Abstract
Background
Ezetimibe inhibits intestinal absorption of cholesterol.
Methods
Multicentered double-blind, randomized, placebo-controlled, crossover study to determine the short-term safety, efficacy, and tolerability of ezetimibe in combination with ongoing statin therapy in HIV-infected adults with elevated low-density lipoprotein cholesterol (LDL-C). Participants on stable HAART with fasting LDL-C at least 130 mg/dl and stable statin were randomized to ezetimibe 10 mg daily or placebo for 12 weeks followed by 4 weeks of washout and then 12 weeks with alternative study assignment. Percentage and absolute change in LDL-C (primary endpoint), total cholesterol, triglyceride, high-density lipoprotein cholesterol (HDL-C), apolipoprotein B, and high sensitivity C-reactive protein were compared. Changes in clinical symptoms and safety laboratory measurements were assessed.
Results
Forty-four participants enrolled: 70% men, median age 49 years, 43% White/Non-Hispanic, median CD4 cell count 547 cells/μl, and 95% HIV RNA less than 50 copies/ml. Median (interquartile range) percentage change in LDL-C was −20.8% (−25.4, −10.7) with ezetimibe and −0.7% (−10.3,18.6) with placebo; the median within-participant effect of ezetimibe was −14.1% (−33.0, −5.0; P < 0.0001). Median difference in absolute LDL-C values between ezetimibe and placebo was −32 mg/dl (−58, −6, P < 0.0001). Significant differences in within-participant effect of ezetimibe were noted for total cholesterol −18.60% (−27.22, −11.67, P < 0.001), non-HDL-C −23.18% (−33.14, −14.36, P < 0.0001), and apolipoprotein B −8.73% (−18.75, 1.99, P = 0.02). No significant changes seen in HDL-C, triglyceride, or high sensitivity C-reactive protein. Ezetimibe was well tolerated. Adverse events were similar between phases.
Conclusion
The present short-term study found adding ezetimibe to ongoing statin therapy was well tolerated and effective in reducing LDL-C, total cholesterol, non-HDL-C, and apolipoprotein B. Adding ezetimibe to statin therapy offers reasonable treatment option for HIV-infected patients with elevated LDL-C.
Keywords: ACTG A5209 study, elevated low-density lipoprotein cholesterol, ezetimibe, ongoing statin therapy
Introduction
Abnormalities of lipid metabolism are common complications of HIV disease and HIV therapy. Since the availability of potent antiretroviral therapy (ART), elevations in triglycerides, low-density lipoprotein cholesterol (LDL-C), and total cholesterol are commonly seen in practice, particularly with the use of protease inhibitors. The AIDS Clinical Trials Group study A5087 found that monotherapy with either pravastatin or fenofibrate for HIV-related dyslipidemia was safe but unlikely to achieve the composite National Cholesterol Educational Program Adult Treatment Panel III (NCEP ATP III) goal [1,2]. The subset of patients in the A5087 trial who went on to receive dual therapy with pravastatin and fenofibrate also infrequently achieved optimal lipid levels, suggesting that other adjunctive lipid-lowering agents are worthy of study in this population [1].
Ezetimibe, an inhibitor of Niemann-Pick C1 Like 1 protein, is a lipid-controlling agent that inhibits the intestinal absorption of dietary and biliary cholesterol [3]. Data from prospective studies on HIV-seronegative individuals showed ezetimibe monotherapy reduced LDL-C by 17–20% compared with placebo [4]. Triglyceride and apolipoprotein B (Apo B) levels were also significantly reduced by 5 and 15%, respectively, and high-density lipoprotein cholesterol (HDL-C) was increased by 2–3% [4,5]. Ezetimibe has the greatest effect when added to statin therapy. Several prospective double-blind, randomized, placebo-controlled studies were completed on the efficacy and safety of ezetimibe added to ongoing statin therapy in individuals with primary hypercholesterolemia [6-9]. Significant reductions in LDL-C ranged from 25 to 60% depending on the baseline dose of statin therapy. Triglyceride levels were reduced by 19–30% compared with statin monotherapy.
Four prospective studies [10-14] have assessed the safety and efficacy of ezetimibe in HIV-infected individuals. Although three of these studies showed that ezetimibe was well tolerated and effective in reducing LDL-C either as monotherapy or in combination with statin therapy, all of these studies were nonrandomized and open labeled. Only the fourth study by Wohl et al. [10] reported a randomized, placebo-controlled, crossover study of ezetimibe monotherapy. Although the study showed that ezetimibe was well tolerated and effective, monotherapy does not reflect the typical clinical use of ezetimibe where it is used in combination with statins. We hypothesized that the addition of ezetimibe to an existing statin regimen would improve the LDL-C response without additional toxicity.
We report the first multicentered double-blind, randomized, placebo-controlled, crossover study designed to determine the short-term safety, efficacy, and tolerability of the addition of ezetimibe therapy in HIV-infected adults with suboptimal LDL-C response to ongoing statin therapy.
Methods
The primary objective of this study was to evaluate the change in directly measured LDL-C after the addition of ezetimibe to a stable background of HAART and statin therapy for 12 weeks compared with the change in LDL-C after 12 weeks of adding placebo. The short-term safety and tolerability of ezetimibe added to a background of stable ART and statin therapy were also assessed. Secondary objectives included evaluation of changes in other lipid parameters such as nonhigh-density lipoprotein cholesterol (non-HDL-C), HDL-C, and triglyceride, and surrogate cardiovascular markers such as Apo B and high sensitivity C-reactive protein (hsCRP).
Participants were HIV-1-infected individuals with directly measured (by ultracentrifugation) LDL-C of at least 130 mg/dl within 30 days of entry. In order to be included, patients must have been maintained on a stable dose of selected statins (pravastatin, atorvastatin, or fluvastatin) and on a stable ART regimen for the past 3 months. A maximal statin dose was not specified to allow for inclusion of participants whose providers had maximized statin therapy for each participant in the context of a background of stable HAART and other concomitant medications received by persons with HIV. Participants were, however, required to remain on both stable statin and stable HAART regimens throughout the study. Patients were excluded if they had previously taken ezetimibe and if they were on any other lipid lowering medications in addition to statin therapy. Other exclusion criteria included diabetes mellitus, history of coronary heart disease or congestive heart disease, and use of anabolic steroids. The study was approved by the institutional review committees of each clinical site and all participants signed an informed consent prior to enrollment.
Participants were randomized to either one of two arms. Arm A consisted of giving ezetimibe 10 mg orally once daily for 12 weeks, undergoing a washout period of 4 weeks, and then receiving placebo orally once daily for 12 weeks. Arm B consisted of giving placebo orally once daily for 12 weeks, undergoing a washout period of 4 weeks, and then receiving ezetimibe 10 mg orally once daily for 12 weeks. Participants were asked to fast at least 8 h prior to blood sampling for lipid determinations. Fasting blood samples were obtained prior to, 4 weeks into, and at the end of each ezetimibe and placebo intervention. Changes in lipid metabolism, Apo B, and hsCRP were assessed. Specifically, total cholesterol, LDL-C, HDL-C, and triglyceride were measured by ultracentrifugation and Apo B and hsCRP levels were measured on batched samples at a central laboratory (Quest Diagnostics, Baltimore, Maryland, USA). Safety laboratory parameters, including complete blood count, serum chemistries, CD4 cell counts, and HIV-1 RNA copies were obtained throughout the study. All participants were advised to maintain their usual diet and exercise throughout the study.
The primary analysis of efficacy was performed by per protocol analysis, limited to participants who had data for baseline and week 12 lipid levels for both periods. A Wilcoxon signed-rank test was used to assess the within-participant differences between the changes over 12 weeks in a given lipid parameter on ezetimibe minus the changes over 12 weeks on placebo. This approach assumed no period or carryover effect. Mixed effects models (MEMs) were used to check for period and carryover effects assuming normality assumption was acceptable. If the MEM demonstrated period or carryover effects, P values were based on two-sample Wilcoxon tests of period 1 only and estimates of the difference between the medians and interquartile range (IQR) were made using the Hodges-Lehmann method [15]. Safety was evaluated by summarizing the nature and rate of adverse events (signs and symptoms and laboratory values) within each arm. Using a per protocol analysis, it was estimated that 31 participants would have a 90% power to detect a 12% decrease in LDL-C with ezetimibe compared with placebo at α value equal to 0.05 (one-sided), assuming that there is no correlation between the two sets of changes. The study planned to recruit 43 participants in total to account for a 15% dropout rate or missing data for each period separately.
Results
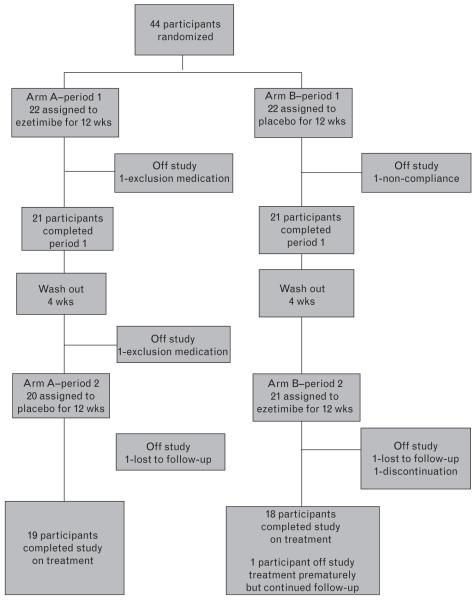
Forty-four participants were enrolled between December 2005 and October 2006 from 18 centers across the United States. Figure 1 shows the flow of participants from study entry to study completion at 28 weeks. Among those enrolled to the study, 37 participants completed the study on treatment and 33 completed the study with all visit evaluations; six participants went off study prematurely and one additional participant went off study treatment prematurely but continued in follow-up. None of the six discontinued the study prematurely due to study drug toxicities. The dropout rate was 13.6%. Among the six participants who were off study prematurely, two were driven by protocol requirements (one due to increase in dosage of pravastatin at week 12 and one discontinued the study treatment at week 16 due to a significant change in ART regimen); one participant discontinued the study at week 20 due to severe debilitation with depression and other confounding medical conditions that were unrelated to the study treatment; one participant was noncompliant with study visit and medications at week 10; two participants were lost to follow-up, one at week 20 and one just prior to week 28. Table 1 displays the summary of selected demographics and baseline clinical and laboratory measures of the 44 participants along with the subset of 33 participants who completed the study with all study evaluations.
Fig. 1. Flow of participants from study entry to completion of the final follow-up assessment.
Table 1. Baseline characteristics of study participantsa.
| Variable | All participants | Participants with complete follow-up data |
|---|---|---|
| n | 44 | 33 |
| Age, years median | 49 (45, 55) | 49 (45, 56) |
| BMI (kg/m2) | 26.14 (23.12, 29.41) | 26.80 (24.06, 29.70) |
| Sex | ||
| Male, n (%) | 31 (70%) | 23 (70%) |
| Female, n (%) | 13 (30%) | 10 (30%) |
| Race/ethnicity | ||
| White, n (%) | 19 (43%) | 14 (42%) |
| Black, n (%) | 18 (41%) | 15 (45%) |
| Hispanic, n (%) | 5 (11%) | 3 (9%) |
| Other, n (%) | 2 (5%) | 1 (3%) |
| CD4 cell count (cells/ml) | 547 (434, 781) | 533 (443, 756) |
| HIV RNA below 50 copies/ml, n (%) | 42 (95%) | 31 (94%) |
| Lipid profile | ||
| Cholesterol, total (mg/dl) | 244 (218, 272) | 243 (222, 267) |
| LDL-C (mg/dl) | 160 (134, 181) | 161 (138, 183) |
| HDL-C (mg/dl) | 49 (42, 56) | 49 (41, 56) |
| Non-HDL-C (mg/dl) | 197 (170, 222) | 197 (172, 218) |
| Triglyceride (mg/dl) | 158 (116, 201) | 168 (116, 203) |
| Apo B (mg/dl) | 118 (108, 130) | 120 (110, 131) |
| hsCRP (mg/dl) | 4.45 (1.15, 8.75) | 4.40 (1.30, 8.50) |
| Statin use, n (%) | ||
| Pravastatin | 22 (50%) | 15 (45%) |
| Atorvastatin | 20 (45%) | 16 (49%) |
| Current antiretroviral medication use | ||
| NRTI, n (%) | 37 (84%) | 27 (82%) |
| NNRTI, n (%) | 29 (66%) | 20 (61%) |
| PI, n (%) | 27 (61%) | 22 (67%) |
Baseline demographic, clinical and laboratory measures are shown for all 44 participants along with the subset of 33 participants who completed the study with all study evaluations. Apo B, apolipoprotein B; HDL-C, high-density lipoprotein cholesterol; hsCRP, high sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Continuous variables are presented as median (1st, 3rd quartile).
The median (IQR) duration of continuous statin use prior to study entry was 15.5 (7.4, 32.9) months. Twenty-seven participants were taking protease inhibitors, the most common being lopinavir/ritonavir (n = 13) and atazanavir (n = 7). The primary analysis of efficacy was performed by per protocol analysis on the 33 participants who had complete data for the entire 28-week study. The baseline characteristics of the 11 participants not evaluated in primary analysis (due to missing of at least one evaluation) were similar to those of the 33 participants. Secondary analysis with mixed model analysis of variance used data from all 44 participants.
Table 2 presents the median and IQR of the within-participant effects for ezetimibe and placebo and the within-participant difference between the two. This is provided for the percentage change from baseline and the change in absolute value (mg/dl) over the 12 weeks on a regimen. When the MEM demonstrated period or carryover effects, only between-arm results for participants evaluable for the first 12-week period of the study were analyzed.
Table 2. Median (interquartile range) percentage change and change in absolute value by treatment and between treatments.
| Ezetimibe | Placebo | Ezetimibe effect | P | ||
|---|---|---|---|---|---|
| LDL-C | % Change, median (IQR) | −20.79 (−25.36, −10.67) | −0.72 (−10.26, 18.63) | −14.11 (−33.01, −5.00) | <0.0001 |
| Change (mg/dl), median (IQR) | −33 (−46, −19) | −1 (−20, 29) | −32 (−58, −6) | <0.0001 | |
| Non-HDL-C | % Change, median (IQR)a | −22.38 (−32.17, −15.83) | 0.59 (−6.25, 4.19) | −23.18 (−33.14, −14.36) | <0.0001 |
| Change (mg/dl), median (IQR) | −41 (−54, −21) | 3 (−19, 13) | −37 (−58, −20) | <0.0001 | |
| TC | % Change, median (IQR)a | −17.57 (−27.06, −13.65) | −1.05 (−3.62, 3.73) | −18.60 (−27.22, −11.67) | <0.0001 |
| Change (mg/dl), median (IQR) | −35 (−57, −23) | −1 (−17, 11) | −38 (−58, −22) | <0.0001 | |
| HDL-C | % Change, median (IQR) | 0 (−9.76, 12.77) | −2.50 (−13.33, 4.26) | 2.38(−5.10, +14.29) | 0.23 |
| Change (mg/dl), median (IQR) | 0 (−5, 5) | −1 (−7, 2) | 0 (−3, 5) | 0.33 | |
| TG | % Change, median (IQR) | −8.42 (−25.14, 3.68) | 0.81 (−21.90, 15.19) | −1.14 (−31.93, 10.16) | 0.26 |
| Change (mg/dl), median (IQR) | −8 (−63, 6) | 1 (−35, 19) | −8 (−43, 18) | 0.23 | |
| Apo B | % Change, median (IQR)a | −12.39 (−18.98, −4.05) | −4.40 (−10.65, 3.81) | −8.73 (−18.75, 1.99) | 0.02 |
| Change (mg/dl), median (IQR) | −12 (−26, −1) | 0 (−10, 4) | −7 (−22, 0) | 0.004 | |
| hsCRP | % Change, median (IQR)a | 0 (−41.83, 25.00) | 0 (−33.33, 34.72) | −2.88 (−50.00, 27.47) | 0.35 |
| Change (mg/dl), median (IQR) | 0 (−1.90, 0.40) | 0.15 (−1.20, 0.40) | −0.10 (−3.25, 2.55) | 0.70 |
Apo B, apolipoprotein B; HDL-C, high-density lipoprotein cholesterol; hsCRP, high sensitivity C-reactive protein; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride.
Measures with period or carryover effects confirmed with mixed effects models present data from period 1 (week 0–12) only, comparing results between N = 21 participants who received ezetimibe with N = 20 participants who received placebo. P values are based on two-sample Wilcoxon rank sum tests. The difference in medians is estimated using the method of Hodges-Lehmann.
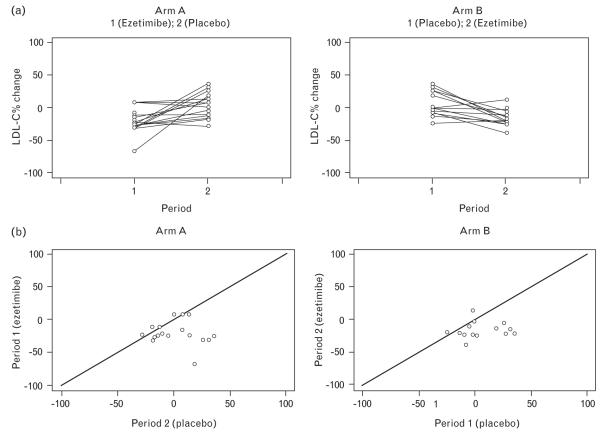
The median within-participant difference between the LDL-C percentage change from baseline with ezetimibe minus the percentage change on placebo is −14.11% (IQR: −33.01%, −5.00%), Wilcoxon P value less than 0.0001. The median difference in absolute change in LDL-C values between ezetimibe and placebo is −32 mg/dl (IQR: −58, −6, P < 0.0001). As noted previously, MEMs provide a formal test of whether there was a period or carryover effect. The models indicated no evidence of period or carryover effects for LDL-C expressed as percentage change or absolute value. Figure 2 demonstrates the results graphically for percentage change from period baseline in LDL-C. Within-participant percentage changes over the first and second treatment period are shown for the two arms in the first row of Fig. 2. The second row of Fig. 2 shows the pattern of percentage change in LDL-C over the ezetimibe period vs. the placebo period within each participant by arm. The diagonal lines indicate equality between the percentage change on ezetimibe and placebo within a participant; points below the lines favor ezetimibe. The points from the two arms are almost identically distributed, demonstrating the absence of period or carryover effects confirmed by the mixed effects modeling.
Fig. 2. Low-density lipoprotein cholesterol percentage change from period baseline.
(a) Profile plots of the within-participant percentage change in low-density lipoprotein cholesterol (LDL-C) over the first and second treatment periods for the two arms. (b) Pattern of percentage change in LDL-C over the ezetimibe period vs. the placebo period within each participant by arm.
Except for total cholesterol, non-HDL-C, and Apo B, the descriptive plots of the lipid parameters were similar to the one presented for LDL-C. Using linear mixed models, it was found that there was evidence of a period effect for total cholesterol, non-HDL-C, and Apo B. Therefore, results for these parameters are based on the first period only. Similar to the changes in LDL-C, the median of the difference in percentage change for total cholesterol, non-HDL-C, and Apo B showed significant reductions as shown in Table 2. By adding ezetimibe to ongoing statin therapy, there were reductions of 18.60% in total cholesterol, 23.18% in non-HDL-C, and 8.73% in Apo B. There were no significant changes in HDL-C, triglyceride, or hsCRP.
Perfect adherence (never missed any study treatment) was reported as 70, 63, 58, and 57% of all participants at weeks 4, 12, 20, and 28, respectively. However, 81, 91, 91, and 92%, respectively, reported not missing a dose ‘within the last week’ at these same time points. Ezetimibe was well tolerated in the study participants. None of the participants discontinued therapy due to protocol-defined toxicities. Adverse events were not significantly increased during the ezetimibe phase compared with placebo. The highest grade of sign, symptom, and/or laboratory toxicity was a grade 3. Five of the 44 participants (11.4%) experienced grade 3 toxicities, including fever (one), decreased absolute neutrophil count (one), increased total bilirubin (two), nausea and vomiting (one). The most common toxicities were ache/pain/discomfort (eight participants with grade 2 toxicity, 18.2%) and gastrointestinal symptoms such as nausea, diarrhea, and distention (five participants with grade 2 toxicity, 11.4%). There were no reports of grade 3 or higher liver enzyme elevations. None of the participants had a serious drug-related adverse event.
There were no significant changes in CD4 cell count between the arms during the two periods. The median (IQR) CD4 cell count was 575 (435, 744) cells/μl after period 1 and 574 (429, 753) cells/μl after period 2. The percentage of individuals with undetectable HIV RNA copies were 93% after period 1 and 91% after period 2. Overall, there were no detrimental effects of ezetimibe on immunologic status or virologic suppression.
Discussion
The present randomized, placebo-controlled, crossover study showed that ezetimibe significantly lowered LDL-C along with non-HDL-C, total cholesterol, and Apo B in HIV-infected participants on stable statin therapy. Our data are in agreement with previous studies from the general population that showed ezetimibe reduced LDL-C, non-HDL-C, total cholesterol, and Apo B when added to statin therapy. The magnitude of change in LDL-C in our study is similar to that seen in open label studies on HIV-infected patients by Negredo et al. [11] and Berg-Wolf et al. [12] in which ezetimibe was administered with low-dose statin therapy reporting 12 and 18% decreases in LDL-C, respectively. Bennett et al. [13] reported a greater reduction in LDL-C of 35% in a retrospective study, but ezetimibe was added to maximally tolerated lipid-lowering therapy that included lipid-lowering medications in addition to statins. Interestingly, the two ezetimibe monotherapy studies on HIV-infected patients showed LDL-C reductions of 11 and 20%, which were similar in magnitude to the changes seen here combining ezetimibe with ongoing statin therapy. Overall, the magnitude of LDL-C changes from all the studies involving HIV-infected patients was not as robust as in the non-HIV-infected population in whom LDL-C was found to be reduced by 25–60% [9,16-18], suggesting that LDL-C may be more difficult to control in the HIV-infected population.
The reported effects of ezetimibe on other lipid measures and hsCRP have been variable. Our study results showed a decrease in total cholesterol similar to the 10–32% reduction cited in the literature [12-14]. We did not see the significant changes in triglyceride and HDL that have been reported by other investigators. HDL-C subclasses were not measured. The large reductions in Apo B reported in non-HIV studies were not seen in our results [4]. This may have been tempered by the short exposure to ezetimibe and the chronic inflammatory state induced by HIV. Of note, at least 50% of participants had a baseline hsCRP over 3 mg/dl. Our study, which is similar to the other ezetimibe studies involving HIV-infected patients, did not show changes in hsCRP in response to lipid-lowering therapy.
Ezetimibe was found to be well tolerated with a clinical safety profile similar to that of placebo. It does not affect absorption of bile acids, fatty acids, or fat-soluble vitamins; its effects are specific to cholesterol and related steroids. An advantage is that it does not inhibit or induce cytochrome P450, including CYP 3A4 or the N-acetyltransferase enzyme systems. Consequently, there is a very low likelihood of drug interactions between ezetimibe and other drugs that are CYP3A4 substrates/inhibitors, including protease inhibitors.
Ezetimibe is well tolerated and effective in controlling dyslipidemia. However, its long-term use has recently been debated. The Effect of Combination Ezetimibe and High-Dose Simvastatin vs. Simvastatin Alone on the Atherosclerotic Process in Patients with Heterozygous Familial Hypercholesterolemia (ENHANCE) study reported that combined therapy with ezetimibe and simvastatin did not result in a significant difference in changes in carotid intima-media thickness, as compared with simvastatin alone, despite decreases in levels of LDL-C and CRP [19]. It must be noted that the ENHANCE study did not have primary clinical endpoints, but rather focused on carotid intima-media thickness, a surrogate marker of atherosclerosis. Changes in carotid intima-media thickness did not differ between individuals receiving statin medication alone and those receiving a statin along with ezetimibe. However, 80% of the patients had already been treated with statins with minimal baseline carotid intima-media thickness, which may have precluded finding differential carotid intima-media thickness changes in just 2 years. Carotid intima-media thickness was improved in individuals on aggressive LDL-C treatment (target LDL-C ≤ 70 mg/dl), many of whom required ezetimibe to achieve the LDL-C goals [20].
Hence, some investigators have suggested the use of ezetimibe in HIV patients as the combination of ezetemibe with any statin is less likely to produce significant drug interactions with HIV therapy than having to use very high-dose statins alone to achieve the same lipid goal [21].
Data on the effect of ezetimibe on clinical cardiovascular outcomes are limited. The Intensive Lipid Lowering with Simvastatin and Ezetimibe in Aortic Stenosis (SEAS) trial did show a small reduction in the incidence of ischemic cardiovascular events in patients with aortic stenosis given simvastatin and ezetimibe, but there was no difference in the primary outcome of combined aortic-valve and ischemic events [22]. Further studies are warranted to determine the clinical cardiovascular outcomes of ezetimibe in the HIV-infected population.
The present study adds to the body of information known about ezetimibe. The strengths of the present study include being an adequately powered prospective, randomized, placebo-controlled, multicenter crossover trial that determined the efficacy and short-term safety of ezetimibe with ongoing statin therapy. In addition, the LDL-C was measured directly using ultracentrifugation of fasting samples, rather than by calculation using the Friedewald formula, which is only valid if the fasting triglyceride level is less than 400 mg/dl.
There are several limitations to this study; primarily that the course of ezetimibe was short and the dose of statin was not specified. The requirement for stable statin therapy was intended to provide a setting close to clinical practice, wherein providers had individualized statin dosing on a background of stable HAART and in the face of other medications required by persons living with HIV. Despite this variation, additional LDL-C lowering was achieved in participants when ezetimibe was added to their stable statin therapy. Drug-drug interactions with concurrent protease inhibitors may have played a role in the dosing of the statins. The unfixed statin dose may have contributed to a lower change in lipid parameters and hsCRP. This study was not designed to evaluate the long-term adverse effects of ezetimibe, such as neoplastic disease potential.
Conclusion
The present short-term study found adding ezetimibe to ongoing statin therapy was well tolerated and effective in reducing LDL-C, total cholesterol, non-HDL-C, and Apo B. Addition of ezetimibe to statin therapy offers a reasonable treatment option in lipid management of HIV-infected patients with elevated LDL-C.
Acknowledgements
We thank the study participants for generously donating their time and effort. We also acknowledge the contributions of the following past and current members of the A5209 team: Courtney Ashton (Frontier Science and Technology Research); Richard Brundage, (University of Minnesota); Susan Fiscus (Professor/Director of the Retrovirology Core Laboratory); Marilyn Foutes (Social & Scientific Systems, Inc.); Linda Naini (Social & Scientific Systems, Inc.); Lauren Queen (Social & Scientific Systems, Inc.); Mapedro Willoughby (Social & Scientific Systems, Inc.); W. Keith Henry (University of Minnesota); Pualani Kondo (University of Hawaii at Manoa); Linda Millar (Frontier Science & Technology Research); Robert A. Parker (Harvard School of Public Health); Joseph Robinson (CCG representative); Paul Tran.(NIH/NIAID/DAIDS); Heather Vezina (University of Minnesota Medical School)
The present work was supported by the AIDS Clinical Trials Group, which is funded by the National Institute of Allergy and Infectious Diseases (AI68636) and SDMC (AI68634). The following ACTG sites participated in A5209:
Carl J. Fichtenbaum, MD and Carol F. Hyc, RN - University of Cincinnati (Site 2401) CTU Grant #AI69513.
Julie Hoffman, RN and Dee Dee Pacheco - UCSD (701) CTU Grant #AI 69432.
Judith A. Aberg, MD and Karen Cavanagh, RN - New York University/NYC HHC at Bellevue Hospital Center (Site 401) CTU Grant #AI069532.
Mykyelle Crawford, RN, BSN and Jolene NoelConnor, RN, BA - HIV Prevention and Treatment (Columbia Presbyterian) (Site 30329) CTU Grant #5U01A1069470-02.
Oluwatoyin Adeyemi, MD and Joanne Despotes, RN - CORE Center, Chicago (Site 2705).
Allan Tenoiro, MD and Kristine L. Richards, RN - Rush University Medical Center. (Site 2702) CTU Grant #U01 A1069471.
Gary Matthew Cox, MD and Caroline Elkins, RN - Duke University Medical Center (Site 1601) CTU Grant #5U01 AI069 484-02.
Judith S. Currier, MD and Maria Palmer, PA - UCLA CARE Center (Site 601) CTU Grant #5U01A1069424-02.
James Raper and Karen Savage - Alabama Therapeutics (Site 5801) CTU Grant #5U01AI069452-02.
Jane Norris, PA-C and Sandra Valle, PA-C - Stanford University (Site 501) CTU Grant #5 U01 AI069556-03.
Karen T. Tashima, MD and Pamela Poethke, RN - The Miriam Hospital-Brown University Partners/Harvard (Site 2951) CTU Grant #A1069472.
Cecilia Shikuma, MD and Debbie Ogata-Arakaki, RN - University of Hawaii at Manoa (Site 5201) CTU Grant #AI-34853.
Michael Dube, MD and Martha Greenwald, NP, MSN - Indiana University School of Medicine (Site 2601) CTU Grant #AI 25859.
Kim Whitely and Robert Kalayjian - MetroHealth CRS (Site 2503) CTU Grant #AI069501-02.
Barbara Ehrgott, RN and Kathy Watson, RN - The Ohio State University (Site 2301) CTU Grant #AI069474.
Frances Van Meter and Sharon Richard - University of Nebraska Medical Center (Site 1505) CTU Grant #AI27661.
Jeffrey T. Schouten, MD, JD and Sheryl S. Storey, PA-C - University of Washington (Site 1401) CTU Grant #AI069434.
Roberto Corales, DO and Christine Hurley, RN - University of Rochester (Site 1108) CTU Grant #U01AI069511-02, GCRC Grant #5-MO1 RR00044.
Deborah McMahon, MD and Carol Oriss, BSN, RN - University of Pittsburgh (Site 1001) CTU Grant #1 U01AI 069494-01.
The following A5209 investigators were supported in part by grants through the National Institutes of Health: D.C. (K23 HL088981) and M.J.G. (K24 AI 78884).
The A5209 study was supported in part by an unrestricted grant fromMerck & Co., Inc, whichalsoprovided the study medication. The sponsor did not have any influence on the design and conduct of the study; the collection or interpretation of the data; the development of the analysis plan; the preparation and conduct of the analysis; or the drafting, critical revision, or approval of the final article.
Footnotes
The present article is presented in part as Poster #712, at 16th Conference on Retroviruses and Opportunistic Infections, 2009, Montreal, Canada. Clinical trials #NCT00099684
References
- 1.Aberg JA, Zackin RA, Brobst SW, Evans SR, Alston BL, Henry WK, et al. A randomized trial of the efficacy and safety of fenofibrate versus pravastatin in HIV-infected subjects with lipid abnormalities: AIDS Clinical Trials Group Study 5087. AIDS Res Hum Retroviruses. 2005;21:757–767. doi: 10.1089/aid.2005.21.757. [DOI] [PubMed] [Google Scholar]
- 2.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 3.Altmann SW, Davis HR, Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, et al. Niemann-Pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 4.Dujovne CA, Ettinger MP, McNeer JF, Lipka LJ, LeBeaut AP, Suresh R, et al. Efficacy and safety of a potent new selective cholesterol absorption inhibitor, ezetimibe, in patients with primary hypercholesterolemia. Am J Cardiol. 2002;90:1092–1097. doi: 10.1016/s0002-9149(02)02798-4. [DOI] [PubMed] [Google Scholar]
- 5.Sudhop T, Lutjohann D, Kodal A, Igel M, Tribble DL, Shah S, et al. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002;106:1943–1948. doi: 10.1161/01.cir.0000034044.95911.dc. [DOI] [PubMed] [Google Scholar]
- 6.Gagne C, Bays HE, Weiss SR, Mata P, Quinto K, Melino M, et al. Efficacy and safety of ezetimibe added to ongoing statin therapy for treatment of patients with primary hypercholesterolemia. Am J Cardiol. 2002;90:1084–1091. doi: 10.1016/s0002-9149(02)02774-1. [DOI] [PubMed] [Google Scholar]
- 7.Ballantyne CM, Houri J, Notarbartolo A, Melani L, Lipka LJ, Suresh R, et al. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation. 2003;107:2409–2415. doi: 10.1161/01.CIR.0000068312.21969.C8. [DOI] [PubMed] [Google Scholar]
- 8.Kerzner B, Corbelli J, Sharp S, Lipka LJ, Melani L, LeBeaut A, et al. Efficacy and safety of ezetimibe coadministered with lovastatin in primary hypercholesterolemia. Am J Cardiol. 2003;91:418–424. doi: 10.1016/s0002-9149(02)03236-8. [DOI] [PubMed] [Google Scholar]
- 9.Davidson MH, McGarry T, Bettis R, Melani L, Lipka LJ, LeBeaut AP, et al. Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J Am Coll Cardiol. 2002;40:2125–2134. doi: 10.1016/s0735-1097(02)02610-4. [DOI] [PubMed] [Google Scholar]
- 10.Wohl DA, Waters D, Simpson RJ, Jr, Richard S, Schnell A, Napravnik S, et al. Ezetimibe alone reduces low-density lipoprotein cholesterol in HIV-infected patients receiving combination antiretroviral therapy. Clin Infect Dis. 2008;47:1105–1108. doi: 10.1086/592116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Negredo E, Molto J, Puig J, Cinquegrana D, Bonjoch A, Perez-Alvarez N, et al. Ezetimibe, a promising lipid-lowering agent for the treatment of dyslipidaemia in HIV-infected patients with poor response to statins. AIDS. 2006;20:2159–2164. doi: 10.1097/01.aids.0000247573.95880.db. [DOI] [PubMed] [Google Scholar]
- 12.Berg-Wolf MV, Klibanov OM, Gaughan JP, Tedaldi EM. Ezetimibe combined with low-dose statin effectively lowers LDL in protease inhibitor treated patients. AIDS Patient Care STDS. 2008;22:483–488. doi: 10.1089/apc.2007.0206. [DOI] [PubMed] [Google Scholar]
- 13.Bennett MT, Johns KW, Bondy GP. Ezetimibe is effective when added to maximally tolerated lipid lowering therapy in patients with HIV. Lipids Health Dis. 2007;6:15. doi: 10.1186/1476-511X-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coll B, Aragones G, Parra S, Alonso-Villaverde C, Masana L. Ezetimibe effectively decreases LDL-cholesterol in HIV-infected patients. AIDS. 2006;20:1675–1677. doi: 10.1097/01.aids.0000238418.43937.3b. [DOI] [PubMed] [Google Scholar]
- 15.Hodges JL, Lehmann EL. Estimates of location based on rank tests. Ann Math Stat. 1963;34:598–611. [Google Scholar]
- 16.Conard SE, Bays HE, Leiter LA, Bird SR, Rubino J, Lowe RS, et al. Efficacy and safety of ezetimibe added on to atorvastatin (20 mg) versus uptitration of atorvastatin (to 40 mg) in hypercholesterolemic patients at moderately high risk for coronary heart disease. Am J Cardiol. 2008;102:1489–1494. doi: 10.1016/j.amjcard.2008.09.075. [DOI] [PubMed] [Google Scholar]
- 17.Leiter LA, Bays H, Conard S, Bird S, Rubino J, Hanson ME, et al. Efficacy and safety of ezetimibe added on to atorvastatin (40 mg) compared with uptitration of atorvastatin (to 80 mg) in hypercholesterolemic patients at high risk of coronary heart disease. Am J Cardiol. 2008;102:1495–1501. doi: 10.1016/j.amjcard.2008.09.076. [DOI] [PubMed] [Google Scholar]
- 18.Pearson TA, Denke MA, McBride PE, Battisti WP, Brady WE, Palmisano J. A community-based, randomized trial of ezetimibe added to statin therapy to attain NCEP ATP III goals for LDL cholesterol in hypercholesterolemic patients: the ezetimibe add-on to statin for effectiveness (EASE) trial. Mayo Clin Proc. 2005;80:587–595. doi: 10.4065/80.5.587. [DOI] [PubMed] [Google Scholar]
- 19.Kastelein JJ, Akdim F, Stroes ES, Zwinderman AH, Bots ML, Stalenhoef AF, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358:1431–1443. doi: 10.1056/NEJMoa0800742. [DOI] [PubMed] [Google Scholar]
- 20.Fleg JL, Mete M, Howard BV, Umans JG, Roman MJ, Ratner RE, et al. Effect of statins alone versus statins plus ezetimibe on carotid atherosclerosis in type 2 diabetes. The SANDS (Stop Atherosclerosis in Native Diabetics Study) trial. J Am Coll Cardiol. 2008;52:2198–2205. doi: 10.1016/j.jacc.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aberg JA. Lipid management in patients who have HIV and are receiving HIV therapy. Endocrinol Metab Clin North Am. 2009;38:207–222. doi: 10.1016/j.ecl.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossebo AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]