Abstract
The current investigation aimed to develop a biomimetic, three-dimensional (3D) culture system for poorly adherent bone metastatic prostate cancer cells (C4-2B) for use as an in vitro platform for anti-cancer drug screening. To this end, hyaluronic acid (HA) derivatives carrying complementary aldehyde (HAALD) and hydrazide (HAADH) groups were synthesized and characterized. In situ encapsulation of C4-2B cells was achieved by simple mixing of HAALD and HAADH in the presence of the cells. Unlike two-dimensional (2D) monolayer culture in which cells adopt an atypical spread morphology, cells residing in the HA matrix formed distinct clustered structures which grew and merged, reminiscent of real tumors. Anti-cancer drugs added to the media surrounding the cell/gel construct diffused into the gel and killed the embedded cells. The HA hydrogel system was used successfully to test the efficacy of anti-cancer drugs including camptothecin, docetaxel, and rapamycin, alone and in combination, including specificity, dose and time responses. Responses of cells to anti-neoplastics differed between the 3D HA hydrogel and 2D monolayer systems. We suggest that the data obtained from 3D HA systems is superior to that from conventional 2D monolayers as the 3D system better reflects the bone metastatic microenvironment of the cancer cells.
Keywords: prostate cancer, bone metastasis, hyaluronic acid, hydrogels, 3D, drug selection
1. Introduction
For prostate cancer, five year survival rates for local disease approach 100% while for bone metastatic disease the five year survival rate is less than 35% [1]. A major reason for the large discrepancy between the survival rate for local and advanced disease is a lack of effective therapies to treat bone metastases. The majority of prostate cancer bone metastases are osteoblastic in nature [2].
Because of the low efficacy of current treatments for bone metastatic disease and the number of potential lives that could be saved or extended with new therapeutics, there is considerable interest in developing new systems to test potential drugs more quickly and accurately [3]. In the late 1980’s the National Cancer Institute (NCI) developed an in vitro drug discovery tool to efficiently test new therapeutics using 60 different well characterized human cancer cell lines. Since its induction, this system has remained the gold standard of anticancer drug discovery [4, 5]. Of the 60 cell lines in the screen, called the NCI-60, only two are derived from prostate cancers, a disproportionately low number when compared to the disease’s prevalence. A promising group of prostate cancer cell lines, called the LNCaP series, was disqualified from the NCI-60 panel because they adhere poorly to plastic, frequently detaching during the standard assay protocol. The LNCaP series is one of the best prostate cancer progression models available. C4-2B cells, a LNCaP subline typical of bony metastases, form osteoblastic lesions when injected into mice [6]. The ability to test new anticancer therapeutics on C4-2B cells offers a great advantage to the development of therapies to treat advanced prostate cancer growing in bone.
For this study, we selected three anti-cancer drugs with different mechanisms of action and clinical relevance in the treatment of bone metastatic prostate cancer to test in the current investigation. Camptothecin (CPT) is a classical chemotherapeutic that is well known to efficiently cause an apoptotic response in prostate cancer cells. Due to adverse side effects and poor solubility, it is not administered clinically, but two analogues have been used to treat advanced prostate cancer [7]. Docetaxel (DOX) is the standard of care for advanced prostate cancer and considerable interest has been expressed for using it as a part of combination therapies [8]. Rapamycin (RAP) is currently in clinical trials for many different types of cancer, including prostate, and shows promise as a part of combination therapies [9]. Comparisons of the mechanisms of action and properties of these three drugs are outlined in Table 1 [9-12].
Table 1. Comparisons of anti-cancer therapeutics used in this study.
| Common Name | Camptothecin | Docetaxel | Rapamycin |
| Trademarked Name | Topotecan, Irinotecan | Taxotere | Sirolimus, Everolimus |
| Molecular Weight | 348.4 g/mol | 807.9 g/mol | 914.2 g/mol |
| Solubility in water | 10.9 μM | 7.4-8.7 μM | 2.8 μM |
| Mechanism of Action | Stabilizes Topoisomerase I preventing DNA re-ligation | Inhibits microtubule depolymerization | Inhibits mTOR pathway |
| Cell Processes Impacted | DNA Replication | Cell Division, Cellular Trafficking | Transcription |
In addition to disqualifying poorly adherent cells, which may in fact better mimic metastatic cancer cells than do cells forming tight focal adhesions to plastic, monolayer drug discovery systems have additional disadvantages. On stiff and planar plastic surfaces typically used for culture of mammalian cell lines, adherent cells adopt a sheet-like morphology which is atypical of solid tumors. This flat morphology affects gene expression, cell behavior, and drug sensitivity [13, 14]. Additionally, the microenvironment of the cancer cells, specifically the chemical composition, nanoscale organization, and physical properties of their extracellular matrix (ECM), affect sensitivity to anticancer drugs [15, 16]. To remedy this, 3D culture systems based on porous synthetic polymer scaffolds [13, 17], reconstituted ECM components [18, 19], and fibroblast-derived 3D matrix [20] have been developed to better simulate the tumor microenvironment. Although tumor cells residing within artificial 3D matrices generally adopt tumor-like tissue morphology [21, 22], limitations exist for these culture systems. While synthetic scaffolds based on poly(lactide) or poly(lactide-co-glycolide) can be used, these are not natural matrices and hence do not provide extracellular cues as would native ECM. Additionally, matrices constructed from isolated biological sources can be variable in composition and hence irreproducible [16].
Hyaluronic acid (HA) is a ubiquitous nonsulfated glycosaminoglycan that is prevalent in the bone marrow [23-25]. HA is highly expressed in malignant tumors, making it an integral component of the microenvironment of bone metastatic cancer cells [26]. Through interaction with its receptors, cluster designation 44 (CD44) and receptor for hyaluronan mediated motility (RHAMM), HA alters the biological activity of cancer cells by activating many signaling pathways including the transforming growth factor β (TGF-β), Rho GTPase, and focal adhesion kinase (FAK) pathways [27-29]. These biological changes may play a role in the cell’s response to chemotherapeutic drugs [30]. Thus, HA-based, covalently cross-linked, 3D hydrogel matrices are attractive candidates for in vitro investigation of novel therapeutics targeting osteoblastic prostate cancer cells. In this study, we present the use of an in situ crosslinkable HA hydrogel system (Figure 1). Here, we performed a systematic comparison of C4-2B cell killing by three therapeutic agents in 2D versus 3D HA matrices.
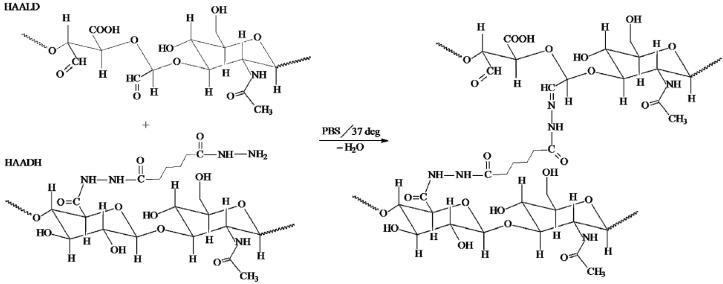
Figure 1. Chemical structures of materials used in HA hydrogels.
Covalent cross-linking of HAALD and HAADH in PBS produces in a stable hydrogel via the formation of hydrazone bonds, with water being the only by-product.
2. Materials and Methods
2.1. Materials
Hyaluronic acid (HA, sodium salt) of high (HMW, 1.1 MDa) and low (LMW, 490 KDa) molecular weights were generously donated by Genzyme Corporation (Cambridge, MA). Sodium periodate (NaIO4), ethylene glycol, adipic dihydrazide (ADH), 1-ethyl-3-[3-(dimethylamino)propyl] carbodiimide (EDC), sodium hydroxide (NaOH), hydrochloric acid (HCl) and 1-hydroxybenzotriazole (HOBt) were purchased from Aldrich (Milwaukee, WI). Dimethyl sulfoxide (DMSO), acetone, acetonitrile and isopropyl alcohol were obtained from Fisher Scientific (Waltham, MA). DOX (LKT Laboratories, Saint Paul, MN), CPT (Sigma-Aldrich, Saint Louis, MO), and RAP (A.G. Scientific, San Diego, CA) all were purchased commercially. All chemicals were used as received. All cell culture reagents were purchased from Invitrogen (Carlsbad, CA). 1X Dulbecco’s phosphate buffered saline (DPBS) was purchased from Cellgro (Herndon, VA).
2.1. Cell Culture
C4-2B cells were maintained in Corning (Lowell, MA) tissue culture 75mm flasks at 37°C in 5.0% (v/v) CO2 in T-medium supplemented with 5% (v/v) fetal bovine serum (FBS) and 100 U/ml penicillin G sodium and 100 μg/ml streptomycin sulfate in 0.085% (w/v) saline (PS). Medium was changed every two days. Cells were routinely passaged using 0.25% (w/v) trypsin with ethylenediaminetetraacetic acid (EDTA) 4*Na.
2.2. Synthesis of HA derivatives
HA derivatives were prepared as previously described with minor adjustments [31, 32]. To synthesize HA aldehyde (HAALD), HMW HA was allowed to react with sodium periodate in aqueous media at room temperature in the dark for 2 hours. The degree of oxidation was 12% as determined by idometry (data not shown) [33]. The excess NaIO4 was deactivated by ethylene glycol before the reaction mixture was subjected to dialysis (MWCO 10,000, Spectra/Por membrane, Spectrum Laboratories, Rancho Dominguez, CA) and freeze drying. The molecular weight of HAALD was 200 kDa as determined by Shimadzu (Columbia, MD) high-performance liquid chromatography (HPLC) system operating in size exclusion chromatography (SEC) mode using a RID-10A refractive index detector and a two TSK gel columns (Tosoh Bioscience, Montgomeryville, PA) calibrated by HA molecular weight standards (Lifecore Biomedical LLC, Chaska, MN). Sodium acetate buffer (pH 6.7, NaCl 0.2M) was used as the mobile phase, the flow rate was 0.5 mL/min and the sample concentration was 1 mg/mL.
Separately, HA adipic dihydrazide (HAADH) was synthesized by carbodiimide mediated coupling of ADH with the carboxyl groups of HA in aqueous media. Specifically, a 30 molar excess of ADH was added to the HA (LMW) solution (2 mg/mL). Solution pH was adjusted to 6.8 using NaOH (0.1 M) and HCl (0.1 M). EDC (5 mmol) and HOBt (5 mmol) were dissolved separately in DMSO/ H2O (1/1 volume ratio, total of 10 mL) and added to the HA solution sequentially. The solution pH was maintained at 6.8 for at least 6 hours. The solution pH was subsequently adjusted to 7.0 and exhaustively dialyzed against deionized (DI) water. NaCl then was added to produce a 5% (w/v) solution, and HAADH was precipitated in ethanol. The precipitate was re-dissolved in H2O and dialyzed again to remove the salt. The purified product was freeze-dried and stored at 4 °C. A 37% ADH incorporation was determined by 1H NMR spectroscopy (AV400 NMR). The molecular weight of HAADH was determined to be 298 kDa following the same procedure described above.
2.3. Culturing cells in HA-hydrogel
HA derivatives were dissolved separately in 1X DPBS at a concentration of 10 mg/mL overnight at 37°C. The HA solutions were sterilized by UV irradiation (254 nm) for 30 minutes prior to cell encapsulation. C4-2B cells were detached from the flask with a 0.25% (w/v) trypsin/EDTA 4Na and the total number of cells was counted using a hemacytometer (Hausser Scientific, Horsham, PA). Cells were pelleted by centrifugation for 1 minute at 5000 RPM and the medium was removed. Cell culture inserts (Millipore, Billerica, MA, diameter: 12 mm, pore size: 0.4μm) were pre-wet in T-medium then placed in the wells of a 24-well culture plate (Beckton-Dickenson Labware, Franklin Lakes, NJ). HAADH (100 μL) was added to the HAALD solution (100 μL) containing dispersed C4-2B cells (1 × 105). After thorough mixing, the solution was pipetted into a cell culture insert and was incubated for approximately 10 minutes at 37°C. T-medium (1 mL) was subsequently added around the insert and the construct was incubated at 37°C and 5.0% (v/v) CO2. Cells cultured on 2D in a 24-well culture plate were used as the control.
2.5. Treating cells with drugs
After 24 hour incubation in hydrogels or on plastic surfaces, cells were treated with DOX, CPT, RAP, or their respective combinations. Drugs were diluted in DMSO to a concentration such that 1μl of each dilution could be added to each of the cultures to produce the desired final concentration. Vehicle control cultures were treated with 1μl DMSO. For cultures growing in monolayer on plastic, drug dilution or DMSO (1μl) was added to the medium on top of the cells. For 3D cultures, the drug or DMSO (1μl) was added to the medium around the gel containing insert. The cultures were swirled gently to evenly disperse the drug.
2.4. Immunostaining
For all immunostaining procedures, cells growing on plastic were cultured in an 8 well Lab-Tek II Chambered Coverglass (Nalge Nunc, Naperville, IL) while cells in HA hydrogel were cultured in 24 well plates as described above. For live/dead staining, SYTO-13 green fluorescent nucleic acid stain and propidium iodide red fluorescent nucleic acid stain, both from Invitrogen, were diluted 1:1000 (v/v) each in DPBS. Medium was removed from cells plated on plastic and the 1 mL of the immunostaining mixture was applied. For HA hydrogel cultures, 1 mL of the immunostaining mixture was applied to an empty well and the hydrogel containing insert was removed from the medium and placed in the immunostaining mixture. The cultures were incubated for 15 minutes then visualized using confocal microscopy on a Zeiss LSM 510 VIS (Carl Zeiss, Maple Grove, MN). To visualize cells in HA hydrogels, the hydrogel-containing insert was transferred to a 1 well Lab-Tek II Chambered Coverglass (Nalge Nunc) prior to imaging on the confocal microscope.
For cytoskeleton visualization of monolayer cultures, medium was removed and chambers were washed with DPBS. Cells were fixed using 4% (v/v) paraformaldehyde (PFA) (Electron Microscopy Sciences, Hatfield, PA) in ddH2O. Excess PFA was removed and the chambers were washed again with DPBS. 0.2% (v/v) Triton X-100 (Fisher Scientific, Waltham, MA) was prepared and applied to chambers for 7 minutes. Excess Triton solution was removed and chambers were again washed with DPBS. Cultures were subsequently blocked in 3% (w/v) bovine serum albumin (BSA) (Sigma-Aldrich) in DPBS at 4°C overnight. Cells were incubated with 1:200 (v/v) solution of AlexaFluor 488 phalloidin (Invitrogen) in 3% BSA at 4°C overnight. A 1:1000 (v/v) solution of Draq5 (Biostatus Limited, Leicestershire, UK) in 3% BSA was applied for 10 minutes at room temperature. Chambers were again washed with DPBS and Gel/Mount™ (Biomeda, Foster City, CA) was added to chambers to prevent photobleaching. Cultures were imaged using confocal microscopy as described above.
For cytoskeleton staining of HA hydrogel cultures, hydrogel-containing inserts were removed from the medium and placed in a well containing DPBS to wash. Inserts then were placed in 4% (v/v) PFA and incubated at room temperature for 20 minutes. Cultures were again washed in DPBS. Cultures were incubated with 0.2% Triton for 15 minutes at room temperature and were washed with DPBS. Cultures were blocked in 3% BSA at 4°C overnight. Culture was moved a 1:200 (v/v) solution of AlexaFluor 488 phalloidin in 3% BSA at 4°C overnight. Inserts then were incubated with a 1:1000 (v/v) solution of Draq5 in 3% BSA at room temperature for 15 minutes. Insert again was washed in DPBS. Gel was removed gently from the insert using a 1mL micropipette and placed into the chambers of an 8 well Lab-Tek II chambered coverglass. Gel/Mount™ was added to the gel within the chambers and cultures were imaged using confocal microscopy as described above.
2.6. Viable/Non-viable Cell Counts
Trypan blue exclusion was used to quantify the relative numbers of viable and non-viable cells cultured on 2D and in 3D. Twelve plastic and hydrogel cultures were prepared and part of medium (600 μl) was changed every 2 days of incubation. Partial medium changes were utilized to minimize cell loss on plastic cultures. At days 1, 3, 5, and 7 after plating, three of each type of culture were treated with bovine testicular hyaluronidase (0.3 mg/mL) for 30 min at 37°C, then cultures were collected and cells were pelleted by centrifuging for 10 minutes at 5000 rpm. The supernatant was removed and 0.25% trypsin EDTA 4•Na (30 μl) was added to cell pellet, then was incubated at 37°C for 5 minutes. 70 μl 0.4% Trypan blue (Sigma-Aldrich) then was added to the trypsinized pellet and viable and non-viable cells were counted using a hemacytometer.
2.7. Apoptosis Assays
To detect apoptosis a Cell Death Detection ELISAPLUS (Roche Applied Science, Mannheim, Germany) was used. Protocol was followed except for the following modifications to allow for use of a 24 well plate and testing of cells cultured in the HA hydrogel. Prior to the assay, cells were collected from the hydrogel and plastic with hyaluronidase and centrifugation as previously described. Colorimetric detection was carried out according to manufacturer’s instructions using MRX microplate reader (Dynex Technologies, Chantilly, VA) at 405 nm excitation / 490 nm emission.
2.8. Drug Diffusion Experiments
HA hydrogels without encapsulated cells were prepared as described above. To avoid interference during the subsequent HPLC analysis, gels were immersed in DPBS instead of T-medium. Twenty-four hours following the gelation, DPBS surrounding the insert was dosed with the respective drugs. For CPT, the drug was dissolved in DMSO to a concentration such that 1 μl of the dilution could be added to reach the desired final concentration. For DOX and RAP, the drug was dissolved in DMSO to a concentration such that 100 μl of the dilution could be added to reach the final concentration. After 6 and 24 hours of drug treatment, the gels were treated with hyaluronidase (0.3 mg/mL) for 30 minutes and the gel component was collected separately from the DPBS component.
The amount of CPT in the hydrogel and media was measured using a FluoroMax-3 spectroflourmeter (Jobin Yvon, NJ) at an excitation wavelength of 370 nm and an emission wavelength of 434 nm. Standard solutions were prepared by diluting the stock solution of CPT (1μg/ml in DMSO) in PBS. The calibration curve was obtained with at a CPT concentration varying from 3 ng/ml to 100 ng/ml. After 10x dilution using PBS, the fluorescence intensity of the test samples (three replicates for each sample) was collected and the CPT concentration was determined using the calibration curve [34, 35].
Shimadzu HPLC (Shimadzu Scientific Instruments, Columbia, MD, USA) equipped with photodiode detector (SPD-M20A) was used for the quantification of DOX and RAP concentration in hydrogels and the media surrounding the gels. [36, 37] Separation of DOX was performed using a Curosil-PFP column (250×4.5 mm, 5 μm particles size) packed with pentafluorophenyl groups chemically bonded to silica particles (Phenomenex, CA, USA). Deionized water served as solvent A and HPLC grade acetonitrile served as solvent B. A linear gradient of 25-65% solvent B over 40 minutes was used as the mobile phase and then the mobile phase was equilibrated with 25% solvent for 10 minutes. Flow rate of the mobile phase was 1.0 ml/minute. The injected volume was 50 μl and eluents were detected at 228 nm. The stock solution of DOX was prepared in DMSO at concentration of 1 mg/ml. Standard solutions were prepared at concentrations of 6.0 μg/ml to 100 μg/ml by diluting the stock solution of DOX in DPBS. All measurements were performed in triplicates.
Separation of RAP was performed using Luna C-18 (2) (250×4.6 mm, 5 μm particles size) packed with silica particles (Phenomenex). Deionized water with 0.1% trifluoroacetic acid (TFA) was used as a solvent A and HPLC grade acetonitrile was used as a solvent B. A linear gradient of 50-90% solvent B over 25 minutes was used as a mobile phase and maintained at 90% solvent B for another 10 minutes. Finally, the mobile phase was equilibrated with 50% solvent for 10 minutes. The flow rate of the mobile phase was 1.0 ml/minute. The injected volume was 100 μl and eluents were detected at 278nm. A stock solution of RAP was prepared in DMSO at concentration of 1mM. Standard solutions were prepared at concentrations of 10.0 μg/ml to 25.0 μg/ml by diluting the stock solution of RAP in DPBS. All measurements were performed in triplicates.
2.9. Statistical Analysis
Error bars on all figures represent standard error of the mean (SEM). Significance was determined using Student’s two sample two-tailed t-tests with a p<0.05 considered significant.
3. Results
3.1. Cell growth, viability, and morphology in 3D HA hydrogel and 2D monolayer
3.1.1. Cell growth in HA hydrogel
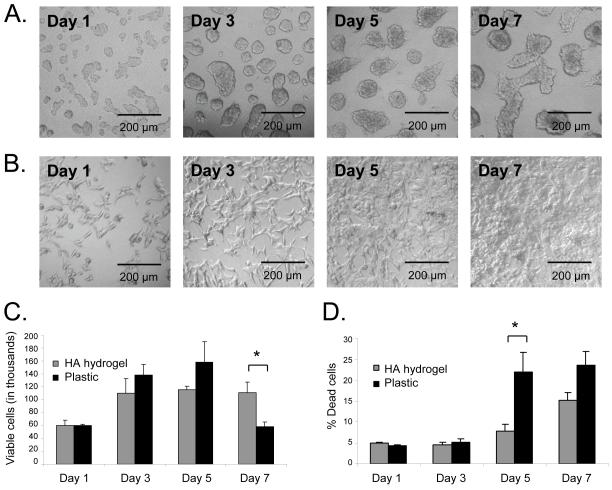
First, we investigated cell growth in the HA hydrogel. Phase-contrast microscope images for C4-2B cells cultured in the 3D matrix displayed the presence of cell clusters composed of greater than 50 cells (Figure 2A) that were absent in 2D monolayer culture system (Figure 2B). The size of these clusters increased over the 7 days of culture during which neighboring clusters were observed to merge. By day 5, we viewed processes at the edges of clusters. By counting viable cells using Trypan blue staining exclusion (Figure 2C), we found that cells plated on 2D monolayer increased in number rapidly between days 1 and 5 then decreased dramatically between days 5 and 7 as they became confluent and released from the flask due to their poor adherence. Interestingly, cells cultured in HA hydrogel increased in number between days 1 and 3, then stayed at a steady number from days 3 to 7. The number of cells was approximately equal on plastic or HA hydrogel from days 1 through 5 for which reason we conducted the drug testing experiments during that time period.
Figure 2. C4-2B cell viability is retained when cultured in the HA hydrogels.
Phase microscope images of cell cluster growth in HA hydrogel over seven days (A). Phase microscope images of cell growth on 2D plastic monolayer over seven days (B). Viable cell numbers in HA hydrogel compared to plastic over seven days (C). Trypan blue positive dead cells were excluded from these counts. Basal level of cell death in HA hydrogel or on plastic over seven days (D). Dead cells were counted after staining with trypan blue. * p<0.05, n = 3, error bars = SEM.
3.1.2. Cell viability in HA hydrogel
Next, we investigated if the HA hydrogel could preserve C4-2B cell viability. By counting total cells and dead cells using Trypan blue staining exclusion (Figure 2D), we observed that the percentage of dead cells in the HA hydrogel remained low and steady from days 1 through 5, after which the percent dead cells increased slightly. On 2D monolayer culture, the percentage of dead cells was approximately the same as those plated in the HA hydrogel from days 1 to 3, while by day 5 the percentage of dead cells increased dramatically compared to the hydrogel and remained the same through day 7. Additionally, by using live/dead staining viewed using confocal microscopy, it was observed that there are few dead (red) cells whether the cells are plated on 2D monolayer (Figure 3C) or in the HA hydrogel (Figure 3D). Again, the number of dead cells was approximately the same between the 2D monolayer culture and the HA hydrogel as determined by live/dead immunostaining.
Figure 3. C4-2B cells form clusters in HA hydrogels but not on plastic.
Phase contrast images of cells cultured on plastic (A) or in HA hydrogel (B) for 2 days. Confocal images of live/dead stained cells cultured on plastic (C) or HA hydrogel (D) for 2 days. Cells were stained with Syto-13 (green) for live cells and PI (red) for dead cells. Confocal images of cells cultured on plastic (E) or HA hydrogel (F) for 2 days stained for F-actin with phalloidin (green) and Draq5 for nuclei (blue).
3.1.3. Cell morphology in HA hydrogel
Finally, we investigated the effects of HA hydrogel culture on cell morphology. We compared the cell morphology of C4-2B cells plated on plastic or in the HA hydrogel using various microscopy techniques. On 2D monolayer, the C4-2B cells showed a spread-out morphology typical of this cell type on tissue culture plastic. This morphology was observed using phase microscopy (Figure 3A) and cytoskeleton and nuclei immunostaining (Figure 3E). The cytoplasm was elongated and nuclei appeared to be flattened against the 2D surface and cell-cell contacts were limited in subconfluent cells. Using the same microscopy techniques that were used to visualize cells in 2D monolayer culture, C4-2B cells plated in the HA hydrogel displayed a rounded, clustered morphology with clearly distinct rounded nuclei evident (Figure 3B and 3F). The cytoplasm was rounded in these cells, giving the illusion of smaller cells when viewed from a single plane, and cell-cell contacts within clusters appeared to be more extensive than the same cells on 2D. These results showed that cells in the hydrogel displayed a rounded, clustered morphology distinct from the spread morphology on 2D monolayer culture.
3.2. Drug diffusion into HA hydrogel system
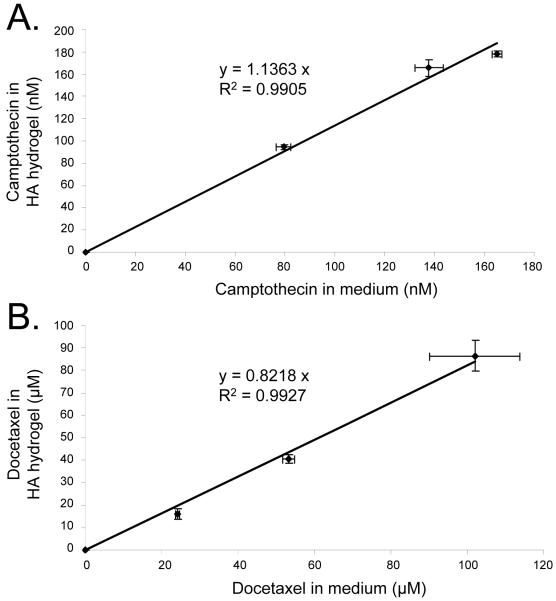
To show that hydrophobic drugs, initially dissolved in DMSO, diffused into the hydrogel matrix when added to the medium surrounding the hydrogel, we measured the drug content within the hydrogel and the medium after 24 hours of the drugs being introduced. The total amount of drug from the gel and the surrounding media were found to equal the total amount of drug initially added. The drug concentration in the 3D matrix was similar to the concentration in the surrounding medium, although the slopes shown in Figure 4 did not achieve a precise slope of 1.0 as they would if the concentrations were completely uniform. CPT diffused into the gel within 24 hours of addition to the medium, and was concentrated in the gel to a factor of 1.14 (Figure 4A). DOX also penetrated the gel within the same time period, but was more concentrated in the medium than in the gel (by a factor of 1.2, Figure 4B). Using these figures, the actual concentration of drug within the hydrogel was calculated. These calculated values were used to analyze data in subsequent sections.
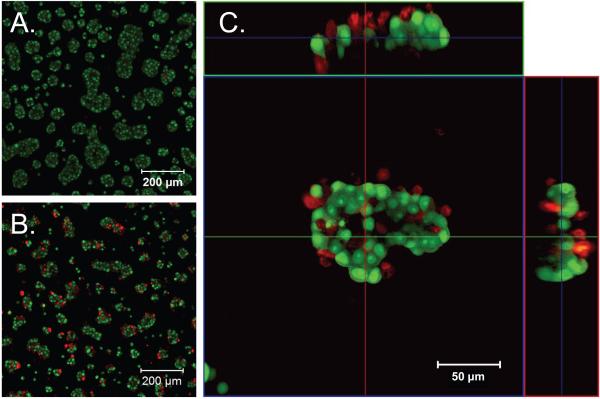
Figure 4. Camptothecin-treated C4-2B cells cultured in HA hydrogel die from the outside in.
Live/dead staining of cells in HA hydrogel treated with DMSO as a vehicle control (A) or treated with 0.574 nM CPT for 24 hours (B). Expanded horizontal and vertical views of a single cluster of cells in HA hydrogel treated with 0.574 nM CPT for 24 hours (C). Live/dead staining as in Figure 2 shows significant cell death (red) beginning on the periphery of the clusters.
HPLC analysis of freshly prepared RAP standard solutions revealed a distinct peak at a retention time (RT) of 19.6 at a maximum UV absorption wavelength of 278 nm (Figure S1), corresponding to the intact RAP. A linear calibration curve was constructed using stock solutions with a RAP concentration of 10-30 μg/mL. The presence of HA, HAase, and PBS did not interfere with RAP quantification. On the other hand, no distinguishable peaks were detected at RT of 19.5-19.6 minutes for both the media and the gel samples after a 24 hour drug treatment (data not shown). HPLC analyses were subsequently performed on a RAP solution (25 μg/ml in PBS/DMSO) that had been incubated at 37°C for varying times. Our results showed that as the incubation time increased, the characteristic RAP peak (RT=19.5-19.6 minutes) decreased in intensity, while two additional peaks RT of 13.1 and 13.9 minutes increased in intensity correspondingly (Figure S1). After 24 hours incubation, the intensity of the original RAP peak has decreased close to the level of background noise.
3.3. Cell killing by CPT in HA hydrogel
To ensure that the drugs can kill the cells within the hydrogels, live/dead staining of CPT or DMSO treated cells was carried out (Figure 5A-B). When cells were treated with CPT in the HA hydrogel, there was an increased number of dead (red) cells compared to DMSO control. The dead cells were evenly spread throughout the viewing area, further showing that the drugs evenly penetrated the HA hydrogels. A magnification of a single cell cluster with horizontal and vertical cross-sections showed that the cell clusters were several cell-layers thick, in addition to being wide and long, and that the CPT treatment first killed cells on the outside surface of the clusters (Figure 5C). Together these results show that the drugs can diffuse readily through the HA-hydrogel, causing cell death, and the drug treatment may kill cells in the clusters from the outside in.
Figure 5. Camptothecin and Docetaxel diffuse from the medium into the HA hydrogel.
Efficiency of diffusion of CPT from the medium into the HA hydrogel after 24 hours (A). Efficiency of diffusion of DOX from the medium into the HA hydrogel after 24 hours (B). Error bars = SEM, n = 3.
3.4. HA hydrogel as a system for testing the efficacy of anti-cancer drugs
3.4.1. Dose response killing of anti-cancer drugs
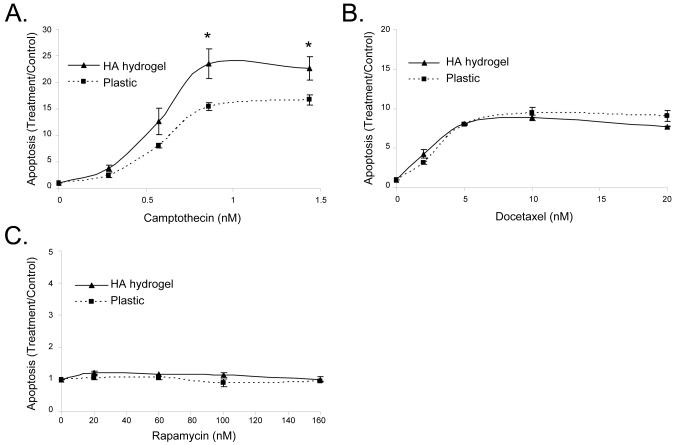
We next tested the applicability of the HA hydrogel as a system for drug selection by comparing the effects of several different anti-cancer drugs on C4-2B cells cultured in 3D HA matrices or on 2D plastic surfaces. For a CPT dose response assay (Figure 6A), both HA hydrogel and plastic cell culture systems showed smooth plateau dose response curves with an IC50 value of 0.574 nM. Interestingly, at higher concentrations of CPT, the HA hydrogel system showed a significantly more robust apoptotic response compared to plastic. For a DOX dose response assay (Figure 6B), both HA hydrogel and plastic cell culture systems showed a smooth plateau dose response curve with the same level of apoptotic response and an IC50 value of 2 nM. As the DOX stock solution aged, cells showed a decreased apoptotic response due to DOX degradation, so results shown were collected within two weeks of preparation of DOX stock solution. RAP treatment (Figure 6C) showed no increase in apoptosis over control for any concentration both in the HA hydrogels or on the plastic surfaces. The x-axes of Figure 6 are the calculated final drug concentrations in the media. The same trend of apoptosis was observed when the drug concentration was converted to the actual concentration in the hydrogel based on the drug diffusion data (data not shown).
Figure 6. C4-2B cells are most sensitive to camptothecin when administered as a single agent.
Dose response curves for killing by DOX and RAP were compared to that for CPT in HA hydrogel or on plastic. Drug induced killing by CPT after a 24 hour treatment; n = 4 (A). Apoptotic response to DOX after 48 hour treatment; n =2 (B). Apoptotic response to RAP after 48 hour treatment; n=2 (C). Error bars = SEM, * p < 0.05 comparing HA hydrogel and plastic cultures.
3.4.2. Time response killing of anti-cancer drugs
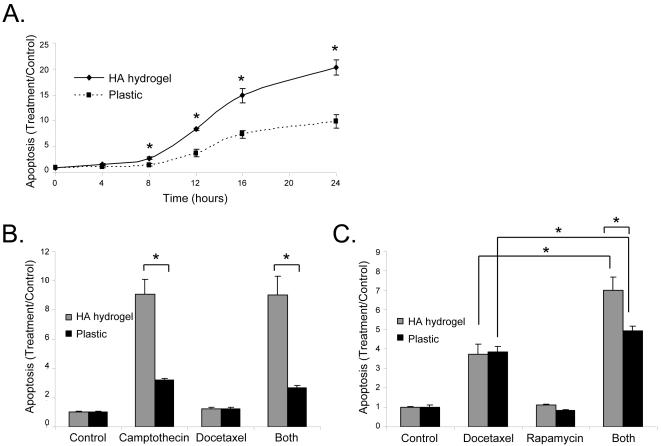
To test the ability of optimized doses of various treatments to kill cells in 2D or 3D cultures over time, we conducted time course response experiments. Over 24 hours treatment with CPT (Figure 7A) cells in the HA hydrogel showed a faster and more robust response to the CPT treatment than cells plated on plastic from 8 through 24 hours of treatment. Both the HA hydrogel and 2D monolayer systems showed smooth plateau time course response curves. No significant cell death was observed when the drug treatment was less than 6 hours, both in 3D hydrogel matrices and on 2D monolayer culture.
Figure 7. Apoptosis in C4-2B cells is higher in HA hydrogel than plastic in response to single or combination drug treatment.
Levels of apoptosis over a 24 hour time course with 1.435 nM CPT (A). Levels of apoptosis after 24 hours with 0.574 nM CPT and 2 nM DOX (B). Levels of apoptosis seen after 48 hours with 2 nM DOX and 20 nM RAP (C). Error Bars = SEM, n = 3, * p < 0.05.
3.4.3. Combined killing effects of anti-cancer drugs
A combination of CPT and DOX (Figure 7B) showed no increase in apoptosis over CPT alone either in the hydrogels or on plastic surfaces. However, a combination of DOX and RAP (Figure 7C) showed a significant (p < 0.05) increase in apoptosis over DOX alone. This trend was observed in both HA hydrogel and 2D monolayer cultures. Additionally, while the level of apoptosis for DOX alone was equal in the HA hydrogel and plastic cultures, the level of apoptosis for the combination therapy was significantly higher in the HA hydrogel than on plastic.
4. Discussion
We describe the use of a novel 3D HA-based hydrogel system for testing the efficacy of anti-cancer drugs on the C4-2B bone metastatic prostate cancer cell line. The hydrogel mimics the bone marrow microenvironment of these cancer cells that is rich in HA [23]. Our HA hydrogel system has the advantage of being able to spontaneously crosslink, allowing the prostate cancer cells to be encapsulated directly, rather than needing to migrate in as required in most other previously described systems [18, 38]. Cell encapsulation provides a useful method for testing drugs on poorly adherent cells, such as C4-2B. This problem has prevented the NCI-60 drug testing system from including some of the most physiologically relevant but poorly adherent prostate cancer cell lines. Finally, the HA components can be synthesized reproducibly and with defined quality controls to prevent the batch-to-batch heterogeneity that other isolated biological matrices show [20] and can be degraded with hyaluronidase application for easy removal of cells from the system [39].
Previous publications have described the use of 3D cell culture systems for testing the efficacy of chemotherapeutic drugs. Research groups growing cancer cells in HA hydrogels [38], collagen/fibrin gels [18], surface engineered porous microparticles [40], and fibroblast derived 3D matrices [20] have shown differential drug sensitivity when compared to the same cells grown on 2D monolayer culture. Many of these current systems have serious limitations in that: a) they require migration of the cells into the hydrogel before drug testing can occur, b) are not highly biomimetic, and/or c) lack a method of consistently producing the same matrix for 3D study. In this study, we address these issues with our own 3D drug testing system.
HA derivatives carrying complementary aldehyde (HAALD) and hydrazide (HAADH) groups were successfully synthesized and characterized. The degree of chemical modification and the molecular weight of the products were monitored closely to ensure reproducibility and to minimize batch-to-batch heterogeneity. Because of the presence of an open-ring structure (Figure 1), HAALD exhibited reduced solution viscosity compared to HAADH of the same concentration and comparable molecular weight, allowing C4-2B cells to be readily dispersed without any noticeable cell damage. Upon addition of HAADH, viscoelastic gels were obtained via the formation of stable hydrazone bond, with water being the only by-product.
We showed that the HA hydrogel is a viable cell culture option to study the sensitivity of bone metastatic prostate cancer cells to chemotherapeutic agents. Cells in the hydrogel remained viable longer than cells plated on plastic. By day 7, a significant reduction in the number of cells was seen on 2D monolayer, due to cell detachment and death. Around day 5, cells in the hydrogel were remodeling the matrix surrounding them by sending out processes and altering their morphology (Figure 2A). This may be attributed to the cells’ production of hyaluronidases and/or the activation of motility pathways due to interactions between HA and its receptors, CD44 and RHAMM [27-29]. Ongoing studies will determine if these molecules play an important role in the cancer cells’ interaction with the HA hydrogel matrix and to characterize the downstream signaling pathways. C4-2B cells cultured in the HA hydrogel showed an interesting change in morphology compared to the same cells plated on plastic. The cells in the hydrogel show a rounded, clustered morphology reminiscent of tumors in vivo, indicating that the cells in the hydrogel may be behaving like true cancer cells in tumors.
Before using the system to test the efficacy of anti-cancer drugs it was imperative to confirm that the drugs could diffuse into the HA hydrogel. We have found that both CPT and DOX readily diffused into the HA hydrogel from the surrounding medium. CPT was concentrated slightly in the gel while DOX was concentrated slightly in the medium surrounding the gel. The actual drug concentration used in the apoptosis assays is beyond the detection limits of HPLC and spectrofluorometer. Accordingly, we used higher concentrations of drugs to confirm diffusion from the medium into the gel and were able to extrapolate from plots (Figure 4 A,B) to lower drug concentrations which were used in the biological experiments. Because molecular diffusion is concentration independent [41], our use of higher concentrations for extrapolation remains valid.
Because RAP has a similar molecular weight and solubility compared to CPT and DOX (Table 1), we believe that that RAP also would diffuse readily into the HA hydrogel from the surrounding medium. RAP has been reported to undergo facile hydrolysis under physiological conditions with a half life of 13 h [42] Our data supports this report showing rapid degradation of RAP within 24 hours (Figure S1). Therefore, the susceptibility of RAP [42] to hydrolytic degradation and the presence of different types of isomers in the degraded products prohibited accurate diffusion measurements. The stability of rapamycin when administered in vivo or when added into cell cultures is increased by the presence of serum proteins [43]. Therefore, these observations do not contradict the results summarized in Figure 7, nor do they contradict our recent detailed studies of the interaction of rapamycin with C4-2B cells grown on 2D [44].
When treated with chemotherapeutic agents, cells cultured in the HA hydrogels died, and did so evenly throughout the gel, as also occurs on 2D monolayer culture. By magnifying a single cluster of CPT treated cells, we showed that in the initial stages of treatment, the drug kills only some of the cells within a single cluster and, additionally, the cells appear to be dying from the outside of the cluster in. This is likely because the drug, which is able to diffuse readily through the hydrogel, cannot easily penetrate the cell clusters and the outer cells of the cluster may protect the inner cells. We have not examined the presence of drug transporters in this study, but differential transport of drug in 2D versus 3D cultures could occur as a result of the clustering and morphological differences. These observations may provide insight into how drug treatment affects tumors in vivo. Similar results were reported by Hicks et al [45] who studied hypoxia targeted anti-tumor drugs in 3D in vitro systems as well as in tumor xenografts.
Cells cultured on plastic and in the HA hydrogel both displayed a smooth plateau dose-response curve for increasing concentrations of CPT and DOX. As expected based on previous reports, no apoptotic response was displayed for RAP treatment on plastic or in the hydrogel [44, 46]. For CPT, cells cultured in the HA hydrogel showed a higher apoptotic response for a given concentration of drug than did those cultured on plastic. Several reasons could account for this observation. Being cultured in a 3D environment or in the presence of HA changes the cells’ expression and activation levels of various proteins. To name a few examples, HA specifically, and flexible 3D networks generally, change levels of focal adhesion kinase (FAK) activation [29, 47]. Additionally, the interaction of HA with CD44 activates RhoA, leading to the regulation of a number of downstream targets [27]. One or more of these changes in expression or activation of proteins likely is responsible for the difference in sensitivity to CPT.
The HA hydrogel cell culture system also can be used successfully to study time courses and responses to combinations of anti-cancer drugs. In the HA hydrogel, C4-2B cells showed a more robust, faster apoptotic response to CPT treatment compared to those cells plated on the 2D monolayer. C4-2B cells cultured in the HA hydrogel were shown to have an increased sensitivity to RAP and DOX in combination. The likely reasons for this are the gene expression and activation changes common when comparing cells cultured in 3D and 2D systems mentioned above. For the combination therapies, CPT and DOX did not function synergistically, while a combination of DOX and RAP showed an enhanced killing effect. This combination of DOX and RAP has been shown previously to have synergistic effects in a mouse model [48] and a phase I clinical trial combining DOX with a soluble analogue of RAP is ongoing.
Collectively, we have shown that a novel, spontaneously crosslinking HA hydrogel system maintains prostate cancer cell growth and viability and provides a good platform for drug testing in dose, time, or combination killing formats. By encapsulating bone metastatic prostate cancer cells which adhere poorly to plastic, this system could be adapted for use in high-throughput screening applications such as in the NCI-60 database. Interestingly, we also have shown that cancer cells cultured in the HA hydrogel system respond to CPT treatment more robustly than the same cells cultured on plastic. These differences could be reflective of how cancer cells respond in vivo, and support previously published reports [13, 14, 22] stressing the difference in expression and activation profiles of cells cultured in a 3D system in the presence of extracellular matrix molecules. Ongoing experiments will examine the differences in expression and activation of genes and proteins in our 3D HA based hydrogel system in comparison to 2D monolayer culture. By specifically investigating pathways responsible for chemotherapy resistance and increased cancer cell motility in poorly adherent cells resembling native tumors, we provide a good next step toward testing of novel anti-cancer drugs.
5. Conclusion
In this report, we describe a HA based hydrogel system to encapsulate cancer cells for drug testing purposes. This HA hydrogel system supports cell growth and viability. In the hydrogels, cancer cells adopt a rounded, clustered morphology reminiscent of tumor tissue in vivo. Drugs readily diffuse into the HA hydrogel to kill cells and the HA hydrogel allows testing of anti-cancer drugs for dose and time dependence. Single agent and combinations of agents were tested. Interestingly, the prostate cancer cells cultured in the HA hydrogel responded differently to therapeutics than did the same cells cultured on plastic. This system provides a useful new alternative to study anti-neoplastics on poorly adherent cell types such as those which have metastasized to the bone marrow and have the potential to expand the list of prostate cancer cells for high-throughput testing.
Supplementary Material
Figure S1. HPLC profiles of rapamycin obtained after 0, 6, 12 and 24 hours incubation at 37°C. Samples were prepared by diluting RAP/DMSO (1 mM) stock solution with PBS to a final concentration of 25 μg/ml.
Acknowledgements
We thank Dr. Jianjun Cheng and Mr. Rong Tong for the insightful discussion on HPLC analysis of docetaxel, Dr. Nicholas Petrelli for the inspiration for this project, and Genzyme for providing HA. This work was partially supported by NIH/NIDCD (Jia, R01DC008965) and NIH/ NCI P01 CA098912 (to LWKC and MCF-C). This publication was made possible by Grant Number 2 P20 RR016472-09 under the INBRE program of the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society . Cancer Facts & Figures 2008. 2008. [Google Scholar]
- 2.Ye XC, Choueiri M, Tu SM, Lin SH. Biology and clinical management of prostate cancer bone metastasis. Front Biosci. 2007;12:3273–3286. doi: 10.2741/2311. [DOI] [PubMed] [Google Scholar]
- 3.Clines GA, Guise TA. Molecular mechanisms and treatment of bone metastasis. Expert Rev Mol Med. 2008;10:e7. doi: 10.1017/S1462399408000616. [DOI] [PubMed] [Google Scholar]
- 4.Boyd MR, Paull KD. Some Practical Considerations and Applications of the National Cancer Institute In Vitro Anticancer Drug Discovery Screen. Drug Develop Res. 1995;34:91–109. [Google Scholar]
- 5.Shoemaker RH. The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer. 2006;6:813–823. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- 6.Thalmann GN, Sikes RA, Wu TT, Degeorges A, Chang SM, Ozen M, et al. LNCaP progression model of human prostate cancer: androgen-independence and osseous metastasis. Prostate. 2000 Jul;44:91–103. doi: 10.1002/1097-0045(20000701)44:2<91::aid-pros1>3.0.co;2-l. 101;144(102) [DOI] [PubMed] [Google Scholar]
- 7.Cragg GM, Newman DJ. A tale of two tumor targets: topoisomerase I and tubulin. The Wall and Wani contribution to cancer chemotherapy. J Nat Prod. 2004;67:232–244. doi: 10.1021/np030420c. [DOI] [PubMed] [Google Scholar]
- 8.Dosso SD, Berthold DR. Docetaxel in the management of prostate cancer: current standard of care and future directions. Expert Opin Pharmacother. 2008;9:1969–1979. doi: 10.1517/14656566.9.11.1969. [DOI] [PubMed] [Google Scholar]
- 9.Abraham RT, Gibbons JJ. The mammalian target of rapamycin signaling pathway: twists and turns in the road to cancer therapy. Clin Cancer Res. 2007;13:3109–3114. doi: 10.1158/1078-0432.CCR-06-2798. [DOI] [PubMed] [Google Scholar]
- 10.Brown DM. Drug Delivery Systems in Cancer Therapy. Humana Press; Totowa, NJ: 2004. [Google Scholar]
- 11.Qiu Y, Gao Y, Hu K, Li F. Enhancement of skin permeation of docetaxel: a novel approach combining microneedle and elastic liposomes. J Control Release. 2008;129:144–150. doi: 10.1016/j.jconrel.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Simamora P, Alvarez JM, Yalkowsky SH. Solubilization of rapamycin. Int J Pharm. 2001;213:25–29. doi: 10.1016/s0378-5173(00)00617-7. [DOI] [PubMed] [Google Scholar]
- 13.Fischbach C, Chen R, Matsumoto T, Schmelzle T, Brugge JS, Polverini PJ, et al. Engineering tumors with 3D scaffolds. Nat Methods. 2007;4:855–860. doi: 10.1038/nmeth1085. [DOI] [PubMed] [Google Scholar]
- 14.Smalley KS, Lioni M, Herlyn M. Life isn’t flat: taking cancer biology to the next dimension. In Vitro Cell Dev Biol Anim. 2006;42:242–247. doi: 10.1290/0604027.1. [DOI] [PubMed] [Google Scholar]
- 15.Kong HJ, Mooney DJ. Microenvironmental regulation of biomacromolecular therapies. Nat Rev Drug Discov. 2007;6:455–463. doi: 10.1038/nrd2309. [DOI] [PubMed] [Google Scholar]
- 16.Prestwich GD. Evaluating drug efficacy and toxicology in three dimensions: using synthetic extracellular matrices in drug discovery. Acc Chem Res. 2008;41:139–148. doi: 10.1021/ar7000827. [DOI] [PubMed] [Google Scholar]
- 17.Sahoo SK, Panda AK, Labhasetwar V. Characterization of porous PLGA/PLA microparticles as a scaffold for three dimensional growth of breast cancer cells. Biomacromolecules. 2005;6:1132–1139. doi: 10.1021/bm0492632. [DOI] [PubMed] [Google Scholar]
- 18.Doillon CJ, Gagnon E, Paradis R, Koutsilieris M. Three-dimensional culture system as a model for studying cancer cell invasion capacity and anticancer drug sensitivity. Anticancer Res. 2004;24:2169–2177. [PubMed] [Google Scholar]
- 19.Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer. 2005;5:675–688. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- 20.Serebriiskii I, Castello-Cros R, Lamb A, Golemis EA, Cukierman E. Fibroblast-derived 3D matrix differentially regulates the growth and drug-responsiveness of human cancer cells. Matrix Biol. 2008;27:573–585. doi: 10.1016/j.matbio.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JB. Three-dimensional tissue culture models in cancer biology. Semin Cancer Biol. 2005;15:365–377. doi: 10.1016/j.semcancer.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Clark BR, Keating A. Biology of bone marrow stroma. Ann N Y Acad Sci. 1995;770:70–78. doi: 10.1111/j.1749-6632.1995.tb31044.x. [DOI] [PubMed] [Google Scholar]
- 24.Meyer K. Chemical structure of hyaluronic acid. Fed Proc. 1958;17:1075–1077. [PubMed] [Google Scholar]
- 25.Schade UM, Nehmann N, Horny HP, Prehm P, Delpech B, Kruger WH, et al. Hyaluronate and its receptors in bone marrow. Acta Histochem. 2006;108:141–147. doi: 10.1016/j.acthis.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 26.Bharadwaj AG, Kovar JL, Loughman E, Elowsky C, Oakley GG, Simpson MA. Spontaneous metastasis of prostate cancer is promoted by excess hyaluronan synthesis and processing. Am J Pathol. 2009;174:1027–1036. doi: 10.2353/ajpath.2009.080501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bourguignon LY, Singleton PA, Zhu H, Diedrich F. Hyaluronan-mediated CD44 interaction with RhoGEF and Rho kinase promotes Grb2-associated binder-1 phosphorylation and phosphatidylinositol 3-kinase signaling leading to cytokine (macrophage-colony stimulating factor) production and breast tumor progression. J Biol Chem. 2003;278:29420–29434. doi: 10.1074/jbc.M301885200. [DOI] [PubMed] [Google Scholar]
- 28.Bourguignon LY, Singleton PA, Zhu H, Zhou B. Hyaluronan promotes signaling interaction between CD44 and the transforming growth factor beta receptor I in metastatic breast tumor cells. J Biol Chem. 2002;277:39703–39712. doi: 10.1074/jbc.M204320200. [DOI] [PubMed] [Google Scholar]
- 29.Fujita Y, Kitagawa M, Nakamura S, Azuma K, Ishii G, Higashi M, et al. CD44 signaling through focal adhesion kinase and its anti-apoptotic effect. FEBS Letters. 2002;528:101–108. doi: 10.1016/s0014-5793(02)03262-3. [DOI] [PubMed] [Google Scholar]
- 30.Toole BP, Slomiany MG. Hyaluronan: a constitutive regulator of chemoresistance and malignancy in cancer cells. Semin Cancer Biol. 2008;18:244–250. doi: 10.1016/j.semcancer.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia XQ, Colombo G, Padera R, Langer R, Kohane DS. Prolongation of sciatic nerve blockade by in situ cross-linked hyaluronic acid. Biomaterials. 2004;25:4797–4804. doi: 10.1016/j.biomaterials.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Jia XQ, Yeo Y, Clifton RJ, Jiao T, Kohane DS, Kobler JB, et al. Hyaluronic acid-based microgels and microgel networks for vocal fold regeneration. Biomacromolecules. 2006;7:3336–3344. doi: 10.1021/bm0604956. [DOI] [PubMed] [Google Scholar]
- 33.Jha AK, Hule RA, Jiao T, Teller SS, Clifton RJ, Duncan RL, et al. Structural Analysis and Mechanical Characterization of Hyaluronic Acid-Based Doubly Cross-Linked Networks. Macromolecules. 2009;42:537–546. doi: 10.1021/ma8019442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Chen L, Li L, Hu X, Cai Y. Steady-state fluorescence study on release of camptothecin from agar hydrogel. Int J Pharm. 2004;287:13–19. doi: 10.1016/j.ijpharm.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe M, Kawano K, Toma K, Hattori Y, Maitani Y. In vivo antitumor activity of camptothecin incorporated in liposomes formulated with an artificial lipid and human serum albumin. J Control Release. 2008;127:231–238. doi: 10.1016/j.jconrel.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Farokhzad OC, Cheng JJ, Teply BA, Sherifi I, Jon S, Kantoff PW, et al. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. P Nat Acad Sci USA. 2006;103:6315–6320. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phenomenex C. Curosil/Taxol: Columns for Taxane Analysis. USA: [Google Scholar]
- 38.David L, Dulong V, Le Cerf D, Cazin L, Lamacz M, Vannier JP. Hyaluronan hydrogel: an appropriate three-dimensional model for evaluation of anticancer drug sensitivity. Acta Biomater. 2008;4:256–263. doi: 10.1016/j.actbio.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 39.Stern R, Kogan G, Jedrzejas MJ, Soltes L. The many ways to cleave hyaluronan. Biotechnol Adv. 2007;25:537–557. doi: 10.1016/j.biotechadv.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Horning JL, Sahoo SK, Vijayaraghavalu S, Dimitrijevic S, Vasir JK, Jain TK, et al. 3-D tumor model for in vitro evaluation of anticancer drugs. Mol Pharm. 2008;5:849–862. doi: 10.1021/mp800047v. [DOI] [PubMed] [Google Scholar]
- 41.Gayet JC, Fortier G. High water content BSA-PEG hydrogel for controlled release device: Evaluation of the drug release properties. J Control Release. 1996;38:177–184. [Google Scholar]
- 42.Nelson FC, Stachel SJ, Eng CP, Sehgal SN. Manipulation of the C(22)-C(27) region of rapamycin: stability issues and biological implications. Bioorg Med Chem Lett. 1999;9:295–300. doi: 10.1016/s0960-894x(98)00735-5. [DOI] [PubMed] [Google Scholar]
- 43.Laplanche R, Meno-Tetang GM, Kawai R. Physiologically based pharmacokinetic (PBPK) modeling of everolimus (RAD001) in rats involving non-linear tissue uptake. J Pharmacokinet Pharm. 2007;34:373–400. doi: 10.1007/s10928-007-9051-7. [DOI] [PubMed] [Google Scholar]
- 44.Opdenaker LM, Farach-Carson MC. Rapamycin selectively reduces the association of transcripts containing complex 5′ UTRs with ribosomes in C4-2B prostate cancer cells. J Cell Biochem. 2009;107:473–481. doi: 10.1002/jcb.22145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hicks KO, Pruijn FB, Secomb TW, Hay MP, Hsu R, Brown JM, et al. Use of three-dimensional tissue cultures to model extravascular transport and predict in vivo activity of hypoxia-targeted anticancer drugs. J Natl Cancer I. 2006;98:1118–1128. doi: 10.1093/jnci/djj306. [DOI] [PubMed] [Google Scholar]
- 46.Lin J, Adam RM, Santiestevan E, Freeman MR. The phosphatidylinositol 3′-kinase pathway is a dominant growth factor-activated cell survival pathway in LNCaP human prostate carcinoma cells. Cancer Res. 1999;59:2891–2897. [PubMed] [Google Scholar]
- 47.Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004;1692:103–119. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 48.Morgan TM, Pitts TE, Gross TS, Poliachik SL, Vessella RL, Corey E. RAD001 (Everolimus) inhibits growth of prostate cancer in the bone and the inhibitory effects are increased by combination with docetaxel and zoledronic acid. Prostate. 2008;68:861–871. doi: 10.1002/pros.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. HPLC profiles of rapamycin obtained after 0, 6, 12 and 24 hours incubation at 37°C. Samples were prepared by diluting RAP/DMSO (1 mM) stock solution with PBS to a final concentration of 25 μg/ml.