Abstract
Objective
Despite cultural pressure to wean when a new pregnancy occurs, some women choose to continue breastfeeding. We determined the effect of an overlap of lactation and late pregnancy on breastfeeding and growth in early infancy.
Methods
We studied 133 Peruvian pregnant women who were ≥18 years of age, had a child <4 years old, and who then had a vaginal birth with a healthy, normal weight infant. Of the 133 women, 68 breastfed during the last trimester of pregnancy (BFP), and 65 had not breastfed during pregnancy (NBFP). On day 2 and at 1-month postpartum, 24-hour intake of breast milk and other liquids was measured. Twice weekly home surveillance documented infant morbidity and dietary intakes. Anthropometry was taken at birth and at 1 month. Maternal anthropometric, health, and socioeconomic status data were collected pre- and postpartum.
Results
Pregnant BFP mothers breastfed 5.3 ± 4.3 times/day. BFP and NBFP infants did not differ in breastfeeding behavior or in colostrum intake on day 2. BFP infants breastfed longer per feed and per 24 hours (35.2 minutes/24 hours) than did NBFP infants; however, 1-month intakes per feed tended to be lower among the BFP infants. After controlling for confounders, BFP infants gained 125 g less than did NBFP infants (about 15% of mean weight gain). A sustained decline would result in a −0.7 z score change in weight-for-age by 6 months.
Conclusions
A lactation-pregnancy overlap had a negative effect on early infant outcomes. Additional studies are needed to determine whether the effect continues past 1 month of age.
Keywords: breastfeeding, pregnancy, overlap, breast milk volume, weight gain, infant feeding, Peru
ABBREVIATIONS: BFP, breastfed during pregnancy; NBFP, did not breastfeed during pregnancy; BMI, body mass index; SES, socioeconomic status; CI, confidence interval; OR, odds ratio
Many women throughout the world breastfeed for as long as possible to give their children the nutritional, immunologic, and emotional benefits of breastfeeding. When lactation overlaps a new pregnancy, some women choose not to wean their toddlers, although there may be strong cultural taboos against continuing to breastfeed.1, 2 The practice of continuing to breastfeed during pregnancy has been reported among US women but might not be discussed with health professionals because of anticipated criticism of the practice.3–5 Breastfeeding during pregnancy is more common in some low-income countries than in the United States because shorter birth intervals increase the likelihood of a pregnancy-lactation overlap.
It has been suggested that an overlap could produce suboptimal outcomes for both pregnancy and subsequent lactation.6–8 Dairy research has demonstrated that a complete overlap of lactation during pregnancy dramatically compromises the total production of mature milk during the next lactation period.9–14 A dry (nonmilking) period of approximately 2 months before calving is usual in the dairy industry. This is the first human study to provide data on the association between the practice of lactating through late pregnancy and breastfeeding and growth outcomes of young infants.
METHODS
Participants
The study was conducted in a poor periurban community of approximately 800 000 on the outskirts of Lima, Peru. The inclusion criteria for mothers were: 1) pregnant; 2) ≥18 years of age; 3) multiparous and living with her child <4 years old; 4) without apparent indicators for elective cesarean section; and 5) either continuing to breastfeed into the third trimester or never breastfed during this pregnancy. Routes to identify possible participants were community census, local prenatal health service registers, and referrals. Between July 1998 and January 2000, 3417 pregnant women were identified. Of these, 601 women were not located at home, and 27 women declined to participate in a screening interview. Field workers screened 2789 pregnant women, and 727 met all of the inclusion criteria: 170 women breastfed their older children during the third trimester of pregnancy (BFP) and 557 women had not breastfed at all during the present pregnancy (NBFP). Breastfeeding was confirmed by direct observation; date of weaning was obtained from those mothers who had weaned during the third trimester and before enrollment.
Postpartum follow-up was confined to women who gave birth to an infant who was a vaginal delivery, full-term (>37 weeks’ gestational age), healthy birth weight (>2500 g), and with no birth defects or complications that would hinder breastfeeding. Of the 170 enrolled BFP women, 70 mother-infant pairs were followed after birth. Reasons for no postpartum follow-up included refusal (N = 40), moved or worked (N = 12), mother not available within 48 hours of birth (N = 13), birth problems (N = 19), and other (eg, twins, older child with problems; N = 16).
Of the 557 NBFP women enrolled during pregnancy, 67 were followed up. Probable data of delivery was recorded for all NBFP mothers. For each BFP infant born, a NBFP woman was selected at random from the pool of women scheduled to give birth that month. The first selected NBFP woman who met the postpartum criteria was included. The reasons for no follow-up were refusal (N = 174), moved or worked (N = 40), not randomly selected (N = 71), not available within 48 hours of birth (N = 99), birth problems (N = 54), and other (N = 52). Of the 137 infants who were studied on day 2 postpartum, 133 infants had a repeated study at 1 month of age. Two BFP and 2 NBFP infants had no repeat study because of refusal (N = 2) and moving (N = 2). This analysis is based on the 133 infants with observations for both days.
Comparison of Included and Excluded Families
The included BFP families had some indicators of a better economic status (eg, housing quality) than the excluded BFP families. The included NBFP mothers were significantly more likely to be younger, born in the mountains, and live with fewer adults as compared with excluded NBFP families. Other family demographic, socioeconomic, and obstetric data that were collected during pregnancy did not differ.
Data Collection
Obstetric History and Birth
At screening, all pregnant women were interviewed about their obstetric history and previous breastfeeding experience. Morbidity data were gathered twice monthly for all enrolled women. Relevant delivery and birth information was collected from clinical records when available and from mothers. For deliveries at health facilities, dystocia (abnormal duration of labor) was defined by practitioners, using the World Health Organization’s partograph to monitor cervical dilatation and fetal descent.15 All home births were assumed to have involved normal labor.
Breastfeeding Behavior
Once a month during pregnancy, BFP mothers recorded their toddlers’ feeding frequency or the date of weaning. After birth at twice-weekly home visits, mothers recalled their breastfeeding practices of the previous days, including whether the infant was breastfed by another woman and whether the mother breastfed another child.
Twenty-four-hour breast milk intakes and duration of feeds of all infants were measured twice: day 2 (41.7 ± 5.7 hour) and 1 month (33 ± 3 days). Milk intake was measured by the test weighing method,16 weighing the infant before and after each feed, using an electronic digital balance sensitive to 1 g (Mettler Toledo Model SB/16 000, Columbus, OH). Total milk intake was corrected for a 3% insensible water loss.17 On both days, only 0.1% of feeds were not weighed because the mother was not at home at the time of the feed. The missed values were imputed using separate multiple regression equations that estimated intake per feed by time since last feed, duration of the missed feed, and child identifier. Milk intake was expressed as g/feeding and g/24 hours.
Intake of Other Liquids/Foods
Nonbreast milk liquids that were consumed during the 24-hour observation period were weighed. Information on reported intake for all other days was collected at twice-weekly visits.
Anthropometry
Birth weights and lengths were collected from health facilities when available. A similar number of BFP and NBFP mothers gave birth at home, and their infants were weighed and measured by the study staff within 3 days. For clarity, both of these measurements are referred to as the first weight or length. At 1 month of age, infants were measured in triplicate without clothes in the field office. Weight was measured on a digital electronic infant scale (Soehnle-Waagen GMbH and Co, KG, Murrhardt, Germany) sensitive to 10 g. Length was measured to the nearest 0.1 cm with a locally made rigid length board. Head circumference was measured to 1 mm with nonstretchable tape measures (Lasso, Child Growth Foundation, London, United Kingdom).
Maternal mid-arm and calf circumferences were measured pre-and postnatally to a precision of 1 mm with the same nonstretchable tape measurer mentioned above. Weight and height were measured at 1-month postnatally, using a Seca adult beam balance with a precision of 100 g and a locally-made stadiometer with a precision of a 0.1 cm. Body mass index (BMI) (weight/height2) was calculated. Field workers were standardized.18
Morbidity
Maternal reports of daily symptoms of infants and any treatment were recorded twice weekly for the first month. Stool character and frequency as well as general and respiratory symptoms were recorded along with maternal illness and treatment. A diarrheal day was a day with 3 or more liquid/semiliquid stools in 24 hours. A dichotomous variable of ever having diarrhea was developed. Prevalence of diarrhea and cough in the infant and any maternal illness was calculated. Other symptoms were infrequent (<6% of days observed) and, therefore, not considered here.
Socioeconomic Data
Socioeconomic data on the quality of housing, hygiene, possessions owned, and education and employment of family members were collected at the screening interview and day 2 postpartum.
Data Analysis
Mean group differences for continuous variables were tested with the Student t test and analysis of variance; Kruskal-Wallis 1-way analysis of variance was used for variables with nonnormal distribution. Frequency differences were tested with the χ2 goodness-of-fit test. Multiple linear regression models were used to estimate the effect of breastfeeding late in pregnancy on day 2 and 1-month breast milk intake and 1-month growth. Logistic regression was used to estimate the effect of an overlap on the risk of very low (<25th percentile) milk intake and growth. Both models controlled for confounding factors including sex, age, first weight, feeding behaviors (duration and frequency of feeds, breastfed by another woman, hours postpartum), and maternal characteristics (age, parity, pregnancy complication, anthropometric measurements). With birth weight or length in an equation predicting 1-month weight or length, the other coefficients reflected their effects on the corresponding increment from birth. Morbidity (eg, prevalence of diarrhea and cough) and socioeconomic status (SES) variables were not significant predictors of the milk and growth outcomes. The effects of BFP on milk intake and growth outcomes in the models discussed here were unchanged when analyses were rerun without 10 children who received breast milk from other mothers.
All analyses were conducted with SYSTAT version 10.19 Data are presented as the mean ± standard deviation and significance for all 2-tailed probability tests was set at P < .05, unless otherwise indicated. This study was approved by the Human Subjects Research Office at Iowa State University, University of Alabama at Birmingham, and the Ethics Committee at the Instituto de Investigación Nutricional; written informed consent was obtained.
RESULTS
Family Baseline Characteristics
NBFP families appeared poorer than BFP households (Table 1). NBFP houses were made of lower quality materials and were less likely to have piped water and a functioning sewage system. Field workers observed human fecal matter on the floor in 3 times more NBFP than BFP homes; however, this did not reach significance.
TABLE 1.
Baseline Family Socioeconomic and Demographic Characteristics by Practice of Breastfeeding During Pregnancy (n = 133)
| Breastfeeding Practice
|
||
|---|---|---|
| BFP (n = 68) | NBFP (n = 65) | |
| N (%) | N (%) | |
| House characteristics and indicators of wealth | ||
| Cement floor** | 41 (60.3) | 27 (41.5) |
| Brick/cement wall* | 43 (63.2) | 31 (47.7) |
| Electricity | 58 (85.3) | 55 (84.6) |
| Daily food expenditures (S/.)† | 13.3 ± 5.3‡ | 12.8 ± 4.5‡ |
| Home environmental health | ||
| Piped water*** | 50 (73.5) | 32 (49.2) |
| Sewage**** | 43 (63.2) | 16 (24.6) |
| Human fecal material visible on floor | 2 (2.9) | 7 (10.8) |
| Family member smokes*** | 33 (48.5) | 15 (23.1) |
| Family member smokes in house | 18 (26.5) | 8 (12.3) |
| Family composition | ||
| Number of members in home | 5.4 ± 2.7‡ | 5.0 ± 2.2‡ |
| Extended family | 27 (39.7) | 20 (30.8) |
| Paternal | ||
| Lived with family** | 59 (86.8) | 64 (98.5) |
| Completed primary school | 57 (83.8) | 60 (92.3) |
| Had a stable income | 20 (29.4) | 28 (43.1) |
Significant difference by breastfeeding practice:
P < .10;
P < .05;
P < .01;
P < .001.
Peruvian currency (S/. = Nuevos Soles).
Mean ± standard deviation.
Maternal and Infant Characteristics
Baseline
BFP and NBFP mothers were similar in age and education (Table 2). Only 2 women (1 BFP and 1 NBFP) worked in the formal sector. Compared with NBFP mothers, twice as many BFP mothers were born on the coast and lived about 3 years longer in Lima. Although there were no differences between groups in parity, the last interbirth interval was longer for NBFP mothers than BFP mothers, as demonstrated by the 10-month difference in the age of the last child.
TABLE 2.
Baseline, Prenatal, and Postpartum Maternal Characteristics by Practice of Breastfeeding During Pregnancy (n = 133)
| Breastfeeding Practice
|
||
|---|---|---|
| BFP (n = 68) | NBFP (n = 65) | |
| Baseline | ||
| Age (y) | 25.9 ± 5.5† | 25.9 ± 4.7 |
| Born on the coast** | 37 (54.4) ‡ | 16 (24.6) |
| Lived in Lima (y)* | 16.6 ± 8.8 | 13.9 ± 8.3 |
| Completed primary school | 60 (88.2) | 54 (83.1) |
| Number of live births | 2.1 ± 1.3 | 2.1 ± 1.1 |
| Age of last child (y)** | 1.9 ± 0.6 | 2.7 ± 0.7 |
| Prenatal§ | ||
| Attended prenatal care at least once | 51 (75.0) | 52 (80.0) |
| Mid-arm circumference (mm) | 26.9 ± 3.1 | 26.5 ± 2.9 |
| Calf circumference (mm) | 33.6 ± 2.3 | 33.5 ± 2.6 |
| Postpartum (1 mo)§ | ||
| Height (cm) | 149.1 ± 5.2 | 150.0 ± 7.1 |
| Mid-arm circumference (mm) | 27.3 ± 3.1 | 26.9 ± 2.6 |
| Calf circumference (mm) | 33.0 ± 2.5 | 32.6 ± 2.8 |
| BMI | 26.2 ± 3.8 | 25.9 ± 3.6 |
P < .10;
P < .001.
Data in this format indicate mean ± standard deviation.
Data in this format indicate n [%].
Sample for anthropometric measurements was at least n = 64 BFP and 64 NBFP.
Prenatal, Birth, and Postpartum Characteristics
About three fourths of all women attended a health facility for prenatal care (Table 2). NBFP mothers were more likely than BFP mothers to seek prenatal care at the governmental health centers rather than private facilities (75.8% vs 55.9%). There were no group differences in prepartum anthropometric measurements, reported pregnancy complications, or smoking during pregnancy (2 BFP and 2 NBFP). BFP mothers breastfed their toddlers 5.3 ± 4.3 (median: 4) times per day. Fourteen mothers breastfed >5 times during the daytime; the maximum frequency reported was 17 feeds/day.
Of those who gave birth in a health facility, abnormally prolonged labor (dystocia) tended to occur more often among the BFP than the NBFP mothers (Table 3). Other birth and newborn characteristics were not different between groups.
TABLE 3.
Birth Events and Newborn Characteristics by Practice of Breastfeeding During Pregnancy
| Breastfeeding Practice
|
||
|---|---|---|
| BFP (n = 68) | NBFP (n = 65) | |
| Birth event | ||
| Born in health facility | 54 (79.4)* | 50 (76.9) |
| Prolonged labor (dystocia)† ‡ | 7 (13.0) | 2 (4.0) |
| Mother received medicine during labor | 18 (26.5) | 15 (23.1) |
| Mother received medicine after birth | 51 (75.0) | 53 (81.5) |
| Newborn characteristics | ||
| Female sex | 33 (48.5) | 32 (49.2) |
| First weight (kg)§ | 3.4 ± 0.4|| | 3.4 ± 0.4 |
| First length (cm)§ | 50.0 ± 1.7 | 50.0 ± 1.6 |
| Gestational age (wk)§ | 39.6 ± 1.0 | 39.6 ± 1.1 |
| Time after birth started breastfeeding (h) | 1.7 ± 2.6 | 2.4 ± 6.0 |
| Did not receive liquids in first 24 h | 61 (89.7) | 61 (93.8) |
Data in this format indicate n (%).
P < .10.
Extracted from clinic records; reflects inadequate progress of cervical dilation and fetal descent, as defined by the World Health Organization’s partogram.15 All births at home were assumed to be normal.
Samples for anthropometric and gestational age measurements were at least n = 59 BFP and 60 NBFP.
Data in this format indicate mean ± standard deviation.
There were no differences in postpartum anthropometry or prevalence of maternal illness in the first month postpartum. Mothers reported being ill <10% of the first 30 days postpartum.
Breastfeeding Characteristics
Breast Milk Intake
There was a wide range of 24-hour intake of colostrum (2g–570 g; Fig 1). When intake was adjusted for time after birth of initiation of study, the higher intake of BFP infants than NBFP infants (195.3 ± 110.7 vs 175.1 ± 110.7 g/24 hour, P = .13; respectively) did not reach significance. There were also no group differences in frequency or total duration of breastfeeding over the 24 hours. Mothers breastfed a mean of 20.7 ± 5.1 times for a total of 305.8 ± 116.9 minutes.
Fig 1.
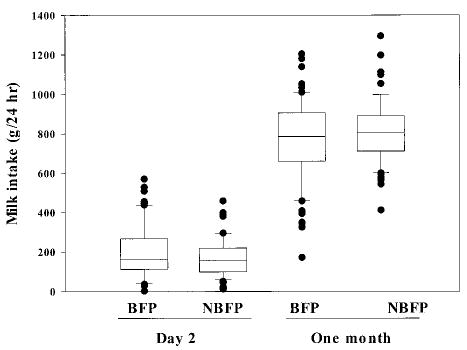
Infants’ 24-hour intake of their mothers’ breast milk by presence of a breastfeeding-pregnancy overlap and day of observation. Upper and lower limits of the box represent the 25th and 75th percentile values; the horizontal line within the box represents the median. Bars represent the standard deviation.
At 1 month of age, the number of feeds per 24 hours decreased to 16.9 ± 3.4. In contrast with the observation shortly after birth, BFP mothers breastfed for 35.2 minutes (95% confidence interval [CI]: 11.5–58.9 minutes) longer than NBFP mothers, for a mean total time of 213.8 ± 80.0 versus 178.6 ± 56.4 minutes (P < .01), respectively. The average duration of a feed was significantly longer among the BFP than the NBFP mothers (12.4 ± 3.9 vs 10.9 ± 2.9 minutes, respectively; P < .01). This increased time did not translate into higher milk intakes (Figs 1 and 2). BFP as compared with NBFP infants had about a 9% decline in intake per feed that tended to be significant (46.2 ± 14.6 vs 50.4 ± 11.2 g/feed; P = .06) and about an 8% lower total milk intake (765.5 ± 212.3 vs 810.5 ± 161.9 g/24 hour; P = .17).
Fig 2.
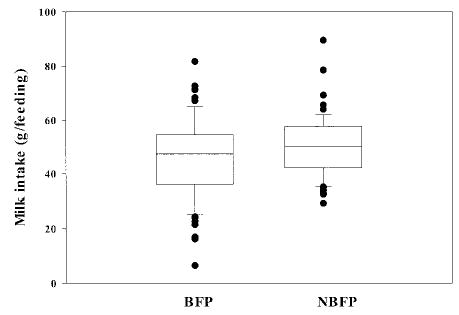
Infants’ mean intake per feed of their mothers’ breast milk at 1 month by presence of a breastfeeding-pregnancy overlap. Upper and lower limits of the box represent the 25th and 75th percentile values; the horizontal line within the box represents the median. Bars represent the standard deviation.
Breast Milk From Other Mothers
Ten infants were observed also to be breastfed by another woman, 6 on day 2 (4 BFP and 2 NBFP) and 4 (all BFP) at 1 month. The odds ratio (OR) of a BFP infant receiving milk from another mother at either observation point was 4.2 (P = .06). During both observation days, the range of milk consumed from other mothers was wide: 1 g to 105 g (median: 30.4 g) on day 2 and 14 g to 209.1 g (median: 54.6 g) at 1 month. When intakes of breast milk from the infant’s mother and other women were summed, the difference in milk intake at 1 month between BFP and NBFP infants was attenuated slightly (770.4 ± 208.4 vs 810.5 ± 161.9 g/24 hour; P = .22). The results (regression coefficients and significance levels for breastfeeding during pregnancy) for breast milk intake and growth discussed below were similar with and without the data from these 10 children who received milk from another woman.
Tandem Breastfeeding
About one third of the BFP mothers breastfed another child in addition to the newborn on day 2 (N = 24; 35.3%) and at 1 month (N = 27; 39.7%) postpartum. On day 2, only 1 NBFP mother breastfed another infant (total = 10.3 g). Milk intakes of the other child ranged from 0 to 384.2 g on day 2 and 0 to 405.8 g at 1 month. When milk intake of the other child was considered as part of total milk production, the difference between BFP and NBFP mothers was reduced. The mean 1-month intakes of infants increased progressively from BFP/no tandem breastfeeding (762.5 ± 232.4 g/24 hour), BFP/tandem breastfeeding (768.6 ± 192.3 g/24 hour), to NBFP (813.0 ± 161.8 g/24 hour), demonstrating that tandem feeding did not account for the low milk intakes of BFP infants.
Use of Other Liquids
Exclusive breastfeeding was short-lived in this community. Ten percent (10.3%) of the BFP infants and 6.2% of the NBFP infants received nonbreast milk liquids during the first 24 hours of life, including sugar water, other milks, and teas. By 1 month, 31.6% of all infants had been given other liquids (32% of liquids were teas or water; 68% were other milks), with no group difference in use. However, the BFP as compared with the NBFP infants had a higher total number of days when other liquids were offered (9.4 ± 7.5 days vs 4.4 ± 3.0 days; P < .001) and higher total 30-day intake of teas and water (3.5 ± 9.4 vs 0.7 ± 2.0 fl oz).
One-Month Infant Characteristics
Morbidity
There was little diarrheal morbidity during the first 30 days of life; 25 mothers reported that their infants had at least 1 day of diarrhea (about 20% in both groups). Significantly more BFP than NBFP infants had a cough for >7 days (35.3% vs 20.0%).
Growth Outcomes
Complete weight data were available for 66 BFP and 64 NBFP infants; length data were available for 63 BFP and 57 NBFP infants. The infants had similar first weights. BFP infants gained 170 g less (95% CI: −0.308 to −0.031) than NBFP infants over the first month of life (Fig 3), a 14% difference in weight gain. By 1 month of age, there was a nonsignificant mean difference of −118 g (95% CI: −313 to −77) in attained weight of BFP as compared with NBFP infants. There was no group difference in length gain (BFP gained 4.6 ± 1.4 and NBFP gained 4.7 ± 1.5 cm), attained length, or head circumference at 1 month.
Fig 3.
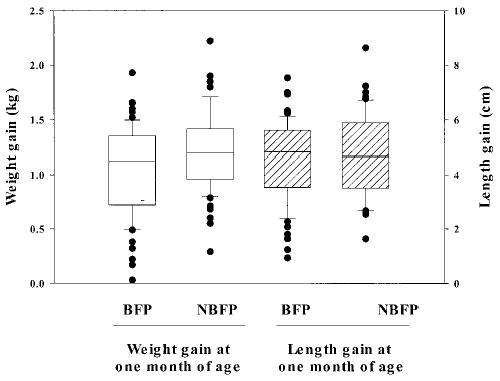
One-month weight (solid) and length (hatched) gain of study infants by presence of a breastfeeding-pregnancy overlap. Upper and lower limits of the box represent the 25th and 75th percentile values; the horizontal line within the box represents the median. Bars represent the standard deviation.
Models to Estimate Breast Milk Intakes From Infant’s Mother
Day 2
Day 2 breast milk intakes were associated with several factors (Table 4). Milk intake decreased about 25 g for each 5-year increment of maternal age and 3 g per 10 additional minutes of breastfeeding. Intake increased 6.5 g per 100-g increase in the first weight. Receiving breast milk from another woman was strongly associated with a lower intake (−96 g) from the infant’s own mother. BFP was not associated with day 2 intake when controlling for these above factors and time of initiation of the study.
TABLE 4.
Multiple Linear Regression Models for 24-Hour Breast Milk Intake on Day 2 and at 1-Month Postpartum*
| Day 2 (Adjusted R = 0.339)
|
1 Month (Adjusted R = 0.366)
|
||||||
|---|---|---|---|---|---|---|---|
| Variable | Coefficient | SE | P Value | Variable | Coefficient | SE | P Value |
| Constant | −98.681 | 99.855 | .325 | Constant | 183.796 | 126.886 | .150 |
| First weight (kg) | 64.986 | 21.927 | .004 | First weight (kg) | 152.094 | 33.624 | .000 |
| BF duration (min) | −0.341 | 0.085 | .000 | BF duration (min) | −0.770 | 0.236 | .001 |
| Initiate study (h) | 168.958 | 35.359 | .000 | Parity (number)† | −17.116 | 17.792 | .338 |
| BF other mother on day 2 | −95.881 | 41.193 | .022 | Pregnancy complication | 55.092 | 27.280 | .046 |
| Mother’s age (y) | −4.971 | 1.719 | .005 | Breastfeeds (number) | 14.039 | 4.904 | .005 |
| BFP (1 = yes) | −40.714 | 27.665 | .144 | ||||
| Parity BFP† | −57.778 | 22.805 | .013 | ||||
BF indicates breastfeeding; SE, standard error.
n = 132; data for 1 birth weight was missing. Intake was adjusted for 3% insensible water loss.
Variable was centered for analysis.
One Month
Parity significantly modified the effect of a breastfeeding-pregnancy overlap on 1-month milk intake (Table 4). Holding the other explanatory variables constant, for each increase of 1 birth, there was an additional 116-g reduction in milk intake among BFP infants but only a 17-g reduction among NBFP infants (interaction term; P = .01).
Milk intake was positively associated with the number of feeds but was negatively associated with duration. The first weight, an indicator of child requirements, was the primary determinant of milk intake; a 1-kilo increase was associated with a 152-g increase in intake. Finally, maternal report of complications during pregnancy was positively associated with milk intake; the explanation of this relationship is not clear. Tandem breastfeeding, receiving milk from another mother, and intake of nonbreast milk liquids did not explain any additional variance.
The risk of a very low breast milk intake (<25th percentile of the study population) at 1 month was fivefold higher among those infants who were breastfed also by another women (OR: 5.3; P = .01). These data do not indicate if this practice negatively affected maternal production or was in response to a previous low production.
Models to Explain 1-Month Infant Weight Gain
After controlling for other factors, the practice of breastfeeding throughout pregnancy was associated with a decrease in 1-month weight gain (Table 5). Holding the continuous explanatory variables at their mean, a BFP infant gained 125 g less (95% CI: 8–241) than NBFP infants, or about 15% of National Center for Health Statistics mean weight gain.20 A sustained decline of this magnitude would result in a −0.7 z score change in weight-for-age by 6 months of life.
TABLE 5.
Multiple Linear Regression Models for 1-Month Weight (kg) and Length (cm) Gain
| Weight (n = 128; Adjusted R = 0.995)
|
Length (n = 120; Adjusted R = 0.999)
|
||||||
|---|---|---|---|---|---|---|---|
| Variable | b | SE | P Value | Variable | b | SE | P Value |
| First weight (kg) | 1.000 | 0.052 | .000 | First length (cm) | 0.993 | 0.022 | .000 |
| Infant’s age (d) | 0.048 | 0.006 | .000 | Infant’s age (d) | 0.137 | 0.033 | .000 |
| 30-d total bovine milk (fl oz) | −0.001 | 0.000 | .002 | Day 2 intake (g) | 0.002 | 0.001 | .052 |
| BFP (1 = yes) | −0.125 | 0.059 | .036 | 30-d total bovine milk (fl oz) | −0.003 | 0.001 | .086 |
| Maternal calf circumference (mm)* | 0.005 | 0.017 | .783 | Maternal calf circumference (mm)* | 0.114 | 0.054 | .038 |
| Sex (1 = female) | −0.174 | 0.058 | .003 | ||||
| Parity (number) | −0.069 | 0.026 | .009 | ||||
| BFP calf circumference* | −0.058 | 0.023 | .013 | ||||
SE indicates standard error.
Variable was centered for analysis.
The negative effect of BFP on weight gain was amplified by an increase in maternal calf circumference (an additional −53 g/mm increase in calf circumference among only BFP infants). For example, for a mother with a calf circumference equivalent to the 75th percentile (34.4 mm), the weight gain in a BFP infant was 175 g less than that of an equivalent NBFP infant, almost 20% of National Center for Health Statistics mean weight gain. Maternal calf circumference had a significant positive correlation with maternal age and BMI at 1 month (r = 0.25 and 0.75, respectively) and may be functioning as a proxy of nutritional stress, age, or other related biological factors.
Intake of bovine milk had a negative effect on weight gain. Lower weight gain was also associated with being female, higher parity, and younger infant age.
Controlling for infant’s age, the risk of a very low weight gain (<25th percentile value of the study population; <0.9 kg) tended to double for BFP infants (OR = 2.193; P = .077) and was threefold higher for females (OR = 2.8; P = .03). Other feeding practices, such as tandem feeding, breastfeeding from another woman, or use of nonbreast milk liquids, did not affect the total weight gain or the risk of a very low weight gain.
Models to Explain 1-Month Infant Length Gain
Similar models were developed for length gain (Table 5). Length gain was positively associated with infant’s age and tended to increase with increased breast milk intake on day 2. BFP as well as other breastfeeding practices did not explain any additional variance in length gain.
No variables were significant explanatory factors for the risk of very low (<25th percentile value of the study population; <3.55 cm) length gain.
DISCUSSION
Lactation during pregnancy is known to have adverse effects on milk production in cows.9–14 The present study is the first human study to document a negative effect of an overlap of lactation and late pregnancy on the breastfeeding and growth success of the subsequent infant. The magnitude of the effect on weight gain is cause for concern. A sustained 15% decline in expected weight gain would move an infant born at the 50th percentile to under the 25th percentile weight-for-age by 6 months of age.
The primary limitation of the study is group self-selection. That is, mothers chose whether they were BFP or NBFP; they were not randomly assigned to a feeding group. Without the benefit of randomization, it is possible that other confounding factors explain our results. For example, if SES were a confounder and the true determinant of poor growth among the BFP children, then one would expect the BFP families to be poorer than the NBFP families. However, a number of indicators suggested that the SES was lower in the NBFP families. It is not clear how the group differences in maternal characteristics, such as place of birth, would affect breastfeeding success unless they reflect breastfeeding support networks. Shorter interbirth intervals among BFP mothers may reflect decreased recuperation of maternal stores.21,22 Milk production may be reduced when maternal diet is extremely limited23,24; however, observed social conditions and maternal BMI levels (mean value in the range of overweight) do not support this mechanism. In addition, there were no group differences in maternal anthropometric indicators pre- or postnatally. It is possible that BFP is a proxy for other maternal psychosocial factors, not measured, that influenced successful mothering and, ultimately, growth.
Butcher (cited in reference 11) estimated a reduction of 585 kg of milk over the course of the lactation in cows that were milked up to 5 to 20 days before parturition. The mechanism of reduction of milk production in cows is not well understood; possibly, with an inadequate nonmilking period before birthing, there is insufficient involution and subsequent rejuvenation of the mammary secretory cells. Species vary in the amount of involution that naturally occurs; those with longer cycles and a functional luteal phase normally retain some of the alveolar structure.11,14 The importance of this mechanism in humans is not well understood.
Healthy infants self-regulate intake to meet their needs.25 Generally, a reduced milk intake in BFP infants could have 3 causes: 1) maternal behaviors that reduce infant access to milk, 2) limited maternal production, and/or 3) reduced infant demand. The similar feeding frequency and increased duration among BFP infants are inconsistent with the first explanation. However, the data were collected at 1 time point and may not reflect feeding behaviors over the entire month. Also, although access was similar, BFP infants may have been less successful at extracting milk or expending more energy doing so. Studies on feeding mechanisms are needed.
Second, dairy research would suggest that the lower intakes were related to lower milk production. However, when total milk production was calculated, those BFP mothers who had additional stimulation because of tandem breastfeeding were able to produce an equal amount of milk as NBFP mothers, suggesting that milk production was not a limitation. The selection bias for tandem breastfeeding is not known. Women who perceived inadequate milk production may have chosen not to tandem breastfeed.
Finally, there are no data to assume a lower demand for milk from the BFP infants. No infant was low birth weight or premature, or had any conditions that would hinder breastfeeding. BFP infants had slightly higher rates of symptoms of upper respiratory tract infections. Difficulty in breathing associated with illness could hinder infants breastfeeding; however, previous studies have demonstrated only a minor effect of respiratory illness (without fever) on breast milk intakes.26,27 Our regression analysis failed to find any association with upper respiratory tract infections and milk intake; it is not clear whether this was because our variable was an insensitive marker of illness.
BFP mothers may perceive feeding difficulties and attempt to increase milk intake by breastfeeding more frequently or by using more nonbreast milk liquids. Given the documented high frequency of feeding events, it seems more likely that mothers would introduce other liquids to supplement a perceived inadequate milk supply than increase the number of feeds. In this study, there was an increased total use of teas, but not bovine milk, among the BFP infants that would support this explanation.
There are cultural taboos against breastfeeding during pregnancy throughout the world.1–5 Despite this, the practice is common.7,8,28,29 In Bangladesh, an estimated 20% of women who were lactating and pregnant were still lactating at the beginning of the ninth month.28 In Guatemala, 50% of pregnant women were breastfeeding an older children.7,8 In Peru, 10% of women with preschoolers continued to breastfeed until the last trimester of pregnancy (G.S.M., unpublished data). Similar prevalence data for US women are not available, but the breastfeeding behavior is present.3–5 Before health professionals can offer appropriate advice to women, the biological consequences of the breastfeeding behavior need to be understood.
Few researchers have looked at the consequences of a lactation-pregnancy overlap. Merchant et al7,8 reported that an overlap was associated with a non-significant decrease of 57 g in birth weight; the effect increased with the overlap duration. Studies with the appropriate design to look at birth outcomes (risk of low birth weight or prematurity) have not been conducted yet. A longer cohort study is needed to determine the cumulative effect of an overlap on exclusive breastfeeding and infant health and growth through 6 months, as well as its effect on the mother and toddler. If future studies confirm a negative effect of an overlap, then consideration needs to be given to the optimal time to wean during pregnancy. However, if additional studies find that infants and mothers adjust their feeding behavior and are able to exclusively breastfeed for 6 months with adequate growth, there is no reason to wean a toddler before a mother wishes to do so. With these additional results, health professionals will be able to provide advice that will lead to optimal health for the infant, toddler, and mother.
Acknowledgments
This work was funded by National Institutes of Health R03 grant HD35183–03.
We thank the field staff of the Instituto de Investigación Nutricional for their outstanding work; the staff at the Instituto Materno Perinatal, the Hospital Materno-infantil, Huascar, and the Ministry of Health facilities in San Juan de Lurigancho in the study community for facilitating access to mothers who gave birth in their units; and the Iowa State University Community Nutrition Research group for their critical review of the manuscript.
References
- 1.Oliveros C, Marquis GS, Ormsby G, Rudatsikira E. Maternal lactation: a qualitative analysis of the breastfeeding habits and beliefs of pregnant women living in Lima, Peru. Int Q Community Health Educ. 1998–99;18:415–434. [Google Scholar]
- 2.Dettwyler KA. Breastfeeding and weaning in Mali: cultural context and hard data. Soc Sci Med. 1987;24:633–644. doi: 10.1016/0277-9536(87)90306-6. [DOI] [PubMed] [Google Scholar]
- 3.Wrigley E, Hutchinson S. Long-term breastfeeding: the secret bond. J Nurse Midwifery. 1990;35:35–41. doi: 10.1016/0091-2182(90)90055-a. [DOI] [PubMed] [Google Scholar]
- 4.Gromada K. Breastfeeding more than one: multiples and tandem breastfeeding. NAACOGS Clin Issues Perinatol Womens Health Nurs. 1992;3:656–666. [PubMed] [Google Scholar]
- 5.Moscone S, Moore M. Breastfeeding during pregnancy. J Hum Lact. 1993;9:83–88. doi: 10.1177/089033449300900219. [DOI] [PubMed] [Google Scholar]
- 6.Garza C. Lactation during pregnancy. In: Gleicher N, editor. Principles of Medical Therapy in Pregnancy. New York, NY: Medical Book Company; 1985. pp. 263–268. [Google Scholar]
- 7.Merchant K, Martorell R, Haas J. Consequences for maternal nutrition of reproductive stress across consecutive pregnancies. Am J Clin Nutr. 1990a;52:616–620. doi: 10.1093/ajcn/52.4.616. [DOI] [PubMed] [Google Scholar]
- 8.Merchant K, Martorell R, Haase J. Maternal and fetal responses to the stresses of lactation concurrent with pregnancy and of short recuperative intervals. Am J Clin Nutr. 1990b;52:280–288. doi: 10.1093/ajcn/52.2.280. [DOI] [PubMed] [Google Scholar]
- 9.Vanlandingham AH, Weakley CE, Ackerman RA, Hyatt G. The relationship of production of heifers milked prepartum to the composition of colostrum. J Dairy Sci. 1949;32:559–564. [Google Scholar]
- 10.Smith VR. Physiology of Lactation. 5th ed. Ames, IA: Iowa State University Press; 1959. [Google Scholar]
- 11.Larson BL. Lactation. Ames, IA: Iowa State University Press; 1985. [Google Scholar]
- 12.Keown J, Everett R. Effect of days carried calf, days dry, and weight of first calf heifers on yield. J Dairy Sci. 1986;69:1891–1896. doi: 10.3168/jds.S0022-0302(86)80615-4. [DOI] [PubMed] [Google Scholar]
- 13.Remond B, Ollier A, Miranda G. Milking of cows in late pregnancy: milk production during this period and during the succeeding lactation. J Dairy Res. 1992;59:233–241. doi: 10.1017/s002202990003051x. [DOI] [PubMed] [Google Scholar]
- 14.Wilde C, Quarrie L, Tonner E, Flint D, Peaker M. Mammary apoptosis. Livestock Prod Sci. 1997;50:29–37. [Google Scholar]
- 15.World Health Organization. Partographic management of labour. Lancet. 1994;343:1399–1404. [PubMed] [Google Scholar]
- 16.Neville MC. Volume and caloric density of human milk. In: Jensen RG, editor. Handbook of Milk Composition. San Diego, CA: Academic Press; 1995. pp. 99–113. [Google Scholar]
- 17.Hendrikson EC, Seacat JM, Neville MC. Insensible weight loss in children under one year of age. Acta Paediatr Scand. 1985;74:678–680. doi: 10.1111/j.1651-2227.1985.tb10012.x. [DOI] [PubMed] [Google Scholar]
- 18.Habicht JP. Estandarización de métodos epidemiológicos cuantitativos sobre el terreno. Bol Sanit Panam. 1974;76:375–384. [PubMed] [Google Scholar]
- 19.SYSTAT version 10.0. Chicago, IL: SPSS, Inc; 2000. [Google Scholar]
- 20.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. Hyattsville, MD: National Center for Health Statistics; 2000. CDC Growth Charts: United States. Advance Data From Vital and Health Statistics. No. 314. [PubMed] [Google Scholar]
- 21.Rasmussen KM. Nutritional consequences of lactation for the mother: definition of issues. In: Picciano MF, Lonnerdal B, editors. Mechanisms Regulating Lactation and Infant Nutrient Utilization. New York, NY: Wiley-Liss; 1992. pp. 97–108. [Google Scholar]
- 22.Winkvist A, Jalil F, Rasmussen KM, Habicht J-P. Maternal energy depletion is buffered among malnourished women in Punjab, Pakistan. J Nutr. 1994;124:2376–2385. doi: 10.1093/jn/124.12.376. [DOI] [PubMed] [Google Scholar]
- 23.Young MC, Rasmussen KM. Effects of varying degrees of chronic dietary restriction in rat dams on reproductive and lactational performance and body composition in dams and their pups. Am J Clin Nutr. 1985;41:979–987. doi: 10.1093/ajcn/41.5.979. [DOI] [PubMed] [Google Scholar]
- 24.Gunther M. Diet and milk secretion in women. Proc Nutr Soc. 1968;27:77–82. doi: 10.1079/pns19680016. [DOI] [PubMed] [Google Scholar]
- 25.Dewey K, Lonnerdal B. Infant self-regulation of breast milk intake. Acta Paediatr Scand. 1986;75:893–898. doi: 10.1111/j.1651-2227.1986.tb10313.x. [DOI] [PubMed] [Google Scholar]
- 26.Martorell R, Yarbrough C, Yarbrough S, Klein RE. The impact of ordinary illnesses on the dietary intakes of malnourished children. Am J Clin Nutr. 1980;33:345–350. doi: 10.1093/ajcn/33.2.345. [DOI] [PubMed] [Google Scholar]
- 27.Brown KH, Black RED, Robertson AD, Becker S. Effects of season and illness on the dietary intake of weanlings during longitudinal studies in rural Bangladesh. Am J Clin Nutr. 1985;41:343–355. doi: 10.1093/ajcn/41.2.343. [DOI] [PubMed] [Google Scholar]
- 28.Huffman SL, Chowdhury A, Charkraborty J, Simpson NK. Breastfeeding patterns in rural Bangladesh. Am J Clin Nutr. 1980;33:144–154. doi: 10.1093/ajcn/33.1.144. [DOI] [PubMed] [Google Scholar]
- 29.Cantrelle P, Leridon H. Breast feeding, mortality in childhood and fertility in a rural zone of Senegal. Popul Stud. 1971;25:505–533. doi: 10.1080/00324728.1971.10405821. [DOI] [PubMed] [Google Scholar]


