Abstract
Introduction
Near-infrared spectroscopy (NIRS) is a non-invasive, real-time bedside modality sensitive to changes in cerebral perfusion and oxygenation and is highly sensitive to physiological oscillations at different frequencies. However, the clinical feasibility of NIRS remains limited, partly due to concerns regarding NIRS signal quantification, which relies on mostly arbitrary assumptions on hemoglobin concentrations and tissue layers. In this pilot study comparing stroke patients to healthy controls, we explored the utility of the interhemispheric correlation coefficient (IHCC) during physiological oscillations in detecting asymmetry in hemispheric microvascular hemodynamics.
Methods
Using bi-hemispheric continuous-wave NIRS, 12 patients with hemispheric strokes and 9 controls were measured prospectively. NIRS signal was band-pass filtered to isolate cardiac (0.7–3 Hz) and respiratory (0.15–0.7 Hz) oscillations. IHCCs were calculated in both oscillation frequency bands. Using Fisher’s Z-transform for non-Gaussian distributions, the IHCC during cardiac and respiratory oscillations were compared between both groups.
Results
Nine patients and nine controls had data of sufficient quality to be included in the analysis. The IHCCs during cardiac and respiratory oscillations were significantly different between patients versus controls (cardiac 0.79 ± 0.18 vs. 0.94 ± 0.07, P = 0.025; respiratory 0.24 ± 0.28 vs. 0.59 ± 0.3; P = 0.016).
Conclusions
Computing the IHCC during physiological cardiac and respiratory oscillations may be a new NIRS analysis technique to quantify asymmetric microvascular hemodynamics in stroke patients in the neurocritical care unit. It allows each subject to serve as their own control obviating the need for arbitrary assumptions on absolute hemoglobin concentration. Future clinical applications may include rapid identification of patients with ischemic brain injury in the pre-hospital setting. This promising new analysis technique warrants further validation.
Keywords: Near-infrared spectroscopy, Cerebrovascular disease, Stroke, Critical care
Introduction
Bedside or out-of-hospital measurement of global or regional cerebral oxygenation, not surrogate markers such as intracranial pressure or point-source oxygenation has thus far not been reliably achieved. Near-infrared spectroscopy (NIRS) is an emerging non-invasive modality which provides real-time, continuous bedside sensitivity to the regional perfusion and oxygenation states of the brain [1]. NIRS is based on local illumination of the head by red-and near-infrared light, and the detection of the diffused light which exits the tissue after propagation through the scalp, skull, and superficial layers of the cortex. By measuring changes in light reflectance at two or more wavelengths, NIRS enables the spectroscopic resolution of oxy-hemoglobin (HbO) and deoxy-hemoglobin (HbR) concentration changes. Consequently, variations in oxygen saturation and total hemoglobin, which is directly proportional to cerebral blood volume, can be measured. NIRS has mostly been applied to measuring cerebral hemodynamic changes associated with brain activation during functional studies (motor, somatosensory, visual, auditory, cognitive, and language) [2–7], as well as to assessing variations in brain oxygenation during cardiac surgery, neurovascular interventions, and stroke [8–11]. The most commonly used NIRS modality, continuous-wave (CW) NIRS, enables measurements of relative changes in hemoglobin concentrations over seconds (as is the case during cerebral activation), but cannot measure absolute concentrations, nor their changes over minutes or hours [12–14]. For this purpose, various assumptions on absolute hemoglobin concentration and tissue layer structures are commonly made [15]. Variability in anatomy makes comparisons between patients difficult and probably incorrect [12].
In this pilot study, we explore a new approach and take advantage of the temporal features of the CW-NIRS signal. CW-NIRS is highly sensitive to spontaneous or induced oscillations in HbO and HbR occurring at different physiological frequencies, including cardiac, respiratory, and lower frequencies [16, 17]. These oscillations may reflect to some degree of cerebrovascular dynamic autoregulation, and NIRS has recently been suggested as a new means to assess dynamic cerebral autoregulation [18–20].
We hypothesized that CW-NIRS derived physiological oscillation patterns may be asymmetric between hemispheres in stroke patients rather than symmetric as observed in healthy controls, and therefore quantification of this asymmetry using the interhemispheric correlation coefficient (IHCC) might detect subtle abnormal hemispheric microvascular hemodynamics without the need for assumptions about absolute hemoglobin values and tissue layers.
Methods
Subjects
The Massachusetts General Hospital (MGH) Institutional Review Board approved the study protocol. Written informed consent was obtained from all patients or their surrogate and all control subjects. Twelve patients with ischemic strokes were recruited from the neurocritical care unit at MGH and measured prospectively, as were nine healthy control subjects (n = 6 female). Healthy controls were recruited with posted and emailed advertisements of the study and did not have a history of stroke or transient ischemic attack. Nine stroke subjects (n = 4 female) met the pre-specified data quality criteria (described below) and were compared to control subjects.
Instrument and Measurement
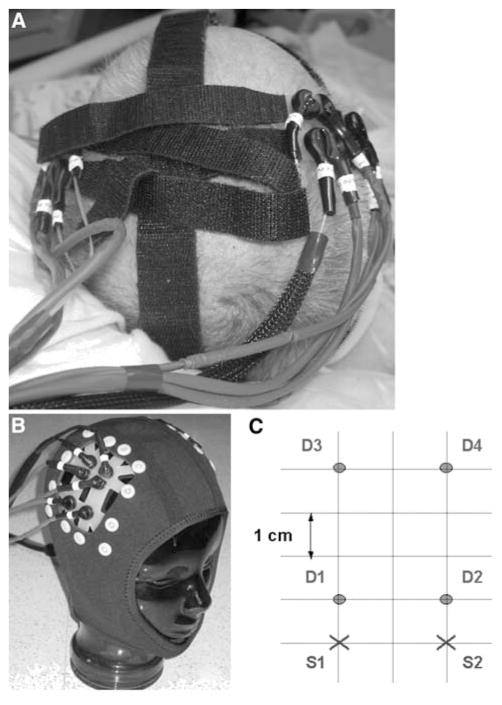
We have developed a portable CW-NIRS system which can be placed on any region of the adult human head and provides very high temporal resolution (up to 200 Hz) [4, 16]. For this pilot study, we used two synchronized CW-NIRS units (NIRS2, TechEn Inc., Milford, MA), one for each cerebral hemisphere. Each unit consisted of four light sources at two different wavelengths (690 and 830 nm) and four avalanche photodiode detectors. The NIRS probes were placed symmetrically on the scalp of the stroke patients at the approximate location overlying the injured area and on the mirror-image location using Velcro® strips or a Neoprene® cap for stabilization (Fig. 1a, b). In healthy controls the probes were placed symmetrically on the scalp of the subjects over the middle cerebral artery territories using Velcro® strips or a Neoprene® cap for stabilization (Fig. 1a, b). The specific probe geometry is shown in Fig. 1c. With this configuration each source was detected at a long distance (4 cm). After optimal probe positioning, 1–10 measurements of 2–15 min each were recorded. The actual recording time depended on the ability of the subject to stay still during the measurement, with the shortest recording times (2 min) in patients with excessive head movements.
Fig. 1.
NIRS probes are attached to a patient’s head at the bedside using Velcro® strips (a) or a neoprene skull cap (b). c The source-detector geometry overlying each hemisphere consists of two source positions (S1, 2) two cm apart, and four detector positions (D1–4) at one and four cm distance from each source
In healthy volunteers, we recorded synchronously with the NIRS signal: arterial saturation and heart rate with a pulse oxymeter (Nonin 8600, Nonin Medical Systems, Inc., Minneapolis, MN), respiration movements with a respiratory belt (Sleepmate, Newlife Technologies, Midlothian, VA), and blood pressure variations non-invasively from a finger measurement (pressure pad/respiration transducer (TSD110) with transducer amplifier (DA100C), Biopac Systems, Inc., Goleta, CA). In stroke subjects, the auxiliary physiological signals were recorded directly from the analog output of their clinical monitoring module (TRAM-Rac 4A, GE Medical Systems, South Burlington, VT). These signals included some or all of the following: heart rate and rhythm, arterial saturation, respiratory rate, and arterial blood pressure from an invasive arterial line. For some patients, not all auxiliary physiological signals were available because measurements were done after discharge from the neurocritical care unit on a regular floor where patients were not monitored physiologically.
Data Analysis
Pre-specified objective criteria for data quality were applied to all raw data in order to remove the channels with excessive motion artifacts (signal to noise ratio above 30, with the noise level defined as the standard deviation of the signal over the recording time). Only data meeting these quality criteria were included in the analysis. Therefore, three stroke patients were excluded (Table 1). We analyzed data obtained from the long source-detector distances (4 cm), because these are more sensitive to changes in the deeper tissue layers (i.e., superficial layers of the cortex of the brain), while data from shorter source-detector distances reflect mostly more superficial extracranial tissues (i.e., scalp). Raw data were converted to changes in optical density (ΔOD) and then band-pass filtered in two different frequency bands to extract the cardiac (0.7–3 Hz) and respiratory (0.15–0.7 Hz) oscillations (Chebitchev low-pass filter and Butterworth high-pass filter). In all subjects, we confirmed that the cardiac and respiratory oscillations were within these frequency bands.
Table 1.
Baseline characteristics of all stroke patients and healthy control subjects
| Subject | Age | Gender | Side | Vessel | Stroke | Character of stroke | Volume (ml) | Ipsilateral ICA stenosis | Contra- lateral ICA stenosis | IV- rtPA given | Measured on post-stroke day |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | M | L | SSS | Venous | Ischemic with hemorrhage | 142 | None | None | No | 3 |
| 2 | 59 | F | R | MCA | Arterial | Ischemic | 72 | Occlusion | Moderate | Yes | 6 |
| 3 | 74 | F | L | MCA | Arterial | Ischemic | 132 | None | None | Yes | 10 |
| 4 | 72 | M | R | MCA | Arterial | Ischemic | 185 | None | Moderate | No | 9 |
| 5 | 89 | F | R | MCA | Arterial | Ischemic with hemorrhage | 39 | None | None | Yes | 3 |
| 6 | 72 | M | R | MCA, ACA | Arterial | Ischemic | 131 | None | Moderate | No | 9 |
| 7 | 55 | F | L | MCA | Arterial | Ischemic | 80 | Occlusion | None | No | 2 |
| 8 | 70 | M | R | MCA | Arterial | Ischemic | 14 | Severe | Moderate | Yes | 2 |
| 9 | 49 | M | R | MCA | Arterial | Ischemic | 243 | Occlusion | None | No | 9 |
| Excluded patients: | |||||||||||
| 10 | 74 | M | R | MCA | Arterial | Ischemic | 149 | Severe | None | No | 5 |
| 11 | 75 | F | L | MCA | Arterial | Ischemic | 85 | None | None | No | 3 |
| 12 | 45 | M | L | MCA | Arterial | Ischemic | 213 | Occlusion | None | No | 5 |
| Healthy control subjects: | |||||||||||
| 1 | 61 | M | |||||||||
| 2 | 51 | F | |||||||||
| 3 | 64 | F | |||||||||
| 4 | 60 | F | |||||||||
| 5 | 74 | M | |||||||||
| 6 | 58 | F | |||||||||
| 7 | 56 | M | |||||||||
| 8 | 62 | F | |||||||||
| 9 | 77 | F | |||||||||
ACA anterior cerebral artery, F female, IA intraarterial, ICA internal cerebral artery, IV intravenous, L left, M male, MCA middle cerebral artery, R right, rtPA recombinant tissue plasminogen activator, SSS superior sagittal sinus
We computed the interhemispheric correlation coefficient (IHCC) within each individual (defined as the zero-lag cross-correlation of the ΔOD signal at 830 nm between symmetrical channels on both hemispheres), averaged over all measurements and channels. An IHCC of 1 indicates that both hemispheres are perfectly symmetric (in phase), while an IHCC of −1 indicates the perfect anti-symmetry (180° phase delay). An IHCC of 0 indicates a 90° phase delay between hemispheres or a complete asymmetry. An example is shown in Fig. 2. The IHCC is insensitive to the relative amplitude of the oscillations; it is therefore a measure of the symmetry of the temporal features of the signal, and not their amplitude.
Fig. 2.
An example calculation of the interhemispheric correlation coefficients (IHCC) is shown in a control and stroke subject. The change in optical density (ΔOD) at 830 nm for a healthy control (top) and a stroke subject (bottom) are shown after data filtering in the cardiac (0.7–3 Hz, left) and the respiratory (0.15–0.7 Hz, right) frequency bands. Note the different time scales. For simplicity, only two channels are presented for each hemisphere. The corresponding IHCCs are shown in the boxes
Additional covariates obtained include age, gender, vascular territory of the stroke, lesion volume as measured by one of the investigators (S.M.) according to the ellipsoid volume estimation method [21] on a CT, or diffusion weighted MRI study on the day of or closest to the day of NIRS-measurement, and degree of ipsi- and contralateral internal carotid artery (ICA) stenosis as read by an independent neuroradiologist on CT-angiogram or MR-angiogram using the North American Symptomatic Carotid Endarterectomy Trial method [22].
Statistical Analysis
Using Fisher’s Z-transform for non-Gaussian distributions, the IHCC during cardiac and respiratory oscillations were compared between stroke and control subjects using a two-sample t-test (mean ± SD) with alpha set at 0.05. All values are expressed as non-transformed mean ± SD.
Results
Baseline characteristics of all subjects are shown in Table 1. Nine patients and nine healthy control subjects were included in the analysis. There was no age difference between stroke patients and control subjects (67 ± 12 years vs. 63 ± 8 years; P = NS [Wilcoxon test]). The stroke lesion volume was 115 ± 68 ml. Representative thumbnail images of the stroke territories are shown in Table 2. Eight patients had embolic arterial strokes and one patient had a venous infarction. One arterial stroke had a small area of hemorrhagic transformation after intravenous recombinant tissue plasminogen activator. The ICA ipsilateral to the stroke was either occluded or severely stenosed in 4/9 (44%) of the patients.
Table 2.
Image thumbnails (either non-contrast CT or diffusion weighted-MRI) are shown for each included stroke subject
 |
For cardiac oscillations, IHCCs were significantly different between stroke patients versus control subjects (0.79 ± 0.18 vs. 0.94 ± 0.07, P = 0.025). Of note, within this frequency band, there was tight interhemispheric correlation in both groups, as reflected by the IHCCs closer to 1. For respiratory oscillations, the IHCCs were also significantly different between stroke patients versus control subjects (0.24 ± 0.28 vs. 0.59 ± 0.3; P = 0.016 [Fig. 3]).
Fig. 3.
Boxplots (mean ± SD) with individual datapoints of the interhemispheric correlation coefficients (IHCC) of the stroke patients (n = 9) and control group (n = 9) are shown during cardiac (left) and respiratory oscillations (right). The asterisks indicate P < 0.05. Mean ± SD IHCC during cardiac oscillations was 0.79 ± 0.18 for the stroke patients and 0.94 ± 0.07 for the control group (P = 0.025). Mean ± SD IHCC during respiratory oscillations was 0.24 ± 0.28 for the stroke patients and 0.59 ± 0.3 for the control group (P = 0.016)
Discussion
In this pilot study, we observed that IHCCs of CW-NIRS at both cardiac and respiratory oscillation frequencies were significantly different between stroke patients and healthy controls. The strength of the study is the application of a novel analysis technique using IHCC that allows each subject to serve as their own control obviating the need for controversial assumptions on absolute baseline hemoglobin concentration and anatomic variations.
Three different NIRS techniques currently exist, each based on a specific illumination type [23, 24]: (1) the CW modality is based on constant illumination of the medium, and simply measures the attenuation of light through the head; (2) frequency-domain (FD) devices illuminate the head with intensity-modulated light, and measure both the attenuation and the phase shift of the emerging light; (3) the time-domain (TD) technology is based on short pulses of light, and the time-resolved detection of the shape of the pulse after propagation through the head. In ascending order, CW, FD, and TD-NIRS involve increased cost and technological complexity, but also offer more detailed information about the studied medium. In particular, FD and TD technologies enable the absolute characterization of the optical properties of the head, from which one can retrieve absolute values of cerebral blood volume and oxygenation. This feature is not available with CW systems, which only enable relative measurements of hemoglobin variations. On the other hand, CW devices provide very high temporal resolution (as fast as a few tens of milliseconds), and offer the advantages of low-cost and portability.
In this study, we propose a different approach by taking advantage of the temporal features of the CW signal: rather than measuring the absolute values of hemoglobin concentrations or the amplitude of the concentration oscillations, our analysis focuses on the temporal characteristics of the oscillations. Measuring NIRS signals at the 4 cm light detector distance limits, but does not entirely eliminate signals from the superficial scalp layers. Despite the contribution from the scalp to these signals, we detected a temporal asymmetry during physiological oscillations in stroke patients, hence demonstrating that these signals most likely arise from cerebral structures.
We did not investigate the cause of the reduced IHCCs during these physiological oscillations, but a plausible hypothesis is that this finding is due to a disruption in dynamic cerebral autoregulation (DCA). This is in concordance with other studies examining DCA. In the healthy state, DCA is elicited during fast, short changes in systemic blood pressure or other hemodynamic parameters. These can be induced by a rapid-step decrease in arterial blood pressure, and have traditionally been studied on a macrovascular level using transcranial Doppler [25]. Several investigators have shown that spontaneous blood pressure oscillations or changes in intrathoracic pressure can be used to study DCA [26–29]. CW-NIRS can assess DCA on a microvascular level and supplements TCD [18]. Other investigators have confirmed that CW-NIRS can non-invasively detect spontaneous oscillations in cerebral blood volume [16, 17, 30] and CW-NIRS is recently emerging as a new modality to assess dynamic autoregulation [20, 30]. The interesting observation in our study was that the difference in the IHCC was larger during the lower frequency oscillations. One explanation could be that cardiac oscillations may be too fast to detect the autoregulatory response. Alternatively, it may be that the respiration-induced fluctuations in end-tidal carbon dioxide (CO2) concentrations produced different degrees of vasodilation on the normal versus injured hemispheres and thereby desynchronized the two hemispheres to a larger degree.
The differences in the IHCCs during physiological oscillations seen in our study could potentially be explained by differences in the degree of carotid disease given that 44% of our patients had high-degree ipsilateral carotid disease. A previous study has shown that perturbations in NIRS patterns may predict misery perfusion in patients with symptomatic carotid disease compared to preserved cerebral perfusion in patients with asymptomatic disease [8]. In a different study of patients with critically stenosed or occluded carotid arteries using TCD and CW-NIRS combined, the authors concluded that carotid disease may lead to perturbations of DCA [18]. Yet, neuroimaging was not performed in these patients, and therefore it is not known whether prior strokes were present and might be contributing to the DCA perturbation.
One of the main limitations of this pilot study is the small sample size which may increase the risk of both type 1 and type 2 error, and does not allow for statistical adjustment of covariates such as the presence or degree of carotid stenosis. Furthermore, although there was no difference in the mean age, the stroke and control patients were not age or disease-matched, and therefore we cannot exclude that age or co-morbid conditions were effect modifiers. Another limitation of the present study is the lack of knowledge about the origin of the blood flow detected by NIRS. While we believe that we are measuring signals derived from the underlying brain with little contribution from the skull based on the characteristic physiological oscillations observed in the NIRS signal, therefore suggesting either residual or collateral flow, we are unable to differentiate between the two with the data currently collected. Therefore, we cannot correlate the origin of the blood flow to the observed asymmetry in the IHC. Further investigation will be required in order to answer this question, notably by correlating perfusion maps from CT perfusion or MR perfusion to the CW-NIRS oscillations. Finally, 25% of the stroke subjects in this pilot study had to be removed from the analysis because the data did not meet the quality criteria, which in all three cases was due to excessive motion artifacts. Further technical improvement in the probe design and stabilization when attached to the scalp is required to enable good contact of the probe with the scalp during several minutes of recording in intensive care unit patients who are agitated, while still maintaining acceptable levels of patient comfort.
In conclusion, our results suggest that CW-NIRS during physiological cardiac and respiratory oscillations may detect asymmetry in microvascular hemodynamics between hemispheres in stroke patients. Calculating the IHCC may be a useful quantification technique of CW-NIRS signals, obviating the need for assumptions on absolute hemoglobin values or extracranial tissues. Further prospective study involving larger numbers of patients and comparing patients with and without carotid disease as well as comparing the effects of deep and cortical strokes is warranted. This technique could be extended to simultaneous measurements over different territories of the head, in particular for patients where the extent of the injury is unknown.
The results could be applied during non-invasive optical monitoring not just of stroke patients but other patients at risk for primary or secondary brain injury. Conventional bedside monitoring techniques for these patients are mostly invasive and only measure surrogate markers such as intracranial pressure or point source oxygenation. They might be supplemented by CW-NIRS with IHCC calculation as a biomarker of regional microperfusion, once this technique has been further validated and proven to provide robust data with negligible artifact. In addition, if this technique could reliably identify patients with ischemic brain injury in the pre-hospital setting, it could potentially be useful in the selected triage of patients for thrombolytic or neuroprotective strategies.
Acknowledgments
This work was supported by the National Institute of Health/National Center for Research Resources (P41RR14075; PI: David Boas).
Footnotes
Disclosure: The use of NIRS2 as described in this study is off-label. David Boas has received royalties from TechEn, Inc. (Milford, MA) on the sales of NIRS instruments. None of the other authors report any conflict of interest.
References
- 1.Jobsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198(4323):1264–7. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- 2.Meek JH, Elwell CE, Khan MJ, et al. Regional changes in cerebral haemodynamics as a result of a visual stimulus measured by near infrared spectroscopy. Proc Biol Sci. 1995;261(1362):351–6. doi: 10.1098/rspb.1995.0158. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe E, Maki A, Kawaguchi F, et al. Non-invasive assessment of language dominance with near-infrared spectroscopic mapping. Neurosci Lett. 1998;256(1):49–52. doi: 10.1016/s0304-3940(98)00754-x. [DOI] [PubMed] [Google Scholar]
- 4.Franceschini MA, Toronov V, Filiaci ME, Gratton E, Fantini S. On-line optical imaging of the human brain with 160-ms temporal resolution. Opt Express. 2000;6:49–57. doi: 10.1364/oe.6.000049. [DOI] [PubMed] [Google Scholar]
- 5.Kotilahti K, Nissila I, Huotilainen M, et al. Bilateral hemodynamic responses to auditory stimulation in newborn infants. Neuroreport. 2005;16(12):1373–7. doi: 10.1097/01.wnr.0000175247.35837.15. [DOI] [PubMed] [Google Scholar]
- 6.Wilcox T, Bortfeld H, Woods R, Wruck E, Boas DA. Using near-infrared spectroscopy to assess neural activation during object processing in infants. J Biomed Opt. 2005;10(1):11010. doi: 10.1117/1.1852551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niederhauser BD, Rosenbaum BP, Gore JC, Jarquin-Valdivia AA. A functional near-infrared spectroscopy study to detect activation of somatosensory cortex by peripheral nerve stimulation. Neurocrit Care. 2008;9(1):31–6. doi: 10.1007/s12028-007-9022-2. [DOI] [PubMed] [Google Scholar]
- 8.Vernieri F, Tibuzzi F, Pasqualetti P, et al. Transcranial Doppler and near-infrared spectroscopy can evaluate the hemodynamic effect of carotid artery occlusion. Stroke. 2004;35(1):64–70. doi: 10.1161/01.STR.0000106486.26626.E2. [DOI] [PubMed] [Google Scholar]
- 9.Damian MS, Schlosser R. Bilateral near infrared spectroscopy in space-occupying middle cerebral artery stroke. Neurocrit Care. 2007;6(3):165–73. doi: 10.1007/s12028-007-0010-3. [DOI] [PubMed] [Google Scholar]
- 10.Calderon-Arnulphi M, Alaraj A, Amin-Hanjani S, et al. Detection of cerebral ischemia in neurovascular surgery using quantitative frequency-domain near-infrared spectroscopy. J Neurosurg. 2007;106(2):283–90. doi: 10.3171/jns.2007.106.2.283. [DOI] [PubMed] [Google Scholar]
- 11.Nollert G, Jonas RA, Reichart B. Optimizing cerebral oxygenation during cardiac surgery: a review of experimental and clinical investigations with near infrared spectrophotometry. Thorac Cardiovasc Surg. 2000;48(4):247–53. doi: 10.1055/s-2000-6895. [DOI] [PubMed] [Google Scholar]
- 12.Muehlschlegel S, Lobato EB. Con: all cardiac surgical patients should not have intraoperative cerebral oxygenation monitoring. J Cardiothorac Vasc Anesth. 2006;20(4):613–5. doi: 10.1053/j.jvca.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Hargroves DR, Tallis RC, Pomeroy VM, Bhalla A. Near-infrared spectroscopy in stroke: from research to clinical practice. Stroke. 2004;35(11):2430–1. doi: 10.1161/01.STR.0000144656.77330.18. (author reply-1) [DOI] [PubMed] [Google Scholar]
- 14.Taillefer MC, Denault AY. Cerebral near-infrared spectroscopy in adult heart surgery: systematic review of its clinical efficacy. Can J Anaesth. 2005;52(1):79–87. doi: 10.1007/BF03018586. [DOI] [PubMed] [Google Scholar]
- 15.Hoshi Y. Functional near-infrared optical imaging: utility and limitations in human brain mapping. Psychophysiology. 2003;40(4):511–20. doi: 10.1111/1469-8986.00053. [DOI] [PubMed] [Google Scholar]
- 16.Franceschini MA, Joseph DK, Huppert TJ, Diamond SG, Boas DA. Diffuse optical imaging of the whole head. J Biomed Opt. 2006;11(5):054007. doi: 10.1117/1.2363365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obrig H, Neufang M, Wenzel R, et al. Spontaneous low frequency oscillations of cerebral hemodynamics and metabolism in human adults. Neuroimage. 2000;12(6):623–39. doi: 10.1006/nimg.2000.0657. [DOI] [PubMed] [Google Scholar]
- 18.Reinhard M, Wehrle-Wieland E, Grabiak D, et al. Oscillatory cerebral hemodynamics—the macro- versus microvascular level. J Neurol Sci. 2006;250(1–2):103–9. doi: 10.1016/j.jns.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Brady KM, Lee JK, Kibler KK, et al. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke. 2007;38(10):2818–25. doi: 10.1161/STROKEAHA.107.485706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steiner LA, Pfister D, Strebel SP, Radolovich D, Smielewski P, Czosnyka M. Near-infrared spectroscopy can monitor dynamic cerebral autoregulation in adults. Neurocrit Care. 2009;10(1):122–8. doi: 10.1007/s12028-008-9140-5. [DOI] [PubMed] [Google Scholar]
- 21.Sims JR, Gharai LR, Schaefer PW, et al. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72(24):2104–10. doi: 10.1212/WNL.0b013e3181aa5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1991;325(7):445–53. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 23.Obrig H, Villringer A. Beyond the visible-imaging the human brain with light. J Cereb Blood Flow Metab. 2003;23(1):1–18. doi: 10.1097/01.WCB.0000043472.45775.29. [DOI] [PubMed] [Google Scholar]
- 24.Gibson AP, Hebden JC, Arridge SR. Recent advances in diffuse optical imaging. Phys Med Biol. 2005;50(4):R1–43. doi: 10.1088/0031-9155/50/4/r01. [DOI] [PubMed] [Google Scholar]
- 25.Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989;20(1):45–52. doi: 10.1161/01.str.20.1.45. [DOI] [PubMed] [Google Scholar]
- 26.Blaber AP, Bondar RL, Stein F, et al. Transfer function analysis of cerebral autoregulation dynamics in autonomic failure patients. Stroke. 1997;28(9):1686–92. doi: 10.1161/01.str.28.9.1686. [DOI] [PubMed] [Google Scholar]
- 27.Diehl RR, Linden D, Lucke D, Berlit P. Phase relationship between cerebral blood flow velocity and blood pressure. A clinical test of autoregulation. Stroke. 1995;26(10):1801–4. doi: 10.1161/01.str.26.10.1801. [DOI] [PubMed] [Google Scholar]
- 28.Kuo TB, Chern CM, Sheng WY, Wong WJ, Hu HH. Frequency domain analysis of cerebral blood flow velocity and its correlation with arterial blood pressure. J Cereb Blood Flow Metab. 1998;18(3):311–8. doi: 10.1097/00004647-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol. 1998;274(1 to 2):H233–41. doi: 10.1152/ajpheart.1998.274.1.h233. [DOI] [PubMed] [Google Scholar]
- 30.Rowley AB, Payne SJ, Tachtsidis I, et al. Synchronization between arterial blood pressure and cerebral oxyhaemoglobin concentration investigated by wavelet cross-correlation. Physiol Meas. 2007;28(2):161–73. doi: 10.1088/0967-3334/28/2/005. [DOI] [PubMed] [Google Scholar]