Summary
The PAT family of lipid droplet proteins includes 5 members in mammals: perilipin, adipose differentiation-related protein (ADRP), tail-interacting protein of 47 kiloDaltons (TIP47), S3-12, and OXPAT. Members of this family are also present in evolutionarily distant organisms, including insects, slime molds and fungi. All PAT proteins share sequence similarity and the ability to bind intracellular lipid droplets, either constitutively or in response to metabolic stimuli, such as increased lipid flux into or out of lipid droplets. Positioned at the lipid droplet surface, PAT proteins manage access of other proteins (lipases) to the lipid esters within the lipid droplet core and can interact with cellular machinery important for lipid droplet biogenesis. Genetic variations in the gene for the best characterized of the mammalian PAT proteins, perilipin, have been associated with metabolic phenotypes, including type 2 diabetes mellitus and obesity. In this review, we discuss how the PAT proteins regulate cellular lipid metabolism both in mammals and in model organisms.
Keywords: lipid droplet, lipolysis, lipogenesis, perilipin, PAT proteins, adipocyte
1. Introduction
1.1 Lipid droplets and cellular lipid metabolism
Lipid droplets are intracellular storehouses of lipid esters. In organisms as diverse as fungi, plants, insects, and mammals, lipid droplets sequester lipids inside cells for later use as metabolic fuel, membrane components, post-translational protein modifications, and signaling molecules [1]. Investigations over the past decade have revealed lipid droplets as regulated organelles of surprising complexity. Lipid droplets contain a core of neutral lipid surrounded by a phospholipid monolayer and coated by specific proteins, including proteins of the PAT family that are the subject of this review. Proteomic studies have identified lipid droplet-associated proteins that are involved in lipid metabolism and transport, intracellular trafficking, signaling, chaperone function, RNA metabolism, and cytoskeletal organization [2–7]. The protein coat of lipid droplets can vary between droplets within a cell, between metabolic conditions, and between cell types. This heterogeneity of the protein coat is consistent with the dynamic changes in morphology and intracellular location that lipid droplets undergo according to the metabolic state or developmental stage of the cell or organism. Lipid droplets and lipid droplet proteins have been the subject of multiple recent reviews [8–14], including many articles within this issue.
1.2 Perilipin and the PAT family of lipid droplet proteins
The spark that ignited interest in mammalian lipid droplets was the discovery by the laboratory of Constantine Londos of perilipin as a lipid droplet protein phosphorylated in response to signals that stimulated breakdown of triacylglycerol (TAG) stored in fat cells (adipocytes) [15–17]. This discovery pointed to the existence of regulatory mechanisms to control fat storage. Subsequent work established perilipin as the gatekeeper of the adipocyte lipid storehouse. Studies in cells and in mice have shown that perilipin can either restrict lipid droplet access of enzymes that hydrolyze lipid esters (lipases) or facilitate their enzymatic activity under appropriate metabolic conditions.
Perilipin is the founding member of a family of proteins that share sequence similarity and the ability to bind lipid droplets (Fig. 1). When first grouped as a family [18], these proteins were termed the “PAT family” after the first three members identified: perilipin, adipocyte differentiation-related protein (ADRP), and tail-interacting protein of 47 kiloDaltons (TIP47). Two other proteins, S3-12 [19, 20] and OXPAT [21–23], round out the family in mice and humans. The members of the family differ from one another in size, tissue expression, affinity for lipid droplets, stability when not bound to lipid droplets, and transcriptional regulation. These differences imply that each PAT protein has distinct cellular functions, but all PAT proteins likely regulate the interface between lipid droplets and their cellular environment. The PAT proteins divide conceptually into those that are expressed in a tissue-restricted manner (perilipin, S3-12, and OXPAT) versus ubiquitous manner (ADRP and TIP47) and into those that are constitutively bound to lipid droplets (perilipin and ADRP, the CPATs) versus those that demonstrate exchangeable lipid droplet binding (TIP47, S3-12, and OXPAT, the EPATs) [24]. The next four sections of this review examine the mammalian PAT proteins in detail, with emphasis on the best-characterized member of the family, perilipin. Other reviews specifically focused on the PAT family have appeared over the past few years [24–26]. For this review our goal is to incorporate important new published findings into a comprehensive overview of this increasingly studied family of proteins.
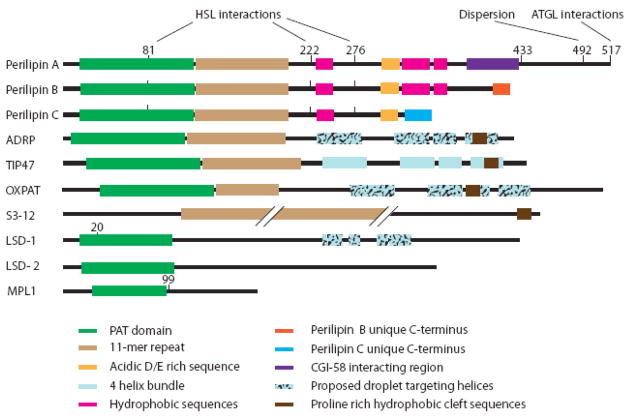
Figure 1. PAT family members have similar predicted structural features.
This cartoon compares the structure of ten members of the PAT family of lipid droplet proteins. The first seven shown (perilipin A through S3-12) are from mammals, the last three from non-mammalian species (flies and fungi, respectively). With the exception of S3-12, these proteins share a ~ 100 amino acid region of high sequence similarity near their N-termini (PAT domain, green). For this figure, we estimated the approximate extent of this domain based on the analysis of Lu and colleagues [30]. Despite the absence of a clear PAT domain, S3-12 retains sequence similarity to the other family members in other parts of the protein, including the 11-mer repeats. Numbers indicate mapped (perilipin A, LSD-1) or predicted (MPL1) PKA sites. For perilipin A, phosphorylation of those sites has been linked to specific functions of the protein, such as interactions with HSL and ATGL or droplet dispersion. All drawings are to scale with the exception of that for S3-12, a 1403 amino acid-protein with a 11-mer repeat that runs ~70 % of the length of the protein (from aa~100 to ~1060). X-ray crystallography has revealed a four-helix bundle domain in TIP47 (shown in light blue). Based on sequence similarity, similar domains have been predicted in ADRP and OXPAT; they are proposed to target the proteins to lipid droplets. A different set of potentially droplet-targeting helical segments has been predicted in LSD-1. The absence of features from a specific protein as depicted in this cartoon does not necessarily mean that these features do not exist, but rather that they have not yet been reported. Specifically, attempts to predict the structural features of LSD-1, LSD-2 and MPL1 are only in their infancy. For example, no published studies critically examine the presence or absence of 11-mer repeats in the non-mammalian family members. The reader is referred to a complementary depiction of the structural features of the mammalian PAT proteins in a recent review [25].
The PAT family proteins have been independently identified, cloned, and characterized by many different labs world-wide, each lab working in different model systems. Accordingly, each PAT protein goes by several different names in the literature, which are summarized in Table 1. It is likely that in the future a consensus nomenclature for the PAT family will be agreed upon by the lipid droplet community. For the purpose of clarity in this review, we have chosen to use the names perilipin, ADRP, TIP47, S3-12, and OXPAT to refer to the mammalian proteins.
Table 1 .
Nomenclature for the PAT proteins
| Names used in this review | Other namesa | Gene symbols (loci)b | References |
|---|---|---|---|
| Perilipin | Peri, PLIN | Human: PLIN (15q26) Mouse: Plin (7 D3) |
[16, 30] |
| ADRP (adipose differentiation-related protein) | Adipophilin, ADPH, ADFP | Human: ADFP (9p22.1) Mouse: Adfp (4 C4) |
[113, 116, 121, 141] |
| TIP47 (tail-interacting protein, 47 kD) | Cargo selection protein TIP47, Mannose-6-phosphate receptor binding protein 1, placental protein 17 (PP17) | Human: M6PRBP1 (19p13.3) Mouse: M6prbp1 (17 D) |
[149, 208] |
| S3-12 | Plasma membrane associated protein, S3-12; K1AA1881 | Human: KIAA1881 (19p13.3) Mouse: S3-12 (17 D) |
[19] |
| OXPAT | Myocardial lipid droplet protein (MLDP), lipid storage droplet protein 5 (LSDP5), PAT-1 | Human: (19p13.3)c Mouse: 2310076L09Rik (17 D) |
[21–23] |
| Fatvg | [27] | ||
| LSD-1 | LSD1, LSDP1, Lipid storage droplet-1 | Drosophila: Lsd-1 (95B) | [18, 30, 173] |
| LSD-2 | LSD2, Lipid storage droplet-2 | Drosophila: Lsd-2 (13A) | [18, 28, 30, 170, 171] |
| MPL1 | [29] |
Other names for these proteins include names used in databases, such as Entrez Gene [209], but not routinely used in research papers or reviews within the lipid droplet field. Names more commonly used in papers are in bold and referenced.
Approved gene names, symbols and loci are derived from online databases as follows: Entrez Gene [209] (http://www.ncbi.nlm.nih.gov/sites/entrez?db=gene), HUGO Gene Nomenclature Committee (http://www.genenames.org/), Mouse Genome Informatics (http://www.informatics.jax.org/), and FlyBase (http://flybase.org/).
The HUGO Genome Nomenclature Committee has not yet indicated an approved gene name for MLDP/OXPAT/LSDP5.
The PAT family is evolutionarily ancient; family members are present in many animal species (like frogs and flies) and even in fungi and slime molds [18, 27–29]. This conservation of the family points to the importance of its function in regulating intracellular lipid stores. Indeed, significant conservation between mammals and insects with respect to the molecular mechanisms of lipid droplet metabolism extends to other proteins, including receptors, enzymes, and co-factors, at the level of sequence and/or function (Fig. 2). This conservation highlights the tremendous potential of using genetically tractable model organisms to discover novel mechanisms for the control of intracellular lipid storage and utilization. Accordingly, this review includes an account of recent discoveries made in non-mammalian systems.
Figure 2. Regulation of lipolysis is conserved in the adipose tissues of mammals (A) and insects (B).
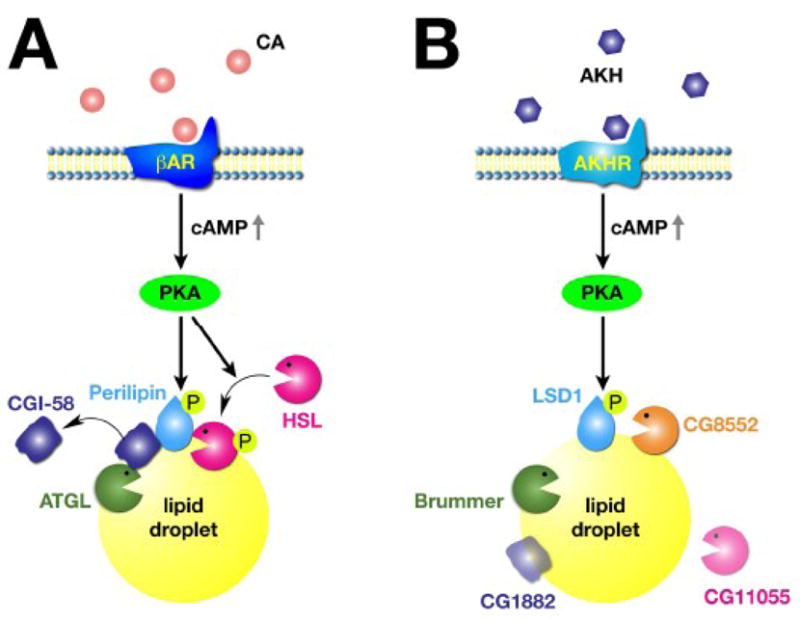
In both cases, circulating hormones (CA = catecholamines; AKH = adipokinetic hormone) stimulate G-protein coupled surface receptors (βAR = β-adrenergic receptor; AKHR = AKH receptor) resulting in the production of the second messenger cAMP. High cAMP levels activate Protein Kinase A (PKA) which in turn phosphorylates PAT proteins on the surface of lipid droplets: perilipin and LSD-1. In mammals, phosphorylated perilipin recruits the lipase HSL to the surface of lipid droplets, stimulating lipolysis. HSL is also a target of PKA. In insects, phosphorylated LSD-1 is thought to activate the lipase CG8552; the possible insect ortholog of HSL (CG11055) has yet to be functionally characterized. A second set of lipases from the ATGL/Brummer family cooperates in lipolysis. In mammals, both perilipin and ATGL interact with CGI-58; these interactions are modulated by perilipin phosphorylation (see Fig. 3 for a closer look at these mechanisms); the function of the insect ortholog (CG1882) is unknown, but this protein has been found associated with lipid droplets.
Aberrant cellular lipid metabolism has been associated with common human conditions that cause significant morbidity and mortality, including atherosclerosis, cardiomyopathy, obesity, type 2 diabetes mellitus, and non-alcoholic fatty liver disease. We anticipate that basic discoveries about how PAT proteins, mammalian and non-mammalian, contribute to the regulation of cellular lipid stores will lead to novel approaches to these pressing issues in human health.
2. Perilipin
In mice and humans, a single perilipin gene gives rise to at least three protein isoforms (perilipin A, B and C) that share a common N-terminal region and differ in their C-terminal tails [30] (Fig. 1). The shared region encompasses the PAT domain, three of the 6 recognized protein kinase A (PKA) sites, a stretch of 16 aspartate and glutamate residues (the acidic loop region), and two of three hydrophobic regions that target the protein to lipid droplets [31]. These regions have been hypothesized to act as hydrophobic fingers that dip into the non-polar core of the lipid droplet [32]. A fourth isoform, perilipin D, has been predicted based on isolation of cDNAs that contain an in-frame stop codon within an unspliced intron [30]; to our knowledge no protein that corresponds to the predicted perilipin D has been identified and reported. Perilipin A is the most abundant isoform [16, 33], and the focus of almost all functional studies on perilipins.
2.1. Perilipin expression
Perilipin is a marker of adipocyte differentiation and thus has been used as a reporter gene to identify regulators of adipogenesis. Expression of the perilipin gene is regulated primarily by peroxisome proliferator activated receptor gamma (PPARγ); however, recent reports also implicate the estrogen receptor related receptor alpha (ERRα) [34–37]. Furthermore, ERRα-dependent perilipin expression is activated by PPARγ coactivator-1 alpha (PGC-1α) and repressed by the small heterodimer partner (SHP) [34]. Perilipin expression also is activated by the constitutive coactivator of PPARγ (CCPG) or repressed by tribbles homolog 3 (TRB3), both acting through PPARγ [38, 39]. By contrast, the pro-inflammatory cytokine tumor necrosis factor alpha (TNFα) induces triacylglycerol (TAG) hydrolysis partially through a decrease in perilipin mRNA and partially through an indirect elevation of cAMP [40, 41]. The effect of TNFα is at least in part mediated by NF-κB and occurs via the ERK pathway [42, 43].
Perilipin associated with droplets has a half-life of 40 hours, while excess perilipin not bound to droplets is rapidly degraded [44]. There is disagreement in the literature as to whether this is due to a proteosomal or lysosomal mechanism. The Londos lab found that ubiquitinated perilipin accumulates in the presence of the proteosomal inhibitors MG-132 and ALLN in CHO cells [45]. The Greenberg lab was unable to replicate these results in either NIH-3T3 fibroblasts or 3T3-L1 cells. Instead, this group found that perilipin degradation was blocked by the lysosomal protease inhibitors leupeptin and ammonium chloride, thereby implicating the lysosomal pathway [46, 47]. Differences in cell type may account for some of the observed dissimilarities. Because all 3 perilipin isoforms are stable only when bound to lipid, the mass of cellular neutral lipid act as a means of post-translational control of perilipin protein levels [44].
2.2. Perilipin is polyphosphorylated by protein kinase A
Perilipin A was originally identified as the major protein kinase A substrate associated with the lipid storage droplet [15]. Murine perilipin A is phosphorylated on up to 6 potential PKA sites. It is currently unclear if other kinases play a role in its function. Upon phosphorylation the role of perilipin A shifts from storage to mobilization of stored neutral lipid, as discussed below. Perilipin A is dephosphorylated by protein phosphatase 1 [48]. The perilipins are the only mammalian PAT family members whose function is known to be acutely regulated by their phosphorylation state; phosphorylation dependent events have not been reported for ADRP, TIP47, OXPAT, or S3-12.
2.3. The functions of perilipin in cellular TAG metabolism
Most functional studies of perilipin have focused on the role of murine perilipin A and have utilized NIH 3T3 fibroblasts, Chinese hamster ovary (CHO) cells, or differentiated 3T3-L1 adipocytes. Each of these model cell systems has different strengths and weaknesses pertinent to these studies. While differentiated 3T3-L1 adipocytes express native perilipin A and many other adipocyte proteins, 3T3 cells and CHO cells lack many of these same proteins, but are relatively easy to culture and transfect. Perilipins function in conjunction with other proteins via protein-protein interactions, for example with CGI-58 and ATGL, as discussed below, and these other proteins may not be present in all cell types. Also, results may vary between host cells of different species. For example, the specific activity of ATGL orthologs towards lipid substrates varies among species [49]. Thus, caution must be exercised in interpreting studies in which perilipins of one species are expressed heterologously in a host cell of another species and when using model cell systems that do not recapitulate the cellular milieu in which perilipins are endogenously expressed. With these caveats in mind, cell-based studies of perilipin have yielded insights into its role in cellular lipid metabolism.
Lipolysis is the hydrolysis of fatty acyl esters and is the means by which stored lipids are mobilized for production of membranes and for provision of metabolic fuel substrates. For the purpose of energy storage, fatty acids are esterified to a glycerol backbone to generate sequentially mono-, di-, and triacylglycerols, whereas cholesteryl esters are made to store excess cholesterol for membrane and hormone biosynthesis. In the adipocyte, catecholamines signal through a β-adrenergic receptor and G-protein coupled signaling cascade to elevate cellular cAMP levels (Fig. 2A). This in turn activates cAMP-dependent PKA, which polyphosphorylates the perilipins and hormone-sensitive lipase (HSL). HSL then translocates from the cytoplasm to the lipid droplet surface (Fig. 3A, B). Recently, another acyl hydrolase, adipocyte triglyceride lipase (ATGL) has emerged as the principal TAG lipase, at least in mice. ATGL catalyzes the initial step in lipolysis (TAG to diacylglycerol), while HSL catalyzes the second step (diacylglycerol to monoacylglycerol), and a soluble monoglyceride lipase catalyzes release of the remaining fatty acyl moiety from the glycerol backbone [50, 51].
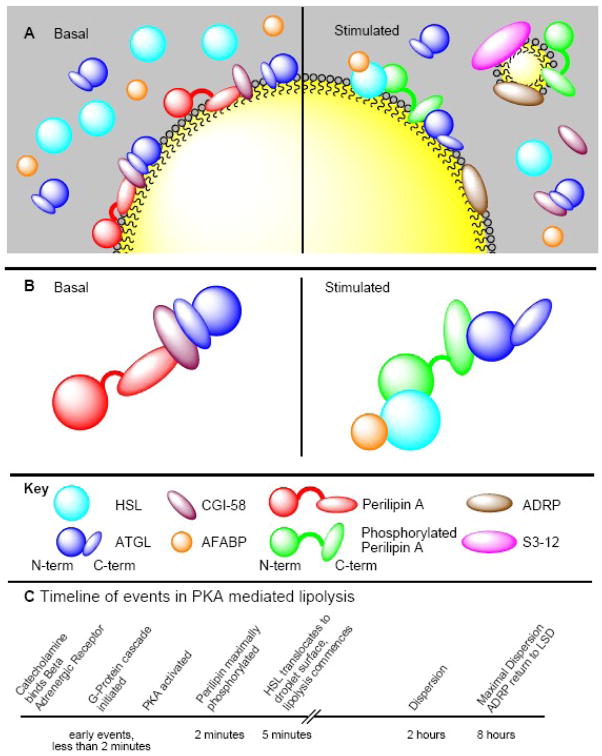
Figure 3. Phosphorylated perilipin A organizes lipolysis in murine adipocytes.
(A and B) In the basal state (left) the lipolytic regulator perilipin A is found at the surface of the lipid droplet in a complex with CGI-58. ATGL may also be in this complex. ATGL activity is kept quiescent through the autoinhibitory C-terminus of the lipase. Upon lipolytic stimulation (right), PKA is activated and phosphorylates up to 6 serine residues on perilipin A (Ser81, 222, 276, 433, 492, and 517) and 2 on HSL (Ser659, and 660). This results in the following rearrangements. 1) CGI-58 dissociates from the droplet surface; it is unclear if ATGL remains associated with GCI-58 in the cytosol. 2) Phosphorylated HSL translocates to the droplet surface and associates with both perilipin A and AFABP. 3) ATGL interacts with perilipin A through phosphorylated Ser517, and lipolysis commences. 4) At a later time (several hours) droplets fragment and disperse, a phenomenon dependent on phosphorylation of perilipin A Ser492. The fragmented microdroplets become coated with ADRP and S3-12, as well as perilipin, and ADRP also associates with the remaining larger droplets. The depiction of perilipin A delineates the acidic loop region and amino- and carboxy-terminal segments for the sake of clarity, but is not meant to show discreetly folding domains. (C) Timeline of lipolysis. Please note that this line is not to scale.
Data from transfected cells have implicated perilipin as an organizational node for the control of lipolysis in adipocytes. In the hypophosphorylated state, perilipin A is a gatekeeper that prevents lipases from gaining access to neutral lipids in the droplet core; thus, under basal conditions, hypophosphorylated perilipin reduces TAG hydrolysis [52–55]. In contrast, once phosphorylated, perilipin A actively facilitates lipase action, in part by recruiting HSL to the droplet surface. Data from perilipin knockout mice are consistent with this model as such mice show increased basal and decreased stimulated lipolysis [56, 57].
There are 6 consensus PKA sites in murine perilipin A. The roles each of these sites play continue to unfold, but it is clear that the mechanisms of perilipin A regulation are complex. The sixth site in perilipin A (Ser517) is emerging as the master regulator of lipolysis [51]. Phosphorylation of this site is essential for activation of ATGL activity and the initial steps in lipolysis. Mutation of this site (S517A) diminishes forskolin stimulated (PKA dependent) lipolysis by 95% without influencing basal lipolysis. Interestingly, a phosphomimetic mutant designed to be constitutively active (S517D) does not elevate basal lipolysis in the absence of PKA activation. However, in the presence of lipolytic stimuli, lipolysis is activated 35 % over wild-type levels. Mutations of other PKA sites reveal that a combination of the three amino terminal sites is required for optimal hormone-sensitive lipase (HSL) catalyzed TAG hydrolysis [54, 55].
Phosphorylation of perilipin A is essential for maximal PKA-stimulated lipolysis. Two groups conducted studies using embryonic fibroblasts created from perilipin null mice. One found that phosphorylation of the 3 amino terminal PKA sites of perilipin was essential for the translocation of HSL [58], while the other found translocation in the absence of phosphorylatable perilipin [59]. Both studies found that phosphorylation of perilipin A was essential for maximal PKA-stimulated lipolysis. Additionally, Miyoshi and colleagues found that PKA activation increased the amount of HSL that could be crosslinked to or immunoprecipitated with perilipin A. When a mutant perilipin lacking all six PKA phosphorylation sites was examined, the total amount of HSL crosslinked or precipitated was diminished [59]. Currently, it is unclear if the three amino terminal PKA sites are responsible solely for translocation or translocation as well as activation of HSL.
Prolonged stimulation of PKA leads to a change in adipocyte lipid droplet morphology wherein relatively few large perinuclear lipid droplets of several microns to tens of microns diameter fragment into thousands of submicron droplets, each coated with perilipins, which disperse throughout the cytoplasm (Fig. 3B). Phosphorylation of the fifth PKA site (Ser492) has been found to be the essential step in this process [60]. Mutation of Ser517 does not appear to influence droplet dispersion [51]. While HSL translocation to the droplet surface is maximal at 5 minutes, fragmentation and dispersion of lipid droplets are noticeable at 2 hours and maximal at 8 hours. These studies were confirmed independently [51]. Fragmentation and dispersion are not dependent on lipid breakdown [60], nor do they contribute to lipolysis in the absence of perilipin phosphorylated on residue 517 [51]. Fragmentation and dispersion of large lipid droplets provides a mechanism by which lipases could gain access to greater surface area to permit higher levels of lipolysis.
The interaction of perilipin with other proteins is important for regulation of lipolysis in adipocytes and is dependent on the phosphorylation state of perilipin. In the basal state, perilipin A is associated with CGI-58 (Fig. 3A). CGI-58 is an activator of the acyl hydrolase activity of ATGL [61–63]. CGI-58 was identified as a lipid droplet protein through proteomic studies and as a PAT protein-interacting protein by yeast 2-hybrid screens [64, 65]. CGI-58 localizes to lipid droplets in cells that express perilipin A but not in cells that express only perilipin B [64]. Interestingly, while CGI-58 is an activator of ATGL, activation of PKA causes dissociation of CGI-58 from the droplet surface, potentially through phosphorylation events in the C-terminus of perilipin A as well as CGI-58 [66]. The presence or absence of CGI-58 does not seem to impact the dispersion of lipid droplets that occurs with the chronic phosphorylation of perilipin A Ser492 [63]. It has not been determined whether perilipin, ATGL, and CGI-58 simultaneously interact directly with one another on the droplet surface.
Recent studies have revealed that human CGI-58 acts as lysophosphatidic acid (LPA) acyltransferase [67], whereby LPA is acylated to form phosphatidic acid, a key intermediate in the biosynthesis of many glycerophospholipids. Mutations in CGI-58 previously reported as associated with the neutral lipid storage disease Chanarin-Dorfman syndrome (Q130P and E260K) and to disrupt the ATGL activation function of CGI-58 have no effect on its LPA acyltransferase activity. The significance, if any, of the LPA acyltransferase of CGI-58, for the control of lipolysis is not clear. One possibility is that the lipase activation and acyltransferase activities of CGI-58 fulfill two discreet and unrelated functions that may have more or less prominent roles in different tissues. However, armed with Occam’s razor, it is tempting to speculate that these two functions are integrated. The origin of the lysophosphatidic acid substrate for CGI-58 is unknown, but kinase activity that generates LPA from monoacylglycerol has been reported [68]. Perhaps CGI-58 mediates a shift of a product of lipolysis, monoacylglycerol, into a phospholipid synthetic pathway [69, 70]. Other possibilities are that the phosphatidic acid generated or a metabolite further downstream serves in a signaling capacity or acts as an allosteric regulator of the proteins involved in lipolysis.
Once phosphorylated by PKA, perilipin A functions as a scaffolding protein about which the lipolytic machinery assembles. Phosphorylated HSL (on serines 659 and 660) requires phosphorylated perilipin (some combination of the three amino terminal most-PKA sites) for optimal activity and targeting to the lipid droplet. In turn, HSL interacts with AFABP (fatty acid binding protein) [71].
Collectively, these data reveal the order of events in the lipolytic stimulation of an adipocyte or adipocyte-like cell line (Fig. 3C). Catecholamines bind β-adrenergic receptors, which signal through a heterotrimeric G-protein to activate adenyl cyclase, thereby elevating cAMP levels and activating PKA. This step takes place in seconds or faster. PKA phosphorylates both perilipin A and HSL. Maximal phosphorylation of perilipin A takes place in less than 2 minutes, and HSL translocation peaks within five minutes. At the same time, CGI-58 dissociates from the lipid droplet. The timing of the ATGL interaction with perilipin is unknown, but most likely occurs concomitantly with perilipin phosphorylation and the departure of CGI-58 from the droplet surface. If the lipolytic stimulus persists, droplets fragment and disperse into perilipin A-coated microdroplets beginning at 2 hours and peaking at 8 hours. These microdroplets are also coated with the other PAT proteins ADRP [4, 46] and S3-12 [4], which do not coat lipid droplets under basal conditions [4, 20, 72]. During prolonged lipolysis, induced either by PKA activation [4] or by trans-10-cis-12 conjugated linoleic acid [73], ADRP also coats the surfaces of larger lipid droplets.
2.4. The role of perilipin in physiology
Two groups independently ablated the perilipin gene in mice and characterized the metabolic phenotype [56, 57]. Perilipin knockout mice are lean and have normal body weight, but their adipose mass is reduced 65–75%. They consume food normally and are resistant to diet-induced obesity, but have increased peripheral insulin resistance at a young age. 14-week-old animals have normal plasma glucose levels [57], but also have insulin levels increased 2-fold over those of wild-type littermates, which suggests that perilipin knockout mice are insulin resistant. The animals are otherwise healthy and reproduce normally.
Metabolic studies on perilipin null mice were conducted using indirect calorimetry. Null animals consume more oxygen than wild type presumably due to increased thermogenesis. Injection of wild-type animals with the β-3 receptor agonist CL316243 results in both an increase in oxygen consumption and a drop in the respiratory exchange ratio (RER), which indicates increased fat as opposed to carbohydrate catabolism [74]. By contrast, perilipin null animals have an increase in oxygen consumption and a blunted fall in RER, consistent with an animal that exhibits elevated basal and decreased stimulated lipolysis. In fact, lipolysis in adipocytes isolated from perilipin null mice exhibit both elevated basal lipolysis and diminished stimulated lipolysis.
Lipodystrophy is a condition of abnormal fat distribution, development or deterioration that may occur in humans on a genetic or acquired basis. In contrast to lipodystrophic mouse models, which often are very sick and typically exhibit severe fatty liver, perilipin knockout mice are far healthier. Two plausible explanations are offered for this observation. Tansey and colleagues found that levels of leptin in the knockout mice failed to correlate with fat pad mass. Plasma leptin levels between knockout and wild-type animals were similar, indicating that adipocytes in the knockout continued to produce and secrete leptin despite not being able to properly store TAG. Other lean mouse models with deficiency of functional adipose tissue are deficient in leptin [75, 76], and administration of leptin either by infusion or transgenic overexpression reverses some of the pathology observed [77, 78]. Thus, preserved leptin secretion in perilipin knockout mice may protect against excessive ectopic lipid accumulation and the associated metabolic complications.
Subsequent studies of perilipin null mice employing microarrays and hyperinsulinemic-euglycemic clamps have provided further explanation for the observed resistance to weight gain of perilipin null mice. Microarray studies were conducted on white adipose tissue (WAT), kidney, liver, heart, and skeletal muscle. Given the tissue distribution of perilipin in mice, it is not surprising that WAT had the greatest number of transcripts affected by perilipin gene ablation (543 downregulated, 270 upregulated) [79]. Elevated levels of transcripts of enzymes involved in beta-oxidation, the Krebs cycle, and the electron transport chain were all observed. Furthermore, expression of steroyl-CoA desaturase-1 and other genes involved in lipid biosynthesis was downregulated. Increased mRNAs for the uncoupling proteins UCP2 and UCP3 in WAT and brown adipose tissue (BAT) were identified [80]. These alterations in gene expression manifested themselves as changes in overall physiology. In clamp studies perilipin knockout mice had peripheral insulin resistance, decreased hepatic glucose production, and increased beta-oxidation [80]. In contrast to the glucose tolerance test results reported by Tansey et al., the animals used in this study displayed no elevation in plasma glucose but did show significant peripheral insulin resistance when tested using a low dose insulin clamp. Differences in the strains of the mice used in these two studies may account for the reported differences. The observed euglycemia in these studies and the decrease in hepatic glucose output are explained in two ways. First, being lean, the perilipin knockout mice have a decreased output of glycerol to serve as a substrate for gluconeogenesis. Second, gluconeogenesis is itself impaired as evidenced by a decreased ability to incorporate pyruvate into glucose. At least two of the key enzymes in gluconeogenesis, phosphoenolpyruvate carboxy kinase (PEPCK) and fructose-1,6-bisphosphatase, are downregulated in these animals. Increases in fatty acid oxidation were found in liver, muscle, and adipose tissues. Hence, the perilipin knockout mice adapt to the elevated basal lipolysis by upregulating catabolic pathways and downregulating anabolic ones.
2.5. Roles of perilipins in human health
Several studies have focused on how perilipin expression changes with obesity. These studies offer conflicting results. Kern and colleagues observed that perilipin A expression in abdominal subcutaneous adipose tissue was positively correlated with obesity, but not insulin resistance in a non-diabetic cohort [81]. The population for the study was of mixed race and gender. In this study perilipin A mRNA levels were increased compared to total body fat percentage. In contrast, Wang found a significant decrease in perilipin A message and protein levels in obese subjects when samples were normalized to total protein or cell size (surface area) [82], but mass of perilipin A per cell did not change. Interestingly, subcutaneous perilipin A protein levels were higher in obese men compared to obese women, which the authors speculated may give rise to the higher levels of basal lipolysis observed in women. In a separate study, Mottagui-Tabar and colleagues also found a significant diminution of perilipin A protein levels (50%) in the abdominal subcutaneous adipose tissue of obese women; men were not included in this study [83]. Both basal and stimulated lipolysis were increased by two- to four-fold. A polymorphism at 11482G/A was commonly found, and subjects homozygous for the A allele had increased basal and stimulated lipolysis along with perilipin protein levels reduced by up to 80%.
Several studies have investigated the relationship between genetic variation in the perilipin locus and human disease. At least 11 different single nucleotide polymorphisms (SNPs) have been noted in the human perilipin gene. Association of these SNPs with human diseases may be complicated by the polygenic nature of the diseases in question (obesity, diabetes, and hypertension), gender effects, and environmental factors (obesity, weight loss, diet, and drug treatment), as well as the frequency of the alleles in question in the selected population. Not all alleles are polymorphic in all populations. Studies of SNPs in the perilipin gene are summarized in Table 2. Several excellent reviews exist on these findings [84, 85]. All of the known perilipin SNPs studied are in non-coding regions.
Table 2.
Single nucleotide polymorphisms in PLIN and metabolic phenotypes in humans
| Polymorphism (db SNP identifier) | Risk Allele | Population (Race and gender) | Phenotype/Linkage | Reference |
|---|---|---|---|---|
| 1243 C/T (rs2304796) | C | Japanese males | Decreased bone density | [210] |
| 6209 T/C (rs2289487) | C | White and Spanish females | Decreased obesity risk | [211] |
| White females | Increased risk of type 2 DM | [212] | ||
| 10171 A/T (rs8179043) | T | White females | Increased risk of type 2 DM | [212] |
| 11482 G/A (rs894160) | A | White and Spanish females | Decreased obesity risk | [83, 211, 212] |
| Whites | Resistance to weight loss | [213] | ||
| Koreans with type 2 DM | Rosiglitizone dependent weight gain | [214] | ||
| Asian and white females | Increased risk of type 2 DM | [215] | ||
| 13041A/G (rs2304795) | A | White females | Increased obesity risk | [216] |
| 14995 A/T (rs1052700) | T | White females | Increased obesity risk | [216] |
| Asian females | Increased obesity risk | [217] | ||
| Increased risk of type 2 DM | [215] |
One of the most consistent and striking observations regarding perilipin gene polymorphisms is the effect of gender on associated metabolic phenotypes. With few exceptions all the observed linkages are only found in women. While the authors of these studies note that there is, at present, no known functional basis for the observed gender association [84], there is clearly a complex interplay between genes, gender, and environment in matters regarding the metabolic syndrome and related pathologies.
Several drugs used in the clinic have been shown to affect perilipin gene expression. The most widely prescribed of these drugs are the thiazolidinediones (TZDs), which are PPARγ agonists. Treatment of a rat model of type 2 diabetes with the TZD rosiglitazone results in increased perilipin mRNA and protein levels in subcutaneous but not visceral adipose pools, which offers one explanation for the fat redistribution seen in patients on these compounds [86]. The experimental thiazolidinedione BRL 49653 reduces TNFα-induced lipolysis, but not catecholamine-stimulated lipolysis, and ameliorates the reductions in adipocyte perilipin that are associated with TNFα treatment [87, 88].
Antiretroviral therapies used to treat HIV infection are often associated with increased rates of lipolysis. This has been most clearly seen with the aspartate-based protease inhibitors nilfinivir and ritonavir. Two different studies have found that decreases in perilipin A protein are at least partially responsible for the increase in lipolysis. The studies suggested significantly different mechanisms how perilipin protein levels are decreased. One study found that the decrease was at the mRNA level [89], while the second found that the increased lipolysis could be directly attributed to increased perilipin degradation in lysosomes [47].
Several nutritional supplements have recently been identified as compounds that may prove to be interesting antiobesity treatments. Trans-10, cis-12 conjugated linoleic acid (CLA) and the soy isoflavone genistein have both been shown to decrease neutral lipid stores [90, 91]. Each of these compounds is thought to act at the transcriptional level to modulate expression of genes involved in neutral lipid metabolism, including the perilipin gene: CLA acting on PPARγ and genestein acting through the estrogen receptor.
2.6. Disease states may affect perilipin expression
The initial papers that characterized the perilipins identified them as components of white and brown adipose tissue and steroidogenic tissues, such as adrenal cortex and Leydig cells of testis. They were not found to any appreciable extent by either immunoblotting or Northern blot analysis in other tissues. With the advent of microarray data and the GEO (Gene Expression Omnibus) profiles database, several hundred studies have entries attributed to perilipin transcripts. Many of these entries come from tissues other than ones previously cited, including various parts of brain, breast tissue, several cancers, and many others. It is possible that in some of these instances the tissue sample in question had adipocytes interspersed in the tissue of interest [92]. Likewise, analysis of complex tissues should be undertaken with the appreciation that microscopy needs to be applied to identify which cells are expressing the protein of interest. Immunoblot analysis of tissues may well have contaminating levels of perilipins from nearby adipose tissue. Additionally, the similarity between PAT family members in terms of their amino acid sequence raises questions of antibody specificity. Several excellent antibodies have been generated against the perilipin sequence with minimal cross reactivity. The specificity of commercially available antibodies to the perilipins may not be known and should not be presumed.
The role perilipin plays in neutral lipid biochemistry has led several investigators to hypothesize that the perilipins are also expressed in macrophages found in atherosclerotic plaques. Several reports indicate that perilipins A and B are expressed at a low level in atheroma or cultured macrophages [93–95]. However, investigators have found ADRP to be the major lipid droplet protein in many of these same tissues [95–99]. It is possible that neutral lipid storage begins with ADRP as it does in 3T3-L1 preadipocytes and that subsequent perilipin expression is a sign of more significant lipid accumulation. The origin of cells (preadipocyte vs. monocyte derived macrophages) expressing these proteins remains in question and may provide clues to some of the divergent results observed.
Initial reports of perilipin expression failed to find significant expression in liver, but it is possible that in pathological states the situation is different. Regenerating liver tissue undergoes lipid deposition. In a partial hepatectomy model, compounds or processes that inhibited lipid deposition (glucocorticoid receptor disruption, leptin treatment, and rosiglitizone treatment) all suppressed hepatic lipid deposition and blunted hepatocyte proliferation [100, 101]. In general, these studies have found that an adipogenic pattern of gene expression is necessary to initiate liver repair. A recent report examined the expression of PAT proteins in human fatty liver and found perilipin A expression in steatotic hepatocytes, but not retinoid storing stellate cells [102]. A strength of this study is that multiple independent techniques, including microscopic and biochemical analysis, were employed to reach this conclusion. It is of interest to note that the authors did not observe perilipin expression in Kupffer cells, the resident macrophages of the liver, under these conditions.
High levels of lipids are damaging to islet cells of the pancreas and have been implicated in the development of type 2 diabetes. Recently, ectopic adipocytes have been found both in the exocrine tissues of the pancreas of diabetic patients and in mice fed high fat diets [103]. In this study perilipin protein was used as a marker of adipocytes and correlated positively with total pancreatic triacylglycerols. The authors concluded that ectopic adipocytes in the pancreas changed the surrounding milieu, presumably leading to elevated free fatty acid levels in tissues surrounding the islet cells, and eventually contributing to β-cell failure.
Perilipin expression has also been detected in sebaceous glands [104] and has recently been used as a marker of sebaceous epithelial-myoepithelial carcinoma of the parotid gland [105].
Collectively these data indicate that perilipin expression may be strongly affected by the metabolic state of the organism and that, in certain disease states, perilipin may be expressed in tissues not identified in initial characterizations.
2.7. Perilipin may not localize exclusively to the surface of lipid storage droplets
The perilipins were originally identified as integral lipid droplet proteins resistant to carbonate washing and found on the surface of the lipid storage droplet [16, 33]. Initial studies were conducted using both biochemical and microscopic analysis. Subsequently, perilipin functions have been studied in a number of cultured cell systems (NIH 3T3, 3T3-L1 adipocytes, CHO, and MEFs), and perilipin has consistently been found at the surface of lipid droplets. Several recent studies call this exclusive localization into question.
Two independent groups have found evidence that perilipin A [106] or B [107] can localize to the plasma membrane. These studies offer biochemical evidence in the form of cell fractionation studies and rely heavily on immunogold electron microscopy and immunofluorescence. It should be noted that the EPATs (S3-12, TIP47, and OXPAT) associate with cellular compartments in addition to lipid droplets. This may be the case for perilipins as well. Similarly, one of these groups reported perilipins in the hydrophobic core of lipid droplets [108]. These studies relied on freeze fracture immunogold electron microscopy. The physiological role perilipins may play in the core of the droplet remains unexplained.
Another group has reported that perilipin, rather than coating the central lipid droplet of adipocytes, coats smaller droplets peripheral to the central lipid droplet [109]. The authors assert that the choice of cells (transformed fibroblasts, 3T3-L1 adipocytes and primary white adipocytes) and culture conditions (3D matrices) better simulate conditions found in vivo. This study also employed a novel approach, whereby constructs coding for perilipin-EYFP and HSL-ECFP fusion proteins were electroporated into suprascapular white adipose tissue in living mice. Using this approach, localization of these proteins was studied after several days in living tissue. The authors found that both fusions target to small lipid droplets and conclude that this pool of small droplets that surround the central lipid storage droplet is the metabolically active pool, separate and distinct from the main droplet. However, they did not identify any of the known lipid droplet coat proteins on the main droplet. One possible explanation is that the fluorescent protein tag is influencing the properties of the fusion protein, yet this does not explain other observations made in this same study. Immunofluorescence studies using cultured cells and antibodies directed against native proteins produced similar results; perilipin A and HSL targeted to droplets peripheral to the main storage depot.
Many of the aforementioned studies employ microscopy as the main means of investigation. The literature offers many caveats when examining PAT proteins by microscopy. As the initial steps in several microscopic techniques are either geared towards delipidating the sample or fixing the cells in solvents that hold the potential to delipidate, there exists a strong possibility that commonly used techniques which may work well on other proteins in other tissues fail to provide satisfactory results when employed to study the perilipins or other PAT proteins. Several works describe in detail the steps that must be taken to conduct microscopic analysis on lipid-laden cells and tissues [110, 111].
One final observation concerns the source of anti-perilipin antibody used in these studies, many of which employ antisera obtained from a single commercial supplier. It may be worthwhile for the research community to compare these antisera to others that have been used in the past to be certain of antibody specificity and to identify specific epitopes that are recognized by the reagent.
3. ADRP/Adipophilin/ADFP/ADPH
ADRP is a 50 kiloDalton protein that was first identified as an RNA transcript significantly induced during differentiation of cultured adipocytes [112, 113]. Sequence similarity of ADRP to the perilipins led to the discovery that ADRP coats small lipid storage droplets in a variety of cell lines, including early differentiating 3T3-L1 adipocytes [114].
3.1 ADRP and adipogenesis
In contrast to ADRP mRNA, ADRP protein decreases as adipocyte differentiation progresses, while perilipin mRNA and protein levels increase. Whereas early adipocytes have smaller, ADRP-coated lipid droplets, mature adipocytes have larger, perilipin-coated lipid droplets. As has been shown for perilipin, ADRP that is not bound to lipid droplets is degraded via the ubiquitin/proteosome pathway [46, 115]. Thus, both perilipin and ADRP generally behave as constitutive lipid droplet proteins, CPATs, (as reviewed in [24]), but perilipin appears to out-compete ADRP for lipid droplet binding in adipocytes. Unlike the restricted tissue distribution of perilipin (adipocytes, steroidogenic cells, and perhaps macrophage foam cells), that of ADRP is more generalized to include many other tissues that accumulate lipids [116], with the major exception being mature human adipocytes.
3.2 ADRP in perilipin null mice
In perilipin-null mice ADRP replaces perilipin on adipocyte lipid storage droplets [57], but ADRP does not functionally compensate for loss of perilipin with respect to regulating lipolysis [56, 57]. Since basal lipolysis is increased in perilipin-null mice, ADRP appears to be a less robust barrier to lipases than perilipin. Also, the blunting of catecholamine-induced lipolysis observed in perilipin-null mice suggests that ADRP does not facilitate stimulated lipolysis to the extent that perilipin does, though protein-protein interaction between ADRP and CGI-58 has been reported [117].
3.3 Studies of ADRP function in cellular lipid metabolism
Studies to elucidate the function of ADRP have included gain- and loss-of-function experiments in cells and gene targeting and silencing in mice. Although the experimental systems and designs have varied from study to study and the results have differed in some respects, the weight of the data supports a role for ADRP in limiting the interaction of lipases with the neutral lipid within the droplets, thereby promoting neutral lipid accumulation. The mechanism by which ADRP hinders lipase targeting to lipid droplets has not been elucidated experimentally.
Overexpression of ADRP has been associated with expansion of lipid droplet pools and increased cellular triacylglycerol (TAG) mass in multiple studies. For examples, over-expression of a green fluorescent protein (GFP)-ADRP fusion protein in Swiss-3T3 cells resulted in increased triacylglycerol (TAG) content, even when the cells were cultured in delipidated serum [118]. Lipid droplet size and number were also increased compared with LacZ control cells. Similarly, stable heterologous expression of ADRP in human embryonic kidney (HEK) 293 cells, which express very little endogenous ADRP, resulted in increased cellular TAG accumulation both with and without oleate supplementation, and there was increased partitioning of oleate-driven TAG into the lipid droplet fraction at the expense of the membrane fraction [119]. As was shown previously for perilipin [52], the effect of ADRP on cellular TAG and lipid droplets appears to have been due at least in part to reduced TAG catabolism. These studies exploited the activity of Triacsin C to block TAG synthesis via inhibition of acyl CoA synthases. When cells are lipid loaded by oleate supplementation and then treated with Triacsin C, the changes in cellular TAG mass over time should reflect the rate of TAG hydrolysis. In the experiments cited above, oleate-loaded HEK 293 cells that expressed exogenous ADRP maintained greater TAG levels over time than did wild-type HEK 293 cells [119]. At least one mechanism for the ability of ADRP to reduce TAG hydrolysis is reduced localization of the major TAG lipase, ATGL, to lipid droplets, as demonstrated by both microscopy and immunoblotting [119]. ADRP expression was also associated with reduced lipid droplet binding of another PAT protein, TIP47, as well as other unidentified proteins. Another group has reported experiments using chimeras of TIP47 and ADRP are consistent with the amino-terminal half of ADRP being responsible for exclusion of TIP47 from lipid droplets in the absence of exogenous oleate [120].
How might ADRP exclude other proteins from the lipid droplet? Listenberger and colleagues have proposed that, in binding to lipid droplets via hydrophobic interactions with the neutral lipid core, ADRP crowds together the phospholipids that form the monolayer around each droplet [119]. Thus, other proteins that also rely on hydrophobic interactions with the lipid droplet core in order to associate with lipid droplets may be denied access, especially if they bind with lower affinity. In this context, several natural mutants of ATGL that are associated with neutral lipid storage disease in humans result in truncation of ~200 amino acids from the ATGL carboxyl-terminus, including most or all of a predicted 45 amino acid hydrophobic region [49]. Notably, these ATGL truncation mutants have increased TAG hydrolase activity, but reduced lipid droplet binding. One possibility is that the presence of ADRP on lipid droplets prevents ATGL from inserting a hydrophobic anchor into the droplet core, but at this point there is no data that specifically implicates the hydrophobic motif of ATGL in lipid droplet binding.
3.4 Mouse models of ADRP deficiency
The significance of ADRP in hepatic lipid metabolism has been established by loss-of-function experiments in mice created by Larry Chan’s group. Chang and colleagues generated mice globally deficient in full-length ADRP by gene knockout [121]. ADRP deficient mice displayed normal adipocyte and adipose tissue differentiation and function, as assessed by histology, adipocyte marker expression, adipocyte TAG mass, white and brown adipose mass, basal lipolysis, and catecholamine-stimulated lipolysis. The mice also had normal plasma lipids, glucose, and insulin levels. These data suggest that ADRP is dispensable with respect to the function of the mature adipocyte and to its development. However, ADRP deficiency was associated with reduced liver TAG content and with attenuation of hepatic steatosis induced by high-fat feeding. The overall reduction in liver TAG was not explained by reduced fatty acid uptake or increased β-oxidation in primary hepatocytes nor by reduced lipogenesis or lipogenic enzyme activity in mouse livers. There was a non-significant trend towards increased TAG secretion from liver, but no change in systemic TAG clearance. The authors noted that VLDL secretion was at least preserved despite reduced intrahepatocyte TAG, but the mechanism for reduced hepatic TAG in ADRP deficient mice remains unclear.
Though lipid droplets in the livers of high-fat fed ADRP deficient mice were reduced in size and number compared to those of high-fat fed wild-type mice, and TAG levels in the cytosol were reduced, TAG levels in the microsomal fraction were increased. Levels of microsomal triglyceride transfer protein (MTP), a protein essential for lipoprotein assembly and secretion, were also increased. As noted by the authors, these data are consistent with ADRP having a role in the formation of lipid droplets, whereby inhibition of lipid droplet biogenesis would lead to accumulation of TAG at the site of initial lipid droplet formation, the endoplasmic reticulum (ER) membrane. The authors proposed that increased hepatic MTP preserves TAG export in the form of VLDL and shift the liver lipid economy during high-fat feeding from storage to export.
Interpretation of these data is complicated by the subsequent finding that the ADRP deficient mouse line studied unexpectedly expresses an amino-terminal truncation of ADRP (termed “Δ2,3 ADPH”), at least in the lactating mammary gland, that may partially replace the function of full-length ADRP in lipid droplet formation and secretion [122]. To our knowledge, it has not been clarified whether this truncated ADRP, which likely corresponds to an ADRP variant endogenously expressed in mammary tissue, is expressed in other tissues of the ADRP deficient mouse model.
Imai and colleagues have reported metabolic effects of ADRP antisense oligonucleotides administered by intraperitoneal injection to two mouse models of hepatic steatosis, ob/ob mice and high-fat fed obese mice [123]. In both cases, reduction of hepatic ADRP protein by antisense treatment was associated with significant hepatic and systemic changes in lipid and glucose metabolism. Similar to the genetic model of ADRP deficiency, hepatic TAG was decreased in the ADRP antisense treated mice, and the mice were protected from hepatic steatosis on a high-fat diet. However, in contrast to the genetic model, expression of lipogenic genes was decreased, and plasma triglycerides and hepatic TAG secretion were reduced. The beneficial effects of antisense-mediated reduction in hepatic ADRP appeared to be due to reduced lipogenesis rather than increased lipolysis or increased TAG export as VLDL. These changes were accompanied by improved insulin sensitivity and glucose tolerance, as well as reduced body fat. Reduction in hepatic ADRP did not promote liver damage; on the contrary, serum alanine aminotransferase, a marker of liver injury, was significantly reduced in both genetic and diet-induced obese models.
How do we explain the differences in phenotype between the ADRP knockout mouse reported by the Chan lab and the mouse with knockdown of hepatic ADRP reported by Imai and colleagues? The experimental approaches employed by each lab resulted in differences in the degree of ADRP deficiency, location (tissues) of ADRP deficiency, and timing of ADRP deficiency during development. These factors may have influenced degree, location, and timing of compensatory changes in expression of other genes involved in lipid and carbohydrate metabolism.
3.5 ADRP protein levels in human skeletal muscle
Interventions associated with improved insulin sensitivity in human, weight loss in obese, non-diabetic subjects and treatment of type 2 diabetic subjects with insulin sensitizers (troglitazone or metformin), have been associated with increased skeletal muscle ADRP protein levels [124].
3.6 Cell-based studies of ADRP and lipoprotein metabolism
The link between ADRP and hepatic lipoprotein synthesis is supported by cell-based studies of hepatoma cell lines. Magnusson and colleagues manipulated ADRP levels in a hepatoma cell line (McA-RH 7777 cells) and in primary rat hepatocytes. Increased ADRP partitioned TAG into lipid storage droplets and reduced secretion of TAG as VLDL [125]. Conversely, silencing of ADRP expression with siRNA resulted in reduced lipid droplets, increased fatty acid β-oxidation, and increased secretion of apoB-48 VLDL1. The reduction in lipid droplets contrasted with results reported for ADRP deficient mouse embryonic fibroblasts (MEFs) [126] derived from the ADRP knockout mice generated by the Chan lab. ADRP null MEFs did not differ from wild-type MEFs in lipid droplet accumulation, uptake of radiolabeled oleate, or release of radiolabeled oleate into the media. Sztalryd and colleagues found increased TIP47 mRNA and protein in the knockout MEFs and suggested that increased TIP47 functionally compensated for ADRP deficiency [126]. The effects of ADRP deficiency differ from cell-type to cell-type and, likely, from tissue to tissue.
3.7 ADRP and atherosclerosis
ADRP deficiency has been associated with reduced lipid accumulation not only in the liver but also in macrophage foam cells in vitro and within the vessel wall [127]. Using the same ADRP knockout mice generated by the Chan laboratory, Paul and colleagues found that lack of full-length ADRP was associated with reduced size of atherosclerotic lesions in atherosclerosis susceptible mice (ApoE−/−), despite unchanged plasma cholesterol and triglycerides. Peritoneal macrophages isolated from ADRP deficient ApoE−/− mice showed increased cholesterol efflux and reduced cholesterol ester accumulation. There was no effect of ADRP deficiency on expression of genes involved in intracellular cholesterol metabolism, on microsomal acyl-CoA:cholesterol acyltransferase (ACAT) activity, nor on the rate of cholesterol ester hydrolysis. These studies of ADRP deficiency suggest that ADRP has a primary role in channeling cholesterol into the stored cholesterol ester pool of macrophage foam cells, as opposed to the pool available for efflux, thereby promoting atherosclerosis in the ApoE−/− mouse model.
3.8 Regulation ADRP expression and function
Given the accumulating in vivo evidence that ADRP facilitates ectopic lipid accumulation in the liver and vessel wall, it is increasingly important to understand the factors that regulate its expression and function. Studies thus far have focused on transcriptional regulation, post-translational modification, post-translational stability, and protein-protein interactions. Like perilipin gene expression in adipocytes [35, 36, 128], ADRP gene expression is regulated by nuclear hormone receptors of the peroxisome proliferator-activated receptor (PPAR) subfamily, including PPARα in hepatocytes [129] and PPARβ/δ in keratinocytes [130]. A PPAR response element (PPRE) has been identified in both the mouse [131] and human [132] ADRP promoters. Ligands for PPAR receptors, including long-chain fatty acids [133], PPAR agonists [36, 134–137], and cyclooxygenase inhibitors [138], have been shown to induce ADRP mRNA, but these effects vary among cell-types and species. ADRP mRNA did not increase significantly in the subcutaneous adipose tissue of humans with impaired glucose tolerance after 10 weeks of PPARγ agonist treatment [22]. Transcriptional activation of the ADRP gene by long-chain fatty acids may provide a feed-forward mechanism, whereby increased ADRP provides a reservoir to sequester the final reaction products of neutral lipid synthesis. This process could drive the synthesis reactions forward and may explain the early observation that overexpression of ADRP increased fatty acid uptake into COS-7 cells [139]. Although the potential to modulate ADRP expression pharmacologically at the transcriptional level is clear, agents that do so affect expression of many other genes as well.
ADRP may be post-translationally modified by phosphorylation, acylation, or ubiquitination. Using mass spectrometry, Bartz and colleagues identified a human ADRP phospho-peptide (serine 291) in the phospho-proteome of lipid droplets isolated from oleate-treated HeLa cells [2]. This serine is conserved in the mouse ADRP. As noted in the discussion of perilipin, phosphorylation of other lipid droplet proteins, including perilipin and HSL, influences the function and/or localization to lipid droplets of these proteins. However, the functional significance of ADRP phosphorylation is not known, nor has the upstream kinase been reported.
ADRP is a component of the milk lipid globule membrane, which surrounds triglyceride droplets in milk [140]. Analysis of ADRP derived from milk lipid globule membranes suggests modification by acylation [140]. Mechanisms of targeting ADRP to lipid droplets in other cells have focused on peptide motifs in the primary sequence, as discussed below, but a role for acylation in lipid droplet localization has not been excluded.
Similar to perilipin, ADRP that is not bound to lipid droplets is covalently modified with ubiquitin and targeted for proteosomal degradation [115]. Compounds that inhibit the targeting of ubiquinated proteins to proteosomes led to increased ADRP protein levels and triacylglycerol levels in Chinese hamster ovary (CHO) cells cultured in lipid-poor conditions, but lysosomal and other protease inhibitors did not [115]. Also, lipid-loading of CHO cells was associated with reduced levels of ubiquitinated ADRP, which is consistent with a model in which increased lipid accumulation protects ADRP from the action of a ubiquitin ligase. Recently, an amino-terminal truncation of ADRP that corresponded to Δ2,3 ADPH (ADRP with residues 1–89 removed) was found to resist proteosomal degradation under lipid-poor culture conditions [120]. Thus, exogenous fatty acids may increase ADRP levels both by activating transcription of the ADRP gene and by promoting triacylglycerol synthesis, thereby providing additional lipid droplet surface to protect ADRP protein from degradation.
The stabilization of ADRP by TAG accumulation in lipid droplets and its destabilization by delipidation explains observations in many different experimental systems in which states of increased fatty acid flux are associated with increased ADRP protein and its association with lipid droplets. For example, overnight fasting of mice was associated with increased lipid accumulation and increased total ADRP and lipid droplet-associated ADRP in liver [22]. In this scenario, delivery of fatty acids liberated from adipose tissue leads to increased TAG accumulation in hepatocytes, thereby providing both fatty acid ligands to activate ADRP gene transcription and increased lipid droplet surface to stabilize ADRP protein. In a contrasting metabolic state, that of prolonged isoproterenol-stimulation of lipolysis in engineered adipocytes that express both perilipin and ADRP, ADRP protein also increased in parallel with ADRP binding to lipid droplets [46]. Notably, perilipin levels remained constant under these conditions. Perhaps the expansion of the lipid droplet surface due to fragmentation and dispersion of droplets during prolonged lipolysis created additional binding sites for ADRP with consequent stabilization of the protein. An alternative explanation suggested by the authors is that ADRP is able to displace perilipin during prolonged lipolysis or to associate with lipid droplets formed during re-esterification of fatty acids [46].
Analysis of the affinity of ADRP for lipid droplet binding has focused on analysis of ADRP truncation or deletion mutant proteins. As is the case for perilipin, no single domain or motif of ADRP has been found to be both necessary and sufficient for lipid droplet binding [141–143]. Rather, regions in both the amino- and carboxyl-terminal halves of the full-length protein appear to contribute to such binding. Although ADRP associates with lipid droplets via hydrophobic interactions, its association with lipid droplets may be regulated by protein complexes. Nakamura and colleagues identified ADP-ribosylation factor 1 (ARF1) as an ADRP-interacting protein by yeast two-hybrid screening and confirmed the interaction by co-immunoprecipitation [144]. ADRP preferentially associated with GDP-bound ARF1 and not GTP-bound ARF1. Expression of a mutant ARF1 to mimic the GDP-bound state (dominant negative) resulted in dissociation of ADRP from lipid droplets. This guanine nucleotide-dependent ADRP-ARF1 interaction suggests a means of regulating the lipid droplet surface proteome that is independent of the accessibility of the hydrophobic droplet core to ADRP targeting domains. Recently, protein complexes of ADRP and SNARE proteins have been identified in NIH 3T3 cells and implicated in homotypic lipid droplet fusion [145]. ADRP also forms complexes with the microtubule minus-end motor protein dynein, and disruption of dynein reduces lipid droplet formation [146].
3.9 ADRP, the constitutive PAT protein for non-adipocytes
In non-adipocytes the steady state protein levels of ADRP correlate with intracellular neutral lipid, and its association with lipid droplets increases during states of increased lipid flux as additional lipid droplet surface is created. Though its precise function remains to be defined, manipulation of ADRP expression both in cells and in organisms is associated with marked changes in lipid metabolism. In some tissues, ADRP may promote lipid accumulation at the cost of reduced lipid export via lipoprotein secretion or reverse transport (hepatic steatosis, atherosclerosis), but in other tissues ADRP may sequester lipid and attenuate lipotoxic damage (skeletal muscle). Despite the proliferation of ADRP studies in the literature, whether and how ADRP can be exploited as a therapeutic target in lipid-associated human disease remain as important questions.
4. TIP47/PP17/M6PBP
4.1 TIP47, the first reported exchangeable lipid droplet protein
Tail-interacting protein of 47 kiloDaltons (TIP47) was identified as a relative of perilipin and ADRP by sequence similarity of their amino-termini [116, 147] (Fig. 1). Whereas perilipin and ADRP are both regulated by PPARs as discussed above, TIP47 is not PPAR-regulated [36]. In contrast to perilipin and similar to ADRP, TIP47 is expressed in almost if not all tissues. The sequence homology between ADRP and TIP47 extends throughout the entire lengths of the proteins, over which they are 43% identical [116, 147] (Fig. 1). Consistent with this sequence similarity, Wolins and colleagues found TIP47 to associate with lipid droplets in cells incubated with oleate [147]. Though initially questioned [148], this result was independently confirmed [18]. In contrast to perilipin and ADRP, TIP47 was stable in the cytosol when HeLa cells were grown in lipid-poor medium and moved onto lipid droplets in response to lipid loading. TIP47 was later shown to behave similarly in 3T3-L1 adipocytes [72] and in mouse liver [22].
4.2 Other proposed roles for TIP47
TIP47 was initially described as directly binding the cytoplasmic “tail” of mannose 6-phosphate receptors and mediating their transport from the endosomal compartment to the trans-Golgi network [149]. Thus, it is possible that lipid-loading of cells may interfere with other functions of TIP47 by its recruitment to lipid droplets. In another context, Sato and colleagues found that expression in a hepatoma cell line of the hepatitis C virus (HCV) core protein, which largely localized to lipid droplets, was associated with redistribution of TIP47 from the cytosol to lipid droplets, perhaps as a result of reduced ADRP expression [6]. The authors hypothesized that changes in lipid metabolism associated with HCV core protein expression in the liver may be due to reduced TIP47 available for endosomal trafficking. In other contexts, TIP47 has been implicated as a cofactor in HIV viral assembly [150], as a mechanism for targeting a protein-tyrosine phosphatase to secretory vesicles [151], and as an inhibitor of retinylester hydrolysis by GS2 lipase and hormone-sensitive lipase in keratinocytes [152].
4.3 TIP47, lipid droplets, and the eye
Recent experiments have extended the potential importance of TIP47 to tissues other than those directly relevant to systemic lipid metabolism. Exposure of mouse eyes to light flashes photobleaches rhodopsin in photoreceptor cells and leads to increased all-trans-retinol, which is taken up, esterified with fatty acids, and stored in droplets within the retinal pigment epithelium. Tsuiki and colleagues found that light flashes to mouse eye and incubation of a cultured human retinal pigment epithelial cell line with all-trans-retinol led to increased lipid droplet formation and TIP47 translocation from the cytosol to lipid droplets [153]. In contrast, ADRP was constitutively associated with lipid droplets. This process was highly reminiscent of TIP47 recruitment to TAG-containing lipid droplets upon oleate-loading. Based on the previous description of TIP47 as a retinylester hydrolase inhibitor, the authors investigated whether TIP47 might function similarly in the eye. However, over 70% knockdown of TIP47 by siRNA did not significantly alter accumulation of retinyl esters.
4.4 Functional studies of TIP47 using RNA interference
The function of TIP47 has also been explored by siRNA-mediated knockdown in ADRP deficient MEFs [126], as discussed above, and in a liver cell line [154]. In ADRP deficient MEFs, TIP47 replaces ADRP as the predominant PAT protein on lipid droplets. MEFs deficient in both TIP47 and ADRP, compared with ADRP deficient MEFs, partitioned more exogenous fatty acids into phospholipids at the expense of triacylglycerol, but total lipid uptake was similar. Combined PAT protein deficiency was also associated with increased basal lipolysis over the first hour [126]. Subsequently, Bell and colleagues knocked down TIP47 and ADRP singly and together in the AML12 liver cell line [154]. Morphologically, TIP47 knockdown cells had smaller, more numerous lipid droplets, whereas ADRP knockdown cells had lipid droplets similar in size and number to control AML12 cells. ADRP knockdown resulted in increased TIP47 on lipid droplets, similar to what was observed in Huh7 cells [155]. Knockdown of both TIP47 and ADRP was associated with lipid droplets that were much larger in size and fewer in number. The authors suggested that the larger droplets were the result of uncontrolled droplet fusion, thereby highlighting the role of PAT proteins in emulsifying intracellular neutral lipids. Combined knockout also resulted in increased lipolysis and increased ATGL and CGI-58 in the fat cake. These effects on lipid metabolism were accompanied by changes at multiple steps of the insulin signaling pathway consistent with decreased insulin sensitivity. Knocking down ATGL in addition to the PAT proteins corrected the defect in insulin-stimulated Akt phosphorylation. These studies, taken together, provide strong evidence that the relative expression of PAT proteins influences cellular lipid metabolism and secondarily influences insulin signaling, most likely by means of controlling access of lipases to stored lipid esters.
4.5 The structure of TIP47
Solution of the crystal structure of the carboxyl 60% of TIP47 has provided insight into how PAT proteins may bind lipid droplets [156]. This structure consists of a four-helix bundle domain and an α/β domain with a hydrophobic cleft between them. The hydrophobic cleft may form a binding pocket for small molecules. Disruption of this cleft by alanine mutagenesis resulted in some TIP47 mutants that localized to lipid droplets even in the absence of oleate loading [155]. The four-helix bundle structure of TIP47 is similar to a structure in the amino-terminal half of apoE that can reversibly open to expose hydrophobic surfaces and bind lipoproteins or close to remain stable in solution. The similarity of the four-helix bundles in apoE and TIP47 suggests a common mechanism for reversible binding to lipoproteins and lipid droplets, respectively, as has been discussed in reviews [24, 25].
5. S3-12 and MLDP/OXPAT/LSDP5
5.1 S3-12 and OXPAT, tandem genes with reciprocal expression
Like TIP47, the most recent additions to the PAT proteins, S3-12 and OXPAT, are EPATs (Fig. 1). S3-12 and OXPAT are encoded by tandem genes on human chromosome 19 and mouse chromosome 17 [22, 23]; the gene for TIP47, the only other mammalian EPAT is located less than 200,000 base pairs upstream. Despite their proximity in the genome, the genes for S3-12 and OXPAT appear to have evolved for specialized functions based on their distribution in mouse tissues. S3-12 is expressed primarily in the tissue most specialized for fat storage, white adipose tissue, and to lesser degree in skeletal muscle and heart [20, 22, 23]. S3-12 is expressed little if at all in brown adipose tissue [20], which is specialized for adaptive thermogenesis and has a high capacity for fat oxidation. OXPAT, on the other hand, is expressed prominently in tissues that exhibit a high capacity for fatty acid oxidation, including heart, brown adipose tissue, liver, and skeletal muscle, especially more oxidative, less glycolytic muscles. The differential expression of S3-12 and OXPAT in white and brown adipose tissues, respectively, suggests that these PAT proteins may package triacylglycerol for different metabolic pathways. S3-12 may primarily serve to facilitate longer-term neutral lipid storage, whereas OXPAT may facilitate lipid storage for near-term utilization through oxidative pathways [22]. Endogenous S3-12 and exogenously expressed OXPAT are stable in the cytosol of cultured cells under lipid-poor conditions, but move to the surface of lipid droplets during lipid-loading with long-chain fatty acids [20, 22, 23, 72]. S3-12 also relocates to lipid droplets during lipolytic stimulation of adipocytes [4]. This phenomenon has not been reported for OXPAT. Localization of endogenous OXPAT protein to lipid droplets has been confirmed by microscopy in mouse primary cardiomyocytes [22] and by cell fractionation in fasted mouse liver [22] and MLTC-1 steroidogenic cells [21].
5.2. S3-12, TIP47, and ADRP in nascent lipid droplet biogenesis
The expression of endogenous S3-12, TIP47, ADRP, and perilipin in 3T3-L1 adipocytes has permitted the investigation of their dynamic association with lipid droplets during rapid TAG synthesis [20, 24, 72]. Within 10 minutes of the addition of oleate, glucose and insulin to the medium, discrete puncta immunoreactive for S3-12 and TIP47 are detected. Over the next 30–60 minutes, these puncta enlarge and become discernable as tiny lipid droplets, which then move centrally. As the droplets continue to grow in size, TIP47 leaves and ADRP becomes detectable on the enlarging nascent droplet surface. At later stages, larger droplets accumulate more centrally that are coated by S3-12, ADRP, and perilipin or perilipin alone. Thus, lipid droplet biogenesis in adipocytes appears as an ordered maturation of the nascent lipid droplet with progressive changes in the PAT proteins that coat its surface as the droplet enlarges, moves centrally, and ultimately becomes or merges with a mature storage droplet.
5.3. S3-12 and the 11-mer repeats: a putative mechanism for lipid droplet binding
S3-12 was initially identified and cloned during an antibody-based screen for cell surface proteins induced during 3T3-L1 adipocyte differentiation [19]. Exoplasmic exposure of S3-12 epitopes has not been confirmed in subsequent studies, but its induction during adipogenesis has been confirmed with two independent antibodies [20]. A notable feature of the primary amino acid sequence of S3-12 is multiple tandem 33-mer repeats that constitute approximately two-thirds of the 160 kiloDalton protein [19]. This repeat motif has sequence similarity to two 33-mer repeats in ADRP [19]. Bussell and Eliezer later identified an 11-mer repeat pattern that is also observed in multiple other proteins that bind lipids, including α-synuclein, S3-12 and the other PAT proteins, and apolipoproteins [157]. The similarity of the 11-mer repeats between the PAT proteins and other protein families is not at the level of primary amino acid sequence but rather predicted secondary and tertiary protein structure. It was proposed that the 11-mer repeats in these proteins fold into elongated α-helices with hydrophobic faces capable of binding lipids and hydrophilic faces soluble in the aqueous environments of the cytosol or plasma. Thus, the 33-mer repeat of S3-12 resolves into an even more basic 11-mer repeat that may contribute to its lipid droplet localization. Notably, a carboxy-terminal deletion of OXPAT that bisects the 33-mer motif disrupts lipid droplet binding [21], but the similar motif in TIP47 is not necessary for binding [155].
Similar to the other PAT proteins, with the exception of TIP47, expression of S3-12 is PPAR-regulated, at least in cultured cells [36].
5.4. OXPAT, regulation by PPARs in mice and humans
Initial identifications and characterizations of OXPAT as a lipid droplet binding protein were reported by three independent groups as myocardial lipid droplet protein (MLDP) [21], OXPAT [22], and lipid storage droplet protein 5 (LSDP5) [23]. These reports were in agreement that OXPAT is expressed in tissues that exhibit high levels of fatty acid oxidation, including heart, skeletal muscle (oxidative > glycolytic), and fasted liver. High expression in brown but not white adipose tissue was also noted [22]. OXPAT RNA and/or protein were inducible by fasting in the heart [21, 23], liver [21–23], and skeletal muscle [23] and by high-fat diet [22] in the gastrocnemius muscle. A physiological role for OXPAT induction during fasting is suggested by its enrichment on fractionated lipid droplets in the fasted liver [22]. Consistent with these effects, OXPAT expression was stimulated in mouse tissues by a pharmacological ligand for PPARα (Wy-14643) in liver, heart, and skeletal muscle [21–23]. Basal OXPAT expression was significantly reduced in the heart and liver of PPARα null mice [21–23]. Whereas two groups found induction of OXPAT gene expression by fasting to be PPARα dependent (that is, significantly reduced in PPARα null mice), one group did not [23]. OXPAT gene expression in mouse epididymal adipose tissue was induced by a PPARγ agonist (rosiglitazone) [22]. Relevance of this last finding to human physiology is suggested by the increased OXPAT RNA levels detected in the subcutaneous fat of humans with impaired glucose tolerance after 10 weeks of treatment with pioglitazone, a PPARγ agonist [22]. Notably, adipose expression of none of the remaining PAT proteins was significantly increased by pioglitazone treatment.
5.5. OXPAT, functional studies in cells
Functional evaluation of OXPAT has been limited thus far to gain-of-function studies in cultured cells. No loss-of-function studies, either by gene knockout or RNAi approaches, have been reported. Heterologous stable expression of OXPAT is associated with increased TAG accumulation in oleate-treated COS-7 and OP9 cells [22]. One explanation for this effect in promoting TAG accumulation is that OXPAT protects lipid droplet TAG from both basal and stimulated lipolysis, as was demonstrated in stable CHO cell lines [23]. The tissues in which OXPAT is expressed have high capacities to oxidize long chain fatty acids, so it is consistent that OXPAT would promote expansion of the storage pool of lipid substrates. However, in the COS-7 and OP9 cells that stably expressed OXPAT, β-oxidation of palmitate was also increased.
Insight into the mechanism underlying the promotion of fatty acid oxidation by OXPAT has been provided by an exciting set of experiments recently reported by Granneman and colleagues [158]. Using transfected cells and fluorescently tagged proteins, these investigators have shown that OXPAT promotes the targeting of CGI-58 to the surface of lipid droplets, as well as the co-localization of CGI-58 with ATGL. Loading of cells with oleate increased the interaction of OXPAT with CGI-58. Fluorescence resonance energy transfer (FRET) and bimolecular fluorescence complementation (BiFC) experiments were consistent with OXPAT and CGI-58 being in close proximity on the lipid droplet surface, if not in direct protein-protein contact. Perhaps most intriguingly, mouse CGI-58 with an E260K mutation, the same mutation reported by Zechner’s group to block the interaction of human CGI-58 with perilipin [61], also blocked its interaction with OXPAT. Thus, OXPAT may function similarly to perilipin in regulating lipase action at the lipid droplet surface, but perhaps without modulation by PKA activation.
The pattern of increased capacity both for TAG accumulation and for fatty acid oxidation that is observed in cells that heterologously express OXPAT is also seen in the skeletal muscle of endurance trained athletes. OXPAT may be part of an adaptive response to increase the availability of lipids for utilization as fuel. In this regard, it is interesting that OXPAT gene expression in the subcutaneous fat of humans is inversely associated with body mass index [22]. Whether a similar association exists between OXPAT expression in skeletal muscle and body mass index is not known. If it does, then perhaps obesity-related OXPAT deficiency in muscle limits the capacity for lipid oxidation in muscle, thereby impairing insulin signaling pathways and promoting peripheral insulin resistance.
6. Non-mammalian PAT proteins
PAT proteins are not restricted to mammals. Proteins with significant sequence similarity have been found in many other animal species (ranging from sea anemones and insects to snails, frogs and sea urchins) and even in unicellular eukaryotes (such as choanoflagellates, fungi, and slime molds) ([18, 27–29] and MAW, unpublished observations). Functional data have been reported only from a few species, but what is available suggests that across this broad phylogenetic spectrum PAT proteins play fundamental roles in lipid homeostasis and lipid-droplet biology. For example, in both the fruit fly Drosophila melanogaster and the pathogenic fungus Metarhizium anisopliae, PAT proteins localize to lipid droplets and promote storage of neutral lipids.
This broad conservation provides rich opportunities to advance our understanding of this protein family. On the one hand, we can use technical advantages in model organisms to dissect the molecular and cellular roles of PAT family members. For example, the ease of gene replacement in fungi makes it possible to quickly analyze many mutant versions of the proteins in vivo. Likewise, the sophisticated genetic techniques in Drosophila should allow screening for novel molecules that function in the same pathways as PAT proteins. Such analyses are expected to yield testable hypotheses about the functions of mammalian PATs. On the other hand, we can employ the insights from studies of mammalian PATs to shed light on the role of PAT proteins, and more generally on the role of lipid droplets, in poorly studied species. For example, there are intriguing hints that PAT proteins might be key virulence factors of certain fungal plant pathogens. Thus, knowledge of the molecular biology of PAT proteins from animals might lead to novel ways of combating fungal infections that threaten crop plants.
6.1. LSD-1 and LSD-2 of Drosophila and other insects
6.1.1. Lipid metabolism in mammals and insects
Recent studies have established insects as surprisingly good models for mammalian physiology. In particular, control of lipid metabolism employs many similar strategies in these two groups of animals (reviewed in [159, 160]): similar to mammalian adipocytes, the insect fat body serves as the major storage depot for neutral lipids, and when lipids need to be mobilized, the neuropeptide AKH (adipokinetic hormone, called the “insect glucagon”) triggers lipolysis in the fat body [161]. Fat is released into the insect bloodstream (the hemolymph) and taken up by oenocytes (akin to mammalian hepatocytes) for energy production [162].
Similar to mammals, mobilization of lipids from fat body stores is coordinated by two lipocatabolic systems [163]. One involves signaling through the AKH receptor (AKHR) and dominates the immediate response to starvation. This pathway acts in part via activation of protein kinase A (PKA). A second system is particularly important after extended food deprivation and involves the Brummer lipase, a homolog of the mammalian adipose triglyceride lipase (ATGL). Mutants in either pathway overstore neutral lipids and are impaired in lipolysis; animals mutant for both AKHR and Brummer cannot mobilize any significant amounts of stored fat and die rapidly upon starvation [163] (Fig. 2).
These extensive similarities suggest that it should be possible to use the technical advantages of insects to uncover generally important paradigms of lipid homeostasis. For the model organism Drosophila melanogaster, such advantages include a short generation time, a wealth of classical genetics information and an ever-expanding toolkit of molecular, genomic and biochemical approaches. In a recent example, two groups performed genome-wide RNAi screens to identify proteins required for lipid-droplet formation and utilization in Drosophila cultured cells [164, 165]. One of the pathways chosen for closer analysis (the COP I trafficking proteins) was then found to have a similar function in mammalian cells, suggesting that even in these evolutionarily quite distant animals lipid-droplet biology shares deep similarities. This notion is also supported by proteomic studies that find many related proteins on lipid droplets from Drosophila [3, 166] and mammalian [4, 167–169] sources.
6.1.2. PAT proteins of insects
Insects express PAT proteins from two distinct subfamilies (LSD-1 and LSD-2; lipid storage droplet protein-1 and -2) [18, 28] (Fig. 1). Emerging evidence suggests that – like mammalian family members – insect PATs can play crucial roles in PKA-triggered lipolysis and promote storage of neutral lipids. Many other features are shared with mammalian PATs: they localize to lipid droplets, they are regulated by phosphorylation (with PKA one of the responsible kinases), and they work together with the ATGL/Brummer family of lipases. In addition, Drosophila LSD-2 regulates the intracellular trafficking of lipid droplets, a role conserved with ADRP and perilipin.
Furthermore, PAT proteins of mammals and insects act in very similar cellular environments. For example, the Drosophila genome encodes potential homologs of proteins known as functional or physical interactors of PAT proteins in mammals. These include CG1882 (a homolog of mammalian CGI-58), Brummer lipase (ATGL), and CG11055 (a possible ortholog of the hormone-sensitive lipase of adipocytes). Proteomic analysis suggests that these candidates reside on lipid droplets [3, 166] and are therefore in the correct intracellular compartment to interact with LSD-1 and LSD-2.
6.1.3. Intracellular distribution of insect PAT proteins
6.1.3.1. LSD-1 and LSD-2 are lipid-droplet proteins
Many independent lines of evidence show that LSD-2 is present in massive amounts on lipid droplets. Its intracellular distribution has been assessed by biochemical fractionation as well as by immunolocalization, and the protein has been detected using several distinct methods (three independently developed anti-sera [28, 170, 171] and mass spectrometry). LSD-2 is present on lipid droplets in cultured cells [171], in nurse cells and oocytes of the adult ovary [170]), in early embryos [171], in fat bodies of both larvae and adults [3, 28], and in larval wing discs (the precursors of the adult wings; [172]). Ectopically expressed GFP-fusion constructs localize to lipid droplets in cultured mammalian and Drosophila cells [3, 18] as well as in fat bodies of Drosophila larvae [18, 28].
Although fewer data are available for LSD-1, they also indicate droplet localization of the protein. For Drosophila, proteomic analysis of biochemically purified lipid droplets found LSD-1 in large amounts on lipid droplets from larval fat bodies [3] and in trace amounts on embryonic lipid droplets [166]. In the moth Manduca sexta (also known as the tobacco hornworm), a major phosphoprotein from fat-body lipid droplets was identified by mass spectrometry as LSD-1 [173], an assignment subsequently confirmed by Western analysis [174]. And ectopically expressed, tagged LSD-1 localizes to lipid droplets in both mammalian and Drosophila cultured cells [3, 18, 164] and in Drosophila larval fat bodies [18].
6.1.3.2. Mechanisms of droplet targeting
Little is yet known about how LSD-1 and LSD-2 attach to lipid droplets. LSD-2 binding to droplets is dominated by hydrophobic interactions since LSD-2 remains attached to embryonic lipid droplets when charge interactions are blocked, but can be washed off with strong detergents [166]. LSD-1 and LSD2 apparently bind directly to lipid since recombinant protein purified from E. coli can be reconstituted into small lipoprotein particles in the absence of any other protein [174, 175]. Computational analysis points to four stretches in the central region of LSD-1 (outside the PAT domain) as possible features mediating droplet targeting; they are predicted to form either largely hydrophobic or highly amphipathic alpha helices [175]. Thus, like in mammalian PATs, several elements may independently target the protein to droplets, together resulting in robust droplet attachment [32].
6.1.3.3 Are LSD-1 and LSD-2 restricted to lipid droplets?
Mammalian PAT proteins fall into two general classes: CPATs are constitutively associated with lipid droplets; EPATs exchange between cytoplasm and lipid droplets depending on the physiological state of the cell [24]. Do LSD-1 and LSD-2 resemble CPATs or EPATs? For LSD-1, studies of fluorescent fusion proteins have only described droplet localization [18, 164], and subcellular fractionation of Manduca sexta fat bodies detected endogenous LSD-1 only in the lipid-droplet fraction [174]. For LSD-2, massive droplet localization is well established, but there are also hints that LSD-2 may sometimes reside elsewhere. To elucidate the full range of LSD-1 and LSD-2 distributions, a future comprehensive analysis will require examining these proteins in various cell types and under different physiological conditions (e.g., during both lipolysis and lipogenesis).
In many cells, LSD-2 is predominately localized to lipid droplets. Biochemical fractionation of embryos and larval fat bodies reveals the majority of LSD-2 in the droplet fraction [28, 171]; whether LSD-2 signal in some non-droplet fractions [3, 28] is due to loss of LSD-2 from the droplets during purification or genuine localization to other compartments has not yet been critically tested. In micrographs of embryos, cultured cells, fat bodies and oocytes, droplet-bound LSD-2 clearly represents the major source of LSD-2 signal; this is true both for endogenous LSD-2 and GFP-LSD-2 fusions [3, 18, 28, 170, 171].
In contrast, two studies report high levels of LSD-2 signal not associated with lipid droplets. In larval wing discs, LSD-2 colocalizes with both lipid droplets and ER markers [172]. For nurse cells of the Drosophila ovary, there are conflicting data: one study employing fluorescence microscopy [176] reports general cytoplasmic distribution of LSD-2 (and exclusion from lipid droplets), while an earlier EM analysis showed LSD-2 highly enriched at the surface lipid droplets [170]. This discrepancy might be due to technical difficulties (e.g., problems with antibody specificity or penetration into the tissue) or biological differences (e.g., the exact stage of oogenesis analyzed or the physiological state of the females). That LSD-2 might exist in non-droplet bound forms is also suggested by the fact that – in contrast to LSD-1 – recombinant LSD-2 from E. coli is soluble in aqueous buffers [177].
6.1.4. Lipid homeostasis
6.1.4.1. LSD-1 phosphorylation is linked to control of lipolysis
In Drosophila, LSD-1’s expression pattern is consistent with roles in lipid metabolism: Its mRNA is expressed in the embryonic fat body; the protein is found on purified lipid droplets from larval fat body [3]; and its mRNA is downregulated in adult flies upon starvation [178]. However, because no LSD-1 mutants have yet been reported, there has been no direct test of LSD-1’s function.
But physiological studies in Manduca point to an important role of LSD-1 in the control of lipolysis in the adult fat body. Enhanced lipolysis observed after AKH stimulation is largely due to a physical change in lipid droplets, since it can be mimicked when droplets purified from stimulated fat bodies are mixed with lipases from unstimulated samples [179]. Lipolytic rates are also enhanced when droplets are phosphorylated by PKA in vitro, suggesting that upregulated lipolysis is due to the phosphorylation of droplet-associated proteins. A leading candidate is LSD-1, since it is the major protein on lipid droplets incorporating phosphates, both after hormone stimulation in vivo and with PKA treatment in vitro. Interestingly, LSD-1 levels in the larval fat body are significantly lower, and here AKH signaling does not induce lipolysis [174]. Drosophila LSD-1 can also be a PKA target; in vitro, PKA phosphorylates recombinant LSD-1 (reconstituted into lipoprotein particles) on Ser20 [175].
How does phosphorylation of droplets promote lipolysis? It has been hypothesized that phosphorylated LSD-1 enhances droplet binding of lipases or stimulates their catalytic activity [179]. However, the main triglyceride-lipase from fat bodies is not detected on purified lipid droplets, neither before nor after hormone stimulation [173], suggesting that it may only weakly or transiently interact with droplets. A new in vitro system may soon make it possible to resolve this puzzle: when purified lipase is exposed to lipoprotein particles reconstituted with recombinant LSD-1, lipase activity is enhanced when the particles are first treated with PKA [175].
6.1.4.2. LSD-2 promotes storage of neutral lipids
Like LSD-1, Drosophila LSD-2 is expressed in an intricate tissue-specific pattern consistent with roles in lipid metabolism [28, 170, 172, 180]. In this case, genetic analysis has confirmed that LSD-2 is required for normal lipid storage. Animals entirely lacking LSD-2 are viable but have impaired lipid accumulation in a number of tissues. In adults, overall triglyceride levels are reduced by about 30% [28, 170]. In wing discs of LSD-2 mutants, neutral lipid accumulation in the wing pouch is dramatically reduced [172]. And in the nurse cells of mutant females, the intracellular distribution of neutral lipids is profoundly altered and eggs laid by these females have reduced amounts of triglycerides [170]. These lower triglyceride levels have severe organismal consequences: LSD-2 mutant adults die more quickly when starved [28], and LSD-2 mutant embryos display lower hatching rates [170].
Conversely, increased levels of LSD-2 allow cells to accumulate more lipid. When LSD-2 is highly overexpressed in the fat body of otherwise wild-type adults, these animals can accumulate up to 50% more triglycerides [28]. Similarly, localized overexpression of LSD-2 in parts of the wing disc promotes excess lipid accumulation [172]. The organism can access these extra lipid stores in times of need since adults overexpressing LSD-2 in the fat body display increased starvation resistance [28]. Therefore, excess LSD-2 does not simply seal those lipids away into inert blobs, but apparently mimics a physiological process.
6.1.4.3. LSD-2 and droplet biogenesis
How does LSD-2 promote lipid storage? LSD-2 might be involved in the biogenesis of lipid droplets since nurse cells lacking LSD-2 display abnormal, patchy distribution of neutral lipids instead of the discrete lipid droplets dispersed throughout wild-type cells [170]. It has been proposed that the large aggregates in the mutants might represent accumulation of neutral lipid in the ER due to inefficient droplet formation [170]. A detailed examination of these structures (by costaining for ER markers and by electron microscopy) will likely provide important clues about LSD-2’s mechanism of action. Indeed, nurse cells may be a particularly auspicious place to examine a role of LSD-2 in droplet biogenesis because these cells generate myriads of lipid droplets over just a few hours, in a tightly orchestrated developmental program [181].
However, LSD-2 is not absolutely required for droplet formation. Oocytes of LSD-2 mutant females are filled with lipid droplets [170], and the resulting embryos have many normal-sized droplets [171]. Does LSD-2 therefore simply make droplet formation more efficient? Or is lack of LSD-2 partially compensated for by other mechanisms? In LSD-2 mutant wing discs, at least, LSD-1 mRNA is upregulated several fold [172], reminiscent of TIP47 upregulation in ADRP deficient MEFs [126]. In both cases, one PAT protein may compensate for the lack of another.
LSD-2 likely has roles beyond droplet formation, as it remains present on droplets long after they have been born. For example, the lipid droplets generated in nurse cells are transferred to the oocyte, are present when the egg is laid and persist well into embryogenesis. LSD-2 is present on those lipid droplets in oocytes [170] and early embryos [171]; indeed, for the first few hours of embryonic development, there is no detectable change in the number or size of these lipid droplets [182] or in the amount of LSD-2 present on them [171].
6.1.4.4. LSD-2 and lipid homeostasis
Clues for a role of LSD-2 in lipid homeostasis come from experiments that examined genetic interactions between LSD-2 and ATGL/Brummer lipase. Modulation of LSD-2 and ATGL expression result in opposite phenotypes [178]: Overexpression of the lipase or lack of LSD-2 cause reduction in triglyceride levels; overexpression of LSD-2 or lack of Brummer result in increased lipid storage. Combined overexpression of the proteins results in intermediate triglyceride levels; thus, LSD-2 and Brummer act in an antagonistic manner [178]. One possibility is that LSD-2 interferes with access of the lipase to the stored lipid. However, that cannot be its only role, since animals lacking both LSD-2 and Brummer do not display the Brummer mutant phenotype, but have wild-type triglyceride levels. It is therefore conceivable that LSD-2 also functions in a second pathway, e.g. in conjunction with the AKHR pathway of lipid mobilization.
If LSD-2 were downstream of AKHR signaling, it might be regulated via phosphorylation, just like perilipin A and LSD-1. In early embryos, LSD-2 does indeed carry multiple phosphates and its phosphorylation state is modulated in response to developmental signaling [171] (though at this developmental stage, LSD-2 controls motion of lipid droplets and no connection to lipid metabolism is apparent, see also below); the relevant kinase(s) have yet to be identified. Given these precedents, a direct examination of LSD-2’s phosphorylation state in larval and adult fat bodies could provide crucial clues about its mechanism of action in lipid homeostasis. It will be particularly important to establish whether LSD-2’s phosphorylation state varies under different physiological conditions and whether it is downstream of AKHR signaling and PKA.
Since LSD-1 is apparently also a target of AKHR-induced PKA, a full understanding of lipid homeostasis in flies will require unraveling the relationship between LSD-1 and LSD-2. Do these proteins act redundantly, or do they manage distinct branches of this signaling pathway? Too little is yet known to address this question, although it is clear that LSD-1 and LSD-2 are co-expressed in the larval fat body [3] and that a single lipid droplet can simultaneously carry both proteins [3].
6.1.5. Lipid droplet motion
In many cells, lipid droplets undergo extensive intracellular trafficking (reviewed in [171]); such droplet motion has been proposed to serve diverse biological roles, including the control of lipid homeostasis. For example, when lipolysis is induced in adipocytes, one large lipid droplet breaks down into many small ones that then disperse throughout the cytoplasm [60]. Dispersion may allow quick delivery of lipids wherever they are needed. During lipogenesis, the motor protein Cytoplasmic Dynein promotes fusion of nascent lipid droplets [146], and thus helps droplets grow in size even without further triglyceride synthesis [183]. Furthermore, it has been hypothesized that droplet motion may facilitate delivery of droplet-associated proteins and lipids to other cellular compartments [184]. A striking example occurs in cells upon infection with Hepatitis C virus: lipid droplets undergo dynein-dependent motion and accumulate around the nucleus [185]. This motion may promote assembly of infectious virus by bringing together newly synthesized viral proteins (that accumulate on lipid droplets) and newly replicated viral RNA (present perinuclearly) [186, 187]. Understanding how and when droplets move may therefore provide new strategies for modulating lipid homeostasis as well as for combating viral infections.
6.1.5.1. PAT proteins and droplet motion
Mechanistic studies of droplet motion are still in their infancy, but it is already clear that PAT proteins play a prominent role. In many mammalian cells, for example, lipid droplets are normally dispersed throughout the cytoplasm but cluster near the nucleus when PAT protein expression is altered, such as when ADRP is knocked down by RNAi in human hepatoma cells [185] or when perilipin is ectopically expressed in mouse fibroblasts [60]. Similarly, when LSD-2 is absent from Drosophila embryos, lipid droplets fail to accumulate near microtubule plus ends [171], and knockdown of the PAT protein Fatvg in frog eggs alters microtubule-based trafficking events [188].
6.1.5.2. LSD-2 determines directionality of droplet motion
In Drosophila embryos, lipid droplets undergo a set of tightly choreographed trafficking events, over a two-hour period [189]. Initially spread throughout the cytoplasm (Phase I), droplets first accumulate near microtubule plus ends (Phase II) and then shift towards microtubule minus ends (Phase III). During all phases, droplets move incessantly back and forth along microtubules; net shifts in distribution come about because the relative contributions of plus- and minus-end travel changes [182].
In the absence of LSD-2, droplets still move vigorously in both directions in all phases, but there is no net plus-end motion in Phase II, and no quantitative change in motion parameters from Phase II to Phase III [171]. Thus, LSD-2 is not directly involved in the machinery that moves droplets, but is required to regulate it in time. It has been proposed that LSD-2 transmits developmental signals to the motors moving the droplets.
Consistent with a regulatory role, there is an intriguing correlation between the phosphorylation state of LSD-2 and droplet motion [171]. In Drosophila embryos, LSD-2 is multiply phosphorylated, and its phosphorylation state changes in a phase-dependent manner. On 2D gels, the most-highly phosphorylated forms disappear in Phase II and reappear in Phase III, raising the possibility that dephosphorylation of a particular residue on LSD-2 favors plus-end motion.
Genetic and biochemical approaches have identified several molecules that cooperate with LSD-2 in droplet transport. The motor machinery controlled by LSD-2 includes the plus-end motor Kinesin-1 [190], the minus-end motor Cytoplasmic Dynein [191] and the dynein binding-protein BicaudalD [192], though how these proteins function together remains to be established. A direct binding partner of LSD-2 is the lipid-droplet protein Klar [171, 193], a novel regulator proposed to coordinate opposite-polarity motors, i.e. turning the plus-end motor off while the minus-end motor is active, and vice versa [182]. A related coordination role has also been proposed for the dynein cofactor Dynactin [194]. Finally, LSD-2 dephosphorylation depends on the transacting factor Halo [171], a small protein specifically expressed in Phase II [195].
6.1.5.3. Parallels to mammals
Several features of droplet motion in Drosophila are conserved in mammals. First, in a number of cell types, minus-end motion of lipid droplets is powered by Cytoplasmic Dynein [146, 185]. Second, Dynactin may coordinate opposite-polarity motors also on mammalian droplets since disruption of Dynactin abolishes minus- as well as plus-end motion [196]. Third, in fibroblasts, distribution of lipid droplets is controlled by the phosphorylation state of perilipin. Here, perilipin-induced droplet aggregation is reversed upon sustained activation of PKA [60]. PKA likely acts through perilipin since under these conditions perilipin is phosphorylated, and a single point mutation in Ser492 (one of the PKA target sites in perilipin) prevents droplet dispersion [60]. Ser492 phosphorylation of perilipin also controls droplet dispersion in adipocytes [51]. Because mutations in other PKA target sites on perilipin are compatible with dispersion [51, 60], it appears that – just as in Drosophila – phosphorylation of a single residue on a PAT protein is the key regulatory switch that controls overall direction of droplet trafficking.
6.2. Fatvg of Xenopus laevis
PAT proteins are common in many vertebrate species. Available genome annotations reveal multiple family members in birds, amphibians and fish. This diversity represents an as-of-yet untapped potential to reveal conserved features in these proteins for droplet localization and interactions with partner proteins and to probe how PAT proteins might be adapted to function optimally in species with vastly different physiology.
As certain species have unique experimental advantages, they may be ideally suited to probe specific properties of this protein family that would be hard to investigate in mammals. Indeed, studies in Xenopus laevis, the African Clawed Frog, have uncovered a surprising role of the PAT family member Fatvg for early embryogenesis [188]. As Xenopus is a major model organism for developmental biology, methods and reagents are readily available to uncover the molecular basis for this phenomenon, an analysis that will provide the foundation to test whether PAT proteins in other animals have similar developmental roles.
6.2.1. Fatvg mRNA is asymmetrically distributed
By sequence similarity, Fatvg is one of two ADRP-like PAT family members in Xenopus laevis. Fatvg mRNA is broadly expressed during development, with particularly high levels in the fat body (the adipose tissue of frogs) [27, 197]. In early embryos, fatvg mRNA is also found in the germ plasm (which gives rise to the primordial germ cells) and highly enriched at the vegetal pole. The latter pattern lead to its name: fatvg = a vegetally localized RNA encoding an adipose-related protein [27].
Targeting of mRNAs is a powerful mechanism to ensure precise intracellular protein localization [198]. It will therefore be interesting to determine whether the distribution of Fatvg protein is similarly polarized in the embryo. In electron micrographs, antibodies against Fatvg protein label the surface of unidentified vesicular structures in oocytes and early embryos [188]. The size and general appearance of these “vesicles” is reminiscent of lipid droplets, but their identity has yet to be established. When the Fatvg antibody is used to stain oocytes of the sturgeon Acipenser gueldenstaedtii, signal is detected both in the cytoplasm and concentrated around lipid droplets [199].
6.2.2. Fatvg is essential for normal embryogenesis
To address the function of Fatvg, oocytes were injected with anti-sense oligonucleotides directed against Fatvg [188], a treatment that induced turnover of the endogenous fatvg mRNA. Embryos developing from these oocytes displayed dramatic, yet specific defects in development, including loss of head structures and eyes as well as a reduction in the number of germ cells. These defects are specifically due to lack of fatvg since they were partially suppressed by simultaneous injection of wild-type fatvg mRNA. Clearly, Fatvg plays a surprisingly specific role in early embryogenesis.
Exactly how Fatvg acts in frog development remains to be established. Since PAT proteins in Drosophila and mammals modulate the intracellular trafficking of lipid droplets, it is particularly intriguing that in the knockdown embryos microtubule-based organelle motion is either disrupted or randomized [188]. One speculative model proposes that Fatvg is required to transport vesicles that carry signals crucial for determining the dorsal side of the embryo [188].
6.2.3. Relevance for mammalian biology
Is the striking developmental role of Fatvg a unique adaptation of amphibian eggs or does it provide insight into general functions of PAT proteins? Embryonic development typically utilizes the same molecular machinery employed in other cells, but the formidable constraints faced by embryos (such as the huge size of eggs and the remarkable speed of embryogenesis) often make it easier to spot the function of such common pathways. The fact that other instances of asymmetrically distributed PAT mRNAs have not yet been reported may simply be due to technical limitations. Recent advances in in-situ hybridization have revealed spatially intricate localization patterns for an astonishingly high proportion of all RNAs examined [200, 201].
But why would PAT mRNAs be unevenly distributed? Emerging evidence suggests that within a single cell different lipid droplets can have distinct protein complements (reviewed in [8, 184]). One mechanism to generate such variation would be localized synthesis of those proteins; the presence of ribosomes and RNAs on lipid droplets [202, 203] has indeed been taken as indication that certain proteins may be translated directly at the droplet surface [7]. The case of fatvg should alert investigators to be on the lookout for unusual intracellular distributions of PAT-encoding mRNAs; such instances, if they can be found, may very well reveal novel pathways for regulating PAT protein functions in particular and the properties of lipid droplets in general.
6.3. MPL1 of Metarhizium and related fungi
It had long been thought that fungi lack PAT proteins since no proteins with significant sequence similarity to animal PATs are present in the genome of the budding yeast Saccharomyces cerevisiae [18, 30] nor among the S. cerevisiae lipid-droplet proteins characterized by proteomics approaches [204, 205]. However, a recent report [29] makes a good case that the MPL1 protein of the pathogenic fungus Metarhizium anisopliae belongs to the PAT family. Proteins with significant sequence similarity to MPL1 are present throughout a major group of fungi (the Pezizomycotina among the Ascomycetes) that contains many medically and economically important members.
6.3.1. MPL1: a droplet protein involved in lipid metabolism
MPL1 (Metarhizium perilipin-like) is a 183 amino-acid protein that displays low, but significant sequence similarity to the N-terminal regions of animal PAT proteins [29] (Fig. 1). Expression of MPL1 mRNA is induced when cells accumulate lipids, and an MPL1-GFP fusion protein colocalizes with lipid droplets. Mutants lacking MPL1 have fewer lipid droplets and reduced levels of intracellular lipid. Apparently, MPL1 promotes lipid storage, yet is not absolutely required for droplet formation. One possibility is that, like perilipin, MPL1 protects the stored lipid from attack by lipases. Consistent with this proposal, MPL1-GFP ectopically expressed in S. cerevisiae localizes to lipid droplets and interferes with lipid breakdown under starvation conditions [29].
6.3.2. MPL1 as a virulence factor
Many pathogenic fungi, including the insect pathogen Metarhizium anisopliae, use specialized cells called appressoria to break through the outer surface of their hosts, such as the cuticle of insects or the epidermis of plants. Appressoria generate enormous intracellular pressure by taking in large amounts of water, drawn in osmotically due to high levels of free glycerol (>3M). One of the sources of this glycerol is thought to be the breakdown of triglycerides from lipid droplets, and indeed lipid droplets are degraded during appressoria maturation [206].
In Metarhizium anisopliae, MPL1 appears to be important for normal function of appressoria and for successful host invasion. MPL1-mutants appressoria have reduced numbers of lipid droplets and generate lower intracellular pressure [29]. Mutant fungi are also significantly less successful at killing their insect hosts. The likely reason is a failure in cuticle penetration since upon direct injection of spores into the insect hemolymph the mutants kill as well as the wild type [29].
Proteins related to MPL1 are found in many economically and medically important fungi [29]. Their functions are generally not known, but in the plant pathogen Colletotrichum gloeosporioides mutants in the MPL1 homolog CAP20 also display reduced virulence. This fungus causes fruit rot in a number of commercially important species, like tomato and avocado, and the CAP20 mutants cause substantially less fruit damage [207]. If the MPL1 homologs of other fungi are also key virulence factors, the insights about PAT protein functions derived from animal studies could provide important clues how to disrupt the life cycles of these fungi.
6.4. Prospects for the study of mammalian PATs
Compared to animals, analysis of fungi has a number of technical advantages. For example, most fungal PAT candidates are encoded by single copy genes and thus knockout phenotypes will not be masked by compensatory expression of other family members. In addition, molecular and genetic techniques are well developed in many fungi, making gene knock-outs or knock-ins easy and quick. In those species, it should be fairly straightforward to determine the consequences of lack of PAT proteins, to introduce mutant versions (e.g. to map the droplet binding domains or to test the relevance of proposed PKA phosphorylation sites) and to test double mutants with candidate interaction proteins. For example, BLAST searches reveal that many fungi encode proteins with high sequence similarity to the mammalian perilipin partner CGI-58. With the power of microbial genetics, it should be fairly quick to establish the molecular mechanisms of fungal PATs; insights from such analyses should then greatly advance the study of animal PATs.
7. Mammalian and non-mammalian PAT proteins, an ancient family for a fundamental need
The discovery of perilipin opened a window into the control of cellular lipid metabolism that many investigators in many fields have now climbed through. These studies have established the PAT family as coat proteins for lipid droplets that regulate access to the lipid ester core. A theme that runs throughout studies of the PATs is that they protect the stored lipid esters from uncontrolled hydrolysis by cytoplasmic lipases. Each of the 5 mammalian PAT proteins likely is specialized for specific functions. Perilipin, alone among the mammalian PATs, is known to change its role upon phosphorylation by PKA. In the basal state, perilipin inhibits hydrolysis of triacylglycerol in adipocytes. But when perilipin is phosphorylated on specific serines, it promotes lipolysis. In fact, ectopic expression of perilipin A in CHO cells is sufficient to make lipolysis in those cells responsive to PKA activation[54]. Even though neither LSD-1 nor LSD-2 in Drosophila are direct orthologs of perilipin, their functions in lipolysis and lipid droplet trafficking, respectively, are similarly modulated by phosphorylation.
The PAT family was originally identified through homology to a highly conserved 100 amino acid domain in the amino terminus of perilipin, the “PAT domain” (Fig. 1). Although all family members can bind to lipid droplets, this droplet targeting is not dependent on the PAT domain. Indeed, there does not appear to be a single domain or motif common to all family members that is responsible for targeting to the droplet surface. Despite its conservation from fungi to mammals, the function of the PAT domain remains unknown. In perilipin A, the PAT domain contains one of the phosphorylation sites thought to be necessary for activation of HSL, yet this phosphorylation site is not conserved in other PAT family members. Finally, the PAT domain is entirely lacking in S3-12; this protein earns its spot in the family based on extensive sequence similarities in other parts of the protein.
Over the past few years, the discoveries of protein-protein interactions between perilipin A and the lipases ATGL and HSL, as well as the ATGL activator CGI-58, have provided clues as to how perilipin might organize lipolysis, but the exact mechanisms remain for detailed elucidation. Now very recent work from Granneman and colleagues has demonstrated the importance of at least functional if not direct physical interactions between OXPAT, CGI-58, and ATGL. It is likely that additional novel protein complexes involving PAT proteins that influence cellular lipid metabolism remain for discovery. Though protein complexes of Drosophila PAT proteins and orthologs of CGI-58, ATGL, and HSL have not yet been reported, these proteins have all been localized to lipid droplets, so it is likely that such complexes play roles in insect lipid metabolism.
It must be remembered that perilipin expression is limited to adipocytes, steroidogenic cells, and perhaps to pathological states of lipid accumulation in vessel macrophages and the liver. Thus, the above observations about the control of lipid stores by perilipins do not apply to most cells in the organism. ADRP is the CPAT protein in these other cells, and its expression at the protein level largely reflects the mass of accumulated neutral lipid. While ADRP may act primarily by excluding lipases from lipid droplet association, as has been suggested for ATGL, it is unclear if there are regulated mechanisms to extract ADRP from the droplet surface, thereby targeting it for proteosomal degradation. An ARF1-dependent mechanism as described by the Fujimoto group is promising [144]. Indeed, two groups have used genome-wide RNAi screens in Drosophila cells to discover an important role for the ARF-1/COPI trafficking system in the regulation of lipolysis [164, 165]. In an elegant translation of this discovery back to mammalian cells, Beller and colleagues validated the role of COPI-mediated transport for lipolysis in a mouse liver cell line and found that this pathway modulates the PAT protein composition of lipid droplets[165]. These studies illustrate the great value of model organisms for the discovery of novel mechanisms in the control of intracellular lipid metabolism.
Intracellular lipid droplets have been compared to both aqueous-cored vesicles and to lipoproteins [24]. It has been hypothesized that some PAT proteins may be involved in the trafficking of neutral lipid from the site of lipid droplet biogenesis to the site of storage in the perilipin-coated droplet of adipocytes. Roles for PAT proteins in intracellular trafficking have indeed been uncovered. Phosphorylation of perilipin is critical for fragmentation and dispersion of lipid droplets during prolonged lipolytic stimulation. ADRP forms protein complexes with ARF1 and with SNARE proteins, and ADRP deficiency promotes lipid droplet aggregation. TIP47 was originally identified as being involved in the retrieval of the mannose-6-phosphate receptor from the endosomal compartment to the Golgi. Fatvg knockdown results in disrupted trafficking of unidentified vesicles. And the phosphorylation state of LSD-2 determines the directionality of lipid droplet transport in Drosophila embryos. Observations like these make it is likely that novel functions for the PAT proteins will involve the movement of lipid within cells, analogous to the role of apolipoproteins in the movement of lipid between cells and tissues.
Data from cells, mice, and humans, as reviewed above, do not give an unequivocal answer to the question of whether more or less ADRP is beneficial. Because ADRP expression appears to reflect the capacity to store neutral lipids, at least in cell-based gain-of-function studies, the answer to the question of whether less is more may depend on whether lipid storage within a specific tissue is beneficial. Within macrophage foam cells, it may be more advantageous to export cholesterol by reverse transport rather than store it as cholesteryl ester in lipid droplets. Conversely, in skeletal muscle it may be more beneficial to sequester fat in droplets rather than to produce lipotoxic fatty acid metabolites. In this last example, it will be interesting to discover whether differential packaging of lipid droplets in skeletal muscle by different PAT proteins (ADRP versus TIP47 versus S3-12 versus OXPAT) explains the paradoxical observation that the muscle of endurance athletes has increased intramyocellular TAG as does that of sedentary humans with type 2 diabetes.
The finding that single nucleotide polymorphisms in the perilipin gene are associated with obesity and diabetes phenotypes strongly supports the relevance of the PAT family proteins to human lipid and carbohydrate metabolism. It remains to be determined whether genetic variations in the other PAT proteins have similar associations. Why the associations are seen in females but not males remains for investigation.
Future efforts to understand the biology of PAT proteins will require integrated approaches. From the perilipin and ADRP knockout mice, we see that alteration of PAT protein expression leads to secondary effects on gene expression or metabolic function that may not be anticipated. Is the phenotype observed due to loss of a function of the targeted protein? This may or may not be the case. As we have seen, less of one PAT protein may lead to more of another PAT protein on lipid droplets. Thus, the observed phenotype alternatively could be due to decreased levels of the targeted PAT protein on lipid droplets or increased levels of another PAT protein on lipid droplets. Another possibility is that sequestration of the non-targeted PAT protein on lipid droplets may lead to loss of its function in its cytoplasmic compartment (for example, loss of TIP47 for endosomal trafficking). Finally, as has been shown for ATGL, the presence or absence of PAT proteins on lipid droplets affects lipid droplet localization of non-PAT proteins, as well. Thus, it will be important to integrate the mouse genetic models with proteomic studies of isolated lipid droplets, as well as with other unbiased approaches like gene expression microarrays and mass spectrometric analysis of lipids and other metabolites.
The high level of similarity between the regulation of lipolysis in mammals and in insects (Fig. 2) highlights the importance of studying the PAT proteins in multiple model systems. Similarities between PAT proteins, both mammalian and non-mammalian (Table 3) suggests that insights gained in insects, fungi, and slime molds can be translated back to mammalian systems, and vice versa. We anticipate that the problems associated with overnutrition in developed countries may be addressed by novel therapeutic strategies identified through study of PAT proteins in mammalian systems and non-mammalian systems. Undernutrition remains a problem in the underdeveloped world. It is possible that the greatest impact on human health will be made through study of lipid droplets as virulence factors in the pathogenic fungi that infect crop plants, especially if the insights developed for mammalian PAT proteins lead to discoveries that target lipid droplets in pathogenic fungi and thus improve crop yields.
Table 3.
Comparisons of the mammalian and non-mammalian PAT proteins1
| PAT Protein | Regulated by phosphorylation | Promotes lipid storage | Recruits lipases or co-lipases | Role in intracellular trafficking | EPAT versus CPAT | PAT domain |
|---|---|---|---|---|---|---|
| Perilipin | Yes (PKA) | Yes | Yes | Yes (droplets) | CPAT | Yes |
| ADRP | Unknown | Yes | Unknown | Yes (droplets) | CPAT | Yes |
| TIP47 | Unknown | Yes | Unknown | Yes (vesicles) | EPAT | Yes |
| S3-12 | Unknown | Unknown | Unknown | Unknown | EPAT | No |
| OXPAT | Unknown | Yes | Yes | Unknown | EPAT | Yes |
| Fatvg | Unknown | Unknown | Unknown | Yes (vesicles) | Unknown | Yes |
| LSD-1 | Correlation (PKA) | Unknown | Inferred | Unknown | Unknown | Yes |
| LSD-2 | Correlation | Yes | Unknown | Yes (droplets) | Unknown | Yes |
| MPL1 | Proposed | Yes | Unknown | No | Unknown | Yes |
This table is intended as a summary of the text. Please refer to the text for details and references.
Acknowledgments
Our studies of PAT proteins and lipid droplets are supported by NIH grants DK068046 to PEB and GM64687 and AG031531 to MAW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murphy DJ. The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog Lipid Res. 2001;40:325–438. doi: 10.1016/s0163-7827(01)00013-3. [DOI] [PubMed] [Google Scholar]
- 2.Bartz R, Zehmer JK, Zhu M, Chen Y, Serrero G, Zhao Y, Liu P. Dynamic activity of lipid droplets: protein phosphorylation and GTP-mediated protein translocation. J Proteome Res. 2007;6:3256–65. doi: 10.1021/pr070158j. [DOI] [PubMed] [Google Scholar]
- 3.Beller M, Riedel D, Jansch L, Dieterich G, Wehland J, Jackle H, Kuhnlein RP. Characterization of the Drosophila lipid droplet subproteome. Mol Cell Proteomics. 2006;5:1082–1094. doi: 10.1074/mcp.M600011-MCP200. [DOI] [PubMed] [Google Scholar]
- 4.Brasaemle DL, Dolios G, Shapiro L, Wang R. Proteomic Analysis of Proteins Associated with Lipid Droplets of Basal and Lipolytically Stimulated 3T3-L1 Adipocytes. J Biol Chem. 2004;279:46835–42. doi: 10.1074/jbc.M409340200. [DOI] [PubMed] [Google Scholar]
- 5.Liu P, Ying Y, Zhao Y, Mundy DI, Zhu M, Anderson RGW. Chinese Hamster Ovary K2 Cell Lipid Droplets Appear to Be Metabolic Organelles Involved in Membrane Traffic. J Biol Chem. 2004;279:3787–3792. doi: 10.1074/jbc.M311945200. [DOI] [PubMed] [Google Scholar]
- 6.Sato S, Fukasawa M, Yamakawa Y, Natsume T, Suzuki T, Shoji I, Aizaki H, Miyamura T, Nishijima M. Proteomic profiling of lipid droplet proteins in hepatoma cell lines expressing hepatitis C virus core protein. J Biochem (Tokyo) 2006;139:921–30. doi: 10.1093/jb/mvj104. [DOI] [PubMed] [Google Scholar]
- 7.Wan HC, Melo RC, Jin Z, Dvorak AM, Weller PF. Roles and origins of leukocyte lipid bodies: proteomic and ultrastructural studies. Faseb J. 2007;21:167–78. doi: 10.1096/fj.06-6711com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ducharme NA, Bickel PE. Minireview: lipid droplets in lipogenesis and lipolysis. Endocrinology. 2008;149:942–9. doi: 10.1210/en.2007-1713. [DOI] [PubMed] [Google Scholar]
- 9.Fujimoto T, Ohsaki Y, Cheng J, Suzuki M, Shinohara Y. Lipid droplets: a classic organelle with new outfits. Histochem Cell Biol. 2008;130:263–79. doi: 10.1007/s00418-008-0449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Listenberger LL, Brown DA. Lipid droplets. Curr Biol. 2008;18:R237–8. doi: 10.1016/j.cub.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Martin S, Parton RG. Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol. 2006;7:373–8. doi: 10.1038/nrm1912. [DOI] [PubMed] [Google Scholar]
- 12.Granneman JG, Moore HP. Location, location: protein trafficking and lipolysis in adipocytes. Trends Endocrinol Metab. 2008;19:3–9. doi: 10.1016/j.tem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Olofsson SO, Bostrom P, Andersson L, Rutberg M, Levin M, Perman J, Boren J. Triglyceride containing lipid droplets and lipid droplet-associated proteins. Curr Opin Lipidol. 2008;19:441–7. doi: 10.1097/MOL.0b013e32830dd09b. [DOI] [PubMed] [Google Scholar]
- 14.Thiele C, Spandl J. Cell biology of lipid droplets. Curr Opin Cell Biol. 2008;20:378–85. doi: 10.1016/j.ceb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Egan JJ, Greenberg AS, Chang MK, Londos C. Control of endogenous phosphorylation of the major cAMP-dependent protein kinase substrate in adipocytes by insulin and beta-adrenergic stimulation. J Biol Chem. 1990;265:18769–75. [PubMed] [Google Scholar]
- 16.Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem. 1991;266:11341–6. [PubMed] [Google Scholar]
- 17.Greenberg AS, Egan JJ, Wek SA, Moos MC, Jr, Londos C, Kimmel AR. Isolation of cDNAs for perilipins A and B: sequence and expression of lipid droplet-associated proteins of adipocytes. Proc Natl Acad Sci U S A. 1993;90:12035–9. doi: 10.1073/pnas.90.24.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miura S, Gan JW, Brzostowski J, Parisi MJ, Schultz CJ, Londos C, Oliver B, Kimmel AR. Functional conservation for lipid storage droplet association among perilipin-, ADRP-, and TIP 47-related proteins in mammals, drosophila, and dictylostelium. J Biol Chem. 2002;277:32253–32257. doi: 10.1074/jbc.M204410200. [DOI] [PubMed] [Google Scholar]
- 19.Scherer PE, Bickel PE, Kotler M, Lodish HF. Cloning of cell-specific secreted and surface proteins by subtractive antibody screening. Nat Biotechnol. 1998;16:581–6. doi: 10.1038/nbt0698-581. [DOI] [PubMed] [Google Scholar]
- 20.Wolins NE, Skinner JR, Schoenfish MJ, Tzekov A, Bensch KG, Bickel PE. Adipocyte Protein S3-12 Coats Nascent Lipid Droplets. J Biol Chem. 2003;278:37713–37721. doi: 10.1074/jbc.M304025200. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi T, Matsushita S, Motojima K, Hirose F, Osumi T. MLDP, a novel PAT family protein localized to lipid droplets and enriched in the heart, is regulated by peroxisome proliferator-activated receptor alpha. J Biol Chem. 2006;281:14232–40. doi: 10.1074/jbc.M601682200. [DOI] [PubMed] [Google Scholar]
- 22.Wolins NE, Quaynor BK, Skinner JR, Tzekov A, Croce MA, Gropler MC, Varma V, Yao-Borengasser A, Rasouli N, Kern PA, Finck BN, Bickel PE. OXPAT/PAT-1 is a PPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes. 2006;55:3418–28. doi: 10.2337/db06-0399. [DOI] [PubMed] [Google Scholar]
- 23.Dalen KT, Dahl T, Holter E, Arntsen B, Londos C, Sztalryd C, Nebb HI. LSDP5 is a PAT protein specifically expressed in fatty acid oxidizing tissues. Biochim Biophys Acta. 2007;1771:210–27. doi: 10.1016/j.bbalip.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Wolins NE, Brasaemle DL, Bickel PE. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 2006;580:5484–91. doi: 10.1016/j.febslet.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 25.Brasaemle DL. The perilipin family of structural lipid droplet proteins: Stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Londos C, Sztalryd C, Tansey JT, Kimmel AR. Role of PAT proteins in lipid metabolism. Biochimie. 2005;87:45–9. doi: 10.1016/j.biochi.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Chan AP, Kloc M, Etkin LD. fatvg encodes a new localized RNA that uses a 25-nucleotide element (FVLE1) to localize to the vegetal cortex of Xenopus oocytes. Development. 1999;126:4943–53. doi: 10.1242/dev.126.22.4943. [DOI] [PubMed] [Google Scholar]
- 28.Grönke S, Beller M, Fellert S, Ramakrishnan H, Jäckle H, Kühnlein RP. Control of Fat Storage by a Drosophila PAT Domain Protein. Curr Biol. 2003;13:603–6. doi: 10.1016/s0960-9822(03)00175-1. [DOI] [PubMed] [Google Scholar]
- 29.Wang C, St Leger RJ. The Metarhizium anisopliae Perilipin Homolog MPL1 Regulates Lipid Metabolism, Appressorial Turgor Pressure, and Virulence. J Biol Chem. 2007;282:21110–5. doi: 10.1074/jbc.M609592200. [DOI] [PubMed] [Google Scholar]
- 30.Lu X, Gruia-Gray J, Copeland NG, Gilbert DJ, Jenkins NA, Londos C, Kimmel AR. The murine perilipin gene: the lipid droplet-associated perilipins derive from tissue-specific, mRNA splice variants and define a gene family of ancient origin. Mamm Genome. 2001;12:741–9. doi: 10.1007/s00335-01-2055-5. [DOI] [PubMed] [Google Scholar]
- 31.Subramanian V, Garcia A, Sekowski A, Brasaemle DL. Hydrophobic sequences target and anchor perilipin A to lipid droplets. J Lipid Res. 2004;45:1983–91. doi: 10.1194/jlr.M400291-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Garcia A, Sekowski A, Subramanian V, Brasaemle DL. The central domain is required to target and anchor perilipin A to lipid droplets. J Biol Chem. 2003;278:625–35. doi: 10.1074/jbc.M206602200. [DOI] [PubMed] [Google Scholar]
- 33.Blanchette-Mackie EJ, Dwyer NK, Barber T, Coxey RA, Takeda T, Rondinone CM, Theodorakis JL, Greenberg AS, Londos C. Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. J Lipid Res. 1995;36:1211–1226. [PubMed] [Google Scholar]
- 34.Akter MH, Yamaguchi T, Hirose F, Osumi T. Perilipin, a critical regulator of fat storage and breakdown, is a target gene of estrogen receptor-related receptor alpha. Biochemical and Biophysical Research Communications. 2008;368:563–568. doi: 10.1016/j.bbrc.2008.01.102. [DOI] [PubMed] [Google Scholar]
- 35.Arimura N, Horiba T, Imagawa M, Shimizu M, Sato R. The peroxisome proliferator-activated receptor gamma regulates expression of the perilipin gene in adipocytes. J Biol Chem. 2004;279:10070–6. doi: 10.1074/jbc.M308522200. [DOI] [PubMed] [Google Scholar]
- 36.Dalen KT, Schoonjans K, Ulven SM, Weedon-Fekjaer MS, Bentzen TG, Koutnikova H, Auwerx J, Nebb HI. Adipose tissue expression of the lipid droplet-associating proteins S3-12 and perilipin is controlled by peroxisome proliferator-activated receptor-gamma. Diabetes. 2004;53:1243–52. doi: 10.2337/diabetes.53.5.1243. [DOI] [PubMed] [Google Scholar]
- 37.Nagai S, Shimizu C, Umetsu M, Taniguchi S, Endo M, Miyoshi H, Yoshioka N, Kubo M, Koike T. Identification of a functional peroxisome proliferator-activated receptor responsive element within the murine perilipin gene. Endocrinology. 2004;145:2346–2356. doi: 10.1210/en.2003-1180. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi Y, Ohoka N, Hayashi H, Sato R. TRB3 suppresses adipocyte differentiation by negatively regulating PPAR gamma transcriptional activity. Journal of Lipid Research. 2008;49:880–892. doi: 10.1194/jlr.M700545-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Li DC, Kang QH, Wang DM. Constitutive coactivator of peroxisome proliferator-activated receptor (PPAR gamma), a novel coactivator of PPAR gamma that promotes adipogenesis. Molecular Endocrinology. 2007;21:2320–2333. doi: 10.1210/me.2006-0520. [DOI] [PubMed] [Google Scholar]
- 40.Prusty D, Park BH, Davis KE, Farmer SR. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma (PPARgamma ) and C/EBPalpha gene expression during the differentiation of 3T3-L1 preadipocytes. J Biol Chem. 2002;277:46226–32. doi: 10.1074/jbc.M207776200. [DOI] [PubMed] [Google Scholar]
- 41.Zhang HH, Halbleib M, Ahmad F, Manganiello VC, Greenberg AS. Tumor necrosis factor-alpha stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular cAMP. Diabetes. 2002;51:2929–35. doi: 10.2337/diabetes.51.10.2929. [DOI] [PubMed] [Google Scholar]
- 42.Laurencikiene J, van Harmelen V, Arvidsson Nordstrom E, Dicker A, Blomqvist L, Naslund E, Langin D, Arner P, Ryden M. NF-kappaB is important for TNF-alpha-induced lipolysis in human adipocytes. J Lipid Res. 2007;48:1069–77. doi: 10.1194/jlr.M600471-JLR200. [DOI] [PubMed] [Google Scholar]
- 43.Souza SC, Palmer HJ, Kang YH, Yamamoto MT, Muliro KV, Paulson KE, Greenberg AS. TNF-alpha induction of lipolysis is mediated through activation of the extracellular signal related kinase pathway in 3T3-L1 adipocytes. J Cell Biochem. 2003;89:1077–86. doi: 10.1002/jcb.10565. [DOI] [PubMed] [Google Scholar]
- 44.Brasaemle DL, Barber T, Kimmel AR, Londos C. Post-translational regulation of perilipin expression - Stabilization by stored intracellular neutral lipids. Journal of Biological Chemistry. 1997;272:9378–9387. doi: 10.1074/jbc.272.14.9378. [DOI] [PubMed] [Google Scholar]
- 45.Xu G, Sztalryd C, Londos C. Degradation of perilipin is mediated through ubiquitination-proteasome pathway. Biochim Biophys Acta. 2006;1761:83–90. doi: 10.1016/j.bbalip.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Gross DN, Miyoshi H, Hosaka T, Zhang HH, Pino EC, Souza S, Obin M, Greenberg AS, Pilch PF. Dynamics of lipid droplet-associated proteins during hormonally stimulated lipolysis in engineered adipocytes: stabilization and lipid droplet binding of adipocyte differentiation-related protein/adipophilin. Mol Endocrinol. 2006;20:459–66. doi: 10.1210/me.2005-0323. [DOI] [PubMed] [Google Scholar]
- 47.Kovsan J, Ben-Romano R, Souza SC, Greenberg AS, Rudich A. Regulation of adipocyte lipolysis by degradation of the perilipin protein: nelfinavir enhances lysosome-mediated perilipin proteolysis. J Biol Chem. 2007;282:21704–11. doi: 10.1074/jbc.M702223200. [DOI] [PubMed] [Google Scholar]
- 48.Clifford GM, McCormick DKT, Londos C, Vernon RG, Yeaman SJ. Dephosphorylation of perilipin by protein phosphatases present in rat adipocytes. Febs Letters. 1998;435:125–129. doi: 10.1016/s0014-5793(98)01052-7. [DOI] [PubMed] [Google Scholar]
- 49.Schweiger M, Schoiswohl G, Lass A, Radner FP, Haemmerle G, Malli R, Graier W, Cornaciu I, Oberer M, Salvayre R, Fischer J, Zechner R, Zimmermann R. The C-terminal region of human adipose triglyceride lipase affects enzyme activity and lipid droplet binding. J Biol Chem. 2008;283:17211–20. doi: 10.1074/jbc.M710566200. [DOI] [PubMed] [Google Scholar]
- 50.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–6. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 51.Miyoshi H, Perfield JW, 2nd, Souza SC, Shen WJ, Zhang HH, Stancheva ZS, Kraemer FB, Obin MS, Greenberg AS. Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J Biol Chem. 2007;282:996–1002. doi: 10.1074/jbc.M605770200. [DOI] [PubMed] [Google Scholar]
- 52.Brasaemle DL, Rubin B, Harten IA, Gruia-Gray J, Kimmel AR, Londos C. Perilipin A increases triacylglycerol storage by decreasing the rate of triacylglycerol hydrolysis. J Biol Chem. 2000;275:38486–93. doi: 10.1074/jbc.M007322200. [DOI] [PubMed] [Google Scholar]
- 53.Souza SC, de Vargas LM, Yamamoto MT, Lien P, Franciosa MD, Moss LG, Greenberg AS. Overexpression of perilipin A and B blocks the ability of tumor necrosis factor alpha to increase lipolysis in 3T3-L1 adipocytes. J Biol Chem. 1998;273:24665–9. doi: 10.1074/jbc.273.38.24665. [DOI] [PubMed] [Google Scholar]
- 54.Tansey JT, Huml AM, Vogt R, Davis KE, Jones JM, Fraser KA, Brasaemle DL, Kimmel AR, Londos C. Functional studies on native and mutated forms of perilipins. A role in protein kinase A-mediated lipolysis of triacylglycerols. J Biol Chem. 2003;278:8401–6. doi: 10.1074/jbc.M211005200. [DOI] [PubMed] [Google Scholar]
- 55.Souza SC, Muliro KV, Liscum L, Lien P, Yamamoto MT, Schaffer JE, Dallal GE, Wang X, Kraemer FB, Obin M, Greenberg AS. Modulation of hormone-sensitive lipase and protein kinase A-mediated lipolysis by perilipin A in an adenoviral reconstituted system. J Biol Chem. 2002;277:8267–72. doi: 10.1074/jbc.M108329200. [DOI] [PubMed] [Google Scholar]
- 56.Martinez-Botas J, Anderson JB, Tessier D, Lapillonne A, Chang BH, Quast MJ, Gorenstein D, Chen KH, Chan L. Absence of perilipin results in leanness and reverses obesity in Lepr(db/db) mice. Nat Genet. 2000;26:474–9. doi: 10.1038/82630. [DOI] [PubMed] [Google Scholar]
- 57.Tansey JT, Sztalryd C, Gruia-Gray J, Roush DL, Zee JV, Gavrilova O, Reitman ML, Deng CX, Li C, Kimmel AR, Londos C. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci U S A. 2001;98:6494–9. doi: 10.1073/pnas.101042998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sztalryd C, Xu G, Dorward H, Tansey JT, Contreras JA, Kimmel AR, Londos C. Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J Cell Biol. 2003;161:1093–103. doi: 10.1083/jcb.200210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miyoshi H, Souza SC, Zhang HH, Strissel KJ, Christoffolete MA, Kovsan J, Rudich A, Kraemer FB, Bianco AC, Obin MS, Greenberg AS. Perilipin promotes hormone-sensitive lipase-mediated adipocyte lipolysis via phosphorylation-dependent and -independent mechanisms. J Biol Chem. 2006;281:15837–44. doi: 10.1074/jbc.M601097200. [DOI] [PubMed] [Google Scholar]
- 60.Marcinkiewicz A, Gauthier D, Garcia A, Brasaemle DL. The phosphorylation of serine 492 of perilipin a directs lipid droplet fragmentation and dispersion. J Biol Chem. 2006;281:11901–9. doi: 10.1074/jbc.M600171200. [DOI] [PubMed] [Google Scholar]
- 61.Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 2006;3:309–19. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 62.Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P, Tornqvist H, Zechner R, Zimmermann R. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem. 2006;281:40236–41. doi: 10.1074/jbc.M608048200. [DOI] [PubMed] [Google Scholar]
- 63.Yamaguchi T, Omatsu N, Morimoto E, Nakashima H, Ueno K, Tanaka T, Satouchi K, Hirose F, Osumi T. CGI-58 facilitates lipolysis on lipid droplets but is not involved in the vesiculation of lipid droplets caused by hormonal stimulation. J Lipid Res. 2007;48:1078–89. doi: 10.1194/jlr.M600493-JLR200. [DOI] [PubMed] [Google Scholar]
- 64.Subramanian V, Rothenberg A, Gomez C, Cohen AW, Garcia A, Bhattacharyya S, Shapiro L, Dolios G, Wang R, Lisanti MP, Brasaemle DL. Perilipin A mediates the reversible binding of CGI-58 to lipid droplets in 3T3-L1 adipocytes. J Biol Chem. 2004;279:42062–71. doi: 10.1074/jbc.M407462200. [DOI] [PubMed] [Google Scholar]
- 65.Yamaguchi T, Omatsu N, Matsushita S, Osumi T. CGI-58 interacts with perilipin and is localized to lipid droplets - Possible involvement of CGI-58 mislocalization in Chanarin-Dorfman syndrome. Journal of Biological Chemistry. 2004;279:30490–30497. doi: 10.1074/jbc.M403920200. [DOI] [PubMed] [Google Scholar]
- 66.Granneman JG, Moore HP, Granneman RL, Greenberg AS, Obin MS, Zhu Z. Analysis of lipolytic protein trafficking and interactions in adipocytes. J Biol Chem. 2007;282:5726–35. doi: 10.1074/jbc.M610580200. [DOI] [PubMed] [Google Scholar]
- 67.Ghosh AK, Ramakrishnan G, Chandramohan C, Rajasekharan R. CGI-58, the causative gene for Chanarin-Dorfman syndrome, mediates acylation of lysophosphatidic acid. J Biol Chem. 2008;283:24525–33. doi: 10.1074/jbc.M801783200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simpson CM, Itabe H, Reynolds CN, King WC, Glomset JA. Swiss 3T3 cells preferentially incorporate sn-2-arachidonoyl monoacylglycerol into sn-1-stearoyl-2-arachidonoyl phosphatidylinositol. J Biol Chem. 1991;266:15902–9. [PubMed] [Google Scholar]
- 69.Igal RA, Coleman RA. Acylglycerol recycling from triacylglycerol to phospholipid, not lipase activity, is defective in neutral lipid storage disease fibroblasts. J Biol Chem. 1996;271:16644–51. doi: 10.1074/jbc.271.28.16644. [DOI] [PubMed] [Google Scholar]
- 70.Igal RA, Coleman RA. Neutral lipid storage disease: a genetic disorder with abnormalities in the regulation of phospholipid metabolism. J Lipid Res. 1998;39:31–43. [PubMed] [Google Scholar]
- 71.Smith AJ, Thompson BR, Sanders MA, Bernlohr DA. Interaction of the adipocyte fatty acid-binding protein with the hormone-sensitive lipase - Regulation by fatty acids and phosphorylation. Journal of Biological Chemistry. 2007;282:32424–32432. doi: 10.1074/jbc.M703730200. [DOI] [PubMed] [Google Scholar]
- 72.Wolins NE, Quaynor BK, Skinner JR, Schoenfish MJ, Tzekov A, Bickel PE. S3-12, Adipophilin, and TIP47 package lipid in adipocytes. J Biol Chem. 2005;280:19146–55. doi: 10.1074/jbc.M500978200. [DOI] [PubMed] [Google Scholar]
- 73.Chung S, Brown JM, Sandberg MB, McIntosh M. Trans-10, cis-12 CLA increases adipocyte lipolysis and alters lipid droplet-associated proteins: role of mTOR and ERK signaling. J Lipid Res. 2005;46:885–95. doi: 10.1194/jlr.M400476-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mclean JA, Tobin G. Animal and Human Calorimetry. 1987. Cambridge University Press; Cambridge: 1988. [Google Scholar]
- 75.Moitra J, Mason MM, Olive M, Krylov D, Gavrilova O, Marcus-Samuels B, Feigenbaum L, Lee E, Aoyama T, Eckhaus M, Reitman ML, Vinson C. Life without white fat: a transgenic mouse. Genes Dev. 1998;12:3168–81. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shimomura I, Hammer RE, Richardson JA, Ikemoto S, Bashmakov Y, Goldstein JL, Brown MS. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev. 1998;12:3182–94. doi: 10.1101/gad.12.20.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ebihara K, Ogawa Y, Masuzaki H, Shintani M, Miyanaga F, Aizawa-Abe M, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Gavrilova O, Reitman ML, Nakao K. Transgenic overexpression of leptin rescues insulin resistance and diabetes in a mouse model of lipoatrophic diabetes. Diabetes. 2001;50:1440–8. doi: 10.2337/diabetes.50.6.1440. [DOI] [PubMed] [Google Scholar]
- 78.Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–6. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 79.Castro-Chavez F, Yechoor VK, Saha PK, Martinez-Botas J, Wooten EC, Sharma S, O’Connell P, Taegtmeyer H, Chan L. Coordinated upregulation of oxidative pathways and downregulation of lipid biosynthesis underlie obesity resistance in perilipin knockout mice: a microarray gene expression profile. Diabetes. 2003;52:2666–74. doi: 10.2337/diabetes.52.11.2666. [DOI] [PubMed] [Google Scholar]
- 80.Saha PK, Kojima H, Martinez-Botas J, Sunehag AL, Chan L. Metabolic adaptations in the absence of perilipin - Increased beta-oxidation and decreased hepatic glucose production associated with peripheral insulin resistance but normal glucose tolerance in perilipin-null mice. Journal of Biological Chemistry. 2004;279:35150–35158. doi: 10.1074/jbc.M405499200. [DOI] [PubMed] [Google Scholar]
- 81.Kern PA, Di Gregorio G, Lu T, Rassouli N, Ranganathan G. Perilipin expression in human adipose tissue is elevated with obesity. Journal of Clinical Endocrinology and Metabolism. 2004;89:1352–1358. doi: 10.1210/jc.2003-031388. [DOI] [PubMed] [Google Scholar]
- 82.Wang YX, Sullivan S, Trujillo M, Lee MJ, Schneider SH, Brolin RE, Kang YH, Werber Y, Greenberg AS, Fried SK. Perilipin expression in human adipose tissues: Effects of severe obesity, gender, and depot. Obesity Research. 2003;11:930–936. doi: 10.1038/oby.2003.128. [DOI] [PubMed] [Google Scholar]
- 83.Mottagui-Tabar S, Ryden M, Lofgren P, Faulds G, Hoffstedt J, Brookes AJ, Andersson I, Arner P. Evidence for an important role of perilipin in the regulation of human adipocyte lipolysis. Diabetologia. 2003;46:789–797. doi: 10.1007/s00125-003-1112-x. [DOI] [PubMed] [Google Scholar]
- 84.Ordovas JM. Gender, a significant factor in the cross talk between genes, environment, and health. Gender Medicine. 2007;4:S111–S122. doi: 10.1016/s1550-8579(07)80052-0. [DOI] [PubMed] [Google Scholar]
- 85.Tai ES, Ordovas JM. The role of perilipin in human obesity and insulin resistance. Current Opinion in Lipidology. 2007;18:152–156. doi: 10.1097/MOL.0b013e328086aeab. [DOI] [PubMed] [Google Scholar]
- 86.Kim HJ, Jung TW, Kang ES, Kim DJ, Ahn CW, Lee KW, Lee HC, Cha BS. Depot-specific regulation of perilipin by rosiglitazone in a diabetic animal model. Metabolism. 2007;56:676–85. doi: 10.1016/j.metabol.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 87.Rosenbaum SE, Greenberg AS. The short- and long-term effects of tumor necrosis factor-alpha and BRL 49653 on peroxisome proliferator-activated receptor (PPAR)gamma2 gene expression and other adipocyte genes. Mol Endocrinol. 1998;12:1150–60. doi: 10.1210/mend.12.8.0151. [DOI] [PubMed] [Google Scholar]
- 88.Souza SC, Yamamoto MT, Franciosa MD, Lien P, Greenberg AS. BRL 49653 blocks the lipolytic actions of tumor necrosis factor-alpha: a potential new insulin-sensitizing mechanism for thiazolidinediones. Diabetes. 1998;47:691–5. doi: 10.2337/diabetes.47.4.691. [DOI] [PubMed] [Google Scholar]
- 89.Adler-Wailes DC, Liu HG, Ahmad F, Feng NP, Londos C, Manganiello V, Yanovski JA. Effects of the human immunodeficiency virus-protease inhibitor, ritonavir, on basal and catecholamine-stimulated lipolysis. Journal of Clinical Endocrinology and Metabolism. 2005;90:3251–3261. doi: 10.1210/jc.2004-2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kennedy A, Chung S, LaPoint K, Fabiyi O, McIntosh MK. Trans-10, Cis-12 conjugated linoleic acid antagonizes ligand-dependent PPAR gamma activity in primary cultures of human adipocytes. Journal of Nutrition. 2008;138:455–461. doi: 10.1093/jn/138.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Park HJ, Della-Fera MA, Hausman DB, Rayalam S, Ambati S, Baile CA. Genistein inhibits differentiation of primary human adipocytes. J Nutr Biochem. 2008 doi: 10.1016/j.jnutbio.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 92.Persson J, Degerman E, Nilsson J, Lindholin MW. Perilipin and adipophilin expression in lipid loaded macrophages. Biochemical and Biophysical Research Communications. 2007;363:1020–1026. doi: 10.1016/j.bbrc.2007.09.074. [DOI] [PubMed] [Google Scholar]
- 93.Faber BC, Cleutjens KB, Niessen RL, Aarts PL, Boon W, Greenberg AS, Kitslaar PJ, Tordoir JH, Daemen MJ. Identification of genes potentially involved in rupture of human atherosclerotic plaques. Circ Res. 2001;89:547–54. doi: 10.1161/hh1801.096340. [DOI] [PubMed] [Google Scholar]
- 94.Forcheron F, Legedz L, Chinetti G, Feugier P, Letexier D, Bricca G, Beylot M. Genes of cholesterol metabolism in human atheroma: overexpression of perilipin and genes promoting cholesterol storage and repression of ABCA1 expression. Arterioscler Thromb Vasc Biol. 2005;25:1711–7. doi: 10.1161/01.ATV.0000174123.19103.52. [DOI] [PubMed] [Google Scholar]
- 95.Larigauderie G, Bouhlel MA, Furman C, Jaye M, Fruchart JC, Rouis M. Perilipin, a potential substitute for adipophilin in triglyceride storage in human macrophages. Atherosclerosis. 2006;189:142–8. doi: 10.1016/j.atherosclerosis.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 96.Wang XK, Reape TJ, Li X, Rayner K, Webb CL, Burnand KG, Lysko PG. Induced expression of adipophilin mRNA in human macrophages stimulated with oxidized low-density lipoprotein and in atherosclerotic lesions. Febs Letters. 1999;462:145–150. doi: 10.1016/s0014-5793(99)01521-5. [DOI] [PubMed] [Google Scholar]
- 97.Chen JS, Greenberg AS, Tseng YZ, Wang SM. Possible involvement of protein kinase C in the induction of adipose differentiation-related protein by sterol ester in RAW 264.7 macrophages. Journal of Cellular Biochemistry. 2001;83:187–199. doi: 10.1002/jcb.1225. [DOI] [PubMed] [Google Scholar]
- 98.Larigauderie G, Furman C, Jaye M, Lasselin C, Copin C, Fruchart JC, Castro G, Rouis M. Adipophilin enhances lipid accumulation and prevents lipid efflux from THP-1 macrophages: potential role in atherogenesis. Arterioscler Thromb Vasc Biol. 2004;24:504–10. doi: 10.1161/01.ATV.0000115638.27381.97. [DOI] [PubMed] [Google Scholar]
- 99.Robenek H, Lorkowski S, Schnoor M, Troyer D. Spatial integration of TIP47 and adipophilin in macrophage lipid bodies. J Biol Chem. 2005;280:5789–94. doi: 10.1074/jbc.M407194200. [DOI] [PubMed] [Google Scholar]
- 100.Shteyer E, Liao YJ, Muglia LJ, Hruz PW, Rudnick DA. Disruption of hepatic adipogenesis is associated with impaired liver regeneration in mice. Hepatology. 2004;40:1322–1332. doi: 10.1002/hep.20462. [DOI] [PubMed] [Google Scholar]
- 101.Turmelle YP, Shikapwashya O, Tu S, Hruz PW, Yan QY, Rudnick DA. Rosiglitazone inhibits mouse liver regeneration. Faseb Journal. 2006;20:2609–2611. doi: 10.1096/fj.06-6511fje. [DOI] [PubMed] [Google Scholar]
- 102.Straub BK, Stoeffel P, Heid H, Zimbelmann R, Schirmacher P. Differential pattern of lipid droplet-associated proteins and de novo perilipin expression in hepatocyte steatogenesis. Hepatology. 2008;47:1936–1946. doi: 10.1002/hep.22268. [DOI] [PubMed] [Google Scholar]
- 103.Pinnick KE, Collins SC, Londos C, Gauguier D, Clark A, Fielding BA. Pancreatic ectopic fat is characterized by adipocyte infiltration and altered lipid composition. Obesity. 2008;16:522–530. doi: 10.1038/oby.2007.110. [DOI] [PubMed] [Google Scholar]
- 104.Muthusamy K, Halbert G, Roberts F. Immunohistochemical staining for adipophilin, perilipin and TIP47. Journal of Clinical Pathology. 2006;59:1166–1170. doi: 10.1136/jcp.2005.033381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shinozaki A, Nagao T, Endo H, Kato N, Hirokawa M, Mizobuchi K, Komatsu M, Igarashi T, Yokoyama M, Masuda S, Sano K, Izumi M, Fukayama M, Mukai K. Sebaceous epithelial-myoepithelial carcinoma of the salivary gland: Clinicopathologic and immunohistochemical analysis of 6 cases of a new histologic variant. American Journal of Surgical Pathology. 2008;32A:913–923. doi: 10.1097/PAS.0b013e318160852a. [DOI] [PubMed] [Google Scholar]
- 106.Robenek H, Robenek MJ, Buers I, Lorkowski S, Hofnagel O, Troyer D, Severs NJ. Lipid droplets gain PAT family proteins by interaction with specialized plasma membrane domains. J Biol Chem. 2005;280:26330–8. doi: 10.1074/jbc.M413312200. [DOI] [PubMed] [Google Scholar]
- 107.Aboulaich N, Vener AV, Stralfors P. Hormonal control of reversible translocation of perilipin B to the plasma membrane in primary human adipocytes. Journal of Biological Chemistry. 2006;281:11446–11449. doi: 10.1074/jbc.C500461200. [DOI] [PubMed] [Google Scholar]
- 108.Robenek H, Robenek MJ, Troyer D. PAT family proteins pervade lipid droplet cores. J Lipid Res. 2005;46:1331–8. doi: 10.1194/jlr.M400323-JLR200. [DOI] [PubMed] [Google Scholar]
- 109.Moore HP, Silver RB, Mottillo EP, Bernlohr DA, Granneman JG. Perilipin targets a novel pool of lipid droplets for lipolytic attack by hormone-sensitive lipase. J Biol Chem. 2005;280:43109–20. doi: 10.1074/jbc.M506336200. [DOI] [PubMed] [Google Scholar]
- 110.DiDonato D, Brasaemle DL. Fixation methods for the study of lipid droplets by immunofluorescence microscopy. Journal of Histochemistry & Cytochemistry. 2003;51:773–780. doi: 10.1177/002215540305100608. [DOI] [PubMed] [Google Scholar]
- 111.Listenberger LL, Brown DA. Fluorescent detection of lipid droplets and associated proteins. Curr Protoc Cell Biol. 2007;Chapter 24(Unit 24):2. doi: 10.1002/0471143030.cb2402s35. [DOI] [PubMed] [Google Scholar]
- 112.Jiang HP, Harris SE, Serrero G. Molecular cloning of a differentiation-related mRNA in the adipogenic cell line 1246. Cell Growth Differ. 1992;3:21–30. [PubMed] [Google Scholar]
- 113.Jiang HP, Serrero G. Isolation and characterization of a full-length cDNA coding for an adipose differentiation-related protein. Proc Natl Acad Sci U S A. 1992;89:7856–60. doi: 10.1073/pnas.89.17.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, Londos C. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res. 1997;38:2249–63. [PubMed] [Google Scholar]
- 115.Xu G, Sztalryd C, Lu X, Tansey JT, Gan J, Dorward H, Kimmel AR, Londos C. Post-translational regulation of adipose differentiation-related protein by the ubiquitin/proteasome pathway. J Biol Chem. 2005;280:42841–7. doi: 10.1074/jbc.M506569200. [DOI] [PubMed] [Google Scholar]
- 116.Heid HW, Moll R, Schwetlick I, Rackwitz HR, Keenan TW. Adipophilin is a specific marker of lipid accumulation in diverse cell types and diseases. Cell Tissue Res. 1998;294:309–21. doi: 10.1007/s004410051181. [DOI] [PubMed] [Google Scholar]
- 117.Yamaguchi T, Omatsu N, Omukae A, Osumi T. Analysis of interaction partners for perilipin and ADRP on lipid droplets. Mol Cell Biochem. 2006;284:167–73. doi: 10.1007/s11010-005-9045-y. [DOI] [PubMed] [Google Scholar]
- 118.Imamura M, Inoguchi T, Ikuyama S, Taniguchi S, Kobayashi K, Nakashima N, Nawata H. ADRP stimulates lipid accumulation and lipid droplet formation in murine fibroblasts. Am J Physiol Endocrinol Metab. 2002;283:E775–83. doi: 10.1152/ajpendo.00040.2002. [DOI] [PubMed] [Google Scholar]
- 119.Listenberger LL, Ostermeyer-Fay AG, Goldberg EB, Brown WJ, Brown DA. Adipocyte differentiation-related protein reduces lipid droplet association of adipose triglyceride lipase and slows triacylglycerol turnover. J Lipid Res. 2007;48:2751–2761. doi: 10.1194/jlr.M700359-JLR200. [DOI] [PubMed] [Google Scholar]
- 120.Orlicky DJ, Degala G, Greenwood C, Bales ES, Russell TD, McManaman JL. Multiple functions encoded by the N-terminal PAT domain of adipophilin. J Cell Sci. 2008;121:2921–9. doi: 10.1242/jcs.026153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chang BH, Li L, Paul A, Taniguchi S, Nannegari V, Heird WC, Chan L. Protection against fatty liver but normal adipogenesis in mice lacking adipose differentiation-related protein. Mol Cell Biol. 2006;26:1063–76. doi: 10.1128/MCB.26.3.1063-1076.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Russell TD, Palmer CA, Orlicky DJ, Bales ES, Chang BH, Chan L, McManaman JL. Mammary glands of adipophilin-null mice produce an N-terminally truncated form of adipophilin that mediates milk lipid formation and secretion. J Lipid Res. 2007:206–216. doi: 10.1194/jlr.M700396-JLR200. [DOI] [PubMed] [Google Scholar]
- 123.Imai Y, Varela GM, Jackson MB, Graham MJ, Crooke RM, Ahima RS. Reduction of hepatosteatosis and lipid levels by an adipose differentiation-related protein antisense oligonucleotide. Gastroenterology. 2007;132:1947–54. doi: 10.1053/j.gastro.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 124.Phillips SA, Choe CC, Ciaraldi TP, Greenberg AS, Kong AP, Baxi SC, Christiansen L, Mudaliar SR, Henry RR. Adipocyte differentiation-related protein in human skeletal muscle: relationship to insulin sensitivity. Obes Res. 2005;13:1321–9. doi: 10.1038/oby.2005.160. [DOI] [PubMed] [Google Scholar]
- 125.Magnusson B, Asp L, Bostrom P, Ruiz M, Stillemark-Billton P, Linden D, Boren J, Olofsson SO. Adipocyte differentiation-related protein promotes fatty acid storage in cytosolic triglycerides and inhibits secretion of very low-density lipoproteins. Arterioscler Thromb Vasc Biol. 2006;26:1566–71. doi: 10.1161/01.ATV.0000223345.11820.da. [DOI] [PubMed] [Google Scholar]
- 126.Sztalryd C, Bell M, Lu X, Mertz P, Hickenbottom S, Chang BH, Chan L, Kimmel AR, Londos C. Functional compensation for adipose differentiation-related protein (ADFP) by Tip47 in an ADFP null embryonic cell line. J Biol Chem. 2006;281:34341–8. doi: 10.1074/jbc.M602497200. [DOI] [PubMed] [Google Scholar]
- 127.Paul A, Chang BH, Li L, Yechoor VK, Chan L. Deficiency of adipose differentiation-related protein impairs foam cell formation and protects against atherosclerosis. Circ Res. 2008;102:1492–501. doi: 10.1161/CIRCRESAHA.107.168070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shimizu M, Takeshita A, Tsukamoto T, Gonzalez FJ, Osumi T. Tissue-selective, bidirectional regulation of PEX11 alpha and perilipin genes through a common peroxisome proliferator response element. Mol Cell Biol. 2004;24:1313–23. doi: 10.1128/MCB.24.3.1313-1323.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Edvardsson U, Ljungberg A, Linden D, William-Olsson L, Peilot-Sjogren H, Ahnmark A, Oscarsson J. PPARalpha activation increases triglyceride mass and adipose differentiation-related protein in hepatocytes. J Lipid Res. 2006;47:329–40. doi: 10.1194/jlr.M500203-JLR200. [DOI] [PubMed] [Google Scholar]
- 130.Schmuth M, Haqq CM, Cairns WJ, Holder JC, Dorsam S, Chang S, Lau P, Fowler AJ, Chuang G, Moser AH, Brown BE, Mao-Qiang M, Uchida Y, Schoonjans K, Auwerx J, Chambon P, Willson TM, Elias PM, Feingold KR. Peroxisome proliferator-activated receptor (PPAR)-beta/delta stimulates differentiation and lipid accumulation in keratinocytes. J Invest Dermatol. 2004;122:971–83. doi: 10.1111/j.0022-202X.2004.22412.x. [DOI] [PubMed] [Google Scholar]
- 131.Chawla A, Lee CH, Barak Y, He W, Rosenfeld J, Liao D, Han J, Kang H, Evans RM. PPARdelta is a very low-density lipoprotein sensor in macrophages. Proc Natl Acad Sci U S A. 2003;100:1268–73. doi: 10.1073/pnas.0337331100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Targett-Adams P, McElwee MJ, Ehrenborg E, Gustafsson MC, Palmer CN, McLauchlan J. A PPAR response element regulates transcription of the gene for human adipose differentiation-related protein. Biochim Biophys Acta. 2005;1728:95–104. doi: 10.1016/j.bbaexp.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 133.Gao J, Ye H, Serrero G. Stimulation of adipose differentiation related protein (ADRP) expression in adipocyte precursors by long-chain fatty acids. J Cell Physiol. 2000;182:297–302. doi: 10.1002/(SICI)1097-4652(200002)182:2<297::AID-JCP19>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 134.Bildirici I, Roh CR, Schaiff WT, Lewkowski BM, Nelson DM, Sadovsky Y. The lipid droplet-associated protein adipophilin is expressed in human trophoblasts and is regulated by peroxisomal proliferator-activated receptor-gamma/retinoid X receptor. J Clin Endocrinol Metab. 2003;88:6056–62. doi: 10.1210/jc.2003-030628. [DOI] [PubMed] [Google Scholar]
- 135.Schadinger SE, Bucher NL, Schreiber BM, Farmer SR. PPARgamma2 regulates lipogenesis and lipid accumulation in steatotic hepatocytes. Am J Physiol Endocrinol Metab. 2005;288:E1195–205. doi: 10.1152/ajpendo.00513.2004. [DOI] [PubMed] [Google Scholar]
- 136.Vosper H, Patel L, Graham TL, Khoudoli GA, Hill A, Macphee CH, Pinto I, Smith SA, Suckling KE, Wolf CR, Palmer CN. The peroxisome proliferator-activated receptor delta promotes lipid accumulation in human macrophages. J Biol Chem. 2001;276:44258–65. doi: 10.1074/jbc.M108482200. [DOI] [PubMed] [Google Scholar]
- 137.Buechler C, Ritter M, Duong CQ, Orso E, Kapinsky M, Schmitz G. Adipophilin is a sensitive marker for lipid loading in human blood monocytes. Biochim Biophys Acta. 2001;1532:97–104. doi: 10.1016/s1388-1981(01)00121-4. [DOI] [PubMed] [Google Scholar]
- 138.Ye H, Serrero G. Stimulation of adipose differentiation related protein (ADRP) expression by ibuprofen and indomethacin in adipocyte precursors and in adipocytes. Biochem J. 1998;330( Pt 2):803–9. doi: 10.1042/bj3300803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gao J, Serrero G. Adipose differentiation related protein (ADRP) expressed in transfected COS-7 cells selectively stimulates long chain fatty acid uptake. J Biol Chem. 1999;274:16825–30. doi: 10.1074/jbc.274.24.16825. [DOI] [PubMed] [Google Scholar]
- 140.Heid HW, Schnolzer M, Keenan TW. Adipocyte differentiation-related protein is secreted into milk as a constituent of milk lipid globule membrane. Biochem J. 1996;320( Pt 3):1025–30. doi: 10.1042/bj3201025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McManaman JL, Zabaronick W, Schaack J, Orlicky DJ. Lipid droplet targeting domains of adipophilin. J Lipid Res. 2003;44:668–73. doi: 10.1194/jlr.C200021-JLR200. [DOI] [PubMed] [Google Scholar]
- 142.Nakamura N, Fujimoto T. Adipose differentiation-related protein has two independent domains for targeting to lipid droplets. Biochem Biophys Res Commun. 2003;306:333–8. doi: 10.1016/s0006-291x(03)00979-3. [DOI] [PubMed] [Google Scholar]
- 143.Targett-Adams P, Chambers D, Gledhill S, Hope RG, Coy JF, Girod A, McLauchlan J. Live cell analysis and targeting of the lipid droplet-binding adipocyte differentiation-related protein. J Biol Chem. 2003;278:15998–6007. doi: 10.1074/jbc.M211289200. [DOI] [PubMed] [Google Scholar]
- 144.Nakamura N, Akashi T, Taneda T, Kogo H, Kikuchi A, Fujimoto T. ADRP is dissociated from lipid droplets by ARF1-dependent mechanism. Biochem Biophys Res Commun. 2004;322:957–65. doi: 10.1016/j.bbrc.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 145.Bostrom P, Andersson L, Rutberg M, Perman J, Lidberg U, Johansson BR, Fernandez-Rodriguez J, Ericson J, Nilsson T, Boren J, Olofsson SO. SNARE proteins mediate fusion between cytosolic lipid droplets and are implicated in insulin sensitivity. Nat Cell Biol. 2007;9:1286–93. doi: 10.1038/ncb1648. [DOI] [PubMed] [Google Scholar]
- 146.Andersson L, Boström P, Ericson J, Rutberg M, Magnusson B, Marchesan D, Ruiz M, Asp L, Huang P, Frohman MA, Boren J, Olofsson SO. PLD1 and ERK2 regulate cytosolic lipid droplet formation. J Cell Sci. 2006;119:2246–57. doi: 10.1242/jcs.02941. [DOI] [PubMed] [Google Scholar]
- 147.Wolins NE, Rubin B, Brasaemle DL. TIP47 associates with lipid droplets. J Biol Chem. 2001;276:5101–8. doi: 10.1074/jbc.M006775200. [DOI] [PubMed] [Google Scholar]
- 148.Barbero P, Buell E, Zulley S, Pfeffer SR. TIP47 is not a component of lipid droplets. J Biol Chem. 2001;276:24348–51. doi: 10.1074/jbc.M102468200. [DOI] [PubMed] [Google Scholar]
- 149.Diaz E, Pfeffer SR. TIP47: a cargo selection device for mannose 6-phosphate receptor trafficking. Cell. 1998;93:433–43. doi: 10.1016/s0092-8674(00)81171-x. [DOI] [PubMed] [Google Scholar]
- 150.Lopez-Verges S, Camus G, Blot G, Beauvoir R, Benarous R, Berlioz-Torrent C. Tail-interacting protein TIP47 is a connector between Gag and Env and is required for Env incorporation into HIV-1 virions. Proc Natl Acad Sci U S A. 2006;103:14947–52. doi: 10.1073/pnas.0602941103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Saito K, Williams S, Bulankina A, Honing S, Mustelin T. Association of protein-tyrosine phosphatase MEG2 via its Sec14p homology domain with vesicle-trafficking proteins. J Biol Chem. 2007;282:15170–8. doi: 10.1074/jbc.M608682200. [DOI] [PubMed] [Google Scholar]
- 152.Gao JG, Simon M. Molecular screening for GS2 lipase regulators: inhibition of keratinocyte retinylester hydrolysis by TIP47. J Invest Dermatol. 2006;126:2087–95. doi: 10.1038/sj.jid.5700327. [DOI] [PubMed] [Google Scholar]
- 153.Tsuiki E, Fujita A, Ohsaki Y, Cheng J, Irie T, Yoshikawa K, Senoo H, Mishima K, Kitaoka T, Fujimoto T. All-trans-retinol generated by rhodopsin photobleaching induces rapid recruitment of TIP47 to lipid droplets in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2007;48:2858–67. doi: 10.1167/iovs.06-0768. [DOI] [PubMed] [Google Scholar]
- 154.Bell M, Wang H, Chen H, McLenithan JC, Gong DW, Yang RZ, Yu D, Fried SK, Quon MJ, Londos C, Sztalryd C. Consequences of lipid droplet coat protein downregulation in liver cells: abnormal lipid droplet metabolism and induction of insulin resistance. Diabetes. 2008;57:2037–45. doi: 10.2337/db07-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ohsaki Y, Maeda T, Maeda M, Tauchi-Sato K, Fujimoto T. Recruitment of TIP47 to lipid droplets is controlled by the putative hydrophobic cleft. Biochem Biophys Res Commun. 2006;347:279–87. doi: 10.1016/j.bbrc.2006.06.074. [DOI] [PubMed] [Google Scholar]
- 156.Hickenbottom SJ, Kimmel AR, Londos C, Hurley JH. Structure of a lipid droplet protein; the PAT family member TIP47. Structure. 2004;12:1199–207. doi: 10.1016/j.str.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 157.Bussell R, Jr, Eliezer D. A structural and functional role for 11-mer repeats in alpha-synuclein and other exchangeable lipid binding proteins. J Mol Biol. 2003;329:763–78. doi: 10.1016/s0022-2836(03)00520-5. [DOI] [PubMed] [Google Scholar]
- 158.Granneman JG, Moore HP, Mottillo EP, Zhu Z. Functional Interactions between Mldp (LSDP5) and Abhd5 in the Control of Intracellular Lipid Accumulation. J Biol Chem. 2009;284:3049–57. doi: 10.1074/jbc.M808251200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Leopold P, Perrimon N. Drosophila and the genetics of the internal milieu. Nature. 2007;450:186–8. doi: 10.1038/nature06286. [DOI] [PubMed] [Google Scholar]
- 160.Schlegel A, Stainier DY. Lessons from “lower” organisms: what worms, flies, and zebrafish can teach us about human energy metabolism. PLoS Genet. 2007;3:e199. doi: 10.1371/journal.pgen.0030199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gäde G, Auerswald L. Mode of action of neuropeptides from the adipokinetic hormone family. Gen Comp Endocrinol. 2003;132:10–20. doi: 10.1016/s0016-6480(03)00159-x. [DOI] [PubMed] [Google Scholar]
- 162.Gutierrez E, Wiggins D, Fielding B, Gould AP. Specialized hepatocyte-like cells regulate Drosophila lipid metabolism. Nature. 2007;445:275–80. doi: 10.1038/nature05382. [DOI] [PubMed] [Google Scholar]
- 163.Grönke S, Müller G, Hirsch J, Fellert S, Andreou A, Haase T, Jäckle H, Kühnlein RP. Dual lipolytic control of body fat storage and mobilization in Drosophila. PLoS Biol. 2007;5:e137. doi: 10.1371/journal.pbio.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Guo Y, Rao CWTM, Stuurman N, Goshima G, Terayama K, Wong JS, Vale RD, Walter P, Farese RV. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature. 2008;453:657–661. doi: 10.1038/nature06928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Beller M, Sztalryd C, Southall N, Bell M, Jackle H, Auld DS, Oliver B. COPI complex is a regulator of lipid homeostasis. PLoS Biol. 2008;6:e292. doi: 10.1371/journal.pbio.0060292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cermelli S, Guo Y, Gross SP, Welte MA. The lipid-droplet proteome reveals that droplets are a protein-storage depot. Curr Biol. 2006;16:1783–95. doi: 10.1016/j.cub.2006.07.062. [DOI] [PubMed] [Google Scholar]
- 167.Wu CC, Howell KE, Neville MC, Yates JR, 3rd, McManaman JL. Proteomics reveal a link between the endoplasmic reticulum and lipid secretory mechanisms in mammary epithelial cells. Electrophoresis. 2000;21:3470–82. doi: 10.1002/1522-2683(20001001)21:16<3470::AID-ELPS3470>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 168.Fujimoto Y, Itabe H, Sakai J, Makita M, Noda J, Mori M, Higashi Y, Kojima S, Takano T. Identification of major proteins in the lipid droplet-enriched fraction isolated from the human hepatocyte cell line HuH7. Biochim Biophys Acta. 2004;1644:47–59. doi: 10.1016/j.bbamcr.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 169.Umlauf E, Csaszar E, Moertelmaier M, Schuetz GJ, Parton RG, Prohaska R. Association of stomatin with lipid bodies. J Biol Chem. 2004;279:23699–709. doi: 10.1074/jbc.M310546200. [DOI] [PubMed] [Google Scholar]
- 170.Teixeira L, Rabouille C, Rorth P, Ephrussi A, Vanzo NF. Drosophila Perilipin/ADRP homologue Lsd2 regulates lipid metabolism. Mech Dev. 2003;120:1071–81. doi: 10.1016/s0925-4773(03)00158-8. [DOI] [PubMed] [Google Scholar]
- 171.Welte MA, Cermelli S, Griner J, Viera A, Guo Y, Kim DH, Gindhart JG, Gross SP. Regulation of Lipid-Droplet Transport by the Perilipin Homolog LSD2. Current Biology. 2005;15:1266–1275. doi: 10.1016/j.cub.2005.06.062. [DOI] [PubMed] [Google Scholar]
- 172.Fauny JD, Silber J, Zider A. Drosophila Lipid Storage Droplet 2 gene (Lsd-2) is expressed and controls lipid storage in wing imaginal discs. Dev Dyn. 2005;232:725–32. doi: 10.1002/dvdy.20277. [DOI] [PubMed] [Google Scholar]
- 173.Patel RT, Soulages JL, Hariharasundaram B, Arrese EL. Activation of the lipid droplet controls the rate of lipolysis of triglycerides in the insect fat body. J Biol Chem. 2005;280:22624–31. doi: 10.1074/jbc.M413128200. [DOI] [PubMed] [Google Scholar]
- 174.Arrese EL, Mirza S, Rivera L, Howard AD, Chetty PS, Soulages JL. Expression of lipid storage droplet protein-1 may define the role of AKH as a lipid mobilizing hormone in Manduca sexta. Insect Biochem Mol Biol. 2008;38:993–1000. doi: 10.1016/j.ibmb.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Arrese EL, Rivera L, Hamada M, Mirza S, Hartson SD, Weintraub S, Soulages JL. Function and structure of lipid storage droplet protein 1 studied in lipoprotein complexes. Arch Biochem Biophys. 2008;473:42–7. doi: 10.1016/j.abb.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Vereshchagina N, Wilson C. Cytoplasmic activated protein kinase Akt regulates lipid-droplet accumulation in Drosophila nurse cells. Development. 2006;133:4731–5. doi: 10.1242/dev.02659. [DOI] [PubMed] [Google Scholar]
- 177.Arrese EL, Rivera L, Hamada M, Soulages JL. Purification and characterization of recombinant lipid storage protein-2 from Drosophila melanogaster. Protein Pept Lett. 2008;15:1027–32. doi: 10.2174/092986608785849191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Grönke S, Mildner A, Fellert S, Tennagels N, Petry S, Müller G, Jäckle H, Kühnlein RP. Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell Metab. 2005;1:323–30. doi: 10.1016/j.cmet.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 179.Arrese EL, Patel RT, Soulages JL. The main triglyceride-lipase from the insect fat body is an active phospholipase A(1): identification and characterization. J Lipid Res. 2006;47:2656–67. doi: 10.1194/jlr.M600161-JLR200. [DOI] [PubMed] [Google Scholar]
- 180.Arbeitman MN, Furlong EE, Imam F, Johnson E, Null BH, Baker BS, Krasnow MA, Scott MP, Davis RW, White KP. Gene expression during the life cycle of Drosophila melanogaster. Science. 2002;297:2270–5. doi: 10.1126/science.1072152. [DOI] [PubMed] [Google Scholar]
- 181.King RC. Drosophila melanogaster. Academic Press, Inc; New York, NY: 1970. Ovarian development. [Google Scholar]
- 182.Welte MA, Gross SP, Postner M, Block SM, Wieschaus EF. Developmental regulation of vesicle transport in Drosophila embryos: forces and kinetics. Cell. 1998;92:547–57. doi: 10.1016/s0092-8674(00)80947-2. [DOI] [PubMed] [Google Scholar]
- 183.Bostrom P, Rutberg M, Ericsson J, Holmdahl P, Andersson L, Frohman MA, Boren J, Olofsson SO. Cytosolic lipid droplets increase in size by microtubule-dependent complex formation. Arterioscler Thromb Vasc Biol. 2005;25:1945–51. doi: 10.1161/01.ATV.0000179676.41064.d4. [DOI] [PubMed] [Google Scholar]
- 184.Welte MA. Proteins under new management: lipid droplets deliver. Trends Cell Biol. 2007;17:363–9. doi: 10.1016/j.tcb.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 185.Boulant S, Douglas MW, Moody L, Budkowska A, Targett-Adams P, McLauchlan J. Hepatitis C Virus Core Protein Induces LIpid Droplet Redistribution in a Microtubule- and Dynein-Dependent Manner. Traffic. 2008;9:1268–1282. doi: 10.1111/j.1600-0854.2008.00767.x. [DOI] [PubMed] [Google Scholar]
- 186.Miyanari Y, Atsuzawa K, Usuda N, Watashi K, Hishiki T, Zayas M, Bartenschlager R, Wakita T, Hijikata M, Shimotohno K. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol. 2007;9:1089–97. doi: 10.1038/ncb1631. [DOI] [PubMed] [Google Scholar]
- 187.Targett-Adams P, Boulant S, McLauchlan J. Visualization of double-stranded RNA in cells supporting hepatitis C virus RNA replication. J Virol. 2008;82:2182–95. doi: 10.1128/JVI.01565-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chan AP, Kloc M, Larabell CA, LeGros M, Etkin LD. The maternally localized RNA fatvg is required for cortical rotation and germ cell formation. Mech Dev. 2007;124:350–63. doi: 10.1016/j.mod.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Welte MA. Bidirectional Transport along Microtubules. Curr Biol. 2004;14:R525–37. doi: 10.1016/j.cub.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 190.Shubeita GT, Tran SL, Xu J, Vershinin M, Cermelli S, Cotton SL, Welte MA, Gross SP. Consequences of motor copy number on the intracellular transport of kinesin-1-driven lipid droplets. Cell. 2008;135:1098–107. doi: 10.1016/j.cell.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gross SP, Welte MA, Block SM, Wieschaus EF. Dynein-mediated Cargo Transport In Vivo: A Switch Controls Travel Distance. J Cell Biol. 2000;148:945–956. doi: 10.1083/jcb.148.5.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Larsen KS, Xu J, Cermelli S, Shu Z, Gross SP. BicaudalD actively regulates microtubule motor activity in lipid droplet transport. PLoS ONE. 2008;3:e3763. doi: 10.1371/journal.pone.0003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Guo Y, Jangi S, Welte MA. Organelle-specific Control of Intracellular Transport: Distinctly Targeted Isoforms of the Regulator Klar. Mol Biol Cell. 2005;16:1406–16. doi: 10.1091/mbc.E04-10-0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gross SP, Welte MA, Block SM, Wieschaus EF. Coordination of opposite-polarity microtubule motors. J Cell Biol. 2002;156:715–24. doi: 10.1083/jcb.200109047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gross SP, Guo Y, Martinez JE, Welte MA. A determinant for directionality of organelle transport in Drosophila embryos. Curr Biol. 2003;13:1660–8. doi: 10.1016/j.cub.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 196.Valetti C, Wetzel DM, Schrader M, Hasbani MJ, Gill SR, Kreis TE, Schroer TA. Role of dynactin in endocytic traffic: effects of dynamitin overexpression and colocalization with CLIP-170. Mol Biol Cell. 1999;10:4107–20. doi: 10.1091/mbc.10.12.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chan AP, Kloc M, Bilinski S, Etkin LD. The vegetally localized mRNA fatvg is associated with the germ plasm in the early embryo and is later expressed in the fat body. Mech Dev. 2001;100:137–40. doi: 10.1016/s0925-4773(00)00517-7. [DOI] [PubMed] [Google Scholar]
- 198.St Johnston D. Moving messages: the intracellular localization of mRNAs. Nat Rev Mol Cell Biol. 2005;6:363–75. doi: 10.1038/nrm1643. [DOI] [PubMed] [Google Scholar]
- 199.Zelazowska M, Kilarski W, Bilinski SM, Podder DD, Kloc M. Balbiani cytoplasm in oocytes of a primitive fish, the sturgeon Acipenser gueldenstaedtii, and its potential homology to the Balbiani body (mitochondrial cloud) of Xenopus laevis oocytes. Cell Tissue Res. 2007;329:137–45. doi: 10.1007/s00441-007-0403-9. [DOI] [PubMed] [Google Scholar]
- 200.Lecuyer E, Yoshida H, Parthasarathy N, Alm C, Babak T, Cerovina T, Hughes TR, Tomancak P, Krause HM. Global analysis of mRNA localization reveals a prominent role in organizing cellular architecture and function. Cell. 2007;131:174–87. doi: 10.1016/j.cell.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 201.Kingsley EP, Chan XY, Duan Y, Lambert JD. Widespread RNA segregation in a spiralian embryo. Evol Dev. 2007;9:527–39. doi: 10.1111/j.1525-142X.2007.00194.x. [DOI] [PubMed] [Google Scholar]
- 202.Dvorak AM, Morgan ES, Lichtenstein LM, Weller PF, Schleimer RP. RNA is closely associated with human mast cell secretory granules, suggesting a role(s) for granules in synthetic processes. J Histochem Cytochem. 2000;48:1–12. doi: 10.1177/002215540004800101. [DOI] [PubMed] [Google Scholar]
- 203.Dvorak AM, Morgan ES, Weller PF. RNA is closely associated with human mast cell lipid bodies. Histol Histopathol. 2003;18:943–68. doi: 10.14670/HH-18.943. [DOI] [PubMed] [Google Scholar]
- 204.Athenstaedt K, Zweytick D, Jandrositz A, Kohlwein SD, Daum G. Identification and characterization of major lipid particle proteins of the yeast Saccharomyces cerevisiae. J Bacteriol. 1999;181:6441–8. doi: 10.1128/jb.181.20.6441-6448.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Binns D, Januszewski T, Chen Y, Hill J, Markin VS, Zhao Y, Gilpin C, Chapman KD, Anderson RG, Goodman JM. An intimate collaboration between peroxisomes and lipid bodies. J Cell Biol. 2006;173:719–31. doi: 10.1083/jcb.200511125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang ZY, Jenkinson JM, Holcombe LJ, Soanes DM, Veneault-Fourrey C, Bhambra GK, Talbot NJ. The molecular biology of appressorium turgor generation by the rice blast fungus Magnaporthe grisea. Biochem Soc Trans. 2005;33:384–8. doi: 10.1042/BST0330384. [DOI] [PubMed] [Google Scholar]
- 207.Hwang CS, Flaishman MA, Kolattukudy PE. Cloning of a gene expressed during appressorium formation by Colletotrichum gloeosporioides and a marked decrease in virulence by disruption of this gene. Plant Cell. 1995;7:183–93. doi: 10.1105/tpc.7.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Than NG, Sumegi B, Than GN, Kispal G, Bohn H. Cloning and sequence analysis of cDNAs encoding human placental tissue protein 17 (PP17) variants. Eur J Biochem. 1998;258:752–7. doi: 10.1046/j.1432-1327.1998.2580752.x. [DOI] [PubMed] [Google Scholar]
- 209.Maglott D, Ostell J, Pruitt KD, Tatusova T. Entrez Gene: gene-centered information at NCBI. Nucleic Acids Res. 2007;35:D26–31. doi: 10.1093/nar/gkl993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yamada Y, Ando F, Shimokata H. Association of polymorphisms in forkhead box C2 and perilipin genes with bone mineral density in community-dwelling Japanese individuals. International Journal of Molecular Medicine. 2006;18:119–127. [PubMed] [Google Scholar]
- 211.Qi L, Corella D, Sorli JV, Portoles O, Shen H, Coltell O, Godoy D, Greenberg AS, Ordovas JM. Genetic variation at the perilipin (PLIN) locus is associated with obesity-related phenotypes in White women. Clin Genet. 2004;66:299–310. doi: 10.1111/j.1399-0004.2004.00309.x. [DOI] [PubMed] [Google Scholar]
- 212.Qi L, Zhang CL, Greenberg A, Hu FB. Common variations in perilipin gene, central obesity, and risk of type 2 diabetes in US women. Obesity. 2008;16:1061–1065. doi: 10.1038/oby.2008.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Corella D, Qi L, Sorli JV, Godoy D, Portoles O, Coltell O, Greenberg AS, Ordovas JM. Obese subjects carrying the 11482G>A polymorphism at the perilipin locus are resistant to weight loss after dietary energy restriction. J Clin Endocrinol Metab. 2005;90:5121–6. doi: 10.1210/jc.2005-0576. [DOI] [PubMed] [Google Scholar]
- 214.Kang ES, Hur KY, Cha BS, Lee HJ, Kim HJ, Shim WS, Ahn CW, Kim SH, Lee HC. The 11482G > A polymorphism in the perilipin gene is associated with weight gain with rosiglitazone treatment in type 2 diabetes. Diabetes Care. 2006;29:1320–1324. doi: 10.2337/dc05-2466. [DOI] [PubMed] [Google Scholar]
- 215.Corella D, Qi L, Tai ES, Deurenberg-Yap M, Tan CE, Chew SK, Ordovas JM. Perilipin gene variation determines higher susceptibility to insulin resistance in Asian women when consuming a high-saturated fat, low-carbohydrate diet. Diabetes Care. 2006;29:1313–1319. doi: 10.2337/dc06-0045. [DOI] [PubMed] [Google Scholar]
- 216.Qi L, Shen H, Larson I, Schaefer EJ, Greenberg AS, Tregouet DA, Corella D, Ordovas JM. Gender-specific association of a perilipin gene haplotype with obesity risk in a white population. Obes Res. 2004;12:1758–65. doi: 10.1038/oby.2004.218. [DOI] [PubMed] [Google Scholar]
- 217.Qi L, Tai ES, Tan CE, Shen H, Chew SK, Greenberg AS, Corella D, Ordovas JM. Intragenic linkage disequilibrium structure of the human perilipin gene (PLIN) and haplotype association with increased obesity risk in a multiethnic Asian population. J Mol Med. 2005;83:448–56. doi: 10.1007/s00109-004-0630-4. [DOI] [PubMed] [Google Scholar]