Abstract
DNA methylation is an important epigenetic mark for transcriptional gene silencing (TGS) in diverse organisms1–6. Recent studies suggest that the methylation status of a number of genes is dynamically regulated by methylation and demethylation7–10. In Arabidopsis, active DNA demethylation is mediated by the ROS1 (repressor of silencing 1) subfamily of 5-methylcytosine DNA glycosylases through a base excision repair pathway8,10–13. These demethylases play critical roles in erasing DNA methylation and preventing TGS of target genes7,8,10. However, it is not known how the demethylases are targeted to specific sequences. We report here the identification of ROS3, an essential regulator of DNA demethylation that contains an RNA recognition motif. Analysis of ros3 mutant and ros1ros3 double mutant suggests that ROS3 acts in the same genetic pathway as ROS1 to prevent DNA hypermethylation and TGS. Gel mobility shift assays and analysis of ROS3 immunoprecipitate from plant extracts showed that ROS3 binds to small RNAs in vitro and in vivo. Immunostaining shows that ROS3 and ROS1 proteins colocalize in discrete foci dispersed throughout the nucleus. These results demonstrate a critical role for ROS3 in preventing DNA hypermethylation and suggest that DNA demethylation by ROS1 may be guided by RNAs bound to ROS3.
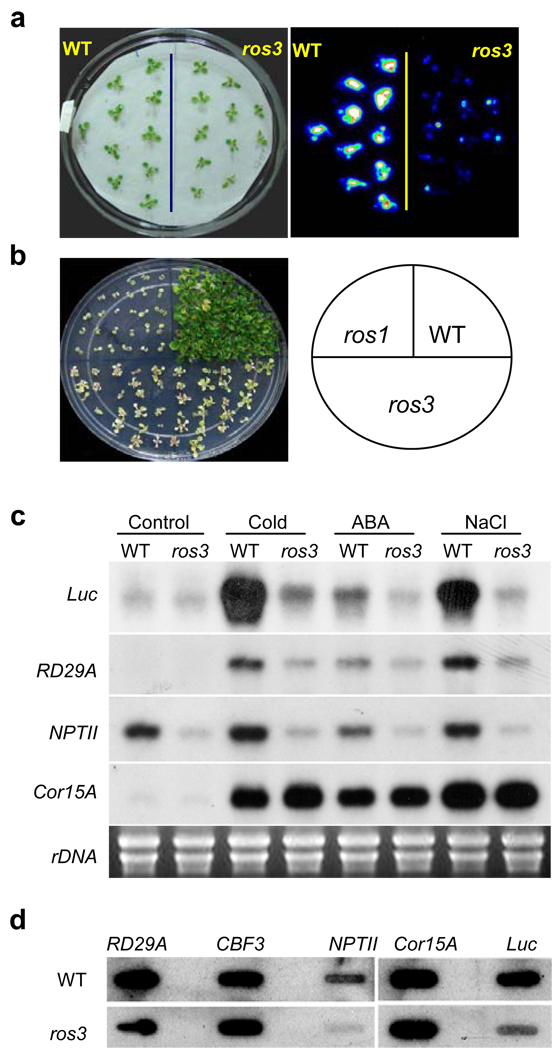
We developed a sensitive assay system in Arabidopsis to genetically dissect active DNA demethylation10,14. The system consists of the RD29A-LUC transgene (firefly luciferase reporter driven by the stress-responsive RD29A promoter) and the non-allelic endogenous RD29A gene. The RD29A promoter is subjected to continuous siRNA-directed DNA methylation such that active DNA demethylation is required to keep the RD29A and RD29A-LUC genes transcriptionally active. In ros1 mutants, the RD29A promoter for both the transgene and endogenous gene becomes hypermethylated and both genes are silenced10. In addition, the 35S-NPTII transgene linked to RD29A-LUC is also silenced such that ros1 mutant plants are sensitive to kanamycin. We isolated the ros3 mutant from a T-DNA mutagenized population15. Like ros1 mutations, ros3 causes a substantial reduction in bioluminescence emission (Fig. 1a and Supplementary Fig. 1a and 1b) as well as sensitivity to kanamycin (Fig. 1b). Genetic analysis indicated that the ros3 mutation is recessive and affects a nuclear gene (data not shown).
Figure 1. The ros3 mutation causes transcriptional gene silencing.
a, Stress-induced expression of the RD29A-LUC transgene in wild type (WT) and ros3 mutant plants after treatment with 300 mM NaCl for 5 hr. b, Like ros1, the ros3 mutant plants are sensitive to kanamycin. c, Northern blots showing that ros3 reduces the transcript levels of LUC, NPTII and endogenous RD29A, but not of the control, COR15A. Plants were either untreated (Control) or treated with cold (4°C) for 24 hr, 100 µM ABA for 3 hr, or 300 mM NaCl for 5 hr. d, Nuclear run-on assay showing the pre-mRNA levels of LUC, NPTII and RD29A genes in wild-type and ros3. COR15A and CBF3 were used as controls.
Northern blot (Fig. 1c) and nuclear run-on (Fig. 1d) assays showed that the RD29A-LUC and 35S-NPTII transgenes and the endogenous RD29A gene are repressed transcriptionally in ros3 plants. Compared with the wild type, both the endogenous (Fig. 2a) and transgene (Fig. 2b) RD29A promoters in ros3 are substantially more heavily methylated at CpG, CpNpG (N is A, T or C) and CpNpN sites (Supplementary Table 1). Southern blot analysis with methylation-sensitive restriction enzymes also indicated DNA hypermethylation in the RD29A promoters in the mutant (Supplementary Fig. 2a and 2b). Treatment with the cytosine methylation inhibitor 5-aza-2’-deoxycytidine increased RD29A-LUC expression in the ros3 mutant to the wild type level (Supplementary Fig. 3). These results suggest that DNA hypermethylation is responsible for the TGS in ros3 mutant plants.
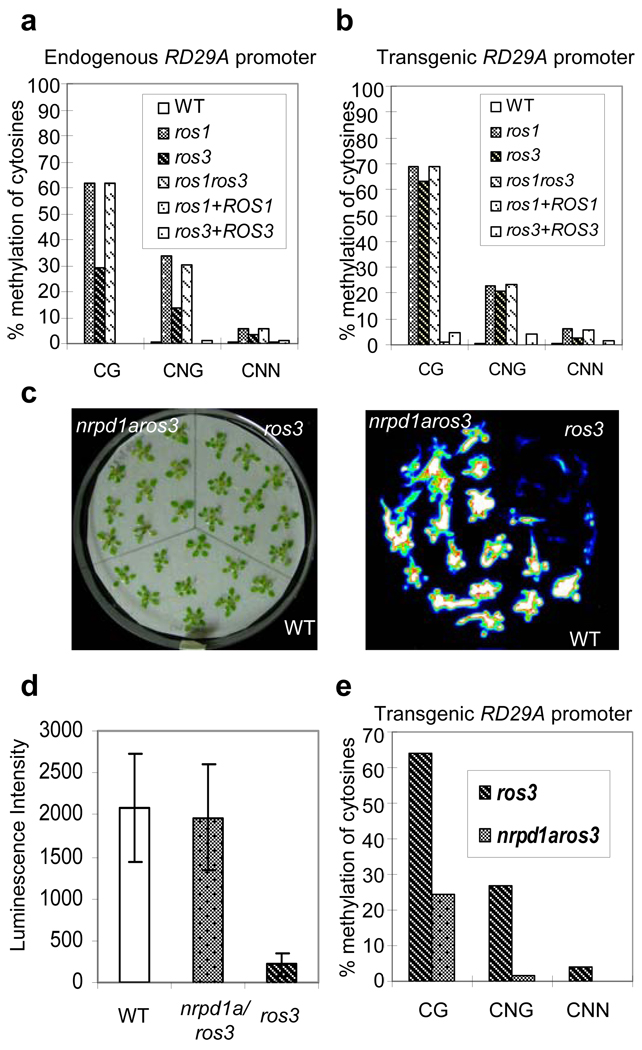
Figure 2. DNA hypermethylation in ros3 and suppression of ros3 by nrpd1a.
a and b Bisulfite sequencing analysis of promoter methylation status of the endogenous RD29A (a) and RD29A-LUC transgene (b) in WT, ros1, ros3, ros1ros3, ros1 complemented with wild type ROS1 transgene, and ros3 complemented with wild type ROS3 transgene. c, Suppression of ros3 by the nrpd1a-1 mutation. Seedlings of WT, ros3 and nrpd1aros3 double mutant grown in MS medium for three weeks were transferred to a filter paper soaked with 300 mM NaCl for 5 hr. Left, picture of the seedlings; right, luminescence image. d, Quantification of luminescence in (c). Error bars represent standard deviation (n=20). e, DNA methylation status at the transgene RD29A promoter in ros3 and nrpd1aros3 plants.
The nrpd1a-1 mutation in the largest subunit of RNA polymerase IVa blocks the accumulation of 24-nt siRNAs corresponding to the RD29A promoter (data not shown). Analysis of nrpd1aros3 double mutant showed that the nrpd1a mutation causes a significant increase in RD29A-LUC expression (Fig. 2c and 2d) and substantial decrease in CpG, CpNpG and CpNpN methylation at the transgene RD29A promoter (Fig. 2e) in the ros3 mutant background. The release of TGS in ros3 by nrpd1a suggests that the RD29A promoter siRNAs are the initial trigger of TGS in the ros3 mutant. The levels of the RD29A promoter siRNAs do not differ substantially between ros3 and the wild type (Supplementary Fig. 1c).
To analyze the genetic interaction between ros3 and ros1, we crossed ros1-1 with ros3 and generated a ros1ros3 double mutant. Analysis of cytosine methylation levels at the endogenous RD29A as well as transgenic RD29A promoters showed that the levels of CpG, CpNpG and asymmetric CpNpN methylation in ros1ros3 are similar to those in the ros1 single mutant (Fig. 2a and 2b, and Supplementary Table 1). DNA methylation at the intergenic 80, At1g23740389-0769, At4g5390602-0993 and At2g1699280-9430 loci is higher in ros1 and ros3 single mutants compared to the wild type, and the double mutant shows a methylation pattern similar to ros1 (Supplementary Fig. 4a–d, and Supplementary Table 2). Therefore, the effects of the ros1 and ros3 mutations on the methylation levels are not additive, indicating that ROS3 and ROS1 function in the same genetic pathway to demethylate DNA.
The second and third pairs of true leaves in ros3 are narrower and slightly lobed compared to those in the wild type (Supplementary Fig. 5a). Based on the leaf morphology and kanamycin sensitivity phenotypes, we mapped the ros3 mutation to a genomic region on Chromosome V (Supplementary Fig. 5b). Sequencing of all annotated genes in this region revealed a deletion of 260 bp in ros3 that includes the first intron and part of the second exon of AT5G58130 (Supplementary Fig. 5c and Supplementary 6). AT5G58130 encodes a protein with an N-terminal RNA recognition motif (RRM), a central COG5406 domain found in the nucleosome binding factor SPN and a C-terminal sequence with a secondary structure similar to those of RRMs (Supplementary Fig. 5c, Supplementary Fig. 7 and Supplementary 8). A wild-type genomic fragment of At5G58130 was found to complement the luminescence, leaf morphology (Supplementary Fig. 5d and 5e) and DNA hypermethylation (Fig. 2a and 2b) phenotypes of the ros3 mutant, confirming that AT5G58130 is ROS3.
Expression of a translational fusion of yellow fluorescence protein (YFP) at the N terminus of ROS3 in onion epidermal cells or Arabidopsis showed a clear nuclear localization of the fusion protein (Supplementary Fig. 9a). Close inspection revealed that the fusion protein is concentrated in discrete foci in the nucleoplasm and is also present in the nucleolus (Supplementary Fig. 9b and 9c). Analysis of GUS reporter gene expression driven by the ROS3 promoter suggested that ROS3 is ubiquitously expressed in plant tissues (Supplementary Fig. 9d).
Because ROS3 contains an RRM domain, we tested whether it may bind to RNA. We expressed recombinant ROS3 or its truncated forms with an N-terminal histidine tag (Fig. 3a and 3b). Electrophoretic mobility-shift assays with single stranded and double stranded RNAs of different sizes (Supplementary Table 3) were carried out. ROS3 was able to bind a single stranded RNA of sequence b (corresponding to the RD29A promoter) that are either 21-, 24- or 26-nt, but it did not bind 21-, 24- or 26-nt single stranded RNA of sequence a (Fig. 3c). It also did not bind a 40-nt single stranded RNA (Fig. 3c). There was no binding (data not shown) to any double stranded RNA or any other single stranded RNA tested (Supplementary Table 3). Removal of the N-terminal RRM domain and the C-terminal region (Fig. 3c, lanes 4, 12 and 14; Fig. 3d, lane 4) or of the N-terminal RRM domain alone (Fig. 3d, lane 2) abolished binding to RNA (b). However, removal of the C-terminal region did not substantially affect RNA binding (Fig. 3d, lane 3). The binding to the 24-nt RNA (b) is ROS3 protein concentration-dependent, and is competed by excess unlabeled small RNA of the same sequence (Fig. 3e). These results show that ROS3 has the capacity to bind specific small RNAs and that the RRM domain is required for this binding. Although ROS3 could bind 21-, 24-and 26-nt single stranded RNAs of sequence b in vitro, RNA blot analysis showed that only an ~24-nt small RNA of sequence b is present in Arabidopsis (Supplementary Fig. 10a).
Figure 3. ROS3 binds small RNAs.
a, Diagram of ROS3 and its truncated mutant forms. b, Coomassie stained SDS-PAGE gel showing the recombinant proteins used for RNA binding assays. c, ROS3 but not ROS3ΔNC binds 21-,24- and 26-nt single stranded RNA of sequence b. Lanes 1, 3, 5, 7, 9, 11 and 13, ROS3; lanes 2, 4, 6, 8, 10, 12 and 14, ROS3ΔNC. d, ROS3ΔC but not ROS3ΔN or ROS3ΔNC binds 24-nt single stranded RNA of sequence b. Lane 1, ROS3; lane 2, ROS3ΔN; lane 3, ROS3ΔC; lane 4, ROS3ΔNC. e, Protein concentration-dependent binding to 24-nt single stranded RNA (b) and competition by unlabeled small RNA. Lanes 1–4, increasing binding to 24-nt (b) small RNA by increasing ROS3 protein concentration; lanes 5–9, competition by increasing amount of cold 24-nt RNA (b). f, ROS3 binds 25-nt RNA (c) and RNA (d) in vitro. RNAs of sequences b and a are used as controls. Lanes 1, 3, 5 and 7, ROS3; lanes 2, 4, 6 and 8, ROS3ΔN.
To determine whether ROS3 may bind small RNAs in vivo, ROS3 protein was immunoprecipitated from Arabidopsis extracts using anti-ROS3 antisera. The immunoprecipitate was fractionated on a polyacrylamide gel and putative ROS3-bound small RNAs of ~15–30 nt were extracted from the gel, cloned and sequenced. Out of 288 clones sequenced, 140 had inserts of 10–30 nt (Supplementary Table 4). Interestingly, the sequences are very rich in Gs. Two 25-nt sequences, RNA (c) (5’-GGGAGUCCGGAGACGUCGGCGGGGG-3’) and RNA (d) (5’-UCGGGAGGGAAGCGGAUGGGGGCCG-3’) were chosen for further analysis. These small RNAs correspond to a genomic region (intergenic between At3G41979 and At3TE58310) that appears to have higher DNA methylation in the ros1dml2dml3 demethylase triple mutant than in the Columbia wild type control plants9 (http://neomorph.salk.edu/epigenome.html). Both RNA (c) and RNA (d) could be detected as ~25 nt small RNAs in plants by Northern blot analysis (Supplementary Fig. 10b and 10c). ROS3 protein is able to bind RNA (c) and RNA (d) in vitro (Fig. 3f). Bisulfite sequencing analysis indicated higher CG, CNG and CNN methylation in ros3 compared to the wild type in the genomic region corresponding to the small RNAs (Supplementary Fig. 11 and Supplementary Table 2). These results suggest that ROS3 binds to small RNAs in vivo, and the small RNAs may direct demethylation of target sequences. Further studies may reveal whether ROS3 is also capable of binding larger RNAs and what specific sequence features it recognizes.
We immunolocalized ROS3 and ROS1 proteins using antibodies recognizing the native proteins or epitope tags fused to ROS1 and ROS3 recombinant proteins. Immunolocalization in Arabidopsis leaf nuclei at interphase revealed that in wild-type plants ROS3 is localized in the nucleoplasm as well as nucleolus (Fig. 4a–c). The ROS3 signals appear as scattered speckle-like structures, ranging in number from 5 to more than 12 per nucleus (Fig. 4, and Supplementary Table 5). This pattern is consistent with the YFP-ROS3 result (Supplementary Fig. 9b and 9c). ROS1 was similarly found to be dispersed throughout the nucleoplasm and nucleolus, although the immunolocalization signals tended to appear somewhat more diffuse and smaller than ROS3 foci (Fig. 4a and 4b, and Supplementary Table 6). In ros3 and ros1 mutants, no significant signals could be obtained, which indicates that the antibodies are specific for ROS3 and ROS1, respectively (Fig. 4a). Moreover, epitope-tagged ROS3 and ROS1 proteins displayed interphase localization patterns very similar to the patterns observed for the wild-type proteins (Supplementary Fig. 12). Simultaneous immunolocalization of cMyc-tagged ROS1 and native ROS3 revealed substantial colocalization of ROS1 and ROS3 (Fig. 4b and Supplementary Table 7).
Figure 4. Co-localization of ROS3 with ROS1 in the nucleus of Arabidopsis mesophyll cells and assay of ROS1 and ROS3 mRNA levels.
a, Localization of ROS3 and ROS1 by their respective antibodies. b, Dual immunolocalization of ROS3 and ROS1 with use of anti-ROS3 and anti-Myc. c, ROS1 localization in ros3 mutant and ROS3 localization in ros1 showing their inter-dependence for appropriate localization. Size bars correspond to 5 µm. d, Quantitative RT-PCR assay of relative ROS1 and ROS3 transcript levels in the various genotypes.
ROS3 and ROS1 immunostaining was also carried out in ros1 and ros3 mutants, respectively. ROS1 localization was found to be severely disrupted in the ros3 mutant background (Fig. 4c). The ROS3 interphase localization pattern was less changed in the ros1 mutant, although ROS3 nucleolar localization was reduced in ros1 (Fig. 4c). These results suggest that ROS1 and ROS3 are inter-dependent for their nuclear, and especially nucleolar, co-localization.
Because ROS1 transcript levels are reduced in mutants defective in DNA methylation16, we tested whether ros3 may prevent demethylation by reducing ROS1 expression. ROS1 mRNA level was reduced in ago6-117 as expected, but was increased in ros3 (Fig. 4d). Similarly, ROS3 mRNA level was decreased in ago6-1 but increased in ros1 (Fig. 4d). The results suggest that ros3 does not reduce ROS1 mRNA levels and that the DNA methylation status at some critical sites may be sensed to regulate the transcript levels of active DNA demethylation factors such as ROS1 and ROS3.
Our results suggest that ROS3 functions in the same DNA demethylation pathway with ROS1. ROS3 is capable of binding small RNAs in vitro and in vivo, suggesting that small RNAs and/or larger RNAs bound to ROS3 may guide sequence-specific DNA demethylation by ROS1. In plants, 24-nt siRNAs bound to Argonaute 4 (AGO4) and AGO6 can direct DNA methylation and TGS5,6,17–21. Recent studies suggest that piwi-interacting small RNAs function to direct de novo DNA methylation and silencing of retrotransposons in mammalian germ cells22. In mammalian cells, promoter-directed siRNAs can also induce TGS23,24. Interestingly, some promoter-directed small RNAs were recently found to cause gene activation in human cells25,26. Our work showing that a protein required for DNA demethylation is capable of binding to small RNAs contributes to understanding the mechanism of DNA demethylation and adds to the expanding role of small RNAs in gene regulation20,21,27. It also sets the stage for future work to determine the relationship between small RNAs directing DNA methylation and TGS and those directing DNA demethylation and gene activation. Although our data suggest that ROS3 with its bound RNAs may guide sequence specific demethylation, it is also possible that ROS3 may suppress DNA methylation by sequestering RNAs that guide DNA methylation, or by promoting ROS1 protein stability or nuclear localization.
METHODS SUMMARY
The ros3 mutant was isolated from a mutagenized population of RD29A-LUC plants by luminescence imaging15. Gene silencing phenotypes of the mutant were examined by RNA blot analysis and nuclear run-on assays. DNA methylation levels were assessed by bisulfite sequencing of genomic DNA or Southern blot analysis. The ros3 mutant was mapped and cloned following previously published strategy10. The subcellular localization of ROS3 protein was determined by confocal imaging of cells or plants expressing YFP-ROS3 (driven by the 35S promoter). The sub-nuclear co-localization of ROS3 and ROS1 proteins was examined by immunostaining using antibodies against the wild type protein or epitope tags. Bacterially expressed recombinant ROS3 protein and its truncated forms were purified and used for gel mobility shift assays to examine RNA binding in vitro. To test in vivo RNA binding by ROS3, ROS3 protein was immunoprecipitated from plant extracts. Small RNAs from the immunoprecipitate were identified by cloning and sequencing.
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.
Supplementary Material
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Acknowledgements
This work was supported by National Institutes of Health grants R01GM070795 and R01GM059138 (J.-K. Zhu) and R01GM077590 and 1R01GM060380 (C.S. Pikaard).
References
- 1.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 2.Martienssen RA, Colot V. DNA methylation and epigenetic inheritance in plants and filamentous fungi. Science. 2001;293:1070–1074. doi: 10.1126/science.293.5532.1070. [DOI] [PubMed] [Google Scholar]
- 3.Tariq M, Paszkowski J. DNA and histone methylation in plants. Trends Genet. 2004;20:244–251. doi: 10.1016/j.tig.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Bender J. DNA methylation and epigenetics. Annu. Rev. Plant Biol. 2004;55:41–68. doi: 10.1146/annurev.arplant.55.031903.141641. [DOI] [PubMed] [Google Scholar]
- 5.Matzke MA, Birchler JA. RNAi-mediated pathways in the nucleus. Nat. Rev. Genet. 2005;6:24–35. doi: 10.1038/nrg1500. [DOI] [PubMed] [Google Scholar]
- 6.Chan SW, Henderson IR, Jacobsen SE. Gardening the genome: DNA methylation in Arabidopsis thaliana. Nat. Rev. Genet. 2005;6:351–360. doi: 10.1038/nrg1601. [DOI] [PubMed] [Google Scholar]
- 7.Zhu J, Kapoor A, Sridhar VV, Agius F, Zhu JK. The DNA glycosylase/lyase ROS1 functions in pruning DNA methylation patterns in Arabidopsis. Curr. Biol. 2007;17:54–59. doi: 10.1016/j.cub.2006.10.059. [DOI] [PubMed] [Google Scholar]
- 8.Penterman J, et al. DNA demethylation in the Arabidopsis genome. Proc. Natl. Acad. Sci. U. S. A. 2007;104:6752–6757. doi: 10.1073/pnas.0701861104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lister R, et al. Highly integrated single base resolution maps of the epigenome in Arabidopsis. Cell. 2008;133:523–536. doi: 10.1016/j.cell.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong Z, et al. ROS1, a repressor of transcriptional gene silencing in Arabidopsis, encodes a DNA glycosylase/lyase. Cell. 2002;111:803–814. doi: 10.1016/s0092-8674(02)01133-9. [DOI] [PubMed] [Google Scholar]
- 11.Agius F, Kapoor A, Zhu JK. Role of the Arabidopsis DNA glycosylase/lyase ROS1 in active DNA demethylation. Proc. Natl. Acad. Sci. U. S. A. 2006;103:11796–11801. doi: 10.1073/pnas.0603563103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morales-Ruiz T, et al. DEMETER and REPRESSOR OF SILENCING 1 encode 5-methylcytosine DNA glycosylases. Proc. Natl. Acad. Sci. U. S. A. 2006;103:6853–6858. doi: 10.1073/pnas.0601109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gehring M, et al. DEMETER DNA glycosylase establishes MEDEA polycomb gene self-imprinting by allele-specific demethylation. Cell. 2006;124:495–506. doi: 10.1016/j.cell.2005.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapoor A, Agius F, Zhu JK. Preventing transcriptional gene silencing by active DNA demethylation. FEBS Lett. 2005;579:5889–5898. doi: 10.1016/j.febslet.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 15.Xiong L, et al. FIERY1 encoding an inositol polyphosphate 1-phosphatase is a negative regulator of abscisic acid and stress signaling in Arabidopsis. Genes Dev. 2001;15:1971–1984. doi: 10.1101/gad.891901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huettel B, et al. Endogenous targets of RNA-directed DNA methylation and Pol IV in Arabidopsis. EMBO J. 2006;25:2828–2836. doi: 10.1038/sj.emboj.7601150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng X, Zhu J, Kapoor A, Zhu JK. Role of Arabidopsis AGO6 in siRNA accumulation, DNA methylation and transcriptional gene silencing. EMBO J. 2007;26:1691–1701. doi: 10.1038/sj.emboj.7601603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wassenegger M. RNA-directed DNA methylation. Plant Mol. Biol. 2000;43:203–220. doi: 10.1023/a:1006479327881. [DOI] [PubMed] [Google Scholar]
- 19.Vaucheret H, Fagard M. Transcriptional gene silencing in plants: targets, inducers and regulators. Trends Genet. 2001;17:29–35. doi: 10.1016/s0168-9525(00)02166-1. [DOI] [PubMed] [Google Scholar]
- 20.Baulcombe D. RNA silencing in plants. Nature. 2004;431:356–363. doi: 10.1038/nature02874. [DOI] [PubMed] [Google Scholar]
- 21.Brodersen P, Voinnet O. The diversity of RNA silencing pathways in plants. Trends Genet. 2006;22:268–280. doi: 10.1016/j.tig.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Aravin AA, Bourc'his D. Small RNA guides for de novo DNA methylation in mammalian germ cells. Genes Dev. 2008;22:970–975. doi: 10.1101/gad.1669408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim DH, Villeneuve LM, Morris KV, Rossi JJ. Argonaute-1 directs siRNA-mediated transcriptional gene silencing in human cells. Nat. Struct. Mol. Biol. 2006;13:793–797. doi: 10.1038/nsmb1142. [DOI] [PubMed] [Google Scholar]
- 24.Janowski BA, et al. Involvement of AGO1 and AGO2 in mammalian transcriptional silencing. Nat. Struct. Mol. Biol. 2006;13:787–792. doi: 10.1038/nsmb1140. [DOI] [PubMed] [Google Scholar]
- 25.Li LC, et al. Small dsRNAs induce transcriptional activation in human cells. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17337–17342. doi: 10.1073/pnas.0607015103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janowski BA, et al. Activating gene expression in mammalian cells with promoter-targeted duplex RNAs. Nat. Chem. Biol. 2007;3:166–173. doi: 10.1038/nchembio860. [DOI] [PubMed] [Google Scholar]
- 27.Carrington JC, Ambros V. Role of microRNAs in plant and animal development. Science. 2003;301:336–338. doi: 10.1126/science.1085242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.