Abstract
Apolipoprotein-E protein is an endogenous immunomodulatory agent that affects both the innate and the adaptive immune responses. Since individuals with the APOE4 gene demonstrate worsened pathology and poorer outcomes in many neurological disorders, we examined isoform specific differences in the response of microglia, the primary cellular component of the brain’s innate immune response, in detail. Our data demonstrate that microglia derived from APOE4/4 targeted replacement mice demonstrate a pro-inflammatory phenotype that includes altered cell morphology, increased NO production associated with increased NOS2 mRNA levels, and higher pro-inflammatory cytokine production (TNFα, IL-6, IL12p40) compared to microglia derived from APOE3/3 targeted replacement mice. The effect is gene dose dependent and increases with the number of APOE4 gene alleles. The APOE genotype-specific immune profile observed in the microglial immune response is also observed in the cortex of aged APOE3/3 and APOE4/4 mice treated with lipopolysacchride (LPS) and in peripheral (peritoneal) macrophages. To determine if APOE4’s action resulted from an isoform specific difference in effective levels of the apolipoproteins, we generated mice expressing only a single allele of APOE3. Immune-stimulated macrophages from APOE3/0 mice demonstrated an increased inflammatory response compared to APOE3/3 mice, but less than in APOE4/4 mice. These data suggest that inhibition of inflammation depends upon the dose of apoE3 protein available and that apoE4 protein may alter inflammation partly by dose effects and partly by being qualitatively different than apoE3 Overall, these data emphasize the important role of apolipoprotein E and of the APOE genotype on the immune responses that are evident in most, in not all, neurological disease.
Keywords: microglia, apolipoprotein E, innate immunity, cytokine, neuroinflammation
1. Introduction
In the early 1980s, an elegant series of studies demonstrated that plasma lipoproteins such as apolipoprotein E (apoE) and apolipoprotein B (apoB), suppressed phytohemagglutinin-stimulated proliferation, inhibited DNA synthesis and reduced phospholipid turnover in T cells [2;20;33]. These data indicated that lymphocyte function and hence, adaptive (humoral) immunity were regulated by a subclass of apolipoproteins including apoE. Recently, apoE has also been shown to play a role in the innate immune response [3;5;8;32;38;45]. Mice that lack an APOE gene and apoE protein demonstrate a large increase in pro-inflammatory cytokines [14;25] suggesting that the presence of apoE regulates macrophage function.
The most prevalent variant of the APOE gene in the human population encodes the apoE3 protein, while other common variants that encode the apoE2 and apoE4 proteins are associated with disease [36;42]. A dose-dependent inheritance of the APOE4 allele is associated with increased risk for developing Alzheimer’s disease (AD) [12;42] and poor outcome from neurological injury [7;12;18;22;24].. Our previous studies have strongly suggested that APOE4’s role in AD and neurological injury may be linked, in part, to an aberrant immunomodulatory role. We have shown that macrophage function is regulated by apoE in an isoform specific manner such that the presence of the APOE4 allele is associated with an enhanced inflammatory response compared to macrophages not expressing an APOE4 allele [5;8;10;13]. Lynch et al [32] and more recently, Ophir et al [38] have shown that either intraperitoneal or intraventricular injection of LPS into the brains of APOE3/3 and APOE4/4 mice result in significantly higher levels of pro-inflammatory cytokines, defense response genes or chemotaxis genes in APOE4/4 mice compared to APOE3/3 mice. The higher production of cytoactive agents by APOE4/4 microglia has been linked to an increased bystander injury to cultured neurons in the presence of activated microglia [35].
Since microglia are a primary cellular component of the brain’s innate immune response, we have examined the APOE genotype-specific differences in microglial production of cytoactive factors in more detail. Our data demonstrate that microglia derived from APOE4/4 targeted replacement mice demonstrate a pro-inflammatory phenotype that includes altered cell morphology and increased production of cytoactive factors. The gentoype specific effects are global in nature and extend to peripheral macrophage function and to whole brain. In addition, the immune effect observed in APOE4 mice is produced by the presence of the APOE4 gene alleles in addition to effective changes in apolipoprotein E levels.
Methods
Reagents
1400W (Alexis Biochemicals, San Diego, CA), an iNOS specific inhibitor, was prepared in sterile 1X PBS and diluted in microglial media to a 5 µM or 25 µM concentration for experiments. Arginine free medium was purchased from ICN Biomedicals, Costa Mesa, CA.
Animals
Homozygous apolipoprotein E targeted replacement (APOE TR) mice contain a targeted insertion of exons 2–4 of the human APOE2, APOE3 or APOE4 gene that replaces the corresponding genomic DNA at the mouse APOE locus [44]. APOE2/4-TR and APOE3/4-TR heterozygotes were bred by crossing APOE2/2 [44] or APOE3/3 TR homozygotes with APOE4/4 TR homozygotes. APOE3/0 mice were produced by crossing APOE3/3 to APOE knockout (APOE0/0) mice. All animals were housed under a standard light:dark cycle and received food and water ad libitum. All experiments were conducted in compliance with rules and regulations of the Duke University Institutional Animal Care and Use Committee.
Preparation of Microglial Cultures
Enriched microglial cultures were prepared from postnatal day 0–2 pups (gender identity unknown). Briefly, whole brains were removed and placed into sterile phosphate buffered saline (PBS) containing 100µg/ml penicillin/streptomycin and 0.5% fungizone (Invitrogen, Rockville, MD). Meninges were removed under a dissecting microscope and cortices were dissociated using the Papain Dissociation System (Worthington Biochemical Corp) as described by the manufacturer. A mixed glial culture was prepared by plating dissociated cells into 75 mm flasks and growing them at 37°C, 5% CO2 in growth media (DMEM containing 10% bovine fetal calf serum (FBS), 2 mM glutamine and 100µg/ml penicillin/streptomycin). After 4–5 days, cultures were enriched for microglia (>95%) by replacing the growth media with fresh growth media containing 10% horse serum in place of fetal bovine serum (FBS). After an additional 3–5 days, loosely adherent cells were removed from the culture by shaking on a rotary shaker (240 rpm) for 2 hours. The cells were pelleted by centrifugation (1000g; 10 min) and resuspended into serum free medium. These cells were then plated into 96 well dishes for NO and cytokine determination or into 24 well dishes for mRNA or protein determinations. In some cases, the mixed glial culture was grown directly into 96 well dishes and the cultures enriched to microglia in the same manner. To determine the number of contaminating cells, separate control wells were periodically assessed for microglia using binding of the lectin, Griffonia simplicifolia (GS-1), coupled to peroxidase for cytochemical microglial identification. Color was developed using nickel-enhanced diaminobenzidine (DAB) according to manufacturer’s instructions (SigmaFast DAB, Sigma) and cells were visualized using a Nikon inverted microscope (Nikon, Melville, NY). Cell viability was assessed using the MTT assay as previously described [5].
Peritoneal macrophage isolation and culture
To promote the entry of circulating monocytic cells into the peritoneum, macrophages from adult mice were elicited prior to isolation by intraperitoneal (ip) injection of each mouse with 5mM sodium periodate. Only adult male mice were used in these studies. After 72 hours, the mice were killed and the peritoneal cavities were flushed 2 times with warm PBS to obtain macrophages. Lavage fluid containing cells was carefully extracted from the peritoneum and the fluid containing cells from 2 to 3 mice of the same genotype was pooled. In this manner a sufficient number of macrophages were obtained for the experimental assays. The cells were pelleted by centrifugation at 1000g and then re-suspended into phenol red–free, serum-free media (high glucose DMEM containing 2 mM glutamine and 50 µg/ml gentamycin). Cells were counted, plated directly into 96 well plates and cultured for 2 days in a humidified 5% CO2, 95% air atmosphere. During this time the macrophages attach to the plastic of the culture plate and spread, resembling typical tissue macrophages.
Immune Stimulation in vitro
Microglial or peritoneal macrophage cultures were immune activated by treatment with varying concentrations of recombinant mouse interferon-γ (IFNγ); polyinosinic:polycytidylic acid Poly I:Poly C (PIC), a double stranded RNA viral mimetic and /or lipopolysaccharide (LPS), a bacterial endotoxin prepared from E.coli serotype O11:B5 (all from Sigma, St. Louis, MO) in serum free media for 20–24 hours. Cell death was routinely measured in similarly- treated sister cultures using the MTT assay and was found to be minimal.
Immune activation in vivo
APOE3/3 and APOE4/4 male mice were injected intravenously (tail vein) with 40 µg/kg body weight LPS. Mice were sacrificed at 0, 3,7 or 17 hrs after injection and the cortex rapidly removed and frozen in liquid nitrogen. Frozen cortical tissue was pulverized on dry ice and samples were used for quantitative mRNA analysis.
Measurement of Nitrite and Protein
The supernatant levels of nitrite, a stable oxidation product of nitric oxide (NO), was used as a measure of microglial NO production. Nitrite levels were measured by chemiluminescence using a Sievers 280 Nitric Oxide Analyzer (Sievers, Boulder CO) and a nitrite standard curve prepared from sodium nitrite. Total protein (µg/well) was measured using the BCA method (Pierce, Rockford, IL) based on the manufacturer’s directions using BSA (µg/ml) as standard. Nitrite levels in each well were normalized to total µg protein and expressed as nmol nitrite/µg protein. The average background level of supernatant nitrite produced by untreated cells for each experimental group was determined and subtracted from the average immune-stimulated values.
Measurement of cytokine levels
Supernatant levels of cyokines were determined using either single cytokine ELISAs (for TNFαIL-6 and IL-18) or the Bio-plex cytokine assay using Luminex Bead technology (for IL-1β, IL-12p40, IL-4; IL-5, IFNγ). Single cytokine assays were carried out as described per the manufacturer’s instructions (BioSource International, Camarillo, CA). TMB-One (Promega, Madison, WI) was used for substrate color development and the reaction was stopped using 1M H2SO4. Absorbance was measured at 450 nm using a Molecular Devices microplate reader (Molecular Devices, Sunnydale, CA).. Multiplex bead assays were performed at the Duke University Medical Center Human Vaccine Institute Core facility (under the guidance of Dr. G. Semposki). All cytokine values were normalized to µg protein.
RNA Extraction and Preparation
RNAs from cell cultures or whole brain lysates were isolated using the RNAEasy Mini Kit (Qiagen, Valencia, CA) according to manufacturer’s instructions (Qiagen). RNA was quantified using a Beckman DV530 Life Science UV/Visible Spectrophotometer (Beckman-Coulter, Fullerton, CA) and was reverse transcribed to cDNA using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA)..
Real time PCR
Real time PCR reactions were performed using 100ng cDNA per reaction with a ABI PRISM 7900 HT Sequence Detection System (Applied Biosystems) and Taqman Assays-on-Demand Gene Expression primer/probe sets and the Taqman Universal PCR master mix (all from Applied Biosystems) for the following mouse genes: nitric oxide synthase 2 (NOS2), NOS1 and NOS3 and eukaryotic 18S, a standard housekeeping gene.. Relative mRNA quantitation was calculated using the 2−ΔΔCT method [31] by normalizing the value of the target gene for each sample to its endogenous 18S control value, and then normalizing these values to a baseline sample, designated as the calibrator. In these experiments, the average APOE3/3 sample served as the calibrator.
Statistical Analysis
Samples were assayed and averaged from at least 3 different litter groups (where a litter group is defined as those cells grown from a single mouse litter of 3–5 mouse pups), with a minimum of triplicate wells analyzed per each experimental paradigm for each litter group. Data were analyzed using the Student’s t-test or one-way or two way analysis of variance (ANOVA) with Bonferroni’s post hoc test where appropriate using the Prism 4.0 software package (Graphpad, San Diego, CA). Significance was set at P < 0.05 in all cases.
Results
Activated APOE4/4 microglia are morphologically distinct from APOE3/3 microglia
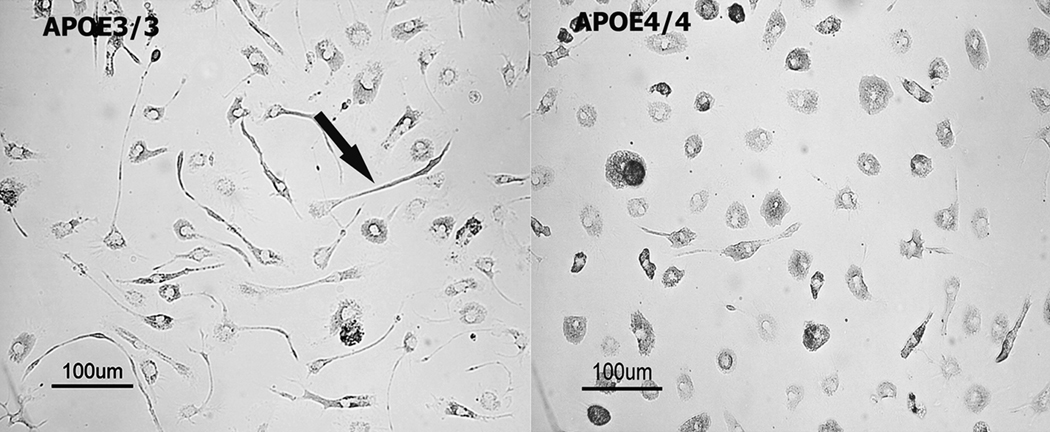
Activated microglia display distinct morphological features largely characterized by an amoeboid morphology and retraction of ramified processes. Bright-field micrographs of APOE3/3 (Figure 1A) and APOE4/4 (Figure 1B) microglia depict two distinct microglial morphologies. APOE3/3 microglia displayed a partial ramified morphology and extended distinct cellular processes while APOE4/4 microglia displayed an amorphous, amoeboid morphology characteristic of both in vivo and in vitro classically activated microglia [39;43].
Figure 1.
Differential morphology in APOE3/3 and APOE4/4 primary microglial cultures. Typical resting (non-immune stimulated) APOE3/3 (left panel) or APOE4/4 (right panel) microglial cultures were viewed under brightfield microscopy. Microglia from APOE4/4 mice were consistently observed to be less branched and demonstrated a rounded, more ameboid appearance compared to APOE3/3 microglia.
Production of inflammatory mediators is increased in APOE4/4 microglial cultures
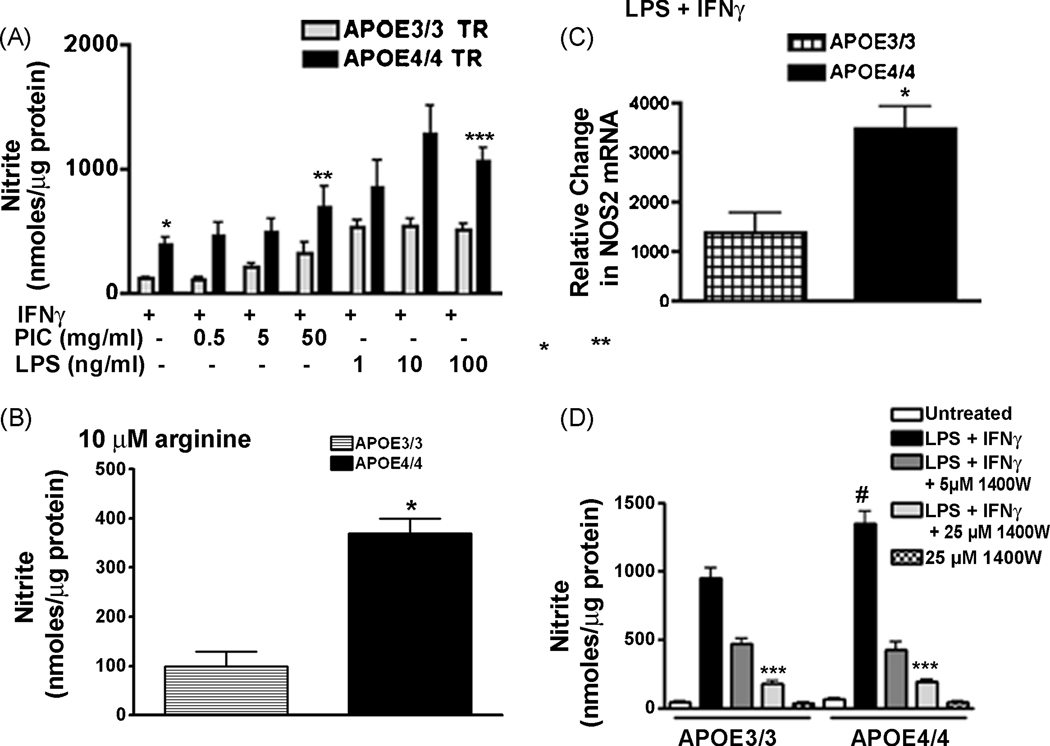
To study the APOE genotype-specific production of inflammatory mediators, we immune stimulated microglia using polycytidylic: polyinosinic acid (PIC), a toll-like receptor -3 (TLR-3) agonist, or lipopolysaccharide (LPS), a TLR-4 agonist. Stimulation was carried out in the presence or absence of interferon γ (IFNγ), a master cytokine that helps to orchestrate inflammation [1]. After 24 hrs the supernatant levels of NO or cytokines were measured and the results were compared across genotypes. Figure 2A shows that nitrite production was significantly higher in APOE4/4 microglia than in APOE3/3 microglia at all doses of either PIC or LPS. The effect of the APOE4 gene was judged as statistically significant for both types of immune activation (p < 0.0001) using 2-way ANOVA. The APOE genotype specific effect was also observed in media that contained levels of arginine similar to the level found in the brain (10 µM) [29]. Normal tissue culture media contains supra-saturating levels of arginine (480 µM), which is the sole substrate of iNOS. Because arginine is rate limiting for iNOS activity, the level of NO produced will change if intracellular arginine levels are reduced as a result of decreasing extracellular arginine concentrations [23]. To determine if an isoform specific difference in NO production was observed at physiological levels of arginine, we pre-incubated microglia in media containing 10 µM arginine for 2 hrs and then immune activated the cells for 24 hrs using LPS + IFNγ. As predicted supernatant nitrite levels were decreased in cells treated with 10 µM arginine compared to cells treated with 480 µM arginine (Fig 2A;B). However, NO production remained significantly greater in microglia from APOE4/4 mice in the presence of 10 µM arginine-containing media compared to similarly treated microglia from APOE3/3 mice (Fig 2B).
Figure 2.
The presence of the APOE4 gene promotes induction of NOS2 mRNA and increased production of NO in immune stimulated microglia: Panel A- Average supernatant levels of nitrite (± SEM) were measured for APOE3/3 or APOE4/4 microglial cultures stimulated for 24 hrs with recombinant murine IFNγ (100 U/ml) plus increasing doses of PIC or LPS. * P <0.04 for IFNγ–treated APOE4/4 microglia compared to IFNγ–treated APOE3/3 microglia; ** p <0.0001 for IFNγ + PIC- treated APOE4/4 microglia compared to APOE3/3 microglia; ***p <0.0001 for IFNγ + LPS - treated APOE4/4 microglia compared to APOE3/3 microglia; all using 2-way ANOVA. A significant effect of dose was observed for PIC treatment (p < 0.001; 2 way ANOVA) but was not observed for LPS treatment. Panel B- Average supernatant levels of nitrite (± SEM) were measured for APOE3/3 or APOE4/4 microglial cultures stimulated for 24 hrs with recombinant murine IFNγ (100 U/ml) + LPS (100 ng/ml) in media containing physiological levels of arginine (10 µm). * p< 0.001 for APOE4/4 compared to APOE3/3 microglia. Panel C- Relative changes in mRNA for NOS2 was determined for LPS (10 ng/ml) + IFNγ (10 U/ml)-treated APOE3/3 microglia compared to APOE4/4 microglia. A significant increase (p < 0.03; unpaired student’s t test) was observed after 6 hrs of treatment in APOE4/4 compared to APOE3/3 microglia. Panel D- A significant decrease (***p < 0.001; 2-way ANOVA) in supernatant nitrite levels was found in both APOE3/3 and APOE4/4 microglia treated with LPS (100 ng/ml) + IFNγ (100 U/ml) in the presence of varying doses of 1400W, an iNOS inhibitor. No effect of 1400W alone was observed. #= p < 0.01 for LPS + IFNγ-treated APOE4/4 microglia compared to APOE3/3 microglia.
To determine if the genotype-specific effect on NO production was generated by changes in the immune-activated induction of NOS genes, we used quantitative RT-PCR to compare the levels of NOS1, NOS2 and NOS3 mRNAs in APOE3/3 and APOE4/4 microglia. As shown in Figure 2C, treatment for 6 hrs with 10ng/ml LPS + 10 U/ml IFNγ produced a 2.5 fold higher level of NOS2 mRNA in APOE4/4 microglia compared to APOE3/3 microglia. mRNAs for NOS1 and NOS3 were below detectable levels. Finally, to confirm the role of iNOS in the NO produced by APOE3/3 and APOE4/4 microglia, we also treated immune-activated microglia with 1400W, a highly specific iNOS inhibitor (Fig 2C) [16]. Simultaneous treatment of microglia with 1400W in the presence of LPS + IFNγ resulted in a dose-dependent suppression of NO production to the same level in the APOE3/3 and APOE4/4 cultures, suggesting that NO production was due to iNOS in both APOE3/3 and APOE4/4 microglia. No effect was observed with 1400W in the absence of LPS + IFNγ.
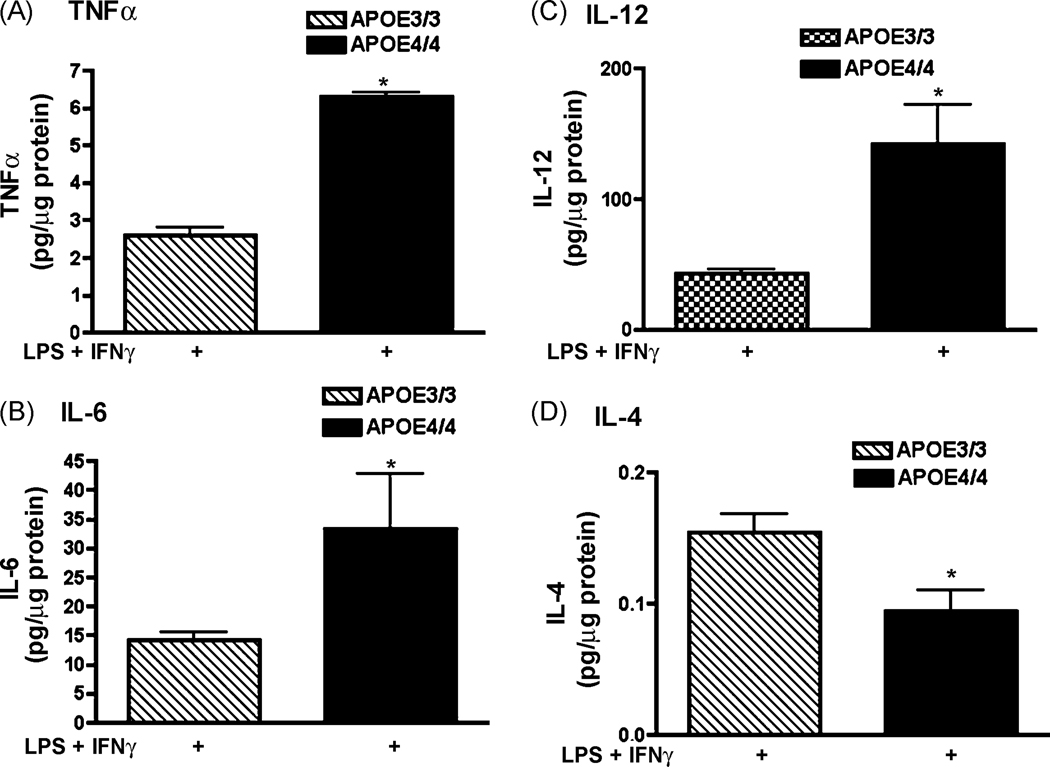
In addition to NO, immune-activated macrophages release cytokines as part of the innate immune response [17;28]. To determine if APOE4/4 microglia also released higher levels of cytokines, microglial supernatants were assayed for both pro-inflammatory (TNFα, IL-1β, IL-6, IL-12p40 and IL-18) and anti-inflammatory cytokine levels using ELISA (IL-4, IL-10). LPS + IFNγ-treatment of APOE4/4 microglia significantly increased TNFα, IL-6 and IL-12p40 levels by approximately 2.4, 2.3 and 3.3 fold, respectively compared to APOE3/3 microglia (Figure 3 A;B;C) while IL-4 levels were significantly lower (Fig 3D; 0.5 fold). No significant difference was observed for IL-18, IL-1β or IL-10 (data not shown).
Figure 3.
Cytokine production in APOE4/4 microglia compared to APOE3/3 microglia. Supernatant levels of TNFα (A); IL-6 (B); IL-12 p40 (C) and IL-4 (D) were measured after 24 hrs of treatment with LPS (100 ng/ml) + IFNγ (100 U/ml). Significantly increased levels for APOE4/4 microglia compared to APOE3/3 microglia were observed for TNFα, IL-6 and IL-12 while a significant decrease was observed for IL-4 (* = p < 0.001; unpaired student’s t test).
An increased pro-inflammatory response is also observed in peripheral macrophages
The APOE genotype-specific effect on immune activation is not limited to the brain but extends to the peripheral innate immune system. Peritoneal macrophages were cultured from adult male APOE3/3 and APOE4/4 mice and stimulated with increasing doses of PIC + IFNγ or LPS + IFNγ. A dose dependent increase was observed in PIC + IFNγ –treated cells, only. However, significantly higher supernatant levels of nitrite and TNFα were observed in macrophages derived from APOE4/4 adult male mice compared to macrophages from APOE3/3 mice (Fig 4).
Figure 4.
Innate immune responses are also increased in cultured peritoneal macrophages derived from adult male APOE4/4 mice. Panel A- Average supernatant levels of nitrite (± SEM) were measured for APOE3/3 or APOE4/4 peritoneal macrophage cultures stimulated for 24 hrs with recombinant murine IFNγ (100 U/ml) plus increasing doses of PIC or LPS. Using 2-way ANOVA, a significant effect of APOE4 genotype (* p < 0.001) was observed for all treatment conditions while a significant effect of dose (p < 0.01) was observed for PIC treatment. Panel B- Average supernatant levels of TNFα (± SEM) were measured after 24 hrs in immune stimulated APOE3/3 and APOE4/4 peritoneal macrophage cultures using ELISA. LPS = 100 ng/ml; IFNγ= 100 U/ml. In each case, a significantly higher level of TNFα was found in APOE4/4 cultures compared to APOE3/3 ( * = p < 0.001; 2 way ANOVA).
The effect of the APOE4 gene allele is dose dependent
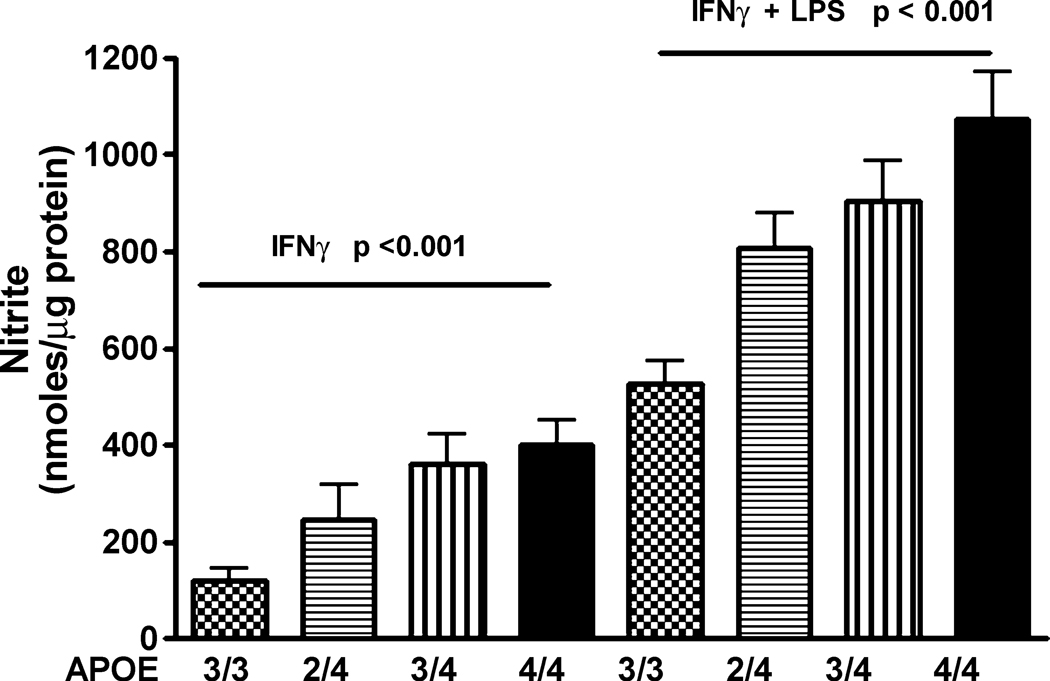
The dose-dependence of the APOE4 gene allele was examined by comparing similarly treated microglia cultured from homozygous APOE3/3 and APOE4/4 mice and heterozygous APOE2/4 and APOE3/4 mice (Fig 5). Significantly higher supernatant nitrite levels were observed in APOE4/4 microglia compared to APOE3/3, APOE3/4, or APOE2/4 microglia. These data suggest that the presence of the APOE4 gene is the primary factor in the increased microglial immune responsiveness.
Figure 5.
The effect of APOE4 is dependent on the allelic dose. Average supernatant levels of nitrite (± SEM) were measured for APOE3/3, APOE2/4, APOE3/4 or APOE4/4 microglial cultures stimulated for 24 hrs with recombinant murine IFNγ (100 U/ml) alone or plus LPS (100 ng/ml). A significant (p < 0.001, 1-way ANOVA) increase in nitrite levels was found for increasing APOE4 gene numbers under both stimulation conditions
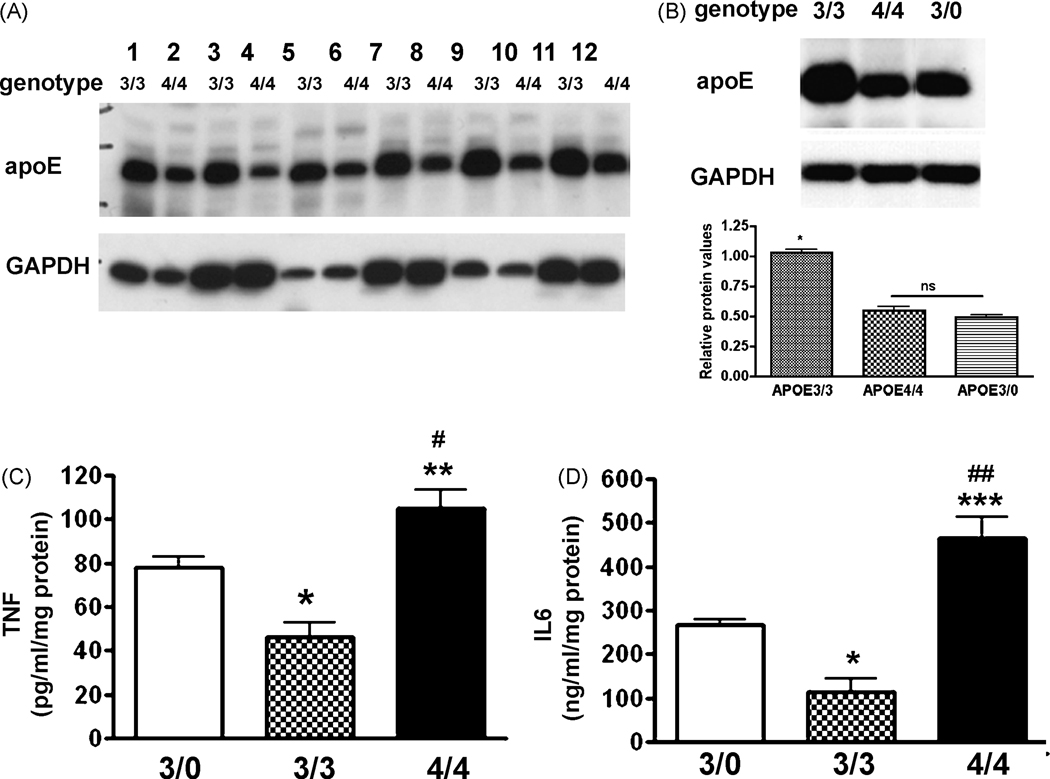
The effect of the APOE4 gene on reduction of apoE protein
Ramassamy et al [40] have recently suggested that isoform specific differences between APOE3/3 and APOE4/4 expressing cells are due to a decreased overall level of apoE protein produced by APOE4 cells compared to APOE3 cells. To confirm and extend this finding, we measured protein expression levels in brain lysates from neonatal and from adult APOE3/3 and APOE4/4 mice using Western blot. We also compared untreated to LPS-treated mice to determine if immune activation affects the isoform specific differences in protein expression. As shown in Figure 6A, protein expression levels in APOE3/3 brain lysates demonstrated significantly higher protein levels than APOE4/4 brain lysates in both neonatal and old (60 wk) mice. Immune stimulation by LPS injection into the tail vein also did not change the relative apoE protein expression pattern. To test the effect of a reduction in apoE protein expression on the immune response of APOE3/3 and APOE4/4 macrophages, we generated an APOE3/0 mouse by crossing APOE null (APOE0/0) mice to APOE3/3 mice, thus generating a hypomorph expressing only a single APOE3 gene allele. Brain lysates were then used to examine the expression level of apoE3 protein. Figure 6B shows representative Western blots for apoE3 protein in the brain lysates from LPS-injected APOE3/0, APOE3/3 and APOE4/4 targeted replacement mice. Protein expression levels were consistently less in the APOE3/0 mice brain lysates than APOE3/3 brains but APOE3/0 levels were not significantly different from the protein expression levels in the APOE4/4 brain. We next examined cytokine production by immune activated peritoneal macrophages derived from APOE3/0 adult male mice and compared these results to identically treated macrophages from APOE3/3 and APOE4/4 adult male mice. The supernatant levels of TNFα and IL6 were significantly higher in the APOE 3/0 mice compared to APOE3/3 mice but were significantly lower than the levels observed in APOE4/4 mice (Fig 6C; D).
Figure 6.
The gene effect of APOE4 is not solely dependent on the level of apoliporotein E protein. Panel A- Western blot depicting the expression of human apoE protein in brain (lanes 1–4; 7–12) and liver (lanes 5,6) lysates from APOE3/3 and APOE4/4 targeted replacement mice. Lower levels of protein were observed in APOE4/4 compared to APOE3/3 mice regardless of age (neonatal mice – lanes 1,2, 9,10; aged to >56 wks- all other lanes) or if immune stimulated with LPS (lanes 7,8). GAPDH was used as a protein loading control. Panel B- Western blot confirms a decreased expression of apolipoprotein E in LPS-treated APOE3/0 brain lysates compared to LPS-treated APOE3/3 brain lysates. Average relative changes in the ratio of apoE protein to GAPDH levels for each genotype are also shown (n= 4 mice/group). * = p < 0.001 for APOE3/3 brain lysates compared to either APOE4/4 or APOE3/0 lysates. Panel C/D- Average supernatant levels (± SEM) of TNFα (C) or IL-6 (D) were measured after 24hrs for immune stimulated APOE3/3 and APOE4/4 peritoneal macrophage cultures using ELISA. LPS = 50 ng/ml; IFNγ = 10 U/ml. * p < 0.001 for APOE3/3 compared to APOE3/0 cells; # p< 0.01 for APOE4/4 compared to APOE3/0 cells; ** p < 0.0001 for APOE4/4 compared to APOE3/3 cells; *** p < 0.001 for APOE4/4 compared to APOE3/3 cells and ## p < 0.001 for APOE4/4 compared to APOE3/0 cells.
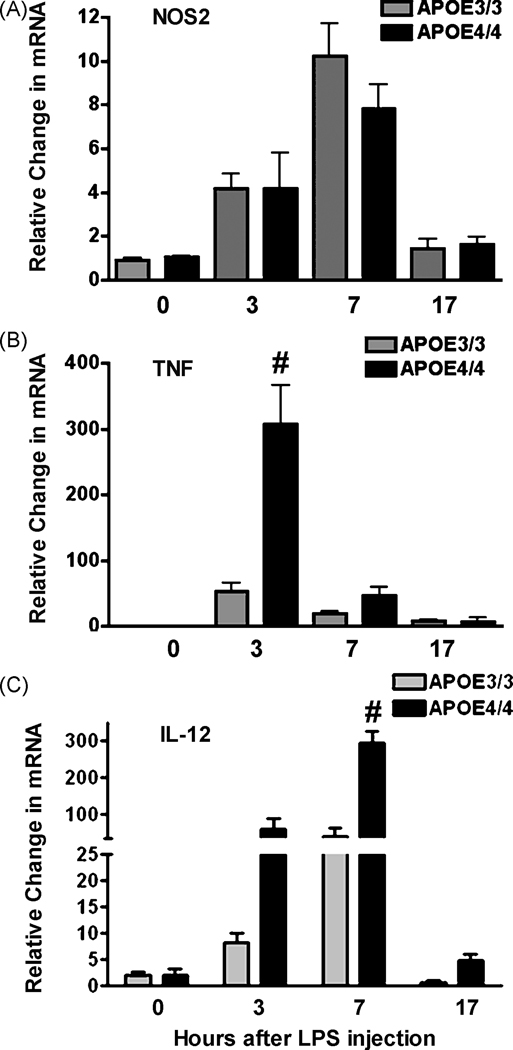
Aged APOE4/4 mice display an enhanced CNS acute innate immune response
To test whether the APOE4/4 genotype is associated with an enhanced neuroinflammatory response in whole animals, we measured NOS2, TNFa and IL-12p40 mRNA expression in brains of aged (60 week old) mice subjected to LPS challenge. Following tail vein injection of LPS, we collected cortical tissue from all mice at 4 specific time points (0,3,7 and 17 hrs post injection) and determined the mRNA profiles for NOS2, TNFa and IL-12p40 mRNA at each time using quantitative RT-PCR. Our results showed that cortical mRNA levels for each of the pro-inflammatory genes increased significantly within 7 hours of LPS injection and returned to baseline values by 24 hrs (Figure 7A;B;C). Interestingly, NOS2 mRNA levels did not demonstrate an APOE genotype specific difference. However, mRNA levels for TNFa and IL-12p40 were significantly elevated in APOE4/4 mice compared to APOE3/3 mice. Two way ANOVA analysis revealed a significant (p < 0.0001) gene affect over the time course studied.
Figure 7.
In vivo changes in the brain’s innate immune response in APOE4/4 and APOE3/3 adult mice. Adult APOE3/3 and APOE4/4 mice were injected with LPS and brains harvested at 0, 3, 7 or 17 hrs after injection. Average relative changes (± SEM) in mRNAs for NOS2 (A), for TNFα (B) and for IL-12 p40 (C) were determined using quantitative RT-PCR. Significantly higher levels of mRNA were observed for TNFα and IL-12p40 (# p < 0.001; 2 way ANOVA).
Discussion
Although APOE4 is a well-known, statistically relevant risk factor for Alzheimer’s disease [11], the widespread correlation of the APOE4 gene with increased pathology and worsened outcomes in many diseases strongly implies that the apoE3 protein isoform plays critical roles in basic cellular mechanisms and that the apoE4 protein isoform is impaired in its ability to orchestrate these mechanisms. Multiple sites of action have been proposed for the physiological effects of apoE3 on a variety of specific cells types, including regulation of lipid distribution, maintenance of redox balance and modulation of mitochondrial energy production [36]. The impact of apoE3 on immune cells such as lymphocytes and macrophages, however, can alter disease outcome in an additional manner by affecting the overall immune status of the organism. This is clearly shown in APOE knockout mice that lack apoE protein. Exposure of mice lacking apoE protein to various disease-producing organisms such as Listeria momocytogenes and Klepsiella pneumoniae results in decreased resistance to infection and increased mortality [14;41]. The addition of recombinant apoE3 holoprotein or of mimetic peptides derived from the receptor binding site of apoE, both reduced LPS- or injury mediated inflammation [26;27]. Thus, these data strongly suggest that the APOE3 gene and apoE3 protein are critical to suppressing inflammation and promoting repair.
The presence of the APOE4 gene, in contrast, is associated with an overactive pro-inflammatory immune phenotype [5;8;19;32;38]. Our data suggest that this is an inherent property of the cells expressing APOE4. For example, microglia derived from APOE4/4 mice show an “activated” morphology. Immune activated microglia are well known to assume a rounded, amoeboid-like morphology when activated both in vitro and in vivo whereas non-activated (resting) microglia show a more branched structure [39;43]. Rounded ameboid morphology is clearly observed in untreated APOE4/4 microglia in culture. The microglia also show an abnormal pro-inflammatory secretory profile when compared to APOE3/3 microglia. Cytokines that are commonly found in the classical stage of an innate immune response such as NO, TNFα, IL-12p40 and IL-6 are significantly elevated in APOE4/4 microglia compared to APOE3/3 microglia while anti-inflammatory cytokines such as IL-4 are significantly depressed. Jofre-Monseny et al [21] have recently demonstrated decreased IL-10 levels in RAW 264.7 cells transfected with APOE4 constructs compared to cells transfected with APOE3 constructs. Since cytokines like IL-4 and IL-10 help to initiate the repair process after injury [17], these data suggest that the shift to the macrophage-orchestrated repair program is blunted or delayed in the presence of the APOE4 gene. Finally, the effects of APOE4 on microglial immune function depend on gene dosage. Microglia from heterozygous APOE3/4 mice express a greater proinflammatory immune response than APOE3/3 microglia but less than microglia from an APOE4/4 mouse.
As predicted by the widespread immunomodulatory role of apoE3 throughout the body, the effect of the APOE4 gene on macrophage activation is not limited to microglia but extends to macrophages in the periphery. Levels of NO and pro-inflammatory cytokines produced by peritoneal macrophages derived from APOE4/4 adult male mice are significantly greater than the levels produced by similarly treated male APOE3/3 mice. Our previous reports, however, have shown that the effect of the APOE4 gene in peripheral macrophages displays gender bias [4–6;9]. A distinct genotype difference between APOE3/3 and APOE4/4 mice is most readily observed in normal adult male mice but is less easily observed in adult female mice [5]. This is due, at least in part, to the exposure of adult macrophages to estrogens in female mice. Circulating levels of estrogen interact with the immune pathways regulated by apoE in a complex fashion which is different for APOE3/3 and APOE4/4 macrophages [4;5].
Abnormal immune modulation mediated by the APOE4 gene is also observed in vivo. Our data show that LPS-injected APOE4/4 mice display a time dependent increase in mRNA for pro-inflammatory cytokines that is significantly greater than the mRNA changes observed in brains of APOE3/3 mice. The dramatic increase in TNFα and IL-12p40 mRNA levels in APOE4/4 mice as shown in our study mirrors the changes observed by Lynch et al [32] in targeted replacement APOE3/3 and APOE4/4 mice and by Ophir et al [38] using transgenic mice that express human APOE3 or APOE4 on a mouse knockout background. Interestingly, we do not see an APOE4>APOE3 genotype- specific difference in NOS2 mRNA expression in brain suggesting that NOS2 mRNA levels in vivo do not necessarily follow in vitro results. This may simply be due to a technical dilution of cells expressing NOS2 when whole brain samples are used. However, Maezawa et al [34] have shown that cultured astrocytes from APOE3/3 and APOE4/4 mice respond in the opposite manner to LPS induction compared to microglia. In astrocytes NO and cytokine production in vitro is greater in APOE3 compared to APOE4 cells [34]. These data suggest that the levels of NOS2 and cytokine mRNA expression as measured from whole brain samples may reflect an integrated profile from multiple cell types. However, our study and that of others [32;38] clearly show higher TNFα and IL6 mRNA and protein levels in brains from APOE4/4 mice compared to APOE3/3 mice. NOS2 mRNA levels remain the exception and suggests that other brain- specific factors regulate NOS2 gene expression in the brain in APOE4/4 mice.
The mechanism by which APOE4 differentially modulates the innate immune response is not clear. As shown by our data from APOE3/0 macrophages, it is unlikely that reduced apoE4 protein levels compared to apoE3 protein levels are entirely responsible for the enhanced immune action observed in APOE4/4 mice. While our data clearly show less apoE protein in APOE4/4 mice, we still find an increased pro-inflammatory response in APOE4/4 mice compared to APOE3/0 mice even when the apoE protein levels are the same. Thus, the presence of the APOE4 gene may also regulate classical immune activation and the production of pro-inflammatory factors in a manner independent of protein concentration. The pro-inflammatory effect of APOE4 is also triggered by multiple immune receptors and may involve multiple signaling pathways. Using genome-wide expression profiling, Ophir et al [38] have shown that the expression of NF-κB regulated genes in the hippocampus was significantly greater in LPS-treated APOE4 Tg mice compared to APOE3 Tg mice [46]. The increased expression of pro-inflammatory genes in the presence of APOE4 compared to APOE3 was associated with increased NF-κB activation. A similar increase in NF-κB activity was observed in stably transfected RAW 264.7 cells expressing APOE4 compared to cells expressing APOE3 [21]. The p38 MAP kinase signaling pathway is also differentially regulated in APOE4 microglia compared to APOE3 microglia. As recently shown by Maezawa et al [35], pharmacological inhibition of p38 MAP kinase abolished APOE4-specific toxicity in LPS-treated hippocampal slices. The finding of altered activation of the NF-κB or p38 MAP kinase signaling pathways in APOE4/4 microglia is consistent with our findings of increased mRNAs for NOS2, IL-12p40 and TNFα in culture and with similar increases in whole brain lysates.
A large number of studies now show that the APOE4 allele plays an important role in human pathophysiology. In addition to its well-characterized role in Alzheimer’s disease (reviewed by [36]), very compelling genetic data show that the APOE4 allele is associated with poor recovery from nervous system insults such as closed head injury, intracranial hemorrhage, and cardiopulmonary bypass. Other genetic associations have been reported between APOE4 and multiple sclerosis, HIV-associated dementia, Parkinson’s disease (PD), and diabetic neuropathy (reviewed by [37]). The common thread linking these diverse disease states is an up-regulation of innate immunity, conditions where the presence of the APOE4 gene is likely to be additive with other gene variants that collectively promote a sustained, altered immune response [30]. As discussed by Finch and Morgan [15], the presence of a global activator of innate immune function such as APOE4, has survival value in populations exposed to high levels of infectious disease. However, an enhanced pro-inflammatory status is clearly detrimental when faced with diseases of advanced age such as AD or PD. Thus, identification of the mechanisms underlying the increased inflammatory response in APOE4 individuals will provide numerous insights and potential therapeutic outcomes for treating multiple inflammatory diseases.
Acknowledgements
This work was supported by a grant from NIH to CAC (AG023802-01), MPV (AG019780), and CMB (Minority Research Supplement to P50 AG05128) and an NSF Predoctoral Fellowship (CMB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure: Dr. Vitek is the founder and principal in Cognosci, Inc. Dr. Brown and Dr. Colton have no conflicts.
Contributor Information
Michael P. Vitek, Email: mikevitek@cognosci.com.
Candice M. Brown, Email: canbrown@u.washington.edu.
Carol A. Colton, Email: glia01@aol.com.
References
- 1.Adams D, Hamilton T. The cell biology of macrophage activation. Ann Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- 2.Avila E, Holdsworth G, Sasaki N, Jackson R, Harmony J. Apolipoprotein E suppresses phytohemagglutinin-activated phospholipid turnover in peripheral blood mononuclear cells. J Biol Chem. 1982;257:5900–5909. [PubMed] [Google Scholar]
- 3.Barger S, Harmon A. Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature. 1997;388:878–881. doi: 10.1038/42257. [DOI] [PubMed] [Google Scholar]
- 4.Brown C, Choi E, Xu Q, Vitek M, Colton CA. The APOE4 genotype alters the response of microglia and macrophages to 17beta-estradiol. Neurobiol Aging. 2007 Jun 4; doi: 10.1016/j.neurobiolaging.2007.04.018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown C, Wright E, Colton C, Sullivan P, Laskowitz D, Vitek M. Apolipoprotein E isoform mediated regulation of nitric oxide release. Free Radic Biol Med. 2002;32:1071–1075. doi: 10.1016/s0891-5849(02)00803-1. [DOI] [PubMed] [Google Scholar]
- 6.Brown C, Xu Q, Okhubo N, Vitek M, Colton CA. Androgen-mediated immune function is altered by the apolipoprotein E gene. Endocrinology. 2007;148:3390. doi: 10.1210/en.2006-1200. [DOI] [PubMed] [Google Scholar]
- 7.Chapman J, Vinokurov S, Achiron A, Karussis D, Mitosek-Sewczyk K, Birnbaum M, Michaelson D, Korczyn A. APOE genotype is a major predictor of long term progression of disability in MS. Neurology. 2001;56:312–316. doi: 10.1212/wnl.56.3.312. [DOI] [PubMed] [Google Scholar]
- 8.Colton C, Brown C, Czapiga M, Vitek M. Apolipoprotein-E allele specific regulation of nitric oxide production. New York Acad Sci. 2002;962:212–225. doi: 10.1111/j.1749-6632.2002.tb04070.x. [DOI] [PubMed] [Google Scholar]
- 9.Colton C, Brown C, Vitek M. Sex steroids, APOE genotype and the innate immune system. Neurobiol Aging. 2005;26:363–372. doi: 10.1016/j.neurobiolaging.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Colton CA, Brown C, Cook D, Needham L, Xu Q, Czapiga M, Saunders A, Schmeckel D, Rasheed K, Vitek MP. APOE and the regulation of microglial nitric oxide production: a link between genetic risk and oxidative stress. Neurobiol Aging. 2002;23:777–785. doi: 10.1016/s0197-4580(02)00016-7. [DOI] [PubMed] [Google Scholar]
- 11.Coon KD, Myers A, Craig D, Webster J, et al. A high-density whole-genome association study reveals that APOE is the major susceptibility gene for sporadic late-onset Alzheimer's disease. J Clin Psychiatry. 2007;68:613–618. doi: 10.4088/jcp.v68n0419. [DOI] [PubMed] [Google Scholar]
- 12.Corder E, Robertson K, Lannfelt L, Bogdanovic N, Eggertsen G, Wilkins J, Hall C. HIV-infected subjects with the E4 allele for APOE have excess dementia and peripheral neuropathy. Nat Med. 1998;4:1182–1184. doi: 10.1038/2677. [DOI] [PubMed] [Google Scholar]
- 13.Czapiga M, Colton CA. Microglial function in human APOE3 and APOE4 transgenic mice: Altered arginine transport. J Neuroimmunol. 2002;4673:1–8. doi: 10.1016/s0165-5728(02)00394-6. [DOI] [PubMed] [Google Scholar]
- 14.deBont N, Netea M, Demacker P, Verschureren I, Kullberg B, van Dijk K, van der Meer J, Stalenhoef AF. Apolipoprotein E knock-out mice are highly susceptible to endotoxemia and Klebsiella pneumoniae infection. J Lipid Res. 1999;40:680–685. [PubMed] [Google Scholar]
- 15.Finch CE, Morgan T. Systemic inflammation, infection, ApoE alleles, and Alzheimer disease: a position paper. Curr Alzheimer Res. 2007;4:185–189. doi: 10.2174/156720507780362254. [DOI] [PubMed] [Google Scholar]
- 16.Garvey E, Oplinger J, Furfine E, Kiff R, Laszlo F, Whittle B, Knowles R. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J Biol Chem. 1997;272:4959–4963. doi: 10.1074/jbc.272.8.4959. [DOI] [PubMed] [Google Scholar]
- 17.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 18.Grocott H, Newman M, El-Moalem H, Bainbridge D, Butler A, Laskowitz D. Apolipoprotein E genotype differentially influences the pro-inflammatory and anti-inflammatory response to cardiopulmonary bypass. J Thoracic Cardiovas Surg. 2001;122:622–623. doi: 10.1067/mtc.2001.115152. [DOI] [PubMed] [Google Scholar]
- 19.Guo L, LaDu M, Van Eldik L. A dual role for apolipoprotein E in neuroinflammation: anti- and pro-inflammatory activity. J Mol Neurosci. 2004;23:205–212. doi: 10.1385/JMN:23:3:205. [DOI] [PubMed] [Google Scholar]
- 20.Hui D, Harmony J, Inneraity T, Mahley R. Immunoregulatory plasma lipoproteins. Role of apoprotein E and apoprotein B. J Biol Chem. 1980;255:11775–11781. [PubMed] [Google Scholar]
- 21.Jofre Monseny L, Lobada A, Wagner AE, Huebbe P, Boesch-Saadatmandi C, Kozkowicz A, Minihane A, Dulak J, Rimbach G. Effects of apoE genotype on macrophage inflammation and heme oxygenase-1 expression. Biochem Biophys Res Commun. 2007;357:319–324. doi: 10.1016/j.bbrc.2007.03.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jordan B, Relkin N, Ravdin L, Jacobs A, Bennett A, Gandy S. Apolipoprotein E epsilon4 associated with chronic traumatic brain injury in boxing. J.Amer.Med.Soc. 1997;278:2143–2145. [PubMed] [Google Scholar]
- 23.Kakuda D, Sweet MJ, MacLeod C, Hume D, Markovich D. CAT2-mediated L-arginine transport and nitric oxide production in activated macrophages. Biochem.J. 1999;340:549–553. [PMC free article] [PubMed] [Google Scholar]
- 24.Kutner K, Erlanger D, Tsai J, Jordon B, Relkin N. Lower cognitive performance of older football players possessing apolipoprotein E epsilon4. Neurosurgery. 2000;47:651–657. doi: 10.1097/00006123-200009000-00026. [DOI] [PubMed] [Google Scholar]
- 25.Laskowitz D, Lee D, Schmechel D, Staats H. Altered immune responses in apolipoprotein E-deficient mice. J Lipid Res. 2000;41:613–620. [PubMed] [Google Scholar]
- 26.Laskowitz D, McKenna S, Song P, Wang H, Durham L, Yeung N, Christensen D, Vitek MP. COG1410, a novel apolipoprotein E-based peptide, improves functional recovery in a murine model of traumatic brain injury. J Neurotrauma. 2007;24:1093–1107. doi: 10.1089/neu.2006.0192. [DOI] [PubMed] [Google Scholar]
- 27.Laskowitz D, Thekdi A, Han S, Myers J, Pizzo S, Bennett E. Down regulation of microglial activation by apolipoprotein E and apoE mimetic peptides. Exp Neurol. 2001;167:74–85. doi: 10.1006/exnr.2001.7541. [DOI] [PubMed] [Google Scholar]
- 28.Lehnardt S, Massillon L, Follett P, Jensen F, Ratan R, Rosenberg P, Volpe J, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll like receptor 4 dependent pathway. Proc Natl Acad Sci. 2003;10:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lerma J, Herranz AS, Herreras O, Abraira V, Martin Del Rio R. In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Res. 1986;384:145–155. doi: 10.1016/0006-8993(86)91230-8. [DOI] [PubMed] [Google Scholar]
- 30.Licastro F, Porcellini E, Caruso D, Lio D, Corder E. Genetic risk profiles for Alzheimer's disease: Integration of APOE genotype and variants that up-regulate inflammation. Neurobiol Aging. 2006 Aug 21; doi: 10.1016/j.neurobiolaging.2006.07.007. 2006. [DOI] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Lynch J, Morgan D, Mance J, Matthew W, Laskowitz D. Apolipoprotein E modulates glial activation and the endogenous central nervous system inflammatory response. J Neuroimmunol. 2001;114:107–113. doi: 10.1016/s0165-5728(00)00459-8. [DOI] [PubMed] [Google Scholar]
- 33.Macy M, Okano Y, Cardin A, Avila E, Harmony J. Suppression of lymphocyte activation by plasma lipoproteins. Cancer Res. 1983;43(5 Suppl):2496s–2502s. [PubMed] [Google Scholar]
- 34.Maezawa I, Maeda N, Montine T, Montine K. Apolipoprotein E-specific innate immune response in astrocytes from targeted replacement mice. J Neuroinflammation. 2006;3:10. doi: 10.1186/1742-2094-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maezawa I, Nivison M, Montine K, Maeda N, Montine T. Neurotoxicity from innate immune response is greatest with targeted replacement of E4 allele of apolipoprotein E gene and is mediated by microglial p38MAPK. FASEB J. 2006;20:797–799. doi: 10.1096/fj.05-5423fje. [DOI] [PubMed] [Google Scholar]
- 36.Mahley R, Weisberger K, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer's disease. Proc Natl Acad Sci U S A. 2006;103:5641–5643. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nathoo N, Chetty R, van Dellen J, Barnett G. Genetic vulnerability following traumatic brain injury: the role of apolipoprotein E. Mol Pathol. 2003;56:132–136. doi: 10.1136/mp.56.3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ophir G, Amariglio N, Jacob-Hirsch J, Elkon R, Rechavi G, Michaelson DM. Apolipoprotein E4 enhances brain inflammation by modulation of the NF-kappaB signaling cascade. Neurobiol Dis. 2005 Dec;20(3):709–718. doi: 10.1016/j.nbd.2005.05.002. 20: 709-718, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Perry VH, Gordon S. Resident macrophages of the central nervous system: Modulation of phenotype in relation to a specialized microenvironment. In: Goetzl E, editor. Neuroimmune Networks: Physiology and Diseases. New York, NY: Alan R. Liss, Inc.; 1989. pp. 119–125. [Google Scholar]
- 40.Ramassamy C, Krzywkowski P, Averill D, Lussier-Cacan S, Theroux L, Christen Y, Davignon J, Poirier J. Impact of apoE deficiency on oxidative insults and antioxidant levels in the brain. Brain Res Mol Brain Res. 2001;86:76–83. doi: 10.1016/s0169-328x(00)00268-0. [DOI] [PubMed] [Google Scholar]
- 41.Roselaar S, Daugherty A. Apolipoprotein E-deficient mice have impaired innate immune responses to Listeria monocytogenes in vivo. J Lipid Res. 1998;39:1740–1743. [PubMed] [Google Scholar]
- 42.Roses AD. Apolipoprotein E, a gene with complex biological interactions in the aging brain. Neurobiol.Dis. 1997;4:170–185. doi: 10.1006/nbdi.1997.0161. [DOI] [PubMed] [Google Scholar]
- 43.Streit W, Colton CA. The Brain's immune system. Sci.Amer. 1995;273:38. doi: 10.1038/scientificamerican1195-54. [DOI] [PubMed] [Google Scholar]
- 44.Sullivan P, Mezour H, Quarfordt SH, Maeda N. Type III hyperlipoproteinemia and spontaneous atherosclerosis in mice resulting from gene replacement of mouse Apoe with human Apoe2. J.Clin.Invest. 1998;102:130–135. doi: 10.1172/JCI2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vitek M, Snell J, Chernyshev O, Colton C. Modulation of nitric oxide production in human macrophages by apolipoprotein E and amyloid beta peptide. Biophys.Biochem Res.Comm. 1997;240:391–394. doi: 10.1006/bbrc.1997.7408. [DOI] [PubMed] [Google Scholar]
- 46.Xu PT, Schmechel D, Rothrock C, Burkhart DS, Qiu HL, Popko B, Sullivan P, Maeda N, Saunders A, Roses AD, Gilbert JR. Human apolipoprotein E2, E3 and E4 isoform-specific transgenic mice: human like pattern of glial and neuronal immunoreactivity in central nervous system not observed in wild type mice. Neurobiol.Dis. 1996;3:229–245. doi: 10.1006/nbdi.1996.0023. [DOI] [PubMed] [Google Scholar]