Abstract
Purpose
Mesothelin is a glycoprotein expressed on normal mesothelial cells and is overexpressed in several histologic types of tumors including pancreatic adenocarcinomas. A soluble form of mesothelin has been detected in patients with ovarian cancer and malignant mesothelioma, and has prognostic value. Mesothelin has also been considered as a target for immune-based therapies. We conducted a study on the potential clinical utility of mesothelin as a biomarker for pancreatic disease and therapeutic target pancreatic cancer.
Experimental Design
Tumor cell-bound and soluble mesothelin in patients was evaluated by immunohistochemistry and ELISA, respectively. The in-vitro cellular immune response to mesothelin was evaluated by INFγ ELISA and intracellular cytokine staining for IFNγ in CD4+ and CD8+ T cells. The level of circulating antibodies to mesothelin was measured by ELISA.
Results
All tumor tissue from patients with pancreatic adenocarcinoma expressed mesothelin (n=10). Circulating mesothelin protein was detected in patients with pancreatic adenocarcinoma (73 of 74 patients) and benign pancreatic disease (5 of 5) but not in healthy individuals. Mesothelin-specific CD4+ and CD8+ T cells were generated from peripheral blood lymphocytes (PBL) of patients with pancreatic cancer in 50% of patients compared to only 20% of healthy individuals. Antibodies reactive to mesothelin were detected in less than 3% of either patients or healthy individuals.
Conclusions
Circulating mesothelin is a useful biomarker for pancreatic disease. Furthermore, mesothelin-specific T cells can be induced in patients with pancreatic cancer. This suggests that mesothelin is a potential target for immune-based intervention strategies in pancreatic cancer.
Keywords: Mesothelin, Pancreatic Cancer, Cellular immunity, Humoral immunity, Biomarker
INTRODUCTION
Pancreatic adenocarcinoma is the fourth leading cause of cancer mortality in the United States(1). The vast majority of patients present with incurable metastatic disease, and there remains a lack of both tests for early diagnosis as well as effective therapies. Recent work has suggested that the immune response plays an important part in the pathogenesis of this malignancy(2–4). In particular, the presence of tumor-infiltrating CD4+ and CD8+ T cells as well as dendritic cells has been associated with improved prognosis(5). Further, it has been demonstrated that this immune response may be mediated in part by tumor-associated antigen(s)(2;4;6). One such candidate antigen is mesothelin(7;8). The mesothelin gene encodes a 71 kDa precursor that is processed into the 40 kDa GPI-anchored protein, mesothelin, and a 31 kDa fragment called megakaryocyte potentiating factor (MPF)(7–10). While its function remains unclear, mesothelin is constitutively expressed by mesothelial cells, but has been found to be over-expressed in pancreatic adenocarcinoma(11) and in a number of other solid tumors, such as lung(12) and ovarian(7;13–15) cancer. Importantly, when over-expressed in patients with ovarian cancer or mesothelioma, circulating mesothelin has been detected(14–20). Due to its limited expression by normal tissues and overexpression on tumors, mesothelin has also been considered as a tumor-associated antigen. Supporting this notion are the findings from various groups demonstrating that anti-mesothelin immune responses can be induced(21;22), even in patients with pancreatic cancer (6;23–25), and several mesothelin peptides have been identified that are capable of inducing T cell responses(23;25–28),
Here, we further explore the potential clinical utility of mesothelin in patients with pancreatic adenocarcinoma. Specifically, we explored its role as a plasma biomarker and investigated the frequency and nature of the anti-mesothelin immune response.
MATERIALS AND METHODS
Patient characteristics
Peripheral blood was collected from 81 patients with pancreatic disease of which 74 (91%) were diagnosed with pancreatic adenocarcinoma, 2 patients with neuroendocrine tumors of the pancreas (2%), and 5 patients with benign pancreatic disease (6%). The median age of the patients was 69 years (range 44 to 97 years) with a male to female ratio of 54 to 27. Clinical staging for patients with pancreatic adenocarcinoma was as follows: Stage IV (25%), Stage III (17%), Stage IIB (42%), Stage IIA (12%), and Stage 1B (4%). Five patients with benign pancreatic disease were included; two males and three females ranging in age from 67 to 71. Four out of five were diagnosed with intraductal papillary mucinous neoplasm and one patient with mucinous cystic neoplasm. All specimens were collected under research protocols approved by the Washington University School of Medicine Human Studies Committee after informed consent was obtained.
Peripheral blood samples
Peripheral blood samples were obtained from patients with pancreatic disease and normal healthy volunteers in part through the Siteman Cancer Center Hereditary Cancer Core. EDTA (plasma) tubes and serum tubes (BD Vacutainer® tubes, BD Biosciences, Franklin Lakes, NJ) were obtained from each patient. EDTA tubes were centrifuged at 2,000×g for 10 minutes at room temperature. The resultant supernatant was spun again at 2,500×g for 15minutes, and plasma was collected. Serum tubes were allowed to clot for 30 minutes at room temperature, then spun at 300×g for 5 minutes. Samples were kept at −80°C until use.
Isolation of peripheral blood mononuclear cells (PBMC)
PBMC were isolated as previously described(29). Briefly, heparinised blood underwent Ficoll density centrifugation, and the buffy coat containing PBMC was harvested and washed twice in PBS. PBMC were subsequently used in functional studies or cryopreserved.
Cell culture and intracellular cytokine analysis
PBMC from each patient were diluted to 2–3×106 cells per ml. Cells were placed in 1ml AIM-V medium (Invitrogen, Carlsbad, CA) supplemented with 2.5% Human AB serum (Sigma), 1% L-glutamine, and 1% penicillin/streptomycin (both Mediatech, Manassas, VA). Cultures were left un-pulsed or were pulsed with purified native mesothelin protein (20µg/ml) or anti-CD3 antibody (OKT3, 1µg/ml). After 5–7 days culture medium was harvested, cells were spun down, and supernatant was frozen at −20°C. Alternatively, one week old mesothelin-stimulated cultures were harvested, spun down, and cells were kept for 24 hours in cytokine-free medium. The next day, cells were re-stimulated at a 1:1 ratio with autologous, irradiated PBMC either unpulsed or pulsed with mesothelin protein, and 24 hours later supernatant was harvested and tested for IFNγ. In follow-up studies, one week old cultures were re-stimulated for a second week. These cultures received interleukin-2 (IL-2) at 50IU/ml every 3 days. After an additional 5–7 days, the cells were washed in medium without IL-2 and placed into culture medium without IL-2 for 24 hours. Subsequently, the cells were re-stimulated for 24 hours at a 1:1 ratio with autologous, irradiated PBMC either un-pulsed or pulsed with mesothelin protein. Brefeldin A (eBioscience, San Diego, CA) was added at 3µg/ml final concentration for the last 5 hours of re-stimulation before harvesting for antibody staining. Cells were generally blocked with mouse serum before incubating with specific staining antibodies. Intracellular staining for IFNγ was performed according to the manufacturer's (eBioscience) reagents and instructions; matched isotypes were used to determine positive and negative cell populations. Briefly, cell suspensions were first stained for CD4 and CD8 (BD Pharmingen, San Jose, CA), fixed at room temperature for 20 minutes then permeabilised in eBioscience Permeabilisation buffer before staining for IFNγ (eBioscience). Cells were analyzed using a FACS Calibur flow cytometer and FlowJo software (TreeStar; Ashland, OR).
IFNγ ELISA
The amounts of IFNγ in the culture supernatants were measured by ELISA using the Human IFN-γ Cytosets™ assay (Biosource, Camarillo, CA) according to the manufacturer’s instructions. A standard curve was included in each assay. The minimum detection level of IFNγ is 7pg/ml.
Mesothelin ELISA
Soluble mesothelin was measured by a validated double determinant sandwich ELISA(18). Detection was measured by absorbance in an ELISA reader (Spectracount Microplate Photometer; Packard, Palo Alto, CA). Monoclonal antibodies (mAbs) to two spatially different epitopes, mAbs OV569 and 4H3 were used to study serum and plasma samples. Mesothelin levels were determined as absorbance according to absorbance measurement by a microplate reader at 450nm (18) and are referred to as absorption units (AU). As reported earlier for this assay(15;17–19), a sample was considered positive if AU ≥ 0.20 at 1:40 dilution, which is 3 standard deviations above the mean absorbance measurement at 450 nm for over 100 healthy controls tested previously(19). Samples were diluted in PBS containing 3% BSA. All assays included either PBS/3% BSA or a healthy volunteer sample as a negative control, and serum from a patient with ovarian carcinoma as positive control on the same ELISA plate(19).
Anti-mesothelin antibody ELISA
Peripheral blood was evaluated for the presence of anti-mesothelin IgG antibodies using a recently developed ELISA(19). In this assay, plates are coated with native purified human mesothelin at 10 µg/ml. After incubation of serum samples, plates are incubated with horseradish peroxidase-conjugated mouse anti-human IgG antibody. Samples were tested at 1:20 and 1:80 dilutions in PBS/3% BSA and absorbance was determined at 450nm. AU ≥ 0.5 was used as cutoff for a positive sample at 1:20 dilution of samples. PBS/3% BSA was added to some wells as negative control, and serum from a patient with ovarian cancer was used as positive control(19).
Mesothelin protein
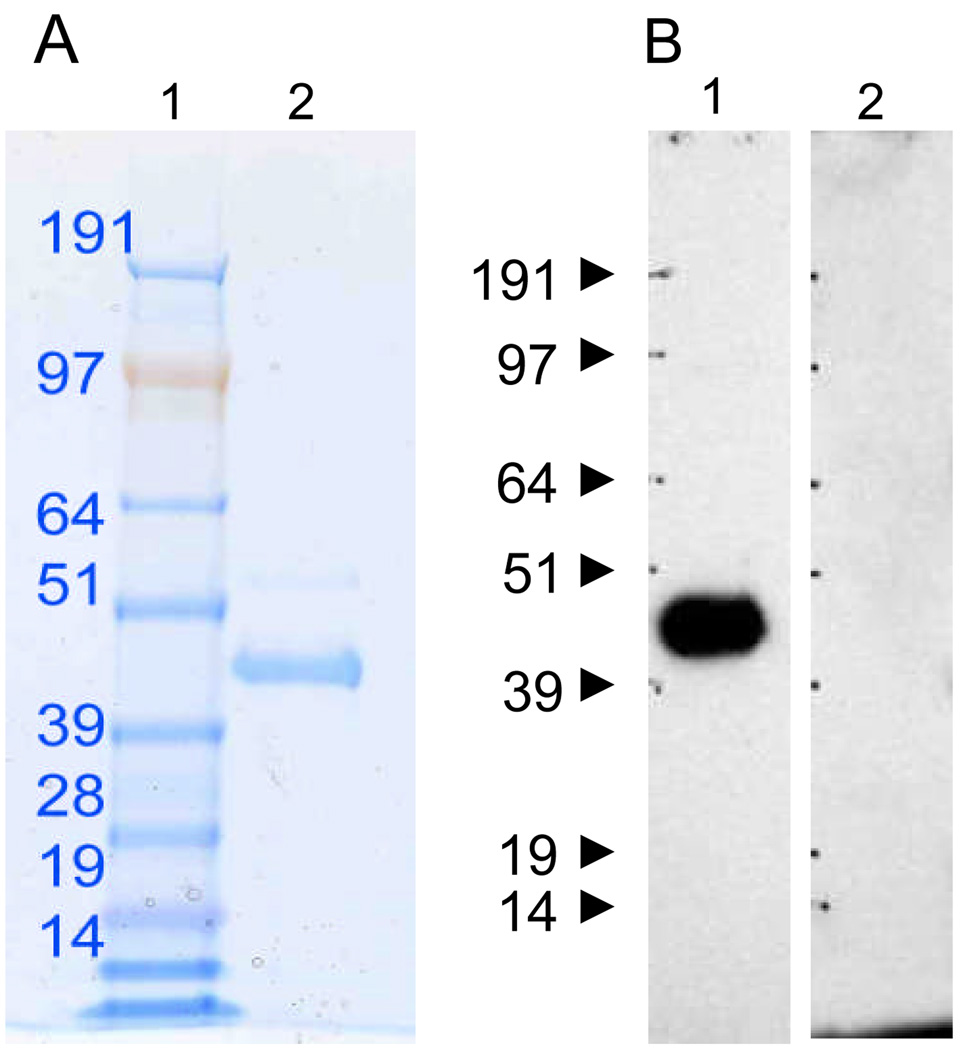
Two sources of mesothelin protein were used in this study. Initial studies were performed with a recombinant human mesothelin-human IgG fusion protein(18;20) with an estimated purity of approximately 60% as determined by gelelectrophoresis and Coomassie staining. The majority of the studies were performed with native human mesothelin purified from either urine of patients with ovarian or pancreatic cancer, or culture medium from a mesothelioma cell line(19). The identity of the purified native mesothelin was earlier confirmed by mass spectrometry(19). The protein solution was further characterized by gel electrophoresis followed by staining of the gel with SYPRO Ruby, and Western blot using the anti-mesothelin (K1) mAb (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) followed by peroxidase-conjugated goat anti-mouse IgG (Figure 1).
Figure 1. Analysis of purified native mesothelin by gel electrophoresis and Western blot.
A. Soluble mesothelin purified from culture supernatant of a mesothelioma cell line or urine from ovarian and pancreatic cancer patients(19) was run on a 4–12% SDS gel (lane 2) and stained by SYPRO-Ruby. Lane 1 was loaded with molecular weight markers. B. Two duplicate lanes were used for further development by Western blot using the anti-human mesothelin mAb, K1 in lane 1 or serum from a patient with pancreatic adenocarcinoma negative for anti-mesothelin antibody (lane 2).
Immunohistochemistry
Paraffin embedded tissue sections of archival human pancreatic ductal adenocarcinoma were evaluated for expression of mesothelin. Detection of mesothelin by immunohistochemistry was performed by using the anti-human mesothelin specific mAb, 5B2 (Novocastra Laboratories, Ltd, Newcastle upon Tyne, UK) following the manufacturer’s instructions. This antibody has been well characterized and been used in immunohistochemistry to assess its diagnostic potential(30). Representative formalin-fixed, paraffin-embedded tissue blocks containing invasive pancreatic ductal adenocarcinoma and normal tissue was chosen for labeling. A positive reaction was defined as discrete localization of the brown chromagen on the cell surface. Detection of immunolabeling was performed by an independent observer (Dr. E.M. Brunt, a pathologist at our institution). All cases demonstrating > 25% labeling were categorized as “positive” and scored on a scale from 1 to 3 with 3 being the highest.
Statistical analysis
The circulating mesothelin levels in patients with benign, primary and metastatic pancreatic cancer were compared to those in healthy volunteers using a nonparametric Mann-Whitney test. Analysis of ELISA results for IFNγ was performed by a Student T-test. Intracellular IFNγ levels in patients versus healthy controls were compared by the nonparametric Mann-Whitney test. In all analyses a p < 0.05 was considered significant.
RESULTS
Pancreatic adenocarcinomas express mesothelin
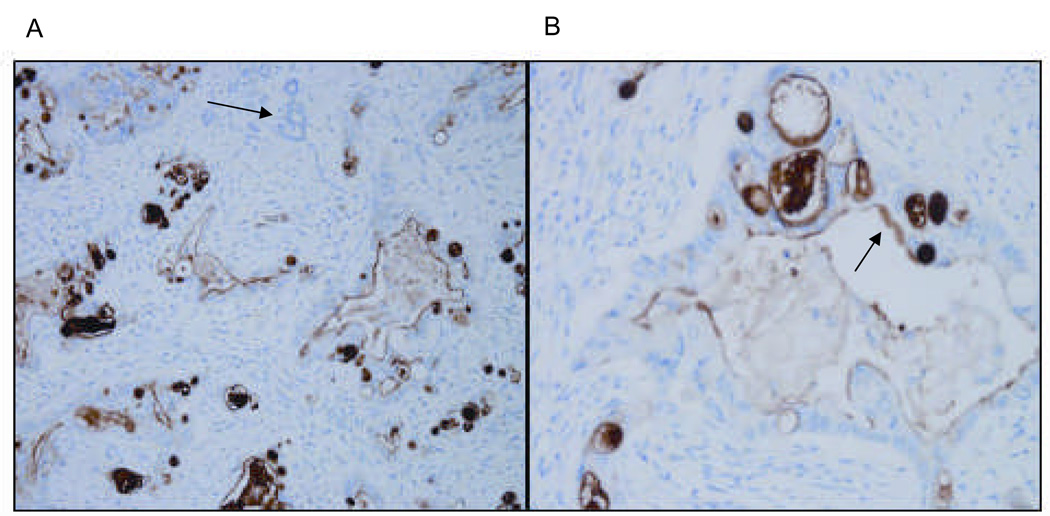
Ten surgically resected specimens of human pancreatic adenocarcinoma were stained for mesothelin (Figure 2). All samples were positive, with immunoreactivity distributed along the plasma cell membrane. The luminal surface of tumor glands but not of normal glands stained positive for mesothelin. This staining pattern is consistent with previous reports of mesothelin expression in pancreatic and other tumors(11;30–32).
Figure 2. Human pancreatic adenocarcinoma expresses mesothelin.
Representative micrographs at low (A) and high (B) power of human pancreatic adenocarcinoma stained for mesothelin. Tissue sections were stained with the anti-human mesothelin mAb, 5B2 and counterstained by H&E. Non-tumor pancreatic glands are nonreactive with the mesothelin antibody, as shown by the arrow in panel A. Only tumor glands (see arrow in B) stain positive for mesothelin. The malignant tumor epithelium is 3+/3 positive in all the tumor glands.
Elevated circulating mesothelin levels in patients with pancreatic disease
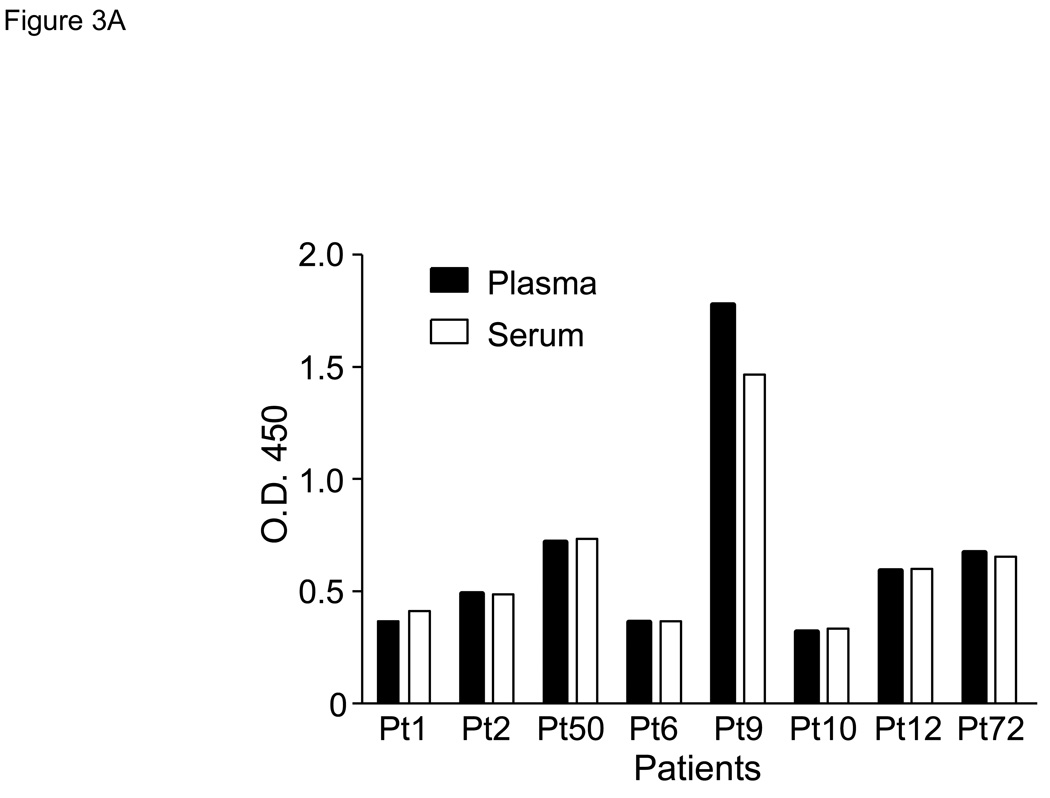
As overexpression of mesothelin in other types of cancer results in soluble mesothelin detectable in peripheral blood, we evaluated patients with pancreatic disease for circulating mesothelin. To test whether the ELISA for circulating mesothelin yields similar results for serum and plasma samples, a side-by-side comparison was performed using serum and plasma samples collected from the same patients. No significant difference was observed in soluble mesothelin levels between plasma and serum samples from the same patients tested at the same dilution (Figure 3A). Using this ELISA, 73 of 74 (99%) patients with pancreatic adenocarcinoma had elevated circulating levels of mesothelin compared with none of 5 healthy controls (Figure 3B). In addition, all 5 patients with biopsy-proven benign pancreatic disease had elevated levels of circulating mesothelin. The median AU in benign disease patients (0.405) was not significantly different from that in patients with either resectable (0.409) or unresectable/metastatic pancreatic cancer (0.497). There was no significant difference in mean AU comparing patients with resectable or unresectable disease. However, the median AU in healthy controls was 0.151 and was significantly lower than the mean AU for the patient populations (p < 0.001).
Figure 3. Soluble mesothelin is detectable in serum and plasma of patients with pancreatic cancer.
Data as determined by ELISA are expressed as absorption units (AU) at 450 nm using a 1:40 dilution. A. Comparison of serum and plasma for soluble mesothelin using samples from patients with metastatic pancreatic cancer. B. Circulating mesothelin levels in healthy volunteers, patients with benign pancreatic disease, patients with primary pancreatic cancer, and patients with unresectable pancreatic cancer. Each symbol represents a patient sample. Median values per group are indicated by a horizontal line. All median values in the patient groups are significantly elevated in comparison to that of healthy volunteers (p<0.05). The horizontal dashed line indicates the upper limit of negative values at AU = 0.2.
Mesothelin specific cellular immune responses
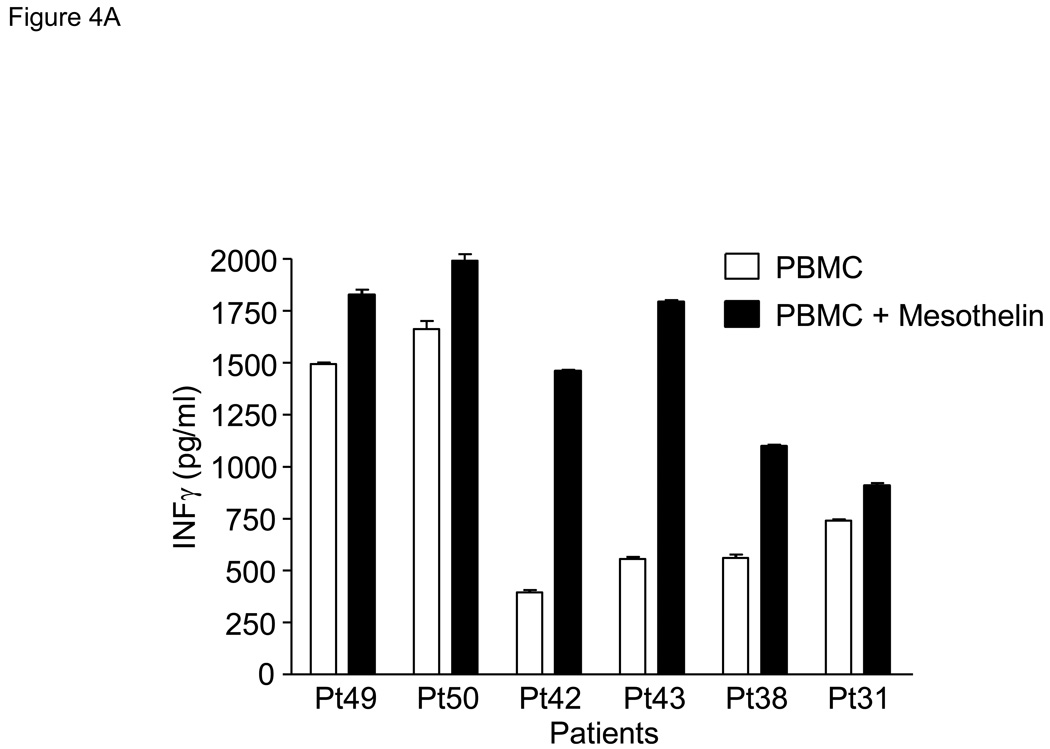
The increased levels of mesothelin protein present in the circulation of patients with pancreatic cancer could potentially have an impact on mesothelin-specific immunity. We therefore performed a series of experiments evaluating the presence of mesothelin-specific T cells in PBMC of pancreatic cancer patients. First, mesothelin protein-pulsed PBMC were evaluated for production of IFNγ after 5–7 days of culture. In 3 of 6 patients, the mesothelin-pulsed PBMC produced markedly increased levels of IFNγ as compared to PBMC alone (Pts 38, 42, and 43, Figure 4A). All six patients produced high levels of IFNγ after anti-CD3 stimulation (data not shown). To further characterize this response, mesothelin-stimulated T cells were rested overnight and restimulated the next day with irradiated, autologous PBMC or PBMC pulsed with either mesothelin or the breast cancer-associated protein, mammaglobin. After 24 hours, supernatant from both cultures were evaluated for the presence of IFNγ. A markedly increased production of IFNγ by the mesothelin-stimulated T cells was observed compared to control PBMC- or mammaglobin-pulsed PBMC-stimulated T cells in 3 of 4 patients with pancreatic cancer by ELISA (Figure 4B).
Figure 4. Patient-derived PBMC stimulated with mesothelin protein produce IFNγ.
A. PBMC were obtained from 10 patients with pancreatic adenocarcinoma including poorly differentiated (Pts 16, 49 and 50), moderately differentiated (Pts18, 43 and 31), moderately to poorly differentiated (Pt42), and well-differentiated (Pts17, 38 and 40). PBMC were stimulated with mesothelin protein and cultured for 5–7 days followed by analysis of culture medium for IFNγ by ELISA. Control PBMC were cultured in medium alone. As a group, the mesothelin cultures contained significantly more IFNγ than the unstimulated cultures (p<0.05). B. After a single stimulation with mesothelin protein, PBMC were taken out of culture, washed, and placed overnight medium without mesothelin. The next day PBMC were restimulated with irradiated PBMC or PBMC pulsed with mesothelin. Culture medium was tested for the presence of IFNγ by ELISA. The mesothelin stimulated cultures but not the mammaglobin-stimulated cultures contained significantly more IFNγ than the unstimulated cultures, p<0.05.
Mesothelin-reactive CD8 and CD4 T cells in pancreatic cancer patients
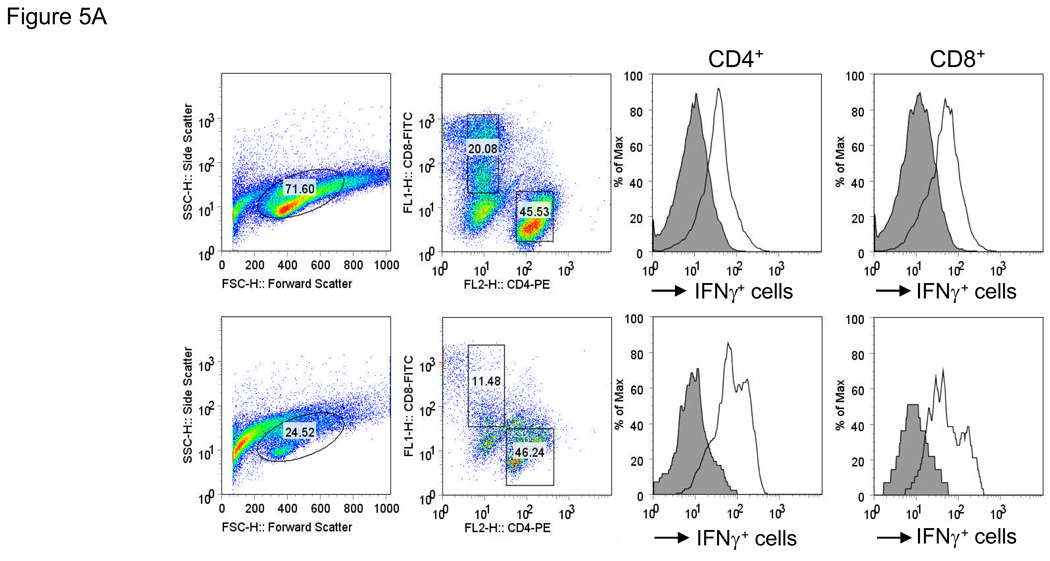
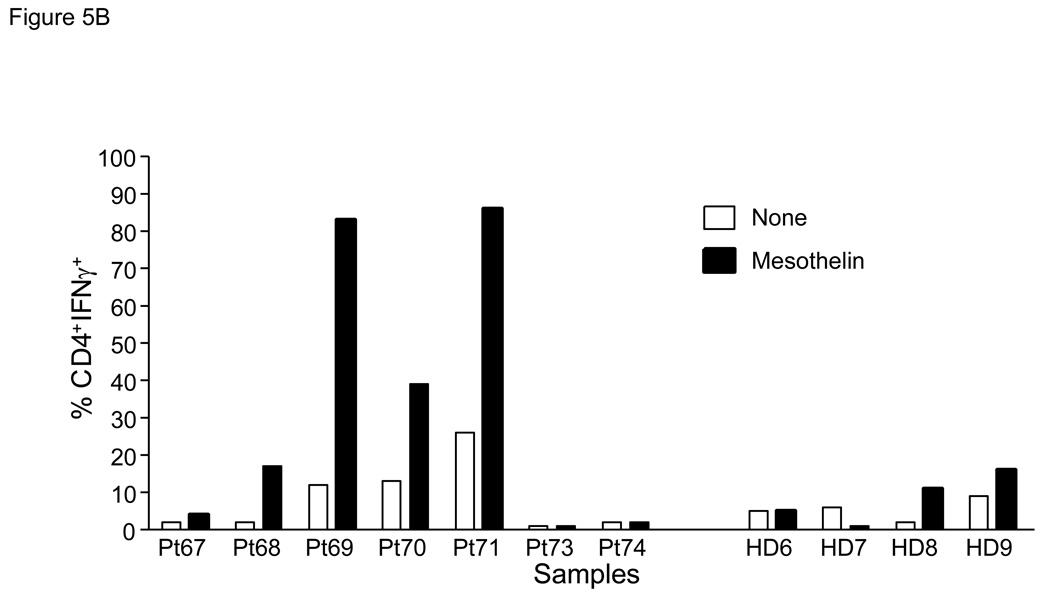
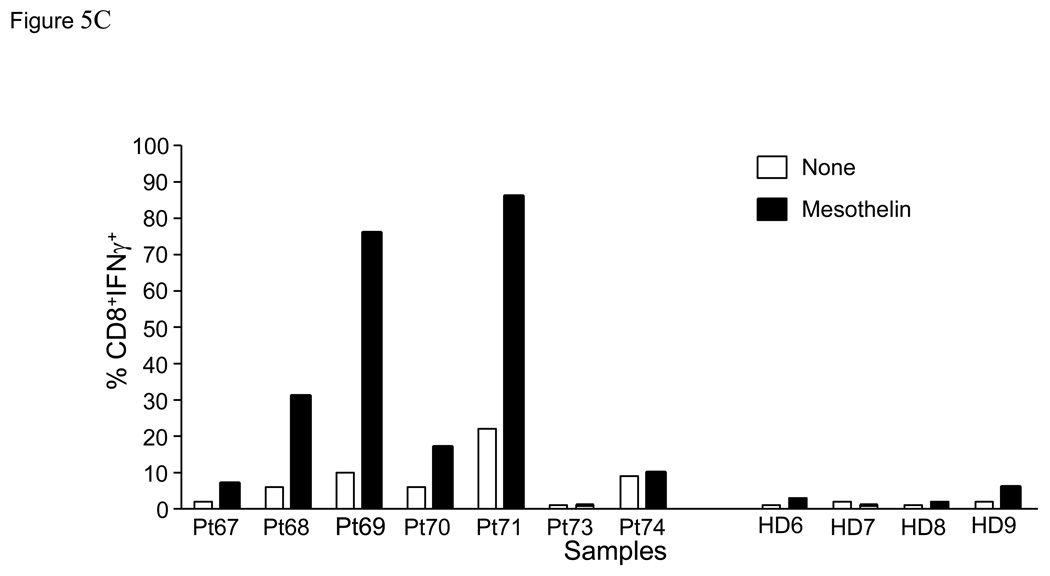
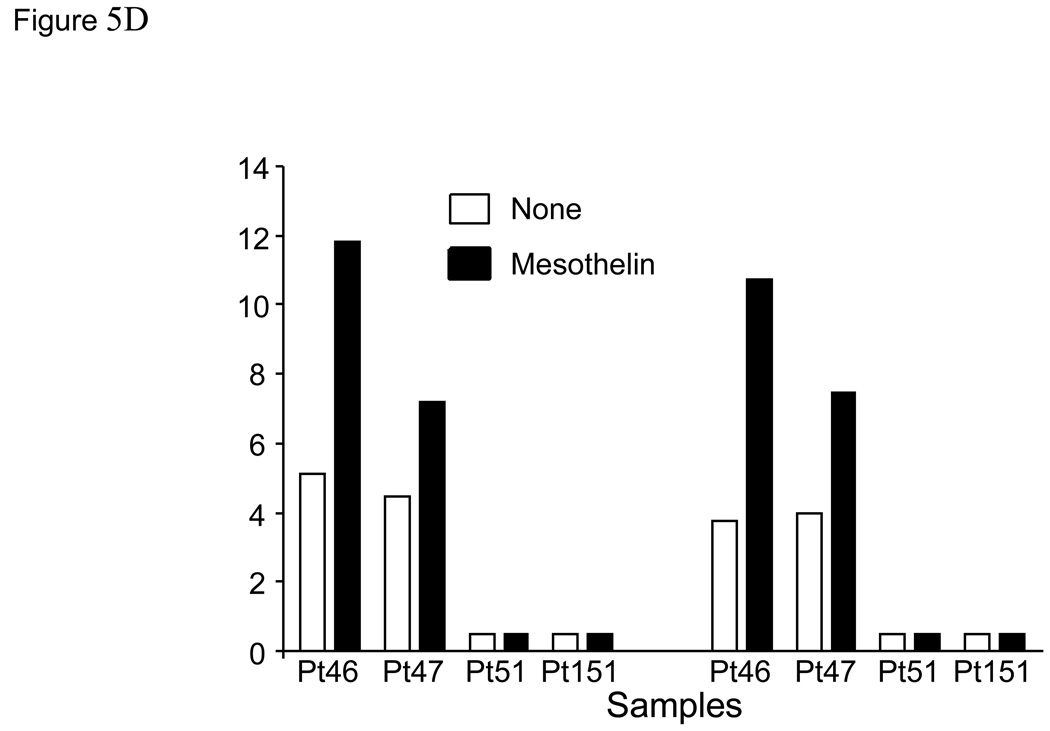
In order to more definitively demonstrate the presence of mesothelin-specific T cells, twice stimulated T cell cultures were rested for 24 hours in culture medium without IL-2. T cells were subsequently re-stimulated with either irradiated autologous PBMC or PBMC pulsed with mesothelin protein, and analyzed for intracellular IFNγ. These studies were performed with PBMC from both patients with pancreatic cancer and healthy controls. Cells were stained for CD4, CD8 and intracellular IFNγ then flow cytometry was performed (Figure 5A). An increased percentage of IFNγ-positive cells was detectable in 4 of 7 patients after mesothelin stimulation compared to control stimulation (Figures 5B, C). The marked increase in IFNγ-positive cells was detected in both the CD4 and CD8 subset. In contrast, in parallel cultures of age-matched, cancer-free controls only minor responses were observed in the CD4 subset of 2 of 4 cultures (Figure 5B) with no IFNγ+ CD8+ T cells detectable in any of the four controls (Figure 5C). Similar studies in an additional 6 healthy volunteers confirmed the observations in the age-matched controls (data not shown). As a group the percentages of IFNγ+ cells in both the CD4 and CD8 subsets of patients were significantly higher than in healthy controls (p < 0.05). Identical studies performed in four patients with benign pancreatic disease, in particular patients with intraductal papillary mucinous neoplasms (IPMN) indicated the presence of mesothelin-specific CD4 and CD8 T cells in two patients (Figure 5D, Pts46 and 47). Of those two, Pt46 was diagnosed with IPMN containing small foci of invasive cancer.
Figure 5. Mesothelin-specific T cells detected by intracellular IFNγ.
PBMC were obtained from seven patients with pancreatic adenocarcinoma including three patients with moderately differentiated tumors (Pts67, 73 and 74), two with moderately to poorly differentiated tumor (Pts69 and 71), and two with poorly differentiated tumors (Pts68 and 70) A. Representative FACS analyses of patient derived mesothelin-specific CD8 and CD4 T cells. The percentage IFNγ+ lymphocytes was determined by gating on either CD4+ or CD8+ cells. Mesothelin-stimulated PBMC were rested overnight and restimulated with autologous PBMC (shaded) or PBMC pulsed with mesothelin protein (unshaded). The percentage IFNγ+ cells was subsequently determined in both cultures. B and C: data summary showing percent double positive cells for INFγ and T cell markers CD4 (B) and CD8 (C). 4/8 patients with pancreatic adenocarcinoma showed significantly elevated levels of IFNγ in both T cell populations. There is a minimal response in 2/4 healthy, age-matched donors in the CD4 cell population. D. Summary data using PBMC from four patients with benign pancreatic lesions, IPMN. Pts47, 51, and 151 contained benign lesions without invasion of tumor whereas the lesion from patient 46 was found to contain small loci of invasive cancer.
Pancreatic cancer patients do not develop increased immunoglobulin G antibodies to mesothelin
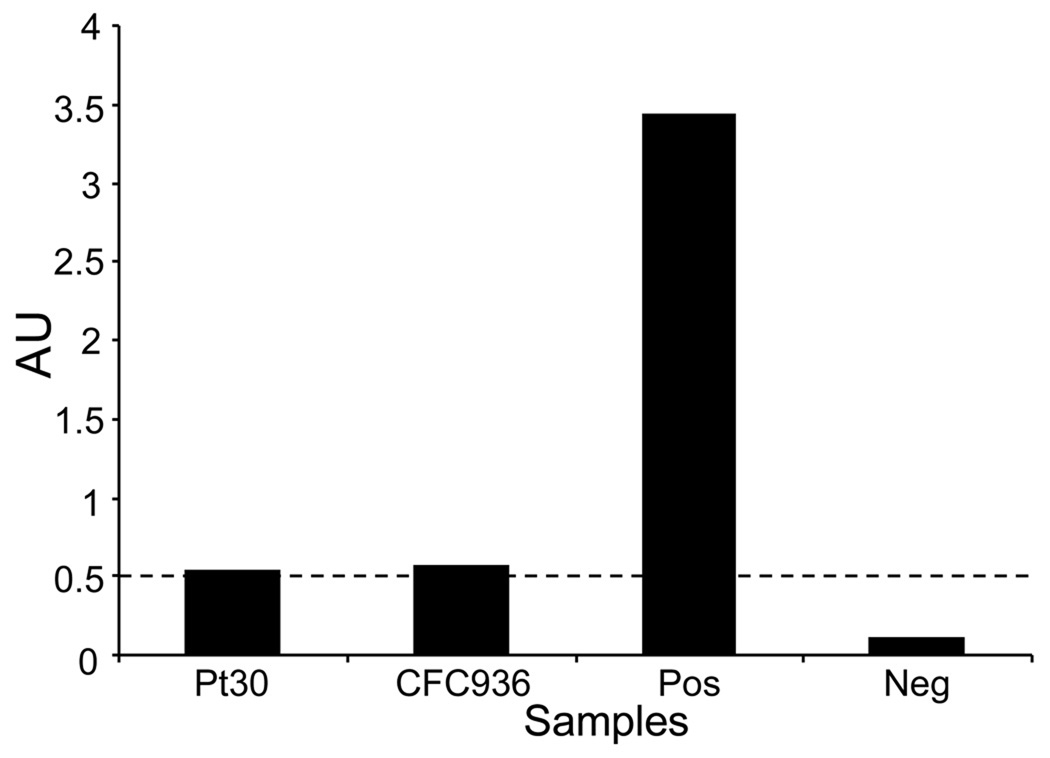
Using an ELISA specific for anti-mesothelin IgG, we determined the presence of specific IgG antibodies to mesothelin present in the serum or plasma of patients with pancreatic adenocarcinoma. A positive reaction was defined as an AU ≥ 0.5 at a 1:20 diluted sample(19). Antibodies to mesothelin were only detected in 1/56 patients (AU = 0.538) that included patients with resectable and unresectable/metastatic pancreatic cancers patients on standard (chemo) therapy, and 1/35 healthy donors (AU = 0.562), 14 of which were age-matched controls (Figure 6).
Figure 6. Detection of anti-mesothelin antibodies in serum of patients with pancreatic adenocarcinoma.
Serum from 56 patients and 35 healthy controls was tested for the presence of anti-human mesothelin IgG antibodies by ELISA. Serum from only one patient and one healthy control tested positive, albeit weakly, defined as an AU ≥ 0.5 at a 1:20 diluted sample(19). BSA was added as a negative control, and validated serum from a patient with ovarian carcinoma(19) was included as a positive control.
DISCUSSION
In this study, we sought to explore the clinical utility of mesothelin as a biomarker and therapeutic target in patients with pancreatic adenocarcinoma. Firstly, we confirmed that mesothelin is expressed in virtually all tumor specimens. Secondly, we demonstrated that patients with pancreatic disease have elevated circulating levels of mesothelin compared to healthy controls, and lastly, we demonstrate that mesothelin-specific CD4 and CD8 T cells can be induced from patient-derived PBMC.
Mesothelin is a cell surface protein expressed in many epithelial cancers(30;33;34). Its unique expression pattern makes it a potential target for immune intervention. Earlier studies in ovarian cancer and mesothelioma have demonstrated that the extracellular, GPI-linked domain of mesothelin can be cleaved off of the cell surface, resulting in a soluble mesothelin protein of approximately 40–45 kDa(9;10;33). Having confirmed previous findings that mesothelin is over-expressed in pancreatic adenocarcinoma, we sought to determine firstly whether circulating mesothelin could be detected in patients with pancreatic disease; secondly, whether soluble levels of mesothelin are elevated in pancreatic cancer patients compared to healthy controls, and, thirdly, whether this is associated with increased anti-mesothelin immunity. Our data show that soluble mesothelin levels are significantly increased in patients with pancreatic disease compared to healthy controls (see Figure 3B). Furthermore, there was no detectable evidence for high affinity anti-mesothelin IgG antibodies in patients. Lastly, mesothelin-specific CD4 and CD8 T cells were readily detectable in pancreatic cancer patient-derived PBMC after in vitro expansion. These data suggest that (1) circulating mesothelin is a biomarker of pancreatic disease, and (2) immune tolerance to mesothelin can be overcome (e.g. by culturing the T cells in the absence of immunosuppressive mechanisms in the tumor-bearing host) and suggest that mesothelin is an attractive target for immune-based therapies.
The normal biological function of cell membrane-bound mesothelin remains elusive. Mutant mice with targeted mesothelin gene inactivation are normal, exhibiting no apparent anatomic, hematologic or reproductive abnormalities(13). It was originally suggested that mesothelin might have a role in cell adhesion(33). Studies in ovarian cancer(35;36) have suggested that by binding to CA125/MUC16 on the surface of tumor cells, mesothelin mediates cell adhesion, and thereby plays a role in metastasis(36). Other studies in pancreatic cancer have suggested a role for mesothelin in tumorigenesis by increasing cell proliferation, migration, and S-phase cell population(8). Thus, from a cellular biological point of view, the apparent redundant homeostatic function of mesothelin combined with growing evidence of its role in carcinogenesis strengthen its candidacy as a tumor antigen worth targeting.
Mesothelin was first described by Pastan and colleagues as the cell surface component of a precursor molecule that also encodes soluble megakaryocyte-potentiating factor. The GPI-anchored mesothelin is shed from tumor cells, as was first demonstrated by Scholler et al(18) who developed a sandwich ELISA for detection of soluble mesothelin. This ELISA has since been used in a number of studies including the studies presented here, and an assay to measure mesothelin has been commercialized as MESOMARK for studies on mesothelioma(37). To our knowledge, this is the first report on detection of soluble mesothelin in patients with pancreatic disease. The assay can be performed with either serum or plasma, as our data suggest (Figure 3B). Elevated levels of soluble mesothelin were detected in the circulation of 73 out of 74 patients tested (Figure 3A), resembling the high frequency of over-expression of mesothelin in pancreatic cancer; in contrast, circulating mesothelin was not detected in healthy controls, which is in agreement with earlier studies on much larger control populations(38). Thus, an increased level of circulating mesothelin indicates pancreatic neoplasm (benign or malignant). Encouraged by these findings, we sought to determine the utility of soluble mesothelin as a marker of pancreatic cancer and more specifically, as a marker of tumor burden. To that end, we first measured mesothelin levels in patients with benign as well as malignant pancreatic disease. Soluble mesothelin was detected in 5/5 patients with benign pancreatic neoplasms at levels not significantly different from patients with malignant pancreatic disease, indicating that measurement of circulating mesothelin does not distinguish between benign and malignant pancreatic neoplasms for which a different approach would be needed. We then attempted to correlate the levels of soluble mesothelin with time to recurrence or, in all patients, time to death. We also evaluated if the level of soluble mesothelin decreased after surgery, and whether the difference between pre – and post surgery levels had prognostic value. Finally, we tested if high levels using an arbitrary cut off either pre – or post surgery were predictive of clinical outcome. However, no statistically significant correlations were detected suggesting that mesothelin does not accurately reflect the individual patient’s tumor burden. This outcome appears disease-related, as the same assay and source of samples does predict clinical outcome in diseases such as ovarian cancer and mesothelioma(17;19;38). It could well be that the rapid recurrence of pancreatic cancer (50% of patients have recurrent disease within one year of surgery) suggests that very few patients are truly disease-free for a period of time and may explain the lack of predictive value of soluble mesothelin in pancreatic cancer.
In addition to evaluation of the diagnostic usefulness of soluble mesothelin we determined if increased levels of soluble mesothelin translated into increased anti-mesothelin immunity. However, only 1/56 patients tested positive for mesothelin antibody (Figure 6). Monoclonal antibodies against mesothelin coupled to toxins are currently being tested in clinical trials of passive immunotherapy in pancreatic and ovarian carcinoma (39–41). It is however possible that anti-mesothelin antibodies themselves have therapeutic activity similar to for example trastuzumab, the anti-Her2/neu antibody. T cell responses to mesothelin were readily detectable in PBMC from pancreatic cancer patients after in vitro expansion with either a purified human mesothelin-Ig fusion protein or native human mesothelin. Both CD4 and CD8 T cell populations contained mesothelin-specific T cells with the ability to produce IFNγ upon activation (Figure 5). Similarly, Thomas et al.(23) showed that three rounds of in vitro stimulation were required to demonstrate CD8 T cell-mediated lysis of mesothelin-expressing tumor lines using T cells from pancreatic cancer patients undergoing immunotherapy. Interestingly, we identified mesothelin specific T cell response in half of the patients with benign pancreatic disease. It is important to note that while IPMN is benign it is a well recognized risk factor for the development of a future pancreatic cancer. These results suggest that an immune response to mesothelin may emerge early in cancer development.
Several T cell epitopes encoded by human mesothelin protein have been identified in vitro (23;25;28). Furthermore, mesothelin-specific T cells were detected in patients with metastatic pancreatic cancer after vaccination with allogeneic GM-CSF-secreting tumor cells with or without cyclophosphamide which favorably correlated with a better clinical course(23;25). Together with our findings here, these data confirm the immunogenicity of mesothelin and support the notion that mesothelin is an attractive target for immune-based therapies in pancreatic cancer.
Statement of Translational Relevance
Novel diagnostic biomarkers and therapeutic agents are much needed for patients with pancreatic cancer. Mesothelin is a differentiation antigen present on normal mesothelial cells. It is also highly expressed in pancreatic cancer. Circulating mesothelin is readily detectable in patients with pancreatic disease using a specific ELISA and thus serves as a useful biomarker for malignant and benign pancreatic tumors. The differential expression of mesothelin in normal versus cancer tissues makes mesothelin an attractive candidate for targeted therapies such as mesothelin-based immune therapies. Understanding the immunologic mechanisms for anti-mesothelin immunity in pancreatic cancer allows for the rational design of therapeutic strategies to improve clinical outcomes for patients afflicted with this deadly disease.
Acknowledgements
We thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, Mo., for the use of the Hereditary Cancer Core, which provided peripheral blood samples of cancer-free donors age 65 or older. The Siteman Cancer Center is supported in part by a NCI Cancer Center Support Grant #P30 CA91842.
Grant Support: This work was supported by grants from the National Institutes of Health, RO1 CA134487 (IH) and T32CA009621 (TJE, FMJ, POS); an AACR-PanCAN Career Development Award in Pancreatic Cancer Research, in Memory of Skip Viragh (WGH), the Barnes-Jewish Hospital Foundation (WGH), the Digestive Diseases Research Core Center grant 5P30 DK052574, and the Frank Cancer Research Fund awarded by the Barnes-Jewish Hospital Foundation (PG).
Footnotes
Presented in part at the annual meeting of the American Association for Cancer Research, San Diego April 2008
Reference List
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J.Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Kubuschok B, Neumann F, Breit R, Sester M, Schormann C, Wagner C, et al. Naturally occurring T-cell response against mutated p21 ras oncoprotein in pancreatic cancer. Clin.Cancer Res. 2006;12:1365–1372. doi: 10.1158/1078-0432.CCR-05-1672. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz-Winnenthal FH, Volk C, Z'graggen K, Galindo L, Nummer D, Ziouta Y, et al. High frequencies of functional tumor-reactive T cells in bone marrow and blood of pancreatic cancer patients. Cancer Res. 2005;65:10079–10087. doi: 10.1158/0008-5472.CAN-05-1098. [DOI] [PubMed] [Google Scholar]
- 4.Schmitz-Winnenthal FH, Escobedo LV, Beckhove P, Schirrmacher V, Bucur M, Ziouta Y, et al. Specific immune recognition of pancreatic carcinoma by patient-derived CD4 and CD8 T cells and its improvement by interferon-gamma. Int.J.Oncol. 2006;28:1419–1428. doi: 10.3892/ijo.28.6.1419. [DOI] [PubMed] [Google Scholar]
- 5.Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28:e26–e31. doi: 10.1097/00006676-200401000-00023. [DOI] [PubMed] [Google Scholar]
- 6.Laheru D, Jaffee EM. Immunotherapy for pancreatic cancer - science driving clinical progress. Nat.Rev.Cancer. 2005;5:459–467. doi: 10.1038/nrc1630. [DOI] [PubMed] [Google Scholar]
- 7.Ho M, Hassan R, Zhang J, Wang QC, Onda M, Bera T, et al. Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clin.Cancer Res. 2005;11:3814–3820. doi: 10.1158/1078-0432.CCR-04-2304. [DOI] [PubMed] [Google Scholar]
- 8.Li M, Bharadwaj U, Zhang R, Zhang S, Mu H, Fisher WE, et al. Mesothelin is a malignant factor and therapeutic vaccine target for pancreatic cancer. Mol.Cancer Ther. 2008;7:286–296. doi: 10.1158/1535-7163.MCT-07-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaguchi N, Hattori K, Oh-eda M, Kojima T, Imai N, Ochi N. A novel cytokine exhibiting megakaryocyte potentiating activity from a human pancreatic tumor cell line HPC-Y5. J.Biol.Chem. 1994;269:805–808. [PubMed] [Google Scholar]
- 10.Kojima T, Oh-eda M, Hattori K, Taniguchi Y, Tamura M, Ochi N, et al. Molecular cloning and expression of megakaryocyte potentiating factor cDNA. J.Biol.Chem. 1995;270:21984–21990. doi: 10.1074/jbc.270.37.21984. [DOI] [PubMed] [Google Scholar]
- 11.Hassan R, Laszik ZG, Lerner M, Raffeld M, Postier R, Brackett D. Mesothelin is overexpressed in pancreaticobiliary adenocarcinomas but not in normal pancreas and chronic pancreatitis. Am.J.Clin.Pathol. 2005;124:838–845. [PubMed] [Google Scholar]
- 12.Cristaudo A, Foddis R, Vivaldi A, Guglielmi G, Dipalma N, Filiberti R, et al. Clinical significance of serum mesothelin in patients with mesothelioma and lung cancer. Clin.Cancer Res. 2007;13:5076–5081. doi: 10.1158/1078-0432.CCR-07-0629. [DOI] [PubMed] [Google Scholar]
- 13.Bera TK, Pastan I. Mesothelin is not required for normal mouse development or reproduction. Mol.Cell Biol. 2000;20:2902–2906. doi: 10.1128/mcb.20.8.2902-2906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassan R, Remaley AT, Sampson ML, Zhang J, Cox DD, Pingpank J, et al. Detection and quantitation of serum mesothelin, a tumor marker for patients with mesothelioma and ovarian cancer. Clin.Cancer Res. 2006;12:447–453. doi: 10.1158/1078-0432.CCR-05-1477. [DOI] [PubMed] [Google Scholar]
- 15.McIntosh MW, Drescher C, Karlan B, Scholler N, Urban N, Hellstrom KE, et al. Combining CA 125 and SMR serum markers for diagnosis and early detection of ovarian carcinoma. Gynecol.Oncol. 2004;95:9–15. doi: 10.1016/j.ygyno.2004.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Creaney J, van B, I, Hof M, Segal A, Musk AW, de Klerk N, et al. Combined CA125 and mesothelin levels for the diagnosis of malignant mesothelioma. Chest. 2007;132:1239–1246. doi: 10.1378/chest.07-0013. [DOI] [PubMed] [Google Scholar]
- 17.Robinson BW, Creaney J, Lake R, Nowak A, Musk AW, de Klerk N, et al. Mesothelin-family proteins and diagnosis of mesothelioma. Lancet. 2003;362:1612–1616. doi: 10.1016/S0140-6736(03)14794-0. [DOI] [PubMed] [Google Scholar]
- 18.Scholler N, Fu N, Yang Y, Ye Z, Goodman GE, Hellstrom KE, et al. Soluble member(s) of the mesothelin/megakaryocyte potentiating factor family are detectable in sera from patients with ovarian carcinoma. Proc.Natl.Acad.Sci.U.S.A. 1999;96:11531–11536. doi: 10.1073/pnas.96.20.11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hellstrom I, Friedman E, Verch T, Yang Y, Korach J, Jaffar J, et al. Anti-mesothelin antibodies and circulating mesothelin relate to the clinical state in ovarian cancer patients. Cancer Epidemiol.Biomarkers Prev. 2008;17:1520–1526. doi: 10.1158/1055-9965.EPI-08-0039. [DOI] [PubMed] [Google Scholar]
- 20.Hellstrom I, Raycraft J, Kanan S, Sardesai NY, Verch T, Yang Y, et al. Mesothelin variant 1 is released from tumor cells as a diagnostic marker. Cancer Epidemiol.Biomarkers Prev. 2006;15:1014–1020. doi: 10.1158/1055-9965.EPI-05-0334. [DOI] [PubMed] [Google Scholar]
- 21.Chang CL, Wu TC, Hung CF. Control of human mesothelin-expressing tumors by DNA vaccines. Gene Ther. 2007;14:1189–1198. doi: 10.1038/sj.gt.3302974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breidenbach M, Rein DT, Everts M, Glasgow JN, Wang M, Passineau MJ, et al. Mesothelin-mediated targeting of adenoviral vectors for ovarian cancer gene therapy. Gene Ther. 2005;12:187–193. doi: 10.1038/sj.gt.3302404. [DOI] [PubMed] [Google Scholar]
- 23.Thomas AM, Santarsiero LM, Lutz ER, Armstrong TD, Chen YC, Huang LQ, et al. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J.Exp.Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J.Clin.Oncol. 2001;19:145–156. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 25.Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin.Cancer Res. 2008;14:1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hung CF, Calizo R, Tsai YC, He L, Wu TC. A DNA vaccine encoding a single-chain trimer of HLA-A2 linked to human mesothelin peptide generates anti-tumor effects against human mesothelin-expressing tumors. Vaccine. 2007;25:127–135. doi: 10.1016/j.vaccine.2006.06.087. [DOI] [PubMed] [Google Scholar]
- 27.Hung CF, Tsai YC, He L, Wu TC. Control of mesothelin-expressing ovarian cancer using adoptive transfer of mesothelin peptide-specific CD8+ T cells. Gene Ther. 2007;14:921–929. doi: 10.1038/sj.gt.3302913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yokokawa J, Palena C, Arlen P, Hassan R, Ho M, Pastan I, et al. Identification of novel human CTL epitopes and their agonist epitopes of mesothelin. Clin.Cancer Res. 2005;11:6342–6351. doi: 10.1158/1078-0432.CCR-05-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J.Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 30.Argani P, Iacobuzio-Donahue C, Ryu B, Rosty C, Goggins M, Wilentz RE, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin.Cancer Res. 2001;7:3862–3868. [PubMed] [Google Scholar]
- 31.Yen MJ, Hsu CY, Mao TL, Wu TC, Roden R, Wang TL, et al. Diffuse mesothelin expression correlates with prolonged patient survival in ovarian serous carcinoma. Clin.Cancer Res. 2006;12:827–831. doi: 10.1158/1078-0432.CCR-05-1397. [DOI] [PubMed] [Google Scholar]
- 32.Chang K, Pastan I, Willingham MC. Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int.J.Cancer. 1992;50:373–381. doi: 10.1002/ijc.2910500308. [DOI] [PubMed] [Google Scholar]
- 33.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc.Natl.Acad.Sci.U.S.A. 1996;93:136–140. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ordonez NG. Application of mesothelin immunostaining in tumor diagnosis. Am.J.Surg Pathol. 2003;27:1418–1428. doi: 10.1097/00000478-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Rump A, Morikawa Y, Tanaka M, Minami S, Umesaki N, Takeuchi M, et al. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J.Biol.Chem. 2004;279:9190–9198. doi: 10.1074/jbc.M312372200. [DOI] [PubMed] [Google Scholar]
- 36.Gubbels JA, Belisle J, Onda M, Rancourt C, Migneault M, Ho M, et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol.Cancer. 2006;5:50. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beyer HL, Geschwindt RD, Glover CL, Tran L, Hellstrom I, Hellstrom KE, et al. MESOMARK: a potential test for malignant pleural mesothelioma. Clin.Chem. 2007;53:666–672. doi: 10.1373/clinchem.2006.079327. [DOI] [PubMed] [Google Scholar]
- 38.Robinson BW, Creaney J, Lake R, Nowak A, Musk AW, de Klerk N, et al. Soluble mesothelin-related protein--a blood test for mesothelioma. Lung Cancer. 2005;49 Suppl 1:S109–S111. doi: 10.1016/j.lungcan.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 39.Hassan R, Broaddus VC, Wilson S, Liewehr DJ, Zhang J. Anti-mesothelin immunotoxin SS1P in combination with gemcitabine results in increased activity against mesothelin-expressing tumor xenografts. Clin.Cancer Res. 2007;13:7166–7171. doi: 10.1158/1078-0432.CCR-07-1592. [DOI] [PubMed] [Google Scholar]
- 40.Hassan R, Ebel W, Routhier EL, Patel R, Kline JB, Zhang J, et al. Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin. Cancer Immun. 2007;7:20. [PMC free article] [PubMed] [Google Scholar]
- 41.Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Willingham MC, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin.Cancer Res. 2007;13:5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]