Abstract
Background
Elevated levels of oxidized phospholipids (OxPL) on apolipoprotein B-100 particles (OxPL/apoB) are associated with cardiovascular disease and predict new cardiovascular events. Elevated lipoprotein (a) [Lp(a)] levels are a risk factor for cardiovascular disease in Whites, and in Blacks if they also carry small apolipoprotein (a) [apo(a)] isoforms. The relationship of OxPL/apoB levels to race, cardiovascular risk factors and apolipoprotein(a) isoforms is not established.
Methods and Results
OxPL/apoB levels were measured in 3481 subjects (1831 Black, 1047 White and 603 Hispanic) in the Dallas Heart Study and correlated with age, gender, cardiovascular risk factors, lipoprotein (a) [Lp(a)] and apolipoprotein(a) isoforms. Significant differences in OxPL/apoB levels were noted among racial subgroups, with Blacks having the highest levels, compared to Whites and Hispanics (p<0.001 for each comparison). OxPL/apoB levels generally did not correlate with age, gender or risk factors. In the overall cohort, OxPL/apoB levels strongly correlated with lipoprotein (a) [Lp(a)] (r=0.85, p<0.001), with the shape of the relationship demonstrating a “reverse L” shape for log-transformed values. The highest correlation was present in Blacks followed by Whites and Hispanics, was dependent on apo(a) isoform size, and became progressively weaker with larger isoforms. The size of the major apolipoprotein(a) isoform (number of kringle type-IV repeats) was negatively associated with OxPL/apoB (r=-0.49, p<0.001) and Lp(a) (r=-0.61, p<0.001), irrespective of racial group. After adjusting for apolipoprotein(a) isoform size, the relationship between OxPL/apoB and Lp(a) remained significant (r=0.67, p<0.001).
Conclusions
OxPL/apoB levels vary according to race, are largely independent of cardiovascular risk factors, and are inversely associated with apolipoprotein(a) isoform size. The association of OxPL with small apolipoprotein(a) isoforms, where a similar relationship is present among all racial subgroups despite differences in Lp(a) levels, may be a key determinant of cardiovascular risk.
Keywords: lipoproteins, oxidation, atherosclerosis, lipoprotein (a), oxidized phospholipids, risk factors
INTRODUCTION
Oxidized lipids play a central role in mediating a variety of immune, pro-inflammatory and plaque destabilizing processes that further amplify the inflammatory response.1 Plasma levels of specific OxPL on apolipoprotein B-100 (apoB) particles (OxPL/apoB) can be measured with the murine monoclonal antibody E06. OxPL/apoB levels are elevated in patients with coronary, carotid and femoral artery disease,2,3 acute coronary syndromes,4 and following percutaneous coronary intervention.5 Interestingly, in human plasma, E06-detectable OxPL are preferentially carried by Lp(a) lipoprotein (a) [Lp(a)], compared to other apoB-100 particles.2-8
We recently showed in the Bruneck population, which is entirely White, that OxPL/apoB and Lp(a) levels were strongly and significantly associated with the presence, extent and interim development of carotid and femoral atherosclerosis from 1995-2000.3 OxPL/apoB and Lp(a) also predicted new cardiovascular events over a 10 year period, independent of other risk factors, and provided additional prognostic information within each Framingham Risk Score tertile.9
The Dallas Heart Study is a unique epidemiological survey of middle-aged, asymptomatic subjects of different racial groups (approximately 50% Blacks, 30% Whites and 20% Hispanic with 56% female and 44% males) with the purpose of evaluating traditional cardiovascular risk factors, biomarkers and non-invasive measures of atherosclerosis.10 The relationship of Lp(a) and apolipoprotein isoform sizes to coronary calcium in this cohort were previously described.11 The purpose of the current study was to determine if racial differences exist in OxPL/apoB levels and evaluate their relationship to cardiovascular risk factors.
METHODS
Study subjects
A description of the Dallas Heart Study (DHS) subjects was previously described in detail.10,11 The DHS is a multiethnic, probability-based sample of the Dallas county population in which Blacks were systematically over-sampled so the final sample was 50% black. In this study, 3,481 blood samples were available from 3 ethnic groups at the baseline timepoint.
Determination of OxPL/apoB Levels
The content of OxPL per apoB-100 particle (OxPL/apoB) was measured as previously described in detail by chemiluminescent ELISA using the murine monoclonal antibody E06, which binds to the phosphocholine (PC) headgroup of oxidized but not native phospholipids.2,3,6,12 As described, equal numbers of apoB-100 particles are captured from each plasma sample and thus the content of OxPL is normalized for apoB-100 in each subject. Thus, by design, the OxPL/apoB measurement is independent of apoB-100 (and LDL-cholesterol) levels. The OxPL/apoB values are expressed as relative lights units reflecting the amount of E06 bound to OxPL on apoB particles captured on microtiter well plates with antibody MB47. It is to be emphasized that the OxPL/apoB measure only represents those OxPL recognized by E06 (i.e. E06 immunoreactivity) and does not represent all OxPL present on apoB particles. In particular, E06 does not recognize lysoPC.12
Determination of lipoprotein (a) levels and apolipoprotein (a) isotypes
Measurement of Lp(a) levels as nmol/L was performed with a well validated assay which is independent of apolipoprotein(a) isoform size.11 If expressed as mg/dl, the values would be ~2.5 fold lower than as in nmol/L, although at extremes of apo(a) size, larger differences would be present at either extreme. Apolipoprotein(a) isoforms were measured as previously described.11 The analyses in this study were based on size of the major apolipoprotein(a) isoform visualized on agarose gel electrophoresis which is directly proportional to the number of Kringle IV repeats.13 In this study, the major apolipoprotein(a) isoform was associated with the smaller of the 2 alleles in 87% of subjects.
Determination of laboratory variables
Total cholesterol, LDL-cholesterol (LDL-C), HDL cholesterol (HDL-C), triglycerides, high sensitivity C-reactive protein (hsCRP), lipoprotein-associated phospholipase A2 (Lp-PLA2) mass and activity were measured as previously described.14,15 Campesterol, lathosterol, sitosterol, homocysteine, monocyte chemoattractant protein-1 (MCP-1), interleukin-18 (IL-18), peptidoglycan recognition protein-1 (PGLYRP-1), brain natriuretic peptide (BNP), troponin and liver fat by magnetic resonance imaging were measured as previously described.15-21
Statistical Analysis
Six groups were formed from 3 ethnic groups and 2 genders. Omnibus tests were performed among these groups on baseline demographic and laboratory variables using chi-square tests for nominal variables. For continuous variables 1-way ANOVA or Kruskal-Wallis tests were used depending on whether or not the variable was normally distributed.
Significance tests for OxPL/apoB and Lp(a) values were computed on log-transformed variables using 3 (racial group) by 2 (gender) ANOVA models. Post-hoc Tukey tests among racial groups and between genders within racial groups were utilized for comparisons. Other tests of OxPL/apoB utilized nonparametric Kruskal-Wallis or Mann-Whitney U tests. Correlations were computed using Spearman's rank-order method to avoid distributional assumptions. Partial correlation was conducted using log-transformed values of OxPL/apoB and Lp(a). Some analyses were stratified based on kringle IV repeats grouped in tertiles.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
RESULTS
Demographic and Laboratory variables
Table 1 shows the baseline demographic and laboratory variables in male and female Black, White, and Hispanic subjects. Significant baseline differences among groups were noted in all variables.
Table 1.
Baseline demographic and laboratory variables of the study participants (N=3481)
| Black Females (n=1058) |
Black Males (n=773) |
White Females (n=546) |
White Males (n=501) |
Hispanic Females (n=351) |
Hispanic Males (n=252) |
P-value | |
|---|---|---|---|---|---|---|---|
| Age, years | 44.4±10.2 | 44.8±10.3 | 45.2± 10.4 | 44.2±9.5 | 40.0±8.9 | 40.0±9.5 | <0.001 |
| BMI kg/m2 | 32.0±8.0 | 28.4±6.4 | 28.6± 7.5 | 28.2±5.2 | 30.1±6.9 | 28.7±4.6 | <0.001 |
| Current smoking, % | 28.8% | 39.6% | 28.1% | 27.5% | 12.3% | 31.7% | <0.001 |
| Hypertension, % | 41.9% | 41.0% | 19.7% | 20.6% | 15.4% | 14.3% | <0.001 |
| Diabetes, % | 14.1% | 14.4% | 7.0% | 6.4% | 11.7% | 12.4% | <0.001 |
| SBP | 129.5±19.1 | 132.0±18.3 | 120.7± 15.1 | 127.4±13.2 | 118.7± 16.4 | 123.9±13.0 | <0.001 |
| DBP | 80.3±9.9 | 79.4±10.2 | 75.9±8.6 | 78.8± 9.0 | 74.6±9.0 | 75.8± 8.8 | <0.001 |
| Laboratory values |
|||||||
| LDL-C, mg/dl | 105.5±36.1 | 103.9±37.4 | 105.2±33.6 | 111.6±35.0 | 103.2±31.6 | 112.5±34.9 | <0.001 |
| HDL-C, mg/dl | 53.9±14.5 | 49.9± 16.0 | 54.2±16.5 | 42.0±10.2 | 48.3±11.5 | 42.1± 9.7 | <0.001 |
| Triglycerides, mg/dl | 97.4±75.5 | 120.8±115.4 | 122.5± 86.6 | 156.9±123.3 | 139.8±129.6 | 166.8±129.9 | <0.001 |
| Glucose, mg/dl | 103.4±46.2 | 107.9±53.1 | 96.8± 33.7 | 98.7± 33.9 | 107.5±48.7 | 104.1±34.5 | <0.001 |
BMI=body mass index, TG-triglyceride, SBP=systolic blood pressure. DBP=diastolic blood pressure.
Absolute OxPL/apoB and Lp(a) levels in different racial groups
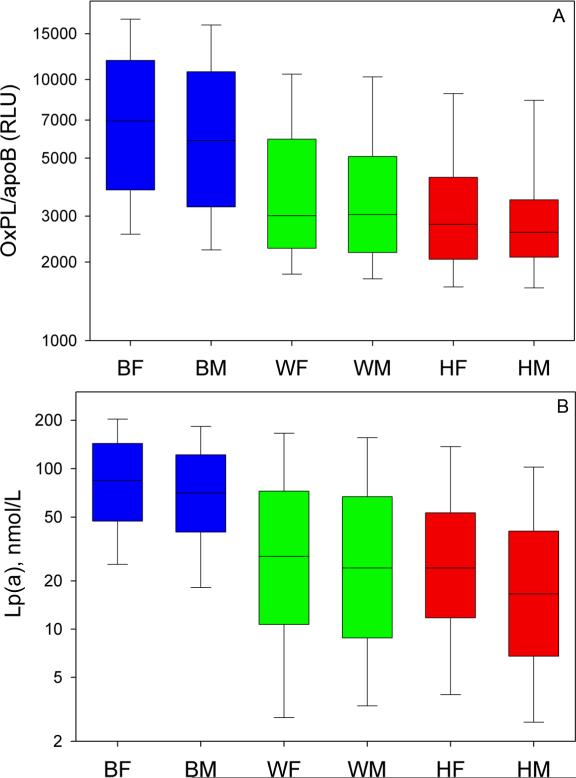
The data for these analyses were evaluated according to sex and race in a 2X3 ANOVA design. There were significant differences in log-transformed OxPL/apoB levels among the racial subgroups, with Blacks having the highest levels, followed by Whites and then by Hispanics (p<0.001 for each comparison, Figure 1A). Differences between males and females in OxPL/apoB within each subgroup were not significant except that Black females had higher OxPL/apoB and Lp(a) values than Black males (p=0.002). Lp(a) levels were higher in Black (p=0.005) and Hispanic (p=0.015) females compared to their male counterparts, but levels were similar among White females and males (p=0.42, Figure 1B). The racial differences remained significant when these groups were stratified by decade age groups (data not shown).
Figure 1.
Levels of OxPL/apoB (A) and Lp(a) (B) categorized by racial group. Boxes indicate medians, 25th and 75th percentile, whiskers indicate 10th and 90th percentile. Differences among racial groups are all significant (p<0.001). BF=Black females, BM- Black males, WF= White females, WM= White Males, HF=Hispanic females, HM= Hispanic males.
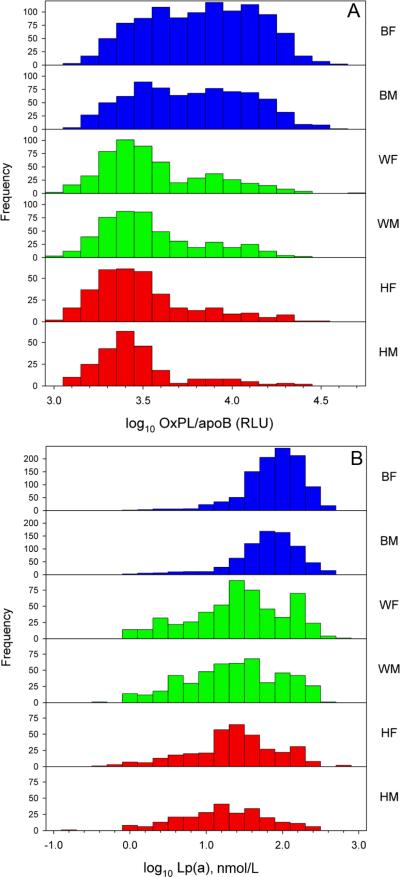
Frequency distribution graphs of log-transformed OxPL/apoB (Figure 2A) and Lp(a) (Figure 2B) levels among the racial groups showed a positive skewness for Hispanics and Whites, whereas less positive skewness was noted in Blacks.
Figure 2.
Frequency distribution of OxPL/apoB (A) and Lp(a) (B) among racial groups. BF=Black females, BM- Black males, WF= White females, WM= White Males, HF=Hispanic females, HM= Hispanic males.
Distribution of Apolipoprotein (a) Isoforms, OxPL/apoB and Lp(a) Levels In the Entire Cohort
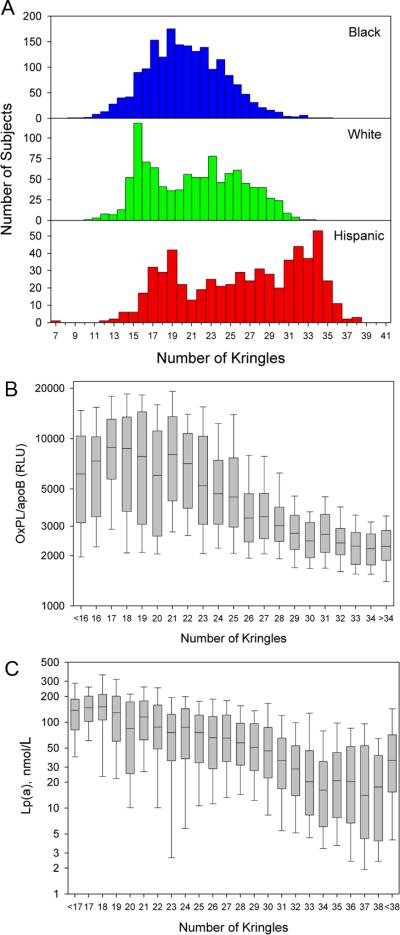
Figure 3A displays the distribution of apo(a) isoforms in the 3 racial groups. In Blacks, a Gaussian distribution is present, whereas a bimodal distribution is more evident in Whites and Hispanics. In the entire cohort with all the 3 racial groups combined, when plotted against the number of kringle IV-repeats, OxPL/apoB (Figure 3B) and Lp(a) (Figures 3C) levels were highest in the subjects with small apo(a) isoforms and lowest in the subjects with large apo(a) isoforms. There was a larger variability in OxPL/apoB values in small apo(a) isoforms, whereas a larger variability in Lp(a) values was present in large apo(a) isoforms.
Figure 3.
Panel A represent the frequency distribution of apo(a) isoform size according to the major apo(a) isoform. Panels B and C represent the distribution of OxPL/apoB and Lp(a) levels, respectively, according to apo(a) isoform size in all the racial groups combined.
Relationship between OxPL/apoB, Lp(a) and apolipoprotein(a) isoforms
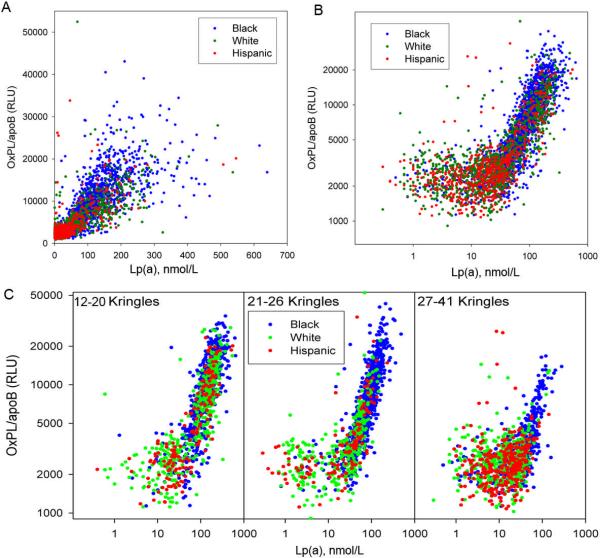
In the overall cohort, OxPL/apoB and Lp(a) levels were highly correlated (Spearman's r=0.85, p<0.001). When plotted on a linear scale, the positive relationship of OxPL/apoB to Lp(a) is noted in the entire cohort (Figure 4A). When plotted on a logarithmic scale (Figure 4B), there was evidence of a “reverse L” shape, with a weaker correlation between OxPL/apoB and Lp(a) (r=0.14, p<0.001) at Lp(a) levels 0<30 nmol/L, and then a stronger correlation (r=0.83, p<0.001) for Lp(a) levels ≥30nmol/L. Furthermore, when evaluating OxPL/apoB versus Lp(a) according to tertiles of apolipoprotein(a) isoform size (number of kringle IV repeats of the major apo(a) isoform: 12-20 repeats, 21-26 repeats and 27-41 repeats), a reverse L relationship was noted in apo(a) isoforms with 12-20 and 21-26 kringle-IV repeats, but less evident in those with 27-41 repeats (Figure 4C).
Figure 4.
This figure displays the correlation between OxPL/apoB and Lp(a). Panel A shows the relationship plotted on a geometric scale. Panel B shows the relationship plotted on a logarithmic scale. Panel C shows the relationship in the entire cohort according to apolipoprotein(a) isoform sizes.
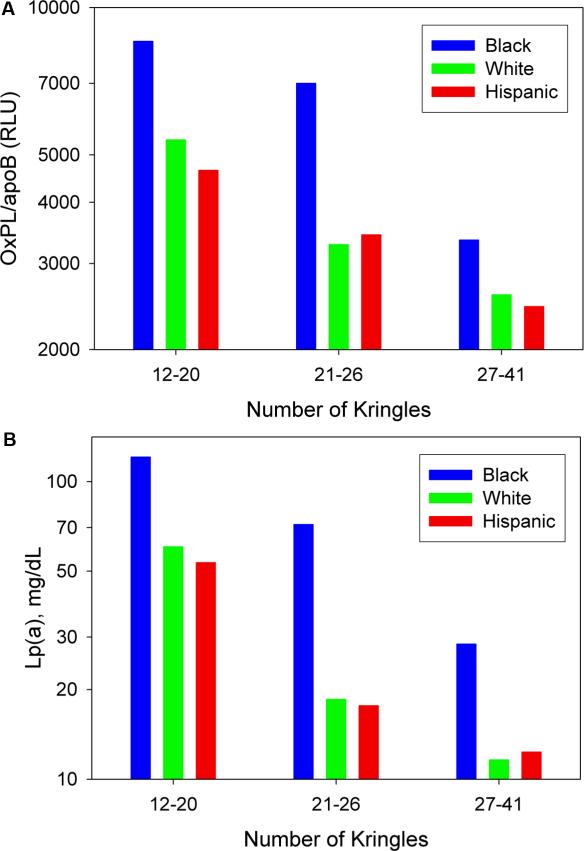
Figure 5 displays the relationship between OxPL/apoB and Lp(a) with apolipoprotein(a) isoform number in different racial groups. It can be appreciated that an inverse relationship is present between OxPL/apoB (Figure 5A) and Lp(a) (Figure 5B) and the number of Kringle IV repeats in Blacks (r= -0.614, p<0.001), Whites (r= -0.567, p<0.001), and Hispanics (r= -0.570, p<0.001). However, within each isoform class, Blacks have higher OxPL/apoB and Lp(a) levels than Whites and Hispanics.
Figure 5.
The bar graph displays the OxPL/apoB (A) and Lp(a) (B) levels according to tertiles of apo(a) isoform size in Blacks, Whites and Hispanics.
Table 2 displays the correlations between OxPL/apoB, Lp(a) and apo(a) isoforms in the overall cohort and in the 3 racial groups. The correlation between OxPL/apoB and Lp(a) was strongest in Blacks, followed by Whites and then Hispanics. When the data were analyzed in groups based on the size of the major apolipoprotein(a) isoform, the racial differences were less marked, with a negative correlation between the major apolipoprotein(a) isoform and OxPL/apoB in all racial subgroups, ranging from r=-0.50 in Black females to r=-0.34 in Hispanic males.
Table 2.
Spearman correlation (r-values) between Lp(a) and OxPL/apoB by race and sex
| Correlation | All | BF | BM | WF | WM | HF | HM |
|---|---|---|---|---|---|---|---|
| Lp(a) vs OxPL/apoB | 0.84* | 0.87* | 0.87* | 0.72* | 0.68* | 0.69* | 0.53* |
| Correlation between major apolipoprotein (a) allele and OxPL/apoB by race-sex | |||||||
| apolipoprotein(a) vs OxPL/apoB | -0.50* | -0.47* | -0.48* | -0.46* | -0.46* | -0.50* | -0.32* |
| Correlation between Lp(a) and OxPL/apoB by race-sex stratified by # of apolipoprotein(a) isoforms in the major allele | |||||||
| 12-20 | 0.85* | 0.81* | 0.84* | 0.84* | 0.85* | 0.85* | 0.80* |
| 21-26 | 0.88* | 0.86* | 0.85* | 0.74* | 0.62* | 0.80* | 0.69* |
| 27-41 | 0.47* | 0.67* | 0.71* | 0.16† | 0.13† | 0.38* | 0.25* |
BF=Black females, BM- Black males, WF= White females, WM= White Males, HF=Hispanic females, HM= Hispanic males.
p < 0.001
p < 0.05
Further analyzing data according to apo(a) tertiles, it is evident that a strong correlation (range r= 0.85-0.79) is noted between OxPL/apoB and Lp(a) in the group with 12-20 kringle IV repeats, irrespective of race. A more modest correlation is noted in all racial subgroups in OxPL/apoB and 21-26 kringle IV repeats (range r-values 0.84-0.43), with the strongest correlations in Blacks and Hispanics. However, weak to no correlations were noted in all racial subgroups with OxPL/apoB and 27-41 kringle IV repeats (Table 2).
In the overall cohort, after adjusting for apolipoprotein(a) isoform size, the relationship between OxPL/apoB and Lp(a) remained significant (r=0.67, p<0.001). Also, the partial correlation adjusting for the sum of the 2 kringle isoforms was 0.686 (p<0.001). For Black, White, Hispanic, the correlations are: 0.729, 0.612, 0.540, respectively, controlling for the sum of the two isoforms and 0.714, 0.582, 0.510, controlling for both major and minor isoforms (all p<0.001).
Adjusting for the socioeconomic status variables of income and education level did not affect any of the above relationships (data not shown).
Relationship of OxPL/apoB to clinical and laboratory variables
In the overall cohort, correlations of OxPL/apoB with blood pressure were very weak (r=0.078 for systolic pressure and r=0.089 diastolic pressure, p<0.001 for both) and not significant with BMI (r=0.019, p=0.27). OxPL/apoB levels were higher in subjects with versus without hypertension (medians of 4910 vs. 3755 RLU, p<0.001) but not in those with versus without diabetes (medians of 4198 vs. 4008 RLU, p=0.135). There were very weak relationships between OxPL/apoB blood glucose levels (Spearman's r = -0.051, p=0.003).
The relationship of OxPL/apoB and Lp(a) to a variety of laboratory variables is shown in Table 3. Although many of these correlations were statistically significant, most were quite weak. Of interest, there was a negative association of both OxPL/apoB and Lp(a) with triglyceride levels and liver fat content measured with MRI. Both OxPL/apoB and Lp(a) correlated very weakly with hsCRP. There were also weak correlations between BNP and OxPL/apoB and Lp(a). There was no relationship between OxPL/apoB or Lp(a) with MCP-1, PGLYRP-S, or troponin levels. Lp-PLA2 mass correlated inversely but weakly with OxPL/apoB and Lp(a) as did Lp-PLA2 activity. No major differences were noted among the racial groups in these associations.
Table 3.
Correlations of OxPL/apoB and Lp(a) with Laboratory Variables
| OxPL/apoB | Lp(a) | |||
|---|---|---|---|---|
| R-Value | P-Value | R-Value | P-Value | |
| TC | 0.024 | 0.07 | 0.086 | <0.001 |
| LDL-C | 0.045 | 0.008 | 0.124 | <0.001 |
| HDL-C | 0.150 | <0.001 | 0.150 | <0.001 |
| TG | -0.156 | <0.001 | -0.150 | <0.001 |
| Liver Fat byMRI | -0.136 | <0.001 | -0.130 | <0.001 |
| Campesterol | 0.085 | 0.001 | 0.055 | 0.001 |
| Lathosterol | -0.091 | <0.001 | -0.091 | <0.001 |
| Sitosterol | 0.050 | 0.003 | 0.003 | 0.87 |
| Homocysteine | 0.056 | <0.001 | 0.018 | 0.29 |
| Lp-PLA2 Mass | -0.061 | <0.001 | -0.037 | 0.033 |
| Lp-PLA2 Activity | -0.120 | <0.001 | -0.077 | <0.001 |
| hsCRP | 0.062 | <0.001 | 0.094 | <0.001 |
| IL-18 | 0.001 | 0.97 | -0.020 | 0.36 |
| MCP-1 | -0.052 | 0.002 | -0.006 | 0.70 |
| PGRP-1 | 0.025 | 0.16 | 0.051 | 0.004 |
| troponin | 0.036 | 0.033 | 0.037 | 0.026 |
| BNP | 0.070 | <0.001 | 0.071 | <0.001 |
DISCUSSION
This large epidemiological study of apparently healthy, young to middle aged individuals demonstrates that significant racial differences exist in OxPL/apoB levels, with Black subjects displaying the highest levels, followed by Whites and then by Hispanics. OxPL/apoB levels were largely independent of most clinical and laboratory variables. However, OxPL/apoB levels were distributed in a manner consistent with genetically determined Lp(a) levels,22,23 and furthermore, were positively correlated with small apolipoprotein(a) isoforms. Interestingly, when the data were analyzed according to the size of the major apolipoprotein(a) isoform, the racial differences in OxPL/apoB were less marked. In fact, in subjects of all racial subgroups with isoform sizes ≥30 kringle IV repeats, no significant relationship was noted between OxPL/apoB and Lp(a). These data suggest that OxPL/apoB levels may reflect a key component of the atherogenicity of elevated Lp(a) levels, particularly in the presence of small apo(a) isoforms.
Distribution of OxPL/apoB levels in Different Racial Groups
Previous studies documenting the relationship between OxPL/apoB and Lp(a) were performed primarily in White populations (MAYO,2 Bruneck3,9 and MIRACL6 Studies). The Dallas Heart Study documents that this relationship also exists in Blacks and Hispanics, but is quantitatively different in these groups compared to Whites. For example, the highest correlation of OxPL/apoB with Lp(a) was present in Blacks and the weakest was present in Hispanics, compared to Whites. High Lp(a) levels are inherited as a dominant quantitative trait and most subjects (~80%) express 2 distinct apolipoprotein(a) alleles, although subjects with one allele or null alleles have been described.24 Elevated Lp(a) levels can be found in a large proportion of individuals in most racial groups, with the prevalence being lowest in Whites and Asians.25,26 The median Lp(a) levels in Black subjects, and in Asian Indians from southern locations,27 are 2-4 fold higher compared to Whites and up to 68% of Blacks have Lp(a) levels >30 mg/dl whereas levels above this threshold are present in ~25% in Whites.23,26,28,29
In Whites, elevated Lp(a) levels are generally associated with small apo(a) isoforms in over 80% of subjects. In Blacks, elevated Lp(a) levels are distributed over a broader range of apo(a) isoform sizes, and plasma Lp(a) levels are higher in Blacks within the same apo(a) isoform sizes as also documented in this study.23,28,30 In particular, differences in Lp(a) levels between Blacks and Whites are prominent in the range of 20-25 kringle IV repeats, but the underlying reasons why Blacks have higher Lp(a) levels than Whites for similar isoform size is not understood.28 The current data are consistent with the interpretation that OxPL/apoB levels are also genetically determined in most subjects in a manner parallel to Lp(a), and also highly reflect apo(a) isoform size.
Relationship of OxPL/apoB and Lp(a) Levels
The OxPL/apoB assay was initially designed as a method to quantitate minimally oxidized phospholipids on apoB particles, as a measure of “oxidized LDL”. Unexpectedly, in all clinical studies done to date, a significant correlation has been found between OxPL/apoB and Lp(a), in the range of R=0.80-0.90.2-7 This finding was also confirmed in the current study, but a more extended analysis of this study provides further, novel insights into this relationship. For example, on a linear scale the relationship of OxPL/apoB to Lp(a) appears positive and roughly linear, but on a logarithmic scale, a “reverse L” shape was noted with a flat relationship up to Lp(a) levels of approximately ~30 nmol/L and then a log-linear relationship at Lp(a) levels >30 nmol/L. Part of this association may be related to the different distribution of Lp(a) levels in this study, where Black patients had highest levels compared to Whites and Hispanics, thus potentially creating an artificial relationship. However, this is not likely the explanation, as a reverse L shape can be seen in all racial groups. This is further substantiated when the data are evaluated by apolipoprotein(a) isoform size demonstrating that this reverse L relationship was evident in all racial groups with isoforms having <26 but not >27 kringle IV repeats. Furthermore, adding both kringle sizes together from each isoform did not substantially change the findings. This also suggests that a threshold effect may occur in this relationship and that a critical value of Lp(a) or a critical number of apolipoprotein(a) particles may be needed to mediate significant binding of OxPL. Further work is required to quantitatively ascertain the extent to which Lp(a) may bind and release OxPL and to identify factors, such as lipoprotein-associated phospholipase A2, that may affect this relationship.31
In support of the ability of Lp(a) as opposed to other lipoproteins in binding OxPL recognized by E06, recent data from our laboratory demonstrated that more than 85% of E06 reactivity (i.e. OxPL) co-immunoprecipitates with Lp(a).8 In lipoprotein ultracentrifugation experiments, nearly all OxPL associated with lipoproteins were found in fractions containing apolipoprotein(a), as opposed to other apolipoproteins. Furthermore, in vitro transfer studies showed that oxidized LDL preferentially donates OxPL to Lp(a), as opposed to LDL, in a time and temperature dependent manner, even in aqueous buffer. Additionally, ~50% of E06 immunoreactivity could be extracted from isolated Lp(a) following exposure of plasma to various lipid solvents. These in vitro studies lend further proof that OxPLs are strongly associated physically with Lp(a) and corroborate the insights derived from clinical populations into its physiological function and mechanisms of atherogenicity.32 Consistent with the above observations, studies from independent laboratories have demonstrated that small dense LDL contains electronegative LDL which is enriched in lipoperoxides and has enhanced susceptibility for generating OxPL.33 Furthermore, electronegative LDL is enriched in Lp-PLA2, which may mediate some of its atherogenic properties.34
Relationship of OxPL/apoB and Apolipoprotein(a) Size
It is well known that elevated Lp(a) levels are associated with increased risk of cardiovascular disease in White subjects, particularly when elevated LDL-C levels are also present,35 but this association has not consistently been demonstrated in Blacks.36,37 In females, Lp(a) appears to be a stronger risk factor when very high Lp(a) levels (>65 mg/dl) are present38 However, apolipoprotein(a) phenotypes, particularly those where apolipoprotein(a) contains <22 kringle IV repeats, appear to be associated with increased cardiovascular risk in both Whites and Blacks and are more predictive of cardiovascular disease than elevated Lp(a) levels.23,28,39,40 In the current study OxPL/apoB levels correlated most strongly with both elevated Lp(a) levels and with smaller apolipoprotein(a) isoforms, irrespective of race, age and gender, whereas they did not correlate in the larger apolipoprotein(a) isoforms, suggesting that this relationship is related to underlying genetic differences in apolipoprotein(a) size and/or number rather than race per se. However, since Blacks had higher Lp(a) levels, they also had correspondingly higher OxPL/apoB levels than Whites and Hispanics. Identifying the potential sites on apolipoprotein(a) and Lp(a) that mediate binding of OxPL and identifying the specific OxPL on these lipoproteins merits further exploration.
Relationship of OxPL and Lp(a) to cardiovascular risk factors and laboratory variables
This is the largest study to examine the relationship of OxPL/apoB levels to cardiovascular risk factors and laboratory variables in an epidemiological cohort. Due to the large number of subjects, there are some statistically significant associations. However, most of these are quite weak or borderline and these correlations are unlikely to exert a significant pathophysiological influence on cardiovascular risk. Notably, OxPL/apoB levels were either not correlated or minimally associated with several inflammatory variables (hsCRP, IL-18, MCP-1, PGLYP-1), markers of myocardial damage (troponin) or increased left ventricular wall stress (BNP). Interestingly, a negative association was noted between both OxPL/apoB and plasma triglyceride level and liver fat content by MRI. This observation has been made previously for Lp(a), although the reasons are not well understood and require further mechanistic insights.41,42 It is also conceivable that anti-PC antibodies may modulate plasma levels of OxPL/apoB. Although such levels were not measured in this study, previous studies have not documented any significant relationship between plasma levels of OxPL/apoB and IgG or IgM autoantibodies to copper oxidized LDL (CuOxLDL), a fraction of which may be anti-PC antibodies, or with autoantibodies to MDA-LDL or to apoB-immune complexes.43 It has been reported in several studies that very low levels of IgM autoantibodies to PC-BSA,44,45 as well as IgM CuOxLDL and MDA-LDL,43 are associated with higher incidence of various manifestations of atherosclerosis and clinical events. It is also possible that such antibodies may also have therapeutic potential through a variety of mechanisms.46 Additional determinants of OxPL/apoB plasma levels besides genetically determined Lp(a) will require future studies.
In a previous publication from the Dallas Heart Study, Lp-PLA2 mass and activity were lowest in Blacks compared to Whites and Hispanics.14 In the current analysis, weak inverse correlations were noted between OxPL/apoB and Lp(a) and both Lp-PLA2 mass and activity. These data are consistent with the physiological action of Lp-PLA2 in cleaving the oxidized fatty acid at the sn-2 position of OxPL and suggest that there may also be an influence of plasma levels of these measures. Furthermore, since Lp(a) is preferentially enriched in Lp-PLA2 activity on an equimolar basis compared to LDL, Lp-PLA2 activity on Lp(a) particles may serve as a mechanism to clear OxPL bound to Lp(a).31
Limitations
This study did not measure clinical outcomes and therefore it cannot be determined if racial differences in OxPL/apoB predict clinical outcomes.
Conclusions
This study documents that elevated levels of pro-inflammatory oxidized phospholipids carried primarily by Lp(a) represent a genetic predisposition to increased oxidative stress. Furthermore, the study suggests that the differences in apolipoprotein(a) isoforms explain some, but not all, of the racial differences in OxPL/apoB and Lp(a) seen in this population. These findings help to understand the mechanistic underpinnings of the potential atherogenicity, and potentially the inhibition of fibrinolysis by Lp(a), which has previously been documented in vitro. Future studies should focus on apolipoprotein(a) isoforms and their relationship to cardiovascular events mediated by OxPL.
Acknowledgment
The authors thank Helen Hobbs for her support and assistance in establishing this investigation.
Funding Sources These studies were supported by grants from the Donald W. Reynolds Foundations to the University of Texas Southwestern, to the Brigham and Women's Hospital and to the University of California San Diego, by the Fondation Leducq and by the General Clinical Research Center, University California, San Diego with funding provided by the National Center for Research Resources, M01RR00827, USPHS.
Footnotes
Disclosures Drs Witztum and Tsimikas are named as inventors in patents and patent applications for the potential commercial use of antibodies to oxidized LDL.
References
- 1.Navab M, Ananthramaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fonarow GC, Vahabzadeh K, Hama S, Hough G, Kamranpour N, Berliner JA, Lusis AJ, Fogelman AM. Thematic review series: The Pathogenesis of atherosclerosis: The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid Res. 2004;45:993–1007. doi: 10.1194/jlr.R400001-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Tsimikas S, Brilakis ES, Miller ER, McConnell JP, Lennon RJ, Kornman KS, Witztum JL, Berger PB. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med. 2005;353:46–57. doi: 10.1056/NEJMoa043175. [DOI] [PubMed] [Google Scholar]
- 3.Tsimikas S, Kiechl S, Willeit J, Mayr M, Miller ER, Kronenberg F, Xu Q, Bergmark C, Weger S, Oberhollenzer F, Witztum JL. Oxidized phospholipids predict the presence and progression of carotid and femoral atherosclerosis and symptomatic cardiovascular disease: five-year prospective results from the Bruneck study. J Am Coll Cardiol. 2006;47:2219–2228. doi: 10.1016/j.jacc.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Tsimikas S, Bergmark C, Beyer RW, Patel R, Pattison J, Miller E, Juliano J, Witztum JL. Temporal increases in plasma markers of oxidized low-density lipoprotein strongly reflect the presence of acute coronary syndromes. J Am Coll Cardiol. 2003;41:360–370. doi: 10.1016/s0735-1097(02)02769-9. [DOI] [PubMed] [Google Scholar]
- 5.Tsimikas S, Lau HK, Han KR, Shortal B, Miller ER, Segev A, Curtiss LK, Witztum JL, Strauss BH. Percutaneous coronary intervention results in acute increases in oxidized phospholipids and lipoprotein(a): Short-term and long-term immunologic responses to oxidized low-density lipoprotein. Circulation. 2004;109:3164–3170. doi: 10.1161/01.CIR.0000130844.01174.55. [DOI] [PubMed] [Google Scholar]
- 6.Tsimikas S, Witztum JL, Miller ER, Sasiela WJ, Szarek M, Olsson AG, Schwartz GG. High-dose atorvastatin reduces total plasma levels of oxidized phospholipids and immune complexes present on apolipoprotein B-100 in patients with acute coronary syndromes in the MIRACL trial. Circulation. 2004;110:1406–1412. doi: 10.1161/01.CIR.0000141728.23033.B5. [DOI] [PubMed] [Google Scholar]
- 7.Rodenburg J, Vissers MN, Wiegman A, Miller ER, Ridker PM, Witztum JL, Kastelein JJ, Tsimikas S. Oxidized low-density lipoprotein in children with familial hypercholesterolemia and unaffected siblings: Effect of pravastatin. J Am Coll Cardiol. 2006;47:1803–1810. doi: 10.1016/j.jacc.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 8.Bergmark C, Dewan A, Orsoni A, Merki E, Miller ER, Shin MJ, Binder CJ, Horkko S, Krauss RM, Chapman MJ, Witztum JL, Tsimikas S. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J Lipid Res. 2008;49:2230–2239. doi: 10.1194/jlr.M800174-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Kiechl S, Willeit J, Mayr M, Viehweider B, Oberhollenzer M, Kronenberg F, Wiedermann CJ, Oberthaler S, Xu Q, Witztum JL, Tsimikas S. Oxidized phospholipids, lipoprotein(a), lipoprotein-associated phospholipase A2 activity, and 10-year cardiovascular outcomes: Prospective results from the Bruneck Study. Arterioscler Thromb Vasc Biol. 2007;27:1788–1795. doi: 10.1161/ATVBAHA.107.145805. [DOI] [PubMed] [Google Scholar]
- 10.Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, Staab JM, Hobbs HH. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 11.Guerra R, Yu Z, Marcovina SM, Peshock R, Cohen JC, Hobbs HH. Lipoprotein(a) and apolipoprotein(a) isoforms: no association with coronary artery calcification in the Dallas Heart Study. Circulation. 2005;111:1471–1479. doi: 10.1161/01.CIR.0000159263.50305.BD. [DOI] [PubMed] [Google Scholar]
- 12.Friedman P, Hörkkö S, Steinberg D, Witztum JL, Dennis EA. Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids: Importance of Schiff base formation and Aldol condensation. J Biol Chem. 2001;277:7010–7020. doi: 10.1074/jbc.M108860200. [DOI] [PubMed] [Google Scholar]
- 13.Marcovina SM, Hobbs HH, Albers JJ. Relation between number of apolipoprotein(a) kringle 4 repeats and mobility of isoforms in agarose gel: basis for a standardized isoform nomenclature. Clinical Chemistry. 1996;42:436–439. [PubMed] [Google Scholar]
- 14.Brilakis ES, Khera A, McGuire DK, See R, Banerjee S, Murphy SA, de Lemos JA. Influence of race and sex on lipoprotein-associated phospholipase A2 levels: Observations from the Dallas Heart Study. Atherosclerosis. 2007 doi: 10.1016/j.atherosclerosis.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deo R, Khera A, McGuire DK, Murphy SA, de PMN, Morrow DA, de Lemos JA. Association among plasma levels of monocyte chemoattractant protein-1, traditional cardiovascular risk factors, and subclinical atherosclerosis. J Am Coll Cardiol. 2004;44:1812–1818. doi: 10.1016/j.jacc.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 16.Wilund KR, Yu L, Xu F, Vega GL, Grundy SM, Cohen JC, Hobbs HH. No association between plasma levels of plant sterols and atherosclerosis in mice and men. Arterioscler Thromb Vasc Biol. 2004;24:2326–2332. doi: 10.1161/01.ATV.0000149140.00499.92. [DOI] [PubMed] [Google Scholar]
- 17.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 18.Rohatgi A, Ayers CR, Khera A, McGuire DK, Das SR, Matulevicius S, Timaran CH, Rosero EB, de Lemos JA. The association between peptidoglycan recognition protein-1 and coronary and peripheral atherosclerosis: Observations from the Dallas Heart Study. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Zirlik A, Abdullah SM, Gerdes N, MacFarlane L, Schonbeck U, Khera A, McGuire DK, Vega GL, Grundy S, Libby P, de Lemos JA. Interleukin-18, the metabolic syndrome, and subclinical atherosclerosis: results from the Dallas Heart Study. Arterioscler Thromb Vasc Biol. 2007;27:2043–2049. doi: 10.1161/ATVBAHA.107.149484. [DOI] [PubMed] [Google Scholar]
- 20.Abdullah SM, Khera A, Das SR, Stanek HG, Canham RM, Chung AK, Morrow DA, Drazner MH, McGuire DK, de Lemos JA. Relation of coronary atherosclerosis determined by electron beam computed tomography and plasma levels of n-terminal pro-brain natriuretic peptide in a multiethnic population-based sample (the Dallas Heart Study) Am J Cardiol. 2005;96:1284–1289. doi: 10.1016/j.amjcard.2005.06.073. [DOI] [PubMed] [Google Scholar]
- 21.Wallace TW, Abdullah SM, Drazner MH, Das SR, Khera A, McGuire DK, Wians F, Sabatine MS, Morrow DA, de Lemos JA. Prevalence and determinants of troponin T elevation in the general population. Circulation. 2006;113:1958–1965. doi: 10.1161/CIRCULATIONAHA.105.609974. [DOI] [PubMed] [Google Scholar]
- 22.Sandholzer C, Saha N, Kark JD, Rees A, Jaross W, Dieplinger H, Hoppichler F, Boerwinkle E, Utermann G. Apo(a) isoforms predict risk for coronary heart disease. A study in six populations. Arterioscler Thromb. 1992;12:1214–1226. doi: 10.1161/01.atv.12.10.1214. [DOI] [PubMed] [Google Scholar]
- 23.Paultre F, Pearson TA, Weil HF, Tuck CH, Myerson M, Rubin J, Francis CK, Marx HF, Philbin EF, Reed RG, Berglund L. High levels of Lp(a) with a small apo(a) isoform are associated with coronary artery disease in African American and white men. Arterioscler Thromb Vasc Biol. 2000;20:2619–2624. doi: 10.1161/01.atv.20.12.2619. [DOI] [PubMed] [Google Scholar]
- 24.Utermann G. Genetic architecture and evolution of the lipoprotein(a) trait. Curr Opin Lipidol. 1999;10:133–141. doi: 10.1097/00041433-199904000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Utermann G. The mysteries of lipoprotein(a) Science. 1989;246:904–910. doi: 10.1126/science.2530631. [DOI] [PubMed] [Google Scholar]
- 26.Hobbs HH, White AL. Lipoprotein(a): intrigues and insights. Curr Opin Lipidol. 1999;10:225–236. doi: 10.1097/00041433-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Geethanjali FS, Luthra K, Lingenhel A, Kanagasaba-Pathy AS, Jacob J, Srivastava LM, Vasisht S, Kraft HG, Utermann G. Analysis of the apo(a) size polymorphism in Asian Indian populations: association with Lp(a) concentration and coronary heart disease. Atherosclerosis. 2003;169:121–130. doi: 10.1016/s0021-9150(03)00143-6. [DOI] [PubMed] [Google Scholar]
- 28.Marcovina SM, Albers JJ, Wijsman E, Zhang Z, Chapman NH, Kennedy H. Differences in Lp[a] concentrations and apo[a] polymorphs between black and white Americans. J Lipid Res. 1996;37:2569–2585. [PubMed] [Google Scholar]
- 29.Kostner KM, Kostner GM. Lipoprotein(a): still an enigma? Curr Opin Lipidol. 2002;13:391–396. doi: 10.1097/00041433-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Marcovina SM, Koschinsky ML, Albers JJ, Skarlatos S. Report of the National Heart, Lung, and Blood Institute Workshop on Lipoprotein(a) and Cardiovascular Disease: recent advances and future directions. Clin Chem. 2003;49:1785–1796. doi: 10.1373/clinchem.2003.023689. [DOI] [PubMed] [Google Scholar]
- 31.Tsimikas S, Tsironis LD, Tselepis AD. New insights into the role of lipoprotein(a)-associated lipoprotein-associated phospholipase A2 in atherosclerosis and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2094–2099. doi: 10.1161/01.ATV.0000280571.28102.d4. [DOI] [PubMed] [Google Scholar]
- 32.Tsimikas S, Witztum JL. The role of oxidized phospholipids in mediating lipoprotein(a) atherogenicity. Curr Opin Lipidol. 2008;19:369–377. doi: 10.1097/MOL.0b013e328308b622. [DOI] [PubMed] [Google Scholar]
- 33.Sevanian A, Hwang J, Hodis H, Cazzolato G, Avogaro P, Bittolo-Bon G. Contribution of an In Vivo Oxidized LDL to LDL Oxidation and Its Association With Dense LDL Subpopulations. Arterioscler Thromb Vasc Biol. 1996;16:784–793. doi: 10.1161/01.atv.16.6.784. [DOI] [PubMed] [Google Scholar]
- 34.Benitez S, Sanchez-Quesada JL, Ribas V, Jorba O, Blanco-Vaca F, Gonzalez-Sastre F, Ordonez-Llanos J. Platelet-activating factor acetylhydrolase is mainly associated with electronegative low-density lipoprotein subfraction. Circulation. 2003;108:92. doi: 10.1161/01.CIR.0000072791.40232.8F. [DOI] [PubMed] [Google Scholar]
- 35.Anuurad E, Boffa MB, Koschinsky ML, Berglund L. Lipoprotein(a): a unique risk factor for cardiovascular disease. Clin Lab Med. 2006;26:751–772. doi: 10.1016/j.cll.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Srinivasan SR, Dahlen GH, Jarpa RA, Webber LS, Berenson GS. Racial (black-white) differences in serum lipoprotein (a) distribution and its relation to parental myocardial infarction in children. Bogalusa Heart Study. Circulation. 1991;84:160–167. doi: 10.1161/01.cir.84.1.160. [DOI] [PubMed] [Google Scholar]
- 37.Moliterno DJ, Jokinen EV, Miserez AR, Lange RA, Willard JE, Boerwinkle E, Hillis LD, Hobbs HH. No association between plasma lipoprotein(a) concentrations and the presence or absence of coronary atherosclerosis in African-Americans. Arterioscler Thromb Vasc Biol. 1995;15:850–855. doi: 10.1161/01.atv.15.7.850. [DOI] [PubMed] [Google Scholar]
- 38.Suk DJ, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), measured with an assay independent of apolipoprotein(a) isoform size, and risk of future cardiovascular events among initially healthy women. JAMA. 2006;296:1363–1370. doi: 10.1001/jama.296.11.1363. [DOI] [PubMed] [Google Scholar]
- 39.Kraft HG, Lingenhel A, Kochl S, Hoppichler F, Kronenberg F, Abe A, Muhlberger V, Schonitzer D, Utermann G. Apolipoprotein(a) kringle IV repeat number predicts risk for coronary heart disease. Arterioscler Thromb Vasc Biol. 1996;16:713–719. doi: 10.1161/01.atv.16.6.713. [DOI] [PubMed] [Google Scholar]
- 40.Seed M, Ayres KL, Humphries SE, Miller GJ. Lipoprotein(a) as a predictor of myocardial infarction in middle-aged men. Am J Med. 2001;110:22–27. doi: 10.1016/s0002-9343(00)00652-5. [DOI] [PubMed] [Google Scholar]
- 41.Schaefer EJ, Lamon-Fava S, Jenner JL, McNamara JR, Ordovas JM, Davis CE, Abolafia JM, Lippel K, Levy RI. Lipoprotein(a) levels and risk of coronary heart disease in men. The Lipid Research Clinics Coronary Primary Prevention Trial. JAMA. 1994;271:999–1003. doi: 10.1001/jama.1994.03510370051031. [DOI] [PubMed] [Google Scholar]
- 42.Tholstrup T, Samman S. Postprandial lipoprotein(a) Is affected differently by specific individual dietary fatty acids in healthy young men. J Nutr. 2004;134:2550–2555. doi: 10.1093/jn/134.10.2550. [DOI] [PubMed] [Google Scholar]
- 43.Gounopoulos P, Merki E, Hansen LF, Choi SH, Tsimikas S. Antibodies to oxidized low density lipoprotein: epidemiological studies and potential clinical applications in cardiovascular disease. Minerva Cardioangiol. 2007;55:821–837. [PubMed] [Google Scholar]
- 44.Su J, Georgiades A, Wu R, Thulin T, de Faire U, Frostegard J. Antibodies of IgM subclass to phosphorylcholine and oxidized LDL are protective factors for atherosclerosis in patients with hypertension. Atherosclerosis. 2006;188:160–166. doi: 10.1016/j.atherosclerosis.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 45.Sjoberg BG, Su J, Dahlbom I, Gronlund H, Wikstrom M, Hedblad B, Berglund G, de Faire U, Frostegard J. Low levels of IgM antibodies against phosphorylcholine-A potential risk marker for ischemic stroke in men. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.07.009. In press. [DOI] [PubMed] [Google Scholar]
- 46.Hartvigsen K, Chou MY, Hansen LF, Shaw PX, Tsimikas S, Binder CJ, Witztum JL. The role of innate immunity in atherogenesis. J Lipid Res. 2008 doi: 10.1194/jlr.R800100-JLR200. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]