Abstract
Background and purpose:
As the use of the 5-HT3 receptor antagonist alosetron (GlaxoSmithKline) and the 5-HT4 receptor agonist tegaserod (Novartis) in patients with irritable bowel syndrome has been associated with cases of ischaemic colitis, the effects of alosetron, cilansetron (Solvay) and tegaserod on the rat splanchnic circulation were evaluated.
Experimental approach:
Phenobarbital-anaesthetised rats were instrumented to record blood flow in the superior mesenteric artery and transverse colon and to calculate mesenteric and colonic vascular conductance.
Key results:
Intravenous alosetron (0.03–0.3 mg·kg−1) did not alter blood pressure or heart rate but reduced mesenteric blood flow and vascular conductance by 15–20%. This activity profile was also seen after intraduodenal alosetron and shared by the 5-HT3 receptor antagonist cilansetron. In contrast, blood flow, vascular conductance and intraluminal pressure in the colon were not modified by alosetron and cilansetron. Intravenous or intraduodenal tegaserod (0.3–1.0 mg·kg−1) had no inhibitory effect on mesenteric and colonic blood flow. Peroral treatment of rats with alosetron or tegaserod for 7 days did not modify mesenteric haemodynamics at baseline and after blockade of nitric oxide synthesis. Mild inflammation induced by dextran sulphate sodium failed to provoke a vasoconstrictor effect of cilansetron in the colon.
Conclusions and implications:
Alosetron and cilansetron, not tegaserod, caused a small and transient constriction of the rat mesenteric vascular bed, whereas blood flow in the colon remained unaltered. The relevance of these findings to the treatment-associated occurrence of ischaemic colitis in patients with irritable bowel syndrome remains open.
Keywords: mesenteric blood flow, colonic blood flow; vascular conductance; mesenteric vasoconstriction; 5-hydroxytryptamine; 5-HT3 receptors; 5-HT4 receptors; alosetron; cilansetron; tegaserod; clonidine; nitric oxide; N-nitro-L-arginine methylester
Introduction
5-Hydroxytryptamine (5-HT) acting via 5-HT3 and 5-HT4 receptors (Alexander et al., 2008) participates in the control of gastrointestinal (GI) motility, epithelial ion and fluid transport as well as sensation (De Ponti, 2004; Gershon and Tack, 2007). As there is evidence that a disturbance of the 5-HT system is a contributory factor in irritable bowel syndrome (IBS), 5-HT3 receptor antagonists such as alosetron (GlaxoSmithKline) and cilansetron (Solvay) as well as 5-HT4 receptor agonists such as tegaserod (Novartis) were developed for the therapy of diarrhoea- and constipation-predominant IBS, respectively (Schoenfeld, 2004; Chey and Cash, 2005; Evans et al., 2007; Andresen et al., 2008; Rahimi et al., 2008). Subsequent clinical observations showed that the most significant adverse effect of the 5-HT3 receptor antagonist alosetron was constipation and, in very rare cases, ischaemic colitis (Miller et al., 2003; Chang et al., 2006; Andresen et al., 2008; Rahimi et al., 2008). Meta-analyses of the clinical trials indicate that 0.15–0.20% of the patients on alosetron may develop ischaemic colitis compared with none under placebo (Chang et al., 2006; Andresen et al., 2008; Rahimi et al., 2008). Later on, a very few cases of ischaemic colitis were also observed in patients under therapy with cilansetron or tegaserod (Brinker et al., 2004; Chey and Cash, 2005; DiBaise, 2005; Andresen et al., 2008). In this context, it is important to consider that patients with IBS or chronic constipation are at greater risk to develop ischaemic colitis than healthy subjects in whom this vascular failure is extremely rare (Higgins et al., 2004; Chang et al., 2008). The mechanisms whereby these drugs may give rise to ischaemic colitis are not known (Camilleri, 2007), but it is worth noting that idiopathic constipation is associated with a reduction of blood flow through the colonic mucosa (Emmanuel and Kamm, 2000). In an experimental study in anaesthetised rats, both acute and short-term alosetron administration failed to significantly alter baseline mesenteric and colonic blood flow (CBF) or to interfere with splanchnic vascular control mechanisms during occlusion and reactive hyperaemia (Grundy et al., 2007). Because cilansetron and tegaserod have not yet been studied in their effects on the splanchnic circulation, it was the overall aim of this study to compare tegaserod, cilansetron and alosetron in their influence on mesenteric and colonic circulation of anaesthetised rats.
By measuring blood flow and vascular conductance in the superior mesenteric artery and within the wall of the transverse colon, which is supplied by the superior mesenteric artery, several factors that might determine the effects of alosetron and tegaserod on the splanchnic circulation were evaluated. The experimental models were validated by their sensitivity to the vasoconstrictor effect of the nitric oxide synthase inhibitor N-nitro-L-arginine methylester (L-NAME, Bachem, Basel, Switzerland) and the vasodilator effect of the α2-adrenoceptor agonist clonidine. In study 1, we set out to explore the dose dependency of any acute effects of alosetron, cilansetron and tegaserod, injected i.v., on CBF measured by the hydrogen gas clearance technique, mesenteric blood flow (MBF) measured by the ultrasonic transit time shift technique and intracolonic pressure. Study 2 was designed to compare the time-dependent effects of alosetron and tegaserod, injected i.v., on CBF measured by laser Doppler flowmetry and MBF in fasted and non-fasted rats. Studies 3 and 4 addressed the possibility that the effects of alosetron and tegaserod on splanchnic haemodynamics depended on the route and duration of administration. In study 3, alosetron and tegaserod were administered i.d., and their acute influence on CBF and MBF was evaluated. Study 4 was designed to test whether peroral treatment of rats with alosetron or tegaserod for 1 week modified the splanchnic circulation at baseline and affected the vasoconstrictor effect of L-NAME. Finally, study 5 addressed the question of whether a vasoconstrictor effect of cilansetron in the colon might be provoked under conditions of mild colitis, given that IBS can be associated with low-grade inflammation in the colon (Bercik et al., 2005; Spiller, 2007; De Giorgio and Barbara, 2008). A preliminary account of some of the findings obtained in study 1 has been published in abstract form (Holzer et al., 2003b).
Methods
Animals
All animal care and experimental procedures complied with the 1986 Directive of the European Communities Council and were approved by an ethics committee at the Federal Ministry of Science and Research of the Republic of Austria. The experiments were conducted in female Sprague Dawley rats (body weight: 180–230 g) (Division of Laboratory Animal Science and Genetics, Department of Biomedical Research, Medical University of Vienna, Himberg, Austria). Unless stated otherwise, the rats were deprived of food for 20 h before blood flow measurement, while tap water was available ad libitum. The animals were anaesthetised with phenobarbital (230 mg·kg−1 injected i.p.) between 7.00 and 8.00 am. After the loss of the righting reflex, they were placed on a thermostated table and, when surgical anaesthesia was achieved, fitted with a tracheal cannula to facilitate spontaneous respiration and to allow for the administration of hydrogen gas. A cannula in a jugular vein was used for continuous infusion of saline (1.5 mL·h−1) for dehydration of the animals to be avoided and for the i.v. administration of drugs.
Induction of mild colitis
Dextran sulphate sodium (DSS, MP Biochemicals, Illkirch, France) was added to the drinking water at a concentration of 3% (w/v) for 7 days. The control animals received normal tap water. The DSS-containing drinking water was made fresh every day. For protocol consistency, the drinking bottles containing normal tap water were also renewed daily.
Measurement of blood pressure and heart rate (HR)
Arterial blood pressure was recorded from a cannula in a common carotid artery, and the blood pressure signal was sampled at a rate of 1 kHz and fed into a personal computer, which calculated mean arterial blood pressure (MAP) and HR online (Heinemann et al., 1998).
Measurement of CBF with the hydrogen gas clearance technique
The hydrogen clearance technique has been established to measure microcirculatory blood flow in the digestive tract and other tissues (Leung et al., 1984; Livingston et al., 1989; Holzer et al., 1991). Based on the washout of inhaled hydrogen, which clears essentially with a single passage of blood through the lungs, the hydrogen clearance method has the advantage of providing absolute estimates of microcirculatory blood flow at the position of a platinum electrode, which catalyses the dissociation of molecular hydrogen into protons and electrons (Guth and Leung, 1987). The major disadvantage of the hydrogen clearance technique is that blood flow recording is discontinuous because periods of hydrogen inhalation alternate with periods of hydrogen washout. For this reason, only changes in blood flow that persist for some time can be recorded.
In the present experiments (study 1), the transverse colon was instrumented with a needle-type platinum electrode, while a reference electrode was placed in the peritoneal cavity. As has been reported for the stomach (Holzer et al., 1991), the platinum electrode was inserted from the serosa tangentially to the colon at an angle of about 30 degrees and positioned in the submucosa of the colon. The reproducibility of placing the needle electrode in the submucosal layer of the colonic wall was confirmed by histology. To this end, the needle electrode was stained with blue ink before being placed in the colonic wall. After removal of the electrode, the tissue was rapidly excised and frozen. Sections (35 µm thick) of the tissue perpendicular to the needle track were taken in a cryostat and examined under a microscope. The needle track was invariably localised to the submucosal layer of the colonic wall, as observed in three rats.
The measurement of CBF via the clearance of inhaled hydrogen gas was discontinuous, as the experimental protocol involved alternating 15 min periods of saturation, and desaturation, of the tissue with hydrogen gas (Holzer et al., 1991; Heinemann et al., 1999). The current representing the actual hydrogen concentration at the site of the electrode was taken up by a polarographic unit, amplified, digitised at a frequency of 1 Hz and recorded on a personal computer with a custom-made software (Heinemann et al., 1999). The washout curve was then fitted to a monoexponential curve, the power of which was used to calculate the average CBF during the 15 min period of desaturation (Livingston et al., 1989). Average values of MAP and HR were determined for the same time periods. In addition, the colonic vascular conductance (CVC) value was calculated as CBF divided by MAP.
Measurement of CBF with laser Doppler flowmetry
The principle of laser Doppler flowmetry is based on the reflectance of monochromatic light by moving particles in the tissue. As the volume of tissue that is sampled is not precisely known, the technique cannot record blood flow in absolute values but in arbitrary perfusion units (PUs). The uncertainty about the tissue volume sampled also makes it difficult to compare recordings taken from different animals with each other because the vascular geometry of the tissue sample (i.e. the relative proportion of arteries, arterioles and capillaries) may differ substantially but cannot be controlled adequately. The major advantages of the technique are that it provides continuous recordings of blood flow and that it is able to detect rapidly occurring and short-lasting changes in this variable following an intervention.
In all studies, except study 1, CBF was measured by laser Doppler flowmetry. In studies 2 and 4, a side delivery endoscopic fibre probe (type: P6asd; external diameter: 2.1 mm; Moor Instruments, Axminster, Devon, UK) was inserted into the lumen of the transverse colon through an incision in the colonic wall at least 1 cm proximally to the site of measurement. The probe was held in place by a ligature at the site of incision and connected to a dual-channel laser Doppler monitor (model MBF3D; Moor Instruments), which fed the data into a personal computer using a custom-made software. In studies 3 and 5, CBF was recorded with a two-channel, side delivery endoscopic fibre probe (415–263 rat intestine probe; external diameter: 3.5 mm; Perimed AB, Stockholm, Sweden) connected to a four-channel laser Doppler monitor (PeriFlux System 5000 equipped with four PF 5010 LDPM Units; Perimed AB). The data (PU) were recorded on a personal computer with the software PSW 2.1 Perisoft for Windows (Perimed AB). The CVC value was calculated as PU divided by MAP.
Measurement of MBF with the ultrasonic transit time shift technique
Blood flow in the superior mesenteric artery was recorded with the ultrasonic transit time shift technique, which measures the net volume of MBF with a factory-calibrated sensor (Heinemann et al., 1998). To this end, the superior mesenteric artery at its exit from the aorta was separated from the surrounding tissue over a length of 4 mm. A perivascular ultrasonic flow probe was placed around the artery and connected to a small animal flowmeter (model T206, Transonic, Ithaca, New York, USA), which calculated the volume of MBF and fed the signal at a rate of 1 Hz into a personal computer. The data acquisition programme calculated the mesenteric vascular conductance (MVC; MBF divided by MAP) online (Heinemann et al., 1998). In addition, the software allowed for the calculation of average values of MAP, HR, MBF, MVC, CBF and CVC during specified periods of time.
Measurement of intraluminal pressure in the colon
Motor activity in the colon was estimated by the method of Wager-Page et al. (1992). The ascending colon was fitted with an inflow cannula (outer diameter: 2.5 mm) through which saline was slowly (0.02 mL·min−1) infused in an aboral direction throughout the experiment. The inflow cannula was connected with a pressure transducer with which the contractile activity of the colon was recorded via changes in the intraluminal pressure. After amplification, the intracolonic pressure signal was digitised at a rate of 1 Hz and fed into a personal computer (Holzer et al., 2003a). Infusion of saline into the colon resulted in an intraluminal pressure of about 400 Pa at equilibrium. Phasic increases in colonic pressure superimposed on the tonic pressure were quantified by averaging the amplitude of the phasic contractions that occurred during the 15 min observation periods and by expressing the average amplitude as Pa·(10 s)−1.
Myeloperoxidase in the colon
The tissue level of myeloperoxidase (MPO) was used to quantify inflammation-associated infiltration of neutrophils and monocytes into the tissue (Krawisz et al., 1984). Full-thickness pieces of the descending colon were excised, shock-frozen in liquid nitrogen and stored at −70°C until assay. After weighing, the frozen tissues were placed, at a ratio of 1 mg: 0.02 mL, in MPO lysis buffer. The composition of this buffer was 200 mmol·L−1 NaCl, 5 mmol·L−1 EDTA, 10 mmol·L−1 Tris, 10% glycine, 0.1 mmol·L−1 phenylmethylsulphonyl fluoride, 1 µg·mL−1 leupeptide and 28 µg·mL−1 aprotinin, pH 7.4. The samples were homogenised on ice with an Ultraturrax (IKA, Staufen, Germany) and then subjected to two centrifugations at 6000 ×g and 4°C for 15 min. The MPO (donor: H2O2 oxidoreductase, EC 1.11.1.7) content of the supernatants was measured with an enzyme-linked immunosorbent assay kit specific for the rat and mouse protein (Hycult Biotechnology, Uden, the Netherlands). The sensitivity of this assay is 1 ng·mL−1 at an intra- and interassay variation of around 10%.
Experimental protocols
After the completion of surgery, the variables under study were monitored for up to 120 min when the cardiovascular parameters had become stable. Thereafter, baseline values were recorded and averaged during a period of 15 min. In study 1, baseline recordings were taken during the period of 25–10 min before i.v. injection of vehicle (1 mL·kg−1), alosetron (0.03, 0.1 or 0.3 mg·kg−1), cilansetron (0.1 or 0.3 mg·kg−1), tegaserod (0.3 or 1 mg·kg−1) or L-NAME (0.02 mmol·kg−1). Post-injection recordings were made during the periods of 5–20 min and 35–50 min. Study 2 was performed to evaluate the effects of select drug doses in fasted and non-fasted rats over a prolonged period of time. After the baseline values during the period of 25–10 min pre injection were recorded, vehicle (1 mL·kg−1), alosetron (0.03 mg·kg−1), tegaserod (1 mg·kg−1) or L-NAME (0.02 mmol·kg−1) was injected i.v.; post-injection recordings were taken for a period of 140 min.
In study 3, the drugs were administered i.d. via a soft infant feeding tube (outer diameter 1.5 mm; Rüsch, Montevideo, Uruguay), which, during surgery, had been passed down through the oesophagus, stomach and pylorus so that its tip was positioned in the duodenum. After baseline recordings of the parameters under study had been made during the period of 15–0 min pre injection, vehicle (1 mL·kg−1), alosetron (0.3 mg·kg−1), tegaserod (30 mg·kg−1) or clonidine (0.03 mg·kg−1) was administered i.d. Post-injection recordings were taken for 90 min.
Study 4 was carried out to investigate whether the splanchnic circulation at baseline and after injection of L-NAME is modified by short-term peroral pretreatment with alosetron or tegaserod for 7 days. Each day, the animals were given vehicle (10 mL·kg−1), alosetron (0.3 mg·kg−1) or tegaserod (1 mg·kg−1) at 7.30–8.00 am, 1.00–1.30 pm and 6.00–6.30 pm. The solutions were administered intragastrically (IG) through a soft infant feeding tube (outer diameter: 2.2 mm; Portex, Hythe, UK). On the eighth day, they received the last dose at 7.30 am, 1 h before they were anaesthetised for blood flow measurement. After baseline recordings had been taken during the period of 25–10 min pre injection, L-NAME (0.02 mmol·kg−1) was injected i.v. Thereafter, the cardiovascular parameters were recorded for a period of 110 min.
In study 5, a group of rats was treated with DSS (3% added to the drinking water) for 7 days before they were anaesthetised for measurement of CBF by laser Doppler flowmetry. The control animals received normal tap water. On day 7, the animals were inspected to calculate a disease activity index based on fur appearance, locomotion, blood on faeces and diarrhoea. Each condition was rated as 0 if no abnormality was observed, or as 1 if the fur appeared neglected, locomotion was reduced, signs of diarrhoea were present and/or traces of blood on the faeces were observed, with the maximum disease activity index scoring as 4. After baseline recordings had been taken during the period of 15–0 min pre injection, cilansetron (0.3 mg·kg−1) was injected i.v., and MAP, HR, CBF and CVC were recorded for 50 min. At the end of the experiments, a segment of the descending colon was excised for determination of the MPO tissue concentration.
In all experiments, only one dose of drug or vehicle was tested in each anaesthetised animal. Rats with a MAP lower than 60 mmHg at the time of vehicle or drug administration were excluded from the study.
Drugs and solutions
For i.v. injection in studies 1 and 2, alosetron (Novartis, Basel, Switzerland) was dissolved at a concentration of 1 mg·mL−1 in saline (0.9% NaCl) whose pH was adjusted to approximately 4 by adding solid tartaric acid. This stock solution was diluted with vehicle (saline of pH 4 adjusted with tartaric acid) to obtain injection solutions containing 0.03 and 0.1 mg·mL−1. Cilansetron (Novartis) was dissolved and diluted with saline. Tegaserod (Novartis) was dissolved in 100% 1-methyl-2-pyrrolidone (NMP) at concentrations of 3 and 10 mg·mL−1 and diluted with saline to yield injection solutions containing 10% NMP. The vehicle control for tegaserod was saline containing 10% NMP.
For i.d. administration in study 3, the vehicle for all drugs was 10% NMP in saline. While alosetron (0.3 mg·mL−1) and clonidine (0.03 mg·mL−1; Sigma, Vienna, Austria) were directly dissolved in the vehicle, tegaserod used in study 3 was suspended in 100% NMP at a concentration of 300 mg·mL−1 and diluted with saline to yield a homogeneous injection suspension of 30 mg·mL−1 tegaserod in 10% NMP. In study 4, the vehicle for all drugs was 1% NMP in saline. In this instance, tegaserod was dissolved in 100% NMP at a concentration of 10 mg·mL−1 and diluted with saline to yield an injection solution of 0.1 mg·mL−1 tegaserod containing 1% NMP.
L-NAME hydrochloride was dissolved in saline at a concentration of 0.02 mmol·mL−1. DSS (molecular weight: 36 000–50 000) was added to the drinking water (tap water) at a concentration of 3% (w/v).
Data presentation and statistics
The cardiovascular parameters (MAP, HR, MBF, MVC, CBF and CVC estimated via hydrogen gas clearance) recorded immediately before i.v. or i.d. administration of vehicle or drugs (baseline values) are given in absolute terms. The parameters measured after i.v. or i.d. administration of vehicle or drugs are presented as a percentage of the baseline values in order to compare drug-induced changes independently of differing baseline values. When CBF and CVC were estimated by laser Doppler flowmetry, only relative changes are given, except in study 5, where absolute values are reported. All data are shown as means ± SEM. Statistical evaluation of the original data (absolute values) was performed with Student's t-test (drug vs. vehicle), one-way analysis of variance (anova; drugs vs. vehicle) or one way anova for repeated measures (post-administration vs. pre-administration) followed by the Bonferroni test, as appropriate. P values less than 0.05 were considered significant.
Results
Dose-dependent effects of i.v. injection of alosetron, cilansetron and tegaserod (study 1)
The rationale of study 1 was to examine whether acute i.v. injections of alosetron, cilansetron and tegaserod have any dose-dependent effect on MAP, HR, mesenteric and colonic haemodynamics as well as intracolonic pressure. In addition, the ability of the vascular recording techniques to pick up vasoconstrictor effects was tested with L-NAME. After baseline recordings of the cardiovascular parameters under study (Table 1) had been taken, vehicle, alosetron, cilansetron, tegaserod or L-NAME was injected i.v. CBF in this study was measured with the hydrogen gas clearance technique. The vehicles for alosetron, cilansetron, tegaserod and L-NAME were devoid of any effect on the cardiovascular parameter and intracolonic pressure recordings (Table 2).
Table 1.
Baseline values of cardiovascular parameters
| Cardiovascular parameter | Study 1: fasted rats | Study 2: fasted rats | Study 2: non-fasted rats | Study 3: fasted rats | Study 4: fasted rats |
|---|---|---|---|---|---|
| MAP (mmHg) | 94.7 ± 1.5 (90) | 84.0 ± 1.4 (85) | 86.5 ± 2.8 (22) | 88.2 ± 1.9 (38) | 81.8 ± 3.6 (21) |
| HR (beats·min−1) | 342 ± 7.2 (81) | 346 ± 7.2 (85) | 357 ± 14.6 (18) | 362 ± 7.1 (38) | 333 ± 12.3 (21) |
| MBF (mL·min−1) | 14.8 ± 0.54 (90) | 12.6 ± 0.31 (85) | 17.4 ± 1.0 (22)** | 13.0 ± 0.45 (38) | 15.7 ± 0.90 (21) |
| MVC [µl·min−1·(mmHg)−1] | 159 ± 6.5 (90) | 153 ± 4.9 (85) | 209 ± 16.2 (22)** | 150 ± 6.5 (38) | 200 ± 13.6 (21) |
| CBFa[mL·min−1·(100 g)−1] | 92.2 ± 4.4 (88) | N.M. | N.M. | N.M. | N.M. |
| CVCa[mL·min−1·(100 g)−1·(mmHg)−1] | 1.0 ± 0.06 (88) | N.M. | N.M. | N.M. | N.M. |
CBF and CVC were measured by the hydrogen gas clearance technique. The cardiovascular parameters were recorded immediately before administration of any drug or vehicle. Means ± SEM, n in parenthesis.
P < 0.01 versus respective values recorded in fasted rats of study 2 (Student's t-test).
CBF, colonic blood flow; CVC, colonic vascular conductance; HR, heart rate; MAP, mean arterial blood pressure; MBF, mesenteric blood flow; MVC, mesenteric vascular conductance; N.M., not measured.
Table 2.
Effect of acute i.v. injection of vehicle, alosetron, cilansetron, tegaserod and L-NAME on MAP, HR, CBF, CVC as well as tonic and phasic pressure in the colon of fasted rats
| Treatment and recording period | MAP (mmHg) | HR (beats·min−1) | CBF [mL·min−1· (100 g)−1] | CVC [mL·min−1· (100 g)−1 (mmHg)−1] | Tonic pressure in colon (Pa) | Phasic pressure in colon (Pa) |
|---|---|---|---|---|---|---|
| Vehicle (alosetron) 25–10 min pre | 93.4 ± 4.75 | 314 ± 8.65 | 72.9 ± 9.64 | 0.76 ± 0.08 | 416 ± 73.5 | 29.7 ± 3.30 |
| Vehicle (alosetron) 5–20 min post | 91.7 ± 8.11 | 335 ± 22.1 | 80.1 ± 13.7 | 0.89 ± 0.12 | 434 ± 72.9 | 32.7 ± 3.25 |
| Vehicle (alosetron) 35–50 min post | 90.2 ± 8.03 | 336 ± 22.9 | 65.0 ± 9.32 | 0.73 ± 0.09 | 429 ± 75.6 | 30.4 ± 3.61 |
| Alosetron (0.03 mg·kg−1) 25–10 min pre | 97.1 ± 4.02 | 338 ± 29.4 | 97.3 ± 7.22 | 1.00 ± 0.07 | 328 ± 58.0 | 26.7 ± 1.73 |
| Alosetron (0.03 mg·kg−1) 5–20 min post | 102 ± 4.19 | 354 ± 41.8 | 107 ± 10.7 | 1.04 ± 0.08 | 345 ± 63.8 | 27.3 ± 2.45 |
| Alosetron (0.03 mg·kg−1) 35–50 min post | 101 ± 4.64 | 369 ± 55.5 | 104 ± 8.85 | 1.03 ± 0.07 | 353 ± 61.0 | 28.6 ± 1.73 |
| Alosetron (0.1 mg·kg−1) 25–10 min pre | 92.4 ± 5.37 | 361 ± 40.3 | 110 ± 13.9 | 1.19 ± 0.14 | 463 ± 91.7 | 23.4 ± 0.37 |
| Alosetron (0.1 mg·kg−1) 5–20 min post | 95.0 ± 4.47 | 347 ± 39.8 | 108 ± 16.4 | 1.13 ± 0.17 | 455 ± 91.8 | 25.0 ± 1.33 |
| Alosetron (0.1 mg·kg−1) 35–50 min post | 89.7 ± 6.83 | 351 ± 32.9 | 97.7 ± 16.2 | 1.19 ± 0.31 | 449 ± 100 | 23.4 ± 1.73 |
| Alosetron (0.3 mg·kg−1) 25–10 min pre | 95.3 ± 6.18 | 321 ± 11.6 | 119 ± 17.0 | 1.28 ± 0.19 | 420 ± 87.6 | 26.0 ± 2.62 |
| Alosetron (0.3 mg·kg−1) 5–20 min post | 99.6 ± 4.53 | 349 ± 28.5 | 117 ± 13.8 | 1.23 ± 0.22 | 440 ± 88.7 | 27.7 ± 3.43 |
| Alosetron (0.3 mg·kg−1) 35–50 min post | 98.1 ± 5.36 | 320 ± 12.4 | 111 ± 10.2 | 1.18 ± 0.17 | 432 ± 88.8 | 24.9 ± 2.54 |
| Vehicle (cilansetron) 25–10 min pre | 87.1 ± 6.17 | 364 ± 25.0 | 106 ± 26.6 | 1.36 ± 0.46 | 427 ± 41.8 | 31.9 ± 3.12 |
| Vehicle (cilansetron) 5–20 min post | 89.0 ± 6.04 | 387 ± 42.7 | 100 ± 26.6 | 1.30 ± 0.48 | 448 ± 47.0 | 29.0 ± 3.54 |
| Vehicle (cilansetron) 35–50 min post | 84.7 ± 8.64 | 350 ± 11.5 | 80.8 ± 19.8 | 1.26 ± 0.56 | 487 ± 54.7 | 31.4 ± 3.51 |
| Cilansetron (0.1 mg·kg−1) 25–10 min pre | 87.9 ± 4.62 | 374 ± 35.7 | 106 ± 14.6 | 1.23 ± 0.19 | 426 ± 60.6 | 43.7 ± 8.66 |
| Cilansetron (0.1 mg·kg−1) 5–20 min post | 93.3 ± 5.27 | 371 ± 32.8 | 104 ± 12.8 | 1.11 ± 0.11 | 473 ± 64.5 | 50.1 ± 9.76 |
| Cilansetron (0.1 mg·kg−1) 35–50 min post | 92.0 ± 6.66 | 382 ± 28.3 | 91.9 ± 7.24 | 1.01 ± 0.07 | 477 ± 46.1 | 47.1 ± 6.92 |
| Cilansetron (0.3 mg·kg−1) 25–10 min pre | 89.2 ± 4.72 | 392 ± 24.5 | 92.7 ± 22.4 | 1.02 ± 0.23 | 464 ± 92.8 | 29.0 ± 4.42 |
| Cilansetron (0.3 mg·kg−1) 5–20 min post | 86.7 ± 2.20 | 376 ± 14.0 | 89.2 ± 18.6 | 1.03 ± 0.22 | 473 ± 96.1 | 28.0 ± 3.67 |
| Cilansetron (0.3 mg·kg−1) 35–50 min post | 85.5 ± 3.84 | 384 ± 18.5 | 80.1 ± 9.44 | 0.95 ± 0.13 | 474 ± 95.5 | 27.7 ± 3.76 |
| Vehicle (tegaserod) 25–10 min pre | 105 ± 4.37 | 359 ± 17.7 | 68.4 ± 11.4 | 0.64 ± 0.10 | 409 ± 114 | 21.3 ± 4.68 |
| Vehicle (tegaserod) 5–20 min post | 103 ± 5.52 | 361 ± 19.2 | 66.8 ± 7.83 | 0.66 ± 0.07 | 409 ± 118 | 21.7 ± 3.84 |
| Vehicle (tegaserod) 35–50 min post | 106 ± 6.02 | 371 ± 20.2 | 66.0 ± 10.8 | 0.63 ± 0.09 | 404 ± 112 | 20.6 ± 2.92 |
| Tegaserod (0.3 mg·kg−1) 25–10 min pre | 94.1 ± 3.19 | 317 ± 17.1 | 66.9 ± 8.97 | 0.75 ± 0.11 | 425 ± 75.1 | 21.6 ± 1.81 |
| Tegaserod (0.3 mg·kg−1) 5–20 min post | 86.6 ± 3.33** | 321 ± 18.0 | 58.4 ± 8.24 | 0.67 ± 0.10 | 437 ± 83.4 | 20.8 ± 1.41 |
| Tegaserod (0.3 mg·kg−1) 35–50 min post | 88.1 ± 3.06* | 316 ± 20.4 | 56.2 ± 9.91 | 0.64 ± 0.12 | 468 ± 83.4 | 24.6 ± 2.54 |
| Tegaserod (1 mg·kg−1) 25–10 min pre | 100 ± 5.41 | 327 ± 23.9 | 75.5 ± 12.8 | 0.68 ± 0.15 | 443 ± 75.1 | 24.8 ± 3.26 |
| Tegaserod (1 mg·kg−1) 5–20 min post | 94.1 ± 4.61 | 293 ± 11.0 | 63.4 ± 11.5 | 0.63 ± 0.14 | 431 ± 73.4 | 23.4 ± 3.06 |
| Tegaserod (1 mg·kg−1) 35–50 min post | 97.3 ± 4.90 | 302 ± 11.1 | 67.9 ± 11.3 | 0.61 ± 0.14 | 445 ± 73.4 | 21.9 ± 2.66 |
| L-NAME (0.02 mmol·kg−1) 25–10 min pre | 89.9 ± 4.80 | 328 ± 16.3 | 106 ± 19.4 | 1.20 ± 0.21 | 392 ± 36.1 | 27.7 ± 3.24 |
| L-NAME (0.02 mmol·kg−1) 5–20 min post | 123 ± 4.19** | 324 ± 14.1 | 78.5 ± 7.99* | 0.66 ± 0.10** | 400 ± 32.6 | 37.4 ± 5.59 |
| L-NAME (0.02 mmol·kg−1) 35–50 min post | 122 ± 5.51** | 333 ± 11.7 | 69.8 ± 12.6** | 0.60 ± 0.14** | 399 ± 32.1 | 30.4 ± 3.07 |
CBF and CVC were measured by the hydrogen gas clearance technique. Note that the vehicle for L-NAME (saline) was the same as for cilansetron. The experimental parameters were recorded and averaged during the pre-injection and post-injection periods as indicated. Means ± SEM, n= 6–9.
P < 0.05,
P < 0.01 versus pre-injection (one-way anova for repeated measures followed by the Bonferroni test).
CBF, colonic blood flow; CVC, colonic vascular conductance; HR, heart rate; L-NAME, N-nitro-L-arginine methylester; MAP, mean arterial blood pressure.
Alosetron (0.03, 0.1 and 0.3 mg·kg−1) failed to alter MAP and HR during the 50 min observation period post injection (Table 2). All three doses of alosetron, though, led to a small and, in some cases, significant decrease of MBF and MVC (Figure 1A,B), whereas CBF and CVC were not significantly altered (Table 2). Like alosetron, cilansetron (0.1 and 0.3 mg·kg−1) did not modify MAP and HR during the 50 min observation period post injection (Table 2). However, both doses of cilansetron caused a slight fall of MBV and MVC, which, in some groups, reached statistical significance (Figure 1C,D). In contrast, both CBF and CVC remained unchanged by either dose of cilansetron (Table 2).
Figure 1.
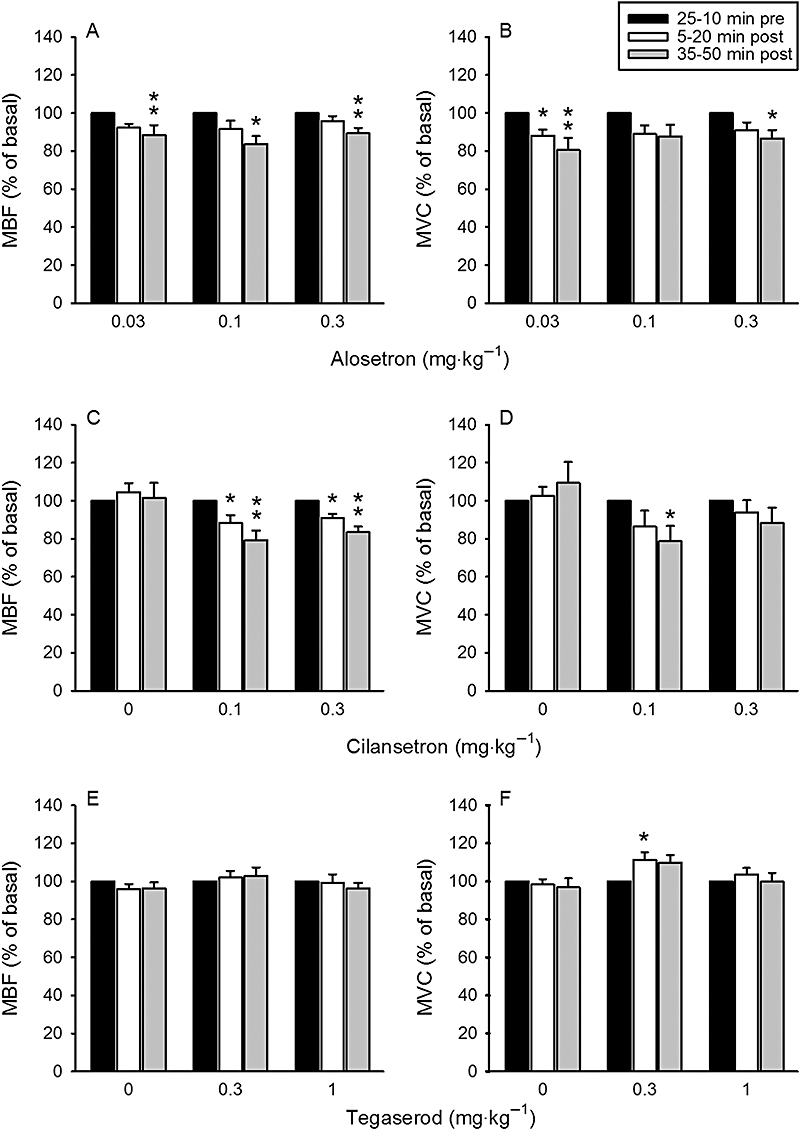
Dose-dependent effects of i.v.-injected (A,B) alosetron, (C,D) cilansetron and (E,F) tegaserod on mesenteric blood flow (MBF, left panels) and mesenteric vascular conductance (MVC, right panels). The doses of the drugs are indicated below the panels: 0 refers to the effects of vehicle. The basal values of the cardiovascular parameters were recorded and averaged during the 25–10 min period pre injection and set as 100%. The values recorded and averaged during the 5–20 min and 35–50 min periods post injection are expressed as a percentage of the baseline recordings. Means + SEM, n= 6–9. *P < 0.05, **P < 0.01 versus basal (one-way anova for repeated measures followed by the Bonferroni test).
After injection of 0.3 mg·kg−1 tegaserod, MAP fell slightly but to a significant extent, whereas, after injection of 1 mg·kg−1 tegaserod MAP did not change significantly (Table 2). HR stayed unaltered by either dose of tegaserod (Table 2). Tegaserod (0.3 and 1 mg·kg−1) also failed to modify MBF and MVC, with the exception of a small but significant rise of MVC during the 5–20 min observation period after administration of 0.3 mg·kg−1 tegaserod (Figure 1E,F). CBF and CVC remained unchanged by either dose of tegaserod (Table 2).
For the current in vivo preparation to be validated, i.v. administration of the vasoconstrictor drug L-NAME was used. Relative to vehicle, L-NAME (0.02 mmol·kg−1) led to a sustained rise of MAP in the absence of any significant change of HR (Table 2). The hypertension caused by L-NAME was accompanied by a pronounced and sustained decrease of MBF and MVC, with the magnitude of these effects being identical to those recorded in study 2 (Figure 2A,B,C,D). CBF and CVC were also significantly attenuated after injection of L-NAME (Table 2).
Figure 2.
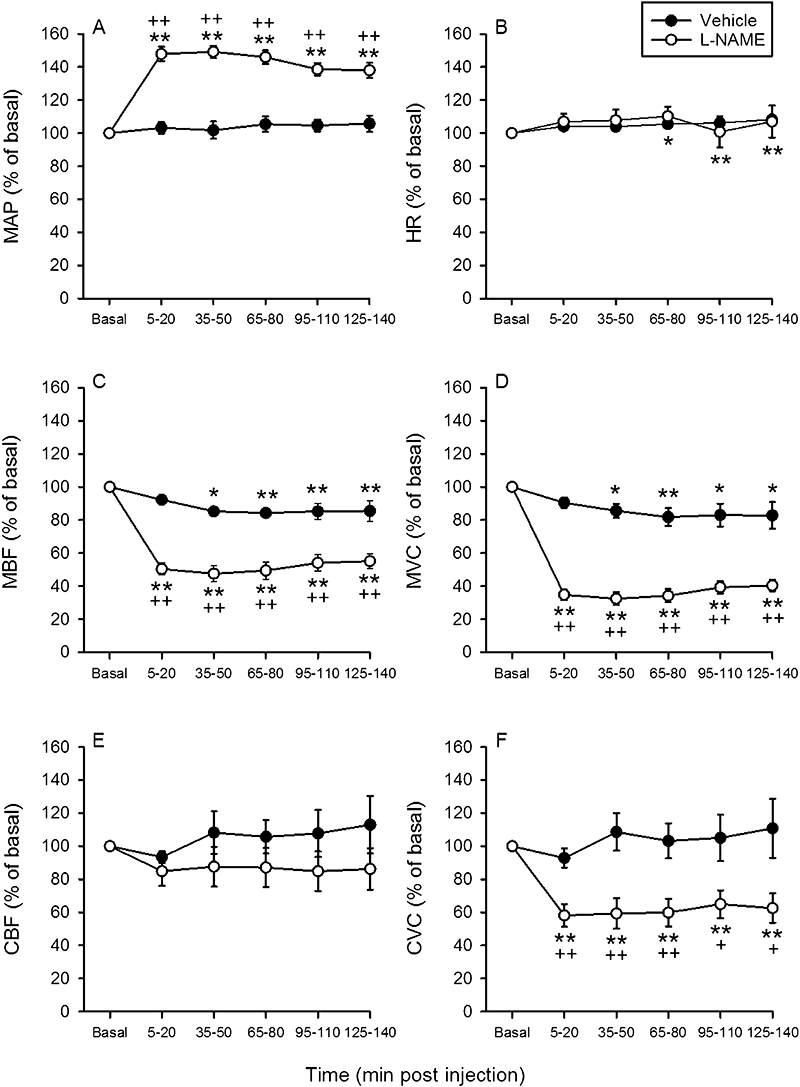
Time-dependent effects of i.v.-injected vehicle and N-nitro-L-arginine methylester (0.02 mmol·kg−1) on (A) mean arterial blood pressure (MAP), (B) heart rate (HR), (C) mesenteric blood flow (MBF), (D) mesenteric vascular conductance (MVC), (E) colonic blood flow (CBF) measured by laser Doppler flowmetry and (F) colonic vascular conductance (CVC). The basal values of the cardiovascular parameters were recorded and averaged during the 25–10 min period pre injection and set as 100%. The values recorded and averaged during the 5–20 min, 35–50 min, 95–110 min and 125–140 min periods post injection are expressed as a percentage of the baseline recordings. Means ± SEM, n= 9–10. *P < 0.05, **P < 0.01 versus basal (one-way anova for repeated measures followed by the Bonferroni test); +P < 0.05, ++P < 0.01 versus vehicle (Student's t-test).
At baseline conditions, the tonic intraluminal pressure in the ascending colon was 424 ± 21.5 Pa (n= 89), and the phasic increases in colonic pressure superimposed on the tonic pressure amounted to 27.5 ± 1.2 Pa·(10 s)−1 (n= 89) Neither the tonic intraluminal pressure nor the phasic pressure increases were altered by L-NAME (0.02 mmol·kg−1), alosetron (0.03, 0.1 and 0.3 mg·kg−1), cilansetron (0.1 and 0.3 mg·kg−1) and tegaserod (0.3 and 1 mg·kg−1) in any consistent manner (Table 2).
Time-dependent effects of i.v. injection of alosetron and tegaserod in fasted and non-fasted rats (study 2)
Study 2 pursued three aims. The first aim was to evaluate whether it takes a prolonged period of time (140 min) to observe changes in colonic haemodynamics after acute i.v. injection of alosetron, tegaserod and, for validation purposes, L-NAME. The second aim was to test whether the drug effects on CBF and CVC recorded with the hydrogen gas clearance technique can be reproduced with laser Doppler flowmetry. The third aim was to examine whether food deprivation before the experiments modifies the effect of acute i.v. injection of alosetron and tegaserod on the splanchnic circulation. These experiments were carried out with only one dose of each drug, which was chosen on the basis of the results of study 1. The dose of 0.03 mg·kg−1 alosetron was chosen because it was most active in reducing MVC in study 1 (Figure 1). As tegaserod had no consistent effects in study 1, the highest dose (1 mg·kg−1) used in those experiments was selected. The cardiovascular parameters recorded at baseline are summarised in Table 1.
Fasted rats
The extension of the recording period to 140 min showed that, following i.v. injection of the vehicles for L-NAME, alosetron and tegaserod, MAP and HR tended to rise over time, whereas MBF, MVC, CBF and CVC tended to fall, and that these changes were statistically significant in some experimental groups (Figures 2–4, Table 3). The prompt effect of L-NAME (0.02 mmol·kg−1) to increase MAP in the absence of any significant change of HR was still evident 90–140 min post injection (Figure 2A,B). This was also true for the action of L-NAME to reduce MBF and MVC (Figure 2C,D). While CBF measured with the laser Doppler flowmetry was not significantly diminished by L-NAME (Figure 2E), CVC was significantly attenuated throughout the 140 min observation period (Figure 2F).
Figure 4.
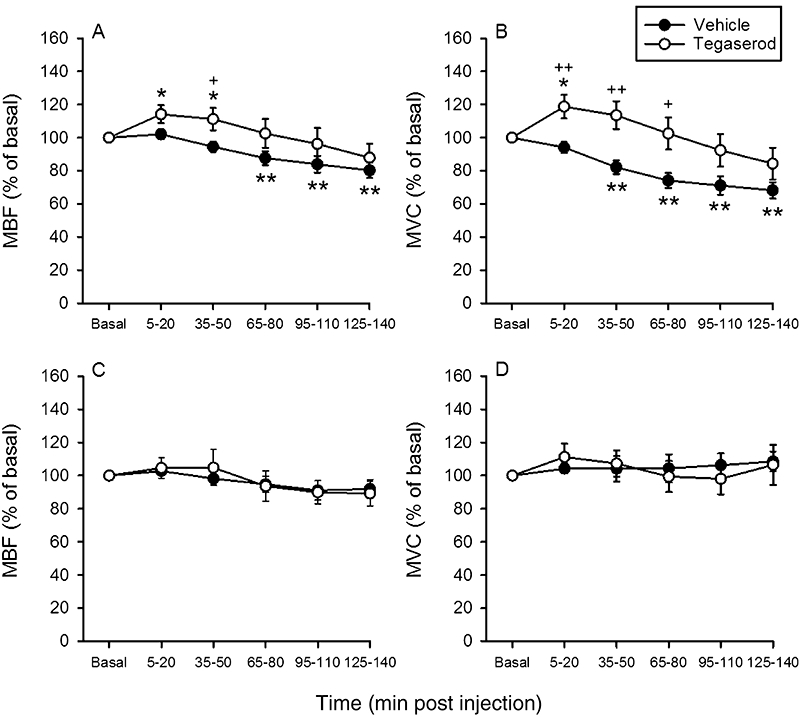
Time-dependent effects of i.v.-injected vehicle and tegaserod (1 mg·kg−1) on mesenteric blood flow (MBF, left panels) and mesenteric vascular conductance (MVC, right panels) in fasted (A,B) and non-fasted (C,D) rats. The basal values of the cardiovascular parameters were recorded and averaged during the 25–10 min period pre injection and set as 100%. The values recorded and averaged during the 5–20 min, 35–50 min, 95–110 min and 125–140 min periods post injection are expressed as a percentage of the baseline recordings. Means ± SEM, n= 7–12. *P < 0.05, **P < 0.01 versus basal (one-way anova for repeated measures followed by the Bonferroni test); +P < 0.05, ++P < 0.01 versus vehicle (Student's t-test).
Table 3.
Effect of acute i.v. injection of vehicle, alosetron and tegaserod on MAP, HR, CBF and CVC of fasted rats during a prolonged period of time (140 min)
| Treatment and recording period | MAP (mmHg) | HR (beats·min−1) | CBF (PU) | CVC [PU·(mmHg)−1] |
|---|---|---|---|---|
| Vehicle (alosetron) 25–10 min pre | 85.5 ± 4.14 | 340 ± 7.47 | 164 ± 49.7 | 1.81 ± 0.45 |
| Vehicle (alosetron) 5–20 min post | 87.9 ± 3.90 | 353 ± 10.2 | 159 ± 51.5 | 1.71 ± 0.45 |
| Vehicle (alosetron) 65–80 min post | 92.3 ± 3.75* | 369 ± 8.07** | 139 ± 40.5 | 1.45 ± 0.36** |
| Vehicle (alosetron) 125–140 min post | 91.0 ± 3.77 | 376 ± 10.5** | 136 ± 32.2 | 1.46 ± 0.31** |
| Alosetron (0.03 mg·kg−1) 25–10 min pre | 86.5 ± 4.86 | 341 ± 32.6 | 155 ± 30.3 | 1.75 ± 0.27 |
| Alosetron (0.03 mg·kg−1) 5–20 min post | 89.0 ± 5.71 | 353 ± 36.6 | 155 ± 31.0 | 1.69 ± 0.26 |
| Alosetron (0.03 mg·kg−1) 65–80 min post | 93.6 ± 4.90 | 376 ± 39.9 | 146 ± 29.6 | 1.52 ± 0.25 |
| Alosetron (0.03 mg·kg−1) 125–140 min post | 91.5 ± 5.45 | 382 ± 37.2* | 141 ± 31.8 | 1.50 ± 0.29 |
| Vehicle (tegaserod) 25–10 min pre | 75.8 ± 2.25 | 344 ± 11.9 | 258 ± 63.0 | 3.45 ± 0.90 |
| Vehicle (tegaserod) 5–20 min post | 83.2 ± 2.09** | 363 ± 14.3 | 233 ± 50.7 | 2.89 ± 0.59 |
| Vehicle (tegaserod) 65–80 min post | 89.9 ± 2.04** | 389 ± 15.5** | 205 ± 44.1 | 2.42 ± 0.54* |
| Vehicle (tegaserod) 125–140 min post | 89.8 ± 2.81** | 391 ± 19.8** | 196 ± 47.6 | 2.26 ± 0.57** |
| Tegaserod (1 mg·kg−1) 25–10 min pre | 89.1 ± 4.53 | 354 ± 18.1 | 216 ± 29.6 | 2.49 ± 0.36 |
| Tegaserod (1 mg·kg−1) 5–20 min post | 86.5 ± 5.22 | 332 ± 15.9* | 218 ± 39.8 | 2.54 ± 0.45 |
| Tegaserod (1 mg·kg−1) 65–80 min post | 87.0 ± 2.57 | 360 ± 15.8 | 203 ± 43.8 | 2.26 ± 0.47 |
| Tegaserod (1 mg·kg−1) 125–140 min post | 93.1 ± 2.48 | 382 ± 14.9** | 184 ± 39.3 | 2.02 ± 0.41 |
CBF and CVC were measured by laser Doppler flowmetry. The experimental parameters were recorded and averaged during the pre-injection and post-injection periods as indicated. Means ± SEM, n= 8–12.
P < 0.05,
P < 0.01 versus pre-injection (one-way anova for repeated measures followed by the Bonferroni test).
CBF, colonic blood flow; CVC, colonic vascular conductance; HR, heart rate; MAP, mean arterial blood pressure; PU, perfusion unit.
Alosetron (0.03 mg·kg−1) did not consistently affect MAP and HR during the 140 min observation period post injection nor did it alter CBF and CVC (Table 3). As found in study 1, MBF and MVC were significantly (P < 0.05) decreased 5–20 min after injection of alosetron when compared with the values measured after vehicle injection (Figure 3A,B). However, this effect of alosetron was no longer seen 35–140 min post injection (Figure 3A,B). Compared with vehicle, tegaserod (1 mg·kg−1) led to an initial rise of MBF and MVC in this series of experiments (Figure 4A,B), an effect that was gone 95–140 min post injection (Figure 4A,B). CBF and CVC did not differ throughout the 140 min observation period after injection of vehicle or tegaserod (Table 3). MAP was not significantly altered by tegaserod, whereas HR first fell and later increased relative to the pre-injection value (Table 3).
Figure 3.
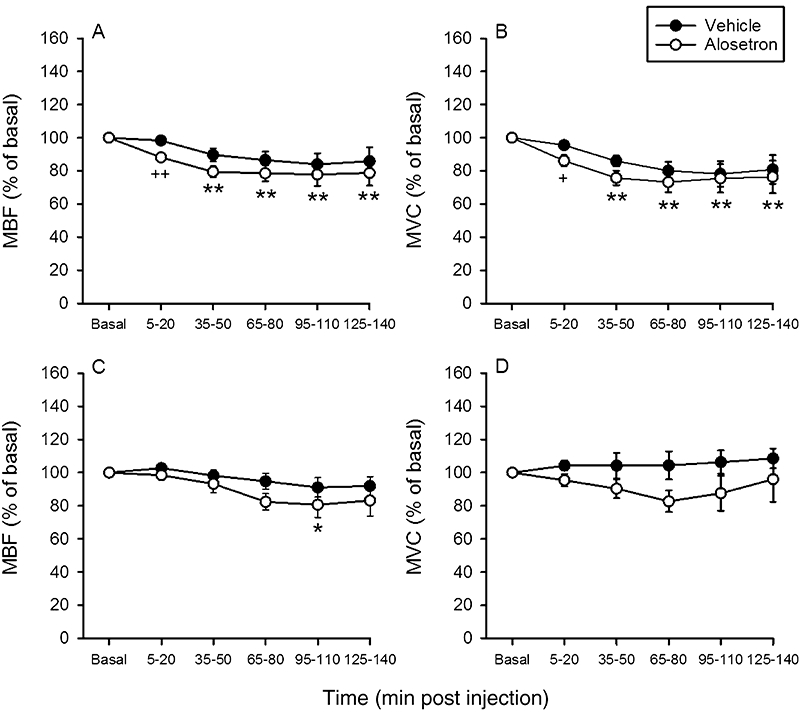
Time-dependent effects of i.v.-injected vehicle and alosetron (0.03 mg·kg−1) on mesenteric blood flow (MBF, left panels) and mesenteric vascular conductance (MVC, right panels) in fasted (A,B) and non-fasted (C,D) rats. The basal values of the cardiovascular parameters were recorded and averaged during the 25–10 min period pre injection and set as 100%. The values recorded and averaged during the 5–20 min, 35–50 min, 95–110 min and 125–140 min periods post injection are expressed as a percentage of the baseline recordings. Means ± SEM, n= 7–10. *P < 0.05, **P < 0.01 versus basal (one-way anova for repeated measures followed by the Bonferroni test); +P < 0.05, ++P < 0.01 versus vehicle (Student's t-test).
Non-fasted rats
In these experiments, CBF and CVC were not estimated because intraluminal placement of the endoscopic laser Doppler flowmetry probe required that the colon be emptied by fasting. The baseline values of MBF and MVC measured in non-fasted rats turned out to be significantly larger than those measured in fasted rats, whereas MAP and HR values did not differ to a significant extent (Table 1). After i.v. administration of alosetron (0.03 mg·kg−1), MBF and MVC tended to fall but, with the exception of one value, did not become significantly different from the values measured after vehicle injection (Figure 3C,D). Like the vehicle, tegaserod (1 mg·kg−1) failed to alter MBF and MVC during the 140 min observation period post injection (Figure 4C,D). MAP and HR did not significantly change after injection of alosetron and tegaserod, while, after administration of vehicle, MAP and HR decreased with a delay of some 125 min (Table 4).
Table 4.
Effect of acute i.v. injection of vehicle, alosetron and tegaserod on MAP and HR of non-fasted rats during a prolonged period of time (140 min)
| Treatment and recording period | MAP (mmHg) | HR (beats·min−1) |
|---|---|---|
| Vehicle (10% NMP) 25–10 min pre | 81.6 ± 5.51 | 362 ± 19.1 |
| Vehicle (10% NMP) 5–20 min post | 80.1 ± 4.85 | 363 ± 20.6 |
| Vehicle (10% NMP) 65–80 min post | 74.0 ± 5.27 | 353 ± 18.9 |
| Vehicle (10% NMP) 125–140 min post | 69.6 ± 5.61** | 342 ± 17.9** |
| Alosetron (0.03 mg·kg−1) 25–10 min pre | 89.8 ± 3.79 | 355 ± 39.2 |
| Alosetron (0.03 mg·kg−1) 5–20 min post | 92.8 ± 3.54 | 356 ± 40.6 |
| Alosetron (0.03 mg·kg−1) 65–80 min post | 90.3 ± 4.65 | 363 ± 42.1 |
| Alosetron (0.03 mg·kg−1) 125–140 min post | 80.9 ± 7.33 | 372 ± 49.1 |
| Tegaserod (1 mg·kg−1) 25–10 min pre | 87.7 ± 5.60 | 354 ± 13.4 |
| Tegaserod (1 mg·kg−1) 5–20 min post | 83.0 ± 5.64 | 333 ± 11.9 |
| Tegaserod (1 mg·kg−1) 65–80 min post | 82.3 ± 4.08 | 347 ± 15.4 |
| Tegaserod (1 mg·kg−1) 125–140 min post | 74.3 ± 3.78 | 352 ± 14.2 |
The experimental parameters were recorded and averaged during the pre-injection and post-injection periods as indicated. Means ± SEM, n= 7–8.
P < 0.01 versus pre-injection (one-way anova for repeated measures followed by the Bonferroni test).
HR, heart rate; MAP, mean arterial blood pressure; NMP, 1-methyl-2-pyrrolidone.
Effects of i.d. injection of alosetron and tegaserod (study 3)
The aim of study 3 was to test whether alosetron and tegaserod are able to modify CBF and vascular conductance after acute i.d. administration. In addition, the ability of the vascular recording techniques to pick up vasodilator effects was tested with clonidine, a drug with proven oral bioavailability that has been found to increase gastric mucosal vascular conductance in a sustained manner (Holzer and Painsipp, 2001). In view of its GI bioavailability in humans (50–60%), the highest dose of alosetron used in study 1 (0.3 mg·kg−1) was selected for i.d. injection. The dose of tegaserod chosen for i.d. administration was 30 mg·kg−1 because the oral bioavailability of this drug in humans is only about 10% (Rivkin, 2003; Evans et al., 2007). Once baseline recordings of the cardiovascular parameters (Table 1) had been made, vehicle, alosetron or tegaserod was administered i.d., whereafter post-injection recordings were taken for 90 min. Following i.d. injection of clonidine (0.03 mg·kg−1), MAP, HR, MBF and CBF (measured by laser Doppler flowmetry) decreased, whereas MVC, and CVC in particular, increased to a significant extent, with these changes being sustained throughout the observation period (Figure 5).
Figure 5.
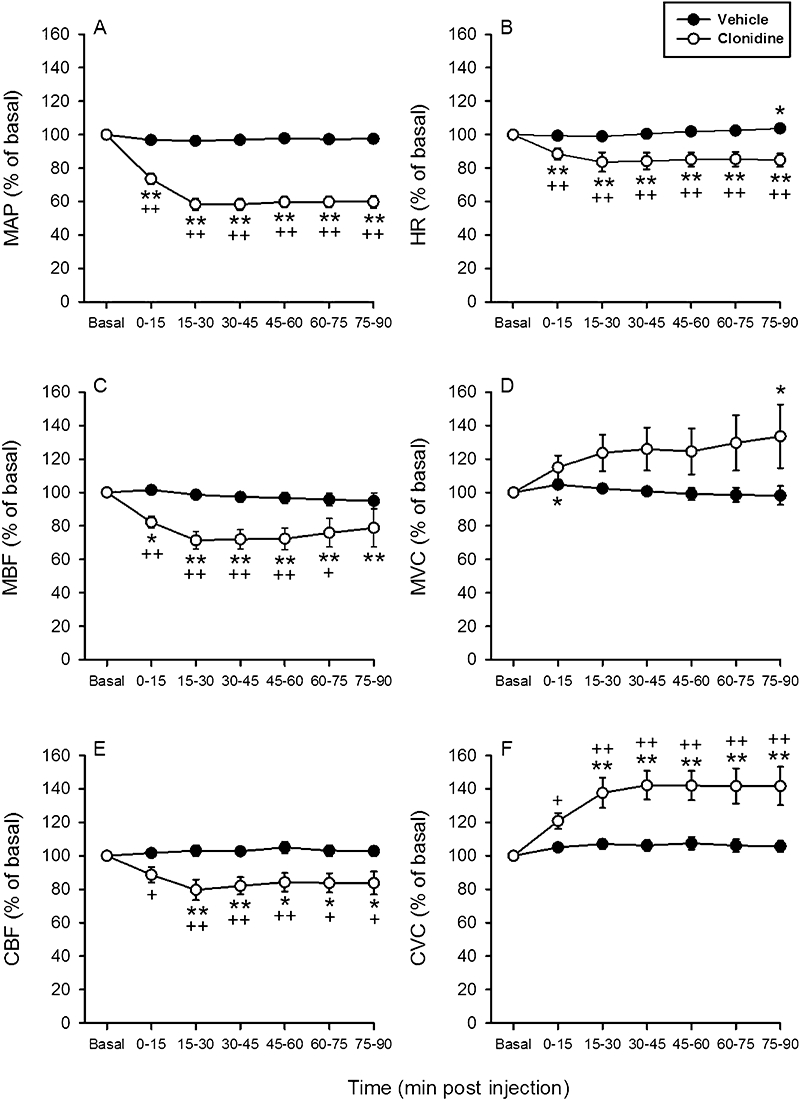
Time-dependent effects of i.d.-injected vehicle and clonidine (0.03 mg·kg−1) on (A) mean arterial blood pressure (MAP), (B) heart rate (HR), (C) mesenteric blood flow (MBF), (D) mesenteric vascular conductance (MVC), (E) colonic blood flow (CBF) measured by laser Doppler flowmetry and (F) colonic vascular conductance (CVC). The basal values of the cardiovascular parameters were recorded and averaged during the 15–0 min period pre injection and set as 100%. The values recorded and averaged during the 0–15 min, 15–30 min, 60–75 min and 75–90 -min periods post injection are expressed as a percentage of the baseline recordings. Means ± SEM, n= 7–8. *P < 0.05, **P < 0.01 versus basal (one-way anova for repeated measures followed by the Bonferroni test); +P < 0.05, ++P < 0.01 versus vehicle (Student's t-test).
Throughout the observation period, i.d. injection of vehicle, alosetron (0.3 mg·kg−1) or tegaserod (30 mg·kg−1) failed to alter MAP, CBF and CVC (Table 5). The HR also remained unchanged, except that there was a late increase of this parameter following vehicle or tegaserod administration (Table 5). Alosetron led to a delayed fall of MBF, which was statistically significant when compared with the pre-injection baseline value (Figure 6A). Relative to vehicle, however, neither alosetron nor tegaserod caused a significant change of MBF and MVC (Figure 6A,B).
Table 5.
Effect of acute i.d. injection of vehicle, alosetron and tegaserod on MAP, HR, CBF and CVC of fasted rats
| Treatment and recording period | MAP (mmHg) | HR (beats·min−1) | CBF (PU) | CVC [PU·(mmHg)−1] |
|---|---|---|---|---|
| Vehicle (10% NMP) 15–0 min pre | 89.3 ± 5.39 | 396 ± 22.1 | 392 ± 48.6 | 4.52 ± 0.73 |
| Vehicle (10% NMP) 0–15 min post | 86.6 ± 5.54 | 393 ± 23.0 | 399 ± 49.8 | 4.75 ± 0.80 |
| Vehicle (10% NMP) 15–30 min post | 86.1 ± 5.64 | 392 ± 22.8 | 405 ± 52.0 | 4.84 ± 0.83 |
| Vehicle (10% NMP) 45–60 min post | 87.1 ± 4.84 | 403 ± 22.7 | 409 ± 51.9 | 4.87 ± 0.88 |
| Vehicle (10% NMP) 75–90 min post | 87.0 ± 5.21 | 422 ± 24.5* | 405 ± 56.3 | 4.86 ± 0.95 |
| Alosetron (0.3 mg·kg−1) 15–0 min pre | 92.7 ± 3.58 | 358 ± 16.8 | 399 ± 51.5 | 4.34 ± 0.59 |
| Alosetron (0.3 mg·kg−1) 0–15 min post | 90.3 ± 4.95 | 352 ± 14.2 | 396 ± 53.3 | 4.46 ± 0.63 |
| Alosetron (0.3 mg·kg−1) 15–30 min post | 91.7 ± 5.63 | 348 ± 14.2 | 395 ± 57.2 | 4.36 ± 0.61 |
| Alosetron (0.3 mg·kg−1) 45–60 min post | 91.7 ± 5.81 | 355 ± 14.4 | 404 ± 53.6 | 4.42 ± 0.55 |
| Alosetron (0.3 mg·kg−1) 75–90 min post | 91.3 ± 4.96 | 357 ± 13.1 | 410 ± 51.0 | 4.50 ± 0.55 |
| Tegaserod (30 mg·kg−1) 15–0 min pre | 81.9 ± 3.37 | 372 ± 8.93 | 392 ± 60.1 | 4.83 ± 0.74 |
| Tegaserod (30 mg·kg−1) 0–15 min post | 79.1 ± 3.86 | 372 ± 11.7 | 391 ± 61.5 | 5.06 ± 0.86 |
| Tegaserod (30 mg·kg−1) 15–30 min post | 79.3 ± 3.60 | 371 ± 11.8 | 370 ± 46.6 | 4.77 ± 0.66 |
| Tegaserod (30 mg·kg−1) 45–60 min post | 84.9 ± 3.70 | 383 ± 14.0 | 377 ± 60.3 | 4.56 ± 0.81 |
| Tegaserod (30 mg·kg−1) 75–90 min post | 85.1 ± 2.72 | 386 ± 13.5** | 379 ± 56.3 | 4.49 ± 0.70 |
The experimental parameters were recorded and averaged during the pre-injection and post-injection periods as indicated. Means ± SEM, n= 7–8.
P < 0.05,
P < 0.01 versus pre-injection (one-way anova for repeated measures followed by the Bonferroni test).
CBF, colonic blood flow; CVC, colonic vascular conductance; HR, heart rate; MAP, mean arterial blood pressure; NMP, 1-methyl-2-pyrrolidone; PU, perfusion unit.
Figure 6.
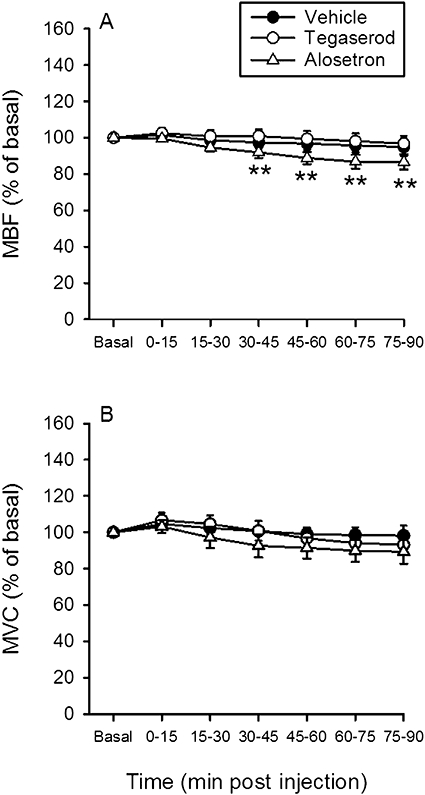
Time-dependent effects of i.d.-injected vehicle, alosetron (0.3 mg·kg−1) and tegaserod (30 mg·kg−1) on (A) mesenteric blood flow (MBF) and (B) mesenteric vascular conductance (MVC). The basal values of the cardiovascular parameters were recorded and averaged during the 15–0 min period pre injection and set as 100%. The values recorded and averaged during the 0–15 min, 15–30 min, 60–75 min and 75–90 min periods post injection are expressed as a percentage of the baseline recordings. Means ± SEM, n= 7–8. **P < 0.01 versus basal (one-way anova for repeated measures followed by the Bonferroni test).
Effects of short-term peroral pretreatment with alosetron and tegaserod (study 4)
The rationale of study 4 was to test whether prolonged peroral administration causes alosetron and tegaserod to induce colonic vasoconstriction and to modify the cardiovascular effects of L-NAME after repeated peroral administration for 1 week. Rats were pretreated for 7 days by thrice-daily IG administrations of vehicle, alosetron (0.3 mg·kg−1) or tegaserod (1 mg·kg−1), the highest doses tested in study 1. The last dose of each drug was administered 1 h before the rats were anaesthetised. Once baseline recordings of the cardiovascular parameters (Table 1) had been made, L-NAME (0.02 mmol·kg−1) was injected i.v., whereafter post-injection recordings were made for 110 min. The baseline parameters for MBF and MVC did not differ significantly between rats that had been pretreated with IG vehicle, alosetron or tegaserod for 7 days (Table 6). As found in study 2 (Figure 2), i.v. injection of L-NAME led to a prompt increase of MAP, which was accompanied by a marked reduction of MBF, MVC and CVC, whereas CBF (measured with laser Doppler flowmetry) was not significantly modified. The magnitude of the hypertensive, mesenteric and colonic vasoconstrictor responses to L-NAME was indistinguishable in rats that had been pretreated with IG vehicle, alosetron or tegaserod for 7 days (Table 6). In contrast, the increase in HR, which, in vehicle-pretreated rats, accompanied the hypertensive response to L-NAME, was absent in rats pretreated with alosetron or tegaserod (Table 6).
Table 6.
Effect of short-term peroral administration of vehicle, alosetron and tegaserod on MAP, HR, MBF, MVC, CBF and CVC of fasted rats at baseline (before) and after acute i.v. injection of L-NAME
| Treatment and recording period | MAP (mmHg) | HR (beats·min−1) | MBF (µL·min−1) | MVC [µL·min−1 (mmHg)−1] | CBF (PU) | CVC [PU (mmHg)−1] |
|---|---|---|---|---|---|---|
| 25–10 min before L-NAME (vehicle pretreatment) | 77.3 ± 4.23 | 316 ± 11.8 | 16.4 ± 0.74 | 215 ± 16.0 | 196 ± 47.8 | 2.68 ± 0.71 |
| 5–20 min post L-NAME (vehicle pretreatment) | 124 ± 3.41** | 350 ± 10.0** | 7.91 ± 0.81** | 64.3 ± 8.26** | 169 ± 45.7 | 1.38 ± 0.38** |
| 95–110 min post L-NAME (vehicle pretreatment) | 125 ± 2.46** | 365 ± 16.2** | 7.59 ± 0.99** | 60.2 ± 7.48** | 183 ± 43.1 | 1.44 ± 0.33** |
| 25–10 min before L-NAME (alosetron pretreatment) | 77.3 ± 5.90 | 316 ± 24.2 | 14.0 ± 1.76 | 192 ± 30.8 | 349 ± 73.9 | 5.05 ± 1.35 |
| 5–20 min post L-NAME (alosetron pretreatment) | 120 ± 2.50** | 340 ± 10.7 | 8.02 ± 1.63** | 67.3 ± 14.4** | 303 ± 64.1 | 2.57 ± 0.58** |
| 95–110 min post L-NAME (alosetron pretreatment) | 116 ± 5.95** | 328 ± 17.1 | 8.33 ± 1.41** | 73.2 ± 12.9** | 428 ± 123 | 4.01 ± 1.28 |
| 25–10 min before L-NAME (tegaserod pretreatment) | 83.1 ± 5.68 | 340 ± 20.8 | 16.0 ± 1.23 | 199 ± 21.4 | 328 ± 94.4 | 3.94 ± 1.22 |
| 5–20 min post L-NAME (tegaserod pretreatment) | 126 ± 2.88** | 331 ± 10.1 | 8.37 ± 0.68** | 66.5 ± 5.69** | 310 ± 93.2 | 2.39 ± 0.70** |
| 95–110 min post L-NAME (tegaserod pretreatment) | 125 ± 2.28** | 342 ± 9.44 | 7.41 ± 0.89** | 59.6 ± 7.51** | 221 ± 63.4 | 1.74 ± 0.48** |
Rats were pretreated for 7 days by 3 daily IG administrations of vehicle, alosetron (0.3 mg·kg−1) or tegaserod (1 mg·kg−1). The cardiovascular parameters were recorded before and after i.v. injection of L-NAME (0.02 mmol·kg−1) and averaged for the periods indicated. Means ± SEM, n= 7.
P < 0.01 versus pre-injection (one way anova for repeated measures followed by the Bonferroni test).
CBF, colonic blood flow; CVC, colonic vascular conductance; HR, heart rate; L-NAME, N-nitro-L-arginine methylester; MAP, mean arterial blood pressure; MBF, mesenteric blood flow; MVC, mesenteric vascular conductance; PU, perfusion unit.
Effect of cilansetron in rats with mild colitis (study 5)
Study 5 was carried out to test whether mild inflammation causes cilansetron to evoke vasoconstriction in the colon. Treatment of rats with DSS (3% added to the drinking water) for 7 days led to a significant increase in the MPO content of the colonic wall (Figure 7A), which was associated with a significant rise of the disease activity index (Figure 7B). Pilot experiments had demonstrated that treatment of rats with 1 or 2% DSS for 7 days (n= 6–9) failed to significantly enhance the colonic MPO level. Baseline MAP (106.5 ± 5.9 mmHg in the control rats, 100.4 ± 5.4 mmHg in the DSS-treated rats, n= 10–11) and baseline HR (334 ± 8.5 beats·min−1 in the control rats, 364 ± 8.7 beats·min−1 in the DSS-treated rats, n= 10–11) did not differ significantly between the control animals and rats with mild colitis. Cilansetron (0.3 mg·kg−1), a dose found to reduce MVC in study 1 (Figure 1), failed to alter any of these parameters in both control and DSS-treated animals. The recordings of CBF and CVC are shown in Figure 7C,D.
Figure 7.
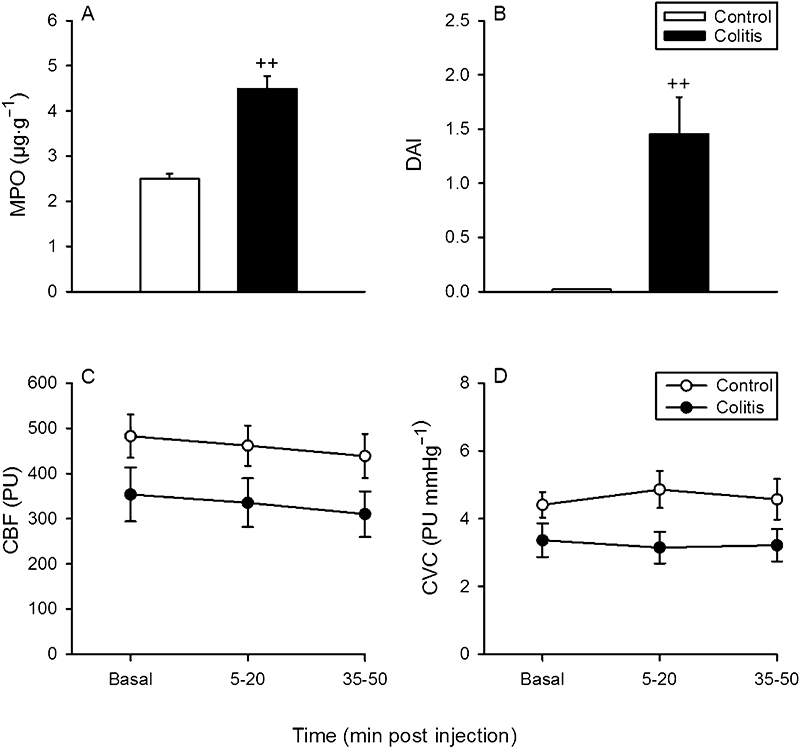
Effect of low-grade colitis on (A) the myeloperoxidase (MPO) content of the colonic wall, (B) the disease activity index (DAI), and the effect of i.v.-administered cilansetron (0.3 mg·kg−1) on (C) colonic blood flow (CBF) measured by laser Doppler flowmetry and (D) colonic vascular conductance (CVC). Low-grade colitis was induced by adding dextran sulphate sodium (3%) to the drinking water for 7 days. The control animals received normal tap water. CBF and CVC were recorded and averaged during the 15–0 min period pre injection and the 5–20 min and 35–50 min periods post injection. It should be noted that the DAI for the control group in panel B was 0. Means ± SEM, n= 10–11. ++P < 0.01 versus control (Student's t-test). PU, perfusion unit.
Discussion
The major results of the current study can be summarised as follows:
Treatment with the 5-HT3 receptor antagonist alosetron had the potential of causing a minor constriction of the vascular bed of the superior mesenteric artery of anaesthetised rats, whereas a minor dilatation of this arterial bed was seen after treatment with the partial 5-HT4 receptor agonist tegaserod.
The small mesenteric vasoconstriction caused by alosetron was observed after both i.v. and i.d. administration of the drug and appears to be a class effect of 5-HT3 receptor antagonists, as it was shared by cilansetron.
The vasoconstrictor response to 5-HT3 receptor antagonism was transient, and the short-term peroral administration of alosetron was without effect on the haemodynamics in the superior mesenteric artery.
The minor vasoconstrictor action of 5-HT3 receptor antagonism on the mesenteric artery was not paralleled by any appreciable change in colonic motor activity.
Alosetron, cilansetron and tegaserod failed to modify blood flow in the colon.
Fasting had no influence on the action profile of alosetron and tegaserod in the mesenteric and colonic vascular beds.
Mild colitis failed to provoke a vasoconstrictor effect of 5-HT3 receptor antagonism in the colon.
These data add significantly to the results of another study in which alosetron did not alter mesenteric and CBF of anaesthetised rats and failed to interfere with splanchnic vascular control mechanisms during occlusion and reactive hyperaemia (Grundy et al., 2007).
Blood flow in the superior mesenteric artery was recorded with the ultrasonic transit time shift technique (Holzer et al., 1994; Heinemann et al., 1999), whereas blood flow in the transverse colon, which is supplied by the superior mesenteric artery, was estimated by hydrogen gas clearance and laser Doppler flowmetry. Both techniques have been validated and used to measure nutrient blood flow in the wall of the GI tract (Johansson, 1988; Livingston et al., 1989; Holzer et al., 1991). By calculating the vascular conductance, it was possible to obtain a measure of dilatation or constriction of the vascular bed under study (Heinemann et al., 1998). The sensitivity of our experimental model to i.v.-administered vasoactive drugs was proved by the vasoconstrictor response to the nitric oxide synthase inhibitor L-NAME (0.02 mmol·kg−1). Intravenous injection of this drug is known to decrease the conductance in various vascular beds including the rat gastric mucosa, which causes sustained hypertension (Gardiner et al., 1990; Holzer et al., 1994). In the current study, L-NAME constricted not only the mesenteric arterial bed but also the colonic vasculature, as revealed by hydrogen gas clearance and laser Doppler flowmetry. The ability of our preparation to respond to i.d.-administered vasoactive drugs was verified by the vasodilator response to clonidine, an α2-adrenoceptor agonist with oral bioavailability (Arndts et al., 1983). In our experiments, clonidine (0.03 mg·kg−1) enhanced the vascular conductance both in the mesenteric and colonic vascular bed. Our in vivo preparation is thus able to reveal constriction and dilatation of the mesenteric and colonic vasculature.
Relative to the pronounced changes in the mesenteric and colonic haemodynamics elicited by L-NAME and clonidine, alosetron, cilansetron and tegaserod caused only minor alterations of the splanchnic circulation. The i.v. doses of alosetron (0.03, 0.01 and 0.3 mg·kg−1), cilansetron (0.1 and 0.3 mg·kg−1) and tegaserod (0.3 and 1 mg·kg−1) used here are considered to be clinically relevant because the standard single doses used in humans are 1 mg alosetron, 2 mg cilansetron and 6 mg tegaserod (Evans et al., 2007; Andresen et al., 2008). For a patient weighing 70 kg, these doses would amount to 0.014 mg·kg−1 alosetron, 0.28 mg·kg−1 cilansetron and 0.086 mg·kg−1 tegaserod. The dose equivalents of these drugs in rodents have not been reported, but it can be assumed that equiactive drug doses in rats are higher than in humans because of faster metabolism processes in small animals. Hence, we suppose that, at the doses used, both alosetron and cilansetron retain their selectivity for 5-HT3 receptors (De Ponti, 2004). In contrast, tegaserod is not only a partial 5-HT4 receptor agonist but also a 5-HT1B and 5-HT2B receptor antagonist (Beattie et al., 2004; Weber et al., 2006).
Our findings with alosetron differ from those made by Grundy et al. (2007), who failed to see an effect of i.v. alosetron (0.01–0.1 mg·kg−1) on MBF and CBF. In contrast, we observed that alosetron consistently reduced MBF and MVC by a maximum of 20%. Following i.v. injection, the mesenteric vasoconstrictor effect of alosetron was prompt and transient because it was seen only during a 50 min post-injection period. Because, therapeutically, alosetron is taken per os, it was also tested after i.d. administration, in which case the effect of alosetron to decrease MBF was delayed, which can be explained by the time taken until alosetron was absorbed from the small intestine. The high potency of alosetron to cause mesenteric vasoconstriction at i.v. doses as small as 0.03 mg·kg−1 is consistent with the high potency of alosetron as a 5-HT3 receptor antagonist (De Ponti, 2004). As the therapeutic dosage of alosetron in humans is also low (De Ponti, 2004), we conclude that the mesenteric vasoconstrictor effect of alosetron in anaesthetised rats is elicited by therapeutically relevant doses of the drug. This conclusion is supported by the results obtained with i.d.-administered alosetron. As the intestinal absorption rate of alosetron in anaesthetised rats has not been published, the haemodynamic effects of i.d.-administered alosetron were tested with a dose (0.3 mg·kg−1) 10 times higher than that found to reduce MBF and MVC after i.v. injection.
The effect of alosetron to reduce MBF and MVC was shared by cilansetron, another 5-HT3 receptor antagonist (Chey and Cash, 2005), which indicates that mesenteric vasoconstriction is a class effect of 5-HT3 receptor antagonists, at least in the rat. In analysing this action, we need to consider that MBF and MVC were attenuated in the absence of any reduction of CBF and CVC. In particular, our measurements of CBF and CVC with hydrogen gas clearance and laser Doppler flowmetry show that nutrient blood flow through the colonic mucosa was not compromised by alosetron and cilansetron. This inference can be made because laser Doppler flowmetry with an endoscopic fibre probe records blood flow primarily in the mucosa, and the hydrogen gas clearance was measured with an electrode positioned close to the submucosal arteriolar plexus, which is critical to the regulation of mucosal blood flow (Granger et al., 1980; Guth and Leung, 1987; Holzer et al., 1991). As about two-thirds of the total resistance in the GI circulation arises from the resistance in the intramural arterioles (Gore and Bohlen, 1977) and about three-quarters of the blood delivered to the GI tract is fed into the submucosal–mucosal arteriolar circuit (Bohlen et al., 1978; Granger et al., 1980; Guth and Leung, 1987), it is, at first sight, surprising that MVC was reduced by alosetron and cilansetron without a concomitant decrease in CVC. However, there are numerous examples that total blood flow to the GI tract and fractional blood flow to its microcirculatory circuits are differentially regulated by endocrine and neural factors as well as by pressure-flow autoregulation, capillary recruitment and opening of arteriovenous anastomoses (Holzer, 2006). As the current study suggests, this is also true for 5-HT3 receptor-mediated modification of the splanchnic circulation.
The observation that MBF and MVC were attenuated by alosetron and cilansetron in the absence of any change of MAP and HR suggests that the haemodynamic alterations in the mesenteric arterial bed are either too small to be reflected by changes of MAP or counterbalanced by alterations in other vascular beds. It was beyond the scope of this study to pinpoint the site of 5-HT3 receptors that are responsible for the mesenteric vasoconstriction caused by alosetron and cilansetron. Mechanistically, this finding implies that endogenous 5-HT exerts a 5-HT3 receptor-mediated dilator action on the mesenteric arterial bed. There is evidence for a local 5-HT-ergic system in the superior mesenteric artery of the rat (Ni et al., 2008), and enteric vasodilator reflexes induced by mucosal stroking are blocked by combined 5-HT3 and 5-HT4 receptor blockade (Reed and Vanner, 2003). However, this finding is likely to reflect that 5-HT released from enterochromaffin cells initiates a vasodilator reflex, but is unlikely to mean that 5-HT released from enteric neurones causes submucosal vasodilatation. 5-HT3 receptors have not yet been firmly localised to mesenteric and GI blood vessels, and little is known of their involvement, if any, in the regulation of the splanchnic circulation (Martin, 1994; Potenza et al., 1998; Gul et al., 2003). In the digestive tract, 5-HT3 receptors are expressed by enteric neurones, extrinsic afferent neurones, endocrine cells and interstitial cells of Cajal (Glatzle et al., 2002) and play a role in reflexes relevant to emesis, GI motility, GI secretion and GI pain (De Ponti, 2004; Gershon and Tack, 2007). It should not go unnoticed, therefore, that 5-HT3 receptor-mediated changes in the GI circulation may be indirect consequences of other changes in GI function. One of these factors was addressed by measuring intraluminal pressure in the colon. The pertinent results indicate that the mesenteric vasoconstrictor response to alosetron and cilansetron is not associated with an increase in intracolonic pressure and, for this reason, unlikely to result from compression of GI blood vessels caused by intestinal muscle contraction.
In another set of experiments, we explored whether the mesenteric vasoconstrictor effect of alosetron differs in fasted versus non-fasted rats, given that intestinal blood flow is regulated according to digestive activity (Granger et al., 1980; Holzer, 2006). As expected, baseline MBF and MVC in non-fasted rats were significantly higher than in fasted rats, which demonstrates that splanchnic blood flow in the interdigestive non-absorbing period of gut activity is reduced compared with that in the post-prandial period. As alosetron also tended to reduce MBF and MVC in non-fasted rats, we conclude that the mesenteric vasoconstrictor effect of alosetron is not related to the post-prandial/interdigestive state of gut function.
Unlike those of alosetron and cilansetron, clinically relevant doses of tegaserod (0.3–1 mg·kg−1) failed to reduce MBF, MVC, CBF and CVC. On the contrary, following i.v. injection of tegaserod, a transient increase in MBF and MVC was noted in some of the experiments. In assessing the effects of this drug, its other activities, besides being a partial 5-HT4 receptor agonist, as a 5-HT1B and 5-HT2B receptor antagonist need to be considered (Beattie et al., 2004; Weber et al., 2006). The mesenteric vasodilator action could arise from the antagonistic property of tegaserod at 5-HT1B receptors that, when activated by endogenous 5-HT, cause mesenteric vasoconstriction (Gul et al., 2003; Weber et al., 2006). The observation that the tegaserod-evoked mesenteric vasodilation was absent in non-fasted rats may be related to the high baseline values of MBF and MVC recorded under these conditions. The absence of intracolonic pressure changes after i.v. injection of tegaserod negates the possibility that the rise of MBF and MVC seen in our experiments was a result of stimulation of intestinal motor activity. This failure of tegaserod to alter colonic motility in our study may seem surprising in view of the ability of this drug to increase intestinal motility in other experimental paradigms and in IBS patients with constipation (Rivkin, 2003; De Ponti, 2004; Evans et al., 2007). It needs, however, to be considered that our experimental set-up involves anaesthesia, laparotomy, incision and multiple instrumentation of the colon. These experimental perturbations are likely to bring about a state of post-operative ileus in which the prokinetic activity of tegaserod may be compromised. In addition, the intrinsic activity of tegaserod at 5-HT4 receptors is only about 0.2 (De Ponti, 2004).
In additional experiments in which the haemodynamic effect of i.d.-administered tegaserod was evaluated, the dose of tegaserod was increased to 30 mg·kg−1 because the oral bioavailability of tegaserod in humans is only about 10% (Rivkin, 2003; Evans et al., 2007) and because we wanted to test whether even a supra-pharmacological dose of tegaserod would be without adverse effect on the splanchnic circulation. However, this dose of tegaserod also remained without effect on systemic, mesenteric and colonic haemodynamics. Similarly, experiments with isolated human and non-human primate mesenteric arteries have revealed that tegaserod is devoid of a constrictor action and fails to modify mesenteric vasodilation induced by acetylcholine, bradykinin or forskolin (Weber et al., 2006). Taken together, our data show that i.v.- or i.d.-administered tegaserod even at supra-therapeutic doses does not compromise the splanchnic circulation in anaesthetised rats.
Because, in humans, alosetron and tegaserod are taken orally, another aims of our study were to mirror this situation in rats and to test whether peroral administration of the two drugs for 1 week affected the rat splanchnic circulation at baseline and following challenge with a vasoconstrictor drug. As already shown for subcutaneous treatment of rats with alosetron (0.5 mg·kg−1 twice daily) for 5 days (Grundy et al., 2007), short-term treatment of rats with therapy-relevant doses of alosetron or tegaserod did not interfere with the cardiovascular system of the rat in general and with the mesenteric and colonic circulation in particular. This outcome is consistent with the other results of the current study and corroborates the conclusion that the mesenteric vasoconstrictor effect of alosetron seen after acute i.v. or i.d. administration is transient and reversible. It was furthermore revealed that the mesenteric and colonic vasoconstriction elicited by L-NAME was not altered by acute i.v. administration of alosetron (Grundy et al., 2007) or short-term treatment with alosetron or tegaserod (this study). Similarly, reactive hyperaemia after a transient occlusion of the superior mesenteric artery remained unabated by acute i.v. and short-term subcutaneous treatment with alosetron (Grundy et al., 2007). We can thus rule out the possibility that acute and short-term exposure to alosetron or tegaserod compromised physiological vasodilator mechanisms, which subsequently resulted in vasoconstriction.
There is emerging evidence that IBS is associated with low-grade colitis (Bercik et al., 2005; Spiller, 2007; De Giorgio and Barbara, 2008) and that the availability of 5-HT is increased in IBS and intestinal inflammation (Camilleri et al., 2002; Coates et al., 2004; Yeo et al., 2004; Gershon and Tack, 2007). This association makes it conceivable that the splanchnic vasoconstrictor effect of 5-HT3 receptor antagonists is exacerbated in patients with IBS. As there may be crosstalk between different 5-HT receptors (Martin, 1994), it has been speculated that, when, in patients with IBS, alosetron prevents 5-HT from binding to 5-HT3 receptors, an excess of 5-HT may stimulate other 5-HT receptors governing intestinal vasoconstriction (Beck, 2001). However, the notion that mild inflammation may unmask a vasoconstrictor effect of 5-HT3 receptor antagonism was refuted by the inability of low-grade colitis induced by DSS (Okayasu et al., 1990) to reveal any cilansetron-induced change of CBF.
Conclusions
The present results have shown that 5-HT3 receptor antagonists can induce a minor constriction of the mesenteric vascular bed but fail to alter haemodynamics in the colonic wall. The inability to change CBF and the transient nature of the mesenteric vasoconstrictor response to 5-HT3 receptor antagonism do not provide any clue as to the potential of alosetron and cilansetron to enhance the incidence of ischaemic colitis in patients with IBS (Miller et al., 2003; Chey and Cash, 2005; Chang et al., 2006; Andresen et al., 2008; Rahimi et al., 2008). In addition, the current results rule out a number of factors that may pose a risk for 5-HT receptor ligands to cause splanchnic vasoconstriction, such as increased intestinal muscle tone, alterations of blood flow related to feeding and fasting, and low-grade inflammation. In view of these data, the question arises as to whether the rat is an appropriate model to study the impact of the 5-HT system on the splanchnic circulation. However, a review of the available information led Gershon and Tack (2007) to conclude that the presence of 5-HT and of 5-HT receptors in the human gut is consistent with that found in laboratory animals including the rat. It need hence be inferred that there are other factors that may shape the impact of 5-HT3 and 5-HT4 receptor ligands on the splanchnic circulation (Camilleri, 2007; Grundy et al., 2007). The challenge is still to identify these factors in order to understand why IBS itself and the pharmacological manipulation of 5-HT3 and 5-HT4 receptors are associated with an increased incidence of ischaemic colitis.
Acknowledgments
This study was supported by Novartis Pharma, the Zukunftsfonds Steiermark (Grant 262) and the Austrian Scientific Research Funds (FWF Grants P14295 and L25-B05). The authors wish to thank Dr. Eckhard Weber and Hans-Jürgen Pfannkuche for their support of the study and critical discussion of the results.
Glossary
Abbreviations:
- CBF
colonic blood flow
- CVC
colonic vascular conductance
- DSS
dextran sulphate sodium
- GI
gastrointestinal
- HR
heart rate
- IBS
irritable bowel syndrome
- i.d.
intraduodenal
- L-NAME
N-nitro-L-arginine methylester
- MAP
mean arterial blood pressure
- MBF
mesenteric blood flow
- MPO
myeloperoxidase
- MVC
mesenteric vascular conductance
- NMP
1-methyl-2-pyrrolidone
Conflict of interest
Except for the fact that this study was financially supported by Novartis Pharma, the authors state no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen V, Montori VM, Keller J, West CP, Layer P, Camilleri M. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol. 2008;6:545–555. doi: 10.1016/j.cgh.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndts D, Doevendans J, Kirsten R, Heintz B. New aspects of the pharmacokinetics and pharmacodynamics of clonidine in man. Eur J Clin Pharmacol. 1983;24:21–30. doi: 10.1007/BF00613922. [DOI] [PubMed] [Google Scholar]
- Beattie DT, Smith JA, Marquess D, Vickery RG, Armstrong SR, Pulido-Rios T, et al. The 5-HT4 receptor agonist, tegaserod, is a potent 5-HT2B receptor antagonist in vitro and in vivo. Br J Pharmacol. 2004;143:549–560. doi: 10.1038/sj.bjp.0705929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck IT. Possible mechanisms for ischemic colitis during alosetron therapy. Gastroenterology. 2001;121:231–232. doi: 10.1053/gast.2001.26046. [DOI] [PubMed] [Google Scholar]
- Bercik P, Verdu EF, Collins SM. Is irritable bowel syndrome a low-grade inflammatory bowel disease? Gastroenterol Clin North Am. 2005;34:235–245. doi: 10.1016/j.gtc.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Bohlen HG, Henrich H, Gore RW, Johnson PC. Intestinal muscle and mucosal blood flow during direct sympathetic stimulation. Am J Physiol. 1978;235:H40–H45. doi: 10.1152/ajpheart.1978.235.1.H40. [DOI] [PubMed] [Google Scholar]
- Brinker AD, Mackey AC, Prizont R. Tegaserod and ischemic colitis. N Engl J Med. 2004;351:1361–1364. doi: 10.1056/NEJM200409233511324. [DOI] [PubMed] [Google Scholar]
- Camilleri M. Is there an experimental basis for the development of ischaemic colitis as a result of 5-HT3 antagonist treatment? Neurogastroenterol Motil. 2007;19:77–84. doi: 10.1111/j.1365-2982.2006.00861.x. [DOI] [PubMed] [Google Scholar]
- Camilleri M, Atanasova E, Carlson PJ, Ahmad U, Kim HJ, Viramontes BE, et al. Serotonin-transporter polymorphism pharmacogenetics in diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2002;123:425–432. doi: 10.1053/gast.2002.34780. [DOI] [PubMed] [Google Scholar]
- Chang L, Chey WD, Harris L, Olden K, Surawicz C, Schoenfeld P. Incidence of ischemic colitis and serious complications of constipation among patients using alosetron: systematic review of clinical trials and post-marketing surveillance data. Am J Gastroenterol. 2006;101:1069–1079. doi: 10.1111/j.1572-0241.2006.00459.x. [DOI] [PubMed] [Google Scholar]
- Chang L, Kahler KH, Sarawate C, Quimbo R, Kralstein J. Assessment of potential risk factors associated with ischaemic colitis. Neurogastroenterol Motil. 2008;20:36–42. doi: 10.1111/j.1365-2982.2007.01015.x. [DOI] [PubMed] [Google Scholar]
- Chey WD, Cash BD. Cilansetron: a new serotonergic agent for the irritable bowel syndrome with diarrhoea. Expert Opin Investig Drugs. 2005;14:185–193. doi: 10.1517/13543784.14.2.185. [DOI] [PubMed] [Google Scholar]
- Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- De Giorgio R, Barbara G. Is irritable bowel syndrome an inflammatory disorder? Curr Gastroenterol Rep. 2008;10:385–390. doi: 10.1007/s11894-008-0073-0. [DOI] [PubMed] [Google Scholar]
- De Ponti F. Pharmacology of serotonin: what a clinician should know. Gut. 2004;53:1520–1535. doi: 10.1136/gut.2003.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBaise JK. Tegaserod-associated ischemic colitis. Pharmacotherapy. 2005;25:620–625. doi: 10.1592/phco.25.4.620.61032. [DOI] [PubMed] [Google Scholar]
- Emmanuel AV, Kamm MA. Laser Doppler flowmetry as a measure of extrinsic colonic innervation in functional bowel disease. Gut. 2000;46:212–217. doi: 10.1136/gut.46.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans BW, Clark WK, Moore DJ, Whorwell PJ. Tegaserod for the treatment of irritable bowel syndrome and chronic constipation. Cochrane Database Syst Rev. 2007;(4) doi: 10.1002/14651858.CD003960.pub3. issue article number: CD003960. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, Compton AM, Kemp PA, Bennett T. Regional and cardiac haemodynamic effects of N-nitro-L-arginine methyl ester in conscious Long Evans rats. Br J Pharmacol. 1990;101:625–631. doi: 10.1111/j.1476-5381.1990.tb14131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Glatzle J, Sternini C, Robin C, Zittel TT, Wong H, Reeve JR, et al. Expression of 5-HT3 receptors in the rat gastrointestinal tract. Gastroenterology. 2002;123:217–226. doi: 10.1053/gast.2002.34245. [DOI] [PubMed] [Google Scholar]
- Gore RW, Bohlen HG. Microvascular pressures in rat intestinal muscle and mucosal villi. Am J Physiol. 1977;233:H685–H693. doi: 10.1152/ajpheart.1977.233.6.H685. [DOI] [PubMed] [Google Scholar]
- Granger DN, Richardson PDI, Kvietys PR, Mortillaro NA. Intestinal blood flow. Gastroenterology. 1980;78:837–863. [PubMed] [Google Scholar]
- Grundy D, McLean P, Stead R. Impact of 5-HT3 receptor blockade on colonic haemodynamic responses to ischaemia and reperfusion in the rat. Neurogastroenterol Motil. 2007;19:607–616. doi: 10.1111/j.1365-2982.2007.00938.x. [DOI] [PubMed] [Google Scholar]
- Gul H, Yildiz O, Simsek A, Balkan M, Ersoz N, Cetiner S, et al. Pharmacologic characterization of contractile serotonergic receptors in human isolated mesenteric artery. J Cardiovasc Pharmacol. 2003;41:307–315. doi: 10.1097/00005344-200302000-00021. [DOI] [PubMed] [Google Scholar]
- Guth PH, Leung FW. Physiology of the gastric circulation. In: Johnson LR, editor. Physiology of The Gastrointestinal Tract, Second Edition. New York: Raven Press; 1987. pp. 1031–1053. [Google Scholar]
- Heinemann A, Wachter CH, Holzer P. Differential regulation of mesenteric and femoral blood flow in the rat as revealed by computerized data acquisition and evaluation. J Auton Pharmacol. 1998;18:39–48. doi: 10.1046/j.1365-2680.1998.1810039.x. [DOI] [PubMed] [Google Scholar]
- Heinemann A, Sattler V, Jocic M, Wienen W, Holzer P. Effect of angiotensin II and telmisartan, an angiotensin1 receptor antagonist, on rat gastric mucosal blood flow. Aliment Pharmacol Ther. 1999;13:347–355. doi: 10.1046/j.1365-2036.1999.00496.x. [DOI] [PubMed] [Google Scholar]
- Higgins PD, Davis KJ, Laine L. Systematic review: the epidemiology of ischaemic colitis. Aliment Pharmacol Ther. 2004;19:729–738. doi: 10.1111/j.1365-2036.2004.01903.x. [DOI] [PubMed] [Google Scholar]
- Holzer P. Neural regulation of gastrointestinal blood flow. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract, Fourth Edition. New York: Academic Press; 2006. pp. 817–839. [Google Scholar]
- Holzer P, Painsipp E. Differential effects of clonidine, dopamine, dobutamine, and dopexamine on basal and acid-stimulated mucosal blood flow in the rat stomach. Crit Care Med. 2001;29:335–343. doi: 10.1097/00003246-200102000-00021. [DOI] [PubMed] [Google Scholar]
- Holzer P, Livingston EH, Guth PH. Sensory neurons signal for an increase in rat gastric mucosal blood flow in the face of pending acid injury. Gastroenterology. 1991;101:416–423. doi: 10.1016/0016-5085(91)90020-l. [DOI] [PubMed] [Google Scholar]
- Holzer P, Wachter C, Jocic M, Heinemann A. Vascular bed-dependent roles of the peptide CGRP and nitric oxide in acid-evoked hyperaemia of the rat stomach. J Physiol (London) 1994;480:575–585. doi: 10.1113/jphysiol.1994.sp020385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P, Painsipp E, Jocic M, Heinemann A. Acid challenge delays gastric pressure adaptation, blocks gastric emptying and stimulates gastric fluid secretion in the rat. Neurogastroenterol Motil. 2003a;15:45–55. doi: 10.1046/j.1365-2982.2003.00382.x. [DOI] [PubMed] [Google Scholar]
- Holzer P, Painsipp E, Weber E, Pfannkuche HJ. 5-HT3 receptor antagonists, alosetron and cilansetron, impair mesenteric blood flow in rats. Gastroenterology. 2003b;124(Suppl. 1):A148–A149. [Google Scholar]
- Johansson K. Gastrointestinal application of laser Doppler flowmetry. An experimental and clinical study in cat and man. Acta Chir Scand Suppl. 1988;545:1–64. [PubMed] [Google Scholar]
- Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- Leung FW, Guth PH, Scremin OU, Golanska EM, Kauffman GL. Regional gastric mucosal blood flow measurements by hydrogen gas clearance in the anesthetized rat and rabbit. Gastroenterology. 1984;87:28–36. [PubMed] [Google Scholar]
- Livingston EH, Reedy T, Leung FW, Guth PH. Computerized curve fitting in the analysis of hydrogen gas clearance curves. Am J Physiol. 1989;257:G668–G675. doi: 10.1152/ajpgi.1989.257.4.G668. [DOI] [PubMed] [Google Scholar]
- Martin GR. Vascular receptors for 5-hydroxytryptamine: distribution, function and classification. Pharmacol Ther. 1994;62:283–324. doi: 10.1016/0163-7258(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Miller DP, Alfredson T, Cook SF, Sands BE, Walker AM. Incidence of colonic ischemia, hospitalized complications of constipation, and bowel surgery in relation to use of alosetron hydrochloride. Am J Gastroenterol. 2003;98:1117–1122. doi: 10.1111/j.1572-0241.2003.07418.x. [DOI] [PubMed] [Google Scholar]
- Ni W, Geddes TJ, Priestley JR, Szasz T, Kuhn DM, Watts SW. The existence of a local 5-hydroxytryptaminergic system in peripheral arteries. Br J Pharmacol. 2008;154:663–674. doi: 10.1038/bjp.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- Potenza MA, Serio M, Montagnani M, Mansi G, Rinaldi R, Genualdo M, et al. Functional evaluation of 5-hydroxytryptamine receptor activity in rat resistance vessels. J Auton Pharmacol. 1998;18:75–81. doi: 10.1046/j.1365-2680.1998.1820075.x. [DOI] [PubMed] [Google Scholar]
- Rahimi R, Nikfar S, Abdollahi M. Efficacy and tolerability of alosetron for the treatment of irritable bowel syndrome in women and men: a meta-analysis of eight randomized, placebo-controlled, 12-week trials. Clin Ther. 2008;30:884–901. doi: 10.1016/j.clinthera.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Reed DE, Vanner SJ. Long vasodilator reflexes projecting through the myenteric plexus in guinea-pig ileum. J Physiol (London) 2003;553:911–924. doi: 10.1113/jphysiol.2003.053140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivkin A. Tegaserod maleate in the treatment of irritable bowel syndrome: a clinical review. Clin Ther. 2003;25:1952–1974. doi: 10.1016/s0149-2918(03)80198-4. [DOI] [PubMed] [Google Scholar]
- Schoenfeld P. Review article: the safety profile of tegaserod. Aliment Pharmacol Ther. 2004;20(Suppl. 7):25–30. doi: 10.1111/j.1365-2036.2004.02182.x. [DOI] [PubMed] [Google Scholar]
- Spiller RC. Irritable bowel syndrome: bacteria and inflammation – clinical relevance now. Curr Treat Options Gastroenterol. 2007;10:312–321. doi: 10.1007/s11938-007-0074-3. [DOI] [PubMed] [Google Scholar]
- Wager-Page SA, Ghazali B, Anderson W, Veale WL, Davison JS. Neuropeptide Y, peptide YY, and pancreatic polypeptide modulate duodenal and colonic motility at a thoracic spinal site in rats. Peptides. 1992;13:807–813. doi: 10.1016/0196-9781(92)90191-5. [DOI] [PubMed] [Google Scholar]
- Weber E, Hamel CT, Bernhard M, Pfannkuche HJ. Tegaserod has no potential for vasoconstriction or impairment of vasodilatation in isolated human and non-human primate mesenteric arteries. Gastroenterology. 2006;130(Suppl. 2):A–489. [Google Scholar]
- Yeo A, Boyd P, Lumsden S, Saunders T, Handley A, Stubbins M, et al. Association between a functional polymorphism in the serotonin transporter gene and diarrhoea predominant irritable bowel syndrome in women. Gut. 2004;53:1452–1458. doi: 10.1136/gut.2003.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]


