Abstract
Background and purpose:
Alcohol produces its behavioural effects in part due to inhibition of N-methyl-d-aspartate (NMDA) receptors in the CNS. Previous studies have identified amino acid residues in membrane-associated domains 3 (M3) and 4 (M4) of the NMDA receptor that influence ethanol sensitivity. In addition, in other alcohol-sensitive ion channels, sedative-hypnotic agents have in some cases been shown to act at sites distinct from the sites of ethanol action. In this study, we compared the influence of mutations at these sites on sensitivity to ethanol and trichloroethanol, a sedative-hypnotic agent that is a structural analogue of ethanol.
Experimental approach:
We constructed panels of mutants at ethanol-sensitive positions in the GluN2A (NR2A) NMDA receptor subunit and transiently expressed these mutants in human embryonic kidney 293 cells. We used whole-cell patch-clamp recording to assess the actions of ethanol and trichloroethanol in these mutant NMDA receptors.
Key results:
Ethanol sensitivity of mutants at GluN2A(Ala825) was not correlated with any physicochemical measures tested. Trichloroethanol sensitivity was altered in two of three ethanol-insensitive mutant GluN2A subunits: GluN2A(Phe637Trp) in M3 and GluN2A(Ala825Trp) in M4, but not GluN2A(Met823Trp). Trichloroethanol sensitivity decreased with increasing molecular volume at Phe637 or increasing hydrophobicity at Ala825 and was correlated with ethanol sensitivity at both sites.
Conclusions and implications:
Evidence obtained to date is consistent with a role of GluN2A(Ala825) as a modulatory site for ethanol and trichloroethanol sensitivity, but not as a binding site. Trichloroethanol appears to inhibit the NMDA receptor in a manner similar, but not identical to, that of ethanol.
Keywords: glutamate receptor, alcohol, sedative-hypnotic, membrane-associated domains, electrophysiology, mutant
Introduction
Trichloroethanol is the active metabolite of chloral hydrate (Butler, 1948; Hobbs et al., 1996), the oldest synthetic sedative-hypnotic agent (Sourkes, 1992), and one that has been widely used in human medicine. Because it is both a structural analogue of ethanol and a sedative-hypnotic agent, with anaesthetic potency and hydrophobicity intermediate to ethanol and general anaesthetics (Breimer, 1977), trichloroethanol has been a useful experimental agent to delineate the mechanisms of modulation and to probe the molecular sites of action of alcohols and anaesthetics on a number of ion channels in the CNS (Lovinger et al., 1993; Li et al., 1994; Peoples and Weight, 1994; 1998; Downie et al., 1995; Lovinger and Zhou, 1998). Results of previous studies have identified ethanol modulatory sites in the M3 and M4 domains of the N-methyl-d-aspartate (NMDA) glutamate receptor (Ronald et al., 2001; Ren et al., 2003; 2007; Honse et al., 2004; Smothers and Woodward, 2006), an important target of alcohol action in the CNS (Peoples, 2003).
In the present study, we investigated the actions of ethanol at Ala825, one of the two sites in the M4 domain of the GluN2A (formerly NR2A) subunit (nomenclature follows Alexander et al., 2008). Although this position can powerfully regulate alcohol sensitivity, our findings did not provide evidence for a direct interaction of ethanol with this site. We also tested the actions of trichloroethanol at Ala825, as well as at Phe637 in the M3 domain and Met823 in the M4 domain. We found that Phe637 and Ala825 also regulated sensitivity to trichloroethanol, but Met823, which appears to be an important site of alcohol action on the NMDA receptor (Ren et al., 2003; 2008;), did not. These results suggest that despite their structural similarity, there are important differences in the action of ethanol and trichloroethanol on the NMDA receptor.
Methods
Site-directed mutagenesis, cell culture and transfection
Site-directed mutagenesis in plasmids containing GluN2A subunit cDNA was performed using the QuikChange kit (Stratagene, La Jolla, CA, USA), and all mutants were verified by double-strand DNA sequencing. Human embryonic kidney (HEK) 293 cells were transfected with GluN1-1a, GluN2A and green fluorescent protein (GFP) at a ratio of 2:2:1 using the calcium phosphate transfection kit (Invitrogen, Carlsbad, CA, USA) and were cultured in the presence of 100 µM ketamine and 200 µM d,l-2-amino-5-phosphonovaleric acid (APV) in the culture medium to prevent excitotoxic cell death. Immediately prior to use in experiments, cells were extensively washed to remove NMDA antagonists. Cells were used in experiments 18–48 h after transfection.
Electrophysiological recording
Whole-cell patch-clamp recording was performed at room temperature using an Axopatch 1D or Axopatch 200B (Axon Instruments Inc., Foster City, CA, USA) amplifier. Patch-pipettes had open tip resistances of 2–8 MΩ following fire polishing. Series resistances of 4–15 MΩ were compensated by 80%. Cells were voltage-clamped at −50 mV and superfused in an external recording solution containing (in mM): 150 NaCl, 5 KCl, 0.2 CaCl2, 10 HEPES, 10 glucose and 10 sucrose (pH 7.4). Low Ca2+ was used to minimize NMDA receptor inactivation. Patch-pipettes were filled with a solution containing (in mM): 140 CsCl, 2 Mg4ATP, 10 BAPTA [1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid] and 10 HEPES (pH 7.2). Solutions of agonists and trichloroethanol were applied to cells using a stepper motor-driven rapid solution exchange apparatus (Warner Instruments, LCC, Hamden, CT, USA) and square glass tubing (600 µm i.d.). Data were filtered at 2 kHz (8-pole Bessel) and acquired at 5 kHz on a computer using a DigiData interface and pClamp software (Axon Instruments).
Calculation of physicochemical properties of amino acids
Molecular (van der Waals) volumes and log octanol : water partition coefficients (Log P) of amino acids were calculated using Spartan Pro (Wavefunction, Inc., Irvine, CA, USA) following structural optimization using the AM1 semi-empirical parameters. Values used for amino acid hydrophilicity and polarity were reported previously (Zimmerman et al., 1968; Hopp and Woods, 1981).
Data analysis
In concentration–response experiments, IC50 and n (slope factor) were calculated using the equation: y=Emax/1 + (IC50/x)n, where y is the measured current amplitude, x is concentration, n is the slope factor, and Emax is the maximal current amplitude. Statistical differences among concentration–response curves were determined by comparing log transformed IC50 values from fits to data obtained from individual cells using analysis of variance (anova) followed by the Dunnett's (for comparison of mutant values to the wild-type value) or Fisher's (for comparisons among mutant values) post hoc tests. Comparisons among mean values of log IC50 for ethanol versus trichloroethanol in the various mutants were made using correlation analysis, and tests for linear relations of these values to amino acid physicochemical scales were made using linear regression analysis. Values for ethanol log IC50 in GluN2A(Phe637) mutants are from Ren et al. (2007). All values are reported as means ± SEM.
Materials
2,2,2-Trichloroethanol (>99%) and all other drugs and chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Results
Effects of trichloroethanol in ethanol-insensitive GluN2A mutant subunits
Previous studies from this laboratory have shown that tryptophan substitution mutations at three sites in the M3 and M4 domains of the GluN2A subunit can markedly reduce ethanol sensitivity. To test whether these sites similarly affect trichloroethanol sensitivity, we performed concentration–response experiments for trichloroethanol inhibition in NMDA receptors containing these mutant subunits. In wild-type GluN1/GluN2A receptors, trichloroethanol inhibited glutamate-activated current with an IC50 of 7.31 ± 0.45 mM (Figure 1). All three mutant subunits were inhibited by trichloroethanol, but the IC50 values of the GluN2A(Phe637Trp) and GluN2A(Ala825Trp) mutant subunits were significantly increased compared with the wild-type value (anova and Dunnett's test, P < 0.01), whereas the IC50 value of the GluN2A(Met823Trp) subunit was unchanged (anova and Dunnett's test, P > 0.05).
Figure 1.
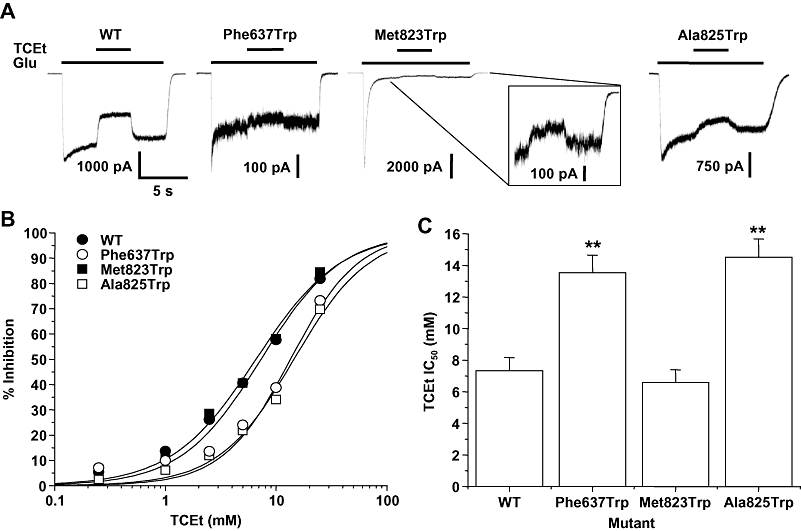
Trichloroethanol inhibition of N-methyl-d-aspartate (NMDA) receptors containing wild-type and mutant GluN2A subunits with tryptophan substitutions at Phe637, Met823, Ala825. (A) Records are currents activated by 10 µM glutamate (Glu) and 50 µM glycine in the absence and presence of 5 mM trichloroethanol (TCEt) in human embryonic kidney (HEK) 293 cells coexpressing GluN1 and wild-type GluN2A (WT) or various substitution mutant subunits. The time scale in the first trace applies to all traces. The inset shows the steady-state current carried by Met823Trp mutant receptors and its inhibition by trichloroethanol on an expanded scale. (B) Concentration–response curves for trichloroethanol inhibition of current activated by 10 µM glutamate and 50 µM glycine in HEK 293 cells coexpressing GluN1 and wild-type GluN2A (WT) or various GluN2A substitution mutant subunits. Data points are means of 5–14 cells. Error bars are not shown to enhance clarity. (C) Bar graph shows IC50 values for trichloroethanol in cells expressing the various GluN2A mutant subunits. Asterisks indicate IC50 values that differed significantly (anova and Dunnett's test; **P < 0.01) from the IC50 for wild-type GluN1/GluN2A subunits.
Effects of mutations at GluN2A(Ala825) on ethanol sensitivity
We previously reported that tryptophan substitution at GluN2A(Ala825) increased the ethanol IC50 over twofold (Honse et al., 2004). To determine how the molecular characteristics of the substituent amino acid at this position influence NMDA receptor ethanol sensitivity, we performed ethanol concentration–response experiments in a panel of substitution mutants at this position. All of the subunits incorporating mutations at this site that were tested, including those with substitutions of the charged amino acids arginine and aspartate, yielded functional receptors (Figure 2). Ethanol inhibited glutamate-activated current in all of the mutant subunits at this position, although the inhibitory effect of 100 mM ethanol was minimal in the GluN2A(Ala825Trp) mutant. Concentration–response analysis yielded ethanol inhibition curves with similar slope factors (anova, P > 0.05), but differing ethanol IC50 values (anova, P < 0.0001). Of the 13 mutants tested, 10 had ethanol IC50 values that were significantly increased compared with that of the wild-type subunit, and three had IC50 values that did not differ from that of the wild-type subunit. Consistent with our previous observations, tryptophan substitution at Ala825 produced an over twofold increase in ethanol IC50 (anova and Dunnett's test, P < 0.01), and this value differed from that for all other mutant subunits (anova and Fisher's test, P < 0.05).
Figure 2.
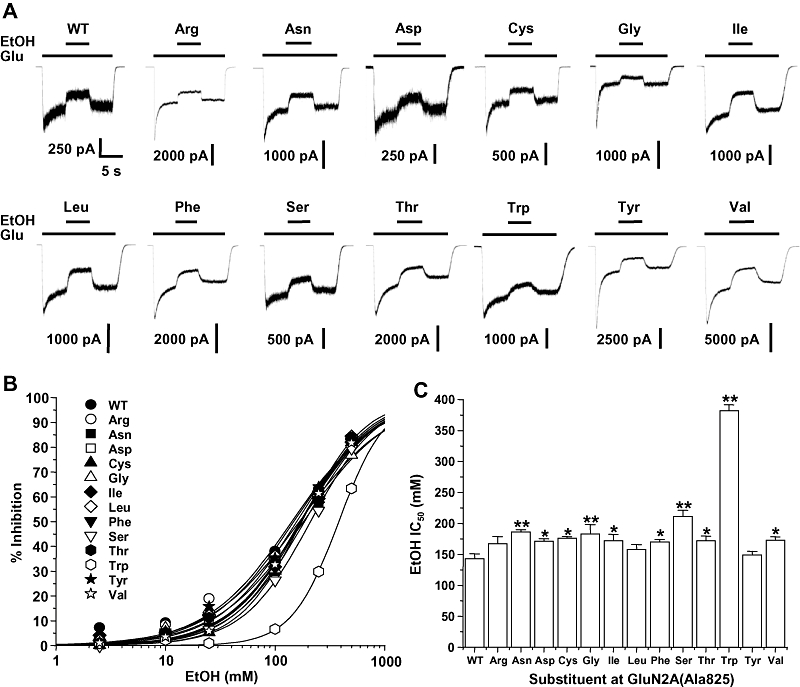
Ethanol inhibition of GluN2A(Ala825) mutant N-methyl-d-aspartate (NMDA) receptors. (A) Records are currents activated by 10 µM glutamate (Glu) and 50 µM glycine in the absence and presence of 100 mM ethanol (EtOH) in human embryonic kidney (HEK) 293 cells coexpressing GluN1 and wild-type GluN2A (WT) or various GluN2A(Ala825) substitution mutant subunits. The time scale in the first trace applies to all traces. (B) Concentration–response curves for ethanol inhibition of current activated by 10 µM glutamate and 50 µM glycine in HEK 293 cells coexpressing GluN1 and wild-type GluN2A (WT) or various GluN2A(Ala825) substitution mutant subunits. Data points are means of 5–10 cells. Error bars are not shown to enhance clarity. (C) Bar graph shows IC50 values for ethanol in cells expressing the various GluN2A(Ala825) mutant subunits. Asterisks indicate IC50 values that differed significantly (anova and Dunnett's test; *P < 0.05, **P < 0.01) from the IC50 for wild-type GluN1/GluN2A subunits. Additional significant differences existed among the various mutants (Fisher's test, P < 0.05).
Relation of ethanol sensitivity to physicochemical measures in GluN2A(Ala825) mutants
Our finding that mutations at GluN2A(A825) caused significant variation in ethanol IC50 values could indicate that ethanol physically interacts with this site, and changes in the characteristics of the side chain at this site influence the binding interaction. If this is true, then the ethanol IC50 value should be linearly related to at least one physicochemical measure of the amino acid substituent at this position. We thus performed linear regression analysis of the ethanol log IC50 versus hydrophilicity, log P, molecular volume and polarity of the substituent (Figure 3). Ethanol sensitivity was not significantly linearly related to any of the measures tested (linear regression analysis, P > 0.05; R2 values: hydrophilicity, 0.0906; log P, 0.0169; molecular volume, 0.125; polarity, 0.00755).
Figure 3.
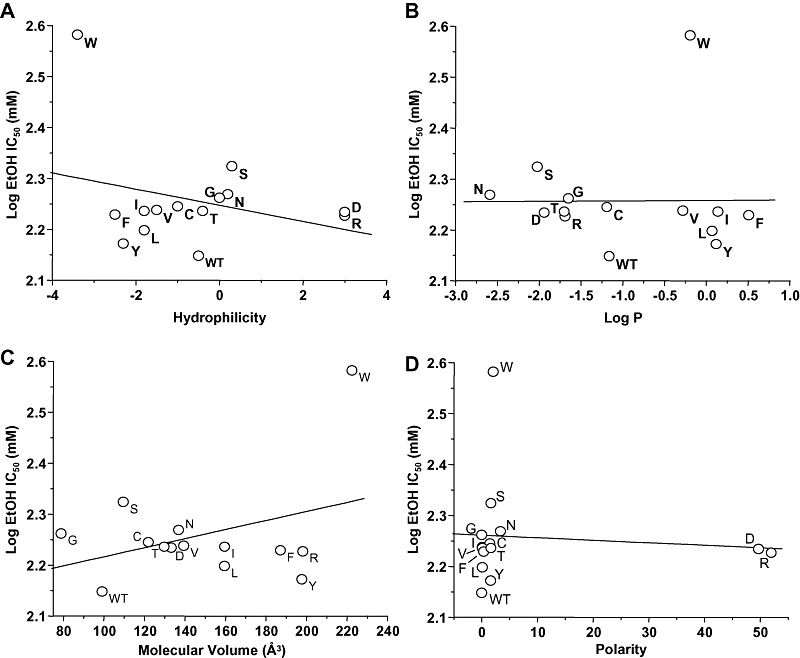
Relation of ethanol sensitivity to physicochemical parameters in N-methyl-d-aspartate (NMDA) receptors containing GluN2A(Ala825) mutant subunits. Graphs plot log IC50 for ethanol (EtOH) versus molecular volume in Å3 (A), log octanol : water partition coefficient (Log P; B), hydrophilicity (C) and polarity (D) for the various substituents at GluN2A(Ala825). Data points are labelled with the substituted amino acid. The lines shown are the least-squares fits to the data. There were no significant linear relations between log EtOH IC50 values and any of the physicochemical measures tested.
Trichloroethanol modulation of GluN2A(Ala825) mutant subunits
Because trichloroethanol sensitivity was significantly altered in the GluN2A(Ala825Trp) subunit, and ethanol sensitivity was altered in a number of substitution mutants at this position, we investigated whether the concentration–response relationship for trichloroethanol inhibition was altered in a panel of substitution mutants at GluN2A(Ala825). As can be seen, trichloroethanol inhibited all of the subunits bearing substitution mutations at this position (Figure 4). Concentration–response analysis revealed that mutations at Ala825 did not alter the slope factor of the curve (anova, P > 0.05), but were able to affect the IC50 value (anova, P < 0.0001). The IC50 values for only the arginine and tryptophan substitution mutants differed significantly from the wild-type value (anova and Dunnett's test, P < 0.01), but there were multiple differences in IC50 values among the various mutant subunits (anova and Fisher's test, P < 0.05).
Figure 4.
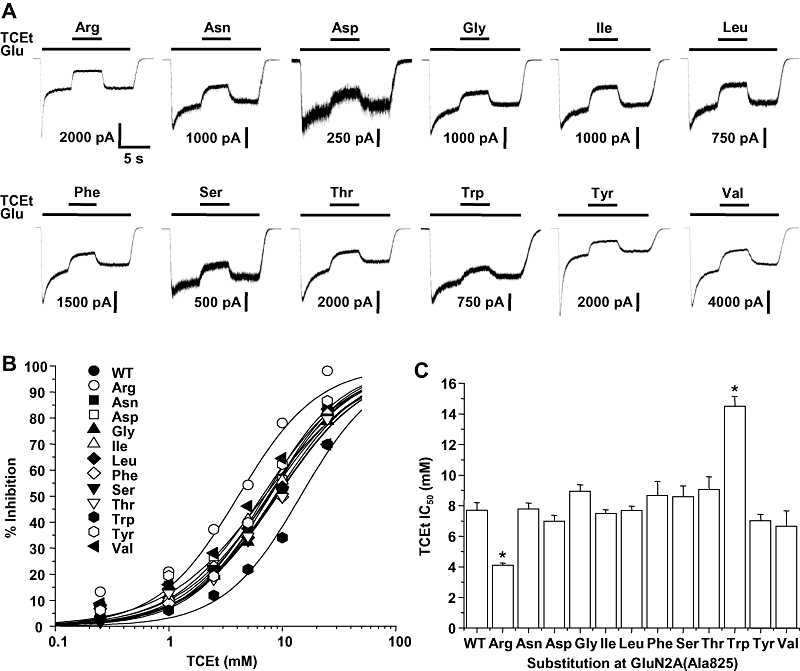
Trichloroethanol inhibition of GluN2A(Ala825) mutant N-methyl-d-aspartate (NMDA) receptors. (A) Records are currents activated by 10 µM glutamate (Glu) and 50 µM glycine in the absence and presence of 5 mM trichloroethanol (TCEt) in human embryonic kidney (HEK) 293 cells coexpressing GluN1 and wild-type GluN2A (WT) or various GluN2A(Ala825) substitution mutant subunits. The time scale in the first trace applies to all traces. (B) Concentration–response curves for trichloroethanol inhibition of current activated by 10 µM glutamate and 50 µM glycine in HEK 293 cells coexpressing GluN1 and wild-type GluN2A (WT) or various GluN2A(Ala825) substitution mutant subunits. Data points are means of 5–14 cells. Error bars are not shown to enhance clarity. (C) Bar graph shows IC50 values for trichloroethanol in cells expressing the various GluN2A(Ala825) mutant subunits. Asterisks indicate IC50 values that differed significantly (anova and Dunnett's test; *P < 0.05) from the IC50 for wild-type GluN1/GluN2A subunits. Additional significant differences existed among the various mutants (Fisher's test, P < 0.05).
Relation of physicochemical characteristics at GluN2A(Ala825) to trichloroethanol sensitivity
We also tested for the presence of linear relations between trichloroethanol IC50 values for mutants at GluN2A(Ala825) and physical chemical measures of the amino acid substituent at this position to evaluate the possibility that the trichloroethanol molecule directly interacts with this site. Linear regression analysis (Figure 5) revealed that the trichloroethanol log IC50 was not linearly related to log P (R2= 0.032, P > 0.05) or molecular volume (R2 < 0.01, P > 0.05), but was negatively linearly related to hydrophilicity (R2= 0.384, P < 0.05) and polarity (R2= 0.346, P < 0.05) of the amino acid at GluN2A(Ala825).
Figure 5.
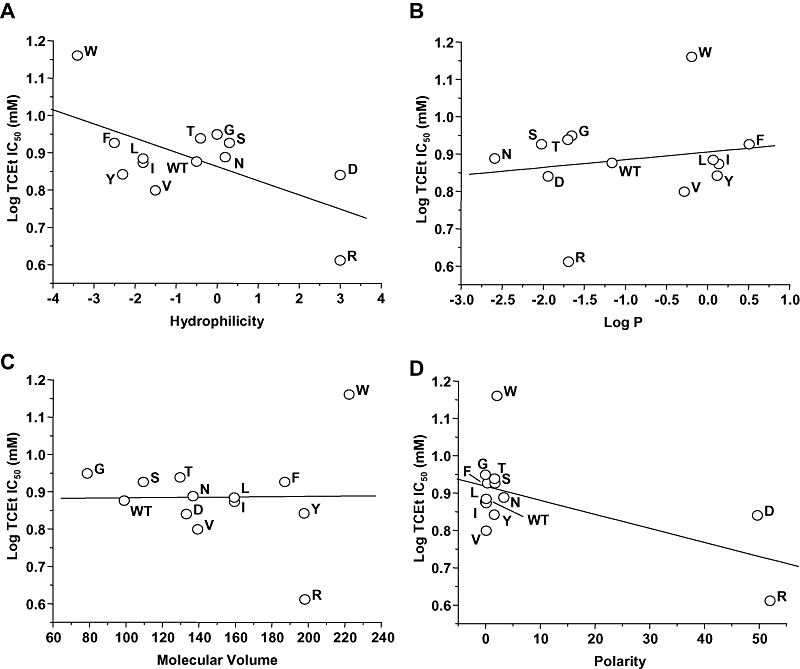
Relation of trichloroethanol sensitivity to physicochemical parameters in N-methyl-d-aspartate (NMDA) receptors containing GluN2A(Ala825) mutant subunits. Graphs plot log IC50 for trichloroethanol (TCEt) versus molecular volume in Å3 (A), log octanol : water partition coefficient (Log P; B), hydrophilicity (C) and polarity (D) for the various substituents at GluN2A(Ala825). Data points are labelled with the substituted amino acid. The lines shown are the least-squares fits to the data. Log TCEt IC50 values were significantly linearly related to both hydrophilicity and polarity, but not molecular volume or Log P.
Trichloroethanol modulation of GluN2A(Phe637) mutant subunits
In order to investigate whether trichloroethanol may be interacting with the substituent at Phe637, we tested the concentration–response relationship for trichloroethanol inhibition in a panel of substitution mutants at this site (Figure 6). The resulting concentration–response curves were parallel to each other, as the slope factors of the curves did not differ significantly (anova, P > 0.05), but the trichloroethanol IC50 values varied significantly among the mutant subunits (anova, P < 0.0001). Of the mutant subunits, only the tryptophan substitution mutant had an IC50 that differed significantly from the wild-type value (anova and Dunnett's test, P < 0.01), although there were multiple significant differences in IC50 values among the remaining mutant subunits (anova and Fisher's test, P < 0.05).
Figure 6.
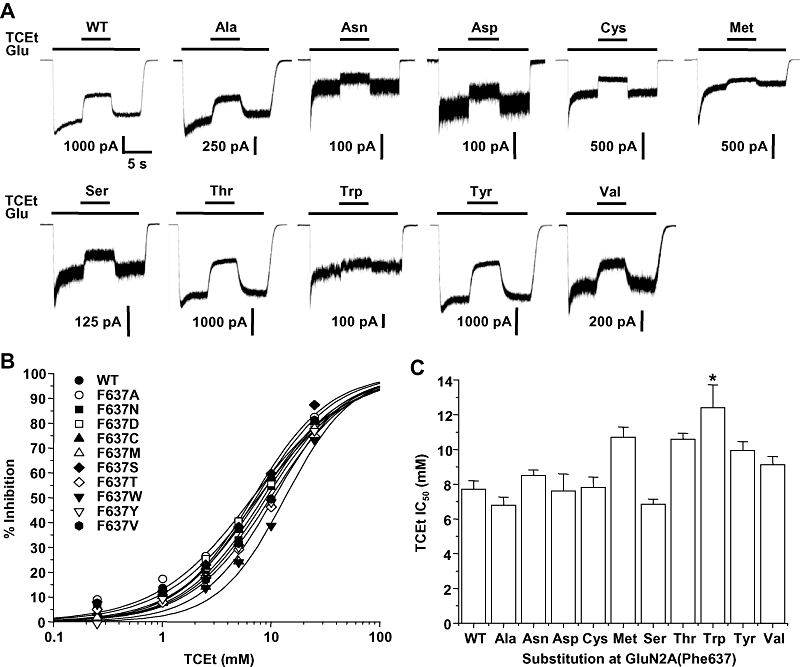
Trichloroethanol inhibition of GluN2A(Phe637) mutant N-methyl-d-aspartate (NMDA) receptors. (A) Records are currents activated by 10 µM glutamate (Glu) and 50 µM glycine in the absence and presence of 5 mM trichloroethanol (TCEt) in human embryonic kidney (HEK) 293 cells coexpressing GluN1 and wild-type GluN2A (WT) or various GluN2A(Phe637) substitution mutant subunits. The time scale in the first trace applies to all traces. (B) Concentration–response curves for trichloroethanol inhibition of current activated by 10 µM glutamate and 50 µM glycine in HEK 293 cells coexpressing GluN1 and wild-type GluN2A (WT) or various GluN2A(Phe637) substitution mutant subunits. Data points are means of 5–14 cells. Error bars are not shown to enhance clarity. (C) Bar graph shows IC50 values for trichloroethanol in cells expressing the various GluN2A(Phe637) mutant subunits. Asterisks indicate IC50 values that differed significantly (anova and Dunnett's test; *P < 0.05) from the IC50 for wild-type GluN1/GluN2A subunits. Additional significant differences existed among the various mutants (Fisher's test, P < 0.05).
Relation of physicochemical characteristics at GluN2A(Phe637) to trichloroethanol sensitivity
If the trichloroethanol molecule is binding to GluN2A(Phe637) to influence ion channel function, then trichloroethanol sensitivity of the receptor should be linearly related to one or more physical chemical measures of the amino acid substituent at this position that influence its interaction with trichloroethanol. To test this, we performed linear regression analysis of the trichloroethanol log IC50 versus hydrophilicity, log P, molecular volume and polarity of the substituent (Figure 7). As can be seen, trichloroethanol sensitivity was not linearly related to hydrophilicity (R2= 0.287, P > 0.05), log P (R2= 0.0940, P > 0.05) or polarity (R2= 0.0552, P > 0.05), but was significantly linearly related to molecular volume (R2= 0.438, P < 0.05) of the amino acid at GluN2A(Phe637).
Figure 7.
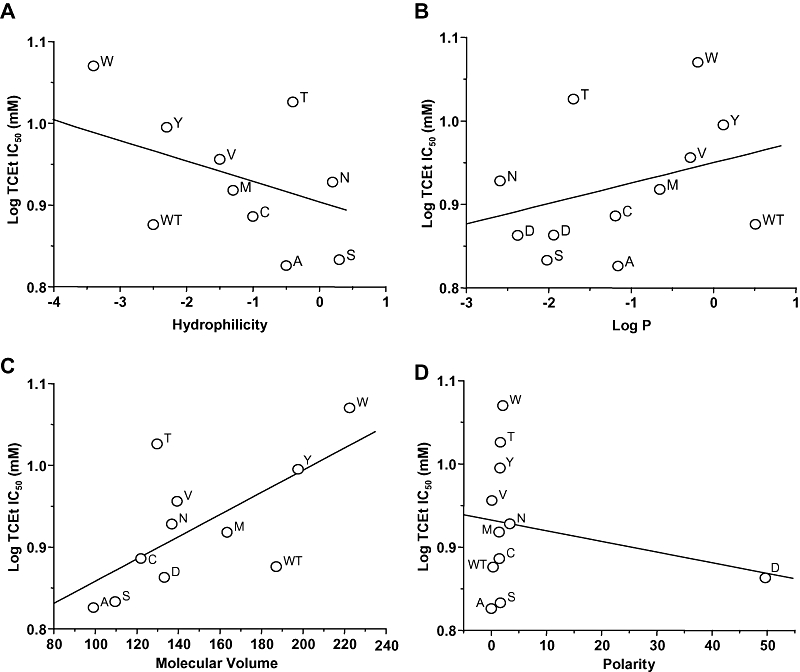
Relation of trichloroethanol (TCEt) sensitivity to physicochemical parameters in N-methyl-d-aspartate (NMDA) receptors containing GluN2A(Phe637) mutant subunits. Graphs plot log IC50 for ethanol versus molecular volume in Å3 (A), log octanol : water partition coefficient (Log P; B), hydrophilicity (C) and polarity (D) for the various substituents at GluN2A(Phe637). Data points are labelled with the substituted amino acid. The lines shown are the least-squares fits to the data. A significant linear relation was obtained between log trichloroethanol IC50 and molecular volume, but not hydrophilicity, Log P or polarity.
Relation of trichloroethanol sensitivity to ethanol sensitivity in GluN2A subunit mutants
The observation that trichloroethanol sensitivity was unaffected by one of three mutations that have been shown to alter ethanol sensitivity is consistent with the view that the actions of trichloroethanol and ethanol on NMDA receptor GluN2A subunits differ in some respects, and raises the possibility that mutations at Phe637 and Ala825 could differentially affect trichloroethanol and ethanol sensitivity. We accordingly compared trichloroethanol log IC50 values to ethanol log IC50 values for mutants at these two sites (Figure 8). There was a strong correlation between sensitivity to trichloroethanol and that to ethanol among GluN2A substitution mutants at both Phe637 (R2= 0.509, P < 0.05) and Ala825 (R2= 0.669, P < 0.001).
Figure 8.
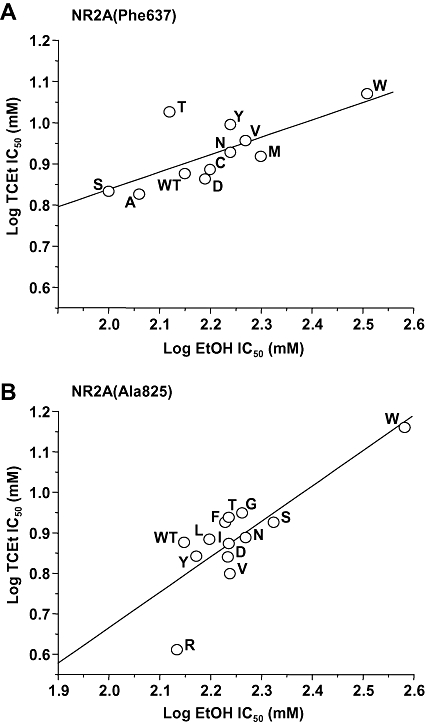
The graph plots log IC50 for trichloroethanol (TCEt) versus log IC50 for ethanol (EtOH) among GluN2A mutants at Phe637 (A) and Ala825 (B). Data points are labelled with the substituted amino acid for the various mutants at either position. The lines shown are the least-squares fits to the data. A significant correlation was obtained between log trichloroethanol IC50 and log ethanol IC50 at each position. Values for ethanol log IC50 in (A) are from Ren et al. (2007).
Discussion
Trichloroethanol, the active metabolite of chloral-derived sedative-hypnotic agents such as chloral hydrate (Hobbs et al., 1996), is a halogenated ethanol analogue that has physical chemical characteristics, such as hydrophobicity, that are generally intermediate to those of ethanol and volatile anaesthetics. Trichloroethanol modulates the activity of a number of ligand-gated ion channels, including GABAA receptor–ion channels (Lovinger et al., 1993; Peoples and Weight, 1994), 5-HT3 receptors (Lovinger and Zhou, 1993; Downie et al., 1995) and NMDA receptors (Peoples and Weight, 1998), with greater potency relative to ethanol. In the present study, trichloroethanol had an IC50 value of 7.3 mM for inhibition of GluN1/GluN2A receptors, which agrees well with the IC50 of 6.4 mM obtained in mouse hippocampal neurons in a previous study (Peoples and Weight, 1998). Previous studies conducted in this laboratory have shown that tryptophan substitution mutations at Phe637, Met823 and Ala825 in the NMDA receptor GluN2A subunit markedly decrease its sensitivity to ethanol (Ren et al., 2003; 2007; Honse et al., 2004). In the present study, we found that two of these three mutations, Phe637Trp and Ala825Trp, also decreased sensitivity to trichloroethanol, whereas trichloroethanol sensitivity was unchanged by tryptophan substitution at the third site, Met823. These observations indicate that ethanol and trichloroethanol interact with the NMDA receptor in a similar, but not identical, manner.
Previous results from this laboratory have demonstrated that Phe637 and Met823 in the GluN2A subunit regulate NMDA receptor alcohol sensitivity in a manner that is dependent upon the characteristics of the amino acid residue at each site. For mutations at Met823, ethanol sensitivity was inversely related to molecular volume of the substituent at the site (Ren et al., 2003). This finding is consistent with a model for a site of alcohol action, first proposed for GABAA and glycine receptors (Wick et al., 1998), in which ethanol acts by occupation of a critical volume in a binding cavity formed in part by the amino acid at this position. In this model, increases in the volume of the amino acid side chain at this position result in a decrease in the volume that must be occupied by ethanol to modulate channel activity, which increases ethanol potency. Similar observations in other ligand-gated ion channels have been interpreted as evidence for a site of ethanol action (Wick et al., 1998; Ye et al., 1998; Koltchine et al., 1999; Yamakura et al., 1999; Kash et al., 2003). In contrast, for mutations at Phe637, there was a significant positive linear relation between ethanol sensitivity and molecular volume at the site, which is not consistent with a simple volume occupation by ethanol at the site (Ren et al., 2007). The results of the present study show that for the third site, Ala825, there were no significant relations found between ethanol sensitivity and any of the physicochemical measures tested. This was despite the observation that 10 of the 13 mutations at this site significantly decreased ethanol sensitivity. The value for the tryptophan mutant appears to be an outlier in each of these analyses; however, removal of this point did not result in a significant linear relation for any measure (results not shown). The observation that most of the substitutions at Ala825 tested decreased sensitivity to ethanol would seem to indicate that the presence of the wild-type residue, alanine, at this site allows for optimal modulation of the receptor by ethanol. The lack of any linear relation between the characteristics of the substituent at the site and ethanol sensitivity of the receptor does not appear to be consistent, however, with a direct interaction of ethanol with this site. Thus it is more likely that Ala825 indirectly influences receptor ethanol sensitivity rather than directly interacting with the ethanol molecule. This agrees well with the results of molecular dynamics simulations, which did not identify Ala825 as an ethanol binding site (Ren et al., 2008).
Studies testing for dependence of trichloroethanol sensitivity in substitution mutants at Phe637 and Ala825 on the characteristics of the amino acid side chain at the site generally yielded results that were similar to those obtained for ethanol sensitivity. For mutations at Phe637, as was observed previously for ethanol (Ren et al., 2007), there was a significant positive linear relation between trichloroethanol sensitivity and molecular volume at the site. Furthermore, among substitution mutants at GluN2A(Phe637), trichloroethanol log IC50 values and ethanol log IC50 values were significantly correlated. Taken together, these results are consistent with the view that the molecular characteristics of the side chain at this position influence ethanol and trichloroethanol sensitivity in a similar manner. In addition, if Phe637 is a site of interaction of the ethanol molecule, as indicated by molecular dynamics simulations (Ren et al., 2008), these results would be predicted if ethanol and trichloroethanol bind to this site in a similar manner. For mutations at Ala825, trichloroethanol log IC50 values were significantly linearly related to hydrophilicity and polarity, despite the absence of a significant linear relation of ethanol log IC50 values to these physicochemical measures. However, the significance of the linear relations was clearly due to the Trp and Arg mutations, suggesting that specific structural features of these residues may have a greater influence than side chain hydrophobicity per se. Nevertheless, log IC50 values for ethanol and trichloroethanol in these mutants were highly correlated, suggesting that changes in the characteristics at this site, whether related to hydrophobicity or molecular structure, produce similar changes in ethanol and trichloroethanol sensitivity.
Results of previous studies have identified ethanol modulatory sites in the M domains of the NMDA receptor. In the present study, we found that two of these three sites in the GluN2A subunit also regulated sensitivity to trichloroethanol, an ethanol analogue and sedative-hypnotic agent with greater molecular volume and hydrophobicity compared with ethanol. These results appear to be consistent with those of a recent study by Ogata et al. (2006), in which mutations at one of these sites, Ala825, and at Phe639 in the GluN1 subunit, the cognate site of GluN2A(Phe637), reduced or eliminated sensitivity to inhibition by hexanol, a straight-chain alcohol with hydrophobicity and molecular volume comparable to that of trichloroethanol. Interestingly, although previous studies have presented evidence for a role for GluN2A(Met823) as a site of alcohol action (Ren et al., 2003; 2008;), in the present study, trichloroethanol did not appear to interact with this site. This is consistent with results of a number of studies on other neurotransmitter-gated ion channels showing different sites of action of ethanol and various sedative-hypnotic agents and anaesthetics (Mihic et al., 1997; Krasowski and Harrison, 2000; Lopreato et al., 2003; Bali and Akabas, 2004; Schofield and Harrison, 2005; Yevenes et al., 2008). These results may also have implications for studies using series of alcohols or anaesthetics with differing molecular size and hydrophobicity: there may be important differences in the actions of smaller, less hydrophobic molecules on the receptor compared with larger, more hydrophobic molecules.
Acknowledgments
We thank David Wagner and Michelle Mynlieff for helpful discussions. These studies were supported by grants from the Alcoholic Beverage Medical Research Foundation (RWP) and the National Institutes of Health (AA015203-01A1, RWP).
Glossary
Abbreviations:
- BAPTA
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- EtOH
ethanol
- TCEt
2,2,2-trichloroethanol
Conflicts of interest
None.
References
- Alexander S, Mathie A, Peters J. Guide to receptors and channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bali M, Akabas MH. Defining the propofol binding site location on the GABAA receptor. Mol Pharmacol. 2004;65:68–76. doi: 10.1124/mol.65.1.68. [DOI] [PubMed] [Google Scholar]
- Breimer DD. Clinical pharmacokinetics of hypnotics. Clin Pharmacokinet. 1977;2:93–109. doi: 10.2165/00003088-197702020-00002. [DOI] [PubMed] [Google Scholar]
- Butler TC. The metabolic fate of chloral hydrate. J Pharmacol Exp Ther. 1948;92:49–58. [PubMed] [Google Scholar]
- Downie DL, Hope AG, Belelli D, Lambert JJ, Peters JA, Bentley KR, et al. The interaction of trichloroethanol with murine recombinant 5-HT3 receptors. Br J Pharmacol. 1995;114:1641–1651. doi: 10.1111/j.1476-5381.1995.tb14952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs WR, Rall TW, Verdoorn TA. Hypnotics and sedatives; ethanol. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. The Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 1996. pp. 361–396. [Google Scholar]
- Honse Y, Ren H, Lipsky RH, Peoples RW. Sites in the fourth membrane-associated domain regulate alcohol sensitivity of the NMDA receptor. Neuropharmacology. 2004;46:647–654. doi: 10.1016/j.neuropharm.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Hopp TP, Woods KR. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci USA. 1981;78:3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Jenkins A, Harrison NL. Molecular volume determines the activity of the halogenated alkane bromoform at wild-type and mutant GABAA receptors. Brain Res. 2003;960:36–41. doi: 10.1016/s0006-8993(02)03748-4. [DOI] [PubMed] [Google Scholar]
- Koltchine VV, Finn SE, Jenkins A, Nikolaeva N, Lin A, Harrison NL. Agonist gating and isoflurane potentiation in the human γ-aminobutyric acid type A receptor determined by the volume of a second transmembrane domain residue. Mol Pharmacol. 1999;56:1087–1093. doi: 10.1124/mol.56.5.1087. [DOI] [PubMed] [Google Scholar]
- Krasowski MD, Harrison NL. The actions of ether, alcohol and alkane general anaesthetics on GABAA and glycine receptors and the effects of TM2 and TM3 mutations. Br J Pharmacol. 2000;129:731–743. doi: 10.1038/sj.bjp.0703087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Peoples RW, Weight FF. Alcohol action on a neuronal membrane receptor: evidence for a direct interaction with the receptor protein. Proc Natl Acad Sci USA. 1994;91:8200–8204. doi: 10.1073/pnas.91.17.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopreato GF, Banerjee P, Mihic SJ. Amino acids in transmembrane domain two influence anesthetic enhancement of serotonin-3A receptor function. Brain Res Mol Brain Res. 2003;118:45–51. doi: 10.1016/s0169-328x(03)00332-2. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Zhou Q. Trichloroethanol potentiation of 5-hydroxytryptamine3 receptor-mediated ion current in nodose ganglion neurons from the adult rat. J Pharmacol Exp Ther. 1993;265:771–776. [PubMed] [Google Scholar]
- Lovinger DM, Zhou Q. Alcohol effects on the 5-HT3 ligand-gated ion channel. Toxicol Lett. 1998;101:239–246. doi: 10.1016/s0378-4274(98)00191-x. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Zimmerman SA, Levitin M, Jones MV, Harrison NL. Trichloroethanol potentiates synaptic transmission mediated by γ-aminobutyric acidA receptors in hippocampal neurons. J Pharmacol Exp Ther. 1993;264:1097–1103. [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MA, Finn SE, et al. Sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- Ogata J, Shiraishi M, Namba T, Smothers CT, Woodward JJ, Harris RA. Effects of anesthetics on mutant N-methyl-D-aspartate receptors Expressed in Xenopus oocytes. J Pharmacol Exp Ther. 2006;318:434–443. doi: 10.1124/jpet.106.101691. [DOI] [PubMed] [Google Scholar]
- Peoples RW. Alcohol actions on glutamate receptors. In: Herman BH, Frankenheim J, Litten RZ, Sheridan PH, Weight FF, Zukin SR, editors. Glutamate and Addiction. Totowa, NJ: Humana Press; 2003. pp. 343–356. [Google Scholar]
- Peoples RW, Weight FF. Trichloroethanol potentiation of γ-aminobutyric acid-activated chloride current in mouse hippocampal neurones. Br J Pharmacol. 1994;113:555–563. doi: 10.1111/j.1476-5381.1994.tb17025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples RW, Weight FF. Inhibition of excitatory amino acid-activated currents by trichloroethanol and trifluoroethanol in mouse hippocampal neurones. Br J Pharmacol. 1998;124:1159–1164. doi: 10.1038/sj.bjp.0701949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Honse Y, Peoples RW. A site of alcohol action in the fourth membrane-associated domain of the NMDA receptor. J Biol Chem. 2003;278:48815–48820. doi: 10.1074/jbc.M302097200. [DOI] [PubMed] [Google Scholar]
- Ren H, Salous AK, Paul JM, Lipsky RH, Peoples RW. Mutations at F637 in the NMDA receptor NR2A subunit M3 domain influence agonist potency, ion channel gating and alcohol action. Br J Pharmacol. 2007;151:749–757. doi: 10.1038/sj.bjp.0707254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Salous AK, Paul JM, Lamb KA, Dwyer DS, Peoples RW. Functional interactions of alcohol-sensitive sites in the N-methyl-D-aspartate receptor M3 and M4 domains. J Biol Chem. 2008;283:8250–8257. doi: 10.1074/jbc.M705933200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald KM, Mirshahi T, Woodward JJ. Ethanol inhibition of N-methyl-D-aspartate receptors is reduced by site-directed mutagenesis of a transmembrane domain phenylalanine residue. J Biol Chem. 2001;276:44729–44735. doi: 10.1074/jbc.M102800200. [DOI] [PubMed] [Google Scholar]
- Schofield CM, Harrison NL. Transmembrane residues define the action of isoflurane at the GABAA receptor alpha-3 subunit. Brain Res. 2005;1032:30–35. doi: 10.1016/j.brainres.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Smothers CT, Woodward JJ. Effects of amino acid substitutions in transmembrane domains of the NR1 subunit on the ethanol inhibition of recombinant N-methyl-D-aspartate receptors. Alcohol Clin Exp Res. 2006;30:523–530. doi: 10.1111/j.1530-0277.2006.00058.x. [DOI] [PubMed] [Google Scholar]
- Sourkes TL. Early clinical neurochemistry of CNS-active drugs. Chloral hydrate. Mol Chem Neuropathol. 1992;17:21–30. doi: 10.1007/BF03159978. [DOI] [PubMed] [Google Scholar]
- Wick MJ, Mihic SJ, Ueno S, Mascia MP, Trudell JR, Brozowski SJ, et al. Mutations of γ-aminobutyric acid and glycine receptors change alcohol cutoff: evidence for an alcohol receptor? Proc Natl Acad Sci USA. 1998;95:6504–6509. doi: 10.1073/pnas.95.11.6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakura T, Mihic SJ, Harris RA. Amino acid volume and hydropathy of a transmembrane site determine glycine and anesthetic sensitivity of glycine receptors. J Biol Chem. 1999;274:23006–23012. doi: 10.1074/jbc.274.33.23006. [DOI] [PubMed] [Google Scholar]
- Ye Q, Koltchine VV, Mihic SJ, Mascia MP, Wick MJ, Finn SE, et al. Enhancement of glycine receptor function by ethanol is inversely correlated with molecular volume at position α267. J Biol Chem. 1998;273:3314–3319. doi: 10.1074/jbc.273.6.3314. [DOI] [PubMed] [Google Scholar]
- Yevenes GE, Moraga-Cid G, Peoples RW, Schmalzing G, Aguayo LG. A selective Gβγ-linked intracellular mechanism for modulation of a ligand-gated ion channel by ethanol. Proc Natl Acad Sci USA. 2008;105:20523–20528. doi: 10.1073/pnas.0806257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JM, Eliezer N, Simha R. The characterization of amino acid sequences in proteins by statistical methods. J Theor Biol. 1968;21:170–201. doi: 10.1016/0022-5193(68)90069-6. [DOI] [PubMed] [Google Scholar]


