Abstract
Locally produced dopamine in the renal proximal tubule inhibits salt and fluid reabsorption, and a dysfunctional intrarenal dopaminergic system has been reported in essential hypertension and experimental hypertension models. Using catechol-O-methyl-transferase knockout (COMT−/−) mice, which have increased renal dopamine due to deletion of the major renal dopamine metabolizing enzyme, we investigated the effect of intrarenal dopamine on the development of hypertension in the deoxycorticosterone acetate/high salt (DOCA/HS) model. DOCA/HS led to significant increases in systolic blood pressure (SBP) in wild type mice (from 115 ± 2 to 153 ± 4 mmHg), which was significantly attenuated in COMT−/− mice (from 114 ± 2 to 135 ± 3 mmHg). In DOCA/HS COMT−/− mice, the D1-like receptor antagonist SCH-23390 increased SBP (156 ± 2 mmHg). DOCA/HS COMT−/− mice also exhibited more urinary sodium excretion (COMT−/− vs. wild type: 3038 ± 430 vs. 659 ± 102 μM/24 h, P < 0.01). Furthermore, DOCA/HS-induced renal oxidative stress was significantly attenuated in COMT−/− mice. COX-2-derived prostaglandins in the renal medulla promote sodium excretion, and dopamine stimulates medullary prostaglandin production. Renal medullary COX-2 expression and urinary PGE2 excretion were significantly higher in COMT−/− than wild type mice after DOCA/HS treatment. In DOCA/HS treated COMT−/− mice, the COX-2 inhibitor SC-58236 reduced urinary sodium and PGE2 excretion and increased SBP (153 ± 2 mmHg). These studies indicate that an activated renal dopaminergic system attenuates the development of hypertension, at least in large part through activating medullary COX-2 expression/activity and also decreases oxidative stress resulting from DOCA/HS.
Keywords: dopamine, hypertension, cyclooxygenase-2, prostaglandin E2, oxidative stress, kidney
INTRODUCTION
Although dopamine is an essential neurotransmitter, extraneural dopamine also serves important physiologic functions. The kidney possesses a robust intrarenal dopaminergic system that is distinct from any neural dopaminergic input. Circulating concentrations of dopamine are in the picomolar range, while dopamine levels in the kidney can reach high nanomolar concentrations.1 The dopamine precursor, L-dihydroxyphenylalanine (L-DOPA), is taken up in the proximal tubule following filtration at the glomerulus and is then converted to dopamine by aromatic amino acid decarboxylase.1–4 Renal dopamine is metabolized predominantly by catechol-O-methyltransferase (COMT), with a smaller contribution by monoamine oxidase.
Dopamine’s cellular actions are mediated by signaling through G protein-coupled seven transmembrane receptors. There are five known renal dopamine receptors, which are divided into two subclasses: D1-like and D2-like receptors. D1-like receptors (D1 and D5) are coupled to Gs and stimulate adenylate cyclase. D2-like receptors (D2, D3 and D4) are coupled predominantly to Gi. In the mammalian kidney, dopamine serves as a major regulator of salt and water reabsorption by inhibiting both proximal and distal solute and water transport, mediated at least in part by inhibition of specific tubule transporter activity: apical(e.g., Na/H exchange and chloride-bicarbonate exchange and Na-P cotransport) and basolateral (e.g., Na-K-ATPase and Na-HCO3 cotransport) transporters in the proximal tubule, apical Na-K-2Cl co-transporter in the thick ascending limb, and apical Na+ channel and aquaporins-2 and -4 in the collecting duct.1–5
Alterations in intrarenal dopamine production and/or activity have been reported in essential hypertension.2, 3 Decreased intrarenal dopamine production, dysfunctional dopamine signaling in the proximal tubule due to abnormalities in GRK4 coupling to D1-like receptors, and decreased D1-like receptors in the medulla have been found in experimental models of hypertension.6–8 Deleting each of the five dopamine receptor subtypes leads to hypertension,4 while increased renal dopamine prevents high salt-induced elevation of blood pressure.9 However, the mechanisms underlying dopamine-mediated antihypertensive effects are not fully understood.
Dopamine-mediated inhibition of salt and water reabsorption in proximal and distal tubules contributes to its antihypertensive effects, and increasing evidence suggests that dopamine also has antioxidant effects, which may contribute to its antihypertensive effects.10, 11 In addition, dopamine can stimulate prostaglandin production in renal medulla.12, 13 Prostaglandins produced in the renal medulla promote sodium and water excretion, and inhibition of prostaglandin production has the potential to increase blood pressure in some individuals.14 In the current studies, we investigated whether intrarenal dopamine can protect against DOCA/HS-induced hypertension and whether dopamine-mediated stimulation of renal medullary prostaglandin production may contribute to any dopamine-mediated antihypertensive effects.
METHODS
Animals
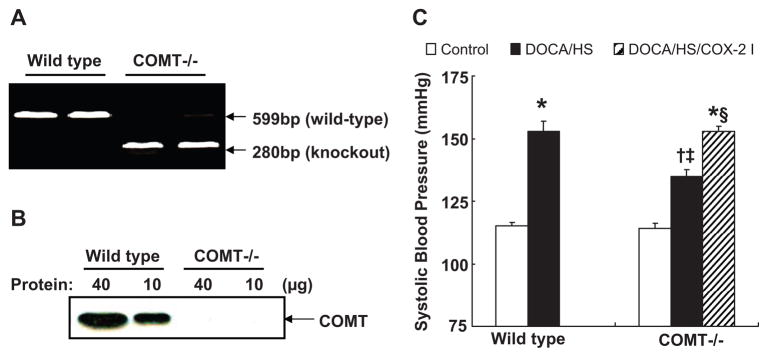
All animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of Vanderbilt University. Wild type and COMT−/− mice on the 129J/sv background were obtained from Dr. Karayiorgou of Rockefeller University.9 All mice were genotyped before use with PCR. The COMT primers 5′-GCAGTGATTCGGGAGTACAG-3′ (forward) and 5′-TAGCGGTCTTTCCAGTGGTC-3′ (reverse) generated a 599-bp product in heterozygous (not shown) and wild type mice (Figure 1A). The neo cassette primers 0IMR013 and 0IMR014 (JAX laboratory) generated a 280-bp product in heterozygous (not shown) and homozygous mice (Figure 1A). Deletion of kidney COMT was also confirmed by western analysis after the animals were sacrificed (Figure 1B). COMT−/− mice have increased dopamine levels in the kidney and urine due to deletion of the major renal dopamine metabolizing enzyme.15 All mice were fed normal pelleted rodent chow with 0.29% sodium (w/w, Harlan). Mice were divided into 6 groups: control wild type, DOCA/HS treated wild type, control COMT−/−, DOCA/HS treated COMT−/− with or without administration of D1-like receptor antagonist or selective COX-2 inhibitor. DOCA pellets (150 mg, 60-d release, Innovative Research of American) were implantedsubcutaneously. HS was achieved by adding 1% NaCl in the drinking water. The COX-2 inhibitor SC-58236 (2 mg/kg) was given by daily gastric gavage (a gift from Searle Monsanto). The D1-like receptor antagonist SCH-23390 (Sigma-Aldrich) was given at a dose of 1 mg/kg per day via osmotic mini-pump (2004; Alzet) implanted subcutaneously under sterile conditionsand ether anesthesia. To collect 24-h urine, the animals were first acclimated individually in metabolic cages, and then 24-h urine was collected.
Figure 1.
DOCA/HS-induced elevations of blood pressure were attenuated in COMT −/− mice. A: Genotyping of COMT −/− mice with PCR. B: Western analysis showed deletion of COMT in COMT −/− mouse kidney. C: Blood pressure was higher in wild type than COMT −/− mice after DOCA/HS treatment. *: P < 0.01 vs. control; †: P < 0.05 vs. control COMT −/−; ‡: P < 0.05 vs. DOCA/HS wild type; §: P < 0.05 vs. DOCA/HS COMT −/−. COX-2 I: COX-2 inhibitor SC58236.
Blood Pressure Measurement Using Tail-cuff and Carotid Catheterization
Systolic blood pressure (SBP) was measured with a tail-cuff microphonic manometer.16 Blood pressure was also measured using carotid catheterization. Mice were anesthetized with 80 μg/g ketamine (Fort Dodge Laboratories) and 8 μg/g inactin (BYK) by i.p. administration. Mice were placed on a temperature-controlled pad. After tracheostomy, PE-10 tubing was inserted into the right carotid artery. The catheter was tunneled under the skin, exteriorized, securedat the back of the neck, filled with heparinized saline and sealed. The catheterized mouse was housed individually and trained three times before measurement of blood pressure with a Blood Pressure Analyzer (Micro-Med, Louisville, KY).17
Determination of Urinary F2-isoprostane and PGE-M
Urinary F2-isoprostane, a well-accepted marker of systemic oxidative stress, and urinary PGE-M, the major metabolite of PGE2, were measured by GC/electron capture/negative chemical ionization MS assay as previously described.18
Immunohistochemistry and Western Blot
The mice were anesthetized with Nembutal (50 mg/kg, i.p.), given heparin (1,000 units/kg, i.p.) to minimize coagulation. One kidney was taken out for western analysis and the other was perfused with FPAS (3.7% formaldehyde, 10 mM sodium m-periodate, 40 mM phosphate buffer, and 1% acetic acid) through the aortic trunk. After fixation, the selected tissues were dehydrated and paraffin-embedded, and immunostained as previously described.19, 20 The kidney sections were immunostained with rabbit anti-murine COX-2 antibody (Cayman Chemicals), rabbit anti-nitrotyrosine antibody (a marker of oxidative stress, Santa Cruz Biotechnology) and monoclonal rat anti-mouse F4/80 (a marker of macrophage, AbD Serotec). Western analysis was carried out as described previously.21 Antibodies used for western analysis included COX-2 antibody (Cayman Chemicals) and COMT antibody (Novus Biologicals).
Quantitative Image Analysis
Macrophage infiltration and nitrotyrosine immunostaining were quantified using the BIOQUANT image analysis system (R & M Biometrics, Nashville, TN).22
Micrography
Bright-field images from a Leitz Orthoplan microscope with DVCdigital RGB video camera were digitized and saved as computer files. Contrast and color level adjustment (Adobe Photoshop) were performed for the entireimage; i.e., no region- or object-specific editing or enhancements were performed.
Statistical Analysis
Values are presented as means ± S.E.M. ANOVA and Bonferroni t-test were used for statistical analysis, and differences wereconsidered significant when P < 0.05.
RESULTS
DOCA/HS-Induced Diuresis and Natriuresis Were Augmented in COMT−/− Mice
Urine volume and urinary Na (UNa) and K (UK) excretion were similar between control wild type and control COMT−/− mice. DOCA/HS led to significant increases in urine volume and UNa in wild type mice, but even more significant increases in COMT−/− mice, resulting in ~ 3.4 fold higher levels in urinary volume and ~ 4.6 fold higher levels of UNa in COMT−/− than wild type mice after DOCA/HS treatment (Table 1). Urinary dopamine excretion was significantly higher in control COMT−/− mice than control wild type mice (7858 ± 1794 versus 4133 ± 670ng/24 hours of control wild-type; P<0.01; n = 4), similar to our previous reports.15 DOCA/HS treatment did not affect urinary dopamine excretion appreciably in COMT−/− mice and wild type mice (6273 ± 608 versus 3015 ± 670ng/24 hours of DOCA/HS wild-type; P<0.01; n = 4).
Table 1.
Effects of DOCA/HS on urine volume, urinary Na and K excretion in wild type and COMT −/− mice
| Wild type |
COMT −/− |
||||
|---|---|---|---|---|---|
| Group | control | DOCA/HS | control | DOCA/HS | DOCA/HS/C2I |
| 24-H urine volume, ml | 1.1 ± 0.1 | 6.4 ± 1.6* | 1.2 ± 0.2 | 21.6 ± 3.3*† | 4.2 ± 1.1*‡ |
| 24-H urinary Na excretion, μM | 123 ± 10 | 659 ± 102* | 131 ± 20 | 3038 ± 430*† | 399 ± 82*‡ |
| 24-H urinary K excretion, μM | 164 ± 20 | 134 ± 19 | 253 ± 36 | 228 ± 42 | 128±16 |
Values are means ± S.E.M. Animals were caged individually for 24 h and urine sample was collected.
P < 0.01 vs. control;
P < 0.01 vs. DOCA/HS wild type;
P < 0.01 vs. DOCA/HS COMT−/−; n = 6 in each group. HS: 1% NaCl in drinking water; C2I: COX-2 inhibitor SC58236.
DOCA/HS-Induced Increases in Blood Pressure Were Attenuated in COMT−/− Mice
When blood pressure was measured by tail-cuff microphonic manometer, we found that although blood pressure was similar between control wild type and control COMT−/− mice (SBP: 112 ± 4 vs. 117 ± 3 mmHg of wild type, n = 6), it was significantly higher in wild type than COMT−/− mice after DOCA/HS treatment (SBP: 149 ± 4 vs. 131 ± 3 mmHg of DOCA/HS COMT−/−, P < 0.05, n = 6). To confirm this attenuated DOCA/HS-induced blood pressure increases in COMT−/− mice, blood pressures were measured using carotid catheterization in another set of mice. As indicated in Figure 1C, blood pressure was significantly higher in DOCA/HS treated wild type mice than DOCA/HS treated COMT−/− mice (SBP: 153 ± 4 vs. 135 ± 3 mmHg, P < 0.05, n = 5).
To investigate whether activation of D1-like receptor contributed to the attenuated DOCA/HS-induced elevation of blood pressure in COMT−/− mice, a subset of DOCA/HS treated COMT−/−mice was treated with the D1-like receptor antagonist SCH-23390. SCH-23390 treatment increased blood pressure in DOCA/HS treated COMT−/− mice (SBP: 156 ± 2 vs. 135 ± 3 mmHg, P < 0.05, n = 5).
DOCA/HS-Induced Increases in Oxidative Stress and Macrophage Infiltration in the Kidney Were Attenuated in COMT−/− Mice
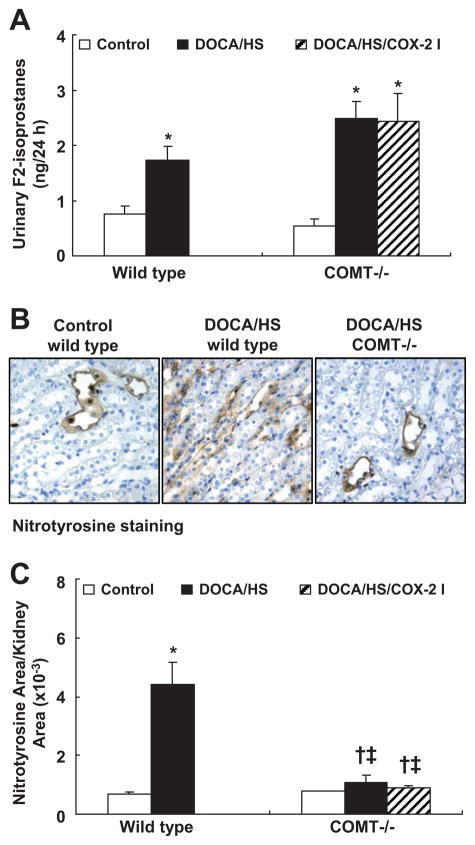
Vasculature-derived oxidative stress may contribute to DOCA/HS-induced hypertension.23–25 Urinary F2-isoprostane levels were similar between control wild type and control COMT−/− mice. DOCA/HS led to similar increases in urinary F2-isoprostane excretion in wild type and COMT−/− mice (wild type: 1.73 ± 0.26 vs. 0.76 ± 0.13 ng/24 h, P < 0.01; COMT−/−: 2.49 ± 0.31 vs. 0.55 ± 0.11 ng/24 h, P < 0.01 vs. control COMT−/− but P > 0.05 vs. DOCA/HS treated wild type, n = 6) (Figure 2A).
Figure 2.
DOCA/HS-induced renal but not systemic elevations of oxidative stress were attenuated in COMT −/− mice. A: DOCA/HS increased urinary F2-isoprostane to similar levels in wild type and COMT −/− mice. *: P < 0.01. B: Representative photomicrographs of nitrotyrosine immunostaining in the outer medulla (original magnification: ×250). C: DOCA/HS induced significant increases in nitrotyrosine in wild type mice, but nitrotyrosinde increases were significantly attenuated in COMT −/− mice. *: P < 0.01 vs. control; †: P < 0.05 vs. control; ‡: P < 0.01 vs. DOCA/HS treated wild type. N = 3 in each group.
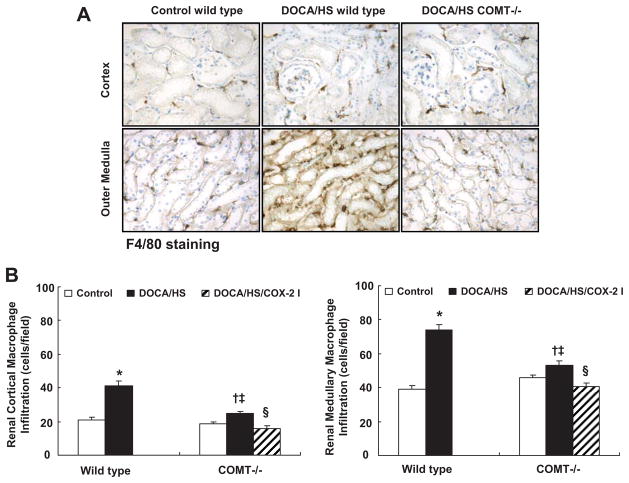
Dopamine has been proposed to exert antioxidant effects in the kidney.10, 11 Renal oxidative stress was evaluated via nitrotyrosine immunostaining. Nitrotyrosine immunostaining was primarily found in the renal medulla. As shown in Figure 2B&C, DOCA/HS led to significant increases in nitrotyrosine immunostaining in wild type mice, but only minimal increases in COMT−/− mice. Macrophage infiltration also increases in response to increased oxidative stress.26 As shown in Figure 3, DOCA/HS led to significant increases in macrophage infiltration in cortex and medulla in wild type mice (cortex: 41.2 ± 2.7 vs. 21.0 ± 1.4 cells/field, P < 0.01; medulla: 73.9 ± 3.3 vs. 39.0 ± 2.2 cells/field, P < 0.01. N = 6), but less significant increases in COMT−/− mice (cortex: 24.8 ± 1.4 vs. 18.7 ± 1.3 cells/field, P < 0.05; medulla: 52.9 ± 2.7 vs. 45.8 ± 1.6 cells/field, P < 0.05. N = 6).
Figure 3.
DOCA/HS-induced renal macrophage infiltration was attenuated in COMT −/− mice. A: Representative photomicrographs of F4/80-positive macrophages in renal cortex and medulla (original magnification: ×250). B: There was significantly less macrophage infiltration in COMT −/−than wild type mice, and COX-2 inhibition reduced macrophage infiltration further in DOCA/HS treated COMT −/− mice.. *: P < 0.01 vs. control; †: P < 0.05 vs. control; ‡: P < 0.01 vs. DOCA/HS treated wild type. §: P < 0.01 vs. DOCA/HS treated COMT −/−.
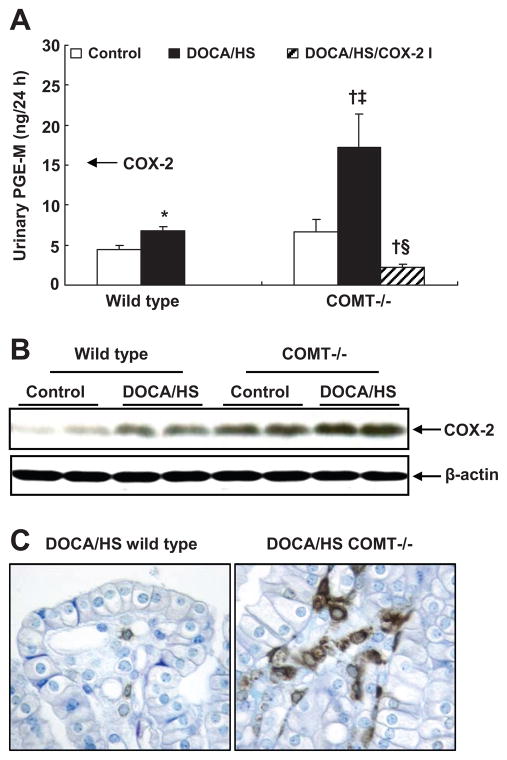
DOCA/HS Treated COMT−/− Mice Have Higher Urinary PGE2 Excretion and Medullary COX-2 Expression
Prostaglandins promote renal sodium excretion,21 and dopamine has been reported to stimulate medullary prostaglandin production.12, 13 As shown in Figure 4A, urinary PGE-M excretion was numerically but not significantly increased in control COMT−/− mice compared with control wild type mice. DOCA/HS led to a modest increase in urinary PGE-M excretion in wild type mice (6.8 ± 0.5 vs. 4.5 ± 0.4 ng/24 h, P < 0.05, n = 5), but a significant increase in COMT−/− mice (17.2 ± 4.2 vs. 6.7 ± 1.5 ng/24 h, P < 0.01, n = 5). Immunoblotting indicated that medullary COX-2 expression was higher in control COMT−/− than control wild type mice. DOCA/HS stimulated medullary COX-2 expression to a greater extent in COMT−/− mice than wild type mice (Figure 4B). Immunostaining confirmed higher COX-2 expression in medullary interstitial cells after DOCA/HS treatment in COMT−/− than wild type mice (Figure 4C).
Figure 4.
COMT −/− mice exhibited increased renal COX-2 expression and activity in response to DOCA/HS treatment. A: DOCA/HS led to a modest increase in urinary PGE-M excretion in wild type mice, but a significant increase in COMT −/− mice. COX-2 inhibition significantly reduced PGE-M excretion in DOCA/HS treated COMT −/− mice. *: P < 0.05 vs. control; †: P < 0.01 vs. control; ‡: P < 0.01 vs. DOCA/HS treated wild type; §: P < 0.01 vs. DOCA/HS treated COMT −/−. B: DOCA/HS stimulated medullary COX-2 expression to a greater extent in COMT −/− mice. C: More medullary interstitial cells were COX-2 positive in COMT −/− than wild type mice after DOCA/HS treatment (original magnification: ×400).
COX-2 Inhibition Increased Blood Pressure in DOCA/HS Treated COMT−/− Mice
To investigate further whether increased medullary COX-2 expression/activity contributed to the protection against DOCA/HS-induced elevation of blood pressure in COMT−/− mice, a subset of DOCA/HS treated COMT−/− mice was treated with the selective COX-2 inhibitor SC-58236. COX-2 inhibition increased blood pressure in DOCA/HS treated COMT−/− mice (SBP: 153 ± 2 vs. 135 ± 3 mmHg, P < 0.05, n = 5) (Figure 1C) and decreased urine volume and UNa (Table 1) and urinary PGE-M excretion (2.2 ± 0.4 vs. 17.2 ± 4.2 ng/24 h, P < 0.01 vs. control and DOCA/HS treated COMT−/− mice, n = 5) (Figure 4A), but had no effect on urinary F2-isoprostane excretion (2.4 ± 0.5 vs. 2.5 ± 0.3 ng/24 h, n = 6) or expression of the intrarenal oxidative stress marker, nitrotyrosine (Figure 2). The COX-2 inhibitor significantly decreased DOCA/HS-induced macrophage infiltration in renal cortex (15.7 ± 1.7 vs. 24.8 ± 1.4 cells/field, P < 0.01, n = 6) and renal medulla (40.6 ± 2.4 vs. 52.9 ± 2.7 cells/field, P < 0.01, n = 6) (Figure 3B), consistent with the anti-inflammatory effect of COX-2 inhibition.
DISCUSSION
The current studies investigated the effects of increased renal dopamine on the development of DOCA/HS-induced hypertension. The major findings include: 1) in COMT−/− mice, which have increased intrarenal dopamine levels15, DOCA/HS-induced elevation of blood pressure was attenuated; 2) COMT−/− mice exhibited augmented diuresis and natriuresis in response to DOCA/HS; 3) DOCA/HS-induced increases in renal oxidative stress and macrophage infiltration were attenuated in COMT−/− mice; and 4) DOCA/HS treated COMT−/− mice had increased urinary PGE2 excretion and medullary COX-2 expression/activity. Of note, administration of a COX-2 inhibitor to DOCA/HS treated COMT−/− mice led to increases in blood pressure and decreases in urinary sodium excretion but did not increase marker of renal and systemic oxidative stress and decreased renal macrophage infiltration. Taken together, these results indicate that an activated intrarenal dopaminergic system may attenuate DOCA/HS-induced elevation of blood pressure by promoting diuresis and natriuresis due to increased medullary COX-2 expression/activity. In addition, DOCA and/or high salt may induce intrarenal oxidative stress directly, rather than secondary to increased blood pressure,27 and dopamine inhibits the oxidative stress directly rather than as a result of decreasing blood pressure and/or increasing sodium excretion.28–32
Cyclooxygenase is a rate-limitingstep in prostaglandin production. Both COX isoforms, COX-1 and COX-2, are expressed at high levels in the inner medulla/papilla.21, 33 In the medulla, prostaglandins act as diuretic and natriuretic agents byincreasing blood flow in the vasa recta, decreasing salt reabsorptionin the medullary thick ascending limbs, and reducing vasopressin-stimulated water reabsorption fromcollecting ducts.34 All of these effects are inhibited by COX-2 inhibitors.14 Inhibition of prostaglandin production by COX-2 inhibitors may cause edema and modest elevations in blood pressure in a minority of subjects and may also exacerbate preexisting hypertension.14 Renal medullary COX-2 expression is stimulated by activation of mineralocorticoid receptors by administration of DOCA or inhibition of 11β hydroxysteroid dehydrogenase-2 activity with glycyrrhizic acid, while COX-1 expression is unaltered.17, 33 Furthermore, we have previously reported that inhibition of COX-2 activity augmented blood pressure elevations in glycyrrhizic acid/HS treated animals while inhibition of COX-1 activity had no effect.17
Dopamine has been shown to stimulate prostaglandin production in isolated rabbit kidney and microsomes isolated from rabbit kidney medulla.12, 13 Infusion of dopamine or the D1-like receptor agonist, fenoldopam, stimulated renal prostaglandin production in normal volunteers.35 Dopamine has also been reported to stimulate PGE2 production through activation of D2-like receptors in cultured inner medulla collecting duct cells.36 In the present studies, renal medullary COX-2 expression and urinary PGE2 excretion were significantly higher with DOCA/HS treatment of COMT−/− mice compared to wild type mice, and these increases were blocked by a highly selective COX-2 inhibitor. These studies suggest that dopamine stimulates prostaglandin production through increasing medullary COX-2 expression and activity and that the increased COX-2 expression and activity are involved in the increased natriuresis seen in the COMT−/− mice with DOCA/HS, but definitive proof of such integrated regulation and interaction will require inhibition of the intrarenal dopaminergic system.
Although blood pressure was similar in DOCA/HS treated wild type and DOCA/HS plus COX-2 inhibitor treated COMT−/− mice, sodium excretion was different (Table 1), suggesting that COX-2 inhibition-induced hypertension is not entirely related to renal sodium handling. Rodriguez et al found that selective COX-2 inhibition led to decreased glomerular filtration rate and sodium excretion and increased blood pressure in dog.37 Therefore, decreased glomerular filtration rate may also contribute to COX-2 inhibition-induced blood pressure elevation in the current studies,
Activation of NADPH oxidase and xanthine oxidase and inactivation of Cu/Zn superoxide dismutase all appear to contribute to increased superoxide anion generation in DOCA/HS hypertensive models.23, 38, 39 Similarly, in cultured human renal proximal tubular cells, aldosterone activated mitochondrial oxidative stress.27 Vascular and intrarenal oxidative stress accompany DOCA/HS-induced elevations in blood pressure, although the role of oxidative stress in development or maintenance of hypertension remains controversial. In mice with genetic deletion of the NADPH oxidase subunit, gp91phox, DOCA/HS treatment did not lead to increases in blood pressure and vascular oxidative stress, compared with increased blood pressure and vascular oxidative stress in the wild type mice.23 In contrast, vascular superoxide anion production did not increase in a hypertension model induced by norepinephrine infusion, suggesting that increased vascular oxidative stress may not be secondary to increased blood pressure per se.25
Previous studies have indicated that activation of D1-like or D2-like receptors can induce antioxidant responses.28, 40 D2 receptor and D5 receptor knockout mice develop ROS-dependent hypertension. In these mice, renal NADPH activity and expression are increased, and inhibition of NADPH oxidase activity normalizes the blood pressure.10, 11 COMT is the major intrarenal dopamine metabolizing enzyme, and COMT−/− mice have increased intrarenal dopamine levels due to the absence of COMT metabolism of dopamine.9 However, plasma dopamine concentrations are similar between wild type and COMT−/− mice while intrarenal and urinary dopamine levels are significantly higher in COMT−/− mice.15 After DOCA/HS treatment, systemic oxidative stress (urinary F2-isoprostane excretion) was similar between wild type and COMT−/− mice, while intrarenal oxidative stress was significantly lower in COMT−/− mice than wild type mice (Figure 2). Western blotting with anti-4-Hydroxynonenal antibody, another biomarker of oxidative stress, also demonstrated that DOCA/HS-induced renal oxidative stress was attenuated in COMT−/− mice (unpublished data). That COX-2 inhibition increased blood pressure without an increase in markers of intrarenal oxidative stress in COMT−/− mice suggests that the intrarenal oxidative stress in response to DOCA and/or high salt is not necessarily the result of increased blood pressure, and that the ability of dopamine to reduce intrarenal oxidative stress is not necessarily only due to increased natriuresis and diuresis.
Abnormalities in dopamine production and receptor function accompany a high percentage of human essential hypertension and several forms of rodent genetic hypertension.4 A general characteristic of essential hypertension is a relative defect in renal sodium and water handling. Intrarenal dopamine may act to protect the kidney from hypertension-induced injury through the following possible mechanisms: 1) inhibition of tubular salt reabsorption. Dopamine directly inhibits net NaCl and fluid reabsorption in the proximal and distal tubules;2, 3, 5, 41, 42 2) interaction with angiotensin II. Intrarenal dopamine antagonizes angiotensin II-induced salt reabsorption in the proximal tubule through decreasing AT1 receptor expression;4, 43 3) interaction with intrarenal renin. Intrarenal dopamine indirectly inhibits renal renin expression through inhibition of COX-2 expression in the macula;15, 22 4) an antioxidant effect;10, 11, 28, 40 and finally 5) stimulation of medullary prostaglandin production.12, 13, 36
Clinical Perspectives
A Guytonian view of hypertension posits that dysfunctional salt and water excretion by the kidney ultimately underlies the development and maintenance of hypertension.44 Normally functioning kidneys respond to increased intravascular volume by inducing pressure natriuresis, by which increased renal perfusion pressure is transmitted to inhibit tubule reabsorption and to increase vasa recta capillary pressure and blood flow, leading to both increased hydrostatic pressure and medullary interstitial osmotic gradient washout.2 Using gene targeting plus renal cross-transplantation technique, Coffman’s group found that deletion of renal AT1 receptors alone is sufficient to reduce blood pressure, and that Ang II causes hypertension primarily through its effects on AT1 receptors in the kidney associated with reduced urinary sodium excretion.45 Our present studies also point out the importance of intrarenal dopamine and renal medullary COX-2 in protecting against the development of hypertension.
Acknowledgments
Sources of Funding
This work was supported by NIH grants DK62794 and DK079341 (to R.C. Harris), CA122620 (to M.-Z. Zhang); and by funds from theVeterans’ Administration (to R.C. Harris).
Footnotes
Disclosures
None.
References
- 1.Zeng C, Sanada H, Watanabe H, Eisner GM, Felder RA, Jose PA. Functional genomics of the dopaminergic system in hypertension. Physiol Genomics. 2004;19:233–246. doi: 10.1152/physiolgenomics.00127.2004. [DOI] [PubMed] [Google Scholar]
- 2.Aperia AC. Intrarenal dopamine: a key signal in the interactive regulation of sodium metabolism. Annu Rev Physiol. 2000;62:621–647. doi: 10.1146/annurev.physiol.62.1.621. [DOI] [PubMed] [Google Scholar]
- 3.Carey RM. Theodore Cooper Lecture: Renal dopamine system: paracrine regulator of sodium homeostasis and blood pressure. Hypertension. 2001;38:297–302. doi: 10.1161/hy0901.096422. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Villar VA, Armando I, Eisner GM, Felder RA, Jose PA. Dopamine, kidney, and hypertension: studies in dopamine receptor knockout mice. Pediatr Nephrol. 208;23:2131–2146. doi: 10.1007/s00467-008-0901-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zelenina M, Zelenin S, Bondar AA, Brismar H, Aperia A. Water permeability of aquaporin-4 is decreased by protein kinase C and dopamine. Am J Physiol Renal Physiol. 2002;283:F309–318. doi: 10.1152/ajprenal.00260.2001. [DOI] [PubMed] [Google Scholar]
- 6.Felder RA, Jose PA. Mechanisms of disease: the role of GRK4 in the etiology of essential hypertension and salt sensitivity. Nat Clin Pract Nephrol. 2006;2:637–650. doi: 10.1038/ncpneph0301. [DOI] [PubMed] [Google Scholar]
- 7.Shin Y, Kumar U, Patel Y, Patel SC, Sidhu A. Differential expression of D2-like dopamine receptors in the kidney of the spontaneously hypertensive rat. J Hypertens. 2003;21:199–207. doi: 10.1097/00004872-200301000-00030. [DOI] [PubMed] [Google Scholar]
- 8.Sidhu A, Kumar U, Uh M, Patel S. Diminished expression of renal dopamine D1A receptors in the kidney inner medulla of the spontaneously hypertensive rat. J Hypertens. 1998;16:601–608. doi: 10.1097/00004872-199816050-00007. [DOI] [PubMed] [Google Scholar]
- 9.Helkamaa T, Mannisto PT, Rauhala P, Cheng ZJ, Finckenberg P, Huotari M, Gogos JA, Karayiorgou M, Mervaala EM. Resistance to salt-induced hypertension in catechol-O-methyltransferase-gene-disrupted mice. J Hypertens. 2003;21:2365–2374. doi: 10.1097/00004872-200312000-00026. [DOI] [PubMed] [Google Scholar]
- 10.Armando I, Wang X, Villar VA, Jones JE, Asico LD, Escano C, Jose PA. Reactive oxygen species-dependent hypertension in dopamine D2 receptor-deficient mice. Hypertension. 2007;49:672–678. doi: 10.1161/01.HYP.0000254486.00883.3d. [DOI] [PubMed] [Google Scholar]
- 11.Yang Z, Asico LD, Yu P, Wang Z, Jones JE, Escano CS, Wang X, Quinn MT, Sibley DR, Romero GG, Felder RA, Jose PA. D5 dopamine receptor regulation of reactive oxygen species production, NADPH oxidase, and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R96–R104. doi: 10.1152/ajpregu.00434.2005. [DOI] [PubMed] [Google Scholar]
- 12.Needleman P, Douglas JR, Jr, Jakschik B, Stoecklein PB, Johnson EM., Jr Release of renal prostaglandin by catecholamines: relationship to renal endocrine function. J Pharmacol Exp Ther. 1974;188:453–460. [PubMed] [Google Scholar]
- 13.Tai HH, Tai CL, Hollander CS. Biosynthesis of prostaglandins in rabbit kidney medulla. Properties of prostaglandin synthase. Biochem J. 1976;154:257–264. doi: 10.1042/bj1540257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris RC. COX-2 and the kidney. J Cardiovasc Pharmacol. 2006;47 (Suppl 1):S37–42. doi: 10.1097/00005344-200605001-00007. [DOI] [PubMed] [Google Scholar]
- 15.Zhang MZ, Yao B, Fang X, Wang S, Smith JP, Harris RC. Intrarenal dopaminergic system regulates renin expression. Hypertension. 2009;53:564–570. doi: 10.1161/HYPERTENSIONAHA.108.127035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng HF, Wang JL, Zhang MZ, Miyazaki Y, Ichikawa I, McKanna JA, Harris RC. Angiotensin II attenuates renal cortical cyclooxygenase-2 expression. J Clin Invest. 1999;103:953–961. doi: 10.1172/JCI5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao B, Harris RC, Zhang MZ. Interactions between 11beta-hydroxysteroid dehydrogenase and COX-2 in kidney. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1767–1773. doi: 10.1152/ajpregu.00786.2004. [DOI] [PubMed] [Google Scholar]
- 18.Morrow JD, Roberts LJ. The isoprostanes: Unique bioactive products of lipid peroxidation. Progress in Lipid Research. 1997;36:1–21. doi: 10.1016/s0163-7827(97)00001-5. [DOI] [PubMed] [Google Scholar]
- 19.Zhang MZ, Yao B, Cheng HF, Wang SW, Inagami T, Harris RC. Renal cortical cyclooxygenase 2 expression is differentially regulated by angiotensin II AT(1) and AT(2) receptors. Proc Natl Acad Sci U S A. 2006;103:16045–16050. doi: 10.1073/pnas.0602176103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang MZ, Wang JL, Cheng HF, Harris RC, McKanna JA. Cyclooxygenase-2 in rat nephron development. Am J Physiol. 1997;273:F994–1002. doi: 10.1152/ajprenal.1997.273.6.F994. [DOI] [PubMed] [Google Scholar]
- 21.Harris RC, McKanna JA, Akai Y, Jacobson HR, Dubois RN, Breyer MD. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest. 1994;94:2504–2510. doi: 10.1172/JCI117620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang MZ, Yao B, McKanna JA, Harris RC. Cross talk between the intrarenal dopaminergic and cyclooxygenase-2 systems. Am J Physiol Renal Physiol. 2005;288:F840–845. doi: 10.1152/ajprenal.00240.2004. [DOI] [PubMed] [Google Scholar]
- 23.Fujii A, Nakano D, Katsuragi M, Ohkita M, Takaoka M, Ohno Y, Matsumura Y. Role of gp91phox-containing NADPH oxidase in the deoxycorticosterone acetate-salt-induced hypertension. European Journal of Pharmacology. 2006;552:131–134. doi: 10.1016/j.ejphar.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 24.Wang HD, Xu S, Johns DG, Du Y, Quinn MT, Cayatte AJ, Cohen RA. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ Res. 2001;88:947–953. doi: 10.1161/hh0901.089987. [DOI] [PubMed] [Google Scholar]
- 25.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen B, Hagiwara M, Yao Y-Y, Chao L, Chao J. Salutary Effect of kallistatin in salt-induced renal injury, inflammation, and fibrosis via antioxidative stress. Hypertension. 2008;51:1358–1365. doi: 10.1161/HYPERTENSIONAHA.107.108514. [DOI] [PubMed] [Google Scholar]
- 27.Zhang A, Jia Z, Guo X, Yang T. Aldosterone induces epithelial-mesenchymal transition via ROS of mitochondrial origin. Am J Physiol Renal Physiol. 2007;293:F723–731. doi: 10.1152/ajprenal.00480.2006. [DOI] [PubMed] [Google Scholar]
- 28.Yasunari K, Kohno M, Kano H, Minami M, Yoshikawa J. Dopamine as a novel antioxidative agent for rat vascular smooth muscle cells through dopamine D(1)-like receptors. Circulation. 2000;101:2302–2308. doi: 10.1161/01.cir.101.19.2302. [DOI] [PubMed] [Google Scholar]
- 29.Cosentino M, Rasini E, Colombo C, Marino F, Blandini F, Ferrari M, Samuele A, Lecchini S, Nappi G, Frigo G. Dopaminergic modulation of oxidative stress and apoptosis in human peripheral blood lymphocytes: evidence for a D1-like receptor-dependent protective effect. Free Radical Biology and Medicine. 2004;36:1233–1240. doi: 10.1016/j.freeradbiomed.2004.02.065. [DOI] [PubMed] [Google Scholar]
- 30.Asghar M, Chillar A, Lokhandwala MF. Renal proximal tubules from old Fischer 344 rats grow into epithelial cells in cultures and exhibit increased oxidative stress and reduced D1 receptor function. Am J Physiol Cell Physiol. 2008;295:C1326–1331. doi: 10.1152/ajpcell.00367.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banday AA, Lau Y-S, Lokhandwala MF. Oxidative stress causes renal dopamine D1 receptor dysfunction and salt-sensitive hypertension in Sprague-Dawley rats. Hypertension. 2008;51:367–375. doi: 10.1161/HYPERTENSIONAHA.107.102111. [DOI] [PubMed] [Google Scholar]
- 32.Zeng C, Villar VAM, Yu P, Zhou L, Jose PA. Reactive oxygen species and dopamine receptor function in essential hypertension. Clinical and Experimental Hypertension. 2009;31:156 – 178. doi: 10.1080/10641960802621283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang MZ, Hao CM, Breyer MD, Harris RC, McKanna JA. Mineralocorticoid regulation of cyclooxygenase-2 expression in rat renal medulla. Am J Physiol Renal Physiol. 2002;283:F509–516. doi: 10.1152/ajprenal.00236.2001. [DOI] [PubMed] [Google Scholar]
- 34.Stokes JB. Integrated actions of renal medullary prostaglandins in the control of water excretion. Am J Physiol. 1981;240:F471–480. doi: 10.1152/ajprenal.1981.240.6.F471. [DOI] [PubMed] [Google Scholar]
- 35.Horton R, Bughi S, Jost-Vu E, Antonipillai I, Nadler J. Effect of dopamine on renal blood flow, prostaglandins, renin and electrolyte excretion in normal and hypertensive humans. Am J Hypertens. 1990;3:108S–111S. doi: 10.1093/ajh/3.6.108s. [DOI] [PubMed] [Google Scholar]
- 36.Huo T, Ye MQ, Healy DP. Characterization of a dopamine receptor (DA2K) in the kidney inner medulla. Proc Natl Acad Sci U S A. 1991;88:3170–3174. doi: 10.1073/pnas.88.8.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez F, Llinas MT, Gonzalez JD, Rivera J, Salazar FJ. Renal changes induced by a cyclooxygenase-2 inhibitor during normal and low sodium intake. Hypertension. 2000;36:276–281. doi: 10.1161/01.hyp.36.2.276. [DOI] [PubMed] [Google Scholar]
- 38.Viel EC, Benkirane K, Javeshghani D, Touyz RM, Schiffrin EL. Xanthine oxidase and mitochondria contribute to vascular superoxide anion generation in DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol. 2008;295:H281–288. doi: 10.1152/ajpheart.00304.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu R, Millette E, Wu L, de Champlain J. Enhanced superoxide anion formation in vascular tissues from spontaneously hypertensive and desoxycorticosterone acetate-salt hypertensive rats. J Hypertens. 2001;19:741–748. doi: 10.1097/00004872-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Yang Z, Asico LD, Yu P, Wang Z, Jones JE, Bai RK, Sibley DR, Felder RA, Jose PA. D5 dopamine receptor regulation of phospholipase D. Am J Physiol Heart Circ Physiol. 2005;288:H55–61. doi: 10.1152/ajpheart.00627.2004. [DOI] [PubMed] [Google Scholar]
- 41.Jose PA, Eisner GM, Felder RA. Dopamine and the kidney: a role in hypertension? Curr Opin Nephrol Hypertens. 2003;12:189–194. doi: 10.1097/00041552-200303000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Pedrosa R, Jose PA, Soares-da-Silva P. Defective D1-like receptor-mediated inhibition of the Cl-/HCO3- exchanger in immortalized SHR proximal tubular epithelial cells. Am J Physiol Renal Physiol. 2004;286:F1120–1126. doi: 10.1152/ajprenal.00433.2003. [DOI] [PubMed] [Google Scholar]
- 43.Cheng HF, Becker BN, Harris RC. Dopamine decreases expression of type-1 angiotensin II receptors in renal proximal tubule. J Clin Invest. 1996;97:2745–2752. doi: 10.1172/JCI118729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guyton AC. Blood pressure control--special role of the kidneys and body fluids. Science. 1991;252:1813–1816. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- 45.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]