Abstract
Background and purpose:
The immunomodulatory effects of α-fetoprotein (AFP) on lymphocytes and macrophages have been described in vitro and in vivo. Recombinant forms of human AFP have been proposed as potential therapeutic entities for the treatment of autoimmune diseases. We examined the effects of embryonic and recombinant human AFP on the spontaneous, UVA- and cytokine-induced pro-inflammatory responses of human keratinocytes.
Experimental approach:
Cultures of primary and immortalized human keratinocytes (HaCaT) and human blood T lymphocytes were used. The effects of AFP on cytokine expression were studied by bioplexed elisa and quantitative reverse transcriptase polymerase chain reaction assay. Kinase and nuclear factor kappa B (NFκB) phosphorylation were quantified by intracellular elisa. Nuclear activator protein 1 and NFκB DNA binding activity was measured by specific assays. Nitric oxide and H2O2 production and redox status were assessed by fluorescent probe and biochemical methods.
Key results:
All forms of AFP enhanced baseline expression of cytokines, chemokines and growth factors. AFP dose-dependently increased tumour necrosis factor alpha-stimulated granulocyte macrophage colony stimulating factor and interleukin 8 expression and decreased tumour necrosis factor alpha-induced monocyte chemotactic protein 1 and IP-10 (interferon gamma-produced protein of 10 kDa) expression. AFP induced a marked activator protein 1 activation in human keratinocytes. AFP also increased H2O2 and modulated nitrite/nitrate levels in non-stimulated keratinocytes whereas it did not affect these parameters or cytokine release from UVA-stimulated cells. Phosphorylation of extracellular signal-regulated kinase (ERK1/2) and Akt1 but not NFκB was activated by AFP alone or by its combination with UVA.
Conclusions and implications:
Exogenous AFP induces activation of human keratinocytes, with de novo expression of a number of pro-inflammatory mediators and modulation of their pro-inflammatory response to cytokines or UVA. AFP may modulate inflammatory events in human skin.
Keywords: AP-1, α-fetoprotein, cytokines, chemokines, human keratinocytes, UVA
Introduction
Human embryonic α-fetoprotein (AFP) is a 70 kDa oncofetal glycoprotein consisting of the 590 amino acid polypeptide and a carbohydrate moiety, and it is detected both fetally and maternally during pregnancy (Mizejewski, 2004). AFP is thought to play a role of an intrinsic immunomodulator synthesized to avoid rejection of the developing embryo by the maternal immune system (Mizejewski, 2004;Dudich, 2007). The intrinsic immunomodulatory properties of the AFP molecule and its ability to regulate pro-inflammatory cytokine gene expression and production (Yamashita et al., 1994; Filella et al., 2001; Cao et al., 2007; Dudich, 2007) strongly suggest that recombinant forms of AFP and AFP-derived peptides could be developed as a new generation of anti-inflammatory drugs effective in a number of autoimmune diseases, such as demyelinizing encephalitis, rheumatoid arthritis, psoriasis, etc. (Irony-Tur-Sinai et al., 2006; Dudich, 2007; Murray and Sawitzke, 2007;Nizri et al., 2007). Numerous in vitro and in vivo studies have shown that AFP-based products exerted a wide spectrum of immunomodulating properties. The earliest studies reported that human AFP could suppress tumour necrosis factor alpha (TNF-α) and interleukin 1β (IL-1β) expression stimulated in human monocytes and a monocyte-derived cell line by a prostaglandin E2-dependent mechanism (Wang et al., 2005). More recent studies have shown that AFP-derived entities suppressed the growth of T cells activated by alloantigen or autoantigen but did not affect resting T lymphocytes. They triggered apoptosis in the activated immune cells (Semenkova et al., 1997; Dudich, 2007) and suppressed pro-inflammatory cytokine gene expression and production (Wang and Alpert, 1995;Filella et al., 2001; Cao et al., 2007). AFP-based entities also abolished the effects of TNF-α by two distinct mechanisms: (i) direct binding to the TNF-α molecule; and (ii) concurrent signalling via nuclear factor κB (NFκB) (Mizejewski, 2002; Cavin et al., 2004). Physiologically relevant concentrations of AFP (10–100 µg·mL−1) protected against TNF-α-induced apoptosis (Semenkova et al., 1997). AFP modulated the immune response of T lymphocytes favouring proliferation and activity of tumour specific transforming growth factor beta-producing CD4(+) cells while suppressing T helper (Th)1, Th2 and Th17 responses (Alisa et al., 2008), which are the characteristics of chronic autoimmune inflammation (Pastore et al., 2006). To our knowledge data on the immunomodulatory effects of AFP-derived products have been confined to T lymphocytes, monocytes, macrophages and tumour cell lines derived from these immune cells (Dudich, 2007).
In the present study we focused on the effects of AFP-derived material on human keratinocytes, which form a major cellular component of skin. Moreover, keratinocytes represent the static part of the skin immune system playing an active role in both the protective immune responses and the immunopathological reactions of the skin (Nickoloff et al., 2006). Non-stimulated resting keratinocytes produce minimal amounts of immune mediators including reactive oxygen and nitrogen species (ROS/RNS), lipid peroxides or pro-inflammatory proteins and cytokines (Pastore et al., 2006; Korkina and Pastore, 2009). Exposure of keratinocytes to physical, chemical or biological stimuli results in an increased release of pro-inflammatory immune response mediators and by the activation of pro-survival mechanisms. Attracted by keratinocyte-derived chemokines, circulating immune cells such as granulocytes, monocytes and lymphocytes penetrate the skin, release their own cytokines and other factors, proliferate and differentiate. The pivotal role of keratinocytes ensures that the skin and ultimately, the entire organism is protected against changes in the external environment (Eming et al., 2007). In chronic inflammatory skin diseases, uncontrolled and exaggerated responses by both keratinocytes and recruited immune cells takes place, which negatively affects keratinocyte structure and function and maintains a local inflammatory state (Pastore et al., 2006). We speculated that human keratinocytes, being rapidly dividing cells with multiple immune functions, may be excellent cellular targets for exogenous AFP, which could modulate their responses to both exogenous and endogenous triggers. The mechanisms of AFP-induced modulation of keratinocyte responses to UVA irradiation, and pro-inflammatory cytokines (TNF-α and interferon gamma, IFN-γ) were assessed by the analysis of a spectrum of cytokines, growth factors and chemokines and activation of phosphorylation pathways. AFPs as potential signalling molecules for the expression of cytokine genes and/or the activation of protein synthesis, hydrogen peroxide (H2O2) and nitric oxide (NO) were also evaluated. The results obtained suggest that AFP may modulate immune responses of human keratinocytes by activator protein 1 (AP-1)-driven pathways through activation of extracellular signal-regulated kinases (ERKs) and Akt1. H2O2 could be implicated in AFP-induced signal transduction.
Methods
Human keratinocyte cultures and exposure to pro-inflammatory cytokines
Primary cultures of keratinocytes were obtained from skin biopsies of healthy volunteers (n= 4), as previously reported (Pastore et al., 1997). Briefly, cells were cultured in serum-free keratinocyte growth medium (KGM, Clonetics, Walkersville, MD, USA) for at least 3–5 days until the cultures reached 60–80% confluence. Keratinocytes were pre-incubated with escalating concentrations of AFP preparations or human serum albumin for 1 h. Then, TNF-α (100 ng·mL−1) or IFN-γ (100 U·mL−1) or combination of these two cytokines were added. The combination of two pro-inflammatory factors leads to maximal expression and release of cytokines and chemokines in keratinocytes (Pastore et al., 2006). After 24 h, concentrations of interleukin 8 (IL-8), interferon gamma-produced protein of 10 kDa (IP-10), granulocyte macrophage colony stimulating factor (GM-CSF) and monocyte chemotactic protein 1 (MCP-1) were determined by elisa assay of the supernatant.
The immortalized human keratinocyte cell line HaCaT was a gift from Dr NE Fusenig (Deutsches Krebsforschungszentrum, Heidelberg, Germany) and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat inactivated fetal bovine serum at 37°C in a humidified atmosphere containing 5% CO2. Before all experiments, HaCaT cells were incubated in serum-free DMEM for 24 h (starvation conditions). Cells were exposed to escalating concentrations of glycosylated recombinant human α-fetoprotein (gly-rhAFP) (25, 50 and 100 µg·mL−1) in fresh serum-free DMEM unless otherwise noted in the text. In the experiments on combined effects of AFP and UVA, cells suspended in phosphate-buffered saline (PBS)-glucose solution were exposed to standardized UVA irradiation without UVB admixture with the following characteristics: irradiance 33 mW·cm−2, total dose 2.5 J·cm−2, emission spectrum from 320 nm and emission peak at 375 nm (Solar Simulator, Dermalight Vario with filter A1, Dr Hoehnle AG, UV Technology, Planegg, Germany) and then, 100 µg·mL−1 of gly-rhAFP was added. At different time points, conditioned medium was collected, and cells were detached by addition of trypsin/EDTA, harvested, washed in PBS, quickly frozen and stored at −80°C.
Effects of AFP on the primary keratinocyte and HaCaT cell growth and viability were assessed by standard assays at 24 h of exposure to maximal concentration of AFP (200 µg·mL−1). To evaluate the keratinocyte proliferation, sub-confluent primary cultures were trypsinized, and cells were counted. Cell proliferation and viability were also determined using the MTT (3-[4,5-dimethylthiazol-2yl]-diphenyl tetrazolium bromid) colorimetric assay. After exposure to AFP, the MTT dye solution was added to cultures for 4 h. Finally, the light absorbance of the cultures was measured using the microplate (elisa) reader at 570 nm. The experiments were performed in duplicate.
Peripheral blood CD4-positive T cell purification
Peripheral blood mononuclear cells from healthy individuals (n= 6) were obtained by blood centrifugation over Hystopaque (d= 1077, Sigma Chemicals) and subsequently left to adhere (6 × 106 cells·mL−1) in Petri dishes in RPMI 1640 complemented with 2 mM glutamine, 1 mM sodium pyruvate, 1% non-essential amino acids, 0.05 mM 2-mercaptoethanol, 100 U·mL−1 penicillin, and 100 µg·mL−1 streptomycin (all from Invitrogen, San Giuliano Milanese, Italy) and 5% human plasma (all from Sigma Chemicals). After 2 h at 37°C, the non-adherent cells (essentially composed of T cells) were collected, and the CD4 fraction was isolated by means of CD4 antibodies attached to magnetic microbeads (Miltenyi Biotec, GmbH, Bergisch Gladbach, Germany). The purity of the CD4-positive T cell preparation (>99%) was verified by cytofluorimetric analysis using a fluorescein isothiocyanate-conjugated anti-CD4 antibody (BD Biosciences, San Jose, CA, USA).
Stimulation of CD4-positive T cells by CD3/CD28 combination
CD4-positive T cells were stimulated by the combination of plate-coated anti-CD3 (R&D Systems, Minneapolis, MN, USA) and soluble anti-CD28 (BD Biosciences) monoclonal antibodies (mAbs), both used at 1 µg·mL−1, in 24-well plates in complete RPMI plus 5% human plasma (Viola et al., 1999) and exposed to escalating doses of AFP. Cell supernatants were collected after 24 h, frozen at −80°C and subsequently analysed by elisa for TNF-α and IFN-γ levels.
elisa assays
Human TNFα and IFN-γelisa kits were from R&D Systems. GM-CSF, IL-8, MCP-1 and IP-10 levels in cell supernatants were measured with elisa kits from BD Biosciences. Samples were assayed in triplicate for each experiment.
Phosphorylation assay
Phosphorylation of NFκB (p65/RelA) at serine 536, protein kinase B (Akt1) and extracellular regulated kinases 1/2 (ERK1/2) was quantified by modified elisa assays without protein isolation from HaCaT cells using CASE™ Kit (SABiosciences Corporation, Frederick, MD, USA). These cell-based elisa kits quantify the amount of activated (phosphorylated) protein relative to total protein. Before analysis, HaCaT cells or primary keratinocytes were fixed with 4% formaldehyde. Results were expressed as percentage of phosphorylated form of the protein relative to its total content.
Cytokine analysis by multiplexed assay
Cytokine levels in conditioned medium were determined by multiplexed analysis using the panel with the Bio-Plex Suspension Array System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The following cytokines were simultaneously detected with this panel: interleukin 1 receptor antagonist (IL-1ra), interleukins IL-6, IL-8, IL-10, granulocyte colony stimulating factor (G-CSF), IP-10, MCP-1, macrophage inflammatory proteins (MIP-1α and MIP-1β), platelet-derived growth factor (PDGFbb), TNF-α and vascular endothelial growth factor (VEGF). The assay was performed according to the manufacturer's instructions. Briefly, conditioned medium was centrifuged for 5 min at 2200×g at 4°C and incubated with beads labelled by antibodies specific to each cytokine for 30 min. Following a wash step, the beads were incubated with the detection antibody cocktail, each specific to a single cytokine. After another wash, the beads were incubated with streptavidin-phycoerythrin for 10 min and finally washed. Cytokine concentrations were determined on a Bio-Rad array reader using linear parts of corresponding calibration curves and expressed in pg·mg−1 cellular protein.
RNA purification and quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was isolated from frozen HaCaT cells using the GenElute™ Mammalian Total RNA Kit from Sigma (Milan, Italy) in accordance to manufacturer's instructions. The amount of RNA was determined by absorbance at 260 nm. Total RNA (1 µg) was reverse transcribed using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) at 25°C for 5 min and 42°C for 30 min, followed by 85°C for 5 min in a final reaction volume of 40 µL. cDNA was amplified with iQTM Supermix using the MiniOpticon Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). All real-time assays were carried out under the following conditions: 35 cycles of denaturation at 95°C for 15 s, annealing and extension at 60°C for 60 s. Melt curve analysis was performed to confirm the specificity of the amplified products. All samples were run in triplicate, and relative expression was determined by normalizing samples to β-actin housekeeping gene. Data were analysed using the comparative Ct method (ΔΔCt) (Livak and Schmittgen, 2001). The primer sets in Table 1 were obtained from Applied Biosystems.
Table 1.
Primers used for quantitative real-time reverse transcription polymerase chain reaction
| n/n | Primers | Sequence | Size | Amplicon size |
|---|---|---|---|---|
| 1 | β-actin sense | 5′-AAATCTGGCACCACACCTTCTAC-3′ | 23 | 171 |
| β-actin antisense | 5′-ATAGCACAGCCTGGATAGCAAC-3′ | 22 | ||
| 2 | iNOS sense | 5′-ACGTGCGTTACTCCACCAACA-3′ | 21 | 113 |
| iNOS antisense | 5′-CATAGCGGATGAGCTGAGCA-3′ | 20 | ||
| 3 | IL-6 sense | 5′-GTGTGAAAGCAGCAAAGAG-3′ | 19 | 133 |
| IL-6 antisense | 5′-CTCCAAAAGACCAGTGATG-3′ | 19 | ||
| 4 | TNF-α sense | 5′- TCCTTCAGACACCCTCAACC-3′ | 20 | 172 |
| TNF-α antisense | 5′- AGGCCCCAGTTTGAATTCTT-3′ | 20 | ||
| 5 | IL-1α sense | 5′-TGGCTCATTTTCCCTCAAAAGTTG-3′ | 24 | 170 |
| IL-1α antisense | 5′-AGAAATCGTGAAATCCGAAGTCAAG-3′ | 25 |
IL, interleukin; iNOS, inducible nitric oxide synthase; TNF-α, tumour necrosis factor alpha.
Hydrogen peroxide and nitric oxide assays
H2O2 in the conditioned medium was determined with Hydroperoxide Assay kit (Sigma, Milan, Italy). The results were expressed as nmol H2O2·mg−1 protein. The nitrate/nitrite levels in the culture medium were measured by the Griess reagent (Cayman Chemicals, Ann Arbor, MI, USA). Intracellular NO production was detected using 4,5-diaminofluorescein diacetate (DAF-DA) as a specific fluorescent probe (Kojima et al., 1998). In brief, HaCaT cells were incubated with DAF-DA (1 h, 2.5 µM), thoroughly washed and finally fixed by 2% paraformaldehyde for further microscopic examination. To quantify the data, HaCaT cells were destroyed by five freeze/thaw cycles and the intensity of fluorescence was measured on a Shimadzu RF-5301 spectrofluorimeter using λex= 488 nm for excitation and λfl= 530 nm for fluorescence. The results were expressed in arbitrary fluorescence units normalized to protein content.
Catalase, superoxide dismutase (SOD), glutathione-S-transferase (GST), reduced glutathione (GSH) and protein SH-group assays
HaCaT cells were lysed by five freeze/thaw cycles, and the post-spin cell lysates were analysed. Total GST activity was measured by the method described previously using chloro-2,3-dinitrobenzene as a substrate (Habig et al., 1974). Total SOD activity was measured spectrophotometrically (Kostyuk and Potapovich, 1989). Catalase activity was detected by Aebi method (Aebi, 1984). GSH and protein SH-groups were determined with ThioGlo™-1 (Tyurina et al., 2004).
Nuclear extracts preparation and Western blot analysis
Levels of c-Fos, which is a component of the AP-1 transcription factor, and of the NFκB subunit p65/RelA were measured in nuclear lysates of keratinocytes, obtained by Schreiber's method (Schreiber et al., 1989). Briefly, keratinocytes were initially lysed in a hypotonic ice-cold buffer: 20 mM HEPES (pH 7.5) with 5 mM NaF, 10 µM Na2MoO4 and 0.1 mM EDTA. The nuclear pellet obtained by gentle centrifugation (340×g, 10 min at 4°C) was resuspended in hypertonic buffer: 20 mM HEPES, 400 mM NaCl, 25% glycerol, 1 mM EDTA, 1 mM EGTA, 0.5 mM Na3VO4 and protease inhibitors. It was incubated on ice for 30 min with vortexing every 5 min and pelleted by centrifugation (14 000×g for 20 min at 4°C) to collect the nuclear extract supernatant. Anti-c-Fos, anti-p65/RelA and anti-lamin B antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Western blot assay was performed by standard procedure.
DNA binding activity of transcription factors
Nuclear factor kappa B (p65/RelA) and AP-1 (c-Fos) specific DNA binding activity were detected in cell nuclear lysates and quantified using the transcription factor-specific TransAM kits (Active Motif (Carlsbad, CA, USA) (Renard et al., 2001).
Protein content was measured according to Bradford using Bio-Rad microplate assay kit (Bradford, 1976).
Data analysis
All measurements were taken from triplicate samples, and data of at least three independent experiments were statistically evaluated. To assess the difference between experimental groups, two-tailed Student's t-test was applied, and P-values < 0.05 were considered to be significant.
α-Fetoprotein preparations
All three AFP preparations were commercially available from BioSystem Ltd., Moscow, Russia. In brief, the human serum embryonic AFP (ehAFP) was isolated from umbilical cord serum by ion exchange, affinity and gel-filtration chromatography (Dudich et al., 1999). Recombinant glycosylated form of human AFP (gly-rhAFP) with a 100% amino acid homology to human AFP was isolated from the culture medium of recombinant yeast Saccharomyces cerevisiae strain YBS723/pKX transformed by plasmid pKX, encoding full-length human AFP gene by affinity and gel-chromatography (Benevolensky et al., 2006). Non-glycosylated recombinant form of AFP (rhAFP) was obtained from recombinant strain of Escherichia coli DH5α/pAfp transformed by plasmid pTrcafp2 containing human AFP gene by purification of rhAFP from inclusion bodies (Boismenu et al., 1997). Human serum albumin used as a control protein was purchased from Sigma Chemicals (Milan, Italy).
Results
AFP preparations modulate cytokine expression in primary cultured human keratinocytes and enhance TNF-α and IFN-γ release by anti-CD3- and anti-CD28-activated human CD4-positive T lymphocytes
In the first set of experiments, the effects of ehAFP and two recombinant forms of hAFP on the growth factor and chemokine expression by non-stimulated, TNF-α-stimulated, IFN-γ-stimulated and a combination of (TNF-α+ IFN-γ)-activated primary cultured human keratinocytes were examined. Non-stimulated keratinocytes responded to gly-rhAFP (10–200 µg·mL−1) by a dose-dependent moderate increase in the release of GM-CSF, IL-8 and MCP-1(Figure 1). The expression of IP-10 was only slightly perturbed by gly-rhAFP. Both ehAFP and non-glycosylated rhAFP exerted similar dose-dependent effects (data not shown). In response to 100 ng·mL−1 TNF-α, keratinocytes released significant amounts of all cytokines studied (Figure 1). The presence of AFP led to additional stimulation of TNF-α-induced expression of GM-CSF and IL-8 depending on the concentration of AFP added (Figure 1A and B). At the same time, AFP dose-dependently inhibited TNF-α-induced release of MCP-1 and IP-10 from keratinocytes (Figure 1C and D). IFN-γ (100 U·mL−1) alone did not affect spontaneous expression of GM-CSF and IL-8 (Figure 1A and B). Addition of IFN-γ to keratinocytes resulted in up-regulation of MCP-1 and IP-10, in particular (Figure 1C and D). There were no statistically significant changes in the IFN-γ-induced expression of the two cytokines upon AFP addition. The combination of 100 ng·mL−1 TNF-α plus 100 U·mL−1 IFN-γ to primary human keratinocytes triggered an extremely high release of all the above inflammatory mediators. In the case of GM-CSF, IL-8 and MCP-1 the release was two orders of magnitude and in the case of IP-10 was four orders of magnitude greater than the corresponding responses to gly-rhAFP (Figure 2). When associated with this optimal pro-inflammatory combination of (TNF-α+ IFN-γ), gly-rhAFP did not significantly affect the release of GM-CSF or the chemokines (Figure 2). Similar data were obtained for embryonic and rhAFP (data not shown). Moreover, the response of HaCaT cells to ehAFP and rhAFP in terms of chemokine modulation closely paralleled that observed on primary human keratinocytes (data not shown). Interestingly, in the same experimental setting, human plasma albumin (used as a control peptide) did not show any effects at equimolar concentrations (data not shown). We also verified the effects of all three AFP preparations on blood-derived immune cells. Purified CD4-positive T cells from the peripheral blood of healthy donors were treated with AFP, and concomitantly co-stimulated with anti-CD3 and anti-CD28 mAbs. Escalating doses of gly-rhAFP alone induced a prominent TNF-α release (Figure 3A) and synergized with simultaneous CD3/CD28 co-stimulation, leading to the release of very high levels of TNF-α and in particular of IFN-γ (Figure 3). Similar results were obtained using embryonic or rhAFP (data not shown). All preparations of AFP studied (200 µg·mL−1, exposure for 24 h) did not affect viability and proliferation of primary keratinocytes as was revealed by microscopic examination and MTT test (data not shown).
Figure 1.
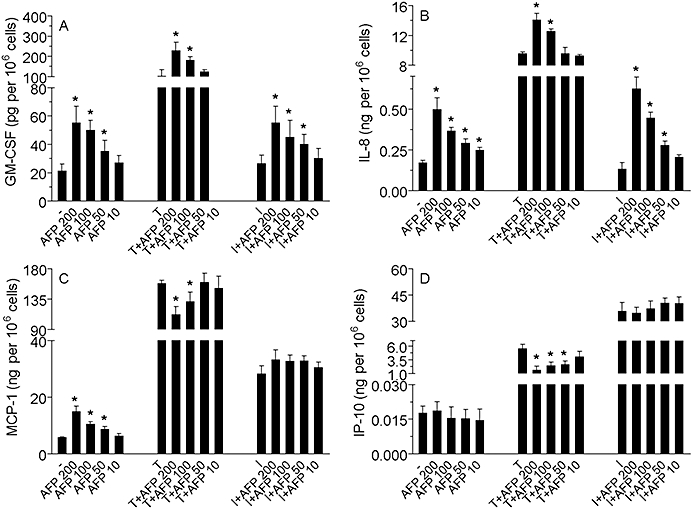
Effect of gly-rhAFP on the release of GM-CSF (A), IL-8 (B), MCP-1 (C) and IP-10 (D) by normal human keratinocytes, used alone in the dose range 10–200 µg·mL−1, or in combination with 100 ng·mL−1 TNF-α (T) or 100 U·mL−1 IFN-γ (I). The results are expressed as the mean ± SD of three independent experiments. *P < 0.05 versus untreated controls (-) or versus stimulated controls (T or I). AFP, α-fetoprotein; gly-rhAFP, glycosylated recombinant human alpha fetoprotein; GM-CSF, granulocyte macrophage colony stimulating factor; IFN-γ, interferon gamma; IL, interleukin; IP-10, interferon gamma-produced protein of 10 kDa; MCP-1, monocyte chemotactic protein 1; TNF-α, tumour necrosis factor alpha.
Figure 2.
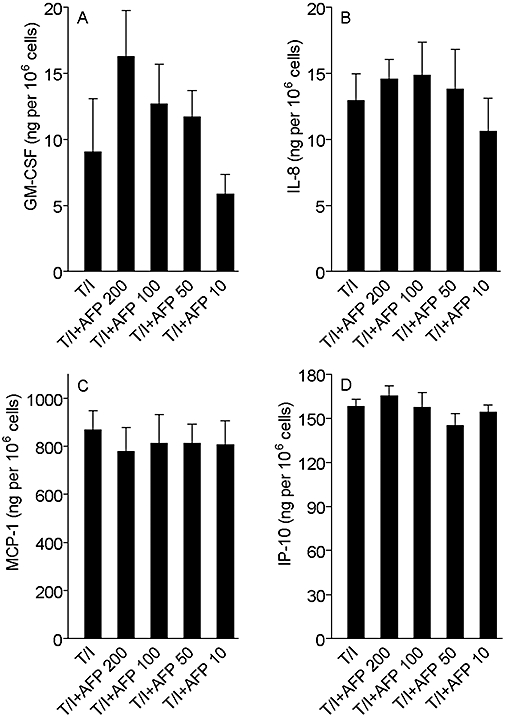
Effect of gly-rhAFP on the release of GM-CSF (A), IL-8 (B), MCP-1 (C) and IP-10 (D) by normal human keratinocytes, used in combination with 100 ng·mL−1 TNF-α and 100 U·mL−1 IFN-γ (T/I). The results are expressed as the mean ± SD of three independent experiments. AFP, α-fetoprotein; gly-rhAFP, glycosylated recombinant human alpha fetoprotein; GM-CSF, granulocyte macrophage colony stimulating factor; IFN-γ, interferon gamma; IL, interleukin; IP-10, interferon gamma-produced protein of 10 kDa; MCP-1, monocyte chemotactic protein 1; TNF-α, tumour necrosis factor alpha.
Figure 3.
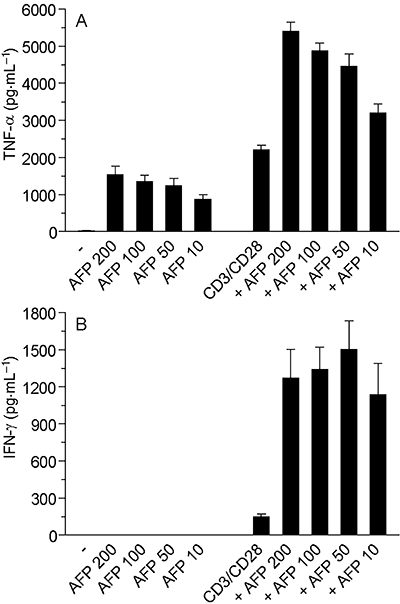
AFP alone induces TNF-α in purified CD4 T cells and boosts the release of IFN-γ (A) and TNF-α (B) due to CD3/CD28 stimulation. T lymphocytes were cultured in 24-well clusters and treated with rhAFP, in the presence or absence of CD3/CD28 stimulation. The results are representative of data obtained with CD4-positive T cells from six distinct blood donors. AFP, α-fetoprotein; IFN-γ, interferon gamma; rhAFP, recombinant human alpha fetoprotein; TNF-α, tumour necrosis factor alpha.
AFP alone and in combination with UVA induce cytokine expression in HaCaT cells
Experiments with bioplexed analysis of 12 cytokines released from HaCaT cells as a result of their incubation with 100 µg·mL−1 gly-rhAFP for 24 h showed that gly-rhAFP significantly induced release and accumulation in the conditioned medium of all cytokines studied: IL-1β, IL-1ra, IL-6, IL-8, IL-10, IP-10, MCP-1, MIP-1α, MIP-1β, PDGFbb, TNF-α and VEGF (Table 2). At the same time, only two of these cytokines, IL-1ra and VEGF, were statistically significantly induced by a moderate dose of UVA (2.5 J·cm−2), although to a lesser extent as compared with AFP. The combination of UVA + gly-rhAFP resulted in cytokine production similar to that induced by AFP alone. We also analysed gene expression for TNF-α, IL-6 and IL-1α using qRT-PCR assay. Table 3 shows that 1 h incubation of HaCaT cells with 100 µg·mL−1 of gly-rhAFP led to remarkable and transient overexpression of these three genes; the expression was significantly decreased after 24 h of cell exposure to AFP. A brief exposure to UVA (75 s, 2.5 J·cm−2) induced the same genes 1 h post irradiation, although to a lesser extent as compared with AFP. The combination of UVA and AFP substantially attenuated gene overexpression induced by either AFP or UVA alone (Table 3).
Table 2.
Effects of gly-rhAFP (100 µg·mL−1) and UVA (2.5 J·cm−2) on the release of cytokines (pg·mg−1 protein) from HaCaT cells within 24 h
| Cytokines | Control | AFP | UVA | UVA+AFP |
|---|---|---|---|---|
| IL-1β | 0.5 ± 0.02 | 1.4 ± 0.07**a | 0.8 ± 0.21 | 1.5 ± 0.22**a |
| IL-1ra | 4 ± 1 | 18 ± 1***a | 12 ± 7*a | 19 ± 3***a |
| IL-6 | 1.1 ± 0.0 | 4.0 ± 0.2**a | 1.6 ± 0.5 | 5.0 ± 0.5**a |
| IL-8 | 6 ± 0 | 16 ± 1***a | 8 ± 1 | 16 ± 2**a |
| IL-10 | 279 ± 19 | 933 ± 86**a | 441 ± 105 | 1063 ± 207**a |
| IP-10 | 4 ± 1 | 13 ± 2**a | 4 ± 2 | 11 ± 3*a |
| MCP-1 | 99 ± 19 | 1195 ± 83**a | 132 ± 54 | 912 ± 84**a,*b |
| MIP-1α | 8 ± 1 | 10 ± 0*a | 10 ± 1 | 12 ± 1 |
| MIP-1β | 1.4 ± 0.1 | 3.9 ± 0.4**a | 2.4 ± 0.8 | 5.2 ± 1.5**a |
| PDGFbb | 3 ± 0 | 17 ± 2***a | 7 ± 4 | 14 ± 2*a |
| TNF-α | 7 ± 2 | 17 ± 2**a | 9 ± 4 | 13 ± 3**a |
| VEGF | 1938 ± 182 | 7432 ± 335***a | 2940 ± 835*a | 7698 ± 759***a |
Values shown in the Table are means ± SD from three independent experiments.
AFP, α-fetoprotein; gly-rhAFP, glycosylated recombinant human alpha fetoprotein; IL, interleukin; IL-1ra, interleukin 1 receptor antagonist; IP-10, interferon gamma-produced protein of 10 kDa; MCP-1, monocyte chemotactic protein 1; MIP, macrophage inflammatory protein; PDGF, platelet-derived growth factor; TNF-α, tumour necrosis factor alpha; VEGF, vascular endothelial growth factor.
P < 0.05;
P < 0.01;
P < 0.001 versus control cells;
P < 0.05 versus cells exposed to gly-rhAFP.
Table 3.
Effects of gly-rhAFP (100 µg·mL−1) and UVA (2.5 J·cm−2) on the cytokine gene expression in HaCaT cells (fold induction related to control)
| Genes | Control | AFP, 1 h | AFP, 24 h | 1 h after UVA | |
|---|---|---|---|---|---|
| Without AFP | With AFP | ||||
| TNF-α | 1.0 ± 0.4 | 5.3 ± 1.7*a | 1.3 ± 0.2 | 3.9 ± 0.2**a | 2.7 ± 0.5*a,*b |
| IL-6 | 1.0 ± 0.3 | 6.7 ± 0.5**a | 1.8 ± 0.2**a | 2.1 ± 0.1*a | 1.2 ± 0.4*a,*b |
| IL-1α | 1.0 ± 0.2 | 4.7 ± 0.9*a | 1.7 ± 0.2*a | 3.4 ± 0.2**a | 1.4 ± 0.6*a,*b |
Values shown in the Table are means ± SD from three independent experiments.
AFP, α-fetoprotein; gly-rhAFP, glycosylated recombinant human alpha fetoprotein; IL, interleukin; TNF-α, tumour necrosis factor alpha.
P < 0.05;
P < 0.01; versus control cells;
P < 0.05 versus cells exposed to UVA.
AFP affected hydrogen peroxide and nitric oxide production and did not influence antioxidant systems in HaCaT cells
HaCaT cells, exposed to physiological concentrations of AFP (25–100 µg·mL−1) for 24 h, released H2O2 time- and dose-dependently (Figure 4A). Maximal H2O2 release was observed after 4 h of incubation with 100 µg·mL−1 of the protein. Control human plasma albumin did not affect H2O2 production by the cultured cells (data not shown). Brief exposure of HaCaT cells to UVA irradiation alone (75 s, 2.5 J·cm−2) increased H2O2 levels in conditioned medium 1 h post irradiation (1.0 ± 0.5 nmol·mg−1 protein; P < 0.05 vs. 0.01 ± 0.08 nmol·mg−1 protein in non-exposed cells). The combination of UVA and gly-rhAFP induced intense H2O2 release equal to a sum of H2O2 production by HaCaT cells (15.2 ± 3.5 nmol·mg−1 protein; P < 0.01 vs. control and P < 0.05 vs. UVA-exposed cells) upon exposure either to gly-rhAFP (12.9 ± 1.9; P < 0.005 vs. control) or to UVA alone (1.0 ± 0.5 nmol·mg−1 protein; P < 0.05 vs. control). During HaCaT incubation in serum-free medium (starvation conditions), the baseline levels of nitrates plus nitrites (NO2−/NO3−) accumulated in conditioned medium gradually increased (Figure 4B). In the presence of gly-rhAFP, low baseline levels of NO2−/NO3− at 1 h were increased depending on the dose of the protein. In contrast, higher extracellular levels of NO2−/NO3− observed at 4 and 24 h of cell starvation were significantly decreased in the presence of gly-rhAFP (Figure 4B). Of note, low baseline levels of NO2−/NO3− in the conditioned medium (0.0 ± 2.1 nmol·mg−1 protein) observed at 1 h of cell starvation were slightly influenced by UVA irradiation. Combining the two factors produced considerable increases compared with single factor treatment [(13.9 ± 5.2; P > 0.05 vs. exposure to AFP alone) obtained either upon UVA irradiation (0.9 ± 1.7; P > 0.05 vs. control) or AFP alone (12.9 ± 7.8; P < 0.01 vs. control; P > 0.05 vs. exposure to UVA + AFP)]. Baseline intracellular NO levels, assessed by the specific fluorescence probe DAF-2DA, were not affected after a 1 h incubation of HaCaT cells with 100 µg·mL−1 gly-rhAFP for (Figure 5A and B). Exposure to UVA alone or in combination with 100 µg·mL−1 gly-rhAFP led to significant increases in intracellular NO levels. At the same time, analysis of inducible nitric oxide synthase (iNOS) gene expression revealed its substantial overexpression in HaCaT cells after 24 h incubation with 100 µg·mL−1 gly-rhAFP (Figure 5A and B). Similar overexpression was observed 1 h after UVA irradiation. However, the combination of the two factors, gly-rhAFP + UVA, completely prevented up-regulation of iNOS mRNA (Figure 5C). Interestingly, gly-AFP did not affect several other parameters of redox status in HaCaT cells, such as GSH and protein SH-group levels as well as SOD, catalase and GST activities (Table 4).
Figure 4.
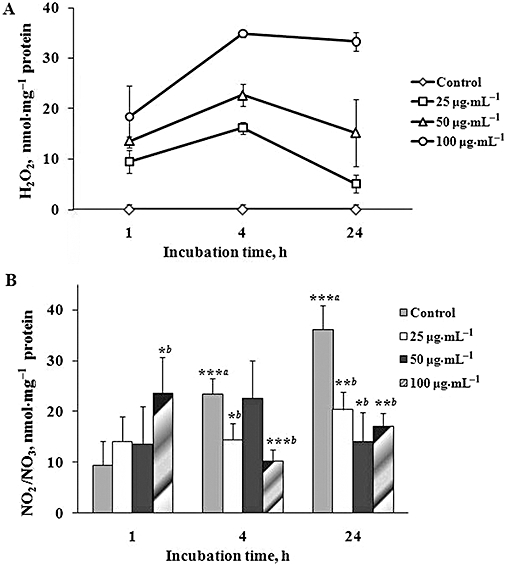
Effects of glycosylated recombinant human alpha fetoprotein (gly-rhAFP) on H2O2 (A) and NO2/NO3 (B) levels in conditioned medium of HaCaT cells (mean ± SD). Cells were treated with various doses of gly-rhAFP (25, 50 and 100 µg·mL−1), and the conditioned medium was analysed at different time points (1, 4 and 24 h). ***aP < 0.005 versus control at 1 h; *bP < 0.05; **bP < 0.01; ***bP < 0.005 versus respective control.
Figure 5.
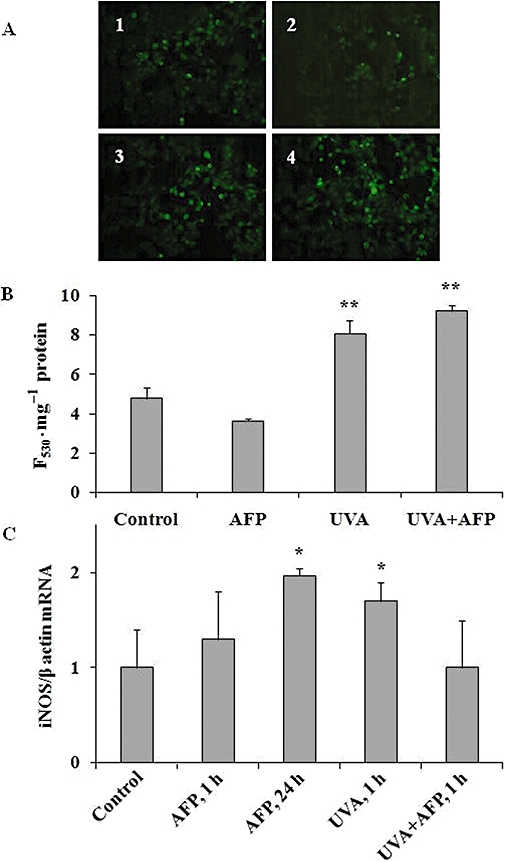
Effects of gly-rhAFP (100 µg·mL−1) and UVA (2.5 J·cm−2) on intracellular NO levels and iNOS gene expression in HaCaT cells (mean ± SD). (A) Typical fluorescence micrographs of HaCaT cells incubated for 1 h with fluorescent probe DAF-2DA: 1 – control cells; 2 – cells exposed to gly-rhAFP; 3 – cells exposed to UVA, 4 – cells exposed to the combination of UVA and gly-rhAFP. (B) Fluorescence intensity (F530·mg−1 protein) in the lysates of DAF-2DA-loaded HaCaT cells. (C) iNOS gene expression expressed as fold induction related to control. *P < 0.05; **P < 0.01 versus control. AFP, α-fetoprotein; DAF-DA, 4,5-diaminofluorescein diacetate; gly-rhAFP, glycosylated recombinant human alpha fetoprotein; iNOS, inducible nitric oxide synthase.
Table 4.
Effects of gly-rhAFP on the activity of catalase, SOD, GST, and the level of GSH and protein SH-groups in HaCaT cells (mean ± SD)
| Gly-rhAFP, µg·mL−1 |
Catalase |
GST |
SOD |
GSH |
Protein SH-groups |
|---|---|---|---|---|---|
| µmol·min−1·mg−1protein | U·mg−1protein | nmol·mg−1protein | |||
| 0a | 1.20 ± 0.28 | 0.94 ± 0.27 | 3.50 ± 0.77 | 203 ± 26 | 19.6 ± 10.4 |
| 25a | 1.28 ± 0.34 | 0.92 ± 0.17 | 3.67 ± 0.48 | 201 ± 18 | 21.3 ± 16.1 |
| 50a | 1.48 ± 0.38 | 0.98 ± 0.27 | 3.86 ± 0.82 | 209 ± 15 | 17.3 ± 20.0 |
| 100a | 1.24 ± 0.35 | 0.94 ± 0.29 | 3.42 ± 0.71 | 194 ± 23 | 21.9 ± 21.1 |
| 0b | 1.07 ± 0.21 | 1.02 ± 0.19 | 3.44 ± 0.28 | 241 ± 29 | 24.8 ± 16.0 |
| 25b | 0.93 ± 0.21 | 0.90 ± 0.13 | 3.22 ± 0.22 | 228 ± 37 | 21.6 ± 9.1 |
| 50b | 0.97 ± 0.22 | 0.95 ± 0.18 | 3.38 ± 0.33 | 236 ± 29 | 25.5 ± 23.2 |
| 100b | 1.14 ± 0.13 | 1.11 ± 0.40 | 3.37 ± 0.58 | 232 ± 41 | 25.7 ± 19.2 |
| 0c | 2.28 ± 0.80 | 1.03 ± 0.08 | 3.38 ± 0.06 | 184 ± 34 | 17.4 ± 5.0 |
| 25c | 2.01 ± 0.57 | 1.07 ± 0.25 | 4.43 ± 1.94 | 177 ± 9 | 26.0 ± 13.0 |
| 50c | 1.86 ± 0.43 | 0.89 ± 0.01 | 4.06 ± 0.60 | 181 ± 20 | 19.7 ± 8.0 |
| 100c | 2.24 ± 0.78 | 1.09 ± 0.21 | 4.19 ± 0.72 | 199 ± 27 | 27.2 ± 18.0 |
Incubation time with gly-rhAFP:
1 h;
4 h;
24 h.
Values shown in the Table are means ± SD from four–five independent experiments.
gly-rhAFP, glycosylated recombinant human alpha fetoprotein; GST, glutathione-S-transferase; SOD, superoxide dismutase.
AFP alone and in combination with UVA induced Akt1 and ERK1/2 but not NFκB phosphorylation in HaCaT cells
Incubation of HaCaT cells with 100 µg·mL−1 gly-rhAFP for 20 min did not induce phosphorylation of NFκB at serine 536 of p65 but did markedly activate phosphorylation of both protein kinase B (Akt1) and ERK1/2 (Figure 6A). Exposure of HaCaT cells to UVA slightly and transiently enhanced p65/RelA phosphorylation at 5 min followed by its decrease at 20 min (Figure 6B). The UVA-induced NFκB activation was abolished in the presence of AFP (Figure 6C). With regard to ERK1/2, AFP significantly and time-dependently enhanced its phosphorylation up to fourfold versus baseline level by 20 min (Figure 6A). UVA alone as well as combination of UVA + AFP exerted similar effects (Figure 6B and C). Protein kinase B was rapidly and markedly phosphorylated in the presence of AFP [1.9-fold and 4.5-fold vs. baseline level by 5 min and 20 min, respectively (Figure 6A)]. UVA-induced phosphorylation of the protein was delayed and much less evident (Figure 6B). UVA and AFP applied together (Figure 6C) exerted an effect lower than that of AFP alone and greater than that of UVA alone.
Figure 6.
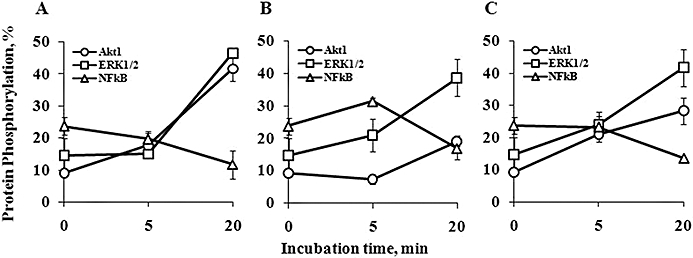
Effects of gly-rhAFP and UVA on phosphorylation of NFκB, Akt1 and ERK1/2 in HaCaT cells (mean ± SD). 0 min – control cells without treatment; 5 and 20 min – time after treatment. (A) cells exposed to 100 µg·mL−1 gly-rhAFP; (B) cells exposed to 2.5 J·cm−2 UVA; (C) cells exposed to UVA + gly-rhAFP. Akt, protein kinase B; ERK, extracellular signal-regulated kinase; gly-rhAFP, glycosylated recombinant human alpha fetoprotein; NFκB, nuclear factor kappa B.
AFP alone and in combination with inflammatory cytokines induced AP-1 but not NFκB activation in primary keratinocyte cultures
In order to detect effects of AFP on two major transcription factors (AP-1 and NFκB) involved in the regulation of cytokine and chemokine expression in keratinocytes, we examined the nuclear translocation of c-Fos and p65/RelA as relevant components of AP-1 and NFκB complex respectively. Either administered alone or in combination with TNF-α or IFN-γ, gly-rhAFP led to a dose-dependent increase in the nuclear level of c-Fos, whereas p65/RelA was only slightly affected by the higher AFP concentration (50 µg·mL−1) (Figure 7A). In keeping with this result, the AP-1-associated DNA binding activity was dose-dependently enhanced by AFP, either alone or in association with the pro-inflammatory cytokines. By contrast, no relevant AFP-dependent effect was observed on NFκB DNA binding activity, which was significantly up-regulated only by TNF-α (Figure 7B).
Figure 7.
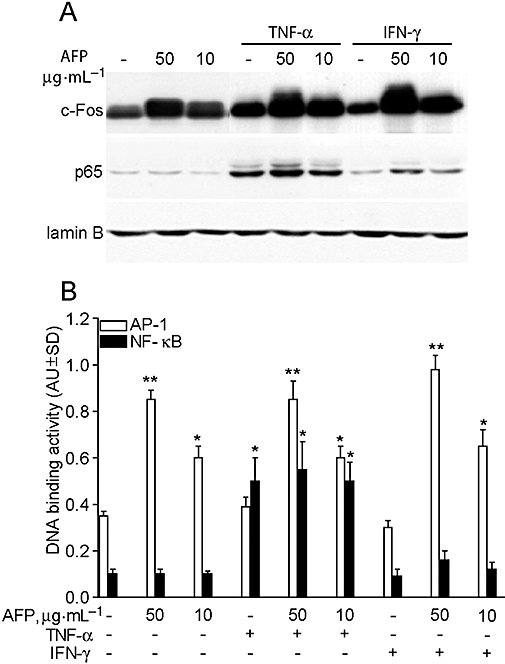
Gly-rhAFP activates AP-1 in normal human keratinocytes. (A) Western blot analysis of c-Fos (AP-1 subunit) and p65 (RelA, NFκB subunit) in nuclear lysates (10 µg per lane) from untreated cultures (-), incubated for 20 min with 50 or 10 µg·mL−1 rhAFP alone, or in the presence of 50 ng·mL−1 TNF-α or 50 U·mL−1 IFN-γ. Lamin B was used to assess equal loading. These results are representative of three independent experiments. (B) AP-1- and NFκB-specific DNA binding activity in keratinocytes following 2 h treatments. **P < 0.01 versus untreated keratinocyte cultures (-).*P < 0.05 versus untreated keratinocyte cultures. AFP, α-fetoprotein; AP-1, activator protein 1; gly-rhAFP, glycosylated recombinant human alpha fetoprotein; IFN-γ, interferon gamma; NFκB, nuclear factor kappa B; TNF-α, tumour necrosis factor alpha.
Discussion and conclusions
Human AFP has long attracted interest for its intrinsic regulating role in embryogenesis, tumorigenesis, tissue regeneration and immune response. Drug development of AFP-based preparations exploits the differential capacity of AFP to affect tumour and normal cells as well as resting and activated immune cells (Mizejewski, 2002; Dudich, 2007). It has been suggested that exogenous AFP may regulate two mechanisms involved in the pathogenesis of chronic immune-mediated inflammatory diseases: apoptotic disorders and immune inflammation (Dudich, 2007; Nizri et al., 2007). Inhibition of immune inflammation has been attributed to AFP-mediated down-regulation of the expression of inflammatory cytokines in activated immune cells by an NFκB-dependent mechanism (Semenkova et al., 1997; Cavin et al., 2004; Li et al., 2007). The immunomodulating properties of AFP have led to the development of AFP-based, injectable anti-inflammatory drugs targeting autoimmune disorders such as autoimmune encephalomyelitis, rheumatoid arthritis, autoimmune uveitis and psoriasis (Irony-Tur-Sinai et al., 2006; Dudich, 2007; Murray and Sawitzke, 2007; Nizri et al. (2007). In the pathogenesis of psoriasis, crosstalk between activated T lymphocytes and keratinocytes mediated by both keratinocyte- and T cell-derived cytokines seems to play a crucial role (Lowes et al., 2007). Because the biological effects of AFP are cell-specific (Mizejewski, 2002), in the present study we, examined the interaction of exogenous AFP preparations with human keratinocytes.
Three human AFP preparations (the embryonic form isolated from umbilical cord blood and two recombinant forms completely homologous to native human AFP: glycosylated and non-glycosylated forms) were studied in order to recognize the role, if any, of a large carbohydrate moiety in the interaction with keratinocytes. It has been previously reported that the sugar moiety is involved in the AFP binding to lectin-like receptors on the immune cell membrane, thus regulating transduction pathways (Mizejewski, 2004). In this study, we did not observe any difference between the three AFP forms studied suggesting that: (i) the carbohydrate moiety of the AFP molecule did not influence the protein–keratinocyte interaction; and (ii) both recombinant and native embryonic forms exert similar effects.
Within the physiologically relevant range of concentrations (10–200 µg·mL−1), all three AFP preparations dose-dependently induced a moderate but significant expression of chemokines active towards granulocytes and monocytes (IL-8 and MCP-1, respectively) and GM-CSF in resting primary keratinocytes (Figure 1). AFP concentration-dependently attenuated the TNF-α-driven release of all the factors under investigation by enhancing GM-CSF and IL-8, while reducing MCP-1 and IP-10. In contrast, no attenuation was apparent when AFP was coupled with IFN-γ. Extremely high overexpression of these cytokines induced by the combination of TNF-α and IFN-γ (Figure 2, Korkina et al., 2007) was not affected by any AFP preparation. As all the AFP preparations added to keratinocyte cultures at concentration of 200 µg·mL−1 for 24 h did not influence either cell viability or proliferation, we concluded that AFP induced cytokine expression specifically and not as a consequence of any cytotoxicity. Cytotoxic effects had been observed in the early work with low concentration (1 µg·mL−1) of non-purified embryonic AFP (Voroteliak et al., 1996). On the grounds of these data, we suggest that AFP has a major impact on the signal transduction pathways implicated in the response to TNF-α. We would like to stress that AFP alone induced TNF-α expression and release by resting peripheral blood-derived CD4 T cells and it synergized with CD3/CD28 co-stimulation in the induction of both TNF-α and IFN-γ (Figure 3), indicating that it exerted a clear pro-inflammatory activity on these cells.
Tumour necrosis factor alpha-driven de novo expression of the inflammatory mediators crucially depends upon the functional activation of the transcription factor NFκB in a variety of cell types, in cooperation with other transcription factors involved in maximal gene induction (Wajant et al., 2003). We have previously demonstrated that activation of the ERK1/2-AP-1 signalling enhanced GM-CSF and IL-8 expression in human keratinocytes due to its synergistic action with NFκB. Conversely it opposed MCP-1 and IP-10 expression (Pastore et al., 2000; Pastore et al., 2005). The profile of AFP-induced effects in our experiments confirms that AFP, independent on its origin and glycosylation state, acts on keratinocytes through activation of the ERK1/2-AP-1 pathway. The analysis of AFP-induced phosphorylation of NFκB component p65, ERK1/2 and Akt1 in human keratinocytes confirmed our hypothesis that AFP acts by inducing kinases involved in the AP-1 transactivation, including ERK1/2 and Akt1/protein kinase B (De Bosscher et al., 2003) (Figure 5B and C). Indeed, moderate doses of UVA known to activate the mitogen-activated protein kinase cascades (Silvers and Bowden, 2002;De Bosscher et al., 2003; Bachelor and Bowden, 2004, Wu et al., 2008) showed effects similar to AFP on both the kinases ERK1/2 and Akt1. Akt1 is involved in cellular survival pathways due to its anti-apoptotic effects (Song et al., 2005) and is thought to be a key signalling molecule to induce protein synthesis necessary for tissue growth (Yang et al., 2004) and physiological angiogenesis (Somanath et al., 2006). The UVA-induced phosphorylation was not attenuated by AFP indicating the absence of competition or synergism between these two factors at the kinase-dependent step of signalling cascade. In agreement with this evidence, we demonstrated that the DNA binding activity of AP-1 was selectively and dose-dependently enhanced by AFP in resting keratinocytes (Figure 7). Activation of the ERK1/2-AP-1 transcription pathway inevitably leads to expression of those genes, which contain AP-1 sites in their promoters. Therefore we evaluated the effects of AFP on the expression of several early immune/inflammatory response genes, such as TNF-α (considered as an early mediator in the induction phase of the inflammatory cascade (Bashir et al., 2009), IL-1β, IL-6 and iNOS (NOS2) (Table 3 and Figure 5). Either AFP or UVA alone markedly induced expression of these genes at 1 h after exposure. The inducing effect of AFP was transient (the gene expression returned practically to baseline level after 24 h) and greater than that of UVA at 1 h. Surprisingly, we observed highly suppressed gene expression in HaCaT cells exposed to a combination of UVA + AFP for 1 h. AFP stimulated release and accumulation in the cell supernatant of a wide array of cytokines, chemokines and growth factors with pro-inflammatory (IL-1β, IL-6, IL-8, IP-10, MIP-1α, MIP-1β and TNF-α), anti-inflammatory (IL-1ra, IL-6, IL-10) and angiogenic (MCP-1, PDGFbb and VEGF) action (Table 2). At the same time, UVA enhanced the levels of VEGF and IL-10. Notwithstanding the fact that UVA induced a fourfold increase in the expression of TNF-α and a twofold increase in the expression of IL-6 (Table 3), the corresponding gene products TNF-α and IL-6 were not accumulated in the UVA-exposed HaCaT cells. Moreover, UVA irradiation did not interfere with the effects of AFP. The discrepancy between UVA-induced gene expression and protein synthesis could be partly explained by either partial block of translation process and/or protein synthesis at a post-transcriptional level or by a lower sensitivity of multiplexed assay versus qRT-PCR.
During the last decade, numerous studies have shown that, at low concentrations, reactive oxygen species (ROS) regulate signal transduction and gene expression processes (Rhee, 1999; Korkina and Pastore, 2009) in a variety of cells including keratinocytes (Epinat and Gilmore, 1999; Thannickal and Fanburg, 2000). In the present study, we observed that AFP induced an early response in HaCaT cells consisting of enhanced H2O2 and NO2−/NO3− (secondary products of NO reaction with superoxide and other oxidants) production (Figure 4). Although substantially enhanced as compared with baseline levels, concentrations of both H2O2 and NO by-products remained within the non-toxic, regulatory, micromolar range (Roy et al., 2006). Conversely, AFP reduced the substantially high levels of NO2−/NO3− in HaCaT cells when cultured in a nutrient-poor environment (Figure 4). Another confirmation of the absence of AFP-associated ROS/RNS toxicity in HaCaT cells came from the observation that protective antioxidant and detoxifying systems did not respond to AFP challenge (Table 4). UVA alone induced selective intracellular NO production. Only a slight increase in the H2O2 and NO2−/NO3− release from HaCaT cells occurred, which probably indicated a selective action of UVA irradiation towards expression and activity of iNOS (Figure 4) and not towards NADPH-oxidase in keratinocytes. This result correlates with previous in vivo observations that shortly after UVA exposure, iNOS protein is strongly up-regulated in both dermis and epidermis, presumably due to increased concentration of local pro-inflammatory cytokines involved in iNOS expression (Cals-Grierson and Ormerod, 2004).
The combined effects of AFP and UVA on ROS/RNS production were merely additive. This response allowed us to suggest that at several points, such as phosphorylation, cytokine gene and protein expression and ROS/RNS production, AFP acts in the same way as moderate non-cytotoxic doses of UVA, and their individual molecular effects are usually additive.
In conclusion, the data obtained appear to provide the first evidence that the pharmacological effects of AFP on human keratinocytes could be closely connected with AFP-associated modulation of their intrinsic immune response to challenges with inflammatory mediators and UVA irradiation. The AFP-induced regulation of cytokine expression is modulated through intracellular ERKs and Akt1 signalling pathways, eventually leading to AP-1-mediated control of gene transcription. This signalling mechanism could also underlie the AFP effect on cytokine expression by resting and CD3/CD28 stimulated CD4-positive T cells (Schafer et al., 2003), although this hypothesis requires further investigation. The AFP-induced response in keratinocytes may be mediated by non-toxic amounts of hydrogen peroxide and secondary nitric oxide metabolites. Although it is difficult to envisage a direct anti-inflammatory action of AFP-containing preparations when applied topically it is possible that they could protect skin by boosting its own immune response.
Acknowledgments
The work was financed by grant RC-2007-IDI IRCCS of Italian Ministry for Health. The authors thank Dr Edward Tatulov, General Director of BioSystem Ltd., for the kind gift of AFP preparations. The excellent technical assistance of Dr Ivan Fini is greatly appreciated.
Glossary
Abbreviations:
- AFP
α-fetoprotein
- Akt
protein kinase B
- AP-1
activator protein 1
- DAF-DA
4,5-diaminofluorescein diacetate
- DMEM
Dulbecco's modified Eagle's medium
- ehAFP
embryonic human alpha fetoprotein
- ERK
extracellular signal-regulated kinase
- gly-rhAFP
glycosylated recombinant human alpha fetoprotein
- GM-CSF
granulocyte macrophage colony stimulating factor
- GST
glutathione-S-transferase
- IFN-γ
interferon gamma
- IL
interleukin
- IL-1ra
interleukin 1 receptor antagonist
- iNOS
inducible nitric oxide synthase
- IP-10
interferon gamma-produced protein of 10 kDa
- mAbs
monoclonal antibodies
- MCP-1
monocyte chemotactic protein 1
- MIP
macrophage inflammatory protein
- MTT
3-[4,5-dimethylthiazol-2yl]-diphenyl tetrazolium bromid
- NFκB
nuclear factor kappa B
- NO2−/NO3−
nitrite/nitrate
- PDGF
platelet-derived growth factor
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- rhAFP
recombinant human alpha fetoprotein
- ROS/RNS
reactive oxygen and nitrogen species
- SOD
superoxide dismutase
- Th
T helper
- T/I
TNF-α/IFN-γ
- TNF-α
tumour necrosis factor alpha
- VEGF
vascular endothelial growth factor
Conflicts of interest
None.
References
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Alisa A, Boswell S, Pathan AA, Ayaru L, Williams R, Behboudi S. Human CD4(+) T cells recognize an epitope within alpha-fetoprotein sequence and develop into TGF-beta-producing CD4(+) T cells. J Immunol. 2008;180:5109–5117. doi: 10.4049/jimmunol.180.7.5109. [DOI] [PubMed] [Google Scholar]
- Bachelor MA, Bowden GT. UVA-mediated activation of signalling pathways involved in skin tumor promotion and progression. Semin Cancer Biol. 2004;14:131–138. doi: 10.1016/j.semcancer.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Bashir MM, Sharma MR, Werth VP. TNF-alpha production in the skin. Arch Dermatol Res. 2009;301:87–91. doi: 10.1007/s00403-008-0893-7. [DOI] [PubMed] [Google Scholar]
- Benevolensky SV, Marchenko AN, Kozlov DG, Zatsepin SS, Shingarova LN, Dudich IV, et al. Recombinant α-fetoprotein, method and means for preparation thereof, compositions on the base thereof and use thereof. WORLD PATENT WO-2006009492: 2006 January 26.
- Boismenu R, Semeniuk D, Murgita R. Purification and characterization of human and mouse recombinant alpha-fetoproteins expressed in Escherichia coli. Protein Expr Purif. 1997;10:10–26. doi: 10.1006/prep.1996.0697. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of dye-binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cals-Grierson MM, Ormerod AD. Nitric oxide function in the skin. Nitric Oxide. 2004;10:179–193. doi: 10.1016/j.niox.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Cao DY, Yang JY, Dou KF, Ma LY, Teng ZH. Alpha-fetoprotein and interleukin-18 gene-modified dendritic cells effectively stimulate specific type-1 CD4- and CD8-mediated T-cell response from hepatocellular carcinoma patients in vitro. Hum Immunol. 2007;68:334–341. doi: 10.1016/j.humimm.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Cavin LG, Venkatraman M, Factor VM, Kaur S, Schroeder I, Mercurio F, et al. Regulation of α-fetoprotein by nuclear factor-κB protects hepatocytes from tumor necrosis factor-α cytotoxicity during fetal liver development and hepatic oncogenesis. Cancer Res. 2004;64:7030–7038. doi: 10.1158/0008-5472.CAN-04-1647. [DOI] [PubMed] [Google Scholar]
- De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-kB or activator protein-1: molecular mechanisms for gene repression. Endocr Rev. 2003;24:488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- Dudich E. MM-093, a recombinant human alpha-fetoprotein for the potential treatment of rheumatoid arthritis and other autoimmune diseases. Curr Opin Mol Ther. 2007;9:603–610. [PubMed] [Google Scholar]
- Dudich IV, Tokhtamysheva N, Semenkova L, Dudich E, Hellman J, Korpela T. Isolation and structural and functional characterization of two stable peptic fragments of human alpha-fetoprotein. Biochemistry. 1999;38:10406–10414. doi: 10.1021/bi990630h. [DOI] [PubMed] [Google Scholar]
- Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- Epinat JC, Gilmore TD. Diverse agents act at multiple levels to inhibit the Rel/NFkappaB signal transduction pathways. Oncogene. 1999;18:6896–6909. doi: 10.1038/sj.onc.1203218. [DOI] [PubMed] [Google Scholar]
- Filella X, Molina R, Alcover J, Coca F, Zarco MA, Ballesta AM. Influence of AFP, CEA and PSA on the in vitro production of cytokines. Tumour Biol. 2001;22:67–71. doi: 10.1159/000050598. [DOI] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Irony-Tur-Sinai M, Grigoriadis N, Lourbopoulos A, Pinto-Maaravi F, Abramsky O, Brenner T. Amelioration of autoimmune neuroinflammation by recombinant human alpha-fetoprotein. Exp Neurol. 2006;198:136–144. doi: 10.1016/j.expneurol.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Korkina L, Pastore S. The role of redox regulation in the normal skin physiology and inflammatory skin diseases. Front Biosci. 2009;E1:123–141. doi: 10.2741/E13. [DOI] [PubMed] [Google Scholar]
- Korkina LG, Mikhal'chik E, Suprun MV, Pastore S, Dal Toso R. Molecular mechanisms underlying wound healing and anti-inflammatory properties of naturally occurring biotechnologically produced phenylpropanoid glycosides. Cell Mol Biol (Noisy-le-grand) 2007;53:84–91. [PubMed] [Google Scholar]
- Kojima H, Nakatsubo N, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, et al. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem. 1998;70:2446–2453. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- Kostyuk VA, Potapovich AI. Superoxide driven oxidation of quercetin and a simple sensitive assay for determination of superoxide dismutase. Biochem Int. 1989;19:1117–1124. [PubMed] [Google Scholar]
- Li M, Zhou S, Liu X, Li P, McNutt MA, Li G. alpha-Fetoprotein shields hepatocellular carcinoma cells from apoptosis induced by tumor necrosis factor-related apoptosis-inducing ligand. Cancer Lett. 2007;249:227–234. doi: 10.1016/j.canlet.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- Mizejewski GJ. Biological role of α-fetoprotein in cancer: prospects for anticancer therapy. Expert Rev Anticancer Ther. 2002;2:709–735. doi: 10.1586/14737140.2.6.709. [DOI] [PubMed] [Google Scholar]
- Mizejewski GJ. Biological roles of α-fetoprotein during pregnancy and perinatal development. Exp Biol Med. 2004;229:439–463. doi: 10.1177/153537020422900602. [DOI] [PubMed] [Google Scholar]
- Murray J, Sawitzke A. MM-093 is safe and well tolerated in an exploratory phase 2 study and patients with the highest serum levels of MM-093 achieved robust clinical responses. Ann Rheum Dis. 2007;66(Suppl. 2):434–440. [Google Scholar]
- Nickoloff BJ, Bonish BK, Marble DJ, Schriedel KA, DiPietro LA, Gordon KB, et al. Lessons learned from psoriatic plaques concerning mechanisms of tissue repair, remodeling, and inflammation. J Investig Dermatol Symp Proc. 2006;11:16–29. doi: 10.1038/sj.jidsymp.5650010. [DOI] [PubMed] [Google Scholar]
- Nizri E, Irony-Tur-Sinai M, Grigoriadis N, Abramsky O, Amitai G, Brenner T. Novel approaches to treatment of autoimmune neuroinflammation and lessons for drug development. Pharmacology. 2007;79:42–49. doi: 10.1159/000097628. [DOI] [PubMed] [Google Scholar]
- Pastore S, Fanales-Belasio E, Albanesi C, Chinni LM, Giannetti A, Girolomoni G. Granulocyte/macrophage colony-stimulating factor is overproduced by keratinocytes in atopic dermatitis. Implications for sustained dendritic cell activation in the skin. J Clin Invest. 1997;99:3009–3017. doi: 10.1172/JCI119496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore S, Giustizieri ML, Mascia F, Giannetti A, Kaushansky K, Girolomoni G. Dysregulated activation of activator protein 1 in keratinocytes of atopic dermatitis patients with enhanced expression of granulocyte/macrophage-colony stimulating factor. J Invest Dermatol. 2000;115:1134–1143. doi: 10.1046/j.1523-1747.2000.00149.x. [DOI] [PubMed] [Google Scholar]
- Pastore S, Mascia F, Mariotti F, Dattilo C, Mariani V, Girolomoni G. ERK1/2 regulates epidermal chemokine expression and skin inflammation. J Immunol. 2005;174:5047–5056. doi: 10.4049/jimmunol.174.8.5047. [DOI] [PubMed] [Google Scholar]
- Pastore S, Mascia F, Mariani V, Girolomoni G. Keratinocytes in skin inflammation. Expert Rev Dermatol. 2006;1:279–291. [Google Scholar]
- Renard P, Ernest I, Houbion A, Art M, Le Calvez H, Raes M, et al. Development of a sensitive multi-well colorimetric assay for active NF-kappaB. Nucleic Acids Res. 2001;29(4):E21. doi: 10.1093/nar/29.4.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG. Redox signaling: hydrogen peroxide as intracellular messenger. Exp Mol Med. 1999;31:53–59. doi: 10.1038/emm.1999.9. [DOI] [PubMed] [Google Scholar]
- Roy S, Khanna S, Nallu K, Hunt TK, Sen CK. Dermal wound healing is subject to redox control. Mol Ther. 2006;13:211–220. doi: 10.1016/j.ymthe.2005.07.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer PH, Gandhi AK, Loveland MA, Chen RS, Man HW, Schnetkamp PP, et al. Enhancement of cytokine production and AP-1 transcriptional activity in T cells by thalidomide-related immunomodulatory drugs. J Pharmacol Exp Ther. 2003;305:1222–1232. doi: 10.1124/jpet.102.048496. [DOI] [PubMed] [Google Scholar]
- Schreiber E, Matthias P, Müller MM, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419–6424. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenkova LN, Dudich EI, Dudich IV, Shingarova LN, Korobko VG. α-Fetoprotein as a TNF resistance factor for the human hepatocarcinoma cell line HepG2. Tumour Biol. 1997;18:30–40. doi: 10.1159/000218013. [DOI] [PubMed] [Google Scholar]
- Silvers AL, Bowden GT. UVA irradiation-induced activation of activator protein-1 is correlated with induced expression of AP-1 family members in the human keratinocyte cell line HaCaT. Photochem Photobiol. 2002;75:302–310. doi: 10.1562/0031-8655(2002)075<0302:uiiaoa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Somanath PR, Razorenova OV, Chen J, Byzova TV. Akt1 in endothelial cell and angiogenesis. Cell Cycle. 2006;5:512–518. doi: 10.4161/cc.5.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signalling. Am J Physiol Lung Cell Mol Physiol. 2000;279:1005–1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- Tyurina YY, Serinkan FB, Tyurin VA, Kini V, Yalowich JC, Schroit AJ, et al. Lipid antioxidant, etoposide, inhibits phosphatidylserine externalization and macrophage clearance of apoptotic cells by preventing phosphatidylserine oxidation. J Biol Chem. 2004;279:6056–6064. doi: 10.1074/jbc.M309929200. [DOI] [PubMed] [Google Scholar]
- Viola A, Schoroeder S, Sakakibara Y, Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–682. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- Voroteliak EA, Vasil'ev AV, Gusev SA, Povalii TM, Terskikh VV. Alfa-fetoprotein suppresses keratinocyte proliferation in vitro. Izv Akad Nauk Ser Biol. 1996;1:117–120. [PubMed] [Google Scholar]
- Wajant H, Pfinzenmaier K, Scheurich P. Tumor necrosis factor signalling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- Wang W, Alpert E. Downregulation of phorbol 12-myristate 13-acetate-induced tumor necrosis factor-alpha and interleukin-1 beta production and gene expression in human monocytic cells by human alpha-fetoprotein. Hepatology. 1995;22:921–928. [PubMed] [Google Scholar]
- Wang YS, Ma XL, Qi TG, Liu XD, Meng YS, Guan GJ. Downregulation of alpha-fetoprotein siRNA inhibits proliferation of SMMC-7721 cells. World J Gastroenterol. 2005;11:6053–6055. doi: 10.3748/wjg.v11.i38.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Gao J, Dinh QT, Chen C, Fimmel S. IL-8 production and AP-1 transactivation induced by UVA in human keratinocytes: roles of D-alpha-tocopherol. Mol Immunol. 2008;45:2288–2296. doi: 10.1016/j.molimm.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Nakane A, Watanabe T, Miyoshi I, Kasai N. Evidence that alpha-fetoprotein suppresses the immunological function in transgenic mice. Biochem Biophys Res Commun. 1994;201:1154–1159. doi: 10.1006/bbrc.1994.1826. [DOI] [PubMed] [Google Scholar]
- Yang ZZ, Tschopp O, Baudry A, Dummler B, Hynx D, Hemmings BA. Physiological functions of protein kinase B/Akt. Biochem Soc Trans. 2004;32:350–354. doi: 10.1042/bst0320350. [DOI] [PubMed] [Google Scholar]


