Summary
Umbilical cord blood has rapidly become a valuable alternative stem cell source for allogeneic hematopoietic stem cell transplantation. Extensive research over the last 20 years has established the safety and efficacy of umbilical cord blood transplantation in both children and adults with a variety of malignant and non-malignant diseases. This research has clearly shown that this stem cell source has several unique characteristics resulting in distinct advantages and disadvantages when compared to transplantation with unrelated bone marrow or peripheral blood stem cells. This article reviews the most recent literature comparing the outcomes after umbilical cord blood transplantation with other alternative stem cell sources.
Keywords: stem cell transplantation, umbilical cord blood, alternative stem cell sources
INTRODUCTION
Over the last 40 years, allogeneic hematopoietic stem cell transplantation (HSCT) has become an increasingly used treatment modality for both malignant and non-malignant disorders. But because nearly two-thirds of patients requiring HSCT will not have a suitable related donor, the applicability of HSCT to larger numbers of patients has been augmented with the increasing availability of unrelated donors. Currently, alternative hematopoietic stem cell (HSC) sources include unrelated donor (URD) bone marrow (BM) or peripheral blood stem cells (PBSC) and unrelated donor umbilical cord blood (UCB). While URD BM and PBSC transplants have a proven track record of success, the search process takes 3-4 months, which is often longer than patients with high risk disease can wait. Despite nearly 13 million registered volunteer donors worldwide, nearly half of patients still do not have a closely human leucocyte antigen (HLA)-matched donor. The applicability of HSCT markedly expanded with the introduction of UCB transplant (UCBT), particularly for racial and ethnic minorities. The advent of public UCB banks in the United States and Europe resulted in the first unrelated transplants in 1993 and 1994. (Kurtzberg, et al 1994, Wagner, et al 1996) Since those first reports, it has become clear that UCB is a safe and effective source of HSC for transplant. With approximately 350,000 units banked worldwide (http://www.bmdw.org), the addition of UCB to the available stem cell sources makes it possible for nearly everyone who requires an HSCT to have a suitable donor available.
Umbilical Cord Blood Transplantation – The Initial Experience
Early clinical studies reporting positive outcomes in patients undergoing UCBT for treatment of high risk diseases initiated the widespread interest and subsequent research endeavors in this field. (Gluckman, et al 1997, Kurtzberg, et al 1994, Kurtzberg, et al 1996, Rubinstein, et al 1998, Wagner, et al 1996) These and subsequent studies indicated that HSCT with UCB is different in many respects when compared to more traditional HSC sources including BM and PBSC. First of all, the time to hematopoietic recovery after UCBT is delayed, with the median time to neutrophil recovery ranging between 20 and 30 days in most reports. In addition, cell dose, which is limited to what can be collected from a single placenta, has been shown to significantly influence the rate and incidence of hematopoietic recovery after UCBT. (Gluckman, et al 1997, Laughlin, et al 2004, Locatelli, et al 2003, Rocha, et al 2004, Rubinstein, et al 1998, Wagner, et al 2002) Because each individual UCB unit has a fixed cell dose, its use in larger patients historically has been restricted given that total nucleated cell dose and CD34+ cells dose are both critical determinants of engraftment and survival after UCBT. (Gluckman, et al 2004, Rubinstein, et al 1998, Wagner, et al 2002)
Secondly, unlike transplantation with BM or PBSC, UCB is less restricted with regards to HLA matching, such that a mismatch at 1 or 2 loci is well tolerated without a significant increase in graft-versus-host disease (GVHD) or impaired survival. Recent studies have shown that the number of nucleated cells infused, and not the degree of HLA disparity, is the most significant predictor of success with UCBT. (Gluckman, et al 2005, Wagner, et al 2002) This permissive HLA mismatching improves the chances of finding a suitable unit for every patient, even those with unusual tissue types.
In addition, these early studies began to delineate other risks and benefits of UCBT. First, UCB is harvested after the delivery of the infant and the placenta and therefore poses no risk to either the mother or child. Secondly, UCB units are HLA-typed and tested for infectious diseases prior to cryopreservation, making them rapidly available as an “off the shelf” product. This becomes especially important when considering transplantation for rapidly progressive diseases in which waiting for an unrelated donor to be available may be too risky. Lastly, UCB is viral pathogen-free with rare exception.
Deciding which type of donor to use for URD HSCT is complicated and many issues must be considered. Ideally an individualized decision is made for each patient. Based on the promising results seen with UCBT in those patients for whom a suitably HLA-matched BM or PBSC donor was not available, the use of UCB has surpassed the use of BM and PBSC in children and is rapidly growing as a major HSC source for adults. However, because each HSC source has unique advantages and disadvantages, the logical next step is to compare the outcomes between the sources. The purpose of this review is to provide the most recent information regarding these comparisons so that we can determine the best options for our patients with hematological malignancies, marrow failure syndromes, immune deficiency states and storage diseases. Because the conclusions differ by underlying disease and age of the patient, the discussion reflects these observations.
HEMATOLOGICAL MALIGNANCIES
Hematological malignancy is the most common indication for allogeneic HSCT in both children and adults. While the choice of HSC source depends on both patient and disease characteristics, with malignant diseases, the speed of availability is often critically important. Because of the rapid availability of units, UCB is a particularly attractive option. However, rapid availability is only an advantage if the outcomes are at least as good for UCB recipients when compared to other HSC sources. Recent studies addressing the outcomes of alternative donor HSCT in children and adults with hematological malignancies are summarized in Table 1.
Table 1.
Comparison of Stem Cell Sources in Hematological Malignancies
| PAEDIATRICS | ||||||||
|---|---|---|---|---|---|---|---|---|
| Reference | HSC source/n | Median Age (years) |
Disease/ n |
Incidence of TRM |
Incidence of Grade II-IV GVHD |
Probability of LFS |
Probability of OS |
Outcomes and Conclusions |
| Eapen et al (2006) | MUCB/12 MMUCB/69 MUBM/61 MMUBM/24 MSD/101 |
0-1.5 | ALL/146 AML/12 1 |
31% 15% 6% |
Acute: RR significantly higher in UBM and UCB (3.03 and 2.45, respectively) compared to MSD (1.0) Chronic: RR significantly higher in URD (3.5) compared to MSD (1.0) |
3-year LFS: CR1 URD 54% MSD 49% >CR1 URD 30% MSD 20% |
3-year OS: CR1 URD 62% MSD 54% >CR1 URD 33% MSD 35% |
• There is no difference in OS and LFS following URD transplant as compared to MSD transplant following adjustment for disease status. • URD HSCT (BM or UCB) should be considered for infants with acute leukemia in CR1 using the same criteria as those with a MSD. |
| Eapen et al (2007) | MUCB/35 MMUCB(1L)/44 MMUCB(1H)/157 MMUCB(2)/267 MUBM/116 MMUBM/166 |
0-16 | ALL/19 AML/16 ALL/36 ALL/8 ALL/88 AML/69 ALL/166 AML/10 1 ALL/80 AML/36 ALL/106 AML/60 |
6% 43% 29% 46% 21% 31% |
Acute: 24% Chronic: 30% Acute: 36% Chronic: 18% Acute: 42% Chronic: 18% Acute: 41% Chronic: 15% Acute: 46% Chronic: 32% Acute: 60% Chronic: 40% |
5-year LFS 60% 5-year LFS 36% 5-year LFS 45% 5-year LFS 33% 5-year LFS 37% 5-year LFS 38% |
NA | • 5-year LFS similar after MUBM, MMUBM, and MMUCB and possibly higher after MUCB. • TRM higher after MMUCB(2) and possibly MMUCB(1L). • Relapse rates lower after MMUCB(2). • Better HLA matching and higher cell doses decrease risk of TRM. • Results support the use of MUCB and MMUCB in children with acute leukemia requiring HSCT. |
| ADULT | ||||||||
|---|---|---|---|---|---|---|---|---|
| Reference | HSC source/n | Median Age (years) |
Disease/n | Incidence of TRM |
Incidence of Grade II-IV GVHD |
Probability of LFS |
Probability of OS |
Outcomes and Conclusions |
| Laughlin et al (2004) | UCB/150 MUBM/367 MMUBM/83 |
16-60 | ALL/45 AML/58 ALL/82 AML/115 ALL/17 AML/27 |
63% 46% 65% |
Acute: 41% Chronic: 51% Acute: 48% Chronic: 35% Acute: 52% Chronic: 40% |
3-year LFS 23% 3-year LFS 33% 3-year LFS 19% |
26% 35% 20% |
• TRM, treatment failure and overall mortality lowest in MUBM group. • No difference between UCB and MMUBM in TRM and treatment failure. • In the absence of MUBM, UCB is an acceptable stem cell source for adults. |
| Rocha et al (2004) | UCB/98 MUBM/584 |
24.5 32 |
ALL/53 AML/45 ALL/267 AML/317 |
69% 63% |
Acute: 26% Chronic: 30% Acute: 39% Chronic: 46% |
2-year LFS 33% 2-year LFS 38% |
36% 42% |
• Grade II-IV aGVHD is lower in UCB compared to MUBM. • No difference in cGVHD, TRM, and LFS. • UCB is an acceptable alternative stem cell source for adults |
| Takahashi et al (2004) | UCB/68 UBM/45 |
36 26 |
ALL/15 AML/39 ALL/8 AML/15 |
1-year 9% 1-year 29% |
Acute: 44% Chronic: 74% Acute: 67% Chronic: 78% |
2-year LFS 74% 2-year LFS 44% |
76% 47% |
• UCB had decreased grades II- IV GVHD and TRM and increased LFS compared to UBM. • UCB is safe and effective for adults with haematological malignancies. |
| Takahashi et al (2007) | UCB/100 RBM or RPBSC/71 |
38 40 |
ALL/20 AML/57 ALL/17 AML/31 |
1-year 8% 1-year 4% |
Acute: 52% Chronic: 71% Acute: 52% Chronic: 74% |
3-year LFS 70% 3-year LFS 60% |
75% 63% |
• No differences in TRM, relapse and LFS between the groups. • UCB is as safe and effective as RBM and RPBSC. |
| Kumar et al (2008) | UCB/19 MRBM/90 MUBM/15 MMUBM/14 |
30 30 32 31 |
ALL/19 ALL/90 ALL/15 ALL/14 |
3-year 34% 3-year 44% 3-year 53% 3-year 86% |
Acute: 32%* Chronic: 16% Acute: 20%* Chronic: 22% Acute: 10%* Chronic:47% Acute: 7%* Chronic: 21% |
3-year LFS 61% 3-year LFS 27% 3-year LFS 13% 3-year LFS 14% |
66% 27% 13% 14% |
• OS and LFS were best and TRM was lowest in the UCB group. • UCB is an acceptable stem cell source for adults with ALL. |
| Eapen et al (2008) | UCB/148 MUBM/243 MMUBM/111 MPBSC/518 MMPBSC/210 |
29 39 35 |
NA | 41% 26% 37% 27% 42% |
NA | 33%** 46%** 34%** 43%** 33%** |
35% 48% 38% 45% 36% |
• TRM was decreased and LFS/OS were increased in MUBM and MPBSC as compared to other sources. • Matched BM or PBSC should be the first choice for adults with leukaemia if time permits, but UCB with an adequate cell dose is a suitable alternative. |
HSC = haematopoietic stem cell, TRM = treatment-related mortality, GVHD = graft-versus-host disease, aGVHD = acute GVHD, cGVHD = chronic GVHD, LFS = leukaemia-free survival, OS = overall survival, UCB = unrelated umbilical cord blood, MUBM = matched unrelated bone marrow, MMUBM = mismatched unrelated bone marrow, UBM = unrelated bone marrow, RBM = related bone marrow, RPBSC = related peripheral blood stem cells, MRBM = matched related bone marrow, MPBSC = matched peripheral blood stem cells, MMPBSC = mismatched peripheral blood stem cells, ALL = acute lymphoblastic leukaemia, AML = acute myeloid leukaemia, NA = information not available
Incidence of Grades III-IV acute graft versus host disease.
LFS with unspecified years of follow-up.
HSC(T) = haematopoietic stem cell (transplantation), TRM = treatment-related mortality, GVHD = graft versus host disease, LFS = leukemia-free survival, OS = overall survival, MUCB = matched unrelated cord blood, MMUCB = mismatched unrelated umbilical cord blood, MUBM = matched unrelated bone marrow , MMUBM = mismatched unrelated bone marrow , MMUCB(1L) = one antigen mismatched umbilical cord blood with low cell dose, MMUCB(1H) = one antigen mismatched umbilical cord blood with high cell dose, MMUCB(2) = two antigen mismatched umbilical cord blood with any cell dose, ALL = acute lymphoblastic leukaemia, AML = acute myeloid leukaemia, CR1 = first complete remission, RR = relative risk, UCB = unrelated umbilical cord blood, (U)BM = (unrelated) bone marrow , MSD = matched sibling donor bone marrow , URD = unrelated donor, NA = information not available.
Pediatrics
Many of the initial studies of UCBT in children were performed on children with hematological malignancies. Though important in establishing the utility of UCBT, more recent analyses have provided more insight on outcomes after alternative donor transplant in children with hematological malignancies.
Infant leukemia is a particularly challenging form of leukemia to treat and the decision of whether to treat with intensive chemotherapy or to proceed with URD transplant when a suitable related donor is not available is a difficult one. On behalf of the Center for International Blood and Marrow Research (CIBMTR), Eapen et al (2006) retrospectively compared the outcomes after URD BM (n=85), UCB (n=81) and HLA-matched sibling BM (n=101) transplantation. Despite higher treatment-related mortality (TRM) in the URD groups (UCB 31%, URD BM 15%, matched sibling donor 6%), there was no difference in overall survival (OS) and leukemia-free survival (LFS) between the groups (3-year OS 62% vs. 54% and LFS 54% vs. 49% in the URD and matched sibling groups respectively). These results support the decision to proceed with URD transplant for infant leukemia using the same criteria used if an HLA-matched sibling was available. (Eapen, et al 2006) Another study examining the use of UCBT for infant leukemia had slightly different conclusions however. Though not comparative in nature, the Cord Blood Transplantation Study (COBLT) was a large, multicentre study sponsored by the National Heart, Lung and Blood Institute. Using a non-radiation containing preparative regimen of busulfan, melphalan and antithymocyte globulin (in contrast to the study by Eapen et al (2006) in which many patients received a radiation-containing regimen), Wall et al (2005) reported that the 2-year LFS was only 28%. Notably, relapse rates and LFS were similar between patients in first complete remission (CR1) and those in second complete remission (CR2) and beyond, indicating a reasonable salvage rate with UCBT for patients in CR2. These authors concluded that UCBT should be limited to those with later stage disease. (Wall, et al 2005)
In contrast, several recent non-comparative studies have demonstrated a benefit of UCBT in older children. In a small single-institution study looking at 26 UCBT in children with high risk acute lymphoblastic leukemia (ALL), Sawczyn et al (2005) showed a 3-year LFS of 62% which compared very favorably to historical outcomes with URD bone marrow transplantation (BMT) that showed long-term LFS of 36-49%. (Al-Kasim, et al 2002, Bunin, et al 2002, Davies, et al 1997) In a similar cohort, Kurtzberg et al (2008) reported results for the COBLT study, which prospectively examined outcomes in 193 pediatric patients with hematological malignancies and found 6-month and 1-year OS rates to be 67.4% and 57.3%, respectively. These results were similar to that reported with related and unrelated donor bone marrow. (Rocha, et al 2001)
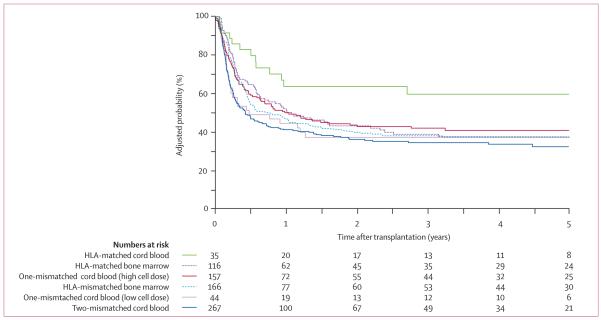
While large, randomized clinical trials would be ideal, such comparative trials between the HSC sources are not likely because of unequal donor availability and investigator preferences. Therefore, retrospective comparative studies are the next best option. Again on behalf of the CIBMTR, Eapen et al (2007) recently reported the outcomes of 785 children with acute leukemia comparing outcomes in recipients of UCB (n=503) and URD BM (n=282). While all transplant-specific outcomes were evaluated, the most notable finding was that UCB compared favorably to the ‘gold standard’ of 8/8 allele-matched unrelated BM. In fact, the 5-year LFS was similar after 8/8 matched unrelated BM (MUBM), mismatched unrelated bone marrow (MMUBM) and mismatched UCB (MMUCB) with higher survival in recipients of matched UCB (MUCB). (Figure 1) The incidence of acute and chronic GVHD was similar between the groups. While TRM was higher after two-antigen MMUCB, a lower risk of relapse resulted in comparable survival outcomes for this cohort. This study was unique in that UCB was compared to the present day standard of allele-level HLA-matched BM donors. These data support the use of HLA-matched or -mismatched UCB in children with high risk acute leukemia who need transplantation. (Eapen, et al 2007)
Figure 1.
Probability of leukemia-free survival after bone marrow and cord blood transplantation adjusted for disease status at transplantation.
Reprinted from The Lancet, 369, Eapen, M., Rubinstein, P., Zhang, M.J., Stevens, C., Kurtzberg, J., Scaradavou, A., Loberiza, F.R., Champlin, R.E., Klein, J.P., Horowitz, M.M. & Wagner, J.E. Copyright 2007 Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet1947-1954. Copyright 2007, with permission from Elsevier.
Adults
In contrast to the outcomes in children, HSCT in adults is typically associated with higher risks of GVHD, infections, delayed immune reconstitution and increased TRM, partly related to a higher likelihood of comorbidities at the time of transplant. In contrast to children, use of UCB in adults has been more restricted due to cell dose limitations. The safety and feasibility of UCBT in adults with hematological malignancies was first reported in 2004. (Laughlin, et al 2004, Rocha, et al 2004) Laughlin et al (2004) compared outcomes in patients with leukemia after UCB (n=150), 6/6 matched unrelated bone marrow (MUBM) (n=367) and mismatched unrelated bone marrow (MMUBM) (n=83) transplants from 1996 to 2001. In this analysis, patients transplanted with MUBM had the lowest TRM, treatment failure and overall mortality with no differences in patients receiving UCB or MMUBM (Laughlin, et al 2004) These results suggested that MUBM may be the preferred URD stem cell source for adults, but at the same time provided evidence that UCB is a reasonable alternative for those without a matched URD or who cannot wait for such a search. Similarly, Rocha et al (2004) reported the outcomes of 682 adults with acute leukemia (98 UCB, 584 MUBM [6/6]) who were transplanted between 1998 and 2002. Notably, their analysis demonstrated similar outcomes in terms of TRM, chronic GVHD, relapse rate and LFS between the two groups (see Figure 1b). Because the UCB group had a significantly lower incidence of acute GVHD and similar survival the conclusion was that UCBT is an acceptable stem cell source for adults with leukemia. (Rocha, et al 2004) More recently, in an abstract published on behalf of the CIBMTR, Eapen at al (2008) examined outcomes in 1240 adults (148 UCB, 243 MUBM, 111 MMUBM, 518 matched PBSC [MPBSC] and 210 mismatched PBSC [MMPBSC]). In contrast to the prior reports (Laughlin et al 2004; Rocha et al (2004), all unrelated donor BM and PBSC grafts were matched at allele level for HLA-A, -B, -C, and –DRB1. In this analysis, TRM was lower and LFS higher when MUBM and MPBSC were used as compared to the other sources, suggesting that these graft sources are preferred when available and time permits. However, partially HLA-matched UCB with an adequate cell dose (≥ 2.5 × 107 nucleated cells/kg) is a suitable alternative when an HLA-matched URD is not available or when the transplant is urgent. (Eapen et al 2008)
Two separate large retrospective single-institution studies compared outcomes in patients with hematological malignancies after transplant with UCB, UBM and related donor BM or PBSC (RBM, RPBSC). (Takahashi, et al 2004, Takahashi, et al 2007) These studies had the benefit of similar evaluation criteria, supportive care measures and preparative therapies between the groups. The first analysis of 113 adult patients (68 UCB, 45 UBM) showed significantly less grade III-IV acute GVHD (6% vs. 27%, p= 0.01) and TRM (9% vs. 29%, p=0.02), in addition to an improved 2-year LFS in the UCB group when compared to the UBM group (74% vs. 44%, p<0.01). (Takahashi, et al 2004) In a follow-up analysis comparing outcomes with UCB and related donors (100 UCB, 71 RBM/RPBSC), Takahashi et al (2007) found that there were no differences in TRM (9% vs. 13%, p=0.13), relapse (17% vs. 26%, p=0.34) and LFS (70% vs. 60%, p=0.26) between the groups. While the high overall LFS rates are encouraging, potential racial and ethnic differences may limit the extrapolation of results to other populations. (Takahashi, et al 2007) In an analysis of a more genetically heterogeneous group of patients, Kumar et al (2008) also showed superior outcomes in UCB recipients relative to those transplanted with other sources of HSC. Patients receiving UCB had the lowest TRM and highest 3-year LFS (61% vs. 27%, 13% and 14% in the matched related donor, matched unrelated donor (MURD) and mismatched unrelated donor (MMURD) groups, respectively). Taken together, these results at least advocate for continued investigations into the use of UCB as an alternative stem cell source for the treatment of adults with hematological malignancy. (Kumar, et al 2008)
In summary, these retrospective studies suggest for children, the first line HSC source would be a 6/6 MUCB provided that the cell dose is adequate. The probability of finding a 6/6 MUCB, however, is low (~10%). However, results in recipients of 8/8 MUBM and 5/6 MMUCB and 4/6 MMUCB are similar, suggesting that any of these options are reasonable. In this case, the decision must be individualized and based on the urgency of the transplant and potential need for future donor lymphocyte infusion (DLI). In adults, cell dose limitations with UCB units give the advantage to HLA matched BM and PBSC. For all patients, if an 8/8 MUBM is not available, no one source stands out. UCB has the advantage of rapid availability while BM and PBSC have the advantage of availability of DLI.
NON-MALIGNANT DISORDERS
Over the years, the use of HSCT as a therapeutic modality has been extended to a variety of non-malignant disorders. Even though these diseases are often not as rapidly life-threatening as malignant disorders, they do cause significant morbidity and mortality. As many of these disorders are inherited, HLA-matched sibling donors (MSD) must also be free of the underlying genetic disease, making the chance of finding a HLA-matched and healthy related donor even less likely. Alternative donor HSCT is therefore often considered.
Hemoglobinopathies
HSCT has curative potential for hemoglobinopathies including sickle cell disease (SCD) and thalassemia. HLA MSD transplantation results in a high survival rate and few transplant-related complications and is an accepted treatment for high-risk disease (Panepinto, et al 2007) (Table 2A). Because of the risks of TRM and GVHD with BM grafts are not insignificant, UCB is an attractive option. Locatelli et al (2003) examined the outcomes in 44 patients who received related UCBT (RUCBT) for either SCD (n=11) or thalassemia (n=33) after a busulfan-based myeloablative (MA) preparative regimen. The 2-year OS was 100% and event-free survival (EFS) was 79% for those with thalassemia and 90% for those with SCD. The rates of GVHD were also low, with only 4 patients developing acute and 2 patients developing chronic symptoms. These results clearly supported the use of RUCB over BM due to the decreased risk of GVHD. (Locatelli, et al 2003) In recent years, researchers have begun to investigate the role of alternative donor transplant in the treatment of these patients; however most reports are small single centre studies. More recently, Adamkiewicz et al (2007) reported a four-centre experience of unrelated donor UCBT in 7 children with SCD who were treated with a busulfan-based MA (n=4) or fludarabine-based reduced-intensity conditioning (RIC) (n=3) preparative regimen. All patients who received RIC and one received MA conditioning failed to engraft and 57% developed acute GVHD. Though the 2- year OS was 86%, EFS was only 43% due to graft failure suggesting improvements are needed. (Adamkiewicz, et al 2007) In a large single-institution analysis of UCBT (21 single, 9 double) for thalassemia, however, Jaing et al reported that more favorable results (3 year OS 82%, EFS 78%) may be attainable when the total nucleated cell (TNC) dose is optimized. (Jaing et al 2008) Until there are retrospective comparative analyses examining alternative donor HSCT for hemoglobinopathies, MUBM remains the gold standard with UCB reserved for those without a MUBM donor. Regardless of the HSC source, the major obstacle in alternative donor HSCT for hemoglobinopathies continues to be graft rejection and future prospective studies are urgently needed.
Table 2.
UCB in Non-Malignant Haematological Disorders
| A: HAEMOGLOBINOPATHIES | ||||||||
|---|---|---|---|---|---|---|---|---|
| Reference | HSC source/n | Median Age (years) |
Disease/ n |
Incidence of TRM |
Incidence of Grade II-IV GVHD |
Graft Failure |
Probability of EFS/OS |
Outcomes and Conclusions |
| Locatelli et al (2003) | RUCB/44 (61% MM, 39% M) |
5 | SCD/11 Thal/33 |
0/44 | Acute:4/44 Chroni c: 2/36* |
SCD/1 Thal/7 |
OS 100% EFS 79-90% (2 years) |
• RUCB transplant offers a good probability of success and is associated with a low risk of GVHD. |
| Adamkiewicz et al (2007) | UCB/7 (100% MM) |
2.4 | SCD/7 | 1/7 | Acute: 57% Chronic: 14% |
3/7 | OS 86% EFS 43% (2 years) |
• Engraftment, GVHD and infection continue to provide challenges. |
| Jaing et al (2008) | UCB/21 dUCB/9 |
5 | Thal/30 | 10% | Acute: 40%** Chronic: 4% |
NA | OS: 82% EFS: 78% (3 years) |
• Favourable clinical results are attainable when TNC is optimized. |
| B: FANCONIAN AEMIA | ||||||||
|---|---|---|---|---|---|---|---|---|
| Reference | HSC source/n | Median Age (years) |
Disease/n | Incidence of TRM |
Incidence of Grade II-IV GVHD |
Neutrophil Recovery |
Probability of OS |
Outcomes and Conclusions |
| Gluckman et al (2007) | UCB/92 (13% M, 87% MM) |
8.6 | FA+AA/81 FA+MDS/8 FA+AL/4 |
NA | Acute: 32% Chronic: 16% |
60% at 60 days. |
3-year OS 40% +Flu 50% −Flu 25% |
• Factors associated with favourable outcome include the use of Flu, ≥ 4.9 × 107/kg infused, recipient CMV negative. |
| Wagner et al (2007) | UBM/98 (78% M, 22% MM) |
12 | FA+AA/75 FA+MDS/14 FA+AL/7 FA/2 |
NA | Acute: 29% ·Non-Flu +TCD 21% −TCD 70% ·Flu 16% Chronic: 31% |
78% at 28 days. |
• Recommend using Flu in the context of TCD grafts and earlier referral (prior to excessive transfusions) |
|
| C: INBORN ERRORS OF METABOLISM | ||||||||
|---|---|---|---|---|---|---|---|---|
| Reference | HSC source/n | Median Age (years) |
Disease/n | Incidence of TRM |
Incidence of Grade II-IV GVHD |
Neutrophil Recovery/ Graft Failure |
Incidence of OS/EFS |
Outcomes and Conclusions |
| Martin et al (2006) | UCB/69 (97% MM, 3% M) |
1.8 | LSD+PSD/69 | NA | Acute: 44% Chronic: 18% |
Neutrophil recovery 84% at 100 days. 4/69 |
OS 72% (1 year) LSD stratum OS 81% EAP stratum OS 64% |
• Because UCB donors are readily available for rapid transplantation, cord blood transplantation should be considered frontline therapy for young patients with LSD and PSD. |
| Boelens et al (2007) | UCB/20 RUCB/3 BM/103 PBSC/20 (66% M, 34% MM) |
1.5 | MPS1/146 | 19/146 (after first transplant) |
Acute: 16% Chronic: NA |
33 patients received a 2nd transplant. 3 patients received a 3rd transplant. |
OS 81% A&E 76% HSCT#1 OS 85% A&E 56% HSCT#2 A&E 79% |
• After HSCT#1, 71% overall achieved full donor chimerism (>95%). In the BM/PBSC group 44/67 (66%), in the UCB group 14/15 (93%). • UCB increased the likelihood of sustained engraftment associated with normal enzyme levels and could be considered a preferential stem cell source for inborn errors of metabolism. |
| Prasad et al (2008) * | UCB/159 (96% MM, 4%M) |
1.5 | LSD+PSD/15 9 |
45/159 | Acute: 40% Chronic: 21% |
Neutrophil recovery 87% at day +42 |
OS 72% High performance status OS 85% (1 year) |
• UCBT is an effective therapy for children with IMD. • Earlier transplantation before a decline is seen in performance status leads to better outcomes. |
HSC = hematopoietic stem cell, TRM = treatment-related mortality, GVHD = graft-versus-host disease, OS = overall survival, EFS = event-free survival, RUCB = related umbilical cord blood, MM = mismatched, M = matched, UCB = unrelated umbilical cord blood, dUCB = double umbilical cord blood, SCD = sickle cell disease, Thal = thalassemia, NA = information not available, TNC = total nucleated cells,.
All Limited chronic GVHD
Grades III-IV
HSC = hematopoietic stem cell, TRM = 1-year treatment-related mortality, GVHD = graft-versus-host disease, OS = overall survival,UCB = unrelated umbilical cord blood, UBM = unrelated bone marrow , M = matched, MM = mismatched, FA = Fanconi anaemia, AA = aplastic anaemia, MDS = myelodysplastic syndrome, AL = acute leukemia, Flu = fludarabine, TCD = T-cell depletion, CMV , cytomegalovirus, NA , not available.
Partial data on 92 of these patients was reported in the COBLT study (Martin et al 2006).
HSC(T) = hematopoietic stem cell (transplantation), TRM = 1-year treatment-related mortality, GVHD = graft-versus-host disease, LFS = leukemia-free survival, OS = overall survival, UCB(T) = unrelated umbilical cord blood (transplantation), RUCB = related umbilical cord blood, BM = bone marrow (match not specified), PBSC = peripheral blood stem cells (match not specified), MM = mismatched, M = matched, LSD = lysosomal storage disorder, PSD = peroxisomal storage disorder, MPS1 = Hurler syndrome, NA = information not available, EAP = expanded access protocol, A&E = alive and engrafted with >10% donor Chimerism and an α-L-iduronidase level > 4.5nmol/h/mg, IMD = Inherited metabolic disease.
Fanconi Anemia
Fanconi anemia (FA) is a rare autosomal recessive disease characterized by excessive chromosomal breakage, congenital abnormalities, progressive bone marrow failure and a predisposition to leukemia and epithelial malignancies. (Gluckman, et al 2007, Gluckman and Wagner 2008, Tan, et al 2006, Wagner, et al 2007) Though historically associated with inferior outcomes, recent improvements in HSCT, including the addition of fludarabine to the conditioning regimen and T-cell depletion of URD grafts, have allowed alternative donor transplant to become a first line treatment modality. (Gluckman, et al 2007) (Table 2B). In a registry-based study of UCBT in 93 FA patients, Gluckman et al (2007) found that higher cell dose (≥ 4.9 × 107 nucleated cells/kg) and the addition of fludarabine to the conditioning regimen positively affected both engraftment and survival. HLA disparity, though not statistically significant, negatively affected engraftment, GVHD and survival. (Gluckman, et al 2007) In comparison, Wagner et al (2007) reported outcomes of 98 recipients of URD BM and observed significantly improved engraftment (89% vs. 69%) and 3-year survival (52% vs. 13%) in those who received fludarabine versus no fludarabine. In addition, T-cell depletion was associated with less acute and chronic GVHD (relative risk [RR] 1.0 vs. 2.95, p=0.003 and RR 1.0 vs. 3.3, p=0.03 respectively). Increased mortality was observed in older patients (> 10 years), cytomegalovirus (CMV) positive patients and those that had received >20 blood product transfusions prior to HSCT. (Wagner, et al 2007) To date, there have been no formal comparisons between the alternative donor sources. As with hemoglobinopathies, URD transplant remains the gold standard until retrospective or prospective comparative trials can be performed. However, for patients who do not have an HLA-matched donor or who cannot wait the time it takes to complete a donor search, UCB is a reasonable alternative.
Metabolic Storage Diseases
Inherited metabolic diseases (IMD) are rare disorders caused by enzyme deficiencies and characterized by the accumulation of toxic metabolites in various tissues. Broadly, these diseases are divided into the mucopolysaccharidoses and sphingolipidoses or leucodystrophies. Allogeneic HSCT has the capacity to halt progression of these diseases by providing a constant source of the missing enzyme through engrafted donor leucocytes. (Martin, et al 2006, Orchard, et al 2007, Prasad and Kurtzberg 2008) (Figure 2) Because many patients lack an unaffected, fully HLA-matched sibling donor, appropriately matched alternative donors are often used. The use of UCBT, in particular, is especially desirable in these patients because the time from diagnosis to definitive treatment is crucial to prevent neurological disease progression and UCB units can be obtained quickly (Table 2C). To investigate the utility of UCBT in these disorders, Martin et al (2006) examined outcomes in 69 patients with lysosomal and peroxisomal storage disorders (LSD and PSD) as part of the COBLT study. Results demonstrated engraftment and 1-year OS rates of 78% and 72%, respectively. Neurocognitive results were not addressed. (Martin, et al 2006)
Figure 2.
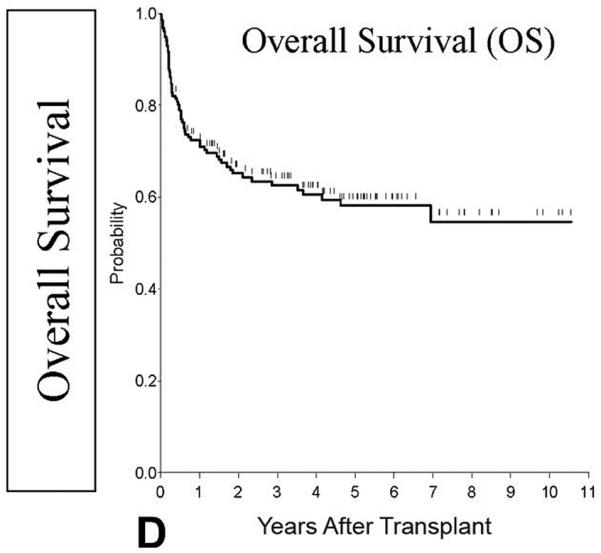
Probability of overall survival
This research was originally published in Blood. Prasad, V., Mendizabal, A., Parikh, S., Szabolcs, P., Driscoll, T., Page, K., Lakshminarayanan, S., Allison, J., Wood, S., Semmel, D., Escolar, M., Martin, P., Carter, S. & Kurtzberg, J. Unrelated donor umbilical cord blood transplantation for inherited metabolic disorders in 159 pediatric patients from a single center: influence of cellular composition of the graft on transplantation outcomes. Blood, (2008) 112, 2979-2989. © American Society of Hematology
Graft failure was high relative to that expected in recipients of unrelated BM and in the treatment of malignancy. Boelens et al (2007) therefore retrospectively examined outcomes in 146 patients with mucopolysaccharidoses (MPS) type 1 (20 UCB, 3RUCB, 103 BM, 20 PBSC) to look at risk factors for graft failure. No difference in graft failure was seen between the stem cell sources. Notably more patients in the UCB group achieved complete chimerism (93% vs. 66% in the combined BM/PBSC group) suggesting UCB may be considered the preferential stem cell source. (Boelens, et al 2007) In a separate study, Prasad et al (2008) reported the results of 159 IMD patients who received UCBT. Engraftment occurred in 87.1% and 1-year OS was 71.8%. Notably, those with high performance status had better OS, 84.5%, emphasizing the importance of definitive treatment early in the course of the disease. (Prasad, et al 2008) Based on these studies, it appears that alternative donor transplant for IMD provides outcomes at least as good as that seen with matched family donor BM and should be considered in patients with IMD not amenable to other therapies. Because the data on HSCT in this patient population have yet to include long-term neurological outcomes it is difficult to suggest one HSC source over another at this time.
REDUCED INTENSITY CONDITIONING
Risk of TRM has limited the use of alternative donor HSCT. RIC has therefore been explored, as an alternative for patients deemed too high-risk for MA conditioning. Whether RIC is as effective in disease control as a fully MA conditioning is as yet unknown and its primary role is to extend transplants to a wider patient population previously excluded from transplantation, such as those who are older (>45 years of age), have been heavily pretreated and/or come to transplant with co-morbidities.
The goal of RIC is to provide sufficient immunosuppression to prevent graft failure, achieve and maintain complete chimerism and to promote a graft-versus-malignancy effect. (Barker, et al 2003, Chen and Spitzer 2008, Satwani, et al 2008) As with traditional HSCT, the optimal donor is a fully HLA-matched sibling, but because this source of stem cells is not available to the majority of patients, alternative donors are often employed. The majority of the experience with alternative donor RIC has been with MURD. In recent years, though, many groups have examined the use of UCB in this setting because of its immediate availability and permissible HLA disparity. (Chen and Spitzer 2008) Though there was initial concern that RIC would not be sufficient enough to allow engraftment of UCB grafts secondary to its reduced alloreactivity as compared to BM or PBSC, the first reports of RIC with UCBT were encouraging. (Barker, et al 2003) (Table 3).
Table 3.
UCB and RIC Transplant
| Reference | Conditioning Regimen |
Disease/ n |
HSC source/n |
Median Age (years) |
Incidence of TRM |
Incidence of Grades II-IV GVHD |
Graft Failure |
Probability of OS/EFS |
Outcomes and Conclusions |
|---|---|---|---|---|---|---|---|---|---|
| Del Toro et al (2004) | Flu based Plus: Bu, Cy, melph, or VP- 16 Plus: ATG or C1-H |
HL/6 NHL/1 NBL/2 Wilms/1 CML/1 AML/1 MDS/2 WAS/1 Thal/2 SAA/2 HLH/1 FA/1 |
UCB/14 (100% mm) RBM/2 RPBSC/4 RBM+PBSC/ 1 |
13 | 10% (n=2) (100 days) |
Acute: 38% Chronic: 0% (median f/u 149 days) |
n=5 (24%) (UCB: 3 early GF, 1 late GF) |
OS: 73% (1 -year) Good risk: 89% High risk: 40% |
• RIC in kids is feasible (≤ 25% GF) and results in = ≥85% of recipients initially achieving >50% donor chimerism. |
| Jacobsohn et al (2004) | Flu/Bu based +/−ATG |
SCD/3 Thal/1 MPS/3 Imm/6 |
UCB/2 PBSC/6 RPBSC/5 |
5.2 | 15% (n=2) (100 days) |
Acute: 8% (n=1) Chronic: 37% (n=3) |
Primary: n=2 Late: n=1 |
OS: 84% (1 -year) |
• RIC provides a good alternative to MA transplant for children with non-malignant disorders, except for hemoglobinopathi es where engraftment is poor. |
| Majhail et al (2006a) | Bu/Flu/TBI(20 0) or Cy/Flu/TBI(20 0) |
HL/21 | UCB/9 (100% mm) MSD/12 (1 mm) |
28 42 |
n=1 (100 days) n=2 (100 days) |
*Acute: 33% Chronic: 11% *Acute: 33% Chronic: 33% |
n=0 n=0 |
OS: 51% EFS: 20% (2-year) OS: 49% EFS: 25% (2-year) |
• Comparable outcomes with UCB and MSD after RIC. |
| Majhail et al (2008) | Flu/Cy/TBI(20 0) Flu/Bu/TBI (n=16) Flu/Clad/TBI (n=5) |
AML/29 ALL/4 CML/3 CLL/5 MDS/16 NHL/21 HL/2 MM/6 Other/2 |
UCB/5 dUCB/38 (93% mm) RPBSC/44 RBM/1 RBM+PBSC/ 2 |
59 58 |
28% 23% (180 days) |
Acute: 49% Chronic: 17% Acute: 42% Chronic: 40% |
89% 100% (Sustain ed donor engraft ment at 42 days) |
OS: 34% EFS: 34% OS: 43% EFS: 30% (3-year) |
• Survival and TRM following RIC UCB and MSD transplant are comparable in adults over 55 years. • Graft type had no impact on TRM or survival. |
Grades III-IV acute GVHD
HSC(T) = haematopoietic stem cell (transplantation), TRM = treatment-related mortality, GVHD = graft-versus-host disease LFS = leukemia-free survival, OS = overall survival, Flu = fludarabine, Bu = busulfan, Cy = cytoxan, melph = melphalan, VP-16 = etoposide, ATG = antithymocyte globulin, C1-H = campath, TBI = total body irradiation, Clad = cladribine, HL = Hodgkin lymphoma, NHL = Non-Hodgkin lymphoma, NBL = neuroblastoma, Wilms = Wilms tumour. CML = chronic myeloid leukaemia,AML = acute myeloid leukaemia, MDS = myelodysplastic syndrome, WAS = Wiskott-Aldrich syndrome, Thal = thalassaemia, SAA = severe aplastic anemia, HLH = haemophagocytic lymphohistiocytosis, FA = Fanconi anaemia,SCD = sickle cell disease, Imm = immune deficiency, MPS = mucopolysaccharidoses, ALL = acute lymphoblastic leukaemia, CLL = chronic lymphocytic leukaemia, MM = multiple myeloma, UCB = unrelated umbilical cord blood, RBM = related bone marrow, (R)PBSC = (related) peripheral blood stem cells, MSD = matched sibling donor, dUCB = double unrelated umbilical cord blood, mm = mismatch, f/u = follow-up, GF = graft failure, RIC = reduced intensity conditioning, MA = myeloablative.
Pediatrics
In general, there is less need for RIC in pediatrics because most children do not have preexisting comorbidities that would preclude them from moving forward with a MA HSCT. However, many children are transplanted for benign conditions and transplantation with RIC could potentially achieve the same goals with less toxicity, making it an attractive alternative. In addition, long-term effects are important in children and RIC could potentially lessen the incidence of these complications. Outcome data on alternative donor HSCT with RIC in children is just beginning to emerge.
Del Toro et al (2004) reported the encouraging results of a pilot study of RIC in 21 children with a variety of malignant and nonmalignant diseases using either UCB (n=14) or related donor BM/PBSC (n=7). In this small cohort, RIC was found to result in more than 85% of children initially achieving >50% chimerism. The 5 graft failures (3 primary, 2 secondary) occurred in patients with nonmalignant disorders, including Beta-thalassemia, hemophagocytic lymphohistiocytosis (HLH), myelodysplastic syndrome (MDS) and severe aplastic anemia (SAA), indicating that there may be a subgroup of primary hematological disorders that may require more intense conditioning. (Del Toro, et al 2004)
Two other recent studies looked at alternative donor RIC exclusively in children with nonmalignant disease. (Jacobsohn, et al 2004, Rao, et al 2005) Rao et al (2005) looked only at MURD and MMURD RIC in 33 children with high risk immune deficiencies and showed that, compared to group of 19 historical controls receiving MA conditioning, the children who underwent less intensive therapy had an improved 1-year OS (94% vs. 47%), mainly secondary to decreased TRM. Jacobsohn et al (2004) reported the results of 13 RIC transplants (2 UCB, 6 PBSC, 5 RPBSC) in children with benign hematological disorders, storage diseases and immune deficiencies. The TRM, incidence of acute GVHD and 1-year OS were encouraging (15%, 8% and 84%, respectively) and the graft failures (2 primary and 1 secondary) occurred in patients with thalassemia and SCD. (Jacobsohn, et al 2004)
These retrospective results in heterogeneous groups of children highlight the feasibility and tolerability of alternative donor RIC HSCT in children and provide a solid base for continued research in this area. It appears that all donor sources, including UCB and BM/PBSC, are equally feasible options.
Adults
As previously stated, use of RIC is most common in adults either because of older age or preexisting co-morbidities. Two groups have recently looked at the use of alternative donor RIC HSCT for the treatment of high risk, heavily pretreated lymphoma. (Majhail, et al 2006a, Yuji, et al 2005) In one report of 20 RIC UCBT (Yuji et al 2005), the OS at 1 year was 50%, which compared very favorably to historical survival rates of 19-30% after RIC transplant with other donor sources. (Robinson, et al 2002) TRM was higher than many previous reports at 41%, but age, prior therapy, disease status probably played a role. (Yuji, et al 2005) Majhail et al (2006a) compared the outcomes after RIC transplant for high risk Hodgkin Lymphoma in 9 recipients of UCB and 12 recipients of MSD grafts. TRM, 2-year EFS and 2-year OS were comparable between the UCB and MSD groups (11% vs. 16%, 20% vs. 25% and 51% vs. 49%, respectively), indicating that UCB is a reasonable alternative even to MSD in this setting. (Majhail, et al 2006a) This may be important as older MSD may be at higher risk for complications during the HSC collection process.
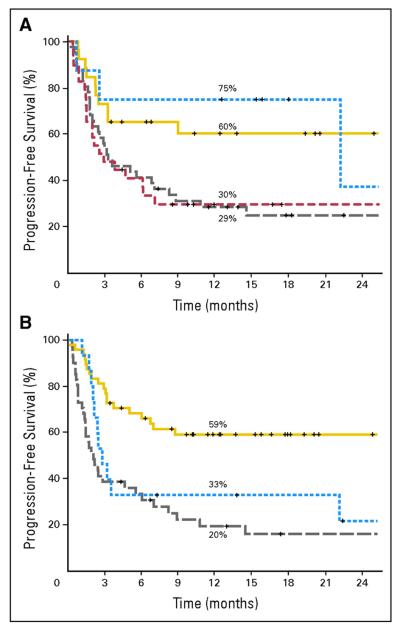
Other recently published studies report the results of RIC UCBT for a variety of malignant disorders in adults. Brunstein et al (2007a) reported results in adults (n=110) transplanted for hematological disease with either 1 or 2 UCB units to achieve a minimum cell dose of 2 × 107 nucleated cells/kg. They reported a 92% incidence of sustained engraftment with 19% TRM and a 3-year OS of 45%, which is similar to those reported with other stem cell sources. (Brunstein, et al 2007a) Similarly, Majhail et al (2008) examined the outcomes of RIC UCBT in adults older than 55 years of age and compared them to outcomes seen after matched related donor transplantation. They observed comparable TRM and 3-year OS in the two groups (28% vs. 23%, p=0.36 and 34% vs. 43%, p=0.57, respectively), though the UCB group had a lower incidence of sustained donor engraftment and chronic GVHD (89% vs. 100%, p=0.05 and 17% vs.40%, p=0.02 respectively). In another study, Uchida looked at whether or not elderly patients (median age 61 years) could tolerate RIC UCBT and found acceptable OS and EFS (23% and 23%, respectively), but with high rates of acute GVHD (61%) with single agent GVHD prophylaxis. They conclude that older age should not be a contraindication to RIC UCBT, but emphasized that GVHD prophylaxis should be optimized. (Uchida, et al 2008) Finally, in a cohort of patients with lymphoid malignancies, Rodrigues et al (2009) found that those patients who received low-dose total body irradiation (TBI) had significantly lower TRM and better EFS and OS when compared to those who received RIC without TBI or MA preparation prior to an UCBT. (Figure 3)
Figure 3.
(A) Estimated progression-free survival according to histological subtype. Patients with indolent non-Hodgkin lymphoma (NHL: yellow line), mantle-cell lymphoma (blue line), aggressive NHL (grey line), and Hodgkin lymphoma (red line). (B) Estimated progression-free survival according to the use of total-body irradiation (TBI). Patients who received low-dose TBI-containing regimens (yellow line), high-dose TBI (blue line), or no TBI (gray line) after umbilical cord blood transplantation for lymphoid malignancies.
From Rodrigues, C et al J Clin Oncol, 27, (2009) 256-263. Reprinted with permission. © 2009 American Society of Clinical Oncology. All rights reserved.
All of these results, though retrospective in nature and from heterogeneous populations, indicate that alternative donor RIC HSCT in adults is feasible with acceptable rates of TRM, GVHD and graft failure, thus extending the availability of transplantation therapy, especially to the elderly and those with co morbid conditions.
INFECTIONS
Since the inception of UCBT there has been considerable concern that the naivety of the neonatal immune system may not only be associated with less GVHD, but also more infection. Serious infection remains a significant cause of morbidity and mortality after unrelated donor transplantation regardless of HSC source. Few detailed analyses of infectious complications after UCBT have been reported. Certainly patients do exhibit immune reconstitution, but the question is whether it is delayed relative to other HSC sources.
Two recent reports in adult recipients of URD transplants have attempted to address the issue of infections after UCBT. In a single centre, retrospective study, Hamza et al (2004) looked at myeloid and lymphocyte recovery and infectious outcomes in 28 UCB and 23 MURD recipients. They found that the median duration of neutropenia was longer after UCBT (29 days vs. 14 days) and that, subsequently, the risk of bacterial infections was higher in the first 50 days post-transplant in the UCB group. There was no difference in the incidence of CMV, fungal or other viral infections between the groups. EFS at 3 years was higher in the MUD group (35% vs. 25%, p=0.014) though incidence of deaths related to infection was not different. (Hamza, et al 2004) But the recipients of UCB were more likely to have intermediate and high risk disease by IBMTR risk assessment and a significantly longer time from diagnosis to transplant, both of which could be confounding the comparison. In addition, the UCB units in this analysis were matched at a minimum of 3/6 HLA loci and the median infused nucleated and CD34+ cell dose was 2.1 × 107/kg and 1.7 × 105/kg, respectively. However, Wagner et al (2002) have shown that a CD34+ cell dose of <1.7 × 105/kg was associated with significantly lower neutrophil engraftment and TRM after UCBT. These two factors may also contribute to the delayed engraftment and subsequent increase in bacterial infections seen in the UCBT group. In another retrospective study, Parody et al (2006) describe severe infections in 192 adult recipients of URD HSCT (48 UCB, 144 BM/PBSC) and report that UCB recipients have delayed count recovery (neutrophils, monocytes, lymphocytes and platelets) and a higher risk of developing any severe infection by 3 years when compared to the BM/PBSC group (85% vs. 69%, respectively p<0.01). However, infection-related deaths and OS were not different between the two groups (Parody, et al 2006)
Infections after URD transplant in 136 children were examined in a single centre, retrospective report (Barker et al 2005). In this analysis, there were 60 UCB, 52 BM and 24 T cell-depleted (TCD) BM grafts. Neutrophil recovery was faster in the TCD group (14.5 days), but similar in the UCB and BM groups (22 vs. 23 days). The cumulative incidence of 1 or more serious infections was comparable between the groups (90% UCB, 81% BM, 83% TCD, p=0.12), but the TCD group had significantly higher incidence of early (<42 days) and late (180 days – 2 years) viral infections and late bacterial infections. Two-year OS, however, was not different between the groups (UCB 43%, BM 45%, TCD 63%, p=0.29). (Barker, et al 2005) These results indicate that serious infection after pediatric UCBT is comparable to that with unmanipulated BM.
Epstein Barr virus (EBV) and post-transplant lymphoproliferative disorder (PTLD) are well-recognized complications of allogeneic HCT that have been associated with unrelated donors, HLA mismatch, antithymocyte globulin (ATG) administration and T cell depletion. (Hoshino, et al 2001, van Esser, et al 2001) There has been some concern that neonatal T cells may be less able to regulate EBV-associated lymphoproliferation than those in volunteer unrelated bone marrow or peripheral blood thus leading to a higher incidence of EBV-related complications after UCBT.However, this does not seem to be the case in the setting of MA transplantation. In a two centre retrospective analysis, the incidence of EBV-PTLD after UCBT was similar to that seen after unrelated BM. (Barker, et al 2001) In a separate study looking at the impact of conditioning intensity on EBV-related complications after UCBT, the relative risk of EBV-PTLD was significantly higher in those patients receiving non-MA conditioning with ATG as compared to those receiving MA conditioning or non-MA conditioning without ATG. (Brunstein, et al 2006)
Serious infection after URD transplant continues to be a major problem regardless of donor source. Recent retrospective studies do not support the theory that serious infection is more common after UCBT in the pediatric population and that, although bacterial infections may be more common early after UCBT in adults, the risk of dying from infection is the same as the risk after other types of transplantation. The only way to determine if a true difference really exists, however, would be to do a large randomized study with prospective collection of infection and laboratory immune reconstitution data. In the meantime, continuing research on immune reconstitution after unrelated donor transplant and its relationship to post transplant infections will help to further define risk.
DONOR-DERIVED LEUKEMIA AFTER ALTERNATIVE DONOR HCT
Donor cell leukemia (DCL) is a rare complication of allogeneic HCT with an unclear aetiology. The first reports examined cases that developed after BM or PBSC transplantation. (Cooley, et al 2000, Hertenstein, et al 2005) Though the aetiology was not clearly delineated, the incidence was notably quite low. In recent years, there have been several case reports of DCL after UCBT (Ando, et al 2006, Fraser, et al 2005, Matsunaga, et al 2005) and it has been hypothesized that the incidence may be higher after UCBT when compared to other stem cell sources. (Greaves 2006) However, considering the fact that there have been more than 10,000 UCBT to date, it is estimated that the risk is still <1%. While DCL needs to be listed as a potential risk with UCBT, there is no data to suggest that the risk is higher than that observed with other stem cell sources.
DOUBLE CORD BLOOD TRANSPLANT
Over time, there has been a growing general consensus that 2.5×107 nucleated cells/kg recipient body weight represents the UCB cell dose threshold necessary for consistent engraftment. While this cell dose is often achievable with a single UCB unit for young children, it is often not possible for adult recipients. This has been a major barrier to its more widespread use. One strategy to overcome to achieve the cell dose threshold and engraftment in adults is the co-infusion of two partially HLA-matched UCB units. Results reported in the last few years indicate that the co-infusion of two partially HLA-matched UCB units is safe and efficacious, regardless of the intensity of the conditioning. (Brunstein, et al 2007b, Majhail, et al 2006b) While there is data suggesting that double UCBT may be associated with a lower risk of relapse(Verneris et al 2005), it is unclear whether it offers any other benefit other than extending the application of UCBT by virtue of greater chance of achieving the cell dose threshold of 2.5 × 107/kg and augmenting the cell dose which is particularly important in recipients of 2 HLA mismatched grafts. Until data demonstrate a clear survival advantage, double UCBT is only recommended for those patients who do not have an adequate single unit.
On the basis of the clinical data demonstrating the increasing importance of cell dose with increasing HLA mismatch (Gluckman 2006), an adequate single unit at the University of Minnesota has been defined as: >3.0 × 107 nucleated cells/kg for 6/6 HLA-matched units, >4.0 × 107 nucleated cells/kg for 5/6 HLA-matched units and >5.0 × 107 nucleated cells/kg for 4/6 HLA-matched units. While the exact cut off criteria for each degree of HLA mismatch is not known, the dose algorithm in principle is clear.
CONCLUSIONS AND FUTURE DIRECTIONS
Decisions regarding HSC source should be based on individual patient needs, including the urgency of the transplant, size of the patient (adequacy of cell dose) and potential need for future DLI, but some broad guidelines can be drawn from the above results. (Table 4) For malignant diseases, a 6/6 HLA-matched UCB with an adequate cell dose, on the rare occasion that it is available, should be considered the first line HSC source. Because 8/8 HLA-matched BM, 5/6 HLA-matched UCB and 4/6 HLA-matched UCB have similar outcomes, any of these are good second line sources. However, it is clear that with UCB, TRM increases with each degree of HLA-mismatch, so higher cell doses are needed with more HLA disparity. In addition, PBSC or BM is often a more realistic HSC option in adults due to cell dose limitations. In nonmalignant diseases, more comparative studies are needed before definitive conclusions can be made, but the studies reported to date indicate that UCB is a feasible alternative HSC source in most patient populations.
Table 4.
General Recommendations
| Malignant Diseases | Non-Malignant Diseases | |||
|---|---|---|---|---|
| Paediatrics | Adults | Paediatrics | Adults | |
| First Choice |
• 6/6 HLA- matched UCB* |
• 6/6 HLA- matched UCB* |
• 8/8 HLA MUBM | • 8/8 HLA-matched PBSC • 8/8 HLA MUBM |
|
| ||||
| Second Choice** |
• 8/8 HLA MUBM • 5/6 HLA- matched UCB* • 4/6 HLA- matched UCB* |
• 8/8 HLA- matched PBSC • 8/8 HLA MUBM • 5/6 HLA- matched UCB* • 4/6 HLA- matched UCB* |
• 6/6 HLA-matched UCB* • 5/6 HLA-matched UCB* |
• 6/6 HLA-matched UCB* • 5/6 HLA-matched UCB* |
|
| ||||
| Third Choice |
• 4/6 HLA-matched UCB* |
• 4/6 HLA-matched UCB* |
||
Provided the unit has an adequate cell dose.
Survival rates are similar; decision should be made based on the urgency of the transplant, adequacy of UCB cell dose and potential need for future donor lymphocyte infusion.
HLA = human leucocyte antigen, UCB = unrelated umbilical cord blood, MUBM = MUBM = matched unrelated bone marrow, PBSC = peripheral blood stem cells.
In general, though, 8/8 HLA-matched BM remains the ‘gold standard’ for alternative donor HSCT, but UCB should be considered a reasonable option in those that do not have such a donor available and for those in whom the time to transplant is critical, such that waiting for an URD BM would not be in the best interest of the patient. Further efforts focused on increasing the number, HLA diversity and quality of stored UCB units as well as addressing cell dose limitations using strategies, such as double UCB transplant and ex vivo expansion of a single unit are needed to continue to advance the field of UCBT.
ACKNOWLEDGEMENTS
This work was in part supported by the National Cancer Institute (PO-CA65493), COBLT N01-HB 67139 and the Children’s Cancer Research Fund.
REFERENCES
- Adamkiewicz T, Szabolcs P, Haight A, Baker K, Staba S, Kedar A, Chiang K, Krishnamurti L, Boyer M, Kurtzberg J, Wagner J, Wingard J, Yeager A. Unrelated cord blood transplantation in children with sickle cell disease: review of four-center experience. Pediatr Transplant. 2007;11:641–644. doi: 10.1111/j.1399-3046.2007.00725.x. [DOI] [PubMed] [Google Scholar]
- Al-Kasim FA, Thornley I, Rolland M, Lau W, Tsang R, Freedman MH, Saunders EF, Calderwood S, Doyle JJ. Single-centre experience with allogeneic bone marrow transplantation for acute lymphoblastic leukaemia in childhood: similar survival after matched-related and matched-unrelated donor transplants. Br J Haematol. 2002;116:483–490. [PubMed] [Google Scholar]
- Ando T, Yujiri T, Mitani N, Takeuchi H, Nomiyama J, Suguchi M, Matsubara A, Tanizawa Y. Donor cell-derived acute myeloid leukemia after unrelated umbilical cord blood transplantation. Leukemia. 2006;20:744–745. doi: 10.1038/sj.leu.2404121. [DOI] [PubMed] [Google Scholar]
- Barker J, Martin P, Coad J, DeFor T, Trigg M, Kurtzberg J, Weisdorf D, Wagner J. Low incidence of Epstein-Barr virus-associated posttransplantation lymphoproliferative disorders in 272 unrelated-donor umbilical cord blood transplant recipients. Biol Blood Marrow Transplant. 2001;7:395–399. doi: 10.1053/bbmt.2001.v7.pm11529490. [DOI] [PubMed] [Google Scholar]
- Barker J, Weisdorf D, DeFor T, Blazar B, Miller J, Wagner J. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood. 2003;102:1915–1919. doi: 10.1182/blood-2002-11-3337. [DOI] [PubMed] [Google Scholar]
- Barker J, Hough R, van Burik J, DeFor T, MacMillan M, O’Brien M, Wagner J. Serious infections after unrelated donor transplantation in 136 children: impact of stem cell source. Biol Blood Marrow Transplant. 2005;11:362–370. doi: 10.1016/j.bbmt.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Boelens J, Wynn R, O’Meara A, Veys P, Bertrand Y, Souillet G, Wraith J, Fischer A, Cavazzana-Calvo M, Sykora K, Sedlacek P, Rovelli A, Uiterwaal C, Wulffraat N. Outcomes of hematopoietic stem cell transplantation for Hurler’s syndrome in Europe: a risk factor analysis for graft failure. Bone Marrow Transplant. 2007;40:225–233. doi: 10.1038/sj.bmt.1705718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunstein C, Weisdorf D, DeFor T, Barker J, Tolar J, van Burik J, Wagner J. Marked increased risk of Epstein-Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood. 2006;108:2874–2880. doi: 10.1182/blood-2006-03-011791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunstein C, Barker J, Weisdorf D, DeFor T, Miller J, Blazar B, McGlave P, Wagner J. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007a;110:3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunstein C, Setubal D, Wagner J. Expanding the role of umbilical cord blood transplantation. Br J Haematol. 2007b;137:20–35. doi: 10.1111/j.1365-2141.2007.06521.x. [DOI] [PubMed] [Google Scholar]
- Bunin N, Carston M, Wall D, Adams R, Casper J, Kamani N, King R. Unrelated marrow transplantation for children with acute lymphoblastic leukemia in second remission. Blood. 2002;99:3151–3157. doi: 10.1182/blood.v99.9.3151. [DOI] [PubMed] [Google Scholar]
- Chen Y, Spitzer T. Current status of reduced-intensity allogeneic stem cell transplantation using alternative donors. Leukemia. 2008;22:31–41. doi: 10.1038/sj.leu.2404932. [DOI] [PubMed] [Google Scholar]
- Cooley L, Sears D, Udden M, Harrison W, Baker K. Donor cell leukemia: report of a case occurring 11 years after allogeneic bone marrow transplantation and review of the literature. Am J Hematol. 2000;63:46–53. doi: 10.1002/(sici)1096-8652(200001)63:1<46::aid-ajh11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Davies S, Wagner J, Shu X, Blazar B, Katsanis E, Orchard P, Kersey J, Dusenbery K, Weisdorf D, McGlave P, Ramsay N. Unrelated donor bone marrow transplantation for children with acute leukemia. J Clin Oncol. 1997;15:557–565. doi: 10.1200/JCO.1997.15.2.557. [DOI] [PubMed] [Google Scholar]
- Del Toro G, Satwani P, Harrison L, Cheung Y, Bradley M. Brigid, George D, Yamashiro D, Garvin J, Skerrett D, Bessmertny O, Wolownik K, Wischhover C, van de Ven C, Cairo M. A pilot study of reduced intensity conditioning and allogeneic stem cell transplantation from unrelated cord blood and matched family donors in children and adolescent recipients. Bone Marrow Transplant. 2004;33:613–622. doi: 10.1038/sj.bmt.1704399. [DOI] [PubMed] [Google Scholar]
- Eapen M, Rubinstein P, Zhang M, Camitta B, Stevens C, Cairo M, Davies S, Doyle J, Kurtzberg J, Pulsipher M, Ortega J, Scaradavou A, Horowitz M, Wagner J. Comparable long-term survival after unrelated and HLA-matched sibling donor hematopoietic stem cell transplantations for acute leukemia in children younger than 18 months. J Clin Oncol. 2006;24:145–151. doi: 10.1200/JCO.2005.02.4612. [DOI] [PubMed] [Google Scholar]
- Eapen M, Rubinstein P, Zhang MJ, Stevens C, Kurtzberg J, Scaradavou A, Loberiza FR, Champlin RE, Klein JP, Horowitz MM, Wagner JE. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- Eapen M, Rocha V, Scaradavou A, Gluckman E, Laughlin M, Stevens C, Horowitz MM, Wagner JE. Effect of Stem Cell Source on Transplant Outcomes in Adults with Acute Leukemia: A Comparison of Unrelated Bone Marrow (BM), Peripheral Blood (PB) and Cord Blood (CB) Blood (ASH Annual Meeting Abstracts) 2008;112:151. [Google Scholar]
- Fraser C, Hirsch B, Dayton V, Creer M, Neglia J, Wagner J, Baker K. First report of donor cell-derived acute leukemia as a complication of umbilical cord blood transplantation. Blood. 2005;106:4377–4380. doi: 10.1182/blood-2005-06-2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman E. Cord blood transplantation. Biol Blood Marrow Transplant. 2006;12:808–812. doi: 10.1016/j.bbmt.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Gluckman E, Wagner J. Hematopoietic stem cell transplantation in childhood inherited bone marrow failure syndrome. Bone Marrow Transplant. 2008;41:127–132. doi: 10.1038/sj.bmt.1705960. [DOI] [PubMed] [Google Scholar]
- Gluckman E, Rocha V, Boyer-Chammard A, Locatelli F, Arcese W, Pasquini R, Ortega J, Souillet G, Ferreira E, Laporte J, Fernandez M, Chastang C. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373–381. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- Gluckman E, Rocha V, Arcese W, Michel G, Sanz G, Chan K, Takahashi T, Ortega J, Filipovich A, Locatelli F, Asano S, Fagioli F, Vowels M, Sirvent A, Laporte J, Tiedemann K, Amadori S, Abecassis M, Bordigoni P, Diez B, Shaw P, Vora A, Caniglia M, Garnier F, Ionescu I, Garcia J, Koegler G, Rebulla P, Chevret S. Factors associated with outcomes of unrelated cord blood transplant: guidelines for donor choice. Exp Hematol. 2004;32:397–407. doi: 10.1016/j.exphem.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Gluckman E, Koegler G, Rocha V. Human leukocyte antigen matching in cord blood transplantation. Semin Hematol. 2005;42:85–90. doi: 10.1053/j.seminhematol.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Gluckman E, Rocha V, Ionescu I, Bierings M, Harris R, Wagner J, Kurtzberg J, Champagne M, Bonfim C, Bittencourt M, Darbyshire P, Fernandez M, Locatelli F, Pasquini R. Results of unrelated cord blood transplant in fanconi anemia patients: risk factor analysis for engraftment and survival. Biol Blood Marrow Transplant. 2007;13:1073–1082. doi: 10.1016/j.bbmt.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Greaves M. Cord blood donor cell leukemia in recipients. Leukemia. 2006;20:1633–1634. doi: 10.1038/sj.leu.2404293. [DOI] [PubMed] [Google Scholar]
- Hamza N, Lisgaris M, Yadavalli G, Nadeau L, Fox R, Fu P, Lazarus H, Koc O, Salata R, Laughlin M. Kinetics of myeloid and lymphocyte recovery and infectious complications after unrelated umbilical cord blood versus HLA-matched unrelated donor allogeneic transplantation in adults. Br J Haematol. 2004;124:488–498. doi: 10.1046/j.1365-2141.2003.04792.x. [DOI] [PubMed] [Google Scholar]
- Hertenstein B, Hambach L, Bacigalupo A, Schmitz N, McCann S, Slavin S, Gratwohl A, Ferrant A, Elmaagacli A, Schwertfeger R, Locasciulli A, Zander A, Bornhäuser M, Niederwieser D, Ruutu T. Development of leukemia in donor cells after allogeneic stem cell transplantation--a survey of the European Group for Blood and Marrow Transplantation (EBMT) Haematologica. 2005;90:969–975. [PubMed] [Google Scholar]
- Hoshino Y, Kimura H, Tanaka N, Tsuge I, Kudo K, Horibe K, Kato K, Matsuyama T, Kikuta A, Kojima S, Morishima T. Prospective monitoring of the Epstein-Barr virus DNA by a real-time quantitative polymerase chain reaction after allogenic stem cell transplantation. Br J Haematol. 2001;115:105–111. doi: 10.1046/j.1365-2141.2001.03087.x. [DOI] [PubMed] [Google Scholar]
- Jacobsohn D, Duerst R, Tse W, Kletzel M. Reduced intensity haemopoietic stem-cell transplantation for treatment of non-malignant diseases in children. Lancet. 2004;364:156–162. doi: 10.1016/S0140-6736(04)16628-2. [DOI] [PubMed] [Google Scholar]
- Jaing TH, Wang B, Gjertson D, Law P, Petz L, Chow R. Unrelated Cord Blood Transplantation (UCBT) for Transfusion-Dependent Thalassemia a CIBMTR Audited Retrospective Analysis of 30 Consecutive Patients from a Single Center. Blood (ASH Annual Meeting Abstracts) 2008;112:131. [Google Scholar]
- Kumar P, Defor T, Brunstein C, Barker J, Wagner J, Weisdorf D, Burns L. Allogeneic hematopoietic stem cell transplantation in adult acute lymphocytic leukemia: impact of donor source on survival. Biol Blood Marrow Transplant. 2008;14:1394–1400. doi: 10.1016/j.bbmt.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzberg J, Graham M, Casey J, Olson J, Stevens C, Rubinstein P. The use of umbilical cord blood in mismatched related and unrelated hemopoietic stem cell transplantation. Blood Cells. 1994;20:275–283. discussion 284. [PubMed] [Google Scholar]
- Kurtzberg J, Laughlin M, Graham M, Smith C, Olson J, Halperin E, Ciocci G, Carrier C, Stevens C, Rubinstein P. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335:157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- Kurtzberg J, Prasad V, Carter S, Wagner J, Baxter-Lowe L, Wall D, Kapoor N, Guinan E, Feig S, Wagner E, Kernan N, on behalf of the COBLT Steering Committee Results of the Cord Blood Transplantation Study (COBLT): clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood. 2008;112:4318–4327. doi: 10.1182/blood-2007-06-098020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin M, Eapen M, Rubinstein P, Wagner J, Zhang M, Champlin R, Stevens C, Barker J, Gale R, Lazarus H, Marks D, van Rood J, Scaradavou A, Horowitz M. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- Locatelli F, Rocha V, Reed W, Bernaudin F, Ertem M, Grafakos S, Brichard B, Li X, Nagler A, Giorgiani G, Haut P, Brochstein J, Nugent D, Blatt J, Woodard P, Kurtzberg J, Rubin C, Miniero R, Lutz P, Raja T, Roberts I, Will A, Yaniv I, Vermylen C, Tannoia N, Garnier F, Ionescu I, Walters M, Lubin B, Gluckman E. Related umbilical cord blood transplantation in patients with thalassemia and sickle cell disease. Blood. 2003;101:2137–2143. doi: 10.1182/blood-2002-07-2090. [DOI] [PubMed] [Google Scholar]
- Majhail N, Weisdorf D, Wagner J, Defor T, Brunstein C, Burns L. Comparable results of umbilical cord blood and HLA-matched sibling donor hematopoietic stem cell transplantation after reduced-intensity preparative regimen for advanced Hodgkin lymphoma. Blood. 2006a;107:3804–3807. doi: 10.1182/blood-2005-09-3827. [DOI] [PubMed] [Google Scholar]
- Majhail N, Brunstein C, Wagner J. Double umbilical cord blood transplantation. Curr Opin Immunol. 2006b;18:571–575. doi: 10.1016/j.coi.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Majhail N, Brunstein C, Tomblyn M, Thomas A, Miller J, Arora M, Kaufman D, Burns L, Slungaard A, McGlave P, Wagner J, Weisdorf D. Reduced-intensity allogeneic transplant in patients older than 55 years: unrelated umbilical cord blood is safe and effective for patients without a matched related donor. Biol Blood Marrow Transplant. 2008;14:282–289. doi: 10.1016/j.bbmt.2007.12.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Carter S, Kernan N, Sahdev I, Wall D, Pietryga D, Wagner J, Kurtzberg J. Results of the cord blood transplantation study (COBLT): outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with lysosomal and peroxisomal storage diseases. Biol Blood Marrow Transplant. 2006;12:184–194. doi: 10.1016/j.bbmt.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Matsunaga T, Murase K, Yoshida M, Fujimi A, Iyama S, Kuribayashi K, Sato T, Kogawa K, Hirayama Y, Sakamaki S, Kohda K, Niitsu Y. Donor cell derived acute myeloid leukemia after allogeneic cord blood transplantation in a patient with adult T-cell lymphoma. Am J Hematol. 2005;79:294–298. doi: 10.1002/ajh.20349. [DOI] [PubMed] [Google Scholar]
- Orchard P, Blazar B, Wagner J, Charnas L, Krivit W, Tolar J. Hematopoietic cell therapy for metabolic disease. J Pediatr. 2007;151:340–346. doi: 10.1016/j.jpeds.2007.04.054. [DOI] [PubMed] [Google Scholar]
- Panepinto J, Walters M, Carreras J, Marsh J, Bredeson C, Gale R, Hale G, Horan J, Hows J, Klein J, Pasquini R, Roberts I, Sullivan K, Eapen M, Ferster A. Matched-related donor transplantation for sickle cell disease: report from the Center for International Blood and Transplant Research. Br J Haematol. 2007;137:479–485. doi: 10.1111/j.1365-2141.2007.06592.x. [DOI] [PubMed] [Google Scholar]
- Parody R, Martino R, Rovira M, Vazquez L, Vázquez M, de la Cámara R, Blazquez C, Fernández-Avilés F, Carreras E, Salavert M, Jarque I, Martín C, Martínez F, López J, Torres A, Sierra J, Sanz G. Severe infections after unrelated donor allogeneic hematopoietic stem cell transplantation in adults: comparison of cord blood transplantation with peripheral blood and bone marrow transplantation. Biol Blood Marrow Transplant. 2006;12:734–748. doi: 10.1016/j.bbmt.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Prasad V, Kurtzberg J. Emerging trends in transplantation of inherited metabolic diseases. Bone Marrow Transplant. 2008;41:99–108. doi: 10.1038/sj.bmt.1705970. [DOI] [PubMed] [Google Scholar]
- Prasad V, Mendizabal A, Parikh S, Szabolcs P, Driscoll T, Page K, Lakshminarayanan S, Allison J, Wood S, Semmel D, Escolar M, Martin P, Carter S, Kurtzberg J. Unrelated donor umbilical cord blood transplantation for inherited metabolic disorders in 159 pediatric patients from a single center: influence of cellular composition of the graft on transplantation outcomes. Blood. 2008;112:2979–2989. doi: 10.1182/blood-2008-03-140830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K, Amrolia P, Jones A, Cale C, Naik P, King D, Davies G, Gaspar H, Veys P. Improved survival after unrelated donor bone marrow transplantation in children with primary immunodeficiency using a reduced-intensity conditioning regimen. Blood. 2005;105:879–885. doi: 10.1182/blood-2004-03-0960. [DOI] [PubMed] [Google Scholar]
- Robinson S, Goldstone A, Mackinnon S, Carella A, Russell N, de Elvira C, Taghipour G, Schmitz N. Chemoresistant or aggressive lymphoma predicts for a poor outcome following reduced-intensity allogeneic progenitor cell transplantation: an analysis from the Lymphoma Working Party of the European Group for Blood and Bone Marrow Transplantation. Blood. 2002;100:4310–4316. doi: 10.1182/blood-2001-11-0107. [DOI] [PubMed] [Google Scholar]
- Rocha V, Cornish J, Sievers E, Filipovich A, Locatelli F, Peters C, Remberger M, Michel G, Arcese W, Dallorso S, Tiedemann K, Busca A, Chan K, Kato S, Ortega J, Vowels M, Zander A, Souillet G, Oakill A, Woolfrey A, Pay A, Green A, Garnier F, Ionescu I, Wernet P, Sirchia G, Rubinstein P, Chevret S, Gluckman E. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. 2001;97:2962–2971. doi: 10.1182/blood.v97.10.2962. [DOI] [PubMed] [Google Scholar]
- Rocha V, Labopin M, Sanz G, Arcese W, Schwerdtfeger R, Bosi A, Jacobsen N, Ruutu T, de Lima M, Finke J, Frassoni F, Gluckman E. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351:2276–2285. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- Rodrigues C, Sanz G, Brunstein C, Sanz J, Wagner J, Renaud M, de Lima M, Cairo M, Fürst S, Rio B, Dalley C, Carreras E, Harousseau J, Mohty M, Taveira D, Dreger P, Sureda A, Gluckman E, Rocha V. Analysis of risk factors for outcomes after unrelated cord blood transplantation in adults with lymphoid malignancies: a study by the Eurocord-Netcord and lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol. 2009;27:256–263. doi: 10.1200/JCO.2007.15.8865. [DOI] [PubMed] [Google Scholar]
- Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio A, Berkowitz R, Cabbad M, Dobrila N, Taylor P, Rosenfield R, Stevens C. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339:1565–1577. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- Satwani P, Cooper N, Rao K, Veys P, Amrolia P. Reduced intensity conditioning and allogeneic stem cell transplantation in childhood malignant and nonmalignant diseases. Bone Marrow Transplant. 2008;41:173–182. doi: 10.1038/sj.bmt.1705923. [DOI] [PubMed] [Google Scholar]
- Sawczyn K, Quinones R, Malcolm J, Foreman N, Garrington T, Gore L, Gao D, Giller R. Cord blood transplant in childhood ALL. Pediatr Blood Cancer. 2005;45:964–970. doi: 10.1002/pbc.20414. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Iseki T, Ooi J, Tomonari A, Takasugi K, Shimohakamada Y, Yamada T, Uchimaru K, Tojo A, Shirafuji N, Kodo H, Tani K, Takahashi T, Yamaguchi T, Asano S. Single-institute comparative analysis of unrelated bone marrow transplantation and cord blood transplantation for adult patients with hematologic malignancies. Blood. 2004;104:3813–3820. doi: 10.1182/blood-2004-03-1001. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Ooi J, Tomonari A, Konuma T, Tsukada N, Oiwa-Monna M, Fukuno K, Uchiyama M, Takasugi K, Iseki T, Tojo A, Yamaguchi T, Asano S. Comparative single-institute analysis of cord blood transplantation from unrelated donors with bone marrow or peripheral blood stem-cell transplants from related donors in adult patients with hematologic malignancies after myeloablative conditioning regimen. Blood. 2007;109:1322–1330. doi: 10.1182/blood-2006-04-020172. [DOI] [PubMed] [Google Scholar]
- Tan P, Wagner J, Auerbach A, Defor T, Slungaard A, Macmillan M. Successful engraftment without radiation after fludarabine-based regimen in Fanconi anemia patients undergoing genotypically identical donor hematopoietic cell transplantation. Pediatr Blood Cancer. 2006;46:630–636. doi: 10.1002/pbc.20538. [DOI] [PubMed] [Google Scholar]
- Uchida N, Wake A, Takagi S, Yamamoto H, Kato D, Matsuhashi Y, Matsumura T, Seo S, Matsuno N, Masuoka K, Kusumi E, Yuji K, Miyakoshi S, Matsuzaki M, Yoneyama A, Taniguchi S. Umbilical cord blood transplantation after reduced-intensity conditioning for elderly patients with hematologic diseases. Biol Blood Marrow Transplant. 2008;14:583–590. doi: 10.1016/j.bbmt.2008.03.003. [DOI] [PubMed] [Google Scholar]
- van Esser J, van der Holt B, Meijer E, Niesters H, Trenschel R, Thijsen S, van Loon A, Frassoni F, Bacigalupo A, Schaefer U, Osterhaus A, Gratama J, Löwenberg B, Verdonck L, Cornelissen J. Epstein-Barr virus (EBV) reactivation is a frequent event after allogeneic stem cell transplantation (SCT) and quantitatively predicts EBV-lymphoproliferative disease following T-cell--depleted SCT. Blood. 2001;98:972–978. doi: 10.1182/blood.v98.4.972. [DOI] [PubMed] [Google Scholar]
- Verneris MR, Brunstein C, DeFor TE, Barker J, Weisdorf DJ, Blazar BR, Miller JS, Wagner JE. Risk of relapse (REL) after umbilical cord blood transplantation (UCBT) in patients with acute leukemia: marked reduction in recipients of two units. Blood (ASH Annual Meeting Abstracts) 2005;106:93a. [Google Scholar]
- Wagner J, Rosenthal J, Sweetman R, Shu X, Davies S, Ramsay N, McGlave P, Sender L, Cairo M. Successful transplantation of HLA-matched and HLA-mismatched umbilical cord blood from unrelated donors: analysis of engraftment and acute graft-versus-host disease. Blood. 1996;88:795–802. [PubMed] [Google Scholar]
- Wagner J, Barker J, DeFor T, Baker K, Blazar B, Eide C, Goldman A, Kersey J, Krivit W, MacMillan M, Orchard P, Peters C, Weisdorf D, Ramsay N, Davies S. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- Wagner J, Eapen M, MacMillan M, Harris R, Pasquini R, Boulad F, Zhang M, Auerbach A. Unrelated donor bone marrow transplantation for the treatment of Fanconi anemia. Blood. 2007;109:2256–2262. doi: 10.1182/blood-2006-07-036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall D, Carter S, Kernan N, Kapoor N, Kamani N, Brochstein J, Frangoul H, Goyal R, Horan J, Pietryga D, Wagner J, Kurtzberg J. Busulfan/melphalan/antithymocyte globulin followed by unrelated donor cord blood transplantation for treatment of infant leukemia and leukemia in young children: the Cord Blood Transplantation study (COBLT) experience. Biol Blood Marrow Transplant. 2005;11:637–646. doi: 10.1016/j.bbmt.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Yuji K, Miyakoshi S, Kato D, Miura Y, Myojo T, Murashige N, Kishi Y, Kobayashi K, Kusumi E, Narimatsu H, Hamaki T, Matsumura T, Kami M, Fukuda T, Masuo S, Masuoka K, Wake A, Ueyama J, Yoneyama A, Miyamoto K, Nagoshi H, Matsuzaki M, Morinaga S, Muto Y, Takeue Y, Taniguchi S. Reduced-intensity unrelated cord blood transplantation for patients with advanced malignant lymphoma. Biol Blood Marrow Transplant. 2005;11:314–318. doi: 10.1016/j.bbmt.2005.01.012. [DOI] [PubMed] [Google Scholar]