Abstract
We found that the selective stimulation of the intracellular, transmembrane G protein-coupled estrogen receptor (GPER), also known as GPR30, acutely lowers blood pressure after infusion in normotensive rats and dilates both rodent and human arterial blood vessels. Stimulation of GPER blocks vasoconstrictor-induced changes in intracellular calcium concentrations and vascular tone, as well as serum-stimulated cell proliferation of human vascular smooth muscle cells. Deletion of the GPER gene in mice abrogates vascular effects of GPER activation and is associated with visceral obesity. These findings suggest novel roles for GPER in protecting from cardiovascular disease and obesity.
Keywords: adipocytes, atherosclerosis, gender differences, vascular disease, metabolic syndrome
Coronary artery disease and stroke remain the leading causes of death in both men and women. The lower cardiovascular risk for premenopausal women as compared to men has been linked to the protective effects of endogenous estrogens on vascular tone, cell growth, and risk factors such as obesity and hypertension.1 Vascular estrogen binding sites include nuclear estrogen receptors ERα and ERβ as well as the novel G protein-coupled estrogen receptor (GPER), also known as GPR30,2, 3 which is localized to the endoplasmic reticulum and mediates non-genomic estrogen signaling.2, 4 The GPER gene maps to chromosome 7p22, a region implicated in arterial hypertension in genetic linkage studies in humans,5 suggesting a role of GPER in blood pressure control. Some studies have proposed that estrogen-dependent intracellular calcium signaling and cell contraction in the cardiovascular system are independent of classical estrogen receptors, ERα and ERβ;6 it has also been demonstrated that non-genomic actions of estrogens involve signaling pathways similar to G protein-coupled receptors.3 Whether GPER, which is highly expressed in human arteries,7 contributes to the regulation of cellular homeostasis or intracellular calcium signaling in the cardiovascular system is unknown.
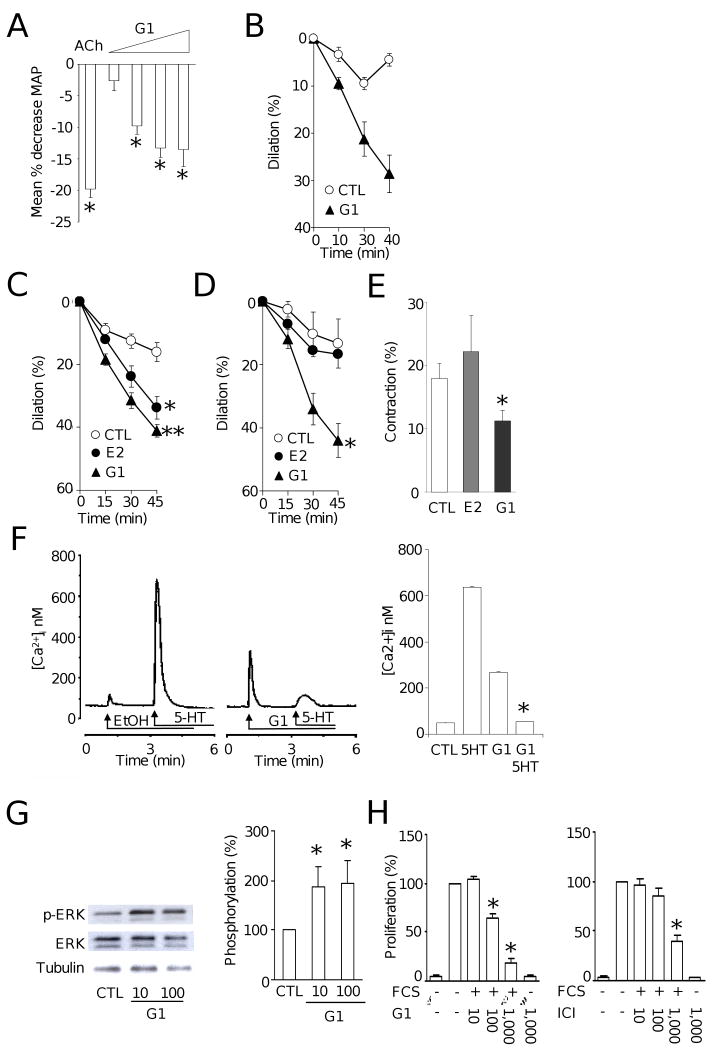
In premenopausal women, endogenous estrogen causes tonic vasodilation and thereby counteracts blood pressure elevation.8 To test whether GPER affects blood pressure, we intravenously infused the selective GPER agonist G-19 into normotonsive Sprague-Dawley rats. G-1 infusion resulted in an acute reduction in mean arterial blood pressure; the response began within approximately 2 minutes, and a maximum effect was typically present 8 minutes after infusion (Figure 1A). Next, using pressurized rat mesenteric resistance arteries of the same model we recorded changes in inner diameter over time (Supplementary Methods), and found that G-1 promotes acute dilation of the preconstricted arteries (Figure 1B). Because human internal mammary arteries dilate in response to 17β-estradiol,10 we next tested whether G-1 also affects vascular tone in these arteries. We found, that in human internal mammary arteries, the relaxant response to G-1 was even stronger than that to 17β-estradiol (P<0.05 vs. E2, Figure 1C). Finally, we investigated effects of G-1 on vascular tone in precontracted murine arteries. 17β-estradiol produced time-dependent relaxation that was only significant from vehicle control in the aorta but not in the carotid artery. In contrast, the same concentration of G-1 caused dilation in both arteries that was more potent than 17β-estradiol (Figure 1D and Online Figure Ia).
Figure 1.
A, Acute effect of intravenous infusion of acetylcholine (ACh) or increasing doses of G-1 (41.2 ng/kg, 412 ng/kg, 4.12 ug/kg and 20.6 ug/kg) into normotensive Sprague Dawley rats. Y axis values are expressed as percent change of mean arterial pressure. G-1 produced a dose-dependent reduction in blood pressure of 2.6±2%, 10±1%, 13±1%, and 13.5±2.2%, respectively. *P<0.05 vs. control.
B, Acute vascular effects of solvent control (ethanol, 0.1%, CTL) and GPER-agonist G-1 (1 μmol/L) on vascular tone in rat mesenteric resistance arteries. Arteries were preconstricted with UTP to induce a stable contraction plateau and exposed to ETOH or G-1, and changes in tone were recorded. At 40 minutes, G-1, reduced vascular tone by 29±4%. n=3-4/group. *P<0.05 vs. control.
C, Acute vascular effects of solvent control, 17β-estradiol, or GPER-agonist G-1 on vascular tone in human internal mammary arteries. Arteries were preconstricted with prostaglandin F2α to induce a stable contraction plateau and exposed to solvent control (ethanol 0.3%, CTL), 17β-estradiol or G-1 (both at 3 μ mol/L), and changes in tone were recorded. Both 17β-estradiol and G-1 induced a relaxant response; however, the relaxation in response to G-1 was more potent *P<0.05 vs. CTL, **P<0.05 vs. 17β-estradiol, unpaired t-test, n=4-7/group.
D, Acute vascular effects of solvent control (ethanol, 0.3%, CTL), 17β-estradiol, or GPER-agonist G-1 on vascular tone in mouse carotid arteries. Arteries were preconstricted with prostaglandin F2α to induce a stable contraction plateau and exposed to 17β-estradiol or G-1 (both at 3 μ mol/L), and changes in tone were recorded. G-1, but not 17β-estradiol, reduced vascular tone by 44±5%. *P<0.05 vs. solvent, unpaired t-test, n=4-7/group;
E, Acute vascular effects of solvent control (ethanol, 0.3%, CTL), 17β-estradiol, or GPER-agonist G-1 on vascular tone in mouse carotid arteries. Arteries were preincubated with 17β-estradiol or G-1 (both at 3 μ mol/L) for 45 minutes and then exposed to serotonin (5HT, 1 μmol/L). G-1 reduced contraction by 39%. *P<0.05 vs. solvent, unpaired t-test, n=4-8/group.
F, Left panel: representative original recordings of the effects of intracellular ligand injection on intracellular calcium concentrations in Fura-2-loaded human aortic vascular smooth muscle cells. Solvent had only small effects on intracellular calcium concentrations. Serotonin (5-HT) produced a strong calcium mobilization response. Intracellular injection of G-1 produced a fast and transient increase in calcium concentration, and pretreatment with G-1 completely blunted the subsequent 5-HT-induced changes in intracellular calcium. Right panel: averaged data of 4 experiments. *P<0.05 vs. 5-HT alone, paired t-test, n=4/group.
G, Effects of G-1 (10 and 100 nmol/L) on ERK-1/2 phosphorylation in human vascular smooth muscle cells expressing only GPER by Western blot. Left panel, representative example; Right, averaged, n=8/group. *P<0.05 vs. solvent, unpaired t-test.
H, Effects of GPER agonists G-1 and ICI 182,780 on serum-stimulated cell proliferation in human vascular smooth muscle cells expressing only GPER. Both agonists reduced cell proliferation between 60% and 80% at concentration of 1000 nmol/L. *P<0.05 vs. solvent, n=6/group
Estrogen also indirectly acts as a vasodilator by blocking the activity of vasoconstrictors, such as platelet-derived serotonin.11 Whereas G-1 reduced serotonin-induced vascular tone, 17β-estradiol did not affect contractions in aorta or carotid arteries (Figure 1E; Online Figure Ib). Similar to murine blood vessels, G-1 also inhibited the contractile response to serotonin in human arteries (12±2 vs. 22±5% of KCl, n=5-6/group P<0.05). Taken together, these data indicate that activation of GPER directly and indirectly mediates acute vasodilation, thus reducing blood pressure.
Calcium mobilization plays an important role in vascular smooth muscle cell relaxation. Activation of GPER leads to phosopholipase C activation, resulting in intracellular calcium mobilization.2 On the other hand, calcium-channel blocking effects of sex steroid hormones have been described. Since estrogen-induced mechanisms independent of of ERα and ERβ have been recently proposed to regulate cardiac myocyte contraction,6 we next determined whether the inhibitory effects of GPER activation on vascular tone involves changes in intracellular calcium concentrations within vascular smooth muscle cells. G-1 effects on intracellular calcium concentrations in the absence and presence of serotonin were determined in single human aortic smooth muscle cells by intracellular injection. The serotonin-induced calcium increase was almost completely abrogated after intracellular injection of G-1 (Figure 1F). Interestingly, when applied extracellularly, G-1 yielded a slow and sustained increase in calcium over a few minutes (Online Figure II), compared with an instantaneous increase on intracellular injection, consistent with an intracellular localization of GPER.2 RNA interference of GPER reduced GPER gene expression (Online Figure IIIa and IIIb), and almost completely abrogated G-1 induced increases in intracellular calcium (Online Figure IIIc). The observation that intracellular injection but not external application of G-1 produced a rapid yet transient calcium increase (Figure 1F) indicates that the dynamics of changes in intracellular calcium concentrations via GPER depend on whether stimulation occurs intra- or extracellularly, consistent with the rather slow dilator response in isolated arterial blood vessels and effects on blood pressure both requiring several minutes. We recently reported that 17β-estradiol mediates phosphorylation of ERK-1/2 in human vascular smooth muscle cells7 and now studied whether GPER also affects ERK-1/2 phosphorylation. We used human umbilical vein smooth muscle cells which lose expression of ERα and ERβ, yet retain GPER expression upon cell culture (Online Figure IV). At nanomolar concentrations of G-1, a robust increase in ERK-1/2 phosphorylation was observed (Figure 1G). In contrast, neither ERα agonist PPT nor ERβ agonist DPN at equimolar concentrations affected ERK-1/2 phosphorylation (Online Figure V).
Since vascular smooth muscle cell proliferation is a prerequisite for the development of atherosclerosis,12 and estrogen inhibits vascular smooth muscle cell growth,1, 12 we next determined if GPER activation plays a role in growth regulation using two agonists for GPER, G-1, and ICI182,780.9, 13 Both compounds had no effect on basal cell growth as determined by 3H-thymidine incorporation; however, serum-stimulated cell growth was inhibited by 60% to 80% (Figure 1H), in line with recently described, growth-inhibitory effects of GPER activation in certain cancer cells.14
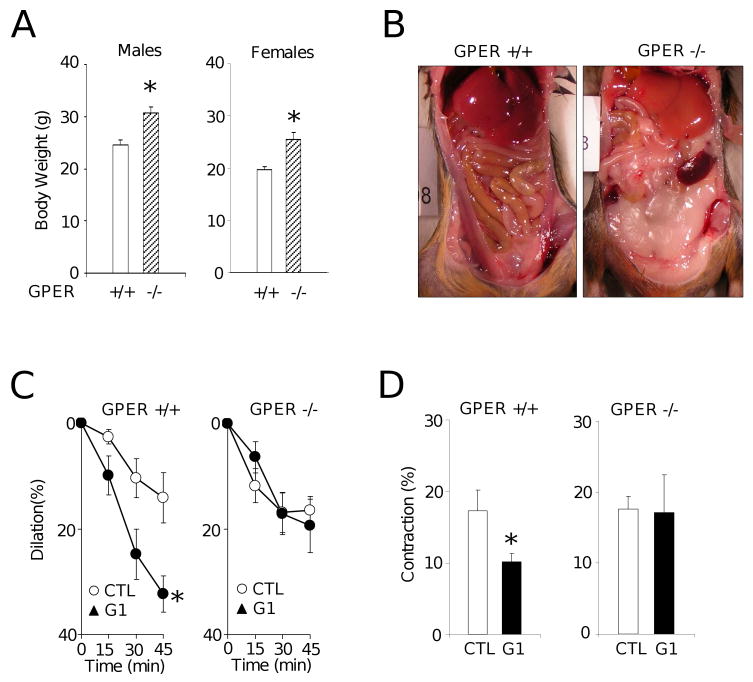
Finally, we examined the effects of GPER deletion on vascular responses in carotid arteries. Arteries from GPER-/- animals showed normal vascular reactivity to contractile stimuli such as potassium chloride (wild-type: 19.94±0.95 mN; GPER-/- 21.12 ±1.03 mN, n.s., n=18-26/group). However, genetic deletion of GPER was associated with increased body weight and visceral adiposity in both male and female animals (P<0.05, Figure 2A and 2B); in addition, the inhibitory effect of G-1 on serotonin-mediated contractions was equally effective in wild-type males (18±2 vs. 27±3%) and females (20±3 vs. 30±4%), suggesting gender-independent, GPER-mediated effects. The different aspects related to obesity in GPER-/- mice, including responses to dietary interventions, have been extensively characterized in a separate study (Dr. D. J. Clegg and collegues, manuscript submitted). Expression of GPER was detected in adipose tissue as well as in carotid artery and aorta of wild-type animals (data not shown). Markers of adipocyte differentiation, C/EBPα and PPARγ, were similarly expressed at the mRNA level in both wild-type and GPER-/- mice (I. Bhattacharya and M. Barton, unpublished observation 2009). As expected for a GPER-mediated response, G-1 had no dilator effects in carotid artery rings of GPER-/- mice (Figure 2C) and attenuated the contractile response to serotonin in wild-type mice (10±1% vs. 17±3%, n=7-8, P<0.05) but not in GPER-/- mice (17±5% vs. 17±2%, n=5-7. n.s., Figure 2D).
Figure 2.
A, Effect of GPER deficiency on body weight in male (left panel) and female (right panel) GPER+/+ (open bars) and GPER-/- mice (hatched bars). *P <0.05 vs. wild-type, Mann-Whitney U test.
B, Representative examples of abdominal fat distribution in GPER+/+ (left) and GPER-/- mice (right). Note the almost complete lack of abdominal fat in the wild-type animal compared to the GPER-/- animal.
C, Acute vascular effects of solvent control (ethanol, 0.1%, CTL), 17β-estradiol, or GPER-agonist G-1 on vascular tone in carotid arteries of GPER+/+ (left) and GPER-/- mice (right). Arteries were preconstricted with prostaglandin F2α to induce a stable contraction plateau and exposed to 17β-estradiol or G-1 (both at 1 μmol/L), and changes in tone were recorded. G-1 reduced vascular tone in GPER+/+ (left, *P <0.05 vs. solvent, unpaired t-test), but not in GPER-/- mice (right, n.s.). n=6-9/group;
D, Contractions to serotonin (100 nmol/L) after preincubation with G-1 (1 μmol/L) in GPER+/+ (left panel) and GPER-/- mice (right panel) *P<0.05 Mann-Whitney U-test, n=5-8/group; *P <0.05 vs. solvent control.
The present findings indicate a novel role for GPER as an intracellular, G protein-coupled estrogen receptor controlling vascular tone and blood pressure as well as body weight. Our findings suggest the possibility that - in contrast to its known direct effects on calcium mobilization via EGF receptor transactivation2, 13 - activation of GPER might also antagonize changes in intracellular calcium evoked by vasoconstrictor agonists such as serotonin, possibly involving ERK-1/2.
Our findings of increased body weight and abdominal obesity in male and female GPER-deficient mice is in contrast to a most recent publication using a different type of GPER-/- mouse generated using a cre/lox approach.15 While we found obesity in GPER-/- mice, these investigators found reduced body growth in female animals only. Moreover, the authors did not detect any expression of GPER in fat tissue,15 while we found expression of GPER in fat tissue of both male and female wild-type animals. The authors also claim slightly increased mean arterial blood pressure levels, however the difference presented was mainly due to a reduction of blood pressure in the wild-type rather than an increase in the knock-out animals, because blood pressure was essentially normal at around 75 mmHg. The contrasting results between the study by Mårtensson et al. and our study are currently unclear. We speculate that the cre/lox approach used by these authors may have involved cryptic or pseudo loxP sites, which may cause unwanted chromosomal translocations.16 Such factors would not be expected to play a role in the GPER-/- used in the present study, which were created using homologous recombination of ES cells (online supplement and the study by Wang et al 17)
In summary, the present study demonstrates for the first time that GPER contributes to regulation of blood pressure, vascular tone, and obesity and suggest the possibility that some of estrogen's known vasculoprotective effects involve GPER activation. Given that selective activation of GPER appears to be highly effective in blocking vasoconstriction and cell growth, GPER could represent a novel target to interfere with the development of vascular disease and obesity in humans.
Supplementary Material
Acknowledgments
Supported by the Swiss National Science Foundation grants 3200-108258/1 and K-33KO122504/1 (to M.B.) and the National Institutes of Health grants HL-90804 (to E.B.), CA-116662 and CA-118743 (to E.R.P), NS-18710 and HL-51314 (to N.J.D.), and HL-77876 and HL-88192 (to T.C.R.), and the IZKF Erlangen, Project A11 (to K.A.).
Footnotes
Disclosures: The University of New Mexico has filed a patent application on compounds utilized in this study.
References
- 1.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60:210–241. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 3.Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (GRAC) Br J Pharmacol. (3rd) 2008;153 2:S1–209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, Soria B. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor alpha and estrogen receptor beta. Proc Natl Acad Sci U S A. 2000;97:11603–11608. doi: 10.1073/pnas.97.21.11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lafferty AR, Torpy DJ, Stowasser M, Taymans SE, Lin JP, Huggard P, Gordon RD, Stratakis CA. A novel genetic locus for low renin hypertension: familial hyperaldosteronism type II maps to chromosome 7 (7p22) J Med Genet. 2000;37:831–835. doi: 10.1136/jmg.37.11.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ullrich ND, Krust A, Collins P, MacLeod KT. Genomic deletion of estrogen receptors ERalpha and ERbeta does not alter estrogen-mediated inhibition of Ca2+ influx and contraction in murine cardiomyocytes. Am J Physiol Heart Circ Physiol. 2008;294:H2421–2427. doi: 10.1152/ajpheart.01225.2007. [DOI] [PubMed] [Google Scholar]
- 7.Haas E, Meyer MR, Schurr U, Bhattacharya I, Minotti R, Nguyen HH, Heigl A, Lachat M, Genoni M, Barton M. Differential effects of 17beta-estradiol on function and expression of estrogen receptor alpha, estrogen receptor beta, and GPR30 in arteries and veins of patients with atherosclerosis. Hypertension. 2007;49:1358–1363. doi: 10.1161/HYPERTENSIONAHA.107.089995. [DOI] [PubMed] [Google Scholar]
- 8.Giannattasio C, Failla M, Grappiolo A, Stella ML, Del Bo A, Colombo M, Mancia G. Fluctuations of radial artery distensibility throughout the menstrual cycle. Arterioscler Thromb Vasc Biol. 1999;19:1925–1929. doi: 10.1161/01.atv.19.8.1925. [DOI] [PubMed] [Google Scholar]
- 9.Bologa CG, Ravenkar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 10.Mügge A, Riedel M, Barton M, Kuhn M, Lichtlen PR. Endothelium independent relaxation of human coronary arteries by 17 beta-oestradiol in vitro. Cardiovasc Res. 1993;27:1939–1942. doi: 10.1093/cvr/27.11.1939. [DOI] [PubMed] [Google Scholar]
- 11.Mügge A, Barton M, Fieguth HG, Riedel M. Contractile responses to histamine, serotonin, and angiotensin II are impaired by 17 beta-estradiol in human internal mammary arteries in vitro. Pharmacology. 1997;54:162–168. doi: 10.1159/000139483. [DOI] [PubMed] [Google Scholar]
- 12.Meyer MR, Haas E, Barton M. Need for research on estrogen receptor function: importance for postmenopausal hormone therapy and atherosclerosis. Gend Med. 2008;5 A:S19–33. doi: 10.1016/j.genm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- 14.Kleuser B, Malek D, Gust R, Pertz HH, Potteck H. 17β-Estradiol inhibits transforming gowth factor-{beta} signalling and function in breast cancer cells via activation of extracellular signal-regulated kinase through the G protein coupled receptor 30. Mol Pharmacol. 2008;74:1533–1543. doi: 10.1124/mol.108.046854. [DOI] [PubMed] [Google Scholar]
- 15.Mårtensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grande PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM. Deletion of the G protein-coupled receptor GPR30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2008 doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt EE, Taylor DS, Prigge JR, Barnett S, Capecchi MR. Illegitimate Cre-dependent chromosome rearrangements in transgenic mouse spermatids. Proc Natl Acad Sci U S A. 2000;97:13702–13707. doi: 10.1073/pnas.240471297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang C, Dehghani B, Magrisso IJ, Rick EA, Bonhomme E, Cody DB, Elenich LA, Subramanian S, Murphy SJ, Kelly MJ, Rosenbaum JS, Vandenbark AA, Offner H. GPR30 contributes to estrogen-induced thymic atrophy. Mol Endocrinol. 2008;22:636–648. doi: 10.1210/me.2007-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.