Abstract
Pulmonary mast cell progenitor (MCp) numbers increase dramatically in sensitized and aerosolized Ag-challenged mice. This increase depends on CD4+ T cells, as no MCp increase occurs in the lungs of sensitized wild-type (WT) mice after mAb depletion of CD4+ but not CD8+ cells before aerosol Ag challenge. Neither the genetic absence of IL-4, IL-4Rα chain, STAT-6, IFN-γ, or IL-12p40 nor mAb blockade of IFN-γ, IL-3, IL-4, IL-5, IL-6, IL-10, IL-13, IL-17A, IL-12p40, or IL-12p40Rβ1 before Ag challenge in WT mice reduces the pulmonary MCp increase. However, sensitized and Ag-challenged IL-9-deficient mice and sensitized WT mice given mAb to IL-9 just before Ag challenge show significant reductions in elicited lung MCp/106 mononuclear cells of 47 and 66%, respectively. CD1d-deficient mice and WT mice receiving anti-CD1d before Ag challenge also show significant reductions of 65 and 59%, respectively, in elicited lung MCp/106 mononuclear cells, revealing an additional requirement for MCp recruitment. However, in Jα18-deficient mice, which lack only type 1 or invariant NKT cells, the increase in the numbers of lung MCp with Ag challenge was intact, indicating that their recruitment must be mediated by type 2 NKT cells. Furthermore, anti-CD1d treatment of IL-9-deficient mice or anti-IL-9 treatment of CD1d-deficient mice does not further reduce the significant partial impairment of MCp recruitment occurring with a single deficiency. These findings implicate type 2 NKT cells and IL-9 as central regulators that function in the same pathway mediating the Ag-induced increase in numbers of pulmonary MCp.
Mast cells (MC)4 have been shown to be beneficial for host defense to microbes and conversely to contribute to immune injury in mouse models for disease. MC are essential for rejection of adult worms in certain intestinal helminth infections involving a Th2-dependent immune response (1– 4) yet also contribute to Ag-elicited Th2 cell-dependent pulmonary inflammation in sensitized mice (5–7). In both settings, intraepithelial mucosal MC undergo T cell-driven hyperplasia. Similarly, connective tissue MC in an innate response can protect against direct instillation of bacteria into the lungs or peritoneal cavity (8 –10) or can participate in autoantibody-mediated arthritis (11, 12). Both mucosal and connective tissue MC arise from a single pool of bone marrow-derived circulating MC progenitors (MCp) in blood (13, 14) that mature after transendothelial migration into peripheral tissues (15). Whereas the small intestine is rich in MCp that home constitutively, presumably to rapidly give rise to mucosal MC, other peripheral tissues such as lung have only minimal numbers of MCp (14, 16, 17).
During allergic pulmonary inflammation, the lung depends on early recruitment of MCp which in turn become mucosal MC (18, 19). After 3 days of aerosol Ag challenge of sensitized mice, the number of MCp in the lung increases more than 10-fold. This rapid recruitment of MCp to the lung requires expression of α4β1 and α4β7 integrins on the MCp, expression of CXCR2 in the lung parenchyma, and induction of VCAM-1 on the endothelium (18, 19). We presumed that Th2 cells would control MCp influx to the lung by providing Th2 cytokines either for VCAM-1 induction (20, 21) and subsequent adherence and transendothelial migration of MCp or for their amplification and chemotaxis (22, 23).
We now find that there is no recruitment of MCp to the lungs of sensitized and aerosolized Ag-challenged mice when CD4+ cells are depleted with mAb at the time of the challenge. This early CD4+ T cell requirement is not dependent on Th2 cell induction because recruitment of MCp to the lungs of mice deficient in IL-4, IL-4Rα, or STAT-6 is equivalent to that in sufficient strains, and mAb blockade of IL-4 or other Th2 cytokines such as IL-3, IL-5, or IL-13 at the time of Ag challenge does not reduce MCp recruitment. Furthermore, the Ag-induced recruitment of MCp to lung does not require the Th1 developmental cytokines IFN-γ or IL-12p40 based on genetic or Ab-blocking approaches. Similarly, neither blocking mAb to IL-6, IL-17A, the IL-12/23 shared p40 component, or the shared IL-12/23 receptor β-chain nor the deficiency of IL-12/23p40 reduces the Ag-induced increase in lung MCp, suggesting that Th17 cells also are not involved (24–26). An effector cytokine is identified by finding significantly reduced MCp recruitment in IL-9-deficient mice and in wild-type (WT) mice receiving blocking anti-IL-9 mAb at the time of challenge. Moreover, when we assess for the involvement of NKT cells, we find that sensitization and challenge of mice lacking CD1d, or treatment of sensitized WT mice with anti-CD1d just before challenge, also significantly reduces recruitment of lung MCp. Finally, mAb blocking of IL-9 in CD1d-deficient mice or mAb blocking of CD1d in IL-9-deficient mice does not further reduce MCp recruitment from the significant, partial reduction observed with either deficiency alone. These latter findings indicate the involvement of CD1d-restricted NKT cells and IL-9 in the same pathway that leads to the aerosolized Ag-induced recruitment of MCp and suggest that CD1d-restricted NKT cells directly or indirectly provide the IL-9.
Materials and Methods
Animals
Male BALB/c 6- to 10-wk-old mice were obtained from Taconic Farms. The athymic BALB/c nudes (BALB/c-nu/nu) and mice deficient in STAT-6 (C.129S2-Stat6tmlGru/J), IL-4 (BALB/c-ll4tm2Nnt/J), IL-4Rα (BALB/c-Il4ratm1Sz/J), IFN-γ (C.129S7(B6)-Ifngtm1Ts/J), Rag2 (C.129S7(B6)-Rag2tmlFwaN12/J), IL-12p40 (C.129S1-Il12btm1Jm/J), CD1d (C.129S2-Cd1tm1Gru/J), and their BALB/c controls were obtained from The Jackson Laboratory. The MC-deficient mouse strains WBB6F1/J-KitW/KitW-v/J and WCB6F1/J KitlSl/KitlSl-d and their WT controls WBB6F1/J and WCB6F1/J, respectively, were obtained from The Jackson Laboratory. The C57BL/6J-KitW-sh and their WT controls C57BL/6J were provided by Dr. H. Katz in our department. IL-9-deficient mice were provided by Dr. A. McKenzie (Medical Research Council Laboratory of Molecular Biology, Cambridge, U.K.). These mice were backcrossed onto the BALB/c background (N7) and bred in-house. The Jα18-deficient mice (27) were provided by Dr. M. Taniguchi (RIKEN Research Center for Allergy and Immunology, Kanagawa, Japan) and bred in-house.
OVA sensitization and OVA aerosol challenge protocol
Individual groups of two to three mice received i.p injections of 10 μg of OVA (Sigma-Aldrich catalog no. A5503) adsorbed to 1 mg of alum (Pierce catalog no. 7161) in 200 μl of sterile HBSS on days 0 and 7. Mice were challenged with 1% aerosolized OVA in HBSS for 30 min per day using a PARI nebulizer. For most experiments, mice were challenged on days 17–19 and were euthanized by isoflurane inhalation on day 20. Immunohistochemistry on challenged lung was performed as previously described (19).
Ab blocking and cell depletions
The monoclonal anti-IL-3 (catalog no. 503906, clone MP2– 8F8), anti-IFN-γ (catalog no. 505812, clone XMG1.2), anti-IL-9 (catalog no. 504802, clone D9302C12), anti-CD1d (catalog no. 123504, clone 1B1), and anti-IL-12p40/IL-23 (catalog no. 505208, clone C15.6) and isotype control Ig were obtained from BioLegend. The anti-IL-4 (catalog no. 554385, clone BVD4-1D11), anti-IL-5 (catalog no. 554391, clone TRFK5), anti-IL-10 (catalog no. 554421, clone JESS2A5), anti-CD212 (anti-IL-12Rβ1; catalog no. 551455, clone 114), and the isotype-matched control Ig were obtained from BD Biosciences. The anti-IL-13 (catalog no. MAB413, clone 38213) and isotype control Ig were obtained from R&D Systems. All mAb were sodium azide free. For blocking, mice were injected i.p. with 100 μg/100 μl of mAb 15 min before each aerosol challenge.
Depletion of various T cells was performed as follows: the mAb anti-CD4 (catalog no. 100416, clone GK1.5) and anti-CD8a (catalog no. 100716, clone 53–6.7) were obtained from BioLegend, and 500 μg of the mAb was injected i.p. on days 15 and 18. For assessment of depletion by flow cytometry, the mAb, FITC-conjugated anti-CD4 (RM4-5, IgG2a; BD Biosciences) and FITC-conjugated anti-CD8a (53–6.7, rat IgG2a; BD Biosciences) were used. For depletion of CD25+ regulatory T (Treg) cells, 1 mg of anti-CD25 mAb (clone PC61) or isotype-matched control (28) diluted in 200 μl of sterile PBS was injected i.p. on days −1, 6, and 16.
Mononuclear cell (MNC) preparation and MCp assessment
Mice were euthanized, the lungs were perfused with 10 ml of HBSS administered via the right ventricle, and both lungs and spleen were harvested. Lung and spleen tissues were placed separately in 20 ml of RPMI 1640 containing 100 U/ml penicillin, 100 μg/ml streptomycin, 10 μg/ml gentamicin, 2 mM L-glutamine, 0.1 mM nonessential amino acids, and 10% heat-inactivated FCS (Sigma-Aldrich catalog no. F2442) and were processed essentially as previously described (14, 29). Briefly, the lungs were finely chopped with scalpels and transferred to 50-ml plastic tubes with 20 ml of complete RPMI 1640 plus 1.25 mg/ml collagenase type 4 (Worthington). Three enzymatic digestions were conducted for ~20 min each at 37°C. The undigested tissue clumps were collected after each digestion and subjected to another enzymatic digestion, while the liberated cells were harvested by centrifugation of the supernatant, resuspended in 44% Percoll (Sigma-Aldrich catalog no. P1644), underlaid with a 67% Percoll layer, and spun at 400 × g for 20 min at 4°C.
The MNC were harvested from the 44/67% Percoll interfaces, and cells from the lungs of the three digestions were pooled and washed in complete RPMI 1640. The number of viable cells was determined by trypan blue dye exclusion on a hemacytometer. The cells were serially 2-fold diluted in complete RPMI 1640, and 100 μl of each dilution was added to each well of standard 96-well flat-bottom microplates (Corning catalog no.) in duplicates. Twenty-four wells were plated for each cell concentration. Lung MNC were plated at concentrations beginning at 20,000 cells/well. Then, each well received 100 μl of γ-irradiated (30 Gy) splenic feeder cells plus cytokines (mouse rIL-3 at 20 ng/ml and mouse recombinant stem cell factor (SCF) at 50 ng/ml). The cultures were placed in humidified 37°C incubators with 5% CO2 for 10–12 days and wells containing MCp colonies were counted with an inverted microscope. The MCp colonies were easily distinguished as large colonies of nonadherent, small- to medium-sized round cells (14, 17, 30). The MCp concentration is expressed as the number of MCp/106 MNC isolated from the tissue. The number of lung MCp per mouse is derived by multiplying the concentration of MCp by the MNC yield from the lung divided by the number of mice if several mice were used to make the pool of MNC as was the case in the evaluation of the IFN−/− mice.
In vitro culture of splenic BALB/c NKT cells with restimulation for assessment of cytokine production
Bone marrow-derived dendritic cells (DC) were obtained by culturing whole bone marrow with GM-CSF (10 ng/ml, catalog no. 315-03; Pepro-Tech) plus IL-4 (20 ng/ml, catalog no. 214-14; PeproTech) for 6 – 8 days. The day before the coculture with the NKT cells, the DC were pulsed with α-galactosylceramide (α-GalCer) at a 100 ng/ml final concentration. Just before coculture, the DC were harvested, irradiated with 35 Gy, and washed with fresh medium.
The NKT cells were purified from the spleens of 10 –12 naive BALB/c mice after depletion of B cells with Dynabeads Mouse pan B (B220) (catalog no. 114.41D; Invitrogen) according to the manufacturer’s protocol. The cells were stained with FITC-anti-TCR-β (hamster IgG2, clone H57-597; BD Biosciences) and PE-conjugated PBS57-loaded CD1d tetramer (National Institutes of Health Tetramer Facility, Atlanta, GA). TCR-β + tetramer+ cells were purified using the Dana-Farber Cancer Institute Flow Cytometry Core. Cells were plated at a concentration of 0.2 × 106/well in 96-well trays. Cultures contained 5 × 105 DC, 25 U/ml human rIL-2 (National Institutes of Health), and 10 ng/ml mouse rIL-7 (catalog no. 217-17; PeproTech). Cells were cultured at 37° C for 4 –5 days and supernatants were collected for determination of cytokine production.
Determination of IL-9 concentrations
The concentration of IL-9 in cell culture supernatants was determined using purified rat anti-mouse IL-9 mAb (clone D8402E8; BD Pharmingen) as a capture Ab at a concentration of 5 μg/ml and biotinylated hamster anti-mouse IL-9 mAb (clone D9302C12; BD Biosciences) as a detection Ab at a concentration of 1 μg/ml. Briefly, Nunc 96-well plates were covered with capture Ab for at least 3 h and then were blocked with 10% FCS. The assay was developed with streptavidin-HRP (BD Biosciences) and tetramethylbenzidine substrate solution (Sigma-Aldrich). Mouse rIL-9 (catalog no. 409-ML; R&D Systems) was used to construct the standard curve and all determinations were performed in duplicate. The limit of sensitivity was ~200 pg/ml IL-9.
Statistical analysis
All experiments were repeated at least three times unless otherwise indicated. Data are expressed as the mean ± SE when derived from three or more values and as mean ± ½ range where n = 2. Significance was determined with a two-tailed Student’s t test where three or more values were available for analysis. Values of p < 0.05 were considered significant.
Results
CD4+ T cells, but not CD8+ T cells, are required for aerosolized OVA-induced increases in pulmonary MCp numbers
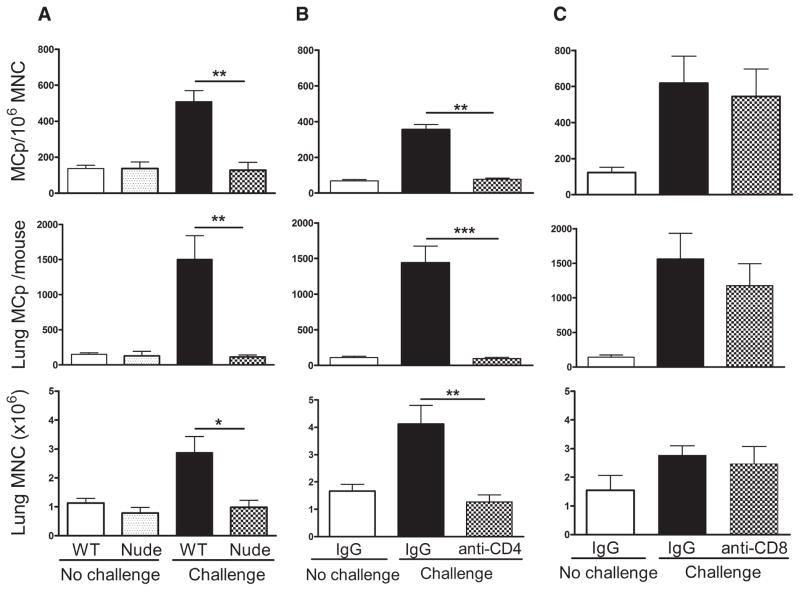
In the lung, the low constitutive levels of MCp are T cell independent (14), whereas the Ag-induced increase requires sensitization and challenge with the same Ag, implying T cell dependence (18). To distinguish the role for T cells in sensitization from a function occurring during Ag challenge, we evaluated the pulmonary recruitment of MCp in athymic and RAG-2−/− mice and in sensitized WT mice depleted of specific T cell subsets during the aerosolized Ag challenge. Sensitized, unchallenged WT and BALB/c nude (athymic) mice had similar numbers of lung MCp and MNC (Fig. 1A). Sensitization and Ag challenge of nude mice elicited no increase in lung MCp or MNC, whereas MCp were recruited in abundance to the lungs of WT mice treated in parallel (Fig. 1A). Similar findings were found for sensitized and challenged RAG-2−/−/BALB/c mice (data not shown), thereby indicating a strict requirement for T cells for the increase in MCp number in the lung in this model of allergic pulmonary inflammation. The protocol and timing of the MCp assay, performed after only three Ag challenges, are designed to avoid the presence of induced mucosal MC in the bronchial or tracheal tissues that would be harvested with the MNC and contaminate the clonogenic assessment of the MCp numbers. This early time point is characterized by modest recruitment of inflammatory cells to the bronchovascular bundles (18) and the infiltrate has few eosinophils based on Congo Red or H&E staining.
FIGURE 1.
The increase in pulmonary MCp numbers with aerosolized Ag challenge requires CD4+ and not CD8+ T cells. A, BALB/c WT and athymic BALB/c (nude) mice were sensitized and half were challenged with aerosolized OVA as indicated. The mean (±SE) concentration of MCp/106 MNC (top panel), the number of lung MCp per mouse (middle panel), and the number of lung MNC per mouse (bottom panel) are shown from four mice in each group. B, Sensitized BALB/c mice were given IgG or anti-CD4 and either not challenged or challenged with aerosolized OVA as indicated. The mean (±SE) concentration of MCp/106 MNC (top panel), the number of lung MCp per mouse (middle panel), and the number of lung MNC per mouse (bottom panel) are shown from five to eight mice. C, The same analysis as in B but using anti-CD8. The mean (±SE) concentration of MCp/106 MNC (top panel), the number of lung MCp per mouse (middle panel), and the number of lung MNC per mouse (bottom panel) are shown from four mice in each group. *, p < 0.05; **, p < 0.01; and ***, p < 0.001.
To determine whether the increase in lung MCp following Ag challenge on days 17–19 was CD4+ cell dependent, BALB/c mice were treated with monoclonal anti-CD4 on days 15 and 18. The anti-CD4 treatment reduced the proportion of CD4+ cells in the harvested lung MNC from 15 ± 2.4% to 0.2 ± 0.1% (mean ± SE, n = 6), as assessed by flow cytometry. The recruitment of MCp, measured as concentration of MCp/106 MNC and as the total number of lung MCp per mouse, was ablated along with the increase in MNC (Fig. 1B). In contrast, treatment with anti-CD8 using the same protocol had no effect on the Ag-induced recruitment of MCp (Fig. 1C), although it reduced the proportion of CD8+ cells in the MNC population from 11.8 ± 5.5% to 3 ± 2.4% (mean ± SE, n = 4), similar to the level of 4% in unchallenged animals (mean of two animals).
Classic Th1 or Th2 development is not required for aerosolized Ag-induced increases in pulmonary MCp numbers
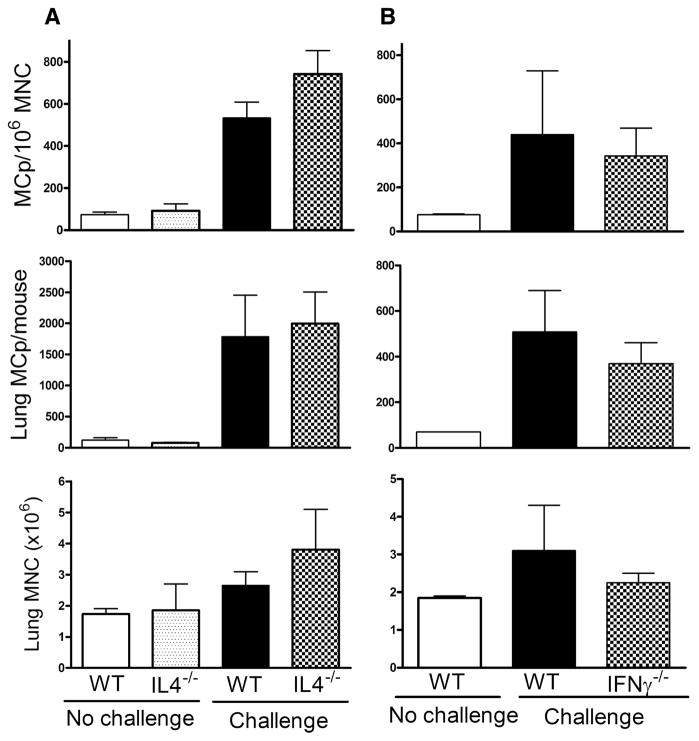
Since CD4+ Th2 cells are implicated in the maturation of MCp to mucosal MC (4, 31), we evaluated the role of Th2 cytokines in the recruitment of MCp by genetic and immunologic approaches. Sensitized, unchallenged WT and IL-4−/− mice had similar numbers of baseline lung MCp and MNC (Fig. 2A). With sensitization and challenge, WT and IL-4−/− mice had similar increments in the concentration of lung MCp/106 MNC, in the number of pulmonary MCp per mouse, and in lung MNC per mouse. Mice lacking the IL-4Rα or its signal transduction molecule STAT-6 also had increments in lung MCp, assessed as MCp/106 MNC or total number of lung MCp per mouse similar to their WT controls treated in parallel (Table I). The lack of production of Ag-specific IgE in these three strains was as expected (data not shown) (32, 33) and eliminated a requirement for Ag-specific IgE in pulmonary accumulation of MCp. Moreover, the administration of mAb to IL-4, IL-5, IL-10, or IL-13 before each of the three daily aerosol Ag challenges also did not inhibit the increase in lung MCp as measured by the concentration of lung MCp/106 MNC or total lung MCp per mouse (Table I). The absence of a requirement for IL-4 signaling, which is needed for robust Th2 cell development (32–36), suggests that recruitment of MCp is Th2 cell independent. The lack of inhibition after IL-5 blockade also indicates that eosinophils are not required for this recruitment.
FIGURE 2.
Ag-induced increases in pulmonary MCp numbers are not attenuated in the absence of IL-4 or IFN-γ. A, BALB/c WT and IL-4-deficient (IL-4−/−) mice were sensitized and either not challenged or challenged with aerosolized OVA as indicated. The mean (±SE) concentration of MCp/106 MNC (top panel), the number of lung MCp per mouse (middle panel), and the number of lung MNC per mouse (bottom panel) are shown from four to eight mice. B, The same analysis as in A with IFN-γ-deficient (IFN-γ−/−) mice on a BALB/c background. Values are the mean (±½range) from two experiments with lung MNC pooled from two mice in each group.
Table I.
Evaluation of the requirement for major Th cytokines for aerosolized Ag-induced recruitment of pulmonary MCp in sensitized BALB/c mice
| Cell and/or Mediator Tested | Deficient strain or mAba | Mean % of Control Lung MCp/106 MNCb (variance) | Mean % of Control Lung MCp/Mouseb (variance) | No. of Expt.c (no. of mice) |
|---|---|---|---|---|
| Null mice | ||||
| Th2/IL-4 | IL-4−/− | 142 (19) | 200 (78) | 5 (7) |
| IL-4Rα−/− | 63 (15) | 118 (18) | 4 (6) | |
| STAT-6−/− | 95 (36) | 99 (21) | 3 (4) | |
| Th1/IFN-γ | IFN-γ−/− | 170 (140) | 90 (50) | 2 (2) |
| Th1/17-IL-12/23 | IL-12p40−− | 71 | 137 | 1 (2) |
| Ab blockade | ||||
| IL-3 | Anti-IL-3 | 148 | 112 | 1 (2) |
| IL-4 | Anti-IL-4 | 64 (3) | 69 (10) | 2 (2) |
| IL-5 | Anti-IL-5 | 218 (27) | 284 (83) | 2 (2) |
| IL-6 | Anti-IL-6 | 126 (11) | 165 (48) | 1 (3) |
| IL-10 | Anti-IL-10 | 98 | 341 | 1 (1) |
| IL-13 | Anti-IL-13 | 106 (23) | 98 (29) | 2 (4) |
| IFN-γ | Anti-IFN-γ | 138 (30) | 155 (100) | 2 (2) |
| IL-12p40 | Anti-IL-12p40 | 78 | 93 | 1 (2) |
| Anti-IL-12Rβ1 | 86 | 88 | 1 (2) | |
| IL-17A | Anti-IL-17A | 111 (29) | 129 (42) | 1 (3) |
| Treg | Anti-CD25 | 146 (46) | 270 (16) | 2 (4) |
All mice listed were on a BALB/c background and were sensitized and challenged. The increment in total lung MCp over baseline was >10-fold in the different experiments. For mAb inhibition, mice were injected with 100 μg of the indicated mAb before each challenge. For Treg depletion, mice were injected with 1 mg of mAb on days −1, 6, and 16.
The values represent the influx of MCp as measured by lung MCp concentration (MCp/106 MNC) or by total lung MCp per mouse as a percentage of the values obtained from control mice treated in parallel. The controls were WT mice for deficient strains and WT mice treated with isotype-matched IgG for WT mice receiving blocking mAb. The values are the mean with the variance in parentheses: SE for three or more experiments or 1/2 range for two experiments.
Number of experiments and in parentheses the number of mice evaluated.
Others have demonstrated an early Th1 response in protocols of Ag-induced lung inflammation (37). Thus, we next assessed whether loss of either the IFN-γ or the IL-12/23 signaling pathway would affect pulmonary MCp numbers. Sensitized and Ag-challenged IFN-γ−/− mice and WT mice developed similar concentrations of lung MCp/106 MNC and total number of lung MCp per mouse (Fig. 2B). Consistent with this result, WT mice treated with anti-IFN-γ showed no attenuation in Ag-induced increases in the number of MCp assessed by the concentration of MCp/106 MNC or total lung MCp per mouse (Table I). Furthermore, IL-12p40−/− mice and WT mice given mAb to IL-12p40 or to IL-12p40Rβ1, which would attenuate Th1 cell development and Th17 cell expansion (24, 26, 38, 39), showed robust increments in concentration and total number of lung MCp, comparable to those values in WT controls treated in parallel (Table I). The administration of blocking mAb to either IL-6 or to IL-17A at the time of Ag challenge did not affect the increase in pulmonary MCp (Table I). Thus, the Ag-induced T cell-dependent increments in pulmonary MCp also do not require either the action of classic Th1 cells or the IL-23 expansion or signature cytokine of Th17 cells.
We then tested the MC-active cytokine IL-3. This cytokine can be generated by either Th1 or Th2 cells (40), plays an important role in helminth-induced MC hyperplasia in the intestine (31, 41), is a potent comitogen for fetal blood MCp (13), and has chemotactic activity for bone marrow-derived immature cultured MC and for peritoneal MC ex vivo (22, 23). Using mAb blockade, we found that mice treated with anti-IL-3 before challenge had no attenuation in the increase in the number of lung MCp measured either by the concentration of MCp/106 MNC or by the total number of lung MCp per mouse, relative to mice given PBS and challenged in parallel (Table I). In one experiment, IL-3−/− mice also showed robust Ag-induced increases in lung MCp (data not shown).
IL-9 regulates pulmonary MCp numbers in aerosolized Ag-challenged mice
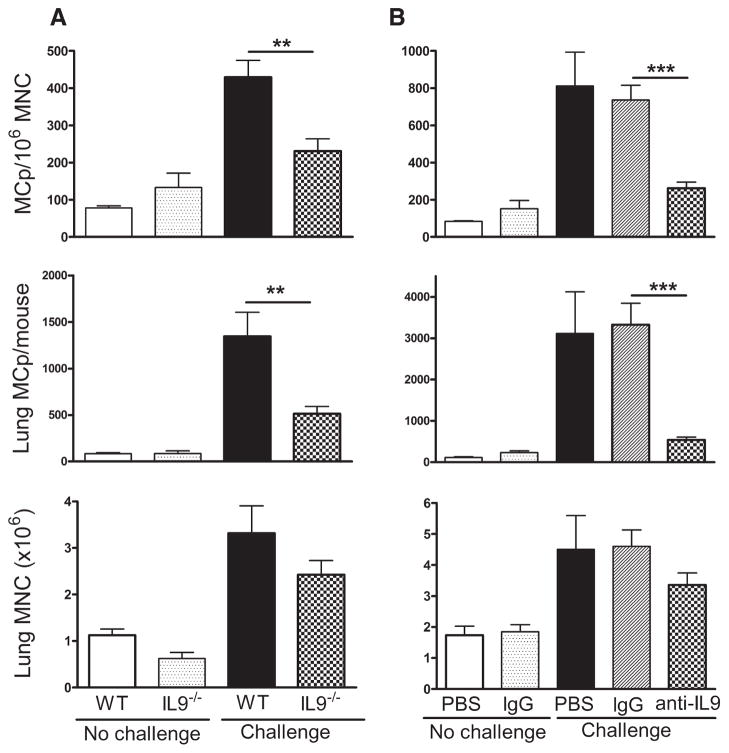
In seeking a MC-active cytokine that did not depend on STAT-6 for signaling, we turned to IL-9. This cytokine signals through STAT-3 and STAT-5 (42, 43), is expressed by Th2 and Treg cells (44, 45) and by MC (46), and can be expressed by lymph node and spleen cells early in an immune response in an IL-4-independent manner (47). Sensitized, unchallenged IL-9-deficient and WT mice had similar numbers of lung MCp and MNC (Fig. 3A). After challenge, the IL-9−/− mice had significant (47 and 62%) reductions in the concentration of lung MCp/106 MNC and in the total number of lung MCp per mouse, respectively (p < 0.02, n = 9), relative to WT mice challenged in parallel. Consistent with these data, WT mice treated with anti-IL-9 at the time of Ag challenge had significant (66 and 76%) reductions in the concentration of lung MCp/106 MNC and in the total number of lung MCp per mouse respectively (p < 0.001, n = 12–14 mice), compared with mice treated with isotype-matched IgG (Fig. 3B). IL-9 deficiency did not significantly affect the level of lung inflammation based on the yields of MNC per mouse (Fig. 3A) or the levels of total IgG1, total IgE, and OVA-specific IgE compared with WT mice (data not shown). Treatment with anti-IL-9 did not change the numbers of MCp/106 MNC in bone marrow in one experiment in which lung MCp/106 MNC was inhibited by 51%, suggesting no effect by this treatment on early lineage progenitors (data not shown). We also saw no diminution in the up-regulation of VCAM-1 expression on the vascular endothelium or in the level of VCAM-1 mRNA expression in lung relative to GAPDH mRNA with Ag challenge (data not shown). These findings are consistent with the reports by Townsend et al. (48) and McMillan et al. (49) who noted that IL-9-deficient mice did not have a diminished inflammatory response as assessed by serum Igs, VCAM-1 expression and collagen deposition in airways, or in airway hyperresponsiveness.
FIGURE 3.
IL-9 regulates the Ag-induced increase in pulmonary MCp numbers. A, BALB/c WT and IL-9-deficient (IL-9−/−) mice were sensitized and half were challenged with aerosolized Ag as indicated. The mean (±SE) concentration of MCp/106 MNC (top panel), the number of lung MCp per mouse (middle panel), and the number of lung MNC per mouse (bottom panel) are shown from 9 to 11 mice in each group. B, BALB/c mice were sensitized and treated with PBS, control IgG, or anti-IL-9, either not challenged or challenged as indicated, and evaluated as in A. The values are the mean (±SE) from 3, 9, 5, 12, and 14 mice in each group, respectively. **, p < 0.02 and ***, p < 0.001.
Early IL-9 expression after Ag exposure has been reported to precede and be independent of IL-4 expression in the local draining lymph nodes (47). This finding led us to determine whether IL-9 played a regulatory role in the robust increments in Ag-induced MCp levels in the lung of the IL-4-deficient strain. Treatment of sensitized and aerosolized Ag-challenged WT and IL-4−/− mice with anti-IL-9 before each challenge reduced MCp recruitment similarly in each strain. Relative to their respective controls, anti-IL-9 treatment in WT and IL-4−/− mice caused a significant reduction of 61 and 78%, respectively (p < 0.05), in the concentration of MCp/106 MNC and a reduction of 77 and 89%, respectively, in the total number of lung MCp per mouse.
Resident tissue MC or Treg cells are not required to elicit increases in pulmonary MCp numbers in aerosolized Ag-challenged mice
Although the requisite IL-9 is likely to be derived from a lymphocyte subset, resident MC can also generate IL-9 (46, 50). Thus, we evaluated resident lung MC as a source of factors essential to the Ag-induced increase in pulmonary MCp by using three different strains of genetically MC-deficient mice. We knew from previous studies that these deficient strains could develop MCp from bone marrow in culture but that either such cells did not develop well in vivo or their distribution could not be sustained in the peripheral tissues (14, 51, 52). With sensitization and challenge, C57BL/6-KitWsh mice with a mutation in the c-Kit promoter region (53) and C57BL/6 mice had increases in the concentration of lung MCp/106 MNC of 7- and 8-fold, respectively. WBB6F1-KitW/KitWv mice with a mutation in the coding region for c-Kit (54) and their WT (WBB6F1) controls responded with an increment in the concentration of MCp/106 MNC of 19- and 13-fold, respectively. WCB6F1-KitSl/KitSld mice, which lack the membrane-bound form of SCF (55), and their controls (WCBB6F1) also responded similarly to Ag challenge with MCp increments of 3- and 4-fold, respectively. These consistent results indicate that mature pulmonary MC are not required for the Ag-driven increase in MCp in this tissue, nor is the SCF-c-Kit interaction needed for the mobilization of these cells to the lung.
The recent observation that CD4+ Treg cells control the level of the immune response in allergen-challenged mice (28) and interact with MC in a tolerogenic response to skin grafts, possibly via the elaboration of IL-9 (45), led us to consider a role for these cells as producers of the IL-9 required for the increment in pulmonary MCp levels. We used anti-CD25 to ablate the Treg cells (28). Compared with sensitized and challenged BALB/c mice receiving isotype-matched Ig, those treated with anti-CD25 had an even greater response in lung MCp/106 MNC and in total lung MCp per mouse (Table I), indicating that recruitment was not dependent on Treg cells or their products. The anti-CD25-treated mice, relative to Ig-treated control mice, had a significant increase in serum IgE concentration (from 9.7 ± 1 to 17 ± 3 μg/ml, p < 0.01, n = 4) and a 53 and 81% reduction (analyzed in two separate mice) in the number of CD4+CD25+ cells in the lung as assessed by flow cytometric analysis of the isolated lung MNC.
CD1d-restricted cells regulate pulmonary MCp numbers in aerosolized Ag-challenged mice
Because several studies implicated NKT cells in early pulmonary responses in mice (56 –58) and others reported that C57BL/6 NKT cells produce IL-9 in vitro (59, 60), we evaluated IL-9 production in NKT cells isolated from BALB/c spleen by sorting for the TCR-β+, α-GalCer-CD1d tetramer+ cells. The purified NKT cells were cultured with α-GalCer-pulsed bone marrow-derived DC (61) for 4 days. The cell supernatants from the NKT cultures contained ~1 ng/ml IL-9 as well as nanogram amounts of IL-4 and IL-17A (data not shown).
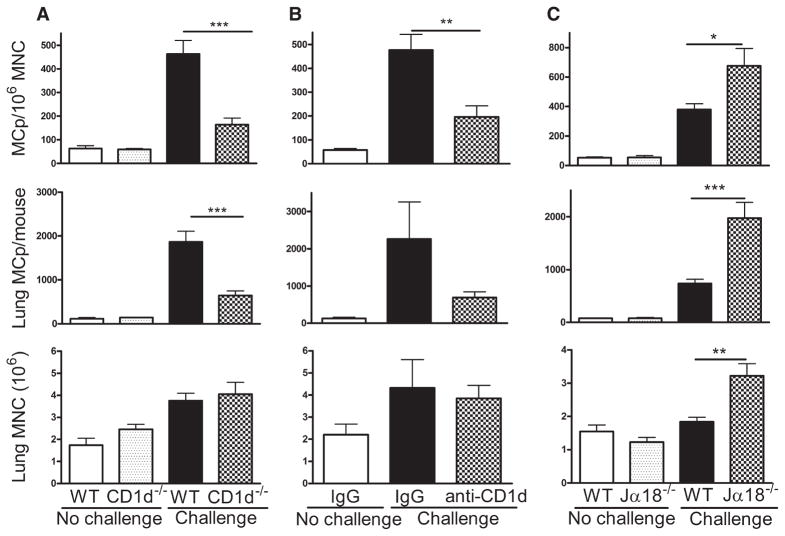
The finding that NKT cells could produce IL-9 prompted an analysis of MCp recruitment to lung in NKT-deficient mice using the CD1d−/− BALB/c strain. Unchallenged, sensitized WT and CD1d−/− mice had similar numbers of lung MCp and MNC (Fig. 4A). Sensitized and Ag-challenged CD1d-deficient mice had significant (65 and 66%) reductions in the numbers of recruited pulmonary MCp/106 MNC and in total pulmonary MCp per mouse, respectively (p < 0.02 for both, n = 7), but no reduction in the number of lung MNC relative to WT BALB/c mice sensitized and challenged in parallel (Fig. 4A). To confirm the requirement for CD1d-restricted cells in the Ag-elicited recruitment of MCp to lung in WT mice, we administered anti-CD1d before each aerosol challenge. The administration of mAb to block CD1d significantly reduced the number of MCp/106 MNC and total lung MCp by 59 and 69%, respectively (p < 0.05, n = 4 – 6), relative to WT BALB/c mice treated with isotype-matched Ig in parallel, and had no effect on the total number of lung MNC (Fig. 4B).
FIGURE 4.
CD1d-restricted NKT cells regulate the Ag-induced increase in pulmonary MCp numbers. A, BALB/c WT and CD1d−/− BALB/c mice were sensitized and half were challenged with aerosolized Ag as indicated. The mean (±SE) concentration of MCp/106 MNC (top panel), the number of lung MCp per mouse (middle panel), and the number of lung MNC per mouse (bottom panel) are shown from six, four, seven, and seven mice in each group, respectively. B, BALB/c mice were sensitized and treated with control IgG or anti-CD1d and either not challenged or challenged as indicated and evaluated as in A. The values are the mean (±SE) from four, four, and six mice in each group, respectively. C, BALB/c WT and Jα18−/− BALB/c mice were sensitized and half were challenged with aerosolized Ag as indicated. The mean (±SE) concentration of MCp/106 MNC (top panel), the number of lung MCp per mouse (middle panel), and the number of lung MNC per mouse (bottom panel) are shown from 7, 4, 14, and 14 mice in each group, respectively. *, p < 0.05; ***, p < 0.01; and ***, p < 0.001.
The genetic loss or Ab blockade of CD1d signaling ablates activation of both the type 1 or invariant NKT (iNKT) and the type 2 NKT cells (56, 57, 62, 63). Thus, Jα18-deficient mice which lack iNKT cells have been used to identify whether a response is dependent or independent of the integrity of this subset (27, 57, 63). In sensitized, unchallenged mice, the number of pulmonary MCp/106 MNC and total lung MCp in Jα18−/− mice was similar to that of WT BALB/c mice (Fig. 4C). In sensitized and aerosol Ag-challenged Jα18−/− mice, the increase in MCp/106 MNC and in total lung MCp was significantly greater than in the WT mice, 675 ± 118 vs 380 ± 39 MCp/106 MNC and 1977 ± 295 vs 736 ± 79 total lung MCp, respectively (mean ± SE, p < 0.001, n = 14 from six experiments; Fig. 4C). In two experiments, blockade with anti-CD1d effected a similar reduction in the number of MCp/106 MNC and in the total number of MCp recruited to the lung in sensitized and Ag-challenged Jα18−/− and BALB/c mice, 63.3 vs 71.4% and 77.2 vs 67.2%, respectively. The same finding of a comparable suppression of MCp recruitment in Jα18−/− and WT mice occurred in a single experiment with anti-IL-9 blockade (data not shown). These data indicate that iNKT cells are not required and that type 2 NKT cells mediate the Ag-induced increase in pulmonary MCp.
Blockade of both CD1d and IL-9 in aerosolized Ag- challenged mice does not further reduce MCp recruitment compared with a single deficiency
To address whether the requirements for CD1d-restricted NKT cells and IL-9 are in the same Ag-induced pathway for recruitment of MCp, we needed a protocol that examined their function during this critical time. We therefore determined whether deficiency and Ab blockade of both together further reduced the impaired Ag-induced recruitment of MCp observed with a single deficiency. IL-9-deficient mice treated with control Ig or with anti-CD1d and WT mice treated with anti-CD1d showed similar, statistically significant reductions in the number of MCp/106 MNC (Fig. 5A). Notably, relative to Ig-treated, Ag-challenged WT mice, the reduction in MCp recruitment per 106 MNC of 54.9% in IL-9-deficient mice treated with anti-CD1d (n = 9, p < 0.001) was not different from the reduction of 54.2% (n = 8, p < 0.001) observed in IL-9−/− mice treated with control Ig or from the 61.5% (n = 7, p < 0.001) in WT mice treated with anti-CD1d. The total number of lung MCp per mouse in IL-9-deficient mice treated with anti-CD1d were also not significantly different from the reductions observed with IL-9 deficiency alone or with anti-CD1d treatment of WT mice (data not shown). To confirm these findings, we compared MCp recruitment in CD1d−/− mice treated with Ig or anti-IL-9 to WT mice treated with Ig or anti-IL-9 in parallel. Relative to the Ag-induced recruitment of MCp/106 MNC in WT mice given control Ig (n = 4), the CD1d−/− mice showed a 57.5% reduction (n = 4, p < 0.05) that was not different from the 54.8% reduction observed after treatment with anti-IL-9 (n = 6, p < 0.05) or from the 52.9% reduction (n = 5, p < 0.05) in WT mice given anti-IL-9 (Fig. 5B). In a separate experiment, the reduction in MCp/106 MNC in WT mice given anti-IL-9 or anti-CD1d alone was not further reduced by treatment with both mAbs at the same time (data not shown).
FIGURE 5.
Blocking both IL-9 and CD1d produces no further reduction in the number of pulmonary MCp. A, BALB/c WT and IL-9−/− mice were sensitized and half were treated with control Ig and the other received anti-CD1d just before challenge with aerosolized Ag as indicated. The mean (±SE) concentration of MCp/106 MNC from six, seven, eight, and nine mice in each group, respectively, is presented. B, BALB/c WT and CD1d−/− mice were sensitized and half were treated with control Ig and the other received anti-IL-9 just before challenge with aerosolized Ag as indicated. The mean (±SE) concentration of MCp/106 MNC from four, five, four, and six mice in each group, respectively is presented.
Discussion
The presence of minimal numbers of constitutive MCp in the lung of sensitized BALB/c mice and the rapid and large (> 10-fold) increase in the number of MCp after three consecutive daily aerosolized Ag challenges provides a window for characterization of this early event in allergic pulmonary inflammation that leads to development of mature intraepithelial/mucosal MC (18, 19). Mature MC provide host defense functions but also are implicated in remodeling of the airways (6). Our previous genetic and immunologic studies demonstrate that this Ag-induced expansion of pulmonary MCp levels involves their influx by transendothelial migration from blood and that this response is observed across strains (18, 19). Although the minimal baseline numbers of MCp in lung of naive mice are T cell independent and do not change with sensitization (14, 18), their expansion with sensitization and aerosolized Ag challenge is absolutely T cell dependent, being absent in T cell-deficient RAG-2−/− and nude mice. Importantly, the T cell requirement for MCp recruitment is separated in time from the T cell role in sensitization because treatment with mAb to CD4 but not to CD8 in sensitized mice at the time of Ag challenge blocks MCp recruitment. In seeking to identify the subclass of the CD4+ T cells and the cytokines regulating the recruitment of MCp in sensitized mice, we found that the Ag-induced expansion of MCp in the lung is dependent on type 2 NKT cells and IL-9 without a requirement for classic polarized Th1 or Th2 cells or the IL-23-dependent expansion of the Th17 subset or their signature cytokine, IL-17A. IL-9 is coproliferative and antiapoptotic for MC in vitro and in vivo (64 – 66) and could increase the numbers of MCp in the circulation or, more likely, in situ after adhesion-based trans-endothelial migration. A requirement for CD1d-restricted type 2 NKT cells is consistent with an innate early host response in which they provide the IL-9. In support of this possibility, we demonstrated that the requirements for CD1d-restricted type 2 NKT cells and IL-9 in Ag-induced MCp recruitment are non-additive in that the absence of either one alone reduced MCp recruitment as much as their combined functional elimination. This indicates a shared pathway in which type 2 NKT cells provide or elicit IL-9 production.
Our findings indicate that the CD4+ T cells effecting the recruitment of the pulmonary MCp are downstream from those required during the sensitization step and are not CD8+, or Th2, Th1, or Treg polarization dependent. Ag-induced accumulation of lung MCp is normal in mice genetically deficient in IL-4, IL-4Rα, or STAT-6, mutations that markedly attenuate Th2 cell development (35, 36, 67, 68). Furthermore, blocking by mAb of two of the Th2 cytokines that promote MC growth, IL-3 and IL-10, also does not reduce the recruitment of pulmonary MCp. Similarly, mice genetically deficient in IFN-γ or in IL-12p40, both of which attenuate Th1 cell development (24, 25), or WT mice receiving blocking mAb to IFN-γ, IL-12p40, or the shared receptor chain IL-12p40Rβ1 show the usual robust increase in pulmonary MCp numbers with sensitization and aerosolized Ag challenge. The CD4+ CD25+ Treg cell was recently reported to be a source of IL-9 yet we found that the recruitment of MCp is not attenuated in mice depleted of the CD25+ cells, eliminating a role for this subset as the critical source of IL-9. We also considered a role for Th17 based on several recent studies implicating these cells and their signature cytokine IL-17A in allergic pulmonary inflammation (69, 70). However, compatible with the lack of effect from the genetic absence of IL-12p40 or mAb blockade of IL-12p40RB1, neither blocking the effector cytokine IL-17A nor blocking IL-6 or IL-23, which are involved in Th17 cell development or expansion, diminishes the recruitment of MCp.
Veldhoen et al. (71) described an IL-9- producing “Th9” cell that requires IL-4 for its development in culture. Our in vivo findings that recruitment of MCp occurs fully in the absence of IL-4 or its receptor do not favor such a cell. However, our findings are consistent with the report of Monteyne et al. (47) showing that after a single immunization IL-9 is expressed early in draining lymph nodes even in IL-4-deficient mice. Our cumulative findings against involvement of classic polarized T cells led to a consideration of a possible role for the NKT subclass which had been shown in vitro to be the source of IL-9 for the C57BL/6 strain (59, 60). Sensitized and challenged CD1d-deficient mice or WT BALB/c mice receiving CD1d-blocking mAb are each ~60% suppressed in recruitment of MCp, a magnitude similar to that occurring in IL-9-deficient or WT mice receiving blocking mAb to IL-9. Since a robust recruitment of MCp was found in Jα18−/− mice which lack the iNKT subpopulation (27), our data indicate that it is the type 2 NKT subpopulation that is critical to this response. That CD1d blockade in IL-9-deficient mice and IL-9 blockade in CD1d-deficient mice do not increase the partial suppression of MCp recruitment occurring with either genetic deficiency alone indicates that IL-9 and CD1d-restricted NKT cells function in the same pathway. The mAb depletion of the CD4+ cells at the time of aerosolized Ag challenge fully abrogated the recruitment of MCp compared with the significant but partial reduction of MCp recruitment in the absence or blockade of CD1d or IL-9. Thus, although we can account for the source of functional IL-9 in MCp recruitment, we cannot exclude a role for two different CD4+ T cells, a type 2 NKT cell and a MHC class II-restricted T cell. The latter could be particularly important for supplying the combination of cytokines needed for the subsequent development of mucosal MC hyperplasia and airway remodeling (6, 72).
The involvement of two classes of CD4+ cells in an in vivo response, with one of them being a NKT cell, has been observed before. Yoshimoto et al. (59) first ascribed a role for NKT cells in concert with another CD4+ T cell in the production of IgE after the i.v. administration of IL-18. In their study, IgE production was absent in CD1d-deficient mice and in MHC class II-deficient mice. The spleen cells of the MHC class II-deficient mice contained a normal number of NKT (CD4+NK1.1+) cells, and these mice were reconstituted for IL-18-induced IgE production after adoptive transfer of conventional MHC class II-restricted (CD4+ NK1.1−) T cells from WT mice. These authors also noted the production of IL-9 by CD4+NK1.1 cells after their stimulation in vitro with IL-2 and IL-18. Others have observed an essential role of iNKT cells in concert with MHC class II-restricted T cells in OVA-induced Th2-type pulmonary inflammation by finding suppression of inflammation in BALB/c and C57BL/6 strains deficient in CD1d-restricted NKT cells or in the Jα18 subpopulation (56, 57). The appearance of IL-13-induced airway hypersensitivity was a characteristic of this response and was directly observed with α-GalCer activation of iNKT in naive mice (56, 58). Administration of mAb to CD1d prevented the OVA-induced Th2-type pulmonary inflammation and appearance of OVA-specific IgE in blood of WT mice, and both findings were reconstituted by adoptive transfer of iNKT cells (57). In contrast, we found that after sensitization and aerosolized Ag challenge, the increase in pulmonary MCp is dependent on type 2 NKT cells as defined by intact recruitment in Jα18−/− mice and a significant reduction in CD1d−/− mice. That the impairments in MCp recruitment with CD1d or IL-9 deficiency are not incremental indicates that the type 2 NKT provide or elicit IL-9 production.
Acknowledgments
We thank Dr. Andrew McKenzie for providing the IL-9−/− mice and Dr. Masaru Taniguchi for providing the Jα18−/− mice.
Footnotes
This work was supported by Grants AI 031599, HL 036110, HL 076383, HL36028, and HL53993 from the National Institutes of Health, a Merit Award from the U.S. Department of Veterans Affairs (to F.D.F.), and a grant from the MedImmune Inc. (to M.F.G.).
Abbreviations used in this paper: MC, mast cell; DC, dendritic cell; α-GalCer, α-galactosylceramide; MCp, MC progenitor; MNC, mononuclear cell; Treg, regulatory T; SCF, stem cell factor; WT, wild type; iNKT, invariant NKT.
Disclosures
T.G.J., J.H., K.F.A., P.A., and F.D.F. have no competing financial interests to disclose. A.H. and T.B. are employees of MedImmune, Inc. M.F.G. received grant support for this study from MedImmune, Inc. and consulted for MedImmune, Inc.
References
- 1.Ruitenberg EJ, Elgersma A. Absence of intestinal mast cell response in congenitally athymic mice during Trichinella spiralis infection. Nature. 1976;264:258–260. doi: 10.1038/264258a0. [DOI] [PubMed] [Google Scholar]
- 2.Kamiya M, Oku Y, Itayama H, Ohbayashi M. Prolonged expulsion of adult Trichinella spiralis and eosinophil infiltration in mast cell-deficient W/Wv mice. J Helminthol. 1985;59:233–239. doi: 10.1017/s0022149x00008002. [DOI] [PubMed] [Google Scholar]
- 3.Knight PA, Wright SH, Lawrence CE, Paterson YY, Miller HR. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J Exp Med. 2000;192:1849–1856. doi: 10.1084/jem.192.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urban JF, Jr, Schopf L, Morris SC, Orekhova T, Madden KB, Betts CJ, Gamble HR, Byrd C, Donaldson D, Else K, Finkelman FD. Stat6 signaling promotes protective immunity against Trichinella spiralis through a mast cell- and T cell-dependent mechanism. J Immunol. 2000;164:2046–2052. doi: 10.4049/jimmunol.164.4.2046. [DOI] [PubMed] [Google Scholar]
- 5.Williams CM, Galli SJ. Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. J Exp Med. 2000;192:455–462. doi: 10.1084/jem.192.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu M, Tsai M, Tam SY, Jones C, Zehnder J, Galli SJ. Mast cells can promote the development of multiple features of chronic asthma in mice. J Clin Invest. 2006;116:1633–1641. doi: 10.1172/JCI25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taube C, Wei X, Swasey CH, Joetham A, Zarini S, Lively T, Takeda K, Loader J, Miyahara N, Kodama T, et al. Mast cells, FcεRI, and IL-13 are required for development of airway hyperresponsiveness after aerosolized allergen exposure in the absence of adjuvant. J Immunol. 2004;172:6398–6406. doi: 10.4049/jimmunol.172.10.6398. [DOI] [PubMed] [Google Scholar]
- 8.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 9.Huang C, De Sanctis GT, O’Brien PJ, Mizgerd JP, Friend DS, Drazen JM, Brass LF, Stevens RL. Evaluation of the substrate specificity of human mast cell tryptase βI and demonstration of its importance in bacterial infections of the lung. J Biol Chem. 2001;276:26276–26284. doi: 10.1074/jbc.M102356200. [DOI] [PubMed] [Google Scholar]
- 10.Thakurdas SM, Melicoff E, Sansores-Garcia L, Moreira DC, Petrova Y, Stevens RL, Adachi R. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J Biol Chem. 2007;282:20809–20815. doi: 10.1074/jbc.M611842200. [DOI] [PubMed] [Google Scholar]
- 11.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 12.Nigrovic PA, Binstadt BA, Monach PA, Johnsen A, Gurish M, Iwakura Y, Benoist C, Mathis D, Lee DM. Mast cells contribute to initiation of autoantibody-mediated arthritis via IL-1. Proc Natl Acad Sci USA. 2007;104:2325–2330. doi: 10.1073/pnas.0610852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodewald HR, Dessing M, Dvorak AM, Galli SJ. Identification of a committed precursor for the mast cell lineage. Science. 1996;271:818–822. doi: 10.1126/science.271.5250.818. [DOI] [PubMed] [Google Scholar]
- 14.Gurish MF, Tao H, Abonia JP, Arya A, Friend DS, Parker CM, Austen KF. Intestinal mast cell progenitors require CD49dβ7 (α4β7 integrin) for tissue-specific homing. J Exp Med. 2001;194:1243–1252. doi: 10.1084/jem.194.9.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arinobu Y, Iwasaki H, Gurish MF, Mizuno S, Shigematsu H, Ozawa H, Tenen DG, Austen KF, Akashi K. Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc Natl Acad Sci USA. 2005;102:18105–18110. doi: 10.1073/pnas.0509148102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guy-Grand D, Dy M, Luffau G, Vassalli P. Gut mucosal mast cells: origin, traffic, and differentiation. J Exp Med. 1984;160:12–28. doi: 10.1084/jem.160.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crapper RM, Schrader JW. Frequency of mast cell precursors in normal tissues determined by an in vitro assay: antigen induces parallel increases in the frequency of P cell precursors and mast cells. J Immunol. 1983;131:923–928. [PubMed] [Google Scholar]
- 18.Abonia JP, Hallgren J, Jones T, Shi T, Xu Y, Koni P, Flavell RA, Boyce JA, Austen KF, Gurish MF. α4 Integrins and VCAM-1, but not MAdCAM-1, are essential for recruitment of mast cell progenitors to the inflamed lung. Blood. 2006;108:1588–1594. doi: 10.1182/blood-2005-12-012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallgren J, Jones TG, Abonia JP, Xing W, Humbles A, Austen KF, Gurish MF. Pulmonary CXCR2 regulates VCAM-1 and antigen-induced recruitment of mast cell progenitors. Proc Natl Acad Sci USA. 2007;104:20478–20483. doi: 10.1073/pnas.0709651104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schleimer RP, Sterbinsky SA, Kaiser J, Bickel CA, Klunk DA, Tomioka K, Newman W, Luscinskas FW, Gimbrone MA, Jr, McIntyre BW. IL-4 induces adherence of human eosinophils and basophils but not neutrophils to endothelium: association with expression of VCAM-1. J Immunol. 1992;148:1086–1092. [PubMed] [Google Scholar]
- 21.Bochner BS, Klunk DA, Sterbinsky SA, Coffman RL, Schleimer RP. IL-13 selectively induces vascular cell adhesion molecule-1 expression in human endothelial cells. J Immunol. 1995;154:799–803. [PubMed] [Google Scholar]
- 22.Matsuura N, Zetter BR. Stimulation of mast cell chemotaxis by interleukin 3. J Exp Med. 1989;170:1421–1426. doi: 10.1084/jem.170.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meininger CJ, Yano H, Rottapel R, Bernstein A, Zsebo KM, Zetter BR. The c-kit receptor ligand functions as a mast cell chemoattractant. Blood. 1992;79:958–963. [PubMed] [Google Scholar]
- 24.Magram J, Connaughton SE, Warrier RR, Carvajal DM, Wu CY, Ferrante J, Stewart C, Sarmiento U, Faherty DA, Gately MK. IL-12-deficient mice are defective in IFNγ production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 25.Bettelli E, V, Kuchroo K. IL-12- and IL-23-induced T helper cell subsets: birds of the same feather flock together. J Exp Med. 2005;201:169–171. doi: 10.1084/jem.20042279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 27.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 28.Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chen BL, Dienger KM, Sproles AA, Shah JS, Kohl J, Belkaid Y, Wills-Karp M. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J Exp Med. 2005;202:1549–1561. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abonia JP, Austen KF, Rollins BJ, Joshi SK, Flavell RA, Kuziel WA, Koni PA, Gurish MF. Constitutive homing of mast cell progenitors to the intestine depends on autologous expression of the chemokine receptor CXCR2. Blood. 2005;105:4308–4313. doi: 10.1182/blood-2004-09-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dillon SB, MacDonald TT. Limit dilution analysis of mast cell precursor frequency in the gut epithelium of normal and Trichinella spiralis infected mice. Parasite Immunol. 1986;8:503–511. doi: 10.1111/j.1365-3024.1986.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 31.Madden KB, Urban JF, Jr, Ziltener HJ, Schrader JW, Finkelman FD, Katona IM. Antibodies to IL-3 and IL-4 suppress helminth-induced intestinal mastocytosis. J Immunol. 1991;147:1387–1391. [PubMed] [Google Scholar]
- 32.Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DA, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 33.Finkelman FD, Morris SC, Orekhova T, Mori M, Donaldson D, Reiner SL, Reilly NL, Schopf L, Urban JF., Jr Stat6 regulation of in vivo IL-4 responses. J Immunol. 2000;164:2303–2310. doi: 10.4049/jimmunol.164.5.2303. [DOI] [PubMed] [Google Scholar]
- 34.Hsieh CS, Heimberger AB, Gold JS, O’Garra A, Murphy KM. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an αβT-cell-receptor transgenic system. Proc Natl Acad Sci USA. 1992;89:6065–6069. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noben-Trauth N, Shultz LD, Brombacher F, Urban JF, Jr, Gu H, Paul WE. An interleukin 4 (IL-4)-independent pathway for CD4+ T cell IL-4 production is revealed in IL-4 receptor-deficient mice. Proc Natl Acad Sci USA. 1997;94:10838–10843. doi: 10.1073/pnas.94.20.10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jankovic D, Kullberg MC, Noben-Trauth N, Caspar P, Paul WE, Sher A. Single cell analysis reveals that IL-4 receptor/Stat6 signaling is not required for the in vivo or in vitro development of CD4+ lymphocytes with a Th2 cytokine profile. J Immunol. 2000;164:3047–3055. doi: 10.4049/jimmunol.164.6.3047. [DOI] [PubMed] [Google Scholar]
- 37.Randolph DA, Carruthers CJL, Szabo SJ, Murphy KM, Chaplin DD. Modulation of airway inflammation by passive transfer of allergen-specific Th1 and Th2 cells in a mouse model of asthma. J Immunol. 1999;162:2375–2383. [PubMed] [Google Scholar]
- 38.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-β induces development of the TH17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 39.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 41.Lantz CS, Boesiger J, Song CH, Mach N, Kobayashi T, Mulligan RC, Nawa Y, Dranoff G, Galli SJ. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392:90–93. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- 42.Demoulin JB, Van Roost E, Stevens M, Groner B, Renauld JC. Distinct roles for STAT1, STAT3, and STAT5 in differentiation, gene induction, and apoptosis inhibition by interleukin-9. J Biol Chem. 1999;274:25855–25861. doi: 10.1074/jbc.274.36.25855. [DOI] [PubMed] [Google Scholar]
- 43.Demoulin JB, Uyttenhove C, Lejeune D, Mui A, Groner B, Renauld JC. STAT5 activation is required for interleukin-9-dependent growth and transformation of lymphoid cells. Cancer Res. 2000;60:3971–3977. [PubMed] [Google Scholar]
- 44.Renauld JC, Houssiau F, Louahed J, Vink A, Van Snick J, Uyttenhove C. Interleukin-9. Adv Immunol. 1993;54:79–97. doi: 10.1016/s0065-2776(08)60533-7. [DOI] [PubMed] [Google Scholar]
- 45.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 46.Hultner L, Kolsch S, Stassen M, Kaspers U, Kremer JP, Mailhammer R, Moeller J, Broszeit H, Schmitt E. In activated mast cells, IL-1 up-regulates the production of several Th2-related cytokines including IL-9. J Immunol. 2000;164:5556–5563. doi: 10.4049/jimmunol.164.11.5556. [DOI] [PubMed] [Google Scholar]
- 47.Monteyne P, Renauld JC, Van Broeck J, Dunne DW, Brombacher F, Coutelier JP. IL-4-independent regulation of in vivo IL-9 expression. J Immunol. 1997;159:2616–2623. [PubMed] [Google Scholar]
- 48.Townsend MJ, Fallon PG, Matthews DJ, Smith P, Jolin HE, McKenzie ANJ. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity. 2000;13:573–583. doi: 10.1016/s1074-7613(00)00056-x. [DOI] [PubMed] [Google Scholar]
- 49.McMillan SJ, Bishop B, Townsend MJ, McKenzie AN, Lloyd CM. The absence of interleukin 9 does not affect the development of allergen-induced pulmonary inflammation nor airway hyperreactivity. J Exp Med. 2002;195:51–57. doi: 10.1084/jem.20011732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stassen M, Muller C, Arnold M, Hultner L, Klein-Hessling S, Neudorfl C, Reineke T, Serfling E, Schmitt E. IL-9 and IL-13 production by activated mast cells is strongly enhanced in the presence of lipopolysaccharide: NF-κB is decisively involved in the expression of IL-9. J Immunol. 2001;166:4391–4398. doi: 10.4049/jimmunol.166.7.4391. [DOI] [PubMed] [Google Scholar]
- 51.Fujita J, Nakayama H, Onoue H, Ebi Y, Kanakura Y, Kuriu A, Kitamura Y. Failure of W/Wv mouse-derived cultured mast cells to enter S phase upon contact with NIH/3T3 fibroblasts. Blood. 1988;72:463–468. [PubMed] [Google Scholar]
- 52.Eklund KK, Ghildyal N, Austen KF, Friend DS, Schiller V, Stevens RL. Mouse bone marrow-derived mast cells (mBMMC) obtained in vitro from mice that are mast cell-deficient in vivo express the same panel of granule proteases as mBMMC and serosal mast cells from their normal littermates. J Exp Med. 1994;180:67–73. doi: 10.1084/jem.180.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamazaki M, Tsujimura T, Morii E, Isozaki K, Onoue H, Nomura S, Kitamura Y. c-kit gene is expressed by skin mast cells in embryos but not in puppies of Wsh/Wsh mice: age-dependent abolishment of c-kit gene expression. Blood. 1994;83:3509–3516. [PubMed] [Google Scholar]
- 54.Geissler EN, Ryan MA, Housman DE. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988;55:185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- 55.Huang E, Nocka K, Beier DR, Chu TY, Buck J, Lahm HW, Wellner D, Leder P, Besmer P. The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell. 1990;63:225–233. doi: 10.1016/0092-8674(90)90303-v. [DOI] [PubMed] [Google Scholar]
- 56.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 57.Lisbonne M, Diem S, Castro Keller A, Lefort J, Araujo LM, Hachem P, Fourneau JM, Sidobre S, Kronenberg M, Taniguchi M, et al. Cutting edge: invariant Vα14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J Immunol. 2003;171:1637–1641. doi: 10.4049/jimmunol.171.4.1637. [DOI] [PubMed] [Google Scholar]
- 58.Meyer EH, Goya S, Akbari O, Berry GJ, Savage PB, Kronenberg M, Nakayama T, DeKruyff RH, Umetsu DT. Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc Natl Acad Sci USA. 2006;103:2782–2787. doi: 10.1073/pnas.0510282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoshimoto T, Min B, Sugimoto T, Hayashi N, Ishikawa Y, Sasaki Y, Hata H, Takeda K, Okumura K, Van Kaer L, et al. Nonredundant roles for CD1d-restricted natural killer T cells and conventional CD4+ T cells in the induction of immunoglobulin E antibodies in response to interleukin 18 treatment of mice. J Exp Med. 2003;197:997–1005. doi: 10.1084/jem.20021701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS, Berzins SP, Smyth MJ, Godfrey DI. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4− NK1.1− NKT cell population. Proc Natl Acad Sci USA. 2008;105:11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watarai H, Nakagawa R, Omori-Miyake M, Dashtsoodol N, Taniguchi M. Methods for detection, isolation and culture of mouse and human invariant NKT cells. Nat Protocols. 2008;3:70–78. doi: 10.1038/nprot.2007.515. [DOI] [PubMed] [Google Scholar]
- 62.Smiley ST, Kaplan MH, Grusby MJ. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science. 1997;275:977–979. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- 63.Terabe M, Swann J, Ambrosino E, Sinha P, Takaku S, Hayakawa Y, Godfrey DI, Ostrand-Rosenberg S, Smyth MJ, Berzofsky JA. A nonclassical non-Vα14Jα18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med. 2005;202:1627–1633. doi: 10.1084/jem.20051381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hultner L, Druez C, Moeller J, Uyttenhove C, Schmitt E, Rude E, Dormer P, Van Snick J. Mast cell growth-enhancing activity (MEA) is structurally related and functionally identical to the novel mouse T cell growth factor P40/TCGFIII (interleukin 9) Eur J Immunol. 1990;20:1413–1416. doi: 10.1002/eji.1830200632. [DOI] [PubMed] [Google Scholar]
- 65.Godfraind C, Louahed J, Faulkner H, Vink A, Warnier G, Grencis R, Renauld JC. Intraepithelial infiltration by mast cells with both connective tissue-type and mucosal-type characteristics in gut, trachea, and kidneys of IL-9 transgenic mice. J Immunol. 1998;160:3989–3996. [PubMed] [Google Scholar]
- 66.Suzuki K, Nakajima H, Watanabe N, Kagami S, Suto A, Saito Y, Saito T, Iwamoto I. Role of common cytokine receptor γ chain (γc)- and Jak3-dependent signaling in the proliferation and survival of murine mast cells. Blood. 2000;96:2172–2180. [PubMed] [Google Scholar]
- 67.Corry DB, Grunig G, Hadeiba H, Kurup VP, Warnock ML, Sheppard D, Rennick DM, Locksley RM. Requirements for allergen-induced airway hyperreactivity in T and B cell-deficient mice. Mol Med. 1998;4:344–355. [PMC free article] [PubMed] [Google Scholar]
- 68.Mattes J, Yang M, Siqueira A, Clark K, MacKenzie J, McKenzie AN, Webb DC, Matthaei KI, Foster PS. IL-13 induces airways hyperreactivity independently of the IL-4R α-chain in the allergic lung. J Immunol. 2001;167:1683–1692. doi: 10.4049/jimmunol.167.3.1683. [DOI] [PubMed] [Google Scholar]
- 69.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 70.Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203:2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Veldhoen M, Uyttenhove C, Van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-β “reprograms” the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 72.Ikeda RK, Miller M, Nayar J, Walker L, Cho JY, McElwain K, McElwain S, Raz E, Broide DH. Accumulation of peribronchial mast cells in a mouse model of ovalbumin allergen induced chronic airway inflammation: modulation by immunostimulatory DNA sequences. J Immunol. 2003;171:4860–4867. doi: 10.4049/jimmunol.171.9.4860. [DOI] [PubMed] [Google Scholar]