Abstract
OBJECTIVE
The objective of this study was to describe lipid profiles and glucose homeostasis in HIV-positive children after initiating or changing antiretroviral therapy and their associations with viral, immune, antiretroviral therapy, and growth factor parameters.
METHODS
Ninety-seven prepubertal HIV-positive children aged 1 month to <13 years were observed for 48 weeks after beginning or changing antiretroviral therapy. Fasting lipid panels, serum glucose, insulin, insulin-like growth factor-1 and binding proteins-1 and -3, plasma viral load, and CD4% were measured. Each child was matched on age, gender, and race/ethnicity to children from the National Health and Nutrition Examination Survey, used to give z scores for each child’s lipid values. Multivariate regression was used to evaluate the association of changes in z scores over 48 weeks with suppression of HIV-1 RNA, change in CD4% and growth factors, and antiretroviral therapy, adjusted for entry z score, CD4%, log10 HIV-1 RNA, Centers for Disease Control and Prevention category, and total fat and cholesterol dietary intake.
RESULTS
Lipid, apolipoprotein, and insulin levels all increased significantly by 48 weeks. Multivariate analysis of changes demonstrated that increased HDL and decreased total-HDL cholesterol ratio were associated with CD4% increase and with insulin-like growth factor-1, which increased to normal (versus remained stable or became low) over 48 weeks. Total cholesterol levels increased among children who achieved HIV-1 RNA of <400 copies per mL. Antiretroviral therapy regimens that included both a protease inhibitor and a non–nucleoside reverse transcriptase inhibitor were associated with greater increases in total-HDL cholesterol ratio than regimens that contained a protease inhibitor or a non–nucleoside reverse transcriptase inhibitor but not both.
CONCLUSIONS
In these HIV-positive children with predominantly mild-to-moderate disease, initiation or change in antiretroviral therapy was associated with significant increases in multiple lipid measures and insulin resistance. Favorable lipid changes were associated with CD4% increases, suggesting a protective effect of immune reconstitution on atherosclerosis, and with increased insulin-like growth factor-1 levels, supporting the theory that reduced growth hormone resistance may be a mechanism by which lipid profiles are improved. Finally, antiretroviral therapy regimens that contain both a non–nucleoside reverse transcriptase inhibitor and a protease inhibitor are associated with worse lipid profiles than regimens that contain 1 but not both of these drug classes.
Keywords: children, cholesterol, glucose, HIV, insulin, insulin resistance, triglycerides, apolipoprotein, viral load, immune reconstitution, insulin like growth factor-1, insulin like growth factor binding protein
Hyperlipidemia and insulin resistance have been noted to occur in HIV-infected individuals1,2 and are sometimes associated with an increase in abdominal fat and/or loss of peripheral fat.3–5 These changes are seen more commonly in individuals who receive highly active antiretroviral therapy (HAART)6–9 but may also occur before treatment.2,7 Individuals who experience these effects seem to be at increased risk for cardiovascular disease,10,11 a particular concern to children who may face lifetime exposure to these complications. Etiologies and interrelationships of these complications are poorly understood. Evidence suggests that alterations in the growth hormone axis may contribute to these metabolic abnormalities; in 1 study, for example, treatment with growth hormone (GH)-releasing factor resulting in increased insulin-like growth factor-1 (IGF-1) levels improved lipid profiles in HIV-infected individuals with central adiposity.12 An increased understanding of risk factors and their relationships to adverse metabolic effects may lead to better insight into the pathophysiology and potential preventive and/or treatment strategies in childhood HIV infection.
Evidence from several studies of children suggests that hyperlipidemia, particularly hypercholesterolemia, is relatively common in HIV-infected children who receive antiretroviral therapy (ART);4,8,9,13,14 less evidence is available on insulin resistance in pediatric patients. Metabolic abnormalities and fat redistribution are more likely to occur in postpubertal children,4,8,15 especially those who are on protease inhibitor (PI) treatment.4,8,16,17 Few studies reported exclusively on prepubertal children. The objectives of this study were to evaluate (1) lipid profiles and glucose homeostasis in HIV-positive prepubertal children on initiating or changing ART and (2) associations of lipid profiles and glucose homeostasis with viral, immune, ART, and growth factor parameters. We hypothesized that treatment-naive patients would have an increased prevalence of lipid abnormalities compared with population-based norms and that both lipid abnormalities and subclinical insulin resistance would increase in prevalence after initiating or changing ART, particularly in children begun on PI therapy. We also hypothesized that lipid profiles and insulin sensitivity would be less favorable in children with abnormalities in the GH axis as measured by IGF-1 and its binding proteins.
METHODS
Subjects and Design
Pediatric AIDS Clinical Trials Group Protocol 1010 (PACTG P1010) was a multisite, prospective, 48-week observational study of HIV-infected, prepubertal children who were aged 1 month to <13 years and were beginning or changing ART. ART criteria for inclusion were (1) beginning any ART when treatment naive, (2) beginning PI-based ART when PI naive, or (3) changing ART because of virologic failure to a regimen that included ≥2 new drugs. Exclusion criteria were concurrent acute illness or fever; treatment within 180 days of entry with corticosteroids, anabolic steroids, megestrol acetate, interleukin, interferon, thalidomide, or GH; malignancy; pubertal development (Tanner stage > 1); and insulin-dependent diabetes. Ethics committee approval was obtained from each participating institution. Written informed consent from the parent or legal guardian and assent from the child when appropriate were also obtained. Accrual began in June 2000 and continued until March 2004.
Visits were at study entry (within 72 hours before ART initiation or change) and at 12, 24, 36, and 48 weeks thereafter. At each visit, the following evaluations were performed by trained staff: interim history and physical examination including Tanner staging, plasma viral load (VL; HIV-1 RNA polymerase chain reaction) and CD4+ T-lymphocyte count, and 3-day diet record (24-hour intake by recall if 3-day record not performed). Blood was drawn for fasting lipid panels, insulin, glucose, and IGF-1 and insulin-like growth factor–binding protein-1 (IGFBP-1) and -3. Insulin resistance was evaluated by calculating the homeostatic model of insulin resistance (HOMA-IR), using the formula: [fasting plasma insulin (mIU/mL) × fasting plasma glucose (mmol/L)]/22.5.18
Fasting cholesterol (total, LDL, HDL, and total-HDL cholesterol ratio) and triglyceride measures from study weeks 0, 24, and 48 were compared with those from age-, gender-, and race/ethnicity-matched control subjects from the National Health and Nutrition Examination Survey 1999–2002 (NHANES IV).19 Z scores were derived from NHANES data by selecting all available control subjects (of the same gender, race/ethnicity, and age [±3 months]) and then calculating the z score as (case patient’s value – mean of matched control subjects)/(SD of matched control subjects). The median (range) number of control subjects per case patient was 27 (7–54) for HDL and total cholesterol and 11 (3–23) for LDL cholesterol and 11 (2–25) for triglycerides. Lipid values (LDL, HDL, and total cholesterol; triglycerides; and the total-HDL cholesterol ratio) were log transformed before calculating z scores because distributions of untransformed values were skewed to higher values. NHANES data were limited in that (1) lipid values (HDL, LDL, and total cholesterol and triglycerides) were measured beginning at 2 years of age, and insulin and glucose were not measured in children who were younger than 12 years; and (2) apolipoprotein A1 and B z scores were obtained from a publication using NHANES III data,20 rather than derived from original data.
Laboratory Analyses
Serum was collected after overnight fasting for lipids (triglycerides and HDL, LDL, and total cholesterol), apolipoproteins A1 and B, insulin, glucose, IGFBP-1, IGF-1, and IGFBP-3. VL and CD4+ lymphocyte quantification were determined at each visit. Serum was separated from clotted blood within 1 hour of collection and shipped overnight to Quest Diagnostics (Baltimore, MD) on frozen cold packs. Routine chemistry analytes (lipid profile, glucose, and insulin) were tested on specimen receipt. Specialty chemistry analytes (apolipoproteins A1 and B, IGF-1, IGFBP-1, and IGFBP-3) were measured on ultrafrozen serum at Quest Diagnostics Nichols Institute (San Juan Capistrano, CA).
Total, HDL, and LDL cholesterol were each measured using routine Food and Drug Administration–approved chemical methods. VLDL cholesterol was calculated using a modified Friedewald formula where VLDL = triglycerides/5; LDL was calculated by subtracting HDL and VLDL cholesterol from total cholesterol when triglycerides were <400 mg/dL; otherwise, it was measured directly using a routine Food and Drug Administration–approved homogeneous immunoassay. Apolipoproteins were measured by turbidimetry, glucose by spectrophotometry, IGFBP-1 and IGFBP-3 by radioimmunoassay, and insulin and IGF-1 by chemiluminescence immunoassays. Laboratories with approved performance in the National Institute of Allergy and Infectious Diseases Division of AIDS Virology and Immunology Quality Assurance Programs conducted HIV-1 RNA and CD4+ cell measurements.
Statistical Analyses
Abnormal fasting values were age and gender specific for apolipoproteins,20 triglycerides,21 and IGF-1, IGFBP-1, and IGFBP-3.22 Other abnormal levels were defined as follows:21,22 total, LDL, and HDL cholesterol ≥200 mg/dL, ≥130 mg/dL, and ≤35 mg/dL, respectively; total-HDL cholesterol >4.0; glucose >100 mg/dL; insulin ≥15 µIU/mL for Tanner stage 1 and ≥30 µIU/mL for Tanner stage 2 and beyond (ie, those who entered puberty during follow-up; and HOMA-IR >3.16 µIU/mL per mmol/L.23
For each of these parameters, conditional logistic regression (taking account of the matching) was used to evaluate the difference between the proportion of P1010 children and the proportion of matched NHANES children with abnormal levels at study entry. Changes in metabolic parameters between entry and week 48 among P1010 children were evaluated using the paired t test to assess whether the mean change was different from 0. McNemar’s test was used to evaluate changes over time in the proportion of P1010 children with an abnormal value in each of these parameters.
For the z scores in fasting cholesterol (total, LDL, HDL, and total-HDL cholesterol ratio) and triglyceride measures (calculated using the matched NHANES IV children) and z scores for apolipoprotein A1 and B (based on published values), t tests were used to evaluate whether the mean z scores at entry were different from 0. Linear regression was used to evaluate bivariate associations of entry z scores and change in z scores over 48 weeks with age (1 to <18 months, 18 months to <3 years, 3 to <8 years, and 8 to <13 years), gender, race/ethnicity, previous therapy (ART naive, PI naive but ART exposed, and PI exposed), Centers for Disease Control and Prevention (CDC) clinical category,24 and entry plasma VL (<3, 3 to <5, and ≥5 log10 copies per mL), CD4% (<15%, 15% to <25%, and ≥25%) and IGF-1, IGFBP-1, and IGFBP-3 category (normal versus less than [IGF-1 and IGFBP-3] or greater than [IGFBP-1] normal). Linear regression was also used to evaluate associations of changes in these z scores at 24 or 48 weeks with changes in CD4%, viral suppression (less than versus more than 400 copies per mL), ART regimen during study follow-up (PI-based versus non–nucleoside reverse transcriptase inhibitor [NNRTI]-based versus both), and changes in IGF-1 and binding proteins.
Multivariate regression analysis was performed to evaluate the associations of entry fasting cholesterol and triglyceride z scores with entry CDC clinical category, CD4%, log10 HIV-1 RNA and previous ART. The associations of changes in z scores with changes in CD4%, log10 HIV-1 RNA, and type of ART taken during the study were simultaneously evaluated in multivariate analyses adjusted for entry z score and CD4%, log10 HIV-1 RNA, and CDC clinical category. Reported analyses were adjusted for lipid intake of P1010 participants reported by caregivers at each visit, dichotomized per American Heart Association guidelines25 as appropriately “heart healthy” or not for each of saturated fat, total fat, and cholesterol; unadjusted analyses gave similar results. Mean dietary intake for each child across all visits was also calculated for each of these categories of fats as an indicator of overall dietary heart healthiness.
Two sample t tests compared mean fasting cholesterol, triglyceride, and apolipoprotein A1 and B z scores at entry for children whose IGF-1 and IGFBP-3 level was below versus above the age-related lower limit of normal22 and for those whose IGFBP-1 level was below versus above the upper limit of normal22 as well as for mean z score changes for children whose IGF-1 or IGFBP-3 level increased from below to above the lower limit of normal or IGFBP-1 level decreased from above to below the upper limit of normal.
For metabolites that were not measured in NHANES (glucose, insulin, and calculated HOMA-IR), actual concentrations of the metabolite were used rather than z scores in the analysis. Otherwise, the methods as described were performed for these values in the same manner.
RESULTS
Study Population
A total of 105 patients were recruited to achieve the desired sample size of 100, because 5 patients were found to be ineligible after study entry as a result of pubarche (n = 3), disallowed medication (n = 1), or withdrawal of consent before initial data collection (n = 1). Three additional patients were excluded because the entry visit occurred subsequent to the change in ART, resulting in a final sample size of 97 children. Six children withdrew from the study before the 48-week visit. Demographic and clinical characteristics of the study population, including viral and immune response and ART regimen on study, are summarized in Table 1 and Table 2. The mean (SD) age at entry was 5.9 (3.6) years, mean CD4% was 24.8 (12.5), and mean HIV-1 RNA level was 4.55 (0.89) log10 copies per mL, corresponding to a geometric mean of 35 338 copies per mL. At study entry, 29% of children were ART naive and an additional 24% were ART exposed but PI naive. At both 24 and 48 weeks, 54% to 55% of children had a VL of <400 copies per mL. Dietary intake data were available for 82 children; mean total fat intake exceeded national recommendations for only 2 (2%) of these children, but mean saturated fat intake exceeded recommendations (<7% of caloric intake) in 81 (99%), or all but 1 child. Approximately one third (37%) of the children had a mean cholesterol intake of ≥300 mg.
TABLE 1.
Demographic Characteristics of PACTG 1010 Study Population
| Characteristic | No. of Children |
%of Childrena |
|---|---|---|
| Ageb | ||
| 1 mo to<18 mo | 9 | 9.3 |
| 18 mo to <3 y | 15 | 15.5 |
| 3 y to <8 y | 33 | 34.0 |
| 8 y to <13 y | 40 | 41.2 |
| Gender | ||
| Male | 45 | 46.4 |
| Female | 52 | 53.6 |
| Race/ethnicity | ||
| White, non-Hispanic | 11 | 11.3 |
| Black, non-Hispanic | 59 | 60.8 |
| Hispanic | 26 | 26.8 |
| Other/unknown | 1 | 1.0 |
| CDC clinical stageb | ||
| A, N | 47 | 48.4 |
| B | 32 | 33.0 |
| C | 18 | 18.6 |
| Previous therapyb | ||
| ART naive | 28 | 28.9 |
| PI naive, ART exposed | 23 | 23.7 |
| PI exposed | 46 | 47.4 |
| ART regimen on study | ||
| NRTI | 4 | 4.0 |
| NRTI/NNRTI | 18 | 18.6 |
| NRTI/NNRTI/PI | 19 | 19.6 |
| NRTI/PI | 55 | 56.7 |
| NRTI/NNRTI/FI | 1 | 1.0 |
NRTI indicates nucleoside reverse transcriptase inhibitor; FI, fusion inhibitor (T20).
Percentage is of children with known status; percentages may not add to 100 because of rounding.
At study entry.
TABLE 2.
Clinical Characteristics of PACTG 1010 Study Population
| Characteristic | No. of Children | % of Childrena | ||
|---|---|---|---|---|
| Entry | 48 wk | Entry | 48 wk | |
| CD4% | ||||
| 0 to <15 | 17 | 8 | 18.9 | 9.1 |
| 15 to <25 | 29 | 14 | 32.2 | 15.9 |
| ≥25 | 44 | 66 | 48.9 | 75.0 |
| Missing | 7 | 9 | ||
| HIV-1 RNA copies per mL | ||||
| <400 | 2 | 46 | 2.2 | 54.1 |
| 400 to <10 000 | 22 | 12 | 23.7 | 14.1 |
| 10 000 to <30 000 | 15 | 11 | 16.1 | 12.9 |
| 30 000 to <100 000 | 26 | 10 | 28.0 | 11.8 |
| ≥100 000 | 28 | 6 | 30.1 | 7.1 |
| Missing | 4 | 12 | ||
Percentage is of children with known status; percentages may not add to 100 because of rounding.
Metabolic Variables
Abnormalities at Study Entry
At study entry, the prevalence of abnormal values in P1010 children was greater than that for control children for HDL cholesterol (21% vs 4%; P < .001) and total-HDL cholesterol ratio (37% vs 17%; P < .001), but the differences in prevalence of abnormal LDL and total cholesterol and triglycerides were not significant. When comparing P1010 children who were ART naive to their matched NHANES children, the differences were even greater: 30% vs 4% with abnormal HDL cholesterol (P < .001) and 45% vs 15% with abnormal total-HDL cholesterol ratio (P = .002).
Metabolic Changes over Time
Mean fasting total, HDL, and LDL cholesterol and apolipoprotein A1 and B levels all increased significantly during the 48 weeks of study observation, whereas mean triglyceride levels increased significantly by 24 weeks of treatment and then decreased slightly between weeks 24 and 48 (Table 3). There was no change in the total-HDL cholesterol ratio at 48 weeks (P = .63). Fasting insulin and the HOMA-IR also increased by 48 weeks of observation, but the change in fasting glucose was not statistically significant (P = .062). In addition, at 48 weeks, the proportion of children with abnormally high total cholesterol concentrations significantly increased, from 6% at entry to 21% (P = .001), and that with abnormally low HDL concentrations significantly decreased from 31% at entry to 16% (P = .004). The proportion of children with abnormally high total-HDL cholesterol ratio decreased slightly from 38% to 32%; this decrease was not statistically significant (P = .13). Abnormal insulin resistance increased from 1% to 3% of the study population, but this increase was not statistically significant (P = 1.00). Changes in percentage of children with abnormal apolipoproteins approached statistical significance, apolipoprotein A1 from 29% to 15% (P = .058) and B from 27% to 36% (P = .059).
TABLE 3.
Mean Metabolic Parameters and Changes During 48 Weeks in P1010 Study Population
| Analyte | Mean Value ± SD (n) | Change From Week 0 to Week 48 | % With Abnormal Values | |||||
|---|---|---|---|---|---|---|---|---|
| Week 0 | Week 48 | Mean±SD (n) | 95% CI | P | Week 0 | Week 48 | Pa | |
| HDL cholesterol, mg/dL | 40.6±10.9 (90) | 48.0±14.3 (85) | 8.3±15.(78) | 14.9 to 11.7 | <.001b | 31 (28/90) | 16 (14/85) | .004b |
| LDL cholesterol, mg/dL | 88.4±32.2 (90) | 102.6±34.8 (85) | 14.0±37.0 (78) | 5.6 to 22.3 | .001b | 13 (12/90) | 21 (18/85) | .110 |
| Total cholesterol, mg/dL | 145.7±35.5 (90) | 172.7±44.1 (86) | 28.1±47.9 (79) | 17.4 to 38.8 | <.001b | 6 (5/90) | 21 (18/86) | .001b |
| Total-HDL cholesterol ratio | 3.8±1.1 (90) | 3.9±1.5 (85) | 0.1±1.5 (78) | −0.3 to 0.4 | .630 | 38 (34/90) | 32 (27/85) | .130 |
| Triglycerides, mg/dL | 93.0±55.5 (90) | 117.7±90.0 (86) | 21.2±86.0 (79) | 2.0 to 40.5 | .031b | 26 (23/90) | 34 (29/86) | .530 |
| Insulin, µ IU/mL | 4.5±3.8 (84) | 6.6±5.0 (79) | 2.1±5.2 (66) | 0.9 to 3.4 | .001b | 1 (1/84) | 3 (2/79) | 1.000 |
| Glucose, mg/dL | 70.3±16.4 (90) | 75.2±17.6 (85) | 4.3±19.9 (78) | −0.2 to 8.7 | .062 | 2 (2/90) | 4 (3/85) | 1.000 |
| HOMA-IR, µ IU/mL per mmol/L | 0.8±0.8 (84) | 1.3±1.2 (78) | 0.5±1.1 (65) | 0.2 to 0.8 | .001b | 1 (1/84) | 8 (7/78) | .480 |
| Apolipoprotein Al, mg/dL | 124.9±25.8 (78) | 137.2±29.6 (72) | 14.4±29.6 (58) | 6.6 to 22.2 | .001b | 29 (16/56) | 15 (8/53) | .058 |
| Apolipoprotein B, mg/dL | 77.9±24.6 (78) | 88.0±29.8 (72) | 10.8±24.4 (58) | 4.4 to 17.3 | .001b | 27 (15/56) | 36 (19/53) | .059 |
n is the number of observations included in the analyses. CI indicates confidence interval.
McNemar’s test was used to assess whether the change in the proportion of children with abnormal values differed from 0.
Significant (P<.05).
Comparison of Lipids With Matched Children From NHANES
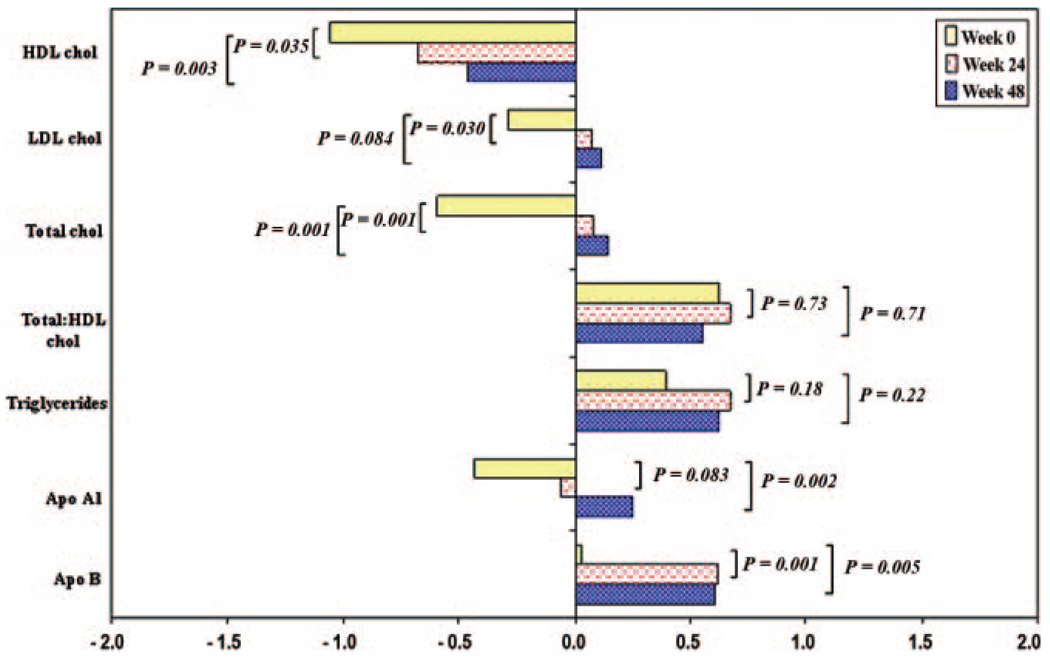
In comparing the study population with the NHANES reference population (restricted to the 71 children who were older than 2 years and had available laboratory data), the mean (SD) z scores for HDL (−1.06 [1.22]; P < .001) and total (−0.60 [1.39]; P = .001) cholesterol were significantly <0 at entry, whereas the mean z scores for triglyceride (0.40 [1.36]; P = .017) and total-HDL cholesterol ratio (0.62 [1.32]; P < .001) were significantly greater (Fig 1). Mean lipid z scores did not differ significantly by age, gender. or race/ethnicity.
FIGURE 1.
Mean metabolic z scores at study weeks 0, 24, and 48 in the P1010 study population.
Multivariate Analysis at Study Entry
In bivariate analyses, HDL cholesterol z scores at study entry were significantly lower in children with lower baseline CD4% (P < .001) or higher baseline HIV-1 RNA (P = .004): mean HDL z scores were −2.05, −0.93, and −0.66 among children with CD4% of <15%, 15% to <25%, and ≥25%, respectively, and −0.55, −1.06, and −1.65 among children with HIV-1 RNA levels of <103, 103 to <105, and ≥105 copies per mL, respectively. In multivariate analysis, however, the association with HIV-1 RNA was not significant (P = .26; Table 4), and the association with CD4% was only marginally significant (P = .053), corresponding to an estimated lower mean z score of 0.28 for each 10% lower CD4% at baseline. No significant associations between other entry lipid z scores and HIV-1 RNA or CD4% were found in bivariate or multivariate analyses. In multivariate analysis of both total and LDL cholesterol, there were, however, significantly lower mean z scores among children with CDC category C disease than among children with less advanced disease.
TABLE 4.
Association of Entry z Scores With Measures of Disease Status and Previous ART in P1010 Population
| Metabolite (n) | CD4% (Per 10% Higher) | HIV RNA (Per 1 log10 Copies per mL Higher) |
CDC Category (vs Category A/N) | Previous ART (vs ART Naive) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted z Score Difference (95% CI) at Entry |
P | Adjusted z Score Difference (95% CI) at Entry |
P | Category B |
Category C |
PI Exposed |
PI Naive |
|||||
| Adjusted z Score Difference (95% CI) at Entry |
P | Adjusted z Score Difference (95% CI) at Entry |
P | Adjusted z Score Difference (95% CI) at Entry |
P | Adjusted z Score Difference (95% CI) at Entry |
P | |||||
| HDL cholesterol (n=65) | 0.28 (0.00 to 0.56) | .053 | −0.26 (−0.72 to 0.20) | .260 | −0.19 (−0.96 to 0.59) | .630 | −0.14 (−1.05 to 0.77) | .760 | −0.23 (−1.11 to 0.66) | .610 | −0.69 (−1.73 to 0.35) | .190 |
| LDL cholesterol (n=66) | 0.18 (−0.19 to 0.54) | .340 | 0.15 (−0.47 to 0.77) | .630 | −0.21 (−1.22 to 0.80) | .680 | −1.78 (−2.96 to−0.60) | .004a | 0.76 (−0.39 to 1.91) | .190 | 0.14 (−1.20 to 1.49) | .830 |
| Total cholesterol (n=65) | 0.01 (−0.22 to 0.41) | .550 | −0.01 (−0.52 to 0.50) | .970 | −0.16 (−1.03 to 0.70) | .700 | −1.20 (−2.21 to−0.18) | .022a | 1.05 (0.06 to 2.04) | .039a | 0.34 (−0.81 to 1.50) | .550 |
| Total-HDL cholesterol ratio | −0.21 (−0.53 to 0.10) | .190 | 0.22 (−0.29 to 0.72) | .400 | 0.13 (−0.73 to 1.00) | .750 | −0.59 (−1.59 to 0.42) | .250 | 0.96 (−0.02 to 1.94) | .056 | 1.01 (−0.14 to 2.16) | .084 |
| (n=65) | ||||||||||||
| Triglycerides (n=65) | −0.22 (−0.55 to 0.12) | .200 | −0.067 (−0.60 to 0.47) | .800 | 0.27 (−0.64 to 1.18) | .560 | 0.85 (−0.22 to 1.92) | .120 | 0.43 (−0.61 to 1.48) | .410 | 0.08 (−1.14 to 1.29) | .900 |
| Apolipoprotein A1 (n=50) | 0.11 (−0.29 to 0.50) | .590 | −0.43 (−1.05 to 0.19) | .170 | −0.10 (−1.12 to 0.92) | .850 | 0.02 (−1.18 to 1.22) | .970 | 0.50 (−0.64 to 1.63) | .380 | −0.17 (−1.58 to 1.24) | .810 |
| Apolipoprotein B (n=50) | −0.15 (−0.61 to 0.31) | .510 | −0.12 (−0.84 to 0.59) | .730 | 0.26 (−0.91 to 1.44) | .660 | −0.76 (−2.14 to 0.62) | .270 | 1.10 (−0.21 to 2.41) | .097 | 0.36 (−1.26 to 1.98) | .650 |
Results are from multivariate models that were adjusted for all variables in table and dietary intake in excess of recommended cholesterol and total fat; there were no significant associations with glucose, insulin, or HOMA-IR n is the number of observations included in the analyses.
Significant (P<.05).
There was a marginally significant difference at study entry in mean total cholesterol z score but not of other lipid levels, according to type of previous antiretroviral therapy (P = .058), reflecting higher levels in children with a history of PI exposure than in PI-naive or ART-naive children (−0.27 vs −1.28 and −0.82). This corresponded to mean z scores that were 1.01 higher in PI-exposed children compared with PI-naive children. In multivariate analysis, the difference according to type of previous therapy was significant (P = .039), primarily reflecting a difference between PI-exposed and ART-naive children (adjusted mean z score difference: 1.05). There were no significant associations found between insulin or glucose concentrations or the calculated HOMA-IR value at entry and entry characteristics found in Table 4.
Multivariate Analysis of Metabolic Changes
During the first 24 weeks after initiating or changing therapy, there were significant increases in mean z scores for HDL (0.38 [1.43]; P = .035), LDL (0.37 [1.34]; P = .030), and total cholesterol (0.68 [1.33]; P < .001), as shown in Fig 1. The mean z score changes from entry were additional increased at week 48 for HDL (0.60 [1.49]; P = .003) and total (0.75 [1.37]; P < .001) cholesterol but were similar for LDL cholesterol (0.40 [1.80]; P = .084). Total-HDL cholesterol ratio and tri-glyceride z scores did not change significantly (−0.07 [1.37; P = .71] and 0.23 [1.44; P = .22] at 48 weeks, respectively). Mean apolipoprotein A1 and B z scores both increased at 24 weeks, apolipoprotein B significantly (0.59 [1.20]; P < .001). Apolipoprotein A1 continued to increase until 48 weeks (0.69 [1.34] z score greater than at entry; P = .002), and B declined slightly.
In multivariate analysis that simultaneously adjusted z scores for changes in CD4%, HIV-1 RNA, and ART on study, mean increase in HDL and decrease in total-HDL cholesterol ratio z scores were significantly associated with increases in CD4% at week 48 (0.67 HDL z score change for each 10% increase in CD4% [P = .044] and −0.71 total-HDL cholesterol ratio z score change[P = .023]; Table 5). There was a similar association of increase in LDL cholesterol z score with increased CD4% at 24 weeks (24-week multivariate analysis not shown), but by 48 weeks, this was no longer seen. There were no significant associations with suppression of HIV-1 RNA to <400 copies per mL at week 24, but children who had undetectable virus at week 48 had higher adjusted mean total cholesterol z score than children with detectable virus (0.90, P = .047). Adjusted for magnitude of CD4% change and suppression of HIV-1 RNA, ART including both a PI and an NNRTI versus an NNRTI without a PI was associated with significantly greater increase in total-HDL cholesterol ratio and triglyceride z scores at both weeks 24 and 48 (1.32 [P = .036] and 1.44 [P = .021], respectively). Similarly, dual NNRTI-PI therapy resulted in greater increase in total-HDL cholesterol ratio than did PI-based regimens without an NNRTI (1.39; P = .018). There were no significant differences in lipid changes between therapies that included a PI versus those that included an NNRTI. In multivariate analysis that simultaneously adjusted the concentrations of metabolites that measure insulin resistance for changes in CD4%, HIV-1 RNA, and ART on study, there were no significant associations with changes in insulin or glucose concentrations or the calculated HOMA-IR value with CD4%, VL changes, or ART classes on study.
TABLE 5.
Association of Changes in z Scores for Metabolic Parameters From Baseline to Week 48 With Measures of Disease Status and ART
| Metabolite (n) | CD4% Change From Baseline to Week 48 (Per 10% Increase) |
HIV RNA <400 Copies per mL at Week 48 (vs ≥400 Copies per mL) |
Antiretroviral Therapy on Study (vs NNRTI Based) |
|||||
|---|---|---|---|---|---|---|---|---|
| NNRTI and PI Based | PI Based | |||||||
| Difference in Adjusted z | P | Difference in Adjusted z | P | Difference in Adjusted z | P | Difference in Adjusted z | P | |
| Score Change (95% CI) | Score Change (95% CI) | Score Change (95% CI) | Score Change (95% CI) | |||||
| From Entry to Week 48 | From Entry to Week 48 | From Entry to Week 48 | From Entry to Week 48 | |||||
| HDL cholesterol (n=53) | 0.67 (0.02 to 1.33) | .044a | −0.17 (−1.10 to 0.76) | .710 | −0.15 (−1.49 to 1.19) | .820 | −0.07 (−1.27 to 1.13) | .910 |
| LDL cholesterol (n=53) | 0.04 (−0.84 to 0.91) | .930 | 0.98 (−0.28 to 2.23) | .120 | 0.09 (−1.76 to 1.94) | .920 | −0.34 (−2.00 to 1.32) | .680 |
| Total cholesterol (n=54) | 0.14 (−0.47 to 0.75) | .650 | 0.90 (0.01 to 1.78) | .047a | 1.06 (−0.20 to 2.33) | .097 | −0.03 (−1.18 to 1.13) | .960 |
| Total-HDL cholesterol ratio (n=53) |
−0.71 (−1.32 to−0.11) | .023a | 0.73 (−0.13 to 1.60) | .094 | 1.32 (0.09 to 2.55) | .036a | −0.07 (−1.17 to 1.02) | .890 |
| Triglycerides (n=54) | 0.02 (−0.55 to 0.60) | .930 | 0.31 (−0.56 to 1.19) | .480 | 1.44 (0.23 to 2.64) | .021a | 0.60 (−0.51 to 1.70) | .280 |
| Apolipoprotein A1 (n=37) | 0.76 (0.00 to 1.52) | .051 | −0.11 (−1.20 to 0.97) | .830 | −1.65 (−3.47 to 0.18) | .074 | −1.78 (−3.62 to 0.05) | .056 |
| Apolipoprotein B (n=37) | −0.59 (−1.42 to 0.25) | .160 | 0.79 (−0.40 to 1.99) | .180 | 0.56 (−1.40 to 2.51) | .560 | −0.81 (−2.77 to 1.15) | .400 |
n is the number of observations included in the analyses; Results are from multivariate models that were adjusted for z score, CD4%, log10 HIV-1 RNA, and CDC category at baseline and dietary intake in excess of recommended cholesterol and total fat; z scores calculated by using age-, gender-, and race/ethnicity-matched control subjects from NHANES (see “Methods”). There were no significant associations with changes in glucose, insulin, or HOMA-IR at 24 or 48 weeks.
Significant (P<.05).
Effect of Diet
Adjustment for total reported fat and cholesterol intake did not substantially change any noted associations, but children with cholesterol intake greater than recommended had greater total cholesterol z score increases at 24 weeks and greater increases in insulin and HOMA-IR at 48 weeks.
Metabolic Associations With IGF-1 and IGFBP-1
At entry, compared with children who had normal IGF-1 levels, children with low levels had lower mean z scores for HDL (−2.08 vs −0.56; P < .001) and total (−1.18 vs −0.32; P < .024) cholesterol and apolipoprotein A1 (−1.44 vs −0.04; P < .0001) and greater mean z scores for total-HDL cholesterol ratio (1.18 vs 0.33; P = .046; Table 6). Similarly, increases in both HDL cholesterol (P = .005) and apolipoprotein A1 (P = .023) z scores and decreases in total-HDL cholesterol ratio (P = .014) at 48 weeks were associated with increased IGF-1 from below to greater than or equal the lower limit of age-adjusted normal values (Table 6). Higher IGFBP-1 at entry was associated with lower triglyceride z scores (P = .048), insulin concentration (P = .007), and HOMA-IR (P = .004; data not shown).
TABLE 6.
Associations of IGF-1 With Lipids in P1010 Study Population
| Metabolite | Mean Metabolite z Score (SD) at Entry (n) | Mean Metabolite z Score (SD) Change at 48 Weeks (n) | ||||
|---|---|---|---|---|---|---|
| Low IGF-1 | Normal IGF-1 | P | IGF-1 Category Stable or Decreased |
IGF-1 Category Increased From Low to Normal |
P | |
| HDL cholesterol | −2.08 (1.03) (21) | −0.56 (1.11) (44) | <.001a | 0.30 (1.50) (42) | 1.96 (1.17) (8) | .005a |
| LDL cholesterol | −0.81 (2.26) (21) | −0.06 (1.29) (44) | .230 | 0.31 (1.79) (42) | 1.01 (2.31) (8) | .340 |
| Total cholesterol | −1.18 (1.66) (21) | −0.317 (1.225) (44) | .024a | 0.66 (1.32) (43) | 1.08 (1.58) (8) | .420 |
| Total-HDL cholesterol ratio | 1.18 (1.44) (21) | 0.33 (1.20) (44) | .046a | 0.16 (1.27) (42) | −1.09 (1.36) (8) | .014a |
| Triglycerides | 0.58 (1.38) (21) | 0.31 (1.41) (44) | .270 | 0.24 (1.46) (43) | −0.10 (1.16) (8) | .550 |
| Apolipoprotein A1 | −1.44 (0.88) (17) | 0.04 (1.28) (37) | <.001a | 0.56 (1.24) (33) | 2.03 (1.67) (5) | .023a |
| Apolipoprotein B | 0.031 (1.58) (17) | 0.06 (1.50) (37) | .820 | 0.57 (1.19) (33) | 0.62 (1.70) (5) | .940 |
n is the number of observations included in the analyses. P values are for Student’s t test difference between group means.
Significant (P<.05).
DISCUSSION
The novel findings of greatest clinical significance in this study are that favorable lipid changes (increased HDL and decreased total-HDL cholesterol ratio z scores) are associated with immune reconstitution and improved GH sensitivity in HIV-infected children who begin or change ART regimens. There was also a trend toward higher HDL cholesterol z score with higher CD4% cell counts at study entry, giving additional credence to the association. In addition, a greater increase in apolipoprotein A1 z score was associated with improved IGF-1 and a trend toward the same with improved CD4%, emphasizing the favorable change in lipid profiles with greater GH sensitivity and immune reconstitution. It is important to note that despite the mean increases in LDL and total cholesterol experienced by these children, the HDL also increased such that there was not a worsening of the cardiovascular risk as measured by total-HDL cholesterol ratio. Accordingly, we were unable to prove our hypothesis that abnormalities in lipid profiles would increase in prevalence in these children. The decrease in HDL abnormalities paralleled the increase in abnormal total cholesterol levels and resulted in a statistically insignificant decrease in the percentage of children with an abnormal total-HDL cholesterol ratio. The hypothesis that treatment-naive patients would have an increased prevalence of lipid abnormalities at entry compared with population-based norms, however, was true, but we were surprised to find that the difference was related to abnormally low HDL cholesterol in the infected children and consequently higher total-HDL cholesterol ratios.
Increases in HDL cholesterol have been described in ART-treated children,26 a finding that we confirmed. We did not, however, find an association of increased total cholesterol with improvements in CD4% counts reported recently in both HAART-treated children9 and adult women.27 Rather, our findings are similar to those from a study in HIV-infected adults in which greater HDL cholesterol was associated with higher CD4+ cell counts.28 We did find a relationship between immune reconstitution and LDL cholesterol only during the first 24 weeks of the study, an association that has been seen in adults.28 It is not clear what biological differences in children would cause a net favorable change in lipid profile with improvements in CD4% not previously noted in adults.
In multivariate analysis, the total cholesterol concentration at baseline in our population was associated with a history of PI use, suggesting a deleterious effect of PI therapy that has been described in multiple previous studies.2,7,28–31 Moreover, we noted that dual NNRTI-PI–based therapy during the study resulted in greater total-HDL cholesterol ratio increases than NNRTI- or than PI-based therapy, suggesting a potential synergistic effect. Greater triglyceride increase was also seen in our study with combined NNRTI and PI therapy, whereas PI therapy without NNRTI use was associated only with greater triglyceride increase at 24 weeks. Greater changes in triglycerides with ART regimens that contain both an NNRTI and a PI compared with regimens that contain just 1 but not both of these drug classes have been previously described.32
Viral suppression was related in multivariate analysis to greater increases in total cholesterol, concurring with the previous report in children of an association between hypercholesterolemia and an undetectable VL.8 Taken together with the finding that the total cholesterol at entry was actually below that of matched population-based control subjects, perhaps viral replication itself interferes with cholesterol metabolism. There is increasing evidence for this concept from in vitro studies. Of particular interest is the recent report that intracellular lipid metabolism is dysregulated in HIV-infected macro-phages.33 It may be that viral suppression unmasks the effect of increased cholesterol related to drug therapy, or it may be a marker for improved adherence and therefore greater ART exposure.
There was a statistically significant increase in insulin and insulin resistance as measured by HOMA-IR in the children our study and a change in fasting blood glucose that approached statistical significance. Unfortunately, there are not available comparison values from NHANES to measure whether these changes were greater than might be expected in the general population. Although there was not a statistically significant change in the number of children with frankly abnormal values (and thus our hypothesis was not confirmed), the mean (SD) increase of HOMA-IR at 48 weeks by 0.48 (1.14 µIU/mL per mmol/L) and the increase in the prevalence of abnormal glucose tolerance as defined by HOMA-IR >3.16 from 1% at entry to 8% at 48 weeks suggest that development of glucose intolerance even in prepubertal children may be of concern and needs additional investigation. We did not identify risk factors for increased insulin resistance with any of the variables in our multivariate analysis, other than cholesterol intake. Previous reports are contradictory regarding whether insulin sensitivity is altered with PI therapy in this age group.30,31 We previously reported that children in this study did not increase their BMI or percentage of body fat z scores,34 suggesting that greater adiposity is unlikely to account for the increase in insulin and HOMA-IR that was seen. The greater increase in insulin resistance associated with excessive cholesterol consumption in this study may suggest that the development of insulin resistance could be prevented, in part, by a heart-healthy diet.
We previously reported that IGF-1 increased over time in a greater number of children in P1010 than would be expected by chance and that these increases were associated with improvements in muscle mass.34,35 This analysis further notes that greater HDL cholesterol and apolipoprotein A1 and lower total-HDL cholesterol ratio concentrations, both at entry and over time, were associated with normal IGF-1 concentrations, confirming our hypothesis. These findings suggest that GH sensitivity is likely related to improved lipid profiles as well as improved anabolism. The improved lipid metabolism that was seen in lipodystrophic HIV-infected adults who were treated with physiologic GH replacement supports the link between GH resistance (or deficiency) and dyslipidemias. 36
This study had several limitations. It is likely the HIV-infected children in our study differed from the overall US population represented in NHANES data in ways for which we could not adjust, such as socioeconomic status, parental health, etc. Furthermore, measures of insulin resistance were not obtained in NHANES, so we had no comparison group for these analytes. Apolipoprotein z values from NHANES were obtained from a publication, rather than derived directly from the data. NHANES itself is a cross-sectional data set and not ideal for comparison of longitudinal data. Longitudinal reference data would allow for calculation of z scores for changes in lipids, etc. We did not have a comparison group of HIV-infected children who were not beginning or changing therapy, so clearly the associations noted are not necessarily causally related and may be different in children who are on long-term therapy. The children in our study also began diverse ART regimens, limiting the power to detect changes that may be associated with specific ART agents or classes. Because therapy on study was not independent of therapy before the study, some caution must be taken when noting associations with the combination of ART classes taken during follow-up. We also cannot be certain that changes that are associated with normalization of IGF-1 are specifically attributable to improved GH sensitivity, although GH insensitivity has been previously demonstrated in HIV-infected children.37 Associations do not demonstrate causality; furthermore, there could be other causes of increased IGF-1, such as an increase in GH secretion. Although we have focused on specific hypotheses concerning the associations between lipid profiles and insulin resistance and improvements in immune status, viral suppression, and ART classes, there remains a risk for inflated type I error rate as a result of multiple comparisons. The major strengths of the study include a sample size larger than most prospective studies of children that have evaluated metabolic changes in this population, evaluation before and after ART initiation or change, and measured indices of the GH axis in addition to those of insulin resistance and lipids.
CONCLUSIONS
In this population of HIV-infected children with predominantly mild-to-moderate disease, initiation or change in ART was associated with significant increases in multiple lipid measures as well as insulin resistance. The total-HDL cholesterol ratio did not change, however, suggesting that there may not be a substantial increase in overall cardiovascular risk. Increased HDL and decreased total-HDL cholesterol ratio are associated with increases in CD4%, suggesting an actual protective effect on risk for atherosclerosis with immune reconstitution specifically. Improvements in HDL and total-HDL cholesterol ratio and apolipoprotein A1 levels also related to increased IGF-1 levels, supporting the theory that reduced GH resistance may be a mechanism by which lipid profiles are improved. Finally, of particular clinical significance, dual NNRTI-PI therapy was associated with worse lipid changes when compared with regimens that contained either but not both of these ART classes.
What’s Known on This Subject.
Hyperlipidemia, particularly hypercholesterolemia, is common in HIV-infected individuals, including children. It occurs more frequently in postpubertal children. Recently, increased HDL cholesterol has also been reported in children. In adults, increased cholesterol levels have been associated with improved CD4% cell counts.
What This Study Adds.
Although lipid levels increased, cardiovascular risk as measured by total-HDL cholesterol ratio did not worsen over time in prepubertal children who were beginning or changing antiretroviral therapy. Improved lipid profiles were associated with immune reconstitution and improvements in IGF-1.
ACKNOWLEDGMENTS
This study was supported in part by the PACTG of the National Institute of Allergy and Infectious Diseases and the Pediatric/Perinatal HIV Clinical Trials Network of the National Institute of Child Health and Human Development, National Institutes of Health (Bethesda, MD).
The following sites and individuals have contributed to this study: Howard University: S. Rana, P. Yu, S. Dangol, and J. Roa; Bronx Lebanon Hospital Center; St Jude Children’s Hospital: M. Donohoe, K. Knapp, N. Patel, and J. Utech; Baylor Texas Children’s Hospital: K. Owl, M. Dobmeier, M. Paul, and C. Hanson; Children’s Hospital Boston; Harlem Hospital: E. Abrams, D. Calo, M. Fere, and S. Champion; North Broward Hospital District; Jacobi Medical Center: A. Wiznia, M. Chin, K. Dorio, and J. Abadi; University of Florida: J. Sleasman, R. Lawrence, C. Delany; Children Hospital of Los Angeles: T. Dunaway and L. Heller; University of Maryland: J. Farley and M. MacFadden; State University of New York at Stony Brook: S. Nachman, M. Davi, C. Seifert, and S. Muniz; Metropolitan Hospital Center: M. Bamji, I. Pathak, and S. Manwani; Childrens Hospital, Oakland: A. Petru, T. Courville, K. Gold, and S. Bessler; Harbor-UCLA Medical Center: M. Keller, K. Zangwill, J. Hayes, and A. Gagajena; Columbia Presbyterian Medical Center: A. Higgins and M. Foca; University of Miami: C. Goldberg, M. Bissainthe, C. Mitchell, and G. Scott; New York University School of Medicine: T. Hastings, M. Mintor, N. Deygoo, and W. Borkowsky; University of Illinois: K. Rich, K. Hayani, and J. Camacho; Children’s Hospital, University of Colorado, Denver: E. McFarland, M. Levin, C. Salbenblatt, and E. Barr; Medical College of Georgia: W. Foshee, C. Mani, C. White, and B. Kiernan; Johns Hopkins University: S. Marvin and A. Ruff; Duke University: R. McKinney, Y. Choi, L. Ferguson, and J. Swetnam; Children’s National Medical Center; San Juan City Hospital: M. Acevedo, M. Gonzales, C. Martinez Betancoult, and F. Pabon; Yale University School of Medicine: D. Schroeder, S. Romano, and M. J. Aquino-de Jesus; Los Angeles County Medical Center: J. Homans, Y. Rodriquez, and A. Kovacs; University of Puerto Rico: I. Febo Rodriquez, L. Lugo, I. Heyer, and C. Martinez; and University of Massachusetts Medical School.
We acknowledge the children who participated in this study and their families and the entire protocol 1010 team for contributions and support.
Abbreviations
- HAART
highly active antiretroviral therapy
- IGF-1
insulin-like growth factor-1
- GH
growth hormone
- ART
antiretroviral therapy
- PI
protease inhibitor
- PACTG
Pediatric AIDS Clinical Trials Group
- VL
viral load
- IGFBP
insulin-like growth factor– binding protein
- NHANES
National Health and Nutrition Examination Survey
- CDC
Centers for Disease Control and Prevention
- NNRTI
non–nucleoside reverse transcriptase inhibitor
Footnotes
Financial Disclosure: During the course of this study (PACTG 1010), Dr Hughes received grant support from Roche and had honoraria or consultancies with Abbott, Boehringer Ingelheim, Bristol Myers Squibb, Chiron, Roche, Tibotec, and Virionyx. These companies are all manufacturers of antiretroviral therapy or other therapy for HIV infection. The other authors have indicated they have no financial relationships relevant to this article to disclose.
This trial has been registered at www.clinicaltrials.gov (identifier NCT00006064).
Reprints, Information about ordering reprints can be found online: http://www.pediatrics.org/misc/reprints.shtml
REFERENCES
- 1.El-Sadr WM, Mullin CM, Carr A, et al. Effects of HIV disease on lipid, glucose and insulin levels: results from a large antiretroviral-naive cohort. HIV Med. 2005;6(2):114–121. doi: 10.1111/j.1468-1293.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 2.Mulligan K, Grunfeld C, Tai VW, Algren H, Pang M, Chernoff DN. Hyperlipidemia and insulin resistance are induced by protease inhibitors independent of changes in body composition in patients with HIV infection. J Acquir Immune Defic Syndr. 2000;23(1):35–43. doi: 10.1097/00126334-200001010-00005. [DOI] [PubMed] [Google Scholar]
- 3.Kosmiski LA, Kuritzkes DR, Lichtenstein KA, et al. Fat distribution and metabolic changes are strongly correlated and energy expenditure is increased in the HIV lipodystrophy syndrome. AIDS. 2001;15(15):1993–2000. doi: 10.1097/00002030-200110190-00012. [DOI] [PubMed] [Google Scholar]
- 4.Beregszaszi M, Dollfus C, Levine M, et al. Longitudinal evaluation and risk factors of lipodystrophy and associated metabolic changes in HIV-infected children. J Acquir Immune Defic Syndr. 2005;40(2):161–168. doi: 10.1097/01.qai.0000178930.93033.f2. [DOI] [PubMed] [Google Scholar]
- 5.Sánchez Torres AM, Munoz Muniz R, Madero R, Borque C, García-Miguel M, Isabel De José Gómez M. Prevalence of fat redistribution and metabolic disorders in human immunodeficiency virus-infected children. Eur J Pediatr. 2005;164(5):271–276. doi: 10.1007/s00431-004-1610-y. [DOI] [PubMed] [Google Scholar]
- 6.Guimarães MM, de Oliveira AR, Jr, Penido MG, et al. Ultra-sonographic measurement of intra-abdominal fat thickness in HIV-infected patients treated or not with antiretroviral drugs and its correlation to lipid and glycemic profiles. Ann Nutr Metab. 2007;51(1):35–41. doi: 10.1159/000100819. [DOI] [PubMed] [Google Scholar]
- 7.Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Coop DA. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet. 1999;353(9170):2093–2099. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- 8.Carter RJ, Wiener J, Abrams EJ, et al. Dyslipidemia among perinatally HIV-infected children enrolled in the PACTS-HOPE cohort, 1999–2004: a longitudinal analysis. J Acquir Immune Defic Syndr. 2006;41(4):453–460. doi: 10.1097/01.qai.0000218344.88304.db. [DOI] [PubMed] [Google Scholar]
- 9.Lapphra K, Vanprapar N, Phongsamart W, Chearskul P, Chokephaibulkit K. Dyslipidemia and lipodystrophy in HIV-infected Thai children on highly active antiretroviral therapy (HAART) J Med Assoc Thai. 2005;88(7):956–966. [PubMed] [Google Scholar]
- 10.d′Arminio A, Sabin CA, Phillips AN, et al. Cardio- and cerebrovascular events in HIV-infected persons. AIDS. 2004;18(13):1811–1817. doi: 10.1097/00002030-200409030-00010. [DOI] [PubMed] [Google Scholar]
- 11.Lorenz MW, Stephan C, Harmjanz A, et al. Both long-term HIV infection and highly active antiretroviral therapy are independent risk factors for early carotid atherosclerosis. Atherosclerosis. 2008;196(2):720–726. doi: 10.1016/j.atherosclerosis.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 12.Falutz J, Allas S, Kotler D, et al. A placebo-controlled, dose-ranging study of a growth hormone releasing factor in HIV-infected patients with abdominal fat accumulation. AIDS. 2005;19(12):1279–1287. doi: 10.1097/01.aids.0000180099.35146.30. [DOI] [PubMed] [Google Scholar]
- 13.Solórzano Santos F, Gochicoa Rangel LG, Palacios Saucedo G, Vazquez Rosales G, Miranda Novales MG. Hypertriglyceridemia and hypercholesterolemia in human immunodeficiency virus-1-infected children treated with protease inhibitors. Arch Med Res. 2006;37(1):129–132. doi: 10.1016/j.arcmed.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Amaya RA, Kozinetz CA, McMeans A, Schwarzwald H, Kline MW. Lipodystrophy syndrome in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 2002;21(5):405–410. doi: 10.1097/00006454-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Viganó A, Mora S, Brambilla P, Schneider L, Merlo M, Monti LD, Manzoni P. Impaired growth hormone secretion correlates with visceral adiposity in highly active antiretroviral treated HIV-infected adolescents. AIDS. 2003;17(10):1435–1441. doi: 10.1097/00002030-200307040-00003. [DOI] [PubMed] [Google Scholar]
- 16.Farley J, Gona P, Crain M, et al. Prevalence of elevated cholesterol and associated risk factors among perinatally HIV-infected children (4–19 years old) in Pediatric AIDS Clinical Trials Group 219C. Acquir Immune Defic Syndr. 2005;38(4):480–487. doi: 10.1097/01.qai.0000139397.30612.96. [DOI] [PubMed] [Google Scholar]
- 17.Jaquet D, Lévine M, Ortega-Rodriguez E, et al. Clinical and metabolic presentation of the lipodystrophic syndrome in HIV-infected children. AIDS. 2000;14(14):2123–2128. doi: 10.1097/00002030-200009290-00008. [DOI] [PubMed] [Google Scholar]
- 18.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. National Center for Health Statistics. [Accessed May 16, 2007];Hyattsville, MD: US Department of Health and Human Services; National Health and Nutrition Examination Survey. Available at: www.cdc.gov/nchs/nhanes/htm.
- 20.Bachorik PS, Lovejoy KL, Carroll MD, Johnson CL. Apolipoprotein B and AI distributions in the United States, 1988–1991: results of the National Health and Nutrition Examination Survey III (NHANES III) Clin Chem. 1997;43(12):2364–2378. [PubMed] [Google Scholar]
- 21.Gunn VL, Nechyba C, editors. The Harriet Lane Handbook: A Manual for Pediatric Houseofficers. 16th ed. Philadelphia, PA: Mosby; 2002. [Google Scholar]
- 22.Fisher DA, editor. Pediatric Endocrinology. San Juan Capistrano, CA: Quest Diagnostics; 2000. [Google Scholar]
- 23.Tresaco B, Bueno G, Pineda I, Moreno LA, Garagorri JM, Bueno M. Homeostatic model assessment (HOMA) index cut-off values to identify the metabolic syndrome in children. J Physiol Biochem. 2005;61(2):381–388. doi: 10.1007/BF03167055. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. 1994 revised classification system for human immunodeficiency virus infection in children less than 13 years of age. MMWR Recomm Rep. 1994;43(RR-12):1–10. [Google Scholar]
- 25.Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114(1):82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 26.Rhoads MP, Smith CJ, Tudor-Williams G, et al. Effects of highly active antiretroviral therapy on paediatric metabolite levels. HIV Med. 2006;7(1):16–24. doi: 10.1111/j.1468-1293.2005.00337.x. [DOI] [PubMed] [Google Scholar]
- 27.Floris-Moore M, Howard AA, Lo Y, Arnsten JH, Santoro N, Schoenbaum EE. Increased serum lipids are associated with higher CD4 lymphocyte count in HIV-infected women. HIV Med. 2006;7(7):421–430. doi: 10.1111/j.1468-1293.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 28.Rose H, Woolley I, Hoy J, et al. HIV infection and high-density lipoprotein: the effect of the disease vs the effect of treatment. Metabolism. 2006;55(1):90–95. doi: 10.1016/j.metabol.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12(7):F51–F58. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Ergun-Longmire B, Lin-Su K, Dunn AM, et al. Effects of protease inhibitors on glucose tolerance, lipid metabolism, and body composition in children and adolescents infected with human immunodeficiency virus. Endocr Pract. 2006;12(5):514–521. doi: 10.4158/EP.12.5.514. [DOI] [PubMed] [Google Scholar]
- 31.Bitnun A, Sochett E, Babyn P, et al. Serum lipids, glucose homeostasis and abdominal adipose tissue distribution in protease inhibitor-treated and naive HIV-infected children. AIDS. 2003;17(9):1319–1324. doi: 10.1097/00002030-200306130-00006. [DOI] [PubMed] [Google Scholar]
- 32.Shlay JC, Bartsch G, Peng G, et al. Long-term body composition and metabolic changes in antiretroviral naive persons randomized to protease inhibitor-, nonnucleoside reverse transcriptase inhibitor-, or protease inhibitor plus nonnucleoside reverse transcriptase inhibitor-based strategy. J Acquir Immune Defic Syndr. 2007;44(5):506–517. doi: 10.1097/QAI.0b013e31804216cf. [DOI] [PubMed] [Google Scholar]
- 33.Mujawar Z, Rose H, Morrow MP, et al. Human immunodeficiency virus impairs reverse cholesterol transport form macrophages. PLoS Biol. 2006;4(11):e365. doi: 10.1371/journal.pbio.0040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chantry C, Hughes M, Alvero C, et al. Growth and body composition in children beginning or changing ART [abstract MOAB0403]. Proceedings of the XVI International AIDS Conference; August 13–18; Toronto, Ontario, Canada. 2006. [Google Scholar]
- 35.Chantry CJ, Hughes MD, Alvero C, et al. Insulin-like growth factor-1 and lean body mass in HIV-infected children. J Acquir Immune Defic Syndr. 2008 doi: 10.1097/QAI.0b013e31817bbe6d. In press. [DOI] [PubMed] [Google Scholar]
- 36.D′Amico S, Shi J, Sekhar RV, et al. Physiologic growth hormone replacement improves fasting lipid kinetics in patients with HIV lipodystrophy syndrome. Am J Clin Nutr. 2006;84(1):204–211. doi: 10.1093/ajcn/84.1.204. [DOI] [PubMed] [Google Scholar]
- 37.Rondanelli M, Caselli D, Arico M, Maccabruni A, Magnani B, Bacchella L. Insulin-like growth factor I (IGF-I) and IGF-binding protein 3 response to growth hormone is impaired in HIV-infected children. AIDS Res Hum Retroviruses. 2002;18(5):331–339. doi: 10.1089/088922202753519106. [DOI] [PubMed] [Google Scholar]