Abstract
Objective
Determine if G-CSF promotes coronary collateral growth (CCG) and decipher the mechanism for this stimulation.
Methods and Results
In a rat model of repetitive episodic myocardial ischemia (RI, 40 sec LAD occlusion every 20 min for 2h20min, 3 times/day for 5 days) CCG was deduced from collateral-dependent flow (flow to LAD region during occlusion). Following RI, G-CSF (100 µg/kg/day) increased CCG (P<0.01) (0.47 ± 0.15) versus vehicle (0.14 ± 0.06). Surprisingly, G-CSF treatment without RI increased CCG (0.57 ± 0.18) equal to G-CSF + RI. We evaluated ROS by dihydroethidine (DHE) fluorescence (LV injection, 60 µg/kg, during two episodes of ischemia). DHE fluorescence was double in G-CSF+RI vs vehicle+RI (P<0.01), and even higher in G-CSF without RI (P<0.01). Interestingly, the DHE signal did not colocalize with myeloperoxidase (immunostaining, neutrophil marker) but appeared in cardiac myocytes. The study of isolated cardiac myocytes revealed the cytokine stimulates ROS which elicit production of angiogenic factors. Apocynin inhibited G-CSF effects both in vivo and in vitro.
Conclusions
G-CSF stimulates ROS production directly in cardiomyocytes, which plays a pivotal role in triggering adaptations of the heart to ischemia including growth of the coronary collaterals.
Keywords: G-CSF, coronary collateral circulation, ROS, cardiomyocytes
Introduction
Granulocyte-colony stimulating factor (G-CSF) is a hormone-like glycoprotein that regulates haematopoietic cell proliferation and differentiation1, and activates cells from the neutrophilic granulocyte lineage2. Bacterial endotoxins, or secondary mediators induced during infections, like tumour necrosis factor-α (TNF-α), interleukin (IL)-1, and interferon-γ (IFN-γ), are major stimulators of G-CSF production in vivo3. G-CSF mediated biological actions are mediated by binding to a specific cell-surface receptor, G-CSFR4, which is found on hematopoietic and non-hematopoietic cells, including myeloid progenitor cells, mature neutrophils, platelets, monocytes, endothelial cells (ECs)5 and adult mouse cardiomyocytes6. One major function of G-CSF is to induce a multiprolonged defence against microbes, stimulating neutrophils to release proteases, DNases, and reactive oxygen species (ROS)7. The production of ROS may also be involved in a large number of reversible regulatory signalling processes8, e.g., coronary collateral development is critically dependent on redox signalling and an optimal amount of ROS9. Furthermore, G-CSF was shown to ameliorate myocardial ischemic injury, by activating various signalling pathways such as Akt, ERK, Janus kinase 2 (Jak2)–signal transducer and activator of transcription 3 (STAT3) (after Myocardial Infarction)6 and eNOS following ischemia/reperfusion10. In this study, we projected G-CSF effects would also translate into the promotion of coronary collateral growth (CCG) under a repetitive episodic ischemia animal model, which could be mediated by the generation of ROS.
Material and Methods
Rat model of collateral growth
Male Sprague-Dawley rats (3–4mo old, 300–350 g) were used for chronic (5 days) implantation of a pneumatic occluder over the left anterior descending coronary artery (LAD), as described by Toyota et al. 11 to produce repetitive ischemia (RI). The RI protocol for rat consisted of eight 40sec occlusions, one every 20min over 2h 20min followed by a period of “rest” for 5h and 40min. This 8h cycle was repeated three times per day over a period of 5 days. Blood was collected from the animals at the beginning and end of surgical procedures for analysis of hematological profile of groups.
Microsphere measurements of myocardial and collateral-dependent blood flow
Flow to the collateral-dependent zone was measured by neutron activated microspheres (5 × 105) labeled with Samarium or Gold. Microspheres were injected into the left ventricle (LV) over 20s during LAD occlusion. Microspheres labelled with FITC were also injected with the first measurement of collateral flow. The collateral (LAD)-dependent zone was indentified by the lack of fluorescent microspheres. Collateral flow was calculated as a ratio between activity (dpm/g) of the tissue samples from the LAD-dependent and normal zones.
Echocardiographic Measurement of Cardiac Function
To determine if the increase in collateral-dependent blood flow led to a functional improvement, echocardiographic measurements of cardiac function were made using a VisualSonics Vevo 770 with a fundamental frequency of 20–35 MHz and a frame rate of 50–70 Hz. Left ventricular cavity dimensions were measured by M-mode echocardiography, using American Society of Echocardiography (ASE) guidelines. Measurements were made from parasternal short-axis views (at the papillary level). LV ejection fraction (EF) was calculated from images obtained during coronary occlusion (Day 5 of the RI protocol) in control rats receiving RI alone, and a group receiving RI+G-CSF.
G-CSF at a dose of 100µg/kg/day (s.c.) was given for 5 days in the specified groups. Collateral flow was measured in the following groups (n=6/group): a sham-operated group that was instrumented but not subjected to RI; a control group/RI; G-CSF, an instrumented group but not subjected to RI; G-CSF+RI; Vehicle+RI (Vehicle=diluting solution of G-CSF: Sodium acetate 10 mM pH 4.2; Sorbitol 200 mM; 0.004% Tween-80); G-CSF+Apocynin (inhibitor of NADPH oxidases, 0.25mg/mL in drinking water, n=3); G-CSF+RI+Apocynin (n=3).
Coronary Microvascular imaging with Cryomicrotome
One of each group of rats was prepared for coronary vascular visualization with micro-CT (n=3/group). Rat heart sectioning was performed with the imaging cryomicrotome as described previously12. In brief, after sacrifice of the animals, perfusion of the rat heart was continued until the efflux was clear of blood. Subsequently the perfusate was replaced by Batson #17 Solution which was made fluorescent by dissolution of 39µg/mL Potomac yellow, infused at 85 mmHg. Next, the heart was embedded in 5cm diameter cylindrical container filled with 5% carboxymethylcellulose solution containing 5% Indian ink, and frozen at −20°C for at least 24 hours. The specimen was placed in the cryomicrotome which was maintained at −20°C, after which serially slices were made of 17µ thickness and the remaining bulk tissue was imaged with a 2000×2000 pixel CCD Camera (Kodak Megaplus 4.2i CCD 8 bits grayscale camera), which was custom fitted with a Nikon Lens (Nikon Zoom Lens 70–180mm). The magnification and slice thickness were set so that each tissue voxel represented 17 cubic micrometers. Fluorescent images were acquired with use of 440/20nm excitation and 505/30nm emission band pass filters (Chroma Technology Corp., Rockingham, VT, USA) in combination with a 250W Xenon lamp. Three dimensional image representations were acquired by using Amira 3.1 Software.
Measurement of Reactive Oxygen Species
Superoxide production was evaluated by using dihydroethidium (DHE) in vitro and in vivo. For the in vitro studies, in freshly isolated cardiomyocytes (see Cardiomyocyte Isolation section for details), G-CSF was administered for 10 min, and DHE was added during the last 20 min of treatment with G-CSF. Cells were then immediately observed under a fluorescent microscope. For in vivo studies, DHE was injected into the LV (60 µg/kg) for 20 min before two consecutive periods of ischemia-reperfusion (40s occlusion followed by 20min reperfusion and another 40s occlusion and 20min reperfusion). Animals were then sacrificed. The heart was removed, frozen in optimum cutting temperature compound on dry ice, and stored at −80°C until sectioning. Sections (5 µm) were made in a cryomicrotome and were mounted on glass slides. DHE fluorescence was detected with excitation/emission at 518/605 nm. All images were analyzed at the same microscope settings, and fluorescence intensity was obtained by Metamorph Software on three hearts (10 sections per heart).
Immunohistochemical analysis
Cryofrozen hearts were sectioned at 5 µm, and mounted on slides. The heart sections were incubated with blocking solutions (10% normal serum from species of secondary antibody in Tris buffer) and then with polyclonal anti-myeloperoxidase (MPO, Abcam) antibody for 1h at room temperature. FITC secondary antibody was added, sections were mounted in anti fading agent. The slides were observed and analyzed using a fluorescence microscope.
Cardiomyocyte isolation
Adult ventricular myocytes were isolated from male Sprague-Dawley rats (250–300 g). Rats were heparinized (300 U ip) and anesthetized with ketamine (90 mg/kg) and xylazine (10 mg/kg). Hearts were removed and retrogradely perfused for 15 min in Krebs-Henseleit buffer (KHB, in mM: 118 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 11 glucose) containing 5 mM pyruvate and Liberase Blendzyme (0.1 mg/ml). Calcium was gradually added during the final 10 min of digestion to a concentration of 1.0 mM. Ventricles were minced and placed in KHB containing Liberase for 10 min in a shaking water bath at 37 °C and dispersed by trituration. The digested tissue was filtered through a 210 µm nylon mesh and the filtrate was centrifuged at 50g for 5 min. Pelleted cells were resuspended in DMEM and plated at a density of 50,000 rod shaped cells per well on 24-well plates precoated with laminin (1µg/cm2). After 2h, wells were washed with DMEM to remove unattached cells and debris.
Inhibitors of ROS Generation
The following metabolic inhibitors and chelators were used to inhibit ROS generation in isolated cardiomyocytes subjected to G-CSF treatment : apocynin (300 µM), Mn TMPyP [Mn(III)Tetrakis(1-methyl-4-pyridyl)porphyrin pentachloride] (100 µM, cell-permeable superoxide dismutase mimetic). Treatments were conducted in n=4 as follows: Control (no treatment), G-CSF (0.05µg/mL), G-CSF (0.1µg/mL), G-CSF (0.3µg/mL), G-CSF (0.3µg/mL) + Apocynin, G-CSF(0.3µg/mL) + MnTMPyP. DHE (5µM) was added in the last 20 min of treatment and cells were observed under a fluorescent microscope.
Cell Culture
Human coronary artery endothelial cells (HCAEC) were purchased from Clonetics and were cultured at low passages (passages 3–8) in Clonetics EGM-2 BulletKit medium (Lonza) that contains 25% FBS, 0.2% hydrocortisone, 2% human FGF-B, 0.5% IGF-I, 0.5% ascorbic acid, 0.5% human EGF, and 0.5% GA-1000. Rat aortic smooth muscle cells were isolated from explants. Once purity was established (anti-smooth muscle α-actin immunostaining; cultures >95% positive), cells were passaged and used in early passage (up to P5). Smooth muscle cells were grown in DMEM at 37°C and 5% CO2 supplemented with 10%FBS.
To determine if G-CSF stimulated endothelial cells and smooth muscle cells to produce superoxide, 25,000 cells were seeded in 25 mm cell culture chambers, and allowed to stablize overnight. Varying doses of G-CSF were added (0.03, 0.3, and 3.0 ug/ml) to the media for 1 hr. During the last 20 minutes of treatment, DHE (5µM) was added and cells were observed under a fluorescent microscope.
Endothelial tube formation promoted by G-CSF cardiomyocyte stimulation media
Adult ventricular myocytes were isolated and plated as described above. Cardiac myocytes were stimulated with G-CSF (0.3µg/mL) for 2 and 24 hours. This stimulation media was isolated and used to evaluate HCAEC tube formation. HCAECs were seeded on Matrigel (BD Biosciences)-coated 24-well plates according to manufacturer instructions in EGM-2 Bullet Kit medium at a density of 30,000 cells/well. Cells were allowed to attach for 24 h before the addition of 50 ng/ml Regular Media (negative control), VEGF (positive control), 2h G-CSF cardiomyocyte stimulation media, 24h G-CSF cardiomyocyte stimulation media, 2h media + Apocynin (300 µM), 24h media + Apocynin. The extent of tube formation was quantified after 24h by superimposing a grid (25mm2/cube) on microscopic images, and the number of squares containing tubes were counted and averaged from five randomly selected fields for each well to obtain the percentage of total field that contained tubes. Treatments were conducted in n=4.
Data analysis
ANOVA followed by t-tests using the Bonferroni Inequality was used for statistical analysis. To evaluate a functional improvement in cardiac function, a paired comparison between ejection fraction prior to RI protocol and at Day 5 were made and the results are expressed as a Δchange in EF. All results are presented as Mean ± SEM. A probability value of P<0.05 was used to determine statistical significance.
Results
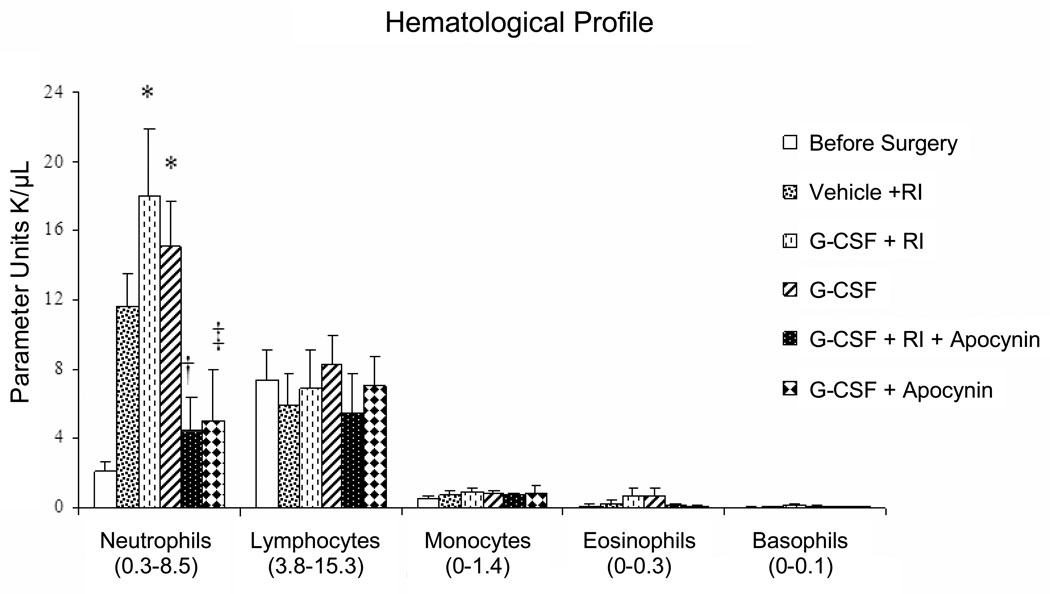
As shown in the haematological profile of groups (Fig. 1), G-CSF (100 µg/Kg/day) induced production of neutrophil without affecting numbers of circulating monocytic cells.
Figure 1.
Groups hematological profile. Normal range is expressed in U K/µL and is given in () below the cell type in the figure. Neutrophils number before surgery (2.13 ± 0.59); after RI+Vehicle (12.17 ± 2.36); RI+G-CSF (18.05 ± 3.84); G-CSF with no RI (15.12 ± 2.61). (n=6/group, means±SE, P<0.01). * vs vehicle; † vs G-CSF+RI; ‡ vs G-CSF No RI.
G-CSF induces Coronary Collateral Growth
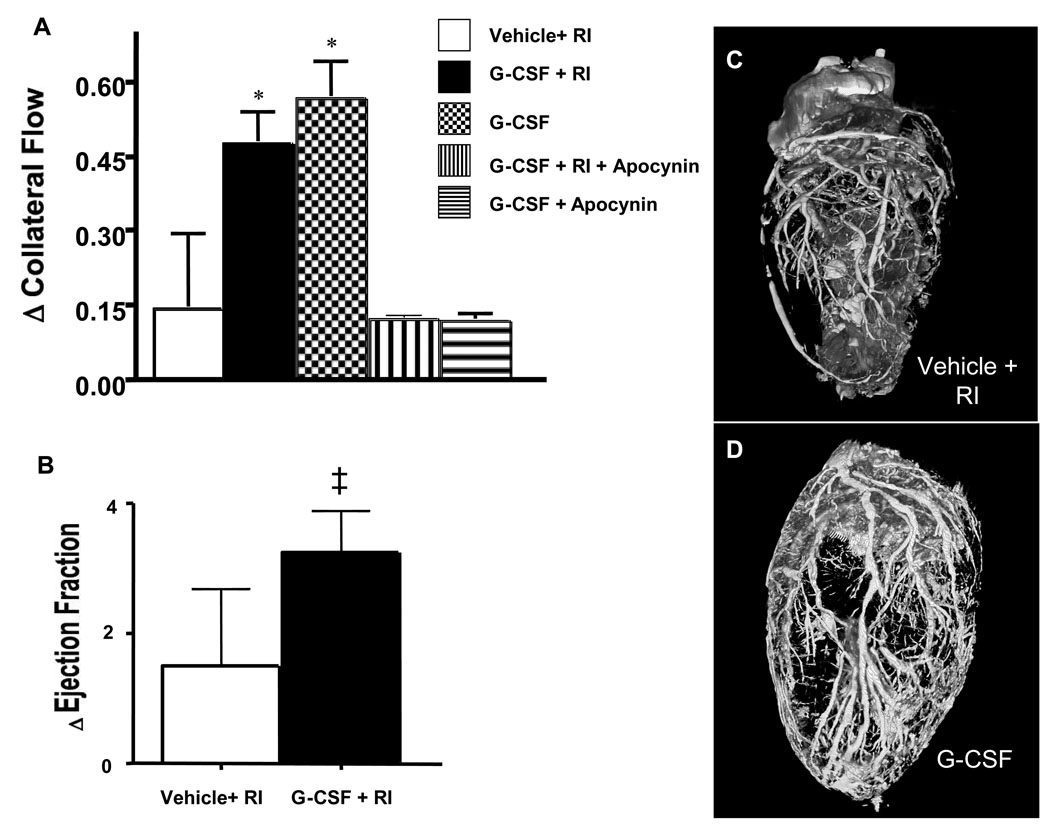
Following RI, G-CSF increased CCG (P<0.01, 0.47 ± 0.15 versus vehicle 0.14 ± 0.06). Surprisingly, G-CSF treatment without RI increased CCG (0.57 ± 0.18, P<0.01 vs vehicle) equal to G-CSF + RI (Figure 2, top). Furthermore, the increase in collateral flow induced by G-CSF translated to less of a reduction in ejection fraction (Figure 2B). Improvement in EF was noted in 1/4 vehicle + RI animals, but occurred in all (4/4) G-CSF+RI rats. The results of collateral flow and function were corroborated by the images of the coronary vasculature where more and larger vessels were observed in the G-CSF+RI hearts (2D) versus the untreated hearts (2C).
Figure 2.
A: Coronary Collateral Blood Flow (CBF) expressed as difference (delta) between relative collateral flows (collateral flow/normal zone flow) first and fifth day of RI protocol. Vehicle+RI (0.14±0.06); G-CSF+RI (0.47±0.15); G-CSF (0.57±0.18); G-CSF+ RI+Apocynin (0.09±0.01); G-CSF+Apocynin (0.09±0.02). n=6/group, *P<0.05 vs vehicle+RI. B: Change in ejection fraction (paired comparison between ejection fraction during a coronary occlusion prior to RI protocol and at Day 5 in RI+vehicle and G-CSF+RI groups). ‡ P<0.05 vs Vehicle+RI. C and D: Micro-CT images of coronary vasculature. Vehicle+RI (C) and G-CSF+RI (D).
G-CSF Induced ROS Production
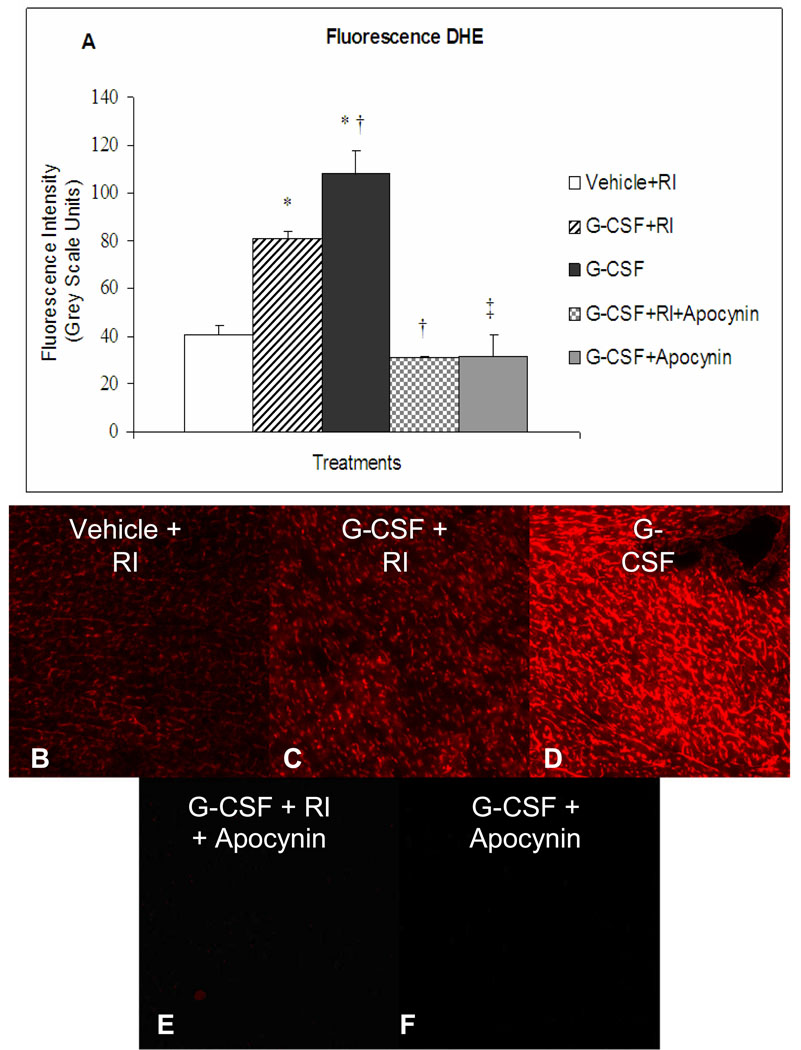
DHE fluorescence intensity was double in G-CSF with RI stimulation (P<0.01) (81.0 ± 2.6) versus vehicle+RI (40.6 ± 3.8). Unexpectedly, even higher levels were found in G-CSF without RI induction (P<0.01) (107.9 ± 9.6) (Fig. 3).
Figure 3.
A: Fluorescence intensity of Dihydroethidium (DHE) injected into the LV (60 µg/kg) before two consecutive periods of repetitive ischemia. Vehicle+RI (40.6±3.8) ; G-CSF+RI (81.0±2.6); G-CSF (107.9±9.6); G-CSF+RI+Apocynin (30.6±0.6); G-CSF+Apocynin (31.6±9.0). B–F: Representative images of DHE fluorescence (Magnification 10×). (n=3/group in 10 sections/heart, means±SE) *vs vehicle, † vs G-CSF+RI; ‡ vs G-CSF, P<0.01
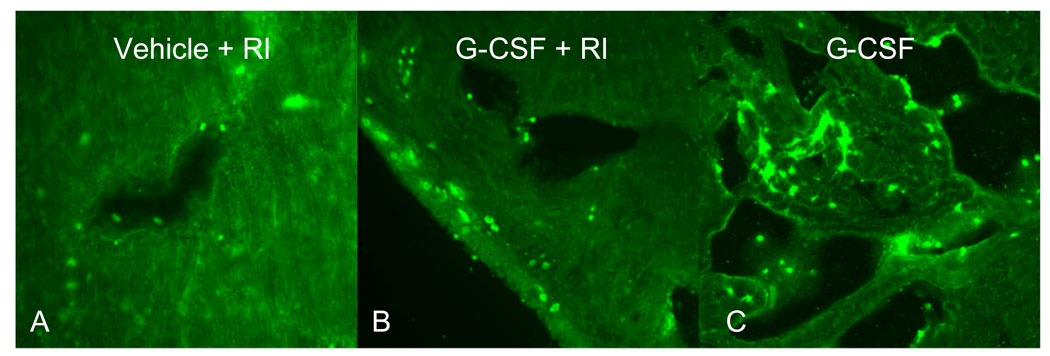
To determine the cell type responsible for the increased DHE signal, myeloperoxidase (MPO, a peroxidase enzyme present in neutrophils granulocytes) immunostaining was undertaken in heart sections of the treated groups (Fig. 4). Interestingly, the DHE signal did not co-localize with MPO, but appeared in cardiac myocytes.
Figure 4.
Immunostaining of Myeloperoxidase (MPO) using a specific antibody in the rat heart. Green dots represent granulocytes in the myocardium. Original magnification is 20× for A, 10× B and C. Data shown is representative of 3 separate experiments.
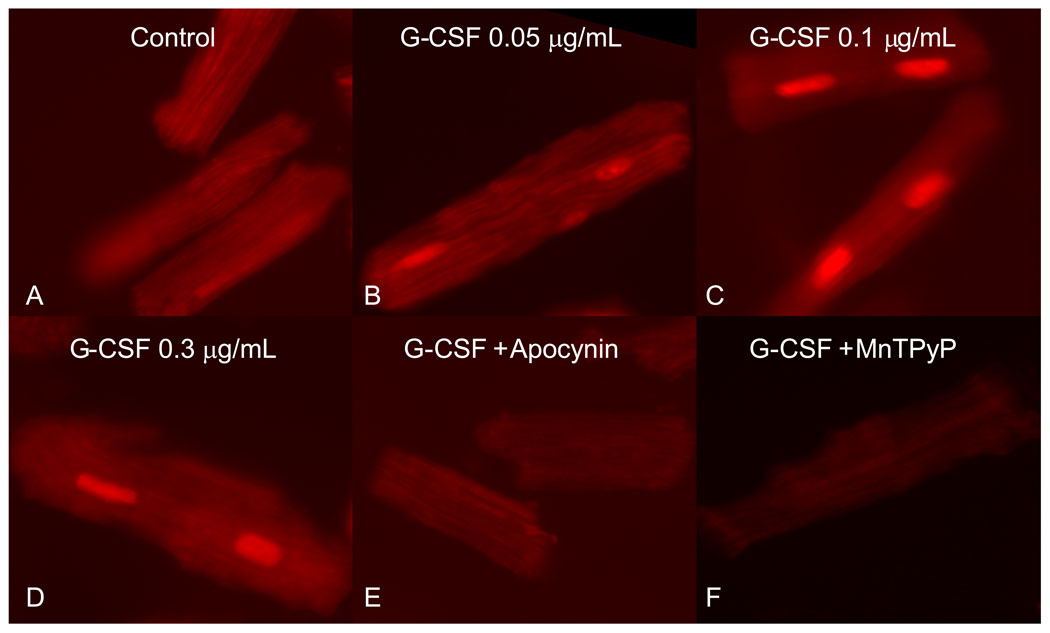
To clearly determine if G-CSF stimulates ROS production in cardiac myocytes, we studied isolated cardiac myocytes. The administration of different concentrations of G-CSF (0.05; 0.1; 0.3 µg/mL) revealed substantial increases in ROS production in comparison with untreated controls. Inhibition of NADPH oxidase by apocynin (300µM) or administration of the cell-permeable superoxide dismutase/catalase mimetic, MnTMPyP (100µM) totally abolished the DHE signal (Fig. 5). G-CSF did not increase DHE fluorescence in smooth muscle cells or endothelial cells (data not shown) suggesting that these cell types do not respond directly to G-CSF.
Figure 5.
Production of ROS by isolated cardiac myocytes revealed by DHE (5 µM). A: Control, not subjected to treatment. B–D: G-CSF in different concentrations (0.05, 0.1, and 0.3 µg/mL). E: G-CSF (0.3 µg/mL) + apocynin (300 µM, inhibitor NADPH oxidase) abolished DHE signal. F: G-CSF (0.3 µg/mL) + MnTMPyP (100µM, superoxide dismutase mimetic) abolished ROS production
To understand if the G-CSF stimulation of ROS in cardiac myocytes was critical for collateral growth, we additionally studied animals given apocynin and subjected to G-CSF. Apocynin prevented coronary collateral growth with or without repetitive occlusions: G-CSF+RI, 0.47± 0.15 vs Apocynin+G-CSF+RI, 0.09 ± 0.01 (P<0.01); G-CSF+No RI, 0.57 ± 0.18 vs Apocynin+G-CSF+No RI, 0.09 ± 0.02 (P<0.01, Fig. 2). Moreover, DHE fluorescence intensity was completely abolished by apocynin in both groups, G-CSF + RI (81.0 ± 2.6 vs 30.6 ± 0.6, P<0.01) and G-CSF+ No RI (107.9 ± 9.6 vs 31.6 ± 9.0, P<0.01) (Fig. 3).
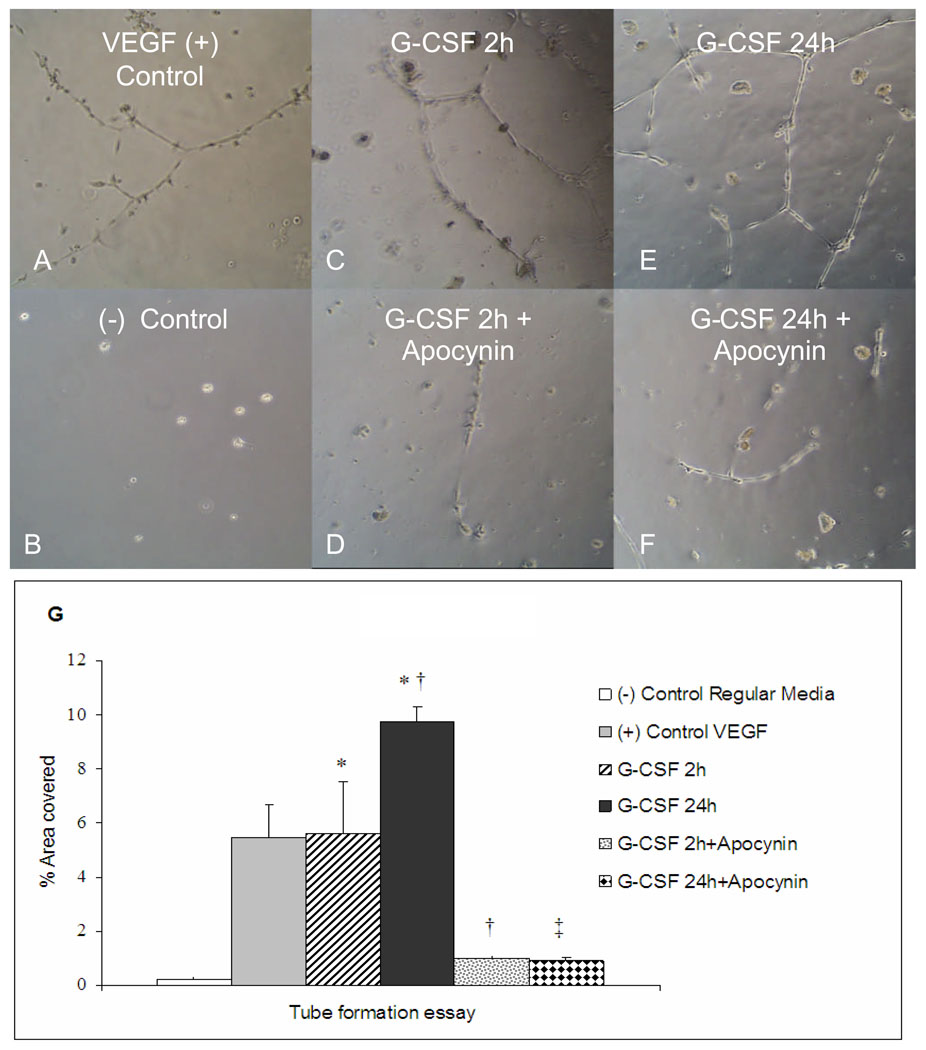
To clarify if cardiomyocytes stimulated by G-CSF would produce a medium rich in angiogenic factors that would promote vascular growth, tube formation assays were undertaken. Isolated cardiomyocytes were stimulated with G-CSF for different periods of time (2 and 24h). This medium was than removed and used to evaluate tube formation in HCAEC cultures. As seen in Fig. 6, 2h conditioned media from the G-CSF-treated cardiomyocytes promoted tube formation to similar values as VEGF positive control (5.6 ± 1.9 vs 5.5 ± 1.2, respectively). Furthermore, 24h of stimulation time further increases the percentage of area covered by new tubes (9.7 ± 0.5). Apocynin clearly abolishes tube formation in both periods of time in comparison with respective groups, 2h (1.0 ±0.1) and 24h (0.9 ± 0.1).
Figure 6.
Human coronary artery endothelial cell (HCAEC) tube formation in Matrigel in response to VEGF or to conditioned media from cardiac myocytes treated with G-CSF for 2 or 24 hrs. A: percentage of area covered with tubes (n=4/group, means±SE). * vs VEGF, † vs G-CSF 2h, ‡ vs G-CSF 24h, P <0.01). B–G: Images of tube formation after addition of G-CSF cardiomyocyte stimulation medium and apocynin (300 µM), as indicated.
Discussion
The major observation of our study is that G-CSF stimulates coronary collateral growth with or without repetitive ischemia. We also found that the improvement in collateral flow by G-CSF was functional in that it enhanced cardiac function during a coronary occlusion. The stimulation of collateral growth in the absence of ischemia was surprising as we had predicted that G-CSF in the absence of ischemia would not initiate coronary collateral growth. However, another observation helped us resolve the dilemma of how G-CSF would initiate collateral growth in the absence of ischemia. Specifically we found that administration of G-CSF increased ROS production in cardiac myocytes, which may mimic the well known event of enhanced ROS production during myocardial ischemia-reperfusion, suggesting that G-CSF to some extent may act as a surrogate for certain aspects of myocardial ischemia. Furthermore, we observed in isolated cardiac myocytes, that G-CSF-induced ROS production is associated with the stimulation of angiogenic factors and promotion of tube formation in HCAECs, which again mimics the actions of the ischemic heart13.
Our observations and conclusions are supported by some cogent work in the literature. In support of the present study, it has been reported that G-CSF has direct and acute protective effects on myocardium against ischemia-reperfusion injury10. It was shown that G-CSF acts directly on cardiomyocytes and induces survival signals in post-MI hearts 6. Here we show for the first time that G-CSF can promote coronary collateral growth, with or without ischemic stimuli. It was shown previously that augmentation in coronary collateral flow reveals an increase in the calibre of the collateral vessels11, therefore the reason for the flow to increase after G-CSF treatment in comparison with RI alone is through growth of these vessels.
Intriguingly, G-CSF in the absence of repetitive ischemia also enhanced the levels of coronary collateral growth, suggesting that the cytokine mimics certain effects of ischemia. Additional results shown in our study and in the literature bear upon this suggestion. We found that treatment of isolated myocytes with G-CSF stimulated the production of growth factors in a ROS-dependent manner. Ischemia-reperfusion is well known to produce a burst of ROS stimulating many adaptations of this ischemic heart including coronary collateral growth9, 14, 15. Furthermore, G-CSF was found to promote neovascularization by releasing vascular endothelial growth factor (VEGF) from neutrophils and bone marrow derived cells of hematopoietic lineage16,17. Toyota et al11 have revealed that VEGF is required for CCG. We opine that these factors are essential for the remodelling that occurs and the promotion of coronary collateral vessels by G-CSF. Zhu and colleagues18 have shown that G-CSF induces ROS production in peripheral blood cells and that G-CSF–induced ROS production is dependent on NADPH oxidase. It has also been demonstrated that coronary collateral development in this specific rat model of RI is critically dependent on an optimal concentration of ROS generated in the myocardium9. Accordingly we believe that redox-dependent signalling, mediated by the stimulation of ROS by G-CSF, initiated coronary collateral development. We also would like to emphasize that our study focussed on the effects of G-CSF in a normal animal without an existing level of oxidative stress, and whether this cytokine would stimulate coronary collateral growth in a model of oxidative stress, e.g., vascular disease, is unknown. We state this in view of our previous observations demonstrating that excessive amounts of ROS (oxidative stress) corrupt collateral growth, but moderate amounts stimulate collateral growth.9,19 Thus, we cannot predict with certainty if larger amounts of ROS would augment or corrupt collateral growth without knowledge of the basal redox state, or the amount of ROS that would be produced by the cytokine.
Although we initially thought that G- CSF would trigger neutrophils to the myocardium under a RI stimulus and that these neutrophils would be responsible for ROS production, we observed that G-CSF directly stimulated cardiomyocytes to generate ROS. Our studies of isolated cardiomyocytes clearly demonstrate G-CSF is acting on cardiomyocytes to produce ROS, and that this ROS generation is critical in the production of growth factors in response to G-CSF stimulation of myocytes. Furthermore, neither ECs or VSMCs responded to G-CSF with increased ROS production, suggesting that in the heart the cytokine targets cardiac myocytes. Indeed, others have suggested directs effects of G-CSF on the heart independent of granulocyte mobilization.20,21 G-CSF is also reported to have direct actions on endothelial cells, resulting in activation of p38 MAPK.22 Related to this we have recently reported a role for p38 MAPK in coronary collateral development,9 so we cannot unequivocally exclude this particular endothelial action of G-CSF in coronary collateral growth. Another possible action by which G-CSF could stimulate coronary collateral growth would be through mobilization of bone marrow stromal and progenitor cells.23–25 Specifically, these observations revealed that G-CSF stimulated angiogenesis and vascular growth in organ systems other than the heart and in tumors. This information may be important in the context of our previous report where multi-potent stromal cells from bone marrow amplified coronary collateral growth.26
Our results are also consistent with the conclusion that G-CSF-mediated induction of cardiomyocyte ROS is dependent on NADPH oxidase, since apocynin, an inhibitor of NADPH oxidase assembly, cancelled the effect promoted by G-CSF both in vivo and in vitro. We are also compelled to think the mechanism by which G-CSF is acting is similar to the one revealed in neutrophils by Zhu et al18, where G-CSF induces ROS production via NADPH oxidase. Further work is needed to verify this mechanism in cardiomyocytes.
In conclusion, we demonstrate the induction of coronary collateral growth by G-CSF, which is mediated by ROS directly produced in cardiomyocytes. Our results offer the hypothesis that G-CSF may be acting as a surrogate for myocardial ischemia in the production of coronary collateral growth.
Acknowledgements
Sources of Funding: This work was supported by Marie-Curie Actions (Carrão, A.C.R.), Boehringer Ingelheim Fonds (Carrão, A.C.R.) and the US Public Health Service (NIH grants HL32788 and HL34286).
We would like to thank Dr. Clemens Gruber for critical reading of the manuscript and Karen Greene for administrative assistance.
Footnotes
Disclosures: The authors have no conflicts or relationships to disclose.
References
- 1.Nagata S, Tsuchiya M, Asano S, Kaziro Y, Yamazaki T, Yamamoto O, Hirata Y, Kubota N, Oheda M, Nomura H, Ono M. Molecular cloning and expression of cDNA for human granulocyte colony-stimulating factor. Nature. 1986;319:415–418. doi: 10.1038/319415a0. [DOI] [PubMed] [Google Scholar]
- 2.Avalos B. Molecular analysis of the granulocyte colony-stimulating factor receptor. Blood. 1996;88:761–777. [PubMed] [Google Scholar]
- 3.Basu S, Hodgson G, Katz M, Dunn AR. Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood. 2002;100:854–861. doi: 10.1182/blood.v100.3.854. [DOI] [PubMed] [Google Scholar]
- 4.Fukunaga R, Ishizaka-Ikeda E, Seto Y, Nagata S. Expression cloning of a receptor for murine granulocyte colony-stimulating factor. Cell Res. 1990;61:341–350. doi: 10.1016/0092-8674(90)90814-u. [DOI] [PubMed] [Google Scholar]
- 5.Calhoun DA, Donnelly WH, Jr, Du Y, Dame JB, Li Y, Christensen RD. Distribution of granulocyte colony-stimulating factor (G-CSF) and G-CSF-receptor mRNA and protein in the human fetus. Pediatr Res. 1999;46:333–338. doi: 10.1203/00006450-199909000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Harada MQY, Takano H, Minamino T, Zou Y, Toko H, Ohtsuka M, Matsuura K, Sano M, Nishi J, Iwanaga K, Akazawa H, Kunieda T, Zhu W, Hasegawa H, Kunisada K, Nagai T, Nakaya H, Yamauchi-Takihara K, Komuro I. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat Med. 2005;11:305–311. doi: 10.1038/nm1199. [DOI] [PubMed] [Google Scholar]
- 7.Sattler M, Verma S, Shrikhande G, Byrne CH, Pride YB, Winkler T, Greenfield EA, Salgia R, Griffin JD. The BCR/ABL tyrosine kinase induces production of reactive oxygen species in hematopoietic cells. J Biol Chem. 2000;275:24273–24278. doi: 10.1074/jbc.M002094200. [DOI] [PubMed] [Google Scholar]
- 8.Bedard K, Krause K-H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 9.Rocic P, Kolz C, Reed R, Potter B, Chilian WM. Optimal reactive oxygen species concentration and p38 MAP kinase are required for coronary collateral growth. Am J Physiol Heart Circ Physiol. 2007;292:H2729–H2736. doi: 10.1152/ajpheart.01330.2006. [DOI] [PubMed] [Google Scholar]
- 10.Ueda KTH, Hasegawa H, Niitsuma Y, Qin Y, Ohtsuka M, Komuro I. Granulocyte colony stimulating factor directly inhibits myocardial ischemia-reperfusion injury through Akt-endothelial NO synthase pathway. Arterioscler Thromb Vasc Biol. 2006;26:e108–e113. doi: 10.1161/01.ATV.0000219697.99134.10. [DOI] [PubMed] [Google Scholar]
- 11.Toyota EFK, Ogasawara Y, Kajita T, Shigeto F, Matsumoto T, Goto M, Kajiya F. Dynamic changes in three-dimensional architecture and vascular volume of transmural coronary microvasculature between diastolic- and systolic-arrested rat hearts. Circulation. 2002;105:621–626. doi: 10.1161/hc0502.102964. [DOI] [PubMed] [Google Scholar]
- 12.Spaan JAtWR, van Teeffelen JW, Streekstra G, Siebes M, Kolyva C, Vink H, Fokkema DS, VanBavel E. Visualisation of intramural coronary vasculature by an imaging cryomicrotome suggests compartmentalisation of myocardial perfusion areas. Med Biol Eng Comput. 2005;43:431–435. doi: 10.1007/BF02344722. [DOI] [PubMed] [Google Scholar]
- 13.Weihrauch D, Tessmer J, Warltier DC, Chilian WM. Repetitive coronary artery occlusions induce release of growth factors into the myocardial interstitium. Am J Physiol. 1998;275:H969–H976. doi: 10.1152/ajpheart.1998.275.3.H969. [DOI] [PubMed] [Google Scholar]
- 14.Hanna IR, Taniyama Y, Szocs K, Rocic P, Griendling KK. NAD(P)H oxidase-derived reactive oxygen species as mediators of angiotensin II signaling. Antioxid Redox Signal. 2002;4:899–914. doi: 10.1089/152308602762197443. [DOI] [PubMed] [Google Scholar]
- 15.Koneru S, Penumathsa SV, Thirunavukkarasu M, Samuel SM, Zhan L, Han Z, Maulik G, Das DK, Maulik N. Redox regulation of ischemic preconditioning is mediated by the differential activation of caveolins and their association with eNOS and GLUT-4. Am J Physiol Heart Circ Physiol. 2007;292:H2060–H2072. doi: 10.1152/ajpheart.01169.2006. [DOI] [PubMed] [Google Scholar]
- 16.Ohki YHB, Sato Y, Akiyama H, Zhu Z, Hicklin DJ, Shimada K, Ogawa H, Daida H, Hattori K, Ohsaka A. Granulocyte colony-stimulating factor promotes neovascularization by releasing vascular endothelial growth factor from neutrophils. FASEB J. 2005;19:2005–2007. doi: 10.1096/fj.04-3496fje. [DOI] [PubMed] [Google Scholar]
- 17.Powell TMPJ, Hill JM, Thompson M, Benjamin M, Rodrigo M, McCoy JP, Read EJ, Khuu HM, Leitman SF, Finkel T, Cannon RO., 3rd Granulocyte colony-stimulating factor mobilizes functional endothelial progenitor cells in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2005;25:296–301. doi: 10.1161/01.ATV.0000151690.43777.e4. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Q-s, Xia L, Mills GB, Lowell CA, Touw IP, Corey SJ. G-CSF induced reactive oxygen species involves Lyn-PI3-kinase-Akt and contributes to myeloid cell growth. Blood. 2006;107:1847–1856. doi: 10.1182/blood-2005-04-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed R, Kolz C, Potter B, Rocic P. The mechanistic basis for the disparate effects of angiotensin II on coronary collateral growth. Arterioscler Thromb Vasc Biol. 2008;28:61–67. doi: 10.1161/ATVBAHA.107.154294. [DOI] [PubMed] [Google Scholar]
- 20.Ueda K, Takano H, Hasegawa H, Niitsuma Y, Qin Y, Ohtsuka M, Komuro I. Granulocyte colony stimulating factor directly inhibits myocardial ischemia-reperfusion injury through Akt-endothelial NO synthase pathway. Arterioscler Thromb Vasc Biol. 2006;26:e108–e113. doi: 10.1161/01.ATV.0000219697.99134.10. [DOI] [PubMed] [Google Scholar]
- 21.Harada M, Qin Y, Takano H, Minamino T, Zou Y, Toko H, Ohtsuka M, Matsuura K, Sano M, Nishi J, Iwanaga K, Akazawa H, Kunieda T, Zhu W, Hasegawa H, Kunisada K, Nagai T, Nakaya H, Yamauchi-Takihara K, Komuro I. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat Med. 2005;11:305–311. doi: 10.1038/nm1199. [DOI] [PubMed] [Google Scholar]
- 22.Fuste B, Mazzara R, Escolar G, Merino A, Ordinas A, Diaz-Ricart M. Granulocyte colony-stimulating factor increases expression of adhesion receptors on endothelial cells through activation of p38 MAPK. Haematologica. 2004;89:578–585. [PubMed] [Google Scholar]
- 23.Takagi Y, Omura T, Yoshiyama M, Matsumoto R, Enomoto S, Kusuyama T, Nishiya D, Akioka K, Iwao H, Takeuchi K, Yoshikawa J. Granulocyte-colony stimulating factor augments neovascularization induced by bone marrow transplantation in rat hindlimb ischemia. J Pharmacol Sci. 2005;99:45–51. doi: 10.1254/jphs.fp0040966. [DOI] [PubMed] [Google Scholar]
- 24.Ohki Y, Heissig B, Sato Y, Akiyama H, Zhu Z, Hicklin DJ, Shimada K, Ogawa H, Daida H, Hattori K, Ohsaka A. Granulocyte colony-stimulating factor promotes neovascularization by releasing vascular endothelial growth factor from neutrophils. Faseb J. 2005;19:2005–2007. doi: 10.1096/fj.04-3496fje. [DOI] [PubMed] [Google Scholar]
- 25.Okazaki T, Ebihara S, Asada M, Kanda A, Sasaki H, Yamaya M. Granulocyte colony-stimulating factor promotes tumor angiogenesis via increasing circulating endothelial progenitor cells and Gr1+CD11b+ cells in cancer animal models. Int Immunol. 2006;18:1–9. doi: 10.1093/intimm/dxh334. [DOI] [PubMed] [Google Scholar]
- 26.Belmadani S, Matrougui K, Kolz C, Pung YF, Palen D, Prockop DJ, Chilian WM. Amplification of Coronary Arteriogenic Capacity of Multipotent Stromal Cells by Epidermal Growth Factor. Arterioscler Thromb Vasc Biol. 2009 doi: 10.1161/ATVBAHA.109.186189. [DOI] [PMC free article] [PubMed] [Google Scholar]