Abstract
Melanin concentrating hormone (MCH) has been implicated in many brain functions and behaviors essential to the survival of animals. The hypothalamus is one of the primary targets where MCH-containing nerve fibers and MCH receptors are extensively expressed and its actions in the brain are exerted. Since the identification of MCH receptors as orphan G protein coupled receptors, the cellular effects of MCH have been revealed in many non-neuronal expression systems (including Xenopus oocytes and cell lines), however, the mechanism by which MCH modulates the activity in the neuronal circuitry of the brain is still under investigation. This review summarizes our current knowledge of electrophysiological effects of MCH on neurons in the hypothalamus, particularly in the lateral hypothalamus. Generally, MCH exerts inhibitory effects on neurons in this structure and may serve as a homeostatic regulator in the lateral hypothalamic area. Given the contrast between the limited data on cellular functions of MCH in the hypothalamus versus a fast growing body of evidence on the vital role of MCH in animal behavior, further investigations of the former are warranted.
Keywords: Synaptic transmission, Ion channel, Action potential, Hypocretin/orexin
1. Introduction
Melanin concentrating hormone (MCH) is a cyclic 17-amino-acid peptide in fish and 19-amino-acid peptide in mammals. It was first isolated from salmon pituitary glands and found to be a regulator of skin color in teleost fish [49]. In mammals, MCH is synthesized in the central nervous system (CNS) mainly by neurons in the lateral hypothalamus (LH) [45]. The expression of the MCH system begins during the late embryo and early postnatal period [9, 63]. MCH-containing efferent axons innervate a wide variety of regions in the CNS from the cortex to the spinal cord [8, 45, 61]. Strikingly, the highest density of MCH axons and boutons is found in the LH [8]. The receptor for MCH has been determined to be the orphan receptor SLC-1 [3, 11, 31, 53], which has a wide distribution throughout the CNS, like the peptide [29, 31, 54], suggesting that MCH may modulate a number of systems in the CNS. In addition, a subtype of the MCH receptor, Slt (or MCHR2), was cloned in humans as well [19, 40].
Currently, MCH is found to be involved in a large number of physiological functions. In lower vertebrates such as reptiles, amphibians, and fish, MCH modulates pigmentation [18, 28]. In fish, MCH plays a strong neuroendocrine role in the inhibition of corticotropin releasing hormone [4, 18]. In mammals, MCH was reported to modify memory retention [39], and to participate in the regulation of the reproductive axis [65] and sleep [25]. Recently, intestinal inflammation has been reported to be mediated by MCH as well [30]. However, one of the most noteworthy functions of MCH that has gathered considerable attention in recent years is its involvement in the regulation of food intake and energy homeostasis. Intracerebral, intraventricular and local administration of MCH directly into the arcuate, paraventricular, or dorsomedial nucleus evokes feeding or elevates food intake in rodents [1, 13, 16, 48, 52]. A chronic infusion of MCH significantly increases food intake, body weight, white adipose tissue (WAT) mass, and liver mass in mice [27]. In addition, chronic activation of MCH receptor-1 (MCH-R1) results in hyperphagia, body weight gain and elevated levels of insulin and leptin [57]. Furthermore, obesity and resistance to insulin have been observed in transgenic mice that overexpress MCH in the LH [34]. There is also evidence that MCH may be involved in positive energy balance promoted by glucocorticoids and hyperphagia induced by lactation in rats [17, 62]. Conversely, MCH-deficient mice have reduced body weight and are lean due to decreased feeding and an enhanced metabolism [58]. The inhibition of MCH neurons contributes to estrogen-induced weight loss [41, 44], and antagonism of MCHR1 leads to sustained reductions in food intake, body weight gain and body fat gain [57]. Consistent with the evidence of a role of MCH in positive energy balance, c-fos expression in MCH neurons is upregulated by starvation and the expression of MCH mRNA is increased by fasting or leptin administration, and in pro-opiomelanocortin (POMC) deficient, obese mice [10, 26, 48]. Long-term fasting elevates MCH mRNA levels in the lateral hypothalamus in rats as well [7].
Despite the wide distribution of MCH-containing nerve fibers and MCH receptors in the CNS as well as multiple facets of MCH-mediated functions and behaviors in animals, the cellular functions of MCH in the brain still remain to be established, particularly in the hypothalamus, where the peptide is synthesized and its primary targets exist. Since MCH-containing neurons are partially GABAergic and GABA may be co-released with MCH in the brain [24, 36], it is clear that the effects of activation of MCH neurons on animal behaviors include contributions from other neurotransmitters and neuromodulators. Therefore, acquiring knowledge of the cellular actions of MCH would, alone, greatly substantiate a role of this peptide in various biological functions and pathological processes in animals.
2. MCH acts as an inhibitory peptide in the hypothalamus
Exactly how MCH exerts its functions at the cellular level in the brain is still under investigation. Two types of receptors (MCH-R1 and MCH-R2) have been cloned in humans, while only one type (MCH-R1) exists in rodents. Since rodents provide a vital avenue for experimental investigations, the MCH-R1 has been studied extensively and our current understanding of the mechanism of MCH-mediated functions is based on these studies.
2.1 Electrophysiological effects of MCH in non-neuronal cell lines
The MCH-R1 (SLC-1) was originally demonstrated to couple to certain G protein pathways in non-neuronal expression systems such as Xenopus oocytes, HEK, and CHO cell lines. MCH induces an intracellular calcium increase and depresses forskolin-induced accumulation of cAMP in these cells [6, 11, 53, 59]. When SLC-1 receptor and G protein-gated inwardly rectifying potassium channels (GIRKs) were co-expressed in Xenopus oocytes, the application of MCH induced strong GIRK currents in a dose-dependent manner [6]. In addition, calcium-induced chloride currents mediated by the activation of the phospholipase C pathway could also be induced in Xenopus oocytes expressing SLC-1 receptor [6]. These results provided the initial clues that MCH could exert its functions by modulating the activity in neurons through coupling to ion channels gated by different G protein (Gi, Go or Gq) pathways.
2.2 Electrophysiological effects of MCH in primary neuronal cultures from the hypothalamus
Although investigations on MCH receptors in non-neuronal expression systems have provided substantial evidence of downstream signaling pathways and associated proteins modulating the functionality of MCH receptors as indicated in the most recent studies [19, 20, 37, 42], it remains to be determined just what effects the activation of MCH receptors mediate in neurons in the CNS, particularly in brain areas responsible for energy homeostasis. In non-neuronal expression systems, cellular responses mediated by MCH receptors vary from inhibitory (i.e., inhibition of cAMP mobilization, induction of GIRK currents) to excitatory effects (i.e., enhancement of intracellular calcium mobilization) depending upon the G protein coupled pathways that are available in these non-neuronal cells [6, 11, 53, 59]. These multiple facets of MCH-mediated effects have not been reported in neurons in situ. However, it may be possible that different G protein-mediated pathways underlie the functions of MCH in various brain areas. Based on this rationale, the examination of cellular functions of MCH in primary neuronal cultures from the hypothalamus was the first step in decoding the cellular functionality of this peptide in the brain [21].
Consistent with the results from non-neuronal expression systems, this first piece of evidence implied that MCH might be inhibitory in neurons from the CNS. First, it was found that the application of MCH significantly inhibits the frequency of spontaneous action potentials in cultured neurons from the LH [21]. This effect is not due to the direct inhibition of LH neurons by MCH, since it does not induce hyperpolarization and changes in membrane conductance in LH neurons. Instead, our data indicate that MCH depresses glutamatergic and GABAergic synaptic transmissions in cultured neurons from the LH [21]. Specifically, MCH depresses both frequency and amplitude of miniature excitatory postsynaptic currents in LH neurons, suggesting that MCH participates in the modulation of glutamate release from presynaptic terminals and postsynaptic glutamate receptors [21]. In addition to its effects at synapses on neurons from the LH, MCH also directly modulates the activity at cell bodies of LH neurons by inhibiting voltage-dependent ion channels in cultured neurons and slices [21, 22]. Voltage-dependent calcium channels (VDCCs) are important membrane proteins responsible for excitation-triggered calcium influx in neurons [14]; the increase in intracellular calcium concentration through the opening of VDCCs serves as a vital signal in the functionality of neurons [14, 46]. It has been shown that MCH attenuates the amplitude of whole cell VDCCs in a dose-dependent manner in cultured LH neurons [22]. The IC50 is 7.84 nM, close to the results reported in non-neuronal cells [6, 11, 53]. Three subtypes of VDCCs (L, N and P/Q) identified at cell bodies of LH neurons are inhibited by MCH. The depletion or inhibition of the Gi/o -mediated pathway abolishes MCH-induced effects on VDCCs in LH neurons. It should be noted that MCH does not induce significant GIRK currents in cultured LH neurons, in which GIRK currents can be easily activated by the GABAB receptor agonist, baclofen [21]. This result implies that MCH receptors do not couple with GIRK currents in these neurons. Recently, additional evidence strengthened the case for an inhibitory role of MCH at the cellular level. MCH inhibits the rise in cAMP induced by the β-adrenergic receptor agonist, isoproterenol in HEK-293 cell lines [47]. The coupling of MCH-R1 to the Gq pathway with a lower affinity than to the Gi pathway has been reported in non-neuronal cell lines [11, 53], but has not been reported in CNS neurons to date.
2.3 Electrophysiological effects of MCH in the medial and paraventricular hypothalamus
MCH is exclusively synthesized in the LH and an extensive innervation by MCH-containing nerve fibers has been identified in the hypothalamus [8]. As a vital structure responsible for the regulation of energy homeostasis, the hypothalamus is suspected to be one of the primary targets of MCH. In brain slices from rats, MCH induces bi-directional responses in spontaneous action potentials in neurons in the ventromedial hypothalamus (VMH) and arcuate nucleus (ARC) [15]. In ad lib fed and fasted rats, MCH both increases and decreases the frequency of action potentials in the VMH and ARC neurons. In overweight and hyperphagic rats, however, MCH mainly increases the firing of action potentials in ARC neurons and attenuates the frequency of action potentials in VMH neurons [15]. Although the neuronal types that respond to MCH and the mechanisms underlying MCH-mediated effects were not identified in this study, it is implied that MCH may regulate neurons responsible for energy homeostasis depending on the nutritional status of the animal. In another study performed by the same group, MCH was shown to inhibit the frequency of action potentials in paraventricular neurons in overweight rats [15]. Considering the role of MCH in energy homeostasis, further examination of the effects of MCH on various neuronal types in these areas is required.
3. Inhibitory effects of MCH on hypocretin neurons at the cellular level in the LH
In the LH, the neuronal populations that synthesize hypocretin/orexin and MCH do not overlap, but generally innervate the same targets in the brain [2] (Figure 1). An accumulating body of evidence indicates that the hypocretin/orexin and MCH systems exert synergistic or antagonistic effects on their common targets in the regulation of energy homeostasis and the sleep/wake cycle [12, 25, 33, 38, 48, 55]. Therefore, it is essential to address the interaction that occurs between the hypocretin/orexin and MCH systems in the LH. In addition, studies in non-neuronal cells have yielded results fundamental to the understanding of signaling pathways downstream to the activation of MCH receptors and the modulation of MCH receptors, themselves [19]. However, since MCH receptors couple to various G protein-mediated pathways available in these cell types, the responses induced by MCH are diverse and cannot account for those occurring in neurons in situ. Although cultured central neurons provide a powerful tool in the characterization of the cellular effects of MCH in the brain as compared with non-neuronal expression systems, the pitfall in this approach is obvious. First, the original connectivity between neurons of interest and their afferent and efferent partners has been lost. Second, the unique neurochemical environment in which neurons of interest reside is absent when these neurons are maintained in culture media. In our recent studies, the effects of MCH in acute brain slices from the LH were examined, in which local circuits centered on neurons of interest and the unique neurochemical environment closely resembling in vivo conditions were preserved [51]. Most importantly, by using transgenic mice expressing green fluorescent protein (GFP) exclusively in hypocretin/orexin neurons, we can examine the cellular effects of MCH on these neurons essential to many brain functions including energy homeostasis, the sleep/wake cycle, stress response, reward and addiction, etc.
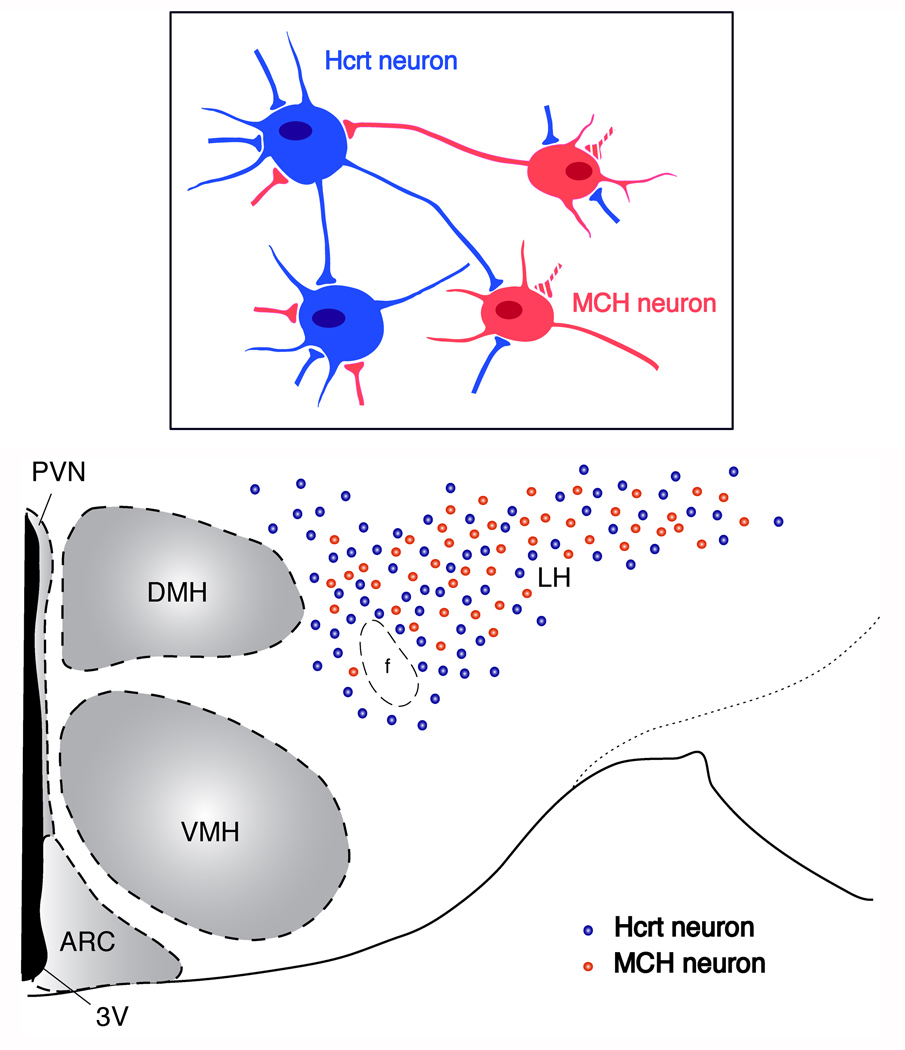
Fig. 1. Schematic representation of localization of hypocretin/orexin (Hcrt)- and MCH-containing neurons in the LH.
Cell bodies of hypocretin/orexin- (blue dots) and MCH-containing (red dots) neurons are mainly localized in the perifornical-lateral hypothalamic area. These two groups of neurons intermingle but do not overlap. Reciprocal synaptic innervations between hypocretin/orexin and MCH neurons are reported as shown in the box. Synaptic contacts among hypocretin/orexin neurons (solid blue boutons on hypocretin/orexin neurons) and among MCH neurons (broken red boutons on MCH neurons) have been reported. Abbreviations: 3V, the third ventricle; PVN, periventricular nucleus; ARC, arcuate nucleus; DMH, dorsomedial hypothalamus; VMH, ventromedial hypothalamus; LH, lateral hypothalamus; f, fornix.
3.1 Up-regulated excitatory inputs onto hypocretin/orexin neurons in the LH in mice with a deficiency in MCHR1
Studies on mice with a deficiency in MCH or its receptors have revealed important implications for a role of this peptide in energy homeostasis, depression, sleep/wake regulation and drug addiction [35, 48, 60]. It has been reported that mice deficient in MCHR1 are hyperphagic, lean, hyperactive, and sensitive to addictive drugs such as amphetamine [35, 60], and that the mesolimbic dopaminergic system is over-sensitive in one strain of KO mice [60]. Recent studies using a different strain of MCHR1 KO mice suggest that the hypocretin/orexin system in the LH is up-regulated in these mice [35, 51]. Compared to their wild type counterparts, MCHR1 KO mice show sensitization to modafinil-mediated arousal-promoting effects. Modafinil (diphenylmethyl-sulfonyl-2-acetamide) is approved by the FDA as a treatment for narcolepsy and many other conditions [5, 43]. Its administration to rats increases c-fos expression in the hypocretin/orexin neurons [56]. Recent results show that modafinil induces synaptic plasticity in hypocretin/orexin neurons, which may underlie its arousal-promoting effects in the brain [50].
The application of modafinil at a low dose (10 mg/kg) induces a significant increase in locomotor activity in the light phase in MCHR1 KO mice but not in WT mice [51]. The sensitization to modafinil at a sub-threshold dose in MCHR1 KO mice is due to an enhanced generation of action potentials in hypocretin/orexin neurons by arousal-promoting molecules, specifically, hypocretin/orexin itself! Since hypocretin/orexin induces action potentials in neurons that synthesize it by increasing the release of glutamate from excitatory synapses on these neurons (a positive feedback) [32], the basal synaptic parameters of glutamatergic synapses on hypocretin/orexin neurons in MCHR1 KO and WT mice were examined [51]. An up-regulation of postsynaptic parameters of glutamatergic synapses on hypocretin/orexin neurons was seen, which includes an enhanced mEPSC amplitude and AMPAR/NMDAR ratio [51]. In addition, the glutamatergic terminals on hypocretin/orexin neurons are sensitive to agonists of D1 dopamine receptor. Taken together, these results suggest that the depletion of the MCH-mediated signaling pathway leads to an up-regulation of glutamatergic synaptic transmission onto hypocretin/orexin neurons, which may be the foundation of the reported behavioral phenotypes seen in MCHR1 KO mice including hyperactivity, an enhanced metabolic rate, sensitivity to amphetamine and modafinil, etc. [35, 51, 60].
3.2 MCH inhibits synaptic transmission in hypocretin neurons
The results collected in MCHR1 KO mice imply that MCH may exert inhibitory effects in the LH in animals with an intact MCH system; the up-regulation of glutamatergic synaptic transmission onto hypocretin/orexin neurons might be the consequence of the removal of MCH-mediated signaling [51]. This hypothesis was tested in hypocretin/orexin neurons in brain slices [51]. In contrast to previous results observed in cultured LH neurons, MCH does not have any effect on the basal spike frequency in hypocretin/orexin neurons; but, rather, after the frequency of spikes has been enhanced by the application of hypocretin-1 to the recorded slices, subsequent application of MCH significantly attenuates the enhanced spike frequency, which is dose dependent and abolished in MCHR1 KO mice [51]. Pre-treatment of brain slices with pertussis toxin (PTX) eliminates MCH-mediated inhibition of spike frequency in hypocretin/orexin neurons, suggesting a mechanism mediated by the Gi/o pathway. Consistent with these results, MCH does not inhibit the basal frequency of mEPSCs in hypocretin/orexin neurons; after the frequency of mEPSCs has been potentiated by hypocretin-1, subsequent application of MCH decreases the frequency of mEPSCs in the presence of hypocretin-1 [51]. This result is consistent with the findings that MCH inhibits glutamate transmission onto NTS neurons [66]. Taken together, these results suggest that in brain slices, MCH attenuates the synaptic efficacy of glutamatergic synapses on hypocretin/orexin neurons presynaptically. It is implied that the inhibitory effects of MCH on the synaptic transmission in hypocretin/orexin neurons may be activity-dependent.
Although the exact mechanisms underlying the activity-dependent inhibition of synaptic efficacy in hypocretin/orexin neurons is not clear, the functional implication is both intriguing and important to the function of the LH. Since reciprocal innervations exist between hypocretin/orexin and MCH neurons in the LH and MCH neurons are activated by hypocretin/orexin both directly and indirectly [23, 64] (Figure 1, box), MCH-induced activity-dependent inhibition of hypocretin/orexin neurons may serve as a homeostatic regulator of hypocretin/orexin neurons [51]. It has been proposed that the crosstalk between the hypocretin/orexin and MCH systems in the LH offers “checks and balances” to maintain sufficient excitability necessary for the LH to function and to prevent over-excitation from leading to a compromised balance between these two systems (Figure 2). This balance may determine a set point for functions and behaviors governed by the LH.
Fig. 2. Schematic illustration of the interaction between hypocretin/orexin (HCRT) and MCH neurons in the LH.
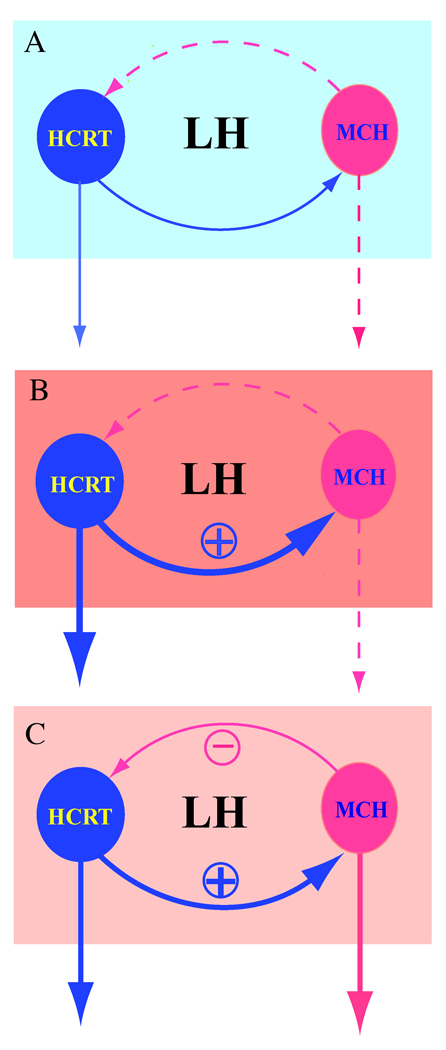
Hypocretin/orexin acts as the major excitatory force in the LH since it may increase excitation in both the hypocretin/orexin and MCH neurons, while MCH works as a feedback regulator. A, under resting conditions, MCH does not inhibit (dashed line) baseline action potential generation and synaptic transmission in hypocretin/orexin neurons (solid line), which may permit the easy activation of hypocretin/orexin neurons. B, intensive activity in hypocretin/orexin neurons resulting from stimulatory inputs leads to an excitatory effect of hypocretin/orexin on MCH neurons (solid line). C, MCH exerts its inhibitory effects on hypocretin/orexin neurons when the excitation of the hypocretin/orexin system reaches a certain level (solid line), thus fine-tuning the final output of the hypocretin/orexin neurons (solid line). It is hypothesized that MCH may work as a homeostatic regulator in such a system of “checks and balances” to maintain the excitability necessary for the brain to function and to prevent over-excitation that might compromise delicately controlled behaviors.
4. Conclusion
Since the identification of MCH receptors as orphan G protein coupled receptors a decade ago, the progress in understanding the cellular effects of MCH in the CNS has lagged far behind the understanding of its role in various brain functions and behaviors. In non-neuronal expression systems, MCH expresses a wide range of cellular actions from enhancement of intracellular calcium mobilization to activation of inhibitory GIRK currents depending on the availability of various G protein-mediated pathways (i.e., Gi, Go and Gq pathways) in these cells [6, 11, 53, 59]. In CNS neurons in primary cultures and brain slices, MCH exerts inhibitory effects by activation of the Gi/o pathway [21, 22, 51]. It is important to note that the mode of action is different between neurons in primary cultures and in acute slices from the hypothalamus, suggesting that the neurochemical environment of the hypothalamic neurons is crucial to MCH-mediated effects as well [21, 22, 51]. The results from our and other laboratories summarized here do not fully address the mechanisms underlying the electrophysiological responses of neurons to MCH in the hypothalamus; instead, many more intriguing and critical questions have been raised. Future investigations should focus on identifying signaling pathways downstream to MCH receptors and associated proteins controlling the desensitization/internalization of MCH receptors in situ in the hypothalamic neurons.
Acknowledgements
This work is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Health (NIH) (grant DK 070723). The author would like to thank Ms. Marya Shanabrough and Mr. Alex H Wang for assistance in preparing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abbott CR, Kennedy AR, Wren AM, Rossi M, Murphy KG, Seal LJ, Todd JF, Ghatei MA, Small CJ, Bloom SR. Identification of hypothalamic nuclei involved in the orexigenic effect of melanin-concentrating hormone. Eur J Pharmacol. 2003;475:37–47. doi: 10.1210/en.2003-0149. [DOI] [PubMed] [Google Scholar]
- 2.Adamantidis A, de Lecea L. Physiological arousal: a role for hypothalamic systems. Cell Mol Life Sci. 2008;65:1475–1488. doi: 10.1007/s00018-008-7521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Audinot V, Beauverger P, Lahaye C, Suply T, Rodriguez M, Ouvry C, Lamamy V, Imbert J, Rique H, Nahon JL, Galizzi JP, Canet E, Levens N, Fauchere JL, Boutin JA. Structure-activity relationship studies of melanin-concentrating hormone (MCH)-related peptide ligands at SLC-1, the human MCH receptor. J Biol Chem. 2001;276:13554–13562. doi: 10.1074/jbc.M010727200. [DOI] [PubMed] [Google Scholar]
- 4.Baker BI, Eberle AN, Baumann JB, Siegrist W, Girard J. Effect of melanin concentrating hormone on pigment and adrenal cells in vitro. Peptides. 1985;6:1125–1130. doi: 10.1016/0196-9781(85)90438-3. [DOI] [PubMed] [Google Scholar]
- 5.Ballon JS, Feifel D. A systematic review of modafinil: Potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006;67:554–566. doi: 10.4088/jcp.v67n0406. [DOI] [PubMed] [Google Scholar]
- 6.Bächner D, Kreienkamp H, Weise C, Buck F, Richter D. Identification of melanin concentrating hormone (MCH) as the natural ligand for the orphan somatostatin-like receptor 1 (SLC-1) FEBS Lett. 1999;457:522–524. doi: 10.1016/s0014-5793(99)01092-3. [DOI] [PubMed] [Google Scholar]
- 7.Bertile F, Oudart H, Criscuolo F, Maho YL, Raclot T. Hypothalamic gene expression in long-term fasted rats: relationship with body fat. Biochem Biophys Res Commun. 2003;303:1106–1113. doi: 10.1016/s0006-291x(03)00481-9. [DOI] [PubMed] [Google Scholar]
- 8.Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon J-L, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- 9.Brischoux F, Fellmann D, Risold PY. Ontogenetic development of the diencephalic MCH neurons: a hypothalamic 'MCH area' hypothesis. Eur J Neurosci. 2001;13:1733–1744. doi: 10.1046/j.0953-816x.2001.01552.x. [DOI] [PubMed] [Google Scholar]
- 10.Challis BG, Coll AP, Yeo GS, Pinnock SB, Dickson SL, Thresher RR, Dixon J, Zahn D, Rochford JJ, White A, Oliver RL, Millington G, Aparicio SA, Colledge WH, Russ AP, Carlton MB, O'Rahilly S. Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-YY(3–36) Proc Natl Acad Sci U S A. 2004;101:4695–4700. doi: 10.1073/pnas.0306931101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers J, Ames RS, Bergsma D, Muir A, Fitzgerald LR, Hervieu G, Dytko GM, Foley JJ, Martin J, Liu WS, Park J, Ellis C, Ganguly S, Konchar S, Cluderay J, Leslie R, Wilson S, Sarau HM. Melanin-concentrating hormone is the cognate ligand for the orphan G-protein-coupled receptor SLC-1. Nature. 1999;400:261–265. doi: 10.1038/22313. [DOI] [PubMed] [Google Scholar]
- 12.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:409–412. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 13.Clegg DJ, Air EL, Benoit SC, Sakai RS, Seeley RJ, Woods SC. Intraventricular melanin-concentrating hormone stimulates water intake independent of food intake. Am J Physiol Regul Integr Comp Physiol. 2003;284:R494–R499. doi: 10.1152/ajpregu.00399.2002. [DOI] [PubMed] [Google Scholar]
- 14.Cohen S, Greenberg ME. Communication between the synapse and the nucleus in neuronal development plasticity, and disease. Annu Rev Cell Dev Biol. 2008;24:183–209. doi: 10.1146/annurev.cellbio.24.110707.175235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidowa H, Li Y, Plagemann A. Hypothalamic ventromedial and arcuate neurons of normal and postnatally overnourished rats differ in their responses to melanin-concentrating hormone. Regul. Pept. 2002;108:103–111. doi: 10.1016/s0167-0115(02)00153-2. [DOI] [PubMed] [Google Scholar]
- 16.Della-Zuana O, Presse F, Ortola C, Duhault J, Nahon JL, Levens N. Acute and chronic administration of melanin-concentrating hormone enhances food intake and body weight in Wistar and Sprague-Dawley rats. Int J Obes Relat Metab Disord. 2002;26:1289–1295. doi: 10.1038/sj.ijo.0802079. [DOI] [PubMed] [Google Scholar]
- 17.Drazen DL, Coolen LM, Strader AD, Wortman MD, Woods SC, Seeley RJ. Differential effects of adrenalectomy on melanin-concentrating hormone and orexin A. Endocrinology. 2004;145:3404–3412. doi: 10.1210/en.2003-1760. [DOI] [PubMed] [Google Scholar]
- 18.Eberle AN. Melanin-concentrating hormone. In: Eberle AN, editor. The Melanotropins. Basel: Karger; 1988. pp. 321–332. [Google Scholar]
- 19.Eberle AN, Mild G, Schlumberger S, Drozdz R, Hintermann E, Zumsteg U. Expression and characterization of melanin-concentrating hormone receptors on mammalian cell lines. Peptides. 2004;25:1585–1595. doi: 10.1016/j.peptides.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 20.Francke F, Ward RJ, Jenkins L, Kellett E, Richter D, Milligan G, Bächner D. Interaction of neurochondrin with the melanin-concentrating hormone receptor 1 interferes with G protein-coupled signal transduction but not agonist-mediated internalization. J Biol Chem. 2006;281:32496–32507. doi: 10.1074/jbc.M602889200. [DOI] [PubMed] [Google Scholar]
- 21.Gao X-B, van den Pol AN. Melanin concentrating hormone depresses synaptic activity of glutamate and GABA neurons from rat lateral hypothalamus. J Physiol (Lond) 2001;533:237–252. doi: 10.1111/j.1469-7793.2001.0237b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao X-B, van den Pol AN. Melanin-concentrating hormone depresses L-, N-, and P/Q-type voltage-dependent calcium channels in rat lateral hypothalamic neurons. J Physiol (Lond) 2002;542:273–286. doi: 10.1113/jphysiol.2002.019372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan JL, Uehara K, Lu S, Wang QP, Funahashi H, Sakurai T, Yanagizawa M, Shioda S. Reciprocal synaptic relationships between orexin- and melanin-concentrating hormone-containing neurons in the rat lateral hypothalamus: a novel circuit implicated in feeding regulation. Int J Obes Relat Metab Disord. 2002;26:1523–1532. doi: 10.1038/sj.ijo.0802155. [DOI] [PubMed] [Google Scholar]
- 24.Harthoorn LF, Sañé A, Nethe M, Van Heerikhuize JJ. Multi-transcriptional profiling of melanin-concentrating hormone and orexin-containing neurons. Cell Mol Neurobiol. 2005;25:1209–1223. doi: 10.1007/s10571-005-8184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassani OK, Lee MG, Jones BE. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc Natl Acad Sci U S A. 2009;106:2418–2422. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Q, Viale A, Picard F, Nahon J, Richard D. Effects of leptin on melanin-concentrating hormone expression in the brain of lean and obese Lepob/Lepob mice. Neuroendocrinol. 1999;l69:145–153. doi: 10.1159/000054413. [DOI] [PubMed] [Google Scholar]
- 27.Ito M, Gomori A, Ishihara A, Oda Z, Mashiko S, Matsushita H, Yumoto M, Ito M, Sano H, Tokita S, Moriya M, Iwaasa H, Kanatani A. Characterization of MCH-mediated obesity in mice. Am J Physiol Endocrinol Metab. 2003;284:E940–E945. doi: 10.1152/ajpendo.00529.2002. [DOI] [PubMed] [Google Scholar]
- 28.Kawauchi H, Kawazoe I, Tsubokawa M, Kishida M, Baker BI. Characterization of melanin-concentrating hormone in chum salmon pituitaries. Nature. 1983;305:321–323. doi: 10.1038/305321a0. [DOI] [PubMed] [Google Scholar]
- 29.Kilduff TS, de Lecea L. Mapping of the mRNAs for the hypocretin/orexin and melanin-concentrating hormone receptors: Networks of overlapping peptide systems. J Comp Neurol. 2001;435:1–5. doi: 10.1002/cne.1189. [DOI] [PubMed] [Google Scholar]
- 30.Kokkotou E, Moss AC, Torres D, Karagiannides I, Cheifetz A, Liu S, O'Brien M, Maratos-Flier E, Pothoulakis C. Melanin-concentrating hormone as a mediator of intestinal inflammation. Proc Natl Acad Sci U S A. 2008;105:10613–10618. doi: 10.1073/pnas.0804536105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lembo PMC, Grazzini E, Cao J, Hubatsch DA, Pelletier M, Hoffert C, St-Onge S, Pou C, Labrecque J, Groblewski T, O'Donnell D, Payza K, Ahmad S, Walker P. The receptor for the orexigenic peptide melanin-concentrating hormone is a G-protein-coupled receptor. Nature Cell Biol. 1999;1:267–271. doi: 10.1038/12978. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Gao XB, Sakurai T, van den Pol AN. Hypocretin/Orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36:1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- 33.Lin L, Faraco J, Li H, Kadotani R, Rogers W, Lin X, Qui X, deJong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 34.Ludwig DS, Tritos NA, Mastaitis JW, Kulkarni R, Kokkotou E, Elmquist J, Lowell B, Flier JS, Maratos-Flier E. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest. 2001;107:379–386. doi: 10.1172/JCI10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marsh DJ, Weingarth DT, Novi DE, Chen HY, Trumbauer ME, Chen AS, Guan XM, Jiang MM, Feng Y, Camacho RE, Shen Z, Frazier EG, Yu H, Metzger JM, Kuca SJ, Shearman LP, Gopal-Truter S, MacNeil DJ, Strack AM, MacIntyre DE, Van der Ploeg LH, Qian S. Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc Natl Acad Sci U S A. 2002;99:3240–3245. doi: 10.1073/pnas.052706899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meister B. Neurotransmitters in key neurons of the hypothalamus that regulate feeding behavior and body weight. Physiol Behav. 2007;92:263–271. doi: 10.1016/j.physbeh.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 37.Miyamoto-Matsubara M, Saitoh O, Maruyama K, Aizaki Y, Saito Y. Regulation of melanin-concentrating hormone receptor 1 signaling by RGS8 with the receptor third intracellular loop. Cell Signal. 2008;20:2084–2094. doi: 10.1016/j.cellsig.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 38.Modirrousta M, Mainville L, Jones BE. Orexin and MCH neurons express c-Fos differently after sleep deprivation vs. recovery and bear different adrenergic receptors. Eur J Neurosci. 2005;21:2807–2816. doi: 10.1111/j.1460-9568.2005.04104.x. [DOI] [PubMed] [Google Scholar]
- 39.Monzon ME, de Souza MM, Izquierdo LA, Izquierdo I, Barros DM, de Barioglio SR. Melanin-concentrating hormone (MCH) modifies memory retention in rats. Peptides. 1999;20:1517–1519. doi: 10.1016/s0196-9781(99)00164-3. [DOI] [PubMed] [Google Scholar]
- 40.Mori M, Harada M, Terao Y, Sugo T, Watanabe T, Shimomura Y, Abe M, Shintani Y, Onda H, Nishimura O, Fujino M. Cloning of a novel g protein-coupled receptor, slt, a subtype of the melanin-concentrating hormone receptor. Biochem Biophys Res Commun. 2001;283:1013–1018. doi: 10.1006/bbrc.2001.4893. [DOI] [PubMed] [Google Scholar]
- 41.Morton GJ, Mystkowski P, Matsumoto AM, Schwartz MW. Increased hypothalamic melanin concentrating hormone gene expression during energy restriction involves a melanocortin-independent, estrogen-sensitive mechanism. Peptides. 2004;25:667–674. doi: 10.1016/j.peptides.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Murdoch H, Feng GJ, Bächner D, Ormiston L, White JH, Richter D, Milligan G. Periplakin interferes with G protein activation by the melanin-concentrating hormone receptor-1 by binding to the proximal segment of the receptor C-terminal tail. J Biol Chem. 2005;280:8208–8220. doi: 10.1074/jbc.M405215200. [DOI] [PubMed] [Google Scholar]
- 43.Myrick H, Malcolm R, Tayloy B, LaRowe S. Modafinil: preclinical, clinical, and post-marketing surveillance—s review of abuse liability issues. Annals of Clinical Psychiatry. 2004;16:101–109. doi: 10.1080/10401230490453743. [DOI] [PubMed] [Google Scholar]
- 44.Mystkowski P, Seeley RJ, Hahn TM, Baskin DG, Havel PJ, Matsumoto AM, Wilkinson CW, Peacock-Kinzig K, Blake KA, Schwartz MW. Hypothalamic melanin-concentrating hormone and estrogen-induced weight loss. J Neurosci. 2000;20:8637–8642. doi: 10.1523/JNEUROSCI.20-22-08637.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nahon JL, Presse F, Bittencourt JC, Sawchenko P, Vale W. The rat melanin-concentrating hormone mRNA encodes multiple putative neuropeptides coexpressed in the dorsolateral hypothalamus. Endocrinol. 1989;125:2056–2065. doi: 10.1210/endo-125-4-2056. [DOI] [PubMed] [Google Scholar]
- 46.Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 47.Pissios P, Trombly DJ, Tzameli I, Maratos-Flier E. Melanin-concentrating hormone receptor 1 activates extracellular signal-regulated kinase and synergizes with G(s)-coupled pathways. Endocrinology. 2003;144:3514–3523. doi: 10.1210/en.2002-0004. [DOI] [PubMed] [Google Scholar]
- 48.Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 49.Rance T, Baker BI. The teleost melanin-concentrating hormone -- a pituitary hormone of hypothalamic origin. Gen Comp Endocrinol. 1979;37:64–73. doi: 10.1016/0016-6480(79)90047-9. [DOI] [PubMed] [Google Scholar]
- 50.Rao Y, Liu ZW, Borok E, Rabenstein RL, Shanabrough M, Lu M, Picciotto MR, Horvath TL, Gao XB. Prolonged wakefulness induces experience-dependent synaptic plasticity in mouse hypocretin/orexin neurons. J Clin Invest. 2007;117:4022–4033. doi: 10.1172/JCI32829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rao Y, Lu M, Ge F, Marsh DJ, Qian S, Wang AH, Picciotto MR, Gao XB. Regulation of synaptic efficacy in hypocretin/orexin-containing neurons by melanin concentrating hormone in the lateral hypothalamus. J Neurosci. 2008;28:9101–9110. doi: 10.1523/JNEUROSCI.1766-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rossi M, Beak SA, Choi SJ, Small CJ, Morgan DGA, Ghatei MA, Smith DM, Bloom SR. Investigation of the feeding effects of melanin concentrating hormone on food intake - action independent of galanin and the melancortin receptors. Brain Res. 1999;846:164–170. doi: 10.1016/s0006-8993(99)02005-3. [DOI] [PubMed] [Google Scholar]
- 53.Saito Y, Nothacker HP, Wang ZW, Lin SHS, Leslie F, Civelli O. Molecular characterization of the melanin-concentrating-hormone receptor. Nature. 1999;400:265–269. doi: 10.1038/22321. [DOI] [PubMed] [Google Scholar]
- 54.Saito Y, Cheng M, Leslie FM, Civelli O. Expression of the melanin-concentrating hormone (MCH) receptor mRNA in the rat brain. J Comp Neurol. 2001;435:26–40. doi: 10.1002/cne.1191. [DOI] [PubMed] [Google Scholar]
- 55.Sakurai T, Amemiya A, MIshii I, Matsuzaki RM, Chemelli H, Tanaka SC, Williams JA, Richardson GP, Kozlowski S, Wilson JRS, Arch RE, Buckingham AC, Haynes SA, Carr RS, Annan McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 56.Scammell TE, Estabrooke IV, McCarthy MT, Chemelli RM, Yanagisawa M, Miller MS, Saper CB. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 2000;20:8620–8628. doi: 10.1523/JNEUROSCI.20-22-08620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shearman LP, Camacho RE, Sloan Stribling D, Zhou D, Bednarek MA, Hreniuk DL, Feighner SD, Tan CP, Howard AD, Van der Ploeg LH, MacIntyre DE, Hickey GJ, Strack AM. Chronic MCH-1 receptor modulation alters appetite, body weight and adiposity in rats. Eur J Pharmacol. 2003;475:37–47. doi: 10.1016/s0014-2999(03)02146-0. [DOI] [PubMed] [Google Scholar]
- 58.Shimada M, Tritos NA, Lowell BB, Flier LS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- 59.Shimomura Y, Mori M, Sugo T, Ishibashi Y, Abe M, Kurokawa T, Onda H, Nishimura O, Sumino Y, Fujino M. Isolation and identification of melanin-concentrating hormone as the endogenous ligand of the SLC-1 receptor. Biochem Biophys Res Commun. 1999;261:622–626. doi: 10.1006/bbrc.1999.1104. [DOI] [PubMed] [Google Scholar]
- 60.Smith DG, Tzavara ET, Shaw J, Luecke S, Wade M, Davis R, Salhoff C, Nomikos GG, Gehlert DR. Mesolimbic dopamine super-sensitivity in melanin-concentrating hormone-1 receptor-deficient mice. J Neurosci. 2005;25:914–922. doi: 10.1523/JNEUROSCI.4079-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skofitsch G, Jacobowitz DM, Zamir N. Immunohistochemical localization of a melanin concentrating hormone-like peptide in the rat brain. Brain Res Bull. 1985;15:635–649. doi: 10.1016/0361-9230(85)90213-8. [DOI] [PubMed] [Google Scholar]
- 62.Sun G, Tian Z, Murata T, Narita K, Honda K, Higuchi T. Central and peripheral immunoreactivity of melanin-concentrating hormone in hypothalamic obese and lactating rats. J Neuroendocrinol. 2004;16:79–83. doi: 10.1111/j.1365-2826.2004.01124.x. [DOI] [PubMed] [Google Scholar]
- 63.van den Pol AN, Patrylo PR, Ghosh PK, Gao X-B. Lateral hypothalamus: Early developmental expression and response to hypocretin (orexin) J Com Neurol. 2001;433:349–363. doi: 10.1002/cne.1144. [DOI] [PubMed] [Google Scholar]
- 64.van den Pol AN, Acuna-Goycolea C, Clark KR, Ghosh PK. Physiological properties of hypothalamic MCH neurons identified with selective expression of reporter gene after recombinant virus infection. Neuron. 2004;42:635–652. doi: 10.1016/s0896-6273(04)00251-x. [DOI] [PubMed] [Google Scholar]
- 65.Viale A, Kerdelhue B, Nahon JL. 17 beta-estradiol regulation of melanin-concentrating hormone and neuropeptide-E-I contents in cynomolgus monkeys: a preliminary study. Peptides. 1999;20:553–559. doi: 10.1016/s0196-9781(99)00007-8. [DOI] [PubMed] [Google Scholar]
- 66.Zheng H, Patterson LM, Morrison C, Banfield BW, Randall JA, Browning KN, Travagli RA, Berthoud HR. Melanin concentrating hormone innervation of caudal brainstem areas involved in gastrointestinal functions and energy balance. Neuroscience. 2005;135:611–625. doi: 10.1016/j.neuroscience.2005.06.055. [DOI] [PubMed] [Google Scholar]