Abstract
BACKGROUND:
Congestive heart failure (CHF) is a common cause of hospitalization and has a poor prognosis. Specialized multidisciplinary clinics are effective in the management of CHF.
OBJECTIVES:
To measure time of admission to the specialized clinics and explore factors related to the time of admission to these clinics.
METHODS:
Patients who were newly admitted to one of six CHF multidisciplinary clinics in the province of Quebec were enrolled in the study. Data were collected from the common clinical database used at these clinics as well as from questionnaires administered to the patients.
RESULTS:
A total of 531 patients with a mean age of 65.9 years were enrolled. Only 26% were women. The median duration of disease before admission to the CHF clinic was 1.2 years. The majority of patients (62%) were referred by a cardiologist or an internist, while 24% were referred by other specialists, and 14% by general practitioners. One-fifth of patients did not have regular follow-up for their CHF before being admitted to the clinic. Factors associated with shorter disease duration at admission to the clinic were referral by a specialist, not having regular medical follow-up for CHF, having a higher income and having visited the emergency room for CHF.
CONCLUSION:
There may be a need to improve dissemination of information regarding availability and benefits of CHF clinics and criteria for referral.
Keywords: Congestive heart failure, Multidisciplinary heart failure clinics, Referral
Abstract
HISTORIQUE :
L’insuffisance cardiaque congestive (ICC) est une cause courante d’hospitalisation et est reliée à un mauvais pronostic. Les cliniques multidisciplinaires spécialisées sont efficaces pour la prise en charge de l’ICC.
OBJECTIFS :
Mesurer le délai d’admission au sein d’une clinique multidisciplinaire spécialisée et explorer les facteurs liés au moment d’admission à ces cliniques.
MÉTHODOLOGIE :
Les patients qui venaient d’être admis à l’une des six cliniques multidisciplinaires d’ICC de la province de Québec ont participé à l’étude. Les données étaient tirées de la base de données cliniques commune utilisée au sein de ces cliniques ainsi que des questionnaires administrés aux patients.
RÉSULTATS :
Au total, 531 patients d’un âge moyen de 65,9 ans ont participé à l’étude. Seulement 26 % étaient des femmes. La durée médiane de la maladie avant l’admission à la clinique d’ICC était de 1,2 an. La majorité des patients (62 %) avaient été aiguillés par un cardiologue ou un interniste, tandis que 24 % l’avaient été par d’autres spécialistes, et 14 % par leur praticien général. Le cinquième des patients n’avaient pas eu de suivi régulier de leur ICC avant l’admission à la clinique. Les facteurs associés à une moins longue durée de la maladie à l’admission à la clinique étaient l’aiguillage par un spécialiste, l’absence de suivi médical régulier pour l’ICC, un revenu plus élevé et la fréquentation de l’urgence en raison de l’ICC.
CONCLUSION :
Il serait peut-être nécessaire d’améliorer la diffusion de l’information au sujet de la disponibilité et des avantages des cliniques d’ICC et des critères d’aiguillage.
Congestive heart failure (CHF) is one of the most common causes of hospitalization among people 65 years of age and older (1). Its prognosis is poor, with high readmission rates (2,3) and mortality (4–9). In the past decade, new developments in treatment have led to improvements in CHF survival, decreased hospital admissions and improved quality of life (6,10). To implement these new treatment regimens and account for the growing number of cases and hospitalizations, specialized multidisciplinary CHF clinics were established. Benefits of these clinics include reductions in the number of emergency room (ER) visits, hospitalization frequency and length of stay, along with reduced cost of care and improvement in quality of life; moreover, there is some evidence to support the impact of these clinics on patient survival (11–22). With increasing evidence of their efficacy, Canadian and American expert committees have introduced such clinic settings in their guideline recommendations (10,23,24). The Canadian Cardiovascular Society stipulates that higher-risk individuals with heart failure, individuals recently hospitalized with heart failure and those with new-onset heart failure would benefit from multidisciplinary heart failure clinics (23,25–27). To optimize clinical outcomes, it is crucial to have access to this type of specialized care.
Despite the guidelines, it is unclear whether all patients with CHF have equal access to specialized CHF clinics. Certain types of patients may be considered better suited for the clinics (28) and physicians may have different referral practices (29). Thus, factors related to earlier admission to the clinics after a first episode of CHF may include the type of physician who has followed the patient (eg, knowledge and attitudes toward referral), patient-related characteristics (eg, age, sex, social situation, ability to attend regular clinic appointments, disease severity and type, and comorbidities) and system-related factors (eg, wait time and service availability). To our knowledge, no study has explored factors associated with the rapidity at which patients are admitted to specialized CHF clinics within the course of their disease. Thus, the objectives of the present study were to determine time to admission of patients with CHF to specialized clinics and explore factors associated with this time interval.
METHODS
Access-Clinic is a prospective cohort study, designed to compare men and women with respect to disease severity at entry, management and outcome. Patients with CHF who were newly admitted to one of six CHF multidisciplinary clinics in the province of Quebec were enrolled. Data were collected from the common clinical database used at these clinics (Vision Cardiologie; Carole Drouin, Claude Sauvé, Charles Brochu and Marc Frenette) as well as from questionnaires administered to the patients. Clinical information extracted from the database for the purposes of the present study included New York Heart Association (NYHA) functional class, left ventricular ejection fraction (LVEF), patient age and sex, and dates patients were seen at the clinic. The questionnaire obtained sociodemographic information, disease history (including initial CHF diagnosis), referral history, health services utilization and self-reported health status, and included the Minnesota Living with Heart Failure Questionnaire (MLHFQ). In addition, each patient underwent the 6min walk test, which measures functional capacity by evaluating submaximal exercise capacity. All patients signed informed consent forms and the study was approved by the institutional review board of each participating institution.
The present study represents cross-sectional data obtained at baseline. Time to admission was defined as the difference in time between the date of the first appointment to the specialized heart failure clinic and the date of diagnosis of CHF. Patient-related variables included sociodemographic characteristics, disease severity, type of CHF condition and presence of comorbid conditions. Severity was measured by NYHA functional class, MLHFQ score and 6 min walk test distance. The NYHA functional classification system consists of four classes based on symptoms reported by patients and evaluated by the cardiologist or clinic nurse during the first visit. Class IV describes those with the greatest disability. The NYHA classification is valuable for establishing the functional status of patients (30–32) and has good prognostic value (33). However, to reduce reliability problems (30), a training session was provided to ensure similar evaluation of this measure. The MLHFQ is a 21-item questionnaire that includes eight items on physical aspects, five on emotional aspects, and eight other items. It measures the patient’s perceptions regarding how CHF symptoms impact their life during the preceding month. Each item is graded on a scale of 0 to 5, with the resultant global summed score ranging between 0 and 105. Higher scores indicate a lower health-related quality of life. Construct validity and internal consistency are high (34–36). Scores are strongly correlated with prognosis (37) and maximal oxygen consumption (38). The 6 min walk test is a measure of submaximal exercise capacity. The patient is instructed by the nurse to walk for 6 min, and may stop and restart as desired. At the end of 6 min, the total distance (in metres) is measured. In general, this test is tolerated well by people with CHF (10,38). There is a good correlation with maximal oxygen consumption (39–42), and a moderate correlation with quality of life and NYHA functional class (39,41,43–45). The 6 min walk test has good reproducibility (39,42,44,46–48). Patients with an LVEF of lower than 40% were classified as having systolic dysfunction heart failure and those with an LVEF of 40% or greater were classified as having heart failure with preserved systolic function (HFPSF). One physician-related factor – type of physician who referred the patient to the clinic – was used. Physicians were classified as one of the following: cardiologist, internist, other specialist (not a cardiologist or internist) or general practitioner (GP). In terms of system-related factors, all patients were seen in specialized clinics in an urban centre and access in terms of service availability was not an issue. Moreover, criteria for admission to the clinics were similar (eg, frequent CHF admissions or ER visits, newly diagnosed CHF admitted to the coronary care unit, heart transplant evaluations and other evaluations for novel heart failure therapies, and patient must have a support system that allows for compliance to benefit from services).
Analysis
The time to admission, defined as time from diagnosis until first appointment in the clinic, was measured. Bivariate statistics (t tests and Pearson correlations) were used to compare time to admission to the clinic with the type of doctor and patient characteristics such as age, sex, socioeconomic status, social situation, level of disease severity, comorbidity and past health care use. The social situation of patients was evaluated based on whether the person was single, lived with someone, or required assistance for organizing or attending medical appointments. The number of comorbid conditions (as described in the database) was assessed and specific comorbidities were explored including chronic obstructive pulmonary disease, diabetes, chronic renal insufficiency and obesity. Health care use included the number of ER visits for CHF and the number of hospital admissions for CHF in the past six months. Disease severity was defined in three ways: using NYHA classification, 6 min walk test distance and health-related quality of life score determined by the MLHFQ. Disease type (systolic dysfunction versus HFPSF) was defined using the cut-off for LVEF at 40%. Multiple logistic regression was used to explore whether time to admission to the clinic (dichotomized at the median) was associated with the type of referring doctor and various patient-related factors. Multiple linear regression was also performed using a log transformation for time from diagnosis to admission to the clinic as the dependent variable.
RESULTS
Of the 582 patients who were newly enrolled in the specialized clinics and approached to participate, 531 participated in the study. The mean (± SD) age of the entire sample was 65.9±11.2 years and 138 (26%) were women. On average, subjects had CHF for 5.2±8.2 years, with a median duration of 1.2 years before admission to the clinic (interquartile range 0.2 to 6.9 years). The mean score on the MLHFQ was 45.1±24.1 and the mean 6 min walk test distance was 277.5±121 m. In the previous six months, more than 70% of patients were admitted to a hospital and nearly 73% visited an ER for their CHF. Table 1 describes the sample in terms of sociodemographic and clinical characteristics. The majority of patients (n=311; 62.3%) were referred by either a cardiologist or an internist, 120 (24.1%) were referred by another specialist and 68 (13.6%) by a family physician. A total of 101 (20.6%) patients had regular follow-up for their CHF before being admitted to the clinic; of these, 52% were referred to the clinic by a cardiologist, 37% by another specialist and 11% by a family physician.
TABLE 1.
Sociodemographic and clinical characteristics of the sample of newly enrolled patients in the multidisciplinary congestive heart failure (CHF) clinics (n=531)
| Characteristic | n | %* |
|---|---|---|
| Women | 138 | 26 |
| Social situation (single) | 169 | 34.3 |
| Requires help to arrange or go to medical appointments | 441 | 89.3 |
| Education level | ||
| Elementary school | 123 | 24.6 |
| High school | 122 | 24.4 |
| College | 92 | 18.4 |
| University | 163 | 35.9 |
| Household income | ||
| <$20,000 | 100 | 24.1 |
| $20,000–$45,0000 | 166 | 40.0 |
| >$45,000 | 149 | 35.9 |
| New York Heart Association functional class | ||
| I | 89 | 17.2 |
| II | 303 | 58.6 |
| III or IV | 125 | 24.2 |
| Left ventricular ejection fraction <40% | 70 | 13.5 |
| Number of emergency room visits in the past 6 months | ||
| 0 | 136 | 27.4 |
| 1 | 184 | 37.0 |
| >1 | 177 | 35.6 |
| Number of hospital admissions for CHF in the past 6 months | ||
| 0 | 143 | 29.0 |
| 1 | 206 | 41.7 |
| >1 | 145 | 29.4 |
| Number of comorbid conditions | ||
| 0 | 21 | 4.0 |
| 1 | 86 | 16.2 |
| 2 | 99 | 18.6 |
| >2 | 325 | 61.2 |
| Diabetes | 189 | 35.6 |
| Chronic obstructive pulmonary disease | 112 | 21.1 |
| Chronic renal insufficiency | 126 | 23.7 |
| Obese | 116 | 21.8 |
| Referring doctor | ||
| Cardiologist | 275 | 55.1 |
| Internist | 36 | 7.2 |
| Other specialist | 120 | 24.0 |
| Family physician | 68 | 13.6 |
Percentages calculated with totals that excluded subjects with missing data
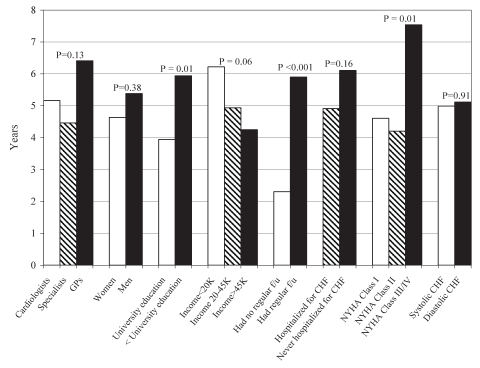
Factors associated with time of admission to the clinic were explored. Bivariate comparisons (Figure 1) indicated that factors associated with admission to the clinic at an earlier point in disease history included not having regular follow-up by a physician in the past for CHF, having a higher level of education and having lower disease severity (ie, lower NYHA functional class). Diabetic patients were referred later than nondiabetic patients (6.4±8.2 years versus 4.5±8.1 years, respectively; P=0.02), as were those with chronic renal insufficiency (8.9±11.3 years versus 4.09±6.6 years, respectively; P<0.0001). There was a significant, although low, positive correlation between disease duration and age (Pearson r=0.2, P<0.001), which suggested that older patients were referred later. This was confirmed when age was stratified by tertile; persons 60 years of age or younger were referred within an average of 3.1 years, those between 61 and 72 years of age were referred within 6.3 years and those older than 72 years of age were referred within 6.3 years (P=0.0005). There was a low negative correlation between disease duration and 6 min walk test distance (Pearson r=–0.3, P<0.001), implying that patients with a shorter 6 min walk distance (more severe functional impairment) were referred later.
Figure 1).
Time from congestive heart failure (CHF) diagnosis until referral to a multidisciplinary clinic. The y-axis displays disease duration in years (ie, time from CHF diagnosis until admission to the clinic). The x-axis displays the various patient groups, defined by specific characteristics. For example, time until referral is described for those persons referred by cardiologists, other specialists and general practitioners (GPs). The corresponding P-values are displayed for the unadjusted differences between groups. f/u Follow-up; NYHA New York Heart Association
The main factors associated with admission to the clinic within 1.2 years of diagnosis of CHF included being referred by a specialist (other than a cardiologist or an internist), not being followed regularly for CHF by a physician, being a woman, having had an ER visit within the preceding six months, and not having chronic renal insufficiency (Table 2). Although not statistically significant, there was a trend toward those with lower household income and older persons to be admitted to the clinic later.
TABLE 2.
Factors associated with later (more than 1.2 years) referral to the clinic
| Factor | Adjusted OR | 95% CI | P |
|---|---|---|---|
| Referring doctor | |||
| General practitioner | 1.9 | 0.9 to 4.2 | 0.1 |
| Other specialist* | 0.6 | 0.3 to 1.0 | 0.05 |
| Internist | 0.7 | 0.2 to 2.0 | 0.5 |
| Cardiologist | Reference | ||
| Regular follow-up for CHF by a physician in the past* | 2.8 | 1.4 to 5.6 | 0.003 |
| Men* | 2 | 1.0 to 3.8 | 0.04 |
| Age, years | |||
| ≤60 | Reference | ||
| ≥61 and ≤72 years | 1.7 | 0.9 to 3.1 | 0.1 |
| >72 years | 1.4 | 0.7 to 2.9 | 0.3 |
| Social situation (single) | 0.7 | 0.4 to 1.3 | 0.3 |
| Needs help for medical appointments | 1.4 | 0.6 to 3.2 | 0.4 |
| Less than university education | 1.1 | 0.7 to 2.0 | 0.6 |
| Household income | |||
| <$20,000 | 2.1 | 1.0 to 4.7 | 0.06 |
| $20,000–$45,0000 | 1.1 | 0.6 to 2.0 | 0.7 |
| >$45,000 | Reference | ||
| New York Heart Association functional class | |||
| III or IV | 1.5 | 0.7 to 3.5 | 0.3 |
| II | 0.7 | 0.4 to 1.3 | 0.3 |
| I | Reference | ||
| Left ventricular ejection fraction <40% | 0.8 | 0.4 to 1.4 | 0.4 |
| Mean HRQOL score | 1 | 1.0 to 1.0 | 0.5 |
| Mean 6 min walk test distance | 1 | 1.0 to 1.0 | 0.4 |
| ≥1 ER visit in past 6 months* | 0.4 | 0.2 to 1.0 | 0.046 |
| ≥1 hospitalization for CHF in past 6 months | 1.3 | 0.5 to 3.2 | 0.6 |
| Chronic renal insufficiency* | 2 | 1.03 to 4.0 | 0.04 |
| Diabetes | 0.8 | 0.5 to 1.5 | 0.6 |
| Chronic obstructive pulmonary disease | 0.8 | 0.4 to 1.5 | 0.5 |
| Obesity | 1.8 | 0.9 to 3.8 | 0.09 |
The statistical analysis used was multiple logistic regression.
P≤ 0.05. CHF Congestive heart failure; ER Emergency room; HRQOL Health-related quality of life
Multiple linear regression analysis (using a logarithmic transformation for time from diagnosis until admission; Table 3) confirmed that earlier admission to a CHF clinic was associated with being referred by a specialist, and not being followed up regularly for CHF by a physician, as well as having more than two comorbid conditions. In addition, those who had a lower annual income (less than $20,000) tended to be admitted later than those with higher incomes, as were those with higher functional disability (lower 6 min walk distance). In Table 3, cardiologists and internists were grouped into one category (reference), maintaining the remaining two categories as other specialists and GPs. When specific comorbid conditions were used in the model, only chronic renal insufficiency was associated with later admission to a CHF clinic (P=0.01).
TABLE 3.
Factors associated with later referral to the clinic
| Factor | Beta | 95% CI | P |
|---|---|---|---|
| Referring doctor | |||
| General practitioner | −0.004 | −0.68 to 0.67 | 0.99 |
| Other specialist* | −0.66 | −1.20 to −0.19 | 0.02 |
| Cardiologist or internist | reference | ||
| Followed regularly for CHF in the past by a physician* | 1.12 | 0.56 to 1.69 | 0.001 |
| Men | 0.43 | −0.15 to 1.00 | 0.15 |
| Age (years) | 0.009 | −0.01 to 0.03 | 0.44 |
| Social situation (single) | −0.47 | −0.99 to 0.05 | 0.08 |
| Needs help for medical appointments | −0.09 | −0.83 to 0.65 | 0.81 |
| Less than university education | 0.17 | −0.30 to 0.65 | 0.48 |
| Household income | |||
| <$20,000* | 0.74 | 0.08 to 1.41 | 0.03 |
| $20,000–$45,000 | 0.28 | −0.25 to 0.81 | 0.3 |
| >$45,000 | reference | ||
| New York Heart Association functional class | |||
| III or IV | 0.25 | −0.52 to 1.01 | 0.58 |
| II | −0.38 | −0.98 to 0.22 | 0.22 |
| I | reference | ||
| Left ventricular ejection fraction <40% | 0.37 | −0.22 to 0.96 | 0.22 |
| Mean HRQOL score | 0.003 | −0.007 to 0.01 | 0.58 |
| Mean 6 min walk test distance* | −0.002 | −0.004 to −0.0003 | 0.02 |
| ≥1 ER visit in past 6 months | −0.51 | −1.23 to 0.20 | 0.16 |
| ≥1 hospitalization for CHF in past 6 months | −0.24 | −1.02 to 0.54 | 0.54 |
| More than 2 comorbid conditions* | 0.51 | 0.09 to 0.92 | 0.02 |
Statistical analyses included logarithmic transformation for time since congestive heart failure (CHF) diagnosis and multiple linear regression. R2=23%.
P≤0.05. ER Emergency room; HRQOL Health-related quality of life
DISCUSSION
The majority of patients newly admitted to specialized CHF clinics were referred by cardiologists. Patients who did not have a regular physician following them for their CHF, those with higher income, those who did not have chronic renal insufficiency and those who had an ER visit for CHF during the preceding six months tended to be referred earlier to the clinic, as were those who were referred by specialists who were not cardiologists.
It is not surprising that cardiologists tended to refer patients to the clinic more often than other types of doctors. It is possible that GPs refer to cardiologists who, in turn, refer to the clinics. For example, GPs may seek diagnostic confirmation from cardiologists or respirologists. Nevertheless, we found that patients with a regular physician who managed their CHF were referred later. Other specialists tended to refer patients sooner than cardiologists or GPs. Specialists are more likely to refer to other specialists when they recognize that the patient’s problem is not within their domain of expertise, whereas a GP may continue to follow the patient, order tests and continue observing the patient over time (49).
Persons with lower incomes were admitted later to CHF clinics. This observation suggests a possible bias in referral of those in higher socioeconomic groups, similar to what was found in the referral of persons with suspected rheumatoid arthritis to rheumatologists (50). Although there are no fees associated with attendance, cost barriers such as those associated with transportation may be an issue. Patients who visited an ER during the preceding six months were referred sooner. On the other hand, persons with more severe CHF (higher NYHA functional class in the bivariate analysis and shorter 6 min walk distance in the multiple linear regression analysis) were referred later. The clinics are designed to promote self-management and are geared toward patients with moderate to severe CHF symptoms. However, persons with very high degrees of disability (also with certain comorbid conditions such as chronic renal insufficiency) may not be regarded as suitable candidates for the clinics and may be less likely to be accepted into these programs. On the other hand, the literature indicates that persons with higher degrees of disability (NYHA functional class III and IV) do benefit (17,22,51–53), which suggests that persons with severe disease may benefit from these multidisciplinary clinics. Criteria for admission to the clinics may need to be better defined and specified.
There was a tendency for men to be admitted to the clinics later than women, although bivariate analysis indicated no significant difference in disease duration at admission between men and women. When we performed separate analyses for men and women (data not shown), men who had lower functional disability (longer 6 min walk distance, P=0.02) were admitted sooner, whereas women with more severe disease (higher NYHA functional class, P=0.07) were admitted sooner. It appears that women may have more severe disease at admission than men, indicating a possible sex bias in referrals.
Older patients tended to be referred later. These patients often have more problems with mobility and would have a more difficult time attending clinic appointments. Home care services for these persons may be a good alternative.
We noted that one-fifth of our cohort had not been receiving regular care for their CHF before being admitted to the clinic. This lack of regular care is discouraging because all patients should have a regular source of primary care to manage their health care needs rather than depending on hospital ERs. Nevertheless, this percentage is slightly lower than that in the general Quebec population, where 26% of people do not have a regular medical doctor (54). Also, these patients were sent to the clinics earlier, possibly because they had no source of regular follow-up. Once they had contact with the health care system, they were referred to the clinic for management of their CHF. Another explanation may be that those who had regular follow-up with a physician for their CHF tended to have well-controlled stable disease and were referred only when their disease became more problematic.
Patients who were followed by their GPs tended to be referred later in the course of their disease, although this factor was not statistically significant. This later referral may be problematic if some doctors are less aware of the existence of the clinics or are reluctant to refer their patients to them. Benefits of such clinics have been clearly demonstrated and physicians should be encouraged to send appropriate CHF patients to the clinics. Appropriate patients (who would benefit from the multidisciplinary clinic) are those who are not cognitively impaired, can cooperate with the self-management and education regimens, and are likely to adhere to the treatment program (28). On the other hand, GPs who refer patients expect to receive information about their patient and if these expectations are not met, the physicians become dissatisfied with the referral process (55). Recent evidence indicates that the establishment of an open access heart failure service at a teaching hospital encouraged GPs to use the service. Referrals can be streamlined by using specific baseline investigations (56). This way, referrals can be both efficient and appropriate.
It must be noted that realistically, it would not be possible to look after all CHF patients at the specialized clinics because of capacity issues. Thus, there must be some selection criteria for appropriate patients. Presently, the clinics operate in a steady state mode, with new admissions roughly equivalent to attrition and mortality.
Limitations
Time of diagnosis was obtained from a questionnaire administered by the nurses to patients newly enrolled in the clinic. There could have been problems with self-report, likely resulting in some random misclassification errors, which may have underestimated the true association between time to admission to the clinic and various factors.
We had information regarding physicians who referred patients to the clinic but not the trajectory of referral (ie, whether a GP referred to a respirologist who, in turn, referred to a cardiologist).
Acceptance of certain types of patients at different clinics may have influenced the results of the present study. Although criteria for referral to multidisciplinary CHF clinics have been established (23), the populations treated in these clinics are likely to reflect the clinical and research interests of the physician, the needs of different communities and the availability of resources (57). Organizational characteristics related to the clinic itself may also contribute to time to admission and were not evaluated in the present study. For example, the wait time to obtain an appointment, staffing and resources at the particular clinic, and administrative requirements (pre-acceptance tests and evaluations) may account for delays in being admitted to the clinics. We cannot be certain of the generalizability of our results. However, there are similarities with other studies of patients attending multidisciplinary CHF clinics in terms of mean age, sex distribution, and the presence of both systolic dysfunction and HFPSF (52).
CONCLUSION
Patients with CHF are more likely to be referred to specialized clinics by cardiologists and specialists than by GPs. The average disease duration before admission to the clinic was five years; however, one-half of the patients were referred after 1.2 years. People with higher socioeconomic status tended to be referred earlier, as were those who did not have a regular source of primary care for their CHF and who recently visited an ER for CHF. Although our study did not address knowledge about CHF clinics among physicians, it may be important to improve the dissemination of information regarding availability and benefits of CHF clinics to primary care practitioners (and emergency medicine doctors) as well as criteria for referral. Strategies such as preceptorships, presentations to family practice conferences and continuing medical education functions may be useful in this regard.
Footnotes
SUPPORT: The present study was supported by a grant from the Canadian Institutes of Health Research. Dr Feldman holds a new investigator career award from The Arthritis Society. Dr Pilote holds an investigator career award from Fonds de la recherche en santé du Québec (FRSQ). Dr Ducharme is supported by the FRSQ.
REFERENCES
- 1.Miller LW, Missov ED. Epidemiology of heart failure. Cardiol Clin. 2001;19:547–55. doi: 10.1016/s0733-8651(05)70242-3. [DOI] [PubMed] [Google Scholar]
- 2.McMurray J, McDonagh T, Morrison CE, Dargie HJ. Trends in hospitalization for heart failure in Scotland 1980–1990. Eur Heart J. 1993;14:1158–62. doi: 10.1093/eurheartj/14.9.1158. [DOI] [PubMed] [Google Scholar]
- 3.Thivierge C, Guerard L, Kapetanakis C, et al. L’insuffisance cardiaque à Montréal-Centre : les faits saillants. Élisabeth Pérès. Montréal: Unité Santé physique de la Direction de la santé publique de la Régie régionale de la santé et des services sociaux de Montréal-Centre, 1999
- 4.Cleland JGF, Clark A. Has the survival of the heart failure population changed? Lessons from trials. Am J Cardiol. 1999;83:112D–9D. doi: 10.1016/s0002-9149(98)01011-x. [DOI] [PubMed] [Google Scholar]
- 5.Cowie MR, Wood DA, Coats AJS, et al. Survival of patients with a new diagnosis of heart failure: A population based study. Heart. 2000;83:505–10. doi: 10.1136/heart.83.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feinglass J, Martin G, Lin E, Johnson M, Gheorghiade M. Is heart failure survival improving? Evidence from 2323 eldery patients hospitalized between 1989–2000. Am Heart J. 2003;146:111–4. doi: 10.1016/S0002-8703(03)00116-9. [DOI] [PubMed] [Google Scholar]
- 7.Ho KKL, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: The Framingham Study. J Am Coll Cardiol. 1993;22:6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 8.Merlo J, Östergren P-O, Broms K, Bjorck-Linné A, Liedholm H. Survival after initial hospitalisation for heart failure: A multilevel analysis of patients in Swedish acute care hospitals. Eur Heart J. 2001;55:323–9. doi: 10.1136/jech.55.5.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Senni M, Tribouilloy CM, Rodeheffer RJ, et al. Congestive heart failure in the community: Trends in incidence and survival in a 10-year period. Arch Intern Med. 1999;159:29–34. doi: 10.1001/archinte.159.1.29. [DOI] [PubMed] [Google Scholar]
- 10.MacIntyre K, Capewell S, Stewart S, et al. Evidence of improving prognosis in heart failure: Trends in case fatality in 66 547 patients hospitalized between 1986 and 1995. Circulation. 2000;102:1126–31. doi: 10.1161/01.cir.102.10.1126. [DOI] [PubMed] [Google Scholar]
- 11.Fonarow GC, Stevenson LW, Walden JA, et al. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J Am Coll Cardiol. 1997;30:725–32. doi: 10.1016/s0735-1097(97)00208-8. [DOI] [PubMed] [Google Scholar]
- 12.Hanumanthu S, Butler J, Chomsky D, Davis S, Wilson JR. Effect of a heart failure program on hospitalization frequency and exercise tolerance. Circulation. 1997;96:2842–8. doi: 10.1161/01.cir.96.9.2842. [DOI] [PubMed] [Google Scholar]
- 13.Hershberger RE, Ni H, Nauman DJ, et al. Prospective evaluation of an outpatient heart failure management program. J Card Fail. 2001;7:64–74. doi: 10.1054/jcaf.2001.21677. [DOI] [PubMed] [Google Scholar]
- 14.McDonald K, Ledwidge M, Cahill J, et al. Heart failure management: Multidisciplinary care has intrinsic benefit above the optimization of medical care. J Card Fail. 2002;8:142–8. doi: 10.1054/jcaf.2002.124340. [DOI] [PubMed] [Google Scholar]
- 15.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190–5. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 16.West JA, Miller NH, Parker KM, et al. A comprehensive management system for heart failure improves clinical outcomes and reduces medical resource utilization. Am J Cardiol. 1997;79:58–63. doi: 10.1016/s0002-9149(96)00676-5. [DOI] [PubMed] [Google Scholar]
- 17.Whellan DJ, Gaulden L, Gattis WA, et al. The benefit of implementing a heart failure disease management program. Arch Intern Med. 2001;161:2223–8. doi: 10.1001/archinte.161.18.2223. [DOI] [PubMed] [Google Scholar]
- 18.Stewart S, Marley J, Horowitz JD. Effects of a multidisciplinary, home-based intervention on planned readmissions and survival among patients with chronic congestive heart failure: A randomised controlled study. Lancet. 1999;354:1077–83. doi: 10.1016/s0140-6736(99)03428-5. [DOI] [PubMed] [Google Scholar]
- 19.Blue L, Lang E, McMurray J, et al. Randomised controlled trial of specialist nurse intervention in heart failure. BMJ. 2001;323:715–8. doi: 10.1136/bmj.323.7315.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cline CM, Israelsson BYA, Willenheimer RB, Broms K, Erhardt LR. Cost effective management programme for heart failure reduces hospitalisation. Heart. 1998;80:442–6. doi: 10.1136/hrt.80.5.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brophy JM, Ducharme A, Doyon O, White M, Rouleau JL.A meta-analysis of randomized trials of congestive heart failure clinics – the importance of cardiovascular specialist Can J Cardiol 200319Suppl SA (Abst) [Google Scholar]
- 22.Ducharme A, Doyon O, White M, Rouleau JL, Brophy JM. Impact of care at a multidisciplinary congestive heart failure clinic: A randomized trial. CMAJ. 2005;173:40–5. doi: 10.1503/cmaj.1041137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu P, Arnold JMO, Belenkie I, et al. The 2002/3 Canadian Cardiovascular Society consensus guideline update for the diagnosis and management of heart failure. Can J Cardiol. 2003;19:347–56. [PubMed] [Google Scholar]
- 24.MacMahon S. Blood pressure and the risk of cardiovascular disease. N Engl J Med. 2000;342:50–2. doi: 10.1056/NEJM200001063420109. [DOI] [PubMed] [Google Scholar]
- 25.Arnold JMO, Howlett JG, Ducharme A, et al. Canadian Cardiovascular Society consensus conference guidelines on heart failure – 2008 update: Best practices for the transition of care of heart failure patients, and the recognition, investigation and treatment of cardiomyopathies. Can J Cardiol. 2008;24:21–40. doi: 10.1016/s0828-282x(08)70545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnold JM, Howlett JG, Dorian P, et al. Canadian Cardiovascular Society consensus conference recommendations on heart failure update 2007: Prevention, management during intercurrent illness or acute decompensation, and use of biomarkers. Can J Cardiol. 2007;23:21–45. doi: 10.1016/s0828-282x(07)70211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross H, Howlett J, Arnold JM, et al. Treating the right patient at the right time: Access to heart failure care. Can J Cardiol. 2006;22:749–54. doi: 10.1016/s0828-282x(06)70290-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houde S, Feldman DE, Pilote L, et al. Are there sex-related differences in specialized, multidisciplinary congestive heart failure clinics? Can J Cardiol. 2007;23:451–5. doi: 10.1016/s0828-282x(07)70783-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forrest CB, Nutting PA, von SS, Rohde C, Starfield B. Primary care physician specialty referral decision making: Patient, physician, and health care system determinants. Med Decis Making. 2006;26:76–85. doi: 10.1177/0272989X05284110. [DOI] [PubMed] [Google Scholar]
- 30.Bennett JA, Riegel B, Bittner V, Nichols J. Validity and reliability of the NYHA classes for measuring research outcomes in patients with cardiac disease. Heart Lung. 2002;31:262–70. doi: 10.1067/mhl.2002.124554. [DOI] [PubMed] [Google Scholar]
- 31.Dunselman PHJM, Kuntze CEE, van Bruggen A, et al. Value of New York Heart Association classification, radionuclide ventriculography, and cardiopulmonary exercises tests for selection of patients for congestive heart failure studies. Am Heart J. 1998;116:1475–82. doi: 10.1016/0002-8703(88)90731-4. [DOI] [PubMed] [Google Scholar]
- 32.Gibelin P, Poncelet P, Gallois H, Sebaoun A, Avierinos C. Évaluation de trois classifications fonctionnelles de l’insuffisance cardiaque: étude multicentrique nationale. Ann Cardiol Angeiol. 1995;44:304–9. [PubMed] [Google Scholar]
- 33.Opasich C, Pinna GD, Mazza A, et al. Six-minute walking performance in patients with moderate-to-severe heart failure; is it a useful indicator in clinical practice? Eur Heart J. 2001;22:488–96. doi: 10.1053/euhj.2000.2310. [DOI] [PubMed] [Google Scholar]
- 34.Middel B, Bouma J, de Jongste M, et al. Psychometric properties of the Minnesota Living with Heart Failure Questionnaire. Clin Rehabil. 2001;15:489–500. doi: 10.1191/026921501680425216. [DOI] [PubMed] [Google Scholar]
- 35.Rector T, Cohn JN. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: Reliability and validity during a randomized, double-blind, placebo-controlled trial of pimobendan. Am Heart J. 1992;124:1017–25. doi: 10.1016/0002-8703(92)90986-6. [DOI] [PubMed] [Google Scholar]
- 36.Sneed NV, Paul S, Michel Y, VanBakel A, Hendrix G. Evaluation of 3 quality of life measurement tools in patients with chronic heart failure. Heart Lung. 2001;30:332–40. doi: 10.1067/mhl.2001.118303. [DOI] [PubMed] [Google Scholar]
- 37.Alla F, Briancon S, Guillemin F, et al. Self-rating of quality of life provides additional prognostic information in heart failure. Insights into the EPICAL study. Eur J Heart Fail. 2002;4:337–43. doi: 10.1016/s1388-9842(02)00006-5. [DOI] [PubMed] [Google Scholar]
- 38.Arena R, Humphrey R, Peberdy MA. Relationship between the Minnesota Living with Heart Failure Questionnaire and key ventilatory expired gas measures during exercise testing in patients with heart failure. J Cardiopulm Rehabil. 2002;22:273–7. doi: 10.1097/00008483-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Cahalin LP, Mathier MA, Semigran MJ, Dec GW, DiSalvo T. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest. 1996;110:325–32. doi: 10.1378/chest.110.2.325. [DOI] [PubMed] [Google Scholar]
- 40.Hendrican MC, McKelvie RS, Smith T, et al. Functional capacity in patients with congestive heart failure. J Card Fail. 2000;6:214–9. doi: 10.1054/jcaf.2000.8830. [DOI] [PubMed] [Google Scholar]
- 41.Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlates and pronostic significance of six-minute walk test in patients with pulmonary hypertension. Am J Respir Crit Care Med. 2000;161:487–92. doi: 10.1164/ajrccm.161.2.9906015. [DOI] [PubMed] [Google Scholar]
- 42.Morales FJ, Martinez A, Méndez M, et al. A shuttle walk test for assessment of functional capacity in chronic heart failure. Am Heart J. 1999;138:291–8. doi: 10.1016/s0002-8703(99)70114-6. [DOI] [PubMed] [Google Scholar]
- 43.Bittner V, Weiner D, Yusuf S, et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. JAMA. 1993;270:1702–7. [PubMed] [Google Scholar]
- 44.Demers C, McKelvie RS, Negassa A, Yusuf S, for the RESOLVD Pilot Study Investigators Reliability, validity, and responsiveness of the six-minute walk test in patients with heart failure. Am Heart J. 2001;142:698–703. doi: 10.1067/mhj.2001.118468. [DOI] [PubMed] [Google Scholar]
- 45.Shah M, Hasselblad V, Gheorghiade M, et al. Prognostic usefulness of the six-minute walk in patients with advanced congestive heart failure secondary to ischemic or nonischemic cardiomyopathy. Am J Cardiol. 2001;88:987–93. doi: 10.1016/s0002-9149(01)01975-0. [DOI] [PubMed] [Google Scholar]
- 46.Guyatt G, Sullivan M, Thompson P, et al. The 6-minute walk: A new measure of exercise capacity in patients with chronic heart failure. CMAJ. 1985;132:919–23. [PMC free article] [PubMed] [Google Scholar]
- 47.Kervio G, Carre F, Ville NS. Reliability and intensity of the six-minute walk test in healthy eldery subjects. Med Sci Sports Exerc. 2003;35:169–74. doi: 10.1097/00005768-200301000-00025. [DOI] [PubMed] [Google Scholar]
- 48.Pinna GD, Opasich C, Mazza A, Tangenti A, Maestri R, Sanarico M. Reproducibility of the six-minute walking test in chronic heart failure patients. Stat Med. 2000;19:3087–94. doi: 10.1002/1097-0258(20001130)19:22<3087::aid-sim628>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 49.Sweeney B. The referral system. BMJ. 1994;309:1180–1. doi: 10.1136/bmj.309.6963.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feldman DE, Bernatsky S, Haggerty J, et al. Delay in consultation with specialists for persons with suspected new-onset rheumatoid arthritis: A population-based study. Arthritis Rheum. 2007;57:1419–25. doi: 10.1002/art.23086. [DOI] [PubMed] [Google Scholar]
- 51.Akosah KO, Schaper AM, Havlik P, Barnhart S, Devine S. Improving care for patients with chronic heart failure in the community: The importance of a disease management program. Chest. 2002;122:906–12. doi: 10.1378/chest.122.3.906. [DOI] [PubMed] [Google Scholar]
- 52.Holst DP, Kaye D, Richardson M, et al. Improved outcomes from a comprehensive management system for heart failure. Eur J Heart Fail. 2001;3:619–25. doi: 10.1016/s1388-9842(01)00164-7. [DOI] [PubMed] [Google Scholar]
- 53.Jain A, Mills P, Nunn LM, et al. Success of a multidisciplinary heart failure clinic for initiation and up-titration of key therapeutic agents. Eur J Heart Fail. 2005;7:405–10. doi: 10.1016/j.ejheart.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 54.Statistics Canada Statistics Canada Survey. <http://www.statcan.ca/Daily/English/040615/d040615b.htm> (Version current at January 21, 2007).
- 55.Piterman L, Koritsas S. Part II. General practitioner-specialist referral process. Intern Med J. 2005;35:491–6. doi: 10.1111/j.1445-5994.2005.00860.x. [DOI] [PubMed] [Google Scholar]
- 56.Shah S, Davies MK, Cartwright D, Nightingale P. Management of chronic heart failure in the community: Role of a hospital based open access heart failure service. Heart. 2004;90:755–9. doi: 10.1136/hrt.2002.006460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gustafsson F, Arnold JM. Heart failure clinics and outpatient management: Review of the evidence and call for quality assurance. Eur Heart J. 2004;25:1596–604. doi: 10.1016/j.ehj.2004.06.023. [DOI] [PubMed] [Google Scholar]