Abstract
Oxidative stress contributes to the pathophysiology of type 2 diabetes mellitus and its complications, including nephropathy. The current study was designed to test the hypothesis that a diet fortified with antioxidants would be beneficial to delay or prevent the progression of this disease. Male and female Zucker fa/fa rats were fed a control or an antioxidant (AO) fortified diet starting at four weeks of age. Metabolic parameters, renal function and renal histopathology were analyzed at 6, 13 and 20 weeks of age. Females on the AO diet had significantly lower blood glucose at 6 and 13 weeks, less severe renal pathology at 20 weeks, and higher glomerular filtration rates (GFR) at 20 weeks than age matched females on the regular diet (p < 0.05). Metabolic parameters including blood glucose, insulin resistance and serum cholesterol, and mean arterial pressure (MAP), worsened with age in both males and females, as expected. GFR decreased and renal pathology also became more severe with age. Finally, females on the AO diet had higher GFRs and lower MAP at 20 weeks than males on the same diet. This may denote a protective effect of the AO diet in females, but not in males. These findings may have implications for the role of antioxidants as therapy in humans with T2DM.
Keywords: Type 2 diabetes mellitus, antioxidants, obese Zucker rat, diabetic nephropathy, oxidative stress
Introduction
Currently, the incidence of type 1 diabetes mellitus (T1DM) remains fairly stable, but type 2 diabetes (T2DM) which accounts for approximately 95% of cases of diabetes (1) and exhibits a world wide prevalence of 150 million, is predicted to increase to 300 million by 2025. In 2006, diabetes accounted for close to 50% of incident end stage renal disease patients in the USA and in 2004, that number was 40% or greater in six of 32 regions worldwide for which data are available (2).
We are using the obese Zucker rat (fa/fa) as a model animal that is genetically programmed to develop T2DM with obesity. This rat model has a genetic mutation in the leptin receptor. The Zucker fa/fa diabetic rat develops obesity, insulin resistance, hyperglycemia, hyperlipidemia and associated nephropathy. We are beginning dietary intervention at four weeks of age before the appearance of overt diabetes. The male fa/fa rat exhibits hyperinsulinemia and hyperlipidemia as early as four to six weeks of age (2, 3). By six weeks of age the oral glucose tolerance test is abnormal in the obese fa/fa rat compared with its lean littermates, Fa/Fa or Fa/fa (4). Urinary albumin excretion is elevated by 12 weeks (5). Basal hyperglycemia is evident by 13–14 weeks (2, 4). At this time glomerular basement membrane width is significantly greater than in age- matched lean controls. People with diabetes get mesangial expansion in their kidneys, called diffuse intercapillary diabetic glomerulosclerosis. In rats the corresponding lesion is focal and segmental glomerulosclerosis (6). Focal and segmental glomerulosclerosis can be seen at 18 weeks in the obese Zucker rat (2, 7).
Controversy exists over whether there are sex differences in severity of the disease in both rats and humans, with some studies showing that males are more affected while others demonstrate that females are more affected (8–14), and the impact of gender on diabetic nephropathy in humans remains controversial. Obese men have a higher incidence of diabetes than do obese women (15). Gender may also affect the prevalence of T2DM related nephropathy, with a higher prevalence in men. According to the European Diabetes and Transplant Association statistics, the percentage of patients with end stage renal disease (ESRD) who are men ranges from 56.5 to 67.5 % while women comprise 32.5 to 44.4% (16). This may indicate a greater susceptibility to renal injury in males than in females, differences in treatment, or other factors.
Several factors that affect the development of nephropathy in T2DM in humans also may play a role in the Zucker rat, including metabolic changes of hyperglycemia, insulin resistance and dyslipidemia (17). Diabetic nephropathy is also associated with oxidative stress and changes in renal nitric oxide (NO) production (18) which leads to endothelial dysfunction and decreased renal perfusion. Circulating levels of nitrite and nitrate (NOx) are increased in patients with T2DM and obese subjects compared with lean, healthy individuals (19). In addition, alterations in nitric oxide (NO) production and utilization can result in NO interaction with O2− to become peroxynitrite which causes cytotoxicity (20). Hypertension associated with oxidative stress may further aggravate the glomerular dysfunction (21).
Results of treatment of human diabetes with antioxidants in the diet have been encouraging but variable (22). However, oxidative imbalance may start well before there is evidence of overt nephropathy and some studies have shown a benefit of antioxidants on glycemic control and the development of diabetic vascular complications (22, 23). When antioxidants are started on the day of streptozotocin induction of T1DM in rats, renal function is preserved (24), and early treatment with vitamins C and E plus insulin has been shown to significantly lower blood glucose in this rat model (25). Therefore it is important to attempt to develop a dietary antioxidant therapy that will work in T2DM as well. Supplementation with antioxidants and factors essential to nitric oxide (NO) production may have the potential to improve endothelial dysfunction and renal perfusion in T2DM (26).
Our strategy was to begin antioxidant therapy at an age prior to the appearance of renal dysfunction, to see if early intervention also works in type 2 diabetes. We evaluated renal structure and function as well as associated metabolic factors including blood glucose and insulin, mean arterial pressure, urinary albumin and NO levels. We compared males and females to evaluate effects of gender and interaction of gender and diet. All measures were done at 6, 13 and 20 weeks of age to test if gender or diet altered the time course of development of metabolic or renal dysfunctions.
Materials and Methods
Animal Groups
All experiments were performed with the approval of the Animal Care and Use Committee of Ohio University. Zucker rats were obtained from Harlan-Sprague Dawley (Indianapolis, IN) at four weeks of age, housed under controlled conditions of lighting, temperature and humidity, and fed ad libitum a constant nutrition diet (rat diet 5012) or an antioxidant (AO) fortified diet (Purina Mills, Inc, St. Louis, MO, USA). A total of 115 animals (Column 5, Table 1) contributed to the study. Food intake was monitored and amount of food ingested per day did not differ between regular and AO diets (data not shown). The antioxidant fortified diet was designed to incorporate a number of substances that could act as antioxidants and fortify oxidative stress reducing enzymes (23, 27) and consisted of rat chow containing 160 IU α-tocopherol/kg food, 1.4 ppm selenium, 150 ppm zinc, 60 ppm copper, 150 ppm manganese and 21.5 ppm β-carotene per kg food, and ascorbic acid fortified water, 1000 U/liter. This diet was designed in consultation with the dieticians at Purina Mills with special attention to avoidance of any known toxicities. The control diet 5012 consisted of regular rat chow containing 32 IU α-tocopherol, 0.23 ppm selenium, 71 ppm zinc, 12 ppm copper, 69 ppm manganese, and 4.3 ppm β-carotene per kg food, and water. We compared obese (fa/fa) male and female littermates at three time points, at 6 weeks of age when hyperglycemia has been reported to be present, at 13 weeks of age when hyperinsulinemia has developed, and at 20 weeks of age when nephropathy has been reported to develop (2, 4).
Table 1.
Systemic and Metabolic Parameters
| Age | Sex | Diet | Body Wt. (g) | N | Kidney Wt. (g) | N | GFR (ml/min) | N | Serum Creatinine (μM/L) | N | Insulin (pmol/L) | N | Cholesterol (mg/dL) | N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 | M | REG | 213±6.73*▵ | 9 | 1.17±0.06*▵ | 9 | 0.76±0.25* | 9 | 37.4±10.8 | 8 | 80.2±38.0*▵ | 7 | 61.9±10.2▵ | 8 |
| 6 | F | REG | 237±46.6*▵ | 9 | 1.31±0.15▵ | 9 | 0.48±0.19 | 9 | 54.4±11.1 | 9 | 148±37.0 | 9 | 93.4±41.8 | 9 |
| 6 | M | AO | 218±4.67*▵ | 10 | 1.17±0.06*▵ | 10 | 0.38±0.24 | 7 | 45.4±18.1 | 8 | 32.6±9.78†*▵ | 9 | 90.7±10.2▵ | 8 |
| 6 | F | AO | 215±9.84*▵ | 11 | 1.15±0.06*▵ | 11 | 0.22±0.07 | 11 | 71.5±14.2 | 11 | 263±81.0 | 11 | 96.8±28.6 | 11 |
| 13 | M | REG | 582±2.50#†▵ | 2 | 1.82±0.10▵ | 2 | 0.06±0.05#▵ | 2 | 85.1±65.2 | 2 | 317±48.1 | 2 | 91.6±51.7 | 2 |
| 13 | F | REG | 446±8.56▵ | 6 | 1.42±0.07▵ | 6 | 0.51±0.18 | 5 | 99.1±31.5 | 6 | 593±192 | 6 | 59.9±11.9▵ | 6 |
| 13 | M | AO | 519±20.1†▵ | 10 | 2.06±0.12† | 10 | 0.69±0.23 | 10 | 33.3±13.6 | 7 | 361±130 | 8 | 71.2±5.50▵ | 7 |
| 13 | F | AO | 440±12.3▵ | 6 | 1.44±0.05▵ | 6 | 0.24±0.07 | 4 | 126±70.7 | 4 | 544±259 | 4 | 73.0±19.0▵ | 4 |
| 20 | M | REG | 647±15.4† | 14 | 2.28±0.08† | 12 | 0.48±0.25 | 13 | 49.1±23.6 | 11 | 392±93.4 | 11 | 188±28.8 | 11 |
| 20 | F | REG | 574±10.4 | 13 | 1.84±0.06 | 13 | 0.06±0.02# | 13 | 67.6±22.1 | 12 | 251±41.7 | 13 | 200±47.1 | 13 |
| 20 | M | AO | 663±7.76† | 12 | 2.35±0.09† | 12 | 0.16±0.05 | 12 | 35.7±12.1 | 11 | 309±96.2 | 11 | 197±31.4 | 11 |
| 20 | F | AO | 552±9.92 | 13 | 1.67±0.07 | 13 | 0.34±0.10 | 12 | 109±40.5 | 13 | 406±122 | 13 | 162±33.6 | 13 |
REG: regular diet; AO: antioxidant diet; GFR: glomerular filtration rate. All values are mean ± SEM.
significantly different vs. AO diet, p < 0.05;
significantly different vs. female gender, p < 0.05;
significantly different vs. 13 week age, p < 0.05;
significantly different vs. 20 week age, p < 0.05.
Fa Genotype Determination
Genomic DNA was isolated from rat livers obtained at the time of euthanasia, using the Qiagen isolation system for animal tissues. The following primers were used to amplify products from 100 ng genomic DNA: 5′-GTT TGC GTA TGG AAG TCA CAG-3′ and 5′-ACC AGC AGA GAT GTA TCC GAG-3′ (28), with a PCR protocol of 32 cycles at 92 °C for 30s, 64 °C for 1 min and 68 °C for 3 min. PCR products were digested with Msp I which distinguishes the Fa (two fragments of 1100 and 700 bp) from the fa allele (three fragments of 950, 700 and 130 bp) (28), and analyzed by 2% agarose gel electrophoresis.
Experimental Measures
At each time point, the following parameters were measured: body weight; kidney weight; mean arterial pressure (MAP); glomerular filtration rate (GFR) using iohexol urinary clearance; blood glucose, serum insulin, creatinine, and cholesterol; and urinary albumin, creatinine and NO. Twenty-four hours prior to the experiment, animals were placed in metabolic cages and fasted, with free access to water.
Metabolic parameters
Blood glucose was measured using the One Touch Lifescan glucometer (Johnson and Johnson). Insulin was measured using a solid phase two site enzyme immunoassay (Rat Insulin EIA, ALPCO). Serum creatinine and total cholesterol were determined using the Jaffe’ reaction (Cayman Chemical Co, USA) and an enzymatic colorimetric assay (Wako Chemicals, USA), respectively. Albumin was measured by enzyme immunoassay (SpiBio, France). The Homeostasis Model Assessment (HOMA), an index of insulin resistance, was calculated as glucose (mM) × insulin (μU/ml)/22.5 (17). NO was measured using the Apollo 4000 electrochemical NO/free radical analyzer (World Precision Instruments, Inc.) (29). A standard ISO-NOP007 sensor, 7 μm in diameter, was used to measure NO in the urine (30). Urinary NO/creatinine (NO/Cr) and albumin/creatinine (Alb/Cr) ratios were also calculated (31, 32).
Renal Histology
Sixty-seven representative renal tissue samples from different groups were studied by light microscopy to assess the renal injury for each group. The kidneys were dissected, sectioned longitudinally, fixed in 10% buffered formalin overnight and embedded in paraffin. Each sample was cut into 4 μm thick sections and stained with hematoxylin and eosin (H&E), periodic acid Schiff (PAS) and Masson’s trichrome stain. Severity of nephropathy was determined according to the degree of glomerulosclerosis and tubulointerstitial damage. Glomerulosclerosis was graded from 1 to 4 according to Li et al. (32). Fifty glomeruli per animal were observed at 200× magnification. Briefly, severity of glomerulosclerosis was evaluated by determining segmental increases in PAS-stained material within glomerular matrix, and collapse and obliteration of the capillary lumen with or without adhesion to Bowman’s capsule. Interstitial damage and fibrosis were scored semiquantitavely from 0 to 3 according to Pichler et al. (33). Interstitial damage was determined according to inflammatory cell infiltration, fibrosis, tubular dilation and/or atrophy and graded as: Score 0: normal interstitium and tubules, Score 1: mild changes, Score 2: moderate changes and Score 3: severe changes. The score was determined at 200× in 30 fields for each animal in the cortex. All histologic and morphometric determinations were performed blindly without knowledge of the experimental group by the same observer.
Statistical analysis
GFR, GFR/g kidney weight (GFR/g KW), MAP, blood glucose, serum creatinine, cholesterol, insulin, HOMA, urinary NO, creatinine, NO/Cr ratio, albumin, Alb/Cr ratio, body weight and kidney weight were compared between groups using factorial analysis of variance with main effects for diet (regular or antioxidant), sex (male or female) and age (6, 13 or 20 weeks) and their interaction (34). Overall correlations between measures were analyzed by both Spearman and Pearson methods. The 0.05 level of probability was used as the criterion of significance.
Results
Genotype Analysis
All obese rats were confirmed to have the fa/fa genotype (Figure 1), which is characterized by three Msp 1 restriction enzyme digestion products from the 1800 bp PCR product. Wild type (Fa) alleles have two bands of 1100 and 700 bp (28).
Figure 1.
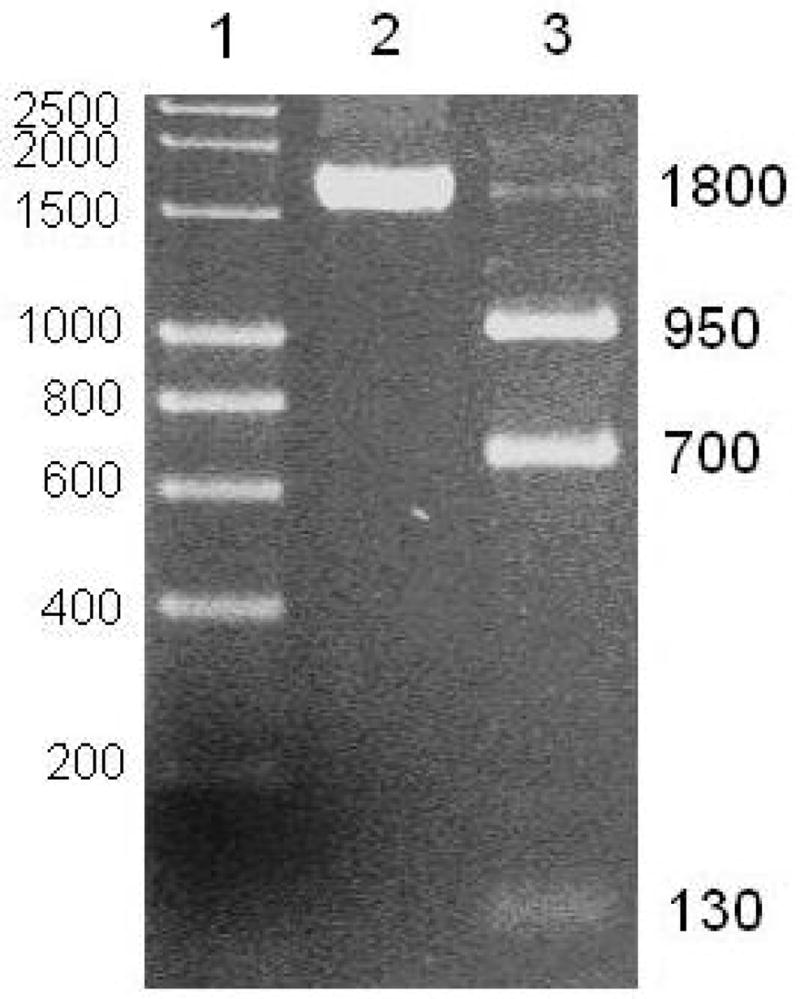
Msp 1 restriction enzyme digestion products of an 1800 base pair PCR product amplified from the leptin receptor gene in obese Zucker rat. Lane 1, molecular weight markers; Lane 2, Obese Zucker rat liver sample before digestion; Lane 3, Obese Zucker rat liver sample, after digestion, showing the characteristic 950, 700 and 130 bp bands for the mutated gene.
Effects of Age on Systemic and Metabolic Parameters and Renal Excretory Function
As shown in Table 1, body weight increased in both males and females on both the regular and AO diets from 6 to 13 and between 13 and 20 weeks of age (p < 0.05). Kidney weights increased in both males and females on both the regular and AO diets between 6 and 13 weeks and between 13 and 20 weeks. These differences were significant (p < 0.05) in all groups except females on the regular diet between 6 and 13 weeks and males on the AO diet between 13 and 20 weeks. There was a significant difference in GFR in males on the regular diet between 6 and 13 weeks and between 13 and 20 weeks with the 13 week values being lower than either 6 or 20 weeks. There was a significant decrease in GFR/gKW (Figure 2) in males on the regular diet between 6 and 13 weeks and in females on the regular diet between 6 or 13 and 20 weeks. Males on the AO diet had lower GFR/gKW at 20 weeks than at 13 weeks; this decrease was not seen in females on the AO diet. There were no significant differences in serum creatinine with age in any group.
Figure 2.
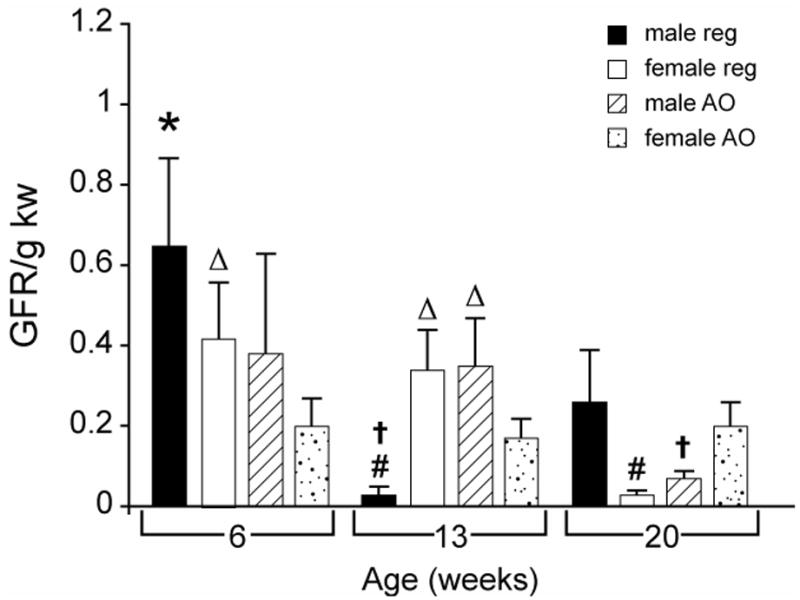
Effects of diet, sex and age on GFR/g KW in obese Zucker rat. All values are mean ± SEM. REG: regular diet; AO: antioxidant diet; GFR: glomerular filtration rate; KW: kidney weight; #:significantly different vs. AO diet, p < 0.05; †:significantly different vs. female gender, p < 0.05; *: significantly different vs. 13 week age, p < 0.05;▵: significantly different vs. 20 week age, p < 0.05.
Overall, MAP (Figure 3) increased with age. It was significantly higher (p < 0.05) in females on the regular diet at 13 and 20 weeks than at 6 weeks, and also at 20 vs. 13 weeks. In males or females on the AO diet, MAP increased significantly at both 13 and 20 weeks compared to 6 weeks of age (p < 0.05). Blood glucose (Figure 4) increased in females on the regular diet at 13 weeks compared with either 6 or 20 weeks (p < 0.05). Blood glucose increased significantly in females on the AO diet between 6 and 20 weeks (p < 0.05). Insulin levels (Table 1) increased with age in males on either the regular diet or AO diet; levels were significantly higher at 13 and 20 weeks than at 6 weeks.
Figure 3.
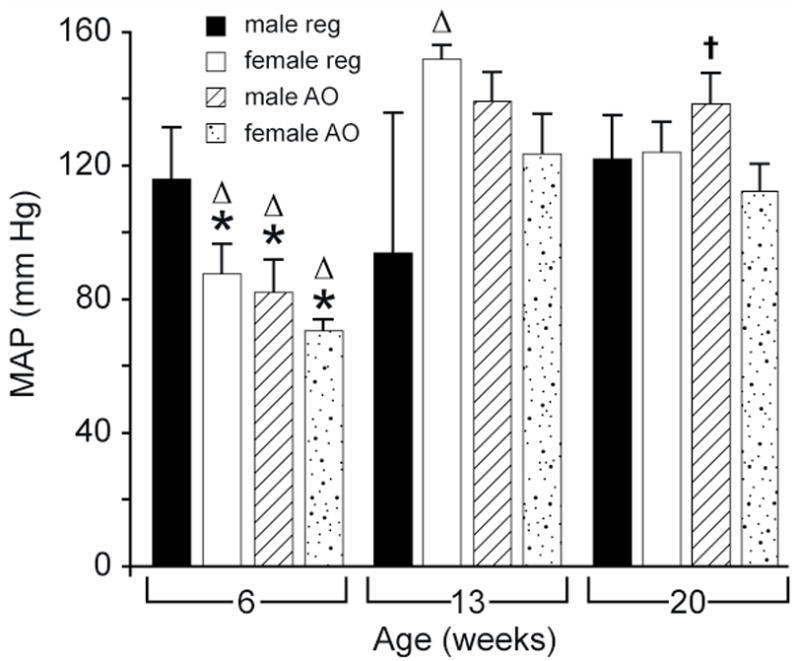
Effects of sex and age on MAP in obese Zucker rat. All values are mean ± SEM. REG: regular diet; AO: antioxidant diet; MAP: mean arterial pressure; †:significantly different vs. female gender, p < 0.05; *: significantly different vs. 13 week age, p < 0.05; ▵: significantly different vs. 20 week age, p < 0.05.
Figure 4.
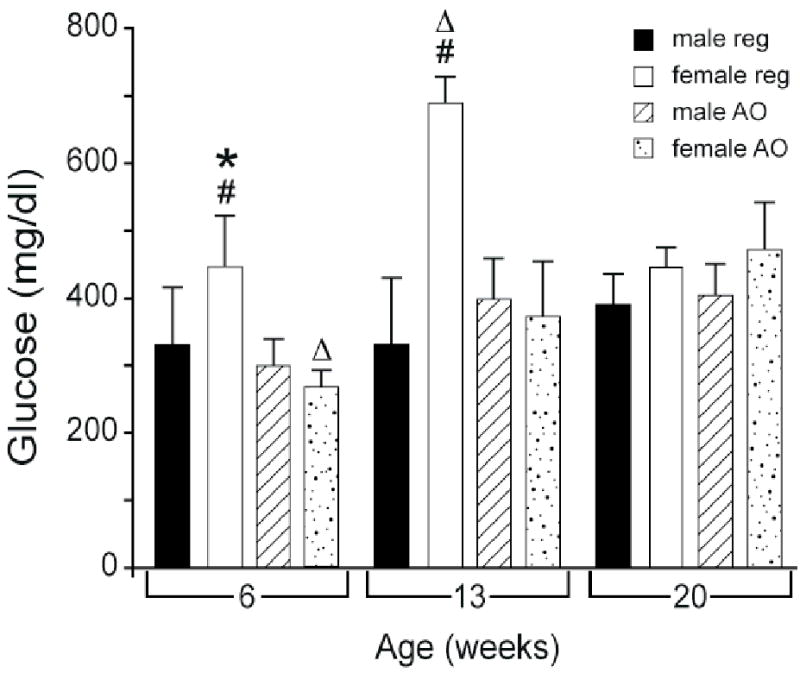
Effects of diet and age on blood glucose in obese Zucker rat. All values are mean ± SEM. REG: regular diet; AO: antioxidant diet; #:significantly different vs. AO diet, p < 0.05; *: significantly different vs. 13 week age, p < 0.05;▵: significantly different vs. 20 week age, p < 0.05.
HOMA (Figure 5) increased with age in both males and females on either the regular or AO diet. This difference was significant in males on the regular or AO diet, and in females on the AO diet between 6 and 20 weeks and in females on the regular diet and males on the AO diet between 6 and 13 weeks (p < 0.05). Cholesterol (Table 1) also increased progressively with age and was higher at 20 weeks than at 6 weeks in males on either the regular or AO diet. It was also higher at 20 weeks than at 13 weeks in females on either the regular or AO diet and in males on the AO diet.
Figure 5.
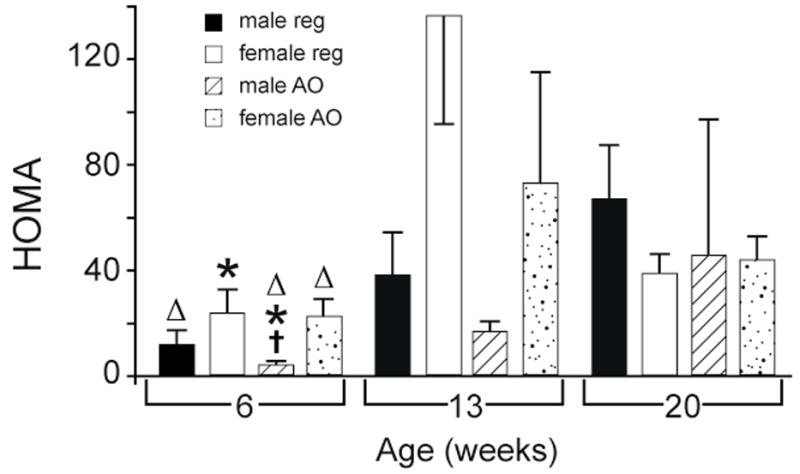
Effects of sex and age on HOMA in obese Zucker rat. All values are mean ± SEM. REG: regular diet; AO: antioxidant diet; HOMA = glucose (mM) × insulin (μU ml−1)/22.5; †:significantly different vs. female gender, p < 0.05; *: significantly different vs. 13 week age, p < 0.05;▵: significantly different vs. 20 week age, p < 0.05.
As shown in Table 2, males and females on the regular diet had a significant increase in urine creatinine between 6 and 20 weeks. Males on the AO diet showed a significant rise in urine creatinine at both 13 and 20 weeks compared to 6 weeks. Urinary NO showed little change with age. The only group to show a change was the males on the regular diet at 13 weeks, where the values were significantly lower than at 6 or 20 weeks. The changes observed in the NO/Cr ratios paralleled those for NO. Males at either 6 or 13 weeks on the regular diet had significantly lower urine albumin than males on the regular diet at 20 weeks of age. Females on the regular diet had lower urine albumin levels at 6 weeks compared to 20 weeks. The Alb/Cr ratios showed parallel differences between groups.
Table 2.
Renal Excretory Function
| Age | Sex | Diet | Urine creatinine (mmol/L) | N | Urine NO (μM) | N | NO/Cr (μg/mg) | N | Urine Albumin (mg/dL) | N | Alb/Cr (μg/mg) | N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 | M | REG | 3.84±0.57#▵ | 9 | 6.56±1.92* | 9 | 0.77±0.33* | 9 | 9.96±2.02▵ | 10 | 2.36±0.66▵ | 9 |
| 6 | F | REG | 3.52±0.76▵ | 8 | 16.8±10.3 | 8 | 1.58±1.18 | 8 | 32.7±13.6#▵ | 8 | 7.91±3.19#▵ | 8 |
| 6 | M | AO | 6.07±0.72▵ | 10 | 22.0±10.7 | 10 | 0.83±0.29 | 10 | 211±169 | 10 | 22.0±14.4 | 10 |
| 6 | F | AO | 5.03±2.04 | 10 | 2.75±1.61 | 10 | 0.31±0.18 | 9 | 256±54.7 | 10 | 68.1±23.9 | 9 |
| 13 | M | REG | 4.34±1.28 | 2 | 0.23±0.18#▵ | 2 | 0.01±0.01#▵ | 2 | 6.36±4.72#▵ | 2 | 1.11±0.98▵ | 2 |
| 13 | F | REG | 4.83±0.49 | 6 | 5.01±2.40 | 6 | 0.30±0.16 | 6 | 270±136 | 7 | 64.3±33.8 | 6 |
| 13 | M | AO | 5.81±1.02▵ | 8 | 9.11±3.90 | 10 | 0.22±0.09 | 8 | 228±86.7 | 9 | 38.0±18.3 | 8 |
| 13 | F | AO | 4.29±0.77 | 6 | 11.9±8.77 | 4 | 0.57±0.33 | 4 | 299±90.9 | 6 | 59.2±13.1 | 6 |
| 20 | M | REG | 6.84±0.90# | 11 | 7.99±3.13 | 11 | 0.32±0.11 | 9 | 644±275 | 11 | 105±38.9 | 11 |
| 20 | F | REG | 6.16±0.90 | 12 | 11.8±4.49 | 11 | 1.10±0.57 | 10 | 237±75.9 | 12 | 51.5±18.2 | 11 |
| 20 | M | AO | 11.4±1.87 | 11 | 15.6±5.22 | 7 | 0.35±0.12 | 7 | 1010±696 | 12 | 144±110 | 10 |
| 20 | F | AO | 7.69±1.68 | 12 | 3.00±1.06 | 11 | 0.14±0.06 | 11 | 797±389 | 12 | 124±56.5 | 12 |
REG: regular diet; AO: antioxidant diet; NO: nitric oxide; Cr: creatinine; NO/Cr: NO/creatinine ratio; Alb/Cr: albumin/creatinine ratio. All values are mean ± SEM.
significantly different vs. AO diet, p < 0.05;
significantly different vs. 13 week age, p < 0.05;
significantly different vs. 20 week age, p < 0.05.
Effect of Age on Renal Histopathology
Histopathological results are shown in Table 3 and Figures 6 and 7. At 20 weeks all animals (100%) showed histological changes in the kidney compatible with diabetic nephropathy, including matrix mesangial expansion identifiable by PAS stain, basement membrane thickening and nodular glomerular lesions. Also at 20 weeks more severe sclerosis and fibrosis could be seen (Figures 6 and 7, bottom row). This demonstrates substantial pathology compared with the 6 week animals.
Table 3.
Effect of Sex and Diet on Renal Histopathology at 20 weeks.
| Sex | Age (wks) | Diet | N (animals) | Glomerulosclerosis score | TI damage (%) | TI damage (score) |
|---|---|---|---|---|---|---|
| F | 20 | AO | 4 | 1.41 | 1.7 | 1 |
| F | 20 | REG | 8 | 1.77 | 11.3 | 2 |
| M | 20 | AO | 7 | 2.53 | 19.2 | 3 |
| M | 20 | REG | 8 | 2.28 | 22.4 | 3 |
Glomerulosclerosis was graded from 1 to 4 according to Li et al. (33). TI: tubulointerstitial. TI damage (%) shows the percentage of fibrosis, infiltration and atrophy related to the total area examined. TI damage (score) is determined by the percent of tubules showing fibrosis and interstitial thickening between tubules as follows: 0 = normal (0%); 1: mild (< 5%); 2: moderate (5–15%); 3: severe alterations (> 15%). Values shown are the average for each group.
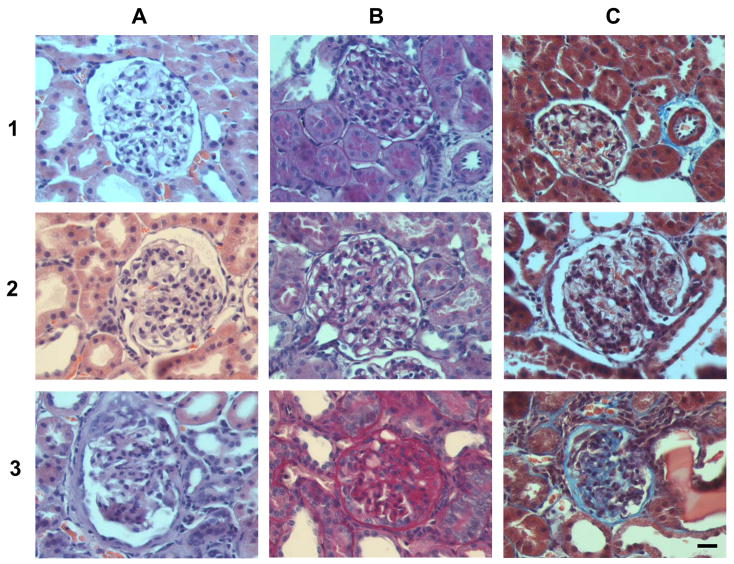
Figure 6. Representative renal images with different scores for glomerulosclerosis. Column A: hematoxylin and eosin stain; column B: PAS stain and C: trichrome stain.
Row 1: sections from a female at 6 weeks on the regular diet. The glomerulus looks normal and the tubulointerstitial areas show normal interstitium and tubules. Row 2: sections from a male at 6 weeks on the regular diet show both glomerulus and interstitium are almost normal. There is slight PAS staining beginning to appear in the glomeruli. Row 3: sections from a female at 20 weeks on the regular diet showing severe glomerulosclerosis, with strong PAS reactivity in the mesangial areas. PAS-stained material within the glomerular matrix is seen in more than 50% of the glomerulus and adhesion to Bowman’s capsule has occurred. The trichrome stain reveals fibrosis in the interstitium (Score 3) and in the glomerulus itself (blue stain). Magnification 400×, bar = 20 μm.
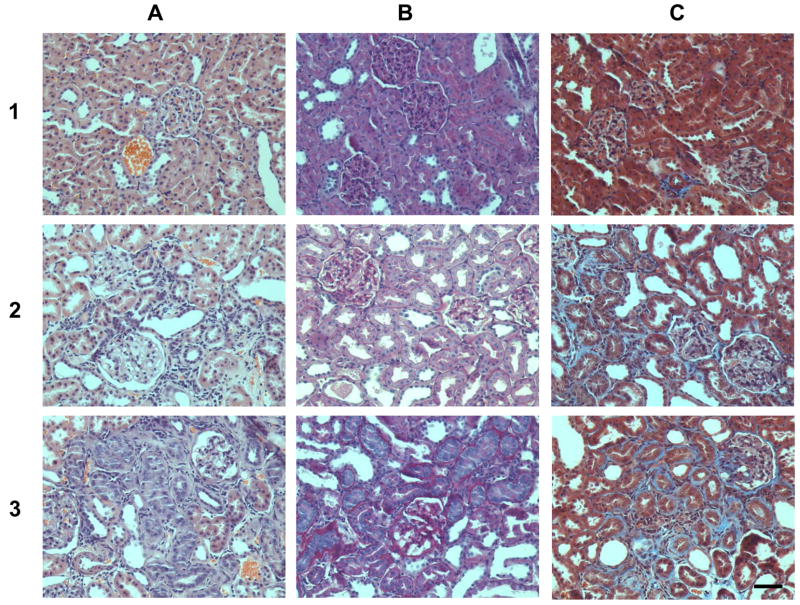
Figure 7. Representative renal images with different grades of nephropathy. Column A: hematoxylin and eosin stain; column B: PAS stain and C: trichrome stain.
Row 1: sections from a female at 6 weeks on the regular diet; Row 2: sections from a female at 20 weeks on the AO diet. In this group there is an increase of PAS positive stain in the mesangium as well as in the basement membrane, the fibrosis in the interstitium is score 1. Row 3: sections from a female at 20 weeks on the regular diet. Glomerulosclerosis and alterations in the tubules and interstitium (score 2) are higher in females at 20 weeks on the regular diet. Magnification 200×, bar = 50 μm.
Sex Differences in Systemic and Metabolic Parameters and Renal Excretory Function
As shown in Table 1, body weight increased progressively with age in males and females on both diets. At 13 weeks and at 20 weeks, males on either the regular or AO diet had significantly higher body weight than females on the same diet. Kidney weight was significantly higher at 13 weeks in males on the AO diet compared to females on the AO diet. At 20 weeks in animals on both the regular and the AO diets, kidney weight was higher in males than in females. There were no significant sex differences in GFR. However GFR/g KW (Figure 2) was lower in males at 13 weeks on the regular diet than in females at 13 weeks on the regular diet. At 20 weeks, GFR/g KW was significantly higher in females on the AO diet than in males on this diet.
Females on either diet and males on the AO diet were normotensive (MAP 70–130 mmHg) at 6 weeks (Figure 3). Females at 20 weeks on the AO diet had significantly lower MAP than males at 20 weeks on the AO diet. There were no sex differences observed in blood glucose or cholesterol (Figure 4 and Table 1). There was only one sex difference in serum insulin (Table 1). At 6 weeks, plasma insulin in males on the AO diet was significantly lower than in females on the same diet. The same difference was seen in HOMA (Figure 5).
There were no significant sex differences observed for urine creatinine, urinary NO, NO/Cr ratio, urinary albumin or Alb/Cr ratio (Table 2).
Effect of Sex on Renal Histopathology
Males showed slight morphological differences from females at age of 6 weeks (Figure 6). Females on both the regular and AO diets were less affected than males at 20 weeks (Figure 6 and Table 3) with males showing more severe alteration than females in both glomerulosclerosis and tubulointerstitial damage.
Effects of AO Diet on Systemic and Metabolic Parameters and Renal Excretory Function
As seen in Table 1, the body weights of males at 13 weeks on the AO diet were significantly lower than those of males on the regular diet. There were no effects of diet on kidney weight. GFR and GFR/g KW (Table 1 and Figure 2) were significantly lower in males at 13 weeks on the regular diet than in males on the AO diet. At 20 weeks females on the AO diet had significantly higher GFRs and GFR/g KW than females on the regular diet.
Blood glucose (Figure 4) was significantly lower at 6 and 13 weeks of age in females on the AO diet compared to the regular diet. There was no effect of diet on serum creatinine (Table 1), MAP, plasma insulin, HOMA or cholesterol (Table 1 and Figures 3 and 5).
At 6 and 20 weeks males on the regular diet had lower urinary creatinine (Table 2) than males on the AO diet. At 13 weeks males on the regular diet had significantly lower urinary NO and NO/Cr ratio (Table 2) than on the AO diet. At 6 weeks of age females on the regular diet had lower urine albumin and Alb/Cr ratio (Table 2) than females on the AO diet and at 13 weeks males on the regular diet had lower urine albumin than males on the AO diet.
Effect of Diet on Renal Histopathology
Females had lower glomerular and tubulointerstitial scores on the AO diet than on the regular diet at 20 weeks (Table 3).
Correlations
When the data are considered as a whole, several interesting correlations are apparent. MAP was positively correlated with blood glucose, insulin and HOMA (p < 0.05). Interestingly, Alb/Cr ratio also correlated positively with insulin and HOMA, while urinary NO showed a negative correlation with insulin and HOMA.
As the animals age and the diabetes progresses, both insulin resistance and blood pressure become more severe. Also as insulin resistance worsens and renal damage becomes more apparent, urinary albumin increases. The negative correlation between insulin resistance and urinary NO may reflect the advancing disruption of NOS activity and decreasing NO bioavailability as hyperglycemia worsens and oxidative imbalance ensues.
Discussion
The obese Zucker rat (fa/fa) as a model of T2DM and the metabolic syndrome (MetS)
The obese Zucker rat has been used as a model of T2DM for almost 40 years. These rats spontaneously develop a type of diabetes with a sequence of events that parallel those hypothesized to result in T2DM in humans (22) including significant weight gain (35), hypertension and hyperglycemia (36). They also develop nephropathy with proteinuria (3) and decreased creatinine clearance (37). By 40 weeks of age, the obese male fa/fa rat exhibits frank proteinuria and severe tubulointerstitial damage (2). We found fasting hyperglycemia at 6 weeks, decreases in GFR by 13 weeks, and abnormalities in renal glomeruli and tubules as early as 20 weeks.
The metabolic syndrome is defined in humans as obesity accompanied by two of the following additional features: hypertrygliceridemia, reduced HDL cholesterol, hypertension and fasting hyperglycemia (38). The fa/fa obese Zucker rat shows elevated basal blood glucose, MAP, and triglycerides and is thus a good model for MetS. We found increases in MAP at 13 and 20 weeks and increased insulin resistance by 13 weeks as well.
Gender Differences in Development of Diabetic Nephropathy
Gender differences in diabetes and diabetic nephropathy have been described, with some studies finding females affected more than males. Albuminuria, which is predictive of development of nephropathy and ESRD, may be more common in female diabetics (8) and female sex has been shown to hasten the decline of renal function in T2DM (9). Diabetic women have an excess cardiovascular disease risk, possibly due to a decrease in estrogen levels that occurs with insulin resistance (10). In addition, in experimental models of renal diseases associated with hypertriglyceridemia, estrogen accelerates progressive renal injury by further elevating triglyceride levels (11) and estrogen has been shown to exacerbate albuminuria and renal pathology in female Zucker rats (12, 13). However other studies have shown a worse prognosis for nephropathy in T2DM in males while others have shown no difference between males and females (14). The role of gender in diabetic nephropathy may be different from that in other forms of chronic renal disease where, overall, men have a higher incidence and prevalence of ESRD (39). Male rats have been shown to be more sensitive to NOS inhibition resulting in proteinuria (40). We found an overall positive correlation between insulin resistance and urine Alb/Cr ratios and a negative correlation between insulin resistance and urinary NO, but no sex difference.
However, sex differences were found in GFR/g KW, MAP, insulin, HOMA, and renal histopathology. The GFR/g KW was lower in males than females at 13 weeks on the regular diet and at 20 weeks on the AO diet. MAP was higher in males on the AO diet than in females on the same diet at 20 weeks. This finding corresponds with better preservation of GFR in the females. In addition at 20 weeks the renal pathologies were worse in males than in females. It has been shown by others that renal dysfunction is associated with increased MAP (41) and hypertension is one factor that aggravates glomerular dysfunction in Wistar fatty rats (21). We also observed a sex difference in insulin levels and HOMA at six weeks with lower values in males. The very low HOMA in males at 6 weeks on the AO diet appears to be a result of the very low insulin levels in this group. In males between 6 and 20 weeks, insulin increased while blood glucose remained fairly stable. In females, insulin remained stable while blood glucose increased. This could imply that insulin secretion is better upregulated in the male Zucker rat than in the female, in response to hyperglycemia. It may also be due to the fact that insulin secretion is already maximal in the females as their insulin levels are uniformly higher in all age groups. The insulin levels determined in our study are consistent with those reported by others (2, 42).
We found sex differences in renal histopathology at 6 and 20 weeks with males being more severely affected. Additional sex differences have been described in the Zucker diabetic rat and several of these have been shown to be modified by gonadectomy or steroid treatment. These include a difference in vascular systemic endothelial and renal microvascular reactivity (36), lower levels of renin activity in females (42), differences in cholecystokinin mediation of food intake (43), differences in levels of hypothalamic neurotransmitters (44) and glucose sensing (45). The mechanistic links between these earlier observations and the present ones remain to be elucidated.
Role of oxidative stress and effect of antioxidants on the development of diabetes and diabetic nephropathy
Oxidative stress contributes to the pathophysiology of T2DM (46) and the development of complications, including nephropathy (47, 48). Diabetes is characterized by both a chronic, growing increase in oxidative stress-favoring stimuli and by a progressive decrease in the efficacy of the antioxidant system (27, 49). Giving a pro-oxidant challenge to the obese male Zucker rat provokes the onset of diabetes (50). This is accompanied by endothelial dysfunction and impaired vascular NO signaling (51). Under hyperglycemic conditions, there is enhanced metabolism of glucose through the sorbitol pathway, with augmented production of superoxide (O2−) radicals. In the presence of O2− radicals, NO is quenched to form peroxynitrite ion (ONOO−), which is a potent oxidant molecule that uncouples iNOS by oxidizing its cofactor, tetrahydrobiopterin. This results in increased levels of superoxide at the expense of NO. Exposure to peroxynitrite during hyperglycemia also produces an uncoupled state of endothelial NOS (eNOS) (52, 53), which causes eNOS to produce O2, further increasing cellular oxidative stress and decreasing NO availability (23). Likewise, nNOS is also uncoupled by reactive oxygen species, including O2− and ONOO− (54). These changes can lead to endothelial dysfunction (55) and local vasoconstriction by unopposed vasoconstrictors including angiotensin II and endothelin-1. The resulting decrease in perfusion can, in turn, lead to cellular damage and renal dysfunction.
Oxidative products also affect mitochondrial function in endothelial cells (48). Hyperglycemia increases the inner mitochondrial membrane proton gradient as a result of overproduction of electron donors by the tricarboxylic acid cycle. This in turn causes increased production of O2− by endothelial cells (23). High glucose-induced reactive oxygen species (ROS) activate signal transduction cascades and transcription factors, leading to upregulation of genes and proteins involved in extracellular matrix remodeling in the kidney (48). In particular, activation of the transcription factor nuclear factor kappa B (NF-κB) has been linked to the development of late diabetic complications. NF-κB enhances NO production (47), which in the presence of superoxide overproduction, favors the formation of peroxynitrite, which again avidly oxidizes tetrahydrobiopterin, this time uncoupling iNOS. Normalizing levels of mitochondrial ROS reduces levels of a number of markers of oxidative stress, including glucose induced activation of protein kinase C, formation of advanced glycation end products, sorbitol accumulation, and activation of NF-κB in endothelial cells.
Given the role of oxidation products in the development of diabetes, dietary antioxidants may prove beneficial in preventing the progression of its complications. It is now clear that a single dietary antioxidant will not be sufficient for prevention of diabetic complications (47, 56, 57). Both vitamins and minerals that are known antioxidants can be added to the diet. We designed an AO fortified diet in consultation with a rodent diet specialist, to avoid any toxicity. Vitamins E and C, β-carotene, zinc, selenium, copper and manganese were added to the diet. Using this combination of antioxidants, we found a protective effect in females on GFR/g KW and renal histopathology at 20 weeks of age and on GFR/g KW in males at 13 weeks of age. There was also improvement in blood glucose in females at 6 and 13 weeks of age, which was no longer the case at 20 weeks. In females, the early improvement in blood glucose may have resulted in the subsequent beneficial effects on renal function and histopathology.
A number of clinical studies have shown that Vitamins C and E increase insulin sensitivity in patients with insulin resistance or T2DM (58). Vitamin E has a major biological role in protecting lipids and other components of cell membranes from oxidation by free radicals. However, it has no effect on other consequences of O2− such as changes in NO bioavailability and increased nitrotyrosine (52). The beneficial effect of Vitamin C or ascorbate, on the other hand, is most likely due to its capacity to scavenge reactive oxygen and reactive nitrogen species, conserving intracellular glutathione and potentiating intracellular NO synthesis (23). Many carotenoids have antioxidant functions, but β-carotene is particularly effective at scavenging singlet oxygen. It is also effective at low partial pressures of oxygen and therefore may be important at the physiological oxygen tensions in tissues (59). A diet supplemented with vitamins C, E and β-carotene has been shown to reverse diabetes-related changes in antioxidant status in kidneys of rats with streptozotocin-induced diabetes (24). A diet supplemented with selenium and vitamin E has been shown to decrease glycemia and delay hyperfiltration and renal lesions in streptozotocin treated rats (60). Parallel reductions in glycemia and renal pathology were seen in this study in female rats given these supplements. A large clinical trial, the HOPE study, showed no benefit of low dose Vitamin E supplementation on the risk of cardiovascular and renal disease in patients with diabetes (61) and a meta-analysis of 19 studies showed that high dose vitamin E actually increased all-cause mortality risk (62). However in the current study, antioxidants were started at a much earlier stage of the disease than is done in most clinical trials and a unique combination of low dose antioxidants was used.
Many enzymes are involved in the reduction of oxidative stress. A number of these enzymes are dependent upon metal ions to catalyze their activity. These include superoxide dismutase (SOD), phospholipid hydroperoxide glutathione peroxidase (PHGPx), and thioredoxin reductase 2 (TrxR2). Within the mitochondria, superoxide anions are converted to hydrogen peroxide (H2O2) by Mn-SOD and later H2O2 is cleared from the mitochondria by PHGPx (63). Changes in these enzymes may contribute to oxidative stress in diabetes (64) and overexpression of these enzymes may reduce oxidative stress damage in renal and cardiovascular systems (65). Cytoplasmic and mitochondrial superoxide dismutases require zinc, copper and manganese to catalyze the removal of superoxide radicals (O2−) produced by the cell (59). PHGPx and TrxR2 are selenium-containing proteins that are involved in the protection against mitochondria-mediated oxidative stress (66). The selenocysteine residues in these enzymes are essential for their activity. Thus, a number of trace metals, including zinc, copper, manganese and selenium, are required for optimal oxidative stress reduction. Inclusion of these trace metals in the AO fortified diet in the present study may have contributed to its beneficial effects.
A number of observations suggest that the pathophysiological process of diabetes and diabetic complications starts long before diabetes is developed and is then further potentiated by increasing glycemia (27). This may explain the mixed results of clinical trials of antioxidants on the progression of micro and macrovascular disease, as dietary intervention is usually initiated well into the disease process. Initiating dietary antioxidants in our rat model as soon after weaning as possible may help explain the observed protective effects on blood glucose, MAP, renal pathology and GFR in females.
In summary, an AO fortified diet may modify the course of T2DM and affect males and females differently. This may be partially due to a gender difference in the production of oxidation products or in the kidney’s response to them. Females on the AO diet had significantly lower blood glucose at 6 and 13 weeks, less severe renal pathology at 20 weeks, and higher GFRs at 20 weeks than age matched females on the regular diet (p < 0.05). Metabolic parameters including blood glucose, insulin resistance and serum cholesterol, and MAP, worsened with age in both males and females, as expected. GFR decreased and renal pathology also became more severe with age. Finally, females on the AO diet had higher GFRs and lower MAP at 20 weeks than males on the same diet. This may denote a protective effect of the AO diet in females, but not in males. These findings may have implications for the role of antioxidants as therapy in humans with T2DM.
Acknowledgments
The authors wish to thank Victor Heh, Ph.D., in the OU-COM Office of Research and Grants for expert assistance with statistical analysis, and R. Balaji, M.S., and K. Coschigano, Ph.D., for critical reading of this manuscript. This work was supported by NIH grant DK073066 to SRI and FVN and by the Diabetes Research Initiative of Ohio University to FVN. Graphical and illustration assistance was provided by the OU-COM Office of Communications.
References
- 1.Moller DE. Nature. 2001;414:821–7. doi: 10.1038/414821a. [DOI] [PubMed] [Google Scholar]
- 2.Coimbra TM, Janssen U, Grone HJ, Ostendorf T, Kunter U, et al. Kidney Int. 2000;57:167–82. doi: 10.1046/j.1523-1755.2000.00836.x. [DOI] [PubMed] [Google Scholar]
- 3.Poirier B, Lannaud-Bournoville M, Conti M, Bazin R, Michel O, et al. Nephrol Dial Transplant. 2000;15:467–76. doi: 10.1093/ndt/15.4.467. [DOI] [PubMed] [Google Scholar]
- 4.Ionescu E, Sauter JF, Jeanrenaud B. Am J Physiol. 1985;248:E500–6. doi: 10.1152/ajpendo.1985.248.5.E500. [DOI] [PubMed] [Google Scholar]
- 5.Kasiske BL, O’Donnell MP, Keane WF. Hypertension. 1992;19:I110–5. doi: 10.1161/01.hyp.19.1_suppl.i110. [DOI] [PubMed] [Google Scholar]
- 6.Myers BD. West J Med. 1986;145:235–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Chander PN, Gealekman O, Brodsky SV, Elitok S, Tojo A, et al. J Am Soc Nephrol. 2004;15:2391–403. doi: 10.1097/01.ASN.0000135971.88164.2C. [DOI] [PubMed] [Google Scholar]
- 8.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, et al. Kidney Int. 2004;65:2309–20. doi: 10.1111/j.1523-1755.2004.00653.x. [DOI] [PubMed] [Google Scholar]
- 9.Garza R, Medina R, Basu S, Pugh JA. Am J Nephrol. 1997;17:59–67. doi: 10.1159/000169073. [DOI] [PubMed] [Google Scholar]
- 10.Resnick HE, Howard BV. Annu Rev Med. 2002;53:245–67. doi: 10.1146/annurev.med.53.082901.103904. [DOI] [PubMed] [Google Scholar]
- 11.Neugarten J. J Am Soc Nephrol. 2002;13:2807–9. doi: 10.1097/01.asn.0000035846.89753.d4. [DOI] [PubMed] [Google Scholar]
- 12.Gades MD, Stern JS, van Goor H, Nguyen D, Johnson PR, Kaysen GA. Kidney Int. 1998;53:130–5. doi: 10.1046/j.1523-1755.1998.00746.x. [DOI] [PubMed] [Google Scholar]
- 13.Stevenson FT, Wheeldon CM, Gades MD, Kaysen GA, Stern JS, van Goor H. Kidney Int. 2000;57:1927–35. doi: 10.1046/j.1523-1755.2000.00042.x. [DOI] [PubMed] [Google Scholar]
- 14.Silbiger S, Neugarten J. Gend Med. 2008;5(Suppl A):S3–S10. doi: 10.1016/j.genm.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Vague J. Am J Clin Nutr. 1956;4:20–34. doi: 10.1093/ajcn/4.1.20. [DOI] [PubMed] [Google Scholar]
- 16.ERA. European Transplant and Dialysis Annual Report. 2005. [Google Scholar]
- 17.Asghar M, Monjok E, Kouamou G, Ohia SE, Bagchi D, Lokhandwala MF. Mol Cell Biochem. 2007;304:93–9. doi: 10.1007/s11010-007-9489-3. [DOI] [PubMed] [Google Scholar]
- 18.Prabhakar S, Starnes J, Shi S, Lonis B, Tran R. J Am Soc Nephrol. 2007;18:2945–52. doi: 10.1681/ASN.2006080895. [DOI] [PubMed] [Google Scholar]
- 19.Kaneki M, Shimizu N, Yamada D, Chang K. Antioxid Redox Signal. 2007;9:319–29. doi: 10.1089/ars.2006.1464. [DOI] [PubMed] [Google Scholar]
- 20.Domenico R. Curr Pharm Des. 2004;10:1667–76. doi: 10.2174/1381612043384709. [DOI] [PubMed] [Google Scholar]
- 21.Tomohiro T, Kumai T, Sato T, Takeba Y, Kobayashi S, Kimura K. Life Sci. 2007;80:1364–72. doi: 10.1016/j.lfs.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 22.Opara EC. J Investig Med. 2004;52:19–23. doi: 10.1136/jim-52-01-22. [DOI] [PubMed] [Google Scholar]
- 23.Vega-Lopez S, Devaraj S, Jialal I. J Investig Med. 2004;52:24–32. doi: 10.1136/jim-52-01-23. [DOI] [PubMed] [Google Scholar]
- 24.Mekinova D, Chorvathova V, Volkovova K, Staruchova M, Grancicova E, Klvanova J, Ondreicka R. Die Nahrung. 1995;39:257–61. doi: 10.1002/food.19950390402. [DOI] [PubMed] [Google Scholar]
- 25.Koo JR, Vaziri ND. Kidney Int. 2003;63:195–201. doi: 10.1046/j.1523-1755.2003.00728.x. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton SJ, Chew GT, Watts GF. Diab Vasc Dis Res. 2007;4:89–102. doi: 10.3132/dvdr.2007.026. [DOI] [PubMed] [Google Scholar]
- 27.Wiernsperger NF. Biofactors. 2003;19:11–8. doi: 10.1002/biof.5520190103. [DOI] [PubMed] [Google Scholar]
- 28.Phillips MS, Liu Q, Hammond HA, Dugan V, Hey PJ, et al. Nat Genet. 1996;13:18–9. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- 29.Levine DZ, Burns KD, Jaffey J, Iacovitti M. Kidney Int. 2004;65:184–9. doi: 10.1111/j.1523-1755.2004.00361.x. [DOI] [PubMed] [Google Scholar]
- 30.Badzynska B, Grzelec-Mojzesowicz M, Sadowski J. Acta Physiol Scand. 2004;182:313–8. doi: 10.1111/j.1365-201X.2004.01364.x. [DOI] [PubMed] [Google Scholar]
- 31.Moriwaki Y, Inokuchi T, Yamamoto A, Ka T, Tsutsumi Z, et al. Acta Diabetol. 2007;44:215–8. doi: 10.1007/s00592-007-0007-6. [DOI] [PubMed] [Google Scholar]
- 32.Li Z, Rodriguez-Iturbe B, Ni Z, Shahkarami A, Sepassi L, Vaziri ND. Kidney Int. 2005;68:2766–72. doi: 10.1111/j.1523-1755.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- 33.Pichler RH, Franceschini N, Young BA, Hugo C, Andoh TF, et al. J Am Soc Nephrol. 1995;6:1186–96. doi: 10.1681/ASN.V641186. [DOI] [PubMed] [Google Scholar]
- 34.Zar JH. Biostatistical Analysis. Prentice Hall; Englewood Cliffs, NJ: 1984. [Google Scholar]
- 35.Bray GA. Fed Proc. 1977;36:148–53. [PubMed] [Google Scholar]
- 36.Ajayi AA, Ogungbade GO, Okorodudu AO. Eur J Clin Invest. 2004;34:349–57. doi: 10.1111/j.1365-2362.2004.01339.x. [DOI] [PubMed] [Google Scholar]
- 37.Kasiske BL, Cleary MP, O’Donnell MP, Keane WF. J Lab Clin Med. 1985;106:598–604. [PubMed] [Google Scholar]
- 38.Alberti KG, Zimmet P, Shaw J. Diabet Med. 2006;23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 39.Collins AJ, Kasiske BL, Herzog C, et al. Am J Kidney Disease. 2007;49:S1–S296. doi: 10.1053/j.ajkd.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 40.Verhagen AM, Attia DM, Koomans HA, Joles JA. Am J Physiol Renal Physiol. 2000;279:F664–70. doi: 10.1152/ajprenal.2000.279.4.F664. [DOI] [PubMed] [Google Scholar]
- 41.Imai G, Satoh T, Kumai T, Murao M, Tsuchida H, et al. Hypertens Res. 2003;26:339–47. doi: 10.1291/hypres.26.339. [DOI] [PubMed] [Google Scholar]
- 42.Riazi S, Madala-Halagappa VK, Hu X, Ecelbarger CA. Gend Med. 2006;3:309–27. doi: 10.1016/s1550-8579(06)80219-6. [DOI] [PubMed] [Google Scholar]
- 43.Strohmayer AJ, Greenberg D. Physiol Behav. 1996;60:273–5. doi: 10.1016/0031-9384(96)83164-7. [DOI] [PubMed] [Google Scholar]
- 44.Svec F, Thompson H, Corll C, Porter J. Nutr Neurosci. 2002;5:321–6. doi: 10.1080/1028415021000033785. [DOI] [PubMed] [Google Scholar]
- 45.Bogacka I, Roane DS, Xi X, Zhou J, Li B, et al. Nutr Neurosci. 2004;7:67–74. doi: 10.1080/10284150410001710401. [DOI] [PubMed] [Google Scholar]
- 46.Mooradian A, Nowak F. In: Cardiovascular Disease in Older People. Kaiser F, Morley J, Coe R, editors. Springer Publishing Co., Inc; New York, NY: 1997. pp. 53–72. [Google Scholar]
- 47.Maritim AC, Sanders RA, Watkins JB., 3rd J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 48.Lee HB, Yu MR, Yang Y, Jiang Z, Ha H. J Am Soc Nephrol. 2003;14:S241–5. doi: 10.1097/01.asn.0000077410.66390.0f. [DOI] [PubMed] [Google Scholar]
- 49.Satoh M, Fujimoto S, Haruna Y, Arakawa S, Horike H, et al. Am J Physiol Renal Physiol. 2005;288:F1144–52. doi: 10.1152/ajprenal.00221.2004. [DOI] [PubMed] [Google Scholar]
- 50.Laight DW, Desai KM, Gopaul NK, Anggard EE, Carrier MJ. Br J Pharmacol. 1999;128:269–71. doi: 10.1038/sj.bjp.0702801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laight DW, Desai KM, Anggard EE, Carrier MJ. Eur J Pharmacol. 2000;402:95–9. doi: 10.1016/s0014-2999(00)00451-9. [DOI] [PubMed] [Google Scholar]
- 52.Ceriello A. Diabetes Care. 2003;26:1589–96. doi: 10.2337/diacare.26.5.1589. [DOI] [PubMed] [Google Scholar]
- 53.Xu J, Xie Z, Reece R, Pimental D, Zou MH. Arterioscler Thromb Vasc Biol. 2006;26:2688–95. doi: 10.1161/01.ATV.0000249394.94588.82. [DOI] [PubMed] [Google Scholar]
- 54.Sun J, Druhan LJ, Zweier JL. Arch Biochem Biophys. 2008;471:126–33. doi: 10.1016/j.abb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thum T, Fraccarollo D, Schultheiss M, Froese S, Galuppo P, et al. Diabetes. 2007;56:666–74. doi: 10.2337/db06-0699. [DOI] [PubMed] [Google Scholar]
- 56.Blakely S, Herbert A, Collins M, Jenkins M, Mitchell G, et al. J Nutr. 2003;133:2838–44. doi: 10.1093/jn/133.9.2838. [DOI] [PubMed] [Google Scholar]
- 57.Faure P. Clin Chem Lab Med. 2003;41:995–8. doi: 10.1515/CCLM.2003.152. [DOI] [PubMed] [Google Scholar]
- 58.Evans JL. Indian J Med Res. 2007;125:355–72. [PubMed] [Google Scholar]
- 59.Garrow J, James W, Ralph A. Human Nutrition and Dietetics. Churchill Livingstone; New York, NY: 2002. [Google Scholar]
- 60.Douillet C, Tabib A, Bost M, Accominotti M, Borson-Chazot F, Ciavatti M. Proc Soc Exp Biol Med. 1996;211:323–31. doi: 10.3181/00379727-211-43976. [DOI] [PubMed] [Google Scholar]
- 61.Brownlee M. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 62.Yim S, Malhotra A, Veves A. Curr Diab Rep. 2007;7:8–13. doi: 10.1007/s11892-007-0003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lastra G, Manrique C. Antioxid Redox Signal. 2007;9:943–54. doi: 10.1089/ars.2007.1615. [DOI] [PubMed] [Google Scholar]
- 64.Tan AL, Forbes JM, Cooper ME. Semin Nephrol. 2007;27:130–43. doi: 10.1016/j.semnephrol.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 65.Lee HB, Seo JY, Yu MR, Uh ST, Ha H. Kidney Int Suppl. 2007;72:S67–70. doi: 10.1038/sj.ki.5002389. [DOI] [PubMed] [Google Scholar]
- 66.Behne D, Kyriakopoulos A. Annu Rev Nutr. 2001;21:453–73. doi: 10.1146/annurev.nutr.21.1.453. [DOI] [PubMed] [Google Scholar]