Abstract
Purpose
Evaluate the efficacy of potential therapeutics in Rdh8−/− Abca4−/−mice, a rodent model of human age-related macular degeneration (AMD).
Methods
Therapeutic efficacy of several antioxidant agents (ascorbic acid, α-lipoic acid, α-tocopherol, Mn(III)-tetrakis(4-benzoic acid)-porphyrin, and butylated hydroxytoluene), an immunosuppressive agent with antivascular endothelial growth factor (VEGF) activity (sirolimus, also known as rapamycin), a retinoid cycle inhibitor (retinylamine), and an artificial chromophore (9-cis-retinyl acetate) were evaluated side by side in a recently described murine model of AMD, the Rdh8−/− Abca4−/−mouse. This animal exhibits a retinopathy caused by delayed all-trans-retinal clearance resulting from the absence of both ATP-binding cassette transporter 4 (Abca4) and retinol dehydrogenase 8 (Rdh8) activities. Drug efficacy was evaluated by retinal histologic analyses and electroretinograms (ERGs).
Results
All tested agents partially prevented atrophic changes in the Rdh8−/− Abca4−/−retina with retinylamine demonstrating the greatest efficacy. A significant reduction of complement deposition on Bruch’s membrane was observed in sirolimus-treated mice, although the severity of retinal degeneration was similar to that observed in antioxidant- and 9-cis-retinyl acetate–treated mice. Sirolimus treatment of 6-month-old Rdh8−/− Abca4−/−mice for 4 months prevented choroidal neovascularization without changing retinal VEGF levels.
Conclusions
Mechanism-based therapy with retinylamine markedly attenuated degenerative retinopathy in Rdh8−/− Abca4−/−mice. Further understanding of pathogenic mechanisms involved in AMD is needed to develop more effective therapeutics.
Age-related macular degeneration (AMD), a leading cause of irreversible human blindness in developed countries, is an etiologically complex disease wherein a multitude of genetic and environmental factors influence disease progression. Although multiple genetic variations have been reported to modulate susceptibility to AMD, only three factors are currently believed to affect the clinical course of established AMD: the complement system, oxidative stress, and lipid metabolism.1 Recently, anomalous reactions in the visual cycle have been shown to cause an AMD-like phenotype in mice lacking both the ATP-binding cassette transporter 4 (Abca4) and retinol dehydrogenase 8 (Rdh8), two enzymes critical for all-trans-retinal clearance from the retina.2–4 Given the pathologic similarities of this model to human AMD, analyses of the Rdh8−/− Abca4−/−mouse have provided strong evidence that aberrant ocular retinoid metabolism can be a critical factor in the development of macular degeneration.5,6
Continuous regeneration of 11-cis-retinal, the chromophore of visual pigments in photoreceptor cells, is essential for vision.7 This process occurs through the retinoid cycle that operates in both the retinal pigmented epithelium (RPE) and photoreceptors.8,9 After 11-cis-retinal is photoisomerized to all-trans-retinal, most of the all-trans-retinal is shuttled to the outside of the photoreceptor discs by ABCA4, a transporter localized in the rims of photoreceptor discs.10 RDH8 is one of the main enzymes that reduces all-trans-retinal to all-trans-retinol in rod and cone outer segments.11 Thus, both ABCA4 and RDH8 are involved in all-trans-retinal clearance in photo-receptors. Any delay in this process leads to accumulation of all-trans-retinal and conjugate products, such as di-retinoid-pyridinium-ethanolamine (A2E), in both photoreceptors and the RPE.12,13 As previously reported, Rdh8−/− Abca4−/−mice display cone–rod dystrophy by the age of 4 to 6 weeks and display light-dependant progressive retinal degeneration as well.4 Retinal degeneration in this mouse model mimics most of the clinical features of human AMD, including lipofuscin accumulation, drusen formation, basal laminar hyaline deposition, RPE cell death, complement activation, and eventual choroidal neovascularization (CNV). Therefore, these animals provide an excellent surrogate model for human AMD in which to test candidate therapeutics.
Advocated AMD treatment modalities include the use of antioxidants14,15 and immune system regulators to suppress oxidative stress and immune activation pathways responsible for AMD progression. Sirolimus (rapamycin), an immunosuppressant used in the management of organ transplantation and ocular inflammatory conditions, inhibits the response to interleukin-2 and thereby blocks T- and B-cell activation.16 It also exhibits cellular antiproliferative effects and anti-tumor/anti-angiogenic activity coupled with a decrease in vascular endothelial growth factor (VEGF) production.17 Recently, it was reported that sirolimus inhibited CNV growth in a laser-induced CNV mouse model.18 Therefore, this agent could be effective in treating AMD, since its pathogenesis includes immune activation and overproduction of VEGF.19 Regeneration of photoreceptor visual pigments with the artificial chromophore, 9-cis-retinal, is an effective method to bypass the visual cycle. Supplementation with this retinoid as a pro drug, 9-cis-retinyl acetate (9cRAc) was effective in rescuing severe early-onset retinal degeneration in mice with impaired visual cycle function.20,21 Because retinal photoreceptor degeneration in AMD is a secondary consequence of primary RPE impairment, artificial visual chromophores such as 9-cis-retinal may prevent the photoreceptor degeneration observed in AMD. Herein, we report the therapeutic effects of differing mechanism-based pharmacologic interventions on the development of retinopathy in a high-fidelity model of human AMD, the Rdh8−/− Abca4−/−mouse.
Materials and Methods
Animals
Rdh8−/− Abca4−/−double-knockout mice were generated and genotyped as previously described.4 Mice with the Leu residue variant at amino acid position 450 of RPE65 were used for the study. All mice used were housed in the animal facility at the School of Medicine, Case Western Reserve University, where they were maintained either under complete darkness or in a 12-hour dim room light (3–5 lux)/12-hour dark cyclic environment. Manipulations in the dark were performed under dim red light transmitted through a filter with transmittance of >560 nm (no.1 Safelight; Eastman, Kodak, Rochester, NY). All animal procedures and experiments were approved by the Case Western Reserve University Animal Care Committees and conformed to recommendations of both the American Veterinary Medical Association Panel on Euthanatization and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Pharmacologic Treatments
Mice were given daily intraperitoneal injections of antioxidants including α-tocopherol (100 mg/kg in vegetable oil), ascorbic acid (250 mg/kg in phosphate-buffered saline [PBS] composed of 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, and 1.4 mM KH2PO4, [pH 7.4]), α-lipoic acid (100 mg/kg in PBS/30% ethanol), and butylated hydroxytoluene (75 mg/kg in PBS). All antioxidants were obtained from Sigma-Aldrich (St. Louis, MO). A metalloporphyrin superoxide dismutase mimetic that protects against intracellular generation of reactive oxygen species, Mn(III)-tetrakis(4-benzoic acid)-porphyrin chloride (MnT-BAP) from AG Scientific (San Diego, CA), was also administered intraperitoneally at 10 mg/kg in PBS. Sirolimus (2 mg/kg in PBS) was injected intraperitoneally each day as an anti-inflammatory/antiproliferative treatment. 9-cis-Retinyl acetate (9cRAc, 50 mg/kg in vegetable oil by oral gavage)20 and retinylamine (Ret-NH2, 10 mg/kg in DMSO intraperitoneally)22 were used as retinoid therapies to bypass or inhibit the visual cycle. The various treatment regimens are summarized in Table 1.
Table 1.
Treatment Regimens Used for Rdh8−/− Abca4−/− Mice
| Drug Group | Medication | Dose | Route of Administration | Interval | Duration | Age Started | Eyes 1* | Eyes 2† |
|---|---|---|---|---|---|---|---|---|
| Antioxidants 0 | Ascorbic acid (vitamin C) α-Lipoic acid MnTBAP |
250 mg/kg in PBS 100 mg/kg in PBS/30% ethanol 10 mg/kg in PBS |
IP | Daily | 4 months | 3 wk | 28 | 0 |
| Antioxidants 1 |
α-Tocopherol (vitamin E) Butylated hydroxytoluene |
100 mg/kg in vegetable oil 75 mg/kg in vegetable oil |
IP | Daily | 4 months | 3 wk | 28 | 20 |
| Antioxidants 2 | Ascorbic acid (vitamin C) α-Lipoic acid |
250 mg/kg in PBS 100 mg/kg in PBS/30% ethanol |
IP | Daily | 4 months | 3 wk | 20 | 18 |
| Antioxidants 0 + 1 | As indicated above | As indicated above | IP | Daily | 4 months | 3 wk | 20 | 0 |
| Immunosupressant | Sirolimus | 2 mg/kg in PBS | IP | Daily | 4 months | 3 wk | 20 | 20 |
| Anti-VEGF | Sirolimus | 2 mg/kg in PBS | IP | Daily | 4 months | 6 mo | 18 | 16 |
| Visual cycle inhibitor | Ret-NH2 | 10 mg/kg in DMSO | IP | Weekly | 4 months | 3 wk | 20 | 20 |
| Artificial chromophore | 9cRAc | 50 mg/kg in vegetable oil | Gavage | 3/Week | 4 months | 3 wk | 20 | 18 |
| Dark-reared | PBS | 50 μL | IP | Daily | 4 months | 3 wk | 20 | 20 |
| Control 1 | PBS | 50 μL | IP | Daily | 4 months | 3 wk | 20 | 20 |
| Control 2 | PBS | 50 μL | IP | Daily | 4 months | 6 mo | 20 | 20 |
Eyes 1, injected number.
Eyes 2, analyzed number.
Induction and Analysis of Light Damage to Retinas of WT Mice
Before exposure to light, BALB/c mice (female, 4 weeks old) were dark-adapted for 48 hours. Light damage was induced in mice without dilated pupils by exposure to 5000 lux of diffuse white fluorescent light (150 W spiral lamp; Commercial Electric Products, Cleveland, OH) for 15 minutes. Light exposure was initiated at 11 AM. These mice were maintained in the dark for 7 days after light exposure and evaluated by histologic study.
Histology and Immunohistochemistry
The histologic and immunohistochemical procedures used in the study are described elsewhere.13 Eyecups for histology were fixed in 2% glutaraldehyde/4% paraformaldehyde and processed for embedding in Epon. Sections were cut at 1 μm and stained with toluidine blue. For immunohistochemistry, eyes were immersion-fixed for 2 hours with freshly prepared 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) and processed for OCT (Miles Laboratories, Elkhart, IN) embedding. The sections were cut at 10 μm and viewed with an inverted laser scanning confocal microscope (LSM 510; Carl Zeiss Meditec, Inc.). 4,6-Diamidino-2-phenylindole (DAPI), Alexa 488–conjugated peanut agglutinin (PNA), and anti-C3 (C3 (H300)) antibody were purchased from Invitrogen (Carlsbad, CA) and Santa Cruz Biotechnology (Santa Cruz, CA). 1D4 (anti-rhodopsin mouse monoclonal antibody) was a generous gift from Robert Molday (University of British Columbia, Vancouver, BC, Canada).
Angiography
Angiograms were performed after a 400 μL intracardiac injection of 10 mg/mL fluorescein isothiocyanate-conjugated high-molecular-weight dextran (FD-2000S; Sigma-Aldrich, St. Louis, MO) into anesthetized mice.23
Electroretinography
All ERG procedures were performed by published methods.13 Briefly, mice under a safety light were anesthetized by intraperitoneal injection of 20 μL/g body weight of 6 mg/mL ketamine and 0.44 mg/mL xylazine diluted with 10 mM sodium phosphate (pH 7.2), containing 100 mM NaCl. Pupils were dilated with 1% tropicamide. A contact lens electrode was placed on the eye and a reference electrode and ground electrode were positioned on the ear and tail, respectively. ERGs were recorded with a computerized system (UTAS E-3000; LKC Technologies, Inc., Gaithersburg, MD).
Single-Flash Recording
The duration of white light flash stimuli (from 20 μs to 1 ms) was adjusted to provide a range of illumination intensities (from −3.7 to 1.6 log cd ·s/m2). Three to five recordings were made at sufficient intervals (from 10 seconds to 10 minutes) between flash stimuli to allow recovery from any photobleaching effects.
Flicker Flash Recording
Recordings were performed with the same procedure used for single-flash recording. Flicker stimuli were applied over a range of intensities (0.62 log cd · s/m2) at 2 different frequencies (20 and 30 Hz) under photopic condition (1.4 log cd · m−2).
A2E Analyses
All experimental procedures related to extraction, derivatization, and separation of retinoids from dissected mouse eyes were performed as formerly described.13 Quantification of A2E by HPLC was performed by comparison with known concentrations of pure synthetic A2E.11
VEGF Quantification
All experimental procedures related to sample preparation from dissected mouse eyes were performed as formerly described.4 Levels of VEGF in the eye were determined with a mouse ELISA kit (R&D Systems, Minneapolis, MN) according to manufacturer’s instructions. Protein concentrations in supernatant solutions were measured by the Bradford method (Bio-Rad, Hercules, CA).
Statistical Analyses
Experimental results were analyzed by the one-way ANOVA. Data are shown as the mean ± SD.
Results
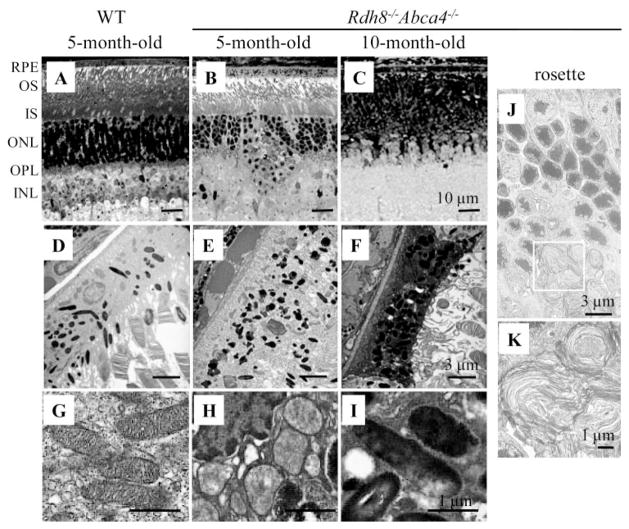
Environmental light intensity was strictly controlled at 3 to 5 lux (dim room light) because Rdh8−/− Abca4−/−mice are prone to light-dependent retinal degeneration.4 Even under these conditions, early retinal degeneration was detected by 6 to 8 weeks of age (data not shown). A reduced number of photoreceptor nuclei along the inferior retinal regions, and severe retinal degeneration relative to 5-month-old wild type (WT) mice were observed in Rdh8−/− Abca4−/−mice at 5 months and 10 months of age, respectively (Figs. 1A–C). Retinal rosette formation was observed in 3-month-old mutant mice with hyaline-like deposits in the rosette structure (Figs. 1J, 1K). Swelling of the RPE and the increased number of pigmented granules that included lipofuscin became evident at 5 months of age, and dead RPE cells lacking mitochondrial inner membrane cristae were visualized at 10 months of age (Fig. 1D–1I).
Figure 1.
Retinal histology of Rdh8−/− Abca4−/− mice. (A–C) Morphology of WT and Rdh8−/− Abca4−/− mouse retinas from 5- and 10-month-old mice kept in dim room light (3–5 lux). Progressive reduction of photoreceptors and loss of the outer nuclear layer compared with WT retina were evident in retinas from Rdh8−/−Abca4−/−mice. (D–F) Electron micrographs of the RPE from 5- and 10-month-old Rdh8−/−Abca4−/−mice kept in room light, showing swollen RPE with increased pigmented (E) and dead cells (F). (G–I) Ultrastructure of mitochondria in Rdh8−/−Abca4−/−mice. Electron micrographs of mitochondria in the RPE cells from 5- and 10-month-old Rdh8−/− Abca4−/−mice kept in room light. Inner membrane cristae were evident in the RPE mitochondria of the 5-month-old WT mice (G), whereas these fine structures were disrupted (H) and then completely lacking (I) in Rdh8−/− Abca4−/−mice at 5 and 10 months of age, respectively. (J, K) Electron micrograph of a local retinal change (rosette) observed in 3-to 5-month-old Rdh8−/− Abca4−/−mice. (K) An enlargement of the square outlined in (J) showing hyaline-like structures. Representative images are shown (n > 5). RPE, retinal pigment epithelium; OS, outer segment; IS, inner segment; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer. Scale bar: (A–C) 10 μm; (D–F, J) 3 μm; (G–I, K) 1 μm.
Both eyes of 117 Abca4−/− Rdh8−/−and 25 WT mice were examined in the study (data summarized in Table 1). Antioxidant administration protocols were based on those used in previous reports.24,25 Unfortunately, Rdh8−/− Abca4−/−mice failed to survive after two daily injections of a five-component antioxidant mixture consisting of α-tocopherol, butylated hydroxytoluene, ascorbic acid, α-lipoic acid, and MnTBAP. The same result was obtained even after halving the initial dose of these agents.24 The same toxicity was also detected in WT mice (i.e., all 10 WT mice tested failed to survive after two daily injections), suggesting that loss of RDH8 and ABCA4 did not affect the toxicity of the tested antioxidants. Revised treatment cocktails consisting of two groups (i.e., α-tocopherol and butylated hydroxytoluene in vegetable oil [antioxidant 1]) or ascorbic acid, α-lipoic acid, and MnTBAP in PBS [antioxidant 0]) were then tested. Four of 14 mice injected daily with antioxidant 1 died during the 4-month treatment period, whereas all 14 mice injected daily with antioxidant 0 died before the third injection. Thus, only antioxidant 1 and a cocktail consisting of ascorbic acid and α-lipoic acid (antioxidant 2) were finally tested (Table 1).
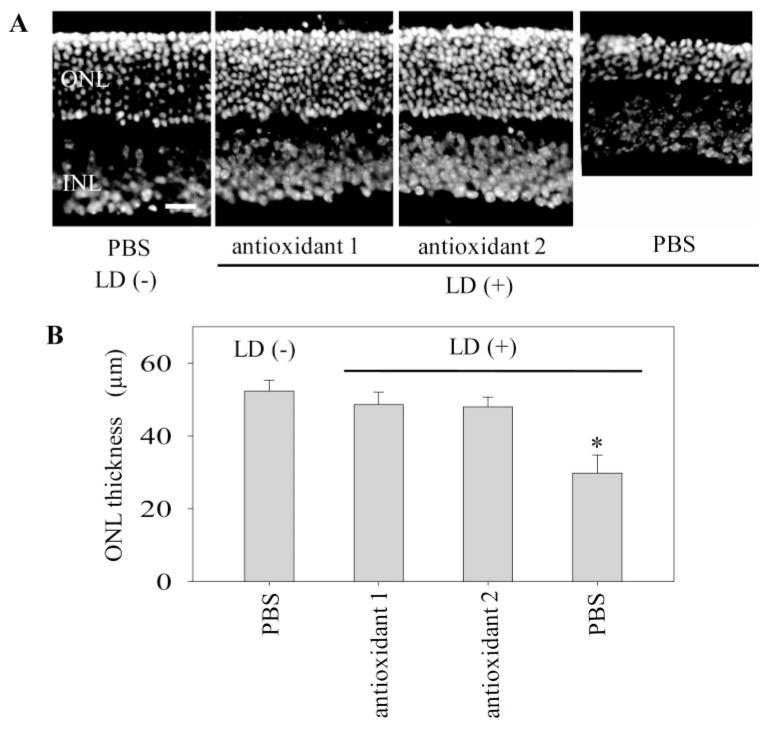
To examine the effects of antioxidants on light-induced retinal degeneration, we exposed WT (BALB/c) mice, which are especially light sensitive,26 to 5000-lux light for 15 minutes. Three hours before light exposure, these mice were treated with either antioxidant 1 or antioxidant 2, and retinal morphology was evaluated 7 days after the exposure. Thickness of the ONL was measured 500 μm away from the optic nerve head (ONH) in the inferior retina. Of note, both antioxidants 1 and 2 ameliorated retinal degeneration in these mice (Fig. 2). These data suggest that treatment with these antioxidants can protect retinas from acute oxidative damage caused by exposure to strong light.
Figure 2.
Effect of antioxidants on light-induced retinal degeneration in WT mice. WT (BALB/c) mice, which are sensitive to light-induced retinal degeneration, were treated with antioxidants 1 and 2 at 3 hours before light exposure (LD; 5000 lux of fluorescent light for 15 minutes). After 7 days of dark adaptation, retinal cross sections were prepared and stained with DAPI to evaluate retinal histology. (A) Representative retinal images from mice treated with antioxidants or PBS are shown. The thickness of the ONL was measured 500 μm from the ONH. Scale bar, 10 μm. (B). The thickness of the ONLs was preserved in retinas from mice treated with antioxidants compared with those from PBS-treated mice (n = 3; mean ± SD; *P <0.01 versus PBS-treated mice).
Histologic Assessment of Retinal Degeneration
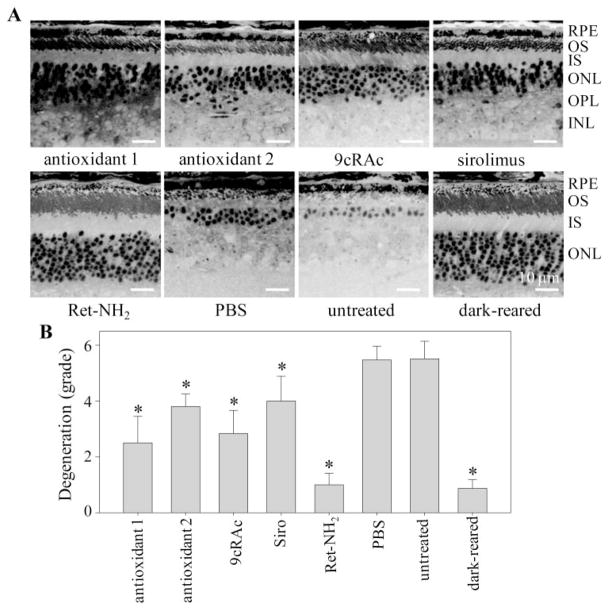
Lengths of photoreceptor outer segments were severely reduced to less than 5 μm in PBS-treated 5-month-old Rdh8−/− Abca4−/−mice, whereas more modest reductions (5–20-μm segments) were found in antioxidant 1, antioxidant 2, 9cRAc, and sirolimus-treated mice. Only regional retinal degeneration was observed in retinylamine-treated and dark-reared mice (Fig. 3A). All tested regimens exhibited some efficacy in preventing retinal degeneration in Rdh8−/− Abca4−/−mice compared with PBS-treated or untreated control Rdh8−/− Abca4−/−mice. To classify the severity of retinal damage, we devised the six-stage grading scheme shown in Table 2. According to this scheme, retinal structures were best preserved in retinylamine-treated Rdh8−/− Abca4−/−mice that displayed morphology similar to that of dark-reared Rdh8−/− Abca4−/−mice; much milder benefits were conferred by the other medications tested in this study (Fig. 3B).
Figure 3.
Effects of various test agents on retinal histology of Rdh8−/−Abca4−/−mice. (A) Retinal structures of Rdh8−/−Abca4−/−mice treated from 3 weeks to 5 months of age with indicated medications are shown. Representative images are presented (n > 10). RPE, retinal pigmented epithelium; OS, outer segment; IS, inner segment; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer. Bar, 10 μm. (B) Effects of various medications on the severity of retinal degeneration are indicated. Severity of retinal degeneration was graded from grade 0 (no degeneration) to grade 6 (severe degeneration) according to criteria described in Table 2. All listed agents conferred partial protection (9cRAc, 9-cis-retinyl acetate; Siro, sirolimus) with Ret-NH2 (retinylamine) exhibiting the greatest efficacy in preventing retinal degeneration in Rdh8−/−Abca4−/−mice (n > 10; bars, SD; *P <0.01 versus PBS-treated mice).
Table 2.
Six-Stage Grading Scheme for Classifying the Severity of Retinal Damage
| Grade 0: No degeneration in the entire retina (whole eyecup cut every 100 μm at 10-μm thickness per section). |
| Grade 1: Less than five punctate changes in the most damaged section of the retina. |
| Grade 2: More than five punctate changes in the most damaged section of the retina. |
| Grade 3: Continuous ONL degeneration (extending 300 μm or more within the most damaged section of the retina). |
| Grade 4: Continuous photoreceptor cell degeneration (≈ 15-μm length of photoreceptor layer extending 500 μm or more in the most damaged section of the retina). |
| Grade 5: Continuous photoreceptor cell degeneration (≈ 10-μm length of photoreceptor layer extending 500 μm or more in the most damaged section of the retina). |
| Grade 6: Continuous photoreceptor cell degeneration (≈ 5-μm length of photoreceptor layer extending 500 μm or more in the most damaged section of the retina). |
Therapeutic Effects on Cone Photoreceptor Survival
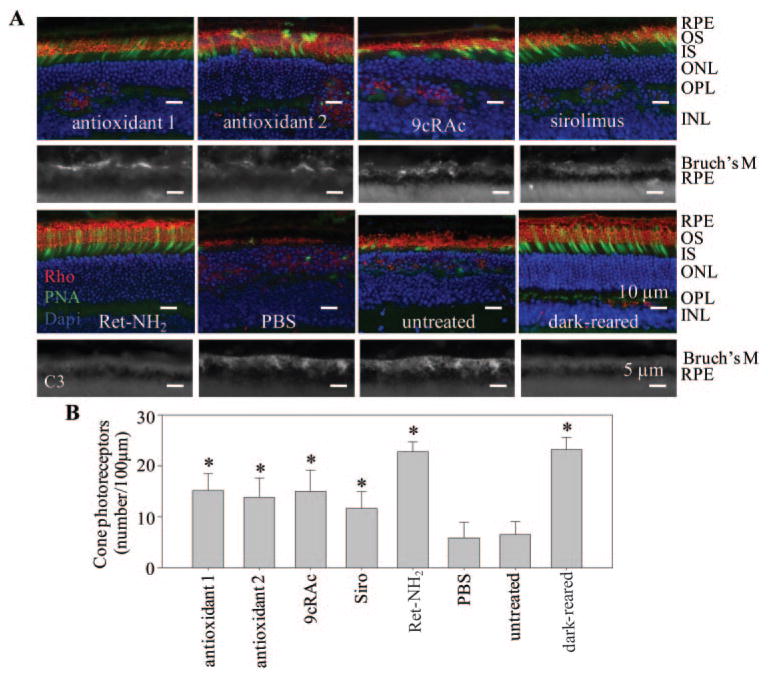
To examine the effects of various agents on cone photoreceptors, we stained the cone photoreceptors with PNA and the number of cells was counted in the inferior central retina (800 μm from the optic nerve head; Figs. 4). This region was selected because it manifests the most severely damaged areas in untreated mice. Retinylamine largely prevented cone photoreceptor degeneration such that the retinal structures of treated mice approximated those of dark-adapted Rdh8−/− Abca4−/−mice (Fig. 4A). Other medications tested also significantly preserved cone photoreceptors, but not as well as retinylamine (Fig. 4B).
Figure 4.
Populations of cone photoreceptors and complement deposition in retinas from Rdh8−/− Abca4−/−mice. (A) Top: retinal structures of Rdh8−/− Abca4−/−mice treated with indicated medications are shown with stained cone photoreceptors (green, PNA), outer segments (red, anti-rhodopsin 1D4), and nuclei (blue, DAPI). RPE, retinal pigmented epithelium; OS, outer segments; IS, inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer. Representative images are shown (n >10). Bottom: complement deposition was examined with anti-C3 antibody after treatment of Rdh8−/− Abca4−/−mice with the indicated medications. Strong C3 signals were observed in Bruch’s membrane of Rdh8−/− Abca4−/−mice that received either PBS or no treatment, whereas weaker signals were noted in mice treated with either antioxidants or 9cRAc. Only a faint signal was seen in sirolimus-treated Rdh8−/− Abca4−/−mice. No signal was detected in either retinylamine-treated or dark-reared mice. Representative images are shown (n >10). Scale bar: (top) 10 μm; (bottom) 5 μm. (B) Number of cone photoreceptors/100 μm is indicated for areas of the inferior central retina located 800 μm from the ONH of Rdh8−/− Abca4−/−mice. Retinylamine (Ret-NH2) prevented cone photoreceptor degeneration, and other medications studied (9cRAc, 9-cis-retinyl acetate; Siro, sirolimus) partially maintained cone photoreceptors (n > 10; mean ± SD; *P <0.01 versus PBS-treated mice).
Complement Activation
Complement activation is believed to be involved in the propagation of human AMD.27 Rdh8−/− Abca4−/−mice also manifested complement deposition on Bruch’s membrane during the late stages of retinal degeneration.4 We assessed complement deposition in the retinas of Rdh8−/− Abca4−/−mice undergoing various treatments by immunohistochemical analyses with anti-C3 antibody and fluorescence microscopy. As shown in Figure 4A, a strong fluorescent signal was observed in PBS-treated and untreated mice. Partial signal reduction was noted in antioxidant 1-, antioxidant 2-, and 9cRAc-treated mice. Only a faint signal was detected in sirolimus-treated Rdh8−/− Abca4−/−mice, even though their retinal structures resembled those of antioxidant 1-, antioxidant 2- and 9cRAc-treated mice. No complement deposition was detected in retinylamine-treated mice.
Retinal Function Evaluated by ERGs
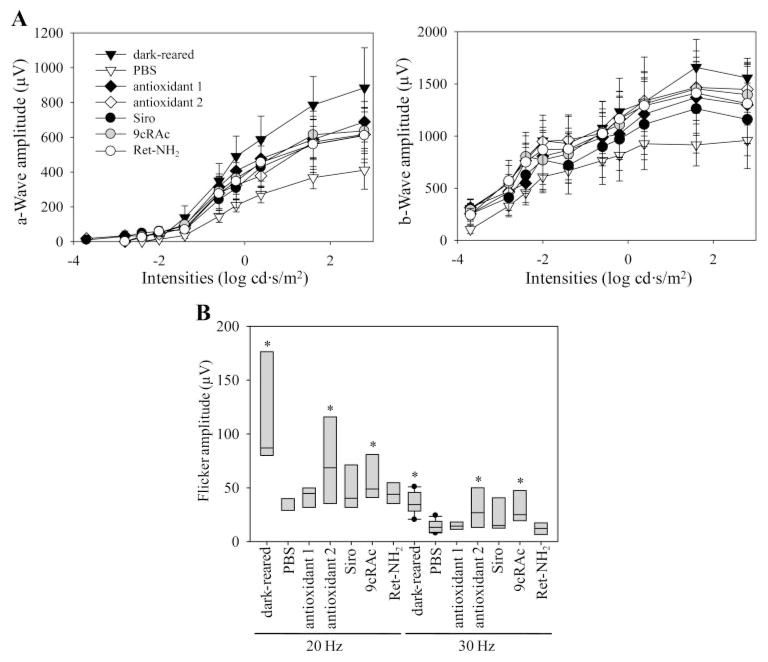
ERG studies were performed to evaluate the therapeutic effects of test compounds on retinal function mediated by rod and cone photoreceptor cells. Thus, single flash ERGs were recorded under scotopic conditions to evaluate rod cell responses (Fig. 5A), and flicker ERGs were performed in photopic conditions to assess cone cell responses (Fig. 5B). With respect to scotopic ERG responses, both a- and b- wave amplitudes were maintained significantly better in medicated than in PBS-treated control mice, and there were no significant differences observed in the effects of different test compounds (Fig 5A). Flicker ERG responses in untreated mice were significantly reduced compared with those of dark-reared mice (Fig. 5B), but intriguingly, responses of both antioxidant 2- and 9cRAc-treated mice were significantly retained after both 20-and 30-Hz stimuli. These observations suggest that these two treatments may have protective effects on cone function in this mouse model. Both scotopic and photopic ERG responses of retinylamine-treated mice were decreased to levels found in saline-treated animals, even though retinal structures in retinylamine-treated mice were comparable with those of dark-reared mice, owing to effects of long-term administration of this compound.28 Decreased ERG responses in retinylamine-treated mice were temporary, recovering to levels in dark-reared animals 2 months after the last administration of retinylamine (data not shown).
Figure 5.
Effects of various regimens on ERGs of Rdh8−/− Abca4−/− mice. Single-flash ERGs under scotopic conditions and flicker ERGs under photopic conditions were recorded to evaluate potential therapeutic effects of various medications on rod and cone photoreceptor function. Single-flash ERG responses of mice treated with indicated medications (Siro, sirolimus; Ret-NH2, retinylamine) were significantly better than those of PBS-treated mice (A, n = 3–4; P < 0.02), whereas flicker ERG responses were better in mice treated with either antioxidant 2 or 9cRAc (B, n = 3–4, boxes indicate SD, P <0.02) compared with responses of PBS-treated mice.
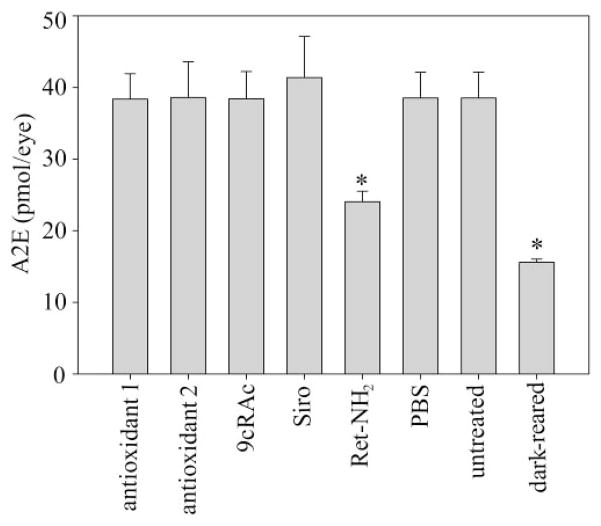
A2E Quantification
A2E, a major fluorophore of lipofuscin produced from all-trans retinal,29 accumulates significantly in the retina/RPE of Rdh8−/− Abca4−/−mice.4 To examine the relationship between the severity of retinal degeneration and A2E accumulation, we quantified A2E in the eyes of Rdh8−/− Abca4−/−mice undergoing various treatment regimens (Fig. 6). Surprisingly, retinylamine was the only compound that significantly decreased A2E accumulation among the medications tested. Even pretreatment with 9cRAc failed to increase A2E accumulation in these mice. Although smaller amounts of A2E were found in retinylamine-treated and dark-reared Rdh8−/− Abca4−/−mice, A2E accumulation did not correlate with the severity of retinal degeneration in either drug-treated or untreated mice.
Figure 6.
Effects of tested medications on A2E accumulation in retinas of Rdh8−/− Abca4−/−mice. A2E in the eyes of Rdh8−/− Abca4−/−mice treated with the indicated medications (9cRAc, 9-cis-retinyl acetate; Siro, sirolimus; Ret-NH2, retinylamine) was quantified by HPLC (n > 3; bars, SD; *P <0.01). Retinylamine inhibited A2E accumulation, whereas other tested medications had no effect.
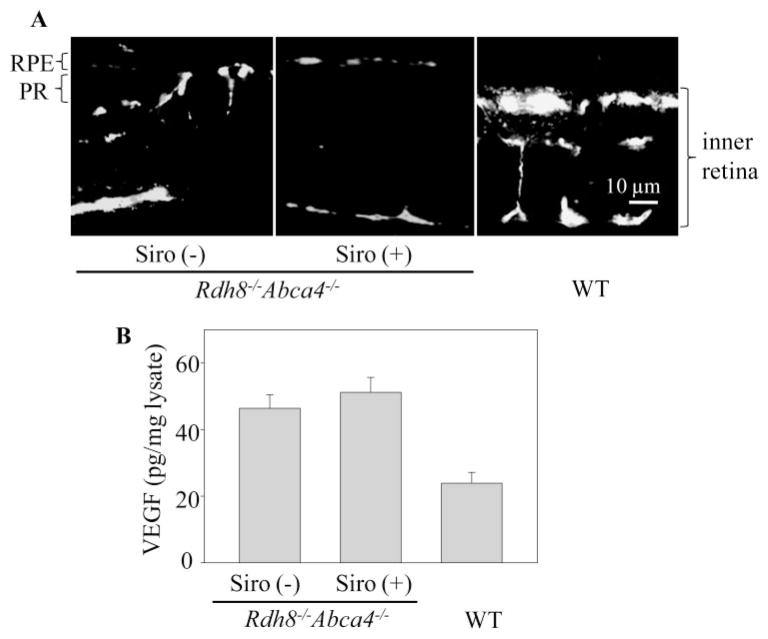
Effects of Sirolimus on Choroidal Neovascularization
CNV is a hallmark of late-stage, wet-type human AMD and it also manifests at the age of 6 months in Rdh8−/− Abca4−/−mice.4 In this study, 6-month-old Abca4−/− Rdh8−/−mice maintained in a 12-hour light (3–5 lux)/12-hour dark cycle environment were injected intraperitoneally with sirolimus, 2 mg/kg daily, for 4 months. No CNV was detected in the eyes of sirolimus-treated mice (0/10) whereas this pathology was seen in 22.2% (2/9) of the eyes of untreated animals (Fig. 7A). Amounts of VEGF were elevated but similar in the eyes of sirolimus-treated and untreated Rdh8−/− Abca4−/−mice (Fig. 7B).
Figure 7.
Effect of sirolimus on development of CNV and high levels of VEGF in eyes of older Rdh8−/− Abca4−/−mice. Sirolimus (Siro, 2 mg/kg) was injected intraperitoneally into 6-month-old Rdh8−/−Abca4−/− mice every day for 4 months. (A) Angiographs of retinas from 10-month-old untreated and sirolimus-treated Rdh8−/− Abca4−/−mice and an untreated 10-month-old WT mouse are shown. CNV, observed in untreated mice, was not detected in the sirolimus-treated Rdh8−/− Abca4−/−mice. Bars, 10 μm. (B) Amounts of VEGF in the eyes were quantified by a specific ELISA. Increased levels of VEGF, noted in 10-month-old Rdh8−/− Abca4−/−compared with WT mice, were unaffected by pretreatment with sirolimus (n > 3; bars, SD; *P <0.01 versus WT mice).
Discussion
Although clinical interventions exist that address the neovascular stage of AMD responsible for 10% of all AMD cases, there is as yet no acceptable treatment for dry AMD. Daily multivitamin therapy, as reported by the Age-Related Eye Disease Study, is effective in slowing progression of the disease in only approximately 20% of people with intermediate to advanced dry AMD.14 Even though more research is needed to slow or stop this debilitating disease, the search for an effective therapy has been hindered by the lack of an appropriate animal model for dry AMD. Recent reports provide evidence that AMD progression is closely related to the activation of innate immunity.30 Accumulation of apolipoproteins, lipids, metals, and other agents is believed to initiate macrophage activation that may lead to the development of retinal degeneration.31–33 In contrast, Rdh8−/− Abca4−/−mice provide strong evidence of a relationship between aberrant retinoid metabolism and severe retinal degeneration with a phenotype similar to human AMD.4,11 This mouse model exhibits accumulation of condensation products of all-trans-retinal and severe retinal degeneration accompanied by complement activation.4 Herein, we report the effects of several categories of pharmacologic agents, including antioxidants, an anti-immune and anti-VEGF drug, an artificial chromophore, and a retinoid cycle inhibitor, on the development of retinopathy in Rdh8−/− Abca4−/−mice.
Research has suggested that oxidative stress, such as that which occurs in SOD1-deficient mice,29,30 plays a significant role in retinal degeneration. Moreover, antioxidants reportedly slow the progression of some types of retinal degeneration including that in rd1/rd1 mice24 and in several human trials.15 Five antioxidants with demonstrated efficacy in preventing progression of congenital retinal degeneration in certain animal models were tested in this study.24 Because a mixture of five different antioxidants displayed lethal toxicity shortly after initiation of the regimen, we simplified the original mixture into 2 antioxidant mixtures: antioxidants 1 and 2.4 Intriguingly, administration of these two antioxidant mixtures showed some beneficial effects on retinal degeneration in Rdh8−/− Abca4−/−mice (Fig. 3) at the same daily dose as that which ameliorated photoreceptor cell death induced by intense light exposure in WT (BALB/c) mice (Fig. 2). This finding suggests that light-induced damage contributes to the retinopathy observed in Rdh8−/− Abca4−/−mice because these animals failed to develop retinopathy when kept in the dark (Fig. 3). But antioxidants only partially preserved retinal histology compared with their counterparts kept in the dark (Figs. 3, 4, 5), even at the nearly lethal doses used. These observations suggest that these compounds may diminish molecular damage to lipids, proteins, and nucleic acids by oxidative stress and thereby decrease apoptotic activation.24,25,34
Sirolimus (rapamycin) demonstrated some efficacy in preserving retinal structure and also inhibited complement deposition on Bruch’s membrane. This macrolide is known to have anti-inflammatory properties that may prevent complement activation. In our study, sirolimus-treated older mice did not develop choroidal neovascularization, indicating that complement activation may be a critical factor in CNV growth, even though the correlation between CNV growth and complement activation is still controversial in clinical studies.35,36 Of interest, sirolimus did not alter levels of VEGF when given to older mice. These findings are consistent with the results of Dejneka et al.,18 who reported that sirolimus inhibited CNV but did not reduce VEGF in a laser-induced CNV mouse model. These observations suggest a possible role for sirolimus in suppressing CNV and may open new venues for its use in treating AMD. Additional studies are needed to investigate the mechanisms of CNV growth and complement activation.
The artificial chromophore precursor 9cRAc also limited the extent of retinal degeneration, supporting the hypothesis that this phenotype in Rdh8−/− Abca4−/−mice is, at a minimum, related to impaired 11-cis-retinal regeneration. Radu et al.37 have reported that vitamin A supplementation increases A2E accumulation in Abca4−/−mice. In our study, mice treated with 9cRAc that evidenced preservation of retinal integrity failed to show increased amounts of A2E. This is contrary to results of studies in which vitamin A was used,20,21 suggesting an advantage for using 9cRAc instead of all-trans isomers such as vitamin A. That 9cRAc as well as retinylamine have efficacy in this model also suggests that some combination of the two artificial retinoids may be optimal.
Among the tested compounds, retinylamine showed the greatest therapeutic potential in the Rdh8−/− Abca4−/−mice. This RPE65 inhibitor slows the conversion of all-trans-retinyl ester to 11-cis-retinal, both in vitro and in vivo.22 Retinylamine treatment has been shown to completely prevent light-induced acute retinal degeneration in mice.38 This compound has the additional advantage of having a long-lasting effect because of its specific storage as a pro-drug in RPE retinosomes.38,39 Therefore, low doses given after prolonged intervals should suffice to achieve RPE65 inhibition. Retinylamine may prove useful for treating dry AMD. However, the extent of delayed all-trans-retinal clearance in human AMD is not known, and so patient-based studies are needed to assess this parameter.
In summary, this study indicates that several medications show some promise in modulating the development of retinal degeneration in Rdh8−/− Abca4−/−mice, a model of human AMD caused by delayed all-trans-retinal clearance. That the retinoid cycle inhibitor, retinylamine, significantly inhibited retinal degeneration in these animals serves as a reminder that understanding the involved pathogenic mechanisms is essential for development of safe and effective therapies for AMD.
Acknowledgments
The authors thank researchers from Case Western Reserve University, Leslie T. Webster Jr. and Philip D. Kiser for comments on the manuscript, Marcin Golczak for retinylamine, and Midori Hitomi for help with the electron microscopy.
Supported by funding from VAMC Career Development Grant, Research to Prevent Blindness, the Ohio Lions Research Foundation, and Grants EY019031, EY09339, P30 EY11373, and EY08123 from the National Institutes of Health.
Footnotes
Disclosure: T. Maeda, QLT, Inc. (C, P), Acucela, Inc. (C); A. Maeda, QLT, Inc. (C), Acucela, Inc. (C); M. Matosky, None; K. Okano, None; S. Roos, None; J. Tang, None; K. Palczewski, QLT, Inc. (C, P), Acucela, Inc. (C, P), Retinagenix, Inc. (I, E, C, P)
References
- 1.Haines JL, Spencer KM, Pericak-Vance MA. Bringing the genetics of macular degeneration into focus. Proc Natl Acad Sci U S A. 2007;104:16725–16726. doi: 10.1073/pnas.0708151104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun H, Smallwood PM, Nathans J. Biochemical defects in ABCR protein variants associated with human retinopathies. Nat Genet. 2000;26:242–246. doi: 10.1038/79994. [DOI] [PubMed] [Google Scholar]
- 3.Rattner A, Smallwood PM, Nathans J. Identification and characterization of all-trans retinal dehydrogenase from photoreceptor outer segments, the visual cycle enzyme that reduces all-trans retinal to all-trans retinal. J Biol Chem. 2000;275:11034–11043. doi: 10.1074/jbc.275.15.11034. [DOI] [PubMed] [Google Scholar]
- 4.Maeda A, Maeda T, Golczak M, Palczewski K. Retinopathy in mice induced by disrupted all-trans retinal clearance. J Biol Chem. 2008;283:26684–26693. doi: 10.1074/jbc.M804505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maeda T, Cideciyan AV, Maeda A, et al. Loss of cone photoreceptors caused by chromophore depletion is partially prevented by the artificial chromophore pro-drug, 9-cis-retinyl acetate. Hum Mol Genet. 2009;18(12):2277–2287. doi: 10.1093/hmg/ddp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maeda A, Maeda T, Golczak M, et al. Involvement of all-trans retinal in acute light-induced retinopathy of mice. J Biol Chem. 2009;284(22):15173–15183. doi: 10.1074/jbc.M900322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palczewski K. G protein-coupled receptor rhodopsin. Annu Rev Biochem. 2006;75:743–767. doi: 10.1146/annurev.biochem.75.103004.142743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Ann Rev Pharmacol Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McBee JK, Palczewski K, Baehr W, Pepperberg DR. Confronting complexity: the interlink of phototransduction and retinoid metabolism in the vertebrate retina. Prog Retin Eye Res. 2001;20:469–529. doi: 10.1016/s1350-9462(01)00002-7. [DOI] [PubMed] [Google Scholar]
- 10.Molday RS. ATP-binding cassette transporter ABCA4: molecular properties and role in vision and macular degeneration. J Bioenerg Biomembr. 2007;39:507–517. doi: 10.1007/s10863-007-9118-6. [DOI] [PubMed] [Google Scholar]
- 11.Maeda A, Maeda T, Sun W, Zhang H, Baehr W, Palczewski K. Redundant and unique roles of retinol dehydrogenases in the mouse retina. Proc Natl Acad Sci U S A. 2007;104:19565–19570. doi: 10.1073/pnas.0707477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 13.Maeda A, Maeda T, Imanishi Y, et al. Role of photoreceptor-specific retinol dehydrogenase in the retinoid cycle in vivo. J Biol Chem. 2005;280:18822–18832. doi: 10.1074/jbc.M501757200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SanGiovanni JP, Chew EY, Clemons TE, et al. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22. Arch Ophthalmol. 2007;125:1225–1232. doi: 10.1001/archopht.125.9.1225. [DOI] [PubMed] [Google Scholar]
- 16.Bierer BE, Mattila PS, Standaert RF, et al. Two distinct signal transmission pathways in T lymphocytes are inhibited by complexes formed between an immunophilin and either FK506 or rapamycin. Proc Natl Acad Sci U S A. 1990;87:9231–9235. doi: 10.1073/pnas.87.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guba M, von Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 18.Dejneka NS, Kuroki AM, Fosnot J, Tang W, Tolentino MJ, Bennett J. Systemic rapamycin inhibits retinal and choroidal neovascularization in mice. Mol Vis. 2004;10:964–972. [PubMed] [Google Scholar]
- 19.Rattner A, Nathans J. Macular degeneration: recent advances and therapeutic opportunities. Nat Rev Neurosci. 2006;7:860–872. doi: 10.1038/nrn2007. [DOI] [PubMed] [Google Scholar]
- 20.Van Hooser JP, Aleman TS, He YG, et al. Rapid restoration of visual pigment and function with oral retinoid in a mouse model of childhood blindness. Proc Natl Acad Sci U S A. 2000;97:8623–8628. doi: 10.1073/pnas.150236297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Batten ML, Imanishi Y, Maeda T, et al. Lecithin retinal acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golczak M, Kuksa V, Maeda T, Moise AR, Palczewski K. Positively charged retinoids are potent and selective inhibitors of the transcis isomerization in the retinoid (visual) cycle. Proc Natl Acad Sci U S A. 2005;102:8162–8167. doi: 10.1073/pnas.0503318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Hu Y, Lu K, Flannery JG, Ma JX. Very low density lipoprotein receptor, a negative regulator of the wnt signaling pathway and choroidal neovascularization. J Biol Chem. 2007;282:34420–34428. doi: 10.1074/jbc.M611289200. [DOI] [PubMed] [Google Scholar]
- 24.Komeima K, Rogers BS, Lu L, Campochiaro PA. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natl Acad Sci U S A. 2006;103:11300–11305. doi: 10.1073/pnas.0604056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komeima K, Rogers BS, Campochiaro PA. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J Cell Physiol. 2007;213:809–815. doi: 10.1002/jcp.21152. [DOI] [PubMed] [Google Scholar]
- 26.Wenzel A, Reme CE, Williams TP, Hafezi F, Grimm C. The Rpe65 Leu450Met variation increases retinal resistance against light-induced degeneration by slowing rhodopsin regeneration. J Neurosci. 2001;21:53–58. doi: 10.1523/JNEUROSCI.21-01-00053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maeda A, Maeda T, Imanishi Y, Golczak M, Moise AR, Palczewski K. Aberrant metabolites in mouse models of congenital blinding diseases: formation and storage of retinyl esters. Biochemistry. 2006;45:4210–4219. doi: 10.1021/bi052382x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sparrow JR, Parish CA, Hashimoto M, Nakanishi K. A2E, a lipofuscin fluorophore, in human retinal pigmented epithelial cells in culture. Invest Ophthalmol Vis Sci. 1999;40:2988–2995. [PubMed] [Google Scholar]
- 30.Ambati J, Anand A, Fernandez S, et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003;9:1390–1397. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- 31.Malek G, Johnson LV, Mace BE, et al. Apolipoprotein E allele-dependent pathogenesis: a model for age-related retinal degeneration. Proc Natl Acad Sci U S A. 2005;102:11900–11905. doi: 10.1073/pnas.0503015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karan G, Lillo C, Yang Z, et al. Lipofuscin accumulation, abnormal electrophysiology, and photoreceptor degeneration in mutant ELOVL4 transgenic mice: a model for macular degeneration. Proc Natl Acad Sci U S A. 2005;102:4164–4169. doi: 10.1073/pnas.0407698102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hahn P, Qian Y, Dentchev T, et al. Disruption of ceruloplasmin and hephaestin in mice causes retinal iron overload and retinal degeneration with features of age-related macular degeneration. Proc Natl Acad Sci U S A. 2004;101:13850–13855. doi: 10.1073/pnas.0405146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dorrell MI, Aguilar E, Jacobson R, et al. J Clin Invest. Antioxidant or neurotrophic factor treatment preserves function in a mouse model of neovascularization-associated oxidative stress. Published online February 2, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chowers I, Meir T, Lederman M, et al. Sequence variants in HTRA1 and LOC387715/ARMS2 and phenotype and response to photodynamic therapy in neovascular age-related macular degeneration in populations from Israel. Molecular vision. 2008;14:2263–2271. [PMC free article] [PubMed] [Google Scholar]
- 36.Kim IK, Ji F, Morrison MA, et al. Comprehensive analysis of CRP, CFH Y402H and environmental risk factors on risk of neovascular age-related macular degeneration. Mol Vis. 2008;14:1487–1495. [PMC free article] [PubMed] [Google Scholar]
- 37.Radu RA, Yuan Q, Hu J, et al. Accelerated accumulation of lipofuscin pigments in the RPE of a mouse model for ABCA4-mediated retinal dystrophies following Vitamin A supplementation. Invest Ophthalmol Vis Sci. 2008;49:3821–3829. doi: 10.1167/iovs.07-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maeda A, Maeda T, Golczak M, et al. Effects of potent inhibitors of the retinoid cycle on visual function and photoreceptor protection from light damage in mice. Mol Pharmacol. 2006;70:1220 –1229. doi: 10.1124/mol.106.026823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imanishi Y, Batten ML, Piston DW, Baehr W, Palczewski K. Non-invasive two-photon imaging reveals retinyl ester storage structures in the eye. J Cell Biol. 2004;164:373–383. doi: 10.1083/jcb.200311079. [DOI] [PMC free article] [PubMed] [Google Scholar]