Abstract
Extracellular signaling “cross-talk” between tissues is an important requirement for development of many organs yet the underlying mechanisms generally remain poorly understood. The anterior segment of the eye, which is constructed from four embryonic lineages, provides a unique opportunity to genetically dissect developmental processes such as signaling “cross-talk” without fear of inducing lethality. In the current review, we summarize recent data showing that PITX2, a homeodomain transcription factor, integrates retinoic acid and canonical Wnt/β-catenin signaling during anterior segment development. Because the requirements for retinoic acid signaling, canonical Wnt/β-catenin signaling, and PITX2 are not unique to the eye, this newly identified pathway may have relevance elsewhere during development and in tissue homeostasis.
Keywords: retinoic acid signaling, canonical Wnt signaling, anterior segment, homeobox
Introduction
In 1901, Hans Spemann reported that the optic cup had the ability to cause overlying head surface ectoderm to form a lens, the first step in development of the ocular anterior segment (Spemann, 1901). Spemann termed this property “induction” and arguably launched the modern era of experimental developmental biology. More than a century later, the anterior segment of the eye remains an important model for investigating basic developmental mechanisms. As with other organs, the mature anterior segment arises from multiple embryonic primordia. Extensive inductive interactions and signaling “cross-talk” between and within the distinct primordia are required for correct patterning and cell specification, which requires cells to integrate multiple signaling inputs. A significant advantage of using the eye as a model system is that it is not required for viability. Using appropriate genetic techniques, one can test developmental processes in great molecular detail without fear of inducing lethality. Advances in understanding basic developmental mechanisms gained while studying the anterior segment are often highly relevant when subsequently applied to the development of other organs or tissues that are required for viability.
All major signaling pathways have been implicated in various aspects of development within the anterior segment and many transcription factors are essential for the process as well. However, only recently has the identification of an initial cohesive pathway that integrates cell signaling between tissues with transcriptional networks emerged from this multitude of factors. Work from several laboratories has now provided evidence linking retinoic acid signaling from the optic cup, the homeodomain transcription factor PITX2 within neural crest and canonical Wnt/β-catenin signaling within the ocular surface ectoderm. Pitx2 is a critical integration node that links the two signaling pathways. This network is required during early eye development to establish the basic framework of general patterning and initial differentiation on which future morphogenesis of the anterior segment depends. How additional cell signaling pathways and transcription factors may contribute to this pathway remains to be determined.
Overview of anterior segment morphogenesis
Major structures/tissues of the mature anterior segment include the lens, ciliary body, iris, cornea, limbus, bulbar conjunctiva, and the eyelid, which is comprised of the palpebral epidermis, palpebral conjunctiva, and eyelid mesenchyme (Fig. 1A). Four embryonic tissues contribute the cellular building blocks for anterior segment structures: neural ectoderm, surface ectoderm, and neural crest and mesoderm mesenchyme (Fig. 1B). Each of these primordia eventually contributes multiple lineages within the mature anterior segment (Fig. 1B). In addition, most mature anterior segment structures are a synthesis of both ectoderm and mesenchyme. For example, in the cornea, the corneal epithelium derives from surface ectoderm whereas the corneal stroma and the corneal endothelium derive from mesenchyme Fig. 1A,B) (Gage et al., 2005). This is fundamentally different from e.g. retinal development, where all differentiated neurons and the Mueller glia differentiate from a single, common pool of neural ectoderm precursors. Work from many model systems has contributed to our current understanding of vertebrate eye development, including the anterior segment (Jacobson and Sater, 1988; Furukawa et al., 1999; Beebe and Coats, 2000; Fuhrmann et al., 2000). Because some interspecies differences exist, mouse will be used as the reference point for summarizing relevant stages of eye development and anterior segment morphogenesis in this review as a prelude to mouse genetics experiments that will be described later.
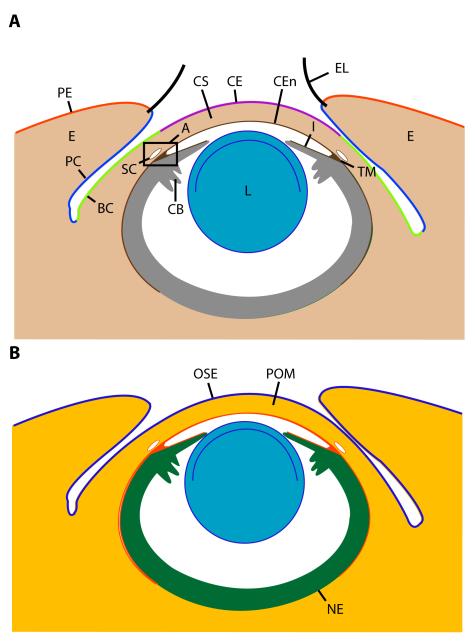
Figure 1. Identities and embryonic origins of major anterior segment tissues.
(A) Adult eye with annotation of the major structures and tissue of the anterior segment. (B) Adult eye illustrating embryonic origins of major tissues from ocular surface ectoderm (blue), periocular mesenchyme (neural crest and mesoderm, red/orange), and neural ectoderm (green). Key: A, iridocorneal angle; BC, bulbar conjunctiva; CB, ciliary body; CE, corneal epithelium; CEn, corneal endothelium; CS, corneal stroma; E, eyelid; EL, eyelash cilium; I, iris; L, lens; OSE, ocular surface ectoderm; PC, palpebral conjunctiva; PE, palpebral epidermis; POM, periocular mesenchyme (both neural crest and mesoderm); SC, Schlemm’s canal; TM, trabecular meshwork.
Several steps in eye development precede and are required for initiation of normal anterior segment morphogenesis. The earliest molecular evidence for the eye primordia is activation of a network of transcription factor genes marking first a single and subsequently two “eye fields” within the anterior neural ectoderm (Chow and Lang, 2001). The optic pits, representing the first morphological evidence of eye development, form as localized thickenings of neural ectoderm within the inner surface of the anterior neural folds at the positions initially marked by the two eye fields (Pei and Rhodin, 1970; Kaufman, 1992). By embryonic day 9 (e9), the optic pits deepen to form the optic vesicles, outgrowths of neural ectoderm on either side of the diencephalon (Fig. 2A) (Pei and Rhodin, 1970; Kaufman, 1992). The optic vesicles continue to expand laterally until they meet the ocular surface ectoderm (Fig. 2A).
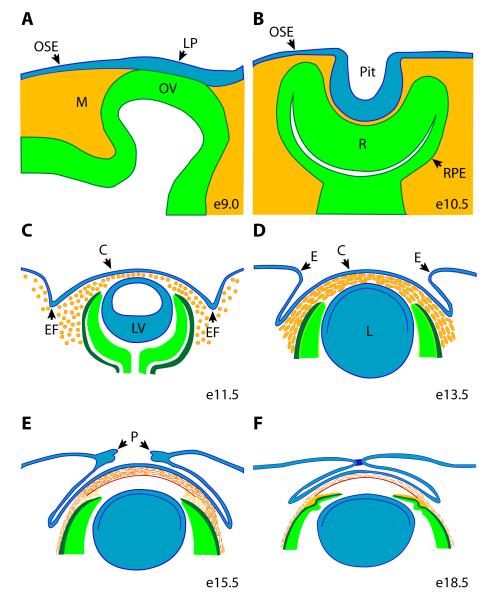
Figure 2. Morphogenesis of the anterior segment.
The anterior segment develops from ocular surface ectoderm (blue), neural ectoderm (green), and neural crest and mesoderm mesenchyme (orange). (A) Anterior segment morphogenesis is initiated when direct interaction of the distal optic vesicle indices formation of the lens placode within the overlying ocular surface ectoderm. (B) The lens pit forms by invagination of the lens pit while the optic vesicle is concurrently converted in the optic cup. The inner layer of optic cup will form the retina, the outer layer will form the retinal pigmented epithelium, and the anterior rim of the optic cup will contribute the epithelial components of the ciliary body and iris. (C) Loosely arranged mesenchyme migrates between the newly formed optic vesicle and anterior optic cup and the overlying presumptive cornea. Eyelid folds emerge as the first morphological evidence of eyelid development. Key: C, cornea; E, eyelid; EF, eyelid folds; L, lens; LP, lens placode; LV, lens vesicle; M, mesoderm; OSE, ocular surface ectoderm; OV, optic vesicle; P, periderm; Pit, lens pit; R, retina; PRE, retinal pigmented epithelium
The ocular surface ectoderm (OSE) is a multipotent patch of head surface ectoderm that, when appropriately patterned, is fated to contribute the lens, all epithelia of the eye surface (cornea, limbus, conjunctiva), ectoderm-derived components of the lacrimal and Harderian (in mice) glands, and the eyelid epidermis (Fig. 1, 2A) (Ashery-Padan et al., 2000). Anterior segment development commences when the distal optic vesicle makes direct physical contact with the overlying OSE on e9.5 in mice, resulting in induction of the lens placode within the OSE (Fig. 2A) (Pei and Rhodin, 1970; Kaufman, 1992). Therefore, the optic vesicle acts early in anterior segment development as an organizing center that is both necessary and sufficient for specifying where within the ocular surface ectoderm the lens primordia will form. The lens placode invaginates towards the head midline, forming a lens pit (Fig. 2B), which then separates from the OSE to form the lens vesicle (e10.5), the precursor to the mature lens (Fig. 2C) (Pei and Rhodin, 1970; Kaufman, 1992). The molecular cascade leading to lens development is known in considerable detail and has been summarized elsewhere (Chow and Lang, 2001; Lang, 2004). Coincident with formation of the lens vesicle, the optic vesicle is converted into an optic cup, the inner layer forming the retina, whereas the outer layer will form the retinal pigmented epithelium (Fig. 2B). The epithelium of the ciliary body and iris, two important structures within the anterior segment, will eventually develop from the anterior rim of the optic cup.
The days following formation of the lens vesicle (e10-13.5) are critically important for anterior segment development, because the general framework of patterning and initial differentiation steps on which the future cornea, limbus, conjunctiva, and eyelids, will depend are laid down. This period is marked by changes in both morphology and gene expression, and coincides with the time at which the newly identified RA/PITX2/Wnt regulatory network is first required (see below). At the beginning of this phase (e10), morphogenesis of the cornea and other central anterior segment tissues starts with migration of largely neural crest mesenchyme into the space between the newly formed lens vesicle and the OSE (Fig. 2C) (Pei and Rhodin, 1970; Kaufman, 1992; Cvekl and Tamm, 2004). The resulting 7-8 cell thick layer of mesenchyme becomes increasingly more organized and condensed, implying a response to local cues (Fig. 2D). The transcription factor genes Foxc1, Foxc2, Lmx1b, and Pitx2 are all initially expressed in specific patterns within the mesenchyme at this time and are subsequently required for anterior segment development (Gage and Camper, 1997; Kidson et al., 1999; Pressman et al., 2000; Kume et al., 2001). Beginning shortly after immigration of the neural crest mesenchyme, distinct regions of the OSE corresponding to the future corneal, limbal, and conjunctival ectoderm, and eyelid epidermis can be identified based on differential expression and sub-cellular localization of Connexin 43, a gap junction protein that is subsequently essential for corneal function (Wolosin et al., 2002). In addition, by e12.5 differential levels of canonical Wnt/β-catenin signaling activity distinguish future elements arising from the OSE (Smith et al., 2005). The appearance of the eyelid grooves within the OSE dorsal and ventral to the optic cup at e11.5 is the first morphological evidence of eyelid development (Fig. 2C). At e14.5, corneal development continues with the emergence of the presumptive corneal endothelium as a continuous sheet of cells from the monolayer of mesenchyme directly overlying the anterior lens and the anterior rim of the optic cup (Pei and Rhodin, 1970; Kaufman, 1992; Cvekl and Tamm, 2004). The maturing corneal endothelium physically dissociates from the anterior lens, forming a space that will become the future anterior chamber once aqueous humor production begins (Pei and Rhodin, 1970; Kaufman, 1992). Keratocytes of the corneal stroma differentiate somewhat later from mesenchyme located between the newly formed corneal endothelium and the overlying presumptive corneal epithelium, which arises from the OSE (Fig. 2E) (Pei and Rhodin, 1970; Kaufman, 1992). By late gestation, keratocytes adopt a lamellar arrangement and begin to secrete a highly specialized extracellular matrix, properties that are required for corneal transparency. After the cornea and lens separate, the anterior rim of the optic cup becomes pigmented and begins to grow into the cavity between the lens and cornea, generating the epithelia of the future ciliary body and iris. A new migration of mesenchyme into the angle formed between the iris and the cornea (iridocorneal angle) provides the precursors that will form the stroma of the ciliary body and iris, as well as components of the future outflow tract within the iridocorneal angle. Development of these later emerging structures continues for several weeks after birth (Gould et al., 2004).
After their patterning and specification, eyelid primordia grow in size and extend toward each other across the cornea until about e15.5 (Fig. 2E). During this period, the eyelid consists of a loose array of mesenchyme sheathed within an outer layer of surface ectoderm, which will form the future palpebral conjunctiva on the inner surface of the eyelid and the palpebral epidermis on the outer surface (Findlater et al., 1993). Regularly spaced eyelash follicles begin to emerge at the interface between the palpebral epidermis and conjunctiva towards the end of eyelid closure. Beginning approximately e15.5, periderm cells located at the leading edge of the each eyelid rim begin to proliferate and migrate across the cornea until they meet and fuse by e16.5 (Fig. 2E,F). The eyelids will ultimately reopen by approximately postnatal day 10 (Findlater et al., 1993; Martin and Parkhurst, 2004; Xia and Karin, 2004).
The increased organization and compaction visible within the periocular mesenchyme (POM) of the anterior segment shortly after migration between the lens vesicle and overlying OSE implies that the POM is responding to local cues. The concurrent activation of specific transcription factor genes within the POM required for anterior segment morphogenesis and corneal development further strengthens this view. Similarly, the differential expression of Cx43 and the coincident emergence of morphological changes demonstrate that patterning within the OSE is established within a similar time window. Disruption of these early processes generally has a negative impact on subsequent steps in anterior segment development. For example, mutations affecting differentiation of the corneal endothelium result in persistent attachment of the posterior cornea to the lens (Blixt et al., 2000; Brownell et al., 2000; Ring et al., 2000; Semina et al., 2001; Ormestad et al., 2002), and disrupted patterning early during development of the OSE can result in the subsequent emergence of ectopic eyelash follicles within the conjunctiva (distichiasis) (Fang et al., 2000; Kriederman et al., 2003). Classic embryology experiments as well as mutant phenotypes in mice and humans implicate the lens and anterior optic cup as likely sources of signaling molecules for organizing the anterior segment, but the identities of the specific factors, as well as the nature of the downstream response, are unknown. Recent data now provide proof that the optic cup acts as a signaling center that orchestrates anterior segment morphogenesis through direct action on the adjacent neural crest, and, indirectly through action of the neural crest, on patterning and morphogenesis within the OSE.
Retinoic acid signaling orchestrates anterior segment morphogenesis during the critical time window following lens vesicle formation
Vitamin A is essential for eye development and exerts its biological effects through retinoic acid (RA), an enzymatic metabolite. The primary activity of RA during development is to act as an autocrine or paracrine signaling molecule (Fig. 3A). In this capacity, RA binds to nuclear receptors, activating these ligand-gated transcription factors within competent cells (Fig. 3A). The nuclear receptors are constituted by proteins from two families, the retinoic acid receptors (RAR) α, β, and γ, and the retinoid X receptors (RXR) α, β, and γ (Chambon, 1996). The functional unit is a RAR/RXR heterodimer and all RAR and RXR proteins are expressed in the developing eye (Ghyselinck et al., 1997; Mori et al., 2001). RAR proteins bind both all-trans- and 9-cis-RA, whereas RXR proteins bind only 9-cis-RA. However, 9-cis-RA is the active factor in cell signaling (Chambon, 1996). Evidence of a requirement for RA signaling in eye development first came from reports of the severe congenital eye defects present in vitamin A deficient (VAD) and/or RA-deficient human and livestock fetuses (Hale, 1933; Warkany and Schraffenberger, 1946; Wilson et al., 1953; Dickman et al., 1997; Dupe et al., 2003). Dysgenesis of the anterior segment is prominent in these individuals, and includes agenesis of the lens, iris and corneal stroma, the corneal endothelium, an absence of the anterior chamber, eyelids open at birth, and (in rodents) no Harderian glands. Additional ocular features include retinal and optic disc coloboma, a foreshortened ventral retina, and agenesis of the sclera. Humans with mutations inhibiting the function of serum retinol binding protein have eye defects, as do mice with a reduced capacity for RA receptor signaling (Lohnes et al., 1994; Seeliger et al., 1999). Genetic ablation of two or more RAR encoding genes in mice results in severe eye defects that mimic those seen in severe VAD (Kastner et al., 1994; Lohnes et al., 1994; Ghyselinck et al., 1997; Kastner et al., 1997; Mascrez et al., 1998). Therefore the competence to respond to RA signaling is critical for normal eye development.
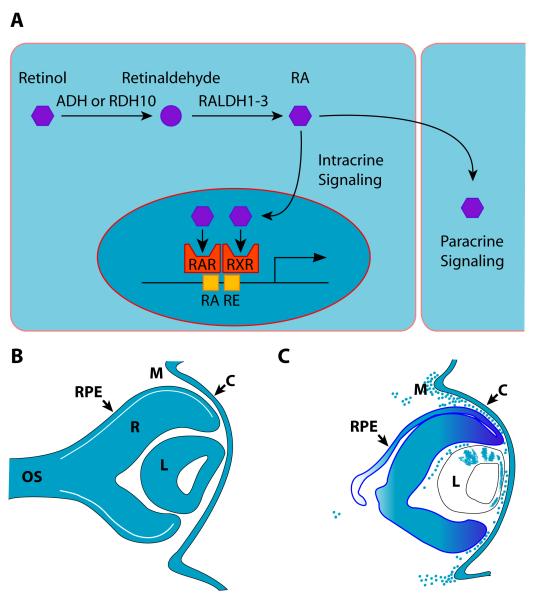
Figure 3. Retinoic acid signaling during development of the anterior segment.
(A) Simplified overview of retinoic acid production and function in intracrine or paracrine signaling. Note: addition factors involved in functions such as transport have been omitted for simplicity. (B) Summary of tissues producing retinoic acid (blue) at e11.5 based on expression of RALDH1 and/or RALDH3. (C) Summary of tissues responding to retinoic acid signaling at e11.5 based on expression pattern of RARE-lacZ reporter transgene. Key: C, cornea; ADH, alcohol dehydrogenase; RAR, retinoic acid receptor; RALDH1-3, retinaldehyde dehydrogenase 1-3; RDH10, retinol dehydrogenase 10; M, mesenchyme; OS, optic stalk; R, retina; PRE, retinal pigmented epithelium; RXR, retinoid X receptor.
Conversion of vitamin A to RA is a two-step process. First, in all cell types, vitamin A is converted to retinaldehyde by the enzyme, alcohol dehydrogenase type 3 (Fig. 3A) (Molotkov et al., 2002). Second, in selected, cell types, retinaldehyde is converted to retinoic acid (either all-trans or 9-cis) by a retinaldehyde dehydrogenase (Fig. 3A) (Duester, 2000). Mammals have four retinaldehyde dehydrogenase proteins (RALDH1-4) encoded by the genes Aldh1a1, Aldh1a2, Aldkh1a3, and Aldh8a1, respectively (Duester, 2000; Lin et al., 2003). Temporal-spatial specificity of RA production, and therefore the capacity for RA signaling, are largely controlled by selective expression of the RALDH proteins during development. In addition, RA signaling can be fine tuned by the activity of RA-degrading enzymes such as CYP26A1 and CYP26C1 (Fujii et al., 1997; Sakai et al., 2004).
RALDH1-3 are expressed in dynamic, overlapping patterns beginning early in eye development, coinciding with conversion of the optic vesicle into the optic cup and formation of the lens vesicle. RALDH2 is transiently expressed in the optic vesicle at e8.5 (Li et al., 2000; Wagner et al., 2000; Mic et al., 2004). By e9.5, RALDH1 is strongly expressed in the dorsal retina while RALDH3 is strongly expressed in the ventral retina (McCaffery et al., 1999; Grun et al., 2000; Li et al., 2000; Mic et al., 2000; Suzuki et al., 2000), prompting the hypothesis that RA signaling may play an essential role in dorsal/ventral patterning of the retina during development (Wagner et al., 2000; Drager et al., 2001; Peters and Cepko, 2002; Sakai et al., 2004). However, functional studies including individual gene ablation have not supported a role for RA signaling in early dorsal/ventral patterning in the retina or specification and morphogenesis of the lens (Matt et al., 2005; Molotkov et al., 2006). Functional redundancy amongst the RALDH proteins does not account for this surprising result, since retinal patterning and lens vesicle formation also appear unaffected in double gene knockout mice lacking any detectable RALDH activity in the eye (Matt et al., 2005; Molotkov et al., 2006). Therefore, RA signaling is not required for lens induction and morphogenesis, the earliest stages of anterior segment development.
In contrast, RA signaling is critically required for the next phase of anterior segment morphogenesis, which includes initiation of all three layers of the cornea, patterning the remaining OSE into presumptive corneal, limbal, and conjunctival ectoderm and eyelid epidermis, and the emergence of the early eyelid primordia. RALDH1 is highly expressed in the corneal ectoderm, lens, and dorsal retina throughout this time period (e10.5-13.5), and at lower levels in the ventral retina beginning by e11.5 (Fig. 3B) (Matt et al., 2005; Molotkov et al., 2006). Concurrently, RALDH3 is strongly expressed in the corneal ectoderm, retinal pigmented epithelium, and ventral retina, with weaker expression in the dorsal retina (Fig. 3B) (Matt et al., 2005; Molotkov et al., 2006). In mice transgenic for a RA-responsive lacZ transgene (RARE-lacZ), β-gal is expressed in the retina, optic nerve, corneal ectoderm, and periocular mesenchyme at this stage, establishing the responsiveness of these tissues to RA (Fig. 3C) (Matt et al., 2005; Molotkov et al., 2006). This same transgene is silent in the eyes of mutant mice lacking both RALDH1 and RALDH3, confirming that these two enzymes account for all RA signaling activity in the eye during this critical period of anterior segment development (Matt et al., 2005; Molotkov et al., 2006).
Genetic ablation of Aldh1a1 (RALDH1) has little effect on expression of the RARE-lacZ transgene or eye development (Matt et al., 2005; Molotkov et al., 2006). Ablation of Aldh1a3 (RALDH3) abolishes expression of the RARE-lacZ reporter in the RPE, ventral corneal ectoderm, ventral retina, and ventral POM while expression in the dorsal tissues is unaffected (Matt et al., 2005; Molotkov et al., 2006). Aldh1a3 mice exhibit mild to moderate ventral defects, including shortening of the ventral retina, ventral rotation of the lens, retrolenticular membrane, and thickening of the ventral POM (Matt et al., 2005; Molotkov et al., 2006). The latter appears to be due to a decrease in apoptosis that normally occurs within the ventral POM (Matt et al., 2005; Molotkov et al., 2006). Similar apoptosis occurs within the dorsal POM of wild type mice but this is unaffected in Aldh1a3 mutants. In contrast to the single mutants, ablation of both Aldh1a1 and Aldh1a3, and the accompanying absence of RA signaling, has profound effects on anterior segment patterning and morphogenesis (Matt et al., 2005; Molotkov et al., 2006). Anterior segment phenotypes include agenesis of the corneal endothelium and stroma, iris stroma, and formation of a thick layer of mesenchyme between the lens and cornea. Invagination of the dorsal eyelid groove fails, leading to the absence of a dorsal eyelid while only a rudimentary eyelid forms ventrally. This ventral eyelid remains small and ultimately fuses with the presumptive corneal ectoderm, forming a hypoplastic conjunctival sac. Additional phenotypes include a persistent retrolenticular membrane, ventral rotation of the lens, shortening of the ventral retina, retinal coloboma, and agenesis of the sclera. Providing the pregnant dam with a RA supplemented diet largely restores normal eye development in the mutants (Molotkov et al., 2006). However, the competence of neural crest to respond to RA by activating Pitx2 expression and other steps required for normal anterior segment development may be transient (See and Clagett-Dame, 2009).
The wild type expression of the RARE-lacZ reporter in multiple ocular tissues combined with the severe Aldh1a1/3 phenotype raises the possibility that normal anterior segment development could require intact RA signaling to the retina, surface ectoderm, and/or POM (Fig. 3C). However, neural crest-specific ablation of two (Rarb and Rarg) or all of the three genes encoding the RAR nuclear receptors results in mice with ocular phenotypes identical to those in the Aldh1a1/3 mice (Matt et al., 2005; Matt et al., 2008). Therefore, neural crest is the primary target of RA signaling in the eye during this critical period of anterior segment patterning and morphogenesis. Loss of RA signaling does not appear to affect neural crest cell survival; on the contrary, apoptosis is reduced in neural crest of the mutant animals (Matt et al., 2005; Molotkov et al., 2006). Absence of RA signaling results in the loss of expression within the neural crest of transcription factor genes (Eya2, Foxc1, and Pitx2) that are required for normal anterior segment morphogenesis (Matt et al., 2005; Molotkov et al., 2006). Therefore, RA signaling to the neural crest orchestrates anterior segment morphogenesis at least in part through the activation of essential transcription factor genes. Whether or not the effects on the target genes are direct or indirect and details regarding additional downstream targets remain unknown.
Canonical Wnt signaling and patterning of the ocular surface ectoderm
Canonical Wnt signaling, mediated by the downstream effector protein β-catenin, is widely required during development, as well as stem cell biology and adult tissue homeostasis (Logan and Nusse, 2004). Temporal-spatial patterns of Wnt pathway activity within a developing tissue or organ are tightly regulated (Logan and Nusse, 2004). Dys-regulation of canonical Wnt/β-catenin signaling leads to abnormal development and plays a prominent role in a number of diseases, including cancer (Nusse, 2005). Canonical Wnt/β-catenin signaling activity is initiated by Wnt ligand binding to a heterodimeric cell surface receptor, resulting in stabilization and translocation of β-catenin to the nucleus where it partners with TCF/LEF transcription factors (Fig. 4A) (Logan and Nusse, 2004; Nusse, 2005). Canonical Wnt/β-catenin signaling can promote gene transcription, cell proliferation, differentiation, cell survival, or developmentally programmed apoptosis (Logan and Nusse, 2004; Nusse, 2005).
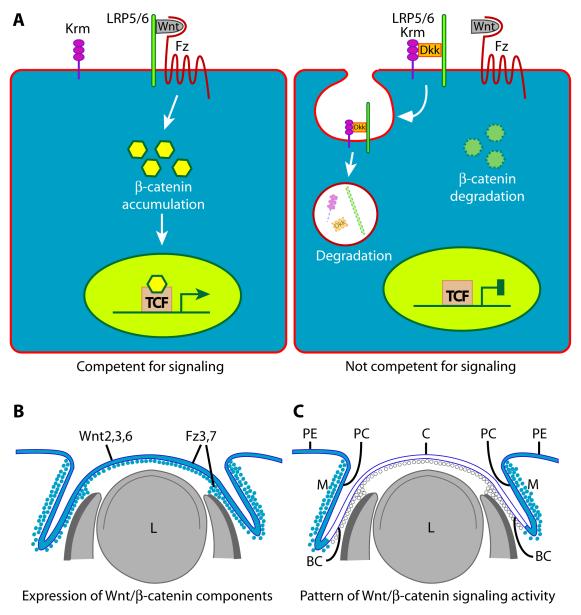
Figure 4. Canonical Wnt/b-catenin signaling during development of the anterior segment.
(A) (Left) Both the LRP5/6 and Fz proteins are present on the surface of a competent cell, providing available Wnt ligand with the full heterodimeric receptor required to transduce the signal. Activation of the full receptor by Wnt ligand induces biochemical changes within the cytoplasm that lead to accumulation of stabilized β-catenin, which is translocated to the nucleus where it activates transcription of target genes. (Right) DKK proteins, when present, render a cell incompetent to respond to Wnt ligand because the LRP5/6 component required for receptor function is removed from the cell membrane. DKK proteins bind LRP5/6, in combination with a third protein called Kremen, and this complex is cleared from the cell surface by endocytosis and subsequently degraded. This leaves the cell incompetent to transduce a signal to the nucleus, even when high quantities of Wnt ligand present, because a fully functional heterodimeric receptor cannot be formed. (B) Summary of Wnt ligand and Frizzled receptor expression sites (blue) in mouse eye tissues at e12.5. (C) Summary of structures/cells exhibiting high (blue) or low (white) canonical Wnt signaling activity in mouse eye tissues at e12.5. Key: BC, bulbar conjunctiva; C, cornea; L, lens; Dkk, Dickkopf; Fz, any frizzled protein; Krm, Kremen; LRP5/6, low density lipoprotein receptor-related protein 5 or 6; M, mesenchyme; PC, palpebral conjunctiva; PE, palpebral epidermis; TCF, transcription factor 4; Wnt, Wnt ligand
A TOPGAL reporter transgene that expresses lacZ in response to canonical Wnt signaling has been used to evaluate pathway activity during eye development (Liu et al., 2003; Smith et al., 2005; Miller et al., 2006). The levels of canonical Wnt/β-catenin signaling activity vary significantly in time and space within the OSE during the critical period for patterning and differentiation of the individual cell fates.
Prior to and during the early phases of lens induction and morphogenesis (e8.5-9.5), canonical Wnt/β-catenin signaling activity is absent from the OSE and remains undetectable during the lens vesicle stage (e10.5) (Miller et al., 2006). Pathway activity remains undetectable in central tissues including the presumptive corneal ectoderm at e12.5, but is markedly elevated peripherally, including in the palpebral epidermis and conjunctiva (Fig. 4C) (Smith et al., 2005). Local activity in the mesenchyme parallels that in the respective overlying ectoderm (Fig. 4C) (Smith et al., 2005).
Manipulating canonical Wnt/β-catenin signaling activity dramatically alters patterning and development of within OSE. Genetically, the pathway can be blocked by ablating the Catnb (β-catenin) gene or activated by rearranging Catnb to produce a highly stable form of β-catenin (Harada et al., 1999; Brault et al., 2001). Generating abnormally high levels of pathway activity throughout the OSE blocks induction of the normal, centrally placed lens placode by the optic vesicle (Smith et al., 2005), whereas ablating pathway activity throughout the OSE results in lentoid bodies forming within the peripheral OSE (Miller et al., 2006). Therefore, the competence of cells within the OSE to form lens correlates with low levels of canonical Wnt/β-catenin signaling activity, suggesting that differences in the levels of signaling activity play an essential role in patterning the OSE. As eye development progresses, mutants with abnormally high canonical Wnt/β-catenin signaling activity throughout the OSE fail to develop a cornea, conjunctiva, or eyelids, indicating that correct levels of pathway activity remain essential for normal anterior segment development in the period following lens formation (Miller et al., 2006). Collectively, these data indicate that normal patterning and differentiation of structures derived from the OSE correlate strongly with achieving the correct temporal/spatial activation of canonical Wnt/β-catenin signaling activity.
Temporally and spatially restricted expression of Wnt ligands and other components required for signal transduction would be one mechanism that could account for the required local activation or suppression of pathway activity during anterior segment development. However, several Wnt ligands that are active in the canonical signaling pathway, as well as multiple Frizzled receptor proteins required for Wnt signaling, are highly expressed in the presumptive corneal ectoderm during the period when pathway activity in the same cells and adjacent mesenchyme is low or undetectable (Fig. 4B) (Liu et al., 2003; Smith et al., 2005). Therefore, expression of the required signaling factors does not correlate with pathway activity levels in the developing OSE through this time period (Liu et al., 2003). This suggests that the pathway may be actively inhibited. Canonical Wnt/β-catenin signaling activity can be inhibited by secreted frizzled-related proteins (SFRP1-4) and Dickkopf proteins (DKK1-4), which antagonize active signaling by binding to and inactivating either the Wnt ligand (SFRP’s) or the Wnt receptor (DKK’s) (Logan and Nusse, 2004; Nusse, 2005; Niehrs, 2006). Although multiple Sfrp and Dkk genes are developmentally expressed in the eye, data on the expression patterns within the relevant time are incomplete and, in some cases, not in agreement (Monaghan et al., 1999; Liu et al., 2003; Ang et al., 2004; Chen et al., 2004). In addition, until recently, no functional analysis on the effects of ablating SFRP’s or DKK’s in the developing eye had been reported. Therefore, the mechanism(s) underlying the essential temporal and regional activation and suppression of canonical Wnt/β-catenin signaling activity within the developing OSE was unknown.
Pitx2 integrates RA and canonical Wnt/β-catenin signaling activity during anterior segment development
Pitx2 encodes a homeodomain transcription factor (PITX2) whose expression is first detectable within the neural crest of the anterior segment beginning at e9.5-10, shortly after lens vesicle formation and early in the critical period for subsequent anterior segment morphogenesis (Hjalt et al., 2000; Gage et al., 2005). These initial data suggest that Pitx2 expression is activated within ocular neural crest in response to local cues, a fact confirmed by the recent demonstration that Pitx2 expression is dependent on RA signaling from the optic cup (Matt et al., 2005; Molotkov et al., 2006). Over the next 1-2 days, Pitx2 expression expands to include neural crest surrounding the optic cup and optic stalk, within the hyaloid space, and extending into the eyelid mesenchyme. Pitx2 is also expressed in mesoderm within the developing eye. Unlike neural crest, Pitx2 expression is high in mesoderm, before these cells associate with the eye primordia, suggesting that Pitx2 activation in mesoderm occurs by a different mechanism than in neural crest. Ultimately, neural crest and mesoderm expressing Pitx2 will contribute to the structures throughout the anterior segment, as well as the sclera, extraocular muscles, and all ocular blood vessels (Gage et al., 2005).
Heterozygous mutations in human PITX2 are one cause of Axenfeld-Rieger Syndrome, a congenital condition resulting in a variable anterior segment dysmorphogenesis and a high risk for glaucoma (Semina et al., 1996; Kulak et al., 1998; Kozlowski and Walter, 2000). The anterior segment defects can affect multiple lineages derived from the POM, including the corneal and iris stroma, corneal endothelium, and trabecular meshwork and Schlemm’s canal within the iridocorneal angle (Semina et al., 1996). Axenfeld-Rieger patients also have elevated intraocular pressure, which likely contributes to their high risk for glaucoma. Ablation of Pitx2 in mice results in agenesis of the corneal endoderm and stroma, iris stroma, and the replacement of these structures by a thick layer of mesenchyme lying between the lens and cornea (Gage et al., 1999; Kitamura et al., 1999; Lin et al., 1999; Lu et al., 1999). In addition, eyelid development is severely affected. Elsewhere, there is shortening of the ventral retina, coloboma formation, ventral rotation of the lens, a severe dysmorphogenesis of the optic nerve, and agenesis of the sclera and extraocular muscles. Mutants in which Pitx2 has been ablated specifically in neural crest recapitulate many ocular features of the global knockout mice, including within the anterior segment (Evans and Gage, 2005). Therefore, Pitx2 function in neural crest is required for the development of multiple ocular tissues, including the anterior segment. Anterior segment defects in global and neural crest-specific Pitx2 knockout mice appear identical to those reported for the loss of RA signaling mice, raising the possibility that Pitx2 is potentially the major downstream effector of RA signaling in the neural crest. (Gage et al., 1999; Matt et al., 2005; Molotkov et al., 2006).
Although the importance of Pitx2 for regulating eye development is well established, little is known regarding the underlying mechanism(s) or key downstream regulatory genes/pathways. Many of the phenotypes in mice and humans affect cell types derived from neural crest and mesoderm, lineages in which Pitx2 is expressed, suggesting that cell autonomous mechanisms, such as regulation of additional transcription factor genes, account for these defects. In contrast, the shortened ventral retina, coloboma, and dysmorphogenesis of the optic nerve all arise from developmental defects in neural ectoderm, a tissue in which Pitx2 is never expressed. These defects must arise out of non-cell autonomous mechanisms, providing the first evidence that critical downstream targets of PITX2 might include genes encoding factors involved in cell signaling pathways.
Dkk2, which encodes a secreted extracellular antagonist of canonical Wnt/β-catenin signaling, was recently identified as an important downstream effector of PITX2 during ocular anterior segment morphogenesis (Gage et al., 2008). Normally, canonical Wnt/β-catenin signaling activity requires the binding of Wnt ligands to heterodimeric cell surface receptors consisting of a Frizzled (FRZ) protein and a LRP5/6 protein (Logan and Nusse, 2004). DKK2 protein antagonizes canonical Wnt/β-catenin signaling by binding LRP5/6 together with a third protein, Kremen, resulting in a trimeric complex that is cleared from the cell surface by endocytosis and degraded within the cell by the proteosome (Niehrs, 2006; Gage et al., 2008). The resulting inability to form a functional heterodimeric receptor makes the cell incompetent to transduce a β-catenin signal even in the presence of high concentrations of Wnt ligand.
Dkk2 expression is first detected in anterior segment POM by e11 and spreads to POM surrounding the optic cup and extending into the emerging eyelids over the next 1-2 days (Monaghan et al., 1999; Gage et al., 2008). Unlike Pitx2, Dkk2 is not expressed in the extraocular muscle primordia (Monaghan et al., 1999; Gage et al., 2008). Although the expression patterns are nearly identical, the timing of Dkk2 activation in the POM lags behind that of Pitx2, consistent with it being a downstream effector (Gage et al., 2008). Dkk2 is absent from eyes of global and neural crest-specific Pitx2 knockout mice and is significantly reduced in Pitx2+/− mice (Gage et al., 2008). In addition, canonical Wnt/β-catenin signaling activity levels are elevated throughout the anterior segment of Pitx2 mutant eyes (Gage et al., 2008). PITX2 protein physically associates with Dkk2 promoter sequences in primary cultures of embryonic POM and a Dkk2 genomic fragment encompassing these sequences is responsive to PITX2 in a dose-dependent manner when linked to a luciferase reporter cassette (Gage et al., 2008). Collectively, these data indicate that Dkk2 is a direct target of PITX2 in neural crest during eye development.
Ablation of Dkk2 results in dys-regulation of canonical Wnt/β-catenin signaling levels, disrupted patterning, and altered fate specification within the OSE (Mukhopadhyay et al., 2006; Gage et al., 2008). Canonical Wnt/β-catenin signaling activity is extremely low to undetectable in developing wild type bulbar conjunctiva, limbal, and corneal ectoderm, tissues that directly overly the mesenchymal cells expressing DKK2 (Liu et al., 2003; Smith et al., 2005; Miller et al., 2006; Gage et al., 2008). In contrast, canonical Wnt/β-catenin signaling activity is elevated throughout the OSE of Dkk2 deficient eyes, including the presumptive bulbar conjunctiva, limbus, and cornea (Mukhopadhyay et al., 2006; Gage et al., 2008). Specific defects include mis-specification of corneal ectoderm as conjunctival goblet cells, ectopic eruption of eyelash follicles from surface ectoderm within the palpebral and bulbar conjunctiva, and limbus, and rudimentary growth of the eyelid (Gage et al., 2008). Therefore, despite uniform high expression of Wnt ligands and other components of the pathway, the paracrine effects of DKK2 represent at least one essential mechanism for negatively regulating canonical Wnt/β-catenin signaling activity that is required for normal patterning and differentiation within the OSE.
Ablation of Dkk2 also results in elevated canonical Wnt/β-catenin signaling activity within the POM (Gage et al., 2008). Similar to the OSE, the greatest increase occurs in cells within the presumptive bulbar conjunctiva, limbus, and cornea. The mesenchyme of the developing conjunctiva and limbus of wild type mice is richly vascularized whereas the cornea is avascular. In contrast, the corneal stroma in Dkk2 mice is richly vascularized prior to birth, similar to mesenchyme underlying the conjunctiva (Gage et al., 2008). The vascularization of the mesenchyme, together with the appearance of goblet cells in the overlying corneal surface ectoderm, provides further evidence that anterior segment patterning is altered in these mice leading this mesenchyme and surface ectoderm to at least partially adopt characteristics of conjunctiva, normally more peripheral fate (Gage et al., 2008). Elevated canonical Wnt/β-catenin signaling within the mutant cornea mirrors that normally found in the conjunctiva, providing evidence that patterning achieved by activation/suppression of elevated canonical Wnt/β-catenin signaling plays a key role in specifying a conjunctiva versus cornea fate. Post-natally in Dkk2 mutant eyes, ectopic blood vessels extend from the iris collarette across the anterior chamber to the central corneal endothelium (Gage et al., 2008). The specific underlying mechanism(s) leading to ectopic blood vessel growth remain to be determined but it is likely that elevated canonical Wnt/β-catenin signaling activity is a general contributing mechanism, since it is angiogenic in other systems (Duplaa et al., 1999; Wright et al., 1999; Longo et al., 2002; Masckauchan et al., 2005; Skurk et al., 2005; Goodwin et al., 2006). Therefore, the abnormally high levels of canonical Wnt/β-catenin signaling activity may directly promote abnormal blood vessel growth within the mutant corneas. Alternatively, the normally low levels of canonical Wnt/β-catenin signaling activity may be required to make the cornea of wild type eyes non-permissive to blood vessel growth. Finally, low levels of canonical Wnt/β-catenin signaling activity levels may be required for expression of known inhibitors of corneal vascularization such as soluble form of VEGF receptor 1 (Ambati et al., 2006)
Current model
Retinoic acid produced from the optic cup and lens serves as a critical mechanism for orchestrating overall morphogenesis and development of specific structures within the anterior segment in the period following formation of the lens vesicle (Fig. 5). The adjacent neural crest within the nascent anterior segment is a primary direct target of RA signaling at this time. The neural crest responds by initiating a transcriptional program that is required for subsequent patterning and development of the cornea, eyelids, and other tissues. Therefore, through production of RA, the optic cup acts as a signaling center nucleating anterior segment morphogenesis. This function is strikingly analogous to the role played by its predecessor, the optic vesicle at an earlier time point in inducing the initiation of lens development at a specific site within the overlying OSE. The transcriptional response within the neural crest includes activation of Eya2, Foxc1, and Pitx2, although it is highly likely that additional important targets are required (Fig. 5).
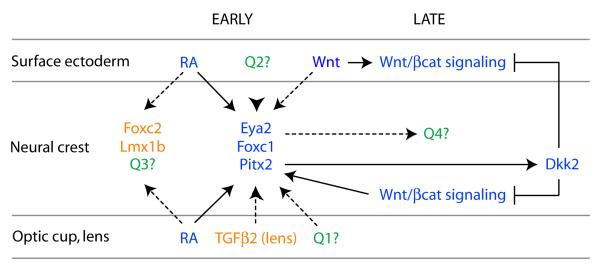
Figure 5. Current model and important questions for future analysis.
Established genetic and molecular pathways are shown in blue. Known candidates that should be tested for potential relevance to the network are shown in orange. More open-ended unknown questions are shown in green. These include what other factors in addition to RA may be required to activate Eya2, Foxc1, and/or Pitx2 (Q1), whether additional factors also signal from the ocular surface ectoderm (Q2), what other targets lie immediately downstream of RA signaling (Q3), and what other genes and classes of genes, in addition to genes for transcription factors Dkk2, are a part of the required response to RA signaling with in the neural crest (Q4). These questions are discussed in greater detail under “Perspectives” in the main text.
The anterior segment phenotypes shared by RA deficient and Pitx2 knockout mice establish that Pitx2 is a critical target of RA signaling in the neural crest. One essential direct target of PITX2 in the neural crest is the gene encoding DKK2, a potent antagonist of canonical Wnt/β-catenin signaling activity (Fig. 5). An important property of DKK2 function is that the target cells are rendered incompetent to mount a canonical Wnt/β-catenin signaling response even in an environment with significant concentrations of Wnt ligand, such as the developing cornea. DKK2 expressed downstream of PITX2 acts by both paracrine (acting on the surface ectoderm) and autocrine (acting on the neural crest) mechanisms to effectively block canonical Wnt/β-catenin signaling activity within the presumptive cornea (Fig. 5). More peripherally, additional phenotypes of Dkk2 deficient mice, including distichiasis and gross defects in eyelid development, provide compelling functional evidence that DKK2 must also act to moderate canonical Wnt/β-catenin signaling activity within these tissues. Therefore, Pitx2 expression within the neural crest acts as a critical node integrating RA signaling from the optic cup, required for morphogenesis of the anterior segment, with activation and suppression of canonical Wnt/β-catenin signaling activity, required to correctly pattern the OSE and mesenchyme.
This model prompts further hypotheses that should be tested in order to provide additional supportive evidence. For example, the model predicts that Dkk2 expression should be lost and canonical Wnt/β-catenin signaling activity should be upregulated in mutant where RA production by ocular structures or the competence of neural crest to respond to RA signaling is lost. These predictions are readily testable by assaying the RA mutants for Dkk2 and Axin2 expression.
Perspectives
Demonstrating the interconnected roles of retinoic acid, PITX2, and canonical Wnt/β-catenin signaling represents a significant breakthrough in understanding anterior segment development, because, for the first time, it provides evidence for how multiple signaling pathways are integrated at the level of transcriptional regulation. However, it is clear that the complete network regulating anterior segment development is significantly more complex. Some candidates that may participate at various levels of the network are already known, while others very likely remain to be identified. The future pursuit of these factors and their integration into the existing model is essential for advancing our understanding of this important process.
Several potential candidates are members of the TGF-β super-family of signaling molecules, a third major essential cell-cell signaling pathway during development. This family is divided into two branches: the TGF-β/activin/nodal branch and the bone morphogenetic protein (BMP)/growth derived factor (GDF) branch (Wu and Hill, 2009). Ablation of TGF-β receptor 2 (Tgfbr2) within the neural crest results in agenesis of the corneal endothelium with associated loss of the anterior chamber, pronounced thinning of the corneal stroma, and a severe reduction or loss of Foxc1 and Pitx2 expression, features that are reminiscent of the RA signaling mutants (Ittner et al., 2005). However, cell death is significantly increased within the corneal mesenchyme of Tfgbr2 mice, a notable difference from RA signaling mutants (Ittner et al., 2005). TGF-β2 appears to be the major signaling ligand acting upon the neural crest as Tfgb2 knockout mice phenocopy the Tgfbr2 knockout mice while anterior segment development appears normal in the absence of TGF-β1 and TGF-β3 (Fig. 5) (Sanford et al., 1997; Saika et al., 2001). Reduction of BMP4 levels in Bmp4+/− mice also results in anterior segment dysgenesis within structures or tissues derived from POM, including iridocorneal adhesions, small or absent Schlemm’s canal, and hypomorphic or absent trabecular meshwork (Chang et al., 2001). The downstream effectors of both TGF-β and BMP signaling are Smad proteins that translocate from the cytoplasm to the nucleus and activate transcription of target genes after phosphorylation by ligand-activated cell surface receptors (Wu and Hill, 2009). Importantly, RAR proteins are able to physically interact with and modify the activity of Smad proteins through various mechanisms in other systems (Roberts and Sporn, 1992; La et al., 2003; Pendaries et al., 2003). Therefore, it will be important to determine not only whether RA and TGF-β super-family signaling act in concert or independently within neural crest of the anterior segment but also the underlying mechanism(s). Additional signaling molecules likely act upon the neural crest and it will be important to identify these molecules and determine their potential relationship to RA signaling (Fig. 5, Q1, Q2).
Our understanding of the response by the neural crest is also in its infancy. Pitx2 and Foxc1 expression is dependent on RA signaling but whether these effects are direct or indirect is unknown (Fig. 5). Although Dkk2 is clearly an important target of Pitx2, the ocular phenotype in Dkk2 mutant mice is significantly less severe than the Pitx2 phenotype. Therefore, Dkk2 likely represents one of what are likely to be many important downstream effectors of PITX2 during anterior segment development and identifying these additional factors remains a high priority. Like Pitx2, Foxc1 is certain to be a key target of RA signaling that should be pursued further because heterozygous and homozygous null mutations cause anterior segment dysgenesis in mice and heterozygous mutations in FOXC1 are a second cause of Axenfeld-Rieger Syndrome in humans (Mears et al., 1998; Kidson et al., 1999; Lehmann et al., 2000). Additional transcription factor genes to test as potential targets of RA signaling in neural crest include Foxc2 and Lmxb1 (Fig. 5, Q3) (Pressman et al., 2000; Smith et al., 2000). FOXC1 and FOXC2 are highly related forkhead transcription factors and mutations affecting FOXC1 cause distichiasis in mice and humans and anterior segment dysgenesis in mice (Mears et al., 1998; Kidson et al., 1999; Fang et al., 2000; Lehmann et al., 2000; Kriederman et al., 2003). Mutations to the homeodomain transcription factor LMX1B cause anterior segment defects in mice and a significant risk for glaucoma in humans (Chen et al., 1998; McIntosh et al., 1998; Vollrath et al., 1998; Pressman et al., 2000). Future studies are needed to determine whether Foxc2 and Lmx1b expression in neural crest is directly or indirectly dependent on RA signaling. RA signaling may regulate expression of additional transcription factor genes in the neural crest and it will be important to identify these, as well as to determine whether they are direct or indirect targets (Fig 5, Q1).
The repertoire of responses by neural crest to RA signaling is likely to include genes encoding proteins with other functions, in addition to transcription factors (Fig. 5, Q4). These additional genes will likely include both direct targets of RA signaling, as well as targets of the transcription factors whose expression is activated in response to RA signaling. The visible changes in organization and compaction that occur within the mesenchyme during its response to RA signaling suggest that one key target set will include genes for cell matrix and/or cell adhesion molecules. Genes encoding additional factors involved in different aspects of cell signaling in addition to Dkk2 may represent a second key target set. These may encode factors that act like DKK2 on the overlying surface or factors involved in signaling back to the optic cup or lens, as well as autocrine factors. They may also include expression of intrinsic factors such as cell surface receptors or cytoplasmic regulators that change the competence of the neural crest to respond to existing or new signaling inputs.
The expression pattern of the RARE-lacZ reporter transgene identifies structures derived from neural ectoderm (anterior optic cup, presumptive ciliary and iris epithelium, RPE) and OSE (presumptive corneal, limbal, conjunctival, and eyelid epithelia), in addition to POM, as competent to respond to RA signaling in the period following lens vesicle formation (Matt et al., 2005; Molotkov et al., 2006). Although neural crest mesenchyme is clearly a critical primary target of RA signaling, these expression data raise the question whether normal anterior segment development may also require a primary response to RA signaling within the neural ectoderm and/or OSE. Appropriate Cre drivers for testing these possibilities are available and should be used to test these possibilities.
This signaling network that has been defined in mouse but its relevance to other organisms remains to be determined. If essential in other experimental organisms such as fish or chickens, this will provide important additional avenues to further explore the pathway as these organisms offer certain experimental advantages that mice do not. Variation in PITX2 gene dose is a central underlying mechanism leading to anterior segment defects in humans. An auto feedback loop between PITX2 and canonical Wnt/β-catenin signaling, if active in humans, would provide an effective means of potentially permitting tissues within the anterior segment some capability to respond to physiological change, such as environmental stressors, and return the system to homeostasis. However, the ability of the system to respond in this fashion, if it exists at all, appears limited since it cannot effectively compensate in patients who have even one hypomorphic allele of PITX2.
This review has focused largely on the interconnected functions of RA and canonical Wnt/β-catenin signaling, together with the homeodomain transcription factor PITX2, in determining overall patterning of the anterior segment in the period following formation of the lens vesicle. Although these functions are essential at this particular developmental stage as a prelude for events that occur later, it is clear that execution of the later events requires additional regulatory steps and it will be important to identify these and determine how they relate to and/or build upon the earlier steps that have been the focus of this review. Unlike the early phase, which appears to play an essential role in relatively broad patterning events within the anterior segment, subsequent regulatory steps are likely to function in more specific developmental processes. As one example, canonical Wnt/β-catenin signaling levels are known to regulate the degree of branching during lacrimal gland development (Dean et al., 2005). FGF10 signaling is required for induction of lacrimal gland development from peripheral OSE where canonical Wnt/β-catenin signaling activity levels are apparently relatively high, suggesting that perhaps FGF signaling together with a particular level of canonical Wnt/β-catenin signaling activity may be required to specify the lacrimal gland fate within the OSE (Makarenkova et al., 2000). However, transgenic expression of FGF10 from the lens is able to induce ectopic lacrimal glands to form within the presumptive cornea (Govindarajan et al., 2000), raising possibility that FGF signaling may be dominant.
RA signaling, canonical Wnt/β-catenin signaling, and PITX2 are all important for normal development of multiple organs. Therefore, aspects of the model presented in Fig. 5 may not be limited to the anterior segment. RA and canonical Wnt/β-catenin signaling interact elsewhere during development and in disease. Induction of a neuronal differentiation program in mouse embryonic stem cells is mediated by activation of Dkk1 downstream of RA signaling and the ensuing suppression of canonical Wnt/β-catenin signaling, a mechanism with clear parallels to events in the anterior segment (Verani et al., 2007). RA and canonical Wnt/β-catenin signaling function in vertebral patterning along the anterior-posterior axis of the embryo by synergistically activating expression of Cdx1 (Prinos et al., 2001). RA and canonical Wnt/β-catenin signaling also interact in breast and colon cancer (Szeto et al., 2001; Tice et al., 2002a; Tice et al., 2002b). In general, genetic evidence for these interactions is strong but the specific underlying mechanisms are unknown. Therefore, it may be that PITX2 or another transcription factor functions as an integration node in these systems, as occurs in the anterior segment. In addition to the eye, Pitx2 is essential for development of the branchial arches, brain, pituitary gland, and heart, all processes that are highly dependent on inductive patterning events, including by RA and canonical Wnt/β-catenin signaling. It will be important to determine whether the interactions between PITX2 and these signaling pathways are replicated during the development or function of these addition organs.
Acknowledgments
We thank Peter Hitchcock for helpful discussions and critical review of the manuscript. This work was supported by NEI/NIH (EY014126, EY007003) (PJG).
References
- Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, Albuquerque RJ, Richter E, Sakurai E, Newcomb MT, Kleinman ME, Caldwell RB, Lin Q, Ogura Y, Orecchia A, Samuelson DA, Agnew DW, St Leger J, Green WR, Mahasreshti PJ, Curiel DT, Kwan D, Marsh H, Ikeda S, Leiper LJ, Collinson JM, Bogdanovich S, Khurana TS, Shibuya M, Baldwin ME, Ferrara N, Gerber HP, De Falco S, Witta J, Baffi JZ, Raisler BJ, Ambati J. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang SJ, Stump RJ, Lovicu FJ, McAvoy JW. Spatial and temporal expression of Wnt and Dickkopf genes during murine lens development. Gene Expr Patterns. 2004;4:289–295. doi: 10.1016/j.modgep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–2711. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe DC, Coats JM. The lens organizes the anterior segment: specification of neural crest cell differentiation in the avian eye. Dev Biol. 2000;220:424–431. doi: 10.1006/dbio.2000.9638. [DOI] [PubMed] [Google Scholar]
- Blixt A, Mahlapuu M, Aitola M, Pelto-Huikko M, Enerback S, Carlsson P. A forkhead gene, FoxE3, is essential for lens epithelial proliferation and closure of the lens vesicle. Genes Dev. 2000;14:245–254. [PMC free article] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Brownell I, Dirksen M, Jamrich M. Forkhead Foxe3 maps to the dysgenetic lens locus and is critical in lens development and differentiation. Genesis. 2000;27:81–93. doi: 10.1002/1526-968x(200006)27:2<81::aid-gene50>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Chambon P. A decade of molecular biology of retinoic acid receptors. Faseb J. 1996;10:940–954. [PubMed] [Google Scholar]
- Chang B, Smith RS, Peters M, Savinova OV, Hawes NL, Zabaleta A, Nusinowitz S, Martin JE, Davisson ML, Cepko CL, Hogan BL, John SW. Haploinsufficient Bmp4 ocular phenotypes include anterior segment dysgenesis with elevated intraocular pressure. BMC Genet. 2001;2:18. doi: 10.1186/1471-2156-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Lun Y, Ovchinnikov D, Kokubo H, Oberg KC, Pepicelli CV, Gan L, Lee B, Johnson RL. Limb and kidney defects in Lmx1b mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet. 1998;19:51–55. doi: 10.1038/ng0598-51. [DOI] [PubMed] [Google Scholar]
- Chen Y, Stump RJ, Lovicu FJ, McAvoy JW. Expression of Frizzleds and secreted frizzled-related proteins (Sfrps) during mammalian lens development. Int J Dev Biol. 2004;48:867–877. doi: 10.1387/ijdb.041882yc. [DOI] [PubMed] [Google Scholar]
- Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001;17:255–296. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Tamm ER. Anterior eye development and ocular mesenchyme: new insights from mouse models and human diseases. Bioessays. 2004;26:374–386. doi: 10.1002/bies.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean CH, Miller LA, Smith AN, Dufort D, Lang RA, Niswander LA. Canonical Wnt signaling negatively regulates branching morphogenesis of the lung and lacrimal gland. Dev Biol. 2005;286:270–286. doi: 10.1016/j.ydbio.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Dickman ED, Thaller C, Smith SM. Temporally-regulated retinoic acid depletion produces specific neural crest, ocular and nervous system defects. Development. 1997;124:3111–3121. doi: 10.1242/dev.124.16.3111. [DOI] [PubMed] [Google Scholar]
- Drager UC, Li H, Wagner E, McCaffery P. Retinoic acid synthesis and breakdown in the developing mouse retina. Prog Brain Res. 2001;131:579–587. doi: 10.1016/s0079-6123(01)31045-2. [DOI] [PubMed] [Google Scholar]
- Duester G. Families of retinoid dehydrogenases regulating vitamin A function: production of visual pigment and retinoic acid. Eur J Biochem. 2000;267:4315–4324. doi: 10.1046/j.1432-1327.2000.01497.x. [DOI] [PubMed] [Google Scholar]
- Dupe V, Matt N, Garnier JM, Chambon P, Mark M, Ghyselinck NB. A newborn lethal defect due to inactivation of retinaldehyde dehydrogenase type 3 is prevented by maternal retinoic acid treatment. Proc Natl Acad Sci U S A. 2003;100:14036–14041. doi: 10.1073/pnas.2336223100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duplaa C, Jaspard B, Moreau C, D’Amore PA. Identification and cloning of a secreted protein related to the cysteine-rich domain of frizzled. Evidence for a role in endothelial cell growth control. Circ Res. 1999;84:1433–1445. doi: 10.1161/01.res.84.12.1433. [DOI] [PubMed] [Google Scholar]
- Evans AL, Gage PJ. Expression of the homeobox gene Pitx2 in neural crest is required for optic stalk and ocular anterior segment development. Hum Mol Genet. 2005;14:3347–3359. doi: 10.1093/hmg/ddi365. [DOI] [PubMed] [Google Scholar]
- Fang J, Dagenais SL, Erickson RP, Arlt MF, Glynn MW, Gorski JL, Seaver LH, Glover TW. Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema-distichiasis syndrome. Am J Hum Genet. 2000;67:1382–1388. doi: 10.1086/316915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlater GS, McDougall RD, Kaufman MH. Eyelid development, fusion and subsequent reopening in the mouse. J Anat. 1993;183(Pt 1):121–129. [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann S, Levine EM, Reh TA. Extraocular mesenchyme patterns the optic vesicle during early eye development in the embryonic chick. Development. 2000;127:4599–4609. doi: 10.1242/dev.127.21.4599. [DOI] [PubMed] [Google Scholar]
- Fujii H, Sato T, Kaneko S, Gotoh O, Fujii-Kuriyama Y, Osawa K, Kato S, Hamada H. Metabolic inactivation of retinoic acid by a novel P450 differentially expressed in developing mouse embryos. Embo J. 1997;16:4163–4173. doi: 10.1093/emboj/16.14.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Morrow EM, Li T, Davis FC, Cepko CL. Retinopathy and attenuated circadian entrainment in Crx-deficient mice. Nat Genet. 1999;23:466–470. doi: 10.1038/70591. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Camper SA. Pituitary homeobox 2, a novel member of the bicoid-related family of homeobox genes, is a potential regulator of anterior structure formation. Hum Mol Genet. 1997;6:457–464. doi: 10.1093/hmg/6.3.457. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Qian M, Wu D, Rosenberg KI. The canonical Wnt signaling antagonist DKK2 is an essential effector of PITX2 function during normal eye development. Dev Biol. 2008;317:310–324. doi: 10.1016/j.ydbio.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage PJ, Rhoades W, Prucka SK, Hjalt TA. Fate maps of neural crest and mesoderm in the mammalian eye. Invest Ophthalmol Vis Sci. 2005;46:4200–4208. doi: 10.1167/iovs.05-0691. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Suh H, Camper SA. Dosage requirement of Pitx2 for development of multiple organs. Development. 1999;126:4643–4651. doi: 10.1242/dev.126.20.4643. [DOI] [PubMed] [Google Scholar]
- Ghyselinck NB, Dupe V, Dierich A, Messaddeq N, Garnier JM, Rochette-Egly C, Chambon P, Mark M. Role of the retinoic acid receptor beta (RARbeta) during mouse development. Int J Dev Biol. 1997;41:425–447. [PubMed] [Google Scholar]
- Goodwin AM, Sullivan KM, D’Amore PA. Cultured endothelial cells display endogenous activation of the canonical Wnt signaling pathway and express multiple ligands, receptors, and secreted modulators of Wnt signaling. Dev Dyn. 2006;235:3110–3120. doi: 10.1002/dvdy.20939. [DOI] [PubMed] [Google Scholar]
- Gould DB, Smith RS, John SW. Anterior segment development relevant to glaucoma. Int J Dev Biol. 2004;48:1015–1029. doi: 10.1387/ijdb.041865dg. [DOI] [PubMed] [Google Scholar]
- Govindarajan V, Ito M, Makarenkova HP, Lang RA, Overbeek PA. Endogenous and ectopic gland induction by FGF-10. Dev Biol. 2000;225:188–200. doi: 10.1006/dbio.2000.9812. [DOI] [PubMed] [Google Scholar]
- Grun F, Hirose Y, Kawauchi S, Ogura T, Umesono K. Aldehyde dehydrogenase 6, a cytosolic retinaldehyde dehydrogenase prominently expressed in sensory neuroepithelia during development. J Biol Chem. 2000;275:41210–41218. doi: 10.1074/jbc.M007376200. [DOI] [PubMed] [Google Scholar]
- Hale F. Pigs born without eyeballs. Journal of Heredity. 1933;24:105–127. [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalt TA, Semina EV, Amendt BA, Murray JC. The Pitx2 protein in mouse development. Dev Dyn. 2000;218:195–200. doi: 10.1002/(SICI)1097-0177(200005)218:1<195::AID-DVDY17>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Ittner LM, Wurdak H, Schwerdtfeger K, Kunz T, Ille F, Leveen P, Hjalt TA, Suter U, Karlsson S, Hafezi F, Born W, Sommer L. Compound developmental eye disorders following inactivation of TGFbeta signaling in neural-crest stem cells. J Biol. 2005;4:11. doi: 10.1186/jbiol29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson AG, Sater AK. Features of embryonic induction. Development. 1988;104:341–359. doi: 10.1242/dev.104.3.341. [DOI] [PubMed] [Google Scholar]
- Kastner P, Grondona JM, Mark M, Gansmuller A, LeMeur M, Decimo D, Vonesch JL, Dolle P, Chambon P. Genetic analysis of RXR alpha developmental function: convergence of RXR and RAR signaling pathways in heart and eye morphogenesis. Cell. 1994;78:987–1003. doi: 10.1016/0092-8674(94)90274-7. [DOI] [PubMed] [Google Scholar]
- Kastner P, Mark M, Ghyselinck N, Krezel W, Dupe V, Grondona JM, Chambon P. Genetic evidence that the retinoid signal is transduced by heterodimeric RXR/RAR functional units during mouse development. Development. 1997;124:313–326. doi: 10.1242/dev.124.2.313. [DOI] [PubMed] [Google Scholar]
- Kaufman MH. The Atlas of Mouse Development. Acad. Press; San Diego: 1992. pp. 346–347. [Google Scholar]
- Kidson SH, Kume T, Deng K, Winfrey V, Hogan BL. The forkhead/winged-helix gene, Mf1, is necessary for the normal development of the cornea and formation of the anterior chamber in the mouse eye. Dev Biol. 1999;211:306–322. doi: 10.1006/dbio.1999.9314. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Miura H, Miyagawa-Tomita S, Yanazawa M, Katoh-Fukui Y, Suzuki R, Ohuchi H, Suehiro A, Motegi Y, Nakahara Y, Kondo S, Yokoyama M. Mouse Pitx2 deficiency leads to anomalies of the ventral body wall, heart, extra- and periocular mesoderm and right pulmonary isomerism. Development. 1999;126:5749–5758. doi: 10.1242/dev.126.24.5749. [DOI] [PubMed] [Google Scholar]
- Kozlowski K, Walter MA. Variation in residual PITX2 activity underlies the phenotypic spectrum of anterior segment developmental disorders. Hum Mol Genet. 2000;9:2131–2139. doi: 10.1093/hmg/9.14.2131. [DOI] [PubMed] [Google Scholar]
- Kriederman BM, Myloyde TL, Witte MH, Dagenais SL, Witte CL, Rennels M, Bernas MJ, Lynch MT, Erickson RP, Caulder MS, Miura N, Jackson D, Brooks BP, Glover TW. FOXC2 haploinsufficient mice are a model for human autosomal dominant lymphedema-distichiasis syndrome. Hum Mol Genet. 2003;12:1179–1185. doi: 10.1093/hmg/ddg123. [DOI] [PubMed] [Google Scholar]
- Kulak SC, Kozlowski K, Semina EV, Pearce WG, Walter MA. Mutation in the RIEG1 gene in patients with iridogoniodysgenesis syndrome. Hum Mol Genet. 1998;7:1113–1117. doi: 10.1093/hmg/7.7.1113. [DOI] [PubMed] [Google Scholar]
- Kume T, Jiang H, Topczewska JM, Hogan BL. The murine winged helix transcription factors, Foxc1 and Foxc2, are both required for cardiovascular development and somitogenesis. Genes Dev. 2001;15:2470–2482. doi: 10.1101/gad.907301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La P, Morgan TA, Sykes SM, Mao H, Schnepp RW, Petersen CD, Hua X. Fusion proteins of retinoid receptors antagonize TGF-beta-induced growth inhibition of lung epithelial cells. Oncogene. 2003;22:198–210. doi: 10.1038/sj.onc.1206100. [DOI] [PubMed] [Google Scholar]
- Lang RA. Pathways regulating lens induction in the mouse. Int J Dev Biol. 2004;48:783–791. doi: 10.1387/ijdb.041903rl. [DOI] [PubMed] [Google Scholar]
- Lehmann OJ, Ebenezer ND, Jordan T, Fox M, Ocaka L, Payne A, Leroy BP, Clark BJ, Hitchings RA, Povey S, Khaw PT, Bhattacharya SS. Chromosomal duplication involving the forkhead transcription factor gene FOXC1 causes iris hypoplasia and glaucoma. Am J Hum Genet. 2000;67:1129–1135. doi: 10.1016/s0002-9297(07)62943-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wagner E, McCaffery P, Smith D, Andreadis A, Drager UC. A retinoic acid synthesizing enzyme in ventral retina and telencephalon of the embryonic mouse. Mech Dev. 2000;95:283–289. doi: 10.1016/s0925-4773(00)00352-x. [DOI] [PubMed] [Google Scholar]
- Lin CR, Kioussi C, O’Connell S, Briata P, Szeto D, Liu F, Izpisua-Belmonte JC, Rosenfeld MG. Pitx2 regulates lung asymmetry, cardiac positioning and pituitary and tooth morphogenesis. Nature. 1999;401:279–282. doi: 10.1038/45803. [DOI] [PubMed] [Google Scholar]
- Lin M, Zhang M, Abraham M, Smith SM, Napoli JL. Mouse retinal dehydrogenase 4 (RALDH4), molecular cloning, cellular expression, and activity in 9-cis-retinoic acid biosynthesis in intact cells. J Biol Chem. 2003;278:9856–9861. doi: 10.1074/jbc.M211417200. [DOI] [PubMed] [Google Scholar]
- Liu H, Mohamed O, Dufort D, Wallace VA. Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev Dyn. 2003;227:323–334. doi: 10.1002/dvdy.10315. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lohnes D, Mark M, Mendelsohn C, Dolle P, Dierich A, Gorry P, Gansmuller A, Chambon P. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994;120:2723–2748. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- Longo KA, Kennell JA, Ochocinska MJ, Ross SE, Wright WS, MacDougald OA. Wnt signaling protects 3T3-L1 preadipocytes from apoptosis through induction of insulin-like growth factors. J Biol Chem. 2002;277:38239–38244. doi: 10.1074/jbc.M206402200. [DOI] [PubMed] [Google Scholar]
- Lu MF, Pressman C, Dyer R, Johnson RL, Martin JF. Function of Rieger syndrome gene in left-right asymmetry and craniofacial development. Nature. 1999;401:276–278. doi: 10.1038/45797. [DOI] [PubMed] [Google Scholar]
- Makarenkova HP, Ito M, Govindarajan V, Faber SC, Sun L, McMahon G, Overbeek PA, Lang RA. FGF10 is an inducer and Pax6 a competence factor for lacrimal gland development. Development. 2000;127:2563–2572. doi: 10.1242/dev.127.12.2563. [DOI] [PubMed] [Google Scholar]
- Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development. 2004;131:3021–3034. doi: 10.1242/dev.01253. [DOI] [PubMed] [Google Scholar]
- Masckauchan TN, Shawber CJ, Funahashi Y, Li CM, Kitajewski J. Wnt/beta-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. Angiogenesis. 2005;8:43–51. doi: 10.1007/s10456-005-5612-9. [DOI] [PubMed] [Google Scholar]
- Mascrez B, Mark M, Dierich A, Ghyselinck NB, Kastner P, Chambon P. The RXRalpha ligand-dependent activation function 2 (AF-2) is important for mouse development. Development. 1998;125:4691–4707. doi: 10.1242/dev.125.23.4691. [DOI] [PubMed] [Google Scholar]
- Matt N, Dupe V, Garnier JM, Dennefeld C, Chambon P, Mark M, Ghyselinck NB. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development. 2005;132:4789–4800. doi: 10.1242/dev.02031. [DOI] [PubMed] [Google Scholar]
- Matt N, Ghyselinck NB, Pellerin I, Dupe V. Impairing retinoic acid signalling in the neural crest cells is sufficient to alter entire eye morphogenesis. Dev Biol. 2008;320:140–148. doi: 10.1016/j.ydbio.2008.04.039. [DOI] [PubMed] [Google Scholar]
- McCaffery P, Wagner E, O’Neil J, Petkovich M, Drager UC. Dorsal and ventral rentinoic territories defined by retinoic acid synthesis, break-down and nuclear receptor expression. Mech Dev. 1999;85:203–214. doi: 10.1016/s0925-4773(99)00132-x. [DOI] [PubMed] [Google Scholar]
- McIntosh I, Dreyer SD, Clough MV, Dunston JA, Eyaid W, Roig CM, Montgomery T, Ala-Mello S, Kaitila I, Winterpacht A, Zabel B, Frydman M, Cole WG, Francomano CA, Lee B. Mutation analysis of LMX1B gene in nail-patella syndrome patients. Am J Hum Genet. 1998;63:1651–1658. doi: 10.1086/302165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears AJ, Jordan T, Mirzayans F, Dubois S, Kume T, Parlee M, Ritch R, Koop B, Kuo WL, Collins C, Marshall J, Gould DB, Pearce W, Carlsson P, Enerback S, Morissette J, Bhattacharya S, Hogan B, Raymond V, Walter MA. Mutations of the forkhead/winged-helix gene, FKHL7, in patients with Axenfeld-Rieger anomaly. Am J Hum Genet. 1998;63:1316–1328. doi: 10.1086/302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mic FA, Molotkov A, Fan X, Cuenca AE, Duester G. RALDH3, a retinaldehyde dehydrogenase that generates retinoic acid, is expressed in the ventral retina, otic vesicle and olfactory pit during mouse development. Mech Dev. 2000;97:227–230. doi: 10.1016/s0925-4773(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Mic FA, Molotkov A, Molotkova N, Duester G. Raldh2 expression in optic vesicle generates a retinoic acid signal needed for invagination of retina during optic cup formation. Dev Dyn. 2004;231:270–277. doi: 10.1002/dvdy.20128. [DOI] [PubMed] [Google Scholar]
- Miller LA, Smith AN, Taketo MM, Lang RA. Optic cup and facial patterning defects in ocular ectoderm beta-catenin gain-of-function mice. BMC Dev Biol. 2006;6:14. doi: 10.1186/1471-213X-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molotkov A, Fan X, Deltour L, Foglio MH, Martras S, Farres J, Pares X, Duester G. Stimulation of retinoic acid production and growth by ubiquitously expressed alcohol dehydrogenase Adh3. Proc Natl Acad Sci U S A. 2002;99:5337–5342. doi: 10.1073/pnas.082093299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molotkov A, Molotkova N, Duester G. Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development. 2006;133:1901–1910. doi: 10.1242/dev.02328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan AP, Kioschis P, Wu W, Zuniga A, Bock D, Poustka A, Delius H, Niehrs C. Dickkopf genes are co-ordinately expressed in mesodermal lineages. Mech Dev. 1999;87:45–56. doi: 10.1016/s0925-4773(99)00138-0. [DOI] [PubMed] [Google Scholar]
- Mori M, Ghyselinck NB, Chambon P, Mark M. Systematic immunolocalization of retinoid receptors in developing and adult mouse eyes. Invest Ophthalmol Vis Sci. 2001;42:1312–1318. [PubMed] [Google Scholar]
- Mukhopadhyay M, Gorivodsky M, Shtrom S, Grinberg A, Niehrs C, Morasso MI, Westphal H. Dkk2 plays an essential role in the corneal fate of the ocular surface epithelium. Development. 2006;133:2149–2154. doi: 10.1242/dev.02381. [DOI] [PubMed] [Google Scholar]
- Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- Ormestad M, Blixt A, Churchill A, Martinsson T, Enerback S, Carlsson P. Foxe3 haploinsufficiency in mice: a model for Peters’ anomaly. Invest Ophthalmol Vis Sci. 2002;43:1350–1357. [PubMed] [Google Scholar]
- Pei YF, Rhodin JA. The prenatal development of the mouse eye. Anat Rec. 1970;168:105–125. doi: 10.1002/ar.1091680109. [DOI] [PubMed] [Google Scholar]
- Pendaries V, Verrecchia F, Michel S, Mauviel A. Retinoic acid receptors interfere with the TGF-beta/Smad signaling pathway in a ligand-specific manner. Oncogene. 2003;22:8212–8220. doi: 10.1038/sj.onc.1206913. [DOI] [PubMed] [Google Scholar]
- Peters MA, Cepko CL. The dorsal-ventral axis of the neural retina is divided into multiple domains of restricted gene expression which exhibit features of lineage compartments. Dev Biol. 2002;251:59–73. doi: 10.1006/dbio.2002.0791. [DOI] [PubMed] [Google Scholar]
- Pressman CL, Chen H, Johnson RL. LMX1B, a LIM homeodomain class transcription factor, is necessary for normal development of multiple tissues in the anterior segment of the murine eye. Genesis. 2000;26:15–25. [PubMed] [Google Scholar]
- Prinos P, Joseph S, Oh K, Meyer BI, Gruss P, Lohnes D. Multiple pathways governing Cdx1 expression during murine development. Dev Biol. 2001;239:257–269. doi: 10.1006/dbio.2001.0446. [DOI] [PubMed] [Google Scholar]
- Ring BZ, Cordes SP, Overbeek PA, Barsh GS. Regulation of mouse lens fiber cell development and differentiation by the Maf gene. Development. 2000;127:307–317. doi: 10.1242/dev.127.2.307. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Sporn MB. Mechanistic interrelationships between two superfamilies: the steroid/retinoid receptors and transforming growth factor-beta. Cancer Surv. 1992;14:205–220. [PubMed] [Google Scholar]
- Saika S, Liu CY, Azhar M, Sanford LP, Doetschman T, Gendron RL, Kao CW, Kao WW. TGFbeta2 in corneal morphogenesis during mouse embryonic development. Dev Biol. 2001;240:419–432. doi: 10.1006/dbio.2001.0480. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Luo T, McCaffery P, Hamada H, Drager UC. CYP26A1 and CYP26C1 cooperate in degrading retinoic acid within the equatorial retina during later eye development. Dev Biol. 2004;276:143–157. doi: 10.1016/j.ydbio.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See AW, Clagett-Dame M. The temporal requirement for vitamin A in the developing eye: mechanism of action in optic fissure closure and new roles for the vitamin in regulating cell proliferation and adhesion in the embryonic retina. Dev Biol. 2009;325:94–105. doi: 10.1016/j.ydbio.2008.09.030. [DOI] [PubMed] [Google Scholar]
- Seeliger MW, Biesalski HK, Wissinger B, Gollnick H, Gielen S, Frank J, Beck S, Zrenner E. Phenotype in retinol deficiency due to a hereditary defect in retinol binding protein synthesis. Invest Ophthalmol Vis Sci. 1999;40:3–11. [PubMed] [Google Scholar]
- Semina EV, Brownell I, Mintz-Hittner HA, Murray JC, Jamrich M. Mutations in the human forkhead transcription factor FOXE3 associated with anterior segment ocular dysgenesis and cataracts. Hum Mol Genet. 2001;10:231–236. doi: 10.1093/hmg/10.3.231. [DOI] [PubMed] [Google Scholar]
- Semina EV, Reiter R, Leysens NJ, Alward WL, Small KW, Datson NA, Siegel-Bartelt J, Bierke-Nelson D, Bitoun P, Zabel BU, Carey JC, Murray JC. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat Genet. 1996;14:392–399. doi: 10.1038/ng1296-392. [DOI] [PubMed] [Google Scholar]
- Skurk C, Maatz H, Rocnik E, Bialik A, Force T, Walsh K. Glycogen-Synthase Kinase3beta/beta-catenin axis promotes angiogenesis through activation of vascular endothelial growth factor signaling in endothelial cells. Circ Res. 2005;96:308–318. doi: 10.1161/01.RES.0000156273.30274.f7. [DOI] [PubMed] [Google Scholar]
- Smith AN, Miller LA, Song N, Taketo MM, Lang RA. The duality of beta-catenin function: A requirement in lens morphogenesis and signaling suppression of lens fate in periocular ectoderm. Dev Biol. 2005;285:477–489. doi: 10.1016/j.ydbio.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Smith RS, Zabaleta A, Kume T, Savinova OV, Kidson SH, Martin JE, Nishimura DY, Alward WL, Hogan BL, John SW. Haploinsufficiency of the transcription factors FOXC1 and FOXC2 results in aberrant ocular development. Hum Mol Genet. 2000;9:1021–1032. doi: 10.1093/hmg/9.7.1021. [DOI] [PubMed] [Google Scholar]
- Spemann H. Über korrelationen in die Entwickelung des Auges. Verh. Anat. Ges. 1901;15:61–79. [Google Scholar]
- Suzuki R, Shintani T, Sakuta H, Kato A, Ohkawara T, Osumi N, Noda M. Identification of RALDH-3, a novel retinaldehyde dehydrogenase, expressed in the ventral region of the retina. Mech Dev. 2000;98:37–50. doi: 10.1016/s0925-4773(00)00450-0. [DOI] [PubMed] [Google Scholar]
- Szeto W, Jiang W, Tice DA, Rubinfeld B, Hollingshead PG, Fong SE, Dugger DL, Pham T, Yansura DG, Wong TA, Grimaldi JC, Corpuz RT, Singh JS, Frantz GD, Devaux B, Crowley CW, Schwall RH, Eberhard DA, Rastelli L, Polakis P, Pennica D. Overexpression of the retinoic acid-responsive gene Stra6 in human cancers and its synergistic induction by Wnt-1 and retinoic acid. Cancer Res. 2001;61:4197–4205. [PubMed] [Google Scholar]
- Tice DA, Soloviev I, Polakis P. Activation of the Wnt pathway interferes with serum response element-driven transcription of immediate early genes. J Biol Chem. 2002a;277:6118–6123. doi: 10.1074/jbc.M111255200. [DOI] [PubMed] [Google Scholar]
- Tice DA, Szeto W, Soloviev I, Rubinfeld B, Fong SE, Dugger DL, Winer J, Williams PM, Wieand D, Smith V, Schwall RH, Pennica D, Polakis P. Synergistic induction of tumor antigens by Wnt-1 signaling and retinoic acid revealed by gene expression profiling. J Biol Chem. 2002b;277:14329–14335. doi: 10.1074/jbc.M200334200. [DOI] [PubMed] [Google Scholar]
- Verani R, Cappuccio I, Spinsanti P, Gradini R, Caruso A, Magnotti MC, Motolese M, Nicoletti F, Melchiorri D. Expression of the Wnt inhibitor Dickkopf-1 is required for the induction of neural markers in mouse embryonic stem cells differentiating in response to retinoic acid. J Neurochem. 2007;100:242–250. doi: 10.1111/j.1471-4159.2006.04207.x. [DOI] [PubMed] [Google Scholar]
- Vollrath D, Jaramillo-Babb VL, Clough MV, McIntosh I, Scott KM, Lichter PR, Richards JE. Loss-of-function mutations in the LIM-homeodomain gene, LMX1B, in nail-patella syndrome. Hum Mol Genet. 1998;7:1091–1098. doi: 10.1093/hmg/7.7.1091. [DOI] [PubMed] [Google Scholar]
- Wagner E, McCaffery P, Drager UC. Retinoic acid in the formation of the dorsoventral retina and its central projections. Dev Biol. 2000;222:460–470. doi: 10.1006/dbio.2000.9719. [DOI] [PubMed] [Google Scholar]
- Warkany J, Schraffenberger S. Congenital malformations induced in rats by maternal vitamin A deficiency. I. Defects of the eye. Arch Ophthalmol. 1946;35:150–169. doi: 10.1001/archopht.1946.00890200155008. [DOI] [PubMed] [Google Scholar]
- Wilson JG, Roth CB, Warkany J. An analysis of the syndrome of malformations induced by maternal vitamin A deficiency. Effects of restoration of vitamin A at various times during gestation. Am J Anat. 1953;92:189–217. doi: 10.1002/aja.1000920202. [DOI] [PubMed] [Google Scholar]
- Wolosin JM, Schutte M, Zieske JD, Budak MT. Changes in connexin43 in early ocular surface development. Curr Eye Res. 2002;24:430–438. doi: 10.1076/ceyr.24.6.430.8599. [DOI] [PubMed] [Google Scholar]
- Wright M, Aikawa M, Szeto W, Papkoff J. Identification of a Wnt-responsive signal transduction pathway in primary endothelial cells. Biochem Biophys Res Commun. 1999;263:384–388. doi: 10.1006/bbrc.1999.1344. [DOI] [PubMed] [Google Scholar]
- Wu MY, Hill CS. Tgf-beta superfamily signaling in embryonic development and homeostasis. Dev Cell. 2009;16:329–343. doi: 10.1016/j.devcel.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Xia Y, Karin M. The control of cell motility and epithelial morphogenesis by Jun kinases. Trends Cell Biol. 2004;14:94–101. doi: 10.1016/j.tcb.2003.12.005. [DOI] [PubMed] [Google Scholar]