Abstract
To investigate whether saccade preparation can modulate processing of auditory stimuli in a spatially-specific fashion, ERPs were recorded for a Saccade task, in which the direction of a prepared saccade was cued, prior to an imperative auditory stimulus indicating whether to execute or withhold that saccade. For comparison, we also ran a conventional Covert Attention task, where the same cue now indicated the direction for a covert endogenous attentional shift prior to an auditory target-nontarget discrimination. Lateralised components previously observed during cued shifts of attention (ADAN, LDAP) did not differ significantly across tasks, indicating commonalities between auditory spatial attention and oculomotor control. Moreover, in both tasks, spatially-specific modulation of auditory processing was subsequently found, with enhanced negativity for lateral auditory nontarget stimuli at cued versus uncued locations. This modulation started earlier and was more pronounced for the Covert Attention task, but was also reliably present in the Saccade task, demonstrating that the effects of covert saccade preparation on auditory processing can be similar to effects of endogenous covert attentional orienting, albeit smaller. These findings provide new evidence for similarities but also some differences between oculomotor preparation and shifts of endogenous spatial attention. They also show that saccade preparation can affect not just vision, but also sensory processing of auditory events.
Keywords: attention, spatial, eye movement, event-related brain potentials, audition
Introduction
To select relevant information from the environment, we often move our eyes toward specific locations, to improve visual detection and discrimination via the fovea. However spatial attention can also be shifted covertly (Eriksen and Colegate, 1971; Eriksen and Hoffman, 1972; Posner et al., 1980). While this indicates that spatial attention can be shifted without any overt eye movement, it has been more controversial whether the converse also applies, i.e. whether or it not it is possible to move our eyes (or even just to plan a saccade) without spatial attention shifting correspondingly.
The exact relation between planned saccades and spatial attention has been debated for at least three decades now. Brain areas activated prior to and during saccadic eye movements are now widely thought to be involved in covert shifts of attention also (see Corbetta, 1998 and Moore et al., 2003, for reviews). Neurophysiological studies on non-human primates have identified a network of cortical areas, including frontal eye fields (FEFs, e.g., Bizzi, 1968; Bruce et al., 1985) and regions in posterior parietal cortex (e.g., Mountcastle et al., 1975; Robinson et al., 1978; Andersen et al., 1987) involved in visually guided saccades, that have now also been implicated in attentional phenomena in the absence of overt eye movements (e.g. Schall et al., 1995; Bushnell et al., 1981; Steinmetz et al., 1994; Colby et al., 1996). More recently, invasive microstimulation of the FEF in monkeys has been shown to affect visual attentional performance (Moore and Fallah, 2001) and visual responses of neurons in occipital regions (Moore and Armstrong, 2003). In humans, transcranical magnetic stimulation (TMS) over the FEF can affect attentionally guided performance in visual search tasks (Muggleton et al., 2003), disrupt shifts of attention (Grosbras and Paus, 2002), and even affect visual activity in occipital cortex as revealed with concurrent TMS-fMRI (Ruff et al., 2006). Several human neuroimaging studies have confirmed an overlapping network of cortical regions (including the FEF and several parietal regions) found both during endogenous covert shifts of visual attention and also for overt eye movements (Astafiev et al., 2003; Beauchamp et al., 2001; Corbetta et al. 1998; Perry and Zeky, 2000).
Such commonality between control of spatial attention (at least for the case of vision) and of oculomotor control has received further recent support from the very different methods of ERP studies, measuring electrophysiological correlates of the cognitive-control processes activated during covert saccade preparation and/or attention shifts (e.g. Eimer et al., 2006; Van der Lubbe et al., 2006; Van der Stigchel et al., 2006; Wauschkuhn et al., 1998). Typically in such studies, a symbolic cue indicated the direction for an upcoming shift of covert attention (e.g. towards the left or right side), or analogously the direction for an upcoming saccade. These ERP works showed that a similar pattern of lateralized ERP components, time-locked to the instructional cue, can be found for both covert orienting of visual attention and saccade preparation. Between ~300-600 ms after cue onset, a negative deflection contralateral to the cued side was observed at anterior electrodes (‘anterior directing attention negativity’, ADAN), followed by a relative positivity over posterior scalp sites contralateral to the cued side (‘late directing attention positivity’, LDAP). These components are thought to reflect brain activity within anterior and posterior regions of a putative attentional-control network. Source localization has implicated dorsal premotor cortex (PMd) (Praamstra et al. 2005) and FEF (Van der Lubbe et al., 2000, 2006) in the ADAN. The LDAP has been attributed to occipitotemporal cortex (Mathews et al., 2006; Praamstra et al., 2005) and/or ventral intraparietal sulcus (VIP, Van der Lubbe et al., 2006). The fact that similar ADAN and LDAP components found both during covert spatial shifts of visual attention and also during saccade preparation suggests not only that similar brain areas may be involved, but also that the temporal dynamics of attentional shifts and saccade plans may be similar (Eimer et al., 2007; Van der Lubbe et al., 2006; but see also Wauschkuhn et al., 1998 for different results).
Such findings appear inconsistent with attentional and saccadic control being entirely separate (cf. Henderson et al., 1989), indicating some anatomical and functional overlap. But the full extent of such overlap, and of any independence, remains debated. Some authors suggest only partial overlap between brain mechanisms for attention and eye movements (e.g. Deubel and Schneider, 1996; Kowler et al., 1995). On this perspective, although attention and saccade plans are functionally coupled when selecting the goal for an eye movement, shifts of attention may occur in the absence of any eye movement activation. Others suggest stronger overlap. On the influential premotor theory of attention, spatial motor plans are considered the primary means of directing spatial attention (Rizzolatti, 1983; Rizzolatti and Camarda, 1987; Rizzolatti et al., 1994). From this perspective, the main difference between saccades and covert shifts of attention is simply that the motor plan is actually executed only in the former case (Rizzolatti et al., 1987).
One central prediction of premotor theory is that attention shifts will be triggered toward the saccade target whenever an oculomotor program is activated. Hence processing of stimuli presented close to the saccade goal should be improved. This prediction has been tested for visual stimuli in a series of neurophysiological, behavioural, and ERP studies. Single-cell studies in monkeys reveal that neural responses to visual stimuli presented close to the saccade-goal location are enhanced, with such enhancements arising before the eyes move (Goldberg and Bushnell, 1981; Robinson et al., 1978; Wurtz and Goldberg, 1972; Wurtz et al., 1982; Wurtz and Mohler, 1976). Behavioural studies in humans have analogously demonstrated superior performance for visual events at saccade-goal locations, prior to the actual gaze shift (e.g., Deubel and Schneider, 1996; Hoffman and Subramaniam, 1995; Irwin and Gordon, 1998). More recent visual studies in our own lab have produced on-line ERP evidence for visual processing benefits at saccade-goal locations (Eimer et al., 2006, 2007). Participants had to prepare a left or right saccade, and peripheral visual probe stimuli were presented during the preparatory interval, at the saccade-goal location, or on the opposite side. Amplitudes of visual N1 components to these probe stimuli were enhanced when probes were delivered at the saccade goal-location.
Given the natural link between eye movements and vision, such effects might, however, be limited to modulation of just visual processing. To assess the generality of any impacts from oculomotor plans upon sensory processing, it is important to study whether saccade preparation can also modulate processing within other modalities such as audition and touch. Unlike visual inputs that can change when the eyes shift, auditory inputs themselves are unaffected by gaze-shifts provided the head is held fixed. If the relationship between oculomotor plans and modulation of visual processing is special, then saccade plans might have no impact on hearing. On the other hand, if oculomotor plans provide one way of shifting crossmodal spatial attention, then audition might also be affected.
Only few physiological studies in animals, using invasive single-cell recordings, have considered such issues to date. Receptive fields (RFs) of multisensory audio-visual neurons in the lateral intra-parietal area (LIP) of the parietal cortex can shift with the direction of gaze (Mazzoni et al., 1996; Stricanne et al., 1996), although the auditory RFs here may depend on overtraining of saccades to sounds. FEF neurons can discharge prior to a saccade either to visual or auditory targets (Russo and Bruce, 1994), though this might just reflect the motor plan rather than an influence on sensory processing per se. Turning to behavioral studies in humans, several studies indicate that the static direction of gaze can affect auditory performance, which may be better for sounds at fixation (Gopher, 1973; Hublet et al., 1976; 1977; Jones and Kabanoff, 1975; Morais et al., 1980; Reisberg et al., 1981); but such static postural manipulations do not involve actual saccades. To our knowledge, just two behavioral studies have examined whether saccade plans can influence hearing (Rorden and Driver, 1999; Lie and Coslett, 2006), with both reporting a positive outcome.
However, no study to date has examined the possible neural correlates of such an influence from saccade plans upon hearing, in humans. Here we applied ERP measures to examine whether oculomotor plans affect the processing of auditory stimuli. For comparison, we also implemented a conventional Covert Attention analogue of the Saccade task, to test if these would show similar or different ERP signatures. In both tasks, each trial contained two auditory stimuli (S1 and S2) separated by a stimulus onset asynchrony (SOA) of 1000 ms. S1 was a symbolic spatial cue, always presented from a central loudspeaker. S2 was an imperative stimulus that required participants to either execute or withhold a response.
In the Covert Attention task, S2 was always presented from a left or right loudspeaker. Participants were instructed to direct their covert auditory attention either to the left or right side as indicated by the pitch (high or low) of the auditory cue (S1), and to respond whenever an auditory target stimulus was presented as S2 at the cued side. They had to ignore auditory nontarget S2 stimuli on the cued side, as well as all S2 stimuli on the uncued side. Nontargets were continuous 100 ms bursts of white noise, while targets were gap stimuli that contained a 30 ms silent interval. In the Saccade task, participants were instructed to prepare a saccade towards a goal location indicated by markers on the left and right side (see Figure 1). Saccade direction was signalled by the pitch of the auditory cue (S1). Saccades had to be executed whenever S2 was presented from the central loudspeaker (go stimulus), but to be withheld whenever S2 was presented from the left or right loudspeaker (nogo stimuli).
Figure1.
Stimulus setup used in this study. Sounds were presented from any one of three loudspeakers. The squares located above each loudspeaker indicate the fixation point (above the central loudspeaker) or the goal locations for leftward or rightward saccades (above the left and right loudspeakers). Response keys were aligned with the central loudspeaker.
The two tasks were thus designed such that participants had to strategically shift auditory attention toward the cued side in the Covert Attention task, since performance of that task required this, but were given no incentive to do so in the Saccade task, as for that task the task-relevant go-stimulus (S2) was always delivered from the central loudspeaker, not peripherally on the cued side. Hence any spatially-specific impact of the saccade plan on ERP responses to a peripheral (no-go) S2 stimulus should reflect the saccadic relevance of the left versus right locations, rather than (as in the Covert Attention task) the auditory relevance of the left versus right sound-sources.
Analyses were conducted for ERPs time-locked to the symbolic central cue (S1), and separately for ERPs elicited in response to auditory S2 stimuli presented on the left or right side (nontarget S2 stimuli in the Covert Attention task and nogo S2 stimuli in the Saccade task). The goal of the first analysis was to identify and compare any lateralised ERP components (e.g. ADAN, LDAP) triggered during covert shifts of auditory spatial attention (Covert Attention task), versus during the preparation of eye movements (Saccade task). This initial analysis (of ERPs time-locked to S1) is analogous to recent ERP comparisons of visual attention and oculomotor preparation (Eimer et al., 2006; 2007; Van der Lubbe et al., 2006), but was now implemented specifically for an auditory rather than visual attention task (see also Green et al., 2005; Green and McDonald, 2006; Seiss et al., 2007, for a recent debate about whether both ADAN and LDAP components are triggered during shifts of unimodal auditory attention).
The second analysis (ERPs time-locked to peripheral auditory S2 stimuli) provides the very first test to our knowledge of whether oculomotor plans can affect human neural responses to sounds in the same way previously established for spatial attention. Endogenous shifts of auditory attention can result in enhanced negativity for sounds at attended locations, commencing on the descending flank of the auditory N1 component (cf., Alho, 1992; Eimer and Schröger, 1998). Typically auditory spatial attention effects show an initial centroparietal maximum, followed by a second more anteriorly distributed phase (cf., Schröger and Eimer, 1993). If saccadic preparation can affect processing of auditory stimuli (as implied behaviourally by Rorden and Driver, 1999; Lie and Coslett, 2006), and if such effects are neurally analogous to attentional modulation, then similar auditory N1 effects should be found here for the Saccade task as well as for the Covert Attention task. On the other hand, if saccade preparation has no such effects on auditory processing, then auditory N1 modulation should be found here only for the Covert Attention task, not the Saccade task.
Results
Behavioural performance
Manual RTs in the Covert Attention task and saccade RTs in the Saccade task did not differ significantly (447 vs. 458 ms, respectively, F<1). RTs did not differ as a function of cued side in the Covert Attention task (450 vs. 443 ms for left and right targets, F<1.5), nor for cued eye-movement direction in the Saccade task (461 vs. 455 ms for left and right eye movements, F<1). There was also no interaction between task and cued side (F<1). In the Covert Attention task, participants missed S2 gap stimuli (targets) on the cued side on 4.6% of such trials. False alarms to S2 gap stimuli on the uncued side were observed on 5% of those trials, and False alarms to S2 non-gap sounds were observed on 1.8% of all nontarget trials. In the Saccade task, participants failed to execute a saccade on 5.4% of all trials where the auditory S2 was presented from the central loudspeaker. Incorrect saccades (i.e. saccades towards the uncued side) were observed on 2.9% of these trials. False alarms (i.e., saccades on nogo trials) occurred on 2% of all trials where the auditory S2 was delivered from the left or right loudspeaker.
Lateralised ERP components in the S1-S2 interval, time-locked to the symbolic cue
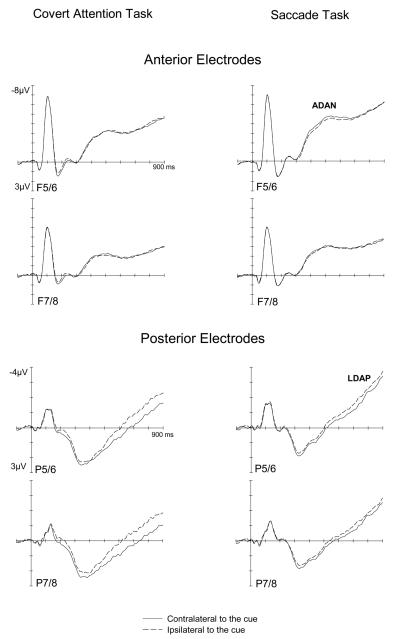
The top half of Figure 2 shows ERPs elicited during the S1-S2 interval in response to the symbolic cue, at lateral anterior electrodes ipsilateral and contralateral to the cued side, separately for the Covert Attention task (left panels) or Saccade task (right panels). The bottom half of Figure 2 shows analogous ERPs during the S1-S2 interval at lateral-posterior electrodes pairs. These figures suggest that the spatial information provided by the cue had systematic effects on ERPs elicited in the S1-S2 interval, similarly for both tasks. Starting about 300 ms after cue onset, ERPs were more negative at anterior electrodes contralateral to the cued side as compared to ipsilateral electrodes (Figure 2, top half). This lateralized component, previously described as anterior directing attention negativity (ADAN), was generally small in amplitude, in particular in the Covert Attention task. At posterior electrodes (Figure 2, bottom half), ERPs were more positive at electrodes contralateral to the cued side between 300 and 900 ms after cue onset, in line with the late directing attention positivity (LDAP) found in previous studies. This LDAP component was clearly present in both tasks, but was larger in the Covert Attention task (left panels) than in the Saccade task (right panels).
Figure 2.
Grand-averaged ERPs elicited during the S1-S2 interval in the 900 ms following onset of the symbolic cue (S1), relative to a 100 ms baseline, for anterior (top panel) and posterior (bottom panel) electrodes ipsilateral (dashed lines) or contralateral (solid lines) to the cued side, shown separately for the Covert Attention task (left) and the Saccade task (right).
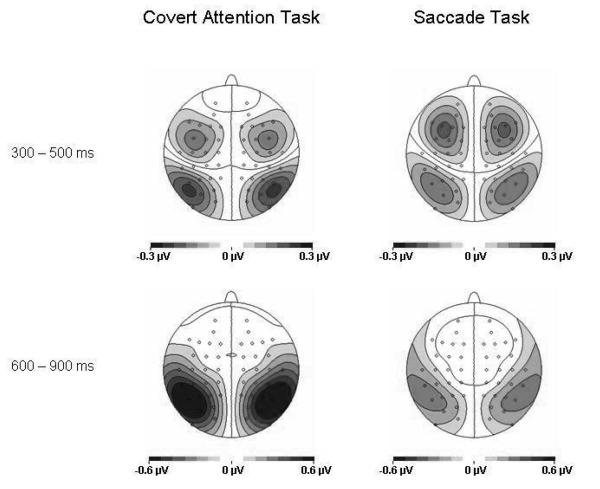
To further visualize the scalp distribution of these lateralized components, the voltage maps of the difference between ipsilateral and contralateral brain activity are shown in Figure 3, separately for the Covert Attention and Saccade tasks (left and right panels respectively). The lateralized activity induced by the cue in the 300-500 ms interval (top row) shows the ADAN component at anterior electrodes, as well as the early phase of the LDAP component at posterior electrodes. In the 600 – 900 ms time window, the maps suggest that the main phase of the LDAP component at posterior electrodes is larger for the Covert Attention relative to the Saccade task (note the different scales used for these two tasks during this time window).
Figure 3.
Topographical voltage maps of lateralized ERP components elicited in the S1-S2 interval in response to auditory spatial cues. Maps are shown separately for the 300-500 ms and 600-900 ms intervals after cue onset, separately for the Covert Attention task (left panels) and the Saccade task (right panel). They were computed by spherical spline interpolation (see Perrin et al., 1989) of difference waves obtained by subtracting ERPs at electrodes ipsilateral to the cued side from those at contralateral ERPs, and then mirroring the difference waveforms to the opposite hemisphere to obtain symmetrical, but inverse, voltage values for both hemispheres. Amplitude scales range from −0.3 to 0.3 μV for the 300-500 ms time interval, and from −0.6 to 0.6 μV for the 600-900 ms time interval.
These observations were confirmed by statistical analyses. In the 300-500 ms interval, a significant main effect of lateralization at lateral anterior electrodes (F(1,11)=10.9, p<.007) reflected the presence of a small but reliable ADAN component across both tasks, as shown in Figures 2 and 3. There was no task × lateralization interaction (F<1), suggesting that the ADAN amplitudes did not differ systematically between the Covert Attention and Saccade tasks. However, follow-up analyses conducted separately for both tasks found a statistically significant ADAN only in the Saccade task (F(1,11)=10.4, p<.008), but not for the Covert Attention task (F<1.5). At posterior electrode pairs, a significant main effect of lateralization (F(1,11)=6.8, p<.025), obtained in the 300-500 ms interval, indicated the presence of the early phase of the LDAP component. The absence of a significant task × lateralization interaction (F<1) suggested that this component was also elicited in an analogous fashion in both tasks. Follow-up analyses conducted separately for both tasks revealed a significant LDAP in the Covert Attention task (F(1,11)=5.6, p<.04), whereas the lateralization effect only approached significance in the Saccade task (F(1,11)=3.5, p=.09).
In the 600-900 ms interval, the presence of the LDAP component was reflected by a main effect of lateralization at lateral posterior electrodes sites (F(1,11)=22.5, p<.001). A significant interaction between task and lateralization (F(1,11)=6.6, p<.03) was also observed, due to the fact that LDAP amplitudes were larger in the Covert Attention than Saccade task (see Figures 2 and 3). Separate analysis conducted for each task revealed significant main effects of lateralization not only in the Covert Attention task (F(1,11)=17.8, p<.001), but also in the Saccade task (F(1,11)=11.7, p<.006), demonstrating that although the LDAP was attenuated in the Saccade task, it was still reliably present. At lateral central electrode sites, a main effect of lateralization was also present during the 600-900 ms interval (F(1,11)=7, p<.023), due to the fact that the LDAP component extended, albeit in an attenuated fashion, to lateral central electrodes (see Figure 3). There was no significant task × lateralisation interaction (F(1, 11)=3.2, p=.1).
In addition to the ADAN and LDAP components, Figure 2 (top panel) also suggests the presence of another early difference between contralateral and ipsilateral ERPs at lateral anterior electrodes in the Covert Attention task. During the 150 - 250 ms post-cue interval, ERPs were more positive at contralateral relative to ipsilateral electrodes in the Covert Attention task, but not the Saccade task. This was reflected by a significant interaction between task and lateralization at anterior electrodes (F(1,11)=6.2, p<.030). Follow-up analyses conducted for each task revealed a main effect of lateralization in the Covert attention task (F(1,11)=11.2, p<.006), but not in the Saccade task (F<1).
ERPs in response to lateral auditory S2 nontarget stimuli
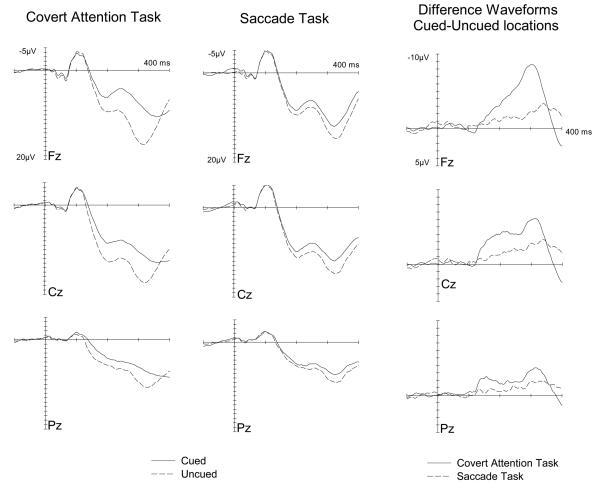
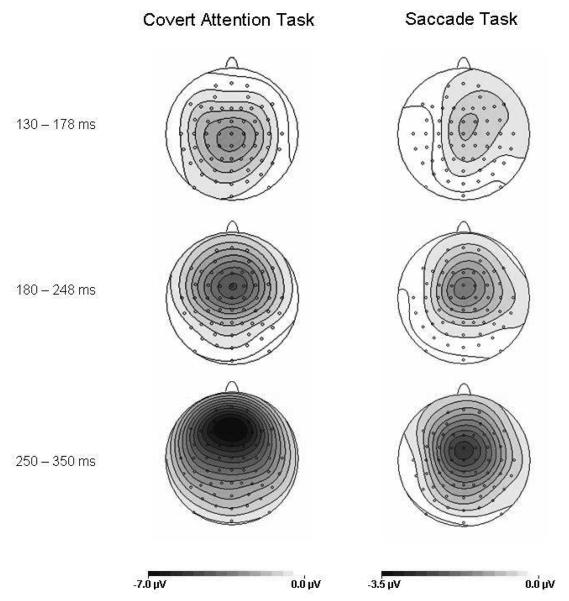
Figure 4 (left and central panels) shows midline ERPs elicited by auditory nontarget S2 stimuli presented from one of the two lateral loudspeakers in the Covert Attention task (left panels) or in the Saccade task (central panels), shown separately for stimuli on the cued or uncued side. The corresponding cued-minus-uncued difference waveforms are shown in Figure 4 (right panel, with solid versus dashed lines in those panels now differentiating the two tasks, rather than cued or uncued sides). Spatial cuing clearly had systematic effects on auditory ERPs in both tasks, with enhanced negativities in response to auditory stimuli at cued versus uncued locations with an onset latency of about 130 ms post-stimulus. The amplitude of these spatial cueing effects appears substantially larger in the Covert Attention task than in the Saccade task, but these effects appear present for both. To further illustrate the scalp topography of spatial cueing effects on auditory ERPs obtained in both tasks, Figure 5 shows maps of the cued-minus-uncued difference-values for three successive time intervals between 130 and 350 ms post-stimulus, separately for either task. Note the voltage-scale difference between the tasks, due to the fact that spatial cueing effects were larger in the Covert Attention task. In that task, spatial cueing effects on auditory ERPs were initially maximal at centroparietal midline electrodes, and later showed a frontocentral distribution. In the Saccade task, these effects appear maximal over central midline electrodes, both during the intermediate (180-248 ms) and late (250-350 ms) time window.
Figure 4.
Grand-averaged ERPs elicited in response to peripheral auditory S2 nontarget stimuli in the 500 ms following stimulus onset (relative to a 100 ms pre-stimulus baseline), at midline electrodes Fz, Cz and Pz. ERPs to stimuli presented on the cued side (solid line) or uncued side (dashed lines) are shown separately for the Covert Attention task (left panel) and the Saccade task (middle panel). The corresponding difference waveforms, obtained by subtracting the ERPs elicited by stimuli presented at uncued locations from ERPs elicited by stimuli presented at cued locations, are shown in the right panel, with solid versus dashed lines now indicating the Covert Attention and Saccade tasks, respectively.
Figure 5.
Topographical voltage maps of spatial cueing effects for auditory ERPs elicited in the Covert Attention task (left panel) and the Saccade task (right panel). Maps display the voltage distributions of difference amplitudes obtained by subtracting ERPs to auditory S2 nontarget stimuli presented on the uncued side from ERPs to stimuli on the cued side, for the 130-178 ms, 180-248 ms, and 250-350 ms intervals after stimulus onset, computed by spherical spline interpolation. Amplitude scales range between −7.0 to 0 μV for the Covert Attention task and −3.5 to 0 μV for the Saccade task.
For the early time window (80-120 ms) that was centred on the peak latency of the auditory N1 component, main effects of task were present at central and posterior electrode sites (both F(1, 11)>6.7, p<.03), due to the fact that N1 amplitudes elicited by auditory stimuli in the Saccade task were slightly enhanced relative to the Covert Attention task. However, there were no main effects of spatial cuing and no interactions between task and spatial cueing on N1 mean amplitudes at frontal, central, or posterior sites (all F<1), confirming that spatial cueing did not modulate N1 peak amplitudes in either task for this time window. Between 130 and 178 ms, main effects of spatial cuing now emerged at frontal, central and posterior sites (all F(1, 11)>12.4; all p<.005). While the task by spatial cuing interaction failed to reach significance at frontal electrode sites (F(1, 11)=2.9; p=.122), it was significant at central and posterior sites (both F(1, 11)>7.2; both p<.021). Follow-up analyses conducted separately for each task confirmed significant main effects of spatial cuing in the Covert Attention task at frontal, central and posterior sites (all F(1, 11)>9.7; all p<.01). In contrast, no significant spatial cueing effects were obtained at these electrode clusters in the Saccade task (all F(1, 11)<4.1; all p>.07).
In the subsequent analysis window (180-248 ms post-stimulus), significant main effects of spatial cuing were obtained at frontal and central electrodes sites (both F(1, 11)>20.7; all p<.001). They were accompanied by interactions between task and spatial cueing at these electrodes (both F(1, 11)>18,9; all p<.001), reflecting the fact that spatial cueing effects were smaller in the Saccade task relative to the Covert Attention task (see also Figure 4). However, follow-up analyses conducted separately for each task revealed that main effects of spatial cuing were present at frontal and central electrodes sites not only in the Covert Attention task (both F(1, 11)>30.3; all p<.001), but also in the Saccade task (both F(1, 11)>6.0; all p<.032). No reliable main effect of spatial cueing (F(1, 11)=3.7; p=.08) and no task × spatial cueing interaction (F(1, 11)=2.2; p=.17) was obtained at posterior electrodes.
In the final analysis window (250-350 ms post stimulus), main effects of spatial cuing were present at frontal, central and posterior electrodes sites (all F(1, 11)>30.5; all p<.001), reflecting enhanced negativities for auditory stimuli at cued versus uncued locations. The interaction between task and spatial cuing was significant at frontal and central electrodes (both F(1, 11)>8.9; both p<.012), due to the fact that spatial cueing effects remained more pronounced for the Covert Attention task than for the Saccade task (see Figure 4). This interaction almost reached significance at posterior electrode sites (F(1, 11)=4.3; p<.062). Follow-up analyses conducted separately for both tasks revealed reliable spatial cueing effects at frontal, central and posterior electrodes in the Covert Attention task (all F(1, 11)>18.5; all p<.001) as well as in the Saccade task (all F(1, 11)>12.3; all p<.004).
As can be seen from Figure 5, spatial cueing effects were maximal at midline electrodes in both tasks. To further explore possible differences in the anterior-posterior distribution of these effects between the two tasks, cued-minus-uncued difference amplitudes obtained from the nine midline electrodes used in this study were compared for each pair of adjacent electrodes, separately for both tasks. During the 130-178 ms time window, enhanced negativities for auditory stimuli at cued versus uncued locations showed a centroparietal maximum in the Covert Attention task (see Figure 5). Spatial cueing effects were significantly larger at Cz relative to FCz and at Pz relative to POz (both t(11)>2.3, all p<.04), but their amplitude did not differ between Cz, CPz and Pz (all t(11)<2, all p>.08). In the Saccade task, reliable spatial cueing effects were present during the 130-178 ms time window at FCz and Cz, whereas they only approached significance at CPz and Fz (see Table 1). However, there were no significant differences in the amplitude of these effects between FCz, Cz, CPz and Pz (all t(11)<1). Larger spatial cueing effects were found at FCz relative to Fz (t(11)=2.3, p<.043) and at Pz relative to POz (t(11)=2.4, p<.034).
Table 1.
| Task × Spatial cuing | Covert Attention Task | Saccade task | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 130-178 | 180-248 | 250-350 | 130-178 | 180-248 | 250-350 | 130-178 | 180-248 | 250-350 | |
| FPz | .793 | .016 | .000 | .786 | .001 | .000 | .260 | .140 | .034 |
| Fz | .331 | .001 | .001 | .036 | .000 | .000 | .116 | .024 | .008 |
| FCz | .044 | .000 | .005 | .007 | .000 | .000 | .033 | .013 | .002 |
| Cz | .006 | .000 | .011 | .002 | .000 | .000 | .045 | .025 | .001 |
| CPz | .006 | .003 | .017 | .001 | .001 | .000 | .081 | .051 | .001 |
| Pz | .023 | .112 | .096 | .002 | .036 | .001 | .160 | .159 | .004 |
| POz | .020 | .195 | .040 | .004 | .152 | .002 | .409 | .579 | .007 |
| Oz | .038 | .405 | .035 | .004 | .333 | .002 | .707 | .729 | .038 |
| Iz | .066 | .935 | .110 | .072 | .649 | .119 | .568 | .517 | .733 |
Reliability of spatial cuing effects as emerged from additional ANOVAs separately conducted for each midline electrode (FPz, Fz, FCz, Cz, CPz, Pz, POz, Oz, Iz) at three different time intervals (130-178 ms, 180-248 ms and 250-350 ms after stimulus onset). Left panel summarizes p-values for the task × spatial cueing interactions obtained in ANOVAs conducted across both tasks, with significant interactions reflecting larger spatial cueing effects in the Covert Attention relative to the Saccade task. P-values for main effects of spatial cueing obtained in ANOVAs conducted separately for Covert attention and Saccade tasks are shown in the middle and right panels respectively. For all analyses significant p-values are highlighted in gray.
During the subsequent 180-248 ms time window, spatial cueing effects had a frontocentral maximum for both tasks (see also Figure 5). In the Covert Attention task, the spatial cuing effect was significantly larger at Cz relative to the two adjacent midline electrodes (FCz and CPz, both t(11)>2.7, all p<.019). In the Saccade task, the amplitude of spatial cuing effects did not differ between FCz and Cz (t(11)=1.5, p=.155), but was significantly larger at FCz relative to Fz (t(11)=2.4, p<.033) and at Cz relative to Cpz (t(11)=2.4, p<.036). During the 250-350 ms time window, the distribution of spatial cueing effects differed across task, with a more anterior maximum for the Covert Attention relative to the Saccade task (see Figure 5). In the Covert Attention task, this effect now peaked at Fz, as confirmed by significant amplitude differences relative to the two adjacent midline electrodes FCz and FPz, (both t(11)>2.4; p<.038). In contrast, the spatial cueing effect remained focused over FCz and Cz in the Saccade task (no significant amplitude difference between these two electrodes: t(11)=1.4, p=.188), as it was reliably larger at FCz relative to Fz (t(11)=3.7, p<.004) and at Cz relative to Cpz (t(11)=3.5, p<.005).
Additional information about the reliability of spatial cueing effects at individual midline electrodes is provided by Table 1, which presents results from additional ANOVAs conducted separately for these electrodes. The left panel shows p-values for the task × spatial cueing interactions obtained in ANOVAs conducted across both tasks, with significant interactions reflecting larger spatial cueing effects in the Covert Attention relative to the Saccade task. The middle and right panels show p-values for main effects of spatial cueing obtained in ANOVAs conducted separately for both tasks.
Discussion
One aim of the present experiment was to determine, via ERPs, whether processing of auditory stimuli can be modulated in a spatially-specific fashion by covert saccade preparation (Saccade task), as suggested by previous behavioural studies (Rorden and Driver, 1999; Lie and Coslett, 2003). Possible influences of saccade preparation on hearing have received much less investigation than analogous influences on vision (see Introduction). For comparison, in a separate task we also manipulated endogenous covert auditory spatial attention per se (Covert Attention task). In both tasks, we examined ERPs time-locked to the symbolic cue (S1) indicating which side to attend to auditorily, or which side to prepare a saccade towards, and also ERPs time-locked to a subsequent peripheral sound on one or other side. This allowed us to directly compared ERPs indicative of covert shifts of auditory spatial attention or of covert saccade preparation. In the Covert Attention task, the central symbolic auditory cue (S1) indicated the direction of a covert endogenous attentional shift for an upcoming auditory target-nontarget (S2) discrimination. In the Saccade task, a comparable cue (S1) signalled the direction of saccade plan that was subsequently to be executed or withheld in response to a subsequently presented auditory go-nogo stimulus (S2).
The ERP results obtained in response to auditory cues in the Saccade task confirmed that lateralised components previously observed during cued shifts of covert spatial attention (ADAN, LDAP) can also be observed during eye movement preparation (see also Eimer et al., 2006; 2007; Van der Lubbe et al., 2006, for similar results). The ADAN emerged between 300 and 500 ms after cue onset at anterior electrodes contralateral to the direction of a cued eye movement. The posterior LDAP already approached significance during this time window, and reached its maximum during the later phase of the S1-S2 interval (see Figure 2 and 3). The fact that these components that are usually associated with covert attentional orienting were elicited during the preparation of saccadic eye movements provides further evidence for a relatively close link between attentional and oculomotor control systems, as expected by the premotor theory of attention (Rizzolatti et al., 1987, 1994). Similar lateralised effects were observed during the S1-S2 interval in the Covert Attention task. A pronounced posterior LDAP was obtained the 600-900 ms post-cue measurement window, and this component was already reliably present during the earlier 300-500 ms measurement interval (see Figure 2). In contrast, the ADAN component was very small in amplitude in the Covert Attention task. Even though there was no significant interaction between lateralization and task for the ADAN, suggesting that ADAN amplitudes did not differ systematically across both tasks, this component was significantly present only in the Saccade task, but not in the Covert Attention task. The absence of a reliable ADAN in this task contrasts with the presence of a small but significant ADAN in the context of a purely unimodal auditory attention task (Seiss et al., 2007; but see also Green et al., 2005). The strong attenuation of this component in the present Covert Attention task may be due to the fact that the posterior LDAP was already reliably present during the early 300-500 ms time window in this task (see Figures 2 and 3). Scalp-recorded ERPs can be modulated by the volume conduction of currents that originate from remote brain regions. When two lateralised ERP components of opposite polarity (such as ADAN and LDAP) are triggered simultaneously at anterior and posterior sites, volume conduction of source currents from anterior to posterior brain regions, or vice versa, can result in an attenuation of component amplitudes.
While ADAN amplitudes did not differ reliably between the two tasks, posterior LDAP amplitudes were significantly larger in the Covert Attention than in the Saccade task. This attenuation of the LDAP during saccade preparation relative to covert spatial orienting accords with previous ERPs studies making a similar comparison but in a visual rather than auditory context (Eimer et al., 2006; 2007; Van der Lubbe et al., 2006). Given that this difference has been further confirmed by the present experiment, it can now be regarded as a reliable and replicable finding. This enhancement of LDAP amplitudes during covert attentional orienting, as compared to saccade programming, might be linked to general differences in task demands between the attention and saccade preparation tasks used in the current and in previous ERP experiments. In the Covert Attention task, participants had to make a target-nontarget discrimination at the cued location, while no such discrimination between laterally presented stimuli was required in the Saccade task. It is possible that the enhanced LDAP amplitudes observed during covert attentional orienting reflect these spatially specific processing requirements in the former task. If so, this may suggest that the neural basis of covert attention shifts and saccade preparation, as reflected by lateralised ERP components, are not strictly identical, but only partially overlapping, as the LDAP may be more sensitive to the specific perceptual requirements of the Covert Attention task.
In addition to the ADAN and LDAP, an additional earlier ERP modulation was observed at lateral anterior electrodes, but only for the Covert Attention task. Here, an enhanced positivity was observed at electrodes contralateral to the direction of a cued shift of auditory attention relative to ipsilateral electrodes (see Figure 2). Because this early lateralization has not been reported in previous studies investigating ERP markers of cued attention shifts, its presence needs to be confirmed in future experiments before it can be interpreted as a new ERP correlate of auditory attention shifts.
A novel question in the present study was whether covert saccade preparation can lead to modulations of auditory processing, and if so whether such modulations are similar to the effects observed as a result of endogenous covert shifts of auditory attention. Recent ERP studies (e.g. Eimer et al., 2006; 2007) have demonstrated that saccade preparation can results in spatially-specific effects on ERPs to task-irrelevant visual probe stimuli, suggesting close links between oculomotor programming and modulation of visual processing. If these effects were mediated by modality-unspecific attentional control mechanisms that are triggered not just during covert orienting, but also during eye movement preparation, such spatially selective modulations of sensory processing should be observed not just for visual stimuli, but also in response to auditory events, as tested here.
This was assessed by comparing ERPs to lateral auditory S2 stimuli at cued versus uncued locations, separately for the Covert Attention and Saccade tasks. In the former, auditory (nontarget) S2 stimuli on the cued side elicited enhanced negativities relative to those on the uncued side. Consistent with previous ERP studies on auditory spatial attention (e.g. Alho, 1992; Eimer and Schröger, 1998; Teder-Sälejärvi et al., 1999), this attentional modulation started on the descending flank of the auditory N1 component. It was initially maximal at centroparietal electrodes, and then shifted to more anterior sites (Figure 5; see also Schröger and Eimer, 1993, for similar results). The critical new question was whether analogous ERP modulations would also be elicited due solely to covert saccade preparation, in spite of the fact that in the Saccade task participants were motivated to keep any focus of auditory attention on the central location, as go stimuli were (like the cues) always centrally presented. One could still argue that the peripheral sounds were also relevant for the Saccade task, since they signalled that no eye movement was to be executed on a specific trial. However, and importantly, these nogo sounds were equally likely to be presented as nogo stimuli on the side cued for eye movement preparation, and on the opposite uncued side. Participants were informed of these probabilities, and therefore had no incentive to intentionally shift auditory attention towards the cued side.
ERP results for peripheral sounds (nogo S2 stimuli) in the Saccade task were clear-cut. Saccade preparation had systematic and reliable effects on auditory ERPs, which were similar, albeit significantly smaller, than the effects observed in the Covert Attention task (see Figure 4). These findings provide new ERP evidence that covert saccade preparation can have spatially specific effects on auditory processing. They suggests not only that eye movement preparation is similar in some respects to attention shifts, but also that saccade plans can result in modality-unspecific modulations of sensory processing, affecting not only vision (Eimer et al., 2006; 2007) but also hearing, as shown for the first time with ERPs here.
The presence of enhanced negativities for auditory stimuli on the cued versus uncued side in the Saccade task accords with the rare previous behavioural studies showing that the direction of an upcoming saccade can affect hearing (Rorden and Driver, 1999; Lie and Coslett, 2006), as well as with a single cell study demonstrating that upcoming eye movements can modulate neural firing to auditory stimuli (Hikosaka and Wurtz, 1983). However, in spite of the analogy between spatial cueing effects on auditory ERPs for the two tasks used here, there were also some notable differences. Attentional modulations of auditory ERPs in the Covert Attention task started earlier and were substantially larger in amplitude than the spatial cueing effects observed in the Saccade task. Topographical maps (Figure 5) showed that the early centroparietal maximum of the spatial cueing effect observed for the Covert Attention task was largely absent in the Saccade task, and that the later phase of this effect had a more anterior focus in the Covert Attention relative to the Saccade task.
These observations suggest that although the spatially specific modulations of auditory ERPs triggered in the two tasks were similar, they were clearly not identical. The fact that spatial cueing effects were attenuated in the Saccade task may relate to differences in S2 processing demands between the two tasks. Lateral auditory S2 stimuli were always nogo stimuli in the Saccade task, regardless of whether they were presented at the cued or uncued side. In contrast, an auditory S2 at the cued side (but not the uncued side) in the Covert Attention task had to be further analysed in order to determine whether it was a target (gap) or a nontarget (non-gap) stimulus. This consideration may explain the larger spatial cueing effects in the Covert Attention task, but makes it all the more remarkable that significant spatial effects were found at all on auditory ERPs in the Saccade task, given that the task-relevance of peripheral sounds was equivalent on either side in the latter task.
Overall, the present findings demonstrate that covert saccade preparation can modulate auditory processing in a spatially specific fashion, even if this modulation is reduced as compared to the effects observed as a result of endogenous auditory attention. It is notable that a similar pattern of results was found in one of our recent ERPs studies, where we likewise directly compared the impact of saccade preparation and covert attentional orienting on ERPs, but in response to lateral visual nontarget stimuli (Eimer et al., 2007), rather than auditory as here. Our new finding that oculomotor plans trigger spatially specific modulations not only of visual but also of auditory processing suggests that modality-unspecific (crossmodal) spatial modulations can be elicited by saccade preparation. Future experiments will have to address the question whether similar links also exist between eye movement preparation and the processing of tactile events (see Rorden et al., 2002, for behavioural evidence for such a link).
In this context, it may be relevant to note that the premotor theory did not only postulate links between covert attention and oculomotor control, but also between attention and other types of actions, such as manual responses (Rizzolatti et al., 1994). We have shown recently that covert preparation of a left or right hand movement can result in spatially-specific modulations of ERPs to task-irrelevant tactile (Eimer et al., 2005) or visual (Eimer and Van Velzen, 2006) stimuli. However, no such effects of unimanual response preparation were present for auditory ERPs (Eimer and Van Velzen, 2006). We had previously suggested that this absence of response preparation effects on auditory ERPs might be due to the generally poorer spatial resolution of audition relative to vision or touch. However given the clear-cut auditory ERP modulations observed in the present experiment as a result of saccade preparation, this possibility can now be dismissed. As an alternative, the lack of manual response preparation effects on auditory ERPs might be related to the fact that auditory information is less relevant than visual and somatosensory information for the on-line control of manual response parameters, while saccade preparation may yield such effects because eye movements are readily triggered by peripheral sounds. Links between response preparation, attention shifts, and modulation of sensory stimulus processing may be constrained by the relative importance of different sensory modalities for the control of specific types of actions. In this respect, it might be interesting also to examine covert preparation of head movements in future studies, given that such movements can be of primary importance for audition, and also of some relevance for vision (e.g. in changing the possible field of view), while arguably having less relevance for touch.
In conclusion, the present study has confirmed recent electrophysiological findings suggesting that shifts of spatial attention are elicited during covert saccade preparation and has provided the first ERP evidence that auditory processing can be modulated in a spatially-selective fashion as a result of saccade preparation. Overall, these results support the premotor theory of attention, and extend the claims of this theory by demonstrating that attention shifts elicited during eye movement preparation affect the processing not only of visual but also of auditory information.
Experimental Procedure
Participants
Fourteen paid volunteers participated. One was excluded because of poor fixation in the cue-target interval (see below) and another due to a large number of eye blinks. Thus twelve participants (8 females), aged 18-36 years (mean 27.7) remained in the sample. Eleven were right-handed, and all reported normal or corrected vision and normal hearing.
Stimuli and Apparatus
The experimental setup is shown in Figure 1. Participants sat in a dimly lit experimental chamber facing a beige cardboard panel (90 cm × 60 cm) at a viewing distance of 57 cm. Three loudspeakers were mounted on the panel, horizontally aligned at an angle of about 30° below eye level. One loudspeaker was located in the centre of the panel, the other two at 40 cm (~40 °) to left and right of the centre. A red square (1 × 1 cm) placed in the centre of the panel (2 cm above the centre of the central loudspeaker) served as the fixation point. To specifically mark saccade target locations, two additional red squares (1 × 1 cm) were used, each placed 2 cm above the centre of one of the peripheral loudspeakers, horizontally aligned with the fixation square. One response key was placed on the table in front of the participants, aligned with the central loudspeaker, to record manual responses in the Covert Attention task.
Central symbolic auditory cues (S1) were high or low tones (500 or 2000 Hz at 72 dB SPL) presented for 100 ms from the central loudspeaker. On each trial, a second sound (S2) was presented from one of the three loudspeakers. This sound was a 100 ms burst of white noise (20 ms rise and fall times, 70 db SPL), which could either be continuous (non-gap sound) or contain a 30 ms silent gap that started 35 ms after sound onset (gap sound).
Procedure
The experiment consisted of 16 experimental blocks with 80 trials per block. Each trial started with an auditory cue stimulus (S1, 100 ms duration) from the central loudspeaker, followed after an interval of 900 ms by a second auditory stimulus (S2, 100 ms duration) from one of the three loudspeakers. Inter-trial interval was 1800 ms. Two tasks (Covert Attention task, Saccade task) were delivered, each consisting of eight successive blocks. The order in which these tasks were delivered was counterbalanced across participants. In the Covert Attention task, participants were instructed to direct their auditory spatial attention to the side indicated by the central symbolic cue (with mapping of high/low cues to left/right side, or vice versa, counterbalanced across participants), and to respond manually (by pressing a key) whenever an auditory S2 target stimulus (with a gap) was presented at the cued side. S2 nontargets (‘non-gap’ sounds) on the cued side, as well as all auditory S2 stimuli (gap and non-gap) on the uncued side were simply to be ignored. Central fixation had to be maintained throughout each trial. Participants responded to target sounds on the cued side, using their right or left index finger in four successive blocks, with order of response-hand balanced across participants.
Each experimental block contained 48 trials with auditory nontargets (non-gap stimuli) presented as S2, comprising twelve trials per block for each combination of cue direction (left versus right) and auditory stimulus side (left versus right). Target S2 stimuli (with a gap) were presented in the remaining 32 trials per block. Twenty-four of these targets were delivered on the cued side (twelve left, twelve right) and thus required a manual response. On eight trials per block, auditory gap-stimuli appeared on the uncued side (four left, four right), and no response was required on these trials.
In the Saccade task, S2 stimuli were always non-gap sounds. Participants were instructed to prepare a saccade towards the side indicated by the symbolic cue S1 (with cue frequency-side mappings identical to the Covert attention task), and to execute this saccade whenever the S2 stimulus was presented from the central loudspeaker (go trials). They had to maintain central fixation on all trials where S2 was presented from elsewhere (from a peripheral loudspeaker) instead (nogo trials). In order to keep the number of response trials as well as the total number of trials per block identical across both tasks, experimental blocks in the Saccade task contained 24 trials where the auditory S2 stimulus was presented centrally and thus required execution of the prepared eye movement (12 trials for cued leftwards and rightwards saccades, respectively). In the remaining 56 nogo trials, S2 was presented with equal probability from the left or right loudspeaker (14 trials for each combination of cued side and auditory stimulus side).
Participants were instructed to use the information provided by the cue either to direct their auditory attention to the cued location (Covert Attention task), or to prepare an eye movement in the cued direction (in the Saccade task) in order to respond as quickly and accurately as possible when a response-relevant auditory S2 stimulus was presented, while withholding responses to all other S2 stimuli. They were explicitly encouraged to maintain central eye fixation in the cue–target interval. Several training blocks were run prior to the beginning of each task. Eye movements were closely monitored during these training blocks. Whenever the horizontal EOG revealed that participants did not maintain central eye fixation, additional training blocks were run until fixation was regarded as satisfactory.
Recording and Data Analysis
EEG was DC-recorded from 63 Ag-AgCl electrodes (AF3, AF4, C1, C2, C3, C4, C5, C6, CP1, CP2, CP3, CP4, CP5, CP6, CPz, Cz, F1, F2, F3, F4, F5, F6, F7, F8, FC1, FC2, FC3, FC4, FC5, FC6, FCz, FP1, FP2, FPz, Fz, Iz, O1, O2, Oz, P1, P10, P2, P3, P4, P5, P6, P7, P8, P9, PO10, PO3, PO4, PO7, PO8, PO9, POz, Pz, T7, T8, TP7, TP8) relative to a left earlobe reference. Horizontal EOG was recorded unipolarly from the outer canthi of both eyes. Electrode impedance was kept below 5 kΩ, and impedances of the earlobe electrodes were kept as equal as possible. Data were recorded with an upper cutoff filter of 40 Hz. EEG and EOG were sampled with a digitization rate of 500 Hz and stored on disk. No additional filters were applied after recording. EEG was digitally re-referenced to the average of the left and right earlobe.
EEG and EOG were epoched offline into 1600 ms periods, starting 100 ms prior to the onset of the cue sound (S1), and ending 500 ms after onset of S2. Separate averages were computed for ERPs time-locked to cues in the S1-S2 interval (relative to a 100 ms baseline preceding cue onset) and for ERPs time-locked to the subsequent S2 stimulus (relative to a 100 ms baseline preceding that onset). ERPs to auditory S2 stimuli were computed only for nontargets trials in the Covert attention task and for no-go trials in the Saccade task, to preclude any contamination by manual responses or eye movements, respectively. Rare trials with manual responses to nontarget S2 sounds in the Covert Attention task, or with eye movement responses to nogo S2 stimuli in the Saccade task, were excluded from analysis, as were trials with eyeblinks (Fpz exceeding ±60 μV relative to baseline), horizontal eye movements (HEOG exceeding ±30 μV relative to baseline), or other artefacts (voltage exceeding ±80 μV at any electrode location relative to baseline). To detect any systematic deviations of eye position indicating residual tendencies to shift the eyes slightly toward the cued location, averaged waveforms in the cue-target interval in response to left versus right cues were examined for each participant, separately for both tasks. One participant showed a residual HEOG deviation exceeding ±3 μV, and was therefore excluded from further analyses.
EEG obtained in the cue target-interval was averaged for all combinations of task (Covert Attention or Saccade) and cue direction (left or right). Mean amplitude values were computed at lateral anterior sites (F3/4, F5/6, F7/8, FC3/4, FC5/6), lateral central sites (C3/4, C5/6, T7/8, CP3/4, CP5/6) and lateral posterior sites (P3/4, P5/6, P7/8, PO3/4, PO7/8) for two successive pre-defined time windows, between 300-500 ms (where the ADAN was previously observed) and between 600-900 ms after cue onset (where the LDAP was previously observed). Mean amplitudes were analysed by repeated measures ANOVAs with the factors of electrode site, task, lateralization (electrode ipsilateral versus contralateral to the cued side), and cued side (left / right). Additional analyses were conducted separately for the Covert Attention and Saccade tasks, where appropriate. In these analyses, the presence of ERP lateralizations sensitive to the side of a cued attention shift (or the side of a prepared eye movement) are reflected by main effects of the lateralization factor.
ERPs elicited by auditory S2 stimuli were separately averaged for all combinations of task (Covert Attention or Saccade), cued side (left or right), and S2 location (left or right). Mean amplitude values were computed within two early post-stimulus latency windows centred on the peak (80-120 ms) and on the descending flank (130-178 ms) of the auditory N1 component, as well as within two subsequent time windows (180-248 and 250-350 ms post stimulus). Mean amplitude values obtained at frontal (F1, F2, Fz, FCz, FC1, FC2), central (C1, C2, Cz, CPz, CP1, CP2) and posterior (P1, P2, Pz, POz, PO3, PO4) electrodes were submitted to separate ANOVAs. An additional analysis was carried out on midline electrodes (FPz, Fz, FCz, Cz, CPz, Pz, POz, Oz, Iz) to compare scalp distribution of spatial cueing effects on auditory ERPs across the two tasks. All analyses included the factors of task, spatial cuing (S2 presented at cued versus uncued location) and electrode site. For all analyses, Green-house-Geisser adjustments to degrees of freedom were applied where appropriate. Preliminary analyses were conducted for ERPs elicited in the cue-target interval and for ERPs in response to auditory S2 stimuli with task order (Covert Attention task followed by Saccade task, or vice versa) as an additional between-subject factor. Because no significant main effects or interactions involving the factor task order were found, this factor was not included in the subsequent analyses.
Saccade onset latencies in the Saccade task were measured on the basis of HEOG waveforms recorded after auditory S2 onset. Saccade onset was defined as the latency (in ms poststimulus) of the first datapoint within this interval exceeding a threshold of ±100 μV (relative to a 100 ms pre-stimulus baseline), with saccade direction (left or right) indicated by the polarity of this value. Saccadic RTs (and manual RTs for the Covert Attention task) were only analysed for correct response trials when a response-relevant auditory S2 stimulus had been presented.
Acknowledgements
This research was supported by the Medical Research Council (UK). ME holds a Royal Society Wolfson Research Merit Award, JD is a Royal Society Leverhulme Trust Senior Research Fellow. The authors thank Alison Eardley, Monika Kiss and Ellen Seiss for technical assistance.
References
- Alho K. Selective attention in auditory processing as reflected by event-related brain potentials. Psychophysiology. 1992;29:247–63. doi: 10.1111/j.1469-8986.1992.tb01695.x. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Essick GK, Siegel RM. Neurons of area 7 activated by both visual stimuli and oculomotor behavior. Exp. Brain Res. 1987;67:316–22. doi: 10.1007/BF00248552. [DOI] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M. Functional Organization of Human Intraparietal and Frontal Cortex for Attending, Looking, and Pointing. J. Neurosci. 2003;23:4689–4699. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Petit L, Ellmore TM, Ingeholm J, Haxby JV. A parametric fMRI study of overt and covert shifts of visuospatial attention. NeuroImage. 2001;14:310–321. doi: 10.1006/nimg.2001.0788. [DOI] [PubMed] [Google Scholar]
- Bizzi E. Discharge of frontal eye field neurons during saccadic and following eye movements in unanesthetized monkeys. Exp. Brain Res. 1968;6:69–80. doi: 10.1007/BF00235447. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME, Bushnell MC, Stanton GB. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J. Neurophysiol. 1985;54:714–734. doi: 10.1152/jn.1985.54.3.714. [DOI] [PubMed] [Google Scholar]
- Bushnell MC, Goldberg ME, Robinson DL. Behavioural enhancement of visual responses in monkey cerebral cortex. I. modulation in posterior parietal cortex related to selective attention. J. Neurophysiol. 1981;46:755–772. doi: 10.1152/jn.1981.46.4.755. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. J. Neurophysiol. 1996;76:2841–2852. doi: 10.1152/jn.1996.76.5.2841. [DOI] [PubMed] [Google Scholar]
- Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: Identical, independent, or overlapping neural systems? Proc. Natl. Acad. Sci. U. S. A. 1998;95:831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Deubel H, Schneider WX. Saccade target selection and object recognition: evidence for a common attentional mechanism. Vision Res. 1996;36:1827–1837. doi: 10.1016/0042-6989(95)00294-4. [DOI] [PubMed] [Google Scholar]
- Eimer M, Schröger E. ERP effects of intermodal attention and cross-modal links in spatial attention. Psychophysiology. 1998;35:313–27. doi: 10.1017/s004857729897086x. [DOI] [PubMed] [Google Scholar]
- Eimer M, Forster B, Van Velzen J, Prabhu G. Covert manual response preparation triggers attentional shifts: ERP evidence for the premotor theory of attention. Neuropsychologia. 2005;43:957–966. doi: 10.1016/j.neuropsychologia.2004.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M, Van Velzen J. Covert manual response preparation triggers attentional modulations of visual but not auditory processing. Clin. Neurophysiol. 2006;117:1063–74. doi: 10.1016/j.clinph.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Eimer M, Van Velzen J, Driver J. Cross-modal interactions between audition, touch, and vision in endogenous spatial attention: ERP evidence on preparatory states and sensory modulations. J. Cogn. Neurosci. 2002;14:254–271. doi: 10.1162/089892902317236885. [DOI] [PubMed] [Google Scholar]
- Eimer M, Van Velzen J, Forster B, Driver J. Shifts of attention in light and in darkness: an ERP study of supramodal attentional control and crossmodal links in spatial attention. Cogn. Brain Res. 2003;15:308–323. doi: 10.1016/s0926-6410(02)00203-3. [DOI] [PubMed] [Google Scholar]
- Eimer M, Van Velzen J, Gherri E, Press C. Manual response preparation and saccade programming are linked to attention shifts: ERP evidence for covert attentional orienting and spatially specific modulations of visual processing. Brain Res. 2006;1105:7–19. doi: 10.1016/j.brainres.2005.10.060. [DOI] [PubMed] [Google Scholar]
- Eimer M, Van Velzen J, Gherri E, Press C. ERP correlates of shared control mechanisms involved in saccade preparation and in covert attention. Brain Res. 2007;1135:154–166. doi: 10.1016/j.brainres.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen CW, Colegate RL. Selective attention and serial processing in briefly presented visual displays. Percept. Psychophys. 1971;10:321–326. [Google Scholar]
- Eriksen CW, Hoffman JE. Temporal and spatial characteristics of selective encoding from visual displays. Percept. Psychophys. 1972;11:301–204. [Google Scholar]
- Goldberg ME, Bushnell MC. Behavioral enhancement of visual responses in monkey cerebral cortex. II. Modulation in frontal eye fields specifically related to saccades. J. Neurophysiol. 1981;46:773–787. doi: 10.1152/jn.1981.46.4.773. [DOI] [PubMed] [Google Scholar]
- Gopher D. Eye-movement patterns in selective listening task of focused attention. Percept. Psychophys. 1973;14:259–264. [Google Scholar]
- Green JJ, McDonald JJ. An event-related brain potential study of supramodal attentional control and crossmodal attention effects. Psychophysiology. 2006;43:161–171. doi: 10.1111/j.1469-8986.2006.00394.x. [DOI] [PubMed] [Google Scholar]
- Green JJ, Teder-Sälejärvi WA, McDonald JJ. Control mechanisms mediating shifts of attention in auditory and visual space: a spatio-temporal ERP analysis. Exp. Brain Res. 2005;166:358–69. doi: 10.1007/s00221-005-2377-8. [DOI] [PubMed] [Google Scholar]
- Grosbras MH, Paus T. Transcranial magnetic stimulation of the frontal eye-field: effects on visual perception and attention. J. Cog. Neurosci. 2002;14:1109–1120. doi: 10.1162/089892902320474553. [DOI] [PubMed] [Google Scholar]
- Henderson JM, Pollatsek A, Rayner K. Covert visual attention and extrafoveal information use during object identification. Percept. Psychophys. 1989;45:196–208. doi: 10.3758/bf03210697. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. I. Relation of visual and auditory responses to saccades. J. Neurophysiol. 1983;49:1230–53. doi: 10.1152/jn.1983.49.5.1230. [DOI] [PubMed] [Google Scholar]
- Hoffman JE, Subramaniam B. The role of visual attention in saccadic eye movements. Percept. Psychophys. 1995;57:787–795. doi: 10.3758/bf03206794. [DOI] [PubMed] [Google Scholar]
- Hublet C, Morais J, Bertelson P. Spatial constraints on focused attention: beyond the right-side advantage. Perception. 1976;5:3–8. doi: 10.1068/p050003. [DOI] [PubMed] [Google Scholar]
- Hublet C, Morais J, Bertelson P. Spatial effects in speech perception in the absence of spatial competition. Perception. 1977;6:461–466. doi: 10.1068/p060461. [DOI] [PubMed] [Google Scholar]
- Irwin DE, Gordon RD. Eye movements, attention, and transsaccadic memory. Vis. Cogn. 1998;5:127–155. [Google Scholar]
- Jones B, Kabanoff B. Eye movements in auditory space perception. Percept. Psychophys. 1975;17:241–245. [Google Scholar]
- Kowler E, Anderson E, Dosher B, Blaser E. The role of attention in the programming of saccades. Vision Res. 1995;35:1897–1916. doi: 10.1016/0042-6989(94)00279-u. [DOI] [PubMed] [Google Scholar]
- Lie E, Coslett HB. The Effect of Gaze Direction on Sound Localization in Brain-Injured and Normal Adults. Exp. Brain Res. 2006;168:322–336. doi: 10.1007/s00221-005-0100-4. [DOI] [PubMed] [Google Scholar]
- Mathews S, Dean PJ, Sterr A. EEG dipole analysis of motor-priming foreperiod activity reveals separate sources for motor and spatial attention components. Clin. Neurophysiol. 2006;117:2675–83. doi: 10.1016/j.clinph.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Mazzoni P, Bracewell RM, Barash S, Andersen RA. Spatially tuned auditory responses in area LIP of macaques performing delayed memory saccades to acoustic targets. J. Neurophysiol. 1996;75:1233–1241. doi: 10.1152/jn.1996.75.3.1233. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM, Fallah M. Visuomotor origins of covert spatial attention. Neuron. 2003;40:671–683. doi: 10.1016/s0896-6273(03)00716-5. [DOI] [PubMed] [Google Scholar]
- Moore T, Fallah M. Control of eye movements and spatial attention. Proc. Natl. Acad. Sci. U. S. A. 2001;9:1273–1276. doi: 10.1073/pnas.021549498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais J, Cary L, Vanhaelen H, Bertelson P. Postural determinants of frontal position advantage in listening to speech. Percept. Psychophys. 1980;27:141–48. doi: 10.3758/bf03204302. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Lynch JC, Georgopoulos A, Sakata H, Acuna C. Posterior parietal association cortex of the monkey: command function for operations within extrapersonal space. J. Neurophysiol. 1975;38:871–908. doi: 10.1152/jn.1975.38.4.871. [DOI] [PubMed] [Google Scholar]
- Muggleton NG, Juan CH, Cowey AZ, Walsh V. Human frontal eye fields and visual search. J. Neurophysiol. 2003;89:3340–3343. doi: 10.1152/jn.01086.2002. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier J. Spherical splines for scalp potential and current density mapping. Electroencephal. Clin. Neurophysiol. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Zeki S. The neurology of saccades and covert shifts in spatial attention: an event-related fMRI study. Brain. 2000;123:2273–2288. doi: 10.1093/brain/123.11.2273. [DOI] [PubMed] [Google Scholar]
- Posner MI, Snyder CRR, Davidson BJ. Attention and the detection of signals. J. Exp. Psychol. Gen. 1980;109:160–174. [PubMed] [Google Scholar]
- Praamstra P, Boutsen L, Humphreys GW. Frontoparietal control of spatial attention and motor intention in human EEG. J. Neurophysiol. 2005;94:764–774. doi: 10.1152/jn.01052.2004. [DOI] [PubMed] [Google Scholar]
- Reisberg D, Scheiber R, Potemken L. Eye position and the control of auditory attention. J. Exp. Psychol. Hum. Percept. Perform. 1981;7:318–323. doi: 10.1037//0096-1523.7.2.318. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G. Mechanisms of selective attention in mammals. In: Ewert JP, editor. Advances in Vertebrate Neuroethology. Plenum; London: 1983. p. 261. [Google Scholar]
- Rizzolatti G, Camarda R. Neural circuits for spatial attention and unilateral neglect. In: Jeannerod M, editor. Neurophysiological and neuropsychological aspects of spatial neglect. Amserdam; North-Holland: 1987. pp. 289–313. [Google Scholar]
- Rizzolatti G, Riggio L, Dascola I, Umilta C. Reorienting attention across the horizontal and vertical meridians: evidence in favor of a premotor theory of attention. Neuropsychologia. 1987;25:31–40. doi: 10.1016/0028-3932(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Sheliga B. Space and selective attention. In: Umilta C, Moscovitch M, editors. Attention and Performance. XV. MIT Press; Cambridge, MA: 1994. pp. 231–265. [Google Scholar]
- Robinson DL, Goldberg ME, Stanton GB. Parietal association cortex in the primate: sensory mechanisms and behavioural modulation. J. Neurophysiol. 1978;41:910–932. doi: 10.1152/jn.1978.41.4.910. [DOI] [PubMed] [Google Scholar]
- Rorden C, Driver J. Does auditory attention shift in the direction of an upcoming saccade? Neuropsychologia. 1999;37:357–377. doi: 10.1016/s0028-3932(98)00072-4. [DOI] [PubMed] [Google Scholar]
- Rorden C, Greene K, Sasine G, Baylis G. Enhanced tactile performance at the destination of an upcoming saccade. Curr. Biol. 2002;20:1429–1434. doi: 10.1016/s0960-9822(02)01039-4. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes J-D, Rees G, Josephs O, Deichmann R, Driver J. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr. Biol. 2006;16:1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Russo GS, Bruce CJ. Frontal eye field activity preceding aurally guided saccades. J. Neurophysiol. 1994;71:1250–3. doi: 10.1152/jn.1994.71.3.1250. [DOI] [PubMed] [Google Scholar]
- Schall JD, Morel A, King DJ, Bullier J. Topography of visual cortex connections with frontal eye field in macaque: convergence and segregation of processing streams. J. Neurosci. 1995;15:4464–87. doi: 10.1523/JNEUROSCI.15-06-04464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröger E, Eimer M. Effects of lateralized cues on the processing of lateralized auditory stimuli. Biol. Psychol. 1993;28:203–26. doi: 10.1016/0301-0511(96)05192-7. [DOI] [PubMed] [Google Scholar]
- Seiss E, Gherri E, Eardley AF, Eimer M. Do ERP components triggered during attentional orienting represent supramodal attentional control? Psychophysiology. 2007;44:987–90. doi: 10.1111/j.1469-8986.2007.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz MA, Connor CE, Constantinidis C, McLaughlin JR. Covert attention suppresses neuronal responses in area 7a of the posterior parietal cortex. J. Neurophysiol. 1994;72:1020–3. doi: 10.1152/jn.1994.72.2.1020. [DOI] [PubMed] [Google Scholar]
- Stricanne B, Andersen RA, Mazzoni P. Eye-centered, headcentered, and intermediate coding of remembered sound locations in area LIP. J. Neurophysiol. 1996;76:2071–2076. doi: 10.1152/jn.1996.76.3.2071. [DOI] [PubMed] [Google Scholar]
- Teder-Sälejärvi WA, Hillyard SA, Röder B, Neville HJ. Spatial attention to central and peripheral auditory stimuli as indexed by event-related potentials. Brain Res. Cogn. Brain Res. 1999;8:213–27. doi: 10.1016/s0926-6410(99)00023-3. [DOI] [PubMed] [Google Scholar]
- Van der Lubbe RHJ, Neggers SFW, Verleger R, Kenemans JL. Spatiotemporal overlap between brain activation related to saccade preparation and attentional orienting. Brain Res. 2006;1072:133–152. doi: 10.1016/j.brainres.2005.11.087. [DOI] [PubMed] [Google Scholar]
- Van der Lubbe RHJ, Wauschkuhn B, Wascher E, Niehoff T, Kömpf D, Verleger R. Lateralized EEG components with direction information for the preparation of saccades versus finger movements. Exp. Brain Res. 2000;132:163–178. doi: 10.1007/s002219900328. [DOI] [PubMed] [Google Scholar]
- Van der Stigchel S, Heslenfeld DJ, Theeuwes J. An ERP study of preparatory and inhibitory mechanisms in a cued Saccade task. Brain Res. 2006;1105:32–45. doi: 10.1016/j.brainres.2006.02.089. [DOI] [PubMed] [Google Scholar]
- Wauschkuhn B, Verleger R, Wascher E, Klostermann W, Burk M, Heide W, Kömpf D. Lateralised human cortical activity for shifting attention and initiating saccades. J. Neurophysiol. 1998;80:2900–2910. doi: 10.1152/jn.1998.80.6.2900. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Goldberg ME. Activity of superior colliculus in the behaving monkey. III. Cells discharging before eye movements. J. Neurophysiol. 1972;35:575–586. doi: 10.1152/jn.1972.35.4.575. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Goldberg ME, Robinson DL. Brain mechanisms of visual attention. Sci. Am. 1982;246:100–7. doi: 10.1038/scientificamerican0682-124. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Mohler CW. Enhancement of visual response in monkey striate cortex and frontal eye fields. J Neurophysiol. 1976;39:766–772. doi: 10.1152/jn.1976.39.4.766. [DOI] [PubMed] [Google Scholar]