Abstract
The objectives of this analysis are to re-examine the foundational studies of the in vivo metabolism of plasma LDL (low-density lipoprotein) particles in humans and, based on them, to reconstruct our understanding of the governance of the concentration of plasma LDL and the maintenance of cholesterol homoeostasis in the hepatocyte. We believe that regulation of cholesterol homoeostasis within the hepatocyte is demonstrably more complex than envisioned by the LDL receptor paradigm, the conventional model to explain the regulation of plasma LDL and the fluxes of cholesterol into the liver, a model which was generated in the fibroblast but has never been fully validated in the hepatocyte. We suggest that the LDL receptor paradigm should be reconfigured as the apoB (apolipoprotein B) paradigm, which states that the rate at which LDL particles are produced is at least an important determinant of their concentration in plasma as the rate at which they are cleared from plasma and that secretion of cholesterol within VLDL (very-low-density lipoprotein) particles is an important mechanism of maintaining cholesterol homoeostasis within the hepatocyte. These two paradigms are not mutually exclusive. The LDL receptor paradigm, however, includes only one critical aspect of the regulation of plasma LDL, namely the rate at which LDL particles are cleared through the LDL receptor pathway, but ignores another – the rate at which LDL particles are added to the plasma compartment. The apoB paradigm includes both and points to a different model of how the hepatocyte achieves cholesterol homoeostasis in a complex metabolic environment.
Keywords: apolipoprotein B, cholesterol, endoplasmic reticulum, low-density lipoprotein, hepatocyte, plasma
Abbreviations: ACAT, acyl-CoA:cholesterol acyltransferase; apoB, apolipoprotein B; ER, endoplasmic reticulum; FCR, fractional catabolic rate; FH, familial hypercholesterolaemia; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein
INTRODUCTION
Few ideas define their time. The LDL (low-density lipoprotein) receptor paradigm certainly achieved that rare rank. The LDL receptor paradigm states that the LDL pathway, the normal route by which LDL particles are removed by plasma, is the principal determinant of the concentration of LDL in plasma and therefore the principal determinant of the risk of coronary disease attributable to LDL [1]. Furthermore, the activity of the LDL pathway, and therefore the concentration of LDL in plasma, is governed by the concentration of cholesterol within a key intracellular regulatory pool. Increased delivery of cholesterol to the cell via the LDL pathway results in the down-regulation of both the synthesis of cholesterol and the synthesis of LDL receptors, whereas reduced delivery of cholesterol results in up-regulation of the synthesis of cholesterol and LDL receptors. The LDL receptor paradigm was developed in fibroblasts and represents a simple action–reaction homoeostatic model whose single objective is to limit the mass of cholesterol entering the cell. However, not only does the fibroblast not play a significant role in total body cholesterol, but also the studies were conducted under experimental conditions which did not allow physiological egress of cholesterol from the fibroblast.
When it comes to the hepatocyte, which is at the centre of all the major cholesterol fluxes within the organism, the LDL receptor paradigm is accurate but incomplete. The apoB (apolipoprotein B) paradigm extends our understanding of the regulation of plasma LDL by taking production as well as clearance of apoB particles into account. The arguments advanced in this article represent novel extensions of previous critiques of the LDL receptor paradigm [2–6] that coalesce with the formulation of the apoB paradigm and our hypothesis as to cholesterol homoeostasis within the hepatocyte.
In brief, we hypothesize that most of the cholesterol that enters the hepatocyte as part of an LDL particle is secreted from it as part of a VLDL (very low-density lipoprotein) particle without coming into full equilibrium with the exchangeable and regulatory pools of cholesterol within the hepatocyte. Consequently, cholesterol and LDL receptor synthesis will not be down-regulated. By contrast, cholesterol that enters the hepatocyte directly or within chylomicron remnant particles will enter the exchangeable and regulatory pools of cholesterol before it can be esterified and secreted from the cell. As such, cholesterol and LDL receptor synthesis will be down-regulated. Therefore the physiological responses of the hepatocyte are governed to an important degree by the route by which cholesterol gains access to the hepatocyte and these responses are important determinants of the regulation of atherogenic particle number in plasma.
DETERMINANTS OF PLASMA LDL PARTICLE NUMBER: LDL-apoB
LDL is a cholesterol-rich lipoprotein particle generated as the end product of the metabolism of VLDL, the triacylglycerol (triglyceride)-rich lipoprotein particle secreted by the liver. For the purposes of this discussion, we will not deal with the fact that both VLDL and LDL particles are heterogeneous in composition, that is, each is present in plasma in multiple forms, some larger containing more core lipid and others smaller containing less. Each VLDL and LDL particle contains one molecule of apoB. Therefore VLDL-apoB equals the number of VLDL particles and LDL-apoB equals the number of LDL particles.
The concentration of LDL particles in plasma, the concentration of LDL-apoB, is determined by the rate at which they are produced and by the rate at which they are cleared from plasma. At steady state, that is, when the concentration of LDL is constant day-to-day, the number of LDL particles produced per day must equal the number cleared per day. Similarly, the mass of cholesterol that enters the LDL density range per day must equal the mass of cholesterol that is cleared in LDL particles per day. Since more than 90% of the removal of LDL takes place in the liver, the liver determines the rates of LDL clearance from plasma. The space of Disse presents no barrier to the movement of plasma lipoproteins from the vascular space to the extravascular space; accordingly, the concentration of LDL outside the hepatocyte is equal to the intravascular concentration of LDL.
LDL particles are irreversibly cleared from plasma either by the LDL receptor pathway or by non-specific pathways. Extensive reviews of the LDL receptor pathway are available [7,8]. This analysis focuses on the kinetic properties of the clearance pathways and the key points are: the binding of a LDL particle to its specific receptor is high-affinity, but the absolute transport capacity of the LDL pathway is limited. Once the LDL pathway is saturated, the number of LDL particles cleared cannot increase. Even so, the activity of the LDL pathway will not automatically be reduced. That will not occur unless the mass of cholesterol within the regulatory pool of cell cholesterol increases, triggering the cascade of metabolic events that results in diminished synthesis of LDL receptors and cholesterol. Unless this occurs, traffic will continue through the LDL receptor pathway at the maximal rate possible.
Non-specific pathways make up the second route of removal of LDL particles from plasma. They are low-affinity, but non-saturable [7,9]. Accordingly, as the plasma LDL increases, so does the mass of LDL removed by the non-specific pathways. Thus the two pathways differ in which characteristic is constant: the non-specific pathways remove a constant fraction of the total number of LDL particles in the plasma pool, whereas the LDL receptor pathway, once saturated, removes a constant number of LDL particles from the plasma pool. By contrast, at higher and higher concentrations of LDL, the non-specific pathways take up more and more LDL particles [9,10].
RELATIONSHIP BETWEEN LDL RECEPTORS, LDL CLEARANCE AND PLASMA LDL CONCENTRATION
Total LDL clearance and recycling of cholesterol in homozygous FH (familial hypercholesterolaemia)
Homozygous FH is, ostensibly, the simplest case to analyse the impact of the LDL receptor pathways on plasma LDL concentration. The reason is that, in homozygous FH, there is no uptake via the LDL receptor pathway and therefore clearance of LDL particles from plasma occurs only through the non-specific pathways. Perhaps counterintuitively, the total clearance of LDL particles from plasma is not reduced in homozygous FH [9]. On the contrary, it is markedly elevated. Thus Bilheimer et al. [9] demonstrated that the total mass of LDL-cholesterol removed per day from plasma in patients with FH was three times that in normal subjects. In spite of that, there is no evidence that cholesterol synthesis in patients with homozygous FH is reduced. Accordingly, even though the uptake of cholesterol into the hepatocyte of FH homozygotes via the non-specific pathways occurs in amounts much larger than normal, this does not appear to result in expansion of the regulatory pool, which would trigger the down-regulation of cholesterol synthesis.
If more cholesterol is entering the hepatocyte than can be secreted in the bile, if the regulatory pool is not expanded, and if cholesterol is not to accumulate within the hepatocyte, there must be recycling of cholesterol by the liver. The bulk of the cholesterol that is taken up by the liver in the patients with homozygous FH must be resecreted from the liver either within apoB particles or via nascent HDL (high-density lipoprotein) particles. Recycling is an obligatory response to prevent accumulation of cholesterol within the liver and almost certainly explains the increased secretion of cholesterol-rich apoB particles in patients with FH [4,9,11–14]. Recycling of cholesterol without expansion of the regulatory pool would also explain why cholesterol synthesis continues in the livers of patients with homozygous FH.
In patients with normal LDL pathways, how does clearance through specific and non-specific pathways vary as plasma levels of LDL increase?
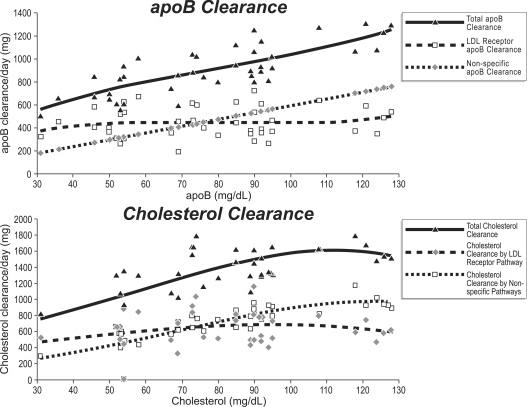
The results in Figure 1 were derived from studies of LDL turnover in individuals with normal and moderately elevated levels of LDL [15,16]. None of these subjects had marked hypercholesterolaemia and therefore none had FH or any other disorder producing marked impairment of clearance through the LDL pathway. Total clearance of LDL-apoB is equal to the sum of the uptakes through the specific and the non-specific pathways. If the FCR (fractional catabolic rate) for non-specific uptake of LDL is 0.17 pools of LDL-apoB per day [9], the FCR for specific uptake of LDL is equal to FCR total LDL clearance–FCR non-specific LDL clearance, or FCR specific LDL pathway=FCR total LDL clearance−0.17.
Figure 1. Results of LDL-apoB clearance and LDL clearance as the total and mass cleared through the specific and non-specific pathways as the concentration of LDL-apoB increases in plasma.
Note that the masses of apoB and cholesterol cleared through the specific LDL pathway do not vary significantly as plasma LDL-apoB increases, whereas the clearance through the non-specific pathways increases linearly. Data were taken from [15,16].
Figure 1 displays the total mass of LDL-apoB cleared per day as well as the mass of LDL-apoB cleared through the specific compared with the non-specific pathways at increasing concentrations of LDL. The total mass of LDL-cholesterol cleared per day is also illustrated as well as the mass of LDL-cholesterol cleared through the specific and non-specific pathways. The first point to note is the positive relationship between the total mass of LDL-apoB cleared per day and the concentration of LDL-apoB in plasma. For clearance to increase as the concentration of LDL increases points to increased production of LDL as the major determinant of the concentration of LDL, an inference that is supported by the formal calculations in these studies [15,16]. Secondly, the mass of LDL-apoB cleared per day through the specific pathway varies little over the entire range of concentrations of LDL-apoB. Thus, as the total mass of LDL-cholesterol cleared per day increases, the mass of LDL-cholesterol cleared through the specific pathway remains constant. Thirdly, because the FCR of the non-specific pathway is constant, the mass of LDL cleared via the non-specific pathway increases linearly as plasma LDL increases. The result is that the total mass of LDLapoB and LDL-cholesterol cleared per day equals the constant amount taken up by the specific pathway plus the constantly increasing amount taken up via the non-specific pathways. These findings from the in vivo turnover studies are supported by the in vitro studies of Havekes and co-workers [17,18].
These findings challenge the LDL receptor paradigm in three critical ways. First, even though the total mass of cholesterol returned to the liver increases, there is no down-regulation of the activity of the LDL receptor pathway. On the contrary, the activity of the LDL receptor pathway appears to be maintained as the plasma concentration of LDL increases and as the total mass of cholesterol transported to the liver increases. Secondly, cholesterol synthesis is sustained and not down-regulated as the LDL receptor paradigm would call for. Thirdly, analogous to homozygous FH, intracellular cholesterol homoeostasis will need to be maintained by secretion of excess cholesterol from the liver.
Moreover, of the two mechanisms that govern the concentration of LDL in plasma, these studies and others [7–9,12] demonstrate that overproduction of LDL particles is a much more common cause of elevated LDL-cholesterol and -apoB than impaired clearance. This finding is not the consequence of study selection, as we will attempt to demonstrate in the next section.
LDL clearance compared with LDL production as determinants of plasma LDL
Table 1 demonstrates the effect on plasma LDL-apoB concentration of progressive reduction in function of the specific LDL pathway to zero in the face of normal LDL production. The example is of a 70 kg male with a plasma volume of 3.5 litres and an LDL production rate of 10 mg·kg−1 of body weight·day−1. These calculations illustrate that, if LDL production remains normal, there must be profound impairment in the functioning of the LDL pathway before LDL levels will be clearly elevated. Even so, even when there is no transport through the LDL pathway, LDL-apoB levels will be only modestly elevated. On the other hand, as shown in Table 2, sequentially increasing the production of LDL-apoB until it is double the normal levels will produce more dramatic effects on plasma LDL-apoB than decreasing the clearance of LDL. These calculations confirm the conclusions reached previously by Meddings and co-workers [19] and Bilheimer and co-workers [9]. They also illustrate that increased production of LDL is a major pathophysiological contributor to the profoundly elevated levels of LDL in homozygous FH as indeed has been repeatedly documented [4,9,11–14]. Most important, as illustrated by the calculations in Table 3, it is the combination of impaired clearance with increased production that produces the most dramatic examples of elevation of plasma LDL-apoB.
Table 1. Effect of decreased clearance by LDL pathway with normal production of LDL.
PR, production rate; NSC, non-specific LDL clearance; SC, specific LDL clearance.
| NSC | SC | ||||||
|---|---|---|---|---|---|---|---|
| PR (mg/day) | FCR (pools/day) | FCR (pools/day) | FCR (mg/day) | FCR (pools/day) | FCR (mg/day) | LDL-apoB pool (mg) | LDL-apoB (mg/dl) |
| 700 | 0.45 | 0.17 | 265 | 0.28 | 435 | 1559 | 45 |
| 700 | 0.42 | 0.17 | 283 | 0.25 | 417 | 1664 | 48 |
| 700 | 0.37 | 0.17 | 322 | 0.20 | 378 | 1894 | 54 |
| 700 | 0.32 | 0.17 | 372 | 0.15 | 328 | 2188 | 63 |
| 700 | 0.27 | 0.17 | 441 | 0.10 | 259 | 2594 | 74 |
| 700 | 0.22 | 0.17 | 541 | 0.05 | 159 | 3182 | 91 |
| 700 | 0.17 | 0.17 | 700 | 0.00 | 0 | 4177 | 118 |
Table 2. Effect of increased production of LDL on plasma LDL-apoB with normal LDL clearance.
PR, production rate; NSC, non-specific LDL clearance; SC, specific LDL clearance.
| NSC | SC | ||||||
|---|---|---|---|---|---|---|---|
| PR (mg/day) | FCR (pools/day) | FCR (pools/day) | FCR (mg/day) | FCR (pools/day) | FCR (mg/day) | LDL-apoB pool (mg) | LDL ApoB (mg/dl) |
| 700 | 0.45 | 0.17 | 264 | 0.28 | 436 | 1553 | 45 |
| 800 | 0.37 | 0.17 | 364 | 0.20 | 436 | 2145 | 61 |
| 900 | 0.33 | 0.17 | 464 | 0.16 | 436 | 2729 | 78 |
| 1000 | 0.30 | 0.17 | 564 | 0.13 | 436 | 3317 | 95 |
| 1100 | 0.28 | 0.17 | 664 | 0.11 | 436 | 3906 | 112 |
| 1200 | 0.27 | 0.17 | 764 | 0.10 | 436 | 4494 | 128 |
| 1300 | 0.26 | 0.17 | 864 | 0.09 | 436 | 5082 | 145 |
| 1400 | 0.25 | 0.17 | 964 | 0.08 | 436 | 5671 | 162 |
Table 3. Effect of increased production of LDL on plasma LDL-apoB with reduced LDL clearance (33%).
PR, production rate; NSC, non-specific LDL clearance; SC, specific LDL clearance.
| NSC | SC | ||||||
|---|---|---|---|---|---|---|---|
| PR (mg/day) | FCR (pools/day) | FCR (pools/day) | FCR (mg/day) | FCR (pools/day) | FCR (mass) | LDL-apoB pool (mg) | LDL ApoB (mg/dl) |
| 700 | 0.29 | 0.17 | 410 | 0.12 | 290 | 2411 | 69 |
| 800 | 0.27 | 0.17 | 510 | 0.10 | 290 | 2941 | 86 |
| 900 | 0.25 | 0.17 | 610 | 0.08 | 290 | 3588 | 103 |
| 1000 | 0.24 | 0.17 | 710 | 0.07 | 290 | 4177 | 119 |
| 1100 | 0.23 | 0.17 | 810 | 0.06 | 290 | 4764 | 136 |
| 1200 | 0.22 | 0.17 | 910 | 0.05 | 290 | 5353 | 153 |
| 1300 | 0.22 | 0.17 | 1010 | 0.05 | 290 | 5941 | 170 |
| 1400 | 0.21 | 0.17 | 1110 | 0.04 | 290 | 6529 | 187 |
A NEW MODEL OF CHOLESTEROL HOMOEOSTASIS WITHIN THE HEPATOCYTE
In this final section, we will present our hypothesis as to the different pathways that cholesterol can take within the hepatocyte and the implications for cholesterol homoeostasis within the hepatocyte. First, we will consider cholesterol that enters the cell directly into the plasma membrane. Cholesterol within the plasma membrane makes up the greatest pool of cholesterol within the cell. Once the capacity to sequester cholesterol within the plasma membrane is exceeded, any excess moves either to acceptors outside the cell or to sites within the cell such as the ER (endoplasmic reticulum) and the mitochondria. This sets off a broad set of regulatory responses. Sequestration of SREBP (sterol-regulatory-element-binding protein) within the ER results in down-regulation of cholesterol and LDL receptor synthesis. An even more immediate reaction may come from increased synthesis of 27-hydroxycholesterol in the mitochondria, which acutely reduces HMG-CoA (3-hydroxy-3-methylglutaryl-CoA) reductase activity by ubiquination of the enzyme [20]. It is also possible that oxysterols generated in the mitochondria may activate the LXRα (liver X receptor α) which, in turn, may induce ABCA1 (ATP-binding-cassette transporter A1) and IDOL (inducible degrader of the LDL receptor) expression to up-regulate cholesterol efflux and LDL receptor degradation respectively [21]. Cholesterol in the ER can also be esterified by ACAT (acyl-CoA:cholesterol acyltransferase) and then either stored within the cytoplasm or secreted from the cell within an apoB lipoprotein particle. In this sequence, all of the appropriate and known regulatory responses are triggered before the cholesterol is esterified.
On the basis of the observations we have presented in the present article, we hypothesize that an alternative sequence is possible, namely that the cholesterol within an LDL particle within a lysosome is delivered directly to ACAT and the bulk of the cholesterol within the particle is esterified before there is a significant increase in the concentration of cholesterol at the ER or the mitochondria. The consequence is that cholesterol taken up within the LDL particle can be resecreted without a significant change in the synthesis of LDL receptors or cholesterol. This would explain the shunting and recycling of cholesterol that was first observed in FH but, as we have shown, is also present in many, if not most, subjects with elevated LDL. We suggest that recycling of cholesterol within the hepatocyte is the key process that permits continued clearance of LDL through the LDL receptor and persistent cholesterol synthesis in the face of increased delivery of cholesterol to the liver. We suspect that the course followed by other lipoprotein particles, such as chylomicron remnants, may correspond more closely to the first route we have outlined rather than the second.
SUMMARY
In summary, the apoB paradigm differs from the LDL receptor paradigm in two critical regards. First, the apoB paradigm reveals that the concentration of LDL particles in plasma is determined not only by the rate at which they are cleared, but also, and generally even more importantly, by the rate at which they are produced. Secondly, in the apoB paradigm, cholesterol synthesis is not constrained as it is in the LDL receptor paradigm. The LDL receptor paradigm demands tight regulation of cholesterol synthesis and preserves the equilibrium of the mass of cholesterol within the organism, whereas the apoB paradigm allows for disequilibrium and expansion of the mass of cholesterol within the organism. The disequilibrium that is a hallmark of the apoB paradigm is much more consistent with the pathophysiology of the major apoB atherogenic dyslipoproteinaemias than is the LDL receptor paradigm.
The reorientation in our understanding called for by the apoB paradigm opens new research and therapeutic opportunities. Different lipoprotein particles are taken up by the hepatocyte by different mechanisms. This almost certainly results in different delivery sites within the hepatocyte. These differences are important because they may determine whether the cholesterol within a lipoprotein particle is delivered to the regulatory or to the shunt pathways. With regard to treatment, therapy for elevated LDL presently focuses on increasing the activity of the LDL receptor pathway. The value of this approach is enormous, but many patients remain undertreated. The apoB paradigm points to production as the major mechanism governing the plasma concentration of LDL. Therefore therapeutic opportunities to diminish apoB secretion should be pursued vigorously [22]. Decreasing VLDL secretion will not necessarily increase total cholesterol mass within the hepatocyte as it may allow more time for equilibration with the regulatory pools and therefore decreased synthesis and uptake. Obviously much work will be necessary to determine the validity of these concepts. Nevertheless, the apoB paradigm appears to provide a more explanatory model of the regulation of plasma LDL and cholesterol homoeostasis in the hepatocyte than the LDL receptor paradigm.
FUNDING
A.D.S. acknowledges the support of the Mike Rosenbloom Foundation.
References
- 1.Brown M. S., Goldstein J. L. A receptor- mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 2.Fisher W. R., Zech L. A., Stacpoole P. W. ApoB metabolism in familial hypercholesterolemia. Inconsistencies with the LDL receptor paradigm. Arterioscler. Thromb. Vasc. Biol. 1994;14:501–510. doi: 10.1161/01.atv.14.4.501. [DOI] [PubMed] [Google Scholar]
- 3.Thompson G. R., Naoumova R. P., Watts G. F. Role of cholesterol in regulating apolipoprotein B secretion by the liver. J. Lipid Res. 1996;37:439–447. [PubMed] [Google Scholar]
- 4.Cummings M. H., Watts G. F., Umpleby M., Hennessy T. R., Quiney J. R., Sönksen P. H. Increased hepatic secretion of very-low-density-lipoprotein apolipoprotein B-100 in heterozygous familial hypercholesterolaemia: a stable isotope study. Atherosclerosis. 1995;113:79–89. doi: 10.1016/0021-9150(94)05430-q. [DOI] [PubMed] [Google Scholar]
- 5.Sniderman A. D., Zhang X., Cianflone K. Governance of the concentration of plasma LDL: a reevaluation of the LDL receptor paradigm. Atherosclerosis. 1999;148:215–229. doi: 10.1016/s0021-9150(99)00282-8. [DOI] [PubMed] [Google Scholar]
- 6.Sniderman A. D., Cianflone K. Substrate delivery as a determinant of hepatic apoB secretion. Arterioscler. Thromb. Vasc. Biol. 1993;13:629–636. doi: 10.1161/01.atv.13.5.629. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein J. L., Brown M. S. The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 2009;29:431–438. doi: 10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown M. S., Goldstein J. L. Cholesterol feedback: from Schoenheimer's bottle to Scap's MELADL. J. Lipid Res. 2009;50:(Suppl.) 15–27. doi: 10.1194/jlr.R800054-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilheimer D. W., Stone N. J., Grundy S. M. Metabolic studies in familial hypercholesterolemia: evidence for a gene-dosage effect in vivo. J. Clin. Invest. 1979;64:524–533. doi: 10.1172/JCI109490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietschy J. M., Turley S. D., Spady D. K. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J. Lipid Res. 1993;34:1637–1659. [PubMed] [Google Scholar]
- 11.Teng B., Sniderman A. D., Soutar A. K., Thompson G. R. Metabolic basis of hyperapobetalipoproteinemia. Turnover of apolipoprotein B in low density lipoprotein and its precursors and subfractions compared with normal and familial hypercholesterolemia. J. Clin. Invest. 1986;77:663–672. doi: 10.1172/JCI112360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Millar J. S., Maugeais C., Ikewaki K., Kolansky D. M., Barrett P. H. R., Budreck E. C., Boston R. C., Tada N., Mochizuki S., Defesche J. C. Complete deficiency of the low-density lipoprotein receptor is associated with increased apolipoprotein B-100 production. Arterioscler. Thromb. Vasc. Biol. 2005;25:560–565. doi: 10.1161/01.ATV.0000155323.18856.a2. [DOI] [PubMed] [Google Scholar]
- 13.Zulewski H., Ninnis R., Miserez A. R., Baumstark M. W., Keller U. VLDL and IDL apolipoprotein B-100 kinetics in familial hypercholesterolemia due to impaired LDL receptor function or to defective apolipoprotein B-100. J. Lipid Res. 1998;39:380–387. [PubMed] [Google Scholar]
- 14.Tremblay A. J., Lamarche B., Ruel I. L., Hogue J. C., Bergeron J., Gagne C., Couture P. Increased production of VLDL apoB-100 in subjects with famililial hypercholesterolemia carrying the same null LDL receptor gene mutation. J. Lipid Res. 2004;45:866–872. doi: 10.1194/jlr.M300448-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Kesanieimi Y. A., Grundy S. M. The significance of low density lipoprotein production in the regulation of plasma cholesterol level in man. J. Clin. Invest. 1982;70:13–22. doi: 10.1172/JCI110585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grundy S. M., Vega G. L., Bilheimer D. W. Kinetic mechanisms determining variability in low density lipoprotein levels and rise with age. Arteriosclerosis. 1985;5:623–630. doi: 10.1161/01.atv.5.6.623. [DOI] [PubMed] [Google Scholar]
- 17.Havekes L. M., de Wit E. C. M., Princen H. M. Cellular free cholesterol in Hep G2 cells is only partially available for down-regulation of low-density-lipoprotein receptor activity. Biochem. J. 1987;247:739–746. doi: 10.1042/bj2470739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Havekes L. M., Verboom H., de Wit E. C. M., Yap S. H., Princen H. M. Regulation of low density lipoprotein receptor activity in primary culture of human hepatocytes by serum lipoproteins. Hepatology. 1986;6:1356–1360. doi: 10.1002/hep.1840060623. [DOI] [PubMed] [Google Scholar]
- 19.Meddings J. B., Dietschy J. M. Regulation of plasma levels of low-density lipoprotein cholesterol: interpretation of data on low-density lipoprotein turnover in man. Circulation. 1986;74:805–814. doi: 10.1161/01.cir.74.4.805. [DOI] [PubMed] [Google Scholar]
- 20.Lange Y., Ory D. S., Ye J., Lanier M. H., Hsu F. F., Steck T. L. Effectors of rapid homeostatic responses of endoplasmic reticulum cholesterol and 3-hydroxy-3-methylglutarylCoA reductases. J. Biol. Chem. 2008;283:1445–1455. doi: 10.1074/jbc.M706967200. [DOI] [PubMed] [Google Scholar]
- 21.Zelcer N., Hong C., Boyadjian R., Tontonoz P. LXR regulates cholesterol uptake through idol-dependent ubiquitination of the LDL receptor. Science. 2009;325:100–104. doi: 10.1126/science.1168974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davidson M. H. Novel nonstatin strategies to lower low-density lipoprotein cholesterol. Curr. Atheroscler. Rep. 2009;11:67–70. doi: 10.1007/s11883-009-0011-0. [DOI] [PubMed] [Google Scholar]