Abstract
Unlike conventional CD8+ T cells, major histocompatibility complex (MHC) class Ib–restricted CD8+ T cells show an activated phenotype in uninfected mice and respond rapidly to foreign invaders. The underlying factors that contribute to these differences are not well understood. We show here that the activated phenotype of MHC class Ib–restricted CD8+ T cells was partially acquired as a result of interactions in the thymus and reflected an increased capacity to be selected via interactions with MHC molecules on hematopoietic cells. Using bone marrow–chimeric mice, we have shown that MHC class Ib–restricted, but not MHC class Ia–restricted, CD8+ T cells specific for Listeria monocytogenes were efficiently selected when MHC class I was expressed only on hematopoietic cells. Thus, the distinct functional properties of MHC class Ib–restricted versus MHC class Ia–restricted CD8+ T cells may result, at least in part, from the different ways in which they are positively selected in the thymus.
Major histocompatibility complex (MHC) class Ib molecules—including H2-M3, Qa-1, Qa-2 and CD1d in mice and HLA-E, HLA-F, HLA-G and CD1a to CD1d in humans—differ from MHC class Ia molecules primarily because they have limited polymorphism1. Nevertheless, many MHC class Ib molecules, like MHC class Ia molecules, associate with β2-microglobulin (β2M) and present antigens to CD8+ T cells. Insight into a distinct role played by MHC class Ib was obtained with the discovery that murine H2-M3 contains a hydrophobic peptide-binding groove with a specific preference for binding N-formylated peptides2. Because bacteria initiate protein synthesis with N-formyl methionine and eukaryotic hosts (except for a few mitochondrial proteins) do not, it was hypothesized that MHC class Ib molecules have evolved to bind distinct microbial products for presentation to T cells3. Indeed, CD8+ T cells recognizing bacteria-derived N-formylated peptides presented by H2-M3 are important mediators of protective immunity against Listeria monocytogenes4–6.
MHC class Ib–restricted CD8+ T cells specific for the antigens of a number of pathogens have been identified7–10. For example, mice infected with Mycobacterium tuberculosis contain H2-M3–restricted CD8+ T cells7, whereas humans infected with M. tuberculosis contain both HLA-E–8 and CD1-restricted cells9,10. Qa-1–restricted CD8+ T cells are abundant in Salmonella typhimurium–infected mice11 and have also been reported in L. monocytogenes–infected mice12. MHC class Ib–restricted CD8+ T cells differ from MHC class Ia–restricted T cells in at least four ways. First, most MHC class Ib–restricted T cells have an activated CD44hi phenotype, even in uninfected mice13. Second, these cells are capable of rapidly producing interferon-γ (IFN-γ) after in vivo stimulation with anti-CD3 and have, therefore, been proposed to be an early source of IFN-γ that promotes T helper type 1 (TH1) priming14. Third, MHC class Ib–restricted CD8+ T cells show a peak immune response that precedes the peak response of MHC class Ia–restricted CD8+ T cells after L. monocytogenes infection13,15. Fourth, these cells participate minimally in the memory response associated with a secondary challenge with L. monocytogenes13,15. Thus, in many ways MHC class Ib–restricted CD8+ T cells behave like innate, rather than adaptive, effector cells.
Using Kb−/−Db−/− mice that lack MHC class Ia, but express MHC class Ib, we extended the above observations and showed that MHC class Ib–restricted CD8+ T cells had an activated phenotype that was distinct from MHC class Ia–restricted CD8+ T cells. We also examined a small population of CD8+ T cells in lethally irradiated β2M−/− mice, which lack both MHC class Ia and Ib, reconstituted with wild-type bone marrow (B6 → β2M−/− chimeras). The CD8+ T cells in these chimeric mice result from positive selection, in the thymus, on MHC class I molecules expressed on hematopoietic cells16, whereas most CD8+ T cells require selection on thymic epithelial cells17. We observed that these unusual CD8+ T cells had a similar activated phenotype to MHC class Ib–restricted T cells in Kb−/−Db−/− mice. These observations led us to hypothesize that MHC class Ib–restricted CD8+ T cells might be positively selected on hematopoietic cells.
We showed here that mature CD8+ thymocytes in Kb−/−Db−/− mice began to express an activated phenotype immediately before they left the thymus, suggesting that this phenotype was acquired as a result of interactions in the thymus, rather than peripheral activation. Using bone marrow chimeras, we also showed that H2-M3–restricted CD8+ T cells that recognize a Listeria-derived N-formylated peptide were selected efficiently on hematopoietic cells, whereas MHC class Ia–restricted T cells were selected poorly. The ability of MHC class Ib–restricted CD8+ T cells to respond quickly to antigen may be a consequence of this unusual pattern of positive selection.
Results
MHC class Ib–restricted T cells
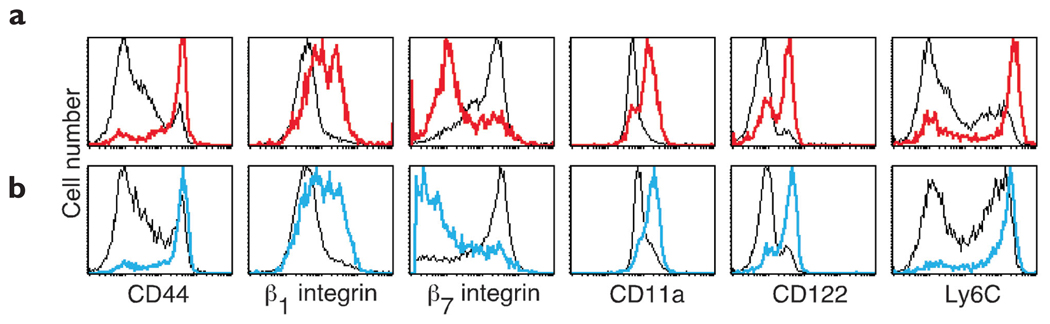
The majority of peripheral MHC class Ib–restricted T cells express an activated CD44hi phenotype, even in naïve Kb−/−Db−/− mice; this confirmed published data13 (Fig. 1a). In contrast, most CD8+ T cells (primarily MHC class Ia–restricted) in C57BL/6 mice have a naïve CD44int phenotype (Fig. 1a). To investigate this difference in more detail, we examined a broad range of activation markers and showed that most peripheral MHC class Ib–restricted CD8+ T cells were β1 integrinhiβ7 integrinloCD11ahiCD122hiLy6Chi (Fig. 1a). In contrast, most MHC class Ia–restricted CD8+ T cells were β1 integrinintβ7 integrinhiCD11aintCD122loLy6Clo (Fig. 1a). However, MHC class Ib– and MHC class Ia–restricted CD8+ T cells expressed identical high amounts of CD62L (data not shown). In addition, CD4+ T cells in C57BL/6 and Kb−/−Db−/− mice showed no marked differences in any of these activation markers (data not shown).
Figure 1. Phenotype of CD8+ T cells in Kb−/−Db−/− and B6 → β2M−/− chimeric mice.
Surface expression of activation markers, gated on CD8+ T cells from lymph nodes. (a) Flow cytometry histograms show CD8+ T cells from B6 (thin black lines) versus Kb−/−Db−/− mice (red lines). Mice were aged 13 weeks. (b) B6 → B6 (thin black lines) versus B6 → β2M−/− chimeras (blue lines). Mice were examined 88 days after irradiation.
T cells selected on hematopoietic cells
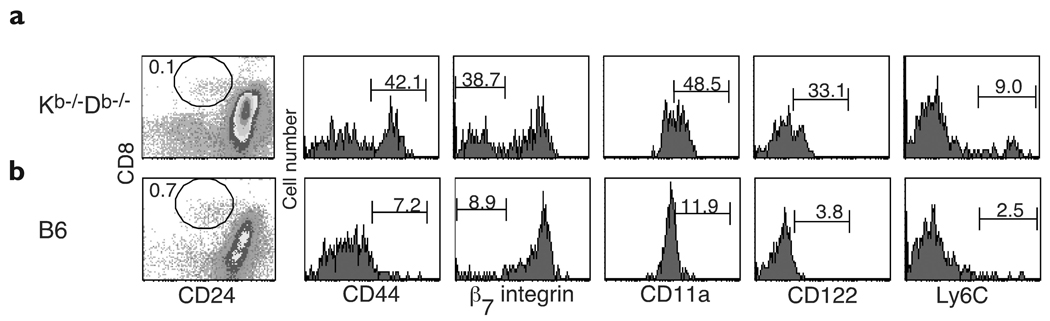
We constructed radiation bone marrow–chimeric mice in which either C57BL/6 or β2M−/− mice were lethally irradiated and reconstituted with C57BL/6 bone marrow (B6 → B6 or B6 → β2M−/− chimeras, respectively). We observed that the small population of CD8+ T cells that developed in B6 → β2M−/− chimeras had an activated phenotype that was almost identical (Fig. 1b) to the one expressed by MHC class Ib–restricted CD8+ T cells in Kb−/−Db−/− mice (Fig. 1a); the majority of these T cells were CD44hiβ1 integrinhiβ7 integrinloCD11ahiCD122hi Ly6Chi. This activated phenotype was not an artifact of irradiation, as CD8+ T cells in B6 → B6 chimeras expressed a phenotype that resembled the pattern seen in unirradiated C57BL/6 mice (Fig. 1b). This unusual population of CD8+ T cells in B6 → β2M−/− chimeras is selected in the thymus by interacting with MHC class I molecules on hematopoietic cells, instead of epithelial cells16. Thus, similarities between these CD8+ T cells and CD8+ T cells in Kb−/−Db−/− mice led us to hypothesize that MHC class Ib–restricted CD8+ T cell are also positively selected on hematopoietic cells.
MHC class Ib–restricted CD8+ T cells in the thymus
If the activated phenotype of peripheral CD8+ T cells in Kb−/−Db−/− mice reflected the nature of their intrathymic selection, we considered the possibility that this phenotype might be expressed by CD4−CD8+ T cells during development in the thymus. Therefore, we examined mature CD8+ thymocytes—defined by their lack of expression of CD2418—in Kb−/−Db−/− mice before they left the thymus. In agreement with published reports18, none of the CD24−CD8+ thymocytes expressed CD4 (data not shown). Some of these mature CD4−CD8+ thymocytes were already expressing the activated phenotype CD44hiβ7 integrinloCD11ahiCD122hi (Fig. 2a), which suggested that this phenotype resulted from intrathymic interactions. This experiment was repeated four times, and age-matched mice that ranged from 5–13 weeks old were used. CD24−CD4−CD8+ thymocytes in Kb−/−Db−/− mice consistently contained a population of activated cells in all experiments; however, the percentage of cells within this population was higher in older mice. As expected, mature CD4−CD8+ thymocytes in C57BL/6 mice expressed a naïve resting phenotype: CD44intβ7 integrinhiCD11aintCD122lo (Fig. 2b).
Figure 2. Phenotype of thymic CD8+ T cells in Kb−/−Db−/− mice.
Surface expression of activation markers gated on CD24−CD8+ thymocytes in (a) Kb−/−Db−/− and (b) C57BL/6 mice. The percentages of each CD24−CD8+ population within total thymus cells and the percentages of CD24−CD4−CD8+ cells displaying each activation marker are shown. Mice were aged 13 weeks.
In contrast to these differences, most mature CD8+ thymocytes in both Kb−/−Db−/− and C57BL/6 mice were Ly6Clo (Fig. 2) and CD45RBlo (data not shown). Our observation that the majority of lymph node CD8+ T cells in Kb−/−Db−/− mice expressed high amounts of Ly6C (Fig. 1a) and CD45RB (data not shown) suggested that the CD8+ cells found in the thymi of Kb−/−Db−/− mice were not simply mature CD8+ T cells that were activated in the periphery and migrated back to the thymus. Instead, these data support the idea that the distinct activated phenotype of MHC class Ib–restricted T cells is due, in part, to a developmental program acquired as a consequence of their intrathymic selection.
T cell selection on hematopoietic cells
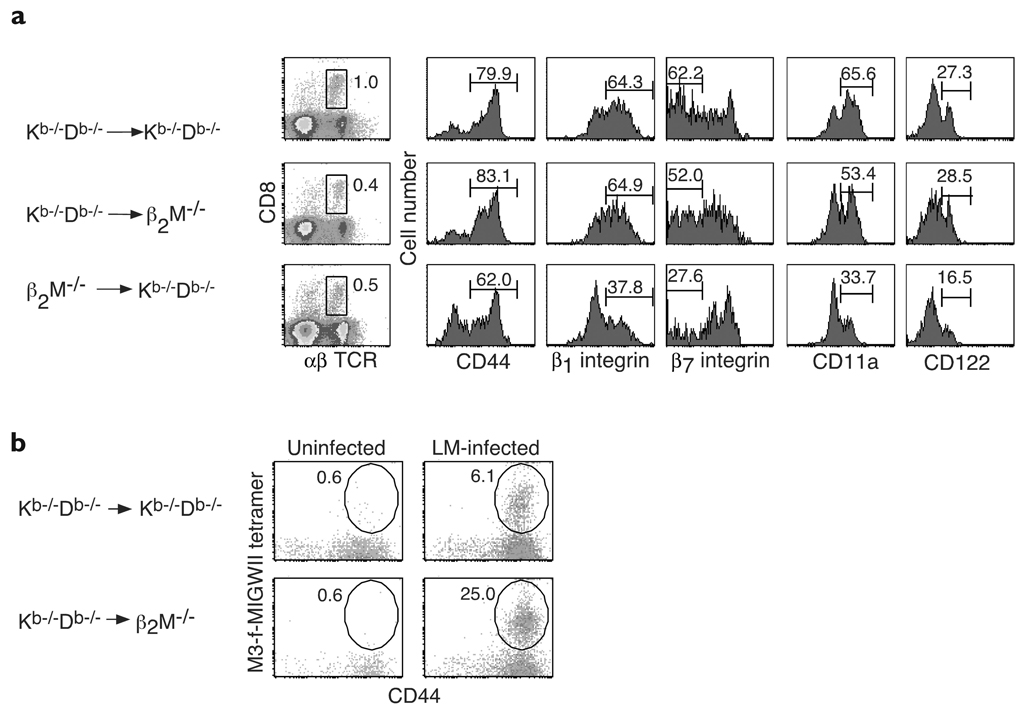
We hypothesized that the activated phenotype of mature CD4−CD8+ thymocytes in Kb−/−Db−/− mice might reflect their capacity to be positively selected on hematopoietic cells. To test this idea, we constructed bone marrow–chimeric mice that expressed MHC class Ib only on hematopoietic cells (Kb−/−Db−/− → β2M−/− chimeras) or only on nonhematopoietic cells (β2M−/− → Kb−/−Db−/− chimeras) and compared the CD8+ T cells in the spleens of each set of chimeric mice. Spleens from control Kb−/−Db−/− → Kb−/−Db−/− chimeras contained a population of CD8+ T cells (1%, Fig. 3a) that was similar in size to the population observed in unirradiated Kb−/−Db−/− mice (data not shown). Slightly smaller populations of CD8+ T cells were present in both Kb−/−Db−/− → β2M−/− and β2M−/− → Kb−/−Db−/− chimeras (0.4% and 0.5%, respectively, Fig. 3a). In comparison, CD8+ T cells comprised only 0.1% of spleen cells in β2M−/− → β2M−/− chimeras (data not shown). Thus, although MHC class Ib–restricted CD8+ T cells seemed to develop most efficiently in mice expressing MHC class Ib on both hematopoietic cells and nonhematopoietic cells, CD8+ T cells could also be selected in chimeras that expressed MHC class Ib only on hematopoietic cells and in chimeras that expressed these molecules only on nonhematopoietic cells.
Figure 3. Selection of MHC class Ib–restricted CD8+ T cells on hematopoietic cells.
(a) Surface expression of activation markers gated on TCRβ+CD8+ spleen cells in Kb−/−Db−/− → Kb−/−Db−/−, Kb−/−Db−/− → β2M−/− and β2M−/− → Kb−/−Db−/− chimeric mice. The percentages of the TCRβ+CD8+ populations within total spleen cells and the percentages of TCRβ+CD8+ cells displaying each activation marker are shown. Chimeric mice represented age-matched mice analyzed 15 weeks after irradiation. (b) Kb−/−Db−/− → Kb−/−Db−/− or Kb−/−Db−/− → β2M−/− chimeric mice were infected intravenously with 2000 CFU L. monocytogenes strain 10403S or were mock infected. Spleen cells were analyzed 6 days after infection. The percentages of CD44+tetramer+ cells within the total CD8+ population are shown. Results are representative of two mice per group.
Next, we compared the cell surface phenotypes of MHC class Ib–restricted CD8+ T cells in each of these chimeras. Like Kb−/−Db−/− → Kb−/−Db−/− controls, the majority of MHC class Ib–restricted CD8+ T cells in both Kb−/−Db−/− → β2M−/− and β2M−/− → Kb−/−Db−/− chimeras were CD44hi (Fig. 3a), although the percentage of CD44+ cells in β2M−/− → Kb−/−Db−/− chimeras was always slightly smaller than the percentage in chimeras expressing MHC class Ib molecules on hematopoietic cells. When we examined integrin expression, we found that most CD8+ T cells in Kb−/−Db−/− → β2M−/− chimeras, like CD8+ T cells in Kb−/−Db−/− → Kb−/−Db−/− controls, were β1 integrinhiβ7 integrinloCD11ahi. In contrast, most CD8+ T cells in β2M−/− → Kb−/−Db−/− chimeras were β1 integrinintβ7 integrinhiCD11aint (Fig. 3a). CD8+ T cells in Kb−/−Db−/− → Kb−/−Db−/− and Kb−/−Db−/− → β2M−/− chimeras also expressed similar amounts of CD122 that were higher than the amounts expressed by CD8+ T cells in β2M−/− → Kb−/−Db−/− chimeras (Fig. 3a). In contrast to these differences, CD8+ T cells in both Kb−/−Db−/− → β2M−/− and β2M−/− → Kb−/−Db−/− chimeras expressed low amounts of Ly6C, whereas CD8+ T cells in Kb−/−Db−/− → Kb−/−Db−/− chimeras expressed high amounts (data not shown). Overall, however, MHC class Ib–restricted CD8+ T cells that were positively selected on hematopoietic cells had a more activated phenotype than MHC class Ib–restricted CD8+ T cells selected on nonhematopoietic cells. These results supported the idea that the activated phenotype of MHC class Ib–restricted CD8+ T cells in Kb−/−Db−/− mice was associated with positive selection on hematopoietic cells.
To further investigate this possibility, we asked whether MHC class Ib–restricted CD8+ T cells that recognized a L. monocytogenes–derived peptide, f-MIGWII, presented by H2-M35 could be positively selected on hematopoietic cells. We infected either Kb−/−Db−/− → Kb−/−Db−/− or Kb−/−Db−/− → β2M−/− chimeric mice with L. monocytogenes and analyzed their spleen cells 6 days later using M3–f-MIGWII tetramers. Kb−/−Db−/− → β2M−/− chimeras showed a marked expansion of M3–f-MIGWII–reactive CD8+ T cells after infection (0.6% of CD8+ T cells in uninfected versus 25% in infected mice, Fig. 3b). Expansion was also seen in Kb−/−Db−/− → Kb−/−Db−/− controls, but the magnitude was less marked (0.6% in uninfected versus 6.1% in infected mice). β2M−/− → Kb−/−Db−/− and β2M−/− → β2M−/− chimeras were also immunized with Listeria, as controls; neither showed any M3–f-MIGWII tetramer staining after infection (data not shown). Our inability to detect M3–f-MIGWII–specific CD8+ T cells in β2M−/− → Kb−/−Db−/− chimeras, however, did not exclude the possibility that these cells might be selected on thymic epithelial cells. This is because expansion of these cells to detectable amounts after infection may require the presence of MHC class Ib on hematopoietic antigen-presenting cells. Nevertheless, these results showed that H2-M3–restricted CD8+ T cells were efficiently positively selected in chimeric mice that expressed MHC class Ib only on hematopoietic cells.
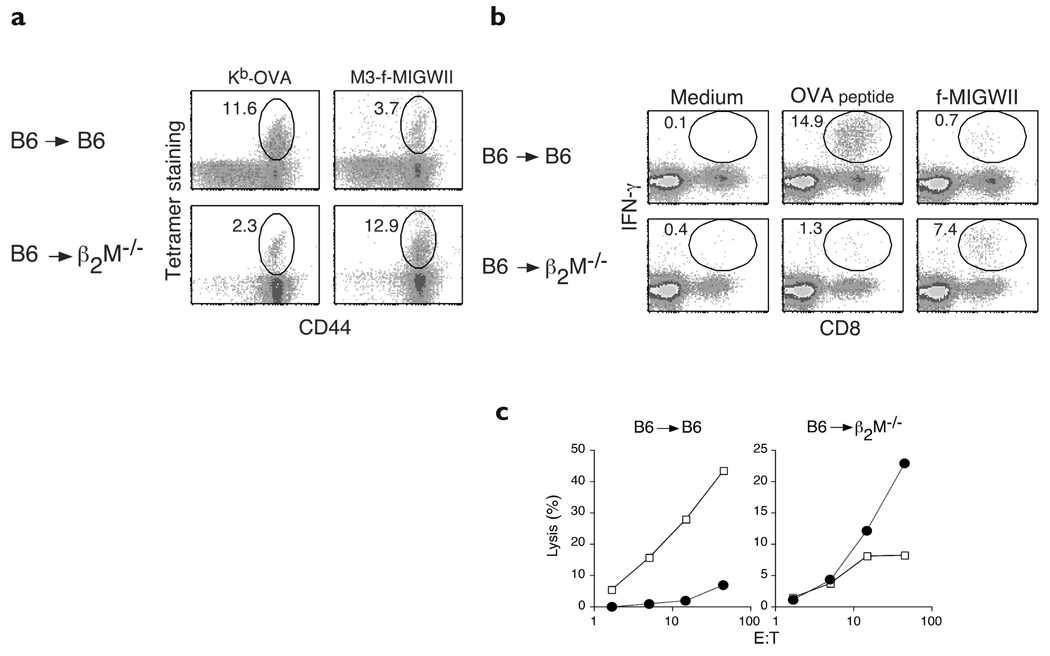
We observed that the majority of CD8+ T cells in B6 → β2M−/− chimeras shared a similar, activated phenotype with CD8+ T cells in Kb−/−Db−/− mice (Fig. 1). Thus, we further hypothesized that MHC class Ib–restricted T cells must have a substantial advantage over MHC class Ia–restricted T cells when positive selection is forced to occur on hematopoietic cells. To directly test this idea, we compared the selection of MHC class Ia– versus MHC class Ib–restricted CD8+ T cells involved in the immune response against L. monocytogenes. Because naturally occurring MHC class Ia–restricted epitopes in H-2b mice are not well characterized, a recombinant L. monocytogenes strain19 expressing secreted ovalbumin (rLM-OVA) was used. Thus, the MHC class Ia–restricted response against an OVA-derived peptide, SIINFEKL, presented by Kb (ref. 20) could be monitored. For comparison, we again followed the response of CD8+ T cells specific for f-MIGWII presented by H2-M3. Using this system, we compared the frequency of MHC class Ia– versus MHC class Ib–restricted CD8+ T cells in B6 → B6 or B6 → β2M−/− chimeric mice after infection with rLM-OVA. The response of OVA-specific CD8+ T cells was dominant in B6 → B6 mice compared to the f-MIGWII–specific response at day 6 after infection (Fig. 4a). The percentage of CD8+ T cells detected with Kb-OVA tetramers was consistently three- to fivefold higher than the percentage detected with M3–f-MIGWII tetramers. In contrast, OVA-specific CD8+ T cells were rare (Fig. 4a) or undetectable (data not shown) in B6 → β2M−/− chimeras, whereas f-MIGWII–specific CD8+ T cells were abundant. CD8+ T cells specific for f-MIGWII were reproducibly five- to tenfold more frequent than OVA-specific CD8+ T cells in chimeras expressing MHC class I molecules only on hematopoietic cells.
Figure 4. Preferential selection of H2-M3–restricted T cells on hematopoietic cells.
(a) Tetramer staining: B6 → B6 or B6 → β2M−/− chimeric mice were infected intravenously with 2000 rLM-OVA and spleen cells were analyzed 6 days after infection. The percentages of CD44+, Kb-OVA or M3–f-MIGWII tetramer+ cells within the total CD8+ population are shown. (b) Intracellular IFN-γ analysis: spleen cells were stimulated with medium, OVA peptide or f-MIGWII and intracellular IFN-γ was analyzed by flow cytometry. The percentages of IFN-γ–expressing cells within the CD8+ population are shown. (c) Cytolysis assay: specific killing of OVA peptide-loaded (open squares) or f-MIGWII–loaded (filled circles) RMA target cells are shown. Analyses in a–c were done at the same time with spleen cells from the same mice. Results are representative of two separate experiments; two mice were used per group in each experiment.
Functional studies were done to determine whether CD8+ T cells selected on hematopoietic cells were capable of producing IFN-γ and mediating cytolysis. Consistent with the frequencies observed with the use of tetramers, CD8+ T cells producing IFN-γ in response to OVA peptide were 20-fold more abundant in infected B6 → B6 chimeras than T cells responding to f-MIGWII peptide (Fig. 4b). In contrast, B6 → β2M−/− chimeras contained at least fivefold more f-MIGWII–reactive than OVA peptide–reactive CD8+ T cells. Similarly, CD8+ T cells from infected B6 → B6 chimeras showed strong cytotoxicity against OVA peptide–loaded target cells and weak cytotoxicity against f-MIGWII–l oaded targets (Fig. 4c), whereas CD8+ T cells from B6 → β2M−/− chimeras showed the opposite pattern: strong cytotoxicity against f-MIGWII–loaded cells and weak cytotoxicity against OVA-peptide–loaded cells. Thus, whereas most MHC class Ia–restricted thymocytes required interactions with MHC class Ia molecules on thymic epithelial cells to mature, our findings showed that functional MHC class Ib–restricted T cells were preferentially selected when MHC class I molecules were expressed only on hematopoietic cells.
Discussion
The results we present here show events that mediate the selection of MHC class Ib–restricted CD8+ T cells differ from those that mediate the selection of MHC class Ia–restricted CD8+ T cells. H2-M3–restricted CD8+ T cells are positively selected on H2-M3 molecules expressed in the thymus21,22, but whether this occurs on epithelial cells or hematopoietic cells had not been examined. We show here that H2-M3–restricted CD8+ T cells are efficiently selected on hematopoietic cells, whereas MHC class Ia–restricted CD8+ T cells are poorly selected.
It was shown previously that a small population of CD8+ T cells can be selected on hematopoietic cells in the thymus, and if these T cells are primed in vivo and boosted in vitro with minor histocompatibility antigens, an MHC class Ia–restricted response can be generated16. This seemed to suggest that CD8+ T cells selected on hematopoietic cells were not a distinct population, but represented a small subset of MHC class Ia–restricted T cells, perhaps those with relatively high affinities. However, the same rules do not seem to apply to CD4+ T cells, as selection of MHC class II–restricted T cells on hematopoietic cells has not been observed23,24. In addition, some MHC class Ia–restricted CD8+ T cells in T cell receptor (TCR)-transgenic mice are unable to be positively selected on hematopoietic cells25,26. Nevertheless, an example of an MHC class Ia–restricted T cell (2C, bearing a Kb-restricted TCR) that can be selected on hematopoietic cells has been demonstrated26. Initially, this finding seemed to support the idea that a subset of CD8+ T cells could be selected via interactions with MHC class Ia molecules expressed on hematopoietic cells. An alternative possibility was suggested by the subsequent finding that the 2C TCR, in addition to recognizing Kb, also recognizes and can be positively selected by an MHC class Ib molecule27. Thus, it is possible that most or all positive selection mediated by hematopoietic cells occurs via interactions with MHC class Ib molecules, but some of these T cells, like 2C, may have dual specificities for MHC class Ia molecules. The observation that positive selection of 2C can occur in lethally irradiated β2M−/− mice reconstituted with Kb−/−Db−/− bone marrow26 suggests the latter possibility, at least for 2C T cells.
Our finding that Listeria-specific H2-M3–restricted CD8+ T cells developed efficiently in B6 → β2M−/− chimeras, but MHC class Ia–restricted T cells did not, supported the idea that MHC class Ib–restricted T cells were preferentially selected on hematopoietic cells. We could not exclude the possibility that a small number of CD8+ T cells could also be positively selected by interacting with MHC class Ia molecules on hematopoietic cells. For example, in most B6 → β2M−/− chimeric mice, a small number of Kb-OVA–specific T cells were usually detected. It is possible that this small population of CD8+ T cells was selected via interactions with MHC class Ia molecules on hematopoietic cells. Alternatively, these Kb-OVA–reactive T cells may actually have been selected on MHC class Ib molecules, but coincidently have a dual specificity for Kb-OVA. Our experiments did not distinguish between these possibilities, but clearly indicated that positive selection on hematopoietic cells favored MHC class Ib–restricted T cells.
The experiments we present here were done to try to explain our finding that CD8+ T cells in Kb−/−Db−/− mice and B6 → β2M−/− chimeric mice show similar activated phenotypes. One possibility that we initially considered was that these small populations of CD8+ T cells may become activated simply as a result of proliferation that can occur in lymphopenic hosts. This explanation seemed unlikely, however, as neither population of CD8+ T cells showed much expansion, but remained at low numbers (1–3% of total spleen cells) for the lifetime of the mice. The finding that large numbers of CD4+ T cells—as are present in both Kb−/−Db−/− mice and B6 → β2M−/− chimeras—inhibit, at least partially, the expansion of CD8+ T cells in hosts with small numbers of CD8+ T cells28,29 may help to explain this lack of expansion. In addition, our observation that CD4−CD8+ thymocytes began to express the activated phenotype in Kb−/−Db−/− mice suggested that this phenotype resulted, in part, from interactions that occurred during development in the thymus, instead of expansion in the periphery.
We considered an alternative possibility, that CD8+ T cells in Kb−/−Db−/− and B6 → β2M−/− mice might be similar because they contained many of the same cells. We hypothesized that this would be true if MHC class Ib–restricted T cells were preferentially selected on hematopoietic cells. The presence of a population of CD8+ T cells in Kb−/−Db−/− → β2M−/− chimeric mice showed that MHC class Ib–restricted CD8+ T cells could be selected on hematopoietic cells; however, the presence of CD8+ T cells in β2M−/− → Kb−/−Db−/− chimeras showed that positive selection of MHC class Ib–restricted CD8+ T cells could also occur on nonhematopoietic cells, most likely thymic epithelial cells. The observation that CD8+ T cells in both Kb−/−Db−/− → β2M−/− and β2M−/− → Kb−/−Db−/− mice were CD44hi indicated that some components of the activated phenotype were intrinsic to MHC class Ib–restricted T cells, regardless of whether they were positively selected on hematopoietic or epithelial cells. In contrast, other markers of the activated phenotype—including expression patterns of β1 integrin, β7 integrin, CD11a and CD122—seemed to be correlated with positive selection on hematopoietic cells. Whether T cells selected on epithelial versus hematopoietic cells represented distinct subsets or whether some T cells had the capacity to be selected on either—but expressed different surface phenotypes depending upon how they were selected—was not directly addressed here. However, the finding that most CD8+ T cells in Kb−/−Db−/− mice expressed the phenotype correlated with selection on hematopoietic cells suggests that this process is important for a large number of MHC class Ib–restricted CD8+ T cells.
We found that MHC class Ib–restricted CD8+ T cells selected on hematopoietic cells (in Kb−/−Db−/− → β2M−/− chimeras) expressed a different set of activation markers compared to MHC class Ib–restricted CD8+ T cells that were selected on epithelial cells (in β2M−/− → Kb−/−Db−/− chimeras). These differences were particularly marked for integrin expression, despite that fact that most CD8+ T cells in both populations expressed an activated CD44hi phenotype. It has been hypothesized that MHC class Ib–restricted CD8+ T cells may be a distinct subset of effector cells that functions in an immune-regulatory role30,31. It seems possible that the differences in integrin expression conferred by positive selection on hematopoietic cells may alter the trafficking of these cells through lymphoid and nonlymphoid tissues, positioning them uniquely for these roles. In addition, the possibility that positive selection on hematopoietic cells functions to give MHC class Ib–restricted T cells many of their innate effector properties also needs to be addressed in future studies.
Why are MHC class Ib–restricted T cells efficiently selected on hematopoietic cells, but not MHC class Ia–restricted T cells? It is likely that the selecting ligands of many MHC class Ia–restricted T cells are expressed on hematopoietic cells and yet selection does not occur. Epithelial cells are generally believed to be specially equipped with cell-surface molecules, or cytokines, that promote T cell development, which makes them the most efficient mediators of positive selection17. It is possible that developing T cells with relatively high affinities for self-MHC molecules have a reduced requirement for these specialized signals. In support of this idea, CD1d-restricted natural killer T (NKT) cells—the only other T cells known to be routinely selected on hematopoietic cells32–36—are self-reactive and are believed to have higher affinities for self-MHC ligands than conventional T cells37. Because MHC class Ib–restricted T cells in Kb−/−Db−/− have an activated phenotype in naïve mice, it seems likely that they also have relatively high affinities for self-MHC–peptide complexes. This idea is supported by the finding that a physiological ligand for positive selection of H2-M3–restricted CD8+ T cells can function as a weak agonist for the activation of the same CD8+ T cells when they are mature38.
As discussed above, NKT cells are the only other T cells known to be routinely selected on hematopoietic cells in the thymus32–36. NKT cells show limited TCR diversity, recognize a nonpolymorphic MHC class Ib–related molecule (CD1d), have an activated phenotype in naïve mice and participate in the early “innate” response to foreign invaders37. These “innate” T cells have been postulated to be evolutionary precursors of conventional T cells involved in adaptive immunity37, which, in contrast, have broad TCR diversity and recognize polymorphic MHC molecules. It is unclear where MHC class Ib–restricted CD8+ T cells fit in the spectrum between “innate” NKT cells and conventional “adaptive” T cells. MHC class Ib–restricted CD8+ T cells, like NKT cells, have an activated phenotype in naïve mice and participate in early immune responses to pathogens. However, despite recognizing MHC molecules with limited polymorphism, MHC class Ib–restricted CD8+ T cells in Kb−/−Db−/− mice have diversity in their Vα and Vβ regions that equals the diversity seen in conventional CD8+ T cells39. However, MHC class Ib–restricted CD8+ T cells are not a pure population, but represent a mixture of cells with specificities for a variety of MHC class Ib molecules, for example, H2-M3, Qa-1, Qa-2 and probably several others. It is possible that if T cells that react with each individual MHC class Ib molecule could be analyzed separately, each population would have limited TCR diversity.
We have demonstrated that H2-M3–restricted CD8+ T cells, like CD1d-restricted NKT cells, have the capacity to be positively selected on hematopoietic cells. In addition, the finding that many CD8+ T cells in Kb−/−Db−/− mice have an activated phenotype that is similar to T cells selected on hematopoietic cells suggests that this may prove to be a general characteristic in the development of many MHC class Ib–restricted T cells. Our results raise the possibility that the distinct properties of MHC class Ib–restricted T cells may result from a different developmental program imprinted by the distinct way in which they are positively selected.
Methods
Mice
C57BL/6 and β2M−/− (backcrossed 11 times to C57BL/6 mice) mice were from Jackson Laboratories (Bar Harbor, ME). Kb−/−Db−/− breeders40 (backcrossed six times to mice) were from J. Forman (Texas Southwestern Medical Center, Dallas, TX) and a colony was maintained at our specific pathogen–free animal facility at the University of Washington. Mice were used at 5–13 weeks of age; all experiments were done according to institutional guidelines.
Bone marrow chimeras
Bone marrow chimeras were prepared by γ-irradiating (1000 rad) 6–8-week-old C57BL/6, β2M−/− or Kb−/−Db−/− recipient mice with a 137Cs source followed by intravenous injection of 107 T-depleted bone marrow cells from C57BL/6, β2M−/− or Kb−/−Db−/− donors. Before injection, T cell depletion was done with CD4 (RL-17241), CD8 (3.16842), Thy1.2 (30-H12, American Type Culture Collection, Manassas, VA) monoclonal antibodies (mAbs) and Low-Tox M rabbit complement (Cedarlane, Hornby, Canada) and was >95% effective. Chimeras were analyzed or infected with L. monocytogenes 8–15 weeks later.
Bacteria and infections
The wild-type L. monocytogenes strain 10403s was from D. Portnoy (University of California at Berkeley, Berkeley, CA) and was grown in brain-heart infusion (BHI) broth. The recombinant L. monocytogenes strain (rLM-OVA), which secretes chicken OVA and contains an erythromycin-resistance marker was from H. Shen (University of Pennsylvania School of Medicine, Philadelphia, PA)19. The rLM-OVA was grown in BHI broth supplemented with 5 µg/ml of erythromycin. Bacteria culture samples were measured by optical density and, at the mid-log growth phase, were diluted in PBS for injection. Chimeric mice were infected with 2000 L. monocytogenes by tail-vein injection. Injected numbers (colony forming units) were more accurately determined by spreading bacterial samples on BHI plates and incubating them overnight at 37 °C. Splenocytes were collected on day 6 or 7 after infection for analysis.
Peptides and tetramers
H-2Kb tetramers bound to the OVA-derived peptide SIIN-FEKL were generated as described43,44. H2-M3 tetramer bound to the Listeria-derived peptide f-MIGWII was a gift of E. Pamer (Sloan-Ketterling, New York, NY). Negative control peptides for both MHC tetramers were prepared to determine the degree of nonspecific background staining, as described15. Splenocytes were resuspended in PBS with fetal calf serum (5%) and NaN3 (0.1%) at a concentration of 107 cells/ml and stained with phycoerythrin (PE)-tetramer, allophycocyanin–anti-CD8 (clone 53.6.7, BD PharMingen, San Diego, CA) and fluorescein isothiocyanate (FITC)–anti-CD44 (clone IM7, BD PharMingen) for 1 h at 4 °C.
Flow cytometry
Single-cell suspensions from spleens, lymph nodes (pooled axillary, brachial, inguinal and mesenteric) or thymi were obtained and red cells were lysed with a hypotonic buffer. The following mAbs were used for flow cytometry: FITC–anti-CD11a (clone 2D7), FITC–anti-CD24 (clone M1/69), FITC–anti-CD44, FITC–anti-CD62L (clone MEL-14), FITC–anti-CD122 (clone TM-β1), FITC–anti- Ly6C (clone AL-21), anti–TCRβ (clone H37-597), FITC–anti-CD8β.2 (clone 53-5.8), PE-anti–β7 integrin (clone M293), biotin–anti-CD29 (β1 integrin, clone Ha2/5), biotin–anti-CD49d (clone R1–2), cychrome–anti-CD4 (clone RM4–5) and allophycocyanin– anti-CD8α (all from BD Pharmingen). FITC-streptavidin (Vector Laboratories, Burlingame, CA) was used as a secondary reagent for biotin-labeled mAb. Flow cytometry was done on a FACSCalibur and analyzed with CELLQuest software (Becton Dickinson, Mountain View, CA).
Intracellular IFN-γ staining
Intracellular IFN-γ staining was done with a kit, as instructed by the manufacturer (Pharmingen). Briefly, in a 24-well plate, 4 × 106 spleen cells per well were stimulated with media alone or 10−8 M of either OVA peptide (SIINFEKL) or f-MIGWII for 6 h in complete RPMI (RPMI 1640 supplemented with 10% fetal calf serum, 2 mM l-glutamine, 10 mM HEPES, 0.5 µM 2-mercaptoethanol, 100 U/ml of penicillin and 100 µg/ml of streptomycin) in the presence of monensin. Cells were washed, stained with allophycocyanin–anti-CD8α, resuspended in permeabilization-fixation buffer and stained with FITC-anti–IFN-γ (clone XMG1.2, Pharmingen). Labeled cells were washed in permeabilization buffer, resuspended in fix buffer and analyzed on a FACSCalibur.
CTL assay
Splenocytes from all experimental mice were assayed ex vivo for their ability to kill peptide-loaded target cells. Serial dilutions (1:3) of effector splenocytes were done in triplicate in 96-well round-bottomed plates. Target cells (a C57BL/6-derived Rauscher leukemia virus (MuLV)-induced T cell lymphoma cell line, RMA) were labeled with 51Cr and cultured with 10 µM OVA or f-MIGWII peptide for 1 h at 37 °C. Negative control target cells were cultured without peptide. Targets were then washed and added to effector cells at 7500 cells/well. Effector cells from each mouse were assayed separately with both peptide-loaded targets. After a 5 h incubation at 37 °C, the plates were centrifuged at 1500 rpm for 5 min and cell supernatants collected. Supernatant samples (100 µl) were counted on a 1470 Wizard automatic γ counter (Perkin Elmer, Shelton, CT) to determine amount of 51Cr release. The percent specific lysis was calculated as 100 × (experimental cpm – spontaneous cpm)/(maximum cpm – spontaneous cpm).
Acknowledgments
We thank X. C. Pan for animal care; B. Dere for tetramer synthesis; J. Yamagiwa for assistance with manuscript preparation; and A. Gallegos and M. Gavin for critical review of the manuscript. Supported by NIH grant K12-HD00850 from the Pediatric Scientist Development Program (to K. B. U.), the Howard Hughes Medical Institute and NIH grant AI19335(to M. J. B.).
Footnotes
Competing interests statement
The authors declare that they have no competing financial interests.
References
- 1.Braud VM, Allan DS, McMichael AJ. Functions of nonclassical MHC and non-MHC-encoded class I molecules. Curr. Opin. Immunol. 1999;11:100–108. doi: 10.1016/s0952-7915(99)80018-1. [DOI] [PubMed] [Google Scholar]
- 2.Loveland B, Wang CR, Yonekawa H, Hermel E, Lindahl KF. Maternally transmitted histo-compatibility antigen of mice: a hydrophobic peptide of a mitochondrially encoded protein. Cell. 1990;60:971–980. doi: 10.1016/0092-8674(90)90345-f. [DOI] [PubMed] [Google Scholar]
- 3.Fischer Lindahl K, Hermel E, Loveland BE, Wang CR. Maternally transmitted antigen of mice: a model transplantation antigen. Annu. Rev. Immunol. 1991;9:351–372. doi: 10.1146/annurev.iy.09.040191.002031. [DOI] [PubMed] [Google Scholar]
- 4.Pamer EG, Wang CR, Flaherty L, Lindahl KF, Bevan MJ. H2-M3 presents a Listeria mono-cytogenes peptide to cytotoxic T lymphocytes. Cell. 1992;70:215–223. doi: 10.1016/0092-8674(92)90097-v. [DOI] [PubMed] [Google Scholar]
- 5.Lenz LL, Dere B, Bevan MJ. Identification of an H2-M3-restricted Listeria epitope: implications for antigen presentation by M3. Immunity. 1996;5:63–72. doi: 10.1016/s1074-7613(00)80310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gulden PH, et al. A Listeria monocytogenes pentapeptide is presented to cytolytic T lymphocytes by the H2-M3 MHC class Ib molecule. Immunity. 1996;5:73–79. doi: 10.1016/s1074-7613(00)80311-8. [DOI] [PubMed] [Google Scholar]
- 7.Chun T, et al. Induction of M3-restricted cytotoxic T lymphocyte responses by N-formylated peptides derived from Mycobacterium tuberculosis. J. Exp. Med. 2001;193:1213–1220. doi: 10.1084/jem.193.10.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewinsohn DM, Briden AL, Reed SG, Grabstein KH, Alderson MR. Mycobacterium tuberculosis-reactive CD8+ T lymphocytes: the relative contribution of classical versus nonclassical HLA restriction. J. Immunol. 2000;165:925–930. doi: 10.4049/jimmunol.165.2.925. [DOI] [PubMed] [Google Scholar]
- 9.Moody DB, et al. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature. 2000;404:884–888. doi: 10.1038/35009119. [DOI] [PubMed] [Google Scholar]
- 10.Moody DB, et al. CD1b-mediated T cell recognition of a glycolipid antigen generated from mycobacterial lipid and host carbohydrate during infection. J. Exp. Med. 2000;192:965–976. doi: 10.1084/jem.192.7.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo WF, et al. Molecular mimicry mediated by MHC class Ib molecules after infection with Gram-negative pathogens. Nature Med. 2000;6:215–218. doi: 10.1038/72329. [DOI] [PubMed] [Google Scholar]
- 12.Bouwer HG, Seaman MS, Forman J, Hinrichs DJ. MHC class Ib–restricted cells contribute to antilisterial immunity: evidence for Qa-1b as a key restricting element for Listeria-specific CTLs. J. Immunol. 1997;159:2795–2801. [PubMed] [Google Scholar]
- 13.Seaman MS, Wang CR, Forman J. MHC class Ib-restricted CTL provide protection against primary and secondary Listeria monocytogenes infection. J. Immunol. 2000;165:5192–5201. doi: 10.4049/jimmunol.165.9.5192. [DOI] [PubMed] [Google Scholar]
- 14.Das G, Sheridan S, Janeway CA., Jr The source of early IFN-γ that plays a role in Th1 priming. J. Immunol. 2001;167:2004–2010. doi: 10.4049/jimmunol.167.4.2004. [DOI] [PubMed] [Google Scholar]
- 15.Kerksiek KM, Busch DH, Pilip IM, Allen SE, Pamer EG. H2-M3-restricted T cells in bacterial infection: rapid primary but diminished memory responses. J. Exp. Med. 1999;190:195–204. doi: 10.1084/jem.190.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bix M, Raulet D. Inefficient positive selection of T cells directed by haematopoietic cells. Nature. 1992;359:330–333. doi: 10.1038/359330a0. [DOI] [PubMed] [Google Scholar]
- 17.Fink PJ, Bevan MJ. Positive selection of thymocytes. Adv. Immunol. 1995;59:99–133. doi: 10.1016/s0065-2776(08)60630-6. [DOI] [PubMed] [Google Scholar]
- 18.Crispe IN, Bevan MJ. Expression and functional significance of the J11d marker on mouse thymocytes. J. Immunol. 1987;138:2013–2018. [PubMed] [Google Scholar]
- 19.Foulds KE, et al. CD4 and CD8 T cells are intrinsically different in their proliferative responses. J. Immunol. 2002;168:1528–1532. doi: 10.4049/jimmunol.168.4.1528. [DOI] [PubMed] [Google Scholar]
- 20.Rotzschke O, et al. Exact prediction of a natural T cell epitope. Eur. J. Immunol. 1991;21:2891–2894. doi: 10.1002/eji.1830211136. [DOI] [PubMed] [Google Scholar]
- 21.Chiu NM, et al. The selection of M3-restricted T cells is dependent on M3 expression and presentation of N-formylated peptides in the thymus. J. Exp. Med. 1999;190:1869–1878. doi: 10.1084/jem.190.12.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berg RE, et al. Positive selection of an H2-M3 restricted T cell receptor. Immunity. 1999;11:33–43. doi: 10.1016/s1074-7613(00)80079-5. [DOI] [PubMed] [Google Scholar]
- 23.Hugo P, Kappler JW, McCormack JE, Marrack P. Fibroblasts can induce thymocyte positive selection in vivo. Proc. Natl. Acad. Sci. USA. 1993;90:10335–10339. doi: 10.1073/pnas.90.21.10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markowitz JS, Auchincloss H, Jr, Grusby MJ, Glimcher LH. Class II-positive hematopoietic cells cannot mediate positive selection of CD4+ T lymphocytes in class II-deficient mice. Proc. Natl. Acad. Sci. USA. 1993;90:2779–2783. doi: 10.1073/pnas.90.7.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kisielow P, Teh HS, Bluthmann H, von Boehmer H. Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature. 1988;335:730–733. doi: 10.1038/335730a0. [DOI] [PubMed] [Google Scholar]
- 26.Zerrahn J, et al. Class I MHC molecules on hematopoietic cells can support intrathymic positive selection of T cell receptor transgenic T cells. Proc. Natl. Acad. Sci. USA. 1999;96:11470–11475. doi: 10.1073/pnas.96.20.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maurice MM, Gould DS, Carroll J, Vugmeyster Y, Ploegh HL. Positive selection of an MHC class-I restricted TCR in the absence of classical MHC class I molecules. Proc. Natl. Acad. Sci. USA. 2001;98:7437–7442. doi: 10.1073/pnas.141143298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rocha B, Dautigny N, Pereira P. Peripheral T lymphocytes: expansion potential and homeostatic regulation of pool sizes and CD4/CD8 ratios in vivo. Eur. J. Immunol. 1989;19:905–911. doi: 10.1002/eji.1830190518. [DOI] [PubMed] [Google Scholar]
- 29.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 30.Shinkai K, Locksley RM. CD1, tuberculosis, and the evolution of major histocompatibility complex molecules. J. Exp. Med. 2000;191:907–914. doi: 10.1084/jem.191.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang H, Chess L. The specific regulation of immune responses by CD8+ T cells restricted by the MHC class Ib molecule, Qa-1. Annu. Rev. Immunol. 2000;18:185–216. doi: 10.1146/annurev.immunol.18.1.185. [DOI] [PubMed] [Google Scholar]
- 32.Bendelac A, Killeen N, Littman DR, Schwartz RH. A subset of CD4+ thymocytes selected by MHC class I molecules. Science. 1994;263:1774–1778. doi: 10.1126/science.7907820. [DOI] [PubMed] [Google Scholar]
- 33.Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J. Exp. Med. 1995;182:2091–2096. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coles MC, Raulet DH. Class I dependence of the development of CD4+ CD8− NK1.1+ thymocytes. J. Exp. Med. 1994;180:395–399. doi: 10.1084/jem.180.1.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coles MC, Raulet DH. NK1.1+ T cells in the liver arise in the thymus and are selected by interactions with class I molecules on CD4+CD8+ cells. J. Immunol. 2000;164:2412–2418. doi: 10.4049/jimmunol.164.5.2412. [DOI] [PubMed] [Google Scholar]
- 36.Ohteki T, MacDonald HR. Major histocompatibility complex class I related molecules control the development of CD4+8− and CD4−8− subsets of natural killer 1.1+ T cell receptor-α/β+ cells in the liver of mice. J. Exp. Med. 1994;180:699–704. doi: 10.1084/jem.180.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 38.Berg RE, Irion S, Kattman S, Princiotta MF, Staerz UD. A physiological ligand of positive selection is recognized as a weak agonist. J. Immunol. 2000;165:4209–4216. doi: 10.4049/jimmunol.165.8.4209. [DOI] [PubMed] [Google Scholar]
- 39.Perarnau B, et al. Single H2Kb, H2Db and double H2KbDb knockout mice: peripheral CD8+ T cell repertoire and anti-lymphocytic choriomeningitis virus cytolytic responses. Eur. J. Immunol. 1999;29:1243–1252. doi: 10.1002/(SICI)1521-4141(199904)29:04<1243::AID-IMMU1243>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 40.Vugmeyster Y, et al. Major histocompatibility complex (MHC) class I KbDb−/− deficient mice possess functional CD8+ T cells and natural killer cells. Proc. Natl. Acad. Sci. USA. 1998;95:12492–12497. doi: 10.1073/pnas.95.21.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ceredig R, Dialynas DP, Fitch FW, MacDonald HR. Precursors of T cell growth factor producing cells in the thymus: ontogeny, frequency, and quantitative recovery in a subpopulation of phenotypically mature thymocytes defined by monoclonal antibody GK-1.5. J. Exp. Med. 1983;158:1654–1671. doi: 10.1084/jem.158.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarmiento M, Glasebrook AL, Fitch FW. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J. Immunol. 1980;125:2665–2672. [PubMed] [Google Scholar]
- 43.Murali-Krishna K, et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 44.Altman JD, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]