Abstract
Background
Patients suffering from chronic heart failure frequently experience skeletal muscle weakness, which limits physical function. The mechanisms underlying muscle weakness, however, have not been clearly defined.
Methods and Results
The present study examined the hypothesis that heart failure promotes a loss of myosin protein from single skeletal muscle fibers, which in turn reduces contractile performance. Ten patients with chronic heart failure and 10 controls were studied. Muscle atrophy was not evident in patients, and groups displayed similar physical activity levels, suggesting that observed differences reflect the effects of heart failure, not muscle atrophy or disuse. In single muscle fibers, heart failure patients showed reduced myosin heavy chain (MHC) protein content (P-values: <0.05) that manifested as a reduction in functional myosin-actin cross-bridges (P<0.05). No evidence was found for a generalized loss of myofilament protein, suggesting a selective loss of myosin. Accordingly, single muscle fiber maximal Ca2+-activated tension was reduced in patients in MHC I fibers (P<0.05). Tension was maintained in MHC IIA fibers in patients, however, because a greater proportion of available myosin heads were bound to actin during Ca2+-activation (P<0.01).
Conclusions
Collectively, our results show that heart failure alters the quantity and functionality of the myosin molecule in skeletal muscle, leading to reduced tension in MHC I fibers. Loss of single fiber myosin protein content represents a potential molecular mechanism underlying muscle weakness and exercise limitation in heart failure patients.
Keywords: heart failure, skeletal muscle, mechanics, myosin
INTRODUCTION
Patients suffering from chronic heart failure have a reduced capacity for physical work. Although cardiac dysfunction is the primary pathological insult, the resulting syndrome of heart failure alters numerous physiological systems to impair functional capacity. Alterations in skeletal muscle are of particular importance, most notably atrophy,1 weakness2,3 and reduced endurance.4 These peripheral skeletal muscle adaptations limit physical function independent of cardiac impairment5 and persist despite correction of cardiac dysfunction.6
Aerobic fitness is commonly assumed to be the primary determinant of physical function in heart failure patients because exertional fatigue and dyspnea are predominant symptoms of the disease. However, aerobic capacity is a relatively poor predictor of performance in activities of daily living.7 This is because most activities are limited instead by skeletal muscle strength.8 Accordingly, functional capacity can be increased in heart failure patients in the absence of alterations in aerobic capacity by improving muscle strength.9 Despite its potential relevance to physical disability in patients with heart failure, few studies have explored the mechanisms underlying reduced skeletal muscle strength.
Skeletal muscle weakness in heart failure is not explained by muscle atrophy2,10 or reduced motor activation,2 suggesting defects in the intrinsic contractile properties of individual muscle fibers. Recent studies in rats11 and humans12 in chemically-skinned single muscle fibers have shown reduced contractile function in heart failure, implicating impaired myofilament protein function in muscle weakness. Because myosin is the most prevalent myofilament protein in muscle13 and is the primary determinant of single fiber contractile mechanics,14 alterations in fiber function can be linked to variation in the quantity or function of the myosin molecule. In this context, our previous work demonstrating a reduction in myosin heavy chain (MHC) protein content in skeletal muscles of heart failure patients15 suggests that contractile dysfunction may result from a reduced quantity of myosin. Indeed, a recent study in rats with heart failure has shown reduced MHC protein content and force production in single diaphragm muscle fibers.11 Additionally, this study found alterations in myosin kinetic properties that could diminish contractile function.11 Whether similar alterations in the quantity and functionality of myosin occur in skeletal muscle of patients with heart failure, however, has not been examined.
The goal of this study was to examine the effect of heart failure on single skeletal muscle fiber myofilament protein composition, function and sarcomeric structure. To accomplish this objective, we evaluated single muscle fibers from the vastus lateralis muscle of patients with chronic heart failure and sedentary controls. Controls were recruited to match the low physical activity levels typically observed in heart failure patients,16 which obviates the effects of muscle activity level on single fiber structure and function.17
METHODS
Subjects
Ten patients (7 men, 3 women) with physician-diagnosed heart failure were recruited. The population consisted of patients with both systolic dysfunction (left ventricular ejection fraction (EF) <40%; n=6; 26.0 ± 2.7%; range: 17–35%) and preserved systolic function (EF>40%; n=4; 46.8 ± 1.8%; range: 45–52%). At the time of study, there were 1 NYHA class I, 5 class II and 4 class III patients. The etiology of heart failure was ischemic in 3 volunteers and non-ischemic in 7. Three patients had Type II diabetes mellitus. All patients were clinically stable and had not been hospitalized for at least 6 months prior to testing. None had evidence of hepatic, renal, or peripheral vascular disease or an active neoplastic process. Patients were receiving angiotensin-converting enzyme (ACE) inhibitors/receptor blockers (100%), β-blockers (90%), diuretics (70%), HMG CoA reductase inhibitors (20%) and one female patient was receiving levothyroxine. Plasma creatine kinase levels were normal in all patients and none were smokers or taking sex steroid replacement therapy.
Controls (n=10; 6 men, 4 women) were recruited who were sedentary or minimally-active, as defined by self-report of ≤2 sessions of ≥30 min of exercise/week and not participating in any exercise training. Controls were non-smokers, had a stable body weight (±2 kg during prior 6 months and not participating in a weight loss program), no signs or symptoms of heart failure, coronary heart disease or diabetes (fasting blood glucose >112 mg/dL), normal left ventricular ejection fraction (>55%), normal complete blood counts and routine biochemical values and were not taking sex steroid replacement therapy. Four controls had a history of hypertension and three were treated with diuretics and one with an ACE inhibitor. All were normotensive at testing and showed no evidence of left ventricular hypertrophy or atrial enlargement by echocardiography. Two controls were on stable doses of HMG CoA reductase inhibitors and one female was on levothyroxine. Plasma creatine kinase levels were normal in all controls. Informed consent was obtained from each volunteer and the protocol was approved by the Committees on Human Research at the University of Vermont. Data showing reduced knee extensor muscle strength in heart failure patients from this cohort have been published.3
Experimental protocol
Eligibility was determined during screening visits, at which time medical history, physical examination, blood samples, whole muscle strength testing, a treadmill test and echocardiography were performed. At least 1 week later, eligible volunteers underwent an inpatient visit. Prior to admission, medications were maintained per normal dosing regimens, except coumadin (n=3), which was stopped 5 days before this visit. On the following morning, in the fasted state, body composition and mid-thigh muscle cross-sectional area was measured, and muscle tissue was obtained via percutaneous biopsy (Bergstrom needle, 5 mm OD) of the vastus lateralis.
Total and regional body composition
Total and regional fat mass and fat-free mass were measured by dual energy x-ray absorptiometry, as described.15 Bone mass data are not reported. Mid-thigh muscle area was measured by computed tomography, as described.18
Peak oxygen consumption (peak VO2)
Peak VO2 was determined using the Naughton protocol.19
Accelerometry
Free-living physical activity was measured using a single-plane accelerometer, as described previously.20
Muscle tissue processing
Approximately two-thirds of the biopsy material was placed immediately into cold (4°C) dissecting solution (see on-line supplement for composition of solutions). Remaining tissue was frozen in liquid nitrogen and stored at −80°C. Muscle fibers were dissected into bundles and tied to glass rods at 4°C, and then placed in skinning solution for 24 h at 4°C, storage solution with 50% (v/v) glycerol for 16 hr at 4°C and finally stored at −20°C until study (within 4 wks).
Tissue homogenate MHC protein content and isoform distribution
Tissue MHC protein content and isoform distribution were determined on frozen muscle tissue, as described previously,21 with minor modifications (see on-line supplement).
Single muscle fiber morphology and MHC protein content
Segments (~3 mm) of single muscle fibers were measured in relaxing solution (20°C) to estimate fiber volume (see on-line supplement for details). Aliquots (1.5 μm3 fiber volume) of sample were analyzed for MHC and actin protein content in triplicate, according to previously published methods,17,22 with modifications (see on-line supplement). Thereafter, each fiber was analyzed for fiber type via MHC isoform expression (see on-line supplement).
Single fiber mechanical measurements
Segments (~2.5 mm) of single fibers were isolated and their ends fixed with glutaraldehyde, as elsewhere,23 with modifications (see on-line supplement). Top and side diameter measurements were made in relaxing solution (pCa 8) at 3 positions to calculate cross-sectional area and the fiber was incubated in dissecting solution containing 1% Triton X-100 (v/v) for 30 min. Fibers were attached to a piezoelectric motor and a strain gauge in relaxing solution, the sarcomere length set to 2.65 μm and unfixed fiber length (~ 1 mm) measured. All mechanical measurements were performed at 15°C. The fiber was transferred to pre-activating solution for 30 s and then to activating solution (pCa 4.5) and tension recorded at plateau. At plateau, sinusoidal length oscillations (10 cycles of 0.125% fiber length at 250 Hz) were imposed to measure fiber dynamic stiffness. Duplicate measurements of maximal Ca2+-activated tension and stiffness were obtained for each fiber. Thereafter, the fiber was placed in rigor solution and, at the plateau of tension, dynamic stiffness was measured. The amplitude of dynamic stiffness in the rigor state is proportional to the total number of available myosin heads that can bind actin (ie, total cross-bridge number), assuming all myosin heads bind to actin in rigor. The ratio of pCa 4.5 to rigor dynamic stiffness, therefore, provided an estimate of the fraction of available myosin heads that bind actin during Ca2+-activation. Following mechanical measurements, single fibers were analyzed for MHC isoform composition to identify fiber type.
Ultrastructural measurements
Electron microscopy measurements were conducted on intact skeletal muscle fiber bundles, as described24 (see on-line supplement for details).
Protein and gene expression
Immunoblotting techniques were used to assess MHC degradation products, according to the method of Ball et al.,25 and the quantity of ubiquitinated MHC (see on-line supplement). MHC isoforms, actin, muscle ring finger-1 (MuRF-1) and atrogin-1 mRNA levels were determined by real-time PCR (see on-line supplement).
Statistics
All data are reported as mean ± SE. Unpaired Student t tests were used to compare groups. Analysis of covariance was used to compare peak VO2 data between groups after adjusting for differences in fat-free tissue mass.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
RESULTS
Groups were similar for age, body size, body mass and total and regional body composition. Peak VO2 adjusted for fat-free mass was lower (P<0.01) in patients (Table 1). Daily physical activity level measured by accelerometry over 7.6 ± 0.4 d was similar between groups.
Table 1.
Clinical characteristics and physical activity levels of controls and heart failure patients.
| Controls | Heart failure | n (control/heart failure) | |
|---|---|---|---|
| Age (yrs) | 69.3 ± 4.4 | 72.2 ± 4.4 | 10/10 |
| Height (cm) | 168.1 ± 3.3 | 170.4 ± 3.0 | 10/10 |
| Body mass (kg) | 81.9 ± 4.0 | 92.4 ± 9.9 | 10/10 |
| Fat mass (kg) | 29.9 ± 2.3 | 32.7 ± 5.3 | 10/9 |
| Fat-free mass (kg) | 49.1 ± 3.3 | 51.8 ± 5.1 | 10/9 |
| Arm fat-free mass (kg) | 5.32 ± 0.50 | 5.27 ± 0.61 | 10/9 |
| Leg fat-free mass (kg) | 15.4 ± 1.8 | 14.8 ± 2.2 | 10/9 |
| Mid-thigh muscle cross-sectional area (cm2) | 108.8 ± 8.8 | 100.9 ± 11.2 | 8/9 |
| Peak oxygen consumption (L/min)† | 1.93 ± 0.12 | 1.24 ± 0.12 * | 9/9 |
| Physical activity level (kcal/d) | 215 ± 32 | 251 ± 45 | 9/10 |
Data are mean ± SE.
peak oxygen consumption data were adjusted for fat-free mass using analysis of covariance.
P<0.01.
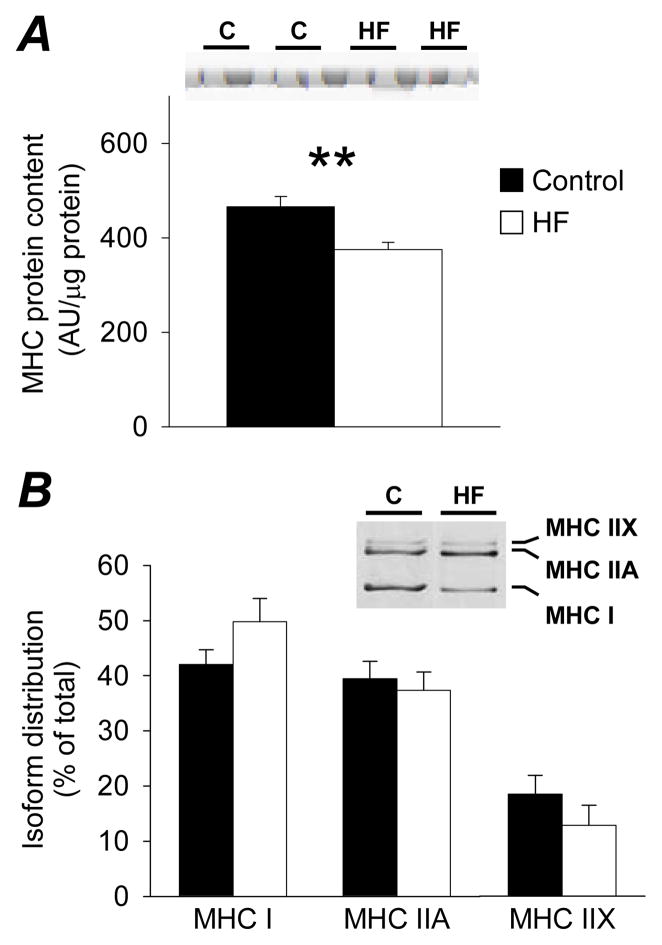
Heart failure patients had lower MHC protein content in tissue homogenates compared to controls (Figure 1A; P<0.01), with no group differences in the relative distribution of MHC isoforms (Figure 1B).
Figure 1.
Tissue homogenate MHC protein content (A; arbitrary densitometry units (AU) per μg of protein) and relative isoform distribution (B; % of total) in controls (C; n=10) and heart failure patients (HF; n=9) with representative sections of gels. Bar graphs represent mean ± SE. **, P<0.01.
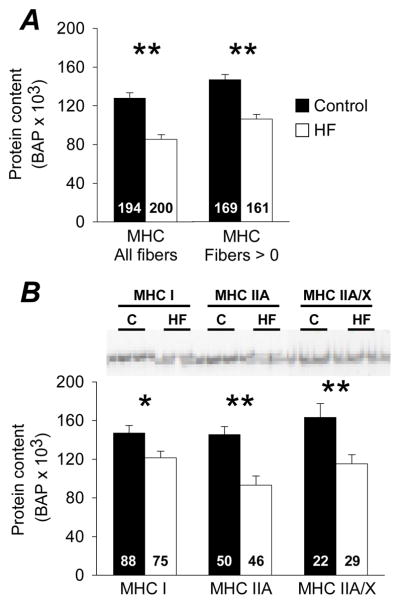
Fibers from patients had greater average cross-sectional area compared to controls (P<0.01; Table 2) when all fibers were pooled, with or without inclusion of those fibers with MHC band densities less than or equal to background. When partitioned into fiber types, patients had greater cross-sectional area in MHC IIA and IIA/X fibers (P<0.01 for both), but not in MHC I fibers.
Table 2.
Average cross-sectional area of single skeletal muscle fibers from controls and heart failure patients.
| Controls | Heart failure | # of fibers (control/heart failure) | |
|---|---|---|---|
| All fibers (μm2) | 5880 ± 145 | 6824 ± 160 * | 194/200 |
| Fibers with detectable MHC content (μm2) | 5916 ± 159 | 6748 ± 169 * | 169/161 |
| MHC I fibers (μm2) | 6613 ± 242 | 6538 ± 247 | 88/75 |
| MHC IIA fibers (μm2) | 5422 ± 210 | 7419 ± 305 * | 50/46 |
| MHC IIA/X fibers (μm2) | 4450 ± 193 | 6091 ± 347 * | 22/29 |
Data are mean ± SE and reflect the average cross-sectional area from top and side diameter measurements every 250 μm along the length of each fiber. Fibers with detectable MHC include only those with band densities greater than background, whereas the All fibers includes those with band densities equal to or less than background. There were too few MHC IIX and I/IIA fibers to permit comparisons between groups.
, P<0.01.
Heart failure patients showed lower MHC protein content in single fibers whether fibers with MHC band densities less than or equal to background (n=39 fibers from patient group; n=25 fibers from control group) were/were not included (P<0.01; Figure 2A). Actin was detected in all fibers examined (see on-line supplement). In fibers with detectable MHC bands, heart failure patients had lower MHC content in MHC I (P<0.05), MHC IIA (P<0.01) and MHC IIA/X (P<0.01) fibers (Figure 2B). There were too few MHC IIX and MHC I/IIA fibers to permit comparisons.
Figure 2.
Single fiber MHC (n=10/group) protein content from controls (C) and heart failure patients (HF) and representative gel images of MHC bands. Data are shown including all fibers measured and only those fibers that contained a measurable MHC band (density > background). The number of fibers is indicated at the base of each bar. Bar graphs represent mean ± SE. *, P<0.05; **, P<0.01.
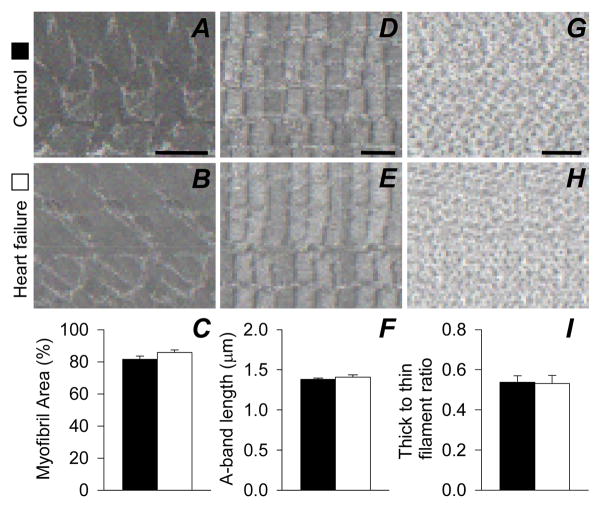
No group differences were evident for myofibrillar volume fraction, A-band length or thick to thin filament ratio (Figure 3A–I). Average sarcomere length also did not differ (C: 2.06 ± 0.09 vs. HF: 2.20 ± 0.13 μm) and no relationship between sarcomere length and the thick to thin filament ratio was found (r=0.022; P=0.94).
Figure 3.
Skeletal muscle fiber ultrastructural data in controls (n=8) and heart failure patients (n=7). Representative cross-sectional (8,000X; A and B; scale bar=1 μm and 60,000X; G and H; bar=100 nm) and longitudinal (5,000X; D and E; scale bar=1 μm) images are presented. Bar graphs in panels C,F and I represent mean ± SE.
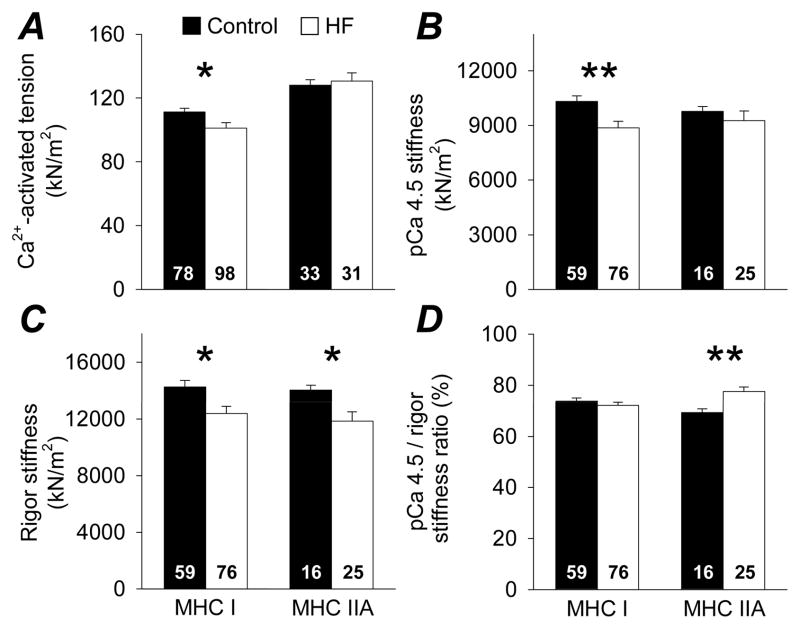
Single muscle fiber Ca2+-activated tension and dynamic stiffness data are shown in Figure 4. Patients showed lower tension in MHC I fibers (P<0.05), while MHC IIA fibers remained similar to controls (Figure 4A). pCa 4.5 stiffness was decreased in MHC I fibers (P<0.01) and unchanged in MHC IIA fibers (Figure 4B), while rigor stiffness was decreased in patients in both MHC I and MHC IIA (both P<0.05; Figure 4C) fibers. The pCa 4.5/rigor stiffness ratio did not differ between groups in MHC I fibers, but was increased in patients in MHC IIA fibers (P<0.01; Figure 4D). Finally, there were a sub-set of fibers in controls (n=7) and patients (n=20) that failed catastrophically (ie, tore) upon Ca2+-activation.
Figure 4.
Single skeletal muscle fiber Ca2+-activated (pCa 4.5) tension (A) and dynamic stiffness data (B, C, D) in controls (n=5) and heart failure patients (n=9). The number of fibers is indicated at the base of each bar. Bar graphs represent mean ± SE. *, P<0.05; **, P<0.01.
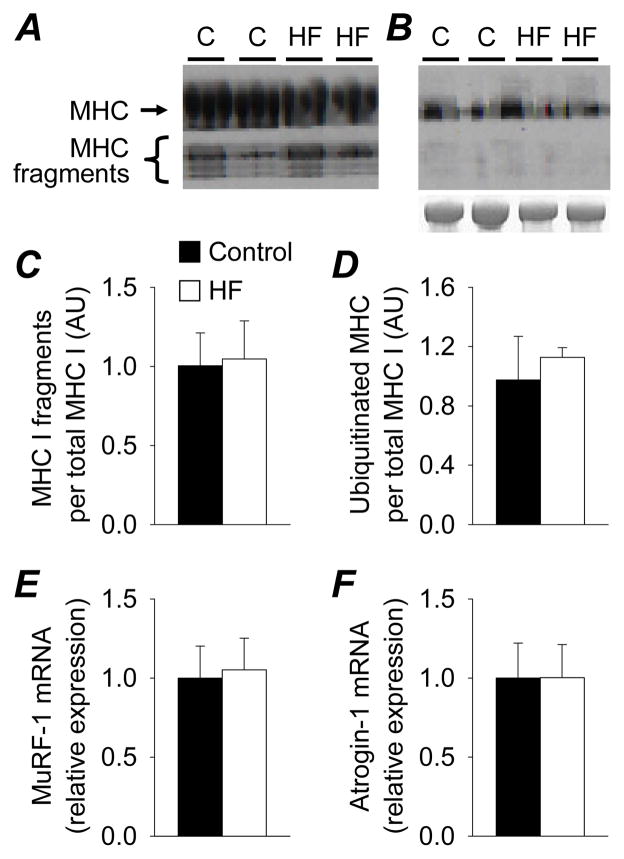
No differences were found between patients (n=7) and controls (n=5) in mRNA abundance for MHC I (C: 1.00 ± 0.19 vs. HF: 1.10 ± 0.33 relative expression), MHC IIA (C: 1.00 ± 0.14 vs. HF: 0.87 ± 0.09), MHC IIX (C: 1.00 ± 0.37 vs. HF: 1.68 ± 0.54) or actin (C: 1.00 ± 0.12 vs. HF: 0.96 ± 0.10). Similarly, no group differences were noted for mRNA of any of these genes when data were analyzed using 18S RNA as the housekeeping gene (see on-line supplement). Additionally, no differences between patients and controls in the amount of MHC I breakdown fragments (Figure 5A,C), ubiquitinated protein corresponding to MHC (Figure 5B,D) or expression of E3 ubiquitin ligases (Figure 5E,F) was found (see on-line supplement for further details).
Figure 5.
MHC I protein breakdown fragments (A,C) and ubiquitinated MHC (B,D) in controls (C; n=4) and heart failure patients (HF; n=4) and E3 ubiquitin ligase expression (E,F; n=6 controls and 4 heart failure). Representative western blots are shown for MHC I breakdown fragments (A) and ubiquitinated MHC (B), including a Simple Blue-stained gel to indicate total MHC protein content. Bar graphs represent mean ± SE.
DISCUSSION
In the present study, we found reduced single fiber MHC protein content in heart failure patients, which was manifest as a decreased number of functional myosin-actin cross-bridges. No evidence was found, however, for a generalized loss of myofibrillar proteins, suggesting a selective loss of myosin protein. In keeping with reduced MHC protein/cross-bridge number, single fiber maximal Ca2+-activated tension (force per cross-sectional area) was reduced in MHC I fibers from patients. Interestingly, tension was maintained in MHC IIA fibers from patients because a greater proportion of myosin heads bound actin during Ca2+-activation, implying altered myosin kinetic properties. Our results represent the first demonstration of an effect of heart failure on the quantity and kinetic properties of the myosin molecule in single skeletal muscle fibers in human heart failure.
Heart failure patients are profoundly inactive relative to age-matched, healthy controls,16 which complicates group comparisons because inactivity modulates muscle structure and function.26 To mitigate the effect of inactivity, we recruited sedentary controls to match patients for muscle use and confirmed that their activity levels were similar to patients. Additionally, patients were tested ≥6 months following inpatient admissions to eliminate any acute effects of muscle disuse on single fiber structure or function. An interesting observation that highlights the importance of these experimental considerations is the similar MHC isoform distribution (ie, fiber type) in patients and controls (Figure 1B). Our fiber type results agree with studies that have similarly controlled the activity status of controls,9,27 but differ from those that have not.15,28,29 In these latter studies, a shift in fiber type towards a fast-twitch phenotype was observed.15,28,29 As muscle disuse increases the number of fast-twitch fibers,30 these results collectively suggest that one of the presumed hallmark muscle adaptations to heart failure--a switch to a fast-twitch phenotype--may simply be a repercussion of the muscle disuse imposed by the disease state. Thus, experimental control for physical activity patterns is necessary to identify alterations in myofilament protein content and function that are specific to heart failure.
At the single fiber level, MHC loss was evident in all fiber types examined from heart failure patients (Figure 2B). These results extend evidence from rat models11 to demonstrate, for the first time, that myosin is lost from single muscle fibers as a consequence of human heart failure. Further reinforcing our MHC content data, we found a decrease in single fiber dynamic stiffness in patients at the plateau of rigor tension (Figure 4C), suggesting a loss of functional myosin heads that can bind actin to form cross-bridges. Because myosin comprises 25% of total skeletal muscle protein and 40% of myofilament protein,13 this reduction may simply reflect a loss of myofilament protein secondary to muscle atrophy. The fact that we found no evidence for muscle atrophy in patients, and no group differences in single fiber actin protein content (see on-line supplement) or myofibrillar volume fraction, however, argues against this conclusion and implies a selective loss of myosin protein. This type of selective myosin depletion is not unique to heart failure and has been observed in acute quadriplegic myopathy patients31 and a rat model of cancer,32 suggesting that this phenotype may be common to a variety of acute and chronic disease states.
From a structural standpoint, loss of myosin could result from a decreased number of thick filaments, shortening of the thick filaments and/or loss of myosin at random points along the thick filament. We found no evidence for a loss of thick filaments (thick-to-thin filament ratio) or shortening of the A-band (ie, thick filament) length in patients, implying a loss of myosin along the entire length of the thick filament. This structural phenotype of myosin depletion agrees with the fact that the thick filament is remodeled by replacing myosin at random points along the length of the filament.33
Single fiber tension is directly related to the number of functional myosin heads, the proportion of these heads bound to actin during Ca2+-activation and the force generated per cross-bridge.34,35 Thus, a loss of MHC protein would result in reduced single fiber tension. Correspondingly, tension was reduced in MHC I fibers from patients (Figure 4A). This reduction in tension is less than the decrement in MHC protein content/cross-bridge number, which may be explained by the fact that the relationship between MHC protein content and tension appears to be non-linear.31 The non-linear nature of this relationship may relate to alterations in cross-bridge kinetics which accompany MHC protein depletion.11 Additionally, variance between single fiber MHC protein content and tension in our study may simply relate to the fact that measurements were preformed on two separate populations of fibers. Regardless of the underlying mechanisms, the diminution of function in MHC I fibers may be relevant to physical disability in heart failure patients in light of the fact that MHC I fibers are recruited for repetitive movements typically encountered in daily activities.36,37 Moreover, diminished function in MHC I fibers may contribute to reduced exercise capacity as these fibers are important determinants of aerobic fitness in heart failure patients.38 In this context, loss of myosin protein represents a potential molecular mechanism underlying physical disability and exercise intolerance in heart failure.
Interestingly, despite the reduction in MHC content, no group differences in tension were found in MHC IIA fibers. The reason for this disparity was revealed when fiber stiffness was evaluated under maximal Ca2+-activated and rigor conditions, which demonstrated a greater proportion of myosin heads bound during Ca2+-activation in MHC IIA fibers in patients (Figure 4D). As detailed above, a greater proportion of myosin heads bound to actin would increase tension. This adaptation could be explained by a change in myosin kinetic parameters,11 which either increases the amount of time myosin is bound to actin and/or decreases the amount of time myosin is detached from actin. Thus, our results suggest that heart failure alters both the quantity and kinetic properties of the myosin molecule in skeletal muscle, with functional consequences that are fiber-type specific.
Our current results in single fibers differ from our previous findings in isolated myosin and thin filaments evaluated using the in vitro motility assay, which showed no effect of heart failure on force production or contractile velocity.39 These differences between studies are likely due to differences in the two assays of myofilament function. In the in vitro motility assay, the quantity of myosin and thin filament proteins used is standardized, which negates the functional effect of MHC protein depletion evident in skinned fibers from heart failure patients. Moreover, in the mixed fiber preparations used in the motility assay in our prior study,39 myosin kinetic properties are dominated by MHC I molecules.40 Hence, alterations in MHC IIA kinetics evident in skinned fibers (Figure 4D) would be masked in the motility assay. Additionally, our results differ somewhat from prior studies that showed large reductions (>30%) in single fiber tension in MHC I and IIA fibers from heart failure patients.12 Here again, direct comparisons between studies are difficult since controls in this prior study were 12 yrs younger than patients and groups were not matched for physical activity level. Thus, the large tension reductions may have been related to aging and/or muscle disuse.17 In contrast, patients and controls in our study were well-matched for age and physical activity, making our findings more reflective of the direct effects of the heart failure syndrome.
In light of the potential relevance of MHC protein depletion, we sought to identify the mechanisms whereby heart failure promotes a loss of skeletal muscle myosin by measuring MHC mRNA abundance and indices of MHC protein degradation. No group differences in MHC mRNA abundance were found, suggesting no alteration in MHC gene transcription. These results differ from our prior work, where we observed a trend towards reduced MHC mRNA in patients.10 This prior finding is likely explained by the fact that we did not match controls and patients for physical activity. Consequently, reduced MHC mRNA was explained entirely by decreased MHC I mRNA,10 which is likely due to inactivity-induced reductions in MHC I gene expression.30 This further emphasizes the importance of considering the activity status of controls. Additionally, using multiple techniques, we found no evidence for elevated MHC protein degradation in patients (Figure 5). These results contrast with recent work in a rat model of heart failure showing that pharmacological treatment with an inhibitor of protein breakdown can prevent the loss of MHC from single diaphragm fibers.41 Reasons for differing results are not clear, but may relate to the fact that we expect the heart failure syndrome to be more severe and rapidly progressive in this animal model compared to well-treated, clinically-stable patients. These differences in disease status between animal models and patients highlight a potential explanation for the MHC protein content depletion observed in our study; specifically, that reduced MHC gene expression and increased proteolysis occur in patients and precipitate MHC protein loss during periods of acute disease exacerbation and hospitalization. These episodes are characterized by neurohumoral/immune activation42,43 and physical inactivity, both of which could contribute to the depletion of MHC protein.17,32
In conclusion, our results suggest selective myosin protein depletion from individual muscle fibers as a potential molecular mechanism contributing to skeletal muscle weakness in heart failure patients. Because controls and patients were similar for age and physical activity level and there was no evidence for muscle atrophy in patients, we believe that this phenotype is reflective of the effects of the heart failure syndrome on skeletal muscle, rather than the effects of aging, muscle atrophy or physical inactivity. Moreover, the fact that these observations were made in well-treated patients with mild to moderate heart failure suggests that single muscle fiber myosin depletion is not merely a manifestation of end-stage disease, but rather a distinct feature of the skeletal muscle myopathy of heart failure.
Supplementary Material
Acknowledgments
We thank all the volunteers who dedicated their valuable time to these studies.
FUNDING SOURCES
This study was funded by grants from the National Institutes of Health HL-077418, AG-031303, RR-16435 and RR-00109. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart Lung and Blood Institute, National Institute on Aging or National Institutes of Health.
Footnotes
CLINICAL PERSPECTIVE
Patients suffering from chronic heart failure frequently experience skeletal muscle weakness, which limits physical function and contributes to their high rates of physical disability. Our study evaluated the mechanisms underlying skeletal muscle weakness in human heart failure by comparing myofilament protein content, function and sarcomeric structure in single skeletal muscle fibers isolated from patients and non-diseased controls. Our results show that heart failure is characterized by a selective loss of the contractile protein myosin from single muscle fibers, which contributed to reduced tension in slow-twitch muscle fibers. This functional impairment may be of clinical significance since slow-twitch fibers are recruited for repetitive movements typically encountered in daily activities and are an important determinant of exercise tolerance. In this context, loss of myosin protein from single muscle fibers represents a potential molecular mechanism underlying physical disability and exercise intolerance in heart failure patients. Moreover, the fact that these observations were made in well-treated patients with mild to moderate heart failure suggests that single muscle fiber myosin depletion is not merely a manifestation of end-stage disease, but rather a distinct feature of the skeletal muscle myopathy of heart failure.
DISCLOSURES
No conflicts of interest to disclose.
References
- 1.Toth MJ, Gottlieb SS, Fisher ML, Poehlman ET. Skeletal muscle atrophy and peak oxygen consumption in heart failure. Am J Cardiol. 1997;79:1267–1269. doi: 10.1016/s0002-9149(97)00098-2. [DOI] [PubMed] [Google Scholar]
- 2.Harrington D, Anker SD, Chua TP, Webb-Peploe KM, Ponikowski PP, Poole-Wilson PA, Coats AJ. Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. J Am Coll Cardiol. 1997;30:1758–1764. doi: 10.1016/s0735-1097(97)00381-1. [DOI] [PubMed] [Google Scholar]
- 3.Toth MJ, Shaw AO, Miller MS, VanBuren P, LeWinter MM, Maughan DW, Ades PA. Reduced knee extensor function in heart failure is not explained by inactivity. Int J Cardiol. doi: 10.1016/j.ijcard.2009.02.040. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minotti JR, Pillay P, Chang L, Wells L, Massie BM. Neurophysiological assessment of skeletal muscle fatigue in patients with congestive heart failure. Circulation. 1992;86:903–908. doi: 10.1161/01.cir.86.3.903. [DOI] [PubMed] [Google Scholar]
- 5.Wilson JR, Mancini DM. Factors contributing to the exercise limitation of heart failure. J Am Coll Cardiol. 1993;22:93A–98A. doi: 10.1016/0735-1097(93)90469-h. [DOI] [PubMed] [Google Scholar]
- 6.Schaufelberger M, Eriksson BO, Lonn L, Rundqvist B, Sunnerhagen KS, Swedberg K. Skeletal muscle characteristics, muscle strength and thigh muscle area in patients before and after cardiac transplantation. Eur J Heart Fail. 2001;3:59–67. doi: 10.1016/s1388-9842(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 7.Neill WA, Branch LG, De Jong G, Smith NE, Hogan CA, Corcoran PJ, Jette AM, Balasco EM, Osberg S. Cardiac disability. The impact of coronary heart disease on patients’ daily activities. Arch Intern Med. 1985;145:1642–1647. doi: 10.1001/archinte.145.9.1642. [DOI] [PubMed] [Google Scholar]
- 8.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 9.Pu CT, Johnson MT, Forman DE, Hausdorff JM, Roubenoff R, Foldvari M, Fielding RA, Singh MA. Randomized trial of progressive resistance training to counteract the myopathy of chronic heart failure. J Appl Physiol. 2001;90:2341–2350. doi: 10.1152/jappl.2001.90.6.2341. [DOI] [PubMed] [Google Scholar]
- 10.Toth MJ, Ades PA, LeWinter MM, Tracy RP, Tchernof A. Skeletal muscle myofibrillar mRNA expression in heart failure: relationship to local and circulating hormones. J Appl Physiol. 2006;100:35–41. doi: 10.1152/japplphysiol.00570.2005. [DOI] [PubMed] [Google Scholar]
- 11.van Hees HW, van der Heijden HF, Ottenheijm CA, Heunks LM, Pigmans CJ, Verheugt FW, Brouwer RM, Dekhuijzen PN. Diaphragm single-fiber weakness and loss of myosin in congestive heart failure rats. Am J Physiol Heart Circ Physiol. 2007;293:H819–828. doi: 10.1152/ajpheart.00085.2007. [DOI] [PubMed] [Google Scholar]
- 12.Szentesi P, Bekedam MA, van Beek-Harmsen BJ, van der Laarse WJ, Zaremba R, Boonstra A, Visser FC, Stienen GJ. Depression of force production and ATPase activity in different types of human skeletal muscle fibers from patients with chronic heart failure. J Appl Physiol. 2005;99:2189–2195. doi: 10.1152/japplphysiol.00542.2005. [DOI] [PubMed] [Google Scholar]
- 13.Yates LD, Greaser ML. Quantitative determination of myosin and actin in rabbit skeletal muscle. J Mol Biol. 1983;168:123–141. doi: 10.1016/s0022-2836(83)80326-x. [DOI] [PubMed] [Google Scholar]
- 14.Bottinelli R. Functional heterogeneity of mammalian single muscle fibres: do myosin isoforms tell the whole story? Pflugers Arch. 2001;443:6–17. doi: 10.1007/s004240100700. [DOI] [PubMed] [Google Scholar]
- 15.Toth MJ, Matthews DE, Ades PA, Tischler MD, VanBuren P, Previs M, LeWinter MM. Skeletal muscle myofibrillar protein metabolism in heart failure: relationship to immune activation and functional capacity. Am J Physiol Endocrinol Metab. 2005;288:E685–692. doi: 10.1152/ajpendo.00444.2004. [DOI] [PubMed] [Google Scholar]
- 16.Toth MJ, Gottlieb SS, Goran MI, Fisher ML, Poehlman ET. Daily energy expenditure in free-living heart failure patients. Am J Physiol. 1997;272:E469–475. doi: 10.1152/ajpendo.1997.272.3.E469. [DOI] [PubMed] [Google Scholar]
- 17.D’Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Saltin B, Bottinelli R. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol. 2003;552:499–511. doi: 10.1113/jphysiol.2003.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toth MJ, Sites CK, Cefalu WT, Matthews DE, Poehlman ET. Determinants of insulin-stimulated glucose disposal in middle-aged, premenopausal women. Am J Physiol Endocrinol Metab. 2001;281:E113–121. doi: 10.1152/ajpendo.2001.281.1.E113. [DOI] [PubMed] [Google Scholar]
- 19.Naughton J, Balke B, Nagle F. Refinements in method of evaluation and physical conditioning before and after myocardial infarction. Am J Cardiol. 1964;14:837–843. doi: 10.1016/0002-9149(64)90011-6. [DOI] [PubMed] [Google Scholar]
- 20.Ades PA, Savage PD, Cress ME, Brochu M, Lee NM, Poehlman ET. Resistance training on physical performance in disabled older female cardiac patients. Med Sci Sports Exerc. 2003;35:1265–1270. doi: 10.1249/01.MSS.0000079044.21828.0E. [DOI] [PubMed] [Google Scholar]
- 21.Toth MJ, Palmer BM, LeWinter MM. Effect of heart failure on skeletal muscle myofibrillar protein content, isoform expression and calcium sensitivity. Int J Cardiol. 2006;107:211–219. doi: 10.1016/j.ijcard.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 22.Geiger PC, Cody MJ, Macken RL, Sieck GC. Maximum specific force depends on myosin heavy chain content in rat diaphragm muscle fibers. J Appl Physiol. 2000;89:695–703. doi: 10.1152/jappl.2000.89.2.695. [DOI] [PubMed] [Google Scholar]
- 23.Hilber K, Galler S. Improvement of the measurements on skinned muscle fibres by fixation of the fibre ends with glutaraldehyde. J Muscle Res Cell Motil. 1998;19:365–372. doi: 10.1023/a:1005393519811. [DOI] [PubMed] [Google Scholar]
- 24.Miller MS, Lekkas P, Braddock JM, Farman GP, Ballif BA, Irving TC, Maughan DW, Vigoreaux JO. Aging enhances indirect flight muscle fiber performance yet decreases flight ability in Drosophila. Biophys J. 2008 doi: 10.1529/biophysj.108.130005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ball RD, Krus DL, Alizadeh B. Myosin degradation fragments in skeletal muscle. J Mol Biol. 1987;193:47–56. doi: 10.1016/0022-2836(87)90625-5. [DOI] [PubMed] [Google Scholar]
- 26.Adams GR, Caiozzo VJ, Baldwin KM. Skeletal muscle unweighting: spaceflight and ground-based models. J Appl Physiol. 2003;95:2185–2201. doi: 10.1152/japplphysiol.00346.2003. [DOI] [PubMed] [Google Scholar]
- 27.Mettauer B, Zoll J, Sanchez H, Lampert E, Ribera F, Veksler V, Bigard X, Mateo P, Epailly E, Lonsdorfer J, Ventura-Clapier R. Oxidative capacity of skeletal muscle in heart failure patients versus sedentary or active control subjects. J Am Coll Cardiol. 2001;38:947–954. doi: 10.1016/s0735-1097(01)01460-7. [DOI] [PubMed] [Google Scholar]
- 28.Vescovo G, Serafini F, Facchin L, Tenderini P, Carraro U, Dalla Libera L, Catani C, Ambrosio GB. Specific changes in skeletal muscle myosin heavy chain composition in cardiac failure: differences compared with disuse atrophy as assessed on microbiopsies by high resolution electrophoresis. Heart. 1996;76:337–343. doi: 10.1136/hrt.76.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sullivan MJ, Duscha BD, Klitgaard H, Kraus WE, Cobb FR, Saltin B. Altered expression of myosin heavy chain in human skeletal muscle in chronic heart failure. Med Sci Sports Exerc. 1997;29:860–866. doi: 10.1097/00005768-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Baldwin KM, Haddad F. Effects of different activity and inactivity paradigms on myosin heavy chain gene expression in striated muscle. J Appl Physiol. 2001;90:345–357. doi: 10.1152/jappl.2001.90.1.345. [DOI] [PubMed] [Google Scholar]
- 31.Larsson L, Li X, Edstrom L, Eriksson LI, Zackrisson H, Argentini C, Schiaffino S. Acute quadriplegia and loss of muscle myosin in patients treated with nondepolarizing neuromuscular blocking agents and corticosteroids: mechanisms at the cellular and molecular levels. Crit Care Med. 2000;28:34–45. doi: 10.1097/00003246-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Acharyya S, Ladner KJ, Nelsen LL, Damrauer J, Reiser PJ, Swoap S, Guttridge DC. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest. 2004;114:370–378. doi: 10.1172/JCI20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franchi LL, Murdoch A, Brown WE, Mayne CN, Elliott L, Salmons S. Subcellular localization of newly incorporated myosin in rabbit fast skeletal muscle undergoing stimulation-induced type transformation. J Muscle Res Cell Motil. 1990;11:227–239. doi: 10.1007/BF01843576. [DOI] [PubMed] [Google Scholar]
- 34.Huxley AF. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- 35.Brenner B. Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci USA. 1988;85:3265–3269. doi: 10.1073/pnas.85.9.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rome LC, Funke RP, Alexander RM, Lutz G, Aldridge H, Scott F, Freadman M. Why animals have different muscle fibre types. Nature. 1988;335:824–827. doi: 10.1038/335824a0. [DOI] [PubMed] [Google Scholar]
- 37.Hennig R, Lomo T. Firing patterns of motor units in normal rats. Nature. 1985;314:164–166. doi: 10.1038/314164a0. [DOI] [PubMed] [Google Scholar]
- 38.Vescovo G, Serafini F, Dalla Libera L, Leprotti C, Facchin L, Tenderini P, Ambrosio GB. Skeletal muscle myosin heavy chains in heart failure: correlation between magnitude of the isozyme shift, exercise capacity, and gas exchange measurements. Am Heart J. 1998;135:130–137. doi: 10.1016/s0002-8703(98)70353-9. [DOI] [PubMed] [Google Scholar]
- 39.Okada Y, Toth MJ, VanBuren P. Skeletal muscle contractile protein function is preserved in human heart failure. J Appl Physiol. 2008;104:952–957. doi: 10.1152/japplphysiol.01072.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris DE, Work SS, Wright RK, Alpert NR, Warshaw DM. Smooth, cardiac and skeletal muscle myosin force and motion generation assessed by cross-bridge mechanical interactions in vitro. J Muscle Res Cell Motil. 1994;15:11–19. doi: 10.1007/BF00123828. [DOI] [PubMed] [Google Scholar]
- 41.van Hees HW, Li YP, Ottenheijm CA, Jin B, Pigmans CJ, Linkels M, Dekhuijzen PN, Heunks LM. Proteasome inhibition improves diaphragm function in congestive heart failure rats. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1260–1268. doi: 10.1152/ajplung.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dzau VJ, Colucci WS, Hollenberg NK, Williams GH. Relation of the renin-angiotensin-aldosterone system to clinical state in congestive heart failure. Circulation. 1981;63:645–651. doi: 10.1161/01.cir.63.3.645. [DOI] [PubMed] [Google Scholar]
- 43.Sato Y, Takatsu Y, Kataoka K, Yamada T, Taniguchi R, Sasayama S, Matsumori A. Serial circulating concentrations of C-reactive protein, interleukin (IL)-4, and IL-6 in patients with acute left heart decompensation. Clin Cardiol. 1999;22:811–813. doi: 10.1002/clc.4960221211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.