Abstract
Background. Integrins are transmembrane αβ heterodimer receptors that function as structural and functional bridges between the cytoskeleton and ECM (extracellular matrix) molecules. The RGD (arginine-glycine-aspartate tripeptide motif)-dependent integrin α8β1 has been shown to be involved in various cell functions in neuronal and mesenchymal-derived cell types. Its role in epithelial cells remains unknown.
Results. Integrin α8β1 was found to be expressed in the crypt cell population of the human intestine but was absent from differentiating and mature epithelial cells of the villus. The function of α8β1 in epithelial crypt cells was investigated at the cellular level using normal HIECs (human intestinal epithelial cells). Specific knockdown of α8 subunit expression using an shRNA (small-hairpin RNA) approach showed that α8β1 plays important roles in RGD-dependent cell adhesion, migration and proliferation via a RhoA/ROCK (Rho-associated kinase)-dependent mechanism as demonstrated by active RhoA quantification and pharmacological inhibition of ROCK. Moreover, loss of α8β1, through RhoA/ROCK, impairs FA (focal adhesion) complex integrity as demonstrated by faulty vinculin recruitment.
Conclusions. Integrin α8β1 is expressed in epithelial cells. In intestinal crypt cells, α8β1 is closely involved in the regulation of adhesion, migration and cell proliferation via a predominant RhoA/ROCK-dependent mechanism. These results suggest an important role for this integrin in intestinal crypt cell homoeostasis.
Keywords: cell migration, cell proliferation, crypt cell, integrin, RhoA
Abbreviations: BrdU, bromodeoxyuridine; Cdc42, cell division cycle 42; CNS, control non-silencing; DAPI, 4′,6-diamidino-2-phenylindole; DPPIV, dipeptidyl peptidase IV; ECM, extracellular matrix; EV, empty vector; FA, focal adhesion; FX, focal complex; GST, glutathione transferase; HIEC, human intestinal epithelial (cells); LSC, laser scanning cytometry; PFA, paraformaldehyde; RGD, arginine-glycine-aspartate tripeptide motif; RNAi, RNA interference; ROCK, Rho-associated kinase; RPLP0, ribosomal protein, large, P0; RT–PCR, reverse transcription–PCR; shRNA, small-hairpin RNA; TNfn3, third type-III fibronectin repeat of tenascin-C; TRITC, tetramethylrhodamine β-isothiocyanate; VSMC, vascular smooth muscle cell
Introduction
Integrins are a superfamily of transmembrane receptors that form heterodimers of non-covalently associated α- and β-subunits. In mammals, more than 24 integrin receptors have been reported after the identification of 18 α- and nine β-subunits. Integrin receptors act as structural and functional bridges between the cytoskeleton and ECM (extracellular matrix) components and mediate intracellular signal transduction (Clark and Brugge, 1995; Bershadsky et al., 2006). Several integrins are present in epithelia throughout the organism, such as α1β1, α2β1, α3β1 and α6β4. The presence of these integrins at the surface of epithelial cells allows recognition and interaction with specific ligands including laminins, collagens and other glycoproteins (Mercurio, 1995; Sheppard, 1996; Beaulieu, 1997). The integrin-dependent connection between the ECM and the cytoplasm promotes the regulation of cell functions such as adhesion, migration, proliferation, apoptosis and differentiation (Giancotti, 1997; Reddig and Juliano, 2005; Lock et al., 2008).
The intestinal epithelium is a valuable system for understanding the relationship between cell state and interactions with the ECM. The functional unit of the small intestinal epithelium is organized as two distinct compartments, the crypts and villi, where proliferative and differentiated epithelial cell populations are located respectively (Arsenault and Menard, 1989; Ménard and Beaulieu, 1994; Babyatsky and Podolsky, 1999). The crypt–villus axis ensures the constant renewal of the epithelial barrier via a tight equilibrium between cell growth, migration and differentiation of mature enterocytes and apoptosis (Babyatsky and Podolsky, 1999; Ménard et al., 2006). In this context, these cell functions are susceptible to regulation by specific interactions between integrins and ECM components (Beaulieu, 1997, 1999; Zargham et al., 2007a). Besides the laminin- and collagen-binding integrins common to most epithelial cells, intestinal epithelial cells have been found to express most other members of the β1 integrin repertoire at one time or another. For example, integrin α9β1, which can bind tenascin-C (Yokosaki et al., 1998) and a cryptic domain of osteopontin (Smith et al., 1996), is transiently expressed in epithelial cells of the intestinal crypts in an EGF (epidermal growth factor)-dependent manner (Desloges et al., 1998) and is re-expressed in colon cancer cells (Basora et al., 1997), whereas expression of the fibronectin receptor α5β1 and the αv-containing integrin(s) was found to be related to intestinal cell proliferation (Beaulieu et al., 1992; Vachon et al., 1995; Zhang et al., 2003).
Integrin α8β1 was first identified in the chick neuronal system prior to the characterization of its human counterpart (Bossy et al., 1991; Schnapp et al., 1995a, 1995b). So far, α8β1 has been shown to be associated with FA (focal adhesion) points where it participates in the regulation of spreading, adhesion, growth and survival in different neuronal and mesenchymal-derived cell types (Muller et al., 1995; Schnapp et al., 1995b; Levine et al., 2000; Bieritz et al., 2003; Wagner et al., 2003; Farias et al., 2005; Zargham and Thibault, 2005; Zargham et al., 2007a). Integrin α8β1 has been shown previously to modulate actin stress fibre assembly (Zargham and Thibault, 2006) and to promote RhoA GTPase activation in VSMCs (vascular smooth muscle cells) (Zargham et al., 2007a, 2007b). However, the existence of an analogous mechanism in an epithelial context has not yet been reported.
In the present study, we report that α8β1 is expressed in the crypt cell population of the human small intestine where it represents one of the major RGD (arginine-glycine-aspartate tripeptide motif)-binding integrins and is a central RhoA/ROCK (Rho-associated kinase)-dependent modulator of cell migration and proliferation, two fundamental functions of intestinal crypt cells.
Results
Expression of the integrin α8 subunit in human intestinal epithelial cells
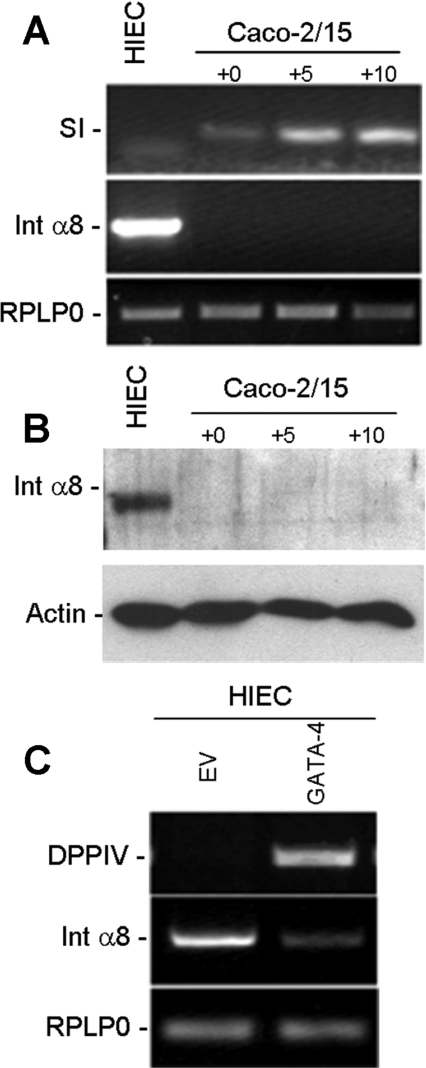
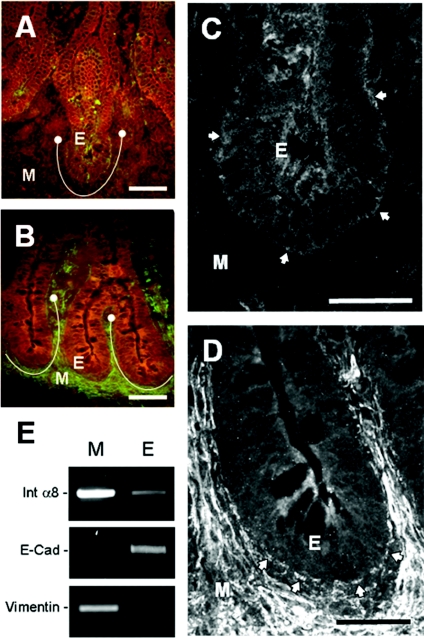
Previous analyses of gene expression profiles in intestinal cells failed to identify integrin subunits that displayed statistically significant levels of differential expression between proliferating and differentiating cells (Tremblay et al., 2006). Rescreening of these experimental models by RT–PCR (reverse transcription–PCR) revealed α8 to be a strongly differentially expressed α-subunit. The α8-subunit was not expressed by differentiating villus-like Caco-2/15 cells but was present in undifferentiated crypt-like HIEC (human intestinal epithelial cells) at the transcript (Figure 1A) and protein levels (Figure 1B). To emphasize a potential relationship between α8β1 expression and the undifferentiated phenotype, we analysed α8 expression in HIEC cells overexpressing GATA-4, a transcription factor well known for its ability to trigger enterocytic differentiation. Induction of the differentiation marker DPPIV (dipeptidyl peptidase IV) in HIEC/GATA-4 cells was accompanied by a dramatic decrease in the α8 subunit level (Figure 1C), suggesting that α8β1 integrin expression was restricted to undifferentiated progenitor epithelial cells. To further study this correlation, we analysed α8 subunit expression in vivo on 14- and 20-week human fetal jejunum specimens by indirect immunofluorescence. In 14-week specimens, the α8 subunit was detected at the base of the proliferative epithelial cells located in the intervillous area (Figures 2A and 2C). At 20 weeks of gestation, the α8 subunit persisted at the base of the proliferative epithelial cell population located in the newly formed crypts, and strong expression of α8 was now also present in the stromal cell population (Figures 2B and 2D). Integrin α8β1 expression in human fetal intestinal epithelial cells was further confirmed by RT–PCR of pure epithelial and mesenchymal fractions isolated from 18–20-week jejunum specimens. As expected, α8 mRNA was readily detected in the mesenchyme, as well as in the epithelial fraction (Figure 2E). Fraction purity was verified by assessing the presence of specific markers for epithelium and mesenchyme, E-cadherin and vimentin respectively (Figure 2E). Taken together, these results showed that integrin α8β1 was specifically expressed in intestinal crypt cells. We then investigated the role of this integrin using HIEC cells.
Figure 1. Expression of the integrin α8 subunit in intestinal cells is lost with differentiation.
(A) Representative RT–PCR, indicating strong expression of the α8 subunit (Int α8) transcript in the progenitor HIEC cells and its absence in differentiating Caco-2/15 cells at various stages of confluence (0, +5 and +10 days post-confluence) as monitored by sucrase-isomaltase (SI) expression. (B) Confirmation of α8 expression in HIEC at the protein level was made by Westernblot analysis. (C) Representative RT–PCR of α8 subunit transcript expression in stable HIEC cell lines with and without ectopic expression of GATA-4. RPLP0 was used as a normalizing gene and DPPIV as a differentiation marker.
Figure 2. Expression and distribution of the α8 integrin subunit in the human small intestine.
Representative immunofluorescence micrographs of human jejunum cryosections at 14 weeks (A, C) and 20 weeks of gestation (B, D) stained with an anti-α8 integrin subunit antibody. In both stages, the α8 subunit was detected in the crypt regions (defined by the brackets in A and B). Higher magnifications of the crypt regions showed that the α8 subunit was present at the basolateral surface (arrows) of epithelial crypt cells (E) at both 14 and 20 weeks (C, D). Weak staining was observed in the mesenchyme (M) at 14 weeks, whereas at 20 weeks, specimens show more prominent staining with the differentiation of smooth muscle cells. Scale bars: (A, B) 50 μm and (C, D) 25 μm. (E) RT–PCR experiments confirm the presence of the α8 transcript (Int α8) in the 18–20 week jejunal epithelium accompanied by strong expression in the mesenchyme. Epithelial (E) and mesenchymal (M) fraction purity was validated by the absence of vimentin and E-cadherin (E-Cad) respectively.
Impact of integrin α8β1 on RGD-dependent cell adhesion
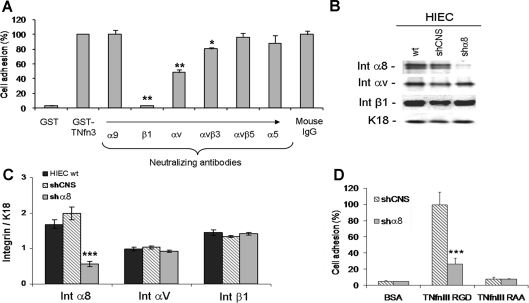
Adhesion assays were performed on a GST (glutathione transferase) fusion peptide containing the TNfn3 (third type-III fibronectin repeat of tenascin-C), which possesses a functional RGD-binding domain for α8β1. Wild-type HIEC cells adhered efficiently to this RGD-containing domain (Figure 3A), and adhesion was completely abolished in the presence of a soluble RGD-containing peptide (results not shown) or by blocking the β1 integrin subunit. The addition of a neutralizing anti-αv antibody reduced adhesion by approx. 50% and neutralizing antibodies directed against the α5 or α9 subunits did not significantly alter this RGD-dependent adhesion (Figure 3A). By elimination, these results suggested the involvement of an additional RGD-dependent β1 integrin, such as α8β1, but we were unable to test this using this assay as no anti-α8 neutralizing antibody is yet available. We therefore opted for the establishment of α8-knockdown and CNS (control non-silencing) stable HIEC cell lines using shRNA (small-hairpin RNA) technology. By Western blotting, we confirmed that this strategy caused a ∼70% reduction in α8 subunit expression in HIEC/shα8 compared with wild-type HIEC and HIEC/shCNS cells (Figures 3B and 3C). In addition, we confirmed that the expression levels of integrin subunits αv and β1 in the three different cell lines were not affected (Figures 3B and 3C). Using these shRNA cell lines we performed adhesion assays on the RGD-containing TNfn3 matrix, and showed a ∼70% decrease in the adhesion of HIEC/shα8 cells compared with control HIEC/shCNS cells (Figure 3D), confirming an important contribution of the α8β1 integrin to RGD-dependent adhesion of intestinal cells. Adhesion on the inactive RAA (arginine-alanine-alanine)-mutated TNfn3 matrix showed only negligible amounts of adherent cells, comparable with assays on control GST-coated plates, for both groups.
Figure 3. Integrin α8β1 and RGD-dependent adhesion of human intestinal crypt cells.
(A) Short-term (1 h) adhesion assays of HIEC cells plated on GST or RGD-containing GST–TNfn3 coating. Cells were treated or untreated with neutralizing antibodies directed against RGD-binding integrins expressed by HIEC. Mouse IgG was used as a negative control (n≥3; *P<0.05; **P<0.01, compared with mouse IgG). (B) Assessment of α8 (Int α8), αv (Int αv) and β1 (Int β1) protein expression levels in wt (wild-type) HIEC and stable cell populations of HIEC/shCNS and HIEC/shα8 by Western blotting. (C) Relative amounts of α8, αv and β1 were detected for each group according to the loading control keratin 18 (K18) (n=3; ***P<0.0001). (D) Long-term (24 h) adhesion assays with HIEC/shCNS and HIEC/shα8 cells plated on GST, RGD-containing GST–TNfn3 or RAA-mutated GST–TNfn3 coating. Cycloheximide (10 μg/ml) was added to the adhesion medium to avoid endogenous fibronectin secretion/deposition (n=4; ***P<0.0001).
Effect of α8 knockdown on focal contact and stress fibre organization
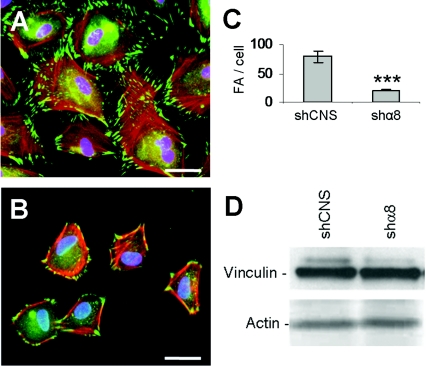
Since integrin α8β1 appeared to be important for the adhesion of epithelial crypt cells, we investigated the impact of α8β1 depletion on focal contact and actin cytoskeleton organization. Control and HIEC/shα8 cells were seeded on serum-coated glass coverslips and analysed by double-labelling immunofluorescence using an anti-vinculin antibody to detect focal contacts and phalloidin to stain actin microfilaments. Knockdown of α8 expression in HIEC led to a reorganization of the actin network, from transverse and parallel stress fibres to a cortical localization (Figures 4A and 4B). Moreover, we observed a significant decrease in the number of vinculin-positive FA points (Figure 4C) and a reduction in spreading in HIEC/shα8 cells, compared with control cells (Figures 4A and 4B), without affecting the overall vinculin expression levels (Figure 4D).
Figure 4. Integrin α8β1 regulates focal contact and stress fibre organization.
Representative micrographs of vinculin immunofluorescence (green) and TRITC–phalloidin staining of actin stress fibres (red) on HIEC/shCNS (A) and HIEC/shα8 (B) 24 h after seeding (n=4; scale bars: 50 μm). (C) Quantification of vinculin-stained FA per cell in HIEC/shCNS and HIEC/shα8 (n=8; ***P<0.0001). (D) Vinculin expression levels in both cell populations were assayed by Western blot (n=3).
Integrin α8β1 and RhoA GTPase activation
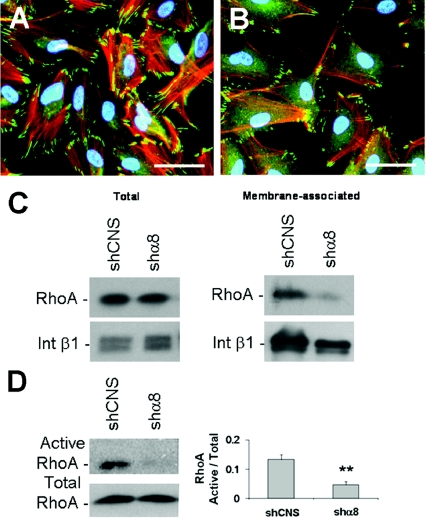
Considering the previously reported relationship between the integrin α8β1, actin network organization and small-GTPase RhoA membrane recruitment/activity (Zargham and Thibault, 2006; Zargham et al., 2007a, 2007b), we treated HIEC cells with the Rho kinase (ROCK 1/2) pharmacological inhibitor Y-27632. ROCK is the principal effector of RhoA GTPase and, once activated, it contributes to cell growth, stress fibre assembly and recruitment of FA components (Loirand et al., 2006). Treatment of wild-type HIEC with Y-27632 at 20 μM for 24 h (Lai et al., 2003) generated a similar phenotype to the HIEC/shα8 cells in terms of stress fibre assembly and vinculin distribution (Figures 5A and 5B). We evaluated RhoA activity in HIEC/shα8 cells by analysing the proportion of membrane-associated RhoA, which is indicative of the GTP-bound active conformation (Zargham et al., 2007a). A significant decrease in membrane-associated RhoA was observed in HIEC/shα8 compared with HIEC/shCNS cells, while the total amount of RhoA remained unchanged between the two groups (Figure 5C and Supplementary Figure S1A at http://www.biolcell.org/boc/101/boc1010695add.htm). We assayed RhoA activity directly by performing a RhoA–GTP pull-down assay using beads coupled with the rhotekin RhoA-binding domain (Cetin et al., 2007). HIEC/shα8 cells demonstrated a significant reduction in the amount of active RhoA compared with HIEC/shCNS (Figure 5D). No significant difference was observed in the total and membrane-associated amounts of Rac1 and Cdc42 (cell division cycle 42) GTPases in HIEC/shCNS and HIEC/shα8 cells (Supplementary Figures S1B and S1C). These observations suggested that integrin α8β1 was central to RhoA functionality in intestinal undifferentiated epithelial cells.
Figure 5. Integrin α8β1 and RhoA GTPase activation in HIEC.
Representative micrograph of vinculin immunofluorescence (green) and TRITC–phalloidin staining of actin stress fibres (red) in normal HIEC (A) and Y-27632-treated (20 μM) HIEC (B) over a 24 h period after seeding (n=3; scale bar: 50 μm). Western-blot analyses of RhoA in total and membrane fractionated protein extracts (C) from HIEC/shCNS and HIEC/shα8 cells (n=3). (D) Active RhoA pull-down experiments using beads coupled with the rhotekin RhoA-binding domain with HIEC/shCNS and HIEC/shα8 (n=4; **P=0.005). Int β1, integrin β1.
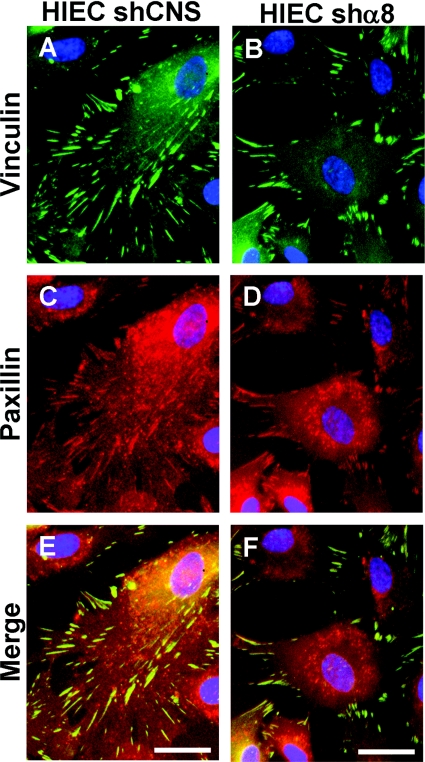
Knockdown of the integrin α8 subunit affects vinculin, but not paxillin, recruitment to FAs
To further characterize the effect of α8 silencing on FA formation we plated control HIEC and HIEC/shα8 on different substrata and quantified the number of vinculin- and paxillin-positive FAs that were generated (Table 1 and Supplementary Figure S2 at http://www.biolcell.org/boc/101/boc1010695add.htm). In wild-type HIEC and HIEC/shCNS cells plated on a serum coating, which provided the RGD-containing fibronectin and vitronectin, vinculin and paxillin displayed abundant (∼70–80 per cell) and broad (mature) FAs in a sparse peripheral pattern (Table 1 and Supplementary Figures S2A and S2B). HIEC/shα8 cells plated on a serum coating resulted in a severe decrease in the number of vinculin-positive FAs, whereas paxillin staining was not affected (Table 1 and Supplementary Figure S2C). Similar observations were made for Y-27632-treated wild-type HIEC cells (Table 1 and Supplementary Figure S2D), suggesting that recruitment of vinculin, but not paxillin, to the developing FA was RhoA/ROCK-dependent. Moreover, when HIEC/shCNS cells were seeded on a type-I collagen matrix, a non-RGD substratum, the number of vinculin-positive FAs was significantly reduced compared with paxillin-positive FAs (Table 1 and Supplementary Figure S2E), closely recreating the phenotype of α8-knockdown and Y-27632-treated wild-type HIEC cells. To further document differences in cellular localization between vinculin and paxillin after α8 silencing, co-immunostaining was performed. Similar localization patterns of vinculin and paxillin were observed in the broad mature FAs at the periphery of control cells, with a few paxillin-positive punctate spots detected in the central region (Figures 6A, 6C and 6E). HIEC/shα8 cells displayed paxillin-stained dots localized more centrally that were apparently devoid of vinculin, whereas only 30% of paxillin-positive FAs were also vinculin-positive and were localized at the periphery of the cells (Figures 6B, 6D and 6F).
Table 1. Quantification of vinculin- and paxillin-marked FAs.
Results shown (means±S.E.M.) are the number of vinculin- and paxillin-positive FAs per cell in wild-type (wt), shCNS-, shα8- and Y-27632-treated HIEC plated on to RGD-containing substratum and HIEC/shCNS on to type I collagen (n≥8).
| Number of FAs per cell | |||||
|---|---|---|---|---|---|
| RGD-containing substratum | Collagen | ||||
| HIEC/wt | HIEC/shCNS | HIEC/shα8 | HIEC/Y-27632 | HIEC/shCNS | |
| Vinculin | 70.83±2.24 | 78.90±9.76 | 20.33±1.19 | 15.13±1.11 | 18.63±2.00 |
| Paxillin | 69.75±5.89 | 73.88±4.38 | 77.25±6.84 | 73.38±12.27 | 76.00±7.45 |
Figure 6. Knockdown of the integrin α8 subunit affects vinculin localization but not paxillin in HIEC.
(A—D) Representative micrographs of vinculin (green) (A, B) and paxillin (red) (C, D) immunofluorescence detection in HIEC/shCNS (A, C) and HIEC/shα8 cells (B, D) 24 h after seeding. (E, F) Merged images of vinculin and paxillin for HIEC/shCNS (E) and HIEC/shα8 (F) illustrate the differential cellular localization of these two proteins under α8-knockdown conditions (n=3; scale bar: 25 μm).
Reduced presence of integrin α8β1 up-regulates cell migration in the HIEC model
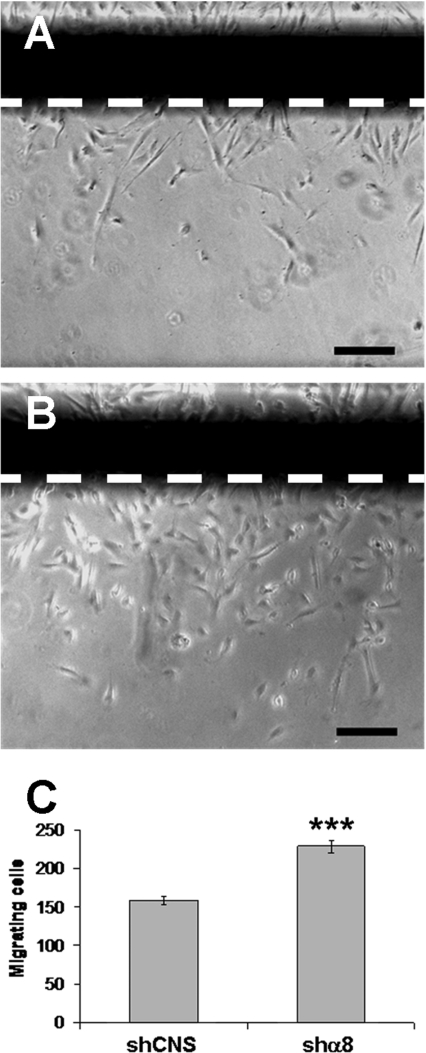
The functional role of RhoA in microfilament organization and its consequent impact on cell motility is well established. By performing wound assays (Figure 7), we demonstrated a 40% increase in the amount of cells that migrated across the wound edge in HIEC/shα8 cell monolayers compared with HIEC/shCNS cells. In these experiments, cell proliferation was blocked by the addition of 2 mM hydroxyurea to the incubation medium. Thus our results confirmed that α8 silencing led to the up-regulation of intestinal epithelial HIEC cell migration.
Figure 7. Silencing of the α8 subunit increases migration in the HIEC cell line.
Phase-contrast micrographs illustrating migrating HIEC/shCNS (A) and HIEC/shα8 cells (B) across the wound edge after 48 h of incubation in the presence of 2 mM hydroxyurea (n=5; scale bar: 100 μm). (C) The histogram represents the number of cells that migrated across the wound edge (n=5; ***P<0.0001).
Knockdown of the integrin α8 subunit impairs cell-cycle progression
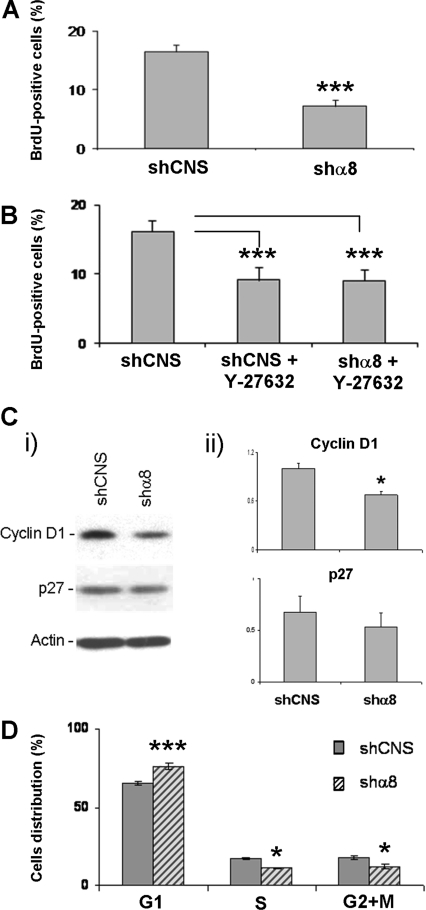
Considering that the RhoA/ROCK pathway affects cell-cycle progression, we assessed the proliferation rate of HIEC/shCNS and HIEC/shα8 cells by BrdU (bromodeoxyuridine) incorporation experiments. These experiments demonstrated a decrease of ∼50% in the number of BrdU-positive cells in HIEC/shα8 cultures compared with control HIEC cells (Figure 8A). Moreover, HIEC/shCNS cells treated with Y-27632 confirmed that ROCK inhibition in these control cultures resulted in a significant decrease in cell growth, comparable with that observed for α8-depleted cells (Figure 8B). Importantly, the treatment of HIEC/shα8 cells with Y-27632 had no additive effect on proliferation inhibition, suggesting that the integrin α8β1 stimulated proliferation via ROCK (Figure 8B). HIEC α8-depleted cells also exhibited a corresponding significant decrease in cyclin D1 expression levels, but no significant changes were noted for p27Kip1 (Figure 8C), two cell-cycle regulators that have been reported to be potential downstream targets of the RhoA/ROCK pathway in various cell models (Croft and Olson, 2006). LSC (laser scanning cytometry) analysis of HIEC/shα8 and HIEC/shCNS populations showed that the decrease in cell growth found in HIEC/shα8 cells was associated with a significantly higher percentage of cells in G1 phase (Figure 8D).
Figure 8. Integrin α8β1 and cell-cycle progression in the HIEC cell lines.
Histograms illustrating the percentage of BrdU-labelled cells for (A) HIEC/shCNS compared with HIEC/shα8 and for (B) non-treated HIEC/shCNS compared with Y-27632-treated HIEC/shCNS and HIEC/shα8 (***P<0.0005). (C) Quantification of the amounts of cyclin D1 and p27Kip1 was performed in HIEC/shCNS and HIEC/shα8. (i) Western-blot analysis; (ii) the relative amounts of cyclin D1 and p27Kip1 detected for each group according to the loading control actin (n=3; *P=0.012). (D) Histogram illustrating the percentage of cells distributed in each of G1, S and G2+M cell-cycle phases for HIEC/shCNS and HIEC/shα8 according to iCys LSC analysis (n=3; *P=0.015, ***P=0.0002).
RNAi (RNA interference)-resistant α8 cDNA restores the wild-type phenotype in HIEC/shα8 cells
To confirm that the observed cell adhesion and proliferation decreases, as well as the cell migration increase in HIEC, were due specifically to the depletion of α8β1 and not because of non-specific off-target effects of the RNAi pathway, we generated an α8 subunit ‘rescue’ vector bearing three silent point mutations in the α8 cDNA that was resistant to RNAi-mediated knockdown. When HIEC/shα8 cells were infected with the α8 subunit rescue lentivirus, the expression levels of the α8 subunit were restored (Supplementary Figure S3A at http://www.biolcell.org/boc/101/boc1010695add.htm) and the cellular adhesion-, migration- and proliferation-related phenotypes were totally abolished and comparable with wild-type levels (Supplementary Figures S3B–S3D). Taken together, these results show that the phenotypes induced by α8 shRNA are the result of specific depletion of the integrin α8 subunit.
Discussion
In the present study, we report for the first time that the integrin α8β1 is expressed by epithelial cells. In the human small intestine, α8β1 was found to be restricted to the crypt cell population in the intact fetal intestine. Expression of α8β1 increases dramatically during development of the smooth musculature from 16 weeks onwards (Beaulieu et al., 1993), including adult tissue (results not shown). Indeed, smooth muscle cells express high levels of α8β1 (Schnapp et al., 1995a). Nevertheless, detection of the α8 transcript in purified epithelial fractions at mid-gestation, as well as in the crypt-like HIEC cells, confirmed the expression of α8β1 in epithelial crypt cells in the functional small intestine.
Additional experiments using HIEC cells revealed that the integrin α8β1 interacted in an RGD-dependent manner and promoted accurate FA and stress fibre assembly through RhoA activation, to enhance cell adhesion and proliferation, accompanied by a decrease in cell migration. Taken together, these findings suggest a role for α8β1 in the stimulation of proliferation while simultaneously restraining migration of these undifferentiated, progenitor epithelial cells towards the terminal differentiation compartment.
Integrin-dependent adhesion involves heterogeneous molecular complexes with the typical composition of anchor proteins such as vinculin and paxillin. The integrin–matrix interactions are followed by the sequential formation of FXs (focal complexes), FAs and FBs (fibrillar adhesions), which leads to linkage of the actin network to the cell membrane (Zaidel-Bar et al., 2004; Zimerman et al., 2004). In adherent and spread cultured cells, stress fibres are associated with FAs and maintain an isometric actomyosin-based tension applied to the ECM (Geiger and Bershadsky, 2001). The regulation of this phenomenon is primarily mediated through the small G-protein RhoA. The positive effect of the integrin α8β1 on the promotion of adhesion is not without precedent since other groups have reported that this RGD-dependent integrin is important for the spreading and generation of strong adhesive forces in mesangial, neuroendocrine, myofibroblastic cells and VSMCs, where interaction of α8β1 with the ECM led to cytoskeleton/FA assembly (Muller et al., 1995; Lu et al., 2002; Bieritz et al., 2003; Zargham et al., 2007b). In HIEC, reduction of adhesion in cells lacking α8 was accompanied by the decreased presence of vinculin in FAs and cortical rearrangement of the actin cytoskeleton. As a plausible explanation, it has been well documented that RhoA activity exerts a control on actin stress fibre assembly through the action of ROCK, the principal effector of this GTPase. This action contributes to decreased cell migration by enhancing adhesion on the ECM (Zaidel-Bar et al., 2003; Cetin et al., 2004; Russo et al., 2005). As reported previously, α8β1 interacts either directly or indirectly with the GTPase RhoA, and facilitates RhoA recruitment to the plasma membrane, allowing its activation by membrane-bound associated proteins (Zargham et al., 2007a). In further investigating this feature, we observed that HIEC α8-knockdown cells displayed a lower amount of RhoA in membrane protein extracts than in control HIEC, while membrane-associated levels of other GTPases such as Rac1 and Cdc42 remained constant. Moreover, pull-down experiments of the RhoA active form revealed a decrease of this activated GTPase in α8-knockdown HIEC cells. These findings suggest that the absence of integrin α8β1 in intestinal epithelial cells impairs the recruitment of RhoA to the membrane and, consequently, its activation.
As stated above, we observed that HIEC α8-knockdown cells displayed a reduced presence of vinculin at the FA, compared with wild-type HIEC, without affecting the expression level of this protein. In contrast, we observed no substantial change in the localization of paxillin, another well-characterized FA component (Turner, 2000), suggesting that α8β1 contribution to FA assembly was specific to vinculin recruitment. Similar observations were made when the control HIEC were grown on the non-RGD substratum collagen I. These observations indicated that vinculin recruitment, but not paxillin, depends on the RGD cell–matrix interaction system through integrin α8β1. Based on the proposed scheme of temporal stages in the formation and organization of cell–matrix adhesions (Zaidel-Bar et al., 2004), vinculin recruitment occurs during the late FX stage, while paxillin is already present in early FX. Thus our results suggest that the knockdown of α8 in HIEC impaired the maturation of focal contacts at the late FX stage.
Other groups have proposed that RhoA activity is involved in the recruitment of some FA components including vinculin (Barry and Critchley, 1994; Zaidel-Bar et al., 2003). Thus our findings strongly suggest that α8β1 is involved, through RhoA activity, in the recruitment of vinculin specifically to the developing focal contacts in intestinal epithelial cells. To emphasize the association between integrin α8β1 and RhoA GTPase in intestinal cells, we have shown that the inhibition of ROCK signalling led to a phenotype similar to that of the α8 knockdown in terms of both actin stress fibre organization and vinculin/paxillin distribution. This supports the hypothesis that the cytoskeleton rearrangement and FA aberrations observed in α8-knockdown cells are mediated largely by the RhoA/ROCK pathway. We also show that loss of α8 is associated with a decrease in cell proliferation, via a RhoA/ROCK mechanism. This signalling pathway has been identified as a modulator of cell-cycle progression through molecular events by which RhoA/ROCK/LIMK (LIM domain kinase) inhibition led to stress fibre disassembly, a decrease in ERK1/2 (extracellular-signal-regulated kinase 1/2) phosphorylation and subsequent cyclin D inhibition (Walker and Assoian, 2005).
Furthermore, Croft and Olson showed that RhoA/ROCK activation led to down-regulation of p27Kip1 and cyclin D1 up-regulation via independent mechanisms (Croft and Olson, 2006). In HIEC cells, the observed antiproliferative effect of α8 knockdown was accompanied by a decrease in cyclin D1 expression, whereas the expression of the CDK (cyclin-dependent kinase) inhibitor p27Kip1 was not affected. In agreement with these findings, the analysis of cell-cycle progression in α8-depleted cells has shown that a significantly higher percentage of the population accumulated in G1 phase when compared with control HIEC. Thus cell-cycle progression analysis indicated that integrin α8β1 facilitated the progression from G1 to S-phase in epithelial progenitor cells of the human intestine. Moreover, inhibition of ROCK in HIEC cells also resulted in a reduction in the amount of proliferative cells compared with α8-knockdown HIEC, suggesting that the α8β1-dependent mechanism of proliferation control acted mainly via RhoA/ROCK signalling.
In addition, we have shown that forced expression of GATA-4, a key transcription factor involved in enterocyte differentiation (Escaffit et al., 2005a; Bosse et al., 2007), led to markedly reduced α8 expression in the HIEC cell model. Mapping of the ITGA8 (integrin α8) human promoter led to the identification of six putative GATA-binding sites in the 2800 bp upstream of the transcription initiation site (results not shown), which could mediate integrin α8 subunit transcriptional repression. These findings are in accordance with the fact that differentiated epithelial cells lack integrin α8β1 (the present study) and that RhoA activity is down-regulated during the intestinal epithelial differentiation process (Gout et al., 2001). Also, our observations regarding α8β1 localization in the crypt–villus axis and the regulation of cell-cycle progression via α8β1/RhoA are concordant with the inhibition of RhoA activity that normally occurs in the differentiating intestinal epithelium, where proliferative cells have to stop cycling prior to terminal differentiation (Pageot et al., 2000). Thus the striking adhesion decrease, due to the loss of α8 expression, could be indicative of an induction of cell migration that occurs normally in the crypt–villus axis after the triggering of the differentiation process in intestinal epithelial cells (Beaulieu, 1999; Jobin et al., 1999; Lussier et al., 2000). As expected, wound assays revealed that the absence of α8β1 led to an increase in cell migration. Considering the antimigratory phenotype resulting from activation of RhoA GTPase in intestinal epithelial cells (Cetin et al., 2004, 2007), our results reinforce RhoA's status as a key element of the intracellular mechanism underlying integrin α8β1 activation.
Materials and methods
Tissues
Specimens of small intestine (jejunum) from fetuses ranging in age from 14 to 20 weeks (post-fertilization) were obtained after legal abortion. The project was performed in accordance with protocols approved by the Institutional Human Research Review Committee of the Université de Sherbrooke for the use of human material. In some experiments, preparations of pure epithelial and mesenchymal fractions were prepared and analysed as previously described (Perreault et al., 1998).
Cell culture
The HIEC cell line was generated and grown as previously described (Perreault and Beaulieu, 1996). These cells express a number of crypt markers and are thus considered to be non-differentiated crypt-like progenitor cells (Quaroni and Beaulieu, 1997; Pageot et al., 2000; Escaffit et al., 2005b; Tremblay et al., 2006). The Caco-2/15 cell line has been generated and characterized previously for its ability to morphologically and functionally differentiate like villus small intestinal cells (Beaulieu and Quaroni, 1991; Vachon and Beaulieu, 1992; Tremblay et al., 2006).
Establishment of α8-knockdown HIEC cells and GATA-4-expressing HIEC cells
Stable shRNA α8 subunit knockdown and CNS (shCNS) HIEC cell populations were established by lentiviral infection. The same strategy was used to generate a stable GATA-4-expressing HIEC cell population. The oligonucleotides used to generate shα8 (5′-GATCCGATCAGAGTTAATGGAACCTTCAAAGAGAGGTTCCATTAACTCTGATCTTTTTTGGAAC-3′ and complement 5′-TCGAGTTCCAAAAAAGATCAGAGTTAATGGAACCTCTCTTGAAGGTTCCATTAACTCTGATCG-3′), shCNS (5′-GATCGTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTT-CGGAGAATTTTTTGGAAC-3′ and complement 5′-TCGAGTTCCAAAAAATTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAAC-3′) and full-length α8 (5′-CGATAAGCTTCTCCTTCCAG-3′and complement 5′-AACATCTGATACGACAACGG-3′) were synthesized by IDT (Coralville, IA, U.S.A.). Silent mutations resistant to α8 shRNA were introduced into full-length human α8 subunit cDNA by PCR mutagenesis. Specifically, nucleotide residues 357 (C→T), 358 (A→C) and 360 (A→T) from the ITGA8 coding sequence (GenBank® accession number NM_003638) were substituted to generate the 355atTCgTgttaatggaacc372 mutant. The oligonucleotide annealed products were subcloned into pLVX-puro (Clontech, CA, U.S.A.). pLPCX/hGATA-4, pLPCX/EV, pLVX/α8-rescue, pLENTIU6/shα8 and pLENTIU6/shCNS vectors (where EV is empty vector) were used to produce viruses in HEK-293T cells [human embryonic kidney cells expressing the large T-antigen of SV40 (simian virus 40)] as previously described (Boudreau et al., 2007). Subconfluent HIEC cells were infected with viral suspensions containing 4 μg/ml polybrene (Sigma–Aldrich) for 48 h at 37°C. The shα8- and shCNS-infected cells were then selected for 10 days with 5 μg/μl blasticidin, while pLPCX/EV- and pLPCX/GATA-4-infected cells were selected for 10 days with 1 μg/μl puromycin to generate stable cell populations. GATA-4- and EV-infected cells were grown as described above for HIEC for 30 days and RNA was extracted for subsequent RT–PCR analysis.
Generation of recombinant tenascin-C peptides: TNfn3 RGD and RAA
cDNA encoding recombinant tenascin-C fragments containing the third fibronectin type-III repeat with the intact RGD motif (TNfn3 RGD) and the mutated site (TNfn3 RAA) were generated by the method of Yokosaki et al (1998).
Antibodies and reagents
The rabbit polyclonal antibody targeting the human α8 cytoplasmic domain (Schnapp et al., 1995b) was used at a 1:500 dilution for indirect immunofluorescence experiments. A rabbit anti-(chick α8) serum (a gift from Dr Louis F. Reichardt, Department of Physiology, University of California San Francisco, San Francisco, CA, U.S.A.) was also used at a 1:100 dilution in initial immunofluorescence experiments (data not shown). Antiserum directed against the α8 integrin subunit was kindly provided by Dr Gaétan Thibault (IRCM, Montreal, QC, Canada) (Zargham and Thibault, 2005), and used at 1:3000 for Western-blot experiments. Other antibodies used for Western blot were polyclonal anti-αv (AV1; 1:1000 dilution; Chemicon), anti-cyclin D1/D2 (1:5000; Santa Cruz Biotechnology), anti-p27Kip1 (1:1000; Santa Cruz Biotechnology), anti-Cdc42 (1:500; Santa Cruz Biotechnology), monoclonal anti-cytokeratin 18 (CY-90; 1:10000; Sigma), anti-vinculin (7F9; 1:10000; Chemicon), anti-RhoA (26C4; 1:200; Santa Cruz Biotechnology), anti-Rac1 (1:1000; BD Transduction Laboratories), anti-β1 integrin [mAb13; 1:1000; a gift from Dr Kenneth M. Yamada, NIH (National Institutes of Health)/NIDCR (National Institute of Dental and Craniofacial Research), Bethesda, MD, U.S.A. (Fogerty et al., 1990)], and anti-actin (C4; 1:75000; Chemicon) antibodies. For immunolocalization, we used the monoclonal antibodies anti-vinculin (7F9; 1:500; Chemicon) and anti-paxillin (1:500; BD Transduction Laboratories). For vinculin/paxillin co-immunolocalization, TRITC (tetramethylrhodamine β-isothiocyanate)-conjugated anti-paxillin (1:500; BD Transduction Laboratories) was used, after vinculin immunodetection. The actin cytoskeleton was stained using TRITC-conjugated phalloidin (1:1000; Chemicon). Blocking anti-integrin antibodies used were anti-β1 (mAb13; 10 μg/ml) and anti-α5 (mAb16; 25 μg/ml) both obtained from Dr K.M. Yamada (Fogerty et al., 1990), anti-α9 [Y9A2; 10 μg/ml; originally obtained as a gift from Dr Dean Sheppard, Lung Biology Center, University of California San Francisco, San Francisco, CA, U.S.A. (Wang et al., 1996) and then purchased from Abcam], anti-αv (mAb2021Z; 25 μg/ml; Chemicon), anti-αvβ3 (LM609; 10 μg/ml; Biogenesis) and anti-αvβ5 (P1F6; 5 μg/ml; Gibco BRL) antibodies. Mouse IgG (Sigma) was used as a negative control at a 10 μg/ml concentration. Y-27632 (Calbiochem), the specific inhibitor of ROCK, was used at a concentration of 20 μM.
Membrane fractionation
For the preparation of fractions enriched in plasma membrane proteins, HIEC cells were treated with ice-cold lysis buffer containing 250 mM sucrose, 10 mM Tris (pH 7.5), 1 mM PMSF and 1% protease inhibitor cocktail (Sigma). After three cycles of freeze-thaw, protein samples were centrifuged at 100000 g at 4°C for 1 h. The supernatant was removed and the pellet was washed twice with lysis buffer and resuspended in 100 μl of lysis buffer containing 0.1% SDS and 1% Triton X-100. The sample was centrifuged to clear cell debris (10000 g at 4°C for 20 min) and the supernatant was harvested and stored at –20°C until used for Western-blot analyses.
RNA extraction and RT–PCR
Total RNA, mRNA and cDNA were prepared as previously described (Dydensborg et al., 2006). Single-stranded cDNA was amplified (Taq polymerase; New England Biolabs) for 30 cycles of denaturation (45 s at 94°C), annealing (45 s at 60°C) and extension (1 min at 72°C). For human α8 subunit detection, we used the forward primer 5′-CAGTTTGGACGAATCCACCT-3′ and the reverse primer 5′-TGCTGTCTGGATTGTCCTTG-3′. Primer sequences for sucrase-isomaltase (oligo-1,6-glucosidase; EC 3.2.1.10), DPPIV and RPLP0 (ribosomal protein, large, P0) detection and the specific conditions used have been previously described (Dydensborg et al., 2006; Escaffit et al., 2006).
Western-blot analysis
Proteins were extracted, separated by SDS/PAGE and transferred on to nitrocellulose membranes as previously described (Perreault and Beaulieu, 1996; Escaffit et al., 2005a). For RhoA–GTP pull-down assays, active RhoA was extracted using the Rho Activation Assay Biochem kit (Cytoskeleton) as previously described (Cetin et al., 2007). Membranes were blocked in 5% (w/v) non-fat dried skimmed milk powder/PBS and 0.1% Tween 20 (Bio-Rad) and then incubated with primary antibodies overnight at 4°C. Primary antibodies were detected with horseradish peroxidase-conjugated secondary antibodies (anti-mouse and anti-rabbit; Amersham) and developed using the Immobilon Western® kit (Millipore).
Indirect immunofluorescence staining
The preparation and OCT (optimal cutting temperature; Canemco-Marivac, Lakefield, QC, Canada) embedding of specimens, cryosectioning as well as staining procedures were performed as previously described (Beaulieu et al., 1992). HIEC cell immunofluorescence was done as previously described (Escaffit et al., 2005b). Cells were seeded on to type I collagen (BD Biosciences) or serum-pretreated coverslips (Fisher) 24 h before fixation. The secondary antibodies used were anti-mouse and anti-rabbit conjugated with FITC (Chemicon). For vinculin/paxillin co-immunolocalization, mouse IgG (Sigma) supplementary blocking and 2% (w/v) PFA (paraformaldehyde) post-fixation steps were found to be sufficient to block potential cross-reactions with FITC-conjugated anti-mouse. Nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole), whereas tissue sections were counterstained with 0.01% Evan Blue. Vinculin/paxillin-stained FXs were counted using the MetaMorph Imaging System (Universal Imaging, West Chester, PA, U.S.A.).
Cell adhesion assays
Adherent HIEC cells were harvested using PBS/0.5 mM EDTA (incubation for 10 min at 37°C) and centrifuged at 100 g for 5 min at 4°C. Cell pellets were resuspended in OptiMEM (Invitrogen) culture medium supplemented with 0.2% FBS (fetal bovine serum) and 0.3 mM MnCl2. For 24 h adhesion assays, cycloheximide was added to the medium at a concentration of 10 μg/ml throughout the assay to avoid endogenous ECM secretion (Vainionpaa et al., 2006). However, for short-term adhesion assays, cycloheximide treatment was omitted since endogenous secretion was considered to be negligible. For assays involving blocking antibodies (short-term assays), suspensions of cells were incubated for 30 min at 37°C with the blocking antibody. Then, 48-well plates were treated with the appropriate coating (40 μg/ml of GST, GST–TNfn3 RGD and GST–TNfn3 RAA in PBS) and blocked with 2% (w/v) BSA. Cells were seeded (5×104 cells per well) and incubated for 1 h for short-term assays (with blocking antibodies) and 24 h for long-term assays (shRNA cells). Non-adherent cells were gently washed away with PBS and the remaining cells were fixed with 2% PFA (pH 7.4) for 1 h at 4°C and permeabilized with 0.1% Triton X-100. Cell nuclei were stained with DAPI and adherent cells were counted manually.
Cell migration assay
HIEC cells were seeded on to 100 mm2 plates and cultured until 2 days post-confluence. Cell monolayers were wounded with a 19 mm plastic cell-scraper (Costar, Corning, NY, U.S.A.). Wounded cell monolayers were incubated for 48 h in OptiMEM with 2 mM hydroxyurea (Sigma) to block cell proliferation (Tetreault et al., 2008). After incubation, DAPI staining was performed and the number of cells that had migrated across the wound edge was manually counted by immunofluorescent microscopy.
BrdU incorporation experiments
Each cell line was seeded on to serum-pretreated coverslips in a 24-well plate (Falcon) at 5×104 cells per well (and treated with Y-27632, where indicated), 24 h before the addition of BrdU labelling reagent (Roche). The procedure and reagents used for detection of S-phase positive HIEC cells were in accordance with the FLUOS® In Situ Cell Proliferation kit protocol (Roche).
Cell-cycle progression analysis by LSC
Each cell line was seeded on to a 12-well plate (Falcon) at 5×104 cells per well, 48 h before methanol fixation and DAPI staining. DAPI-stained cells were scanned with an iCys imaging cytometer (Compucyte, Cambridge, MA, U.S.A.) to measure DNA content using violet diode laser excitation (405 nm) and appropriate filters (463/39 nm) for fluorescence detection. DNA content was measured for at least 3000 isolated nuclei per sample in three separate experiments to assess cell-cycle distribution.
Statistical analysis
Results were expressed as means±S.E.M. Each experiment was repeated at least three times and representative results are shown. Values from adhesion assays and BrdU labelling experiments were subjected to the mixed linear model. All other values were subjected to the two-tailed Student's t test. P<0.05 was considered to be statistically significant.
Online data
Acknowledgements
We thank Dr Louis F. Reichardt, Dr Dean Sheppard, Dr Gaétan Thibault and Dr Kenneth M. Yamada for the gift of antibodies. We also thank Marie-Pierre Garant and Aimé Ntwari for statistical analysis and Elizabeth Herring and Michael Dionne for valuable suggestions on the manuscript and technical support. Special thanks are due to Dominique Jean and Eric Tremblay for molecular cloning and virus production. LSC was performed at the Cell Imaging Facility located in the Service of Genetics of the Department of Pediatrics, Faculty of Medicine and Health Sciences, Université de Sherbrooke.
Funding
The Cell Imaging Facility is funded by grants from the Canadian Foundation for Innovation and from the Centre de recherche clinique Étienne Le Bel of the Centre hospitalier universitaire de Sherbrooke (CHUS). This work was supported by a grant from the Canadian Institutes of Health Research [grant number MOP-62914]. J.-F.B. is the recipient of the Canada Research Chair in Intestinal Physiopathology and is a member of the Fonds de la recherche en santé du Québec (FRSQ)-funded Centre de Recherche Clinique Étienne Le Bel of the Centre hospitalier universitaire de Sherbrooke (CHUS).
References
- Arsenault P., Menard D. Cell proliferation during morphogenesis of the human colon. Biol. Neonate. 1989;55:137–142. doi: 10.1159/000242908. [DOI] [PubMed] [Google Scholar]
- Babyatsky M.W., Podolsky D.K. Growth and development of the gastrointestinal tract. In: Yamada T., editor. Textbook of Gastroenterology. Philadelphia: J.B. Lippincott; 1999. pp. 547–584. [Google Scholar]
- Barry S.T., Critchley D.R. The RhoA-dependent assembly of focal adhesions in Swiss 3T3 cells is associated with increased tyrosine phosphorylation and the recruitment of both pp125FAK and protein kinase C-delta to focal adhesions. J. Cell Sci. 1994;107:2033–2045. doi: 10.1242/jcs.107.7.2033. [DOI] [PubMed] [Google Scholar]
- Basora N., Vachon P.H., Herring-Gillam F.E., Perreault N., Beaulieu J.F. Relation between integrin alpha7Bbeta1 expression in human intestinal cells and enterocytic differentiation. Gastroenterology. 1997;113:1510–1521. doi: 10.1053/gast.1997.v113.pm9352853. [DOI] [PubMed] [Google Scholar]
- Beaulieu J.F. Extracellular matrix components and integrins in relationship to human intestinal epithelial cell differentiation. Prog. Histochem. Cytochem. 1997;31:1–78. doi: 10.1016/s0079-6336(97)80001-0. [DOI] [PubMed] [Google Scholar]
- Beaulieu J.F. Integrins and human intestinal cell functions. Front. Biosci. 1999;4:D310–D321. doi: 10.2741/beaulieu. [DOI] [PubMed] [Google Scholar]
- Beaulieu J.F., Quaroni A. Clonal analysis of sucrase-isomaltase expression in the human colon adenocarcinoma Caco-2 cells. Biochem. J. 1991;280:599–608. doi: 10.1042/bj2800599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu J.F., Millane G., Calvert R. Developmental expression of two antigens associated with mouse intestinal crypts. Dev. Dyn. 1992;193:325–331. doi: 10.1002/aja.1001930405. [DOI] [PubMed] [Google Scholar]
- Beaulieu J.F., Jutras S., Durand J., Vachon P.H., Perreault N. Relationship between tenascin and alpha-smooth muscle actin expression in the developing human small intestinal mucosa. Anat. Embryol. 1993;188:149–158. doi: 10.1007/BF00186248. [DOI] [PubMed] [Google Scholar]
- Bershadsky A., Kozlov M., Geiger B. Adhesion-mediated mechanosensitivity: a time to experiment, and a time to theorize. Curr. Opin. Cell Biol. 2006;18:472–481. doi: 10.1016/j.ceb.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Bieritz B., Spessotto P., Colombatti A., Jahn A., Prols F., Hartner A. Role of alpha8 integrin in mesangial cell adhesion, migration, and proliferation. Kidney Int. 2003;64:119–127. doi: 10.1046/j.1523-1755.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- Bosse T., Fialkovich J.J., Piaseckyj C.M., Beuling E., Broekman H., Grand R.J., Montgomery R.K., Krasinski S.D. Gata4 and Hnf1alpha are partially required for the expression of specific intestinal genes during development. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G1302–G1314. doi: 10.1152/ajpgi.00418.2006. [DOI] [PubMed] [Google Scholar]
- Bossy B., Bossy-Wetzel E., Reichardt L.F. Characterization of the integrin alpha 8 subunit: a new integrin beta 1-associated subunit, which is prominently expressed on axons and on cells in contact with basal laminae in chick embryos. EMBO J. 1991;10:2375–2385. doi: 10.1002/j.1460-2075.1991.tb07776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau F., Lussier C.R., Mongrain S., Darsigny M., Drouin J.L., Doyon G., Suh E.R., Beaulieu J.F., Rivard N., Perreault N. Loss of cathepsin L activity promotes claudin-1 overexpression and intestinal neoplasia. FASEB J. 2007;21:3853–3865. doi: 10.1096/fj.07-8113com. [DOI] [PubMed] [Google Scholar]
- Cetin S., Ford H.R., Sysko L.R., Agarwal C., Wang J., Neal M.D., Baty C., Apodaca G., Hackam D.J. Endotoxin inhibits intestinal epithelial restitution through activation of Rho-GTPase and increased focal adhesions. J. Biol. Chem. 2004;279:24592–24600. doi: 10.1074/jbc.M313620200. [DOI] [PubMed] [Google Scholar]
- Cetin S., Leaphart C.L., Li J., Ischenko I., Hayman M., Upperman J., Zamora R., Watkins S., Ford H.R., Wang J., Hackam D.J. Nitric oxide inhibits enterocyte migration through activation of RhoA-GTPase in a SHP-2-dependent manner. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G1347–G1358. doi: 10.1152/ajpgi.00375.2006. [DOI] [PubMed] [Google Scholar]
- Clark E.A., Brugge J.S. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Croft D.R., Olson M.F. The Rho GTPase effector ROCK regulates cyclin A, cyclin D1, and p27Kip1 levels by distinct mechanisms. Mol. Cell. Biol. 2006;26:4612–4627. doi: 10.1128/MCB.02061-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desloges N., Basora N., Perreault N., Bouatrouss Y., Sheppard D., Beaulieu J.F. Regulated expression of the integrin alpha9beta1 in the epithelium of the developing human gut and in intestinal cell lines: relation with cell proliferation. J. Cell Biochem. 1998;71:536–545. [PubMed] [Google Scholar]
- Dydensborg A.B., Herring E., Auclair J., Tremblay E., Beaulieu J.F. Normalizing genes for quantitative RT–PCR in differentiating human intestinal epithelial cells and adenocarcinomas of the colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G1067–G1074. doi: 10.1152/ajpgi.00234.2005. [DOI] [PubMed] [Google Scholar]
- Escaffit F., Boudreau F., Beaulieu J.F. Differential expression of claudin-2 along the human intestine: Implication of GATA-4 in the maintenance of claudin-2 in differentiating cells. J. Cell Physiol. 2005a;203:15–26. doi: 10.1002/jcp.20189. [DOI] [PubMed] [Google Scholar]
- Escaffit F., Perreault N., Jean D., Francoeur C., Herring E., Rancourt C., Rivard N., Vachon P.H., Pare F., Boucher M.P., et al. Repressed E-cadherin expression in the lower crypt of human small intestine: a cell marker of functional relevance. Exp. Cell Res. 2005b;302:206–220. doi: 10.1016/j.yexcr.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Escaffit F., Pare F., Gauthier R., Rivard N., Boudreau F., Beaulieu J.F. Cdx2 modulates proliferation in normal human intestinal epithelial crypt cells. Biochem. Biophys. Res. Commun. 2006;342:66–72. doi: 10.1016/j.bbrc.2006.01.128. [DOI] [PubMed] [Google Scholar]
- Farias E., Lu M., Li X., Schnapp L.M. Integrin alpha8beta1-fibronectin interactions promote cell survival via PI3 kinase pathway. Biochem. Biophys. Res. Commun. 2005;329:305–311. doi: 10.1016/j.bbrc.2005.01.125. [DOI] [PubMed] [Google Scholar]
- Fogerty F.J., Akiyama S.K., Yamada K.M., Mosher D.F. Inhibition of binding of fibronectin to matrix assembly sites by anti-integrin (alpha 5 beta 1) antibodies. J. Cell Biol. 1990;111:699–708. doi: 10.1083/jcb.111.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Bershadsky A. Assembly and mechanosensory function of focal contacts. Curr. Opin. Cell Biol. 2001;13:584–592. doi: 10.1016/s0955-0674(00)00255-6. [DOI] [PubMed] [Google Scholar]
- Giancotti F.G. Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr. Opin. Cell Biol. 1997;9:691–700. doi: 10.1016/s0955-0674(97)80123-8. [DOI] [PubMed] [Google Scholar]
- Gout S.P., Jacquier-Sarlin M.R., Rouard-Talbot L., Rousselle P., Block M.R. RhoA-dependent switch between alpha2beta1 and alpha3beta1 integrins is induced by laminin-5 during early stage of HT-29 cell differentiation. Mol. Biol. Cell. 2001;12:3268–3281. doi: 10.1091/mbc.12.10.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobin C., Behrns K.E., Brenner D.A. Molecular and cellular biology of the small intestine. Curr. Opin. Gastroenterol. 1999;15:103–107. doi: 10.1097/00001574-199903000-00003. [DOI] [PubMed] [Google Scholar]
- Lai J.M., Hsieh C.L., Chang Z.F. Caspase activation during phorbol ester-induced apoptosis requires ROCK-dependent myosin-mediated contraction. J. Cell Sci. 2003;116:3491–3501. doi: 10.1242/jcs.00660. [DOI] [PubMed] [Google Scholar]
- Levine D., Rockey D.C., Milner T.A., Breuss J.M., Fallon J.T., Schnapp L.M. Expression of the integrin alpha8beta1 during pulmonary and hepatic fibrosis. Am. J. Pathol. 2000;156:1927–1935. doi: 10.1016/s0002-9440(10)65066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock J.G., Wehrle-Haller B., Stromblad S. Cell-matrix adhesion complexes: master control machinery of cell migration. Semin. Cancer Biol. 2008;18:65–76. doi: 10.1016/j.semcancer.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Loirand G., Guerin P., Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ. Res. 2006;98:322–334. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- Lu M., Munger J.S., Steadele M., Busald C., Tellier M., Schnapp L.M. Integrin alpha8beta1 mediates adhesion to LAP-TGFbeta1. J. Cell Sci. 2002;115:4641–4648. doi: 10.1242/jcs.00145. [DOI] [PubMed] [Google Scholar]
- Lussier C., Basora N., Bouatrouss Y., Beaulieu J.F. Integrins as mediators of epithelial cell-matrix interactions in the human small intestinal mucosa. Microsc. Res. Tech. 2000;51:169–178. doi: 10.1002/1097-0029(20001015)51:2<169::AID-JEMT8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Ménard D., Beaulieu J.F. Human intestinal brush border membrane hydrolases. In: Bkaily G., editor. Membrane Physiopathology. Kluwer Academic; 1994. pp. 319–341. [Google Scholar]
- Ménard D., Beaulieu J.-F., Boudreau F., Perreault N., Rivard N., Vachon P.H. Gastrointestinal tract. In: Krieglstein K., Unsicker K., editors. Cell Signaling and Growth Factors in Development: From Molecules to Organogenesis, volume 2. Wiley-VCH; 2006. pp. 755–790. [Google Scholar]
- Mercurio A.M. Laminin receptors: achieving specificity through cooperation. Trends Cell Biol. 1995;5:419–423. doi: 10.1016/s0962-8924(00)89100-x. [DOI] [PubMed] [Google Scholar]
- Muller U., Bossy B., Venstrom K., Reichardt L.F. Integrin alpha 8 beta 1 promotes attachment, cell spreading, and neurite outgrowth on fibronectin. Mol. Biol. Cell. 1995;6:433–448. doi: 10.1091/mbc.6.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pageot L.P., Perreault N., Basora N., Francoeur C., Magny P., Beaulieu J.F. Human cell models to study small intestinal functions: recapitulation of the crypt–villus axis. Microsc. Res. Tech. 2000;49:394–406. doi: 10.1002/(SICI)1097-0029(20000515)49:4<394::AID-JEMT8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Perreault N., Beaulieu J. Use of the dissociating enzyme thermolysin to generate viable human normal intestinal epithelial cell cultures. Exp. Cell Res. 1996;224:354–364. doi: 10.1006/excr.1996.0145. [DOI] [PubMed] [Google Scholar]
- Perreault N., Herring-Gillam F.E., Desloges N., Belanger I., Pageot L.P., Beaulieu J.F. Epithelial vs mesenchymal contribution to the extracellular matrix in the human intestine. Biochem Biophys. Res. Commun. 1998;248:121–126. doi: 10.1006/bbrc.1998.8919. [DOI] [PubMed] [Google Scholar]
- Quaroni A., Beaulieu J.F. Cell dynamics and differentiation of conditionally immortalized human intestinal epithelial cells. Gastroenterology. 1997;113:1198–1213. doi: 10.1053/gast.1997.v113.pm9322515. [DOI] [PubMed] [Google Scholar]
- Reddig P.J., Juliano R.L. Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 2005;24:425–439. doi: 10.1007/s10555-005-5134-3. [DOI] [PubMed] [Google Scholar]
- Russo J.M., Florian P., Shen L., Graham W.V., Tretiakova M.S., Gitter A.H., Mrsny R.J., Turner J.R. Distinct temporal-spatial roles for Rho kinase and myosin light chain kinase in epithelial purse-string wound closure. Gastroenterology. 2005;128:987–1001. doi: 10.1053/j.gastro.2005.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp L.M., Breuss J.M., Ramos D.M., Sheppard D., Pytela R. Sequence and tissue distribution of the human integrin alpha 8 subunit: a beta 1-associated alpha subunit expressed in smooth muscle cells. J. Cell Sci. 1995a;108:537–544. doi: 10.1242/jcs.108.2.537. [DOI] [PubMed] [Google Scholar]
- Schnapp L.M., Hatch N., Ramos D.M., Klimanskaya I.V., Sheppard D., Pytela R. The human integrin alpha 8 beta 1 functions as a receptor for tenascin, fibronectin, and vitronectin. J. Biol. Chem. 1995b;270:23196–23202. doi: 10.1074/jbc.270.39.23196. [DOI] [PubMed] [Google Scholar]
- Sheppard D. Epithelial integrins. BioEssays. 1996;18:655–660. doi: 10.1002/bies.950180809. [DOI] [PubMed] [Google Scholar]
- Smith L.L., Cheung H.K., Ling L.E., Chen J., Sheppard D., Pytela R., Giachelli C.M. Osteopontin N-terminal domain contains a cryptic adhesive sequence recognized by alpha9beta1 integrin. J. Biol. Chem. 1996;271:28485–28491. [PubMed] [Google Scholar]
- Tetreault M.P., Chailler P., Beaulieu J.F., Rivard N., Menard D. Epidermal growth factor receptor-dependent PI3K-activation promotes restitution of wounded human gastric epithelial monolayers. J. Cell Physiol. 2008;214:545–557. doi: 10.1002/jcp.21239. [DOI] [PubMed] [Google Scholar]
- Tremblay E., Auclair J., Delvin E., Levy E., Menard D., Pshezhetsky A.V., Rivard N., Seidman E.G., Sinnett D., Vachon P.H., Beaulieu J.F. Gene expression profiles of normal proliferating and differentiating human intestinal epithelial cells: a comparison with the Caco-2 cell model. J. Cell. Biochem. 2006;99:1175–1186. doi: 10.1002/jcb.21015. [DOI] [PubMed] [Google Scholar]
- Turner C.E. Paxillin and focal adhesion signalling. Nat. Cell Biol. 2000;2:E231–E236. doi: 10.1038/35046659. [DOI] [PubMed] [Google Scholar]
- Vachon P.H., Beaulieu J.F. Transient mosaic patterns of morphological and functional differentiation in the Caco-2 cell line. Gastroenterology. 1992;103:414–423. doi: 10.1016/0016-5085(92)90829-n. [DOI] [PubMed] [Google Scholar]
- Vachon P.H., Simoneau A., Herring-Gillam F.E., Beaulieu J.F. Cellular fibronectin expression is down-regulated at the mRNA level in differentiating human intestinal epithelial cells. Exp. Cell Res. 1995;216:30–34. doi: 10.1006/excr.1995.1004. [DOI] [PubMed] [Google Scholar]
- Vainionpaa N., Kikkawa Y., Lounatmaa K., Miner J.H., Rousselle P., Virtanen I. Laminin-10 and Lutheran blood group glycoproteins in adhesion of human endothelial cells. Am. J. Physiol. Cell Physiol. 2006;290:C764–C775. doi: 10.1152/ajpcell.00285.2005. [DOI] [PubMed] [Google Scholar]
- Wagner T.E., Frevert C.W., Herzog E.L., Schnapp L.M. Expression of the integrin subunit alpha8 in murine lung development. J. Histochem. Cytochem. 2003;51:1307–1315. doi: 10.1177/002215540305101008. [DOI] [PubMed] [Google Scholar]
- Walker J.L., Assoian R.K. Integrin-dependent signal transduction regulating cyclin D1 expression and G1 phase cell cycle progression. Cancer Metastasis Rev. 2005;24:383–393. doi: 10.1007/s10555-005-5130-7. [DOI] [PubMed] [Google Scholar]
- Wang A., Yokosaki Y., Ferrando R., Balmes J., Sheppard D. Differential regulation of airway epithelial integrins by growth factors. Am. J. Respir. Cell Mol. Biol. 1996;15:664–672. doi: 10.1165/ajrcmb.15.5.8918373. [DOI] [PubMed] [Google Scholar]
- Yokosaki Y., Matsuura N., Higashiyama S., Murakami I., Obara M., Yamakido M., Shigeto N., Chen J., Sheppard D. Identification of the ligand binding site for the integrin alpha9 beta1 in the third fibronectin typeIII repeat of tenascin-C. J. Biol. Chem. 1998;273:11423–11428. doi: 10.1074/jbc.273.19.11423. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R., Ballestrem C., Kam Z., Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J. Cell Sci. 2003;116:4605–4613. doi: 10.1242/jcs.00792. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R., Cohen M., Addadi L., Geiger B. Hierarchical assembly of cell-matrix adhesion complexes. Biochem. Soc. Trans. 2004;32:416–420. doi: 10.1042/BST0320416. [DOI] [PubMed] [Google Scholar]
- Zargham R., Thibault G. alpha8beta1 Integrin expression in the rat carotid artery: involvement in smooth muscle cell migration and neointima formation. Cardiovasc. Res. 2005;65:813–822. doi: 10.1016/j.cardiores.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Zargham R., Thibault G. Alpha 8 integrin expression is required for maintenance of the smooth muscle cell differentiated phenotype. Cardiovasc. Res. 2006;71:170–178. doi: 10.1016/j.cardiores.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Zargham R., Wamhoff B.R., Thibault G. RNA interference targeting alpha8 integrin attenuates smooth muscle cell growth. FEBS Lett. 2007a;581:939–943. doi: 10.1016/j.febslet.2007.01.069. [DOI] [PubMed] [Google Scholar]
- Zargham R., Touyz R.M., Thibault G. alpha 8 Integrin overexpression in de-differentiated vascular smooth muscle cells attenuates migratory activity and restores the characteristics of the differentiated phenotype. Atherosclerosis. 2007b;195:303–312. doi: 10.1016/j.atherosclerosis.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Zhang J., Li W., Sanders M.A., Sumpio B.E., Panja A., Basson M.D. Regulation of the intestinal epithelial response to cyclic strain by extracellular matrix proteins. FASEB J. 2003;17:926–928. doi: 10.1096/fj.02-0663fje. [DOI] [PubMed] [Google Scholar]
- Zimerman B., Volberg T., Geiger B. Early molecular events in the assembly of the focal adhesion-stress fibre complex during fibroblast spreading. Cell Motil. Cytoskeleton. 2004;58:143–159. doi: 10.1002/cm.20005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.