Abstract
Inclusion body myopathy associated with Paget’s disease and frontotemporal dementia (IBMPFD) is caused by mutations in the valosin containing protein (VCP) gene. The disease is associated with progressive proximal muscle weakness, inclusions and vacuoles in muscle fibers, malfunction in the bone remodeling process resulting in Paget disease, and premature frontotemporal dementia. VCP is involved in several cellular processes related to the endoplasmic reticulum associated degradation of proteins. To understand the pathological mechanisms underlying the myopathy in IBMPFD, we have studied the cellular consequences of VCP mutations in human primary myoblasts. Our results revealed that patients’ myoblasts accumulate large vacuoles. Lysosomal membrane proteins Lamp1 and Lamp2 show increased molecular weights in patients’ myoblasts due to differential N-glycosylation. Additionally, mutant myoblasts show increased autophagy when cultured in the absence of nutrients, as well as defective cell fusion and increased apoptosis. Our results elucidate that VCP mutations result in disturbances in several cellular processes, which will help us in the understanding of the pathological mechanisms resulting in muscle weakness and other features of VCP associated disease.
Keywords: IBMPFD, inclusion body myopathy, Paget’s disease of the bone, frontotemporal dementia, VCP, vacuoles, myoblasts
1. INTRODUCTION
Inclusion body myopathy associated with Paget’s disease of the bone and frontotemporal dementia (IBMPFD, OMIM 167320) is a progressive condition with an onset typically in the 30s. It is inherited as an autosomal dominant manner causing weakness and atrophy of the skeletal muscles of the pelvic and shoulder girdle muscles. Histologically the disease is characterized by the presence of rimmed vacuoles and ubiquitin positive inclusion bodies in the muscle fibers [1–3]. Cardiac failure and cardiomyopathy have been observed in later stages. Early-onset Paget’s disease (PDB) typically begins in the 30s to 40s, and is caused by excessive osteoclastic activity and increased bone turnover. It typically leads to spine and/or hip pain as well as pathological fractures [1]. Premature frontotemporal dementia (FTD) [4] seen in the mid 50s, is characterized by dysnomia, dyscalculia, auditory comprehension deficits for even one-step commands, alexia, agraphia, and later stages by inability to speak. FTD is more common among women and is associated with one or two alleles of APOE4 [5]. Affected individuals die from progressive muscle weakness, as well as from cardiac and respiratory failure typically in their 40s to 60s.
IBMPFD is caused by mutations in the valosin containing protein (VCP) gene [6], and therefore it is also referred to as VCP disease. The VCP protein is highly conserved in evolution and belongs to the family of type II AAA (ATPases associated with a variety of cellular activities) having four domains: the N-terminal domain, which binds specific ubiquitin substrates through cofactors such as Ufd1, Npl4, and p47, two ATPase domains D1 and D2, and the C-terminal domain, which binds cofactors such as the multiubiquitination enzyme Ufd2 [7, 8]. The clinical observations in IBMPFD suggest an important role for the VCP protein in skeletal muscle, bone and brain cells. It is involved in several cellular activities including homotypic membrane fusion, transcription activation, nuclear envelope reconstruction, postmitotic organelle reassembly, cell cycle control, DNA repair, apoptosis, and endoplasmic reticulum associated degradation of proteins (ERAD) [9–11]. VCP forms homohexamers with a central channel and undergoes conformational changes during its function of binding and removal of specific ubuiquitinated substrates from protein complexes or the endoplasmic reticulum (ER) membrane [12, 13]. More than 50% of IBMPFD families have a mutation at the amino acid position 155 resulting in either the R155H (major mutation), R155P or R155C change. R155 is located in the N-terminal CDC48 domain, which is involved in ubiquitin binding and protein-protein interactions [14, 15]. The most common VCP mutation R155H has been shown to increase overall level of ubiquitin-conjugated proteins, formation of cytoplasmic aggregates and impair the ERAD activity despite normal hexameric structure in transiently transfected cells over-expressing the mutant VCP-gene [16]. Although Weihl et al. (2006) [16] report normal ATPase activity, recent studies have shown increased ATPase activity associated with VCP mutations [17].
The role of the lysosome/autophagy system in VCP associated myopathy is unknown. There is evidence for impaired lysosomal function in various myopathies [18, 19]. Autophagy is a bulk degradative process that results in the breakdown of cytoplasm within lysosomes in response to a variety of cellular stresses [20]. In response to stress signals such as starvation, cytoplasmic proteins or organelles are enwrapped by specialized double membrane structures called autophagosomes, which are subsequently delivered to lysosomes for degradation by lysosomal proteases. Autophagy is considered a catabolic mechanism, which primarily allows cells to generate energy and new materials in order to adapt to environmental or developmental changes and to maintain cellular homeostasis. To date, more than 30 genes have been implicated in this process and are collectively termed autophagy-related (ATG) genes; some play a role in autophagy induction, and others function as a degradative machinery [20].
To elicit more detailed insight into the muscle pathology of the disease, we have studied the molecular and cellular consequences of the VCP disease mutations in patients’ primary myoblast cell lines. Our analyses revealed that myoblasts with VCP mutations accumulate enlarged vacuoles. Mutant cells also revealed increased apoptosis and were defective in the maturation process to myotubes. Understanding the role of these pathways in muscle pathology is critical to understand the pathogenesis of IBMPFD, and offers promising targets for the development of effective new treatments for skeletal muscle diseases.
2. MATERIALS AND METHODS
2.1. Biological materials and reagents
Mutant cell lines with the heterozygous R155H and R155S mutations were obtained from The Muscle Tissue Culture Collection (MTCC)/EuroBioBank (Munich, Germany). These mutant cell lines were generated from the muscle biopsies of patients showing typical clinical phenotype and histological findings of IBMPFD (Table 1). A mutant cell line from a presymptomatic patient with the heterozygous R159C mutation was generated by Dr. Charlotte Peterson (University of Kentucky, Lexington, KY). Two control myoblast cell lines were from MTCC/EuroBioBank (Munich, Germany) and five control myoblast lines were from The Telethon Network of Genetic Biobanks/EuroBioBank (Milan, Italy). All control myoblast cell lines were generated from age-matched control subjects without any pathological findings in histology. Cells were maintained in Skeletal Muscle Cell Growth Medium (PromoCell, Heidelberg, Germany) supplemented with the supplement mix (PromoCell), 10% FBS, Gentamicin (Gibco, Carlsbad, CA) and GlutaMAX-1 (Gibco) in 5% CO2 at 37ºC. The purity of cultures was confirmed by morphological analyses and immunocytochemical stainings (see below).
Table 1.
Patients and controls used for the myoblast generation
| Sample | Sex | Age at onset |
Age at biopsy |
Muscle | Mutation | Microscopic findings | Clinical findings |
|---|---|---|---|---|---|---|---|
| 307/98 | M | 50y | 56y | TA | R155H | neurogenic atrophy; EM: mitochondrial aggregates, minimal intermediate filament accumulation in subsarcolemmal regions, no 14–18 nm filaments |
first symptom: stepping gait; weakness in distal and proximal leg muscles and shoulder girdle, scapular winging, M. Paget, no congnitive impairment |
| 421/07 | F | 46y | 48y | Q | R155S | myofiber size variability, no rimmed vacuoles, EM: normal |
first symptom: weakness in pelvic girdle muscles, weakness in facial and proximal leg muscles, no M. Paget or congnitive impairment |
| 24/002 | F | - | 59y | Q | R159C | NA | no evident muscle weakness or neuropathy, no Paget, normal motor exam, good general health |
| 330/98 | M | - | 48y | Q | - | Normal | Normal |
| 110/06 | M | - | 39y | Q | - | Normal | Normal |
| 9099 | M | - | 34y | Q | - | Normal | Normal |
| 9109 | M | - | 49y | Q | - | Normal | Normal |
| 9311 | F | - | 29y | Q | - | Normal | Normal |
| 9314 | F | - | 25y | Q | - | Normal | Normal |
| 9364 | M | - | 42y | Q | - | Normal | Normal |
307/98 = R155H, 421/07 = R155S, 330/98 = Wild-type A, 110/06 = Wild-type B
M = male, F = female
TA = tibialis anterior, Q = quardriceps/vastus lateralis
Na = not available
Primary antibodies were purchased from the following companies: VCP and Lamp1/Affinity BioReagents (Golden, CO); actin, Lamp2, troponin C, myogenin and M-cadherin/Santa Cruz Biotechnology (Santa Cruz, CA); LC3/Novus Biologicals (Littleton, CO). All secondary antibodies were purchased from Sigma-Aldrich (St. Louis, MO), and PNGaseF was purchased from New England Biolabs (Ipswich, MA).
2.2 Immunofluorescence microscopy
To determine the intracellular distribution of proteins in human myoblasts, cells were plated on collagen coated coverglasses one day prior to analyses. Next day, cells were fixed with 4% paraformaldehyde (Sigma-Aldrich) for 30 min at room temperature, and blocked and permeabilized with 0.5% bovine serum albumin (BSA, Fraction V, Sigma-Aldrich)/0.2% saponin (Sigma-Aldrich) for 15 min at room temperature. Cells were labeled with protein-specific antibodies, washed with the blocking solution, and incubated with fluorescein-conjugated secondary antibodies. After washing with phosphate-buffered saline (PBS, Amresco, Solon, OH), the cells were mounted with Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA) and viewed with a fluorescence microscope (Carl Zeiss, Thornwood, NY) using an AxioVision image capture system (Carl Zeiss).
2.3 Electron microscopy
Electron microscopy was applied to analyze the ultra-structures of the wild-type and mutant myoblasts. Cultured cells were fixed with 4% paraformaldehyde/0.1% glutaraldehyde in 0.1M PBS for 24h at 4ºC, 1% glutaraldehyde overnight at 4ºC and with 1% Osmium for 1hr at 4°C followed by serial dehydration in ethanol. Thereafter, cells were embedded in Eponate 12 resin at 65°C for 24–36h. Ultrathin (60~80 nm) sections were cut with a diamond knife, and the sections were stained with 1% uranyl acetate for 30 min at r.t., followed by lead citrate incubation for 7–10 min at r.t. Sections were examined with a Philips CM10 transmission electron microscope (Philips, Omaha, NE). Electron micrographs were taken with a Gatan UltraScan US1000 digital camera (Philips).
2.4 Western blotting
Protein expression levels were determined by Western blotting. Cultured myoblast cells were harvested using RIPA lysis buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 1% Igepal, 0.5% deoxycholic acid, and 0.1% SDS) supplemented with protease inhibitors (Halt Protease Inhibitor Cocktail Kit, Pierce, Rockford, IL). Protein concentrations were determined using the Bio-Rad Protein Assay kit (Bio-Rad, Hercules, CA) according to the manufacturer’s protocols. Equal amount of proteins were separated on SDS-PAGE gels, and the Western blotting results were obtained using protein-specific antibodies. Enhanced Chemiluminescence (Pierce, Rockford, IL) was used for the protein detection. Equal protein loading was confirmed by actin staining (shown only when necessary for the interpretation of results). PNGaseF treatments of protein lysates were performed according to the manufacturer’s guidelines.
2.5 Apoptosis assays
To test if mutations in the VCP gene result in increased apoptosis in myoblast cells, we analyzed DNA fragmentation by the DeadEnd Fluorometric TUNEL System (Promega, Madison, WI) and by the Colorimetric CaspACE Assay System (Promega) following the manufacturer’s guidelines. Briefly, for TUNEL analysis, cells were plated on collagen coated coverglasses one day prior to the experiments. Next day, the cells were fixed with 4% paraformaldehyde, washed with PBS, permeabilized with 0.2% Triton X-100 (Sigma-Aldrich) solution in PBS, rinsed with PBS, equilibrated with equilibration buffer, and labeled with nucleotide mix/rTdT enzyme solution. After washing with 2 × SSC, cells were washed with PBS and mounted in Vectashield with DAPI. The results were analyzed by immunofluorescence microscopy. For Caspase-3 assay, cells were plated in 6-well plates one day before harvesting them with cell lysis buffer. To complete the lysis, cells were frozen and thawed three times, followed by centrifugation. Supernatants were transferred into fresh tubes, and protein concentrations were determined using the Bio-Rad Protein Assay kit. Equal amount of proteins were analyzed in 96-well plates in triplicate, in the presence of 2% DMSO, 100 mM DTT, and 0.2 mM DEVD-pNA substrate. Reaction mixtures were incubated at 37ºC for 4h, and the results were obtained by measuring the absorbance at 405 nm.
2.6 Autophagy assays
To analyze if nutrient deprivation results in increased autophagy, cells were first starved by culturing them in Skeletal Muscle Cell Growth Medium in the absence of serum and supplement mix for 30 min. Proteins were extracted from cells with RIPA-buffer and lysates were subjected to Western blotting using an LC3-specific antibody
3. RESULTS
3.1 Primary IBMPFD myoblasts demonstrate enlarged ubiquitin-positive vacuoles
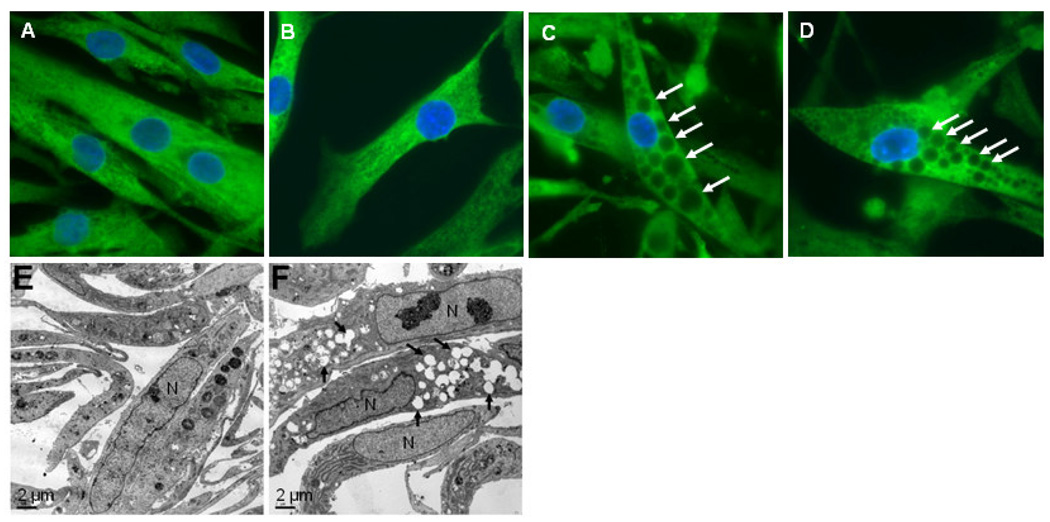
To determine the consequences of the mutations on the subcellular localization of the VCP proteins, the primary myoblasts were plated on coverglasses, fixed, stained with a VCP-specific antibody, and analyzed by immunofluorescence microscopy. These analyses showed that both wild-type and mutant VCP were distributed throughout the cytoplasm (Figure 1A–D). Mutant cell lines contained large vacuoles, which were not observed in control cells. The vacuole containing cells tended to be more abundant when the passage number increased; in newly plated cultures with 3 to 4 passages approximately 10–20% of cells were vacuolated, whereas with 10 to12 passages 70–80% of cells contained enlarged vacuoles. The absence of vacuoles in wild-type myoblast cells was confirmed by analyzing 5 additional control cell lines. None of these control cell lines showed any vacuolization seen in the mutant cell lines (data not shown). To confirm these light microscopic results and to analyze the ultra-structures of cultured myoblasts, we examined the wild-type and mutant myoblasts by electron microscopy. These analyses demonstrated the accumulation of large vacuoles in mutant cell lines, further confirming the results obtained by immunofluorescence microscopy (Figure 1E–F).
Figure 1. Vacuolization of IBMPFD myoblasts.
A–D) Human myoblasts were fixed, stained with a VCP-specific antibody, and viewed with an immunofluorescence microscope. Nuclei were stained with DAPI. Some of the vacuoles are indicated by arrows (C, D). A = wild-type A, B = wild-type B, C = R155H, D = R155S. Magnification 630x. E–F) Electron micrographs of the human myoblasts. E = wild-type A, F = R155H. Some of the vacuoles are indicated by arrows (F). Magnification 900x. Scale bar = 2µm.
3.2 Lamp1 and Lamp2 are differentially N-glycosylated in mutant myoblasts
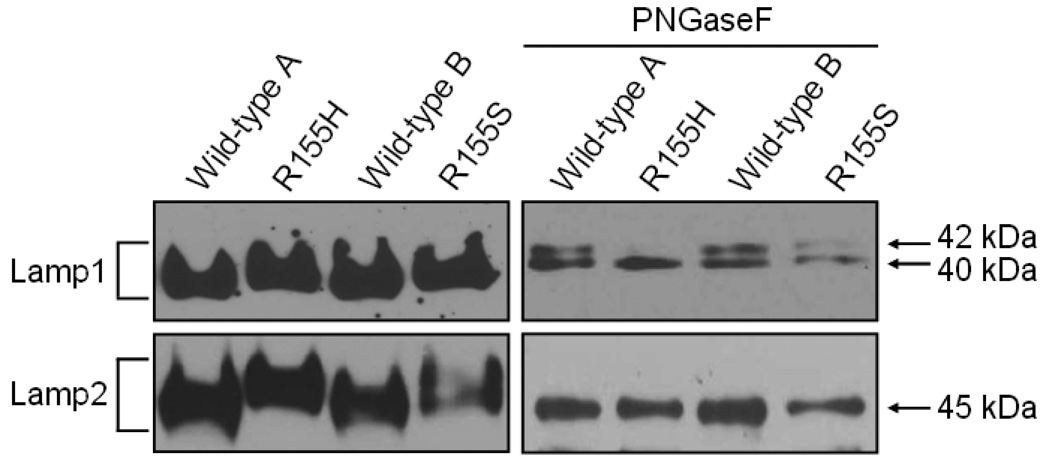
Next we analyzed the properties of the Lamp-proteins using Western blotting analyses of protein lysates from wild-type and mutant myoblasts. The Western blotting results revealed that mutant cells have Lamp1 and Lamp2 proteins of higher molecular weights when compared to the wild-type proteins (Figure 2). This may be caused either by differential post-translational modifications or proteolytic trimming of polypeptides. Since both proteins are known to be heavily N-glycosylated, we removed N-glycans from wild-type and mutant polypeptides by the PNGaseF treatment, and analyzed the results by Western blotting using Lamp1 and Lamp2-specific antibodies. The Lamp1 analyses of wild-type and mutant cell lines resulted in lower molecular weight band of approximately 40 kDa suggesting that the observed difference in the Lamp1 molecular weight is due to differential N-glycosylation. Wild-type cell lines showed also a higher molecular weight band of approximately 42 kDa. Interestingly, the R155H cells did not show this 42 kDa band after the PNGaseF treatment, and the R155S cells showed only a very faint 42 kDa band. The PNGaseF treatment of Lamp2 proteins resulted in a band of 45 kDa in every cell line studied.
Figure 2. Differential N-glycosylation of Lamp1 and Lamp2 proteins in mutant myoblasts.
Proteins were harvested from cultured myoblasts and analyzed by Western blotting using a Lamp1-specific antibody (upper panels) or a Lamp2-specific antibody (lower panel). Panels on the right show the results after PNGaseF treatments, and panels on the left show the results without treatments. Genotypes of the cell lines are indicated above. The brackets on the left indicate the glycosylated forms of Lamp1 and Lamp2 (110–130 kDa), and molecular weights of non-glycosylated Lamp1 and Lamp2 are shown on the right.
3.3 Mutant myoblast cells are defective in maturation to myotubes
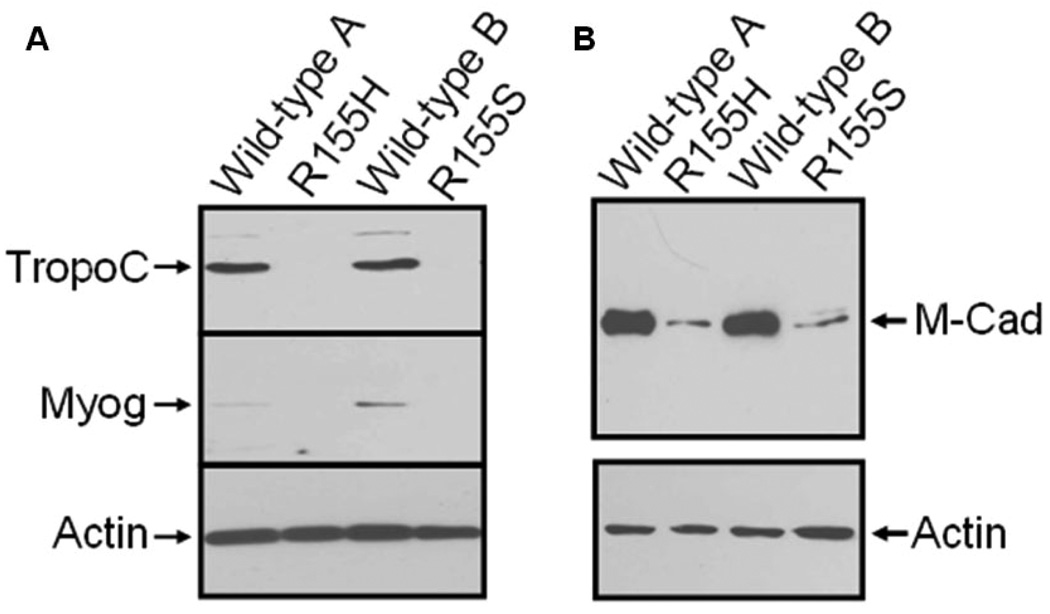
To further characterize the patients’ myoblast cells, we analyzed the maturation processes of cells to myotubes by inducing cells with 2% equine serum for 7 days. Protein lysates of the cell lines were analyzed by Western blotting using troponin C and myogenin-specific antibodies, both of which are expressed in differentiated myotubes. The Western blotting results revealed that both proteins are expressed in control cell lines, but not in mutant cell lines (Figure 3A).
Figure 3. Down-regulation of troponin C, myogenin and M-cadherin in mutant myoblasts.
Human myoblasts were induced with equine serum for 7 days. A) Proteins were harvested from cultured myoblasts and analyzed by Western blotting using a troponin C-specific antibody (upper panel), a myogenin-specific antibody (middle panel) or an actin-specific antibody (lower panel). The arrows on the left indicate the troponin C (TropoC), myogenin (Myog), and actin bands. Cell lines are indicated above. B) Proteins were harvested from cultures myoblasts and analyzed by Western blotting using an M-cadherin-specific antibody (upper panel) or an actin-specific antibody (lower panel). Genotypes of the cell lines are indicated above, and the arrows on the right indicate the M-cadherin (M-Cad), and actin bands.
Defective cell fusion may be caused by altered expression levels of cell surface proteins that are involved in cell adhesion. To study if this hypothesis holds true, we determined the expression levels of the cell surface protein M-cadherin, which is needed for normal myoblast cell fusion. Our Western blotting results demonstrated significantly reduced M-cadherin expression in the mutant myoblast cell lines when compared to the control cell lines (Figure 3B).
3.4 Mutant myoblasts show increased apoptosis and autophagy
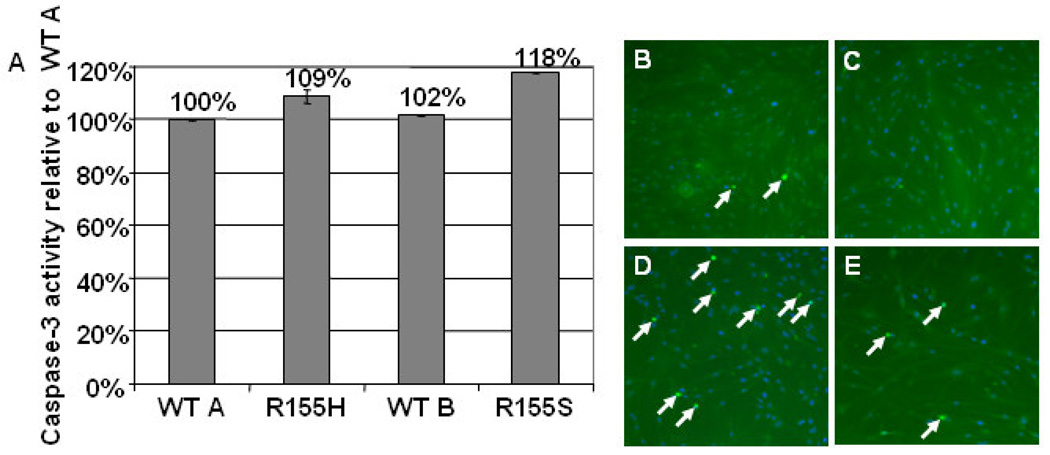
To analyze if cell death is increased in mutant myoblasts, we examined the level of apoptosis by TUNEL staining and the Caspase-3 assay. Mutant cell lines showed increased apoptosis, which was demonstrated by increased Caspase-3 activity; the increase in the R155H cell line was 9% and in the R155S cell line 18% when compared to the wild-type cell lines (Figure 4A). Additionally, the TUNEL-staining demonstrated significantly more apoptotic cell nuclei in mutant cell lines when compared to the control cell lines (Figure 4B–E).
Figure 4. Increased apoptosis in mutant myoblasts.
A) Caspase-3 activity was measured using a CaspACE Assay System. Results were obtained by a spectrophotometer and are presented as relative values to wild-type A in the Y-axis. Cell lines are indicated in the X-axis. B–E) Nuclear DNA degradation of wild-type (B = wild-type A, C = wild-type B) and mutant (D = R155H, E = R155S) cells was analyzed by a TUNEL-system kit. Results were analyzed with an immunofluorescence microscope. Nuclei were stained with DAPI. Apoptotic cell nuclei are shown by arrows. Note that panel E has lower cell density than other panels. Magnification 100x.
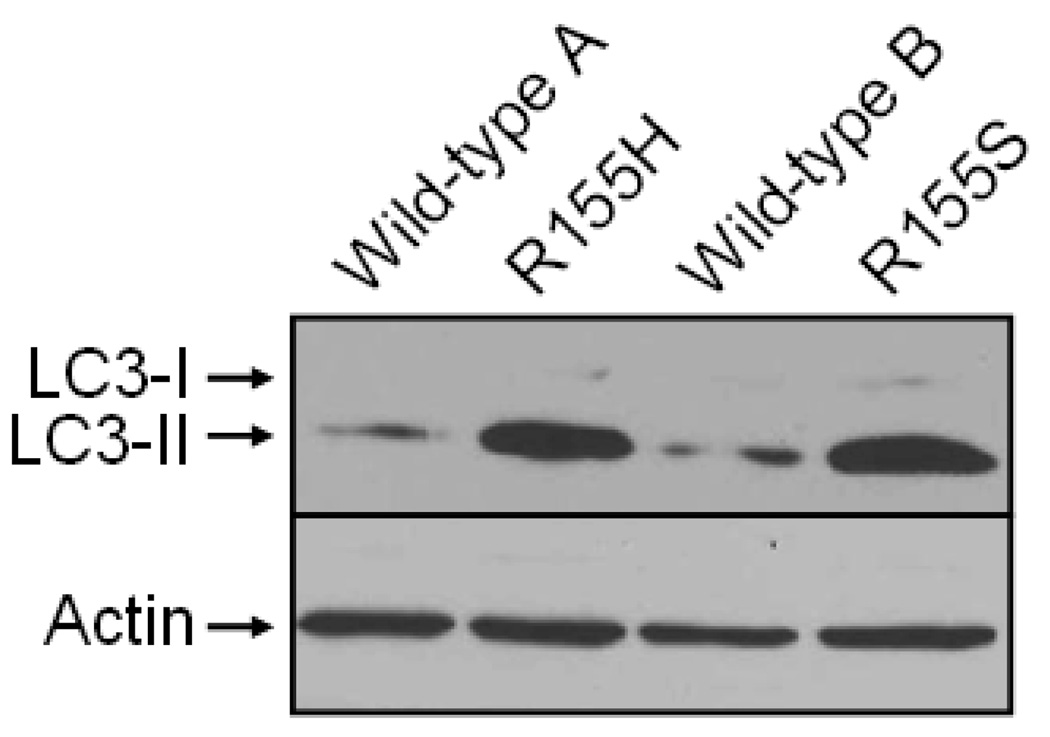
Finally, we analyzed if nutrient deprivation results in increased autophagy in mutant myoblasts. For this purpose, we starved wild-type and mutant myoblasts in the Skeletal Muscle Cell Growth Medium lacking serum and the supplement mix, and stained Western blots of myoblast cell lysates with an LC3-specific antibody. The Western blotting analysis demonstrated that the protein lysates extracted from the mutant cells have significantly increased amount of LC3-II when compared to the wild-type cell lines (Figure 5).
Figure 5. Increased autophagy in starved mutant myoblasts.
Proteins were harvested from starved, cultured myoblasts and analyzed by Western blotting using an LC3-specific antibody (upper panel) or an actin-specific antibody (lower panel). Cytosolic (LC3-I) and autophagic (LC3-II) forms of LC3, as well as actin bands are indicated on the left. Cell lines are indicated above.
4. DISCUSSION
VCP associated disease results in primarily proximal vacuolar inclusion body myopathy, Paget’s disease of the bone, and FTD [6, 21]. To date, several disease mutations have been reported, all of which are missense mutations causing an amino acid change in the N-terminal part of the polypeptide [22]. The high evolutionary conservation suggests an important role for the VCP protein in the normal cellular functions. This is further supported by the finding that homozygous knock-out mice lacking both VCP alleles die in early embryogenesis [23]. On the other hand, the transgenic mice over-expressing the R155H mutation show muscle weakness and muscle tissue pathology similar to human IBMPFD patients [24]. The pathological mechanisms resulting in the clinical and cellular phenotypes in the muscle tissues of the human and mouse are still to be resolved. To begin to resolve these mechanisms, we have analyzed human primary myoblast cell lines. By analyzing patients’ cells that express the mutant VCP at an endogenous level, one can preclude the possibility of over-expression artifacts, and therefore these cells can be considered a better model to study cellular pathogenesis of the disease than over-expressing cell models.
Immunocytochemical results showed that the mutant protein is expressed at the similar level to the wild-type protein, and is targeted to the cytoplasm but for an unknown reason it cannot fulfill its proper cellular function at its final destination. This hypothesis is further supported by earlier studies showing normal hexamer formation and normal to elevated ATPase activities for the mutant VCP protein [16, 17]. Therefore, it is possible that synthesis, processing and targeting of the mutant polypeptides are intact but when hexamers with at least one mutant molecule are formed, their function is severely affected resulting in defective cellular processes. The generation of a knock-out mouse model having only one functional VCP gene also favors this hypothesis, since heterozygous mice having one wild-type allele and one deleted allele are indistinguishable from their wild-type littermates [23].
Vacuolization has previously been reported in many cell types (COS, HeLa and PC12) over-expressing the VCP mutant K524A, which affects the ATPase activity [25]. Here we report the findings of large intracellular vacuoles in mutant myoblast cultures. Ubiquitin or VCP did not accumulate in the vacuoles but other, still unidentified molecules fill the lumen of these organelles. Similar cellular pathology has been reported in Danon disease, another genetic disease characterized by myopathy with vacuolar accumulation seen in 10% of the skeletal muscle fibers [26], heart disease, and mental retardation [27]. This disease is caused by mutations in the Lamp2 gene that encodes a lysosomal membrane protein [28]. Danon disease is considered an autophagic vacuolar myopathy showing intracytoplasmic vacuoles, which are believed to be autolysosomes [29]. These similarities in clinical and cellular phenotypes urged us to analyze patients’ myoblasts in regard to lysosomal membrane proteins. The Western blotting analysis showed increased molecular weights for Lamp1 and Lamp2 in mutant myoblast cells, which was due to defective N-glycosylation processes of these two proteins. Based on these findings, we hypothesize that the N-glycosylation of Lamp-proteins is disturbed in VCP disease patients’ myoblasts, which may be involved in the defective degradation of vacuolar contents.
Myotube formation is a multi-step process that is regulated by two types of fusion events [30]. First, myoblasts fuse together to form myotubes containing few nuclei. Subsequently, additional fusion between myoblasts and nascent myotubes results in the formation of large myotubes containing multiple nuclei. These myoblast fusion processes can be studied by analyzing the expression of proteins that are specific for myotubes. Our Western blotting analyses using antibodies raised against myotube specific proteins troponin C and myogenin suggest that the VCP disease mutations result in defective cell fusion processes in myoblast cultures. The observed defective cell fusion may be caused by affected expressions of cell surface proteins. We tested this hypothesis by analyzing the expression of the transmembrane cell adhesion molecule M-cadherin, of which intact expression is required for normal myoblast fusion. M-cadherin is predominantly expressed in developing skeletal muscle, and in mature skeletal muscle it is detectable in satellite cells and on the sarcolemma of myofibers underlying satellite cells [31–33]. The observed significant down-regulation of M-cadherin in patients’ myoblasts suggests that it may play an important role in the defective myotube formation in the mutant myoblasts. However, we cannot exclude the possibility of the involvement of other cell adhesion molecules in this multi-step process of myotube formation. Similar findings are seen in Oculopharyngeal muscular dystrophy (OPMD), which is an adult-onset autosomal dominant disease associated with drooping of eyelids and swallowing problems around the age of 50 years. The disease is associated with an expansion in the GCG trinucleotide repeat of the nuclear poly(A)-binding protein (PABPN1) gene [34]. The hallmark of OPMD is the presence of intranuclear inclusions in the skeletal muscles of patients, and ectopic expression of the mutant PABPN1 produced inclusions in a muscle cell culture model and reduced expression of several muscle-specific proteins including α-actin, troponin C, and myogenic transcription factors, myogenin and MyoD [35].
Lastly, we analyzed autophagy and the fate of cultured myoblasts. Autophagy is a process that degrades long-lived proteins and cytoplasmic components within vesicles which deliver the contents to the lysosome/vacuole for degradation. Upon activation of autophagy, the 18 kDa cytosolic LC3 (LC3-I) undergoes proteolytic cleavage followed by a lipid modification and is converted to the 16 kDa membrane-bound form (LC3-II), which is specifically localized to the autophagosomal membranes [36]. The conversion from LC3-I to LC3-II can be used as a sensitive marker for distinguishing autophagy in mammalian cells. The increased LC3-II form in mutant myoblasts cultured in starvation condition suggests that mutant cells are prone to the increased level of autophagy, and muscle cell defects can be worsened by insufficient nutrition. Increased autophagy has also been reported in another myopathy, hereditary inclusion body myopathy (hIBM), which is caused by mutations in the UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase (GNE). The analyses of patients’ tissues revealed that the observed cellular organelles represent autophagic vacuoles [37, 38]. Based on these similarities between IBMPFD and hIBM, it is likely that these two adult-onset myopathies share similar pathological pathways, and the vacuolization plays an important role in the development of muscle weakness. The defective processing of Lampproteins in patients’ myoblasts may be connected to the increased vacuolization. This hypothesis is supported by the observation that the mice that are double deficient in Lamp1 and Lamp2 show a much higher frequency of cytoplasmic vacuoles, which are often identified as autophagic vacuoles [39]. It is possible that although the initial maturation of autophagosomes including the fusion with endosomes is functional, the final maturation steps of late autophagic vacuoles may be retarded.
The Caspase-3 assay and TUNEL staining demonstrated increased apoptosis in patients’ myoblasts. This may indicate that the accumulation of storage material in enlarged vacuoles and defective autophagy in patients’ myoblasts is harmful to the normal functioning of differentiated muscle cells. This, in turn, may result in increased apoptosis of patients’ myoblasts. Myotubes are terminally differentiated cells that have lost their ability to proliferate, and therefore the dysfunctions of their cellular processes make them prone to cell death. Similar hypothesis has been proposed for a group of genetic neurodegenerative diseases, e.g. Neuronal Ceroid Lipofuscinoses (NCL) (for review see [40]), which are characterized by accumulation of storage material in patients’ cells. Despite the accumulation of inclusions in many cell types, only neurons are affected resulting in increased apoptosis of neurons in patients’ central nervous system, whereas other, still dividing cells seem to be unaffected. Thus, both IBMPFD and NCL are characterized by accumulation of storage material in terminally differentiated cells types, which results in death of patients’ cells and manifestation of clinical symptoms later in the progression of the disease.
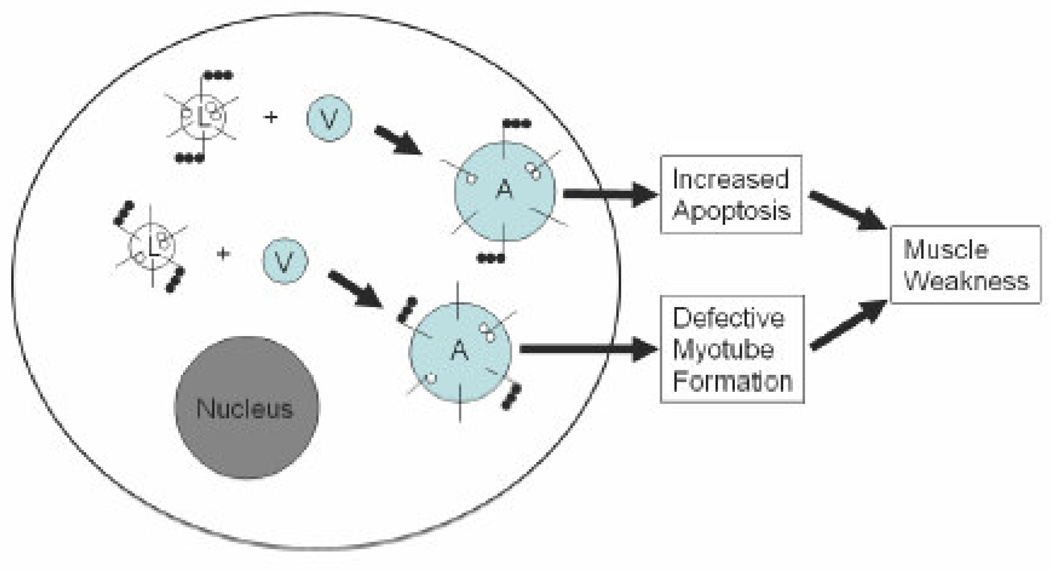
In conclusion, we have shown that the VCP mutations result in vacuolization of patients’ myoblasts. These vacuoles are, for unknown reason, unable to degrade the vacuolar contents. We also observed that the vacuolization increases with the age of the mutant cells. This possibly results in disturbed cellular processes in patients’ muscle cells including defective myotube formation, increased apoptosis, and increased autophagy (Figure 6). These defects may partially explain the observed myopathy in patients with VCP associated disease. Therefore, patients’ myoblasts demonstrate a useful cell model that can be utilized in our future studies to clarify the detailed molecular pathways resulting in myopathy and also for the development of novel treatments to alleviate these pathological manifestations in IBMPFD patients.
Figure 6. Potential pathogenesis of the IBMPFD muscle.
Lamp-proteins (short black lines) of the mutant cells are differentially glycosylated (small white balls in the lumen) and ubiquitinated (small black balls in the cytosol) in the lysosomes (L). These lysosomes are able to fuse with vacuoles (V) but are unable to degrade the vacuolar contents resulting in the accumulation of enlarged autophagosomes (A). The accumulation of undegraded material causes increased apoptosis and defective myotube formation, which eventually lead to progressive muscle weakness observed in IBMPFD patients. Nucleus is shown in grey.
ACKNOWLEDGMENTS
We thank the families, their physicians and our many collaborators for their contributions to this work. Human myoblast cell lines were obtained from the Muscle Tissue Culture Collection at the Friedrich-Baur-Institute (Department of Neurology, Ludwig-Maximilians-University, Munich, Germany) and Dr. Charlotte Peterson (University of Kentucky, Lexington, KY). The Muscle Tissue Culture Collection, which is a part of the German network on muscular dystrophies (MD-NET, service structure S1, 01GM0601) is funded by the German ministry of education and research (BMBF, Bonn, Germany). The Muscle Tissue Culture Collection is a partner of Eurobiobank (www.eurobiobank.org). The Telethon Network of Genetic Biobanks (GTB07001F to M. Mora) and EuroBioBank are gratefully acknowledged for providing five human control myoblast cell lines. This work was supported by the National Institute of Health (R01AR050236) and the Muscular Dystrophy Association (Development grant for J. Vesa).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kimonis VE, Kovach MJ, Waggoner B, et al. Clinical and molecular studies in a unique family with autosomal dominant limb-girdle muscular dystrophy and Paget disease of bone. Genet Med. 2000;2:232–241. doi: 10.1097/00125817-200007000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimonis VE, Mehta SG, Fulchiero EC, et al. Clinical studies in familial VCP myopathy associated with Paget disease of bone and frontotemporal dementia. Am J Med Genet. 2008;A146:745–757. doi: 10.1002/ajmg.a.31862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weihl CC, Temiz P, Miller SE, et al. TDP-43 accumulation in inclusion body myopathy muscle suggests a common pathogenic mechanism with frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2008;79:1186–1189. doi: 10.1136/jnnp.2007.131334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller BL, Ikonte C, Ponton M, et al. A study of the Lund-Manchester research criteria for frontotemporal dementia: clinical and single-photon emission CT correlations. Neurology. 1997;48:937–942. doi: 10.1212/wnl.48.4.937. [DOI] [PubMed] [Google Scholar]
- 5.Mehta SG, Watts GD, Adamson JL, et al. APOE is a potential modifier gene in an autosomal dominant form of frontotemporal dementia (IBMPFD) Genet Med. 2007;9:9–13. doi: 10.1097/gim.0b013e31802d830d. [DOI] [PubMed] [Google Scholar]
- 6.Watts GD, Wymer J, Kovach MJ, et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36:377–381. doi: 10.1038/ng1332. [DOI] [PubMed] [Google Scholar]
- 7.Wang Q, Song C, Li CC. Molecular perspectives on p97-VCP: progress in understanding its structure and diverse biological functions. J Struct Biol. 2004;146:44–57. doi: 10.1016/j.jsb.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Braun RJ, Zischka H. Mechanisms of Cdc48/VCP-mediated cell death: from yeast apoptosis to human disease. Biochim Biophys Acta. 2008;1783:1418–1435. doi: 10.1016/j.bbamcr.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Rabouille C, Kondo H, Newman R, Hui N, Freemont P, Warren G. Syntaxin 5 is a common component of the NSF- and p97-mediated reassembly pathways of Golgi cisternae from mitotic Golgi fragments in vitro. Cell. 1998;92:603–610. doi: 10.1016/s0092-8674(00)81128-9. [DOI] [PubMed] [Google Scholar]
- 10.Rabinovich E, Kerem A, Frohlich KU, Diamant N, Bar-Nun S. AAA-ATPase p97/Cdc48p, a cytosolic chaperone required for endoplasmic reticulum-associated protein degradation. Mol Cell Biol. 2002;22:626–634. doi: 10.1128/MCB.22.2.626-634.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarosch E, Geiss-Friedlander R, Meusser B, Walter J, Sommer T. Protein dislocation from the endoplasmic reticulum--pulling out the suspect. Traffic. 2002;3:530–536. doi: 10.1034/j.1600-0854.2002.30803.x. [DOI] [PubMed] [Google Scholar]
- 12.Kondo H, Rabouille C, Newman R, et al. p47 is a cofactor for p97-mediated membrane fusion. Nature. 1997;388:75–78. doi: 10.1038/40411. [DOI] [PubMed] [Google Scholar]
- 13.Meyer HH, Shorter JG, Seemann J, Pappin D, Warren G. A complex of mammalian ufd1 and npl4 links the AAA-ATPase, p97, to ubiquitin and nuclear transport pathways. Embo J. 2000;19:2181–2192. doi: 10.1093/emboj/19.10.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai RM, Li CC. Valosin-containing protein is a multi-ubiquitin chain-targeting factor required in ubiquitin-proteasome degradation. Nat Cell Biol. 2001;3:740–744. doi: 10.1038/35087056. [DOI] [PubMed] [Google Scholar]
- 15.Rape M, Hoppe T, Gorr T, Kalocay M, Richly H, Jentsch J. Mobilization of processed, membrane-tethered SPT23 transcription factor by CDC48(UFD1/NPL4), a ubiquitin-selective chaperone. Cell. 2001;107:667–677. doi: 10.1016/s0092-8674(01)00595-5. [DOI] [PubMed] [Google Scholar]
- 16.Weihl CC, Dalal S, Pestronk A, Hanson PI. Inclusion body myopathy-associated mutations in p97/VCP impair endoplasmic reticulum-associated degradation. Hum Mol Genet. 2006;15:189–199. doi: 10.1093/hmg/ddi426. [DOI] [PubMed] [Google Scholar]
- 17.Kakizuka A. Roles of VCP in human neurodegenerative disorders. Biochem Soc Trans. 2008;36:105–108. doi: 10.1042/BST0360105. [DOI] [PubMed] [Google Scholar]
- 18.Bechet D, Tassa A, Taillandier D, Combaret L, Attaix D. Lysosomal proteolysis in skeletal muscle. Int J Biochem Cell Biol. 2005;37:2098–2114. doi: 10.1016/j.biocel.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 19.Malicdan MC, Noguchi S, Nonaka I, Saftig P, Nishino I. Lysosomal myopathies: an excessive build-up in autophagosomes is too much to handle. Neuromuscul Disord. 2008;18:521–529. doi: 10.1016/j.nmd.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nature reviews. 2007;8:931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- 21.Kimonis V, Watts G. GeneTests. Seattle: University of Washington; 2007. Inclusion Body Myopathy Associated with Paget Disease of Bone and/or Frontotemporal Dementia. www.genetests.org. [Google Scholar]
- 22.Kimonis VE, Fulchiero E, Vesa J, Watts G. VCP disease associated with myopathy, Paget disease of bone and frontotemporal dementia: Review of a unique disorder. Biochim Biophys Acta. 2008;1782:744–748. doi: 10.1016/j.bbadis.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Muller JM, Deinhardt K, Rosewell I, Warren G, Shima DT. Targeted deletion of p97 (VCP/CDC48) in mouse results in early embryonic lethality. Biochem Biophys Res Commun. 2007;354:459–465. doi: 10.1016/j.bbrc.2006.12.206. [DOI] [PubMed] [Google Scholar]
- 24.Weihl CC, Miller SE, Hanson PI, Pestronk A. Transgenic expression of inclusion body myopathy associated mutant p97/VCP causes weakness and ubiquitinated protein inclusions in mice. Hum Mol Genet. 2007;16:919–928. doi: 10.1093/hmg/ddm037. [DOI] [PubMed] [Google Scholar]
- 25.Hirabayashi M, Inoue K, Tanaka K, et al. VCP/p97 in abnormal protein aggregates, cytoplasmic vacuoles, and cell death, phenotypes relevant to neurodegeneration. Cell Death Differ. 2001;8:977–984. doi: 10.1038/sj.cdd.4400907. [DOI] [PubMed] [Google Scholar]
- 26.Lobrinus JA, Schorderet DF, Payot M, et al. Morphological, clinical and genetic aspects in a family with a novel LAMP-2 gene mutation (Danon disease) Neuromuscul Disord. 2005;15:293–298. doi: 10.1016/j.nmd.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Danon MJ, Oh SJ, DiMauro S, et al. Lysosomal glycogen storage disease with normal acid maltase. Neurology. 1981;31:51–57. doi: 10.1212/wnl.31.1.51. [DOI] [PubMed] [Google Scholar]
- 28.Nishino I, Fu J, Tanji K, et al. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease) Nature. 2000;406:906–910. doi: 10.1038/35022604. [DOI] [PubMed] [Google Scholar]
- 29.Sugie K, Noguchi S, Kozuka Y, et al. Autophagic vacuoles with sarcolemmal features delineate Danon disease and related myopathies. J Neuropathol Exp Neurol. 2005;64:513–522. doi: 10.1093/jnen/64.6.513. [DOI] [PubMed] [Google Scholar]
- 30.Horsley V, Pavlath GK. Forming a multinucleated cell: molecules that regulate myoblast fusion. Cells Tissues Organs. 2004;176:67–78. doi: 10.1159/000075028. [DOI] [PubMed] [Google Scholar]
- 31.Moore R, Walsh FS. The cell adhesion molecule M-cadherin is specifically expressed in developing and regenerating, but not denervated skeletal muscle. Development. 1993;117:1409–1420. doi: 10.1242/dev.117.4.1409. [DOI] [PubMed] [Google Scholar]
- 32.Rose O, Rohwedel J, Reinhardt S, et al. Expression of M-cadherin protein in myogenic cells during prenatal mouse development and differentiation of embryonic stem cells in culture. Dev Dyn. 1994;201:245–259. doi: 10.1002/aja.1002010308. [DOI] [PubMed] [Google Scholar]
- 33.Cifuentes-Diaz C, Nicolet M, Alameddine H, et al. M-cadherin localization in developing adult and regenerating mouse skeletal muscle: possible involvement in secondary myogenesis. Mech Dev. 1995;50:85–97. doi: 10.1016/0925-4773(94)00327-j. [DOI] [PubMed] [Google Scholar]
- 34.Brais B, Bouchard JP, Xie YG, et al. Short GCG expansions in the PABP2 gene cause oculopharyngeal muscular dystrophy. Nat Genet. 1998;18:164–167. doi: 10.1038/ng0298-164. [DOI] [PubMed] [Google Scholar]
- 35.Wang Q, Bag J. Ectopic expression of a polyalanine expansion mutant of poly(A)-binding protein N1 in muscle cells in culture inhibits myogenesis. Biochem Biophys Res Commun. 2006;340:815–822. doi: 10.1016/j.bbrc.2005.12.078. [DOI] [PubMed] [Google Scholar]
- 36.Mizushima N, Yamamoto A, Matsui M, Yoshimori M, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishino I. Autophagic vacuolar myopathies. Curr Neurol Neurosci Rep. 2003;3:64–69. doi: 10.1007/s11910-003-0040-y. [DOI] [PubMed] [Google Scholar]
- 38.Tsuruta Y, Furuta A, Furuta K, Yamada T, Kira J, Iwaki T. Expression of the lysosome-associated membrane proteins in myopathies with rimmed vacuoles. Acta Neuropathol. 2001;101:579–584. doi: 10.1007/s004010000329. [DOI] [PubMed] [Google Scholar]
- 39.Eskelinen EL, Schmidt CK, Neu S, et al. Disturbed cholesterol traffic but normal proteolytic function in LAMP-1/LAMP-2 double-deficient fibroblasts. Mol Biol Cell. 2004;15:3132–3145. doi: 10.1091/mbc.E04-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peltonen L, Savukoski M, Vesa J. Genetics of the neuronal ceroid lipofuscinoses. Curr Opin Genet Dev. 2000;10:299–305. doi: 10.1016/s0959-437x(00)00086-1. [DOI] [PubMed] [Google Scholar]