Abstract
Sphingolipid phosphate analogues bearing 7-(diethylamino)coumarin (DECM) and 4-bromo-5-hydroxy-2-nitrobenzhydryl (BHNB) groups in a photolabile ester bond were synthesized. The ability of the “caged” ceramide 1-phosphate analogues to release the bioactive parent molecule upon irradiation at 400–500 nm was demonstrated by stimulation of macrophage cell proliferation.
“Caging” is a strategy employed in biochemistry, neurobiology, and physiology for the in vitro investigation of the cellular activity of cell-impermeable bioactive molecules.1 The ionic groups in charged, hydrophilic molecules are temporarily masked by a covalent link to a photolabile moiety, thereby facilitating cell delivery and bypass of cell-surface receptors. The bioactive molecule is released in the cytosol on photolysis using light that does not damage cellular components. The first caged biomolecules reported, the intracellular messengers cyclic-AMP2a and ATP,2b incorporated o-nitrobenzyl phosphate esters as the photolabile moiety. Subsequently, the 1-(2-nitrophenyl)ethyl (NPE) group and derivatives containing additional hydroxy or methoxy substituents were used as caging addends.1b,2c Since the NPE chromophore has only weak absorption above 350 nm, cages with more attractive photophysical properties have been developed. For example, derivatives of coumarins3 and quinolines4 are rapidly uncaged using two-photon activation with IR laser light in tiny excitation volumes and may be used in vivo, since the photolytic conditions are noninvasive and permit spatial and temporal control of the uncaging process.
The phosphorylated sphingolipid metabolites sphingosine 1-phosphate (S1P) and ceramide 1-phosphate (C1P) are mediators of a multitude of cellular activities.5 Most of the activities of S1P arise from its action as an extracellular signaling molecule following binding to a family of five G-protein coupled receptors at the cell surface.6 In contrast to S1P, C1P functions as a lipid messenger mainly at the intracellular level.7 Natural C1P is not taken up readily by cells in culture unless it is dispersed in organic solvents such as EtOH/dodecane (49:1), which may allow incorporation of other extracellular compounds. C1P regulates diverse cellular functions, including arachidonic acid release, mast cell degranulation, Ca2+ mobilization, translocation of lipid-metabolizing enzymes, vesicular trafficking, cell proliferation, and cell survival.8 Our investigations of cellular responses to intracellular C1P have been limited to the addition of unnatural short-chain amide analogues of C1P, such as N-acetyl- and N-octanoyl-C1P, as no caged C1P derivative has yet been reported. In fact, the only caged sphingolipid derivatives that have been reported are S1P analogues that contain the NPE chromophore.9
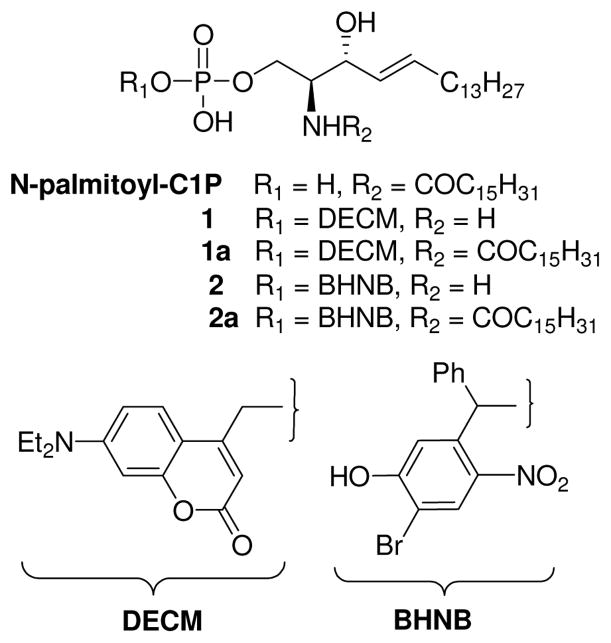
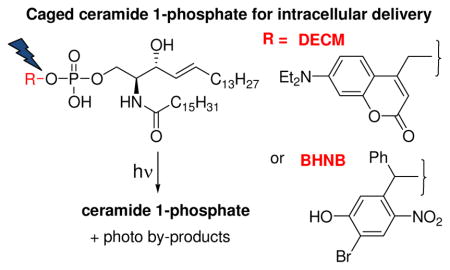
We report here the synthesis of caged S1P (1, 2) and C1P (1a, 2a) analogues in which the phosphate headgroup is esterified to a 7-(diethylamino)coumarin (DECM) or 4-bromo-5-hydroxy-2-nitrobenzhydryl (BHNB) group (Figure 1). We assessed the ability of caged C1P conjugates 1a and 2a to deliver C1P into mammalian cell cultures on exposure to visible light. Photochemical uncaging of C1P may be particularly useful in cells that cannot be transfected with specific enzymes as in primary bone marrow derived macrophage (BMDM) cell types,10 and to distinguish between intracellular and cell surface receptor-mediated activities.
Figure 1.
Structures of C1P, DECM- and BHNB-Caged S1P Analogues 1 and 2, and C1P Analogues 1a and 2a.
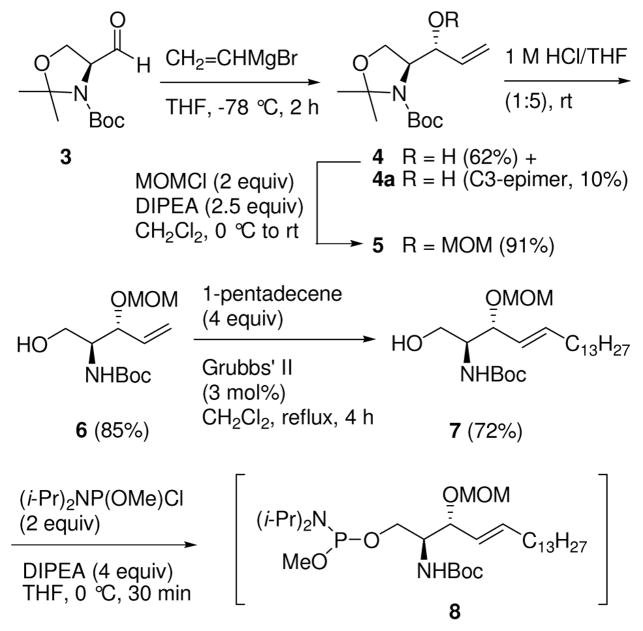
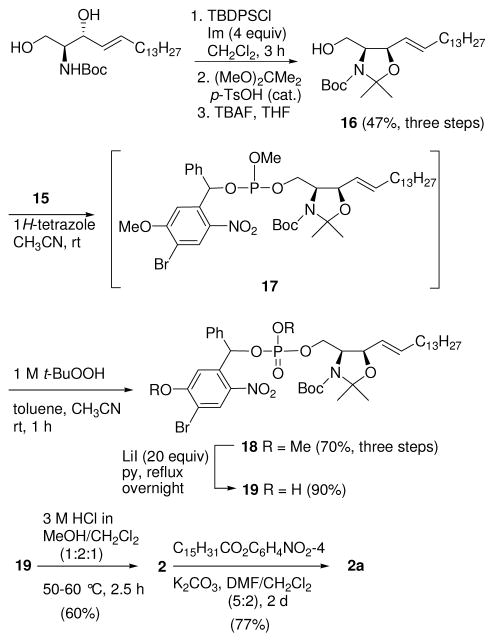
For the synthesis of the DECM-caged S1P 1 and C1P 1a, (S)-Garner aldehyde 311 (Scheme 1) was treated with vinylmagnesium bromide at −78 °C to provide a 6:1 mixture of erythro alcohol 412 and its C3-epimer 4a. Alcohol 4 was purified by chromatography (hexane/EtOAc 5:1), and the hydroxy group was protected as its MOM ether 5. The oxazolidine group was selectively deprotected using 1 M HCl in THF at rt to give N-Boc-3-O-MOM-protected alcohol 6. An E-selective cross metathesis of olefin 6 with 1-pentadecene using Grubbs’ second-generation catalyst13 afforded the 3-MOM ether of (2S,3R)-N-Boc-sphingosine 7.14 Sphingosine derivative 7 was treated with i-Pr2NP(OMe)Cl in the presence of DIPEA to provide phosphoramidite intermediate 8.
Scheme 1.
Synthesis of Phosphoramidite 8
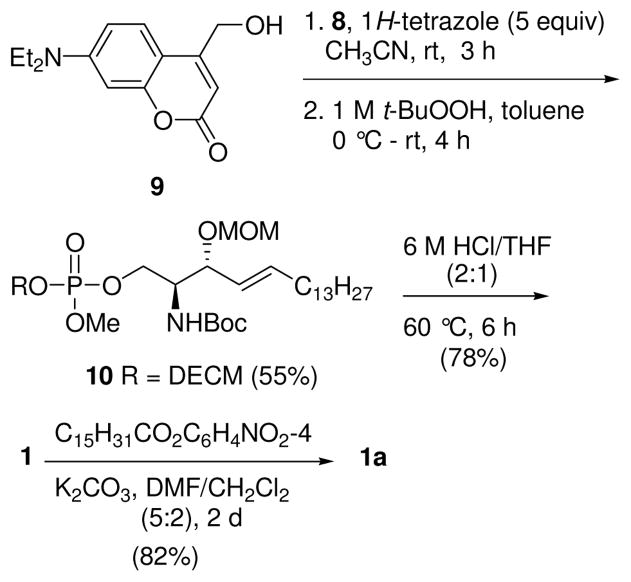
7-(Diethylamino)-4-hydroxymethylcoumarin (9) was prepared by oxidation of 4-methyl-7-(diethylamino)coumarin with SeO2, and subsequent reduction with NaBH4.3d,15 Reaction of alcohol 9 with phosphoramidite 8 in the presence of 1H-tetrazole provided a phosphite intermediate, which was oxidized in the same pot with anhydrous TBHP to afford phosphate 10 (Scheme 2). Removal of the protecting groups required some exploration. An initial attempt to deprotect both the Boc and MOM groups with TFA resulted in the loss of the Boc group in 1 h; however, only 50% of the MOM group was removed even after stirring for 3 days at rt. Reaction with Me3SiBr in CH2Cl2, rather than removing the methyl ester of the phosphate, resulted instead in the loss of the coumarin moiety from the lipid. Fortunately, we found that when 10 was heated in 6 M HCl/THF (2:1) at 60 °C for 6 h, all three groups, Boc, MOM, and the phosphate methyl ester, were removed to provide DECM-S1P (1), which on neutralization and N-acylation using p-nitrophenyl palmitate in DMF/CH2Cl216 gave C1P analogue 1a in good yield.
Scheme 2.
Synthesis of DECM-C1P Analogue 1a
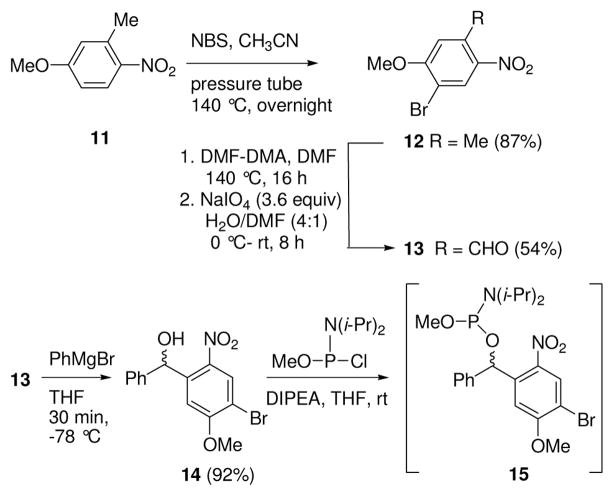
To provide caged phospholipids with a high molar absorptivity at longer wavelengths than 1 and 1a,17 we prepared BHNB-caged compounds 2 and 2a. Bromo and nitro groups were selected as the electron-withdrawing groups, with the nitro group positioned ortho to the benzhydryl carbon to facilitate photorelease of the phosphorylated sphingolipid.18 The first step in the preparation of the BHNB cage was bromination of 4-methoxy-2-methyl-1-nitrobenzene (11, Scheme 3). When 11 was heated with NBS overnight at reflux in MeCN, we observed a substantial amount of the starting material along with the product. However, when a pressure tube was used for this reaction, heating at 140 °C overnight resulted in a mixture of the desired brominated product 12 together with unseparable by-products, and no starting material. We used a known method for the conversion of a methyl group in the ortho position to a nitro group to an aldehyde; thus, reaction of 12 with DMF dimethyl acetal (DMF-DMA), followed by oxidation of the intermediate enamine with NaIO4,19 successfully converted 12 to aldehyde 13. After the by-products were removed by chromatography, addition of PhMgBr to 13 afforded the desired caged benzhydryl alcohol 14. A reaction time of 30 min at −78 °C is critical for this addition reaction, as decomposition of compound 14 was observed after 30 min.
Scheme 3.
Synthesis of Benzhydryl Alcohol 14 and Phosphoramidite 15
After alcohol 14 was converted to phosphoramidite 15 with i-Pr2NP(OMe)Cl (Scheme 3), coupling with sphingosine derivative 169b (Scheme 4) in the presence of 1H-tetrazole afforded 17. Phosphite 17 was purified by flash chromatography prior to oxidation because the corresponding phosphate co-eluted with excess alcohol 16. Oxidation of 17 with TBHP afforded phosphate 18 in 70% yield over three steps. Several reagents (BBr3 in CH2Cl2 at −78 °C, 33% HBr in AcOH at 0 °C, 1 M HCl/THF (1:1) at rt, and TMSBr in CH2Cl2) to remove the protecting groups in 18 were screened unsuccessfully. Use of excess LiI in pyridine at reflux was successful, resulting in O-demethylation of both the anisole methyl group and phosphate methyl ester groups of 18, affording 19 in 90% yield. Reaction of 19 with 3 M HCl/MeOH/CH2Cl2 (1:2:1) at gentle reflux for 2.5 h provided BHNB-caged S1P 2. N-Acylation with p-nitrophenyl palmitate gave C1P analogue 2a in 77% yield.16
Scheme 4.
Synthesis of BHNB-caged C1P Analogue 2a
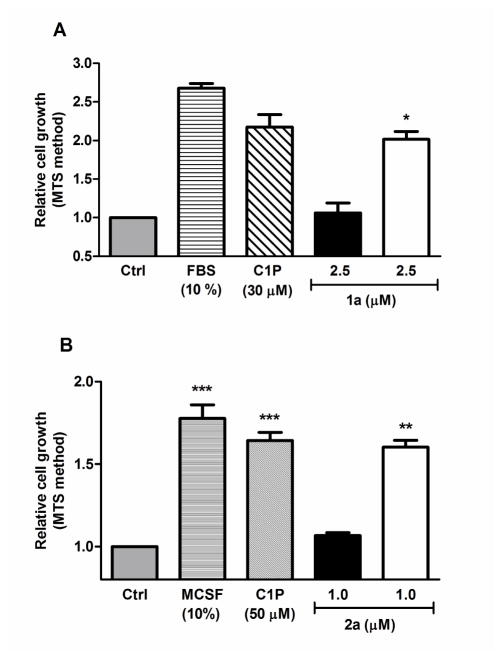
Figure 2 shows a comparison of the effects of adding growth factors,20 N-palmitoyl-C1P (30 or 50 μM, as an aqueous dispersion), 1a (2.5 μM in EtOH), and 2a (1.0 μM in EtOH) to RAW264.7 (panel A) or primary BMDM (panel B). Compounds 1a and 2a stimulated cell division, as determined by the MTS assay,21 at 2.5 and 1.0 μM, respectively, on exposure of the cells to light. To achieve the same relative level of RAW264.7 and BMDM cell growth with exogenous C1P, optimum concentrations of 30 and 50 μM8b were required, respectively. 1a, at 2.5 μM, was as potent as exogenous C1P at 30 μM (see Supporting Information). The putative photo by-product22 formed on photolysis of 1a (compound 9) did not stimulate cell proliferation, nor did an analogue of the photo by-product 20 formed on photolysis of 2a.23 No growth stimulation was observed in the dark.
Figure 2.
Delivery of 1a and 2a into RAW 264.7 macrophages (panel A) and primary BMDM (panel B) stimulates cell growth. Open bars: Cells were exposed to 400–500 nm light in a transilluminator (see Supporting Information). Filled bars: Cells were incubated in the dark with the compounds. The cells were incubated for 48 h in the absence of FBS (A) or with 1.5% MCSF for 24 h (B) prior to the addition of the compounds. Mean ±S.D. of 3 independent experiments.
In conclusion, 1a and 2a, the first caged analogues of C1P, were prepared and found to release C1P intracellularly, as demonstrated by stimulation of macrophage proliferation at significantly lower concentrations than exogenous C1P. These compounds, and their N-acyl variants, are likely to find utility as research tools for investigating the intracellular activities of this important lipid second messenger.
Experimental Section
(4S,5R,1″E)-tert-Butyl-4-((((4′-bromo-5′-hydroxy-2′-nitrophenyl)(phenyl)methoxy) (hydroxy)phosphoryloxy)methyl)-2,2-dimethyl-5-(pentadec-1″-enyl)oxazolidine-3-carboxylate (19)
A solution of 18 (48 mg, 0.056 mmol) and anhydrous LiI (150 mg, 1.12 mmol) in dry py (5 mL) was heated at reflux overnight. The reaction was quenched with saturated aq NH4Cl solution (15 mL), and the product was extracted with CH2Cl2 (3 × 15 mL), dried (Na2SO4), concentrated, and purified by chromatography (CHCl3/MeOH 3:1) to afford 19 (37 mg, 90%): Rf 0.60 (CHCl3/MeOH 3:1); 1H NMR (CD3OD) δ 0.78 (t, 3H, J = 6.6 Hz), 1.18 (m, 31H), 1.38 (m, 6H), 1.90 (m, 2H), 3.79 (m, 3H), 4.40 (m, 1H), 5.42 (m, 1H), 5.69 (m, 1H), 6.99 (m, 1H), 7.23 (m, 6H), 7.53 (m, 1H), 8.20 (m, 1H); 31P NMR (CD3OD) δ −2.92, −2.03; ESI-HRMS [M+Na]+ C39H5881BrN2O10PNa calcd for m/z 849.2889, found 849.2894.
(2S,3R,4E)-2-Amino-3-hydroxyoctadec-4-enyl-(4′-bromo-5′-hydroxy-2′-nitrophenyl)(phenyl)methyl Hydrogen Phosphate (2)
A solution of 19 (25 mg, 0.030 mmol) in 3 M HCl/MeOH/CH2Cl2 (1:2:1, 4 mL) was stirred at gentle reflux (50–60 °C) for 2.5 h. The reaction mixture was diluted with CH2Cl2 (30 mL) and washed with brine (10 mL). The organic layer was dried (Na2SO4) and concentrated. Column chromatography (CHCl3/MeOH 6:1, then 3:1), followed by removal of suspended silica gel by filtration of a solution of 2 in CHCl3 through a 0.45-μm syringe filter, afforded 2 (12 mg, 60%) as a yellow solid: Rf 0.35 (CHCl3/MeOH 3:1); 1H NMR (CD3OD) δ 0.89 (t, 3H, J = 7.0 Hz), 1.28 (m, 22H), 2.03 (m, 2H), 2.95 (m, 1H), 3.59 (m, 1H), 3.75 (m, 1H), 4.07 (m, 1H), 5.28 (dd, 1H, J = 6.5, 15.4 Hz), 5.70 (dt, 1H, J = 6.7, 14.7 Hz), 7.02 (m, 1H), 7.25 (m, 5H), 7.44 (m, 1H), 8.25 (m, 1H); 13C DEPT-45 NMR (CD3OD) δ 14.5, 23.7, 30.2, 30.4, 30.5, 30.6, 30.7, 30.8, 33.1, 33.3, 57.0, 63.3, 70.6, 76.4, 116.8, 127.8, 128.8, 129.0, 129.2, 129.3, 129.4, 131.8, 136.3, 136.8; 31P NMR (CD3OD) δ −1.41, −1.20; ESI-HRMS [M-H]− C31H45BrN2O8P calcd for m/z 683.2096, found 683.2095; UV: λmax 406 nm (ε = 12,000 M−1cm−1), 278 nm (ε = 6,294 M−1cm−1) in 50% aq EtOH, 50% 10 mM Tris, pH 7.4.
Supplementary Material
Acknowledgments
Financial support from NIH grant HL083187 (RB) and BFU2006-13689/BFI (AGM) is gratefully acknowledged. We thank Dr. Lisardo Boscá for providing RAW264.7 macrophages.
Footnotes
Supporting Information Available: Experimental procedures and NMR spectra for the reported compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Footnotes
- 1.For recent reviews of caged biomolecules, see: Mayer G, Heckel A. Angew Chem, Int Ed. 2006;45:4900. doi: 10.1002/anie.200600387.Ellis-Davies GCR. Nature Methods. 2007;4:619. doi: 10.1038/nmeth1072.Ellis-Davies GCR. Chem Rev. 2008;108:1603. doi: 10.1021/cr078210i.
- 2.(a) Engels J, Schlaeger EJ. J Med Chem. 1977;20:907. doi: 10.1021/jm00217a008. [DOI] [PubMed] [Google Scholar]; (b) Kaplan JH, Forbush B, 3rd, Hoffman JF. Biochemistry. 1978;17:1929. doi: 10.1021/bi00603a020. [DOI] [PubMed] [Google Scholar]; (c) Nerbonne JM, Richard S, Nargeot J, Lester HA. Nature. 1984;310:74. doi: 10.1038/310074a0. [DOI] [PubMed] [Google Scholar]
- 3.For references about coumarin-4-yl methyl esters of nucleotides and amino acid derivatives, see: Shembekar VR, Chen Y, Carpenter BK, Hess GP. Biochemistry. 2007;46:5479. doi: 10.1021/bi700280e. and references cited therein.
- 4.For references about the use of 8-bromo-2-hydroxyquinoline derivatives as cages, particularly for carboxylic acids, see: Davis MJ, Kragor CH, Reddie KG, Wilson HC, Zhu Y, Dore TM. J Org Chem. 2009;74:1721. doi: 10.1021/jo802658a.An HY, Ma C, Nganga JL, Zhu Y, Dore TM, Phillips DL. J Phys Chem A. 2009;113:2831. doi: 10.1021/jp809586h.
- 5.(a) Gómez-Muñoz A, Steinbrecher UP. Recent Res Dev Lipids. 2004;7:65. [Google Scholar]; (b) Kihara A, Mitsutake S, Mizutani Y, Igarashi Y. Prog Lipid Res. 2007;46:126. doi: 10.1016/j.plipres.2007.03.001. [DOI] [PubMed] [Google Scholar]; (c) Hannun YA, Obeid LM. Nature Rev Molec Cell Biol. 2008;9:139. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 6.S1P signaling also appears to take place by nonreceptor mechanisms when intracellularly generated S1P binds to unidentified cytosolic targets: Van Brocklyn, J. R.; Lee, M. J.; Menzeleev, R.; Olivera, A.; Edsall, L.; Cuvillier, O. J. Cell Biol. 1998, 142, 229. For a recent review of dual activities of S1P, see: Pyne NG, Long JS, Lee SC, Loveridge C, Gillies L, Pyne S. Adv Enzyme Regul. 2009;49:214. doi: 10.1016/j.advenzreg.2009.01.011.
- 7.Addition of exogenous C1P is not an ideal method for increasing the intracellular C1P concentration. C1P may interact with a cell-surface receptor, triggering effects independent of C1P intracellular accumulation. For a report of C1P-mediated cell migration via interaction with a putative plasma membrane receptor, see: Granado MH, Gangoiti P, Ouro A, Arana L, González M, Trueba M, Gómez-Muñoz A. Cell Signalling. 2009;21:405. doi: 10.1016/j.cellsig.2008.11.003.
- 8.(a) Chalfant CE, Spiegel S. J Cell Sci. 2005;118:4605. doi: 10.1242/jcs.02637. [DOI] [PubMed] [Google Scholar]; (b) Gangoiti P, Granado MH, Wang SW, Kong JK, Steinbrecher UP, Gómez-Muñoz A. Cell Signalling. 2008;20:726. doi: 10.1016/j.cellsig.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 9.For references on NPE derivatives of sphingosine and S1P, see: Zehavi U. Chem Phys Lipids. 1997;90:55. doi: 10.1016/s0009-3084(97)00083-2.Qiao L, Kozikowski AP, Olivera A, Spiegel S. Bioorg Med Chem Lett. 1998;8:711. doi: 10.1016/s0960-894x(98)00112-7.Scott RH, Pollock J, Ayar A, Thatcher NM, Zehavi U. Methods Enzymol. 2000;312:387. doi: 10.1016/s0076-6879(00)12924-6.
- 10.Gómez-Muñoz A. Biochim Biophys Acta. 2006;1758:2049. doi: 10.1016/j.bbamem.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Mengel A, Reiser O. Chem Rev. 1999;99:1191. doi: 10.1021/cr980379w. [DOI] [PubMed] [Google Scholar]
- 12.(a) Bedia C, Casas J, Garcia V, Levade T, Fabriàs G. ChemBiochem. 2007;8:642. doi: 10.1002/cbic.200600533. [DOI] [PubMed] [Google Scholar]; (b) Srivastava AK, Panda G. Chem Eur J. 2008;14:4675. doi: 10.1002/chem.200701991. [DOI] [PubMed] [Google Scholar]; (c) Ullrich T, Ghobrial M, Peters C, Billich A, Guerini D, Nussbaumer P. ChemMedChem. 2008;3:356. doi: 10.1002/cmdc.200700285. [DOI] [PubMed] [Google Scholar]
- 13.Scholl M, Ding S, Lee CW, Grubbs RH. Org Lett. 1999;1:953. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
- 14.Examples of the CM approach to sphingosines with various building blocks: Yamamoto T, Hasegawa H, Hakogi T, Katsumura S. Org Lett. 2006;8:5569. doi: 10.1021/ol062258l.Torssell S, Sonfai P. Org Biomol Chem. 2004;2:1643. doi: 10.1039/b403568b.Rai AN, Basu A. J Org Chem. 2005;70:8228. doi: 10.1021/jo051069y.Peters C, Billich A, Ghobrial M, Högenauer K, Ullrich T, Nussbaumer P. J Org Chem. 2007;72:1842. doi: 10.1021/jo062347b.Pham VT, Joo JE, Tian YS, Oh CY, Ham WH. Arch Pharm Res. 2007;30:22. doi: 10.1007/BF02977774.
- 15.Ito K, Nakajima K. J Heterocycl Chem. 1988;25:511. [Google Scholar]
- 16.Bittman R, Verbicky CA. J Lipid Res. 2000;41:2089. [PubMed] [Google Scholar]
- 17.UV/vis data: 1, λmax 376 (ε = 1.58 × 104 M−1cm−1) in EtOH; 1a, λmax 376 (ε = 1.55 × 104 M−1cm−1) in CH2Cl2.
- 18.The uncaging mechanism postulated for photolysis of o-nitrobenzylic alcohol and NPE derivatives involves participation of an oxygen atom of the nitro group: Riguet E, Bochet CG. Org Lett. 2007;9:5453. doi: 10.1021/ol702327c.
- 19.(a) Vetelino MG, Coe JW. Tetrahedron Lett. 1994;35:219. [Google Scholar]; (b) Caron S, Vazquez E, Stevens RW, Nakao K, Koike H, Murata Y. J Org Chem. 2003;68:4104. doi: 10.1021/jo034274r. [DOI] [PubMed] [Google Scholar]; (c) Naffziger MR, Ashburn BO, Perkins JR, Carter RG. J Org Chem. 2007;72:9857. doi: 10.1021/jo070740r. [DOI] [PubMed] [Google Scholar]
- 20.FBS, fetal bovine serum; MCSF, macrophage colony-stimulating factor.
- 21.Hundal RS, Gómez-Muñoz A, Kong JY, Salh BS, Marotta A, Duronio V, Steinbrecher UP. J Biol Chem. 2003;278:24399. doi: 10.1074/jbc.M209179200. [DOI] [PubMed] [Google Scholar]
- 22.Schade B, Hagen V, Schmidt R, Herbrich R, Krause E, Eckardt T, Bendig J. J Org Chem. 1999;64:9109. [Google Scholar]
- 23.The photo by-product of 2a was identified as 4-bromo-5-hydroxy-2-nitroso-benzophenone (20), after isolation by TLC and HRMS analysis. We synthesized the 2-nitro analogue of 20 and found that it did not stimulate macrophage cell growth.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.