Abstract
Regulatory T cells (Tregs) may play an important role in the immunopathology of chronic HIV-1 infection due to their potent suppressive activity of both T cell activation and effector function. To investigate the correlation between Tregs and immune activation during untreated chronic HIV-1 infection, we conducted a nested case–control study within the Multicenter AIDS Cohort Study (MACS). Twenty HIV-1-infected fast progressors (FP) and 40 slow progressors (SP) were included in our study using risk-set sampling. Nine age-matched HIV-1-uninfected men (UI) were also included. Cryopreserved peripheral blood mononuclear cells (PMBCs) were tested using flow cytometry analyses. We identified Tregs as Foxp3+CD25+CD4+ T cells and assessed the activation of CD4+ and CD8+ T cells by the expression of CD38, HLADR, or both markers simultaneously. There is a relative expansion of Tregs during HIV-1 infection, which is associated with disease progression. The increased CD38 expression on both CD4+ and CD8+ T cells expressed as either percentage or median fluorescence intensity (MFI) and the elevated proportion of CD8+ T cells that is HLADR+CD38+ were all associated with rapid HIV-1 progression. Counter to the assumed role of Tregs as the suppressors of activation, the expansion of Tregs was positively correlated with CD4+ T cell activation among HIV-1-infected fast progressors. The high level of Tregs associated with rapid HIV progression may suggest a detrimental role of these cells in the immune control of HIV-1 infection.
Introduction
HIV-1 infection is associated with a state of excessive T cell activation, which has been shown to be a strong prognostic indicator for disease progression at different stages of HIV-1 infection.1–4 Studies on nonhuman primates and transgenic mice have clearly demonstrated that chronic immune hyperactivation is directly linked to progressive immunodeficiency, independent of viremia control or even viral infection.5–8 Heightened immune activation during HIV-1 infection may be responsible in part for CD4+ T cell depletion by activation-induced cell death or increased viral replication and infection. Despite extensive research, it is still unclear how this immune activation is induced during HIV-1 infection, and how the immune system responds to this hyperactivation state.
Recent studies have suggested that regulatory T cells (Tregs), a small subpopulation of CD4+ T cells, might play a role in the dynamics of immune activation during HIV-1 infection.9,10 Tregs were initially characterized by the expression of CD25. CD25+CD4+ T cells can suppress the CD4+ and CD8+ T cell activation and effector function both in vitro and in vivo,11 and have been shown to be directly involved in preventing, or inhibiting, autoimmune and inflammatory disorders both in mice and humans.12–14 Although Tregs are more common in the CD25+ cell population, this marker is not specific for Tregs.15,16 Recent studies have provided strong evidence that the forkhead transcription factor, Foxp3, predominantly expressed on human CD25+CD4+ T cells, may be the best marker to define Tregs.17 Foxp3 expression is required for the development of Tregs and correlates with the suppressive activity of these cells, irrespective of CD25 expression.17–22 In both mice and human models, a lack of functional Foxp3 has been shown to lead to autoimmune and inflammatory diseases, whereas enforced expression of Foxp3 on conventional CD4+ T cells conferred suppressive capacity on these cells.10,18,19,23–27 In light of the critical role of Tregs in the regulation of immune cells, it is possible that this cell population may play an important role in immunopathology during chronic HIV-1 infection. To our knowledge, the change in Tregs associated with HIV-1 disease progression has never been assessed, though there has been some limited, and inconsistent data regarding the changes in Tregs during HIV-1 infection.9,11,28–30 Even those data are hard to interpret because of the inconsistent phenotypes used to define Tregs. We are the first group that assessed the relationship between Tregs, immune activation, and the rate of HIV-1 disease progression in a longitudinal study, by using Foxp3+CD25+CD4+ as the phenotype for Tregs.31 We found that the proportion of Tregs was elevated during the course of HIV-1 infection and the magnitude of this elevation was associated with disease progression. In addition, the proportion of Tregs was positively correlated with CD4+ T cell activation in HIV-1-infected fast progressors. Defining Tregs as Foxp3+CD4+ T cells regardless of CD25 expression did not change the above findings. Our data suggest that the relative increase of Tregs during HIV-1 infection may play a detrimental role in disease progression.
Materials and Methods
Study subjects and samples
A nested case–control study was carried out within the Multicenter AIDS Cohort Study (MACS), which enrolled 4954 homosexual men between March 1984 and April 1985 from four centers located in Baltimore, Chicago, Los Angeles, and Pittsburgh. Semiannually, participants in the MACS cohort return to one of the four centers for a follow-up visit and specimen collection. Other details regarding the recruitment and characteristics of the MACS cohort have been reported elsewhere.32
We selected 60 men from the MACS participants who were HIV-1 seropositive at the time of enrollment, using risk-set sampling as follows: first, we identified 20 cases from those who developed AIDS within 4 years after enrollment (termed fast progressors, FP); then for each case, at the time of his AIDS diagnosis, two controls were randomly selected from those who were free of AIDS at that calendar date and had a total AIDS-free time of at least 8 years after enrollment (termed slow progressors, SP). The two controls (SP) were matched to the index case (FP) on age (±2 years) and CD4+ T cell count (±100) at an early visit. Thus, our 60 seropositive samples consisted of 20 triplets, for which each set had one FP matched with two SP. Immunologic parameters were measured on cryopreserved peripheral blood mononuclear cells (PBMCs) collected at a single time point, called the index visit, which was approximately 1 year before the AIDS diagnosis for each FP, and the same calendar time for his two matched SP. All participants were antiretroviral therapy naive at the time of sample evaluation. Our study also included 9 HIV uninfected individuals (UI) from the MACS, who were frequency matched with the 60 HIV-1-seropositive men on age. Cryopreserved PBMC samples were collected from UI during the same time period as our HIV-1-seropositive samples.
Immunophenotypic analysis
The cryopreserved PBMCs were thawed by the standard method.33 The median viability of the thawed cells, assessed by Trypan blue exclusion, was 82%. To test the expression of CD38, CD28, and HLA-DR markers, cell surface staining was performed. In brief, aliquots of 5 × 105 PBMCs were incubated with appropriate fluorochrome-conjugated antibodies, including anti-CD3 (PerCP), anti-CD4 (PE-Cy7), anti-CD8 (APC-Cy7), anti-CD28 (APC), anti-CD38 (PE), and anti-HLA-DR (FITC), for 30 min at 4°C in the dark. Following staining, all aliquots were subjected to red cell lysis using NH4Cl. Cells were then washed once and were ready for flow cytometry analysis. To measure the expression of CD25 and Foxp3, we used the Foxp3 Staining Buffer Set (eBioscience, San Diego, CA) following the manufacturer's protocol. Briefly, 1 × 106 PBMCs were surface stained by incubation with anti-CD3 (PerCP), anti-CD4 (PE-Cy7), anti-CD8 (APC-Cy7), anti-CD45RO (APC), and anti-CD25 (PE) for 30 min at 4ºC. Cells were then washed twice in staining buffer and incubated in Fixation/Permeabilization buffer. Cells were then washed once with staining buffer and twice with permeabilization buffer. After a 15-min preincubation in 2 μl of normal rat serum blocking reagent, cells were stained with anti-Foxp3 (FITC, clone PCH101), washed twice with permeabilization buffer, and resuspended in staining buffer for flow cytometry analysis. Tregs were defined as FOXP3+CD25brightCD4+CD3+ T cells (Fig. 1a). T cell activation was determined by the percentage of T cells expressing both HLA-DR+ and CD38+ (see Fig. 3a). The cursor setting used to define CD38-positive expression on CD8+ T cells was set at a median of 4100 PE molecules, a setting previously established in our laboratory as discriminating between CD38 expression levels on resting/naive CD8+ T cells and activated levels observed in HIV-1-infected individuals.2,3,34 QuantiBRITE beads (BD Biosciences) were used to define the equivalent number of PE molecules measured at the fixed cursor setting,35 and a single lot of Quantibrite CD38-PE was used for all testing. The cursor setting used to define CD38 positive expression on CD4– T cells was established using isotype control settings. The fixed cursor settings were used for all samples and are shown in Fig. 3a. In addition, we measured CD38 expression as median fluorescence intensities (MFI) with no cursors set. All measurements in our study were made in one laboratory by one person, who was blinded to the progression status of the participants. Samples belonging to the same triplet (one FP and two matched SP) were always assayed in the same analytical batch. Stained PBMCs were analyzed on a FACSCanto flow cytometer (BDIS) appropriately compensated daily using freshly stained PBMCs. The sensitivity of the fluorescence detectors was standardized daily using fixed chicken red blood cells (Biosure, Grass Valley, CA). Data analysis was performed using FACSDiva software (BDIS). PBMCs were also stained with 7-aminoactinomycin D (Calbiochem, La Jolla, CA) for dead cell discrimination; live cell gating regions yielded a purity of at least 98%. All antibodies with the exception of anti-Foxp3 (FITC) were purchased from Becton Dickinson Immunocytometry Systems (BDIS), San Jose, CA.
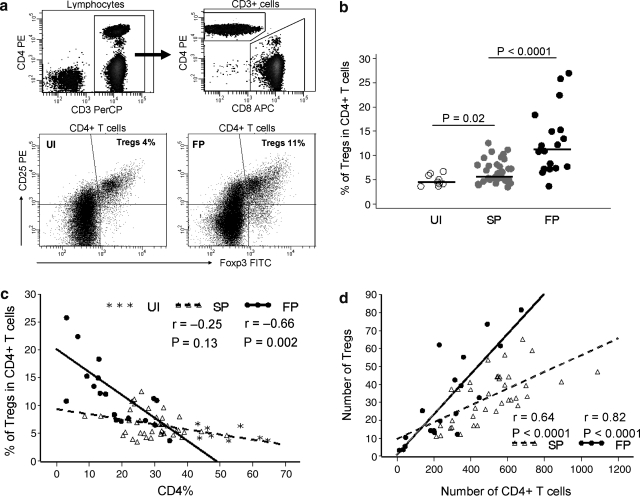
FIG. 1.
Selective expansion of Tregs during HIV-1 infection and progression (a). Gating of CD4 and CD8 T cells (top) and expression of Tregs from representative UI and FP (bottom). (b). Comparison of Tregs in three groups of MACS participants. Bars represent medians. (c). Percentage of Tregs is inversely correlated with the percentage of CD4+ T cells in FP. (d). Absolute numbers of Tregs are positively correlated with the CD4+ T cell counts in FP and SP. Solid line, regression line for the FP. Dotted line, regression line for the SP.
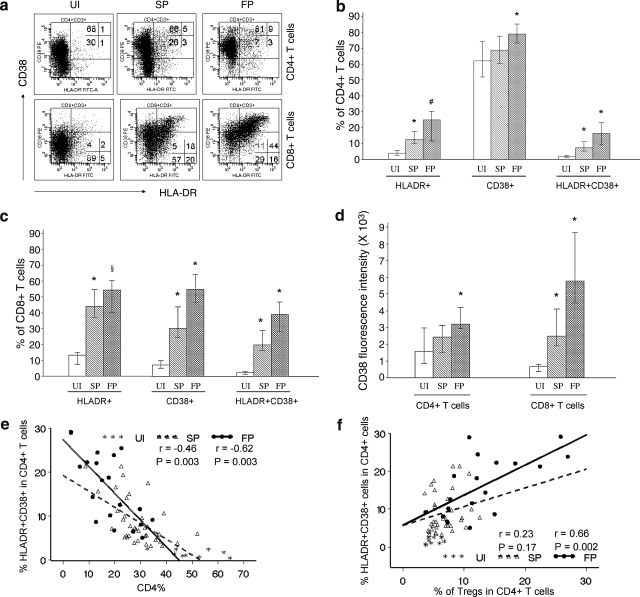
FIG. 3.
Activation of T cells and in relation to Tregs (a). Example flow cytometry histograms showing staining for HLA-DR and CD38 on CD4+ or CD8+ T cells for one set of UI, SP, and FP. (b) Proportion of HLA-DR+, CD38+, or HLA-DR+CD38+ cells among CD4+ T cells. (c) Proportion of HLA-DR+, CD38+, or HLA-DR+CD38+ cells among CD8+ T cells. (d) CD38 median fluorescence intensity of CD4+ and CD8+ T cells. (e) CD4+ T cell activation is inversely correlated with the proportion of CD4+ T cells in HIV-1-infected individuals. (f) Positive correlation between the proportion of activated CD4+ T cells and Tregs among the FP. Depicted in bar plots are medians and interquartile ranges. Significance in comparison of FP vs. SP or comparison of SP vs. UI: *p < 0.0001; #p = 0.0002, §p = 0.03. Solid line, regression line for the FP. Dotted line, regression line for the SP.
Statistical analysis
Medians were compared using the Wilcoxon rank-sum test or Wilcoxon signed-rank test. Fisher's exact test was used to compare two percentages. Bivariate correlations were determined by the Spearman rank correlation test. A regression line was calculated by the least-squares method. Conditional logistic regression models were used to obtain odds ratios and 95% confidence intervals. p values of 0.05 or less were considered significant. The analysis was performed using the SAS program.
Results
Characteristics of participants
We identified two groups of HIV-1-infected men with distinct subsequent AIDS-free and survival times after enrollment into the MACS (Table 1). The twenty FP all developed AIDS within 4 years after enrollment into the MACS, with a median AIDS-free time of 3.0 years; in contrast, only 19 patients out of the 40 SP had developed AIDS by January 2006, the time of sample selection, with a median AIDS-free time of 9.3 years. All 20 FP died from AIDS-related conditions with a median survival of 4.2 years after enrollment, as compared to 16 AIDS-related deaths with a median survival of 10 years out of the 40 SP. Thus, the incidence of both AIDS and death was significantly higher in the FP vs. the SP group after enrollment in the MACS. FP and SP were well matched by set on age. Data of CD4+ and CD8+ T cell count measured on fresh whole blood were retrieved from MACS records. Comparing within each risk-set, the CD4+ cell count of FP at study entry was similar to that of SP (p = 0.36). However, at the time of sample evaluation, i.e., 1 year before AIDS onset for FP, comparing within each risk-set, the CD4+ cell counts of FP was lower than that of SP by a median of 230 CD4+ T cells (p < 0.0001) reflecting accelerated damage to the immune system in FP.
Table 1.
Comparison of the Basic Demographic and Immunologic Parameters at the Index Visita
| FP (n = 20) | SP (n = 40) | UI (n = 9) | pb | |
|---|---|---|---|---|
| Developed AIDS by 01/2006 (n, %) | 20, 100% | 19, 47.5% | NA | <0.0001 |
| AIDS-free time after enrollment among those developing AIDS (years) | 3.0 (1.7–3.8) | 9.3 (8.4–16.7) | NA | <0.0001 |
| Death by 01/2006 (n, %) | 20, 100% | 16, 40% | NA | <0.0001 |
| Survival time after enrollment among those dying (years) | 4.2 (2.6–7.2) | 10.0 (8.3–18.2) | NA | <0.0001 |
| Age | 36.2 (23.3–50.5) | 35.5 (23.9–50.2) | 36.6 (24.4–47.1) | 0.97 |
| CD4+ T cells/mm3 at study entry (visit 3) | 371 (204–619) | 391 (198–707) | 979 (387–1352) | 0.36 |
| Parameters at the index visit | ||||
| CD4+ % | 19.3 (2.9–29) | 28.0 (7.5–52) | 46.0 (34–59) | <0.0001 |
| CD4+ T cells/mm3 | 266 (13–675) | 519 (213–1090) | 995 (387–1195) | <0.0001 |
| CD8+ % | 49.0 (24.2–74.0) | 47.0 (23.7–78.4) | 34.0 (23–45) | 0.70 |
| CD8+ T cells/mm3 | 606 (112–2793) | 826 (169–5673) | 529 (323–857) | 0.006 |
If unspecified, values are presented as medians (range); FP, fast progressors; SP, slow progressors; UI, uninfected individuals.
Wilcoxon signed-rank test and Fisher's exact test were used to compare group medians or percentages between two seropositive groups.
Selective expansion of Tregs during HIV-1 infection and progression
Our data showed that Tregs constitute a small fraction of the CD4+ T cells in the UI with a median of 4.6%. This percentage was slightly increased to 5.6% in SP, and significantly increased to 11.3% in FP (Fig. 1b). This significant increase of Tregs among FP is inversely correlated with the lower proportion of CD4+ T cells (Fig. 1c). Our result is consistent with previous studies29,31 showing that HIV-1-infected individuals with low CD4+ T cell counts have, on average, an elevated percentage of Tregs compared to those with a high CD4 level. We then determined the absolute number of Tregs by multiplying the proportion of Tregs by the CD4+ T cell count. Consistent with previous studies;9,31 the number of Tregs positively correlates with CD4+ T cell count in both FP and SP (Fig. 1d). However, the number of Tregs was not significantly different in the SP compared to the UI (p = 0.15), or when comparing the FP to SP (p = 0.45). Taken together, our data indicate that Tregs appear to decline at a slower rate compared with other CD4+ T cell subpopulations, resulting in a relative expansion of this cell subset in FP. In addition, we observed a greater variability in the proportion of Tregs among FP (Fig. 1b) compared to SP and UI. Furthermore, although Foxp3 was predominantly expressed on CD25+ cells, we observed a clear increase in the proportion of Foxp3+CD25–CD4+ T cells in SP compared to UI (p < 0.0001) and in FP as compared to SP (p < 0.0001). Increases in the proportion of Foxp3+CD25+CD4+ and Foxp3+CD25–CD4+ T cells were highly correlated in FP (r = 0.85, p < 0.0001, data not shown), indicating a parallel upregulation of Foxp3 expression on both CD25+ and CD25– cells among FP. In keeping with previous data,36 we did not observe a difference in CD25+CD4+ T cells regardless of HIV-1 infection/progression status.
Foxp3 expression on CD8+ T cells
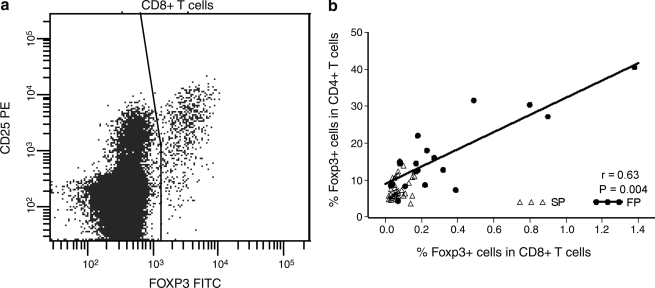
Foxp3 is thought to be a specific marker for CD4+ Tregs, and consistent with this, we observed low Foxp3 expression on CD8+ T cells in most individuals; however, we also observed a clear increase of Foxp3+CD8+CD4– T cells in some FP. Most of these cells coexpressed CD25 (Fig. 2a). Among FP, Foxp3 expression on CD8+ T cells showed greater variability and appeared to be correlated with Foxp3 expression on CD4+ T cells (Fig. 2). Thus, there appears to be a parallel upregulation of Foxp3 expression in both CD4+ and CD8+ T cells among the FP at the advanced disease stage.
FIG. 2.
Foxp3 expression on CD8+ T cells (a) Foxp3+CD8+ T cells in one representative FP. (b) Foxp3 expression in CD4+ and CD8+ T cells is correlated in FP, but not in SP. Solid line, regression line for the FP.
CD4+ but not CD8+ T cell activation correlates with the proportion of Tregs
T cell activation was measured by the expression of CD38 and HLA-DR. Within the CD4+ T cell compartment, HLA-DR+ cells increased stepwise from 3.8% in UI to 12.5% in SP and 24.8% in FP (Fig. 3b). Furthermore, CD38 expression gradually increased with HIV-1 infection and progression as shown by the percentage of CD38+CD4+ T cells (Fig. 3b) as well as the MFI (Fig. 3d). Simultaneous expression of HLA-DR and CD38 on CD4+ T cells increased from 1.5% in UI to 7.2% in SP and 16.3% in FP. The upregulation of activation markers was more overt in CD8+ T cells than in CD4+ T cells in terms of both percentage of positive cells and the fluorescence intensity of CD38. Within the CD8+ T cell subset, HLA-DR+ cells increased significantly from 13% in UI to 44% in SP and 54% in FP (Fig. 3c). There was a remarkable increase in both the percentage of CD38+ cells (Fig. 3c) and the CD38 intensity (Fig. 3d) in the CD8+ T cell subset. Simultaneous expression of HLA-DR and CD38 in CD8+ T cells increased gradually from 2.4% in UI to 20% in SP and 39% in FP (Fig. 3c). We further found that CD4+, but not CD8+, T cell activation was inversely correlated with the proportion of CD4+ T cells in HIV-1-infected individuals, especially in FP (Fig. 3e).
We assessed the relationship between Tregs and the activation of CD4+ or CD8+ T cells (Fig. 3f). There was a positive correlation between the proportion of activated CD4+ T cells and Tregs among the FP, and this association remained significant in multivariate analysis after controlling for the effect of CD4 level (fitted as CD4+ T cell count or percentage, data not shown). The proportions of Tregs and activated CD8+ T cells were not correlated in any of the three groups. This is despite the fact that on a group level, individuals with high T cell activation will, on average, tend to have higher levels of Tregs than those with lower levels of activation.
Expansion of Tregs and immune activation are associated with HIV-1 progression
Table 2 shows the association btween different immunological parameters with HIV-1 progression calculated from conditional logistic regression. Adjustments were made for matching variables (age and CD4+ T cell count at study entry) or for matching variables plus CD4+ T cell count of the index visit. We found that an increase in the proportion of Tregs was correlated with faster disease progression. Increased CD38 expression, expressed as either percentage or MFI, correlates strongly with faster progression. The increase in CD4+ or CD8+ T cells that express HLA-DR and CD38 simultaneously was associated with rapid HIV-1 progression, whereas the increase in cells that lack both markers was associated with a delayed disease progression. Furthermore, within CD8+ T cells, HLA-DR–CD38+ T cells were correlates of fast progression, while HLA-DR+CD38– T cells were correlates of slow progression. These results indicate that CD38 is the critical activation marker in determining HIV-1 progression, irrespective of HLA-DR expression.
Table 2.
Effects of Immunological Markers on HIV-1 Disease Progressiona
| Variable | Increment of increase | OR (95% CI)b | pb | OR (95% CI)c | pc |
|---|---|---|---|---|---|
| % Foxp3+CD25+ in CD4+ | 5% | 7.57 (1.91–29.93) | 0.004 | 6.69 (1.21–37.0) | 0.03 |
| % HLA-DR+ in CD4+ | 5% | 1.78 (1.19–2.67) | 0.005 | 1.72 (0.95–3.12) | 0.08 |
| % HLA-DR+ in CD8+ | 5% | 1.20 (0.97–1.48) | 0.1 | 1.16 (0.88–1.53) | 0.28 |
| % CD38+ in CD4+ | 5% | 1.95 (1.22–3.10) | 0.005 | 2.10 (1.13–3.92) | 0.02 |
| % CD38+ in CD8+ | 5% | 2.19 (1.26–3.80) | 0.005 | 1.92 (1.12–3.31) | 0.02 |
| CD38 MFI of CD4+ | 500 units | 1.86 (1.21–2.89) | 0.005 | 1.80 (1.04–3.10) | 0.03 |
| CD38 MFI of CD8+ | 500 units | 1.45 (1.12–1.87) | 0.004 | 1.34 (1.05–1.71) | 0.02 |
| % HLA-DR+CD38+ in CD4+ | 5% | 3.0 (1.41–6.38) | 0.005 | 3.31 (0.93–11.83) | 0.07 |
| % HLA-DR–CD38– in CD4+ | 5% | 0.36 (0.18–0.74) | 0.005 | 0.35 (0.14–0.85) | 0.02 |
| % HLA-DR+CD38+ in CD8+ | 5% | 2.11 (1.27–3.50) | 0.004 | 2.06 (1.10–3.87) | 0.02 |
| % HLA-DR–CD38– in CD8+ | 5% | 0.65 (0.49–0.86) | 0.002 | 0.70 (0.50–0.98) | 0.04 |
| % HLA-DR–CD38+ in CD8+ | 5% | 2.35 (1.27–4.35) | 0.007 | 2.15 (0.03–4.52) | 0.04 |
| % HLA-DR+CD38– in CD8+ | 5% | 0.16 (0.04–0.61) | 0.007 | 0.02 (0.001–0.75) | 0.03 |
Listed in tables are OR (odds ratio), 95% CI (confidence interval), and p value. p values <0.05 are highlighted in bold.
ORs and p values were calculated from conditional regression model 1 controlling for matching variables.
ORs and p values were calculated from conditional regression model 2 controlling for matching variables and CD4 counts at the index visit.
Discussion
Previous studies2,4,37,38 have reported that an elevated proportion of CD38+ T cells in CD4+ and CD8+ T cells as well as increased CD38 MFI on CD8+ T cells are associated with HIV-1 disease progression. Our study confirmed previous findings and further revealed that increased CD38 intensity on CD4+ T cells, measured at the late stage of HIV-1 infection, is also associated with fast progression to AIDS and death, independent of CD4+ T cell count and age. Although the coexpression of HLA-DR and CD38 correlates with fast disease progression independently, HLA-DR alone was not found to be associated with the rate of progression. Furthermore, we found that elevation of HLA-DR+CD38–CD8+ T cells is a marker of delayed HIV-1 progression, consistent with a previous report that documented that HIV-1-infected individuals who had prolonged stable CD4+ T cell counts after seroconversion, as well as those long-term survivors, had high levels of HLA-DR+CD38–CD8+ T cells.39 These observations suggest a dominant role of CD38 as an activation marker in the relationship between immune activation and HIV-1 progression.
One of the unique findings from our study is that the expansion of Tregs during HIV-1 infection is associated with a rapid disease progression. How Tregs expand within the CD4+ T cell compartment during HIV-1 infection remains unclear. Tregs have been shown to be difficult to proliferate in vitro.10 If Tregs in vivo also have this characteristic; then the peripheral proliferation is not likely to be responsible for the expansion of Tregs during HIV-1 infection. Although it is assumed that Tregs may be partially spared from direct HIV-1 infection and killing due to their low rates of cell division, Tregs have been shown to have a susceptibility to HIV-1 infection comparable to conventional memory T cells.10 However, studies have shown that upon activation, conventional non-Tregs (Foxp3–CD25–CD4+) can convert to Tregs (Foxp3+CD25+CD4+),17,40 which alters the generation rate of Tregs while providing a new avenue of cell loss from the conventional CD4+ T cell compartment. This would result in a relatively faster loss of conventional CD4+ T cells and a somewhat blunted loss of Tregs. As Tregs are a small population, this phenomenon could easily increase the proportion of Tregs within the CD4+ T cell compartment. Thus, the high proportion of Tregs observed among HIV-1-infected individuals may be partially due to the high rate of conversion from non-Tregs to Tregs, resulting from intensive T cell activation associated with HIV-1 infection. This hypotheses is consistent with the fact that Tregs share many activation markers with activated non-Tregs.10,41 It also provides a reasonable explanation for our observation of a positive correlation between Tregs and CD4+ T cell activation in fast progressors. The fact that this correlation was not seen in HIV-1-infected slow progressors, or uninfected individuals, suggests that the immune activation needs to be increased to a high threshold before a measurable effect on Tregs induction is reached. In addition, the relative expansion of Tregs could also be attributed to a longer lifespan of Tregs42 and the sparing of Tregs from the activation-induced apoptosis of uninfected cells due to their inability to proliferate.
An important and unanswered question is whether Tregs play a protective or detrimental role in the immunopathology of HIV-1 infection. On one hand, Tregs are known to profoundly inhibit T cell activation, which is a major contributor to HIV-1 progression. Some researchers observed a depletion of Tregs during HIV-1 infection and suggested that this may contribute to the hyperactivation of T cells and subsequently lead to rapid disease progression.9,10 However, as observed in our study, the positive correlation between the proportion of Tregs and CD4+ T cell activation among fast progressors, and the lack of any association between Tregs and CD8+ T cell activation, does not support an effective suppressive role of Tregs on immune activation. On the other hand, some investigators have proposed that an increase of Tregs may impair the immune control of HIV-1 infection, based on the in vitro evidence that Tregs could suppress virus-specific T cell responses, and that removal of Tregs resulted in increased antigen-specific immune responses from HIV-1-specific CD4+ and CD8+ T cells.11,28,36,43–45 This hypothesis is more in keeping with our observation that HIV-1-infected individuals with a rapid disease course had significantly increased Tregs within the CD4+ T cell compartment. The significant expansion of Tregs relative to other CD4+ T cell subsets in advanced HIV-1 infection may establish an increased suppressor-to-helper ratio, which could lead to suppressed T cell immune responses to HIV-1 and other pathogens, resulting in a rapid progression to AIDS. However, we cannot rule out the possibility that the relative expansion of Tregs can be a by-product of immune activation and has no specific role in HIV-1 disease progression. Considering that a large proportion of T cells appears to be under the regulation of very few Tregs, it would be promising to develop strategies aimed at manipulating this cell population, which plays a potent role in immunoregulation. However, this effort must be preceded by a thorough understanding of the role of Tregs during HIV-1 infection and the underlying mechanisms by which Tregs operate.
Another unique and interesting finding form our study is that we observed an increased Foxp3 expression in CD8+ T cells among fast progressors. Although Foxp3 is thought to be primarily expressed in CD4+ T cells with regulatory activity, CD8+ T cells that express Foxp3 have also been shown to have suppressive activity to other T cells.46,47 Our finding suggests that there is an expansion of CD8+ T cells with regulatory properties during HIV-1 infection. Whether the increased Foxp3 expression in both CD4+ and CD8+ T cells shares similar induction mechanisms and functional consequences needs further investigation.
In summary, our findings suggest that in the absence of therapy, HIV-1-infected individuals with rapid progression to AIDS have a high proportion of Tregs and/or activated T cells. In addition, there is a close correlation between the expansion of Tregs and CD4+ T cell activation, but only among HIV-1-infected fast progressors in advanced disease. Further studies will have to address the question of whether Tregs measured at an early time point of infection have prognostic value for rapid HIV-1 progression.
Our study is limited by the lack of data on HIV-1 viral load. The PBMC samples we used were collected in the early stage of the MACS study, when measures of HIV-1 RNA on heparinized plasma samples were not done. To our knowledge, only one study has suggested a positive association between level of Tregs in tonsils and HIV-1 viral load. However, the result was limited by a small sample size (n = 10).48 The influence of viral load on the level of Tregs is unclear. In addition, immune sequestration may impact our findings, as suggested by our previous report49 that correlations between viral load and immune parameters, such as CD8+ T cell activation, can differ somewhat when looking within lymph nodes as opposed to peripheral blood.
Acknowledgments
We thank Mary Ann Hausner and Marianne Chow for their technical help. We also would like to thank the staff and participants of the Multi-center AIDS Cohort Study. This work was supported by NIH Grants U01 AI03540 and R01 AI058845 (B.D.J. P.I.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Hazenberg MD. Otto SA. van Benthem BH, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17(13):1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 2.Giorgi JV. Hultin LE. McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179(4):859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 3.Liu Z. Cumberland WG. Hultin LE. Prince HE. Detels R. Giorgi JV. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16(2):83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 4.Giorgi JV. Lyles RH. Matud JL, et al. Predictive value of immunologic and virologic markers after long or short duration of HIV-1 infection. J Acquir Immune Defic Syndr. 2002;29(4):346–355. doi: 10.1097/00126334-200204010-00004. [DOI] [PubMed] [Google Scholar]
- 5.Broussard SR. Staprans SI. White R. Whitehead EM. Feinberg MB. Allan JS. Simian immunodeficiency virus replicates to high levels in naturally infected African green monkeys without inducing immunologic or neurologic disease. J Virol. 2001;75(5):2262–2275. doi: 10.1128/JVI.75.5.2262-2275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McClure HM. Anderson DC. Fultz PN. Ansari AA. Lockwood E. Brodie A. Spectrum of disease in macaque monkeys chronically infected with SIV/SMM. Vet Immunol Immunopathol. 1989;21(1):13–24. doi: 10.1016/0165-2427(89)90126-8. [DOI] [PubMed] [Google Scholar]
- 7.Rey-Cuille MA. Berthier JL. Bomsel-Demontoy MC, et al. Simian immunodeficiency virus replicates to high levels in sooty mangabeys without inducing disease. J Virol. 1998;72(5):3872–3886. doi: 10.1128/jvi.72.5.3872-3886.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tesselaar K. Arens R. van Schijndel GM, et al. Lethal T cell immunodeficiency induced by chronic costimulation via CD27-CD70 interactions. Nat Immunol. 2003;4(1):49–54. doi: 10.1038/ni869. [DOI] [PubMed] [Google Scholar]
- 9.Eggena MP. Barugahare B. Jones N, et al. Depletion of regulatory T cells in HIV infection is associated with immune activation. J Immunol. 2005;174(7):4407–4414. doi: 10.4049/jimmunol.174.7.4407. [DOI] [PubMed] [Google Scholar]
- 10.Oswald-Richter K. Grill SM. Shariat N, et al. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol. 2004;2(7):E198. doi: 10.1371/journal.pbio.0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss L. Donkova-Petrini V. Caccavelli L. Balbo M. Carbonneil C. Levy Y. Human immunodeficiency virus-driven expansion of CD4+ CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood. 2004;104(10):3249–3256. doi: 10.1182/blood-2004-01-0365. [DOI] [PubMed] [Google Scholar]
- 12.Sakaguchi S. Regulatory T cells: Key controllers of immunologic self-tolerance. Cell. 2000;101(5):455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 13.Shevach EM. Regulatory T cells in autoimmmunity. Annu Rev Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 14.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 15.Curotto de Lafaille MA. Lafaille JJ. CD4(+) regulatory T cells in autoimmunity and allergy. Curr Opin Immunol. 2002;14(6):771–778. doi: 10.1016/s0952-7915(02)00408-9. [DOI] [PubMed] [Google Scholar]
- 16.Furtado GC. Olivares-Villagomez D. Curotto de Lafaille MA. Wensky AK. Latkowski JA. Lafaille JJ. Regulatory T cells in spontaneous autoimmune encephalomyelitis. Immunol Rev. 2001;182:122–134. doi: 10.1034/j.1600-065x.2001.1820110.x. [DOI] [PubMed] [Google Scholar]
- 17.Walker MR. Kasprowicz DJ. Gersuk VH, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+ CD25- T cells. J Clin Invest. 2003;112(9):1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fontenot JD. Gavin MA. Rudensky AY. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 19.Khattri R. Cox T. Yasayko SA. Ramsdell F. An essential role for Scurfin in CD4+ CD25+ T regulatory cells. Nat Immunol. 2003;4(4):337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 20.Schubert LA. Jeffery E. Zhang Y. Ramsdell F. Ziegler SF. Scurfin (FOXP3) acts as a repressor of transcription and regulates T cell activation. J Biol Chem. 2001;276(40):37672–37679. doi: 10.1074/jbc.M104521200. [DOI] [PubMed] [Google Scholar]
- 21.Hori S. Nomura T. Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 22.Fontenot JD. Rasmussen JP. Williams LM. Dooley JL. Farr AG. Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22(3):329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Godfrey VL. Wilkinson JE. Russell LB. X-linked lymphoreticular disease in the scurfy (sf ) mutant mouse. Am J Pathol. 1991;138(6):1379–1387. [PMC free article] [PubMed] [Google Scholar]
- 24.Brunkow ME. Jeffery EW. Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27(1):68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 25.Wildin RS. Ramsdell F. Peake J, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27(1):18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 26.Bennett CL. Christie J. Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27(1):20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 27.Lahl K. Loddenkemper C. Drouin C, et al. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204(1):57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinter AL. Hennessey M. Bell A, et al. CD25(+)CD4(+) regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4(+) and CD8(+) HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J Exp Med. 2004;200(3):331–343. doi: 10.1084/jem.20032069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsunemi S. Iwasaki T. Imado T, et al. Relationship of CD4+ CD25+ regulatory T cells to immune status in HIV-infected patients. AIDS. 2005;19(9):879–886. doi: 10.1097/01.aids.0000171401.23243.56. [DOI] [PubMed] [Google Scholar]
- 30.Apoil PA. Puissant B. Roubinet F. Abbal M. Massip P. Blancher A. FOXP3 mRNA levels are decreased in peripheral blood CD4+ lymphocytes from HIV-positive patients. J Acquir Immune Defic Syndr. 2005;39(4):381–385. doi: 10.1097/01.qai.0000169662.30783.2d. [DOI] [PubMed] [Google Scholar]
- 31.Montes M. Lewis DE. Sanchez C, et al. Foxp3+ regulatory T cells in antiretroviral-naive HIV patients. AIDS. 2006;20(12):1669–1671. doi: 10.1097/01.aids.0000238415.98194.38. [DOI] [PubMed] [Google Scholar]
- 32.Kaslow RA. Ostrow DG. Detels R. Phair JP. Polk BF. Rinaldo CR:, Jr The Multicenter AIDS Cohort Study: Rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126(2):310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 33.Gjerset G. Nelson KA. Michael D. Methods for cryopreserving cells. In: Rose NR, editor; De MacArio EC, editor; Fahey JL, editor; Friedman H, editor; Penn GM, editor. Manual of Clinical Laboratory Immunology. 4th. American Society of Microbiology; Washington DC: 1992. pp. 61–67. [Google Scholar]
- 34.Hultin LE. Matud JL. Giorgi JV. Quantitation of CD38 activation antigen expression on CD8+ T cells in HIV-1 infection using CD4 expression on CD4+ T lymphocytes as a biological calibrator. Cytometry. 1998;33(2):123–132. doi: 10.1002/(sici)1097-0320(19981001)33:2<123::aid-cyto6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 35.Iyer SB. Hultin LE. Zawadzki JA. Davis KA. Giorgi JV. Quantitation of CD38 expression using QuantiBRITE beads. Cytometry. 1998;33(2):206–212. doi: 10.1002/(sici)1097-0320(19981001)33:2<206::aid-cyto15>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 36.Aandahl EM. Michaelsson J. Moretto WJ. Hecht FM. Nixon DF. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J Virol. 2004;78(5):2454–2459. doi: 10.1128/JVI.78.5.2454-2459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Z. Hultin LE. Cumberland WG, et al. Elevated relative fluorescence intensity of CD38 antigen expression on CD8+ T cells is a marker of poor prognosis in HIV infection: Results of 6 years of follow-up. Cytometry. 1996;26(1):1–7. doi: 10.1002/(SICI)1097-0320(19960315)26:1<1::AID-CYTO1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 38.Kestens L. Vanham G. Vereecken C, et al. Selective increase of activation antigens HLA-DR and CD38 on CD4+ CD45RO+ T lymphocytes during HIV-1 infection. Clin Exp Immunol. 1994;95(3):436–441. doi: 10.1111/j.1365-2249.1994.tb07015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giorgi JV. Ho HN. Hirji K, et al. CD8+ lymphocyte activation at human immunodeficiency virus type 1 seroconversion: Development of HLA-DR+ CD38- CD8+ cells is associated with subsequent stable CD4+ cell levels. The Multicenter AIDS Cohort Study Group. J Infect Dis. 1994;170(4):775–781. doi: 10.1093/infdis/170.4.775. [DOI] [PubMed] [Google Scholar]
- 40.Chen W. Jin W. Hardegen N, et al. Conversion of peripheral CD4+ CD25- naive T cells to CD4+ CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baecher-Allan C. Brown JA. Freeman GJ. Hafler DA. CD4+ CD25 high regulatory cells in human peripheral blood. J Immunol. 2001;167(3):1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 42.Nilsson J. Boasso A. Velilla PA, et al. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood. 2006;108(12):3808–3817. doi: 10.1182/blood-2006-05-021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vahlenkamp TW. Tompkins MB. Tompkins WA. Feline immunodeficiency virus infection phenotypically and functionally activates immunosuppressive CD4+ CD25+ T regulatory cells. J Immunol. 2004;172(8):4752–4761. doi: 10.4049/jimmunol.172.8.4752. [DOI] [PubMed] [Google Scholar]
- 44.Dittmer U. He H. Messer RJ, et al. Functional impairment of CD8(+) T cells by regulatory T cells during persistent retroviral infection. Immunity. 2004;20(3):293–303. doi: 10.1016/s1074-7613(04)00054-8. [DOI] [PubMed] [Google Scholar]
- 45.Estes JD. Li Q. Reynolds MR, et al. Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency virus infection. J Infect Dis. 2006;193(5):703–712. doi: 10.1086/500368. [DOI] [PubMed] [Google Scholar]
- 46.Cosmi L. Liotta F. Lazzeri E, et al. Human CD8+ CD25+ thymocytes share phenotypic and functional features with CD4+ CD25+ regulatory thymocytes. Blood. 2003;102(12):4107–4114. doi: 10.1182/blood-2003-04-1320. [DOI] [PubMed] [Google Scholar]
- 47.Manavalan JS. Kim-Schulze S. Scotto L, et al. Alloantigen specific CD8+ CD28- FOXP3+ T suppressor cells induce ILT3+ ILT4+ tolerogenic endothelial cells, inhibiting alloreactivity. Int Immunol. 2004;16(8):1055–1068. doi: 10.1093/intimm/dxh107. [DOI] [PubMed] [Google Scholar]
- 48.Andersson J. Boasso A. Nilsson J, et al. The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J Immunol. 2005;174(6):3143–3147. doi: 10.4049/jimmunol.174.6.3143. [DOI] [PubMed] [Google Scholar]
- 49.Yang OO. Ferbas JJ. Hausner MA, et al. Effects of HIV-1 infection on lymphocyte phenotypes in blood versus lymph nodes. J Acquir Immune Defic Syndr. 2005;39(5):507–518. [PubMed] [Google Scholar]