Abstract
Humoral immune responses occur following exposure to Adeno-associated virus (AAV) or AAV vectors. Many studies characterized antibody responses to AAV, but human IgG subclass responses to AAV have not been previously described. In this study, IgG subclass responses were examined in serum samples of normal human subjects exposed to wild-type AAV, subjects injected intramuscularly with AAV vectors and subjects injected intravascularly with AAV vectors. A diversity of IgG subclass responses to AAV capsid were found in different subjects. IgG1 was found to be the dominant response. IgG2, IgG3 and IgG4 responses were also observed in most normal human subjects; IgG2 and IgG3 each represented the major fraction of total anti-AAV capsid IgG in a subset of normal donors. Subjects exposed to AAV vectors showed IgG responses to AAV capsid of all four IgG subclasses. IgG responses to AAV capsid in clinical trial subjects were inversely proportional to the level of pre-existing anti-AAV antibody and independent of the vector dose. The high levels of anti-AAV capsid IgG1 can mask differences in IgG2, IgG3 and IgG4 responses that were observed in this study. Analysis of IgG subclass distribution of anti-AAV capsid antibodies indicates a complex, non-uniform pattern of responses to this viral antigen.
Keywords: Immunoglobulin, subclass distribution, AAV, humoral immunity, gene therapy
Introduction
Adeno-associated virus is a helper-dependent virus of the family parvoviridae, subfamily parvovirinae, genus erythrovirus, species adeno-associated virus. It requires a helper virus for replication, so natural infections take place in the context of infection with a helper virus such as adenovirus. Infection with adeno-associated virus causes no known pathologies. Adeno-associated virus (AAV) vectors are scalable, efficient, non-cytopathic gene delivery vehicles used primarily for the treatment of genetic diseases [Warrington and Herzog, 2006]. Their ability to transduce non-dividing cells and persist episomally results in long-term transgene expression in animals. A broad spectrum of animal models of human diseases has been successfully treated by AAV vectors, including diseases of the brain, heart, lung, eye and liver [Warrington and Herzog, 2006]. Hemophilia B is an approachable target for the use of gene transfer vectors because therapeutic benefits can be realized through expression of as little as 1–2% of wild-type levels of Factor IX (hFIX) [High, 2005].
Pre-clinical studies showed that intramuscular delivery of AAV-canine FIX vector in a canine model of Hemophilia B resulted in stable expression of circulating canine FIX at therapeutic levels for the life of the animals [Herzog et al., 1999] (KAH, unpublished data). Two clinical trials were initiated to test the safety and efficacy of AAV-hFIX vector treatment of hemophilia B in human subjects [Manno et al., 2003; Manno et al., 2006]. In humans injected intramuscularly with AAV-hFIX, stable expression of hFIX resulted, but only sub-therapeutic levels of hFIX were achieved [Manno et al., 2003]. Subsequently, a liver-directed AAV-hFIX clinical trial was initiated to treat hemophilia B through a vascular delivery route [Manno et al., 2006]. An hFIX transgene under the control of a liver-specific promoter was used to ensure that transgene expression was restricted exclusively to hepatocytes. In pre-clinical studies, expression of canine FIX was more efficient when AAV vectors were targeted to the liver rather than the muscle in canine models of hemophilia B [Mount et al., 2002]. Indeed, this finding extrapolated to human subjects as well. In the second clinical trial using liver-directed AAV vectors, one of two subjects tested at the highest dose achieved therapeutic levels of hFIX expression which persisted for four weeks before declining to baseline levels [Manno et al., 2006]. Additionally a self-limited transient transaminitis was observed during the decline of hFIX levels. The transient nature of expression of hFIX observed in the clinical study was not expected based on animal studies in mice, dogs and non-human primates, where expression had been long term [Jiang et al., 2006].
Subsequent work identified a CD8+ T cell response against AAV capsid that arose concomitantly with the decline in hFIX levels [Mingozzi et al., 2007]. These data supported a hypothesis that capsid specific CD8+ T cells were activated by the infused vector and responded to the vector-transduced hepatocytes as they would to virus-infected cells. CD8+ T cells that respond to AAV capsid epitopes were also found among normal human subject PBMCs [Mingozzi et al., 2007]. These recent findings give rise to questions regarding the natural infection process of AAV virus in humans, and underscore the dearth of knowledge in this area. While it is established that the first exposure to AAV usually occurs in childhood, the frequency of AAV re-infections, the tissue distribution of AAV during infection and the duration of AAV infections are all unknown at this time [Blacklow et al., 1968; Blacklow et al., 1971].
For many viruses, studies of the IgG subclasses that arise against viral antigens can give insight into the nature and duration of the exposure or infection. Antibodies to Hepatitis B vary in subclass specificity based on whether the primary exposure was to recombinant protein (IgG1), recombinant DNA (IgG1 and IgG2) or a natural infection (IgG1 and IgG3) [Borzi et al., 1992; Carotenuto et al., 1995; Wang et al., 2005]. Moreover, carriers of hepatitis (IgG1>IgG3) can be distinguished from hepatitis-infected individuals undergoing liver cirrhosis (IgG3>IgG1) based on IgG subclass profiles [Akbar et al., 1990; Torgano et al., 1995]. Primary and secondary exposure to viruses can also be distinguished by IgG subclasses in the case of herpesvirus, respiratory syncytial virus, measles virus and dengue virus [El Mubarak et al., 2004; Hashido and Kawana, 1997; Koraka et al., 2001; Wagner et al., 1989]. In the case of human parvovirus B19, closely related to AAV virus, the relative levels of IgG3 and IgG4 can be used as a highly sensitive and accurate measure of the time since acute infection [Franssila et al., 1996].
Antibodies to AAV2 capsid are prevalent in the general population. Estimates of the exact frequency of antibodies to AAV2 capsid vary widely, ranging from 35% to 80% [Erles et al., 1999; Sprecher-Goldberger et al., 1971; Tobiasch et al., 1994]. IgG, IgM and IgA frequencies were measured at different times, but there is no published report of the IgG subclass distribution in humans exposed to AAV capsid [Chirmule et al., 1999; Erles et al., 1999]. Several animal studies did examine the IgG subclass distribution following exposure to AAV vectors. Mice administered AAV vectors intramuscularly exhibited distinct IgG subclass patterns that were discernible across strains. Both IgG2a and IgG3 responses were evident in Balb/C mice exposed to the same vector, while only an IgG2a response was observed in C57/Bl6 mice [Chirmule et al., 2000]. Similarly, intravenous administration of AAV vector in C57/Bl6 mice resulted in IgG2a and IgG2b responses [Zhang et al., 2005]. In contrast to mice, IgG subclass profiles in non-human primates exposed to AAV vector are distinctly dependent upon the route of administration. Intramuscular administration of AAV vectors into non-human primates resulted in only an IgG2 anti-AAV capsid antibody response [Chirmule et al., 2000], while intravascular administration of AAV vector into non-human primates resulted in only IgG1 anti-AAV capsid antibody formation [Nathwani et al., 2006; Nathwani et al., 2007]. The caveat to this disparity is that the intramuscular injections were performed with AAV2, while the findings with intravascular administration pertain to serotypes AAV5 and AAV8.
Given the complete lack of knowledge regarding IgG subclass responses to AAV capsid in humans, this study was initiated to characterize IgG subclass profiles in normal human subjects, human subjects injected intramuscularly with AAV vector, and human subjects injected intravascularly with AAV vector.
Results
IgG subclass distribution in normal human subjects
To determine the relative levels of IgG subclass responses to AAV capsid in normal human subjects, serum samples were collected from 32 normal donors. These 32 samples were then used to determine both IgM and IgG subclass specific levels of anti-AAV2 capsid antibodies (Table 1). ELISA assays were used to determine the anti-AAV2 capsid antibody levels for each subclass. Empty AAV vector particles were coated directly onto ELISA plates. Serum samples were incubated overnight with the coated plates, and detection was performed using subclass specific biotinylated secondary antibodies followed by a streptavidin-conjugated HRP tertiary. To control for differences in the different subclass-specific detection antibodies, purified IgG from the matched subclass was serially diluted and coated directly onto the same plate as the vector particles. The purified IgG was used to generate a standard curve for conversion of O.D. measurements to quantitative values of antibody concentration.
Table 1.
IgG Subclass Distribution and Neutralizing Titer of anti-AAV2 Capsid Antibodies in Normal Human Subjects
| Donor | IgG11 | IgG21 | IgG31 | IgG41 | IgM1 | Neutralizing titer2 |
|---|---|---|---|---|---|---|
| 1 | 862 | 46 | 53 | 166 | 707 | <1:3.1 |
| 2 | 13209 | 1854 | 766 | 0 | 2119 | 1:310–1:1000 |
| 3 | 4258 | 1378 | 776 | 105 | 738 | 1:31–1:100 |
| 4 | 7534 | 70 | 355 | 0 | 413 | 1:10–1:31 |
| 5 | 1405 | 0 | 46 | 0 | 143 | 1:3–1:10 |
| 6 | 1056 | 182 | 36 | 36 | 413 | <1:3.1 |
| 7 | 1071 | 126 | 175 | 0 | 433 | <1:3.1 |
| 8 | 6826 | 195 | 228 | 0 | 317 | 1:31–1:100 |
| 9 | 1050 | 1715 | 2618 | 534 | 598 | ND |
| 10 | 7285 | 0 | 503 | 45 | 208 | ND |
| 11 | 482 | 475 | 1202 | 222 | 411 | ND |
| 12 | 6796 | 1476 | 902 | 278 | 465 | 1:316–1:1000 |
| 13 | 1125 | 0 | 1430 | 78 | 672 | <1:3.1 |
| 14 | 8633 | 1735 | 1794 | 65 | 1012 | ND |
| 15 | 2928 | 1480 | 553 | 565 | 630 | ND |
| 16 | 6142 | 0 | 549 | 0 | 4710 | ND |
| 17 | 635 | 1385 | 0 | 451 | 364 | ND |
| 18 | 6667 | 553 | 253 | 0 | 521 | ND |
| 19 | 3000 | 2511 | 629 | 65 | 2016 | 1:100–1:310 |
| 20 | 1 | 653 | 488 | 40 | 258 | <1:3.1 |
| 21 | 819 | 659 | 574 | 35 | 596 | 1:3.1–1:10 |
| 22 | 0 | 366 | 491 | 11 | 894 | <1:3.1 |
| 23 | 1734 | 583 | 544 | 17 | 1209 | <1:3.1 |
| 24 | 366 | 738 | 496 | 54 | 1005 | <1:3.1 |
| 25 | 3000 | 6220 | 572 | 159 | 534 | 1:31–1:100 |
| 26 | 3000 | 773 | 592 | 82 | 626 | 1:10–1:31 |
| 27 | 3000 | 900 | 511 | 62 | 626 | 1:10–1:31 |
| 28 | 1 | 554 | 487 | 38 | 766 | <1:3.1 |
| 29 | 3000 | 1376 | 480 | 1285 | 794 | 1:10–1:31 |
| 30 | 1920 | 692 | 550 | 16 | 403 | 1:3.1–1:10 |
| 31 | 2624 | 1089 | 495 | 100 | 674 | 1:3.1–1:10 |
| 32 | 714 | 2910 | 782 | 123 | 1609 | 1:3.1–1:10 |
| Average | 3160.7 | 1021.7 | 622.8 | 144.8 | 840.1 | N/A |
Values shown represent antibody concentrations in ng/mL. ND is not determined.
Neutralizing titer represents the highest dilution at which greater than or equal to 50% transduction occurs compared to controls.
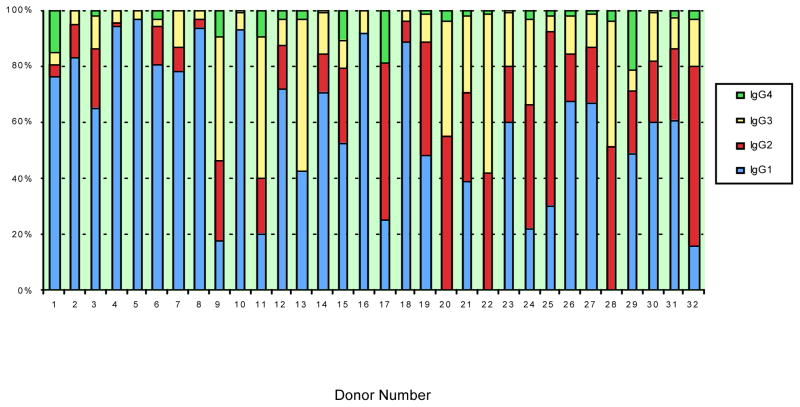
The results, shown in Table 1, establish that antibodies from each IgG subclass were detected in a subset of the samples. Antibody responses for each normal donor are shown for each IgG subclass and IgM. In terms of total levels of IgG produced, the dominant IgG subclass response to AAV2 capsid was IgG1 (Table 1). The frequencies of anti-AAV2 capsid antibodies for each subclass were: 29 out of the 32 subjects were positive for IgG1 (>100 ng/mL), 26 out of the 32 subjects were positive for IgG2 (>100ng/mL), 28 out of the 32 subjects were positive for IgG3 (>100 ng/mL), 25 out of the 32 subjects were positive for IgG4 (>10 ng/mL), and 32 out of 32 were positive for IgM (>100 ng/mL). Figure 1 presents the data for each IgG subclass as a percentage of the total IgG specific for AAV2 capsid. The major fraction of anti-AAV2 capsid IgG antibody was IgG1 for 22 out of the 32 donors, IgG2 was the largest fraction for 6 donors and IgG3 was the largest fraction for 4 donors.
Figure 1. Distribution of IgG subclass responses to AAV capsid in normal human subjects.
The percent of total capsid-specific IgG is shown for each IgG subclass for 32 normal human subjects.
Additionally, neutralizing antibody titers were determined for 24 of the 32 normal donors (Table 1). Consistent with previous reports, 9 of the 24 donor samples exhibited undetectable levels of neutralizing activity (37.5%) [Erles et al., 1999; Sprecher-Goldberger et al., 1971; Tobiasch et al., 1994]. The neutralizing titers ranged from undetectable to 1:1000, as shown in Table 1. High levels of neutralizing antibody correlated with higher levels of IgM, IgG1 and IgG2 while no relationship was observed between neutralizing antibody levels and total levels of IgG3 and IgG4 anti-AAV2 capsid antibody.
Humoral response to intramuscular administration of AAV vector
To compare the results from normal human donors to subjects receiving direct intramuscular injections of AAV vector, we analyzed samples collected from subjects enrolled in a clinical trial to test the safety and efficacy of AAV2-hFIX vector administration for the treatment of hemophilia B. Serial collection of plasma samples from 4 different subjects was performed for anti-AAV2 capsid IgG subclass analysis. Subject 01-01 received a vector dose of 2×1011 vector genomes per kg (vg/kg), subjects 02–05 and 01–06 received a vector dose of 6×1011 vg/kg, and subject 02–07 received a vector dose of 2×1012 vg/kg.
Results are shown in Figure 2 and Table 2 for all four subjects. Subject 01–06 experienced a relatively rapid increase in anti-AAV capsid antibodies. In this subject, anti-AAV2 IgG1 rose almost 10-fold from a baseline level of 920 ng/mL to 8,803 ng/mL by day 2 after vector administration. This subject also experienced a rapid increase in anti-AAV2 capsid IgG3 levels, from a baseline of 46 ng/mL to 1120 ng/mL on day two post-exposure, while a smaller rise in anti-AAV2 capsid IgG2 and IgG4 levels was evident as early as seven days post-exposure. IgM levels rose from a baseline level of 222 ng/mL to a peak of 956 ng/mL one week post-exposure before stabilizing at a level approximately 3-fold higher than the baseline level. For the other three subjects, IgG1 levels were higher at baseline (>18,000ng/mL) and remained relatively unchanged for the duration of the study (<20% increase). Interestingly, in spite of relatively stationary IgG1 levels, the remaining IgG subclasses showed increases in anti-AAV capsid responses over time. This effect was most pronounced in subject 02–05, who experienced a rise in IgG3 from undetectable levels at baseline to 4,828 ng/mL at week 12 post-exposure. Subject 02–05 also showed increases in anti-AAV capsid IgG2 antibodies from a baseline level of 596 ng/mL to a maximum of 2,344 ng/mL at week 4 post-exposure, and in anti-AAV capsid IgG4 antibodies from a baseline level of 373ng/mL to a maximum of 1,256 ng/mL at week 4 post-exposure. Subject 02–07 showed increased levels of anti-AAV capsid antibody for subclasses IgG2, IgG3 and IgG4 while subject 01-01 only exhibited an increase in IgG4 antibody levels. IgM levels rose less than 3-fold over the course of the study for subjects 01-01 and 02–05, while subject 02–07 exhibited over a 7-fold increase from a baseline level of 312 ng/mL to 2244 ng/mL one week post-exposure. This increase in IgM levels for subject 02–07 occurred concurrently with the rise in IgG2 and IgG4 anti-AAV capsid antibody levels seen in this subject.
Figure 2. IgG Subclass responses to human subjects receiving intramuscular AAV vector injection.
Results of ELISA assays measuring AAV capsid-specific IgG levels for each subclass are shown for subjects 01-01 (A), 01–06 (B), 02–05 (C) and 02–07 (D).
Table 2.
IgG Subclass Distribution of anti-AAV2 Capsid Antibodies Following Intramuscular Administration of AAV2-hFIX Vector
| Subject 01-01 (dose 2×1011 vg/kg) | ||||||
|---|---|---|---|---|---|---|
| Time Point | IgG11 | IgG21 | IgG31 | IgG41 | IgM1 | Neutralizing Titer2,3 |
| Baseline | 28213 | 954 | 0 | 235 | 579 | 1:100 |
| Week 3 | 30137 | 1051 | 0 | 293 | 666 | 1:5000 |
| Week 7 | 26056 | 1100 | 0 | 310 | 1083 | ND |
| Week 10 | 29558 | 1161 | 0 | 441 | 918 | ND |
| Week 12 | 29639 | 1075 | 0 | 437 | 1073 | 1:1000 |
| Subject 01–06 (dose 6×1011 vg/kg) | ||||||
| Time Point | IgG11 | IgG21 | IgG31 | IgG41 | IgM1 | Neutralizing Titer2,3 |
| Baseline | 920 | 0 | 46 | 0 | 222 | 1 |
| Day 2 | 8803 | 0 | 1120 | 0 | 581 | ND |
| Week 1 | 11753 | 461 | 1444 | 60 | 956 | ND |
| Week 2 | 9952 | 403 | 1449 | 58 | 298 | ND |
| Week 4 | 9665 | 453 | 3719 | 58 | 505 | 1:1000 |
| Week 8 | 24850 | 651 | 8141 | 59 | 619 | ND |
| Subject 02–05 (dose 6×1011 vg/kg) | ||||||
| Time Point | IgG11 | IgG21 | IgG31 | IgG41 | IgM1 | Neutralizing Titer2,3 |
| Baseline | 20103 | 596 | 0 | 373 | 316 | 1:100 |
| Day 3 | 18418 | 825 | 0 | 458 | 333 | ND |
| Week 1 | 22996 | 1778 | 294 | 1123 | 774 | ND |
| Week 4 | 21377 | 2344 | 4442 | 1256 | 736 | 1:1000 |
| Week 8 | 27396 | 2065 | 3329 | 606 | 517 | ND |
| Week 12 | 23813 | 1375 | 4828 | 594 | 546 | 1:1000 |
| Subject 02–07 (dose 2×1012 vg/kg) | ||||||
| Time Point | IgG11 | IgG21 | IgG31 | IgG41 | IgM1 | Neutralizing Titer2,3 |
| Baseline | 18205 | 678 | 0 | 378 | 312 | 1:100 |
| Day 1 | 19621 | 518 | 0 | 304 | 302 | ND |
| Week 1 | 19763 | 1497 | 0 | 709 | 2244 | ND |
| Week 2 | 23021 | 2270 | 0 | 782 | 1238 | ND |
| Week 3 | 23975 | 1973 | 0 | 779 | 1411 | 1:10000 |
| Week 12 | 20108 | 2298 | 1130 | 0 | 762 | 1:10000 |
Values shown represent antibody concentrations in ng/mL.
Neutralizing titer represents the highest dilution at which greater than or equal to 50% transduction occurs compared to controls. ND is not determined.
These data were previously published [Manno et al., 2003] and are added here for comparison.
Humoral response to intravascular administration of AAV vector
To assess the role of route of vector administration, serum samples were also serially collected from 4 subjects enrolled in a clinical trial to test the safety and efficacy of AAV2-hFIX vector administration into the hepatic artery for the treatment of hemophilia B. These samples were used to determine the IgG subclass profile following systemic AAV2-hFIX vector administration.
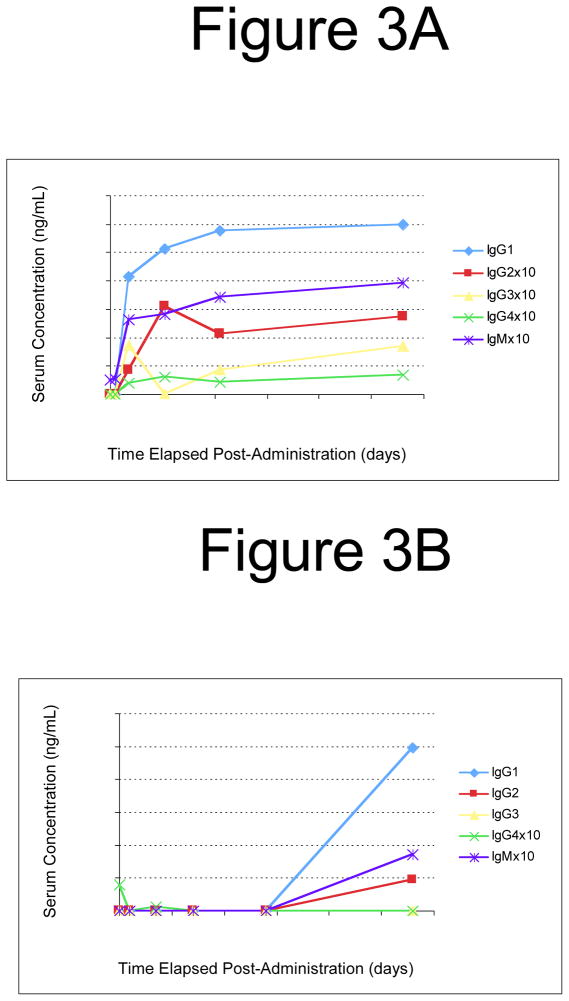
Results obtained from these plasma samples are shown in Figure 3 and Table 3. Subjects A and B received a vector dose of 8×1010 vector genomes per kg (vg/kg), subject D received a vector dose of 4×1011 vg/kg and subject F received a vector dose of 2×1012 vg/kg. In this trial, levels of neutralizing antibodies against AAV2 were measured prior to vector administration. A baseline level of pre-existing neutralizing antibodies to AAV2 (neutralizing titer of 1:17) was evident for one of the four subjects (Subject F), while the remaining three subjects had undetectable levels of neutralizing antibodies at the time of vector administration. Consistent with findings from subjects receiving intramuscular administration of vector, subject F who had the highest pre-existing anti-AAV antibody levels experienced less than a 20% increase in IgG1 levels from a baseline level of 4096 ng/mL of anti-AAV2 capsid IgG1. Also consistent with the previous findings, levels of IgG2 and IgG3 rose significantly in this subject in spite of a relatively modest increase in anti-AAV2 IgG1. Anti-AAV2 capsid IgG2 increased from undetectable levels at baseline to 453 ng/mL at week 16 post-exposure and IgG3 rose from 674 ng/mL at baseline to 1963 ng/mL at week 16 post-exposure. IgM levels in Subject F also remained relatively stable, showing less than a 3-fold increase from baseline levels through the course of study.
Figure 3. IgG Subclass responses to human subjects receiving hepatic artery AAV vector injection.
Results of ELISA assays measuring AAV capsid-specific IgG levels for each subclass are shown for subjects A (A), B (B), D (C) and F (D).
Table 3.
IgG Subclass Distribution of anti-AAV2 Capsid Antibodies Following Hepatic Artery Administration of AAV2-hFIX Vector
| Subject A (dose 8×1010 vg/kg) | ||||||
|---|---|---|---|---|---|---|
| Time Point | IgG11 | IgG21 | IgG31 | IgG41 | IgM1 | Neutralizing Titer2,3 |
| Baseline | 0 | 0 | 0 | 0 | 253 | ND |
| Day 2 | 0 | 0 | 0 | 0 | 269 | ND |
| Day 7 | 20697 | 433 | 874 | 197 | 1319 | 1:316 – 1:3.1×103 |
| Week 3 | 25698 | 1548 | 0 | 312 | 1412 | 1:103 – 1:104 |
| Week 6 | 28864 | 1073 | 429 | 212 | 1716 | 1:103 – 1:104 |
| Week 16 | 29910 | 1366 | 843 | 337 | 1972 | 1:316 – 1:1000 |
| Subject B (dose 8×1010 vg/kg) | ||||||
| Time Point | IgG11 | IgG21 | IgG31 | IgG41 | IgM1 | Neutralizing Titer2,3 |
| Baseline | 0 | 0 | 0 | 162 | 0 | 1:2 |
| Day 2 | 0 | 0 | 0 | 0 | 0 | ND |
| Day 7 | 0 | 0 | 0 | 26 | 0 | 1:10 – 1:31 |
| Week 2 | 0 | 0 | 0 | 0 | 0 | 1:10 – 1:31 |
| Week 4 | 0 | 0 | 0 | 0 | 0 | ND |
| Week 8 | 9916 | 1904 | 0 | 0 | 343 | ND |
| Subject D (dose 4×1011 vg/kg) | ||||||
| Time Point | IgG11 | IgG21 | IgG31 | IgG41 | IgM1 | Neutralizing Titer2,3 |
| Baseline | 0 | 0 | 0 | 61 | 228 | 1:11 |
| Week 1 | 0 | 0 | 108 | 95 | 414 | <1:100 |
| Week 2 | 0 | 510 | 0 | 299 | 795 | 1:100 – 1:316 |
| Week 3 | 6950 | 672 | 0 | 8 | 1346 | 1:3.1×103 – 1:105 |
| Week 5 | 27289 | 481 | 1227 | 45 | 819 | 1:3.1×103 – 1:105 |
| Week 12 | 35274 | 1078 | 3507 | 54 | 956 | ND |
| Subject F (dose 2×1012 vg/kg) | ||||||
| Time Point | IgG11 | IgG21 | IgG31 | IgG41 | IgM1 | Neutralizing Titer2,3 |
| Baseline | 4096 | 0 | 674 | 56 | 228 | 1:17 |
| Week 5 | 5083 | 0 | 1284 | 1 | 468 | 1:3.1×104 –1:106 |
| Week 6 | 5404 | 0 | 1492 | 82 | 535 | ND |
| Week 7 | 4605 | 0 | 1485 | 0 | 269 | ND |
| Week 8 | 5285 | 0 | 1433 | 0 | 401 | ND |
| Week 16 | 5538 | 453 | 1963 | 0 | 415 | ND |
Values shown represent antibody concentrations in ng/mL.
Neutralizing titer represents the highest dilution at which greater than or equal to 50% transduction occurs compared to controls.
These data were previously published [Manno et al., 2006] and are added here for comparison.
Subject F exhibited the smallest overall increase in anti-AAV2 capsid IgG despite receiving the largest vector dose. In contrast, subject A received the smallest vector dose and exhibited the largest overall increase in anti-AAV2 capsid IgG as well as the most rapid response. No anti-AAV2 capsid IgG antibodies were detected in serum from subject A at baseline. By one week after exposure, capsid specific IgG1 levels rose to 20,697 ng/mL, IgG2 levels rose to 433 ng/mL, IgG3 levels rose to 874 ng/mL and IgG4 levels rose to 197 ng/mL. In contrast to IgG, anti-AAV capsid IgM antibodies were detectable in the baseline sample of Subject A. Levels of anti-AAV capsid IgM rose concurrently with the rise in IgG antibodies in this subject. Subject D also experienced increases in all 4 subclasses of anti-AAV2 capsid IgG, but with a longer delay from the time of exposure. In this subject, the appearance of anti-AAV2 capsid IgG2, IgG3 and IgG4 occurred by two weeks after vector administration while anti-AAV2 capsid IgG1 antibody was not detectable until three weeks after vector administration. Of note, the levels of anti-AAV2 capsid IgG1, IgG2 and IgG3 were all found to continually increase from baseline through the final twelve week time point in subject D. It is not known whether anti-AAV2 capsid levels peaked by twelve weeks post-exposure. Subject D also exhibited a rise from a baseline level of 228 ng/mL anti-AAV capsid IgM to a peak of 1346 ng/mL three weeks post-exposure. In a manner similar to subject D, subject B exhibited a late increase in anti-AAV2 capsid IgG. Anti-AAV2 capsid IgG1 and IgG2 were undetectable at baseline and remained undetectable until eight weeks after exposure, at which point anti-AAV2 capsid IgG1 (9916 ng/mL) and IgG2 (1904 ng/mL) were detected. IgM levels also rose from undetectable levels to a level of 343 ng/mL eight weeks post-exposure, mirroring the rise seen in IgG1 and IgG2.
Discussion
Exposure to AAV capsid, through natural infection or through vector administration, can result in production of antibodies from all four IgG subclasses. Although IgG1 is the predominant isotype found following natural AAV infection, IgG2 and IgG3 were each found to be the most prevalent subclass in a subset of normal donors. Serum samples from donors that underwent clinical administration of AAV vectors also exhibited increases in all four IgG subclasses. As with the natural route of exposure, IgG1 was the predominant response against AAV capsid antigen, but in most subjects studied, increases in IgG2, IgG3 and IgG4 antibodies against AAV capsid were also apparent. Moreover, when vector was administered to subjects with high levels of pre-existing anti-AAV capsid IgG1, the relative increases observed in anti-AAV capsid IgG2, IgG3 and IgG4 were proportionally far greater than the rise in anti-AAV capsid IgG1.
The diverse patterns of humoral immunity observed against wild-type AAV could be a product of the diversity of contexts in which the immune system encounters AAV capsid antigens. Adenovirus, herpesvirus and human papilloma virus can all provide the helper functions necessary for completion of the AAV replication cycle [Geoffroy and Salvetti, 2005]. Thus, AAV antigen could be encountered at many different local sites within the body and in the context of many different innate and adaptive immune responses directed against the helper viruses.
Typically, IgG1 and IgG3 arise during an acute viral infection while IgG2 and IgG4 arise following chronic exposure to an antigen. A relevant example of this is the changing pattern of anti-B19 IgG subclass distribution following B19 parvovirus infection. The acute onset of symptoms following B19 infection is typically resolved within a month after the infection occurs [Heegaard and Brown, 2002]. An accompanying rise in anti-B19 IgG1 and IgG3 antibodies peaks by 4 weeks post-infection and is believed to prevent subsequent reinfection [Heegaard and Brown, 2002]. As shown here for AAV, IgG1 is the dominant IgG subclass detected against B19 virus. In spite of the relatively rapid resolution of symptoms following infection, B19 viral replication can persist for much longer periods of time [Lundqvist et al., 1999]. CD8+ T cell responses against B19 epitopes can increase in magnitude for up to one year following infection, and the expanding cells are of the activated, cytolytic phenotype [Isa et al., 2005; Norbeck et al., 2005]. Viral persistence is also supported by the study of IgG subclass responses to B19 virus following infection, where IgG4 antibody levels increase over time while IgG3 levels decline [Franssila et al., 1996]. An inflection point occurs three to five months after infection. IgG2 and IgG4 are typically associated with chronic exposure to an antigen. Given the persistent nature of the infection, it is not surprising that over time levels of IgG4 surpass levels of IgG3 anti-B19 antibody. Interestingly, this study shows that neutralizing antibody levels against AAV capsid following wild-type AAV infection may correlate with levels of IgM, IgG1 and IgG2, but not IgG3 or IgG4 (Table 1). This correlation is not always observed, however, as seen in subjects 22–24 and subject 32 in Table 1.
Whether the detection of IgG2 and IgG4 antibodies against AAV capsid indicates a chronic or persistent infection remains to be shown. AAV DNA sequences were detected by PCR in 7 of 101 human tonsil samples and 2 of 74 other human tissue samples, but whether these sequences represent acute infection or persistent infection remains unclear [Chen et al., 2005; Schnepp et al., 2005]. At a minimum, the presence of all four IgG subclasses in normal donors and clinical trial subjects suggests a complex, atypical and non-uniform immune response following exposure to AAV capsid antigen. This is in contrast to non-human primate IgG subclass responses to AAV vectors which were primarily monotypic [Chirmule et al., 2000; Nathwani et al., 2006; Nathwani et al., 2007].
Comparing anti-AAV antibody levels between naturally exposed subjects (Table 1) and those receiving AAV vector injections (Tables 2 and 3), several differences were observed. The average levels of anti-AAV capsid IgG1 and IgG3 were greater following vector exposure than following natural exposure. Using the last time point tested for each clinical trial subject, average IgG1 and IgG3 antibody levels were 22,381 ng/mL and 2556 ng/mL respectively, compared to average levels of 3161 ng/mL and 623 ng/mL for normal donors. Notwithstanding higher baseline levels of anti-AAV capsid IgG1 in several of the clinical trial subjects, this finding is consistent with a strong, acute response to vector capsid. Indeed, average levels of anti-AAV2 IgG2 and IgG4 levels were not significantly different when compared in the same way – 1275 ng/mL and 185 ng/mL respectively for clinical trial subjects compared with 1022 ng/mL and 145 ng/mL respectively for normal donors. In agreement with these data, the highest observed levels of anti-AAV2 IgG1 and IgG3 antibodies were in clinical trial subjects (35,274 ng/mL and 8,141 ng/mL respectively), while the highest observed levels of anti-AAV2 IgG2 and IgG4 antibodies were found in normal donors (6,220 ng/mL and 1285 ng/mL respectively).
It is difficult to draw conclusions based upon small sample sizes, but these were the only subjects from which samples were available for analysis. No obvious distinctions within IgG subclasses were noted in comparing anti-AAV antibody responses following intramuscular (Table 2) or intravenous (Table 3) administration of AAV vector in human subjects. It is interesting to note that in subjects B and D, who received systemic vector administration (Table 3), antibody responses to AAV were not observed until several weeks after vector administration. In subject D, antibody levels were still rising 12 weeks after vector administration. This too could indicate a persistent antigen, in spite of the replication-defective nature of the vector. These data are supported by previously published neutralizing antibody data on these two subjects. In subject B (Figure 3B and Table 3), neutralizing antibodies rose slightly from a baseline titer of 1:2 to a titer of 1:10–1:31 two weeks after vector administration [Manno et al., 2006]. However, 24 weeks after vector administration the neutralizing antibody titer was greater than three logs higher than at the two week timepoint [Manno et al., 2006]. Similarly for subject D (Figure 3D and Table 3), neutralizing antibodies rose one log from a baseline titer of 1:10 by two weeks after vector administration, but an additional two log increase was observed between two and four weeks after vector administration. This is in accordance with the large rise in IgG1 and IgG3 antibody levels seen between week two and week five post-vector administration (Figure 3D and Table 3). The reason for these delayed antibody responses is not clear. One possibility is that AAV capsid antigen persists for long periods of time in vivo. Indeed, CD8+ T-cell responses were also delayed following vector injection in human subjects [Manno et al., 2006]. Together these findings suggest a prolonged period of antigen degradation following AAV transduction in humans. Unpublished studies by our own laboratory and others confirm the persistence of intact vector particles in cultured cells and in mice. Additional studies to refine and clarify these findings are ongoing.
Antibody production in response to vector injection was not dose-dependent. Rather, stronger antibody responses were observed in those subjects with lower pre-existing antibody levels. This finding was not only true across subjects, but within individual subjects across IgG subclasses. In subjects 02–05 and 02–07, little increase in high pre-existing IgG1 anti-AAV antibodies was observed, whereas low pre-existing levels of IgG3 anti-AAV capsid antibodies increased substantially following vector administration. Of note, all of the doses used in these trials are likely to be far higher than the amount of virus exposure that would occur at the time of a natural infection. A different outcome might be expected in instances in which a lower vector dose is used, such as in a retinal gene transfer study.
This study highlights the diverse IgG subclass distributions found in anti-AAV antibody responses to AAV infection and to AAV gene transfer vectors. Crude measurement of total IgG antibody levels fails to capture this data and may veil important differences in immune responses to AAV vectors amongst clinical trial subjects, and between vector-infused and virally-infected human subjects. Additional analysis of IgG subclasses in vector-infused subjects may reveal correlates of immune responses and outcomes in clinical trials.
Materials and Methods
Samples
To study normal donors, serum samples were collected from non-hemophilic individuals. Additional samples were obtained from the Cooperative Human Tissue Network. Collection of all samples from normal donors was approved by the Institutional Review Board of the Children’s Hospital of Philadelphia. All donors were between the ages of 18 and 77. Collection of samples from hemophilic subjects enrolled in each of the two clinical trials was described previously [Manno et al., 2003; Manno et al., 2006]. Samples from all clinical trial subjects in the study were collected as part of protocols approved by the Institutional Review Boards of the Children’s Hospital of Philadelphia and the University of Pittsburgh Medical Center.
Antibody Subclass ELISA
Empty AAV particles were diluted in coating buffer (13mM sodium carbonate, 35mM sodium bicarbonate buffer, pH 9.2) to a final concentration of 1×1011 vector particles per milliliter. Fifty microliters was added to each well in a 96-well Nunc Polysorp Immunoplate (Fisher Scientific, Newark, DE). To create a standard curve, purified antibody of each isotype was coated in two-fold dilutions beginning from a concentration of 10,000 ng/mL for IgG1, 2,000 ng/mL for IgG2, 1,000 ng/mL for IgG3, 1,000 ng/mL for IgG4 and 1000ng/mL for IgM. All purified subclass specific antibodies were purchased from Sigma (St. Louis, MO). Plates were then incubated at four degrees overnight. The next day, plates were washed three times with wash buffer (.05% Tween-20 in PBS) and then blocked with blocking buffer (1% BSA in wash buffer) in the case of IgG2, IgG3, IgG4 and IgM or with Starting Block blocking buffer (Pierce, Rockford, IL) in the case of IgG1. Plates were blocked for a minimum of two hours. Plates were then washed three times with wash buffer and serum samples were added at dilutions of 1:10 and 1:20 in triplicate. Plates were again incubated overnight. The next day plates were washed four times with wash buffer and then subclass specific biotinylated antibodies were added at a concentration of 1:4000 (Sigma). Plates were incubated at 37 degrees for two hours. Plates were wa[Akbar et al., 1990]shed four times and then incubated with streptavidin-HRP at a dilution of 1:2000 for two hours at room temperature. Finally, plates were washed four times with wash buffer and developed with an OPD sodium citrate buffer (1mg/mL) (Sigma) containing hydrogen peroxide. Measurements were made at 450 wavelength using a Spectramax M2 (Molecular Devices, Sunnyvale, CA).
Neutralizing Antibody Assay
This assay was described previously [Manno et al., 2006]. Briefly, 2V6.11 cells (ATCC, Manassas, VA) were seeded into 96-well cell culture plates at a density of 5×104 cells per well and induced with Ponasterone A (Invitrogen, Carlsbad, CA) at a concentration of 1ug/mL. Serum samples were diluted (1:2, 1:3.1, 1:10, 1:31, 1:100, 1:310, 1:1000, 1:3100, 1:5000, 1:104, 1:3.1×104, 1:105 and 1:106) in heat-inactivated normal mouse serum (Sigma) and mixed directly with AAV-LacZ vector in triplicate for each dilution. Mixtures were incubated at 37 degrees for one hour. Mixtures were then added to cells and allowed to incubate overnight. The next day the medium was removed and cells were lysed in 0.005% SDS solution. Cells were then incubated with an ONPG buffer (4mg/mL) for one hour with shaking at 37 degrees. OD measurements were made using a Spectramax M2 and used to determine the neutralizing activity.
References
- Akbar SM, Onji M, Masumoto T, Ohta Y. Subclass restriction of immunoglobulin G in liver cirrhosis. Journal of gastroenterology and hepatology. 1990;5(6):627–632. doi: 10.1111/j.1440-1746.1990.tb01116.x. [DOI] [PubMed] [Google Scholar]
- Blacklow NR, Hoggan MD, Kapikian AZ, Austin JB, Rowe WP. Epidemiology of adenovirus-associated virus infection in a nursery population. American journal of epidemiology. 1968;88(3):368–378. doi: 10.1093/oxfordjournals.aje.a120897. [DOI] [PubMed] [Google Scholar]
- Blacklow NR, Hoggan MD, Sereno MS, Brandt CD, Kim HW, Parrott RH, Chanock RM. A seroepidemiologic study of adenovirus-associated virus infection in infants and children. American journal of epidemiology. 1971;94(4):359–366. doi: 10.1093/oxfordjournals.aje.a121331. [DOI] [PubMed] [Google Scholar]
- Borzi RM, Dal Monte P, Honorati MC, Facchini A. IgG subclass distribution of anti-HBs antibodies following vaccination with cDNA HBsAg. Journal of immunological methods. 1992;146(1):17–23. doi: 10.1016/0022-1759(92)90043-s. [DOI] [PubMed] [Google Scholar]
- Carotenuto P, Quinti I, Pontesilli O, Hall AJ, DeLange GG, Whittle HC, D’Amelio R, Aiuti F. Response to hepatitis B vaccine in a cohort of Gambian children. The Pediatric infectious disease journal. 1995;14(3):215–220. doi: 10.1097/00006454-199503000-00009. [DOI] [PubMed] [Google Scholar]
- Chen CL, Jensen RL, Schnepp BC, Connell MJ, Shell R, Sferra TJ, Bartlett JS, Clark KR, Johnson PR. Molecular characterization of adeno-associated viruses infecting children. Journal of virology. 2005;79(23):14781–14792. doi: 10.1128/JVI.79.23.14781-14792.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene therapy. 1999;6(9):1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- Chirmule N, Xiao W, Truneh A, Schnell MA, Hughes JV, Zoltick P, Wilson JM. Humoral immunity to adeno-associated virus type 2 vectors following administration to murine and nonhuman primate muscle. Journal of virology. 2000;74(5):2420–2425. doi: 10.1128/jvi.74.5.2420-2425.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mubarak HS, Ibrahim SA, Vos HW, Mukhtar MM, Mustafa OA, Wild TF, Osterhaus AD, de Swart RL. Measles virus protein-specific IgM, IgA, and IgG subclass responses during the acute and convalescent phase of infection. Journal of medical virology. 2004;72(2):290–298. doi: 10.1002/jmv.10553. [DOI] [PubMed] [Google Scholar]
- Erles K, Sebokova P, Schlehofer JR. Update on the prevalence of serum antibodies (IgG and IgM) to adeno-associated virus (AAV) Journal of medical virology. 1999;59(3):406–411. doi: 10.1002/(sici)1096-9071(199911)59:3<406::aid-jmv22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Franssila R, Soderlund M, Brown CS, Spaan WJ, Seppala I, Hedman K. IgG subclass response to human parvovirus B19 infection. Clinical and diagnostic virology. 1996;6(1):41–49. doi: 10.1016/0928-0197(96)00156-0. [DOI] [PubMed] [Google Scholar]
- Geoffroy MC, Salvetti A. Helper functions required for wild type and recombinant adeno-associated virus growth. Current gene therapy. 2005;5(3):265–271. doi: 10.2174/1566523054064977. [DOI] [PubMed] [Google Scholar]
- Hashido M, Kawana T. Herpes simplex virus-specific IgM, IgA and IgG subclass antibody responses in primary and nonprimary genital herpes patients. Microbiology and immunology. 1997;41(5):415–420. doi: 10.1111/j.1348-0421.1997.tb01872.x. [DOI] [PubMed] [Google Scholar]
- Heegaard ED, Brown KE. Human parvovirus B19. Clinical microbiology reviews. 2002;15(3):485–505. doi: 10.1128/CMR.15.3.485-505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog RW, Yang EY, Couto LB, Hagstrom JN, Elwell D, Fields PA, Burton M, Bellinger DA, Read MS, Brinkhous KM, Podsakoff GM, Nichols TC, Kurtzman GJ, High KA. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nature medicine. 1999;5(1):56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- High K. Gene transfer for hemophilia: can therapeutic efficacy in large animals be safely translated to patients? J Thromb Haemost. 2005;3(8):1682–1691. doi: 10.1111/j.1538-7836.2005.01460.x. [DOI] [PubMed] [Google Scholar]
- Isa A, Kasprowicz V, Norbeck O, Loughry A, Jeffery K, Broliden K, Klenerman P, Tolfvenstam T, Bowness P. Prolonged activation of virus-specific CD8+T cells after acute B19 infection. PLoS medicine. 2005;2(12):e343. doi: 10.1371/journal.pmed.0020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Couto LB, Patarroyo-White S, Liu T, Nagy D, Vargas JA, Zhou S, Scallan CD, Sommer J, Vijay S, Mingozzi F, High KA, Pierce GF. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108(10):3321–3328. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koraka P, Suharti C, Setiati TE, Mairuhu AT, Van Gorp E, Hack CE, Juffrie M, Sutaryo J, Van Der Meer GM, Groen J, Osterhaus AD. Kinetics of dengue virus-specific serum immunoglobulin classes and subclasses correlate with clinical outcome of infection. Journal of clinical microbiology. 2001;39(12):4332–4338. doi: 10.1128/JCM.39.12.4332-4338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist A, Tolfvenstam T, Bostic J, Soderlund M, Broliden K. Clinical and laboratory findings in immunocompetent patients with persistent parvovirus B19 DNA in bone marrow. Scandinavian journal of infectious diseases. 1999;31(1):11–16. doi: 10.1080/00365549950161817. [DOI] [PubMed] [Google Scholar]
- Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR, Tai SJ, Ragni MV, Thompson A, Ozelo M, Couto LB, Leonard DG, Johnson FA, McClelland A, Scallan C, Skarsgard E, Flake AW, Kay MA, High KA, Glader B. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101(8):2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B, Dake M, Kaye R, Razavi M, Zajko A, Zehnder J, Rustagi PK, Nakai H, Chew A, Leonard D, Wright JF, Lessard RR, Sommer JM, Tigges M, Sabatino D, Luk A, Jiang H, Mingozzi F, Couto L, Ertl HC, High KA, Kay MA. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nature medicine. 2006;12(3):342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE, Ragni MV, Manno CS, Sommer J, Jiang H, Pierce GF, Ertl HC, High KA. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nature medicine. 2007;13(4):419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- Mount JD, Herzog RW, Tillson DM, Goodman SA, Robinson N, McCleland ML, Bellinger D, Nichols TC, Arruda VR, Lothrop CD, Jr, High KA. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002;99(8):2670–2676. doi: 10.1182/blood.v99.8.2670. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Gray JT, McIntosh J, Ng CY, Zhou J, Spence Y, Cochrane M, Gray E, Tuddenham EG, Davidoff AM. Safe and efficient transduction of the liver after peripheral vein infusion of self complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2006 doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani AC, Gray JT, Ng CY, Zhou J, Spence Y, Waddington SN, Tuddenham EG, Kemball-Cook G, McIntosh J, Boon-Spijker M, Mertens K, Davidoff AM. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2007;107(7):2653–2661. doi: 10.1182/blood-2005-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbeck O, Isa A, Pohlmann C, Broliden K, Kasprowicz V, Bowness P, Klenerman P, Tolfvenstam T. Sustained CD8+ T-cell responses induced after acute parvovirus B19 infection in humans. Journal of virology. 2005;79(18):12117–12121. doi: 10.1128/JVI.79.18.12117-12121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnepp BC, Jensen RL, Chen CL, Johnson PR, Clark KR. Characterization of adeno-associated virus genomes isolated from human tissues. Journal of virology. 2005;79(23):14793–14803. doi: 10.1128/JVI.79.23.14793-14803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher-Goldberger S, Thiry L, Lefebvre N, Dekegel D, de Halleux F. Complement-fixation antibodies to adenovirus-associated viruses, cytomegaloviruses and herpes simplex viruses in patients with tumors and in control individuals. American journal of epidemiology. 1971;94(4):351–358. doi: 10.1093/oxfordjournals.aje.a121330. [DOI] [PubMed] [Google Scholar]
- Tobiasch E, Rabreau M, Geletneky K, Larue-Charlus S, Severin F, Becker N, Schlehofer JR. Detection of adeno-associated virus DNA in human genital tissue and in material from spontaneous abortion. Journal of medical virology. 1994;44(2):215–222. doi: 10.1002/jmv.1890440218. [DOI] [PubMed] [Google Scholar]
- Torgano G, Vecchi M, Podda M, Zuin M, Arosio E, Battezzati PM, de Franchis R. Primary biliary cirrhosis is associated with specific changes in liver IgG-bearing cell subpopulations. Journal of hepatology. 1995;22(5):545–550. doi: 10.1016/0168-8278(95)80449-8. [DOI] [PubMed] [Google Scholar]
- Wagner DK, Muelenaer P, Henderson FW, Snyder MH, Reimer CB, Walsh EE, Anderson LJ, Nelson DL, Murphy BR. Serum immunoglobulin G antibody subclass response to respiratory syncytial virus F and G glycoproteins after first, second, and third infections. Journal of clinical microbiology. 1989;27(3):589–592. doi: 10.1128/jcm.27.3.589-592.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Lin SJ, Tsai JH, Tsai CH, Tsai CC, Yang CC. Anti-hepatitis B surface antigen IgG1 subclass is predominant in individuals who have recovered from hepatitis B virus infection, chronic carriers, and vaccinees. Medical microbiology and immunology. 2005;194(1–2):33–38. doi: 10.1007/s00430-004-0225-2. [DOI] [PubMed] [Google Scholar]
- Warrington KH, Jr, Herzog RW. Treatment of human disease by adeno-associated viral gene transfer. Hum Genet. 2006;119(6):571–603. doi: 10.1007/s00439-006-0165-6. [DOI] [PubMed] [Google Scholar]
- Zhang HG, High KA, Wu Q, Yang P, Schlachterman A, Yu S, Yi N, Hsu HC, Mountz JD. Genetic analysis of the antibody response to AAV2 and factor IX. Mol Ther. 2005;11(6):866–874. doi: 10.1016/j.ymthe.2005.02.014. [DOI] [PubMed] [Google Scholar]