Abstract
PURPOSE
Inflammatory cytokines have been implicated in the progression of HNSCC. Herein we investigate the mechanisms by which IL-1β might contribute to EMT in HNSCC.
EXPERIMENTAL DESIGN
We evaluated the effect of IL-1β on the molecular events of EMT in surgical specimens and HNSCC cell lines. We examined the correlation with tumor histologic features, and a SCID xenograft model was used to assess the effects of Snail overexpression.
RESULTS
COX-2-dependent pathways contribute to the modulation of E-cadherin expression in HNSCC. An inverse relationship between COX-2 and E-cadherin was demonstrated in situ by double immunohistochemical staining of human HNSCC tissue sections. Treatment of HNSCC cells with IL-1β, caused the downregulation of E-cadherin expression and upregulation of COX-2 expression. This effect was blocked in the presence of COX-2 shRNA. IL-1β -treated HNSCC cell lines demonstrated a significant decrease in E-cadherin mRNA and an increase in the mRNA expression of the transcriptional repressor Snail. IL-1β exposure led to enhanced Snail binding at the chromatin level. ShRNA-mediated knockdown of Snail interrupted the capacity of IL-1β to downregulate E-cadherin. In a SCID xenograft model, HNSCC Snail overexpressing cells demonstrated significantly increased primary and metastatic tumor burdens.
CONCLUSIONS
IL-1β modulates Snail and thereby regulates COX-2-dependent E-cadherin expression in HNSCC. This is the first report indicating the role of Snail in the inflammation-induced promotion of EMT in HNSCC. This newly defined pathway for transcriptional regulation of E-cadherin in HNSCC has important implications for targeted chemoprevention and therapy.
Introduction
Head and neck squamous cell carcinoma (HNSCC), is the sixth most common cancer in the world, and affects 50,000 Americans annually. Patients with HNSCC are at considerable risk of mortality, with more than 300,000 deaths attributable to the disease per year (1). The major causes of HNSCC-related deaths are cervical node and distant metastasis. The 5-year overall survival is reduced by approximately 50% in patients with cervical lymph node metastases (2). Delineation of the mechanisms involved in these metastases and identification of molecular markers that can pinpoint patients with biologically aggressive tumors will be of the utmost importance for effective management of HNSCC patients.
Inflammatory mediators and inflammatory cells are dysregulated in smokers and patients with tobacco related malignancies such as HNSCC (3). A chronic increase in inflammatory mediators in the oral cavity and oropharynx can lead to increased tumor promotion, invasion, angiogenesis and metastasis (4). Inflammatory cytokines, growth factors and mediators released in the tumor microenvironment include prostaglandin E2 (PGE2) and interleukin-1 (IL-1). IL-1 has been shown to induce activation of signal transduction pathways that regulate several early transcription factors involved in the transcription of proinflammatory cytokine genes. IL-1α is known to induce the activation of immediate-early transcription factors and genes that promote the survival and proliferation of HNSCC (5, 6, 7). This suggests that IL-1α may serve as an important autocrine and/or exocrine factor in coordinating expression of this repertoire of cytokines in HNSCC. IL-1β has also been implicated in the progression of HNSCC. Increased secretion of IL-1B has been shown to be the profile of resistant or progressing oral tumors (8, 9). IL-1β is one of several cytokines known to potently up regulate COX-2 expression in a variety of cells (5, 6, 10, 11). Tumor COX-2 and its metabolite PGE2 play important roles in regulating diverse cellular functions under physiological and pathological conditions (12, 13, 14).
Loss of E-cadherin is frequently observed at sites of EMT during cancer development and progression, and is closely correlated with poor prognosis (15, 16, 17, 18). Several E-cadherin transcriptional repressors have been characterized (ZEB1, Snail, E12/E47, Slug, Twist, and SIP-1). In head and neck tissues, both malignancy and local recurrence following treatment have been associated with a gene expression signature that includes the zinc-finger E-box-binding transcriptional inhibitor Snail (19). Recently, Lyons et al reported that Snail up regulates proinflammatory mediators in oral keratinocytes, which have been shown to correlate with malignancy (20). Herein, we demonstrate that proinflammatory mediators up regulate Snail, thus further defining the cycle by which inflammation promotes tumor progression. We report that IL-1β upregulates Snail and suppresses E-cadherin in a Cox-2-dependent manner. Immunohistochemical staining of HNSCC tissue sections confirm that these relationships exist in situ. This is the first report implicating inflammation dependent regulation of E-cadherin transcriptional repressors in head and neck cancer.
Materials and Methods
Reagents and cell lines
Recombinant human IL-1β was purchased from (BD Bioscience). 16,16-dimethyl-PGE2 was purchased from Cayman Chemicals (Ann Arbor, MI). Recombinant human IL-1α was purchased from ProSpec Protein Specialists (Rehovot, Israel). IL-1β and IL-1α were dissolved in the diluent 0.1% BSA in 1x PBS. Other reagents were purchased from Sigma Chemicals (St. Louis, MO) unless otherwise specified. HNSCC cells utilized in this study included: Tu686, Tu212 (generously provided by Dr. D. Shin (21); and OSC, HOC, and TSU (generously provided by. Dr. M. Nagayama (22). SNAIL sense (SNAIL-S) and pLHCX (vector alone) clones were generated for Tu686 and OSC cell lines using retroviral transfection as previously described (13, 14). Briefly, for each cell line, an approximate 10- fold higher level of SNAIL was noted in SNAIL-S compared to parental or vector controls (13). These cells were then expanded for further studies. The following cell line terminology is used in the text: 1) Tu-686 SNAIL-S and OSC SNAIL-S are the cell lines transfected with SNAIL in the sense orientation and (2) Tu686-V and OSC-V are the cells transfected with the expression vector pLHCX alone. E-cadherin over expressing cells were generated as follows: wild-type E-cadherin cDNA pcDNA3.1 (a generous gift from A.S.T.Wong and B.M.Gumbiner, University of Virginia, Charlottesville, VA) was excised from the plasmid with HindIII and XbaI and subcloned into PCR3.1 vector (Invitrogen, Carlsbad, CA). 2) A 2.7-kb E-cadherin cDNA was further excised from PCR3.1 construct with PmeI and HindIII and subcloned into the retrovirus vector pLHCX (Clontech, Mountain view, CA), which contains the CMV promoter for controlling transcription of the cDNA insert and hygromycin (Mediatech, Herndon, VA) resistance gene for selection (13). Snail over expressing cells were generated as follows: wild-type Snail cDNA pcDNA3 (a generous gift from Dr. E. Fearon, University of Michigan) was excised from the plasmid with HindIII and EcoRV and subcloned into the retrovirus vector pLHCX. All constructs were verified by restriction endonuclease digestion. For virus production, 70 percent confluent 293T cells were cotransfected with pLHCX-Snail or pLHCX-E-cadherin and pLHCX (vector alone). Tumor cells were then transduced with high-titer supernatants producing either Snail, E-cadherin or pLHCX virus. Following transduction, the tumor cells were characterized by Western blot for expression of Snail or E-cadherin.
Western Blot analysis
HNSCC cells were washed with PBS and whole cell lysate was prepared with modified RIPA buffer at 4° C for 15 min. The cell lysates were centrifuged at 13,000 rpm for 10 min and the supernatant collected. Protein concentration was measured with a protein assay reagent (Bio-Rad, Hercules,CA). Protein for E-cadherin (20µg), COX-2 (20 µg), and Snail (50 µg) were resolved by SDS/PAGE and analyzed by Western blot using PVDF membranes (Millipore, Bedford, CA) according to the manufacturer’s instructions. Membranes were blocked with 5% non-fat dry milk in TBS + 0.1% Tween 20 (TBST). The membranes were probed with anti-E-cadherin antibody (BD Biosciences Pharmingen/Transduction Laboratories, San Jose, CA) at 1:2,500 dilution and anti-Cox-2 antibody (Santa Cruz Biotechnology) at 1: 1,000 dilution in TBST containing 1.0% nonfat dry milk. The membranes were developed by the ECL chemiluminescence system (Amersham Pharmacia Biotech, Piscataway, NJ) and exposed to x-ray film (Optimum Brand X-Ray Film). Equal loading of samples was confirmed by probing the membranes with β-actin or GAPDH antibody.
Total RNA preparation, cDNA synthesis, and real-time PCR
To analyze the COX-2/PGE2 dependent regulation of E-cadherin, Snail mRNA expression, total RNA from 1×106 control and Il-1β (200 U) treated HNSCC cells were extracted using Trizol reagent according to the manufacturer’s instructions (Invitrogen). The cDNA was prepared with a kit (Invitrogen) according to the manufacturer’s instructions. E-cadherin and Snail mRNA levels were quantified by real time RT-PCR using the SYBR Green quantitative PCR kit from Bio Rad in a MyiQ Cycler (Bio Rad) following the manufacture’s protocol. Amplification was carried out in a total volume of 20 µl for 40 cycles of 15 seconds at 95° C, 20 seconds at 60°C and 30 seconds at 72°C. Samples were run in triplicate and their relative expression was determined by normalizing expression of each target either to glyceraldehyde-3-phosphate dehydrogenase (G3PDH) or β-actin. These were then compared to the normalized expression in a reference sample to calculate a fold change value. Primers were designed as previously described (23). Primer sequences were as follows: Human G3PDH 5’ TGCACCACCAACTGCTTAGC -3’, and 5’GGCATGGACTGTGGTCATGAG-3’; β-actin 5' GATGAGATTGGCATGGCTTT- 3'; and 5' -CACCTTCACCGTTCCAGTTT- 3'; human E-cadherin 5’ CGGGAATGCAGTTGAGGATC-3’ and 5’AGGATGGTGTAAGCGATGGC3’; and human Snail 5’ CGCGCTCTTTCCTCGTCAG-3’ and 5’-TCCCAGATGAGCATTGGCAG-3’.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation analysis was performed using the ChIP –IT Enzymatic kit (Active Motif, Carlsbad, CA) following the manufacturer’s protocol. Briefly, control Tu686, Il-1β treated Tu686 cells, control OSC cells, and Il-1β treated OSC, were grown to 70–80% confluence on 100mM dishes. The cells were first fixed with 1.0- % formaldehyde and were used for chromatin preparation as described in the manufacturer’s protocol. Chromatin were then pre-cleared with protein G beads for 2 hours at 4°C. Antibodies to Snail and control IgG (normal goat IgG) were then added to the pre-cleared chromatin and incubated overnight at 4°C. Subsequently, protein G beads were added to the immunoprecipitated chromatin DNA and incubated for 1.5 hours at 4°C. The beads were then collected by centrifugation and washed extensively. The cross-linked DNA then eluted from protein G beads. The eluted cross-linked protein-DNA complexes were treated with RNase A at 65°C overnight for the removal of RNA and then treated with proteinase K at 42°C for 2 hours to reverse protein –DNA complex. The resulting DNA was purified by columns and then subjected to PCR analysis. PCR reactions were performed with the following human E-cadherin promoter primers: forward 5 ’–GGCCGGCAGGTGAACCCTCA- 3 ’, reverse 5 ’-GGGCTGGAGTCTGAACTGA-3’ (Accession number L34545).
RNA Interference with shRNA
The Hush 29mer shRNA constructs against Human Snail1 were purchased from ORIGENE (Rockville, MD 20850, Cal # TG309226). The shRNA constructs against Human Cox-2 were purchased from GeneCopoeia (Germantown, Maryland). The plasmids were co-transfected with the Amphotropic (package plasmid) into 293T cells using the Calcium Phosphate Transfection Kit (Invitrogen). The supernatant was harvested 36 hours after transfection and used to infect HNSCC cells (Tu212 and Tu686) for 6 hours. The cells were then selected in 1ug/ml puromycin containing medium until all the control cells died.
Immunohistochemistry
With Institutional Review Board (IRB) approval, immunohistochemistry was performed on formalin-fixed, paraffin-embedded HNSCC tissues from the UCLA Pathology Department archives. Twenty-four head and neck squamous cell carcinoma specimens were obtained anonymously and randomly. They comprised 8 well differentiated, 8 moderately differentiated, and 8 poorly differentiated tumors. Tissue sections (4µm-thick) were cut, deparaffinized in xylene, rehydrated in alcohols and washed twice with water. Samples were then incubated in 0.01M Citrate buffer (pH 6.0) for 25 minutes in a steamer to unmask antigens as previously described (24, 25). Following cooling to room temperature and rinsing with dH2O, samples were treated for 15 minutes with 3% H2O2 diluted in methanol. Tissue sections were washed in dH2O, then PBS, then blocked with 10% normal horse serum for 30 minutes at room temperature. For COX-2 and E-cadherin co-staining, the sections were first stained for COX-2, followed by E-cadherin. The sections were incubated with goat anti-human COX-2 polyclonal IgG 1.0 µg/ml (Santa Cruz Biotechnology) overnight at 4° C, rinsed, and incubated for 40 minutes at room temperature with horse anti-goat IgG-biotin 7.5 µg/ml (Vector Laboratories, Inc., Burlingame, CA) (26). Samples were then incubated for 30 minutes at room temperature, with Avidin-HRP diluted 1/1,000 in PBS (Vector Laboratories), washed, and treated with Nickel DAB (DAB kit, Vector laboratories) for black color development to augment contrasting color in double stained slides. Samples were extensively washed in PBS (3 ×, 5 minutes each) in preparation for E-cadherin staining. Samples were incubated overnight at 4° C with 250 µg/ml mouse anti-human E-cadherin diluted in normal horse serum (BD Transduction Biosciences, San Diego, CA). After extensive rinsing with PBS, samples were incubated for 40 minutes with 7.5 µg/ml horse anti-mouse IgG-biotin (Vector Laboratories) and rinsed with PBS. Samples were then incubated for 30 minutes at room temperature with the Vectastain ABC- kit (Vector Laboratories), followed by PBS washing, and then incubated with an alkaline phosphatase substrate kit (Vector Laboratories). Color development was followed under the microscope for 20 minutes. The color reaction was stopped by rinsing with dH2O. Samples were counterstained with hematoxylin. Normal human kidney was used as a positive control for both COX-2 and E-cadherin staining. Negative controls included incubation with non-immune pooled rabbit or goat IgG (rabbit IgG was from Vector Laboratories and goat IgG was from Zymed, Invitrogen) at the same concentration as the primary antibody.
Single staining for Snail and COX-2 and double staining for Snail and E-cadherin were performed (n=24) essentially as described above with the following modifications. Goat anti-human Snail polyclonal IgG (1:50 dilution, Abcam) was utilized for Snail immunohistochemistry. All slides were reviewed by two of the investigators (MCF and CL). The following findings were recorded for each slide: 1) % cells positive for each stain 2) intensity of stain (0 to +3). We first examined the relationship between E-cadherin and Snail using the ordinal IHC results (0+, 1+, etc) and found that they are significantly negatively correlated (rho=−0.52, p=0.02). Next, the staining for E-cadherin and Snail were dichotomized as positive (2+ or 3+) or negative (0+,1+). Using Fisher’s exact test we find that E-cadherin and Snail had a significant association (p=0.0006) after adding tumor differentiation status.
In vivo Mouse Model of HNSCC metastasis
Pathogen-free SCID Beige CB17 (8–12 weeks of age) mice were obtained from Charles River Laboratories (Wilmington, MA), and maintained in the West Los Angeles VA Animal Research vivarium. All studies were approved by the institution’s animal studies review board. Five million HNSCC (Tu686-S or Tu686-V) were implanted via subcutaneous injection on the right supra scapular area of the SCID mice. Tumor growth was assessed three times each week following tumor implantation. Two bisecting diameters of each tumor were measured with calipers, and the volume was calculated using the formula 0.4 × ab2, where a represents the larger diameter, and b represents the smaller diameter. Each group consisted of eight animals. At the conclusion of the experiment (day 48), the animals were sacrificed and tumors removed for CXCL8 (antibody pairs, R&D) CXCL5 (antibody pairs, R&D) and VEGF (Chemicon kit) quantification by ELISA in the homogenized tumor lysates.
To assess the metastatic potential of the HNSCC cells in SCID mice, a single cell suspension was prepared from lungs and livers in collagenase digestion buffer (type IV collagenase, 1mg/ml (Sigma), DNase 50U/ml, in RPMI media) for 45 minutes at 37°C. A single cell suspension of lungs or livers from tumor bearing or naïve non-tumor controls were stained by CXCR4 (R&D) to detect HNSCC by FACScan flow cytometer (Becton Dickinson, San Jose, CA) at the UCLA Jonsson Comprehensive Cancer Center Flow Cytometry Core facility. 10,000 gated events were collected and analyzed using CellQuest software (BD). Unstained cells and cells stained by isotype-matched antibodies served as controls.
Statistics
Animal experiments were repeated twice. All other experiments presented were repeated at least three times and measurements were performed in triplicate. When applicable, data are present as the mean ± s.d. The significance of the difference between groups was evaluated with the Student’s t-test or χ2 test. P < 0.05 was considered significant. The biostatistics program S-plus version 8 (Insightful) was utilized for all statistical analyses.
Results
IL-1β up regulates Snail and down regulates E-cadherin expression in HNSCC
Interleukin IL-1β has been implicated in the progression of tobacco related malignancies and is one of several cytokines known to potently up regulate COX-2 expression in a variety of cells (10, 11). We examined the effects of adding IL-1β on COX-2 expression in Tu686, Tu212, and OSC HNSCC cells. These HNSCC cell lines are used for all of the subsequent experiments unless otherwise specified. IL-1β caused the up regulation of COX-2 expression in these cell lines in a concentration-dependent manner (Figure 1A). In addition to assessing COX-2 expression using Western blot analysis, we performed PGE2 assays. Upon treatment with IL-1β, significant increases in PGE2 levels were noted (Table 1). As IL-1α has been shown to induce the activation of immediate-early transcription factors and genes that promote the survival and proliferation of cytokines that mediate inflammatory responses (7, 8, 9), we also examined the effect of IL-1α on E-cadherin, COX-2, and Snail levels in the Tu686, Tu212, and OSC HNSCC cell lines. We repeated the exact protocols we used for IL-1β, but we saw no change in the expression levels of E-cadherin, COX-2, or Snail (data not shown).
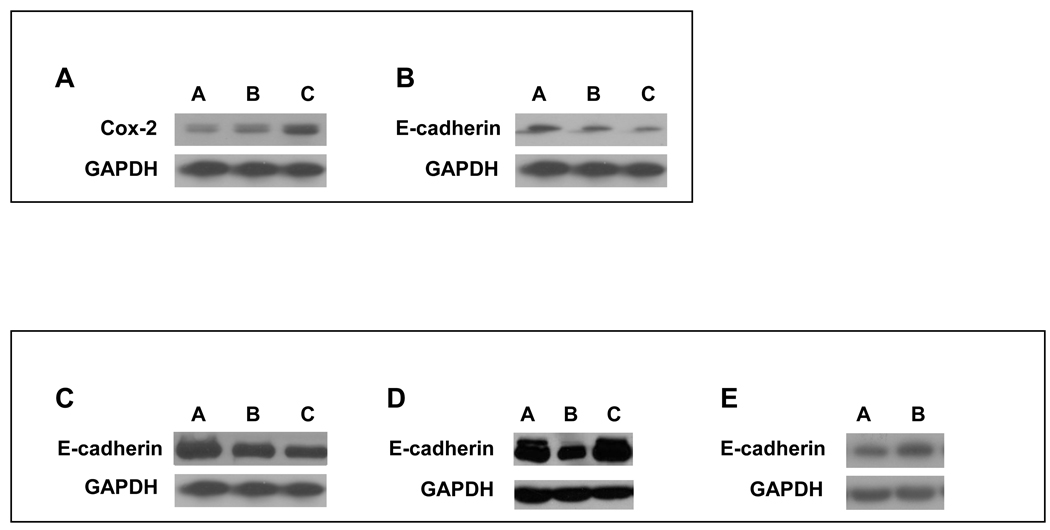
Figure 1. IL-1β -dependent regulation of COX-2 and E-cadherin in HNSCC.
IL-1β regulates expression of COX-2 and E-cadherin in a dose dependent manner. Tu686 cells were treated with the indicated concentrations of IL-1β for 18 h. Protein from whole cell lysates of was analyzed for COX-2 and E-cadherin expression by Western Blot as described in Materials and Methods. (A) IL-1β up-regulates COX-2 expression in a concentration-dependent manner. Lane A: no treatment; Lane B: IL-1β (100 U/ml); Lane C: IL-1β (200 U/ml). (B) IL-1β causes down-regulation of E-cadherin expression in a concentration-dependent manner. Lane A: no treatment; Lane B: IL-1β (100 U/ml); Lane C: IL-1β (200 U/ml). (C) Upon addition of celecoxib, E-cadherin is no longer down-regulated in Tu686 HNSCC cells indicating that functional COX-2/PGE2 is required for its down regulation. Lane A: IL-1β (100 U/ml) + celecoxib (1µM); Lane B: diluent (0.1% BSA in 1x PBS) alone; Lane C: IL-1β (100 U/ml). (D) In the presence of COX-2 shRNA, E-cadherin is no longer down-regulated in Tu686 HNSCC cells indicating that functional COX-2/PGE2 is required for its down regulation. Lane A: diluent (0.1% BSA in 1x PBS) alone; Lane B: IL-1β (100 U/ml); Lane C: IL-1β (100 U/ml) + COX-2 shRNA. (E) PGE2 causes the down-regulation of E-cadherin expression in Tu686 cell lines. Lane A: PGE2 (10µg/ml); Lane B: diluent (0.1% BSA in 1x PBS) alone.
Table 1. IL-1β-dependent regulation of COX-2/PGE2.
HOC, TSU, and Tu686 HNSCC cells were stimulated with IL-1β (100 and 200 U/ml) for 18 hours. PGE2 concentration was then measured by EIA. Upon treatment with IL-1β, significant increases in PGE2 levels were noted Mean values are reported. All measurements were made in triplicate and repeated in at least 3 separate experiments.
| PGE2 levels (pg/ml) |
|||
|---|---|---|---|
| Cell Line | Control | IL-1β 100 U/ml | IL-1β 200 U/ml |
| HOC | 0 | 366 ± 13a | 440 ± 11a,b |
| TSU | 70 ± 3.5 | 303 ± 10a | 413 ± 9.6a,b |
| Tu686 | 8 ± 0.6 | 15 ± 1a | 35 ± 1.9a,b |
p<0.001 vs. Control
p<0.01 vs. 100U/ml
We examined E-cadherin levels in HNSCC cells treated with IL-1β and discovered that its expression was down-regulated in a concentration-dependent manner (Figure 1B). To establish that the down-regulation of E-cadherin was due to a COX-2 dependent mechanism, we added the COX-2 inhibitor celecoxib (1 µM) to cells prior to treatment with IL-1β. Upon addition of celecoxib, E-cadherin was no longer down-regulated indicating that functional COX-2 is required for its down regulation (Figure 1C).
In order to determine more definitively the import of COX-2 in the IL-1β induced reduction of E-cadherin, we used shRNA to knockdown COX-2 expression in Tu686 and Tu212 cells. When the HNSCC cells were transfected with COX-2 shRNA, E-cadherin was no longer down-regulated (Figure 1D). This supports the celecoxib data and more definitively indicates that functional COX-2 is required for IL-1βmediated E-cadherin down regulation. We also treated HNSCC cells directly with PGE2 (16,16-dimethyl-PGE2: 2µg/ml, 5µg/ml, and10µg/ml) and consistent with the celecoxib results, noted a significant decrease in E-cadherin levels (Figure 1E). This decrease in E-cadherin levels was associated with an increase in N-cadherin levels (data not shown). Immunohistochemical staining of oral tongue squamous cell carcinoma tissue sections confirmed that these relationships exist in situ. There is reciprocal expression of E-cadherin and COX-2 in HNSCC in these specimens (Figure 2).
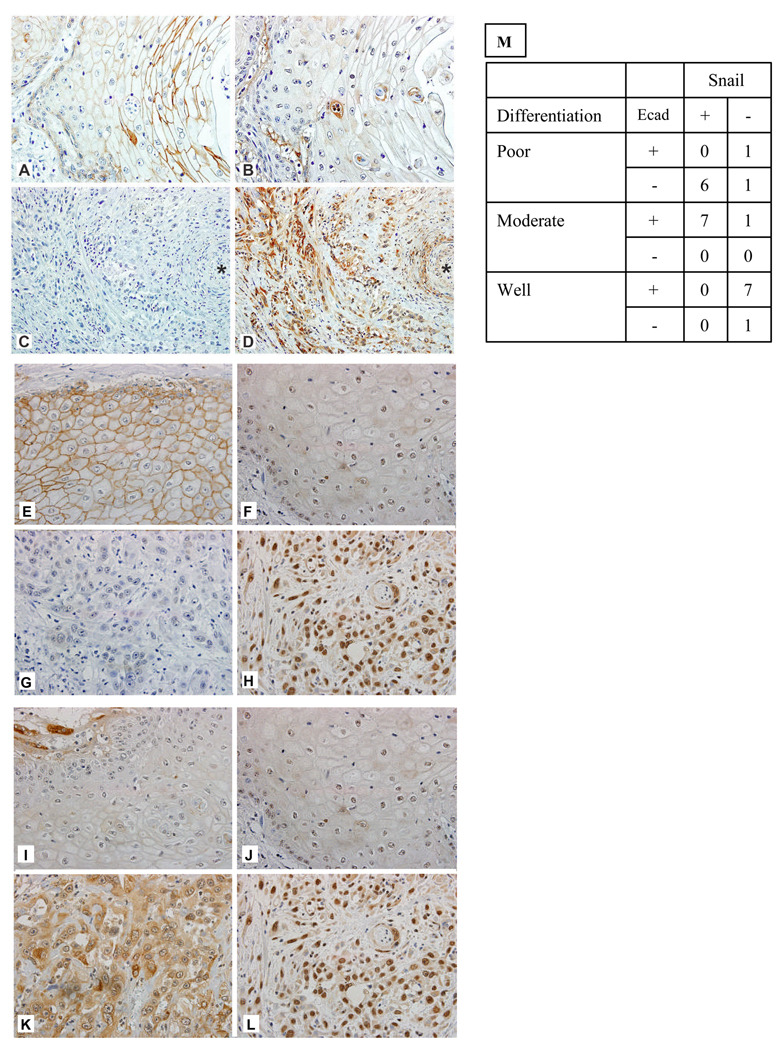
Figure 2. Distinct patterns of COX-2, E-cadherin and Snail expression in HNSCC.
Reciprocal expression of E-cadherin and COX-2; E-cadherin and Snail; and co expression of COX-2 and Snail is evident in HNSCC cells in situ. (Panels A–D): Distinct patterns of COX-2 and E-cadherin expression in HNSCC. (A) E-cadherin immunohistochemical staining of a well differentiated squamous cell carcinoma (original magnification, 400x) demonstrates strong membranous staining, especially as the cells become more squamoid. (B) Cox-2 immunohistochemical staining of corresponding section of previously shown well-differentiated squamous cell carcinoma (original magnification, 400x) exhibits faint, nonspecific intracytoplasmic staining.
(C) E-cadherin immunohistochemical staining of a poorly differentiated squamous cell carcinoma (original magnification, 200x) shows no staining of tumor cells. (D) Cox-2 immunohistochemical staining of corresponding section of previously shown poorly differentiated squamous cell carcinoma (original magnification, 200x) reveals strong, intracytoplasmic staining of tumor cells. Asterisks (*) for C and D indicate the same artery for each of the corresponding sections. (Panels E–H): Distinct patterns of E-cadherin and Snail expression in HNSCC. (E) E-cadherin immunohistochemical staining of a well differentiated squamous cell carcinoma (original magnification, 400x) demonstrates strong membranous staining, especially as the cells become more squamoid. (F) Snail immunohistochemical staining of corresponding section of previously shown well-differentiated squamous cell carcinoma (original magnification, 400x) exhibits faint, nonspecific staining.
(G) E-cadherin immunohistochemical staining of a poorly differentiated squamous cell carcinoma (original magnification, 200x) shows no staining of tumor cells. (H) Snail immunohistochemical staining of corresponding section of previously shown poorly differentiated squamous cell carcinoma (original magnification, 200x) reveals strong staining of tumor cells. (Panels I–L). Distinct patterns of COX-2 and Snail expression in HNSCC. (I) Cox-2 immunohistochemical staining of a well differentiated squamous cell carcinoma (original magnification, 400x) exhibits faint, nonspecific intracytoplasmic staining. (J) Snail immunohistochemical staining of corresponding section of previously shown well-differentiated squamous cell carcinoma (original magnification, 400x) exhibits faint, nonspecific staining. (K) Cox-2 immunohistochemical staining of corresponding section of previously shown poorly differentiated squamous cell carcinoma (original magnification, 200x) reveals strong, intracytoplasmic staining of tumor cells. (L) Snail immunohistochemical staining of corresponding section of previously shown poorly differentiated squamous cell carcinoma (original magnification, 200x) reveals strong staining of tumor cells.
(M) The following findings were recorded for each slide: 1) % cells positive for each stain 2) intensity of stain (0 to +3). We first examined the relationship between E-cadherin and Snail using the ordinal IHC results (0+, 1+, etc) and found that they are significantly negatively correlated (rho=−0.52, p=0.02). Next, the staining for E-cadherin and Snail were dichotomized as positive (2+ or 3+) or negative (0+,1+). Using Fisher’s exact test we find that E-cadherin and Snail had a significant association (p=0.0006) after adding tumor differentiation status.
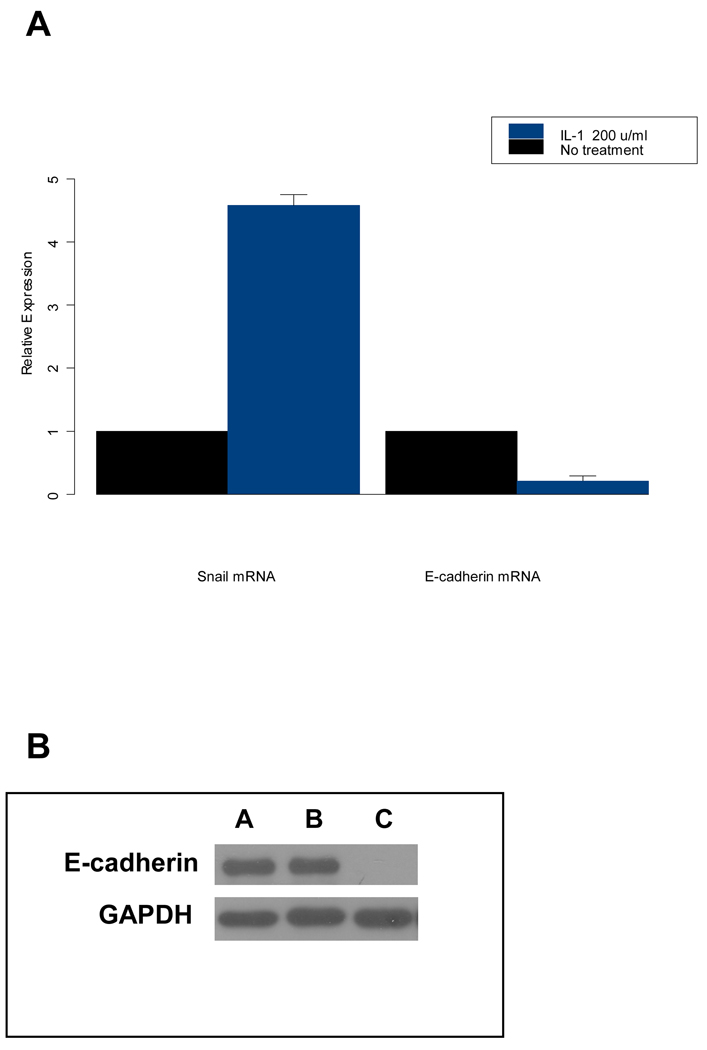
We next determined the Snail and E-cadherin mRNA expression by real-time RT-PCR in control and IL-1β treated HNSCC cell lines. When these lines were exposed to IL-1β, Snail mRNA expression levels were elevated. Consistent with these findings, E-cadherin mRNA expression was decreased under these conditions (Figure 3A). As determined by quantitative real time PCR, IL-1β down regulated E-cadherin and up regulated Snail, in a dose-dependent manner in all three cell lines.
Figure 3. IL-1β dependent regulation of E-cadherin and Snail in HNSCC.
(A) IL-1β regulates expression of E-cadherin and its transcriptional repressor Snail. Tu686 cells were treated with the indicated concentrations of IL-1β for 18 h and levels of mRNA for E-cadherin and Snail were evaluated by quantitative real time PCR analysis as described in Materials and Methods. Upon addition of IL-1β, Snail mRNA expression levels were elevated and E-cadherin mRNA expression was decreased significantly in HNSCC cell lines. Data shown for mean of three experiments; bars, SE P < 0.05 (B) Transfection of Snail into the Tu686 HNSCC cell line (that constitutively expresses high levels of E-cadherin), completely diminishes E-cadherin expression. Lane A: parental cell line; Lane B: vector control; Lane C: Snail
In order to further define the role of Snail in HNSCC, we generated genetically modified Snail over-expressing (Snail-S) HNSCC cells (as well as vector controls). We have determined that in these HNSCC cell lines that constitutively express high levels of E-cadherin, the introduction of Snail down regulates the expression of E-cadherin (Figure 3B).
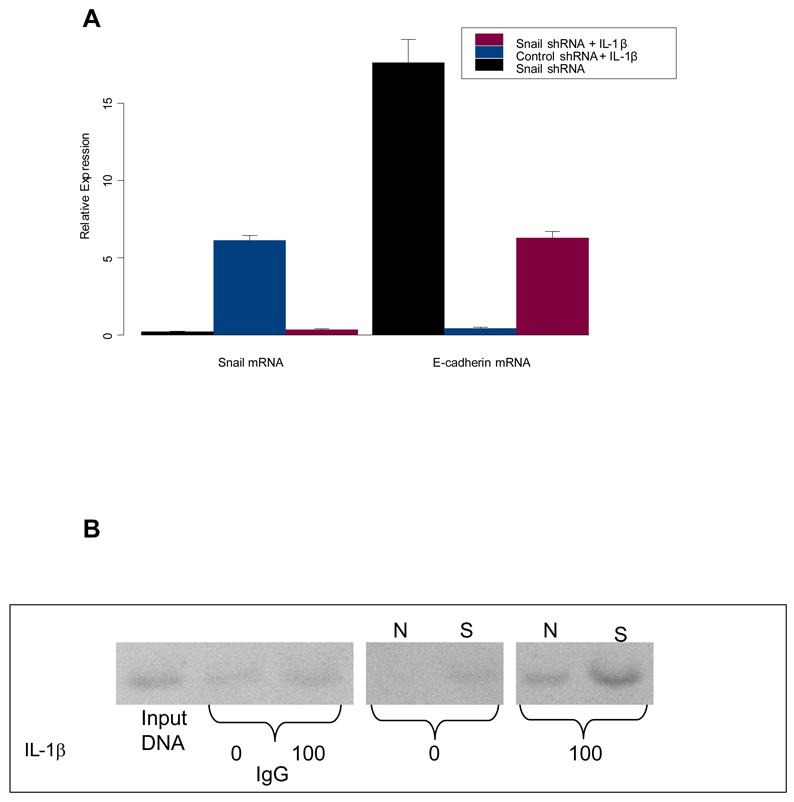
To determine the importance of Snail in the IL-1β -mediated downregulation of E-cadherin, we used small hairpin RNA (shRNA) to knockdown Snail expression in Tu686 and Tu212 cells (Figure 4A). Transfection with Snail shRNA resulted in a 4 fold reduction of Snail mRNA levels compared with control transfected cells. Concomitantly, shRNA-mediated knockdown of Snail led to an 18 fold increase in E-cadherin mRNA (Figure 4A). Treatment of HNSCC cells with IL-1β increased Snail mRNA 6 fold and decreased E-cadherin mRNA expression (0.45 fold). Knockdown of Snail expression prevented the IL-1β -mediated downregulation of E-cadherin (Figure 4A). These findings implicate IL-1β, as an autocrine or paracrine modulator of Snail and define a pathway by which COX-2 decreases E-cadherin expression in HNSCC.
Figure 4.
(A) Inhibition of Snail interrupts the IL-1β -mediated downregulation of E-cadherin in HNSCC. To determine the importance of Snail in the IL-1β -mediated down regulation of E-cadherin, we used small hairpin RNA (shRNA) to knockdown Snail expression in HNSCC cells. E-cadherin and Snail expression levels were evaluated by quantitative real time PCR analysis as described in Materials and Methods. Transfection with Snail shRNA resulted in a 4 fold reduction of Snail mRNA levels compared with control transfected cells and an 18 fold increase in E-cadherin mRNA. Treatment with IL-1β increased Snail mRNA 6 fold and decreased E-cadherin mRNA expression. Knockdown of Snail expression prevented the IL-1β -mediated down regulation of E-cadherin. The data represent the mean ± S.D. of triplicate determinations in one representative experiment out of three separate experiments; bars, SE P < 0.05.
(B) IL-1β-dependent enhancement in binding of Snail to the E-box sequences of the E-cadherin promoter. ChIP assay, Snail associates with the E-cadherin promoter at the chromatin level. Tu686 and Tu212 control and IL-1β- (100 U ˜/ml) treated cells were subjected to ChIP analysis using antibodies to Snail and control IgG as described in methods. IL-1β-(treatment resulted an amplified E-cadherin promoter fragment. S: Snail, N: Negative Control.
Snail binds to E-box elements of the E-cadherin promoter
Snail is known to bind the E-boxes present in the E-cadherin promoter and thus repress E-cadherin transcription. In order to test whether the IL-1β -mediated elevation in Snail is associated with an increase in binding to the E-cadherin promoter, we examined the binding of Snail to E-boxes in ChIP studies in HNSCC cells. Anti-SNAIL antibody directed against the N-terminal domain efficiently pulled down SNAIL protein complexes with the chromatin fragment comprising the −84 to + 64 E-cadherin promoter region (Figure 4B). Control goat IgG did not precipitate the E-cadherin promoter region. Importantly, an enhanced expression of Snail in IL-1β treated cells resulted in an increase in chromatin binding as shown by the increase in intensity of the band in Figure 4B. Our CHIP assays demonstrate that Snail specifically binds to the E-cadherin promoter region.
Snail over expression increases metastasis and up regulates proinflammatory mediators in murine models of HNSCC
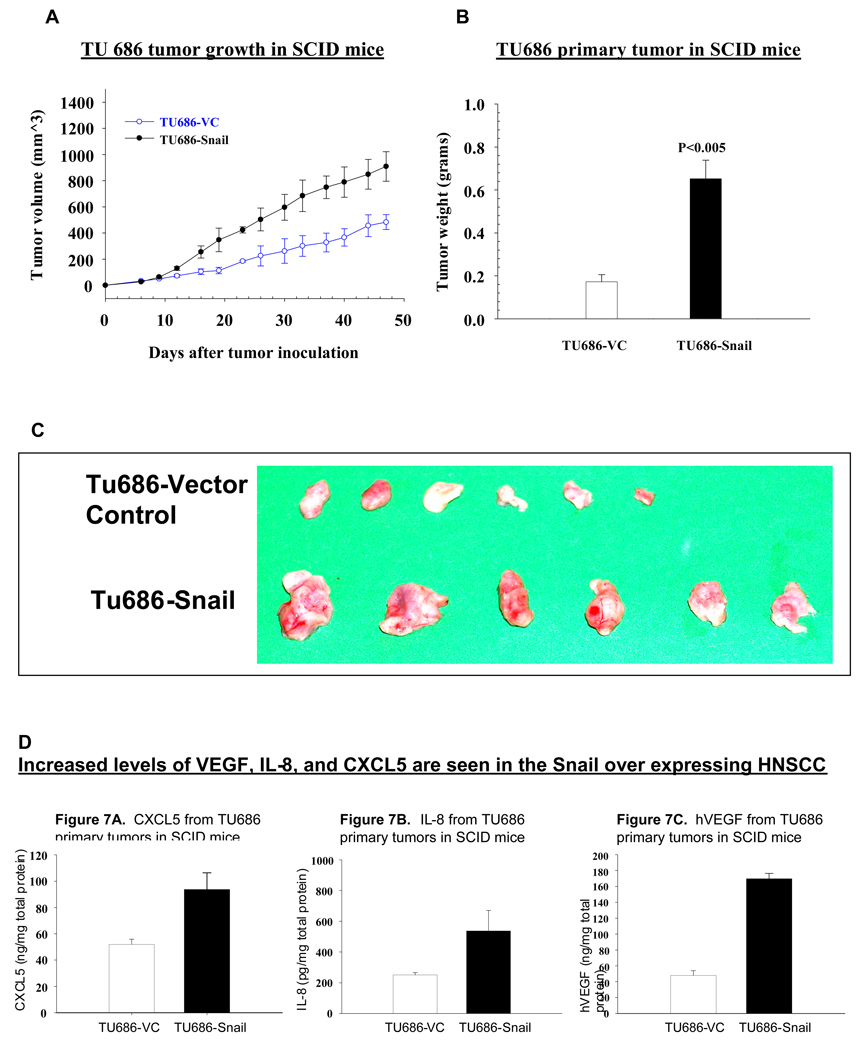
In order to further understand the role of Snail in metastasis and angiogenesis in HNSCC, we made use of xenograft murine models. Tu686 and OSC HNSCC cell lines were genetically altered to produce stable Snail over-expressing lines. Five million HNSCC (Tu686-Snail over expressing or Tu686-vector controls; OSC-Snail over expressing or OSC-vector controls) were implanted via subcutaneous injection on the right suprascapular area of the SCID mice.
Primary tumor burden increased significantly in Snail over expressing tumors as compared to vector controls (p < 0.005, Figure 5 A, B & C). Pulmonary and hepatic metastases were also significantly greater in mice bearing Snail over-expressing tumors (P<0.001). ELISA studies revealed increased levels of the angiogenic and proinflammatory mediators: VEGF, IL-8, and CXCL5 in the Snail over expressing tumors (Figure 5D). These in vivo studies further support our hypothesis that Snail may be an important factor in the growth and metastasis of HNSCC, and therefore is a potential target for therapy.
Figure 5. In the SCID xenograft model, HNSCC Snail overexpressing cells demonstrated significantly increased primary and metastatic tumor burdens.
Tu686 and OSC HNSCC cell lines were genetically altered to produce stable Snail over-expressing lines. Five million HNSCC (Tu686-Snail over expressing or Tu686-vector controls; OSC-Snail over expressing or OSC-vector controls) were implanted via subcutaneous injection on the right suprascapular area of the SCID mice as detailed in methods. Primary tumor burden increased significantly in Snail over expressing tumors as compared to vector controls (p < 0.005, Figure 5 A, B, C).
(D) In the SCID xenograft model, HNSCC Snail overexpressing cells demonstrated increased levels of VEGF, IL-8, and CXCL5. ELISA studies on tumors removed form the animals revealed increased levels of the angiogenic and proinflammatory mediators: VEGF, IL-8, and CXCL5 in the Snail over expressing tumors.
Expression of COX-2, Snail and E-cadherin in human HNSCC tissue sections
In Figure 2 we demonstrated that reciprocal expression of E-cadherin and COX-2 is evident in HNSCC cells in situ. The COX-2-dependent reciprocal expression of Snail and E-cadherin observed in vitro prompted us to determine if this relationship is also present in human neoplasm sections obtained from surgical specimens. Reciprocal expression of Snail and E-cadherin in histologic sections of human HNSCC was evident in a manner analogous to that seen with E-cadherin and COX-2 staining (Figure 2). Low-grade carcinomas showed decreased expression of SNAIL and COX-2 compared to high-grade neoplasms. Imunohistochemical staining of high-grade carcinomas with a solid pattern showed stronger staining for Snail and COX-2 (Figure 2).
Consistent with our in vitro findings, the examination of serial sections indicated that tumor cells that were positive for COX-2, were predominantly also Snail positive. We examined the relationship between E-cadherin and Snail using the ordinal IHC results (0+, 1+, etc) and found that they are significantly negatively correlated (rho=−0.52, p=0.02). Next, the staining for E-cadherin and Snail were dichotomized as positive (2+ or 3+) or negative (0+,1+). Using Fisher’s exact test we find that E-cadherin and Snail had a significant association (p=0.0006) after adding tumor differentiation status (Figure 2M). We found that the COX-2 staining paralleled Snail staining and was inversely proportional to E-cadherin staining. Thus, to summarize the findings of the immunohistochemical studies in human oral squamous cell carcinomas there is: 1) reciprocal expression of E-cadherin and COX-2; 2) reciprocal expression of E-cadherin and Snail; 3) co-expression of COX-2 and Snail.
Discussion
Several studies of E-cadherin in thyroid carcinomas have shown that its reduction or loss is generally related to features that correlate with tumor aggressiveness, such as poorly differentiated or anaplastic histology and widely invasive growth (27, 28, 29, 30). Distant metastasis-free survival was significantly worse in tumors showing reduced E-cadherin expression (31). In HNSCC patient samples, expression of Snail in primary tumors correlates with a higher probability of metastasis and a poor prognosis (23, 32). Snail is expressed at the invasive front of epidermoid carcinomas (33), and has been associated with the lymph node status and/or invasiveness of various carcinomas, as well as local recurrences (26, 34, 35, 36).
Herein, we investigated the mechanisms by which Snail might contribute to the pathogenesis of HNSCC using molecular analyses and in vivo modeling. Inflammation is commonly associated with cancer, and the up regulation of proinflammatory mediators has been observed in HNSCCs (37). We demonstrate that Il-1β and PGE2 lead to a COX-2 dependent up regulation of Snail. This then leads to Snail binding to the E-cadherin promoter, with resultant down regulation of E-cadherin expression. These results support our previous findings in NSCLC (12). When HNSCC cell lines that over express Snail are injected into SCID mice, the primary tumor and metastatic burdens are significantly greater than HNSCC vector controls. It is quite important to highlight that the metastatic model used in this study is not an orthotopic model. Based on Paget’s “seed and soil” theory, this model may not be an ideal one to study the metastasis of HNSCC, but is still informative in terms of the metastatic capacity of each of the cell lines (38).
In HNSCC, IL-1 can induce activation of signal transduction pathways that regulate several early transcription factors involved in the transcription of proinflammatory cytokine genes. IL-1α and IL-1β have been reported to play a prominent role in enhancing the transcription and expression of cytokines IL-6 and Il-8 during the activation of the cytokine cascade. It has been reported that human HNSCCs constitutively express IL-1α and a repertoire of proinflammatory and proangiogenic cytokines that are potentially IL-1 inducible, however, IL-1β was not detected in any of the HNSCC cell lines tested (39). IL-1α contributes to the transcriptional activation of NFkB, to the expression of IL-8, and to cell survival and the growth of HNSCC in vitro (5, 6, 7, 39, 40). Herein, we have define a new role for IL-1β in EMT. The role of IL-1 in enhancing activation of a cascade of proinflammatory cytokine mediators and responses suggests that IL-1 may serve as an important autocrine and/or exocrine factor in coordinating expression of this repertoire of cytokines in HNSCC.
Proinflammatory mediators are upregulated in Snail overexpressing tumors, including IL-8. IL-8 plays an important role in the stimulation of angiogenesis, proliferation, and chemotaxis of granulocytes and macrophages, which are prominent constituents in the stroma of HNSCCs. The up-regulation of IL-8 has been observed in HNSCC patients and is linked to recurrence and metastasis (41). Lyons et al have recently shown that Snail can up regulate proinflammatory cytokines in oral keratinocytes (20). Our work demonstrates that proinflammatory mediators up regulate Snail, thus further defining the cycle by which inflammation promotes tumor progression.
Loss of E-cadherin and gain of the expression of Snail are associated with resistance to EGFR TK inhibitors (42, 43). This evidence supports a key role for Snail as an inducer of tumor invasion as well as a potential contributor to tumor growth and/or chemoresistance mechanisms. Thus, there is a dual purpose in studying pathways that regulate E-cadherin expression in HNSCC: Maintenance of E-cadherin expression may promote sensitivity to targeted therapy and prevent invasion and metastases (42, 43).
The presence of regional metastases in HNSCC patients is a common and adverse event associated with poor prognosis. Understanding the molecular mechanisms that mediate HNSCC invasion and metastasis may enable identification of novel therapeutic targets for the prevention and management of metastasis. Here, we provide the first report indicating the role of E-cadherin transcriptional repressors in the inflammation-induced promotion of EMT in HNSCC.
We also document COX-2-dependent transcriptional regulation of E-cadherin in HNSCC. Furthermore, in human oral squamous cell carcinoma we confirm a reciprocal relationship between COX-2 and E-cadherin as well as Snail and E-cadherin. The studies presented here also indicate a positive correlation between COX-2 and Snail in human oral squamous cell carcinoma. These findings suggest that therapies targeting the cyclooxygenase pathway may diminish the propensity for tumor metastasis in HNSCC by blocking the PGE2-mediated induction of E-cadherin transcriptional repressors. This newly defined pathway for transcriptional regulation of E-cadherin in HNSCC has important implications for chemoprevention as well as therapies utilizing COX-2 inhibitors in combination with other agents. COX-2 inhibitors may enhance HNSCC E-cadherin expression and may therefore augment sensitivity to EGFR TKI therapy. The tailoring of individual treatment strategies to aggressively treat HNSCC will improve long-term survival.
STATEMENT OF TRANSLATIONAL RELEVANCE.
The presence of regional metastases in HNSCC patients is a common and adverse event associated with poor prognosis. Understanding the molecular mechanisms that mediate HNSCC metastasis may enable identification of novel therapeutic targets. Here, we provide the first report indicating the role of E-cadherin transcriptional repressors in the inflammation-induced promotion of EMT in HNSCC. We also document COX-2-dependent transcriptional regulation of E-cadherin in HNSCC. These findings suggest that therapies targeting the cyclooxygenase pathway may diminish the propensity for tumor metastasis in HNSCC by blocking the PGE2-mediated induction of E-cadherin transcriptional repressors. This newly defined pathway for transcriptional regulation of E-cadherin in HNSCC has important implications for chemoprevention as well as therapies utilizing COX-2 inhibitors in combination with other agents. COX-2 inhibitors may enhance HNSCC E-cadherin expression and may therefore augment sensitivity to EGFR TKI therapy. The tailoring of individual treatment strategies to aggressively treat HNSCC will improve long-term survival.
Acknowledgements
This study was supported by the American Academy of Otolaryngology-American Head & Neck Society Surgeon Scientist Career Development Award (MSJ), the Tobacco-Related Disease Research Program of the University of California (MSJ), the STOP Cancer Foundation (MSJ), The Jonsson Cancer Center, the VA Merit Review Research Funds (SD), and NCI RO1 CA111851 (SD).
Footnotes
Acknowledge Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Ferlay J, Bray F, Pisani P, Parkin DM GLOBOCAN. Cancer incidence, mortality and prevalence worldwide, version 1.0. IARC Cancer Base No 5. 2000 [Google Scholar]
- 2.Zender CA, Petruzzelli GJ. Why do patients with head and neck squamous cell carcinoma experience distant metastases: can they be prevented? Curr Opin Otolaryngol Head Neck Surg. 2005;2:101–104. doi: 10.1097/01.moo.0000156171.70521.dc. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, St. John MA, Zhou X, et al. Salivary transcriptome diagnostics for oral cancer detection. Clinical Cancer Res. 2004;24:8442–8450. doi: 10.1158/1078-0432.CCR-04-1167. [DOI] [PubMed] [Google Scholar]
- 4.Lin DT, Subbaramaiah K, Shah JP, Dannenberg AJ, Boyle JO. Cyclooxygenase-2: a novel molecular target for the prevention and treatment of head and neck cancer. Head & Neck. 2002;8:792–799. doi: 10.1002/hed.10108. [DOI] [PubMed] [Google Scholar]
- 5.Bancroft CC, Chen Z, Yeh J, et al. Effects of pharmacologic antagonists of epidermal growth factor receptor, PI3K and MEK signal kinases on NF-kappaB and AP-1 activation and IL-8 and VEGF expression in human head and neck squamous cell carcinoma lines. Int J Cancer. 2002;99:538–548. doi: 10.1002/ijc.10398. [DOI] [PubMed] [Google Scholar]
- 6.Wolf JS, Chen Z, Dong G, et al. IL (interleukin)-1alpha promotes nuclear factor-kappaB and AP-1-induced IL-8 expression, cell survival, and proliferation in head and neck squamous cell carcinomas. Clin Cancer Res. 2001;7:1812–1820. [PubMed] [Google Scholar]
- 7.Chen Z, Colon I, Ortiz N, et al. Effects of interleukin-1alpha, interleukin-1 receptor antagonist, and neutralizing antibody on proinflammatory cytokine expression by human squamous cell carcinoma lines. Cancer Res. 1998;58:3668–3676. [PubMed] [Google Scholar]
- 8.Mukhopadhyay P, Ali MA, Nandi A, Carreon P, Choy H, Saha D. The cyclin-dependent kinase 2 inhibitor down-regulates interleukin-1beta-mediated induction of cyclooxygenase-2 expression in human lung carcinoma cells. Cancer Res. 2006;66:1758–1766. doi: 10.1158/0008-5472.CAN-05-3317. [DOI] [PubMed] [Google Scholar]
- 9.Teruel A, Romero M, Cacalano NA, Head C, Jewett A. Potential contribution of naïve immune effectors to oral tumor resistance: role in synergistic induction of VEGF, IL-6, and IL-8 secretion. Cancer Immunol Immunother. 2008;57:359–366. doi: 10.1007/s00262-007-0375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endo T, Ogushi F, Sone S, et al. Induction of cyclooxygenase-2 is responsible for interleukin-1 beta-dependent prostaglandin E2 synthesis by human lung fibroblasts. Am J Resp Cell Mol Biol. 1995;12:358–365. doi: 10.1165/ajrcmb.12.3.7873203. [DOI] [PubMed] [Google Scholar]
- 11.Tsai CC, Chen CC, Lin CC, et al. Interleukin-1 beta in oral submucous fibrosis, verrucous hyperplasia and squamous cell carcinoma tissues Kaohsiung. J Med Sci. 1999;9:513–519. [PubMed] [Google Scholar]
- 12.Dohadwala M, Yang SC, Luo J, et al. Cyclooxygenase-2-dependent regulation of E-cadherin: prostaglandin E(2) induces transcriptional repressors ZEB1 and snail in non-small cell lung cancer. Cancer Res. 2006;66:5338–5345. doi: 10.1158/0008-5472.CAN-05-3635. [DOI] [PubMed] [Google Scholar]
- 13.Dohadwala M, Luo J, Zhu L, et al. Non small cell lung cancer cyclooxygenase-2-dependent invasion is mediated by CD44. J Biol Chem. 2001;276:20809–20812. doi: 10.1074/jbc.C100140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dohadwala M, Batra RK, Luo J, et al. Autocrine/paracrine prostaglandin E2 production by non-small cell lung cancer cells regulates matrix metalloproteinase-2 and CD44 in cyclooxygenase-2-dependent invasion. J Biol Chem. 2002;277:50828–50833. doi: 10.1074/jbc.M210707200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Z, Zhang X, Li M, et al. Simultaneously targeting epidermal growth factor receptor tyrosine kinase and cyclooxygenase-2, an efficient approach to inhibition of squamous cell carcinoma of the head and neck. Clinical Cancer Res. 2004;10:5930–5939. doi: 10.1158/1078-0432.CCR-03-0677. [DOI] [PubMed] [Google Scholar]
- 16.Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Bremnes RM, Veve R, Hirsch FR, Franklin WA. The E-cadherin cell-cell adhesion complex and lung cancer invasion, metastasis and prognosis. Lung Cancer. 2002;36:115–124. doi: 10.1016/s0169-5002(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 18.Conacci-Sorrell M, Zhurinskey J, Ben-Ze’ev A. The cadherin catenin adhesion system in signaling and cancer. J Clin Invest. 2002;109:987–991. doi: 10.1172/JCI15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ginos MA, Page GP, Michalowicz BS, et al. Identification of a gene expression signature associated with recurrent disease in squamous cell carcinoma of the head and neck. Cancer Res. 2004;64:55–63. doi: 10.1158/0008-5472.can-03-2144. [DOI] [PubMed] [Google Scholar]
- 20.Lyons JG, Patel V, Roue NC, et al. Snail up-regulates proinflammatory mediators and inhibits differentiation in oral keratinocytes. Cancer Res. 2008;68:4525–4530. doi: 10.1158/1078-0432.CCR-07-6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Chen ZG, Choe MS, et al. Tumor growth inhibition by simultaneously blocking epidermal growth factor receptor and cyclooxygenase-2 in a xenograft model. Clinical Cancer Res. 2005;11:6261–6269. doi: 10.1158/1078-0432.CCR-04-2102. [DOI] [PubMed] [Google Scholar]
- 22.Hoteiya T, Hayashi E, Satomura K, Kamata N, Nagayama M. Expression of E-cadherin in oral cancer cell lines and its relationship to invasiveness in SCID mice in vivo. J Oral Pathol Med. 1999;28:107–111. doi: 10.1111/j.1600-0714.1999.tb02006.x. [DOI] [PubMed] [Google Scholar]
- 23.Yokoyama K, Kamata N, Fujimoto R, et al. Increased invasion and matrix metalloproteinase-2 expression by Snail-induced mesenchymal transition in squamous cell carcinomas. Int J Oncol. 2003;22:891–898. [PubMed] [Google Scholar]
- 24.Sarantopoulos GP, Gui D, Shintaku P, et al. Immunohistochemical analysis of lung carcinomas with pure or partial bronchioloalveolar differentiation. Arch Pathol Lab Med. 2004;128:406–414. doi: 10.5858/2004-128-406-IAOLCW. [DOI] [PubMed] [Google Scholar]
- 25.Krysan K, Merchant FH, Zhu L, et al. COX-2 dependent stabilization of surviving in non-small cell lung cancer. Faseb J. 2004;18:206–208. doi: 10.1096/fj.03-0369fje. [DOI] [PubMed] [Google Scholar]
- 26.Moody SE, Perez D, Pan TC, et al. The transcriptional repressor snail promotes mammary tumor recurrence. Cancer Cell. 2005;8:197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Brabant G, Hoang-Vu C, Cetin Y, et al. E-cadherin: a differentiation marker in thyroid malignancies. Cancer Res. 1993;53:4987–4993. [PubMed] [Google Scholar]
- 28.Naito A, Iwase H, Kuzushima T, Nakamura T, Kobayashi S. Clinical significance of E-cadherin expression in thyroid neoplasms. J Surg Oncol. 2001;76:176–180. doi: 10.1002/jso.1031. [DOI] [PubMed] [Google Scholar]
- 29.Rocha AS, Soares P, Fonseca E, Cameselle-Teijeiro J, Oliveira MC, Sobrinho-Simoes M. E-cadherin loss rather than beta-catenin alterations is a common feature of poorly differentiated thyroid carcinomas. Histopathology. 2003;42:580–587. doi: 10.1046/j.1365-2559.2003.01642.x. [DOI] [PubMed] [Google Scholar]
- 30.Kato N, Tsuchiya T, Tamura G, Motoyama T. E-cadherin expression in follicular carcinoma of the thyroid. Pathol Int. 2002;52:13–18. doi: 10.1046/j.1440-1827.2002.01310.x. [DOI] [PubMed] [Google Scholar]
- 31.Brecelj E, Frkovic GS, Auersperg M, Bracko M. Prognostic value of E-cadherin expression in thyroid follicular carcinoma. Eur J Surg Oncol. 2005;31:544–548. doi: 10.1016/j.ejso.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Yokoyama K, Kamata N, Hayashi E, et al. Reverse correlation of E-cadherin and snail expression in oral squamous cell carcinoma cells in vitro. Oral Oncol. 2001;37:65–71. doi: 10.1016/s1368-8375(00)00059-2. [DOI] [PubMed] [Google Scholar]
- 33.Cano A, Perez-Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 34.Takes RP, Baatenburg De Jong RJ, Alles MJ, et al. Markers for nodal metastasis in head and neck squamous cell cancer. Arch Otolaryngol Head Neck Surg. 2002;128:512–518. doi: 10.1001/archotol.128.5.512. [DOI] [PubMed] [Google Scholar]
- 35.Blanco MJ, Moreno-Bueno G, Sarrio D, et al. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene. 2002;21:3241–3246. doi: 10.1038/sj.onc.1205416. [DOI] [PubMed] [Google Scholar]
- 36.Sugimachi K, Tanaka S, Kameyama T, et al. Transcriptional repressor snail and progression of human hepatocellular carcinoma. Clinical Cancer Res. 2003;9:2657–2664. [PubMed] [Google Scholar]
- 37.Loercher A, Lee TL, Ricker JL, et al. Nuclear factor-kappaB is an important modulator of the altered gene expression profile and malignant phenotype in squamous cell carcinoma. Cancer Res. 2004;64:6511–6523. doi: 10.1158/0008-5472.CAN-04-0852. [DOI] [PubMed] [Google Scholar]
- 38.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–573. [PubMed] [Google Scholar]
- 39.Chen Z, Malhotra PS, Thomas GR, et al. Expession of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clinical Cancer Res. 1999;5:1369–1379. [PubMed] [Google Scholar]
- 40.Ondrey FG, Dong G, Sunwoo J, et al. Constitutive activation of transcription factors NF-(kappa)B, AP-1, and NF-IL6 in human head and neck squamous cell carcinoma cell lines that express pro-inflammatory and pro-angiogenic cytokines. Mol Carcinog. 1999;26:119–129. doi: 10.1002/(sici)1098-2744(199910)26:2<119::aid-mc6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 41.St. John MA, Li Y, Zhou X, et al. Interleukin 6 and interleukin 8 as potential biomarkers for oral cavity and oropharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130:929–935. doi: 10.1001/archotol.130.8.929. [DOI] [PubMed] [Google Scholar]
- 42.Witta SE, Gemmill RM, Hirsch FR, et al. Restoring E-cadherin expression increases sensitivity to epidermal growth factor receptor inhibitors in lung cancer cell lines. Cancer Res. 2006;66:944–950. doi: 10.1158/0008-5472.CAN-05-1988. [DOI] [PubMed] [Google Scholar]
- 43.Krysan K, Lee JM, Dohadwala M, et al. Inflammation, epithelial to mesenc hymal transition, and epidermal growth factor receptor tyrosine kinase inhibitor resistance. J Thorac Oncol. 2008;3:107–110. doi: 10.1097/JTO.0b013e3181630ece. [DOI] [PubMed] [Google Scholar]