Abstract
Background and purpose:
The aim of this study was to identify the actions of H2S on ion transport across rat distal colon.
Experimental approach:
Changes in short-circuit current (Isc) induced by the H2S-donor, NaHS, were measured in Ussing chambers. Cytosolic Ca2+ concentration was evaluated using fura-2.
Key results:
NaHS concentration-dependently induced a change in Isc, that was only partially inhibited by the neurotoxin, tetrodotoxin. Lower concentrations (≤10−3 mol·L−1) of NaHS induced a monophasic increase in Isc, whereas higher concentrations induced an additional, secondary fall of Isc, before a third phase when Isc rose again. Blockers of H2S-producing enzymes (expression demonstrated immunohistochemically) decreased basal Isc, suggesting that endogenous production of H2S contributes to spontaneous anion secretion. The positive Isc phases induced by NaHS were due to Cl− secretion as shown by anion substitution and transport inhibitor experiments, whereas the transient negative Isc induced by higher concentrations of the H2S-donor was inhibited by mucosal tetrapentylammonium suggesting a transient K+ secretion. When applied from the serosal side, glibenclamide, an inhibitor of ATP-sensitive K+ channels, and tetrapentylammonium, a blocker of Ca2+-dependent K+ channels, suppressed NaHS-induced Cl− secretion suggesting different types of K+ channels are stimulated by the H2S-donor. NaHS-induced increase in cytosolic Ca2+ concentration was confirmed in isolated, fura-2-loaded colonic crypts. This response was not dependent on extracellular Ca2+, but was inhibited by blockers of intracellular Ca2+ channels present on Ca2+ storage organelles.
Conclusions and implications:
H2S induces colonic ion secretion by stimulation of apical as well as basolateral epithelial K+ channels.
Keywords: chloride secretion, electrolyte transport, NaHS, potassium secretion, rat colon
Introduction
The intestinal epithelium is able both to absorb and secrete water and electrolytes. The switching between the two transport directions is controlled by neurotransmitters from the enteric nervous system, paracrine substances produced within the intestinal wall, and by hormones released from enteroendocrine cells (for review see Binder and Sandle, 1994). In contrast to most of these extracellular messengers, which bind to membrane receptors, so-called ‘gasotransmitters’ such as nitric oxide (NO) can easily diffuse into cells and directly affect intracellular targets, for example, enzymes like the soluble guanylate cyclase (Waldman and Murad, 1987). Another gas serving as extracellular regulator is hydrogen suphide (H2S). H2S is produced from the amino acid L-cysteine by the enzymes cystathionine-β-synthase and cystathionine-γ-lyase (Wang, 2002). It plays an important role in the regulation of circulation, as it induces vasodilatation, decreases cardiac inotropy and inhibits the proliferation of vascular smooth muscle cells (for references, see Geng et al., 2007). In the gut, H2S is known to relax the smooth muscle of various species (Teague et al., 2002), to exert an antinociceptive effect (measured e.g. by colorectal distension; Distrutti et al., 2006) and to induce anion secretion (Schicho et al., 2006).
In the intestinal wall, H2S has been shown to be produced spontaneously in the rat ileum (Zhao et al., 2003). The key enzymes for H2S formation have been found in enteric neurones from guinea-pig and human colon (Schicho et al., 2006). In the colon of these species, an exogenous H2S-donor such as NaHS induces Cl− secretion via stimulation of secretomotor submucous neurones, an effect probably mediated by capsazepine-sensitive transient receptor potential vanilloid 1 channels, whereas direct actions at the epithelium have not been observed. Furthermore, sulphate-reducing bacteria (such as e.g. Desulfovibrio or Desulfomonas species) are able to reduce SO42−, contained in food or in intestinal secretions, to sulphides including H2S (Florin et al., 1991) and thereby contribute to the permanent exposure of the epithelium, especially of the colon, to this gaseous compound.
Two main intracellular sites of action of H2S are known from different cell systems. This includes the ability of H2S to stimulate ATP-sensitive K+ channels, an effect that is well characterized at smooth muscle cells from rat aorta, resulting in a lowering of blood pressure (Zhao et al., 2001), or at rat insulinoma cells, where H2S affects insulin secretion (Yang et al., 2005). H2S has also been demonstrated to increase the cytosolic Ca2+ concentration in microglial cells from the rat (Lee et al., 2006). In these cells, H2S stimulates both the release of stored Ca2+ from thapsigargin-sensitive stores as well as the influx of extracellular Ca2+, although the transporters responsible for the release of intracellular Ca2+ are unknown.
Potassium channels play a dominant role in the control of intestinal secretion. An opening of apical K+ channels is a prerequisite for colonic K+ secretion involved in the regulation of the homeostasis of this cation (Schultheiss and Diener, 1997). Vice versa, basolateral K+ channels generate the driving force for Cl− secretion via apical Cl− channels by maintaining the negative membrane potential, which is dominated by a K+ diffusion potential (Strabel and Diener, 1995; Warth and Barhanin, 2003). In the light of the known effects of H2S on different types of K+ channels, it seemed of interest to study the effect of NaHS, a H2S-donor (Lee et al., 2006), on ion transport across the rat distal colon, where information about the possible actions of this gasotransmitter is lacking.
Methods
Solutions
The standard solution for the Ussing chamber experiments was a buffer solution containing (mmol·L−1): NaCl 107, KCl 4.5, NaHCO3 25, Na2HPO4 1.8, NaH2PO4 0.2, CaCl2 1.25, MgSO4 1 and glucose 12. The solution was gassed with carbogen (5% CO2 in 95% O2, v·v−1); pH was 7.4. In the Cl−-free buffer, NaCl and KCl were substituted by Na gluconate and K gluconate respectively. The Ca2+ concentration in the Cl−-free buffer was increased to 5.75 mmol·L−1 in order to compensate for the Ca2+-buffering properties of gluconate (Kenyon and Gibbons, 1977). For the Ca2+-free buffer, CaCl2 was omitted in the serosal buffer without addition of a chelator.
For the experiments carried out with isolated crypts, the following buffers were used. The EDTA (ethylenediamino-tetraacetic acid) solution for the isolation contained (mmol·L−1): NaCl 107, KCl 4.5, NaHCO3 25, Na2HPO4 1.8, NaH2PO4 0.2, glucose 12.2, EDTA 10 and 1 g·L−1bovine serum albumin (BSA). It was gassed with carbogen; pH was adjusted by tris-base [tris(hydroxymethyl)-aminomethane] to 7.4. The isolated crypts were stored in a high-potassium Tyrode solution consisting of (mmol·L−1): K gluconate 100, KCl 30, HEPES [N-(2-hydroxyethyl)piperazine-N′-2-ethanesulphonic acid] 10, NaCl 20, MgCl2 1, CaCl2 1.25, glucose 12.2, sodium pyruvate 5 and 1 g·L−1 BSA; pH was 7.4. For superfusion of the isolated crypts during the fura-2 experiments, the following buffer was used (in mmol·L−1): NaCl 140, KCl 5.4, CaCl2 1.25, MgSO4 1, HEPES 10, glucose 12.2; pH was 7.4.
For the immunhistochemical experiments, a 100 mmol·L−1phosphate buffer was used containing 80 mmol·L−1 Na2HPO4 and 20 mmol·L−1 NaH2PO4; pH was 7.4. 4′,6-Diamidino-2-phenylindoldilactate (DAPI) was dissolved in a phosphate-buffered saline (PBS) solution containing (in mmol·L−1): sodium phosphate buffer 10, NaCl 120, KCl 2.7; pH was 7.4.
Tissue preparation and crypt isolation
Wistar rats of either sex were used with a weight of 180–240 g. The animals had free access to water and a standard rat diet until the day of the experiment. Animals were killed by a blow on the head followed by exsanguination (approved by Regierungspräsidium Gießen, Gießen, Germany). The serosa and muscularis propria were stripped away to obtain a mucosa-submucosa preparation of the distal colon. The distal colon was distinguished from the proximal colon by the absence of palm leaf-like striae (Lindström et al., 1979). Briefly, the colon was placed on a small plastic rod with a diameter of 5 mm. A circular incision was made near the anal end with a blunt scalpel, and the serosa together with the lamina propria was gently removed in a proximal direction. Two segments of the distal colon of each rat were prepared. In general, one tissue served to measure the control response evoked by NaHS, and the other was treated with putative antagonists before NaHS was applied. If the antagonist had to be administered in a solvent, the control tissue was pretreated with the solvent, too.
For the isolation of intact crypts, the mucosa-submucosa was fixed on a plastic holder with tissue adhesive and transferred for about 7 min to the EDTA solution. The mucosa was vibrated once for 30 s in order to obtain crypts (Schultheiss et al., 2002a). They were collected in a high-K+ gluconate Tyrode buffer.
Short-circuit current measurement
The mucosa-submucosa preparation was fixed in a modified Ussing chamber, bathed with a volume of 3.5 mL on each side of the mucosa. The tissue was incubated at 37°C and short-circuited by a computer-controlled voltage-clamp device (Ingenieur Büro für Mess- und Datentechnik Mussler, Aachen, Germany) with correction for solution resistance. Tissue conductance (Gt) was measured every minute by the voltage deviation induced by a current pulse (±50 µA, duration 200 ms) under open-circuit conditions. Short-circuit current (Isc) was continuously recorded on a chart recorder. Isc is expressed as µEq·h−1·cm−2, that is, the flux of a monovalent ion per time and area, with 1 µEq·h−1·cm−2= 26.9 µA·cm−2. In some figures and in Table 1, the maximal change in Isc (ΔIsc) induced by NaHS or pinacidil is given as the difference from the baseline just before administration of the drug.
Table 1.
Effect of transport inhibitors on NaHS-induced short-circuit current (Isc)
|
ΔIsc (µEq.h−1·cm−2) induced by NaHS |
|||
|---|---|---|---|
| First peak (1) | Decrease (2) | Secondary increase (3) | |
| With tetrodotoxin | 0.34 ± 0.24# | −1.88 ± 0.20* | −0.19 ± 0.63 |
| Without tetrodotoxin | 1.19 ± 0.26* | −1.04 ± 0.29 | 1.25 ± 0.80 |
| Chloride-free | 0.032 ± 0.035# | −0.46 ± 0.059*# | 0.17 ± 0.22# |
| With chloride | 0.85 ± 0.21* | −1.82 ± 0.52* | 2.94 ± 0.91* |
| With bumetanide | 0.27 ± 0.07* | −0.53 ± 0.17*# | 1.22 ± 0.16* |
| Without bumetanide | 0.60 ± 0.24 | −1.58 ± 0.37* | 2.48 ± 0.72* |
| With mucosal glibenclamide | 0.039 ± 0.038# | −1.56 ± 0.23* | 0.89 ± 0.25*# |
| Without glibenclamide | 1.30 ± 0.33* | −1.31 ± 0.10* | 2.35 ± 0.49* |
| With serosal glibenclamide | 0.10 ± 0.003*# | −0.38 ± 0.004*# | 0.35 ± 0.11# |
| Without glibenclamide | 1.42 ± 0.30* | −0.93 ± 0.10* | 2.03 ± 0.50* |
| With serosal tolbutamide | 0.69 ± 0.35* | −0.73 ± 0.15* | 2.74 ± 0.27* |
| Without tolbutamide | 1.15 ± 0.39* | −1.01 ± 0.18* | 3.37 ± 0.73* |
| With mucosal TPeA | 1.28 ± 0.26* | −0.42 ± 0.13*# | 1.62 ± 0.17* |
| Without TPeA | 1.32 ± 0.45* | −0.97 ± 0.16* | 1.42 ± 0.53* |
| With serosal TPeA | 0.12 ± 0.058# | −0.091 ± 0.055# | 0.086 ± 0.052 |
| Without TPeA | 1.36 ± 0.33* | −0.89 ± 0.11* | 0.77 ± 0.25* |
| With serosal Ba2+ | 0.033 ± 0.055# | −0.64 ± 0.079*# | −0.46 ± 0.062*# |
| Without Ba2+ | 1.42 ± 0.17* | −1.53 ± 0.11* | −1.32 ± 0.14 |
| Serosal Ca2+-free | 0.97 ± 0.30* | −0.56 ± 0.094* | 1.47 ± 0.34* |
| With serosal Ca2+ | 2.01 ± 0.60* | −0.95 ± 0.25* | 2.15 ± 1.08* |
| With cyclopiazonic acid | −0.05 ± 0.025# | −2.53 ± 0.31*# | 0.11 ± 0.25# |
| Without cyclopiazonic acid | 0.69 ± 0.22* | −0.94 ± 0.20* | 2.79 ± 0.75* |
| With 2-APB | 0.31 ± 0.12*# | −1.23 ± 0.35* | 1.66 ± 0.46* |
| Without 2-APB | 1.50 ± 0.13* | −0.80 ± 0.39 | 2.42 ± 0.63* |
| With ruthenium red | 1.72 ± 0.29* | −0.00 ± 0.14# | 2.96 ± 0.57*# |
| Without ruthenium red | 1.37 ± 0.30* | −0.69 ± 0.18* | 1.41 ± 0.27* |
Effect of NaHS (10−2 mol·L−1 at the serosal side) under control conditions, in the absence of Cl− (at the mucosal and the serosal side) or serosal Ca2+, and in the presence of tetrodotoxin (10−6 mol·L−1 at the serosal side), bumetanide (10−4 mol·L−1 at the serosal side), glibenclamide (5 × 10−4 mol·L−1 at the mucosal side, or 10−3 mol·L−1 at the serosal side), tolbutamide (5 × 10−3 mol·L−1 at the serosal side), tetrapentylammonium (TPeA; 10−4 mol·L−1at the mucosal or the serosal side), Ba2+ (10−2 mol·L−1 at the serosal side), cyclopiazonic acid (10−5 mol·L−1 at the serosal side), 2-aminoethoxydiphenylborate (2-APB; 10−5 mol·L−1 at the serosal side) and ruthenium red (5 × 10−5 mol·L−1 at the serosal side). The experiments with Ba2+ were performed in HEPES-buffered Tyrode solution in order to avoid precipitation of barium carbonate. The response to NaHS is given as first peak (maximal increase in Isc 0–5 min after administration of NaHS), as secondary decrease in Isc (minimum in Isc 3–10 min after administration of NaHS) and as second peak (maximal increase in Isc 10–25 min after administration of NaHS) marked with (1) to (3) as in Figure 2A. Values are given as difference from the baseline just prior to administration of NaHS and are means ± SEM, n= 6–9.
P < 0.05 versus baseline
P < 0.05 versus response in the absence of the respective inhibitor or in the presence of Cl−.
For the desensitization experiments, NaHS was administered three times to an individual tissue. The compartment, where the H2S-donor had been applied, was exchanged three times with 5× the chamber volume, before the next dose of NaHS was administered.
Imaging experiments
Relative changes in the intracellular Ca2+ concentration were measured using fura-2, a Ca2+-sensitive fluorescent dye (Grynkiewicz et al., 1985). The crypts were pipetted into the experimental chamber with a volume of about 3 mL. They were attached to the glass bottom of the chamber with the aid of poly-L-lysine (0.1 g·L−1). The crypts were incubated for 60 min with 2.5 µmol·L−1 fura-2/acetoxymethylester. Then the dye ester not taken up by the cells was washed away. The preparation was superfused hydrostatically throughout the experiment with 140 mmol·L−1 NaCl Tyrode. Perfusion rate was about 1 mL·min−1.
Changes in the cytoplasmic Ca2+ concentration were monitored as changes in the fura-2 ratio (R; emission at an excitation wave length of 340 nm divided by the emission at an excitation wave length of 380 nm). Experiments were carried out with an inverted microscope (Olympus IX-50; Olympus, Hamburg, Germany) equipped with an epifluorescence set-up and an image analysis system (Till Photonics, Martinsried, Germany). Several regions of interest, each the size of about one cell, were placed over an individual crypt. The emission above 420 nm was measured from the regions of interest. Data were sampled at 0.2 Hz. The baseline in the fluorescence ratio of fura-2 was measured for several minutes before drugs were administered.
Immunohistochemistry
The tissue was rinsed with phosphate buffer and embedded in gelatine (gelatine type A from porcine skin; 100 g·L−1). Then the tissue was cryofixed in N2-cooled 2-methylbutane. Sections (6 µm thick) were cut and mounted on glass slides coated with gelatine containing chrome alum [chromium(III) potassium sulphate; 0.5 g·L−1].
Immunohistochemical staining was performed using an indirect immunofluorescence technique. After rehydration in phosphate buffer, the sections were incubated in phosphate buffer containing 2 mL·L−1 Triton-X-100, 30 g·L−1 BSA and 100 mL·L−1 goat serum to block non-specific binding. Then the blocking solution was removed, and the sections were incubated with the respective primary antibody against cystathionine-β-synthase (antibody H00000875, mouse monoclonal antibody raised against a partial recombinant enzyme; final dilution 1:200), or cystathionine-γ-lyase (antibody H00001434, mouse monoclonal antibody raised against a partial recombinant enzyme; final dilution 1:200).
The antibody was dissolved in phosphate buffer containing 1 mL·L−1 Triton-X-100, 5 g·L−1 milk powder, 10 g·L−1 BSA and 10 mL·L−1 goat serum. After being rinsed with phosphate buffer, the sections were incubated with the secondary antibody (Cy3-conjugated affinipure goat anti-mouse IgG; working dilution 1:800) for 1 h at room temperature. After a further rinse with phosphate buffer, the sections were incubated for 5 min with 3 × 10−7 mol·L−1 DAPI dissolved in PBS. As negative controls, some sections were incubated with a solution that did not contain the primary antibodies (Figure 4, rows 2 and 4).
Figure 4.
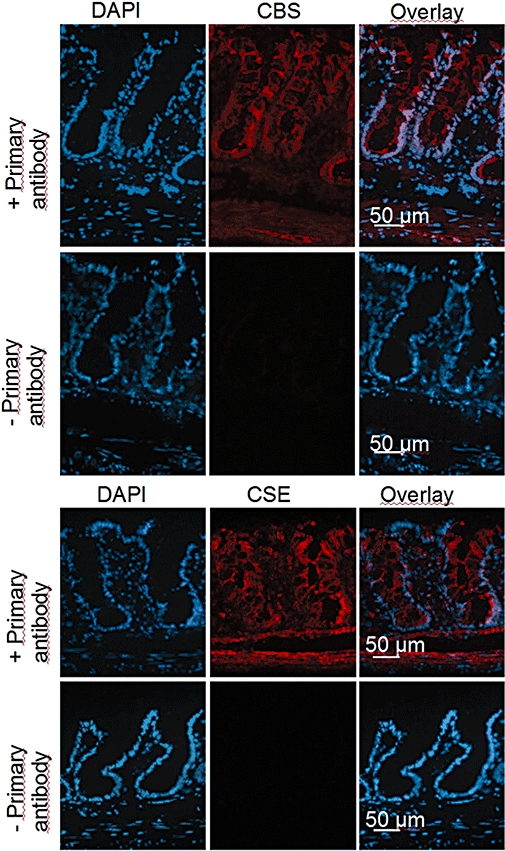
Distribution of cystathionine-β-synthase (CBS) and cystathionine-γ-lyase (CSE) immunoreactivity in rat colonic wall. The orientation of each picture is: lower part: muscularis propria; upper part: surface region of the colonic epithelium. Left column: nuclear staining with 4′,6-diamidino-2-phenylindoldilactate (DAPI) (blue); middle column: CBS signal (first row; Cy3-labelled, red) or CSE signal (third row; Cy3-labelled, red); right column: overlay of both. Second and fourth row: negative controls obtained in the absence of primary antibody against the respective enzyme. Typical results from three independent experiments.
The preparations were examined on a fluorescence microscope (Nikon 80i). Digital images were taken with a B/W camera (DS-2M B/Wc) using the NIS Elements 2.30 software (all from Nikon, Düsseldorf, Germany) to finally adjust brightness, colour and contrast.
Drugs
The drug and molecular target nomenclature followed the guide to receptors and channels of the British Journal of Pharmacology (Alexander et al., 2008). 2-Aminoethoxydiphenylborate (2-APB; Calbiochem, Bad Soden, Germany), cyclopiazonic acid (CPA; Tocris, Bristol, UK), pinacidil and tolbutamide were dissolved in dimethylsulphoxide (DMSO; final maximal concentration 2 mL·L−1). Bumetanide, forskolin and glibenclamide were generous gifts from Boehringer Mannheim (Mannheim, Germany) and were dissolved in ethanol (final maximal concentration 2.5 mL·L−1). Tetrodotoxin was dissolved in 2 × 10−2 mol·L−1 citrate buffer. Barium chloride, NaHS (prepared fresh just before the experiment and kept in a sealed vial), ruthenium red (Alfa Aesar, Karlsruhe, Germany) and tetrapentylammonium chloride were dissolved in aqueous stock solutions. Fura-2 was obtained from Molecular Probes (Leiden, the Netherlands) and poly-L-lysine from Biochrom KG (Berlin, Germany). The goat serum was from Chemicon (Temecula, CA, USA), the primary antibodies against cystathionine-β-synthase and cystathionine-γ-lyase were obtained from Abnova (Heidelberg, Germany), and the Cy3-conjugated affinipure goat anti-mouse IgG was from Dianova (Hamburg, Germany). DAPI was from Molecular Probes (Leiden, the Netherlands). If not indicated differently, the drugs were from Sigma (Taufkirchen, Germany).
Statistics
Values are given as means ± one SEM. In the case that means of several groups had to be compared, an analysis of variance was performed followed by Tukey's post hoc test. For comparisons between two groups, either Student's t-test or Mann–Whitney U-test was applied. An F-test was used to decide which test method should be applied. Both paired and unpaired two-tailed Student's t-tests were applied as appropriate. P < 0.05 was considered to be statistically significant.
Results
Effect of NaHS on the short-circuit current
The H2S-donor, NaHS, induced a concentration-dependent change in Isc, which amounted to 1.24 ± 0.13 µEq·h−1·cm−2 (n= 6) under basal conditions. Lower concentrations of NaHS (≤10−3 mol·L−1) evoked a more or less monophasic increase in Isc (Figure 1A), whereas at higher concentrations (≥5 × 10−3 mol·L−1) the time course of the NaHS effect was composed of at least three phases (Figure 1B). They consisted of (1) an initial rapid increase (peak) in Isc (preceded by a transient decrease, which was not considered further as it was not consistent), followed (2) by a decrease in Isc, and (3) a secondary rise in current (Figure 2A). The concentration–response curve for all three phases after serosal administration of NaHS is given in Figure 1C and that after mucosal administration, which evoked nearly similar responses, in Figure 1D. This current response was concomitant with a reversible increase in tissue conductance (Gt), which increased from 11.5 ± 0.5 mS·cm−2 to 18.4 ± 4.9 mS·cm−2 (P < 0.05, n= 6) at the highest concentration of NaHS used.
Figure 1.
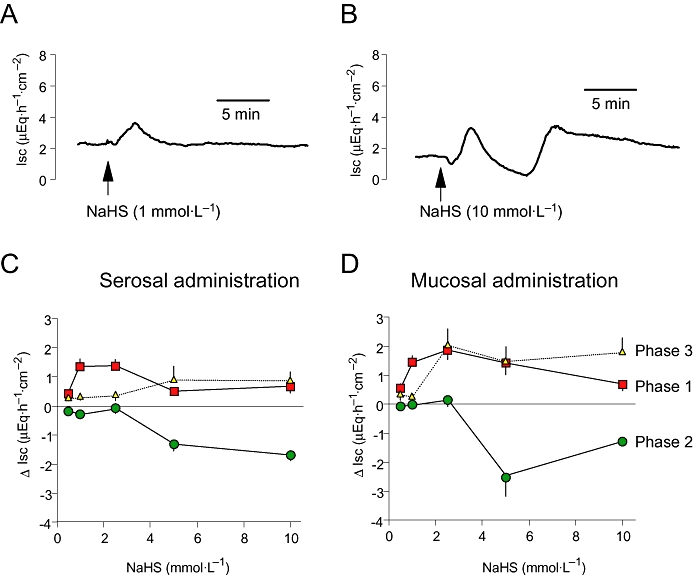
(A) Monophasic increase in short-circuit current (Isc) evoked by NaHS at a concentration of 10−3 mol·L−1at the serosal side: typical trace. (B) Polyphasic change in Isc evoked by NaHS at a concentration of 10−2 mol·L−1at the serosal side: typical trace. (C and D) Concentration-dependent change in Isc across rat distal colon induced by serosal (C) or mucosal (D) administration of NaHS. The rapid increase in Isc (phase 1), the secondary decrease (phase 2) and the final increase in Isc (phase 3) induced by NaHS are shown and correspond to (1) to (3) in Figure 2A. Each tissue was exposed to only one concentration of NaHS to avoid desensitization. Values are given as difference from the baseline Isc just prior to administration of NaHS (ΔIsc) and are means (symbols) ± one SEM (vertical bars), n= 6–8.
Figure 2.
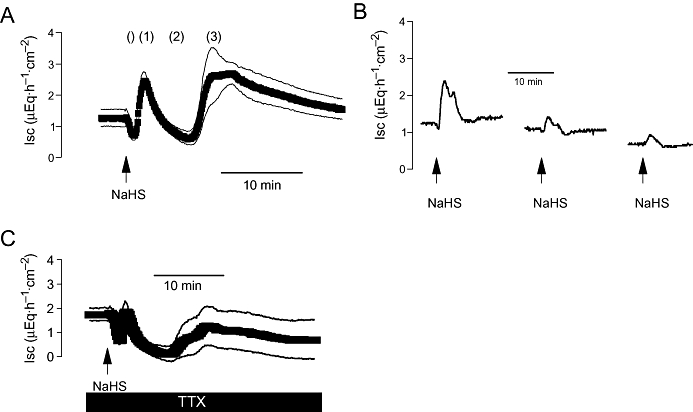
(A) Triphasic effect of a high concentration of NaHS (10−2 mol·L−1at the serosal side; arrow) on short-circuit current (Isc). The three main phases of the Isc response are labelled (1) to (3); the inconsistent early fall in Isc, which was not further analysed, is marked (). Values are means (symbols) ± SEM (lines), n= 8. (B) Desensitization induced by repeated administration of NaHS (10−3 mol·L−1 at the serosal side, arrows). The line interruptions are caused by omission of current artefacts induced by washing the serosal compartment three times with 5× the chamber volume, before the next administration of NaHS followed. Typical trace of eight experiments with similar results. On average, the first administration evoked an increase in Isc above baseline (ΔIsc) of 0.86 ± 0.39 µEq·h−1·cm−2, the second administration of 0.23 ± 0.07 µEq·h−1·cm−2and the third of 0.18 ± 0.02 µEq·h−1·cm−2(n= 8). (C) Effect of NaHS (10−2 mol·L−1at the serosal side; arrow) in the presence of tetrodotoxin (TTX; 10−6 mol·L−1 at the serosal side; solid bar). Values are means (symbols) ± SEM (lines), n= 6. For statistics, see Table 1.
When NaHS (10−3 mol·L−1 at the serosal side) was administered repeatedly to the same tissue, a strong desensitization was observed, even when the compartment, where the drug had been administered, was washed several times with fresh buffer (Figure 2B). The second administration caused an increase in Isc (measured at the initial peak) of only 27%, and the third administration produced only 21% of the response induced by the first administration of the H2S-donor. This desensitization was not due to a non-specific (e.g. toxic) action of NaHS. When tissues had been exposed three times to NaHS (10−2 mol·L−1 at the serosal side with intermediate washing procedures), the activator of adenylate cyclase, forskolin (5 × 10−6 mol·L−1) evoked an increase in Isc of 6.39 ± 1.12 µEq·h−1·cm−2above baseline (n= 8). In time-dependent control experiments, which had not been pretreated with NaHS, forskolin evoked an increase in Isc of 8.10 ± 0.50 µEq·h−1·cm−2above baseline (n= 6; difference not significant), that is, the secretory capacity of the tissue was still preserved after repeated administration of NaHS.
Due to the desensitization, in all further experiments NaHS was administered only once to an individual tissue, and a high concentration (10−2 mol·L−1) was selected to study all phases of the current induced by the drug. When all responses to NaHS (10−2 mol·L−1 at the serosal side) under control conditions, that is, in the absence of any inhibitors, were pooled, the H2S-donor evoked an initial increase in Isc of 1.25 ± 0.10 µEq·h−1·cm−2, followed by a secondary decrease of −1.06 ± 0.08 µEq·h−1·cm−2, finally followed by a secondary increase of 2.17 ± 0.20 µEq·h−1·cm−2 compared with baseline (for all phases: P < 0.05 vs. baseline just before administration of NaHS; n= 91).
The first phase of the Isc induced by H2S was significantly inhibited when the tissues were pretreated with the neurotoxin, tetrodotoxin (10−6 mol·L−1 at the serosal side; Figure 2C; Table 1), which blocks the propagation of action potentials (Catterall, 1980), suggesting the participation of secretomotor neurons in the mediation of this part of the Isc response.
In order to test, whether endogenous H2S produced within the colonic wall might contribute to the induction of basal Isc, which represents basal anion secretion (Strabel and Diener, 1995), the effect of inhibitors of enzymes responsible for H2S production on Isc was tested. Indeed, both aminooxyacetate, an inhibitor of cystathionine-β-synthase, as well as β-cyano-L-alanine, an inhibitor of cystathionine-γ-lyase (for references to both inhibitors, see Zhao et al., 2003), induced a concentration-dependent decrease in basal Isc (Figure 3).
Figure 3.
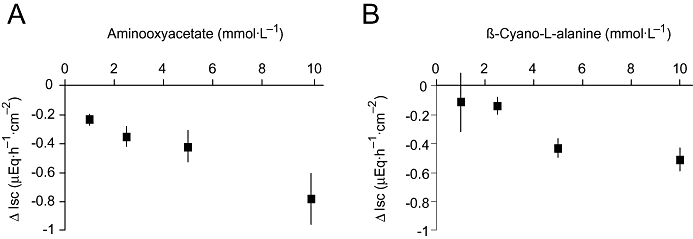
Concentration-dependent decrease in short-circuit current (Isc) induced by the inhibitor of the enzyme cystathionine-β-synthase, aminooxyacetate (A), or the inhibitor of the enzyme cystathionine-γ-lyase, β-cyano-L-alanine (B). The inhibitors were applied in a cumulative manner. Values are given as difference from the basal Isc just prior to the first administration of the respective inhibitor (ΔIsc) and are means (symbols) ± SEM (lines), n= 7.
These data suggest both H2S-producing enzymes have endogenous activity. The presence of these enzymes within the colonic wall was confirmed immunohistochemically. Cystathionine-β-synthase immunoreactivity was observed in the smooth muscle cells of the lamina muscularis propria and, more intensively, within the colonic epithelium (Figure 4, row 1). No gradient in the immunoreactivity was observed along the crypt axis, that is, the signal was found both at the surface epithelium and within the crypts. A similar pattern (with a more intensive contribution of the smooth muscle cells from the lamina muscularis propria and the lamina muscularis mucosae) was found for the second H2S-producing enzyme, cystathionine-γ-lyase (Figure 4, row 3).
In order to test whether any spontaneous production of endogenous H2S might already desensitize the tissue to the H2S-donor, the response to an intermediate concentration of NaHS (10−3 mol·L−1at the serosal side) was tested in the combined presence of the cystathionine-β-synthase inhibitor, aminooxyacetate, and the cystathionine-γ-lyase inhibitor, β-cyano-L-alanine (2.5 × 10−3 mol·L−1of each inhibitor at the serosal side). Compared with tissues, which received mannitol as an osmotic control (5 × 10−3 mol·L−1at the serosal side), the Isc evoked by NaHS was increased by a factor of 2.5 (Figure 5).
Figure 5.
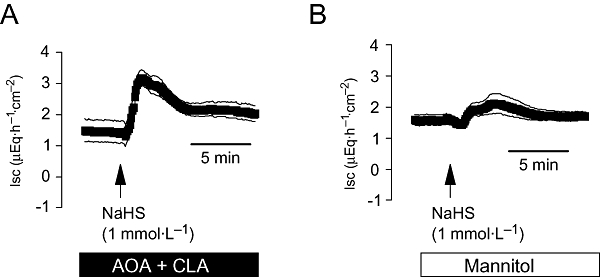
(A) Potentiation of the short-circuit current (Isc) induced by an intermediate concentration of NaHS (10−3 mol·L−1 at the serosal side; arrow) after inhibition of endogenous cystathionine-β-synthase and cystathionine-γ-lyase activity with a combination (solid bar) of aminooxyacetate (AOA; 2.5 × 10−3 mol·L−1 at the serosal side) and β-cyano-L-alanine (CLA; 2.5 × 10−3 mol·L−1 at the serosal side) compared with tissues, which received mannitol (5 × 10−3 mol·L−1 at the serosal side) as osmotic control (open bar; B). Values means (symbols) ± SEM (lines), n= 8. On average, NaHS induced an increase in Isc above baseline of 1.91 ± 0.46 µEq·h−1·cm−2 in the presence of mannitol and of 0.73 ± 0.27 µEq·h−1·cm−2 in the combined presence of AOA and CLA (P < 0.05, n= 8).
Cl− and K+ secretion contribute to the NaHS response
All phases of the NaHS-induced Isc were nearly suppressed, when the drug was administered in the absence of Cl− (Figure 6A,B; Table 1). A similar inhibition was observed in the presence of bumetanide (10−4 mol·L−1 at the serosal side), an inhibitor of the Na+-K+-2Cl−-cotransporter responsible for the uptake of K+ and Cl− ions during colonic secretion (Binder and Sandle, 1994). In the presence of this blocker, NaHS only induced a delayed increase in Isc, whereas the initial peak and the secondary decrease in Isc were abolished (Figure 6C,D). These results are consistent with the assumption that both a stimulation of Cl− secretion, which results in a positive Isc, as well as a stimulation of K+ secretion, which results in a negative Isc, contribute to the triphasic current response evoked by NaHS.
Figure 6.
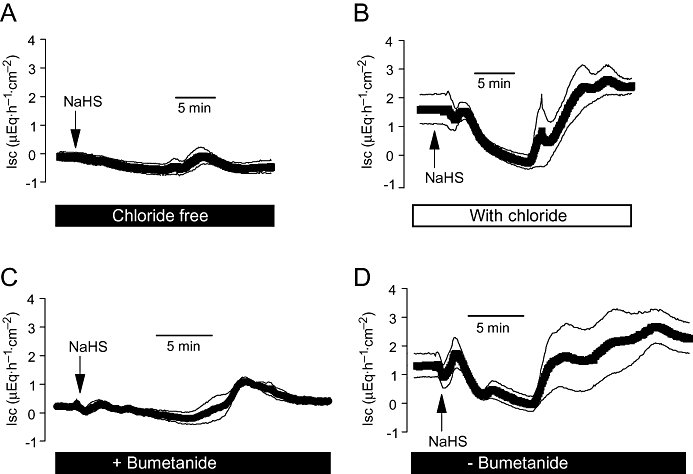
Effect of NaHS (10−2 mol·L−1at the serosal side; arrow) in the nominal absence (A) or presence (B) of chloride, or the presence (C) or absence (D) of bumetanide (10−4 mol·L−1 at the serosal side; solid bar). The bumetanide series were performed in the presence of Cl−. Values are means (symbols) ± SEM (lines), n= 6–7. For statistics, see Table 1. Isc, short-circuit current.
The dominant Cl− channel in the apical membrane involved in colonic Cl− secretion is the CFTR (cystic fibrosis transmembrane regulator) channel (Greger, 2000), which is inhibited by glibenclamide. Glibenclamide (5 × 10−4 mol·L−1 at the mucosal side) suppressed the initial peak in Isc and caused a significant (P < 0.05) reduction in the secondary increase in Isc evoked by NaHS (Figure 7A,B) suggesting that a CFTR-mediated flux of anions via the apical membrane is responsible for the increase in Isc.
Figure 7.
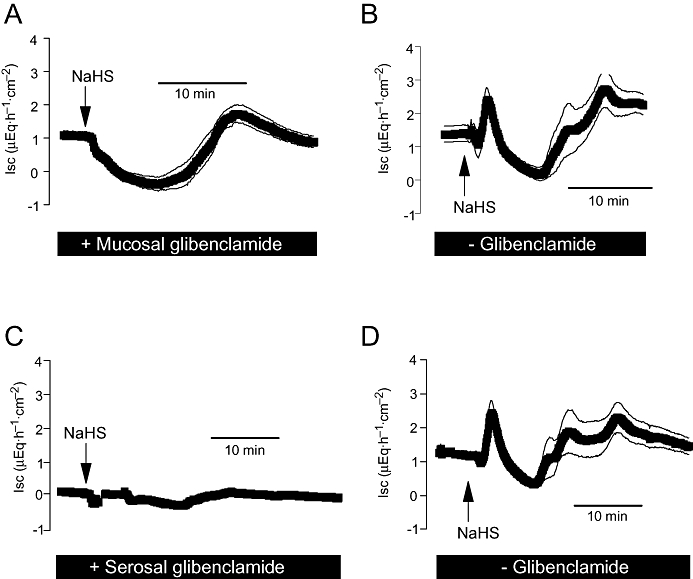
Effect of NaHS (10−2 mol·L−1at the serosal side; arrow) in the presence of mucosal glibenclamide (5 × 10−4 mol·L−1, A) or serosal glibenclamide (10−3 mol·L−1, C). (B and D) Results from the control experiments performed in parallel in the absence of glibenclamide. Values are means (symbols) ± SEM (lines), n= 7–9. For statistics, see Table 1. Isc, short-circuit current.
Glibenclamide also inhibits ATP-sensitive K+ channels, which have been found in the basolateral membrane of rat distal colon (Maguire et al., 1999) and are known to be activated by H2S in other tissues (see e.g. Zhao et al., 2001). In the presence of glibenclamide (10−3 mol·L−1) at the serosal side, all phases of the Isc induced by NaHS were nearly abolished (Figure 7C,D), suggesting an activation of basolateral K+ channels by the H2S-donor as one of the key mechanisms driving an enhanced efflux of Cl− (via the hyperpolarization of the membrane) across the apical membrane (Strabel and Diener, 1995). Interestingly, the inhibition induced by serosal glibenclamide was not mimicked by tolbutamide (5 × 10−3 mol·L−1 at the serosal side; Table 1; see Discussion).
Another type of K+ channel involved in the regulation of colonic ion transport consists of Ca2+-dependent K+ channels in the apical and the basolateral membrane (Schultheiss and Diener, 1997), which are sensitive to tetrapentylammonium (Maguire et al., 1999). When applied from the mucosal side, this inhibitor (10−4 mol·L−1) suppressed the second phase of the Isc induced by NaHS, that is, the fall in Isc, consistent with the assumption that this phase is caused by K+ secretion (Figure 8A,B). When the inhibitor was applied from the serosal side at the same concentration, it suppressed the complete NaHS response in a similar manner to serosal glibenclamide (Figure 8C,D) indicating the central role of basolateral K+ channels as targets for H2S. This conclusion is underlined by the observation that Ba2+ (10−2 mol·L−1 at the serosal side), a non-selective K+ channel blocker (Cook and Quast, 1990), also strongly inhibited the effect of NaHS on Isc (Table 1).
Figure 8.
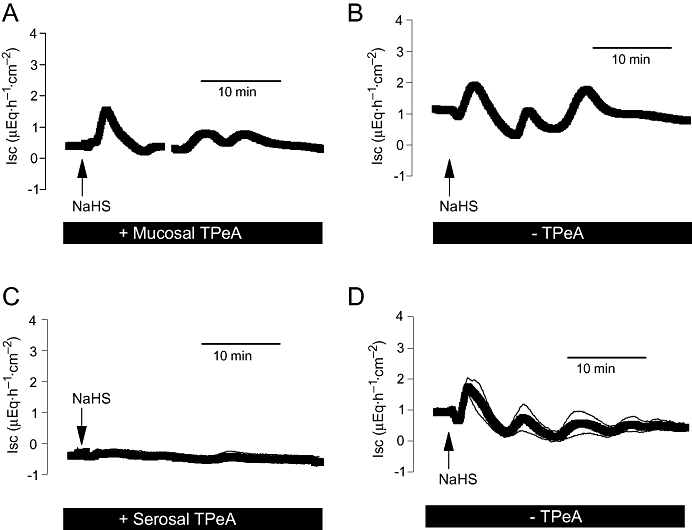
Effect of NaHS (10−2 mol·L−1at the serosal side; arrow) in the presence of mucosal tetrapentylammonium (TPeA; 10−4 mol·L−1, A) or serosal tetrapentylammonium (10−4 mol·L−1, C). (B and D) The corresponding control experiments performed in the absence of tetrapentylammonium. The reason for the ‘undulating’ current increase evoked by NaHS (only observed in this series of experiments) is unknown. Values are means (symbols) ± SEM (lines), n= 8. For statistics, see Table 1. Isc, short-circuit current.
In order to test for the presence of ATP-sensitive K+ channels by an agonist experiment, pinacidil, an opener of this type of cation channel (see Geng et al., 2007), was used. When the K+ channel opener was administered at the serosal side, it caused a concentration-dependent increase in Isc, consistent with the expected Cl− secretion occurring after hyperpolarization of the basolateral membrane. In contrast, when pinacidil was administered to the mucosal side, the drug induced the opposite action, that is, a negative Isc as has to be expected for K+ secretion after opening of apical K+ channels (Figure 9). In contrast to the effect of NaHS, the response to pinacidil was always monophasic, that is, consisted only of a negative Isc after mucosal and of only a positive Isc after serosal administration. These functional data suggest the presence of one of the known targets for H2S, that is, ATP-sensitive K+ channels, on both sides of the epithelium.
Figure 9.
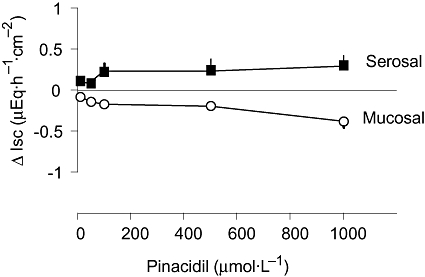
Concentration-dependent effect of the opener of ATP-dependent K+ channels, pinacidil, after serosal or mucosal administration. Pinacidil was applied in a cumulative manner. Values are given as difference from the basal short-circuit current (Isc) just prior to the first administration of pinacidil (ΔIsc) and are means (symbols) ± SEM (lines), n= 6–7.
Involvement of intracellular Ca2+
Tetrapentylammonium, which depending on the side it is administered, inhibits different phases of the NaHS response, is a K+ channel blocker preferentially inhibiting Ca2+-dependent K+ channels (Maguire et al., 1999). Consequently, we investigated whether the H2S-donor affected the cytosolic Ca2+ concentration of fura-2-loaded colonic crypts. Indeed NaHS induced a concentration-dependent increase in the fura-2 ratio signal (Figure 10A,B). The effect started at a concentration of 10−4 mol·L−1 and was maximal at a concentration of 10−3 mol·L−1, that is, the changes in cytosolic Ca2+ concentration were more sensitive to NaHS than the Isc response (see Figure 1C,D). Typically, the first administration of NaHS caused an initial decrease in the fura-2 ratio signal, that is, a fall of the cytosolic Ca2+ concentration, before a long lasting increase evolved (Figure 10C). Due to the high sensitivity of the isolated crypts to NaHS, all further fura-2 experiments were conducted with a concentration of 10−4 mol·L−1.
Figure 10.
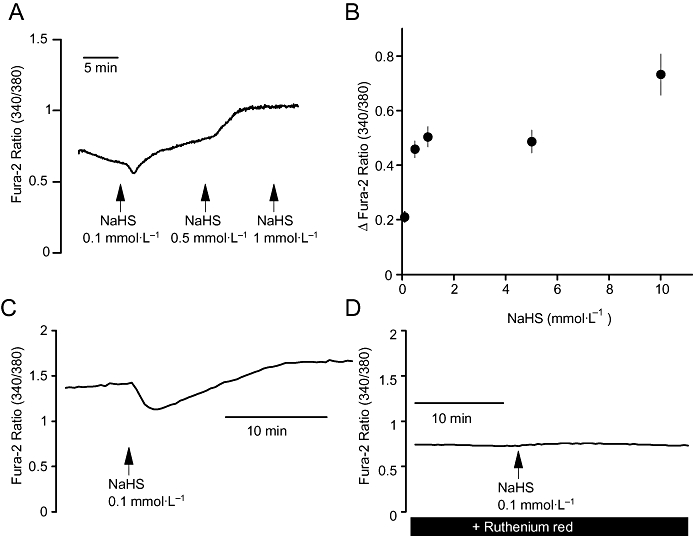
(A) Typical experiment demonstrating the effect of NaHS (arrows) on the fura-2 signal at isolated colonic crypts. (B) Concentration-dependent increase in the fura-2 signal induced by NaHS. Values are given as difference from the baseline just prior to administration of NaHS (Δfura-2 ratio) and are means ± SEM, n= 94. NaHS was administered in a cumulative manner. (C) and (D) Time course of the effect of NaHS (10−4 mol·L−1) on the fura ratio under control conditions (C) and after pretreatment with ruthenium red (5 × 10−5 mol·L−1; D); typical tracings. For statistics, see Table 2.
Superfusion of the crypts with an extracellular Ca2+-free buffer did not significantly reduce the increase in the fura-2 ratio signal and only moderately reduced the initial decrease (Table 2), suggesting that an influx of extracellular Ca2+ does not play a major role in this H2S effect. Ruthenium red (5 × 10−5 mol·L−1), an inhibitor of ryanodine receptors (Xu et al., 1999) expressed by the colonic crypt cells (Prinz and Diener, 2008), significantly inhibited both phases of the fura-2 response evoked by the H2S-donor (Figure 10D; Table 2). 2-APB, an inhibitor of inositol-1,4,5-trisphosphate (IP3) receptors (Maruyama et al., 1997), that is, the second type of intracellular Ca2+ channels present within the colonic crypts (Siefjediers et al., 2007), was ineffective. Surprisingly, pretreatment with cyclopiazonic acid, an inhibitor of sarcoplasmic-endoplasmic Ca2+-ATPases (SERCA; Plenge-Tellechea et al., 1997), reduced the initial decrease, but potentiated the secondary increase in the fura-2 ratio signal induced by NaHS (Table 2; see Discussion).
Table 2.
Effect of NaHS on the fura-2 ratio signal
|
ΔFura-2 ratio induced by NaHS |
|||
|---|---|---|---|
| Initial decrease | Late increase | n | |
| Control | −0.102 ± 0.0069a | 0.161 ± 0.014a | 224 |
| Ca2+-free | −0.062 ± 0.0077b | 0.145 ± 0.019a,b | 86 |
| +2-APB | −0.099 ± 0.0095a | 0.197 ± 0.022a | 108 |
| +Ruthenium red | −0.003 ± 0.0009c | 0.056 ± 0.017b | 82 |
| +Cyclopiazonic acid | −0.035 ± 0.0066b,c | 0.347 ± 0.045c | 84 |
Effect of NaHS (10−4 mol·L−1) in the absence of any inhibitors (control), in the nominal absence of extracellular Ca2+, or in the presence of 2-aminoethoxydiphenylborate (2-APB; 10−5 mol·L−1), ruthenium red (5 × 10−5 mol·L−1), or cyclopiazonic acid (10−5 mol·L−1). The response to NaHS is given as initial decrease (minimal ratio within 4 min after administration of NaHS) and secondary increase (maximum reached after administration of NaHS). Values are given as difference from the baseline in the fura-2 ratio just prior to administration of NaHS and are means ± SEM. Statistically homogeneous groups are indicated by the same letter
analysis of variances followed by Tukey's test).
Finally, we investigated the possible involvement of Ca2+ in the change in Isc induced by NaHS. Removal of extracellular Ca2+ at the serosal side did not cause a significant inhibition of any of the three phases of the Isc response induced by NaHS (10−2 mol·L−1 at the serosal side; Table 1). A strong inhibition of the first and the third phases was observed when intracellular Ca2+ stores were depleted with cyclopiazonic acid, confirming the importance of intracellular Ca2+ release in the mediation of the response, whereas the secondary fall in Isc was paradoxically enhanced (Table 1). In contrast to the fura-2 experiments, in intact mucosa, the response to NaHS, especially the initial rapid increase in Isc, was inhibited by 2-APB, whereas ruthenium red suppressed the second phase, that is, the induction of a negative Isc, and even enhanced the final prolonged increase in current (Table 1).
Discussion
The present results demonstrate that a H2S-donor, NaHS, induces a polyphasic change in Isc, which exhibits a strong desensitization upon repeated administration (Figure 2). This response is composed of a biphasic, Cl−-dependent increase in current representing Cl− secretion, which can be blocked by a Cl− channel blocker such as mucosal glibenclamide (Figure 7A). The increase in Isc is interrupted by a negative Isc [labelled with (2) in Figure 2A], which is sensitive against an apical K+ channel blocker, tetrapentylammonium, indicating that a transient K+ secretion carries this phase of the NaHS response (Figure 8A). Most probably, a different time course of the induction of Cl− and K+ secretion with their opposite effects on Isc (a secretion of anions induces a positive Isc, a secretion of cations a negative Isc) may be responsible for the observation that the initial increase in Isc was inconsistently preceded by a transient negative current [labelled with () in Figure 2A]. Whether K+ and Cl− secretion derive from the same epithelial cell or from different subpopulations (see e.g. Halm and Halm, 2001) is still an unresolved question. The concentrations of NaHS (used here in the millimolar range) needed to evoke these secretory responses are much higher than those needed to produce responses in other systems, especially in in vivo models. For example, the 50% effective dose for inhibition of leukocyte adherence amounts to 43 µmol·kg−1 (Zanardo et al., 2006) and NaHS inhibits gastrointestinal nociception in concentrations as low as 30 µmol·kg−1(Distrutti et al., 2006). However, this may indicate that the continuous production of H2S by the colonic microbial flora (see Introduction) may cause some desensitization, which makes the tissue less sensitive to an exogenous H2S-donor.
In contrast to observations in the guinea-pig and human colon (Schicho et al., 2006), only parts of this response, that is, the initial transient Cl− secretion, proved to be sensitive to the neurotoxin, tetrodotoxin (Figure 2C). In the former species, the effect of NaHS (up to 10−3 mol·L−1) was strongly reduced by this neurotoxin. Different concentrations (we used up to 10−2 mol·L−1of the H2S-donor) may be responsible for this discrepancy. However, when a lower concentration of NaHS (10−3 mol·L−1) was administered to the rat colon, it induced a peak increase of Isc of 1.28 ± 0.28 µEq·h−1·cm−2(n= 6) in the absence and 1.12 ± 0.28 µEq·h−1·cm−2(n= 8) in the presence of tetrodotoxin (difference not significant). So probably species differences must account for the observed discrepancy. However, the Ussing chamber data together with that from the fura-2 experiments in isolated crypts clearly indicate that in rat colon, H2S, in addition to its actions at secretomotor submucosal neurones, exerts an action directly at the intestinal epithelium.
The results obtained with the K+ channel blockers suggest the involvement of basolateral K+channels (to drive Cl− secretion during the early and the late increase in Isc induced by NaHS) and of apical K+ channels (to drive K+ secretion during the decrease in Isc induced by NaHS) in the response to the H2S-donor. Whereas Ca2+-dependent K+ currents have already been demonstrated in both membranes (Schultheiss and Diener, 1997), this information is lacking for ATP-sensitive K+ channels, which up to now have only been shown functionally in the basolateral membrane (Maguire et al., 1999). The opposing actions of pinacidil, an opener of ATP-sensitive K+ channels (Geng et al., 2007), on Isc, that is, an increase in Isc after serosal and a decrease in Isc after mucosal administration (Figure 9) suggest the presence of this type of cation channel(s) at both membranes, although this conclusion should be verified by future experiments with K+ channel blockers and other transport inhibitors, which was, however, outside the scope of the present study. ATP-sensitive K+ channels are a well-characterized target of H2S. For example, the vasorelaxation induced by H2S has been shown to be mediated by the activation of this type of K+ conductance in smooth muscle cells from rat aorta. This action is thought to be mediated by a reduction of disulphide bonds within the channel protein (Zhao et al., 2001) by H2S, which acts as a reducing agent (Reiffenstein et al., 1992). In a pancreatic islet cell line, the effect of H2S on ATP-sensitive K+ channels has been investigated on single channels: H2S acts by increasing the open probability of the channel without altering the single channel conductance (Yang et al., 2005).
ATP-sensitive K+channels, which serve to couple membrane potential to the metabolic state of the cell, that is, the cytosolic ATP : ADP ratio, are octomers, composed of four channel forming subunits (Kir 6) and four regulatory subunits, the sulphonylurea receptors (SUR). Two subtypes of the Kir subunit (Kir 6.1 and Kir 6.2), which form an inwardly rectifying K+ channel (Kir), are known. The SUR subunits (SUR1 and SUR2 with the two splice variants, SUR2A and SUR2B) contain the nucleotide binding site and are the action site for inhibitors such as sulphonylurea derivatives (e.g. glibenclamide or tolbutamide) or activators such as pinacidil (for review, see Seino and Miki, 2003). Consequently, ATP-sensitive K+ channels are heterogeneous due to a different subunit composition. Variations in the subunit composition are known to be responsible for the different sensitivity of ATP-sensitive K+ channels to individual sulphonylurea derivatives (Seino and Miki, 2003) and may explain why in the present experiments the strong inhibition of the H2S response by glibenclamide was only weakly (and insignificantly; Table 1) mimicked by tolbutamide. From the known molecular constituents of ATP-sensitive K+ channels, immunohistochemically Kir 6.1 and SUR2A have been found in the small intestinal epithelium of man and the rat, with a relatively distinct localization in the region of the tight junctions (Jöns et al., 2006). Whether they also exist at enteric neurones, which can be involved in the mediation of the secretory response evoked by H2S (Schicho et al., 2006), is unknown.
A further action site of NaHS at rat colonic epithelium seems to be a Ca2+-dependent K+ conductance because tetrapentylammonium, a K+ channel blocker with some preference for this type of K+ channel (Maguire et al., 1999), inhibited all phases of the NaHS response after serosal administration and reduced the negative current (i.e. the K+ secretion) after mucosal administration (Figure 8; Table 1). Both glibenclamide and tetrapentylammonium, when administered from the serosal side, inhibited more than 80% of the initial or the late increase in Isc, that is, of the Cl− secretion, induced by NaHS (Table 1). This obvious overadditive action may be explained by the well-known dependency of Cl− secretion on the basolateral membrane potential, which acts as a driving force for Cl− exit across apical anion channels: if the basolateral membrane is depolarized by one of these inhibitors, the driving force for Cl− secretion is reduced (Strabel and Diener, 1995).
An increase in the cytoplasmic Ca2+ concentration of the epithelium was confirmed in the fura-2 experiments in isolated colonic crypts (Figure 10). This response was not dependent on the presence of extracellular Ca2+, but was inhibited by ruthenium red (Table 2), suggesting the intracellular Ca2+ release is induced via activation of ryanodine receptors, which have been identified in the apical cytoplasm of rat colonic epithelium (Prinz and Diener, 2008). Interestingly, the increase in the fura-2 ratio signal was preceded by a reproducible fall. This decrease was significantly reduced after blockade of SERCA with cyclopiazonic acid (Table 2), from which the colonic epithelium expresses two types (SERCA2b and 3) as shown by RT-PCR and immunofluorescence staining (G. Prinz and M. Diener, unpubl. obs.). This suggests a transient stimulation of this Ca2+ pump by H2S. Such an effect might also explain why after SERCA blockade the increase in the fura-2 ratio induced by H2S was paradoxically enhanced, as under these conditions the Ca2+ release induced by H2S would not be partially counteracted by the simultaneous enhancement of Ca2+ uptake into the Ca2+ storage organelles. Interestingly, oxidative agents are known to inactivate SERCAs (Pereira et al., 1996), so their stimulation by a reducing agent such as H2S is a distinct possibility. An obvious discrepancy in the present data lies in the observation that 2-APB, an inhibitor of IP3 receptors, was ineffective in reducing the changes of the fura-2 signal induced by NaHS in the isolated crypts (Table 2), but significantly inhibited the first initial increase in Isc induced by NaHS in the mucosa-submucosa preparation (Table 1). However, this apparent contradiction may be explained by the finding that this phase of Isc was also inhibited by the neurotoxin, tetrodotoxin (Figure 2C), suggesting that NaHS stimulates the neuronal IP3 receptors.
Several observations indicate that endogenously produced H2S contributes to the basal secretory activity responsible for the generation of baseline Isc, in rat colon. By using an immunohistochemical technique, two enzymes, cystathionine-β-synthase and cystathionine-γ-lyase, responsible for the production of H2S from cysteine (Wang, 2002), were found within the muscle and the epithelial layer of the colonic wall (Figure 4). Aminooxyacetate and β-cyano-L-alanine, inhibitors of these two enzymes, each caused a concentration-dependent decrease in basal Isc, that is, an inhibition of spontaneous anion secretion (Figure 3). Furthermore, a combination of both inhibitors potentiated the action of an intermediate concentration (10−3 mol·L−1 at the serosal side) of NaHS (Figure 5), suggesting that the apparent sensitivity of the tissue to NaHS is reduced by an ongoing desensitization to spontaneously produced H2S. These data suggest that H2S, together with, for example, prostaglandins (Strabel and Diener, 1995), are involved in the induction of spontaneous anion secretion across rat colonic epithelium in the Ussing chamber, mainly via stimulation of apical as well as basolateral epithelial K+ channels.
The question arises as to whether H2S may also have pathophysiological significance. In a model of caerulein-induced pancreatitis, an up-regulation of cystathionine-β-synthase and cystathionine-γ-lyase with a subsequent increase in H2S formation, which may contribute to the development of the disease, has been observed (Tamizhselvi et al., 2007). However, from experiments with mice, it has also been concluded that H2S has anti-inflammatory actions after experimental induction of colitis (Fiorucci et al., 2007). Interestingly, a similar controversy about pro/anti-inflammatory or proabsorptive/prosecretory actions exists for a second gasotransmitter, NO (see e.g. Schirgi-Degen and Beubler, 1995; Schultheiss et al., 2002b), which might indicate that depending on differences in time, location or amplitude of the production of these gasotransmitters the physiological response may differ fundamentally.
Acknowledgments
The diligent care of Mrs B Brück, E Haas, A Metternich and B Schmitt is a pleasure to acknowledge. Supported by Deutsche Forschungsgemeinschaft, grant Di 388/9-1.
Glossary
Abbreviations:
- 2-APB
2-aminoethoxydiphenylborate
- DAPI
4′,6-diamidino-2-phenylindoldilactate
- SERCA
sarcoplasmic-endoplasmic Ca2+-ATPases
- SUR
sulphonylurea receptor
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edition (2008 revision) Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder HJ, Sandle GJ. Electrolyte transport in the mammalian colon. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. 3rd edn. New York: Raven Press; 1994. pp. 2133–2171. [Google Scholar]
- Catterall WA. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- Cook NS, Quast U. Potassium channels. Structure, classification, function and therapeutic potential. In: Cook NS, editor. Potassium Channel Pharmacology. New York: Ellis Horwood; 1990. pp. 181–255. [Google Scholar]
- Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Antonelli E, et al. Evidence that hydrogen sulfide exerts antinociceptive effects in the gastrointestinal tract by activating KATP channels. J Pharmacol Exp Ther. 2006;316:325–335. doi: 10.1124/jpet.105.091595. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Orlandi S, Mencarelli A, Caliendo G, Santagada V, Distrutti E, et al. Enhanced activity of a hydrogen sulphide-releasing derivative of mesalamine (ATB-429) in a mouse model of colitis. Br J Pharmacol. 2007;150:996–1002. doi: 10.1038/sj.bjp.0707193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin T, Neale G, Gibson GR, Christl SU, Cummings JK. Metabolism of dietary sulphate: absorption and excretion in humans. Gut. 1991;32:766–773. doi: 10.1136/gut.32.7.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng B, Cui YY, Zhao J, Yu F, Zhu Y, Xu G, et al. Hydrogen sulfide downregulates the aortic L-arginine/nitric oxide pathway in rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1608–R1618. doi: 10.1152/ajpregu.00207.2006. [DOI] [PubMed] [Google Scholar]
- Greger R. Role of CFTR in the colon. Annu Rev Physiol. 2000;62:467–491. doi: 10.1146/annurev.physiol.62.1.467. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Halm DR, Halm ST. Prostanoids stimulate K secretion and Cl secretion in guinea pig distal colon via distinct pathways. Am J Physiol Gastrointest Liver Physiol. 2001;281:G984–G996. doi: 10.1152/ajpgi.2001.281.4.G984. [DOI] [PubMed] [Google Scholar]
- Jöns T, Wittschieber D, Beyer A, Meier C, Brune A, Thomzig A, et al. K+-ATP-channel-related protein complexes: potential transducers in the regulation of epithelial tight junction permeability. J Cell Sci. 2006;119:3087–3097. doi: 10.1242/jcs.03041. [DOI] [PubMed] [Google Scholar]
- Kenyon JL, Gibbons WR. Effects of low-chloride solutions on action potentials of sheep cardiac purkinje fibers. J Gen Physiol. 1977;70:635–660. doi: 10.1085/jgp.70.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Hu YS, Hu LF, Lu Q, Dawe GS, Moore PK, et al. Hydrogen sulphide regulates calcium homeostasis in microglial cells. Glia. 2006;54:116–124. doi: 10.1002/glia.20362. [DOI] [PubMed] [Google Scholar]
- Lindström CG, Rosengren JE, Fork FT. Colon of the rat. An anatomic, histologic and radiographic investigation. Acta Radiol Diagn. 1979;20:523–536. doi: 10.1177/028418517902000314. [DOI] [PubMed] [Google Scholar]
- Maguire D, MacNamara B, Cuffe JE, Winter D, Doolan CM, Urbach V, et al. Rapid responses to aldosterone in human distal colon. Steroids. 1999;64:51–63. doi: 10.1016/s0039-128x(98)00096-8. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba M. 2-APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- Pereira C, Ferreira C, Carvalho C, Oliveira C. Contribution of plasma membrane and endoplasmic reticulum Ca2+-ATPases to the synaptosomal [Ca2+]i increase during oxidative stress. Brain Res. 1996;713:269–277. doi: 10.1016/0006-8993(95)01554-x. [DOI] [PubMed] [Google Scholar]
- Plenge-Tellechea F, Soler F, Fernadez-Belda F. On the inhibition mechanism of sarcoplasmic or endoplasmic reticulum Ca2+-ATPases by cyclopiazonic acid. J Biol Chem. 1997;272:2794–2800. doi: 10.1074/jbc.272.5.2794. [DOI] [PubMed] [Google Scholar]
- Prinz G, Diener M. Characterization of ryanodine receptors in the rat colonic epithelium. Acta Physiol. 2008;193:151–162. doi: 10.1111/j.1748-1716.2007.01802.x. [DOI] [PubMed] [Google Scholar]
- Reiffenstein RJ, Hulbert WC, Roth SH. Toxicology of hydrogen sulfide. Annu Rev Pharmacol Toxicol. 1992;1992:109–134. doi: 10.1146/annurev.pa.32.040192.000545. [DOI] [PubMed] [Google Scholar]
- Schicho R, Krueger D, Zeller F, von Weyhern CWH, Frieling T, Kimura H, et al. Hydrogen sulfide is a novel prosecretory neuromodulator in the guinea-pig and human colon. Gastroenterology. 2006;131:1542–1552. doi: 10.1053/j.gastro.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Schirgi-Degen A, Beubler E. Significance of nitric oxide in the stimulation of intestinal fluid absorption in the rat jejunum in vivo. Br J Pharmacol. 1995;114:13–18. doi: 10.1111/j.1476-5381.1995.tb14899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss G, Diener M. Regulation of apical and basolateral K+ conductances in the rat colon. Br J Pharmacol. 1997;122:87–94. doi: 10.1038/sj.bjp.0701353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss G, Kocks SL, Diener M. Methods for study of ionic currents and Ca2+ signals in isolated colonic crypts. Biol Proced Online. 2002a;3:70–78. doi: 10.1251/bpo25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss G, Seip G, Kocks SL, Diener M. Ca2+-dependent and Ca2+-independent Cl− secretion stimulated by the nitric oxide donor, GEA 3162, in rat colonic epithelium. Eur J Pharmacol. 2002b;444:21–30. doi: 10.1016/s0014-2999(02)01600-x. [DOI] [PubMed] [Google Scholar]
- Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol. 2003;81:133–176. doi: 10.1016/s0079-6107(02)00053-6. [DOI] [PubMed] [Google Scholar]
- Siefjediers A, Hardt M, Prinz G, Diener M. Characterization of inositol 1,4,5-trisphosphate (IP3) receptor subtypes at rat colonic epithelium. Cell Calcium. 2007;41:303–315. doi: 10.1016/j.ceca.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Strabel D, Diener M. Evidence against direct activation of chloride secretion by carbachol in the rat distal colon. Eur J Pharmacol. 1995;274:181–191. doi: 10.1016/0014-2999(94)00728-p. [DOI] [PubMed] [Google Scholar]
- Tamizhselvi R, Moore PK, Bhatia M. Hydrogen sulfide acts as a mediator of inflammation in acute pancreatitis: in vitro studies using isolated mouse pancreatic acinar cells. J Cell Mol Med. 2007;11:315–326. doi: 10.1111/j.1582-4934.2007.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teague B, Asiedu S, Moore PK. The smooth muscle relaxant effect of hydrogen sulphide in vitro: evidence for a physiological role to control intestinal contractility. Br J Pharmacol. 2002;137:139–145. doi: 10.1038/sj.bjp.0704858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman SA, Murad F. Cyclic GMP synthesis and function. Pharmacol Rev. 1987;39:163–196. [PubMed] [Google Scholar]
- Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- Warth R, Barhanin J. Function of K+ channels in the intestinal epithelium. J Membrane Biol. 2003;193:67–78. doi: 10.1007/s00232-002-2001-9. [DOI] [PubMed] [Google Scholar]
- Xu L, Tripathy A, Pasek DA, Meissner G. Ruthenium red modifies the cardiac and skeletal muscle Ca2+ release channels (ryanodine receptors) by multiple mechanisms. J Biol Chem. 1999;274:32680–32691. doi: 10.1074/jbc.274.46.32680. [DOI] [PubMed] [Google Scholar]
- Yang W, Yang G, Jia X, Wu L, Wang R. Activation of KATP channels by H2S in rat insulin-secreting cells and the underlying mechanisms. J Physiol. 2005;569:519–531. doi: 10.1113/jphysiol.2005.097642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanardo RCO, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Ndisang JS, Wang R. Modulation of endogenous production of H2S in rat tissues. Can J Physiol Pharmacol. 2003;81:848–853. doi: 10.1139/y03-077. [DOI] [PubMed] [Google Scholar]


