Abstract
Reduced HDL (high-density lipoprotein) concentration in the MetS (metabolic syndrome) is associated with increased risk of cardiovascular disease and is related to defects in HDL-apoA-II (apolipoprotein A-II) kinetics. Dietary restriction is the most commonly used weight loss strategy. In the present study, we examined the effect of weight loss on HDL-apoA-II kinetics in men with the MetS at the start and end of a 16-week intervention trial of a hypocaloric low-fat diet (n=20) compared with a weight maintenance diet (n=15), using a stable isotope technique and compartmental modelling. The low-fat diet achieved a significant reduction (P<0.01) in BMI (body mass index), abdominal fat compartments and HOMA (homoeostasis model assessment) score compared with weight maintenance. Weight loss also significantly (P<0.05) decreased both the production rate (−23%) and FCR (fractional catabolic rate) (−12%) of HDL-apoA-II, accounting for a net decrease in apoA-II concentration (−9%). Reductions in the HDL-apoA-II production rate were significantly associated with changes in body weight (r=0.683, P<0.01), plasma triacylglycerols (triglycerides) (r=0.607, P<0.01) and, to a lesser extent, plasma insulin (r=0.440, P=0.059) and HOMA-IR (HOMA of insulin resistance) (r=0.425, P=0.069). Changes in the apoA-II FCR were also significantly associated with reductions in visceral adipose tissue mass (r=0.561, P=0.010). In conclusion, in obese men with the MetS, short-term weight loss with a low-fat low-caloric diet lowers plasma apoA-II concentrations by decreasing both the production and catabolism of HDL-apoA-II. The cardiometabolic significance of this effect on HDL metabolism remains to be investigated further.
Keywords: apolipoprotein, cardiovascular disease, dietary restriction, lipoprotein kinetics, obesity, weight loss
Abbreviations: apoA etc., apolipoprotein A etc; ATM, adipose tissue mass; BMI, body mass index; CETP, cholesteryl ester transfer protein; CVD, cardiovascular disease; FCR, fractional catabolic rate; FFM, fat-free mass; HDL, high-density lipoprotein; HL, hepatic lipase; IR, insulin resistance; HOMA-IR, homoeostasis model assessment of IR; LDL, low-density lipoprotein; LPL, lipoprotein lipase; MetS, metabolic syndrome; NEFA, non-esterified fatty acid; PLTP, phospholipid transfer protein; VLDL, very-low-density lipoprotein
INTRODUCTION
The MetS (metabolic syndrome) portends diabetes and CVD (cardiovascular disease) [1]. Dyslipoproteinaemia, reflected by elevated plasma triacylglycerol (triglyceride) and reduced HDL (high-density lipoprotein) concentrations, is a cardinal feature of the MetS that independently predicts CVD [2] and is accordingly a therapeutic target for risk reduction [3].
Apo (apolipoprotein) A-I and apoA-II are the major apolipoproteins of HDL, which are extremely diverse in structure and function [4]. Compelling evidence supports the anti-atherogenic role of apoA-I in preventing CVD [5]. By contrast, the function of apoA-II is less consistent [6]. Animal studies have suggested that apoA-II may promote atherosclerosis [7,8]. Some, but not all, epidemiological studies have demonstrated a positive association with CVD in humans [9–13]. In insulin resistance, hepatic overproduction of VLDL (very-low-density lipoprotein), together with decreased LPL (lipoprotein lipase) activity, results in expansion in the VLDL-triacylglycerol pool and enhances CETP (cholesteryl ester transfer protein)-mediated hetero-exchange of neutral lipids among lipoproteins, leading to increased HDL triacylglycerol concentrations. Subsequent hydrolysis by HL (hepatic lipase), which is overactive in insulin resistance and obesity, results in a thermodynamically unstable HDL particle that is catabolized rapidly by the liver and kidney [14,15]. Using stable isotopes and multi-compartmental modelling, we have shown previously an increased catabolism of HDL-apoA-I and -apoA-II particles in male subjects with MetS and IR (insulin resistance) [16].
Weight reduction by dietary restriction is associated with improvements in a number of CVD factors, including dyslipidaemia and IR [17]. We have demonstrated previously that in obese men weight reduction between 5 to 10 kg with a low-fat low-caloric diet effectively decreases hepatic VLDL-apoB secretion and increased LDL (low-density lipoprotein)-apoB catabolism [18]. Our previous findings also suggest that weight loss influences the kinetics of HDL by decreasing both catabolism and production of apoA-I without significantly altering HDL-apoA-I and HDL-cholesterol concentrations [19]. However, there are no kinetic data on the effect of weight loss on apoA-II metabolism in these subjects.
We therefore extend our previous study by investigating the effect of short-term weight loss on HDL-apoA-II kinetics in subjects with the MetS. Given that both apoA-I and apoA-II share similar metabolic pathways, we hypothesized that weight loss would decrease the FCR (fractional catabolic rate) and production rate of HDL-apoA-II.
MATERIALS AND METHODS
Subjects and study design
The details of subject selection and study design have been described previously [19]. Briefly, 35 men with the MetS were randomized to either a hypocaloric low-fat diet for 14 weeks, immediately followed by a 2-week weight stabilization period, or to weight maintenance on an isocaloric diet for 16 weeks. HDL-apoA-II kinetics were measured after a 14 h fast using primed (1 mg/kg of body weight) and constant (1 mg·kg−1 of body weight·h−1) intravenous infusion of 1-[13C]leucine (99.5% enrichment; Tracer Technologies) for 10 h [19]. Body composition was estimated, as described previously [19], using a Holtain body composition analyser from which total fat mass and FFM (fat-free mass) were derived. Abdominal visceral ATM (adipose tissue mass) and subcutaneous ATM were estimated following magnetic resonance imaging [20]. Subjects were requested to maintain their usual level of physical activity and alcohol intake. Dietary intakes were assessed by 7-day recall questionnaires and alcohol diaries during weight loss and weight maintenance. Three day dietary diaries were completed every 3 weeks by both groups, and these were analysed using DIET 4 nutrient calculation software (Xyris Software). All procedures were repeated after the 16-week intervention. The study was approved by the Royal Perth Hospital Ethics Committee, and all participants provided written informed consent.
Isolation and measurement of isotopic enrichment of HDL-apoA-II
HDL-apoA-II were isolated from plasma by sequential ultracentrifugation, separated by SDS/PAGE and blotted on to a PVDF membrane; apoA-II bands were excised from the PVDF membrane, hydrolysed overnight (6 mol/l HCl, 110°C) and dried for derivatization [16]. Isotopic enrichment of apoA-II was determined using negative chemical ionization by GC/MS.
Biochemical measurements
Fasting plasma lipid and lipoprotein concentrations were determined by standard methods. Plasma glucose and NEFAs (non-esterified fatty acids) were measured by enzymatic colorimetric methods, and insulin was determined by immunoenzymometry. These methods have been described elsewhere [19]. HOMA-IR (homoeostasis model assessment of IR) score was used as an estimate of IR [21]. Plasma lathosterol concentration (a surrogate marker of cholesterol synthesis) was measured by GC/MS [22].
Model of apoA-II metabolism
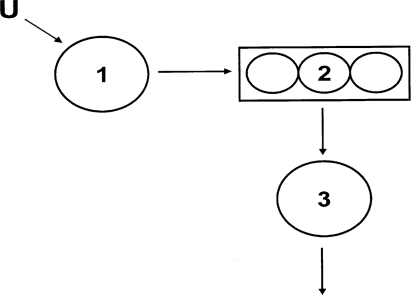
Tracer-to-tracee ratios were modelled using SAAM-II (University of Washington, Seattle, WA, U.S.A.) from which FCRs of HDL-apoA-II were estimated from the best fit of the model to the data. The apoA-II compartmental model consisted of three compartments (Figure 1). Compartment 1 represents the tracer input (plasma leucine enrichment), which is incorporated into an intrahepatic compartment (compartment 2) that accounts for the synthesis and secretion of apoA-II into the HDL fraction (compartment 3). HDL-apoAII transport rate was calculated by multiplying the FCR by pool size (mg·kg−1 of FFM·day−1).
Figure 1. Compartmental model describing HDL-apoA-II tracer kinetics.
Compartment 1 represents the tracer input which is incorporated into an intrahepatic compartment (compartment 2) that accounts for the synthesis and secretion of apoA-II into the HDL fraction (compartment 3).
Statistical analyses
All analyses were carried out using SPSS. Skewed data were log-transformed where appropriate. Treatment effects of the weight loss group relative to the weight maintenance group were analysed using general linear modelling with adjustments for baseline covariates. Associations between absolute changes in variables in the weight loss group were examined using simple regression model. Group differences at baseline were analysed using independent Student t tests. Statistical significance was defined as P<0.05.
RESULTS
Table 1 shows the clinical and biochemical characteristics of the subjects studied. On average, they were middle-aged, centrally obese, normotensive, insulin-resistant and dyslipidaemic (elevated plasma triacylglycerols and total apoB, and low HDL-cholesterol). There were no significant group differences in any of the variables at baseline. Average daily energy and nutrient intake of the 35 obese subjects studied was: 10045±2406 kJ, 36±6% energy from fat, 38±8% energy from carbohydrates, 20±3% energy from protein and 6±6% energy from alcohol (values are means±S.D.). Nutrient intake did not differ between patients randomized to weight loss or weight maintenance.
Table 1. Anthropometric characteristics, plasma lipids and lipoproteins and measures of insulin resistance before and after weight loss and during weight maintenance.
Values are means±S.E.M. Effect of weight loss was tested using general linear modelling after adjusting for the weight maintenance group; ‡P<0.05, †P<0.01 and *P<0.001.
| Weight loss group (n=20) | Weight maintenance group (n=15) | |||
|---|---|---|---|---|
| Characteristic | Week 0 | Week 16 | Week 0 | Week 16 |
| Weight (kg) | 109±2 | 96±3* | 105±3 | 109±2 |
| BMI (kg/m2) | 35±1.0 | 31±0.7* | 33±0.7 | 35±0.9 |
| Waist circumference (cm) | 112±2 | 103±2* | 113±2 | 113±2 |
| Mean blood pressure (mmHg) | 95.4±2.8 | 86.4±2.8† | 96.6±3.0 | 94.7±3.1 |
| Total fat mass (kg) | 42.6±2.7 | 30.0±1.9* | 38.8±1.8 | 44.1±2.8 |
| FFM (kg) | 65.4±1.9 | 62.5±2.0 | 63.9±1.7 | 64.0±1.8 |
| Visceral ATM (kg) | 7.1±0.5 | 5.4±0.4* | 6.9±0.4 | 6.7±0.4 |
| Total subcutaneous ATM (kg) | 8.4±0.7 | 6.5±0.4* | 9.6±0.7 | 9.9±0.7 |
| Cholesterol (mmol/l) | 6.0±0.3 | 5.2±0.2† | 6.0±0.2 | 6.0±0.2 |
| Triacylglycerol (mmol/l) | 3.5±0.6 | 2.0±0.2* | 2.9±0.6 | 2.7±0.4 |
| HDL-cholesterol (mmol/l) | 1.0±0.04 | 1.1±0.05 | 1.0±0.04 | 1.0±0.04 |
| LDL-cholesterol (mmol/l) | 3.3±0.2 | 3.0±0.2‡ | 3.9±0.2 | 3.9±0.29 |
| Non-HDL-cholesterol (mmol/l) | 4.9±0.3 | 4.2±0.2† | 4.8±0.2 | 4.9±0.2 |
| ApoB-100 (g/l) | 1.2±0.06 | 1.0±0.06† | 1.2±0.06 | 1.2±0.05 |
| ApoA-I (g/l) | 1.3±0.05 | 1.3±0.04 | 1.2±0.04 | 1.2±0.02 |
| ApoA-II (g/l) | 0.33±0.01 | 0.30±0.01‡ | 0.31±0.01 | 0.32±0.02 |
| Lathosterol (μmol/l) | 17.4±3.4 | 11.9±2.4‡ | 14.5±2.1 | 14.4±2.0 |
| Glucose (mmol/l) | 5.7±0.2 | 5.3±0.1 | 5.4±0.2 | 5.5±0.3 |
| Insulin (milli-units/l) | 14±2 | 8±1* | 18±3 | 16±2 |
| HOMA-IR score | 3.7±0.5 | 2.0±0.2† | 4.6±0.8 | 4.0±0.6 |
Compared with the weight maintenance group, the weight loss group achieved significant reductions in body weight (−12%, P<0.001), BMI (body mass index; −13%, P<0.001), waist circumference (−9%, P<0.001), mean arterial pressure (−9%, P<0.01), total fat mass (−30%, P<0.001), visceral ATM (−24%, P<0.001) and subcutaneous ATM (−23%, P<0.001) following the weight loss intervention. Compared with the weight maintenance group, weight loss also resulted in significant decreases (P<0.05) in plasma cholesterol (−12%), triacylglycerols (−43%), non-HDL-cholesterol (−14%), LDL-cholesterol (−8%), total apoB (−16%), apoA-II (−9%) and lathosterol (−23%), as well as insulin (−41%) and HOMA-IR score (−46%). There were no significant effects of weight loss on plasma concentrations of HDL-cholesterol, apoA-I and glucose when compared with the weight maintenance group.
Compared with the weight maintenance group, subjects in the weight loss group significantly (P<0.001 for both) reduced their total energy (−37%; mean±S.E.M., 9782±438 compared with 6143± 363 KJ) and fat intake (−30%; 37±1 compared with 26±2%), and increased their carbohydrate consumption (+30%; 37±2 compared with 48±2%), all changes being statistically significant (P<0.001), during the weight loss period. Alcohol intake in the weight loss group was not significantly different before or after weight loss. Nutrient intake did not change in the subjects in the weight maintenance group during the 16-week intervention. In the weight loss group, there were no significant correlations between changes in dietary intake and changes in apoA-II kinetics during weight loss or at weight maintenance. In the weight loss group, there was no significant associations between changes in apoA-II kinetics and changes in nutrient intake during weight maintenance at end of study. There was also no change in reported physical activity levels during the study in either the weight loss or weight maintenance groups (results not shown).
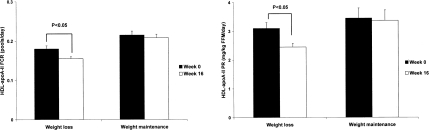
Figure 2 shows the kinetic indices for HDL-apoA-II metabolism after weight loss compared with the weight maintenance group. There were no significant group differences in lipoprotein kinetics at baseline. Compared with weight maintenance, weight loss significantly decreased the FCR (−12%) and production rate (−23%) of HDL-apoA-II, accounting for a net decrease in apoA-II concentration (−9%; see Table 1).
Figure 2. HDL-apoA-II FCR and production rate before and after weight loss and weight maintenance.
In univariate analyses, the change in plasma apoA-II concentration with weight loss was significantly associated with corresponding changes in HDL-apoA-II production rate (r=0.563, P<0.02), but not with changes in FCR (r=−0.246, P>0.05). There was also a significant correlation (r=0.574, P<0.01) between the reduction in HDL-apoA-II production rate and the decrease in HDL-apoA-II FCR. Using HDL-apoA-I kinetic data from the same subjects [19], there were no significant associations between changes in FCRs in HDL-apoA-I and -apoA-II in the weight loss group (r=0.356, P=0.135) nor between the changes in their production (r=0.184, P=0.438); changes in apoA-II kinetics did not correlate with HDL-cholesterol and apoA-I levels.
Reductions in plasma apoA-II concentration and HDL-apoA-II production rate were also associated with changes in body weight (r=0.463, P<0.05 and r=0.683, P<0.01 respectively), BMI (r=0.509, P<0.05 and r=0.672, P<0.01 respectively) and plasma triacylglycerols (r=0.494, P<0.05 and r=0.607, P<0.01 respectively). The association between changes in the apoA-II concentration (partial r=0.484, P=0.042) and apoA-II production rate (partial r=0.643, P=0.004) with weight reduction was independent of dietary fat and carbohydrate intake at weight maintenance. In the weight loss group, there was a trend to a significance in the association between the reduction in HDL-apoA-II production rate and the decreases in plasma insulin (r=0.440, P=0.059) and HOMA-IR (r=0.425, P=0.069). Changes in apoA-II FCR were significantly associated with reductions in visceral ATM (r=0.561, P=0.010). Neither changes in apoA-II concentration nor HDL-apoA-II FCR were associated with corresponding changes in plasma insulin or HOMA-IR.
DISCUSSION
In the present study, we provide new results on the effect of weight loss with a moderately low-fat diet on HDL-apoA-II metabolism. We demonstrate that weight loss principally decreased the production rate of HDLapoA-II with a lesser effect on its FCR. As a consequence, we found a net decrease in apoA-II concentration with weight loss. These effects of weight loss may be related to its favourable impact on visceral ATM, insulin sensitivity and plasma triacylglycerol concentrations.
Previous kinetic studies have only examined the effects of changing the type or content of fat on HDL-apoA-II transport kinetics [23–25]. Vélez-Carrasco et al. [23] reported that the consumption of low saturated fat and cholesterol (Step 2 diet) had no effect on HDL-apoA-II FCR or secretion rate. Brinton et al. [24] found that subjects on a low-fat diet increased HDL-apoA-II FCR without altering apoA-II levels or its secretion rate. Desroches et al. [25] observed that a low-fat/high-carbohydrate diet decreased apoA-II concentration with no effect on HDL-apoA-II kinetics. However, most of these kinetic studies were of small sample size, achieved minimal weight loss and did not employ a weight control group nor a weight stabilization period at the end of the study. In addition, these studies were not restricted to obese subjects with dyslipidaemia. In a placebo-controlled study with the use of stable isotopes, we have examined previously the effects of weight loss on apoB and apoA-I kinetics in subjects with the MetS [19]. We have now extended the study by investigating its impact on HDL-apoA-II metabolism.
It is likely that the combination of central obesity, insulin resistance and hypertriacylglycerolaemia collectively account for the dysregulation of HDL metabolism. Therefore weight loss with a low-fat diet could correct the abnormalities in HDL metabolism potentially by improvements in insulin sensitivity and a reduction in body weight. Consistent with this, we found that the decrease in plasma apoA-II concentration was associated with a reduction in plasma triacylglycerol, body weight and, to a lesser extent, HOMA-IR.
HDL-apoA-II FCR
We have demonstrated previously that weight loss by a low-fat low-caloric diet lowers plasma triacylglycerols by a reduction in VLDL production [18,19]. As described earlier, this in turn decreases the VLDL-triacylglycerol pool and subsequent CETP-mediated hetero-exchange of neutral lipids among lipoproteins, thereby delaying the uptake of HDL by the liver. This notion is consistent with our present findings that weight loss decreased HDL-apoA-II FCR; however, we found no significant correlation between the changes in HDL-apoA-II FCR and plasma triacylglycerols. Given that apoA-I and apoA-II are attached to HDL particles, one would anticipate that the FCRs of HDL-apoA-I and -apoA-II are tightly correlated. We did not find a significant association between the changes in FCRs of HDL-apoA-I and -apoA-II with weight loss nor between the changes in apoA-II FCR and HDL-cholesterol concentrations. Taken together, it is likely that different mechanisms underlie these alterations in HDL catabolism following weight loss. That there was a significant correlation between the changes in HDL-apoA-II FCR and visceral ATM indicates a potential role for adiposity in the regulation of HDL-apoA-II catabolism. Whether the change in HDL-apoA-II FCR was driven by the corresponding changes in various adipocytokines merits investigation.
HDL-apoA-II production rate
The mechanism responsible for the decreased production of HDL-apoA-II with weight loss in the MetS remains unclear. Given the tight correlation between changes in catabolism and production of HDL-apoA-II following weight loss, it is possible that a ‘balancing feedback’ mechanism, as we observed in other interventional kinetic studies [26,27], could account for the reduction in apoA-II production rate. In contrast with apoA-II FCR, we found that the decrease in the production rate of HDL-apoA-II accounted for the reduction in plasma apoA-II concentration. A change in apoA-II production rate following weight loss was also associated with reductions in plasma triacylglycerol concentration, body weight and insulin resistance. This observation suggests that weight loss by dietary restriction could have a direct influence on apoA-II production. Using VLDL-apoB kinetic data from the same subjects [19], we found that the off-treatment production rates of VLDL-apoB and HDL-apoA-II were significantly correlated (r=0.498, P=0.027), and that the fall in VLDL-apoB production with weight loss was significantly and positively correlated with the reduction in the HDL-apoA-II production rate (r= 0.506, P=0.023). Moreover, in our previous cross-sectional studies [28], we also observed significant associations between the production rates of VLDL-apoB, VLDL-apoC-III and HDL-apoA-I. Taken together, it is likely that the global effects of insulin resistance and/or obesity drive the secretion of these lipoproteins and these are reversed by weight loss. This notion is consistent with our present findings.
Limitations
There are limitations to our present study. We acknowledge that the effects of weight loss on apoA-II kinetics cannot be fully dissociated from dietary effect, in particular, changes in fat and carbohydrate intake. However, we found no significant associations between changes in nutrient intake and changes in apoA-II concentration and kinetics. In addition, our kinetic studies were carried out during a weight stable period and on an isocaloric diet. The alcohol intake in our men was relatively high and the findings with apoA-II kinetics may not necessarily translate to those with lesser intake of alcohol intake. We only examined the short-term effect of weight loss followed by a 2-week isocaloric weight-stabilizing period, but we have shown favourable effects on lipoprotein metabolism with this regimen. More prolonged periods of weight maintenance can lead to rebound changes in plasma lipids that could mask the full benefit of weight loss. HDL particles are subjected to modifications by several lipase and lipid transfer proteins, particularly, LPL, HL, CETP and PLTP (phospholipid transfer protein). We have reported previously that weight loss had no effect on CETP and PLTP activity [19]. Additional measurements of LPL and HL activities in plasma may help to formally corroborate our findings. Only obese Caucasian men were studied, and it is possible that the kinetic effects of weight loss might have been different in women and other ethnic groups.
Implications
Recent evidence suggests that the cardioprotective effect of the HDL system may relate chiefly to both the apoA-I and apoA-II content of HDL particles. Higher apoA-I remains an independent negative predictor of cardiovascular risk [5]. An analysis from Epic-Norfolk showed that, in apparently healthy people, increased plasma apoA-II levels were predictive of lower coronary risk [9]. This challenges the previous notion that apoA-II is pro-atherogenic [7,8]. Whether or not the apoA-II-lowering effect with weight loss is anti-atherogeneic remains to be elucidated and must also be viewed in light of the alterations in apoB and apoA-I metabolic pathways. Importantly, how these effects impact upon the other functions of HDL, including cholesterol efflux and whole-body cholesterol turnover, merits further investigation.
In conclusion, we demonstrate that, in men with the MetS, short-term weight loss with a low-fat diet lowers the plasma apoA-II concentration by decreasing both the production and catabolism of HDL-apoA-II. Further investigations should explore the incremental effect of other pharmacotherapies (e.g. fibrates, fish oils or insulin sensitizers) added to a weight loss regimen on the functionality of HDL in these subjects.
ACKNOWLEDGEMENTS
We thank the nursing staff of the Clinical Research Studies Unit of the School of Medicine and Pharmacology (Royal Perth Hospital, University of Western Australia, Perth, Australia) for providing expert clinical assistance.
FUNDING
This work was supported by the National Heart Foundation of Australia; the National Health and Medical Research Foundation; and Medical Research Foundation. T. W. K. N. is an Athelstan and Amy Saw medical research fellow of University of Western Australia. D. C. C. is a National Health and Medical Research Foundation Career Development Fellow. P. H. R. B. is a National Health and Medical Research Foundation Senior Research Fellow.
References
- 1.Grundy S. M. Metabolic syndrome: a multiplex cardiovascular risk factor. J. Clin. Endocrinol. Metab. 2007;92:399–404. doi: 10.1210/jc.2006-0513. [DOI] [PubMed] [Google Scholar]
- 2.Alexander C. M., Landsman P. B., Teutsch S. M., Haffner S. M. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210–1214. doi: 10.2337/diabetes.52.5.1210. [DOI] [PubMed] [Google Scholar]
- 3.Rader D. J. Mechanisms of disease: HDL metabolism as a target for novel therapies. Nat. Clin. Pract. Cardiovasc. Med. 2007;4:102–109. doi: 10.1038/ncpcardio0768. [DOI] [PubMed] [Google Scholar]
- 4.Puchois P., Kandoussi A., Fievet P., Fourrier J. L., Bertrand M., Koren E., Fruchart J. C. Apolipoprotein A-I containing lipoproteins in coronary artery disease. Atherosclerosis. 1987;68:35–40. doi: 10.1016/0021-9150(87)90091-8. [DOI] [PubMed] [Google Scholar]
- 5.Van der Steeg W. A., Holme I., Boekholdt S. M., Larsen M. L., Lindahl C., Stroes E. S. G., Tikkanen M. J., Wareham N. J., Faergeman O., Olsson A. G., et al. High-density lipoprotein cholesterol, high-density lipoprotein particle size, and apolipoprotein A-I: significance for cardiovascular risk: The IDEAL and EPIC-Norfolk studies. J. Am. Coll. Cardiol. 2008;51:634–642. doi: 10.1016/j.jacc.2007.09.060. [DOI] [PubMed] [Google Scholar]
- 6.Blanco-Vaca F., Escolà-Gil J. C., Martín-Campos J. M., Julve J. Role of apoA-II in lipid metabolism and atherosclerosis: advances in the study of an enigmatic protein. J. Lipid Res. 2001;42:1727–1739. [PubMed] [Google Scholar]
- 7.Warden C. H., Hedrick C. C., Qiao J. H., Castellani L. W., Lusis A. J. Atherosclerosis in transgenic mice overexpressing apolipoprotein A-II. Science. 1993;261:469–471. doi: 10.1126/science.8332912. [DOI] [PubMed] [Google Scholar]
- 8.Escolà-Gil J. C., Marzal-Casacuberta À., Julve-Gil J., Ishida B. Y., Ordóñez-Llanos J., Chan L., González-Sastre F., Blanco-Vaca F. Human apolipoprotein A-II is a pro-atherogenic molecule when it is expressed in transgenic mice at a level similar to that in humans: evidence of a potentially relevant species-specific interaction with diet. J. Lipid Res. 1998;39:457–462. [PubMed] [Google Scholar]
- 9.Birjmohun R. S., Dallinga-Thie G. M., Kuivenhoven J. A., Stroes E. S. G., Otvos J. D., Wareham N. J., Luben R., Kastelein J. J. P., Khaw K. T., Boekholdt S. M. Apolipoprotein A-II is inversely associated with risk of future coronary artery disease. Circulation. 2007;116:2029–2035. doi: 10.1161/CIRCULATIONAHA.107.704031. [DOI] [PubMed] [Google Scholar]
- 10.Buring J. E., O'Connor G. T., Goldhaber S. Z., Rosner B., Herbert P. N., Blum C. B., Breslow J. L., Hennekens C. H. Decreased HDL2 and HDL3 cholesterol, Apo A-I and Apo A-II, and increased risk of myocardial infarction. Circulation. 1992;85:22–29. doi: 10.1161/01.cir.85.1.22. [DOI] [PubMed] [Google Scholar]
- 11.Roselli della Rovere G., Lapolla A., Sartore G., Rossetti C., Zambon S., Minicuci N., Crepaldi G., Fedele D., Manzato E. Plasma lipoproteins, apoproteins and cardiovascular disease in type 2 diabetic patients: a nine-year follow-up study. Nutr. Metab. Cardiovasc. Dis. 2003;13:46–51. doi: 10.1016/s0939-4753(03)80167-9. [DOI] [PubMed] [Google Scholar]
- 12.Syvanne M., Kahri J., Virtanen K. S., Taskinen M. R. HDLs containing apolipoproteins A-I and A-II (LpA-I:A-II) as markers of coronary artery disease in men with non-insulin-dependent diabetes mellitus. Circulation. 1995;92:364–370. doi: 10.1161/01.cir.92.3.364. [DOI] [PubMed] [Google Scholar]
- 13.Sweetnam P. M., Bolton C. H., Downs L. G., Durrington P. N., Mackness M. I., Elwood P. C., Yarnell J. W. Apolipoproteins A-I, A-II and B, lipoprotein(a) and the risk of ischaemic heart disease: the Caerphilly study. Eur. J. Clin. Invest. 2000;30:947–956. doi: 10.1046/j.1365-2362.2000.00725.x. [DOI] [PubMed] [Google Scholar]
- 14.Lamarche B., Rashid S., Lewis G. F. HDL metabolism in hypertriglyceridemic states: an overview. Clin. Chim. Acta. 1999;286:145–161. doi: 10.1016/s0009-8981(99)00098-4. [DOI] [PubMed] [Google Scholar]
- 15.Taskinen M. R. Diabetic dyslipidaemia: from basic research to clinical practice. Diabetologia. 2003;46:733–749. doi: 10.1007/s00125-003-1111-y. [DOI] [PubMed] [Google Scholar]
- 16.Ji J., Watts G. F., Johnson A. G., Chan D. C., Ooi E. M. M., Rye K. A., Serone A. P., Barrett P. H. R. High-density lipoprotein transport in the metabolic syndrome: application of a new model for HDL particle kinetics. J. Clin. Endocrinol. Metab. 2006;91:973–979. doi: 10.1210/jc.2005-1895. [DOI] [PubMed] [Google Scholar]
- 17.Van Gaal L. F., Wauters M. A., De Leeuw I. H. The beneficial effects of modest weight loss on cardiovascular risk factors. Int. J. Obes. Relat. Metab. Disord. 1997;21:S5–S9. [PubMed] [Google Scholar]
- 18.Riches F. M., Watts G. F., Hua J., Stewart G. R., Naoumova R. P., Barrett P. H. R. Reduction in visceral adipose tissue is associated with improvement in apolipoprotein B-100 metabolism in obese men. J. Clin. Endocrinol. Metab. 1999;84:2854–2861. doi: 10.1210/jcem.84.8.5925. [DOI] [PubMed] [Google Scholar]
- 19.Ng T. W., Watts G. F., Barrett P. H. R., Rye K. A., Chan D. C. Effect of weight loss on LDL and HDL kinetics in the metabolic syndrome: association with changes in plasma retinol-binding protein-4 and adiponectin levels. Diabetes Care. 2007;30:2945–2950. doi: 10.2337/dc07-0768. [DOI] [PubMed] [Google Scholar]
- 20.Watts G. F., Chan D. C., Barrett P. H. R., Hua J., Swong S. Adipose tissue compartments and the kinetics of very-low-density-lipoprotein B-100 in overweight/obese men. Obesity Res. 2003;11:152–159. [Google Scholar]
- 21.Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., Turner R. C. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 22.Mori T. A., Croft K. D., Puddey I. B. Analysis of native and oxidized low-density lipoprotein oxysterols using gas chromatography-mass spectrometry with selective ion monitoring. Redox Rep. 1996;2:25–31. doi: 10.1080/13510002.1996.11747023. [DOI] [PubMed] [Google Scholar]
- 23.Vélez-Carrasco W., Lichtenstein A. H., Welty F. K., Li Z., Lamon-Fava S., Dolnikowski G. G., Schaefer E. J. Dietary restriction of saturated fat and cholesterol decreases HDL apoA-I secretion. Arterioscler. Thromb. Vasc. Biol. 1999;19:918–924. doi: 10.1161/01.atv.19.4.918. [DOI] [PubMed] [Google Scholar]
- 24.Brinton E. A., Eisenberg S., Breslow J. L. A low-fat diet decreases high density lipoprotein (HDL) cholesterol levels by decreasing HDL apolipoprotein transport rates. J. Clin. Invest. 1990;85:144–151. doi: 10.1172/JCI114405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desroches S., Paradis M. E., Pérusse M., Archer W. R., Bergeron J., Couture P., Bergeron N., Lamarche B. Apolipoprotein A-I, A-II, and VLDL-B-100 metabolism in men: comparison of a low-fat diet and a high-monounsaturated fatty acid diet. J. Lipid Res. 2004;45:2331–2338. doi: 10.1194/jlr.M400287-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Watts G. F., Barrett P. H. R., Ji J., Serone A. P., Chan D. C., Croft K. D., Loehrer F., Johnson A. G. Differential regulation of lipoprotein kinetics by atorvastatin and fenofibrate in subjects with the metabolic syndrome. Diabetes. 2003;52:803–811. doi: 10.2337/diabetes.52.3.803. [DOI] [PubMed] [Google Scholar]
- 27.Chan D. C., Watts G. F., Nguyen M. N., Barrett P. H. R. Factorial study of the effect of n−3 fatty acid supplementation and atorvastatin on the kinetics of HDL apolipoproteins A-I and apoA-II in men with abdominal obesity. Am. J. Clin. Nutr. 2006;84:37–43. doi: 10.1093/ajcn/84.1.37. [DOI] [PubMed] [Google Scholar]
- 28.Chan D. C., Nguyen M. N., Watts G. F., Barrett P. H. Plasma apolipoprotein C-III transport in central obese men: association with low density lipoprotein apolipoprotein B and high-density lipoprotein metabolism. J. Clin. Endocrinol. Metab. 2008;93:557–564. doi: 10.1210/jc.2006-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]