Abstract
Objective
To identify the genes controlling body fat, we carried out a quantitative trait locus (QTL) analysis using C57BL/6J (B6) and 129S1/SvImJ (129) mice, which differ in obesity susceptibility after consuming an atherogenic diet.
Methods
Mice were fed chow until 8 weeks and an atherogenic diet from 8 to 16 weeks; body fatness was measured by X-ray absorptiometry in 528 (B6x129) F2 at 8 and 16 weeks. A high-density genome scan was performed using 508 polymorphic markers. After identifying the genetic loci, we narrowed the QTL using comparative genomics and bioinformatics.
Results
The percentage of body fat was significantly linked to loci on chromosomes (Chr) 1 (22, 68 and 173 Mb), 4 (74 Mb), 5 (73 Mb), 7 (88 Mb), 8 (43 and 80 Mb), 9 (55 Mb), 11 (115 Mb) and 12 (32 Mb); three suggestive loci on Chrs 6 (76 Mb), 9 (30 Mb) and 16 (26 Mb) and two pairs of interacting loci (Chr 2 at 99.8 Mb with Chr 7; Chr 1 at 68 Mb with Chr 11). Comparative genomics narrowed the QTL intervals by 20–57% depending on the chromosome; in most cases, haplotype analysis further narrowed them by about 90%.
Conclusions
Our analysis identified 15 QTL for percentage of body fat. We narrowed the QTL using comparative genomics and haplotype analysis and suggest several candidate genes: Apcs on Chr 1, Ppargc1a on Chr 5, Ucp1 on Chr 8, Angptl6 on Chr 9 and Lpin1 on Chr 12.
Keywords: quantitative trait loci, comparative genomics, haplotype, SNP, fat mass
Introduction
Although environmental effects such as diet and exercise are important in the obesity phenotype, variations in genes affecting energy balance, metabolism and behavior are believed to be involved in the regulation of body weight and body composition in mammals.1 Thus, genetic etiology of obesity is complex, involving multiple genes and environmental factors, each of which may give rise to only a small quantitative difference in the phenotype, so that identifying relevant genes in humans is difficult. The mouse is an excellent model for dissecting the genes underlying obesity,2 because inbred strains differ widely in body size and adiposity (www.jax.org/phenome) and diet and environmental factors can be controlled. Furthermore, different genetic and genomic tools available for the mouse make gene discovery more feasible in the mouse than in humans.
Gene-targeted mice are usually initially created on a 129 background (several 129 substrains are used), and the null mutation subsequently transferred into C57BL/6 (B6), a process that carries some region of DNA flanking the targeted gene from 129 into the B6 background. A difference in fat mass between the gene-targeted mice and B6 would usually be attributed to the effects of the targeted gene but in fact it might be caused by the flanking linked locus if a nearby allelic difference between 129 and B6 affected fat mass, thus leading to incorrect conclusions.3 Thus, it is quite important to know the quantitative trait loci (QTL) causing a difference in obesity between B6 and 129 when evaluating the impact of a gene deficiency in targeted mice. Although previous crosses evaluated obesity QTL between B6 and 129,4 including one from our own laboratory,5 one cross evaluated only QTL found in chow-fed mice and the other only QTL found in atherogenic diet-fed female mice. Thus, to obtain a complete picture of the obesity QTL between B6 and 129, we generated a cross using the same parental strains, but made the following changes: the cross is considerably larger (528 compared to 294 mice), both sexes were included allowing us to test for the dependence of genetic effects on sex, more dense genotyping was used (508 single nucleotide polymorphism (SNP) markers compared to 111 Mit markers) and fat mass was measured both in chow-fed and atherogenic diet-fed mice. The atherogenic diet used contained 50 kcal% glucose, 32 kcal% fat (30 kcal% butter fat and 2 kcal% corn oil), 1% (w/w) cholesterol and 0.5% cholic acid.
The ultimate goal of QTL analysis is to identify the underlying gene. However, the confidence intervals (CI) of the QTL identified from intercrosses are often quite large, and identifying the underlying genes by positional cloning is challenging. Although several classic breeding strategies can be used to resolve QTL,6 such as congenic lines, advanced intercross lines and recombinant inbred segregation test, their usefulness is limited by the available resources, such as time, animal space and money. Bioinformatics tools are an efficient and economical approach for narrowing QTL and prioritizing candidate genes,7 which has been made possible by publicly available sequence, genotype and expression databases. These tools, including comparative genomics and haplotype analysis, allow stepwise narrowing of a QTL interval, often reducing the list of candidate genes to a few, each of which can be tested.
The current study confirmed several of the QTL found previously, reports new genetic loci and characterizes the interaction of QTL with sex. In addition, all significant loci were narrowed using bioinformatics methods, and we suggest several candidate genes.
Methods
Mice and diets
C57BL/6J (B6) and 129S1/SvImJ (129) inbred mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). B6 female were mated to 129 male mice to produce the F1 progeny, which were intercrossed to produce 528 F2 progeny (269 female and 259 male mice). These F2 mice and separate B6, 129 and (B6 x 129)F1 were phenotyped as described. Mice were housed in a climate-controlled facility with a 14:10 h light–dark cycle. After weaning, mice were maintained on a chow diet containing 19% protein and 6% fat (LabDiet 5K52) and given unrestricted access to food and acidified water throughout the experiment. At week 8, the mice were switched to an atherogenic diet containing 50 kcal% glucose, 32 kcal% fat (30 kcal% butter fat and 2 kcal% corn oil), 1% (w/w) cholesterol and 0.5% cholic acid as described previously 8 until they were euthanized at 16 weeks of age. All applicable institutional and governmental regulations concerning the ethical use of animals were followed during this research. The study was approved by the Institutional Animal Care and Use Committee of The Jackson Laboratory.
Measurements of body fat mass
Body fat mass was measured at 8 weeks of age in chow-fed mice and at 16 weeks of age in mice fed atherogenic diet. The fat mass of each mouse was measured using peripheral dual-energy X-ray absorptiometry (DEXA) in a Lunar PixiMus II densitometer (PIXImus; GE-Lunar, Madison, WI, USA), which was calibrated daily according to the manufacturer’s instructions using a quality control phantom (phantom values: percentage of fat=10.0%). Mice anesthetized with 2% tribromoethanol were placed on a positioning tray ventral side down with the legs extended away from the body. The DEXA method has been validated in normal and obese mice as an accurate measure.9
DNA isolation and genotyping
DNA was isolated from tail tips using a genomic DNA purification kit (Gentra Systems, Minneapolis, MN, USA). Genotyping was performed at the Mouse SNP Genotyping Service, Division of Genetics, Brigham and Women’s Hospital, Boston, MA, USA (http://www.brighamandwomens.org/research/Genetics/moran.asp) using a custom mouse SNP array containing 508 SNPs polymorphic between B6 and 129. On average, for each mouse, 98.1 ± 1.3% (mean s.d.) of the SNPs were assigned a genotype. For mapping purposes, the megabase (Mb) position of each SNP was ascertained from the National Center for Biotechnology Information mouse genome build 36. Information about each SNP was retrieved from the dbSNP database (http://www.ncbi.nlm.nih.gov/SNP/index.html), and the sequence surrounding the SNP was used in a BLAT search (http://genome.ucsc.edu/cgi-bin/hgBlat?command=start) against the mouse genome to verify its position.
Statistical analysis
Data were analyzed using R/qtl10 (v. 1.07–12 available at http://www.rqtl.org) and Pseudomarker (v. 2.03 available at http://www.jax.org/staff/churchill/labsite) software packages. R/qtl was used to calculate the recombination frequency and markers that were not placed appropriately were evident by visual plotting of the recombination frequency for each chromosome.
A three-step QTL analysis was conducted to search for main effects and pair-wise gene interactions and then to integrate all the main and interacting QTL into a multiple regression. In single locus scans, the sex was first included as an additive covariate to account for overall differences in phenotypes between the sexes. A second set of scans included an interaction between sex and the putative QTL at each locus to identify sex-specific QTL. The difference in LOD scores (ΔLOD) between these two scans constitutes a test for sex-specific effects. We applied a significance threshold of LOD > 2.0 corresponding to P < 0.01, based on 2 d.f. chi- square distribution of the log-likelihood ratio, for QTL-by-sex interactions.11 A multiple imputation algorithm was used to account for missing marker genotypes. QTL were deemed significant if they either met or exceeded 95% genome-wide adjusted threshold, which was assessed by 1000 permutation analysis for each trait; they were deemed suggestive if they either met or exceeded the 37% genome-wide adjusted threshold but were not significant. QTL CI were calculated according to posterior probability.12 Simultaneous pair-wise genome scans were performed to search for pairs of interacting loci.12 In the regression analysis, we combined all significant and suggestive QTL and interactions in a multiple regression model. Terms that did not meet the nominal 0.01 level in the regression were eliminated in a backward stepwise manner with the exception that main effect terms involved in a significant interaction were retained. Final models were reported for each trait.
To assess whether there were separate QTL on the same chromosome, we conditioned the trait value on one of the markers and computed the LOD score at the second marker using the residual values and vice versa. The LOD score difference between 2 and 1-QTL ( LOD) was used to judge whether there were two separate QTL on the same chromosome. Threshold LOD differences were computed by permutation tests and the significant LOD is 1.99 (P<0.05).
One-way analysis of variance was used for determining whether the mean phenotype values of progeny with different genotypes at a specific marker were significantly different. Pearson’s correlation analysis was performed to assess the association of various traits. Data were analyzed using GraphPad Prism (Windows v. 5.00; GraphPad Software, San Diego, CA, USA).
Comparative genomics and haplotype analysis
Homologous chromosomal regions between mouse and human were found at http://www.informatics.jax.org/reports/homologymap/mouse_human.shtml. When human and mouse QTL were located in homologous locations, we assumed they were caused by the same gene and reduced the mouse QTL region to that homologous to the human QTL. If a CI was not provided in the original report, we used a 1-LOD score drop from the peak; if no chromosomal LOD score plot was provided, we used a CI of 20 Mb on either side of the reported peak.
The region that was obtained after comparative genomics was further reduced by interval-specific haplotyping. To perform interval-specific haplotype analysis, the genotype data covering the QTL interval can be grouped into the strains that carry the high allele and those that carry the low allele. Any region with genotypes that are shared between the high-allele strains and different from the low-allele strains is considered a haplotype region likely to contain the QTL gene. For example, the B6 strain conferred decreased obesity at the Obq25 locus at distal chromosome (Chr) 1 in B6 x129, B6 x C3H and B6 x A/J. We found the region where the strains 129, C3H and A/J all conferring increased obesity, shared an identical haplotype and this haplotype differed from the B6 strain conferring decreased obesity. At Obq29 on Chr 7, B6 mice conferred increased obesity in two crosses, B6x129 and B6xKK. Here, we used 129 and KK as low-allele strains and B6 as a high-allele strain. SNPs were obtained from the extensive public database of SNPs such as Broad SNPs (www.broad.mit.edu/snp/mouse) or the Mouse Phenome Database (www.jax.org/phenome).
Candidate gene analysis
We extracted gene lists for the narrowed QTL from Ensembl (www.ensembl.org) and searched SNP databases (http://www.jax.org/phenome/snp.html) for any nonsynonymous SNPs between B6 and 129 leading to an amino-acid difference in the candidate genes. The high-priority candidate genes were then selected based on the gene with known obesity-related functions and the presence of nonsynonymous SNPs between the parental strains.
Results
Percentage of fat mass in the parental, F1 and F2 mice
The percentage of fat (%fat) for the parental, (B6 x 129) F1, and 528 F2 mice are summarized in Table 1. When mice were fed the chow diet, %fat was 19.6% (P = 1 × 10−4) higher in 129 male as compared with B6 male mice, but similar in female mice of both strains. After mice consumed the atherogenic diet, %fat was 65.4% (P = 8 × 10−11) higher in 129 male and 40.6% (P = 4 × 10−6) higher in 129 female mice compared with sex-matched B6 mice. F1 mice tended to be intermediate between the parents but closer to B6 than 129 mice, and in the case of female F1 fed chow mice, the %fat was lower than B6 female mice. The %fat in F2 progeny fed both diets was normally distributed. Regression analyses revealed significant positive correlation between the %fat in the mice fed chow and the atherogenic diets (r = 0.62, P < 0.0001).
Table 1.
%fat in B6, 129, F1 and F2 progeny fed chow diet for 8 weeks and atherogenic diet for 8 weeks
| Chow diet | Atherogenic diet | |||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| B6 (n=10) | 22.6 ± 2.5 | 17.9 ± 1.9 | 22.4 ± 2.4 | 18.5 ± 1.5 |
| 129(n=10) | 23.5 ± 2.4 | 21.4 ± 1.2a | 31.5 ± 3.7a | 30.6 ± 2.5a |
| (B6 x 129) F1 (n=10) | 20.5 ± 1.1b | 18.2 ± 1.0c | 24.5 ± 3.3c | 24.1 ± 2.4b |
| F2 (n=528) | 21.6 ± 2.5 | 17.8 ± 2.2 | 28.7 ± 6.5 | 24.5 ± 5.8 |
Data are presented as the means ± s.d.
P<0.01 vs. B6.
P<0.01 vs B6 and 129.
P<0.01 from 129 but not significantly different from B6.
Identifying main-effect QTL in the F2 population
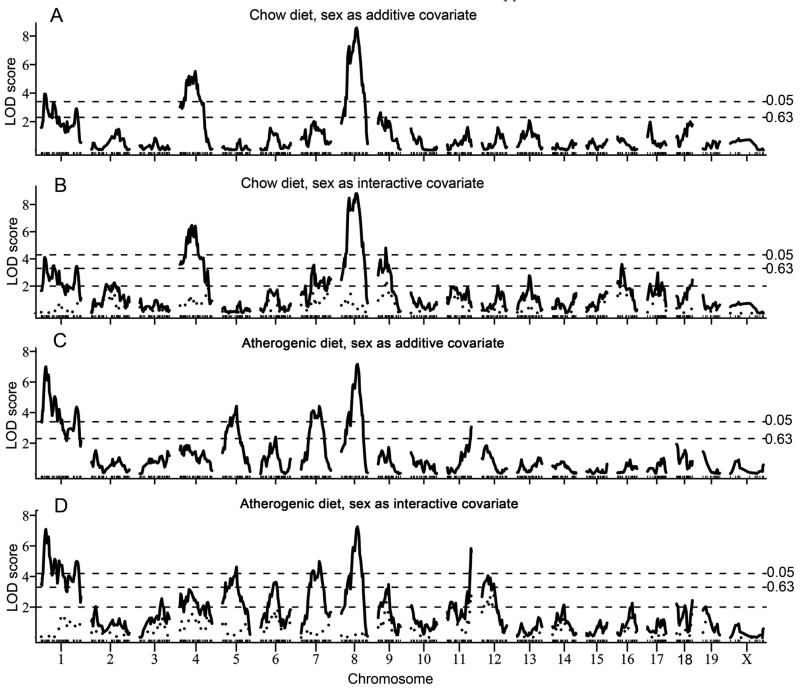
Single-locus genome scans with sex as an additive covariate were performed to account for overall average differences between the sexes (Figures 1a and d). To identify sex-specific QTL, we carried out a second set of single-locus genome scans with sex as an interactive covariate (Figures 1b and e). The difference in LOD (ΔLOD) between the scans with sex as an additive or interactive covariate constitutes a test for QTL-by-sex interaction (Figures 1c and f). Details of the QTL were including peak marker locus, LOD score, 95% CI, allele conferring greater obesity and significance as determined by 1000 permutation tests are summarized in Table 2.
Figure 1.
Genome-wide scans for the percentage of fat mass for mice fed chow or atherogenic diets. Sex as additive covariate (a, d); sex as interactive covariate (b, e). The horizontal dashed lines represent suggestive (P = 0.63) and significant (P = 0.05) levels as determined by 1000 permutation tests. The ΔLOD between sex as an additive and sex as an interactive covariate is depicted in c and f (scans a and b for chow-fed mice, and d and e for atherogenic diet-fed mice. ΔLOD > 2.0 (denoted by the horizontal dashed line) indicates a quantitative trait locus (QTL) that differs significantly between sexes (P < 0.05).
Table 2.
QTL identified for single genome-wide scan of F2 mice
| QTLa | Chr | Peak (Mb) |
95% CI | LODb Chow |
LODb Ath |
Nearest Marker |
High allele |
Human homologous region with obesity QTLc |
Coincident mouse QTLd |
|---|---|---|---|---|---|---|---|---|---|
| Obq23 | 1 | 22 | 10–34 | 3.9 | 7.0 | rs3711079 | 129 | 8q13.1-21.11 | LGxSM |
| Obq24 | 1 | 68 | 58–80 | 3.2 | 5.0 | rs3697638 | 129 | 2q33.1-36.3 | LGxSM, NZOxSM |
| Obq25 | 1 | 173 | 162–184 | 2.9 | 4.3 | rs31507136 | 129 | 1q23.1-23.3 | B6xA/J, B6xC3H |
| Obq26 | 4 | 74 | 32–100 | 5.5 | 3.2 | rs13477785 | 129 | 9q21.31-33.2 1p32.1-31.2 | B6xD2 |
| Obq27 | 5 | 73 | 31–80 | - | 4.4 | rs6409508 | 129 | 2p23, 18p11.32, 4p16.1-15.1, 4p13, 4q12-13.1 | NZOxSJL |
| Obq28e | 6 | 76 | 48–85 | - | 3.6 | rs3710429 | B6/129 | 7p15.3-14.3, 1p31.2, 2p13.3-11.2 | NZOxSM |
| Obq29 | 7 | 88 | 50–100 | 3.5 | 4.5 | rs3658777 | B6 | 15q11-13, 15q25-26, 11q13.4 | B6xKK, LGxSM |
| Obq30 | 8 | 43 | 34–50 | 7.2 | 4.0 | rs13479731 | 129 | 8p23.1-22, 19p13.2-13.1, 16q12-22 | B6xA/J, LGxSM |
| Obq31 | 8 | 80 | 63–100 | 8.6 | 7.2 | rs13479840 | 129 | 8p22-21,19p13.1-12,4q31.1-31.23,19p13.2-13.1, 16q12-22 | LGxSM |
| Obq5f | 9 | 30 | 0–40 | 2.6 | - | rs13480120 | 129 | 19p13.2,7p14.3-14.2,11q24.1-25 | B6xKK |
| Obq32f | 9 | 55 | 50–70 | 4.8 | 3.5 | rs6206488 | 129 | 11q22.3-23.1 | LGxSM |
| Obq33e | 11 | 115 | 112–118 | - | 5.8 | rs13481245 | 129 | 17q24.3-25.2 | LGxSM |
| Obq34e | 12 | 32 | 7–71 | - | 4.1 | rs13481380 | 129 | 2p25.3-23.3,7p21.3-21.1,7q22.3,7q31.1,14q12-13.1 | LGxSM, B6x129 |
| Obq35f | 16 | 26 | 16–46 | 3.6 | - | rs4165503 | B6 | 22q11.21,3q27.1-29,3q13.2-21.2 | B6xKK, LGxSM |
Abbreviations: Ath, atherogenic; Chr, chromosome; CI, confidence interval; Mb, megabase; QTL, quantitative trait locus.
QTL were named if they were significant or if they were suggestive but confirmed previously reported QTL. They were given the same name if the crosses shared at least one common parental strain and a new name if the crosses identifying them involved no common strains.
LOD scores of the analysis with sex as additive covariate are given with the exception of sex-specific QTL. Suggestive and significant LODs are 2.2/3.2 for chow diet and 2.2/3.0 for atherogenic diet using sex as additive covariate. Significant LOD scores are in bold. Chow and atherogenic refer to chow-fed or atherogenic diet-fed mice.
Human homologous region of the mouse QTL were retrieved from Genome Orthology Map at http://www.informatics.jax.org/reports/homologymap/mouse_human.shtml. Human obesity QTL were retrieved from obesity gene map.13
The mouse obesity QTL are reviewed recently by Wuschke et al. 15 The strains conferring high alleles are bolded.
Female- and
Male-specific QTL.
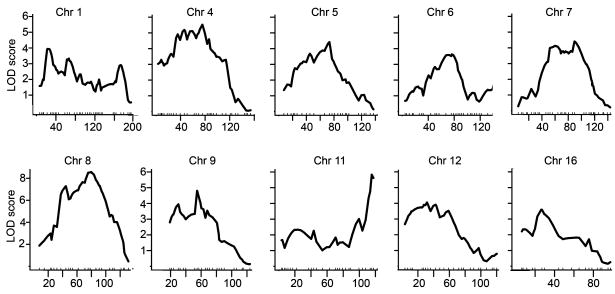
QTL analysis revealed 11 significant and three suggestive QTL for %fat, mapping to Chrs 1, 4, 5, 6, 7, 8, 9, 11, 12 and 16 in the genome scan (Table 2 and Figure 2). QTL on proximal Chrs 9 and 16 were reduced on the atherogenic diet while QTL on Chrs 5, 6, 11 and 12 were specific to the atherogenic diet. Three significant QTL on Chr 9 at 55 Mb, Chr 11 at 115 Mb and Chr 12 at 32 Mb, as well as all three suggestive loci on Chr 6 at 76 Mb, Chr 9 at 30 Mb and Chr 16 at 26 Mb present strong evidence for sex-specific effects. QTL on Chrs 6 (Figure 3a), 11 (Figure 3b) and 12 (Figure 3c) affect the %fat mass only in female mice, whereas QTL on Chr 9 (Figures 3d and e) and Chr 16 (Figure 3f) only affected male mice.
Figure 2.
Chromosomal LOD score plots for significant and suggestive quantitative trait locus (QTL). The x axis depicts the marker positions in megabase (Mb) for each chromosome, and the y axis depicts the LOD score. The evidence indicates three QTL on chromosome (Chr) 1 (Δ LOD = 2.1) and two QTL on Chr 8 for both diets and both sexes (ΔLOD = 2.4). When testing for multiple QTL on Chr 9 on the chow diet, we allowed the QTL to have sex interaction and found evidence (ΔLOD = 2.2) for two QTL.
Figure 3.
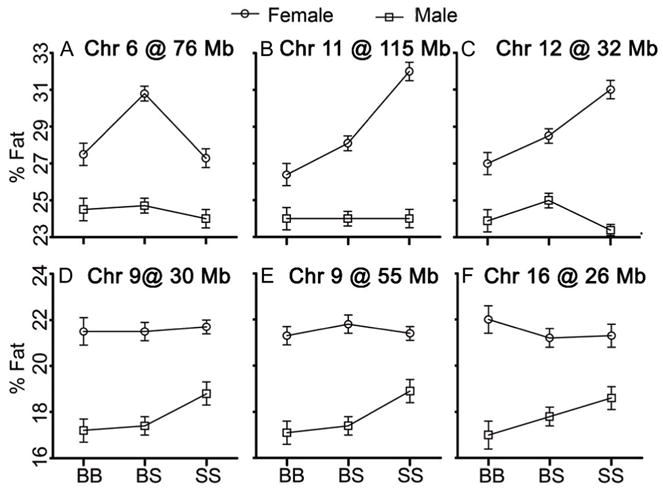
The allele effects of the sex-specific quantitative trait locus (QTL). The impact of different alleles in the F2 offspring at the peaks of chromosomes (Chrs) 6 (a), 11(b) and 12 (c) found in females and Chrs 9 (d, e) and 16 (f) found in males. Chromosome number and the QTL position in megabase (Mb) are given for each QTL. BB represents homozygosity for B6 alleles, SS is homozygosity for 129 alleles and BS is heterozygosity.
Resolution of multiple-linked loci
The shape of the LOD curves of Chrs 1, 8 and 9 in the single-locus genome scans suggested that multiple QTL might be present (Figure 2). To resolve the multiple-linked QTL, we compared models with one, two and three QTL. Maximum LOD scores were obtained from each model by scanning the QTL simultaneously to find their optimal locations. Changes in LOD scores (ΔLOD) between models were used to construct tests for multiple QTL. The evidence indicates three QTL on Chr 1 (ΔLOD = 2.1), two QTL on Chr 8 for both diets and both sexes (ΔLOD = 2.4) and two QTL on Chr 9 that were male specific (ΔLOD = 2.2).
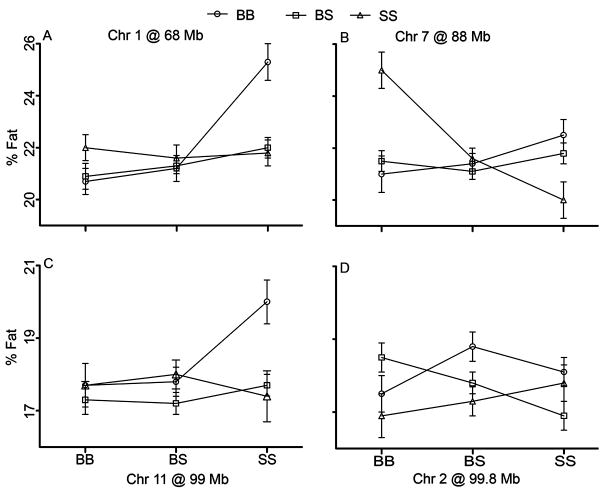
Epistasis and interacting QTL
Pair-wise genome scans with sex as an additive covariate revealed two significant epistatic interactions in mice fed chow diet. The main scan QTL on Chr 1 (68 Mb) interacted with Chr 11 (Figures 4a and b). The locus on Chr 11 did not affect fat mass by itself on the chow diet, but its combined effect with the main scan QTL on Chr 1 on fat mass was strong. When the Chr 1 locus was homozygous B6, 129 alleles at Chr 11 increased the fat content to 25.3% in female (Figure 4a) and 20% in male mice (Figure 4b). A second significant interaction was found between loci on Chrs 2 and 7 in a sex-specific fashion (Figures 4c and d). When the Chr 2 locus was homozygous B6, 129 alleles at Chr 7 increased fat mass to 25% in female mice (Figure 4c); no such increase occurred in male mice (Figure 4d).
Figure 4.
The effects of gene interactions detected by the pair-wise genome scan. The interactions are from females (a, c) and from males (b, d). BB represents homozygosity for B6 alleles, SS is homozygosity for 129 alleles and BS is heterozygosity. Mean values of %fat are shown on y axes, error bars represent s.e.m.
Regression analysis
To evaluate the relative contributions of each QTL when considered together, we combined all significant, suggestive and interacting QTL in a multiple regression model, eliminating terms that did not meet the 0.01 significance level based on the multiple regression F-test (Table 3). In addition to these QTL, sex contributes significantly to the multilocus model on both diets accounting for 38.7 and 13.7% of the total variance in chow and atherogenic diets, respectively. These models confirm the importance of the QTL-by-sex and QTL-by-QTL interactions. The results showed that we could account for 56.1% of variation for mice fed chow and 33.6% for mice fed atherogenic diets (Table 3).
Table 3.
Multiple regression analyses of variance for %fat in F2 progeny fed chow diet or atherogenic diet
| Chr (Mb) | d.f.a | % varianceb | F -value | P-value |
|---|---|---|---|---|
| Chow diet | ||||
| Sex | 1 | 38.7 | 411.6 | <2×10−16 |
| Chr1@68 | 6 | 2.8 | 5.0 | 5.2×10−5 |
| Chr2@99.8 | 6 | 1.9 | 3.3 | 3.2×10−3 |
| Chr4@75.6 | 2 | 2.0 | 10.6 | 3.2×10−5 |
| Chr7@88 | 6 | 1.9 | 3.4 | 3.0×10−3 |
| Chr8@79.6 | 2 | 3.0 | 15.8 | 2.2×10−7 |
| Chr9@54.6 | 2 | 1.0 | 5.1 | 6.7×10−3 |
| Chr11@99.4 | 6 | 2.4 | 4.3 | 3.4×10−4 |
| Chr1@62.6:Chr11@99.4 | 4 | 1.8 | 4.7 | 1.0×10−3 |
| Chr2@99.8:Chr7@88 | 4 | 1.3 | 3.5 | 7.3×10−3 |
| Total | 23 | 56.1 | ||
| Atherogenic diet | ||||
| Sex | 3 | 13.7 | 32.7 | <2×10−16 |
| Chr1@28.6 | 2 | 4.1 | 14.7 | 6.7 ×10−7 |
| Chr5@73.4 | 2 | 3.4 | 12.2 | 6.7 ×10−6 |
| Chr7@89.4 | 2 | 4.3 | 15.4 | 3.1 ×10−7 |
| Chr8@79.6 | 2 | 3.8 | 13.6 | 1.7 ×10−6 |
| Chr11@117.4 | 2 | 1.2 | 4.5 | 0.01 |
| Chr12@31.6 | 4 | 2.0 | 3.6 | 6.2 ×10−3 |
| Chr12@31.6:sex | 2 | 1.6 | 5.8 | 3.4 ×10−3 |
| Total | 15 | 33.6 | ||
Abbreviations: Chr, chromosome; d.f., degree of freedom; Mb, megabase.
d.f. includes main effect and any interactions.
%Variance indicates the percentage of the total F2 phenotypic variance associated with each marker.
Narrowing the QTL interval using bioinformatics tools
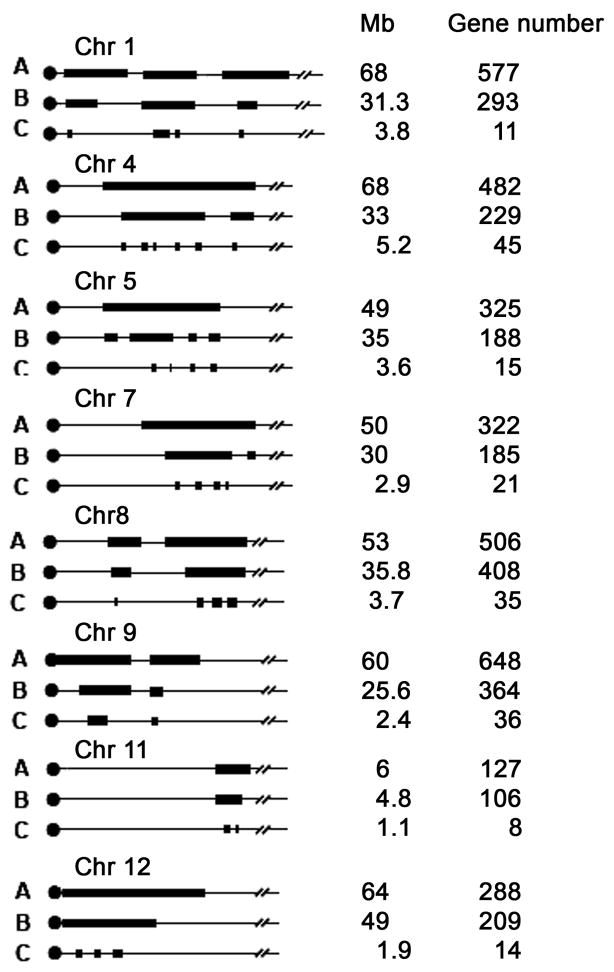
All the QTL for the %fat mass identified in this study are homologous to human QTL for body fat, body weight or body mass index (BMI);13, 14 and all have been also identified in other mouse crosses15 (Table 2). These homologous human QTL and colocalizing mouse QTL combined with the completely sequenced genomes of the B6 and 129 strains, facilitate the narrowing of the QTL regions using bioinformatics.7 Such narrowing is based on the assumptions that the same gene underlies the QTL in different crosses and the same gene accounts for homologous QTLs between mouse and human. We narrowed all significant mouse QTL regions by first reducing the QTL to the interval in which they overlap with the homologous human QTL (Figure 5 step B), a step that narrowed the QTL by a minimum of 20% for Chr 11 to a maximum of 57% for Chr 9. We then further reduced the QTL by using SNPs of all the strains that were parents of the QTL crosses and finding those regions where the strains carrying the high allele at that locus were identical but differed from the strains carrying the low allele at that locus (Figure 5 step C). This step was particularly effective and further narrowed the QTL regions on each chromosome leaving between 8 and 45 genes depending on the QTL (Supplementary Table 1).
Figure 5.
Narrowing quantitative trait locus (QTL) by bioinformatics. (A) The 95% confidence interval (CI) and the number of genes in each QTL identified in cross B6 x 129; (B) Comparative genomics: the QTL narrowed by homology with human obesity QTL; (C) Haplotype analysis: each QTL was reduced by haplotype analysis of the strains involved in the QTL crosses.
Proposed candidate genes
A QTL could result from a difference between parental strains in the expression or function of a protein.16 A functional difference would result from a sequence difference in the coding region. To evaluate which of the genes on our lists had a polymorphism changing an amino acid (cSNP), we examined the SNPs in the Mouse Phenome Database (http://www.jax.org/phenome/snp.html) on a gene-by-gene basis. The genes containing cSNPs are listed in Supplementary Table 2. The most likely candidate genes, based on known function, are in bold.
Discussion
The genetic basis of obesity in the B6x129 cross was clearly complex, involving multiple genetic loci. Females and males share nearly identical genetic information, but vary widely with respect to the phenotype. The findings in the present and previous17 studies highlighted the importance of taking sex into account in the analysis of body fat QTL, because three loci (Obq28, Obq33 and Obq34) affected only female mice and three (Obq5, Obq32 and Obq35) affected only male mice. The strong sex effects were also seen in the analysis of the multiple-QTL models. Sex contributes significantly to the multilocus model on both the chow and the atherogenic diet, which accounts for 38.7 and 13.7% of the total variance, respectively. To identify sex-specific QTL, it is not sufficient to carry out separate analyses of males and females. This approach can be misleading, as the subdivided populations will be much smaller than the combined population, and this increases the likelihood that real QTL effects would fail to be detected in one or both subsets of the data.11 Furthermore, separate analyses would not allow for the detection of QTL that have opposing, or sex-antagonistic, effects in females and males and would hinder the detection of QTL specific to one sex. Analysis of the entire population with and without a QTL-by-sex interaction provides a more appropriate basis for interpretation. This strategy also applies for humans, where QTL analysis is commonly carried out on mixed-sex populations.
Although the %fat in mice fed chow diet and the atherogenic diet were highly related (r=0.62), they had somewhat separate traits, which was also reflected in the genetic loci controlling the %fat. Most loci were common for the %fat for both diets; however, QTL on proximal Chrs 9 and 16 are specific to the chow diet while QTL on Chrs 5, 6, 11 and 12 are specific to the atherogenic diet. This emphasizes the effect of interaction between genetic etiology and environmental factors on the obesity phenotype.
Previously, QTL for individual adipose depot weights were reported in a C57BL/6ByJ x 129P3/J cross.4, 18 There are several differences with our study. We did not observe the loci on Chrs 2, 10, 14 and 15 identified in the C57BL/6ByJ x 129P3/J cross, and although the loci on Chrs 11 and 16 were identified in both crosses, they appear to be at different locations. The QTL are at the most distal Chr 11 and proximal Chr 16 in our cross, whereas they are in the middle of Chr 11 and at distal Chr 16 in the C57BL/6ByJ x 129P3/J cross.4 Several reasons could account for the differences. First, different substrains of C57BL/6 and 129 were used. In contrast with C57BL/6J, strain C57BL/6ByJ is resistant to diet-induced hypercholesterolemia,19 and the 129 strains (129S1/SvImJ, 129P3/J and 129X1/SvJ) are genetically more distinct,20 and exhibit different physical activity.21 Second, the age of the mice when they were phenotyped was different. The mice were much younger in the present study (2 and 4 months of age) than those in the C57BL/6ByJ x129P3/J cross (8 or 9 months of age). Third, the diets differed between the two studies. We used a chow diet containing 6% fat compared to a chow diet with 4.4% fat. Finally, the phenotype was measured in different ways. We measured the whole body fat mass, while the regional adiposity was measured in the C57BL/6ByJ x 129P3/J cross.
The ultimate goal of QTL mapping is to identify the underlying genes. All loci for fat content identified in this study have been found in human homologous genomic locations.13 Because rodent and human QTL for the same trait often map to homologous genomic locations, comparison of these homology maps may reduce the QTL size in both species, if one assumes that homologous QTL are caused by the same gene in both species. To date, there are many examples of correspondence between alleles in particular genes and phenotypes in mice and human. For instance, in the mouse, mutations in the leptin gene cause early-onset, extreme obesity,22 and the same is true in humans.23 However, the pitfall of this strategy arises when homologous QTL are caused by different genes in humans and mice. Since closely linked QTL are often found, there may be circumstances where two genes control a trait on the same chromosome, but one gene is polymorphic in the mouse and the other gene is polymorphic in humans. In such a case, we would fail to find the QTL genes by assuming the same gene caused the QTL in both species.
Because the majority of genetic variation among inbred mouse strains is ancestral, regions that are identical by descent (IBD) between the parental strains of a cross can be identified using SNP genotype data. Such regions are unlikely to contain QTL genes especially if the QTL has been found in multiple crosses. Therefore, it is possible to narrow the QTL by excluding genetic segments that are IBD between parental strains of the crosses by haplotype analysis. The B6 and 129 strains are well genotyped, which facilitates the identification of any polymorphism causing amino-acid change between strains. The combination of amino-acid variant and the known obesity-related function allows prioritizing of the candidate genes. The pitfall of this strategy is that if the QTL is caused by the differential expression levels produced by polymorphisms in regulatory regions, such as the promoter or UTR between strains, we may fail to identify the genes.
The distal Chr 1 locus (Obq25) was reduced to only one gene, Apcs, by comparative genomics and haplotype analysis (Supplementary Table 1). Several lines of evidence support the candidacy of this gene, it is located at 174.7 Mb and the QTL peak is at 173.4 Mb; it contains an amino-acid variant Ile43Ala (B6, 129); Apcs encodes serum amyloid P-component (SAP), the QTL peak for plasma SAP concentrations and body weight are colocalized on the Apcs position in a cross B6 x C3H,24 both 129 and C3H have identical haplotype in the Apcs region; and most recently it has been reported that human SAP concentrations were positively associated with BMI.25
Three of the genes at the Chr 5 locus (Obq27) contain cSNPs (Supplementary Table 2). The strongest candidate is Ppargc1a, encoding the transcriptional coactivator peroxisome proliferator-activated receptor- γ coactivator-1 α. Ppargc1α contains one amino-acid variant Arg675His (B6, 129), Ppargc1a knockout mice develop abnormally increased body fat (more severe in females),26 a QTL for obesity in humans contains Ppargc1a,27 and Ppargc1a polymorphisms are associated with type 2 diabetes and obesity in various human populations.28, 29, 30, 31
The distal Chr 8 locus (Obq31) was reduced to 35 genes, 7 of which contain cSNPs (Supplementary Table 2). Several lines of evidence support the candidacy of Ucp1-encoding uncoupling protein 1. Ucp1 contains the amino-acid variant Asn385Ser (B6, 129), which is mainly expressed in brown adipose tissue and is important in energy homeostasis in rodents and neonates of larger mammals including human,32 and promoter polymorphisms are associated with body fat in several human studies.33, 34, 35, 36
Three genes at the proximal Chr 9 (Obq5) locus contain cSNPs. The strongest candidate is Angptl6-encoding angiopoietin-like 6. This gene contains an amino-acid variant Ala80Val (B6, 129). Surviving Angptl6 knockout mice develop marked obesity, insulin resistance and reduced energy expenditure, and hepatic overexpression leads to a significant body weight loss and increased insulin sensitivity in mice fed a high-fat diet.37
The Chr 12 locus (Obq34) was reduced to 14 genes, 6 of which contain cSNPs. The highest-priority candidate gene is Lpin1 encoding Lipin 1. This gene contains an Ala406Thr polymorphism (B6, 129), Lipin deficiency impairs adipocyte differentiation and causes lipodystrophy,38 and overexpression in either adipose tissue or skeletal muscle promotes obesity.39
In addition to the list of genes for which a known amino-acid change existed between parental strains of the QTL crosses and an obesity phenotype was found in knockout mice, we also suggest some other candidate genes based on known function. For the middle Chr 1 locus (Obq24), the likely candidate gene is Klf7 encoding Kruppel-like zinc-finger transcription factor. It is located at 63.9 Mb and the QTL peak is at 67.8 Mb; the haplotypes of B6 and 129 differ, and other KLF proteins have been implicated in adipogenesis. KLF2, for instance, is expressed in preadipocytes but not in mature adipocytes; it is a negative regulator of adipocyte differentiation, and its overexpresion inhibits adipocyte differentiation and Pparg, C/ebpa and Add1/Srebp1c expression.40 KLF5 is a key component of the transcription factor network controlling adipocyte differentiation,41 and overexpression stimulates the differentiation of preadipocytes into adipocytes.42 The Chr 4 locus was not effectively narrowed, and 7 of the 45 positional candidates contain cSNPs. A possible candidate gene is Xpa-encoding xeroderma pigmentosum complementation group A. Double mutant mice lacking Xpa and Xpd were completely free of body fat.43 The locus on Chr 11 was reduced to nine genes. The likely candidate gene, Gga3, contains a cSNP, is under the QTL peak (at 115 Mb), and encodes the Golgi-associated gamma-adaptin ear-containing ARF-binding protein 3. Adiponectin secretion is dependent on GGA proteins.44
In summary, we identified QTL for fat content and some are sex specific. Furthermore, we narrowed the QTL using bioinformatics and suggested some strong candidate genes. These genes need to be tested further to determine if they are indeed the genes underlying these QTL.
Supplementary Material
Acknowledgments
This work was funded by US National Institutes of Health grants CA034196, GM076468, HL 81162, HL74086 and HL77796, and the American Heart Association grant 0725905T (to SZ). We thank Harry Whitmore and Fred Rumill for their invaluable help in mouse husbandry, Jesse Hammer for graphical assistance and Dr Ed Leiter for helpful comments with regard to the paper.
References
- 1.Bell CG, Walley AJ, Froguel P. The genetics of human obesity. Nat Genet Rev. 2005;6:221–234. doi: 10.1038/nrg1556. [DOI] [PubMed] [Google Scholar]
- 2.Brockmann GA, Bevova MR. Using mouse models to dissect the genetics of obesity. Trends Genet. 2002;18:367–376. doi: 10.1016/s0168-9525(02)02703-8. [DOI] [PubMed] [Google Scholar]
- 3.Lusis AJ, Yu J, Wang SS. The problem of passenger genes in transgenic mice. Arterioscler Thromb Vasc Biol. 2007;27:2100–2103. doi: 10.1161/ATVBAHA.107.147918. [DOI] [PubMed] [Google Scholar]
- 4.Reed DR, McDaniel AH, Li X, Tordoff MG, Bachmanov AA. Quantitative trait loci for individual adipose depot weights in C57BL/6ByJ x 129P3/J F2 mice. Mamm Genome. 2006;17:1065–1077. doi: 10.1007/s00335-006-0054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishimori N, Li R, Kelmenson PM, Korstanje R, Walsh KA, Churchill GA, et al. Quantitative trait loci that determine plasma lipids and obesity in C57BL/6J and 129S1/SvImJ inbred mice. J Lipid Res. 2004;45:1624–1632. doi: 10.1194/jlr.M400098-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Flint J, Valdar W, Shifman S, Mott R. Strategies for mapping and cloning quantitative trait genes in rodents. Nat Rev Genet. 2005;6:271–286. doi: 10.1038/nrg1576. [DOI] [PubMed] [Google Scholar]
- 7.DiPetrillo K, Wang X, Stylianou IM, Paigen B. Bioinformatics toolbox for narrowing rodent quantitative trait loci. Trends Genet. 2005;21:683–692. doi: 10.1016/j.tig.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Nishina PM, Verstuyft J, Paigen B. Synthetic low and high fat diets for the study of atherosclerosis in the mouse. J Lipid Res. 1990;31:859–869. [PubMed] [Google Scholar]
- 9.Nagy TR, Clair AL. Precision and accuracy of dual-energy X-ray absorptiometry for determining in vivo body composition of mice. Obes Res. 2000;8:392–398. doi: 10.1038/oby.2000.47. [DOI] [PubMed] [Google Scholar]
- 10.Broman KW, Wu H, Sen S, Churchill GA. R/qtl: QTL mapping in experimental crosses. Bioinformatics. 2003;19:889–890. doi: 10.1093/bioinformatics/btg112. [DOI] [PubMed] [Google Scholar]
- 11.Solberg LC, Baum AE, Ahmadiyeh N, Shimomura K, Li R, Turek FW, et al. Sex- and lineage-specific inheritance of depression-like behavior in the rat. Mamm Genome. 2004;15:648–662. doi: 10.1007/s00335-004-2326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sen S, Churchill GA. A statistical framework for quantitative trait mapping. Genetics. 2001;159:371–387. doi: 10.1093/genetics/159.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B, et al. The human obesity gene map: the 2005 update. Obesity (Silver Spring) 2006;14:529–644. doi: 10.1038/oby.2006.71. [DOI] [PubMed] [Google Scholar]
- 14.Dai F, Keighley ED, Sun G, Indugula SR, Roberts ST, Aberg K, et al. Genome-wide scan for adiposity-related phenotypes in adults from American Samoa. Int J Obes (Lond) 2007;31:1832–1842. doi: 10.1038/sj.ijo.0803675. [DOI] [PubMed] [Google Scholar]
- 15.Wuschke S, Dahm S, Schmidt C, Joost HG, Al-Hasani H. A meta-analysis of quantitative trait loci associated with body weight and adiposity in mice. Int J Obes (Lond) 2007;31:829–841. doi: 10.1038/sj.ijo.0803473. [DOI] [PubMed] [Google Scholar]
- 16.Abiola O, Angel JM, Avner P, Bachmanov AA, Belknap JK, Bennett B, et al. The nature and identification of quantitative trait loci: a community’s view. Nat Rev Genet. 2003;4:911–916. doi: 10.1038/nrg1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang S, Yehya N, Schadt EE, Wang H, Drake TA, Lusis AJ. Genetic and genomic analysis of a fat mass trait with complex inheritance reveals marked sex specificity. PLoS Genet. 2006;2:e15. doi: 10.1371/journal.pgen.0020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reed DR, Li X, McDaniel AH, Lu K, Li S, Tordoff MG, et al. Loci on chromosomes 2, 4, 9, and 16 for body weight, body length, and adiposity identified in a genome scan of an F2 intercross between the 129P3/J and C57BL/6ByJ mouse strains. Mamm Genome. 2003;14:302–313. doi: 10.1007/s00335-002-2170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mouzeyan A, Choi J, Allayee H, Wang X, Sinsheimer J, Phan J, et al. A locus conferring resistance to diet-induced hypercholesterolemia and atherosclerosis on mouse chromosome 2. J Lipid Res. 2000;41:573–582. [PubMed] [Google Scholar]
- 20.Simpson EM, Linder CC, Sargent EE, Davisson MT, Mobraaten LE, Sharp JJ. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat Genet. 1997;16:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- 21.Almind K, Kahn CR. Genetic determinants of energy expenditure and insulin resistance in diet-induced obesity in mice. Diabetes. 2004;53:3274–3285. doi: 10.2337/diabetes.53.12.3274. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 23.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 24.Su Z, Li Y, James JC, Matsumoto AH, Helm GA, Lusis AJ, et al. Genetic linkage of hyperglycemia, body weight and serum amyloid-P in an intercross between C57BL/6 and C3H apolipoprotein E-deficient mice. Hum Mol Genet. 2006;15:1650–1658. doi: 10.1093/hmg/ddl088. [DOI] [PubMed] [Google Scholar]
- 25.Jenny NS, Arnold AM, Kuller LH, Tracy RP, Psaty BM. Serum amyloid P and cardiovascular disease in older men and women: results from the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 2007;27:352–358. doi: 10.1161/01.ATV.0000254150.97741.fe. [DOI] [PubMed] [Google Scholar]
- 26.Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stone S, Abkevich V, Hunt SC, Gutin A, Russell DL, Neff CD, et al. A major predisposition locus for severe obesity, at 4p15-p14. Am J Hum Genet. 2002;70:1459–1468. doi: 10.1086/340670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esterbauer H, Oberkofler H, Linnemayr V, Iglseder B, Hedegger M, Wolfsgruber P, et al. Peroxisome proliferator-activated receptor-gamma coactivator-1 gene locus: associations with obesity indices in middle-aged women. Diabetes. 2002;51:1281–1286. doi: 10.2337/diabetes.51.4.1281. [DOI] [PubMed] [Google Scholar]
- 29.Vimaleswaran KS, Radha V, Anjana M, Deepa R, Ghosh S, Majumder PP, et al. Effect of polymorphisms in the PPARGC1A gene on body fat in Asian Indians. Int J Obes (Lond) 2006;30:884–891. doi: 10.1038/sj.ijo.0803228. [DOI] [PubMed] [Google Scholar]
- 30.Pihlajamaki J, Kinnunen M, Ruotsalainen E, Salmenniemi U, Vauhkonen I, Kuulasmaa T, et al. Haplotypes of PPARGC1A are associated with glucose tolerance, body mass index and insulin sensitivity in offspring of patients with type 2 diabetes. Diabetologia. 2005;48:1331–1334. doi: 10.1007/s00125-005-1800-9. [DOI] [PubMed] [Google Scholar]
- 31.Ridderstrale M, Johansson LE, Rastam L, Lindblad U. Increased risk of obesity associated with the variant allele of the PPARGC1A Gly482Ser polymorphism in physically inactive elderly men. Diabetologia. 2006;49:496–500. doi: 10.1007/s00125-005-0129-8. [DOI] [PubMed] [Google Scholar]
- 32.Lowell BBVSS, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- 33.Oppert JM, Vohl MC, Chagnon M, Dionne FT, Cassard-Doulcier AM, Ricquier D, et al. DNA polymorphism in the uncoupling protein (UCP) gene and human body fat. Int J Obes Relat Metab Disord. 1994;18:526–531. [PubMed] [Google Scholar]
- 34.Heilbronn LK, Kind KL, Pancewicz E, Morris AM, Noakes M, Clifton PM. Association of -3826 G variant in uncoupling protein-1 with increased BMI in overweight Australian women. Diabetologia. 2000;43:242–244. doi: 10.1007/s001250050036. [DOI] [PubMed] [Google Scholar]
- 35.Fumeron F, Durack-Bown I, Betoulle D, Cassard-Doulcier AM, Tuzet S, Bouillaud F, et al. Polymorphisms of uncoupling protein (UCP) and beta 3 adrenoreceptor genes in obese people submitted to a low calorie diet. Int J Obes Relat Metab Disord. 1996;20:1051–1054. [PubMed] [Google Scholar]
- 36.Kim KS, Cho DY, Kim YJ, Choi SM, Kim JY, Shin SU, et al. The finding of new genetic polymorphism of UCP-1 A-1766G and its effects on body fat accumulation. Biochim Biophys Acta. 2005;1741:149–155. doi: 10.1016/j.bbadis.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 37.Oike Y, Akao M, Yasunaga K, Yamauchi T, Morisada T, Ito Y, et al. Angiopoietin-related growth factor antagonizes obesity and insulin resistance. Nat Med. 2005;11:400–408. doi: 10.1038/nm1214. [DOI] [PubMed] [Google Scholar]
- 38.Phan J, Peterfy M, Reue K. Lipin expression preceding peroxisome proliferator-activated receptor-gamma is critical for adipogenesis in vivo and in vitro. J Biol Chem. 2004;279:29558–29564. doi: 10.1074/jbc.M403506200. [DOI] [PubMed] [Google Scholar]
- 39.Phan J, Reue K. Lipin, a lipodystrophy and obesity gene. Cell Metab. 2005;1:73–83. doi: 10.1016/j.cmet.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Banerjee SS, Feinberg MW, Watanabe M, Gray S, Haspel RL, Denkinger DJ, et al. The Kruppel-like factor KLF2 inhibits peroxisome proliferator-activated receptor-gamma expression and adipogenesis. J Biol Chem. 2003;278:2581–2584. doi: 10.1074/jbc.M210859200. [DOI] [PubMed] [Google Scholar]
- 41.Oishi Y, Manabe I, Tobe K, Tsushima K, Shindo T, Fujiu K, et al. Kruppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab. 2005;1:27–39. doi: 10.1016/j.cmet.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Mori T, Sakaue H, Iguchi H, Gomi H, Okada Y, Takashima Y, et al. Role of Kruppel-like factor 15 (KLF15) in transcriptional regulation of adipogenesis. J Biol Chem. 2005;280:12867–12875. doi: 10.1074/jbc.M410515200. [DOI] [PubMed] [Google Scholar]
- 43.de Boer J, Andressoo JO, de Wit J, Huijmans J, Beems RB, van Steeg H, et al. Premature aging in mice deficient in DNA repair and transcription. Science. 2002;296:1276–1279. doi: 10.1126/science.1070174. [DOI] [PubMed] [Google Scholar]
- 44.Xie L, Boyle D, Sanford D, Scherer PE, Pessin JE, Mora S. Intracellular trafficking and secretion of adiponectin is dependent on GGA-coated vesicles. J Biol Chem. 2006;281:7253–7259. doi: 10.1074/jbc.M511313200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.