Abstract
Background and purpose:
Δ9-tetrahydrocannabinol (THC), the main psychoactive constituent of cannabis, accumulates in adipose tissue where it is stored for long periods of time. Here we investigated whether conditions that promote lipolysis can liberate THC from adipocytes to yield increased blood levels of THC.
Experimental approach:
In vitro studies involved freshly isolated rat adipocytes that were incubated with THC before exposure to the lipolytic agent adrenocorticotrophic hormone (ACTH). A complementary in vivo approach examined the effects of both food deprivation and ACTH on blood levels of THC in rats that had been repeatedly injected with THC (10 mg·kg−1) for 10 consecutive days. Lipolysis promoted by ACTH or food deprivation was indexed by measurement of glycerol levels.
Key results:
ACTH increased THC levels in the medium of THC-pretreated adipocytes in vitro. ACTH also enhanced THC release from adipocytes in vitro when taken from rats repeatedly pretreated with THC in vivo. Finally, in vivo ACTH exposure and 24 h food deprivation both enhanced the levels of THC and its metabolite, (-)-11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THC-COOH) in the blood of rats that had been pre-exposed to repeated THC injections.
Conclusions and implications:
The present study shows that lipolysis enhances the release of THC from fat stores back into blood. This suggests the likelihood of ‘reintoxication’ whereby food deprivation or stress may raise blood THC levels in animals chronically exposed to the drug. Further research will need to confirm whether this can lead to functional effects, such as impaired cognitive function or ‘flashbacks’.
Keywords: ACTH, adipocytes, cannabis, food deprivation, lipolysis, Δ9-tetrahydrocannabinol, (-)-11-nor-9-carboxy-Δ9-tetrahydrocannabinol
Introduction
Δ9-tetrahydrocannabinol (THC), the main psychoactive constituent of cannabis, is a highly lipophilic drug that is rapidly absorbed and preferentially stored in the fat deposits of the body. For example, THC accumulated in rodent gonadal fat at significantly higher concentrations than in other organs such as the liver, brain, lungs and other fatty tissue (Rawitch et al., 1979). Further, THC was detected in rat epididymal fat tissue for up to 2 weeks after a single 4 mg·kg−1 injection and with repeated administration, THC significantly accumulated in this tissue (Kreuz and Axelrod, 1973). Similarly in humans, THC was observed in fat biopsies up to 28 days following the final exposure to the drug (Johansson et al., 1989). The long-term storage of THC in fat is consistent with the observation that heavy cannabis users continue to give positive urine samples (>20 ng·mL−1) after 77 days of drug abstinence (Ellis et al., 1985).
Under normal conditions, THC appears to passively diffuse from fat back into blood, thus explaining its long elimination half-life. However it is possible that under conditions of enhanced fat metabolism (lipolysis), THC might be released from fat at much higher concentrations than normal. Interestingly, there have been anecdotal forensic reports of anomalously high levels of THC in post-mortem blood samples of victims of traumatic death such as drowning (Collins et al., 1997). We have also received recent anecdotal forensic reports of high THC levels in the blood of ex-cannabis users who have lost significant body weight immediately prior to test sampling. Stress and food deprivation (FD) are reliable promoters of lipolysis, and this can be largely ascribed to activation of the sympathetic nervous system (Meisner and Carter, 1977; Palou et al., 1981; Nonogaki, 2000). Activation of the sympathetic nervous system increases the release of hormones such as adrenaline and adrenocorticotrophic hormone (ACTH), which in turn interact with adipocyte membrane-bound receptors such as β-adrenoceptors and melanocortin receptors (MC) that stimulate hormone-sensitive lipases (HSL) to hydrolyse triglycerides into free fatty acids (FFA) and glycerol (Meisner and Carter, 1977; Langin, 2006).
Here we investigated whether conditions that promote lipolysis may cause THC to be released from adipocytes at a higher concentration than under normal, non-lipolytic conditions. Firstly, we examined whether a lipolytic concentration of ACTH enhances the release of THC from adipocytes in vitro that had been pretreated with different concentrations of THC. We also determined whether ACTH enhances THC release from ex vivo adipocytes dissected from rats pretreated with 10 daily injections of THC. Finally, we investigated whether FD or a single systemic dose of ACTH can increase the blood concentration of THC and its non-psychoactive metabolite, (-)-11-nor-9-carboxy-Δ9-THC (THC-COOH) in vivo.
Methods
Animals
A total of 105 male Australian Albino Wistar (AAW) rats (Animal Resource Centre, Perth, Australia) were used for all experiments with a weight range of 200–400 g (see figure legends for the weight ranges used for specific experiments). Rats were housed in large plastic tubs in groups of five to eight in a temperature- and humidity-controlled colony room maintained on a reverse 12 h light/12 h dark cycle (lights off at 07 h 30 min). All experimental procedures were approved by The University of Sydney Animal Ethics Committee in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. Procedures conformed to the guidelines set out by the National Health and Medical Research Council (NH&MRC).
Drug preparation and administration
Δ9-tetrahydrocannabinol was dissolved in absolute ethanol before being added to an equal amount of Tween 80 and diluted in saline to give a final stock solution of ethanol/Tween 80/saline (1:1:18). THC was administered i.p. at a dose of 10 mg·kg−1 for in vivo experiments. THC used in vitro was diluted in Dulbecco's Modified Eagle medium (DMEM) supplemented with 10% foetal bovine serum (FBS) to reach concentrations of 1 and 10 µM. ACTH was diluted in DMEM/10% FBS to reach a concentration of 2 nM in vitro. ACTH used in vivo was diluted in saline and administered i.p. at a dose of 0.2 mg·kg−1. All i.p. drug injections were administered at a volume of 1 mL·kg−1.
Adipocyte isolation
Adipocytes from epididymal adipose tissue were isolated using Rodbell's original procedure with some minor modifications (Rodbell, 1964). In brief, adipose tissue was finely chopped and digested in a Krebs-Ringer (pH 7)/collagenase solution [1.5 mg collagenase·(g tissue)−1] containing 5.6 mM glucose and vigorously shaken at 37°C for 40 min. The upper layer containing the adipocytes was passed through a 400 µm sieve filter before being washed with HEPES/Krebs-Ringer solution. The adipocytes were added to complete DMEM/10% FBS and maintained as freshly isolated cells.
Glycerol analysis
Adipocytes and plasma samples were analysed for glycerol using a glycerol detection kit with a glycerol standard range of 0–3.4 mM. This is a colorimetric assay where the samples are incubated with a detection reagent that contains enzymes such as glycerol kinase, glycerol phosphate oxidase and horseradish peroxidase in the presence of a colorimetric substrate to form a chromophore complex that is detectable in the UV/visible range of the light spectrum particularly at 450 nm.
Detection and quantification of THC and THC-COOH in blood samples
A 50 µL quantity of D3-THC : D3-THC-COOH (0.5 mg·L−1 : 1 mg·L−1) internal standard solution was added to each 0.5 mL sample of whole blood. Acetate buffer was added (pH 4.5) and THC and THC-COOH extracted with a hexane/ethyl acetate (9:1) solvent mixture. Following their extraction and complete drying under a nitrogen stream, the samples underwent derivatization of the polar functional groups (COOH, OH) with bis(trimethylsilyl)trifluroacetamide (BSTFA). To quantify (5 ng·mL−1 limit of quantification) the derivatized extract it was analysed by gas chromatography-mass spectrometry (GC-MS) (Agilent 6890/5973 GC-MS system) using electron impact ionization in selective ion mode. Calibration standards were prepared by spiking drug-free horse blood at concentrations of 5–50 ng·mL−1 for Δ9-THC and 10–100 ng·mL−1 for THC-COOH. The standards were vortexed and treated identically to other blood samples.
Detection and quantification of THC and THC-COOH from freshly isolated adipocytes
Solid-phase extraction was used to concentrate THC from the medium of the freshly isolated adipocyte samples; 1 mL DMEM/10% FBS from each adipocyte sample was passed through a Certify II extraction column according to the manufacturer's instructions and spiked with the same internal standard as that described above. The column was washed with 40% methanol and water before it was eluted using a methanol: ethyl acetate (1:9) solution to extract the THC. Samples were then dried to completeness under nitrogen gas and prepared for GC-MS analysis as described above. Calibration standards were prepared by spiking drug-free DMEM/10% FBS at a concentration range of 5 ng·mL−1–2 mg·mL−1 of THC and treated identically to the other samples.
In vitro experimental procedure
Rats were killed via rapid decapitation and their epididymal fat pads removed. Adipocyte cells were isolated as described above and maintained in DMEM/10% FBS medium. Freshly isolated adipocyte samples were incubated with different concentrations of THC (1 and 10 µM) at 37°C for 90 min. These samples were then thoroughly washed three times with Krebs-Ringer solution to remove residual THC from the medium before being replenished with fresh DMEM/10% FBS. The samples were then incubated at 37°C for a further 2 h with either 2 nM ACTH or a water vehicle (VEH) solution. Finally the medium was collected and prepared for GC-MS analysis of THC and THC-COOH concentrations.
In vivo/in vitro experimental procedure
Rats were administered daily injections of THC (10 mg·kg−1 i.p.) for 10 days before being killed via rapid decapitation 2 or 7 days after their final drug administration. Epididymal fat pads were removed and adipocytes isolated. Cells were either treated with VEH or 2 nM ACTH for 2 h at 37°C. A 50 µL aliquot was taken from the medium for glycerol analysis, and the rest of the sample underwent solid-phase extraction and derivatization before GC-MS analysis of THC and THC-COOH concentrations.
In vivo experimental procedure
Rats were administered daily injections of THC (10 mg·kg−1 i.p.) for 10 days and then given a 2 or 7 day washout period. One cohort of rats received an i.p. injection of VEH or 0.2 mg·kg−1 ACTH at either 48 h or 168 h after their final injection of THC. Thirty minutes after VEH or ACTH injections, the rats were killed via rapid decapitation, trunk blood was collected in EDTA-coated tubes, and epididymal fat pads were removed. Another cohort of rats received either no FD (NFD) or 24 h of FD that occurred between 24–48 h or 144–168 h after their final THC injection. These rats were then killed via rapid decapitation, trunk blood was collected in EDTA-tubes, and epididymal fat pads were removed. A small aliquot of blood was immediately centrifuged at 13 400×g for 10 min at 4°C to separate plasma for glycerol analysis. Fat pads that were not used immediately were snap frozen in isopentane cooled on dry ice before being stored at −80°C.
Statistical analysis
All statistical tests were performed using either the STATVIEW 5.0.1 software program for Windows (SAS Institute, UK) or PRISM 5.0 (GraphdPad Software, USA). Data from in vitro experiments were analysed using a two-way analysis of variance (anova). The two factors were the effect of THC concentration (1 µM and 10 µM) and the effect of ACTH treatment (VEH and ACTH) on concentrations of THC in the medium of freshly isolated adipocytes. A Tukey-Kramer post hoc test (where THC and ACTH treatment was split by the corresponding factor) was used to compare within each group when a significant two-way anova was found. A Pearson's correlation analysis was performed to correlate the levels of THC and glycerol found in the medium of freshly isolated adipocytes. Data for all other experiments were analysed using two-tailed unpaired t-tests.
Drugs and materials
Δ9-tetrahydrocannabinol and ACTH were obtained from Sigma-Aldrich (St Louis, MI, USA) (THC Pharm, Frankfurt, Main, Germany); DMEM and FBS were from Gibco (Carlsbad, CA, USA); the collagenase solution was from Worthington Inc. (Lakewood, NJ, USA); the glycerol detection kit from Cayman (Ann Arbor, MI, USA); D3-THC : D3-THC-COOH was obtained from Novachem (Collingwood, Victoria, Australia), and the Certify II extraction column was from Varian (Mulgrave, Victoria, Australia).
Results
The effect of ACTH on the release of THC and glycerol from adipocytes pretreated with THC
Two-way anova revealed that 2 nM ACTH significantly increased the release of THC from adipocytes previously treated with THC (Figure 1A), indicated by a significant effect of ACTH treatment (F(1,19) = 12.27, P < 0.01) on the levels of THC in the medium. Further, this effect was concentration-dependent as supported by an effect of THC concentration (F(1,19) = 44, P < 0.001). There was no significant interaction between ACTH treatment and THC concentration (P > 0.05). Tukey-Kramer post hoc tests showed THC concentrations were significantly higher in the medium of ACTH-treated adipocytes than in that of VEH-treated adipocytes when pretreated with either 1 µM (P < 0.01) or 10 µM THC (P < 0.01).
Figure 1.
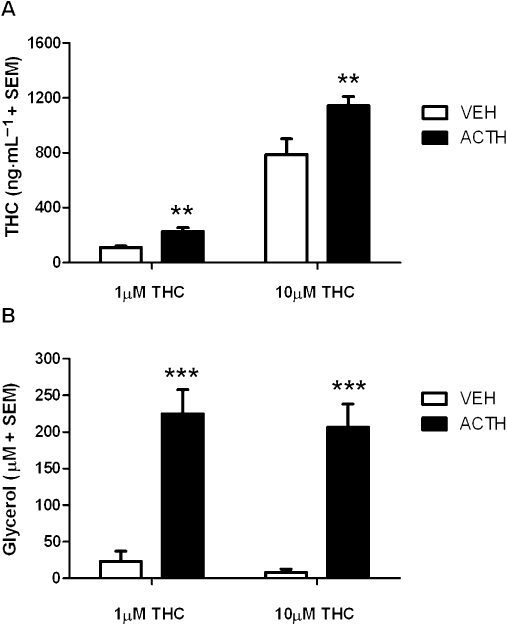
The effect of ACTH (2 nM) on the release of (A) THC and (B) glycerol from adipocytes (105 cells per sample) that were pretreated with either 1 or 10 µM THC (n= 5 per group). **P < 0.01 and ***P < 0.001, significant difference between ACTH and VEH. Adipocytes were isolated from rats weighing between 250 and 300 g. ACTH, adrenocorticotrophic hormone; THC, Δ9-tetrahydrocannabinol; VEH, vehicle.
Two-way anova showed that 2 nM ACTH promoted lipolysis, as demonstrated by increased levels of glycerol in the medium of freshly isolated adipocytes treated with ACTH (F(1,19) = 70.18, P < 0.001) (Figure 1B). THC concentration did not affect ACTH-induced glycerol levels, and no significant interaction was observed between ACTH treatment and THC concentration (P > 0.05). Tukey-Kramer post hoc tests indicated that ACTH increased glycerol levels in the medium similarly in both 1 µM and 10 µM THC-pretreated adipocytes (P < 0.001 and P < 0.001, respectively). A Pearson's correlation analysis revealed that THC levels significantly correlated with glycerol levels in the medium of adipocytes pretreated with 1 µM THC (r= 0.73, P < 0.05) or 10 µM THC (r= 0.70, P < 0.05) and then challenged with VEH or 2 nM ACTH.
The effect of ACTH on the release of THC and glycerol from freshly isolated adipocytes of rats repeatedly pretreated with THC in vivo
Figure 2A shows the effect of ACTH on the release of THC from adipocytes taken from rats pretreated with THC (10 mg·kg−1 i.p.). A t-test showed that 2 nM ACTH significantly increased the release of THC from adipocytes into the medium compared with VEH treatment (t(8) = 7.18, P < 0.001).
Figure 2.
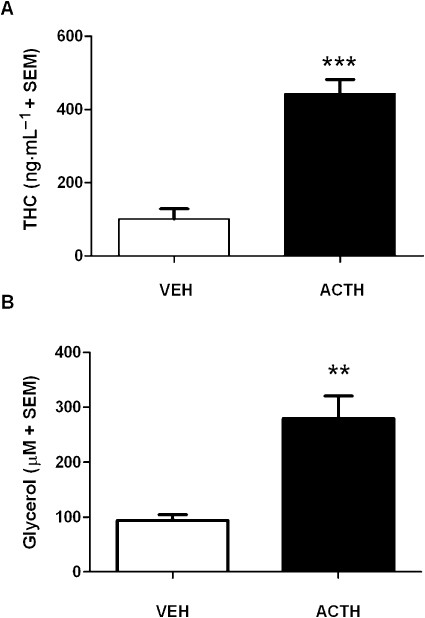
The effect of ACTH (2 nM) on the release of (A) THC and (B) glycerol from adipocytes (105 cells per sample) that were dissected from rats repeatedly pretreated with THC (10 mg·kg−1·day−1) for 10 days before a 2 day washout period (n= 5 per group). **P < 0.01 and ***P < 0.001, significant difference between ACTH and VEH. Adipocytes were isolated from rats weighing between 300 and 350 g. ACTH, adrenocorticotrophic hormone; THC, Δ9-tetrahydrocannabinol; VEH, vehicle.
The results for glycerol are shown in Figure 2B. A t-test indicated that 2 nM ACTH significantly increased glycerol concentrations in the medium compared with the VEH treatment (t(8) = 4.45, P < 0.01). A Pearson's correlation analysis revealed that THC levels significantly correlated with glycerol levels in the medium of adipocytes taken from rats pretreated with THC for 10 days and challenged with either VEH or 2 nM ACTH (r= 0.86, P < 0.001).
The effect of systemically administered ACTH on blood THC and THC-COOH levels in rats repeatedly pretreated with THC in vivo
The results are shown in Figure 3. A t-test indicated that i.p. injection of ACTH (0.2 mg·kg−1) significantly increased blood concentrations of THC (t(14) = 3.81, P < 0.001) and THC-COOH (t(14) = 1.79, P < 0.05), relative to those in VEH-treated animals, in rats that had previously been treated for 10 consecutive days with 10 mg·kg−1 THC i.p. before a 2 day washout period.
Figure 3.
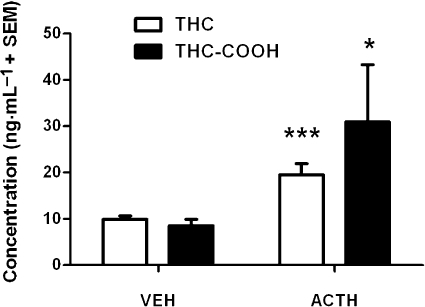
The effect of ACTH (0.2 mg·kg−1) on blood THC and THC-COOH concentrations in rats (200–250 g) that were repeatedly pretreated with THC (10 mg·kg−1·day−1) for 10 days before a 2 day washout period (n= 8 per group). *P < 0.05 and ***P < 0.001, significant difference between ACTH and VEH. ACTH, adrenocorticotrophic hormone; THC, Δ9-tetrahydrocannabinol; THC-COOH, (-)-11-nor-9-carboxy-Δ9-tetrahydrocannabinol; VEH, vehicle.
The effect of FD on blood THC, THC-COOH and glycerol concentration 48 h after the final day of repeated THC treatment
Animals repeatedly pretreated with THC (10 mg·kg−1·day−1 i.p. for 10 days) and deprived of food for 24 h had significantly increased blood THC concentrations compared with NFD controls (t(19) = 2.98, P < 0.05) (Figure 4A). Similarly, the blood THC-COOH concentration was significantly increased in FD rats compared with NFD rats (t(18) = 2.93, P < 0.01).
Figure 4.
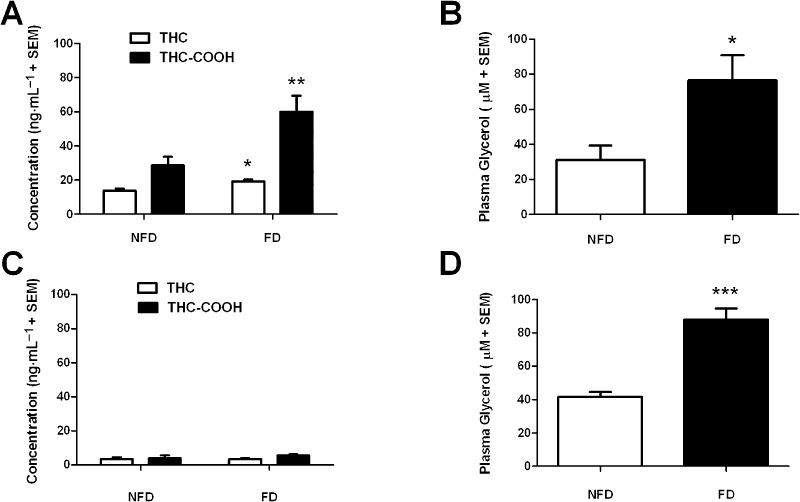
The effect of 24 h FD on (A) blood THC and THC-COOH concentrations and (B) plasma glycerol in rats (300–400 g) that were repeatedly pretreated with THC (10 mg·kg−1·day−1) for 10 days before a 2 day washout period (n= 10 per group). The effect of 24 h FD on (C) blood THC and THC-COOH concentrations and (D) plasma glycerol in rats that were repeatedly pretreated with THC (10 mg·kg−1·day−1) for 10 days and given a 7 day washout period (n= 7–8 per group). *P < 0.05, **P < 0.01 and ***P < 0.001, significant difference between FD and NFD group. FD, food deprivation; NFD, non-food deprived; THC, Δ9-tetrahydrocannabinol; THC-COOH, (-)-11-nor-9-carboxy-Δ9-tetrahydrocannabinol.
Food deprivation also significantly increased the plasma glycerol concentrations (t(14) = 2.77, P < 0.05) compared with levels observed in NFD rats (Figure 4B). Chronic THC treatment per se did not significantly affect FD-induced enhancement of blood glycerol concentrations as equivalent FD-induced glycerol levels were observed in animals that had received 10 daily i.p. injections of VEH (data not shown).
The effect of FD on the release of blood THC, THC-COOH and glycerol 7 days following the final day of repeated THC injections
There was no effect of FD on blood THC concentrations in rats deprived of food from 144–168 h after the final THC injection (P > 0.05, Figure 4C). Similarly, FD caused no significant change in the blood THC-COOH concentration compared with NFD rats (P > 0.05). Figure 4D shows that the plasma glycerol concentration was significantly increased in FD rats compared with NFD rats (t(12) = 6.21, P < 0.001).
Effects of ACTH on THC release from ex vivo adipocytes of FD and NFD rats
As FD following a 48 h washout period increased blood THC concentration, we were interested in whether there was an inverse relationship between THC levels in blood and the amount of THC remaining in these animals' adipocytes. That is, if THC was being released from fat following FD, then THC levels should be lower in the adipocytes of FD rats compared with NFD rats (see Figure 5).
Figure 5.
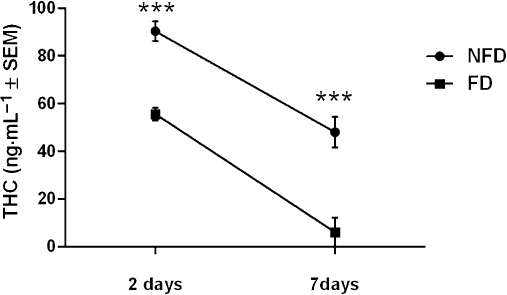
ACTH-induced THC release from adipocytes (104 cells per sample) of FD or NFD rats (300–400 g) repeatedly pretreated with THC (10 mg·kg−1·day−1) for 10 days after a 2 (n= 8 per group) or 7 day (n= 6 per group) washout period. ***P < 0.001, significant difference between FD and NFD group. ACTH, adrenocorticotrophic hormone; FD, food deprivation; NFD, non-food deprived; THC, Δ9-tetrahydrocannabinol.
Two-way anova showed there was a significant overall effect of FD on THC concentration in the medium of freshly isolated adipocytes (F(1,16) = 41.09, P < 0.001) and a significant effect of the number of days of washout (F(1,16) = 58.84, P < 0.001) but no significant interaction between FD and the washout period. Tukey-Kramer post hoc tests showed that ACTH treatment induced higher levels of THC in the medium of adipocytes from NFD compared with FD rats that had received either a 2 (P < 0.001) or 7 day (P < 0.001) washout period. It was also observed that less THC was released from freshly isolated adipocytes of rats that received a 7 day washout compared with those that received a 2 day washout period. ACTH-induced enhancement of glycerol levels in the medium was not significantly different between FD and NFD rats whether they received a 2 or 7 day washout period (data not shown).
Discussion
Collectively the data obtained in the present study demonstrate that lipolysis significantly enhances the release of THC from fat tissue resulting in increased levels in the blood. The lipolytic agent ACTH enhanced the release of THC from adipocytes previously treated in vitro with THC. This effect correlated with ACTH-induced lipolysis. Similarly, ACTH enhanced the release of THC from ex vivo adipocytes of rats that were repeatedly pretreated with systemic injections of THC. Complementary in vivo experiments showed that systemic ACTH or 24 h of FD both increased the blood concentrations of THC and its metabolite THC-COOH in rats pretreated in vivo with THC. Importantly, the increased blood THC levels following FD are probably due to THC release from fat stores because ex vivo adipocytes from the food deprived rats contained less THC than those from animals that had not been deprived of food.
Adrenocorticotrophic hormone proved to be a very potent lipolytic agent in vitro and enhanced THC release from adipocytes that were directly pretreated with known THC concentrations and also from ex vivo adipocytes dissected from rats previously repeatedly treated with THC. We observed a significant, positive correlation between THC release and lipolysis, which suggests that THC stored in fat is released under such conditions. As ACTH-induced lipolysis enhanced the release of THC directly from freshly isolated adipocytes, we then aimed to demonstrate that such a local, in vitro enhancement in THC release translates into augmented concentrations when sampled from the blood in vivo. Indeed, systemic acute administration of ACTH was found to increase the levels of THC and THC-COOH in the blood of THC-pretreated rats. This presumably occurred due to ACTH-induced lipolysis in vivo. Previous studies, as well as the current one, have shown that systemic administration of ACTH increases lipolysis, as gauged by increased plasma levels of glycerol (Spirovski et al., 1975; Opmeer et al., 1978; Boston and Cone, 1996).
In addition, we determined whether FD, another robust in vivo promoter of lipolysis (Migliorini et al., 1997), also enhances the release of THC from fat stores. It was found that rats, abstinent for 2 days following chronic THC treatment, deprived of food for 24 h had increased levels of THC and THC-COOH in their blood and this directly correlated with a significant increase in plasma glycerol concentrations. Furthermore, ACTH-treated adipocytes from these FD rats showed a decreased level of THC release into the medium compared with ACTH-treated adipocytes from NFD rats. This indicates that at least some of the increase in blood THC levels following FD in THC-pretreated rats can be attributed to the release of THC from the epididymal fat pads. While previous studies have shown that cannabinoids accumulate in the epididymal fat pads at higher concentrations than other fat depots in the body (Rawitch et al., 1979), it is likely that lipolysis in other fat depots may also contribute the enhancement of cannabinoid levels in the blood of FD rats.
When comparing the cannabinoid blood profiles promoted by ACTH and FD it is clear that FD caused a higher blood level of THC-COOH than that seen in the ACTH-treated animals. This difference is probably due to the different times of blood sampling in FD rats compared with those that received an ACTH injection. FD occurred over a 24 h period before blood was sampled, whereas blood was collected only 30 min after ACTH injection. Following the smoking of a cannabis cigarette, blood THC levels have been shown to peak approximately 10 min after administration of the drug, whereas THC-COOH reaches a peak much later at around 80 min (Huestis et al., 1992). In addition we have obtained preliminary data showing that after a single THC injection to rats (10 mg·kg−1 i.p.), blood levels of THC peaked at 30 min while THC-COOH levels peaked at 6 h post injection (Arnold JC, and McGregor IS, unpubl. obs.). Therefore, THC released from adipocytes 30 min after ACTH injection is unlikely to have had sufficient time to be metabolically converted to the levels of THC-COOH observed following 24 h of FD.
Food deprivation did not significantly increase blood THC and THC-COOH concentrations in rats that received the last of 10 daily THC injections 6 days before the 24 h of FD. Clearly then, 7 days of washout from THC exposure resulted in most THC being passively diffused from fat stores and subsequently excreted so that FD did not induce a measurable increase in the very low THC levels found in the blood of NFD rats. Interestingly, however, ex vivo studies of THC levels in the adipocytes of these FD and NFD rats showed a similar effect of FD to that observed for the 2 day washout animals, albeit with much lower THC levels being recorded, since again FD rats showed less ACTH-induced THC release compared with NFD rats.
While the present study suggests that lipolysis-induced THC release from fat stores may only occur after a relatively short (2 day) cessation of THC exposure, it is possible that extending the THC dosing regimen beyond 10 consecutive days might lead to THC accruing in fat deposits at much higher levels and for longer periods than that suggested by the current study. Indeed, cannabis users may administer the drug on a daily basis for years, and significant levels of THC have been sampled in human fat samples up to 28 days following abstinence from cannabis use (Johansson et al., 1989).
Adrenocorticotrophic hormone and FD appear to promote lipolysis via distinct mechanisms. ACTH is an agonist for MC particularly of the MC2 subtype that are expressed in murine adipocytes (Boston, 1999; Voisey et al., 2003). Activation of MC2 receptors increases intracellular cAMP levels to activate HSL that hydrolyse and release the triglyceride content of the cells in the form of glycerol and FFA (Schwartz and Jungas, 1971). While it is clear ACTH promotes lipolysis in rodents, it is less clear that ACTH can induce triglyceride breakdown in human cells (Xue et al., 1998; Kiwaki and Levine, 2003). In any case, FD induces lipolysis irrespective of species via the release of many different hormones that interact in a complex fashion to breakdown triglycerides. One of the most important ways food restriction promotes lipolysis is by increasing sympathetic activity to release adrenaline and noradrenaline into the circulation, thus activating adrenoceptors in the membrane of adipocytes (Migliorini et al., 1997). In rodents, β3-adrenoceptors are the most active in increasing cAMP levels that in turn activate HSL, which hydrolyse triglycerides to glycerol and FFA for release from the adipocyte. Accordingly, future investigations could examine whether catecholamine and selective β3-adrenoceptor agonist-induced lipolysis similarly enhances the release of THC from fat stores.
It is interesting to speculate on the mechanism whereby lipolysis dislodges THC from its storage in adipocyte tissue. It is understood that cannabinoids being lipophilic compounds bind to triglycerides explaining why they are sequestered in adipocyte tissue. It appears that no metabolism of THC occurs in the adipocyte and that most of the cannabinoid content of adipocytes can be attributed to unchanged THC or fatty acid conjugates of 11-hydroxy-THC produced in the liver (Kreuz and Axelrod, 1973; Leighty et al., 1976). When triglycerides are hydrolysed the cannabinoids are expelled from the fat cell bound to FFA and make their way into the blood circulation. We speculate that THC is more likely to be bound to hydrophobic FFA than glycerol in the intracellular aqueous environment of the fat cell, as glycerol is a much more polar molecule. Thus, this THC–FFA complex may then diffuse out of the cell, a process facilitated by adipocyte fatty acid binding protein, which has recently been shown to assist in FFA transport from adipocytes (Smith et al., 2007; Jocken and Blaak, 2008). Future studies are required to address the specific nature of the sequestration of THC in adipocytes and how lipolysis acts to liberate THC from its storage site in fat.
As FD and ACTH increase blood THC levels in THC-pretreated rats, it is possible that THC concentrations achieved in the blood reach sufficient levels to cause cannabinoid-related cognitive deficits. Administration of exogenous cannabinoids causes disruption of working memory and other higher cognitive functions (Nakamura et al., 1991; Lichtman and Martin, 1996; Varvel et al., 2001). Future research might therefore explore whether a flashback or ‘reintoxication’ phenomenon might occur, whereby cognitive function is disrupted when stress or FD temporarily increases blood THC levels in currently abstinent cannabis users (Niveau, 2002).
Taken together, our results demonstrate for the first time that lipolysis, induced under two distinct experimental conditions (ACTH treatment or FD), may enhance the release of THC from adipocytes and increase the levels of cannabinoids present in the blood. Future studies could attempt to observe whether other lipolysis-promoting conditions such as exercise or, physical or psychological stress, are able to enhance the release of THC from fat stores at sufficient levels to promote typical cannabinoid behavioural effects. Furthermore, by extending the period of cannabinoid exposure it might be possible to enhance lipolysis-induced THC release from fat, promoting even higher levels in blood long after use of the drug has ceased. These results may provide a mechanism of cannabinoid ‘flashback’ and might help to explain anomalous cases where prior cannabis users, that were exposed to extreme stress or had undergone intensive weight loss, tested positive for THC a long time after they had refrained from cannabis use.
Acknowledgments
This work was supported by a National Health and Medical Research Council (NH&MRC) Project Grant awarded to ISM and JCA, and also a NH&MRC Equipment Award to ISM and JCA for the purchase of a new GC-MS system. An Australian Postgraduate Award supported NG. We would also like to thank Bina Sheriff, Julia Surgeon, David Bosanquet and Keith Lewis for their earlier contributions to this project. Finally, we dedicate this manuscript to the memory of Allan Hodda, a forensic toxicologist and originator of the idea that traumatic stress might dislodge THC from fat stores.
Glossary
Abbreviations:
- ACTH
adrenocorticotrophic hormone
- BSTFA
bis(trimethylsilyl)trifluroacetamide
- FD
food deprivation
- GC-MS
gas chromatography-mass spectrometry
- HSL
hormone-sensitive lipases
- MC
melanocortin receptors
- NFD
non-food deprived
- THC
Δ9-tetrahydrocannabinol
- THC-COOH
(-)-11-nor-9-carboxy-Δ9-tetrahydrocannabinol
- VEH
vehicle
Conflict of interest
None.
References
- Boston BA. The role of melanocortins in adipocyte function. Ann N Y Acad Sci. 1999;885:75–84. doi: 10.1111/j.1749-6632.1999.tb08666.x. [DOI] [PubMed] [Google Scholar]
- Boston BA, Cone RD. Characterization of melanocortin receptor subtype expression in murine adipose tissues and in the 3T3-L1 cell line. Endocrinology. 1996;137:2043–2050. doi: 10.1210/endo.137.5.8612546. [DOI] [PubMed] [Google Scholar]
- Collins M, Easson J, Hansen G, Hodda A, Lewis K. GC-MS-MS confirmation of unusually high delta 9-tetrahydrocannabinol levels in two postmortem blood samples. J Anal Toxicol. 1997;21:538–542. doi: 10.1093/jat/21.7.538. [DOI] [PubMed] [Google Scholar]
- Ellis GM, Jr, Mann MA, Judson BA, Schramm NT, Tashchian A. Excretion patterns of cannabinoid metabolites after last use in a group of chronic users. Clin Pharmacol Ther. 1985;38:572–578. doi: 10.1038/clpt.1985.226. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol. 1992;16:276–282. doi: 10.1093/jat/16.5.276. [DOI] [PubMed] [Google Scholar]
- Jocken JW, Blaak EE. Catecholamine-induced lipolysis in adipose tissue and skeletal muscle in obesity. Physiol Behav. 2008;94:219–230. doi: 10.1016/j.physbeh.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Johansson E, Noren K, Sjovall J, Halldin MM. Determination of delta 1-tetrahydrocannabinol in human fat biopsies from marihuana users by gas chromatography-mass spectrometry. Biomed Chromatogr. 1989;3:35–38. doi: 10.1002/bmc.1130030109. [DOI] [PubMed] [Google Scholar]
- Kiwaki K, Levine JA. Differential effects of adrenocorticotropic hormone on human and mouse adipose tissue. J Comp Physiol [B] 2003;173:675–678. doi: 10.1007/s00360-003-0377-1. [DOI] [PubMed] [Google Scholar]
- Kreuz DS, Axelrod J. Delta-9-tetrahydrocannabinol: localization in body fat. Science. 1973;179:391–393. doi: 10.1126/science.179.4071.391. [DOI] [PubMed] [Google Scholar]
- Langin D. Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol Res. 2006;53:482–491. doi: 10.1016/j.phrs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Leighty EG, Fentiman AF, Jr, Foltz RL. Long-retained metabolites of delta9- and delta8-tetrahydrocannabinols identified as novel fatty acid conjugates. Res Commun Chem Pathol Pharmacol. 1976;14:13–28. [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. Delta 9-tetrahydrocannabinol impairs spatial memory through a cannabinoid receptor mechanism. Psychopharmacology (Berl) 1996;126:125–131. doi: 10.1007/BF02246347. [DOI] [PubMed] [Google Scholar]
- Meisner H, Carter JR., Jr Regulation of lipolysis in adipose tissue. Horiz Biochem Biophys. 1977;4:91–129. [PubMed] [Google Scholar]
- Migliorini RH, Garofalo MA, Kettelhut IC. Increased sympathetic activity in rat white adipose tissue during prolonged fasting. Am J Physiol. 1997;272:R656–R661. doi: 10.1152/ajpregu.1997.272.2.R656. [DOI] [PubMed] [Google Scholar]
- Nakamura EM, da Silva EA, Concilio GV, Wilkinson DA, Masur J. Reversible effects of acute and long-term administration of delta-9-tetrahydrocannabinol (THC) on memory in the rat. Drug Alcohol Depend. 1991;28:167–175. doi: 10.1016/0376-8716(91)90072-7. [DOI] [PubMed] [Google Scholar]
- Niveau G. Cannabis-related flash-back, a medico-legal case. Encephale. 2002;28:77–79. [PubMed] [Google Scholar]
- Nonogaki K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia. 2000;43:533–549. doi: 10.1007/s001250051341. [DOI] [PubMed] [Google Scholar]
- Opmeer FA, van Ree JM, de Wied D. ACTH-induced lipolysis in rat adipocytes: structure-activity relationships. Naunyn Schmiedebergs Arch Pharmacol. 1978;302:31–36. doi: 10.1007/BF00586593. [DOI] [PubMed] [Google Scholar]
- Palou A, Remesar X, Arola L, Herrera E, Alemany M. Metabolic effects of short term food deprivation in the rat. Horm Metab Res. 1981;13:326–330. doi: 10.1055/s-2007-1019258. [DOI] [PubMed] [Google Scholar]
- Rawitch AB, Rohrer R, Vardaris RM. delta-9-Tetrahydrocannabinol uptake by adipose tissue: preferential accumulation in gonadal fat organs. Gen Pharmacol. 1979;10:525–529. doi: 10.1016/0306-3623(79)90019-3. [DOI] [PubMed] [Google Scholar]
- Rodbell M. Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem. 1964;239:375–380. [PubMed] [Google Scholar]
- Schwartz JP, Jungas RL. Studies on the hormone-sensitive lipase of adipose tissue. J Lipid Res. 1971;12:553–562. [PubMed] [Google Scholar]
- Smith AJ, Thompson BR, Sanders MA, Bernlohr DA. Interaction of the adipocyte fatty acid-binding protein with the hormone-sensitive lipase: regulation by fatty acids and phosphorylation. J Biol Chem. 2007;282:32424–32432. doi: 10.1074/jbc.M703730200. [DOI] [PubMed] [Google Scholar]
- Spirovski MZ, Kovacev VP, Spasovska M, Chernick SS. Effect of ACTH on lipolysis in adipose tissue of normal and adrenalectomized rats in vivo. Am J Physiol. 1975;228:382–385. doi: 10.1152/ajplegacy.1975.228.2.382. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Hamm RJ, Martin BR, Lichtman AH. Differential effects of delta 9-THC on spatial reference and working memory in mice. Psychopharmacology (Berl) 2001;157:142–150. doi: 10.1007/s002130100780. [DOI] [PubMed] [Google Scholar]
- Voisey J, Carroll L, van Daal A. Melanocortins and their receptors and antagonists. Curr Drug Targets. 2003;4:586–597. doi: 10.2174/1389450033490858. [DOI] [PubMed] [Google Scholar]
- Xue B, Moustaid N, Wilkison WO, Zemel MB. The agouti gene product inhibits lipolysis in human adipocytes via a Ca2+-dependent mechanism. FASEB J. 1998;12:1391–1396. [PubMed] [Google Scholar]


