1002/ijc.11193. [DOI] [PubMed] [Google Scholar]
- 54.Schairer C., Hill D., Sturgeon S. R., Fears T., Pollak M., Mies C., Ziegler R. G., Hoover R. N., Sherman M. E. Serum concentrations of IGF-I, IGFBP-3 and c-peptide and risk of hyperplasia and cancer of the breast in postmenopausal women. Int. J. Cancer. 2004;108:773–779. doi: 10.1002/ijc.11624. [DOI] [PubMed] [Google Scholar]
- 55.Verheus M., Peeters P. H., Rinaldi S., Dossus L., Biessy C., Olsen A., Tjønneland A., Overvad K., Jeppesen M., Clavel-Chapelon F., et al. Serum C-peptide levels and breast cancer risk: results from the European Prospective Investigation into Cancer and Nutrition (EPIC) Int. J. Cancer. 2006;119:659–667. doi: 10.1002/ijc.21861. [DOI] [PubMed] [Google Scholar]
- 56.Falk R. T., Brinton L. A., Madigan M. P., Potischman N., Sturgeon S. R., Malone K. E., Daling J. R. Interrelationships between serum leptin, IGF-1, IGFBP3, C-peptide and prolactin and breast cancer risk in young women. Breast Cancer Res. Treat. 2006;98:157–165. doi: 10.1007/s10549-005-9144-1. [DOI] [PubMed] [Google Scholar]
- 57.Del Giudice M. E., Fantus I. G., Ezzat S., McKeown-Eyssen G., Page D., Goodwin P. J. Insulin and related factors in premenopausal breast cancer risk. Breast Cancer Res. Treat. 1998;47:111–120. doi: 10.1023/a:1005831013718. [DOI] [PubMed] [Google Scholar]
- 58.Hirose K., Toyama T., Iwata H., Takezaki T., Hamajima N., Tajima K. Insulin, insulin-like growth factor-I and breast cancer risk in Japanese women. Asian Pacific J. Cancer Prev. 2003;4:239–246. [PubMed] [Google Scholar]
- 59.Lawlor D. A., Smith G. D., Ebrahim S. Hyperinsulinaemia and increased risk of breast cancer: findings from the British Women's Heart and Health Study. Cancer Causes Control. 2004;15:267–275. doi: 10.1023/B:CACO.0000024225.14618.a8. [DOI] [PubMed] [Google Scholar]
- 60.Gunter M. J., Hoover D. R., Yu H., Wassertheil-Smoller S., Rohan T. E., Manson J. E., Li J., Ho G. Y., Xue X., Anderson G. L., et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J. Natl. Cancer Inst. 2009;101:48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kabat G. C., Kim M., Caan B. J., Chlebowski R. T., Gunter M. J., Ho G. Y., Rodriguez B. L., Shikany J. M., Strickler H. D., Vitolins M. Z., Rohan T. E. Repeated measures of serum glucose and insulin in relation to postmenopausal breast cancer. Int. J. Cancer. 2009;125:2704–2710. doi: 10.1002/ijc.24609. [DOI] [PubMed] [Google Scholar]
- 62.Bruning P. F., Bonfrèr J. M., van Noord P. A., Hart A. A., de Jong-Bakker M., Nooijen W. J. Insulin resistance and breast-cancer risk. Int. J. Cancer. 1992;21:511–516. doi: 10.1002/ijc.2910520402. [DOI] [PubMed] [Google Scholar]
- 63.Yang G., Lu G., Jin F., Dai Q., Best R., Shu X. O., Chen J. R., Pan X. Y., Shrubsole M., Zheng W. Population-based, case-control study of blood C-peptide level and breast cancer risk. Cancer Epidemiol. Biomarkers Prev. 2001;10:1207–1211. [PubMed] [Google Scholar]
- 64.Malin A., Dai Q., Yu H., Shu X. O., Jin F., Gao Y. T., Zheng W. Evaluation of the synergistic effect of insulin resistance and insulin-like growth factors on the risk of breast carcinoma. Cancer. 2004;100:694–700. doi: 10.1002/cncr.20023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weiderpass E., Brismar K., Bellocco R., Vainio H., Kaaks R. Serum levels of insulin-like growth factor-I, IGF-binding protein 1 and 3, and insulin and endometrial cancer risk. Br. J. Cancer. 2003;89:1697–1704. doi: 10.1038/sj.bjc.6601312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Troisi R., Potischman N., Hoover R. N., Siiteri P., Brinton L. A. Insulin and endometrial cancer. Am. J. Epidemiol. 1997;146:476–482. doi: 10.1093/oxfordjournals.aje.a009301. [DOI] [PubMed] [Google Scholar]
- 67.Gunter M. J., Hoover D. R., Yu H., Wassertheil-Smoller S., Manson J. E., Li J., Harris T. G., Rohan T. E., Xue X., Ho G. Y., et al. A prospective evaluation of insulin and insulin-like growth factor-I as risk factors for endometrial cancer. Cancer Epidemiol. Biomarkers Prev. 2008;17:921–929. doi: 10.1158/1055-9965.EPI-07-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lukanova A., Zeleniuch-Jacquotte A., Lundin E., Micheli A., Arslan A. A., Rinaldi S., Muti P., Lenner P., Koenig K. L., Biessy C., et al. Prediagnostic levels of C-peptide, IGF-I, IGFBP -1, -2 and -3 and risk of endometrial cancer. Int. J. Cancer. 2004;108:262–268. doi: 10.1002/ijc.11544. [DOI] [PubMed] [Google Scholar]
- 69.Cust A. E., Allen N. E., Rinaldi S., Dossus L., Friedenreich C., Olsen A., Tjønneland A., Overvad K., Clavel-Chapelon F., Boutron-Ruault M. C., et al. Serum levels of C-peptide, IGFBP-1 and IGFBP-2 and endometrial cancer risk; results from the European prospective investigation into cancer and nutrition. Int. J. Cancer. 2007;120:2656–2664. doi: 10.1002/ijc.22578. [DOI] [PubMed] [Google Scholar]
- 70.Stattin P., Söderberg S., Hallmans G., Bylund A., Kaaks R., Stenman U. H., Bergh A., Olsson T. Leptin is associated with increased prostate cancer risk: a nested case-referent study. J. Clin. Endocrinol. Metab. 2001;86:1341–1345. doi: 10.1210/jcem.86.3.7328. [DOI] [PubMed] [Google Scholar]
- 71.Hubbard J. S., Rohrmann S., Landis P. K., Metter E. J., Muller D. C., Andres R., Carter H. B., Platz E. A. Association of prostate cancer risk with insulin, glucose, and anthropometry in the Baltimore longitudinal study of aging. Urology. 2004;63:253–258. doi: 10.1016/j.urology.2003.09.060. [DOI] [PubMed] [Google Scholar]
- 72.Borugian M. J., Spinelli J. J., Sun Z., Kolonel L. N., Oakley-Girvan I., Pollak M. D., Whittemore A. S., Wu A. H., Gallagher R. P. Prediagnostic C-peptide and risk of prostate cancer. Cancer Epidemiol. Biomarkers Prev. 2007;16:2164–2165. doi: 10.1158/1055-9965.EPI-07-0495. [DOI] [PubMed] [Google Scholar]
- 73.Hsing A. W., Chua S. J., Gao Y. T., Gentzschein E., Chang L., Deng J., Stanczyk F. Z. Prostate cancer risk and serum levels of insulin and leptin: a population-based study. J. Natl. Cancer Inst. 2001;93:783–789. doi: 10.1093/jnci/93.10.783. [DOI] [PubMed] [Google Scholar]
- 74.Hammarsten J., Högstedt B. Hyperinsulinaemia: a prospective risk factor for lethal clinical prostate cancer. Eur. J. Cancer. 2005;41:2887–2895. doi: 10.1016/j.ejca.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 75.Stolzenberg-Solomon R. Z., Graubard B. I., Chari S., Limburg P., Taylor P. R., Virtamo J., Albanes D. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA, J. Am. Med. Assoc. 2005;294:2872–2878. doi: 10.1001/jama.294.22.2872. [DOI] [PubMed] [Google Scholar]
- 76.Michaud D. S., Wolpin B., Giovannucci E., Liu S., Cochrane B., Manson J. E., Pollak M. N., Ma J., Fuchs C. S. Prediagnostic plasma C-peptide and pancreatic cancer risk in men and women. Cancer Epidemiol. Biomarkers Prev. 2007;16:2101–2109. doi: 10.1158/1055-9965.EPI-07-0182. [DOI] [PubMed] [Google Scholar]
- 77.Tande A. J., Platz E. A., Folsom A. R. The metabolic syndrome is associated with reduced risk of prostate cancer. Am. J. Epidemiol. 2006;164:1094–1102. doi: 10.1093/aje/kwj320. [DOI] [PubMed] [Google Scholar]
- 78.Stocks T., Lukanova A., Rinaldi S., Biessy C., Dossus L., Lindahl B., Hallmans G., Kaaks R., Stattin P. Insulin resistance is inversely related to prostate cancer: a prospective study in Northern Sweden. Int. J. Cancer. 2007;120:2678–2686. doi: 10.1002/ijc.22587. [DOI] [PubMed] [Google Scholar]
- 79.Moore S. C., Leitzmann M. F., Weinstein S. J., Snyder K., Albanes D., Virtamo J., Graubard B. I., Mayne S. T., Yu H., Peters U., MJ. G. Insulin resistance-related gene polymorphisms and risk of prostate cancer. Cancer Epidemiol. Biomarkers Prev. 2007;16:1315–1317. doi: 10.1158/1055-9965.EPI-07-0191. [DOI] [PubMed] [Google Scholar]
- 80.Gunter M. J., Hayes R. B., Chatterjee N., Yeager M., Welch R., Schoen R. E., Yakochi L., Schatzkin A., Peters U. Insulin resistance-related genes and advanced left-sided colorectal adenoma. Cancer Epidemiol. Biomarkers Prev. 2007;16:703–708. doi: 10.1158/1055-9965.EPI-06-0849. [DOI] [PubMed] [Google Scholar]
- 81.Fall K., Stark J. R., Mucci L. A., Chan J., Stampfer M. J., Kurth T., Febbo P. G., Kantoff P., Ma J. No association between a polymorphic variant of the IRS-1 gene and prostate cancer risk. Prostate. 2008;68:1416–1420. doi: 10.1002/pros.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Colangelo L. A., Gapstur S. M., Gann P. H., Dyer A. R., Liu K. Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer Epidemiol. Biomarkers Prev. 2002;11:385–391. [PubMed] [Google Scholar]
- 83.Ahmed R. L., Schmitz K. H., Anderson K. E., Rosamond W. D., Folsom A. R. The metabolic syndrome and risk of incident colorectal cancer. Cancer. 2006;107:28–36. doi: 10.1002/cncr.21950. [DOI] [PubMed] [Google Scholar]
- 84.Bowers K., Albanes D., Limburg P., Pietinen P., Taylor P. R., Virtamo J., Stolzenberg-Solomon R. Z. A prospective study of anthropometric and clinical measurements associated with insulin resistance syndrome and colorectal cancer in male smokers. Am. J. Epidemiol. 2006;164:652–664. doi: 10.1093/aje/kwj253. [DOI] [PubMed] [Google Scholar]
- 85.Stürmer T., Buring J. E., Lee I. M., Gaziano J. M., Glynn R. J. Metabolic abnormalities and risk for colorectal cancer in the Physicians' Health Study. Cancer Epidemiol. Biomarkers Prev. 2006;15:2391–2397. doi: 10.1158/1055-9965.EPI-06-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pasanisi P., Berrino F., De Petris M., Venturelli E., Mastroianni A., Panico S. Metabolic syndrome as a prognostic factor for breast cancer recurrences. Int. J. Cancer. 2006;119:236–238. doi: 10.1002/ijc.21812. [DOI] [PubMed] [Google Scholar]
- 87.Lund Håheim L., Wisløff T. F., Holme I., Nafstad P. Metabolic syndrome predicts prostate cancer in a cohort of middle-aged Norwegian men followed for 27 years. Am. J. Epidemiol. 2006;164:769–774. doi: 10.1093/aje/kwj284. [DOI] [PubMed] [Google Scholar]
- 88.Yancik R., Wesley M. N., Ries L. A., Havlik R. J., Edwards B. K., Yates J. W. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA, J. Am. Med. Assoc. 2001;285:885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 89.Calle E. E., Rodriguez C., Walker-Thurmond K., Thun M. J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 90.Hammarsten J., Högstedt B. Clinical, haemodynamic, anthropometric, metabolic and insulin profile of men with high-stage and high-grade clinical prostate cancer. Blood Press. 2004;13:47–55. doi: 10.1080/08037050310025735. [DOI] [PubMed] [Google Scholar]
- 91.Wright M. E., Chang S. C., Schatzkin A., Albanes D., Kipnis V., Mouw T., Hurwitz P., Hollenbeck A., Leitzmann M. F. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer. 2007;109:675–684. doi: 10.1002/cncr.22443. [DOI] [PubMed] [Google Scholar]
- 92.Ma J., Li H., Giovannucci E., Mucci L., Qiu W., Nguyen P. L., Gaziano J. M., Pollak M., Stampfer M. J. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol. 2008;9:1039–1047. doi: 10.1016/S1470-2045(08)70235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vigneri P., Frasca F., Sciacca L., Pandini G., Vigneri R. Diabetes and cancer. Endocr. Relat. Cancer. 2009 doi: 10.1677/ERC-09-0087. doi: 10.1677/ERC-09-0087. [DOI] [PubMed] [Google Scholar]
- 94.Schimmack G., Defronzo R. A., Musi N. AMP-activated protein kinase: Role in metabolism and therapeutic implications. Diabetes Obes. Metab. 2006;8:591–602. doi: 10.1111/j.1463-1326.2005.00561.x. [DOI] [PubMed] [Google Scholar]
- 95.Xiang X., Saha A. K., Wen R., Ruderman N. B., Luo Z. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem. Biophys. Res. Commun. 2004;321:161–167. doi: 10.1016/j.bbrc.2004.06.133. [DOI] [PubMed] [Google Scholar]
- 96.Evans J. M., Donnelly L. A., Emslie-Smith A. M., Alessi D. R., Morris A. D. Metformin and reduced risk of cancer in diabetic patients. Br. Med. J. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bowker S. L., Majumdar S. R., Veugelers P., Johnson J. A. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–258. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 98.Currie C. J., Poole C. D., Gale E. A. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 99.Donadon V., Balbi M., Ghersetti M., Grazioli S., Perciaccante A., Della Valentina G., Gardenal R., Dal Mas M., Casarin P., Zanette G., Miranda C. Antidiabetic therapy and increased risk of hepatocellular carcinoma in chronic liver disease. World J. Gastroenterol. 2009;15:2506–2511. doi: 10.3748/wjg.15.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ben Sahra I., Laurent K., Loubat A., Giorgetti-Peraldi S., Colosetti P., Auberger P., Tanti J. F., Le MarchandBrustel Y., Bost F. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576–3586. doi: 10.1038/sj.onc.1211024. [DOI] [PubMed] [Google Scholar]
- 101.Vazquez-Martin A., Oliveras-Ferraros C., Menendez J. A. The antidiabetic drug metformin suppresses HER2 (erbB-2) oncoprotein overexpression via inhibition of the mTOR effector p70S6K1 in human breast carcinoma cells. Cell Cycle. 2009;8:88–96. doi: 10.4161/cc.8.1.7499. [DOI] [PubMed] [Google Scholar]
- 102.Yang Y. X., Hennessy S., Lewis J. D. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology. 2004;127:1044–1050. doi: 10.1053/j.gastro.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 103.Chung Y. W., Han D. S., Park K. H., Eun C. S., Yoo K. S., Park C. K. Insulin therapy and colorectal adenoma risk among patients with Type 2 diabetes mellitus: a case-control study in Korea. Dis. Colon Rectum. 2008;51:593–597. doi: 10.1007/s10350-007-9184-1. [DOI] [PubMed] [Google Scholar]
- 104.Hemkens L. G., Grouven U., Bender R., Günster C., Gutschmidt S., Selke G. W., Sawicki P. T. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia. 2009;52:1732–1744. doi: 10.1007/s00125-009-1418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jørgensen L. N., Dideriksen L. H., Drejer K. Carcinogenic effect of the human insulin analogue B10 Asp in female rats. Diabetologia. 1992;35(Suppl 1):A3. [Google Scholar]
- 106.Milazzo G., Sciacca L., Papa V., Goldfine I. D., Vigneri R. ASPB10 insulin induction of increased mitogenic responses and phenotypic changes in human breast epithelial cells: evidence for enhanced interactions with the insulin-like growth factor-I receptor. Mol. Carcinog. 1997;18:19–25. doi: 10.1002/(sici)1098-2744(199701)18:1<19::aid-mc3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 107.Weinstein D., Simon M., Yehezkel E., Laron Z., Werner H. Insulin analogues display IGF-I-like mitogenic and anti-apoptotic activities in cultured cancer cells. Diabetes Metab. Res. Rev. 2009;25:41–49. doi: 10.1002/dmrr.912. [DOI] [PubMed] [Google Scholar]
- 108.Jonasson J. M., Ljung R., Talbäck M., Haglund B., Gudbjörnsdòttir S., Steineck G. Insulin glargine use and short-term incidence of malignancies-a population-based follow-up study in Sweden. Diabetologia. 2009;52:1745–1754. doi: 10.1007/s00125-009-1444-2. [DOI] [PubMed] [Google Scholar]
- 109.Colhoun H. M. (SDRN, Epidemiology and Group). Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia. 2009;52:1755–1765. doi: 10.1007/s00125-009-1453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hansen B. F. Insulin analogues with increased mitogenic potency–are they safe? Horm. Metab. Res. 2008;40:431–433. doi: 10.1055/s-2008-1062740. [DOI] [PubMed] [Google Scholar]
- 111.Sandow J. Growth effects of insulin and insulin analogues. Arch. Physiol. Biochem. 2009;115:72–85. doi: 10.1080/13813450902835690. [DOI] [PubMed] [Google Scholar]
- 112.Monami M., Lamanna C., Pala L., Bardini G., Cresci B., Francesconi P., Balzi D., Marchionni N., Rotella C. M., Mannucci E. Treatment with insulin secretagogues and cancer-related mortality in type 2 diabetic patients a retrospective cohort study. Exp. Clin. Endocrinol. Diabetes. 2008;116:184–189. doi: 10.1055/s-2007-992157. [DOI] [PubMed] [Google Scholar]
- 113.Stout R. W., Vallence-Owen J. Insulin and atheroma. Lancet. 1969;i:1078–1080. doi: 10.1016/s0140-6736(69)91711-5. [DOI] [PubMed] [Google Scholar]
- 114.Heuson J. C., Coune A., Heimann R. Cell proliferation induced by insulin in organ culture of rat mammary carcinoma. Exp. Cell Res. 1967;45:351–360. doi: 10.1016/0014-4827(67)90185-1. [DOI] [PubMed] [Google Scholar]
- 115.Heuson J. C., Legros N. Effect of insulin and of alloxan diabetes on growth of the rat mammary carcinoma in vivo. Eur. J. Cancer. 1970;6:349–351. doi: 10.1016/0014-2964(70)90100-3. [DOI] [PubMed] [Google Scholar]
- 116.Lawlor M. A., Alessi D. R. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J. Cell Sci. 2001;114:2903–2910. doi: 10.1242/jcs.114.16.2903. [DOI] [PubMed] [Google Scholar]
- 117.Weijzen S., Velders M. P., Kast W. M. Modulation of the immune response and tumor growth by activated Ras. Leukemia. 1999;13:502–513. doi: 10.1038/sj.leu.2401367. [DOI] [PubMed] [Google Scholar]
- 118.Belfiore A., Frittitta L., Costantino A., Frasca F., Pandini G., Sciacca L., Goldfine I. D., Vigneri R. Insulin receptors in breast cancer. Ann. N.Y. Acad. Sci. 1996;784:173–188. doi: 10.1111/j.1749-6632.1996.tb16235.x. [DOI] [PubMed] [Google Scholar]
- 119.King G. L., Kahn C. R., Rechler M. M., Nissley S. P. Direct demonstration of separate receptors for growth and metabolic activities of insulin and multiplication-stimulating activity (an insulinlike growth factor) using antibodies to the insulin receptor. J. Clin. Invest. 1980;66:130–140. doi: 10.1172/JCI109826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gliozzo B., Sung C. K., Scalia P., Papa V., Frasca F., Sciacca L., Giorgino F., Milazzo G., Goldfine I. D., Vigneri R., Pezzino V. Insulin-stimulated cell growth in insulin receptor substrate-1-deficient ZR-75–71 cells is mediated by a phosphatidylinositol-3-kinaseindependent pathway. J. Cell. Biochem. 1998;70:268–280. doi: 10.1002/(sici)1097-4644(19980801)70:2<268::aid-jcb12>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 121.Goalstone M. L., Draznin B. Effect of insulin on farnesyltransferase activity in 3T3-L1 adipocytes. J. Biol. Chem. 1996;271:27585–27589. doi: 10.1074/jbc.271.44.27585. [DOI] [PubMed] [Google Scholar]
- 122.Goalstone M., Leitner J. W., Draznin B. GTP loading of farnesylated p21Ras by insulin at the plasma membrane. Biochem. Biophys. Res. Commun. 1997;239:42–45. doi: 10.1006/bbrc.1997.7413. [DOI] [PubMed] [Google Scholar]
- 123.Goalstone M. L., Leitner J. W., Berhanu P., Sharma P. M., Olefsky J. M., Draznin B. Insulin signals to prenyltransferases via the Shc branch of intracellular signaling. J. Biol. Chem. 2001;276:12805–12812. doi: 10.1074/jbc.M009443200. [DOI] [PubMed] [Google Scholar]
- 124.Kao A. W., Waters S. B., Okada S., Pessin J. E. Insulin stimulates the phosphorylation of the 66- and 52-kilodalton Shc isoforms by distinct pathways. Endocrinology. 1997;138:2474–2480. doi: 10.1210/endo.138.6.5203. [DOI] [PubMed] [Google Scholar]
- 125.Migliaccio E., Mele S., Salcini A. E., Pelicci G., Lai K. M., Superti-Furga G., Pawson T., Di Fiore P. P., Lanfrancone L., Pelicci P. G. Opposite effects of the p52shc/p46shc and p66shc splicing isoforms on the EGF receptor-MAP kinase-fos signalling pathway. EMBO J. 1997;16:706–716. doi: 10.1093/emboj/16.4.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Goalstone M. L., Leitner J. W., Wall K., Dolgonos L., Rother K. I., Accili D., Draznin B. Effect of insulin on farnesyltransferase. Specificity of insulin action and potentiation of nuclear effects of insulin-like growth factor-1, epidermal growth factor, and platelet-derived growth factor. J. Biol. Chem. 1998;273:23892–23896. doi: 10.1074/jbc.273.37.23892. [DOI] [PubMed] [Google Scholar]
- 127.Leitner J. W., Kline T., Carel K., Goalstone M., Draznin B. Hyperinsulinemia potentiates activation of p21Ras by growth factors. Endocrinology. 1997;138:2211–2214. doi: 10.1210/endo.138.5.5240. [DOI] [PubMed] [Google Scholar]
- 128.Goalstone M. L., Wall K., Leitner J. W., Kurowski T., Ruderman N., Pan S. J., Ivy J. L., Moller D. E., Draznin B. Increased amounts of farnesylated p21Ras in tissues of hyperinsulinaemic animals. Diabetologia. 1999;42:310–316. doi: 10.1007/s001250051156. [DOI] [PubMed] [Google Scholar]
- 129.Goalstone M. L., Draznin B. What does insulin do to Ras? Cell. Signalling. 1998;10:297–301. doi: 10.1016/s0898-6568(97)00132-0. [DOI] [PubMed] [Google Scholar]
- 130.Goalstone M. L., Natarajan R., Standley P. R., Walsh M. F., Leitner J. W., Carel K., Scott S., Nadler J., Sowers J. R., Draznin B. Insulin potentiates platelet-derived growth factor action in vascular smooth muscle cells. Endocrinology. 1998;139:4067–4067. doi: 10.1210/endo.139.10.6270. [DOI] [PubMed] [Google Scholar]
- 131.Giorgino F., Belfiore A., Milazzo G., Costantino A., Maddux B., Whittaker J., Goldfine I. D., Vigneri R. Overexpression of insulin receptors in fibroblast and ovary cells induces a ligand-mediated transformed phenotype. Mol. Endocrinol. 1991;5:452–459. doi: 10.1210/mend-5-3-452. [DOI] [PubMed] [Google Scholar]
- 132.Frittitta L., Vigneri R., Stampfer M. R., Goldfine I. D. Insulin receptor overexpression in 184B5 human mammary epithelial cells induces a ligand-dependent transformed phenotype. J. Cell. Biochem. 1995;57:666–669. doi: 10.1002/jcb.240570411. [DOI] [PubMed] [Google Scholar]
- 133.Papa V., Pezzino V., Costantino A., Belfiore A., Giuffrida D., Frittitta L., Vannelli G. B., Brand R., Goldfine I. D., Vigneri R. Elevated insulin receptor content in human breast cancer. J. Clin. Invest. 1990;86:1503–1510. doi: 10.1172/JCI114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Milazzo G., Giorgino F., Damante G., Sung C., Stampfer M. R., Vigneri R., Goldfine I. D., Belfiore A. Insulin receptor expression and function in human breast cancer cell lines. Cancer Res. 1992;52:3924–3930. [PubMed] [Google Scholar]
- 135.Finlayson C. A., Chappell J., Leitner J. W., Goalstone M. L., Garrity M., Nawaz S., Ciaraldi T. P., Draznin B. Enhanced insulin signaling via Shc in human breast cancer. Metab. Clin. Exp. 2003;52:1606–1611. doi: 10.1016/s0026-0495(03)00311-1. [DOI] [PubMed] [Google Scholar]
- 136.Frittitta L., Vigneri R., Papa V., Goldfine I. D., Grasso G., Trischitta V. Structural and functional studies of insulin receptors in human breast cancer. Breast Cancer Res. Treat. 1993;25:73–82. doi: 10.1007/BF00662403. [DOI] [PubMed] [Google Scholar]
- 137.Frasca F., Pandini G., Scalia P., Sciacca L., Mineo R., Costantino A., Goldfine I. D., Belfiore A., Vigneri R. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol. Cell. Biol. 1999;19:3278–3288. doi: 10.1128/mcb.19.5.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mountjoy K. G., Finlay G. J., Holdaway I. M. Abnormal insulin-receptor down regulation and dissociation of down regulation from insulin biological action in cultured human tumor cells. Cancer Res. 1987;47:6500–6504. [PubMed] [Google Scholar]
- 139.Jhun B. H., Meinkoth J. L., Leitner J. W., Draznin B., Olefsky J. M. Insulin and insulin-like growth factor-I signal transduction requires p21ras. J. Biol. Chem. 1994;269:5699–5704. [PubMed] [Google Scholar]
- 140.Draznin B., Chang L., Leitner J. W., Takata Y., Olefsky J. M. Insulin activates p21Ras and guanine nucleotide releasing factor in cells expressing wild type and mutant insulin receptors. J. Biol. Chem. 1993;25:19998–20001. [PubMed] [Google Scholar]
- 141.Burgering B. M., Snijders A. J., Maassen J. A., van der Eb A. J., Bos J. L. Possible involvement of normal p21 H-ras in the insulin/insulinlike growth factor 1 signal transduction pathway. Mol. Cell. Biol. 1989;9:4312–4322. doi: 10.1128/mcb.9.10.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yu H., Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J. Natl. Cancer Inst. 2000;92:1472–1489. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 143.Wu Y., Yakar S., Zhao L., Hennighausen L., LeRoith D. Circulating insulin-like growth factor-I levels regulate colon cancer growth and metastasis. Cancer Res. 2002;62:1030–1035. [PubMed] [Google Scholar]
- 144.Bhalla V., Joshi K., Vohra H., Singh G., Ganguly N. K. Effect of growth factors on proliferation of normal, borderline, and malignant breast epithelial cells. Exp. Mol. Pathol. 2000;68:124–132. doi: 10.1006/exmp.1999.2294. [DOI] [PubMed] [Google Scholar]
- 145.Hadsell D. L., Murphy K. L., Bonnette S. G., Reece N., Laucirica R., Rosen J. M. Cooperative interaction between mutant p53 and des(1–3)IGF-I accelerates mammary tumorigenesis. Oncogene. 2000;19:889–898. doi: 10.1038/sj.onc.1203386. [DOI] [PubMed] [Google Scholar]
- 146.Wu Y., Cui K., Miyoshi K., Hennighausen L., Green J. E., Setser J., LeRoith D., Yakar S. Reduced circulating insulin-like growth factor I levels delay the onset of chemically and genetically induced mammary tumors. Cancer Res. 2003;63:4384–4388. [PubMed] [Google Scholar]
- 147.Kari F. W., Dunn S. E., French J. E., Barrett J. C. Roles for insulin-like growth factor-1 in mediating the anti-carcinogenic effects of caloric restriction. J. Nutr. Health Aging. 1999;3:92–101. [PubMed] [Google Scholar]
- 148.Oh J. S., Kucab J. E., Bushel P. R., Martin K., Bennett L., Collins J., DiAugustine R. P., Barrett J. C., Afshari C. A., Dunn S. E. Insulin-like growth factor-1 inscribes a gene expression profile for angiogenic factors and cancer progression in breast epithelial cells. Neoplasia. 2002;4:204–217. doi: 10.1038/sj.neo.7900229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ibrahim Y. H., Yee D. Insulin-like growth factor-I and cancer risk. Growth Horm. IGF Res. 2004;14:261–269. doi: 10.1016/j.ghir.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 150.Kato S., Masuhiro Y., Watanabe M., Kobayashi Y., Takeyama K. I., Endoh H., Yanagisawa J. Molecular mechanism of a cross-talk between oestrogen and growth factor signalling pathways. Genes Cells. 2000;5:593–601. doi: 10.1046/j.1365-2443.2000.00354.x. [DOI] [PubMed] [Google Scholar]
- 151.Bailyes E. M., Navé B. T., Soos M. A., Orr S. R., Hayward A. C., Siddle K. Insulin receptor/IGF-I receptor hybrids are widely distributed in mammalian tissues: quantification of individual receptor species by selective immunoprecipitation and immunoblotting. Biochem. J. 1997;327:209–215. doi: 10.1042/bj3270209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Flier J. S., Usher P., Moses A. C. Monoclonal antibody to the type I insulin-like growth factor (IGF-I) receptor blocks IGF-I receptor-mediated DNA synthesis: clarification of the mitogenic mechanisms of IGF-I and insulin in human skin fibroblasts. Proc. Natl. Acad. Sci. U.S.A. 1986;83:664–668. doi: 10.1073/pnas.83.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wolk A., Mantzoros C. S., Andersson S. O., Bergstrom R., Signorello L. B., Lagiou P., Adami H. O., Trichopoulos D. Insulin-like growth factor 1 and prostate cancer risk: a population- based, case-control study. J. Natl. Cancer Inst. 1998;90:911–915. doi: 10.1093/jnci/90.12.911. [DOI] [PubMed] [Google Scholar]
- 154.Chan J. M., Stampfer M. J., Giovannucci E., Gann P. H., Ma J., Wilkinson P., Hennekens C. H., Pollak M. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 155.Hankinson S. E., Willett W. C., Colditz G. A., Hunter D. J., Michaud D. S., Deroo B., Rosner B., Speizer F. E., Pollak M. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351:1393–1396. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 156.Ma J., Pollak M. N., Giovannucci E., Chan J. M., Tao Y., Hennekens C. H., Stampfer M. J. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J. Natl. Cancer Inst. 1999;91:620–625. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- 157.Yu H., Spitz M. R., Mistry J., Gu J., Hong W. K., Wu X. Plasma levels of insulin-like growth factor-I and lung cancer risk: a case-control analysis. J. Natl. Cancer Inst. 1999;91:151–156. doi: 10.1093/jnci/91.2.151. [DOI] [PubMed] [Google Scholar]
- 158.Leung K. C., Doyle N., Ballesteros M., Waters M. J., Ho K. K. Insulin regulation of human hepatic growth hormone receptors: divergent effects on biosynthesis and surface translocation. J. Clin. Endocrinol. Metab. 2000;85:4712–4720. doi: 10.1210/jcem.85.12.7017. [DOI] [PubMed] [Google Scholar]
- 159.Roberts C. T. J., Brown A. L., Graham D. E., Seelig S., Berry S., Gabbay K. H., Rechler M. M. Growth hormone regulates the abundance of insulin-like growth factor I RNA in adult rat liver. J. Biol. Chem. 1986;261:10025–10028. [PubMed] [Google Scholar]
- 160.Zapf J., Walter H., Froesch E. R. Radioimmunological determination of insulinlike growth factors I and II in normal subjects and in patients with growth disorders and extrapancreatic tumor hypoglycemia. J. Clin. Invest. 1981;68:1321–1330. doi: 10.1172/JCI110379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pandini G., Vigneri R., Costantino A., Frasca F., Ippolito A., Fujita-Yamaguchi Y., Siddle K., Goldfine I. D., Belfiore A. Insulin and insulin-like growth factor-I (IGF-I) receptor overexpression in breast cancers leads to insulin/IGF-I hybrid receptor overexpression: evidence for a second mechanism of IGF-I signaling. Clin. Cancer Res. 1999;5:1935–1944. [PubMed] [Google Scholar]
- 162.Freier S., Weiss O., Eran M., Flyvbjerg A., Dahan R., Nephesh I., Safra T., Shiloni E., Raz I. Expression of the insulin-like growth factors and their receptors in adenocarcinoma of the colon. Gut. 1999;44:704–708. doi: 10.1136/gut.44.5.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Frasca F., Pandini G., Sciacca L., Pezzino V., Squatrito S., Belfiore A., Vigneri R. The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch. Physiol. Biochem. 2008;114:23–37. doi: 10.1080/13813450801969715. [DOI] [PubMed] [Google Scholar]
- 164.Pandini G., Frasca F., Mineo R., Sciacca L., Vigneri R., Belfiore A. Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J. Biol. Chem. 2002;277:39684–39695. doi: 10.1074/jbc.M202766200. [DOI] [PubMed] [Google Scholar]
- 165.Oh Y., Müller H. L., Lamson G., Rosenfeld R. G. Insulin-like growth factor (IGF)-independent action of IGF-binding protein-3 in Hs578T human breast cancer cells. Cell surface binding and growth inhibition. J. Biol. Chem. 1993;268:14964–14971. [PubMed] [Google Scholar]
- 166.Oh S. H., Kim W. Y., Kim J. H., Younes M. N., El-Naggar A. K., Myers J. N., Kies M., Cohen P., Khuri F., Hong W. K., Lee H. Y. Identification of insulin-like growth factor binding protein-3 as a farnesyl transferase inhibitor SCH66336-induced negative regulator of angiogenesis in head and neck squamous cell carcinoma. Clin. Cancer Res. 2006;15:653–661. doi: 10.1158/1078-0432.CCR-05-1725. [DOI] [PubMed] [Google Scholar]
- 167.Rajah R., Valentinis B., Cohen P. Insulin-like growth factor (IGF)-binding protein-3 induces apoptosis and mediates the effects of transforming growth factor-1 on programmed cell death through a p53- and IGFindependent mechanism. J. Biol. Chem. 1997;272:12181–12188. doi: 10.1074/jbc.272.18.12181. [DOI] [PubMed] [Google Scholar]
- 168.Williams A. C., Collard T. J., Perks C. M., Newcomb P., Moorghen M., Holly J. M., Paraskeva C. Increased p53-dependent apoptosis by the insulin-like growth factor binding protein IGFBP-3 in human colonic adenoma-derived cells. Cancer Res. 2000;60:22–27. [PubMed] [Google Scholar]
- 169.Villafuerte B. C., Zhang W. N., Phillips L. S. Insulin and insulin-like growth factor-I regulate hepatic insulin-like growth factor binding protein-3 by different mechanisms. Mol. Endocrinol. 1996;10:622–630. doi: 10.1210/mend.10.6.8776722. [DOI] [PubMed] [Google Scholar]
- 170.Snyder D. K., Clemmons D. R. Insulin-dependent regulation of insulin-like growth factor-binding protein-1. J. Clin. Endocrinol. Metab. 1990;71:1632–1636. doi: 10.1210/jcem-71-6-1632. [DOI] [PubMed] [Google Scholar]
- 171.Powell D. R., Suwanichkul A., Cubbage M. L., DePaolis L. A., Snuggs M. B., Lee P. D. Insulin inhibits transcription of the human gene for insulin-like growth factor-binding protein-1. J. Biol. Chem. 1991;266:18868–18876. [PubMed] [Google Scholar]
- 172.Clemmons D. R., Snyder D. K., Busby W. H. J. Variables controlling the secretion of insulin-like growth factor binding protein-2 in normal human subjects. J. Clin. Endocrinol. Metab. 1991;73:727–733. doi: 10.1210/jcem-73-4-727. [DOI] [PubMed] [Google Scholar]
- 173.Rajaram S., Baylink D. J., Mohan S. Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr. Rev. 1997;18:801–831. doi: 10.1210/edrv.18.6.0321. [DOI] [PubMed] [Google Scholar]
- 174.Baxter R. C. Insulin-like growth factor binding proteins in the human circulation: a review. Horm. Res. 1994;42:140–144. doi: 10.1159/000184186. [DOI] [PubMed] [Google Scholar]
- 175.Hussain S. P., Harris C. C. Inflammation and cancer: an ancient link with novel potentials. Int. J. Cancer. 2007;121:2373–2380. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- 176.Federico A., Morgillo F., Tuccillo C., Ciardiello F., Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer. 2007;121:2381–2386. doi: 10.1002/ijc.23192. [DOI] [PubMed] [Google Scholar]
- 177.Mantovani A., Allavena P., Seca A., Balkwill F. Cancer-related inflamation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 178.Nishikawa T., Araki E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid. Redox Signaling. 2007;9:343–353. doi: 10.1089/ars.2006.1458. [DOI] [PubMed] [Google Scholar]
- 179.Martien S., Abbadie C. Acquisition of oxidative DNA damage during senescence: the first step toward carcinogenesis? Ann. N.Y. Acad. Sci. 2007;1119:51–63. doi: 10.1196/annals.1404.010. [DOI] [PubMed] [Google Scholar]
- 180.King G. L., Loeken M. R. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem. Cell. Biol. 2004;122:333–338. doi: 10.1007/s00418-004-0678-9. [DOI] [PubMed] [Google Scholar]
- 181.Nakamura S., Takamura T., Matsuzawa-Nagata N., Takayama H., Misu H., Noda H., Nabemoto S., Kurita S., Ota T., Ando H., et al. Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J. Biol. Chem. 2009;284:14809–14818. doi: 10.1074/jbc.M901488200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shoelson S. E., Lee J., Goldfine A. B. Inflammation and insulin resistance. J. Clin. Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yuan M., Konstantopoulos N., Lee J., Hansen L., Li Z. W., Karin M., Shoelson S. E. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkβ. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 184.Cuzick J., Otto F., Baron J. A., Brown P. H., Burn J., Greenwald P., Jankowski J., La Vecchia C., Meyskens F., Senn H. J., M T. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10:501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 185.Tegeder I., Pfeilschifter J., Geisslinger G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J. 2001;15:2057–2072. doi: 10.1096/fj.01-0390rev. [DOI] [PubMed] [Google Scholar]
- 186.Ricote M., Li A. C., Willson T. M., Kelly C. J., Glass C. K. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 187.Kitamura S., Miyazaki Y., Shinomura Y., Kondo S., Kanayama S., Matsuzawa Y. Peroxisome proliferator-activated receptor γ induces growth arrest and differentiation markers of human colon cancer cells. Jpn J. Cancer Res. 1999;90:75–80. doi: 10.1111/j.1349-7006.1999.tb00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Housa D., Housová J., Vernerová Z., Haluzík M. Adipocytokines and cancer. Physiol. Res. 2006;55:233–244. doi: 10.33549/physiolres.930848. [DOI] [PubMed] [Google Scholar]
- 189.Hotamisligil G. S., Arner P., Caro J. F., Atkinson R. L., Spiegelman B. M. Increased adipose tissue expression of tumour necrosis factor-α in human obesity and insulin resistance. J. Clin. Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xu W. H., Matthews C. E., Xiang Y. B., Zheng W., Ruan Z. X., Cheng J. R., Gao Y. T., Shu X. O. Effect of adiposity and fat distribution on endometrial cancer risk in Shanghai women. Am. J. Epidemiol. 2005;161:939–947. doi: 10.1093/aje/kwi127. [DOI] [PubMed] [Google Scholar]
- 191.Folsom A. R., Kaye S. A., Prineas R. J., Potter J. D., Gapstur S. M., Wallace R. B. Increased incidence of carcinoma of the breast associated with abdominal adiposity in postmenopausal women. Am. J. Epidemiol. 1990;131:794–803. doi: 10.1093/oxfordjournals.aje.a115570. [DOI] [PubMed] [Google Scholar]
- 192.Kaaks R., Van Noord P. A., Den Tonkelaar I., Peeters P. H., Riboli E., Grobbee D. E. Breast-cancer incidence in relation to height, weight and body-fat distribution in the Dutch “DOM” cohort. Int. J. Cancer. 1998;76:647–651. doi: 10.1002/(sici)1097-0215(19980529)76:5<647::aid-ijc6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 193.Huang Z., Willett W. C., Colditz G. A., Hunter D. J., Manson J. E., Rosner B., Speizer F. E., SE H. Waist circumference, waist:hip ratio, and risk of breast cancer in the Nurses' Health Study. Am. J. Epidemiol. 1999;150:1316–1324. doi: 10.1093/oxfordjournals.aje.a009963. [DOI] [PubMed] [Google Scholar]
- 194.Leyva F., Godsland I. F., Ghatei M., Proudler A. J., Aldis S., Walton C., Bloom S., Stevenson J. C. Hyperleptinaemia as a component of a metabolic syndrome of cardiovascular risk. Arterioscler. Thromb. Vasc. Biol. 1998;18:928–933. doi: 10.1161/01.atv.18.6.928. [DOI] [PubMed] [Google Scholar]
- 195.Sáinz N., Rodríguez A., Catalán V., Becerril S., Ramírez B., Gómez-Ambrosi J., Frühbeck G. Leptin administration favors muscle mass accretion by decreasing FoxO3a and increasing PGC-1α in ob/ob mice. PLoS One. 2009;4:e6808. doi: 10.1371/journal.pone.0006808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ray A., Nkhata K. J., Cleary M. P. Effects of leptin on human breast cancer cell lines in relationship to estrogen receptor and HER2 status. Int. J. Oncol. 2007;30:1499–1509. [PubMed] [Google Scholar]
- 197.Somasundar P., McFadden D. W., Hileman S. M., Vona-Davis L. Leptin is a growth factor in cancer. J. Surg. Res. 2004;116:337–349. doi: 10.1016/j.jss.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 198.Magoffin D. A., Weitsman S. R., Aagarwal S. K., Jakimiuk A. J. Leptin regulation of aromatase activity in adipose stromal cells from regularly cycling women. Ginekol. Pol. 1999;70:1–7. [PubMed] [Google Scholar]
- 199.Kadowaki T., Yamauchi T., Kubota N., Hara K., Ueki K., Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sugiyama M., Takahashi H., Hosono K., Endo H., Kato S., Yoneda K., Nozaki Y., Fujita K., Yoneda M., Wada K., et al. Adiponectin inhibits colorectal cancer cell growth through the AMPK/mTOR pathway. Int. J. Oncol. 2009;34:339–344. [PubMed] [Google Scholar]
- 201.Ouchi N., Kihara S., Arita Y., Okamoto Y., Maeda K., Kuriyama H., Hotta K., Nishida M., Takahashi M., Muraguchi M., et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-κB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 202.Key T. J., Appleby P. N., Reeves G. K., Roddam A., Dorgan J. F., Longcope C., Stanczyk F. Z., Stephenson H. E. J., Falk R. T., Miller R., et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J. Natl. Cancer Inst. 2003;95:1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 203.Plymate S. R., Matej L. A., Jones R. E., Friedl K. E. Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J. Clin. Endocrinol. Metab. 1988;67:460–464. doi: 10.1210/jcem-67-3-460. [DOI] [PubMed] [Google Scholar]
- 204.Pugeat M., Crave J. C., Elmidani M., Nicolas M. H., Garoscio-Cholet M., Lejeune H., Déchaud H., Tourniaire J. Pathophysiology of sex hormone binding globulin (SHBG): relation to insulin. J. Steroid Biochem. Mol. Biol. 1991;40:841–849. doi: 10.1016/0960-0760(91)90310-2. [DOI] [PubMed] [Google Scholar]
- 205.Reed M. J., Purohit A. Breast cancer and the role of cytokines in regulating estrogen synthesis: an emerging hypothesis. Endocr. Rev. 1997;18:701–715. doi: 10.1210/edrv.18.5.0314. [DOI] [PubMed] [Google Scholar]
- 206.Wood J. G., Rogina B., Lavu S., Howitz K., Helfand S. L., Tatar M., Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 207.Brooks C., Gu W. How does SIRT1 affect metabolism, senescence and cancer? Nat. Rev. Cancer. 2009;9:123–128. doi: 10.1038/nrc2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Liang F., Kume S., Koya D. SIRT1 and insulin resistance. Nat. Rev. Endocrinol. 2009;5:367–373. doi: 10.1038/nrendo.2009.101. [DOI] [PubMed] [Google Scholar]
- 209.Luo J., Nikolaev A. Y., Imai S., Chen D., Su F., Shiloh A., Guarente L., Gu W. Negative control of p53 by Sir2α promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 210.Nemoto S., Fergusson M. M., Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306:2105–2108. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- 211.Brunet A., Sweeney L. B., Sturgill J. F., Chua K. F., Greer P. L., Lin Y., Tran H., Ross S. E., Mostoslavsky R., Cohen H. Y., et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;26:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 212.Firestein R., Blander G., Michan S., Oberdoerffer P., Ogino S., Campbell J., Bhimavarapu A., Luikenhuis S., de Cabo R., Fuchs C., et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS One. 2008;3:e2020. doi: 10.1371/journal.pone.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]