Abstract
Objectives
Single-dose nevirapine (SDNVP) for prevention of mother-to-child HIV transmission selects mutations conferring resistance to non-nucleoside reverse transcriptase inhibitor (NNRTI)-based therapy. We investigated mortality and virologic and clinical outcomes following introduction of antiretroviral treatment (ART) among a cohort of women given SDNVP.
Methods
When ART programs were introduced in 2004 in Lusaka, Zambia, we were completing a trial of infant feeding which involved following HIV-infected women who received SDNVP between 2001 and 2005. Women still in follow-up or who could be contacted were evaluated for eligibility for ART (CD4 count <200 or <350 and WHO stage ≥ 3) and started on NNRTI-based therapy if eligible. We compared mortality in the cohort of women before and after ART access, and examined, among women initiating ART, whether virologic response was better allowing a longer time to elapse between SDNVP and treatment initiation.
Results
In the cohort of 872 women, mortality more than halved after ART became available (relative hazard [RH] = 0.46 95% CI: 0.23–0.91 p=0.03). Of 161 SDNVP-exposed women followed on NNRTI-based ART, 70.8% suppressed (viral load <400 copies/ml). Only 3/8 (37.5%) women SDNVP-exposed <6 months of starting therapy suppressed compared to 13/22 (59.1%) who started 6–12 months, 44/61 (72.1 %) 12–24 months, and 54/70 (77.1%) >24 months post-exposure (chi-square trend p=0.01).
Conclusions
Most SDNVP-exposed women respond well to NNRTI-based therapy but there was an attenuation of therapy efficacy that persisted to 12 months after exposure. Women should be screened for ART eligibility during pregnancy and started on effective regimens before delivery.
Introduction
At the time that antiretroviral therapy (ART) became available in Lusaka, Zambia, we were completing a clinical trial of infant feeding options which involved following a cohort of HIV-infected mothers and their children who had received single-dose nevirapine (SDNVP) to prevent mother-to-child HIV transmission (PMTCT).1 SDNVP, while a simple and effective means of reducing transmission,2 readily selects viral mutations associated with resistance to non-nucleoside reverse transcriptase inhibitors (NNRTI).3 First-line regimens in the Zambian program were NNRTI-based.4 Previous studies have observed reductions in the efficacy of NNRTI-based ART if started within 6 months of exposure to nevirapine used with zidovudine for PMTCT.5,6 In a study of SDNVP from Zambia, no effect of past exposure on CD4 counts or clinical response to ART was detected if therapy was started more than 6 months after delivery.7 Virologic response may, however, be a more sensitive and/or earlier indicator of whether response to ART is attenuated. We therefore sought: (1) to determine the impact that ART access had on mortality among HIV-infected women in our cohort and, (2) to investigate, among women who initiated ART, whether virologic response to NNRTI-based therapy would correlate with the length of time that elapsed between SDNVP and treatment initiation.
Methods
Between May 2001 and September 2004, 1,378 HIV-infected women were recruited at two antenatal clinics in Lusaka, Zambia. Those who delivered live-born infants and who met other eligibility criteria were randomized to either short or long duration breast feeding and were followed with their infants to 24 months post-delivery.1 All women received SDNVP for PMTCT and records were maintained concerning women’s adherence to this intervention.8 Women provided written informed consent for their participation. The study and its modifications were approved by the Research Ethics Committees of the investigators’ institutions.
In May 2004, local health authorities began to implement an HIV care and treatment program in Lusaka.4 Prior to this time, ART had not been available. Study clinicians asked participants in active follow-up at this time whether they would like to join the HIV treatment program and conducted outreach to participants who had completed their follow-up in the study. Services were provided primarily by study nurse-counselors and clinical officers supervised by study physicians. Clinicians were trained as part of the local program and followed local protocols for screening, treating and keeping records. ART became available at one of the sites in November 2004 and at the other in April 2005.
At the time that ART became available, 418 participants were still in active follow-up. All of these participants were offered enrollment into the treatment program at scheduled study visits. In addition, participants who had completed the study were re-contacted if possible and offered enrollment into the treatment program. Women with the lowest CD4 count when tested during pregnancy were contacted first. Immediate relatives of study participants, including spouses, children, and other dependents, were also offered access to treatment. Overall 534 women elected to enroll in the HIV treatment program.
Women were screened for eligibility for ART using local guidelines4 and 269 women met eligibility criteria for therapy. Screening included a CD4 count and clinical history/examination to determine WHO disease stage. Eligibility for treatment included CD4 counts <200 cells/μL, WHO stage 4, or WHO stage 3 and CD4 count <350 cells/μL. Those who were eligible for ART had liver and kidney function tests and were screened for tuberculosis. Women were counseled about adherence and if not in need of co-treatment for tuberculosis, were prescribed nevirapine, lamivudine and either zidovudine or stavudine depending on hemoglobin levels. Therapy was usually deferred if tuberculosis treatment was needed but if clinical circumstances indicated efavirenz was given in place of nevirapine. All participants were offered multivitamins, and women with CD4 counts <200 cells/μL or stage 4 were prescribed cotrimoxazole. The cost of laboratory tests, vitamin supplements, and medication was borne by the HIV treatment program. In total, 217 women initiated ART.
As part of the local protocol, blood was drawn every 3 months for CD4 count determination. For the 217 women who initiated ART as part of our study activities, blood collected pre-treatment and at 6 monthly intervals post-treatment was stored for later viral load testing to determine virologic response to antiretroviral therapy. Adherence was strongly emphasized and the home visit teams and peer support groups, already in place for the infant feeding study, were extended to include the HIV treatment program.
As part of the infant feeding trial, blood samples had been collected during pregnancy for CD4 count (BD Immunocytometry Systems, San Jose, CA) and plasma viral load (Roche Amplicor® version 1.5, Branchburg, NJ) determination. At this time, women had been asked about HIV-related clinical conditions and WHO clinical stage9 was assigned based on physical examination and these self-reports. Information about demographic, clinical, educational, economic, and social characteristics of the women was collected during pregnancy.
We restricted analyses pertaining to mortality among women to deaths that occurred after 6 weeks post-delivery in order to exclude pregnancy and delivery-related mortality unlikely to be affected by ART. We censored mortality analyses 24 months after delivery. Kaplan-Meier life-table methods were used to describe pre-ART mortality censoring events and follow-up time accrued after the program started. Cox Proportional Hazards models were used to quantify reduction in mortality after the ART program started using a time-dependent dichotomous variable coding “after” as all time accruing one month after program initiation at each research site and “before” as all time accruing before program initiation.
For women who initiated ART, we restricted analyses to those who were not known to have discontinued therapy. We examined the percentage who had suppressed to <400 copies/ml in the sample collected closest to 6 months post-therapy and investigated factors associated with suppression. Chi-squared tests were used to determine associations between categorical predictor variables and Wilcoxon Sum Rank tests for continuous variables. Multivariable analysis to examine the role of potential confounders was done using logistic regression. Analysis was done utilizing SAS Software (SAS version 9.1.3 Cary, NC).
Results
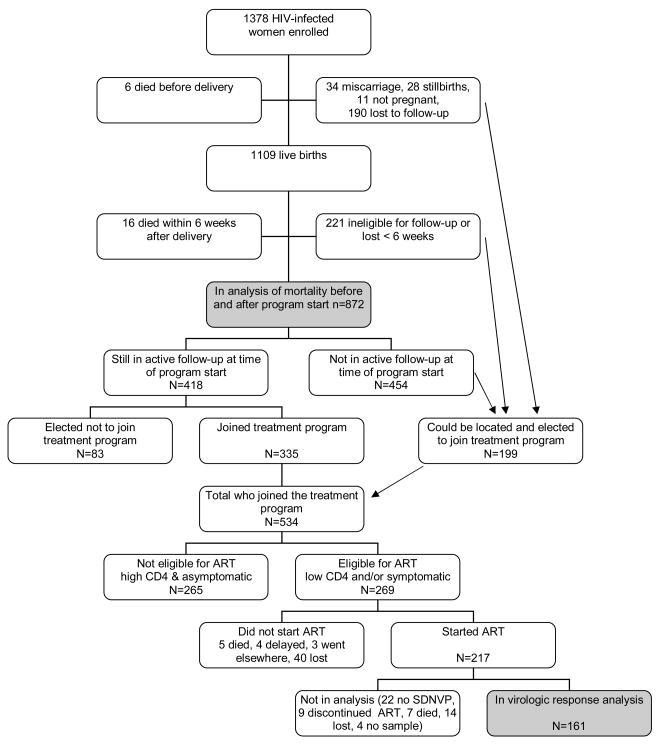
At the time the HIV treatment program began, 418 HIV-infected women were still in active follow-up as part of the infant feeding trial and 335 (80.1%) agreed to be screened for ART eligibility. Factors associated with electing to be screened for ART included higher parity, survival of index child, clinic A, fewer missed study visits, clinical stage ≥2 and disclosure of HIV status (data not shown). Of those who agreed to be screened, 162 (48.4%) met eligibility criteria and 131 (80.9%) initiated therapy. An additional 199 women not in active follow-up at the time of program roll-out could be located and agreed to be screened, 107 (53.8%) met eligibility criteria, and 86 (80.4%) initiated therapy. Of the 52 women who were eligible but did not begin therapy, 5 died, 4 had treatment delayed due to tuberculosis treatment, 3 received ART elsewhere and the others actively refused or passively did not return for visits (Figure 1).
Figure 1.
Flow chart showing the disposition of study participants over the course of the study.
Mortality among HIV-infected women in our cohort was reduced by more than half (relative hazard (RH) = 0.46 95% CI: 0.23–0.91 p=0.03) in the time period once ART became available. Of 872 HIV-infected women in the cohort surviving and still in follow-up 6 weeks after delivery, 53 died before 24 months post-delivery in the period prior to the availability of ART and 11 died in the period after. The probability of death by 12 months was 4.3% and by 24 months 10.3% in the pre-ART era. Reduction in mortality associated with access to ART remained significant (RH=0.47 95% CI: 0.23–0.93) after adjustment for CD4 count (RH=0.57 per each 100 cells/mL increase 95% CI: 0.45–0.71) and viral load (RH 2.14 per each log10 copies/mL increase 95% CI: 1.36–3.37) measured during pregnancy in a multivariate Cox model.
To investigate whether the length of time that had elapsed since SDNVP would influence ART outcomes, we focused on 217 women who initiated ART. Of these, 22 were excluded because they did not receive SDNVP (6 because ART was initiated during pregnancy and 16 because SDNVP was not used in the index pregnancy due to miscarriage [3], stillbirth [5] or noncompliance [8]). Of the remaining 195 women, 9 (4.6%) stopped all therapy, 14 (7.2%) were lost to follow-up and 7 (3.6%) died before 6 months post-therapy. Of 165 women who were not known to have discontinued ART and who were followed to 6 months, the median time between SDNVP and ART initiation was 23 months (min 3 weeks, max 54 months, inter-quartile range 15 to 32 months). Most women (54.5%) initiated therapy with nevirapine, lamivudine and zidovudine, 40.6% with nevirapine, lamivudine and stavudine, and 4.8% with efavirenz, lamivudine and either zidovudine or stavudine.
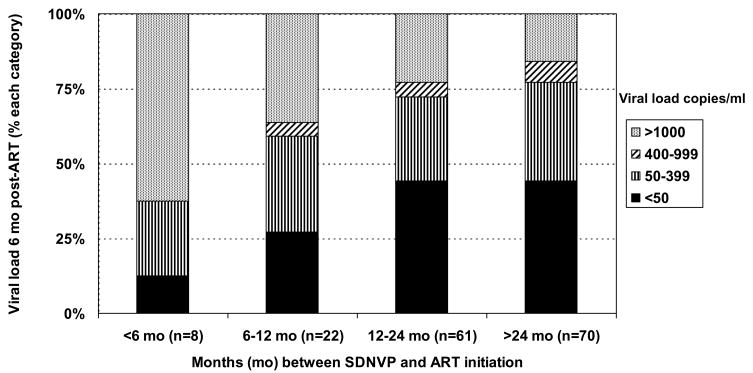
Of 161 SDNVP-exposed women still on treatment at 6 months, 70.8% achieved a viral load <400 copies/ml and 40.4% a viral load <50 copies/ml. Four had no sample stored for viral load testing. The longer the duration of time between SDNVP exposure and treatment initiation, the greater the percentage who achieved viral suppression. Of 8 women exposed to SDNVP within 6 months of starting therapy, only 3 (37.5%) achieved a viral load of <400 copies/ml by 6 months post-therapy, compared to 59.1% of 22 women who started within 6–12 months post-exposure, 72.1 % of 61 who started within 12–24 months, and 77.1% of 70 who started more than 24 months after exposure (p=0.01). The patterns were similar using different cut-offs to define suppression (Figure 2).
Figure 2.
Percentage of women who had viral loads of <50 copies/ml, 50–399 copies/ml, 400–999 copies/ml and ≥1000 copies/ml at 6 months post-therapy initiation by number of months since single-dose nevirapine exposure.
Other factors found to be significantly associated with better viral suppression were being a non-transmitter (child uninfected 76.9% suppressed; child infected 59.7% suppressed p=0.02) and being unemployed (employed 57.1% suppressed; unemployed 78.1% suppressed p=0.005). The association between a longer time since SDNVP and better viral suppression remained significant after adjusting for transmission and employment status. The association between longer time since SDNVP and response to therapy was attenuated and became non-significant if adjusted for pre-treatment viral load, although this latter factor was not significantly associated with virologic response. The association between time since SDNVP exposure and viral suppression was strongest among those whose viral load during the pregnancy in which SDNVP was received was ≥100,000 copies/ml (SDNVP exposure occurred ≤ 12 months earlier 35.3% suppressed; vs. > 12 months earlier 72.9% suppressed, p=0.006), and was not apparent among those with a viral load < 100,000 copies/ml (SDNVP exposure occurred ≤ 12 months earlier 75.0% suppressed; vs. > 12 months earlier 75.9% suppressed, p>0.1), but the interaction term was not significant (p=0.09).
With HIV treatment programs now in place, women should be screened for ART during pregnancy. Based on symptom checklists, examinations and CD4 count data collected during pregnancy on the 1378 women enrolled, 38.3% of women should have started ART during pregnancy (i.e. their CD4 counts were <200 or <350 with stage III conditions). Mortality in women who met ART eligibility criteria was high with 23.7% mortality by 24 months (11.9% by 12 months) in the era before ART became available. Among those not yet eligible for ART during pregnancy, only 6.0% met eligibility criteria when re-tested 12 months after delivery in the era before ART became available. If only CD4 counts are used to determine ART eligibility, 55.8% had a CD4 count <350 during pregnancy and 11.8% of those with CD4 counts above 350 during their pregnancy had CD4 count <350 when re-tested 12 months after delivery.
Discussion
In our cohort of HIV-infected women who received SDNVP, access to an HIV treatment program that provided NNRTI-based ART was associated with >50% reduction in mortality. Services were provided primarily by non-physicians and followed simplified structured protocols. This bodes well for the success of HIV treatment services when expanded to sites with even fewer health resources and for settings were SDNVP has been widely used.
Within our established research cohort, about 20% of women were reluctant to make use of HIV treatment services when they became available. A further 20% of those who agreed to be screened as part of the treatment services and met clinical and immunologic criteria for ART did not initiate therapy. A further 10% of those who initiated ART discontinued treatment within the first 6 months. Taking this attrition together, less than half of those in need actually received ART when it was offered. Much attention has focused on adherence to treatment10 but less attention has been given to attrition at these other stages. Some programs have identified that those with the most advanced disease are over-represented11 which may account for why we observed substantial reductions in mortality despite the limited coverage of the program. Population effects of HIV treatment programs could be improved if more attention is given to uptake and retention.
The majority (71%) of women who initiated NNRTI-based therapy achieved viral suppression (<400 copies/ml) after 6 months of treatment despite past exposure SDNVP but only 40% achieved viral suppression at <50 copies/ml. The percentage suppressing is less than that observed in the Thailand and Botswana studies5,6 but is similar to what has been observed in trials in the U.S. and Europe.12 Highly variable rates of suppression are reported from routine clinical programs in sub-Saharan Africa.13,14
Among SDNVP-exposed women, we observed a significant gradient in the proportion suppressing by the time elapsed between SDNVP and treatment initiation. Consistent with prior studies,6 those women who started therapy within 6 months of SDNVP exposure were the least likely to suppress. However, those who started therapy 6 to 12 months post-SDNVP exposure were also significantly less likely to suppress than those who started therapy more than 12 months later. Our results suggest that some reduction in the efficacy of NNRTI-based treatment regimens may be expected even with SDNVP exposure 12 months previously.
Our cohort was recruited largely before ART became available providing an “accident of history” to examine this question which would pose ethical challenges to investigate in any other way. However, prior to the availability of treatment, mortality among women eligible for ART was high. This may introduce a survivor bias with women with more advanced disease under-represented among those more distant in time from delivery, thus artificially creating the association we and others have observed.
We also observed that women who reported having a full- or part-time job in the formal or informal sector were less likely to suppress. Usually having a job is taken as a marker of higher socioeconomic position but we hypothesize that in this community, where jobs are mostly low paying and with poor working conditions and inflexible hours, having a job may interfere with receiving health care. Being a non-transmitter also improved the likelihood of virologic suppression, an association we hypothesize to be related to viral pathogenicity not fully accounted for by viral load and CD4 count. The association between time since exposure and viral suppression persisted after adjustment for these two factors, but was attenuated if adjusted for baseline viral load.
Any long-lasting effects of past exposure to SDNVP on ART response are only relevant if ART is available. If ART is available, pregnant women should be prioritized and started on therapy if eligible as a matter of urgency.15 In our study population, a large proportion (38%) of pregnant women met ART eligibility criteria and, for this group in the ART era, exposure to SDNVP is irrelevant. In our study, only 6% of women who were ineligible during pregnancy became eligible 12 months later. Thus the numbers of women who would need to start ART within the first 12 months after delivery are small. PMTCT protocols providing extended prophylaxis to reduce resistance16 should reduce this already small proportion even further. However, if pregnant women are not screened during pregnancy, then the impact of SDNVP on ART outcomes is larger. These results emphasize the importance of establishing appropriate referrals and coordination between services so that pregnant HIV-infected women can be triaged for ART if appropriate.17
Acknowledgments
We would like to thank the Zambian families who participated in the research and all the study staff and volunteers. We gratefully acknowledge assistance with aspects of the design and conduct of the study from: Drs. Marc Bulterys, Elwyn Chomba, Lynne Mofenson, Stuart Reid, Kevin Ryan, Aisha Siebert, Jeffrey Stringer, Cheswa Vwalika, and Jan Walter. The study was supported by grants from the National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH) (R01 HD 39611 and R01 HD 40777). Additional funding was received through the President’s Emergency Plan for AIDS Relief (PEPFAR) for provision of antiretroviral treatment services; Elizabeth Glaser Pediatric AIDS Foundation (EGPAF) (GMA is a recipient of the EGPAF Scientist Award); and the Doris Duke Charitable Foundation Operations Research for AIDS Care and Treatment Award (ORACTA) No. 2005041.
Reference List
- 1.Kuhn L, Aldrovandi GM, Sinkala M, et al. Effects of early, abrupt cessation of breastfeeding on HIV-free survival of children in Zambia. N Engl J Med. 2008;359:130–141. doi: 10.1056/NEJMoa073788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson JB, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003;362:859–868. doi: 10.1016/S0140-6736(03)14341-3. [DOI] [PubMed] [Google Scholar]
- 3.Arrive E, Newell ML, Ekouevi DK, et al. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: a meta-analysis. Int J Epidemiol. 2007;36:1009–1021. doi: 10.1093/ije/dym104. [DOI] [PubMed] [Google Scholar]
- 4.Stringer JS, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 5.Jourdain G, Ngo-Giang-Huong N, Le Coeur S, et al. Intrapartum exposure to nevirapine and subsequent maternal responses to nevirapine-based antiretroviral therapy. N Engl J Med. 2004;351:229–240. doi: 10.1056/NEJMoa041305. [DOI] [PubMed] [Google Scholar]
- 6.Lockman S, Shapiro RL, Smeaton LM, et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med. 2007;356:135–147. doi: 10.1056/NEJMoa062876. [DOI] [PubMed] [Google Scholar]
- 7.Chi BH, Sinkala M, Stringer EM, et al. Early clinical and immune response to NNRTI-based antiretroviral therapy among women with prior exposure to single-dose nevirapine. AIDS. 2007;21:957–964. doi: 10.1097/QAD.0b013e32810996b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albrecht S, Semrau K, Kasonde P, et al. Predictors of nonadherence to single-dose nevirapine therapy for the prevention of mother-to-child HIV transmission. Journal of Acquired Immune Deficiency Syndromes: JAIDS. 2006;41:114–118. doi: 10.1097/01.qai.0000179425.27036.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Geneva: 2005. Interim WHO Clinical Staging of HIV/AIDS and HIV/AIDS case definitions for surveillance. http://www.who.int/hiv/pub/guidelines/clinicalstaging.pdf. [Google Scholar]
- 10.Mills EJ, Nachega JB, Buchan I, et al. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. JAMA. 2006;296:679–690. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]
- 11.Lawn SD, Myer L, Harling G, et al. Determinants of mortality and nondeath losses from an antiretroviral treatment service in South Africa: Implications for program evaluation. Clin Infect Dis. 2006;43:770–776. doi: 10.1086/507095. [DOI] [PubMed] [Google Scholar]
- 12.Gupta R, Hill A, Sawyer AW, Pillay D. Emergence of drug resistance in HIV type 1-infected patients after receipt of first-line highly active antiretroviral therapy: A systematic review of clinical trials. Clin Infect Dis. 2008;47:712–722. doi: 10.1086/590943. [DOI] [PubMed] [Google Scholar]
- 13.Weidle PJ, Malamba S, Mwebaze R, et al. Assessment of a pilot antiretroviral drug therapy programme in Uganda: patients’ response, survival, and drug resistance. Lancet. 2002;360:34–40. doi: 10.1016/S0140-6736(02)09330-3. [DOI] [PubMed] [Google Scholar]
- 14.Laurent C, Kouanfack C, Koulla-Shiro S, et al. Effectiveness and safety of a generic fixed-dose combination of nevirapine, stavudine, and lamivudine in HIV-1-infected adults in Cameroon: open-label multicentre trial. Lancet. 2004;364:29–34. doi: 10.1016/S0140-6736(04)16586-0. [DOI] [PubMed] [Google Scholar]
- 15.Dao H, Mofenson LM, Ekpini R, et al. International recommendations on antiretroviral drugs for treatment of HIV-infected women and prevention of mother-to-child HIV transmission in resource-limited settings: 2006 update. Am J Obstet Gynecol. 2007;197:S42–S55. doi: 10.1016/j.ajog.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Chi BH, Sinkala M, Mbewe F, et al. Single-dose tenofovir and emtricitabine for reduction of viral resistance to non-nucleoside reverse transcriptase inhibitor drugs in women given intrapartum nevirapine for perinatal HIV prevention: an open-label randomised trial. Lancet. 2007;370:1698–1705. doi: 10.1016/S0140-6736(07)61605-5. [DOI] [PubMed] [Google Scholar]
- 17.Myer L, Rabkin M, Abrams EJ, et al. Focus on women: linking HIV care and treatment with reprodictive health services in the MTCT-Plus initiative. Reproductive Health Matters. 2005;13:136–146. doi: 10.1016/s0968-8080(05)25185-6. [DOI] [PubMed] [Google Scholar]