Abstract
Lung cancer has become increasingly common in women, and gender differences in the physiology and pathogenesis of the disease have suggested a role for estrogens. In the lung recent data have shown local production of estrogens from androgens via the action of aromatase enzyme and higher levels of estrogen in tumor tissue as compared with surrounding normal lung tissue. High levels of aromatase expression are also maintained in metastases as compared with primary tumors. Consistent with these findings, clinical studies suggest that aromatase expression may be a useful predictive biomarker for prognosis in the management of non-small cell lung cancer (NSCLC), the most common form of lung malignancy. Low levels of aromatase associate with a higher probability of long-term survival in older women with early stage NSCLC. Treatment of lung NSCLC xenografts in vivo with an aromatase inhibitor (exemestane) alone or combined with standard cisplatin chemotherapy elicits a significant reduction in tumor progression as compared to paired controls. Further, lung cancer progression is also governed by complex interactions between estrogen and growth factor signaling pathways to stimulate the growth of NSCLC as well as tumor-associated angiogenesis. We find that combination therapy with the multitargeted growth factor receptor inhibitor vandetanib and the estrogen receptor antagonist fulvestrant inhibit tumor growth more effectively than either treatment administered alone. Thus, incorporation of antiestrogen treatment strategies in standard antitumor therapies for NSCLC may contribute to improved patient outcome, an approach that deserves to be tested in clinical trials.
Keywords: non-small cell lung cancer (NSCLC), aromatase, CYP19, estrogen receptor (ER), epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF) receptor, anastrazole, exemestane, fulvestrant, vandetanib
Introduction
Lung cancer is currently the leading cause of cancer-related mortality worldwide. Despite recent advances in the clinical management of lung cancer, the prognosis for lung cancer patients remains unacceptably poor.1 A causal relationship between smoking and lung cancer is well established, but differences in smoking patterns of men and women are not sufficient to explain apparent biological differences between genders in lung cancer incidence and prognosis.2 Among patients who never smoked women seem to have a higher incidence of lung cancer and to clinically respond better to use of epidermal growth factor receptor (EGFR) inhibitors such as gefitinib and erlotinib.3 Such differences in the clinicopathological characteristics of non-small cell lung cancer (NSCLC) between men and women suggest that gender-dependent factors may play a role in the etiology and progression of NSCLC.4,5
Emerging evidence suggests that estrogen signaling is involved in the pathogenesis of lung cancer, but the exact role of estrogens in this process is unclear. Women are more likely to develop NSCLC, especially adenocarcinoma, and they generally present with the disease at an earlier age and less advanced stage than that found in men.6 It is reported that both endogenous and exogenous estrogens may play a role in the etiology and progression of lung cancer. However, studies investigating exogenous estrogen as a risk factor for lung cancer development have been controversial, with some studies demonstrating an increased incidence of lung cancer with the use of hormone replacement therapy (HRT)7–9 and others suggesting a protective effect.10–12 Schwartz et al.10 determined that postmenopausal hormone exposure is associated with a reduced risk for the later development of NSCLC expressing estrogen receptor-alpha (ER-α) and estrogen receptor-beta (ER-β). In contrast, Ganti et al.9 report that the survival of women with lung cancer who actively took HRT was significantly lower than the survival of those women with lung cancer who were not exposed to exogenous estrogens.9 Thus, it is possible that differences in the tumorigenic effects of estrogens in these studies may be related to the time of administration of estrogens relative to the development or presence of malignancy.
Lung cancer progression is also governed by complex interactions between estrogen and growth factor signaling pathways to stimulate the growth of NSCLC as well as tumor-associated angiogenesis. In the present study we have examined how estrogen signaling can influence NSCLC growth and how interactions with growth factors signaling pathways promote lung cancer. Further, new findings are presented to show that selective targeting of these complex, intersecting pathways can effectively inhibit the growth of human NSCLC.
Materials and Methods
Cell Culture and Cell Proliferation Assays
Human NSCLC cells NCI-H23 and NCI-A549 were obtained from the American Type Culture Collection (ATCC; Manassas, Virginia). Cell lines were routinely maintained in RPMI 1640 medium with 10% fetal bovine serum (FBS, Invitrogen/Life Technologies) and 1% antibiotic–antimycotic solution 100X, (Mediatech, Herndon, Virginia). For estrogen-free conditions, media were changed 48 h before experiments to phenol-red free RPMI 1640 with 0.1% dextrancoated, charcoal-treated (DCC) FBS, as before.13
For proliferation assays cells were cultured in estrogen-free conditions for 48 h, then treated with vehicle, 10 nM estradiol-17β, 1 μM fulvestrant (Faslodex, ICI 182,780; AstraZeneca), or a combination of both agents. After 72 h, cells were counted manually to estimate rates of cell proliferation, with data from four independent experiments.
Assay of Aromatase by Immunohistochemistry in Human Lung Tumors
Patient specimens and information were collected under Institutional Review Board-approved and Health Insurance Portability and Accountability Act (HIPAA)-compliant protocols at the University of California at Los Angeles Medical Center.
Formalin-fixed, paraffin-embedded tissue specimens were cut in 4-μm sections and placed on slides. Standard immunohistochemical (IHC) procedures were then followed for staining.14 In brief, sections were deparaffinized in xylene and hydrated in graded alcohols. Antigen recovery was done in 10 mM sodium citrate buffer (pH 6.0) in a heated water bath at 90°C for 30 min. Slides were incubated with anti-aromatase antibody C-16 (Santa Cruz Biotechnology) for 1 h. Primary antibody detection was accomplished using a secondary anti-goat-peroxidase antibody followed by incubation with the DAB500 chromogen system (Biocare Medical).
Aromatase Activity Assay
Cells were grown to 50% confluence and then starved for 48 h in phenol-red free RPMI with 0.1% DCC FBS. After serum starvation, cells were treated with experimental agents for 48 h. Aromatase activity was assessed by use of radiolabeled substrate, [1β-3H]androst-4-ene-3,17-dione (Perkin-Elmer, Boston, Massachusetts) with established methods.15 Controls included use of cells without [1β-3H]androst-4-ene-3,17-dione and with deletion of cells or tissues.
Western Blot
Before each experiment NSCLC cells were maintained in estrogen-free conditions as described before. After 48 h, cells were treated with experimental agents for 24 h. Then, 40 μg of total protein lysates were separated by SDS-PAGE and transferred to nitrocellulose membranes. Immunodetection was done with anti-aromatase antibody C-16 at a 1:100 dilution (Santa Cruz Biotechnology). For a loading control, membranes were probed with anti-actin antibody. Visualization was done with enhanced chemiluminescence reagents from Amersham Biosciences.
In Vivo Growth of Human NSCLC Xenografts in Nude Mice
Ovariectomized nude mice at 6 weeks of age were obtained from Harlan Sprague Dawley. To prepare human lung tumor xenografts, 2 × 108-H23 NSCLC cells were injected subcutaneously in each nude mouse. When tumors grew to 50–75 mm3, animals were randomized to different treatment groups. In experiments to determine the antitumor effects of fulvestrant (Faslodex, AstraZeneca) and vandetanib (Zactima, AstraZeneca) alone and in combination, mice were primed with extended-release pellets of estradiol-17β (Innovative Research of America) before cells were implanted subcutaneously. Then, at the time of randomization for therapy, mice were treated with control, fulvestrant (5 mg subcutaneous weekly for 21 days), vandetanib (75 mg/kg/day by oral gavage daily for 21 days), or a combination of both agents. In experiments to assess the antitumor activity of exemestane (Aromasin; Pfizer) and cisplatin (Platinol; Bristol-Meyers Squibb), mice were injected with androstenedione (0.1 mg/mouse) subcutaneously (sc) daily throughout the experiment to provide substrate for aromatase. Cisplatin (6 mg/kg/week IP for 21 days) and exemestane (0.15 mg/mouse/day sc for 6 weeks) were then administered either alone or as a dual therapy.
Tumor volumes for mice in experimental and control groups were measured every 3 to 4 days, with tumor volume calculated by (l × w × h), for tumor length l, tumor width w, and tumor height h in mm. Data were presented as the mean ±SEM for tumor volumes measured in cubic mm. Data were analyzed by use of Student’s t-test and ANOVA statistical approaches as before.13,16,17 All studies with animals were approved by our institutional Animal Research Committee.
Results
Estrogen Stimulates Growth of Human NSCLC
Both ER-α and ER-β have been demonstrated to be expressed and active in several NSCLC cell lines and in normal and tumor specimens from the clinic.17–23 As shown in Figure 1, treatment with estradiol promotes a significant twofold increase in the growth of human NSCLC cells grown in vitro. Moreover, this estrogen-induced effect is blocked by the pure antiestrogen fulvestrant, indicating the specificity of this action (Fig. 1).
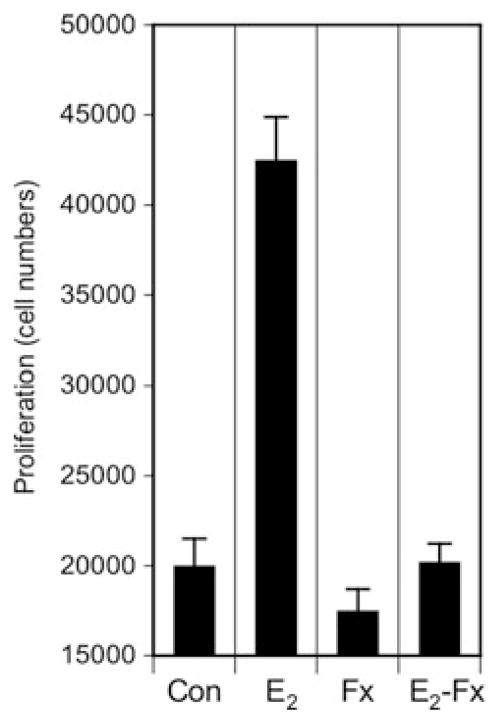
Figure 1.
Estradiol-17β stimulates proliferation of human lung NSCLC cell growth in vitro. H23 cells were grown in estrogen-free conditions 48 h before proliferation studies. Cells were treated with control vehicle (Con), 10 nM estradiol-17β (E2), 1 μM fulvestrant (Fx) or with estradiol plus fulvestrant (E2-Fx) for 48 h before cell counts. Estradiol increased cell numbers to more than 2 times control, and this estrogen-induced effect was blocked by the antiestrogen fulvestrant (both results significantly different from control at P < 0.01 (Student’s t-test). H23 cells express ER.16
Aromatase Is Expressed in NSCLC Tumors and Their Loco-Regional Metastasis
We previously showed aromatase expression by immunohistochemistry in human NSCLC tumor specimens. In order to determine if aromatase is also expressed in metastatic tissues, we evaluated aromatase expression in primary tumors and their loco-regional metastases (Fig. 2). Specific staining for aromatase is present in primary and paired metastatic lesions, with representative examples in Figure 2. Moreover, aromatase expression is enriched in 60% of the metastases as compared with that detected in primary tumor from the same patient.
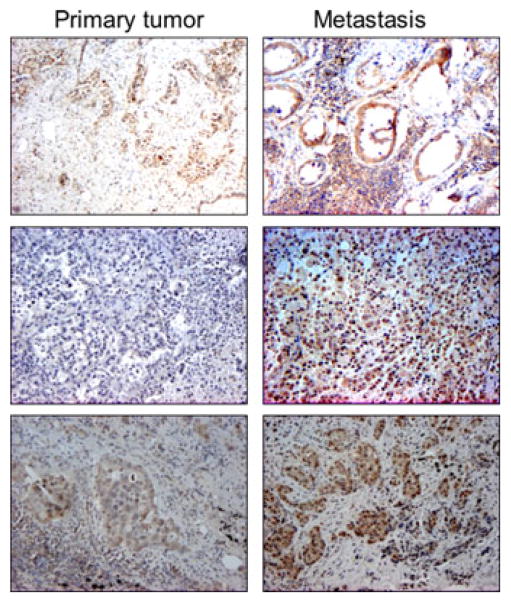
Figure 2.
Immunohistochemistry for aromatase in primary NSCLC (left column of panels) and metastatic lesions (right column of panels) from the same patients. Formalin-fixed, paraffin-embedded tumors were processed for IHC using aromatase antibody (C-16, Santa Cruz Biotechnology). Appropriate tissue and reagent controls were done to confirm specificity of the staining. Aromatase is expressed in both primary and metastatic tumor pairs from 10 patients and is enriched in 60% of metastasis. Representative examples of staining patterns are shown for 3 patients. In cases evaluated to date, enrichment of aromatase expression associates with tumor spread to loco-regional sites.
Dual Cisplatin and Exemestane Block in Vivo Growth of Lung Tumor Xenografts and Enhance Time to Tumor Progression
Aromatase enzyme is critical in the synthesis of estrogens in vivo, and blockade of aromatase activity may adversely impact estrogen signaling. Thus, in preclinical laboratory studies the nonsteroidal aromatase inhibitor anastrazole reduced lung tumor cell growth in vitro and in human NSCLC xenografts in vivo.14 To assess the in vivo antitumor effect of a steroidal aromatase inhibitor, exemestane, and to compare its activity with that of cisplatin, a standard chemotherapy for NSCLC,24 H23 lung tumor cells were grown as xenografts in ovariectomized nude mice treated daily with aromatase substrate androstenedione (Fig. 3). After tumors grew to a limiting size, mice were divided into four treatment groups including control, exemestane, cisplatin, or a combination of both agents and then treated. As expected, NSCLC growth was significantly suppressed by administration of cisplatin in vivo. Notably, growth of tumors was also significantly reduced by treatment with exemestane (P < 0.001) alone, and administration of exemestane together with cisplatin chemotherapy elicited an apparent synergistic effect in blocking NSCLC progression as compared to that achieved with either treatment given alone (P < 0.001).
Figure 3.
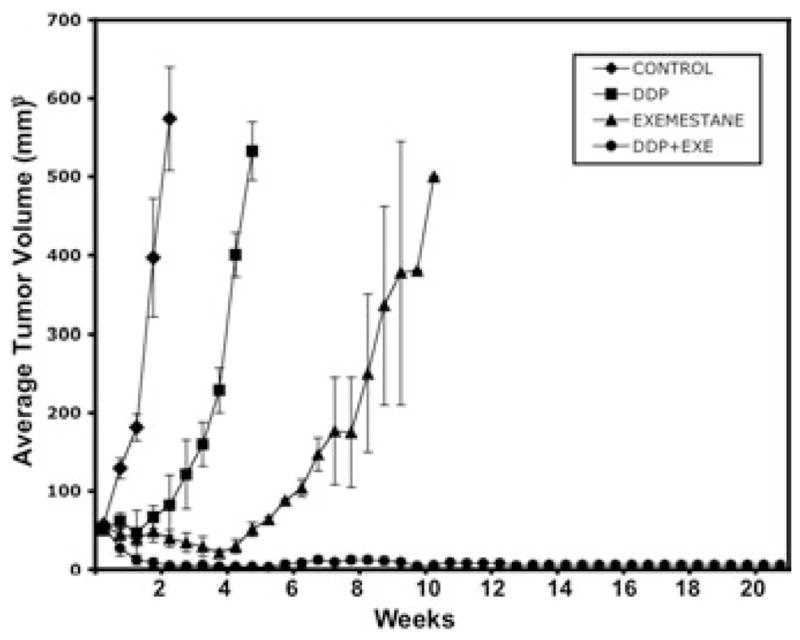
Combination therapy with exemestane and cisplatin blocks growth of NSCLC tumor xenografts. Ovariectomized nude mice (6 weeks of age) were implanted with human H23 tumor xenografts. Mice were supplemented with androstenedione sc each day throughout the experiment.16 When tumors reached 50–100 mm3 in size, mice were randomized to different treatment groups, including control [◆], cisplatin, (6 mg/kg/week IP for 21 days) [DDP, ■], exemestane (0.15 mg/mouse/day sc for 6 wks) [▲], and a combination group with cisplatin and exemestane [●]. Tumor volumes and the time to tumor progression were then recorded for each group. Time to tumor progression was significantly enhanced among those mice treated with the combination of cisplatin and exemestane as compared with animals given either control, cisplatin alone, or exemestane alone (P < 0.001). Data are presented as the mean ±SEM for tumor volumes measured in mm3. Data were analyzed by use of Student’s t-test and ANOVA statistical approaches, as before.13
EGF Increases Expression and Activity of Aromatase
To further investigate interactions between growth factor receptor and ER signaling pathways in NSCLC,16,17,20,21,41 we assessed the influence of growth factor signaling pathways on the expression and activity of aromatase. H23 lung tumor cells were treated in vitro with EGF alone, estrogen alone, or a combination of both agents. The results show that EGF and estrogen both acted to increase aromatase expression, and these effects were inhibited by the multitargeted growth factor receptor inhibitor vandetanib (Fig. 4A). Further, aromatase activity was determined in A549 lung tumor cells treated with EGF alone or in the presence of the EGFR inhibitor erlotinib. As shown in Figure 4B, EGF elicits a marked increase in aromatase activity (P < 0.001), and this EGF-induced effect is significantly suppressed by erlotinib (P < 0.001) (Fig. 4B). Thus, another link between growth factor receptor and estrogen signaling may depend on regulation of aromatase by growth factor signaling in NSCLC.
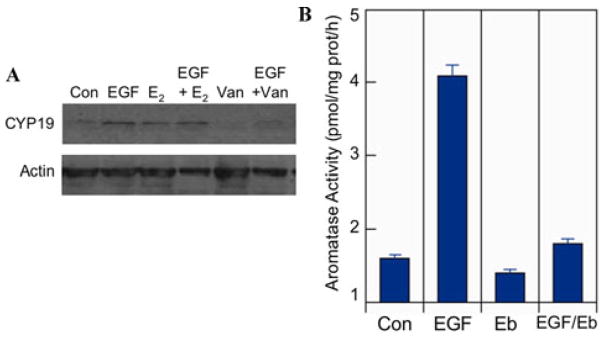
Figure 4.
EGF increases aromatase expression and activity. (A) H23 cells were serum-starved and then treated with control (Con), 10 nM estradiol-17β (E2), 10 nM EGF, a combination of both (EFG+ E2), 5 μM vandetanib (Van) and vandetanib plus EGF (EGF/Van). Western blots with anti-aromatase antibody after 24 h treatment showed increased levels of aromatase after EGF treatment that was inhibited by vandetanib. (B) A549 cells were cultured and preincubated in growth factor-depleted medium prior to treatment with control (Con), 10 nM EGF (EGF), 10 μM erlotinib (Eb), or both agents (EGF/Eb). After 48 h, aromatase activity was measured by conversion of androstenedione substrate to estrogens using a radioassay as before.14 Data on aromatase activity are given as pmol/mg protein/h (n = 3).
Dual Antiestrogen and Antigrowth Factor Therapies Inhibit Growth of Lung Tumor Xenografts
To assess the potential benefit of combining multitargeted therapies in NSCLC management, we tested the antitumor activity of dual administration of vandetanib and the estrogen receptor antagonist, fulvestrant (Faslodex; ICI 182, 780). Ovariectomized female nude mice were injected with 2 × 108H23 NSCLC cells after implantation of estradiol pellets. When tumors reached 50–100 mm3 in size, mice were randomized to different treatment groups and treatment was initiated with control, vandetanib, fulvestrant, or a combination of both agents (Fig. 5). Inhibition of tumor growth was observed after therapy with either vandetanib or fulvestrant alone as compared with controls, but the maximal antitumor response and time to tumor progression was achieved with dual treatment with vandetanib and fulvestrant (P < 0.001). At about 3 months after termination of antitumor drug treatments, animals in the combination therapy group remained free of recurrent disease (Fig. 5).
Figure 5.
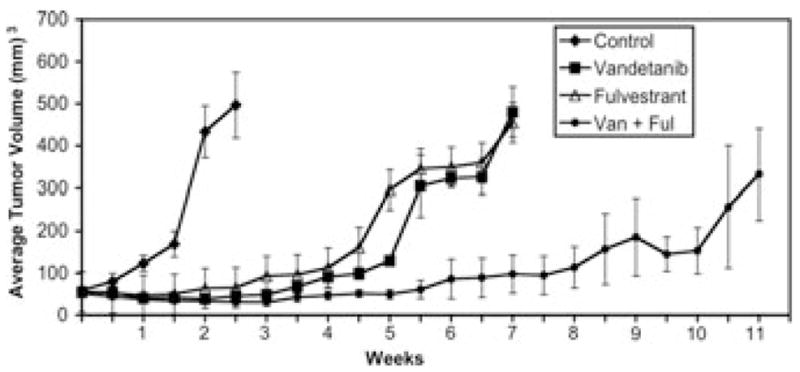
Combination therapy with vandetanib and fulvestrant blocks growth of NSCLC tumor xenografts and enhances time to tumor progression. Ovariectomized nude mice were primed with extended-release estradiol-17β pellets (Innovative Research of America). H23 NSCLC cells were then implanted as subcutaneous xenografts in mice as before.17 When tumors reached 50–100 mm3, mice were randomized to different treatment groups, including control [◆], vandetanib [■] (75 mg/kg/day, by oral gavage, daily for 21 days), fulvestrant [△] (5 mg subcutaneous, weekly, for 21 days), and a combination group with fulvestrant and vandetanib [●]. Data are presented as the mean ± SEM for tumor volumes measured in mm3. Data were analyzed by use of Student’s t-test and ANOVA statistical approaches, as reported before.13
Discussion
Although the lung was not previously considered a target organ for sex steroids, new evidence clearly shows that estrogen signaling plays an important role in lung biology.9,16–18,20–23,35 In studies of specific [3H]-estradiol binding in subcellular fractions of human NSCLC cells after controlled homogenization and quantitative cell fractionation,20 specific estrogen binding was enriched predominantly in cell nuclei, but significant binding also occurred in extranuclear fractions. Estrogen binding in NSCLC cells was saturable, ligand-specific and displayed high-affinity characteristics of an estrogen receptor. These findings are consistent with earlier work showing high-affinity estrogen binding in rat lung25 and are confirmed by recent findings of high-affinity, limited capacity ER in human NSCLC cells20 and by significant nuclear expression of ERα and ERβ in archival NSCLC specimens from the clinic.17,19–22
Studies with the ER-β knockout mouse show that ER-β is important in normal lung physiology and homeostasis. Female ER-β−/− mice have markedly abnormal lung structure and systemic hypoxia.26,27 More recently, studies with a genetically defined mouse model of lung adenocarcinoma indicated that estrogen significantly increases tumor number and volume in ovariectomized females and in males. A difference was also observed between ovary-intact females that exhibited higher grade tumors than did males or ovariectomized females.28 In this laboratory model both normal and tumorous lung tissues expressed ER-β, as demonstrated on Western blots by a prominent band at 59 kDa. Further, it has been reported in carcinogen-induced animal models that females have higher tumor multiplicity as compared with male animals.29,30 Thus, current evidence suggests a basis for sex-related differences in normal lung and lung cancer, and further investigation is clearly needed to better understand the role of estrogens in lung cancer.8,22,31
Aromatase is one of the cytochrome P450 enzymes that catalyze the synthesis of estrogens from androgens. It is responsible for aromatization of the A-ring of C19 steroids, resulting in formation of the phenolic A-ring characteristic of estrogens.32 It is expressed in a number of tissues such as ovary, testis, brain, bone, and adipose tissue of both males and females.33 More recently, it has also been shown to be expressed in lung, in particular in NSCLC.14,22,34,35 Aromatase occurs in about 86% of female and male NSCLC as determined by immunohistochemistry, and aromatase activity was found to be higher in tumor tissue as compared to that in surrounding normal tissue.14 Further, we have now evaluated aromatase expression in archival primary human tumors and their loco-regional metastases. Aromatase as assessed by IHC is not only maintained in metastatic lesions as compared with primary sites from the same patient, but aromatase is enriched in most metastases examined to date. This suggests that estrogens synthesized in metastatic tumors are available to further promote malignant progression that may, in turn, be susceptible to aromatase inhibitors. This finding is important for future targeting of advanced NSCLC using endocrine therapies.14 Further, aromatase expression in NSCLC as assessed by immunohistochemistry was found to be a strong prognostic factor by Mah et al.,35 with lower levels of aromatase predicting a greater chance of long-term survival in women 65 years and older. This association seems very significant for women with no history of smoking in which lower aromatase levels were a strong predictor of survival.35 These findings implicate aromatase as an early-stage predictor of survival and suggest that aromatase inhibitors may prove useful in the clinic for the treatment of lung cancer.
Consistent with these findings, Niikawa et al. recently reported on intratumoral levels of estrogen in NSCLC.22 Intratumoral estradiol concentration was significantly associated with aromatase expression, but not with expression of 17β-hydroxy steroid dehydrogenase type 1 (17βHSD1) or type 2 (17βHSD2), as measured by RT/PCR.22 The steroid concentration in NSCLC was significantly higher (2.2-fold) than that found in non-neoplastic tissue. This finding was similar to that previously reported in tissue specimens from breast cancer patients.31 The intratumoral concentration of estradiol in NSCLC was about 20 times lower than that detected in breast carcinomas of postmenopausal women. However, the relative ratio of intratumoral estradiol to that in corresponding non-neoplastic tissue of the same patient was similar between these two carcinomas (2.2 in NSCLC and 2.3 in breast carcinoma).
In breast cancers this local tissue source of estrogens is not necessarily related to circulating levels of the hormone and provides a stimulus for the growth of ER-positive tumors. It is also notable that plasma concentrations of testosterone and androstenedione are higher in men than in postmenopausal women, and circulating androgens are major precursor substrates for local estradiol production by aromatase-expressing tissues.22 Consistent with this notion, Coombes et al. reported that the development of lung cancers was reduced in breast cancer patients treated with long-term administration of the steroidal aromatase inhibitor exemestane, suggesting that aromatase and local estrogen production may be associated with lung cancer risk in the clinic.36 Thus, a new approach to suppress NSCLC may be to block estrogen signaling (using aromatase inhibitors or antiestrogens) in combination with current approved chemotherapies or inhibitors of growth factor receptor signaling.
In preclinical laboratory studies the non-steroidal aromatase inhibitor anastrazole reduced cell growth in vitro as it did in lung tumor xenografts in vivo.14 Additional studies now demonstrate that the steroidal aromatase inhibitor exemestane also suppresses the growth of NSCLC xenografts in nude mice. In addition, we find that administration of exemestane in combination with cis-platin elicits an apparent synergistic effect in blocking NSCLC progression. These findings point toward a potential new strategy to manage NSCLC in future clinical trials. In breast cancer and other estrogen target tissues, the pathways that regulate aromatase expression still need to be fully elucidated. Immunoreactivity of aromatase is detected in various types of breast tissue cells including stromal cells, carcinoma cells, and normal duct epithelial cells,37 and stromal–tumor cell interactions are postulated to contribute to aromatase regulation. In addition, prostaglandin E2 (PGE2) produced in breast cancer cells stimulates aromatase expression, and several reports suggest that cyclooxigenase-2 (COX-2) and PGE2 may act locally in breast to stimulate aromatase expression and activity.38,39 In NSCLC preliminary findings suggest that growth factors such as EGF and TGF-β can induce COX-2 and consequently increase PGE2 levels in lung.40 It will be important in future work to find if this pathway contributes to the regulation of aromatase and estrogen signaling in NSCLC.
Other significant interactions between EGFR and estrogen signaling have been documented and are important in promoting NSCLC growth.17,20,41,42 Moreover, clinical data showing that combined overexpression of EGFR and ER-α correlate with poor outcome in patients with lung cancer43 offer further support for cooperative interactions of EGFR and ER in lung cancer.
As noted above, cooperative interactions between growth factor receptor and ER signaling pathways have been identified in both breast and NSCLC.41 The EGFR/HER family of receptors as well as the insulin-like growth factor receptor (IGFR), TGF-α, and TGF-β are all implicated in lung cancer pathogenesis.40,44–47 Signaling from these several receptors is associated with mitogenesis, progression of malignancy, inhibition of apoptosis, and angiogenesis. Moreover, EGFR/HER receptors regulate ligand-independent estrogen receptor activation.13,17,41,48–51 Serine phosphorylation of steroid receptors by growth factor-activated signaling kinases triggers activation of ER-dependent transcription.52 Recent data reveal that cross-communication between growth factor and steroid receptors occurs in a bidirectional way at both transcriptional and nontranscriptional levels. In addition to well-characterized nuclear ER signaling in target tissues, membrane-associated ER forms also appear to elicit rapid signaling in cooperation with various kinase cascades, including the EGFR and its downstream effectors, such as mitogen-activated protein kinase (MAPK) and PI3K/AKT-kinase.17,42,53 In lung, estrogen treatment also induces the late expression of membrane receptor tyrosine kinase ligands, such as EGF and insulin-like growth factor (IGF-I).42 Functional interactions between ER-β and EGFR have been reported in lung cancer cells. For example, EGFR-dependent activation of phospho-p44/p42 MAP kinase occurs in response to estrogen treatment in NSCLC cells that express ER-β.17,42 Of special note, we find that EGF signaling increases aromatase expression and activity in NSCLC cells. Thus, EGFR signaling likely contributes to increased estrogen levels in tumor cells and suggests yet another mode of bidirectional cross-talk between ER and EGFR in NSCLC.42 We and others have shown that dual targeting of EGFR and ER signaling in lung cancer models can enhance inhibition of cell proliferation in vitro and in vivo.17,41,42 Recently, Traynor et al. published a pilot study using the combination of gefitinib and fulvestrant in the treatment of postmenopausal women with advanced NSCLC. The combination therapy was determined to be well-tolerated and suggested antitumor efficacy of the tandem treatment.54 Of special note, a phase II clinical trial of therapy with the EGFR inhibitor erlotinib together with fulvestrant in patients with advanced NSCLC is now underway.55
Lung cancer growth also depends, in part, on an adequate blood supply, and tumor-associated angiogenesis is reported to have prognostic value in NSCLC.56 Vascular endothelial growth factor (VEGF) activates endothelial signaling pathways leading to tumor angiogenesis and promotion of cancer progression.56–58 VEGF production and secretion by tumors is elicited by activation of both ER and EGFR/HER signaling pathways in tumors.58,59 Thus, it is plausible that disruption of ER and EGFR/HER signaling may elicit direct and indirect antitumor effects leading to suppression of tumor angiogenesis and inhibition of tumor growth.
EGFR blockade can cause inhibition of the secretion of VEGF and other angiogenic growth factors, including basic fibroblast growth factor, interleukin-8, and TGF-α.58,60–62 Additionally, blockade of VEGF receptor-2, the receptor considered the key signaling receptor for endothelial cell permeability, proliferation, and differentiation, could result in blockade of the VEGF-induced endothelial cell proliferation and subsequent tumor angiogenesis.62
Dual blockade of VEGF receptor-signaling, as well as EGFR signaling, appears to be a promising therapeutic strategy. Vandetinib (ZD6474), a novel, selective dual inhibitor of the VEGF receptor and EGFR pathways,63–65 has been tested in several randomized, controlled phase II clinical trials, and these studies indicate that this is a promising new agent for treatment of patients with advanced NSCLC.66–71 Our studies to assess the antitumor efficacy of the multitargeted growth factor receptor inhibitor vandetanib in combination with the antihormone therapy fulvestrant further indicate the potential for such dual treatment strategies in NSCLC.
In conclusion, emerging evidence on biologic signaling pathways in human NSCLC have established a strong rationale to move forward in targeting the ER–EGFR axis in this disease. This work may help to unravel the mystery of why patient outcomes and responses to treatment of NSCLC are significantly affected by sex.4,5 Current data indicate that patients managed with standard therapy for NSCLC have a worse clinical outcome if their serum estrogen levels are high.72 This finding is consistent with the observation that women with tumors expressing high levels of aromatase (and consequent high local estrogen levels) similarly have a worse prognosis.35 As in breast cancer therapy with hormonal agents, it may be possible in the future to prescreen patients (i.e., based on ER and aromatase positivity) to select those most likely to respond to antiestrogens and aromatase inhibitors.
Acknowledgments
This work was supported by grants from the National Cancer Institute Lung Cancer SPORE Program NIH CA090388, the National Lung Cancer Partnership, Iris Cantor–UCLA Women’s Health Center/UCLA National Center of Excellence in Women’s Health, and the Stiles Program in Integrative Oncology (in vitro studies). Dr. Charles Stark of Pfizer Pharmaceuticals and Sandy McKnight of AstraZeneca Pharmaceuticals helped to provide reagents used in these studies. We thank our colleagues at UCLA, Hermes Garbán, Olga Weinberg, Steven Dubinett, and Eugene Tsai for advice and assistance in the conduct of these studies.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Youlden DR, Cramb SM, Baade PD. The International Epidemiology of Lung Cancer: Geographical distribution and secular trends. J Thorac Oncol. 2008;3:819–831. doi: 10.1097/JTO.0b013e31818020eb. [DOI] [PubMed] [Google Scholar]
- 2.Wu AH, Henderson BE, Pike MC, Yu MC. Smoking and other risk factors for lung cancer in women. J Natl Cancer Inst. 1985;74:747–751. [PubMed] [Google Scholar]
- 3.Zang EA, Wynder EL. Differences in lung cancer risk between men and women: Examination of the evidence. J Natl Cancer Inst. 1996;88:183– 192. doi: 10.1093/jnci/88.3-4.183. [DOI] [PubMed] [Google Scholar]
- 4.Wakelee HA, Gomez SL, Chang ET. Sex differences in lung-cancer susceptibility: A smoke screen? Lancet Oncol. 2008;9:609–610. doi: 10.1016/S1470-2045(08)70162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hede K. Lung cancer may be different for men and women, but researchers ponder what to do? J Natl Cancer Inst. 2007;99:1830–1832. doi: 10.1093/jnci/djm284. [DOI] [PubMed] [Google Scholar]
- 6.Cerfolio RJ, Bryant AS, Scott E, et al. Women with pathologic stage I, II, and III non-small cell lung cancer have better survival than men. Chest. 2006;130:1796–1802. doi: 10.1378/chest.130.6.1796. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Inoue M, Sobue T, Tsugane S. Reproductive factors, hormone use and the risk of lung cancer among middle-aged never-smoking Japanese women: A large-scale population-based cohort study. Int J Cancer. 2005;117:662–666. doi: 10.1002/ijc.21229. [DOI] [PubMed] [Google Scholar]
- 8.Taioli E, Wynder EL. Endocrine factors and adenocarcinoma of the lung in women. J Natl Cancer Inst. 1994;86:869–870. doi: 10.1093/jnci/86.11.869. [DOI] [PubMed] [Google Scholar]
- 9.Ganti AK, Sahmoun AE, Panwalkar AW, et al. Hormone replacement therapy is associated with decreased survival in women with lung cancer. J Clin Oncol. 2006;24:59–63. doi: 10.1200/JCO.2005.02.9827. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz AG, Wenzlaff AS, Prysak GM, et al. Reproductive factors, hormone use, estrogen receptor expression and risk of non small-cell lung cancer in women. J Clin Oncol. 2007;25:5785–5792. doi: 10.1200/JCO.2007.13.3975. [DOI] [PubMed] [Google Scholar]
- 11.Schabath MB, Wu X, Vassilopoulou-Sellin R, et al. Hormone replacement therapy and lung cancer risk: A case-control analysis. Clin Cancer Res. 2004;10(1 Pt 1):113–123. doi: 10.1158/1078-0432.ccr-0911-3. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez C, Spencer Feigelson H, Deka A, et al. Postmenopausal hormone therapy and lung cancer risk in the cancer prevention study II nutrition cohort. Cancer Epidemiol Biomarkers Prev. 2008;17:655–660. doi: 10.1158/1055-9965.EPI-07-2683. [DOI] [PubMed] [Google Scholar]
- 13.Pietras R, Arboleda J, Reese D, et al. HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene. 1995;10:2435–2446. [PubMed] [Google Scholar]
- 14.Weinberg O, Márquez D, Chen H-W, et al. Aromatase inhibitors in human lung cancer therapy. Proc AACR. 2005 doi: 10.1158/0008-5472.CAN-05-2737. (abstract) [DOI] [PubMed] [Google Scholar]
- 15.Kinoshita Y, Chen S. Induction of aromatase (CYP19) expression in breast cancer cells through a nongenomic action of estrogen receptor alpha. Cancer Res. 2003;63:3546–3555. [PubMed] [Google Scholar]
- 16.Weinberg OK, Márquez-Garbán DC, Fishbein MC, et al. Aromatase inhibitors in human lung cancer therapy. Cancer Res. 2005;65:11287–11291. doi: 10.1158/0008-5472.CAN-05-2737. [DOI] [PubMed] [Google Scholar]
- 17.Márquez-Garbán DC, Chen HW, Fishbein MC, et al. Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids. 2007;72:135–143. doi: 10.1016/j.steroids.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beattie CW, Hansen NW, Thomas PA. Steroid receptors in human lung cancer. Cancer Res. 1985;45:4206–4214. [PubMed] [Google Scholar]
- 19.Omoto Y, Kobayashi Y, Nishida K, et al. Expression, function, and clinical implications of the estrogen receptor beta in human lung cancers. Biochem Biophys Res Commun. 2001;285:340–347. doi: 10.1006/bbrc.2001.5158. [DOI] [PubMed] [Google Scholar]
- 20.Pietras RJ, Márquez DC, Chen HW, et al. Estrogen and growth factor receptor interactions in human breast and non-small cell lung cancer cells. Steroids. 2005;70:372–381. doi: 10.1016/j.steroids.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Stabile LP, Davis AL, Gubish CT, et al. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res. 2002;62:2141–2150. [PubMed] [Google Scholar]
- 22.Niikawa H, Suzuki T, Miki Y, et al. Intra-tumoral estrogens and estrogen receptors in human non-small cell lung carcinoma. Clin Cancer Res. 2008;14:4417–4426. doi: 10.1158/1078-0432.CCR-07-1950. [DOI] [PubMed] [Google Scholar]
- 23.Mollerup S, Jorgensen K, Berge G, Haugen A. Expression of estrogen receptors alpha and beta in human lung tissue and cell lines. Lung Cancer. 2002;37:153–159. doi: 10.1016/s0169-5002(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 24.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 25.González-Arenas A, Neri-Gomez T, Guerra-Araiza C, Camacho-Arroyo I. Sexual dimorphism in the content of progesterone and estrogen receptors, and their cofactors in the lung of adult rats. Steroids. 2004;69:351–356. doi: 10.1016/j.steroids.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Patrone C, Cassel TN, Pettersson K, et al. Regulation of postnatal lung development and homeostasis by estrogen receptor beta. Mol Cell Biol. 2003;23:8542–8552. doi: 10.1128/MCB.23.23.8542-8552.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morani A, Barros RP, Imamov O, et al. Lung dysfunction causes systemic hypoxia in estrogen receptor beta knockout (ERbeta−/−) mice. Proc Natl Acad Sci USA. 2006;103:7165–7169. doi: 10.1073/pnas.0602194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammoud Z, Tan B, Badve S, Bigsby RM. Estrogen promotes tumor progression in a genetically defined mouse model of lung adenocarcinoma. Endocr Relat Cancer. 2008;15:475–483. doi: 10.1677/ERC-08-0002. [DOI] [PubMed] [Google Scholar]
- 29.Imai T, Yasuhara K, Tamura T, et al. Inhibitory effects of cinnamaldehyde on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung carcinogenesis in rasH2 mice. Cancer Lett. 2002;175:9–16. doi: 10.1016/s0304-3835(01)00706-6. [DOI] [PubMed] [Google Scholar]
- 30.Singh SV, Benson PJ, Hu X, et al. Gender-related differences in susceptibility of A/J mouse to benzo[a]pyrene-induced pulmonary and forestomach tumorigenesis. Cancer Lett. 1998;128:197–204. doi: 10.1016/s0304-3835(98)00072-x. [DOI] [PubMed] [Google Scholar]
- 31.Chetrite GS, Cortes-Prieto J, Philippe JC, et al. Comparison of estrogen concentrations, estrone sulfatase and aromatase activities in normal, and in cancerous, human breast tissues. J Steroid Biochem Mol Biol. 2000;72:23–27. doi: 10.1016/s0960-0760(00)00040-6. [DOI] [PubMed] [Google Scholar]
- 32.Simpson ER, Mahendroo MS, Means GD, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994;15:342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- 33.Simpson ER. Aromatase: biologic relevance of tissue-specific expression. Semin Reprod Med. 2004;22:11–23. doi: 10.1055/s-2004-823023. [DOI] [PubMed] [Google Scholar]
- 34.Oyama T, Sugio K, Isse T, et al. Expression of cytochrome P450 in non-small cell lung cancer. Front Biosci. 2008;13:5787–5793. doi: 10.2741/3116. [DOI] [PubMed] [Google Scholar]
- 35.Mah V, Seligson DB, Li A, et al. Aromatase expression predicts survival in women with early-stage non small cell lung cancer. Cancer Res. 2007;67:10484–10490. doi: 10.1158/0008-5472.CAN-07-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coombes RC, Hall E, Gibson LJ, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:1081–1092. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki T, Miki Y, Nakamura Y, et al. Sex steroid-producing enzymes in human breast cancer. Endocr Relat Cancer. 2005;12:701–720. doi: 10.1677/erc.1.00834. [DOI] [PubMed] [Google Scholar]
- 38.Subbaramaiah K, Howe LR, Port ER, et al. HER-2/neu status is a determinant of mammary aromatase activity in vivo: Evidence for a cyclooxygenase-2-dependent mechanism. Cancer Res. 2006;66:5504–5511. doi: 10.1158/0008-5472.CAN-05-4076. [DOI] [PubMed] [Google Scholar]
- 39.Prosperi JR, Robertson FM. Cyclooxygenase-2 directly regulates gene expression of P450 Cyp19 aromatase promoter regions pII, pI.3 and pI.7 and estradiol production in human breast tumor cells. Prostaglandins Other Lipid Mediat. 2006;81:55–70. doi: 10.1016/j.prostaglandins.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Liu M, Yang SC, Sharma S, et al. EGFR signaling is required for TGF-beta 1 mediated COX-2 induction in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2007;37:578–588. doi: 10.1165/rcmb.2007-0100OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pietras RJ, Márquez-Garbán DC. Membrane-associated estrogen receptor signaling pathways in human cancers. Clin Cancer Res. 2007;13:4672–4676. doi: 10.1158/1078-0432.CCR-07-1373. [DOI] [PubMed] [Google Scholar]
- 42.Stabile LP, Lyker JS, Gubish CT, et al. Combined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effects. Cancer Res. 2005;65:1459–1470. doi: 10.1158/0008-5472.CAN-04-1872. [DOI] [PubMed] [Google Scholar]
- 43.Kawai H, Ishii A, Washiya K, et al. Combined overexpression of EGFR and estrogen receptor alpha correlates with a poor outcome in lung cancer. Anticancer Res. 2005;25:4693–4698. [PubMed] [Google Scholar]
- 44.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 45.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 46.Dziadziuszko R, Camidge DR, Hirsch FR. The insulin-like growth factor pathway in lung cancer. J Thorac Oncol. 2008;3:815–818. doi: 10.1097/JTO.0b013e31818180f5. [DOI] [PubMed] [Google Scholar]
- 47.Wu W, O’Reilly MS, Langley RR, et al. Expression of epidermal growth factor (EGF)/transforming growth factor-alpha by human lung cancer cells determines their response to EGF receptor tyrosine kinase inhibition in the lungs of mice. Mol Cancer Ther. 2007;6:2652–2663. doi: 10.1158/1535-7163.MCT-06-0759. [DOI] [PubMed] [Google Scholar]
- 48.Campbell RA, Bhat-Nakshatri P, Patel NM, et al. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: A new model for anti-estrogen resistance. J Biol Chem. 2001;276:9817–9824. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- 49.Marquez DC, Lee J, Lin T, Pietras RJ. Epidermal growth factor receptor and tyrosine phosphorylation of estrogen receptor. Endocrine. 2001;16:73–81. doi: 10.1385/ENDO:16:2:073. [DOI] [PubMed] [Google Scholar]
- 50.Font de Mora J, Brown M. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol Cell Biol. 2000;20:5041–5047. doi: 10.1128/mcb.20.14.5041-5047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aronica SM, Kraus WL, Katzenellenbogen BS. Estrogen action via the cAMP signaling pathway: stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proc Natl Acad Sci USA. 1994;91:8517–8521. doi: 10.1073/pnas.91.18.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weitsman GE, Li L, Skliris GP, et al. Estrogen receptor-alpha phosphorylated at Ser118 is present at the promoters of estrogen-regulated genes and is not altered due to HER-2 overexpression. Cancer Res. 2006;66:10162–10170. doi: 10.1158/0008-5472.CAN-05-4111. [DOI] [PubMed] [Google Scholar]
- 53.Richards RG, DiAugustine RP, Petrusz P, et al. Estradiol stimulates tyrosine phosphorylation of the insulin-like growth factor-1 receptor and insulin receptor substrate-1 in the uterus. Proc Natl Acad Sci USA. 1996;93:12002–12007. doi: 10.1073/pnas.93.21.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Traynor A, Schiller J, Stabile L, et al. Combination therapy with gefitinib and fulvestrant (G/F) for women with non-small cell lung cancer (NSCLC) ASCO. 2005;23:676S. [Google Scholar]
- 55.Garon E, Sadeghi S, Kabbinavar F, et al. Interim safety analysis of a phase II study of erlotinib (E) alone or combined with fulvestrant (F) in previously treated patients with advanced non-small cell lung cancer (NSCLC). J Clin Oncol; ASCO Annual Meeting Proceedings 2007; 2007. p. 19091. [Google Scholar]
- 56.Meert AP, Paesmans M, Martin B, et al. The role of microvessel density on the survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer. 2002;87:694–701. doi: 10.1038/sj.bjc.6600551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li D, Williams JI, Pietras RJ. Squalamine and cisplatin block angiogenesis and growth of human ovarian cancer cells with or without HER-2 gene overexpression. Oncogene. 2002;21:2805–2814. doi: 10.1038/sj.onc.1205410. [DOI] [PubMed] [Google Scholar]
- 58.Petit AM, Rak J, Hung MC, et al. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: Angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol. 1997;151:1523–1530. [PMC free article] [PubMed] [Google Scholar]
- 59.Pietras RJ. Interactions between estrogen and growth factor receptors in human breast cancers and the tumor-associated vasculature. Breast J. 2003;9:361–373. doi: 10.1046/j.1524-4741.2003.09510.x. [DOI] [PubMed] [Google Scholar]
- 60.Bruns CJ, Solorzano CC, Harbison MT, et al. Blockade of the epidermal growth factor receptor signaling by a novel tyrosine kinase inhibitor leads to apoptosis of endothelial cells and therapy of human pancreatic carcinoma. Cancer Res. 2000;60:2926–2935. [PubMed] [Google Scholar]
- 61.Ciardiello F, Caputo R, Bianco R, et al. Inhibition of growth factor production and angiogenesis in human cancer cells by ZD1839 (Iressa), a selective epidermal growth factor receptor tyrosine kinase inhibitor. Clin Cancer Res. 2001;7:1459–1465. [PubMed] [Google Scholar]
- 62.Ferrara N. The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat. 1995;36:127–137. doi: 10.1007/BF00666035. [DOI] [PubMed] [Google Scholar]
- 63.McCarty MF, Wey J, Stoeltzing O, et al. ZD6474, a vascular endothelial growth factor receptor tyrosine kinase inhibitor with additional activity against epidermal growth factor receptor tyrosine kinase, inhibits orthotopic growth and angiogenesis of gastric cancer. Mol Cancer Ther. 2004;3:1041–1048. [PubMed] [Google Scholar]
- 64.Herbst RS, Heymach JV, O’Reilly MS, et al. Vandetanib (ZD6474): An orally available receptor tyrosine kinase inhibitor that selectively targets pathways critical for tumor growth and angiogenesis. Expert Opin Investig Drugs. 2007;16:239–249. doi: 10.1517/13543784.16.2.239. [DOI] [PubMed] [Google Scholar]
- 65.Tamura T, Minami H, Yamada Y, et al. A phase I dose-escalation study of ZD6474 in Japanese patients with solid, malignant tumors. J Thorac Oncol. 2006;1:1002–1009. [PubMed] [Google Scholar]
- 66.Kiura K, Nakagawa K, Shinkai T, et al. A randomized, double-blind, phase IIa dose-finding study of Vandetanib (ZD6474) in Japanese patients with non-small cell lung cancer. J Thorac Oncol. 2008;3:386–393. doi: 10.1097/JTO.0b013e318168d228. [DOI] [PubMed] [Google Scholar]
- 67.Arnold AM, Seymour L, Smylie M, et al. Phase II study of vandetanib or placebo in small-cell lung cancer patients after complete or partial response to induction chemotherapy with or without radiation therapy: National Cancer Institute of Canada Clinical Trials Group Study BR.20. J Clin Oncol. 2007;25:4278–4284. doi: 10.1200/JCO.2007.12.3083. [DOI] [PubMed] [Google Scholar]
- 68.Heymach JV, Johnson BE, Prager D, et al. Randomized, placebo-controlled phase II study of vandetanib plus docetaxel in previously treated non small-cell lung cancer. J Clin Oncol. 2007;25:4270–4277. doi: 10.1200/JCO.2006.10.5122. [DOI] [PubMed] [Google Scholar]
- 69.Lee CB, Socinski MA. Vascular endothelial growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer: A review of recent clinical trials. Rev Recent Clin Trials. 2007;2:117–120. doi: 10.2174/157488707780599401. [DOI] [PubMed] [Google Scholar]
- 70.Ciardiello F, Bianco R, Caputo R, et al. Antitumor activity of ZD6474, a vascular endothelial growth factor receptor tyrosine kinase inhibitor, in human cancer cells with acquired resistance to anti-epidermal growth factor receptor therapy. Clin Cancer Res. 2004;10:784–793. doi: 10.1158/1078-0432.ccr-1100-03. [DOI] [PubMed] [Google Scholar]
- 71.Morelli MP, Cascone T, Troiani T, et al. Antitumor activity of the combination of cetuximab, an anti-EGFR blocking monoclonal antibody and ZD6474, an inhibitor of VEGFR and EGFR tyrosine kinases. J Cell Physiol. 2006;208:344–353. doi: 10.1002/jcp.20666. [DOI] [PubMed] [Google Scholar]
- 72.Albain KS, Unger J, GCC, et al. Toxicity and survival by sex in patients with advanced non-small cell lung carcinoma (NSCLC) on modern Southwest Oncology Group (SWOG) trials. J Clin Oncol; ASCO Annual Meeting Proceedings 2007; 2007. p. 7549. Part I. [Google Scholar]