Abstract
Lateral thoracotomy is the traditional surgical approach for preclinical animal testing of various ventricular assist devices. Median sternotomy, however, is regarded from a functional standpoint as the most appropriate approach for cardiac surgical procedures, particularly for device implantation. The purpose of this study was to evaluate the outcomes of performing a median sternotomy in chronic bovine studies. Three chronic studies using the sternotomy approach were performed. Surgical access was compared to the lateral thoracotomy approach used in three other animal experiments. Postoperative speed of recovery, pain management, sternotomy incision, and monitoring line exit site healing and infection were also evaluated. With sternotomy, better surgical access to all cardiac chambers and great vessels and more room for device placement were achieved. The recovery time was similar to that using the lateral thoracotomy approach, with no additional difficulties observed in standing or recumbency and no need for increased pain management. At the time of autopsy, the sternum was well healed without any sign of infection. In conclusion, these studies showed that a median sternotomy can be used successfully for chronic bovine studies. This approach will be used for our future biventricular assist device implantation surgeries.
Keywords: Ventricular assist device, Circulatory support, Thoracotomy, Animal models, Sternotomy
Introduction
The beneficial effect of ventricular assist device (VAD) implantation for patients with end-stage heart failure as bridge to transplantation, destination therapy, or even myocardial recovery has been clearly demonstrated.1 Over the past few decades, several new devices have been developed, each with unique properties and distinctive modes of action. Of necessity, all in vivo testing is performed using animal subjects prior to clinical application. Animal models provide considerable insight into the design and biocompatibility of such devices. It is generally accepted that no single animal model exactly replicates all human parameters. Calves are the most frequently used animal species in the current era of preclinical device testing, in part because of historical developments.2–4 The use of larger devices in the early stages of VAD development was achievable only in the wide, deep space of the calf chest cavity. Thus, due to intrathoracic space considerations required for proper functioning of the implanted devices, the majority of investigators preferred using calves in their experiments. Moreover, calves are also easier to handle following surgery and show fewer postoperative complications compared with other species such as sheep.
The lateral thoracotomy approach is the traditional surgical approach for preclinical animal studies for VAD implantation. Some investigators have reported on the right thoracotomy approach as an alternative procedure.5 The three primary concerns expressed regarding a calf sternotomy included (1) anticipated excessive pain in the recumbent position and in transitioning from standing to lying down, (2) increased incidence and severity of infection at the sternal incision caused by exposure to feces on the cage floors, and (3) sternum instability concerns caused by the calf's weight bearing down on the sternal wound when the calf was in the recumbent position. Median sternotomy is regarded as the most appropriate surgical approach for cardiac procedures. It allows better surgical access to cardiac chambers and great vessels. It also facilitates simultaneous implantation of both a right and left ventricular assist device (RVAD, LVAD). The aim of this study was to evaluate the outcomes of performing a median sternotomy in chronic bovine studies and to compare the postoperative outcomes of these procedures with those of studies using a lateral thoracotomy approach. The feasibility of biventricular assist device implantation through a resternotomy procedure was tested as well in one case.
Material and Methods
Animal Model
This study was approved by the Cleveland Clinic's Institutional Animal Care and Use Committee. Median sternotomy procedures were conducted on three Jersey calves (mean weight 78 ± 10 kg, range 68.4–88.6 kg). The results of these studies were compared with the outcomes of experiments done on three Jersey calves (mean weight 71 ± 9 kg, range 68.2–80.6 kg) using a lateral thoracotomy approach. All animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington, D.C., 1996) and institutional guidelines.
Surgical Procedures
All experiments were performed as part of a different study to evaluate drug-induced heart failure in a bovine model. Anesthesia was initiated with intramuscular ketamine (10 mg/kg) and maintained with inhaled isoflurane (1–2%). After the median sternotomy was performed, fluid-filled pressure monitoring lines and a Transonic ultrasonic perivascular flow probe (Transonic Systems, Inc., Ithaca, NY) were inserted. Using an awl, the sternum was closed with eight Ethicon surgical wires (Ethicon, Inc., Somerville, NJ). Interrupted 0-Ethibond sutures were used for the deep muscular layer, continuous 0-Ethibond suture was used for the subcutaneous tissue, and finally the skin was closed using interrupted 0 Prolene sutures.
On postoperative day 54 of study 3, the animal was moved to the operating room and an acute simultaneous implantation of an LVAD and RVAD was performed through a resternotomy. The continuous flow CorAide LVAD and DexAide RVAD were used for this purpose.6, 7 After dissection of the scar tissues, the outflow graft was anastomosed to the brachiocephalic trunk and pulmonary artery for the LVAD and RVAD, respectively. The inflow cannula of the LVAD was inserted into the left ventricular apex; the RVAD inflow cannula was inserted into the diaphragmatic surface of the right ventricle (Figure 1). Both devices were secured to the diaphragm. The increased intrathoracic access in the sternotomy approach allowed chest closure without compressing the devices.
Figure 1.
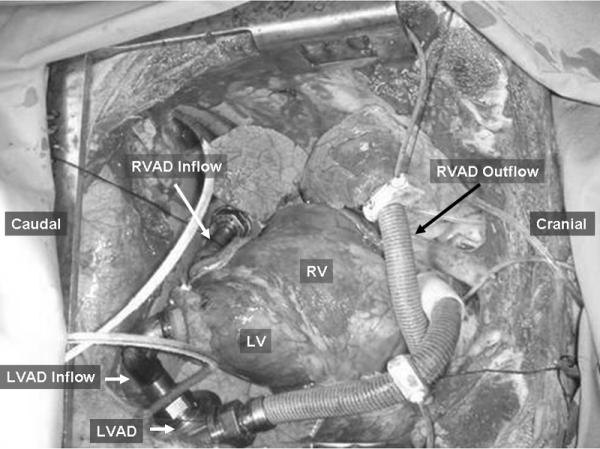
BVAD Implantation using a median sternotomy approach. LVAD, left ventricular assist device; RVAD, right ventricular assist device; RV, right ventricule; LV, left ventricle.
The thoracotomy procedures were performed through the fifth intercostal space. Similar to the sternotomy procedures, pressure monitoring lines and a perivascular flow probe were implanted, and chest closure was performed in the usual manner. Local anesthesia was injected intraoperatively in the corresponding intercostal space to limit postoperative pain after the thoracotomy procedure.
Postoperative Management and Data Acquisition
All animals were closely monitored 24 hours a day for any signs of distress following the surgery. As it is not possible to directly measure the postoperative pain in these animals, we recorded respiration rate, heart rate, and the animal's ability to change posture as indirect measures of assessing postoperative pain. The cage was cleaned several times a day to avoid wound contamination. Intravenous cefazolin (2 gm qid) and gentamycin (160 mg tid) were administered during the first postoperative week as prophylaxis against infection. Intravenous butorphanol (20 mg prn) and Flunixamine™ (100 mg tid) were administered regularly during the first postoperative week to reduce postoperative pain. Hemodynamic variables were recorded hourly by animal monitors. Rectal temperature was also recorded during the entire postoperative period. Complete blood count and comprehensive metabolic panel were performed on a weekly basis postoperatively.
The only change in postoperative management after the sternotomy procedures from that used for the traditional thoracotomy approach was the application of betadine paste to the sternotomy surgical wound at least five times a day. After thoracotomy procedures, betadine spray was applied to the thoracotomy wound instead. To monitor postoperative wound infection, the sternotomy wound and sternum stability was checked daily by the same surgeon.
At the end of each experiment, the animal was sacrificed using intravenous pentobarbital (50 mg/kg) and potassium chloride (120 mEq) in compliance with the Panel on Euthanasia of the American Veterinary Medical Association. Unpaired Student's t test was used to compare the variables between the sternotomy and thoracotomy study groups. Data are expressed as mean ± standard deviation (SD).
Results
All animals were extubated within hours after the sternotomy procedures and were able to stand up and eat immediately. Table 1 shows average data from these studies compared with studies using a lateral thoracotomy approach. There were no statistically significant differences in these values between the two study groups except for the heart rate. The mean heart rate in the first postoperative week after sternotomy experiments was 129 ± 2 bpm vs. 113 ± 5 bpm in thoracotomy procedures (p < 0.05). The average respiratory rate after sternotomy procedures was 33 ± 5 breaths/min, while the average respiratory rates in thoracotomy procedures was 36 ± 3 breaths/min. A mean rectal temperature of 39.4 ± 0.7 °C was recorded in the first postoperative week after sternotomy procedures, which is comparable to 38.5 ± 0.1 °C recorded after the thoracotomy procedures. The mean amount of postoperative chest drainage at the time of chest tube removal was 990 ± 308 ml for median sternotomy vs. 527 ± 380 ml for thoracotomy. This difference was not statistically significant (p = 0.2). The recovery time after sternotomy procedures was similar to that after lateral thoracotomy, with no additional difficulties observed in standing or recumbency and no need for increased pain management.
Table 1.
Average Values of Sternotomy vs. Thoracotomy Approach
| Parameter* | Sternotomy Approach (Mean ± SD) | Thoracotomy Approach (Mean ± SD) | P Value* |
|---|---|---|---|
| Heart rate (bpm) | 129 ± 2 | 113 ± 5 | <0.05 |
| Respiration rate (breaths/min) | 33 ± 5 | 36 ± 3 | NS |
| Rectal temperature (°C) | 39.4 ± 0.7 | 38.5 ± 0.1 | NS |
| Chest drainage content at removal (ml) | 990 ± 308 | 527 ± 380 | NS |
Data represent values taken in the first postoperative week; bpm, beats per minute; NS, statistically not significant.
Student's t test.
In study 3, the feasibility of biventricular assist device implantation was successively tested through a resternotomy approach. Although extensive fibrous tissue was detected from the prior sternotomy procedure, uneventful biventricular assist device implantation and testing were performed (Figure 1).
Figure 2 shows typical sternal wound healing at postoperative day 54 in study 3. The sternal wires were intact, and no sternum dehiscence was observed in any of the studies at the time of autopsy. Except for a small (1 cm × 0.5 cm) superficial infectious pocket at the lower edge of the sternotomy wound in study 3, no superficial or deep infection in the sternotomy wound was observed in any of the three experiments. In study 1, infection of the percutaneous monitoring line penetration sites was observed 4 weeks after the surgery and was treated with local betadine paste and systemic cefazolin and gentamycin antibiotics. This infection occurred due to contamination of the penetration sites caused by placement on the ventral side of the chest. No similar infection was encountered in studies 2 and 3 after we opted to locate the penetration sites to above the midline of the chest. No infection was recorded in any of the thoracotomy procedures.
Figure 2.
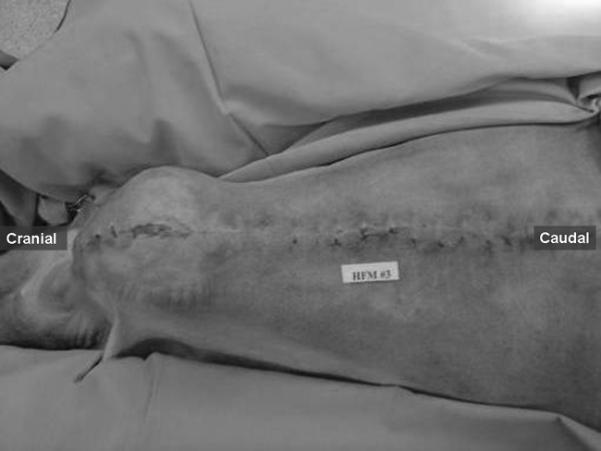
Typical sternal wound healing post sternotomy. Study 3 at postoperative day 54.
Table 2 shows the average complete blood count and differentials after the sternotomy procedures. These values were sustained within normal limits after the surgery. The mean postoperative white blood cell (WBC) count and neutrophil percentage after sternotomy were 12 ± 2 K/μL and 25 ± 10%, respectively. These values are comparable with the preoperative values of 12 ± 2 K/μL and 24 ± 1%, respectively. The mean postoperative WBC count and neutrophil percentage after thoracotomy procedures were 8 ± 2 K/μL and 43 ± 4%, respectively. The relatively higher mean WBC count after the sternotomy surgeries stems from the aforementioned monitoring line penetration site infection in study 1. The hematocrit value was stable after the sternotomy procedures, with a mean value of 30 ± 3%. The mean hematocrit after the thoracotomy procedures was 30 ± 10%.
Table 2.
Postoperative Indices of Infection after Sternotomy Procedures
| Parameter | Postoperative Day* |
||||||
|---|---|---|---|---|---|---|---|
| Pre | 7 | 14 | 28 | 50 | 65 | 77 | |
| WBC (K/μL) | 12 ± 2 | 9 ± 3 | 11 ± 1 | 12 ± 1 | 12 ± 2 | 13 | 11 |
| Hb(g/dL) | 12 ± 0.8 | 9 ± 1 | 10 ± 1 | 11 ± 1 | 11 ± 2 | 11 | 10 |
| HCT (%) | 35 ± 1 | 27 ± 4 | 29 ± 3 | 31 ± 2 | 32 ± 5 | 31 | 28 |
| Neutrophils (%) | 24 ± 8 | 27 ± 13 | 16 ± 1 | 27 ± 6 | 17 ± 1 | 40 | 39 |
| Lymphocytes (%) | 71 ± 5 | 61 ± 15 | 66 ± 11 | 54 ± 1 | 64 ± 4 | 46 | 49 |
| Total bilirubin (mg/dL) | 0.1 ± 0 | 0.1 ± 0 | 0.1 ± 0 | 0.1 ± 0 | 0.1 ± 0 | 0.1 | 0.1 |
| BUN (mg/dL) | 6.3 ± 2.1 | 4.0 ± 2.6 | 3.5 ± 0.7 | 6.0 ± 0 | 9.0 ± 0 | 4.0 | 5.0 |
| Creatinine (mg/dL) | 0.5 ± 0 | 0.3 ± 0 | 0.4 ± 0 | 0.4 ± 0 | 0.5 ± 0.3 | 0.5 | 0.3 |
Data after postoperative day 50 were obtained from study 1 only.
Data after postoperative day 15 includes only studies 1 and 3.
Pre, preoperative; WBC, white blood cell; Hb, hemoglobin, HCT, hematocrit; BUN, blood urea nitrogen.
Discussion
This study showed comparable outcomes using a median sternotomy vs. lateral thoracotomy approach in calves for chronic experiments without adding additional risk to the overall outcome. The postoperative course in this study was found to be similar to that using a thoracotomy approach. Several indirect measures of postoperative pain were recorded to estimate the degree of pain experienced by these animals, including standing and eating, respiration rate, and heart rate. All these values were comparable to those in the experiments using the thoracotomy, except for a significantly higher heart rate after the sternotomy procedures compared to thoracotomy procedures. It is hard to attribute the relatively higher heart rate in these animals to the sternotomy procedure alone because there are several other factors in the immediate postoperative period that may have led to an accelerated heart rate.
No sternal dehiscence was detected in any of the studies. The sternal wound remained clean during the entire postoperative period, except for a small localized superficial infection pocket at the sternotomy wound caudal end of study 3. This superficial infection occurred because two of the skin sutures had opened. No other sternal wound or systemic infections were encountered in the other studies. The infection of the pressure line penetration site occurred in the first study only. This finding is explained by contamination of these penetration sites due to their placement on the ventral side of the chest. In studies 2 and 3, the skin penetration site was located above the midline of the chest, and no similar infection sites were encountered thereafter.
To obtain reproducible results from animal experiments during preclinical studies, testing of the physiological interaction of implantable devices with the body is necessary. Traditionally, the majority of preclinical studies have been performed in the bovine model via a lateral thoracotomy approach. We believe that using the median sternotomy approach for bovine device implantation better simulates the clinical surgical approach and helps to obtain more reproducible physiological data, particularly with regard to LVAD placement. Using the thoracotomy approach limits the space for device placement, particularly when RVAD and LVAD are simultaneously implanted. Among the major advantages of the median sternotomy approach over the thoracotomy approach is better surgical exposure and the presence of adequate space for device placement without interfering with chest closure or necessitating rib resection. It is noteworthy that regardless of the surgical approach, direct anastomosis of the LVAD outflow graft to the ascending aorta is very challenging in the bovine model, because of the relatively short ascending aorta segment in these animals. From a physiological point of view, anastomosis of the LVAD outflow graft to the brachiocephalic trunk simulates to a higher extent the clinical implantation procedures used for an LVAD. It has been shown that at moderate levels of pump support, the amount of blood flow distal to the descending aortic outflow anastomosis may be decreased by up to 25% due to regurgitate blood flow back into the aorta.8 Moreover, the likelihood of detecting neurological findings caused by device-related thrombogenicity is higher when the LVAD outflow graft is anastomosed to the brachiocephalic trunk compared to the descending aorta anastomosis. Because of the aforementioned sternotomy approach advantages, we are currently developing a heart failure model in calves and intend to use the sternotomy approach in these animals for biventricular assist device placement in chronic studies.
In the study reported here, the chest drainage content was higher, yet statistically not significant, in sternotomy vs. thoracotomy procedures. This finding may be explained by differences in the surgical procedures and incision size between these two approaches. A larger incision site is required for sternotomy procedures to allow complete sternotomy. However, no major blood loss was detected in any of the sternotomy procedures, and no blood administration was necessary in any of these procedures.
Our extensive literature search failed to produce any similar studies looking at the use of a median sternotomy in calf chronic animal models except for an abstract by Frazier et al.9 The investigators combined median sternotomy with tracheostomy in chronic bovine experiments after implantation of biventricular assist devices to facilitate weaning from the ventilator. However, in our study, excellent outcomes were observed without performing simultaneous tracheotomy. As mentioned above, weaning of these animals and extubation was achieved without complications within hours of completion of the surgery.
In conclusion, these studies showed good outcomes by performing a median sternotomy for chronic bovine experiments. This surgical approach allows greater access and space for single or multiple device placements without adding additional risks of sternum dehiscence or infection to the study outcome. We also showed the possibility of performing resternotomy and biventricular assist device implantation in these animals.
Acknowledgments
Disclosure: This study was supported by Bioengineering Research Partnerships grant 5R01HL074896 (to K.F.), issued by the National Heart, Lung, and Blood Institute, National Institutes of Health.
Footnotes
Disclaimer Line: None
References
- 1.Lietz K, Long JW, Kfoury AG, et al. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: implications for patient selection. Circulation. 2007;116:497–505. doi: 10.1161/CIRCULATIONAHA.107.691972. [DOI] [PubMed] [Google Scholar]
- 2.Fossum TW, Morley D, Olsen DB, et al. Complications common to ventricular assist device support are rare with 90 days of DeBakey VAD support in calves. ASAIO J. 2001;47:288–92. doi: 10.1097/00002480-200105000-00026. [DOI] [PubMed] [Google Scholar]
- 3.Nonaka K, Linneweber J, Ichikawa S, et al. Development of the Baylor Gyro permanently implantable centrifugal blood pump as a biventricular assist device. Artif Organs. 2001;25:675–82. doi: 10.1046/j.1525-1594.2001.06855.x. [DOI] [PubMed] [Google Scholar]
- 4.Wilson DV, Kantrowitz A, Pacholewicz J, et al. Perioperative management of calves undergoing implantation of a left ventricular assist device. Vet Surg. 2000;29:106–18. doi: 10.1111/j.1532-950x.2000.00106.x. [DOI] [PubMed] [Google Scholar]
- 5.Son HS, Sun K, Hwang CM, et al. Ventricular assist device implantation using a right thoracotomy. ASAIO J. 2006;52:386–90. doi: 10.1097/01.mat.0000227692.75032.86. [DOI] [PubMed] [Google Scholar]
- 6.Doi K, Golding LA, Massiello AL, et al. Preclinical readiness testing of the Arrow International CorAide left ventricular assist system. Ann Thorac Surg. 2004;77:2103–10. doi: 10.1016/j.athoracsur.2003.07.048. [DOI] [PubMed] [Google Scholar]
- 7.Fukamachi K, Horvath DJ, Massiello AL, et al. Development of a small implantable right ventricular assist device. ASAIO J. 2005;51:730–5. doi: 10.1097/01.mat.0000181031.66900.b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Litwak KN, Kihara S, Kameneva MV, et al. Effects of continuous flow left ventricular assist device support on skin tissue microcirculation and aortic hemodynamics. ASAIO J. 2003;49:103–7. doi: 10.1097/00002480-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Frazier OH, Tuzun E, Cohn WD, et al. The use of median sternotomy and tracheostomy in total heart replacement with dual continuous flow pumps: a pilot experimental study. ASAIO J. 2006;52:42A. doi: 10.1097/01.mat.0000196827.61241.07. Abstract. [DOI] [PubMed] [Google Scholar]


