Abstract
Dishevelled (Dvl) proteins are key transducers of Wnt signaling encoded by members of a multi-gene family in vertebrates. We report here the divergent, tissue-specific expression patterns for all three Dvl genes in Xenopus embryos, which contrast dramatically with their expression patterns in mice. Moreover, we find that the expression patterns of Dvl genes in the chick diverge significantly from those of Xenopus. In addition, in hemichordates, an outgroup to chordates, we find that the one Dvl gene is dynamically expressed in a tissue-specific manner. Using knockdowns, we find that Dvl1 and Dvl2 are required for early neural crest specification and for somite segmentation in Xenopus. Most strikingly, we report a novel role for Dvl3 in the maintenance of gene expression in muscle and in the development of the Xenopus sclerotome. These data demonstrate that the expression patterns and developmental functions of specific Dvl genes have diverged significantly during chordate evolution.
Introduction
Dishevelled (Dvl) is a multi-functional adaptor protein that transduces both canonical Wnt/β-catenin and planar cell polarity (PCP) signaling pathways, and recent studies also suggest roles for Dvl in vesicle trafficking and microtubule organization (Wallingford and Habas, 2005). As a component of the canonical Wnt pathway, Dvl functions downstream of Frizzled to govern the activity of axin and GSK3, thereby impacting β-catenin stability and nuclear localization (Baltzinger et al., 1999; Smalley et al., 1999; Amit et al., 2002; Bilic et al., 2007). Dvl has been implicated in a plethora of Wnt-mediated cell-fate specification events in multi-cellular animals including cnidarians, ecdysozoans, and deuterostomes (Noordermeer et al., 1994; Sokol et al., 1995a; Sokol et al., 1995b; Weitzel et al., 2004; Lee et al., 2007). Dvl-dependent PCP signaling controls a variety of polarized cell behaviors, including wing hair outgrowth in Drosophila (Theisen et al., 1994; Krasnow et al., 1995), and convergent extension cell movements, neural tube closure, and ciliogenesis in vertebrates (Sokol, 1996; Wallingford et al., 2000; Wallingford and Harland, 2002; Oishi et al., 2006; Wang et al., 2006; Park et al., 2008).
Wnt ligands and their Frizzled receptors are encoded by multi-gene families, and the precise expression patterns of the many Wnt and Frizzled genes have been well documented in animals ranging from vertebrates to cnidarians (Hotta et al., 2003; Kusserow et al., 2005; Garriock and Krieg, 2007). Indeed, the developmental and evolutionary significance of the individual Wnt genes has been the subject of intensive studies (e.g. (van Amerongen and Berns, 2006)). Like the Wnts and Frizzleds, vertebrate Dvls are also encoded by a multi-gene family, and the recent explosion of genome data has revealed that the number of Dvl genes varies among deuterostomes from one in ascidians and sea urchins to three discrete Dvl genes in most vertebrates genomes (Hotta et al., 2003). Based on current models of vertebrate genome evolution, the presence of three vertebrate paralogs is most likely the result of two rounds of genome duplication followed by gene loss of one of the paralogs (Sidow, 1996; Sidow et al., 1999; Wolfe, 2001; Dehal and Boore, 2005). Unlike the situation with Wnt and Frizzled genes however, the precise expression patterns and the developmental significance of the various Dvl genes has remained largely unexplored outside of the mouse.
Null mutants for Dvl1 in the mouse exhibit deficits in social interaction and sensorimotor gating (Lijam et al., 1997), and these mice display defects in synaptogenesis and in dendritic arborization (Rosso et al., 2005; Ahmad-Annuar et al., 2006). On the other hand, mouse Dvl2 is essential for normal cardiac morphogenesis, somite segmentation, and neural tube closure in the mouse (Hamblet et al., 2002). Dvl3 single mutants develop with heart defects (Etheridge et al., 2008). Mouse Dvl1, Dvl2, and Dvl3 are functionally redundant at least in part; double nulls and even trans-heterozygotes display more severe phenotypes than the single mutant Dvl2 or Dvl3 mice alone (Hamblet et al., 2002; Etheridge et al., 2008).
Our knowledge of the distinct developmental roles played by discrete Dvl genes in other vertebrates is almost completely lacking. Specific knockdown of Xenopus Dvl2 (previously called Xdsh, see below) has been shown to cause defects in the positioning of retinal progenitors (Lee et al., 2006), but the vast majority of experiments concerning Dvl function in other vertebrates have been carried out using dominant-negative constructs that are thought to disrupt the function of all three Dvls. As such, the specific roles of the different genes and the degree of redundancy among these genes, has not been investigated in vertebrate animals other than the mouse.
Dvl gene expression patterns have been reported for the mouse, where they are very broadly expressed (Sussman et al., 1994; Klingensmith et al., 1996; Steitz et al., 1996; Tissir and Goffinet, 2006). The broad expression patterns reported for Dvl genes in the mouse have been paraphrased as being ubiquitous, and so the transcriptional control of Dvl genes has been entirely ignored. Here, we show that the three Dvl genes have divergent and specific expression patterns in early embryogenesis in Xenopus and that these patterns differ significantly from those in the mouse. Moreover, we show that expression patterns of Dvl genes in the chick differ substantially from those in Xenopus and mouse. For a broader evolutionary perspective, we investigated the expression of the single copy of Dvl during the development of hemichordates, an outgroup to chordates, and show it is also developmentally dynamic and tissue-specific.
To better understand the developmental control of Wnt signaling mechanisms, we will need to correlate specific Wnt-mediated developmental events with the various Dvl genes in a variety of vertebrate animals. We therefore report here on the embryonic phenotypes that result from knockdown of specific Dvl genes in Xenopus. As described for mice, we find key roles for Dvl1 and Dvl2 in somite segmentation, while in contrast to the mouse, we observed specific roles for Dvl1 and Dvl2 in early neural crest specification. Finally, we identify a novel role for Dvl3 as a regulator of myotome and sclerotome cell fates in Xenopus. These Xenopus-specific functions for Dvl3 may reflect the evolutionarily derived mechanisms of muscle and sclerotome development in amphibia (Keller, 2000; Scaal and Wiegreffe, 2006; Handrigan and Wassersug, 2007).
Together, these data reveal a surprising divergence in the expression, and therefore, the developmental function, of specific Dvl genes in the vertebrates. Moreover, the data suggest that transcriptional control of Dvl gene expression may be an important regulator of Wnt-dependent developmental events in vertebrates, and when considered in combination with the spatially and temporally regulated expression of Dvl in hemichordates, this likely represents a common regulatory mechanism within the deuterostomes.
Methods and materials
Sequence analyses
Sequence alignment was performed using ClustalX (Jeanmougin et al., 1998). Neighbor-joining trees were constructed using PAUP* (Poe and Swofford, 1999; Rogers and Swofford, 1999). Non-parametric bootstrap proportions for clades were assessed with 1000 pseudo-replicates.
Xenopus embryos, in situ hybridization, and manipulations
Xenopus laevis embryos were obtained and cultured by standard methods (Sive, 2000). Embryos were allowed to develop in 0.3× Marc's modified Ringer (MMR) solution and staged according to the normal table of (Nieuwkoop and Faber, 1967).
Morpholino antisense oligonucleotides were obtained from GeneTools, LLC:
Dvl1MO, 5′-GGTAGATGATTTTGGTCTCAGCCAT-3′
5mis Dvl1MMMO, 5′-GcTAGATcATTTTcGTCTgAGCgAT-3′
Dvl2MO, 5′-TCACTTTAGTCTCCGCCATTCTGCG-3′
As described previously (Lee et al., 2006).
Dvl3MO, 5′-GGTAGATGACCTTGGTCTCCCCCAT-3′
Dvl3 Alternate MO, 5′-GGCTTAGCCCCAAAATCTGTCATAC-3′
5mis Dvl3 MMMO, 5′-GcTAGATcACCTTcGTCTgCCCgAT-3′
Embryos were allowed to develop until desired stage and then fixed for 2 h in MEMFA, except for the fibronectin antibody where embryos fixed in Dent's fixative. In situ hybridizations were carried out with RNA probes labeled with digoxigenin-UTP by using the technique previously described (Sive et al., 2000). Probes used were Pax3, (Mariani et al., 2001); XMyoD, (Hopwood and Gurdon, 1990); Dvl1, CD362087(ATCC); Dvl2{Sokol, 1995 #3404}; Dvl3, XL190g16 (NIBB); XMyf5, (Hopwood et al., 1991); XSlug, (Mancilla and Mayor, 1996); XTwist, (Hopwood et al., 1989); Myoskeletin, (Zhao et al., 2007); XParaxis, (NIBB); and XPax1, XL085n23 (NIBB). The muscle-specific 12/101 monoclonal antibody was used to visualize differentiated somites (Kintner and Brockes, 1984) (Developmental Studies Hybridoma Bank, University of Iowa). 12/101 was used at 1:5, as described (Sive et al., 2000), along with anti-mouse 2° Alexafluor 488 (Molecular Probes). For combined in situ hybridization and immunostaining, in situ staining was performed first.
Chick embryos and in situ hybridization
Fertilized Leghorn eggs (Ideal Poultry, Texas) were incubated at 38°C in a forced draft-humidified chamber. Embryos were staged according to Hamburger and Hamilton (Hamburger and Hamilton, 1951).
Embryos were harvested between embryonic day 2 and 3 and immersion-fixed in 4% paraformaldehyde. Digoxigenin-conjugated antisense riboprobes were prepared from chick cDNAs for chick DVL1 and DVL3. Whole-mount in situ hybridizations were conducted using NBT (Roche) tetrazolium histochemistry according to previously established protocols (Agarwala et al., 2001; Agarwala and Ragsdale, 2002). Probe fragments for chick DVL1 and DVL3 were generated by polymerase chain reaction from a pool of E2 whole embryo cDNA and then subcloned into the pCR-TOPO II vector (Invitrogen). Primers were designed using MacVector based on sequences submitted to NCBI (chick Dvl1: NM001030873 gi-71894888 and chick Dvl3: XM422756 gi-118095208). The primer pairs are as follows:
Chick DVL1
Forward: 5′-GGATTGGACAACGAAACAGGAAC-3′
Reverse: 5′-TGTGGAAGTGGAGCAAGGGTATC-3′
Chick DVL3
Forward: 5′-AATACTGGGGGGAGTCGGGAAAAC-3′
Reverse: 5′-TTCACGGTGTGTCGGATGTAGC-3′
Hemichordate embryos and in situ hybridization
In situ hybridizations were done according to (Lowe et al., 2004), but post-fixation was done in formaldehyde rather than in Bouin's fixative.
Results and Discussion
Divergence of Dvl genes during chordate evolution
The genus Xenopus includes several species, and extensive sequence data are available for two of these, X. tropicalis and X. laevis. Examination of the X. tropicalis genome and of the EST collections for both X. tropicalis and X. laevis reveal that Xenopus has three Dvl genes, as in mammals. We assigned Xenopus Dvl gene names based upon comparison of Xenopus sequence to known human and mouse orthologues. The original Xdsh clone has thus been re-assigned as Dvl2 (U31552){Sokol, 1995 #3404}. We obtained full-length EST clones for Dvl1 and Dvl3 (CD362859 and BJ636385, respectively).
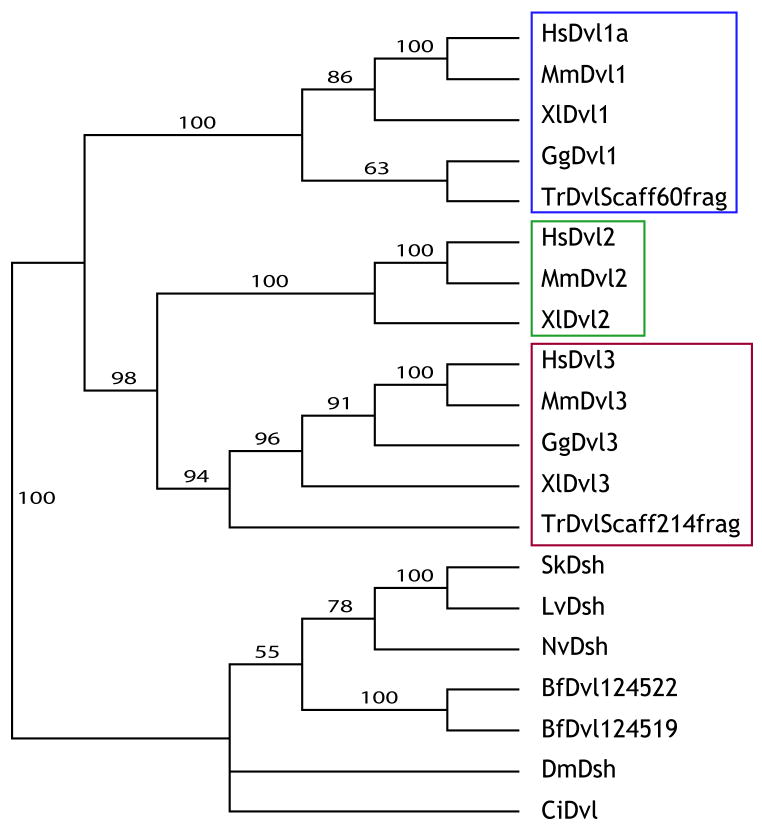
Phylogenetic analyses comparing Xenopus, human and mouse Dvl protein sequences clearly placed the three Xenopus Dvl genes into three different clades with orthologous mouse and human Dvls, strongly supporting orthology of each gene (Fig. 1).
Figure 1. Phylogenic analysis of Dishevelled (Dvl) family genes.
Blue box outlines Dvl1 family genes. Green box outlines Dvl2 family genes. Red box outlines Dvl3 family genes. Hs- Homo sapiens; Mm- Mus musculus; Xl- Xenopus laevis; Gg- Gallus gallus; Tr- Takifugu rubripes; Sk- Saccoglossus kowalevskii; Lv- Lytechinus variegates; Nv-Nematostella vetensis; Bf- Branchiostoma floridae; Dm- Drosophila melanogaster; Ci-Ciona intestinalis.
The genomes of deuterostomes vary in their number of Dvl genes, from one in hemichordates, urchins, and ascidians, to three in most vertebrates. We therefore examined sequence information to begin to understand the divergence of Dvl genes in the deuterostomes. Hemichordates and sea urchins are basal deuterostomes, and like the non-deuterostome Drosophila, each has only one Dvl gene (Fig. 1). This is consistent with a model in which the common ancestor of deuterostomes had only one Dvl gene. The other deuterostomes, the chordates, are divided into three groups: the urochordates (including ascidians), cephalochordates (including Amphioxus) and vertebrates. Ascidians have only one Dvl gene, but interestingly, searches of EST data identify two Dvl genes in the genome assembly of the cephalochordate amphioxus (Branchiostoma fl. (Putnam et al., 2008)). Phylogenetic analyses place these two genes within their own clade, separate from the vertebrate Dvls (Fig. 1), indicating these genes are a product of a duplication within the cephalochordate lineage, independent of gene duplications in the vertebrate lineage. Together, these data suggest there was one Dvl gene in the common ancestor of chordates.
Of the three Dvl genes in most vertebrates, phylogenetic analysis suggests Dvl2 and Dvl3 form a clade distinct from Dvl1 (Fig. 1A). This suggests that within the vertebrate lineage, the ancestral Dvl duplicated once to produce two lineages represented by Dvl1 and Dvl2/3, and a secondary duplication in the latter gene lineage produced Dvl2 and Dvl3. If these duplications are a result of the two whole-genome duplications early within the vertebrate lineage, then another paralogue within the Dvl1 subfamily (Dvl4) was subsequently lost.
The patterns of Dvl1, Dvl2, and Dvl3 expression differ substantially between mouse and Xenopus
To understand the roles played by Dvl1, Dvl2 and Dvl3 during Xenopus development, we first examined their expression patterns by in situ hybridization. We observed that all three Dvls were equivalently expressed in the marginal zone mesoderm and somewhat more weakly in the ectoderm at early gastrulation stages (Supp. Fig. 1). However, at later stages, the expression patterns of the three genes diverged considerably. The pattern of Dvl1 expression in Xenopus shares some similarity with the expression of Dvl1 in the mouse, in particular the robust expression in the neural tube and somites (Fig. 2; Supp. Figs. 2, 3)(Sussman et al., 1994). Moreover, later strong expression of Dvl1 in the cranial placodes of Xenopus (Supp. Fig. 3) may reflect expression of mouse Dvl1 in cranial ganglia (Sussman et al., 1994; Tissir and Goffinet, 2006).
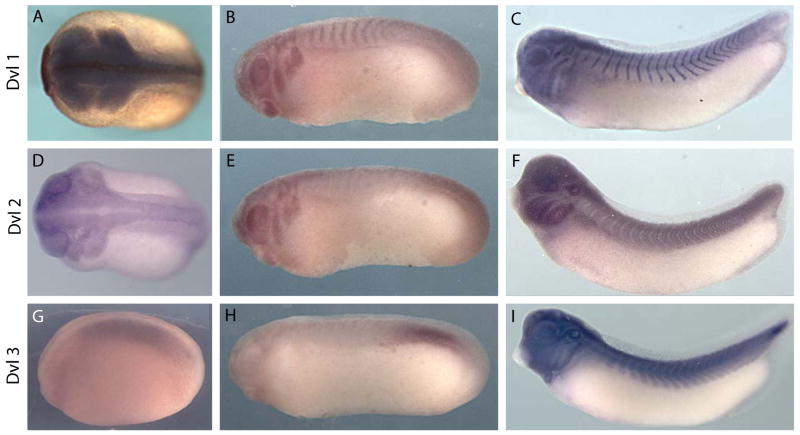
Figure 2. Divergent expression patterns of Dvl1, Dvl2, and Dvl3 in Xenopus embryos.
(A): Dorsal view of a stage 20 embryo exhibits Dvl1 expression in neural crest, optic placodes, and neural folds. (B): Lateral view of a stage 23 embryo shows continued Dvl1 expression in the streaming cranial neural crest and the developing eye, as well as the otic placodes and the segmenting somites. (C): Lateral view of a stage 29/30 embryo expressing Dvl1 in the somites and in multiple cranial placodes (see Supp Fig. 2). (D): Dorsal view of a stage 22 embryo exhibiting Dvl2 expression in the streaming cranial neural crest, developing eyes, and neural folds. (E): Lateral view of a stage 23 embryo expressing Dvl2 in the otic placodes, neural crest and eyes. (F): Lateral view of a stage 29/30 embryo exhibits Dvl2 staining in the somites, developing eye, and brancial arches. (G): Lateral view of a stage 18 embryo expressing Dvl3 in paraxial mesoderm. (H): Lateral view of a stage 23 embryo exhibits Dvl3 expression in the presomitic mesoderm and developing somitomeres. (I): Lateral view of a stage 35/36 embryo expressing Dvl3 in mature somites, multiple head placodes, and the developing heart.
To our surprise however, there were several important points of divergence between the expression patterns of Dvl1 in Xenopus and mouse. For example, while Dvl1 is not expressed in the mouse node (Sussman et al., 1994), Dvl1 was robustly expressed during gastrulation in involuting dorsal tissues, with pan-dorsal expression during neurulation (Fig. 2A-C; Supp. Fig. 3). The most significant difference was the strong and specific expression of Dvl1 in the developing neural crest and in the otic placodes (Fig. 2A, B; Supp. Fig. 3).
Divergence in expression patterns between mouse and Xenopus was even more pronounced for Dvl2. In the mouse, embryonic Dvl2 expression has been reported to be essentially ubiquitous (Klingensmith et al., 1996; Tissir and Goffinet, 2006). By contrast, Dvl2 expression was highly upregulated in dorsal tissues during Xenopus neurulation, and strong expression of Dvl2 was observed in the migrating neural crest at early tailbud stages. At later tailbud stages, Dvl2 is very strongly expressed in the branchial arches, otic placodes and also in the somites (Fig. 2F; Supp. Fig. 4).
Most surprising was the divergence of the Xenopus Dvl3 expression patterns from those of other Dvl family members in Xenopus and also the divergence between Dvl3 expression patterns in Xenopus and mouse. In the mouse, early expression of Dvl3 is ubiquitous, but Dvl3 transcription is subsequently upregulated in neural tissue and somites, before again becoming ubiquitous (Tsang et al., 1996; Tissir and Goffinet, 2006). Dvl3 expression in Xenopus is entirely different. During neurulation, we consistently observed restriction of Dvl3 gene expression to the paraxial mesoderm and then later to presomitic mesoderm (Fig. 2G, H; Supp. Fig. 5). This pattern is maintained well into the late tailbud stages, at which time Dvl3 is also expressed in the developed somites. At these stages, we also observed strong Dvl3 expression in the heart and in a subset of cranial placodes (Fig. 2I; Supp. Fig. 5E).
Dvl expression patterns in the chick differ substantially from those in Xenopus
Given that Dvl gene expression in the mouse has been thought to be somewhat ubiquitous, we found the divergent, tissue-specific expression patterns for Dvl genes in Xenopus to be striking. To ask whether or not such tissue-specific expression of Dvl genes was a general feature of vertebrate animals, we examined Dvl gene expression in the chick. In contrast to Xenopus and mouse, we identified only two Dvl homologues in the chick genome, consistent with previous reports (Sweetman et al., 2008). Based upon primary sequence, the two chick Dvls corresponded to Dvl1 and Dvl3 (Fig. 1).
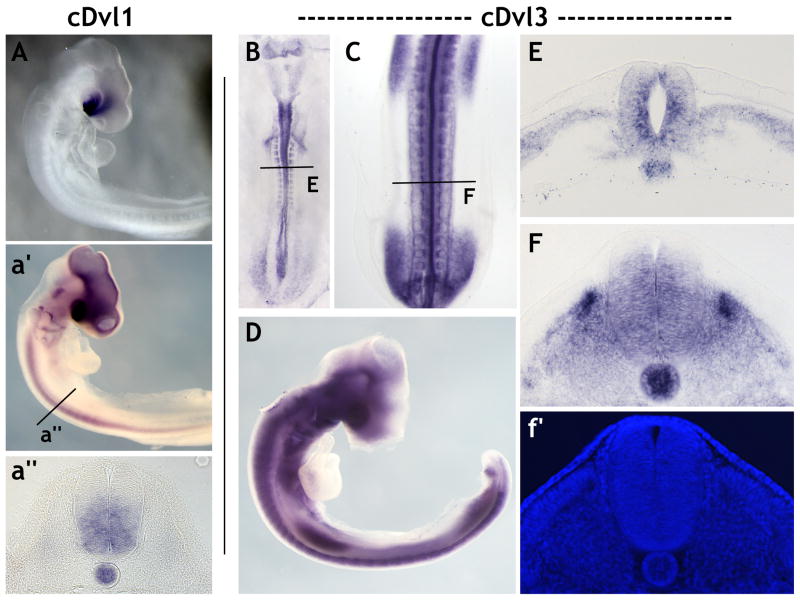
The expression patterns of these two Dvl genes differed dramatically from those observed in Xenopus. Dvl1 was not detected by in situ hybridization in embryonic day 2 (E2) chick embryos (not shown), consistent with previous reports that it does not mediate the PCP-dependent cell movements during chick gastrulation (Hardy et al., 2008; Sweetman et al., 2008). By E3, Dvl1 is very highly expressed, but its expression is largely restricted to the optic stalk and ventral forebrain (Fig. 3A). Upon longer exposure, Dvl1 was also detected throughout the ventral spinal cord and the notochord (Fig. 3a′, 4a″). (We also observed staining in the midbrain and forebrain, but these are most likely artifacts of trapping of the chromogenic substrate in the lumen of the brain.) We observed no expression of Dvl1 in the somites or neural crest in the chick.
Figure 3. Divergent expression patterns of Dvl1 and Dvl3 in the chick.
(A): Lateral view of Dvl1 expression in an E3 chicken embryo. Dvl1 transcripts are strongly localized to the optic stalk and the ventral forebrain. (a′): Extending the length of the color development reveals Dvl1 expression throughout the midbrain, forebrain, eye, spinal cord and notochord. (a″): A cross-section through the spinal cord at the level indicated in (a′). Dvl1 expression is restricted to the notochord and the ventral spinal cord. (B): Dorsal view of Dvl3 expression in an E2 chicken embryo. Dvl3 is expressed in the somites, the tail bud, and throughout the neural tube. (C): Dorsal view of Dvl3 expression in the posterior half of an E3 chicken embryo. Dvl3 expression remains within the neural tube and somites, but is also present in the fore- and hind-limb buds. (D): Lateral view of Dvl3 expression in an E3 embryo. (E): A cross-section through an E2 embryo as indicated in (B). Dvl3 is expressed in the neural tube, the notochord and presomitic mesoderm. Note the elevated level of Dvl3 expression within the cells of the ventricular layer. (F): A cross-section through an E3 embryo as indicated in (C). Dvl3 transcripts are observed in the notochord and the entire spinal cord except for the dorsal-most region. Dvl3 expression in the developing somites is restricted to the ventral dermomyotome, the dorsal sclerotome and the myotome itself. Note the increased level of Dvl3 expression in the medial dermomyotome. (f′): DAPI staining of the section shown in (F) clearly reveals the organization of the somite and the cells within.
Figure 4.
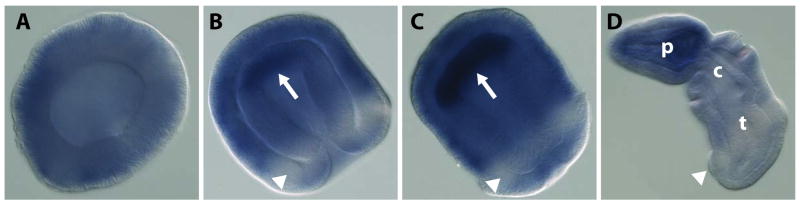
Wholemount in situ hybridization of S. kowalevskii Dvl at the following stages: a) blastula, b) gastrula, c) elongating gastrula, and d) prehatching juvenile. All embryos are shown with anterior to the upper left, and the juvenile embryo has dorsal positioned to the upper right. White arrows indicate Dvl up regulation in the anterior mesoderm, and the white arrowheads note the location of the ectodermal ciliated band. p: proboscis, c: collar, t: trunk.
In stark contrast to the very localized expression of Dvl3 in Xenopus, Chick Dvl3 was quite broadly expressed. At E2, Dvl3 was observed in the brain, spinal cord and in the somites (Fig. 3B). Examination in cross-sections revealed an elevated level of Dvl3 expression in the ventricular layer of cells in the spinal cord in addition to its localization in the presomitic mesoderm and the notochord (Fig. 3E). By E3, Dvl3 expression remained within the neural tube, notochord, and the somites but also was observed in the eye and the fore- and hind-limb buds (Fig. 3C, D). Interestingly, Dvl3 expression in the developing somites was restricted to the sclerotome as well as part of the dermomyotome (Fig. 3F, f′).
Dvl transcription is tightly regulated in the embryos of the basal deuterostome, Saccoglossus kowalevskii
Because mouse, frog and chick homologs of Dvl have such divergent expression domains, we decided to examine Dvl in a non-chordate deuterostome phylum. The hemichordate Saccoglossus kowalevskii is a good outgroup for examinations of chordate Dvl genes (Lowe, 2008), and we found one Dvl ortholog by searching the hemichordate EST library and genome trace archives. Sequence analysis shows that it is a homolog to vertebrate Dvl genes (Fig. 1). In situ hybridization of this Dvl gene shows expression is limited to specific tissues during early development (Fig. 4). In blastulae, Dvl is expressed ubiquitously, but by gastrulation Dvl is downregulated in the posterior ectoderm surrounding the ciliated band (Fig. 4A, B). During late gastrulation, Dvl is heavily upregulated in anterior mesoderm, but downregulated in endoderm and posterior mesoderm. In juveniles, Dvl is expressed heavily in the anterior, including the ectoderm and mesoderm, but in posterior tissues is either expressed at lower levels or is completely absent (Fig. 4C, D).
Thus, expression patterns of S. kowalevskii Dvl are highly regulated and tissue-specific, including expression within the ectoderm and mesoderm. Since both a non-chordate and vertebrates show extensive regulation of Dvl genes at the level of transcription, it is highly likely that ancestral chordate Dvl gene was subject to temporal and spatial transcriptional regulation during early development.
Knockdown of Dvl1 and Dvl2, but not Dvl3, disrupts early neural crest specification in Xenopus
Because the Dvl genes have divergent expression patterns in Xenopus embryos as compared to mouse embryos, we next sought to uncover the developmental functions of the three Dvls in Xenopus and to ask if the assignment of specific Dvl genes to specific developmental events varied between Xenopus and mice. We have generated translation-blocking morpholinos (MOs) that specifically target each Dvl gene in Xenopus (Table 1). The specificity of these MOs for particular Xenopus Dvls has been demonstrated previously using in vitro translated proteins and western blotting of embryo lysates with anti-Dvl antibodies (Park et al., 2008).
Table 1. Complementary base-pairing of Dvl genes to MO used in this study.
Number indicates the number of mismatched base-pairs for each MO and each gene. Green values indicate that the MO should effectively target the gene. Red values indicate 4- or 5- Bp mismatches between a given MO and a given Dvl gene mRNA sequence. These can serve as controls of MO specificity. (Note: For Dvl2 and Dvl3, our the sequences targeted by the MOs are perfectly conserved for both alloalleles, as identified from EST data; for Dvl1, only a single EST exists.)
| Gene → | Dvl1 | Dvl2 | Dvl3 |
|---|---|---|---|
| Morpholinos: | |||
| Dvl1 | 0 | 5 | 4 |
| Dvl1 MM | 5 | 7 | 9 |
| Dvl2 | 9 | 0 | 9 |
| Dvl3 | 4 | 5 | 0 |
| Dvl3B | 12 | 11 | 0 |
| Dvl3 MM | 9 | 7 | 5 |
Dorsally-targeted triple knockdown of all Xenopus Dvls resulted in defects consistent with previous reports using dominant-negative to disrupt Dishevelled function (Sokol et al., 1995a; Sokol et al., 1995b; Sokol, 1996). We observed failure of convergent extension and defects in the anterior-posterior pattern of the central nervous system (Supp. Fig. 6). The aim of this study, however, is to understand gene-specific Dvl functions and how they differ between vertebrates. One key difference in Dvl gene expression between mouse and Xenopus is the strong and specific expression of Dvl1 and Dvl2 in the Xenopus neural crest. Dominant-negative studies have implicated Dvl in mediating PCP signals that govern neural crest migration in Xenopus (De Calisto et al., 2005), but the role of Dvl in early neural crest specification has not been directly examined. Canonical Wnt signaling is known to be crucial to Xenopus neural crest induction (LaBonne and Bronner-Fraser, 1998b; Monsoro-Burq et al., 2005), though this mechanism may not be conserved in amniotes (Schmidt et al., 2007).
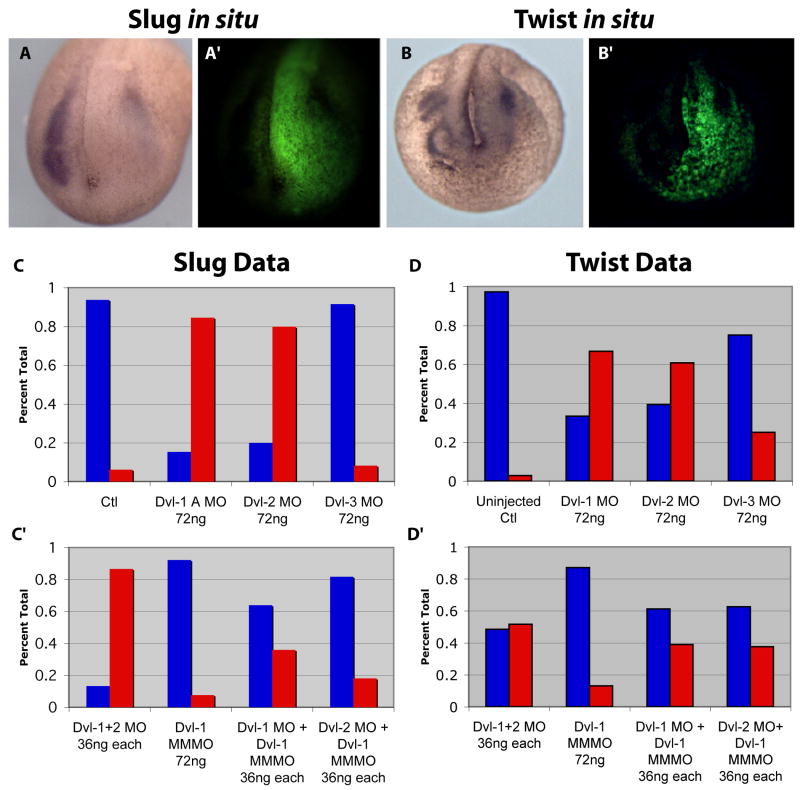
To probe the significance of the strong expression of Dvl in Xenopus neural crest, we injected Dvl1 MO to one dorsal-lateral blastomere at the 16-cell stage of development in order to unilaterally affect the presumptive neural crest (Moody and Kline, 1990). MOs were co-injected with a fluorescent dextran lineage tracer, and only properly targeted embryos (Fig. 5A′,B′) were processed for in situ hybridization for Slug as a marker of early neural crest and Twist as a marker of later neural crest (Aybar et al., 2003). Injection of the Dvl1 MO at a high dose disrupted the expression of Slug in over 80% of embryos (Fig. 5A, C). At later stages, the Dvl1 morphants showed similar disruptions of Twist expression (Fig. 5B, D).
Figure 5. Neural crest induction in Xenopus requires Dvl1 and Dvl2, but not Dvl3.
(A-A″): Unilateral injection of MOs + GFP mRNA targeted to one dorsal lateral blastomere of a 16 cell embryo. Slug expression is lost on the injected side due to Dvl1 and Dvl2 morpholinos. (B-B″): Dvl1 and Dvl2 MOs eliminate expression of Twist. (C-C′): Graphs of combined morpholino data for Slug in situ hybridizations. Data shown are percent of embryos showing normal or abnormal expression of Slug or Twist. Uninjected Control N=16; Dvl-1MO 72ng N=26; Dvl-2MO 72ng N=10; Dvl-3MO 72ng N=12; Dvl-1MO + Dvl-2MNO 36ng each N=15; Dvl-1MMMO 72ng N=13; Dvl-1MO + Dvl-1MMMO 36ng each N=25; Dvl-2MO + Dvl-1MMMO 36 ng each N=11. (D and D′): Graphs of combined data for Twist in situ hybridizations. Uninjected Control N=36; Dvl-1MO 72ng N=84; Dvl-2MO 72ng N=28; Dvl-3MO 72ng N=20; Dvl-1MO + Dvl-2MNO 36ng each N=31; Dvl-1MMMO 72ng N=54; Dvl-1MO + Dvl-1MMMO 36ng each N=18; Dvl-2MO + Dvl-1MMMO 36 ng each N=24.
To demonstrate the specificity of this neural crest phenotype, we designed a 5-bp mismatch MO to Dvl1 (Dvl1MMMO). Injection of this Dvl1MMMO at a high dose had no effect on Slug or Twist expression (Fig. 5C′, D′). Moreover, the effects of Dvl1MO were dose-dependent; co-injection of one-half doses of Dvl1MO and the Dvl1MMMO resulted in a dramatic reduction in the number of embryos with disrupted Slug or Twist expression as compared to injection of a high dose of Dvl1MO alone (Fig. 5C, D).
Dvl2 is co-expressed in the Xenopus neural crest with Dvl1, so we next tested the role of Dvl2 in neural crest specification. Like Dvl1 morphants, Dvl2 morphants also failed to express Slug or Twist (Fig. 5C, D). Consistent with functional redundancy between Dvl1 and Dvl2, we observed that injection of a combined half-dose of Dvl1MO and Dvl2MO (36ng each) resulted in neural crest defects equivalent to injection of a full dose (72ng) of either MO alone. In contrast, injection of a half doses of the Dvl2MO and Dvl1MMMO(s) resulted in far fewer disruptions of slug or twist expression (Fig. 5C′, D′). These data indicate that the MO phenotypes are specific and that Dvl1 and Dvl2 share some functional redundancy in mediating neural crest induction in Xenopus.
As a final control, we injected our MO targeted against Dvl3. Consistent with the absence of Dvl3 expression in early neural crest (Fig. 3G, H), injection of a full dose of the Dvl3MO had no effect on Slug expression (Fig. 5C). Dvl3 MO had a very modest effect on the later expression of Twist, consistent with Dvl3's low level expression in the crest at later stages (Supp. Fig 5D). Serendipitously, our Dvl3MO differs from our Dvl1 MO by only 4bps (Table 1), so the Dvl3 MO serves as an important control for specificity. Injection of the Dvl3MO did not disrupt early neural crest induction, but did consistently elicit specific defects in somite development that were not observed with Dvl1MO or Dvl2MO (see below). Thus, dose-dependent MO phenotypes for each Dvl gene correlated with sites of expression for each Dvl, while mismatched MOs had no effect.
Taken together, these data suggest that Dvl1 and Dvl2, but not Dvl3, are necessary to mediate the Wnt-dependent signals that control neural crest specification in Xenopus (e.g. (LaBonne and Bronner-Fraser, 1998a; Monsoro-Burq et al., 2005)). This result is important, as it highlights the divergent roles of the three vertebrate Dvl genes in the development of different animals. No neural crest-specific expression has been reported for any mouse Dvl. Dvl1 mutant mice do not display neural crest defects (Lijam et al., 1997), but neural crest does contribute importantly to the development of the cardiac outflow tracts, and these are defective in mice lacking Dvl2 (Hamblet et al., 2002). These results also suggest a lack of redundancy for Dvl1 and Dvl2 in neural crest development; lack of Dvl gene redundancy has also been observed for certain phenotypes in the n mouse (Etheridge et al., 2008).
A conserved role for Dvl1 and Dvl2 in somite segmentation
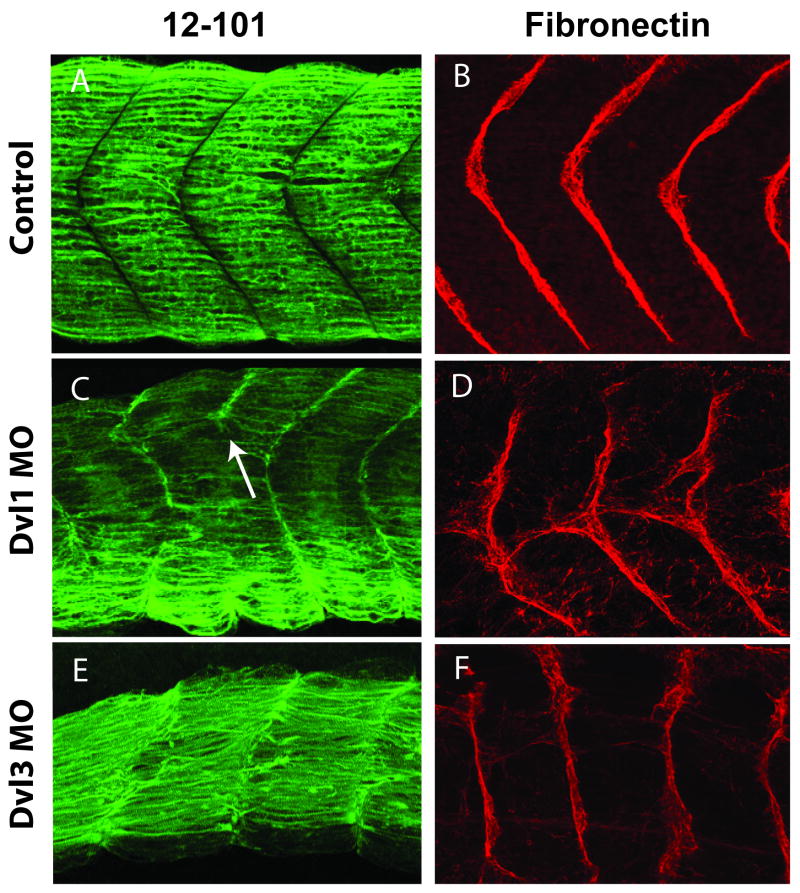
Xenopus Dvl genes are expressed in divergent patterns in the developing somite and presomitic mesoderm (Fig. 2). In order to study the role of Dvls in somitogenesis while avoiding early morphogenetic defects, we targeted our MOs to a subset of the presumptive somites using ventral-vegetal injections (Moody and Kline, 1990; Afonin et al., 2006). Targeted injections of the Dvl1 or Dvl2 MOs alone or a combination of Dvl1 and Dvl2 MOs resulted in embryos that were short in length and that displayed defects in somite boundary formation. Using Immunostaining for 12/101 and for fibronectin, we observed both failure of boundary formation, and ectopic boundary formation in Dvl1 and Dvl2 morphants (Fig. 6C, D; data not shown). In these morphants, the expression of MyoD was maintained throughout the somite (Fig. 7B). This phenotype is consistent with studies in mice lacking Dvl1 and Dvl2 (Hamblet et al., 2002).
Figure 6. Divergent, gene-specific somite defects in Dvl morphants.
Lateral confocal projections displaying somite morphology of Dvl morphants with a muscle specific mouse anti-12/101 antibody (green) and the somite boundaries with a mouse anti-fibronectin antibody (red). (A): Normal morphology of the medial flank of a stage 29/30 embryo (B): Normal fibronectin deposition in somite boundaries stage 29/30 embryo. (C): Dvl1 morphant stage 29/30 embryo displaying segmentation defect (white arrow) (D): Dvl1 morphant stage 29/30 embryo displays defects in the deposition of fibronectin (red) while “chevron” shape is conserved. (E): Dvl3 morphant stage 29/30 embryo displaying un-“chevroned” somites and multiple cells are disrespecting the established somite boundaries. (F): Dvl3 morphant stage 29/30 embryo displaying normal deposition of fibronectin between un-“chevroned” boundaries. Somite defects were observed in 50-90% of morphants, depending upon the experiment.
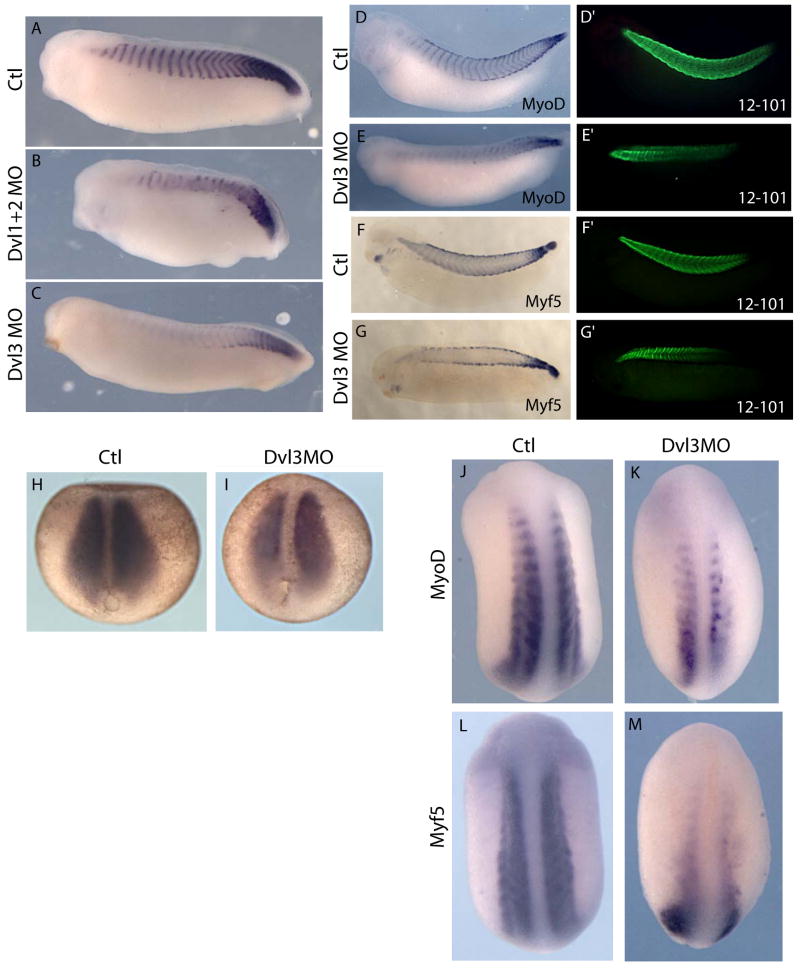
Figure 7. Knockdown of distinct Dishevelled genes generates distinct somite phenotypes.
(A): In situ hybridization to MyoD at control stage 29/30. (B): Injection of Dvl1+2MO cocktail yields shorter embryos that exhibit a disruption of the normal MyoD pattern. (C): Dvl3 morphants are normal in length, but embryos exhibit diminished anterior MyoD expression. (D): St. 32, normal MyoD (purple) in situ hybridization and (D′) 12/101 (green) antibody staining. (E): In Dvl3 morphants, MyoD expression is reduced anteriorly but 12/101 (E′) is maintained. 12/101 staining is increased at the somite boundaries (red arrows). (F): Normal Myf5 expression (purple) within somites and along both epaxial and hypaxial somite edges and (F′) normal 12/101 staining. (G): Myf5 staining of the hypaxial and epaxial somite edges appear unaffected in Dvl3 morphants, but expression is lost within the somites, though 12/101 is maintained (G′). (H): Control stage 13 in situ hybridization for MyoD. (I): Sibling stage 13 Dvl3 morphant embryo in situ hybridized to MyoD (purple). (J, L): Normal expression patterns of bHLH myogenic transcription factors; MyoD and Myf5 at stage 22. (K, M): Reduced anterior expression of assayed myogenic markers in Dvl3 morphants at stage 22.
A novel role for Dvl3 in the maintenance of gene expression in the Xenopus myotome
The most striking difference between mouse and Xenopus Dvls was the strong and specific expression of Dvl3 in the Xenopus presomitic and somitic mesoderm (Fig. 2). This unique pattern of Dvl3 expression is interesting given that somite development is highly derived in amphibians in general, and that somitogenesis is variable even among the anurans, being particularly derived in Xenopus (Keller, 2000; Scaal and Wiegreffe, 2006; Handrigan and Wassersug, 2007). It is thought that the derivation of somite development in amphibians is an adaptation to their initially-aquatic lifestyle; the tadpole tail lacks an osseous skeleton and is more flexible that that of most fishes (Hoff and Wassersug, 2000; Handrigan and Wassersug, 2007). To better understand the role of this unique domain of Dvl3 expression in Xenopus, we examined the effects of targeted Dvl3 knockdown in the somites.
In contrast to Dvl1 and Dvl2, Dvl3 knockdown had little effect on somite segmentation (Fig. 6). Reflecting their highly derived nature, Xenopus somites are formed from mesenchymal, rather than epithelial, cells. These cells are initially oriented perpendicular to the anteroposterior axis; the cells then rotate en mass to take on their final orientation parallel to the long axis (Afonin et al., 2006; Handrigan and Wassersug, 2007). However, we observed anteroposteriorly aligned cells in Dvl3 morphants, suggesting no role for Dvl3 in somite rotation (Fig. 6E).
We did however consistently observe an attenuation of MyoD expression in the anterior somites of Dvl3 morphants (Fig. 7C, E). Curiously, this decrease of anterior MyoD expression was not accompanied by a decrease in anterior 12/101 staining (Fig. 7E′), suggesting that somites had been patterned normally, but that MyoD expression was not maintained. We observed a similar phenotype when we examined Myf5. Myf5 expression in the body of the somite was reduced anteriorly, though expression in epaxial and hypaxial muscle was unaffected by these injections (Fig. 7G). As with MyoD, the reduction of Myf5 was not accompanied by loss of 12/101staining (Fig. 7G′).
Several lines of evidence demonstrate that this somite phenotype was specific for Dvl3. First, we observed the same loss of anterior MyoD expression with a second, independent Dvl3 MO (Table 1; data not shown). Second, a 5bp mismatched MO for Dvl3 had no effect on MyoD expression (data not shown). Third, and most compellingly, our Dvl1 MO differs from the Dvl3 mRNA sequence by only 4bp (Table 1), but injection of Dvl1 MO consistently disrupted somite segmentation and did not attenuate anterior MyoD expression (Fig. 7B; Fig. 6C).
We next asked if the reduction in MyoD expression resulted from a failure to initiate or a failure to maintain expression. We assessed the expression of myogenic regulatory factors at a variety of stages in Dvl3 morphants. When assessed at stage 13, Dvl3 morphants displayed no significant loss of MyoD or Myf5 (Fig. 7I; data not shown). By contrast, we found that expression of MyoD, Myf5, and also MyoS (another bHLH myogenic regulator) was clearly disrupted in anterior regions at early tailbud stages in Dvl3 morphant embryos (Fig. 7K, M; data not shown). These data reveal a novel, Dvl3-specific function in maintaining expression of the myogeneic regulatory transcription factors in Xenopus. We have found no reports of a similar phenotype following manipulation of Wnt signaling in other animals, so this result may reflect the highly derived nature of somitogenesis in Xenopus. It is tempting to speculate that this Xenopus-specific role for Dvl3 in controlling gene-expression in muscle cells may be related in some way to the delayed multi-nucleation of myocytes in anuran amphibians or to the fact that multi-nucleation in anurans involves nuclear divisions rather than the cell fusions observed in amniotes (Handrigan and Wassersug, 2007).
A novel role for Dvl3 in sclerotome development in Xenopus
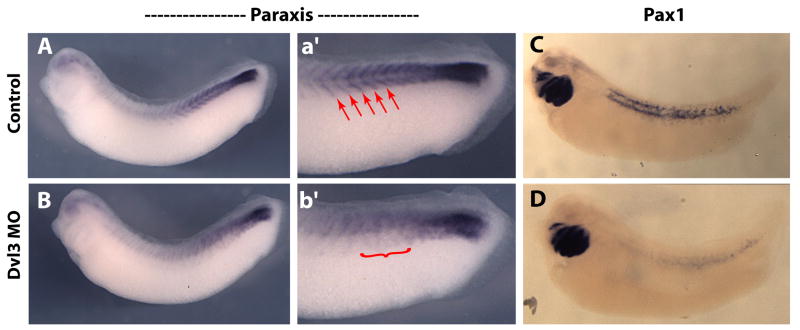
In addition to the curious mode of myotome development in amphibians, the nature of sclerotome development of amphibian embryos is also evolutionarily derived. Though this tissue has received very little attention, it is clear that multiple modes of sclerotome development exist even within the amphibia (Scaal and Wiegreffe, 2006). In contrast to the chick, the sclerotome of amphibians is extremely small and difficult to visualize (Handrigan and Wassersug, 2007). In Xenopus the small number of sclerotome cells segregates from the myotome and takes up a peri-notochordal position (Youn et al., 1980; Handrigan and Wassersug, 2007). The molecular basis of sclerotome development in amphibia is almost entirely unexplored, so we asked if the divergent, specific expression of Dvl3 might be related to sclerotome development. Paraxis is a known Wnt target gene in chick and is involved in sclerotome development (Linker et al., 2005; Takahashi et al., 2007), so we were encouraged to find that Paraxis expression was decreased and disordered in Dvl3 morphants (Fig. 8A, B).
Figure 8. Knockdown of Dvl3 in presomitic mesodermal tissues disrupts normal expression of both paraxis and Pax-1 genes.
(A-B): single stage 29/30 tailbud stage embryo. (A): Control uninjected side displays normal paraxis expression in the posterior presomitic mesoderm and within individual somites. (a′) A magnified image of posterior half of the (A) side of the embryo. (B): morpholino injected side displays a loss of paraxis expression within individual somites as well as diminished expression in the unsegmented mesoderm. (b′) A magnified image of the posterior half of the (B) side of the embryo. (C): Normal expression of Pax-1 in a wild type stage 37 embryo in the branchial arches and perinotochordal region. (D): Dvl3 morphants lack any appreciable Pax-1 staining surrounding the notochord (targeted by injection) while the brachial arches (not targeted by injection) retain Pax-1 expression. Red arrows point to paraxis expression within individual somites. Red bracket highlights defects in both expression and patterning of paraxis transcript expression.
We next examined the expression of Pax-1, which is expressed in the sclerotome of most vertebrates, including Xenopus (Handrigan and Wassersug, 2007). Pax-1 was strongly expressed perinotochordally, consistent with the location of Xenopus sclerotome cells (Fig. 8C). In Dvl3 morphants, perinotochordal Pax1 expression was almost entirely eliminated, though expression in the branchial arches (not targeted by injection) was unaffected (Fig. 8D). These results suggest that the unique and specific expression of Xenopus Dvl3 in the presomitic region is required for the derived mode of sclerotome development in this animal.
Conclusions
Wnts and Frizzleds are members of large gene families, and the developmental functions of these proteins are controlled in part by the restricted expression patterns of the genes that encode them. For example, Wnt11 plays key roles in early dorsal patterning in Xenopus, while ventrally-expressed Wnt8 is central to patterning ventral structures (Christian et al., 1991; Tao et al., 2005). Indeed, a role for patterned expression of Wnt genes is conserved from vertebrates (Garriock et al., 2007) to basal metazoans, such as cnidarians and sponges (Kusserow et al., 2005).
By contrast, the Dvls form a three-member gene family in most vertebrates, but the expression patterns and developmental functions of Dvls have received little attention. Here, we have reported detailed expression patterns for all Dvl genes in Xenopus and in the chick. We find that in contrast to the mouse, Dvl genes' expression is spatially and temporally dynamic, and remarkably divergent between these two vertebrates. Moreover, we have expanded the comparative scope of the project by investigating Dvl expression outside of vertebrates, during the development of a basally branching deuterostome, the hemichordate S. kowalevskii. Dvl expression is also dynamic and tissue-specific in this animal. These data demonstrate that tissue specific, temporal and spatial regulation of Dvl transcription may be a general feature of deuterostomes. Furthermore, our data strongly suggest there was one Dvl gene in the common ancestor of chordates and that this gene was duplicated independently in both cephalochordate and vertebrate lineages.
We have also systematically knocked down the three Dvls in Xenopus. Consistent with previous experiments using dominant-negative constructs, we show a role for Dvl in axis elongation and in anteroposterior patterning of the central nervous system in Xenopus. Moreover, we show that the specific role for Dvl1 and Dvl2 in somite segmentation identified in the mouse is conserved in Xenopus. We also find that Dvl1 and Dvl2 specifically transduce the canonical Wnt signals that specify neural crest in Xenopus, a role not conserved in mice. Canonical Wnt signals also specify neural crest in the chick (Garcia-Castro et al., 2002), so in light of the expression data shown here, we would predict that Dvl3 mediates that signal in the chick.
Finally, we report here that the loss-of-function phenotype for Dvl3 in Xenopus is entirely divergent from that of Dvl3 in the mouse (Etheridge et al., 2008). We report a novel role for Dvl3 in somite patterning in Xenopus, where our data suggest a specific requirement for Dvl3 in mediating Wnt-dependent signals that maintain muscle cell identity and direct sclerotome development. This Xenopus-specific function for Dvl3 likely reflects the highly derived mechanism of amphibian somite development (Scaal and Wiegreffe, 2006; Handrigan and Wassersug, 2007). Moreover, the result suggests that evolutionary changes to Dvl paralogue function during vertebrate diversification have resulted in a range of developmental roles for the lineage specific patterns reported here.
This study demonstrates that Dvl gene transcription is, as a general rule, highly regulated during vertebrate development and identifies specific developmental phenotypes resulting from knockdown of Dvl1, Dvl2, and Dvl3 in Xenopus. A recent study has found that the different Dvl proteins have subtly differing abilities to transduce Wnt signals (Lee et al., 2008). However, other studies have suggested that the different Dvl proteins behave interchangeably (Rothbacher et al., 1995). The dramatically different phenotypes we observed following Dvl knockdowns correlate with the temporal and spatial domains of Dvl gene transcription. We think it likely that the result of this study reflect an important, but previously ignored, role for transcriptional control of Dvl gene expression as an important regulator of Wnt-dependent patterning events in vertebrate embryos. Furthermore, it is likely that tight transcriptional regulation of Dvl expression is a common feature of, at least deuterostomes, and possibly all metazoans.
Supplementary Material
Stage 10 embryos bi-sected through the dorsal lip and in situ hybridized with Dvl probe as indicated. Red arrowhead marks the involuting dorsal lip.
(A): stage 12 embryo exhibiting staining in the involuting dorsal marginal zone. (B): stage 13/14 embryo displaying ubiquitous staining of the dorsal tissues. (C): stage 16/17 embryo shows an attenuation of staining to the neural folds. The neural crest exhibit increased in situ staining (black arrowhead). This over stained embryo exhibits the presence of spotted ectodermal staining. The black bracket outlines peeling of the epithelium. (D): Stage 20 dorsal view unbleached embryo exhibits staining in the developing forebrain, eye cups, and the streaming neural crest (red arrow). (E): Stage 21 embryo dorsal view exhibits staining in the streaming neural crest (red bracket), eyecups, and the developing forebrain. The anterior somites (red arrowhead) are beginning to express Dvl1 mRNA. (F): Stage 23 embryo exhibits continued staining in the streaming neural crest as well as the otic vesicle and the segmenting somites. (G): Stage 29/30 embryo shows continued nuclear somite expression as well as epidermal placodes.
Close up image of a 29/30 embryo in situ hybridized for Dvl1: olfactory placode (pOI), trigeminal placode (pV), anterodorsal lateral line placode (pAD), middle lateral line placode (pM), posteriorlateral line placode (pP), facial epibrachial placodes (epVII), glossopharygeal placodes (epIX), first vagal epibrancial placode (epXI), and the fused second and third vagal epibrachial placodes (epX2/3).
(A): Stage 13/14 embryo exhibits general expression in dorsal tissue akin to Dvl1 expression. (B): Stage 21 dorsal view exhibits staining in the streaming neural crest (red bracket) as well as the developing forebrain and eye cups. (C): Stage 23 embryo exhibits staining in the otic vesicle, streaming cranial neural crest, and eyecups. (D): Stage 29/30 embryo exhibits staining in the somites, developing eyes, and branchial arches.
(A): Lateral view stage 18 embryo exhibits staining in the presomitic mesoderm. (B): Dorsal view of a stage 18 embryo exhibits staining of the presomitic mesoderm. (C): Stage 23 embryo exhibits staining in the presomitic mesoderm and the developing somitomeres. (D): Stage 26/27 embryo exhibits staining in the otic vesicle and the somites as well as continued expression in the pre-somitic mesoderm. (E): Stage 35/36 embryos exhibit continued expression in somites, the developing head region, and the developing heart (red bracket).
(A): control embryo in situ hybridized with a riboprobe mixture of Otx2, Krox20, and HoxB9. Otx2 is expressed in the developing eye, otic vesicle and brain region, as well as in the somites. Krox20 is expressed in rhombomeres 3 and 5. HoxB9 is expressed in the spinal cord. (B): Dvl123 morphant embryo displaying a mild convergent-extension phenotype yet displaying no drastic changes in anterior-posterior patterning. (C): representative Dvl123 morphant embryo displaying a complete loss or reduction (49% and 45% respectively) in Krox20 expression while maintaining normal Otx2 and HoxB9 expression levels. (D) Control Keller sandwhich explants display morphology consistent with normal convergent and extension movements. (E) Dvl123 morpholino injected Keller sandwhich explants display defects in convergence and extension. (F) Quantification of the length to width ratio of the Keller sandwhich explant represented in (D) and (E). Red arrowhead points to defects in Krox-20 patterning (B) or expression (C).
Acknowledgments
We thank P. Abitua and E. Herrington for technical assistance, Anne-Helene Monsoro-Burq for plasmids, and B.L. Martin and J. Gross for critical reading and discussion. This work was supported by The Burroughs-Wellcome Fund, The March of Dimes, and the NIH/NIGMS.
References
- Afonin B, Ho M, Gustin JK, Meloty-Kapella C, Domingo CR. Cell behaviors associated with somite segmentation and rotation in Xenopus laevis. Dev Dyn. 2006;235:3268–3279. doi: 10.1002/dvdy.20979. [DOI] [PubMed] [Google Scholar]
- Ahmad-Annuar A, Ciani L, Simeonidis I, Herreros J, Fredj NB, Rosso SB, Hall A, Brickley S, Salinas PC. Signaling across the synapse: a role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release. J Cell Biol. 2006;174:127–139. doi: 10.1083/jcb.200511054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, Ben-Neriah Y, Alkalay I. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 2002;16:1066–1076. doi: 10.1101/gad.230302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aybar MJ, Nieto MA, Mayor R. Snail precedes slug in the genetic cascade required for the specification and migration of the Xenopus neural crest. Development. 2003;130:483–494. doi: 10.1242/dev.00238. [DOI] [PubMed] [Google Scholar]
- Baltzinger M, Mager-Heckel AM, Remy P. Xl erg: expression pattern and overexpression during development plead for a role in endothelial cell differentiation. Dev Dyn. 1999;216:420–433. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<420::AID-DVDY10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- Christian JL, McMahon JA, McMahon AP, Moon RT. Xwnt-8, a Xenopus Wnt-1/int-1-related gene responsive to mesoderm-inducing growth factors, may play a role in ventral mesodermal patterning during embryogenesis. Development. 1991;111:1045–1055. doi: 10.1242/dev.111.4.1045. [DOI] [PubMed] [Google Scholar]
- De Calisto J, Araya C, Marchant L, Riaz CF, Mayor R. Essential role of non-canonical Wnt signalling in neural crest migration. Development. 2005;132:2587–2597. doi: 10.1242/dev.01857. [DOI] [PubMed] [Google Scholar]
- Dehal P, Boore JL. Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol. 2005;3:e314. doi: 10.1371/journal.pbio.0030314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, Tsang M, Greer J, Kardos N, Wang J, Sussman DJ, Chen P, Wynshaw-Boris A. Murine Dishevelled 3 Functions in Redundant Pathways with Dishevelled 1 and 2 in Normal Cardiac Outflow Tract, Cochlea, and Neural Tube Development. PLoS Genetics. 2008 doi: 10.1371/journal.pgen.1000259. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Castro MI, Marcelle C, Bronner-Fraser M. Ectodermal Wnt function as a neural crest inducer. Science. 2002;297:848–851. doi: 10.1126/science.1070824. [DOI] [PubMed] [Google Scholar]
- Garriock RJ, Krieg PA. Wnt11-R signaling regulates a calcium sensitive EMT event essential for dorsal fin development of Xenopus. Dev Biol. 2007;304:127–140. doi: 10.1016/j.ydbio.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriock RJ, Warkman AS, Meadows SM, D'Agostino S, Krieg PA. Census of vertebrate Wnt genes: isolation and developmental expression of Xenopus Wnt2, Wnt3, Wnt9a, Wnt9b, Wnt10a, and Wnt16. Dev Dyn. 2007;236:1249–1258. doi: 10.1002/dvdy.21156. [DOI] [PubMed] [Google Scholar]
- Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, Mei L, Chien KR, Sussman DJ, Wynshaw-Boris A. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129:5827–5838. doi: 10.1242/dev.00164. [DOI] [PubMed] [Google Scholar]
- Handrigan GR, Wassersug RJ. The anuran Bauplan: a review of the adaptive, developmental, and genetic underpinnings of frog and tadpole morphology. Biol Rev Camb Philos Soc. 2007;82:1–25. doi: 10.1111/j.1469-185X.2006.00001.x. [DOI] [PubMed] [Google Scholar]
- Hardy KM, Garriock RJ, Yatskievych TA, D'Agostino SL, Antin PB, Krieg PA. Non-canonical Wnt signaling through Wnt5a/b and a novel Wnt11 gene, Wnt11b, regulates cell migration during avian gastrulation. Dev Biol. 2008;320:391–401. doi: 10.1016/j.ydbio.2008.05.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff K, Wassersug RJ. Tadpole locomotion: axial movement and tail functions in a largely vertebraeless vertebrate. American Zoology. 2000;40:62–76. [Google Scholar]
- Hopwood ND, Gurdon JB. Activation of muscle genes without myogenesis by ectopic expression of MyoD in frog embryo cells. Nature. 1990;347:197–200. doi: 10.1038/347197a0. [DOI] [PubMed] [Google Scholar]
- Hopwood ND, Pluck A, Gurdon JB. MyoD expression in the forming somites is an early response to mesoderm induction in Xenopus embryos. Embo J. 1989;8:3409–3417. doi: 10.1002/j.1460-2075.1989.tb08505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood ND, Pluck A, Gurdon JB. Xenopus Myf-5 marks early muscle cells and can activate muscle genes ectopically in early embryos. Development. 1991;111:551–560. doi: 10.1242/dev.111.2.551. [DOI] [PubMed] [Google Scholar]
- Hotta K, Takahashi H, Ueno N, Gojobori T. A genome-wide survey of the genes for planar polarity signaling or convergent extension-related genes in Ciona intestinalis and phylogenetic comparisons of evolutionary conserved signaling components. Gene. 2003;317:165–185. doi: 10.1016/s0378-1119(03)00700-5. [DOI] [PubMed] [Google Scholar]
- Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ. Multiple sequence alignment with Clustal X. Trends Biochem Sci. 1998;23:403–405. doi: 10.1016/s0968-0004(98)01285-7. [DOI] [PubMed] [Google Scholar]
- Keller R. The origin and morphogenesis of amphibian somites. Curr Top Dev Biol. 2000;47:183–246. doi: 10.1016/s0070-2153(08)60726-7. [DOI] [PubMed] [Google Scholar]
- Kintner CR, Brockes JP. Monoclonal antibodies identify blastemal cells derived from dedifferentiating limb regeneration. Nature. 1984;308:67–69. doi: 10.1038/308067a0. [DOI] [PubMed] [Google Scholar]
- Klingensmith J, Yang Y, Axelrod JD, Beier DR, Perrimon N, Sussman DJ. Conservation of dishevelled structure and function between flies and mice: isolation and characterization of Dvl2. Mech Dev. 1996;58:15–26. doi: 10.1016/s0925-4773(96)00549-7. [DOI] [PubMed] [Google Scholar]
- Krasnow RE, Wong LL, Adler PN. Dishevelled is a component of the frizzled signaling pathway in Drosophila. Development. 1995;121:4095–4102. doi: 10.1242/dev.121.12.4095. [DOI] [PubMed] [Google Scholar]
- Kusserow A, Pang K, Sturm C, Hrouda M, Lentfer J, Schmidt HA, Technau U, von Haeseler A, Hobmayer B, Martindale MQ, Holstein TW. Unexpected complexity of the Wnt gene family in a sea anemone. Nature. 2005;433:156–160. doi: 10.1038/nature03158. [DOI] [PubMed] [Google Scholar]
- LaBonne C, Bronner-Fraser M. Induction and patterning of the neural crest, a stem cell-like precursor population. J Neurobiol. 1998a;36:175–189. doi: 10.1002/(sici)1097-4695(199808)36:2<175::aid-neu6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- LaBonne C, Bronner-Fraser M. Neural crest induction in Xenopus: evidence for a two-signal model. Development. 1998b;125:2403–2414. doi: 10.1242/dev.125.13.2403. [DOI] [PubMed] [Google Scholar]
- Lee HS, Bong YS, Moore KB, Soria K, Moody SA, Daar IO. Dishevelled mediates ephrinB1 signalling in the eye field through the planar cell polarity pathway. Nat Cell Biol. 2006;8:55–63. doi: 10.1038/ncb1344. [DOI] [PubMed] [Google Scholar]
- Lee PN, Kumburegama S, Marlow HQ, Martindale MQ, Wikramanayake AH. Asymmetric developmental potential along the animal-vegetal axis in the anthozoan cnidarian, Nematostella vectensis, is mediated by Dishevelled. Dev Biol. 2007;310:169–186. doi: 10.1016/j.ydbio.2007.05.040. [DOI] [PubMed] [Google Scholar]
- Lee YN, Gao Y, Wang HY. Differential mediation of the Wnt canonical pathway by mammalian Dishevelleds-1, -2, and -3. Cell Signal. 2008;20:443–452. doi: 10.1016/j.cellsig.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijam N, Paylor R, McDonald MP, Crawley JN, Deng CX, Herrup K, Stevens KE, Maccaferri G, McBain CJ, Sussman DJ, Wynshaw-Boris A. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90:895–905. doi: 10.1016/s0092-8674(00)80354-2. [DOI] [PubMed] [Google Scholar]
- Linker C, Lesbros C, Gros J, Burrus LW, Rawls A, Marcelle C. beta-Catenin-dependent Wnt signalling controls the epithelial organisation of somites through the activation of paraxis. Development. 2005;132:3895–3905. doi: 10.1242/dev.01961. [DOI] [PubMed] [Google Scholar]
- Lowe CJ. Molecular genetic insights into deuterostome evolution from the direct-developing hemichordate Saccoglossus kowalevskii. Philos Trans R Soc Lond B Biol Sci. 2008;363:1569–1578. doi: 10.1098/rstb.2007.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe CJ, Tagawa K, Humphreys T, Kirschner M, Gerhart J. Hemichordate embryos: procurement, culture, and basic methods. Methods Cell Biol. 2004;74:171–194. doi: 10.1016/s0091-679x(04)74008-x. [DOI] [PubMed] [Google Scholar]
- Mancilla A, Mayor R. Neural crest formation in Xenopus laevis: mechanisms of Xslug induction. Dev Biol. 1996;177:580–589. doi: 10.1006/dbio.1996.0187. [DOI] [PubMed] [Google Scholar]
- Mariani FV, Choi GB, Harland RM. The neural plate specifies somite size in the Xenopus laevis gastrula. Dev Cell. 2001;1:115–126. doi: 10.1016/s1534-5807(01)00018-1. [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq AH, Wang E, Harland R. Msx1 and Pax3 cooperate to mediate FGF8 and WNT signals during Xenopus neural crest induction. Dev Cell. 2005;8:167–178. doi: 10.1016/j.devcel.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Moody SA, Kline MJ. Segregation of fate during cleavage of frog (Xenopus laevis) blastomeres. Anat Embryol (Berl) 1990;182:347–362. doi: 10.1007/BF02433495. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis. New York: Garland; 1967. [Google Scholar]
- Noordermeer J, Klingensmith J, Perrimon N, Nusse R. dishevelled and armadillo act in the wingless signalling pathway in Drosophila. Nature. 1994;367:80–83. doi: 10.1038/367080a0. [DOI] [PubMed] [Google Scholar]
- Oishi I, Kawakami Y, Raya A, Callol-Massot C, Izpisua Belmonte JC. Regulation of primary cilia formation and left-right patterning in zebrafish by a noncanonical Wnt signaling mediator, duboraya. Nat Genet. 2006;38:1316–1322. doi: 10.1038/ng1892. [DOI] [PubMed] [Google Scholar]
- Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet. 2008;40:871–879. doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe S, Swofford DL. Taxon sampling revisited. Nature. 1999;398:299–300. doi: 10.1038/18592. [DOI] [PubMed] [Google Scholar]
- Putnam NH, Butts T, Ferrier DE, Furlong RF, Hellsten U, Kawashima T, Robinson-Rechavi M, Shoguchi E, Terry A, Yu JK, Benito-Gutierrez EL, Dubchak I, Garcia-Fernandez J, Gibson-Brown JJ, Grigoriev IV, Horton AC, de Jong PJ, Jurka J, Kapitonov VV, Kohara Y, Kuroki Y, Lindquist E, Lucas S, Osoegawa K, Pennacchio LA, Salamov AA, Satou Y, Sauka-Spengler T, Schmutz J, Shin IT, Toyoda A, Bronner-Fraser M, Fujiyama A, Holland LZ, Holland PW, Satoh N, Rokhsar DS. The amphioxus genome and the evolution of the chordate karyotype. Nature. 2008;453:1064–1071. doi: 10.1038/nature06967. [DOI] [PubMed] [Google Scholar]
- Rogers JS, Swofford DL. Multiple local maxima for likelihoods of phylogenetic trees: a simulation study. Mol Biol Evol. 1999;16:1079–1085. doi: 10.1093/oxfordjournals.molbev.a026197. [DOI] [PubMed] [Google Scholar]
- Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat Neurosci. 2005;8:34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- Rothbacher U, Laurent MN, Blitz IL, Watabe T, Marsh JL, Cho KW. Functional conservation of the Wnt signaling pathway revealed by ectopic expression of Drosophila dishevelled in Xenopus. Dev Biol. 1995;170:717–721. doi: 10.1006/dbio.1995.1249. [DOI] [PubMed] [Google Scholar]
- Scaal M, Wiegreffe C. Somite compartments in anamniotes. Anat Embryol (Berl) 2006;211 1:9–19. doi: 10.1007/s00429-006-0127-8. [DOI] [PubMed] [Google Scholar]
- Schmidt C, McGonnell IM, Allen S, Otto A, Patel K. Wnt6 controls amniote neural crest induction through the non-canonical signaling pathway. Dev Dyn. 2007;236:2502–2511. doi: 10.1002/dvdy.21260. [DOI] [PubMed] [Google Scholar]
- Sidow A. Gen(om)e duplications in the evolution of early vertebrates. Curr Opin Genet Dev. 1996;6:715–722. doi: 10.1016/s0959-437x(96)80026-8. [DOI] [PubMed] [Google Scholar]
- Sidow A, Bulotsky MS, Kerrebrock AW, Birren BW, Altshuler D, Jaenisch R, Johnson KR, Lander ES. A novel member of the F-box/WD40 gene family, encoding dactylin, is disrupted in the mouse dactylaplasia mutant. Nat Genet. 1999;23:104–107. doi: 10.1038/12709. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, et al. Early Development of Xenopus laevis: A Laboratory Manual. Cold Spring Harbor, N.Y.: Cold Spring Harbor, N.Y., Cold Spring Harbor Press; 2000. [Google Scholar]
- Smalley MJ, Sara E, Paterson H, Naylor S, Cook D, Jayatilake H, Fryer LG, Hutchinson L, Fry MJ, Dale TC. Interaction of axin and Dvl-2 proteins regulates Dvl-2-stimulated TCF-dependent transcription. Embo J. 1999;18:2823–2835. doi: 10.1093/emboj/18.10.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol SY. Analysis of Dishevelled signalling pathways during Xenopus development. Current Biology. 1996;6:1456–1467. doi: 10.1016/s0960-9822(96)00750-6. [DOI] [PubMed] [Google Scholar]
- Sokol SY, Klingensmith J, Perrimon N, Itoh K. Dorsalizing and neuralizing properties of Xdsh, a maternally expressed Xenopus homolog of dishevelled. Development. 1995;121:3487. doi: 10.1242/dev.121.10.3487. [DOI] [PubMed] [Google Scholar]
- Steitz SA, Tsang M, Sussman DJ. Wnt-mediated relocalization of dishevelled proteins. In Vitro Cell Dev Biol Anim. 1996;32:441–445. doi: 10.1007/BF02723007. [DOI] [PubMed] [Google Scholar]
- Sussman DJ, Klingensmith J, Salinas P, Adams PS, Nusse R, Perrimon N. Isolation and characterization of a mouse homolog of the Drosophila segment polarity gene dishevelled. Dev Biol. 1994;166:73–86. doi: 10.1006/dbio.1994.1297. [DOI] [PubMed] [Google Scholar]
- Sweetman D, Wagstaff L, Cooper O, Weijer C, Munsterberg A. The migration of paraxial and lateral plate mesoderm cells emerging from the late primitive streak is controlled by different Wnt signals. BMC Dev Biol. 2008;8:63. doi: 10.1186/1471-213X-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Takagi A, Hiraoka S, Koseki H, Kanno J, Rawls A, Saga Y. Transcription factors Mesp2 and Paraxis have critical roles in axial musculoskeletal formation. Dev Dyn. 2007;236:1484–1494. doi: 10.1002/dvdy.21178. [DOI] [PubMed] [Google Scholar]
- Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie CC, Lin X, Heasman J. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005;120:857–871. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Theisen H, Purcell J, Bennett M, Kansagara D, Syed A, Marsh JL. dishevelled is required during wingless signaling to establish both cell polarity and cell identity. Development. 1994;120:347–360. doi: 10.1242/dev.120.2.347. [DOI] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Expression of planar cell polarity genes during development of the mouse CNS. Eur J Neurosci. 2006;23:597–607. doi: 10.1111/j.1460-9568.2006.04596.x. [DOI] [PubMed] [Google Scholar]
- Tsang M, Lijam N, Yang Y, Beier DR, Wynshaw-Boris A, Sussman DJ. Isolation and characterization of mouse dishevelled-3. Dev Dyn. 1996;207:253–262. doi: 10.1002/(SICI)1097-0177(199611)207:3<253::AID-AJA2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Berns A. Knockout mouse models to study Wnt signal transduction. Trends Genet. 2006;22:678–689. doi: 10.1016/j.tig.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132:4421–4436. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Harland RM. Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development. 2002;129:5815–5825. doi: 10.1242/dev.00123. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, Fraser SE, Chen P, Wallingford JB, Wynshaw-Boris A. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006;133:1767–1778. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzel HE, Illies MR, Byrum CA, Xu R, Wikramanayake AH, Ettensohn CA. Differential stability of beta-catenin along the animal-vegetal axis of the sea urchin embryo mediated by dishevelled. Development. 2004;131:2947–2956. doi: 10.1242/dev.01152. [DOI] [PubMed] [Google Scholar]
- Wolfe KH. Yesterday's polyploids and the mystery of diploidization. Nat Rev Genet. 2001;2:333–341. doi: 10.1038/35072009. [DOI] [PubMed] [Google Scholar]
- Youn BW, Keller RE, Malacinski GM. An atlas of notochord and somite morphogenesis in several anuran and urodelean amphibians. J Embryol Exp Morphol. 1980;59:223–247. [PubMed] [Google Scholar]
- Zhao H, Rebbert ML, Dawid IB. Myoskeletin, a factor related to Myocardin, is expressed in somites and required for hypaxial muscle formation in Xenopus. Int J Dev Biol. 2007;51:315–320. doi: 10.1387/ijdb.062260hz. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Stage 10 embryos bi-sected through the dorsal lip and in situ hybridized with Dvl probe as indicated. Red arrowhead marks the involuting dorsal lip.
(A): stage 12 embryo exhibiting staining in the involuting dorsal marginal zone. (B): stage 13/14 embryo displaying ubiquitous staining of the dorsal tissues. (C): stage 16/17 embryo shows an attenuation of staining to the neural folds. The neural crest exhibit increased in situ staining (black arrowhead). This over stained embryo exhibits the presence of spotted ectodermal staining. The black bracket outlines peeling of the epithelium. (D): Stage 20 dorsal view unbleached embryo exhibits staining in the developing forebrain, eye cups, and the streaming neural crest (red arrow). (E): Stage 21 embryo dorsal view exhibits staining in the streaming neural crest (red bracket), eyecups, and the developing forebrain. The anterior somites (red arrowhead) are beginning to express Dvl1 mRNA. (F): Stage 23 embryo exhibits continued staining in the streaming neural crest as well as the otic vesicle and the segmenting somites. (G): Stage 29/30 embryo shows continued nuclear somite expression as well as epidermal placodes.
Close up image of a 29/30 embryo in situ hybridized for Dvl1: olfactory placode (pOI), trigeminal placode (pV), anterodorsal lateral line placode (pAD), middle lateral line placode (pM), posteriorlateral line placode (pP), facial epibrachial placodes (epVII), glossopharygeal placodes (epIX), first vagal epibrancial placode (epXI), and the fused second and third vagal epibrachial placodes (epX2/3).
(A): Stage 13/14 embryo exhibits general expression in dorsal tissue akin to Dvl1 expression. (B): Stage 21 dorsal view exhibits staining in the streaming neural crest (red bracket) as well as the developing forebrain and eye cups. (C): Stage 23 embryo exhibits staining in the otic vesicle, streaming cranial neural crest, and eyecups. (D): Stage 29/30 embryo exhibits staining in the somites, developing eyes, and branchial arches.
(A): Lateral view stage 18 embryo exhibits staining in the presomitic mesoderm. (B): Dorsal view of a stage 18 embryo exhibits staining of the presomitic mesoderm. (C): Stage 23 embryo exhibits staining in the presomitic mesoderm and the developing somitomeres. (D): Stage 26/27 embryo exhibits staining in the otic vesicle and the somites as well as continued expression in the pre-somitic mesoderm. (E): Stage 35/36 embryos exhibit continued expression in somites, the developing head region, and the developing heart (red bracket).
(A): control embryo in situ hybridized with a riboprobe mixture of Otx2, Krox20, and HoxB9. Otx2 is expressed in the developing eye, otic vesicle and brain region, as well as in the somites. Krox20 is expressed in rhombomeres 3 and 5. HoxB9 is expressed in the spinal cord. (B): Dvl123 morphant embryo displaying a mild convergent-extension phenotype yet displaying no drastic changes in anterior-posterior patterning. (C): representative Dvl123 morphant embryo displaying a complete loss or reduction (49% and 45% respectively) in Krox20 expression while maintaining normal Otx2 and HoxB9 expression levels. (D) Control Keller sandwhich explants display morphology consistent with normal convergent and extension movements. (E) Dvl123 morpholino injected Keller sandwhich explants display defects in convergence and extension. (F) Quantification of the length to width ratio of the Keller sandwhich explant represented in (D) and (E). Red arrowhead points to defects in Krox-20 patterning (B) or expression (C).