Abstract
Hesperidin is an abundant flavanone glycoside in citrus fruits and has been reported to possess a wide range of biological activities. However, hesperidin has poor bioavailability. Here, we test the hypothesis that hesperetin found in Chenpi will have a better bioavailability than hesperidin and that treatment of hesperidin with glucosidase-like yeast Bg1A protein will increase its bioavailability. The results indicate that hesperidin in pure or extract form is hydrolyzed by BglA protein extracted from S. singularis or expressed in E coli BL21 (DE3). This biotransformation affected the plasma pharmacokinetics of total hesperetin in rats, in that the plasma Tmax was significantly shorter after administration of BglA protein-treated hesperidin than after administration of hesperidin extract. In addition, the AUC values for total hesperetin after administration of Bg1A-treated hesperidin were approximately 4-fold higher by oral administration and 3-fold higher by intravenous administration, respectively. In contract, the plasma clearance value and volume of distribution after administration of Bg1A-treated hesperidin extract or pure hesperetin was significant smaller than after administration of untreated hesperidin extract or pure hesperidin In conclusion, this is a first study that systemically determines the absolute bioavailability of hesperidin and hesperetin simultaneously, and the study shows clearly that hesperetin is more bioavailable than hesperidin regardless of the route of administration, and that prior transformation of hesperidin to hesperetin via fermentation should significantly increase its bioavailability because of the action of yeast glycosidase-like protein BglA.
Keywords: hesperidin, chenpi, biotransformation, glycosidase, pharmacokinetics, bioavailability
INTRODUCTION
Hesperidin is an abundant flavanone glycoside found in citrus fruits, and is often consumed in the form of orange juices 1. Hesperidin has been reported to possess activities of anti-inflammatory, antioxidant and regulation of hepatic cholesterol synthesis 2-4. Other reported activities of these flavonoids include lowering cancer risk and inhibiting bone resorption 5-7. However, hesperidin, like many other flavonoids, has poor bioavailability 8-12. The reasons for this poor bioavailability include low water solubility of hesperidin, and disposition via the phase II enzymes 13, 14. Additionally, glycosides of hesperetin have been shown to be effluxed in Caco-2 cells 15, consistent with the observations that many flavonoid glycosides are effluxed by the enterocytes 16-18. The bioavailability is also highly variable, perhaps because inter-individual differences in human intestinal microflora that are capable of hydrolyzing hesperidin 19, 20.
In Western countries, hesperidin represents a major flavonoid consumed daily, and some estimates suggest that it could even be responsible for up to 50% of daily dietary intake of flavonoids in a few developed countries 5. Hesperidin is a major active ingredient in Chinese traditional medicinal herb called Chenpi, which is made from tangerine peel and has been used for than 10 centuries. Chenpi is also used in the traditional medicines of other Asian countries such as Japan, where it is called Chinpi 13. Chenpi has expectorant activities and broncho-dilative effect, and is usually used to treat upper respiratory tract infections in traditional Chinese medicinal formula. Chenpi is prescribed as an herbal medicine for inflammation, allergy and hepatopathy in Japan 13. Chenpi is prepared using a well-documented, and ancient 9-step fermentation procedures, because Chenpi so produced appeared to have greater bioactivities than air- or sun-dried tangerine peel. In preliminary studies, we isolated a yeast strain from Chenpi and identified it to be a yeast strain closely related to Sporobolomyces singularis. This later strain has glycosidase activities which were similar to other yeast strains identified previously to produce a glycosidase-like BglA protein 21, 22.
We were interested to determine the presence of BglA in S. singularis because this glucosidase-like protein, an enzyme isoform belonging to the family 1 glycosyl hydrolases, can hydrolyze hesperidin abundant in citrus peels to hesperetin. Since hesperetin is absorbed much faster than hesperidin 15, we hypothesized that ancient Chinese fermentation methods increase the bioavailability of Chenpi by removing the glycosides and thereby increasing the bioavailability of hesperidin (Fig. 1). Testing this hypothesis allows us to provide a mechanistic explain why fermentation-prepared Chenpi may have higher activities, which until now is unclear. To test this hypothesis, we biotransformed hesperidin extract with BglA protein extracted from S. singularis or expressed in E coli BL21 (DE3) in vitro. We also determined how the BglA protein influenced the plasma pharmacokinetics of total hesperetin in rats after administration of BglA protein-treated or untreated hesperidin extracts. For comparison purpose, pharmacokinetic studies were also conducted using pure hesperetin and its glycoside hesperidin.
Fig. 1.
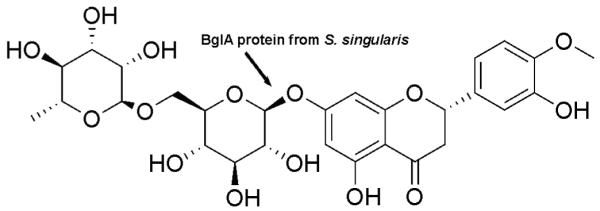
The structure of hesperidin and the proposed site of hydrolysis by BglA protein
MATERIALS AND METHODS
Materials
Chenpi was obtained from a local drugstore. Standard hesperidin was purchased from National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Standard hesperetin, β-glucuronidase type VII-A and H-5, β-glucosidase, and p-nitrophenyl-β-D-glucopyranoside (PNP-Glc) were purchased from Sigma Chemical Co. (St. Louis, USA). All other chemicals were of analytical or HPLC grade. S. Singularis (ATCC 24193) were obtained from American Type Culture Collection (Manassas, Virginia, USA) and were cultured in YM medium contained 3 g of yeast extract, 3g of malt extract, 5 g of peptone, 10 g of dextrose and 20 g of agar in 1.0 liter of distilled water (pH 5.0), and were maintained aerobically at 24°C.
Extraction of Hesperidin from Chenpi
The hesperidin was extracted from Chenpi with an ultrasonic method adapted from a published report 23. Briefly, 4 g Chenpi were ground to the size of 0.5~1mm, blended with 200 ml methanol, and placed in the ultrasonic machine (KQ-100A, Kunming, China) running at 60mHz for 60 min at 40°C. After initial extract, the solvent was recovered and then replace with fresh methanol and the process was then repeated. At the end of second extraction procedure, the methanol is then separated from the Chenpi and then combined with the first methanol extract solution. The combined extract solution was filtered, and the filtrate was evaporated to dryness. The residues were again dissolved in medium at a maximum of 5% (v/v) propylene glycol with a concentration of 10mg/ml. Then the solutions were acidified with 0.1 ml of 0.01M oxalic acid and quantified by HPLC.
Extraction and Purification of BglA Protein from S. Singularis
The S. Singularis yeast cells in mid-exponential phase were harvested, and centrifuged (2,000 × g, 5 min). The pellet was washed twice with 50 mM sodium phosphate buffer (pH 7.0) and cells were resuspended in 50 mM phosphate citrate buffer (pH 4.0) and treated with 1% (w/v) Usukizyme (Kyowa, Japan) for 2 h at 37°C. The soluble fraction of lysate was mixed with the supernatant prepared by homogenization to give a crude extract. The purification steps were carried out according to previous reports 24. Briefly, ammonium sulfate was added to the cell extracts, and then centrifuged (10,000× g, 30 min). The pellet was resuspended in 50 mM potassium phosphate buffer (pH7.0) and loaded onto a DEAE Sepharose CL-6B column (50 × 200 mm, Pharmacia, Swedish). Further purification was done by gel filtration on a Sephacryl S-300 HR column (15 × 900 mm, Pharmacia, Swedish). The eluent was 50 mM sodium phosphate buffer containing 0.1 M NaCl (pH7.2). The β-glucosidase activity of the eluted fractions was measured as described later.
Cloning, Expression and Purification of the BglA Gene in E. coli BL21 (DE3)
S. Singularis were harvested and total RNA was extracted with TRIzol reagent (Invitrogen, USA). PCR was performed using the following procedure: initial denaturation, 94°C for 2 min; 30 cycles with 94°C for 30 s, 58°C for 30 s, and 72°C for 2 min; final extension at 72°C for 5 min. PCR products were resolved by 1.5% agarose gel electrophoresis, stained with ethidium bromide, and visualized with the UV light. A pair of primers; 5′-CGC GGATCC ATGATGCTGCATGCGGCAC -3′ (forward primer) and 5′-CCC AAGCTT TCAGAGGTGGTTGCGACC-3′ (backward primer) were designed and synthesized with a BamH I and a Hind III restriction site added to the primers (underlined). PCR product was digested with BamH I and Hind III. The digested fragments were purified by gel extraction, and then ligated to the pQE30-Xa vector (Qiagen, Germany) to generate the recombinant expression vector, named pQE30-Xa-BglA. The ligated sequence was then confirmed by sequencing (not shown).
Subsequently, pQE30-Xa-BglA was used to transfect the E. coli BL21 (DE3) strain. Briefly, a single transfected colony was picked from a selective plate, and inoculated into LB medium supplemented with ampicillin (200μg/ml). When OD600 of the cultured media reached 0.6, isopropyl-beta-D-thiogalactopyranoside (IPTG) was added to the media at a final media concentration of 1 mM. After additional 4 hr of incubation, a portion of the bacteria were suspended in 200 μl of sample buffer and boiled for 5 min at 90°C. The expression of BglA was detected by SDS-PAGE, carried out according to the method of Laemmli 25. Proteins were stained with the Bio-Safe Coomassie G250 stain (Amresco, USA) according to the instruction of the manufacturer. The remaining bacteria were harvested, and washed twice with 50 mM potassium phosphate buffer (pH 7.0) to pellet the bacteria using centrifugation (4,000 × g, 20 min).
To purify the BglA protein form the bacteria, each gram of cell pellet was resuspended in 3 ml of TE buffer. Following the addition of lysozyme to a final concentration of 0.2 mg/ml, the cell suspension was incubated for 20 min at 37°C with gentle shaking. Then cell extracts were obtained by centrifugation (12,000 ×g, 30 min) and the β-glucosidase activity of the crude BglA protein was measured as described below. Additional purification step, as described previously using various columns, was needed to further purify the BglA protein from bacteria to higher specific activities.
Assay for β-Glucosidase Activity of BglA Protein
The β-glucosidase activity was measured using PNP-Glc as the substrate 24. The incubation mixture (total volume=1 ml) contained 1.5 mM PNP-Glc, 50 mM phosphate buffer (pH6.0), and 0.1 ml solution of crude or purified enzyme preparation. The reaction was carried out at 37°C for 4h and then terminated by adding 4 ml of 0.25 M Na2CO3. The absorbance at 420 nm, subtracting the blank, was used as a measure of the amount of p-nitrophenol (PNP) released. One unit of β-glucosidase activity was defined as the amount of enzyme that hydrolyzes 1 μmol of PNP-Glc per minute under the assay conditions.
In Vitro Biotransformation of Hesperidin by BglA Protein
Each of the reaction mixture (total = 1 ml) containing 0.5 μM hesperidin, 50 mM phosphate citrate buffer (pH6.0) and 0.01 mg of enzyme equivalent of BglA protein either extracted from S. Singularis or expressed in E. coli BL21 (DE3). The mixture without enzyme was pre-incubated at 40°C for 5 min and the reaction was started by the addition of enzyme. After incubated at 40°C for 30 min, the reaction was stopped by boiling for 5 min. Another two mixtures were incubated under the same condition, which were the positive control of β-glucosidase and the mock control. The hesperidin and hesperetin in the reaction mixture were analyzed by HPLC. Samples removed from the reaction mixture for HPLC application was centrifuged (3,000 × g, 20 min) and extracted with 500 μl methanol from the supernatant. The organic layer was transferred to a microcentrifuge tube and dried under vacuum. The residue was reconstituted with 100 μl methanol and centrifuged (3,000 × g, 10 min). Then the supernatant was transferred for HPLC analysis. Experiments were carried out in triplicate for each experiment.
HPLC Assays of Hesperidin and Hesperetin
The concentrations of hesperidin and hesperetin were analyzed by HPLC as describe bellow; system, Shimadzu LC-6 with variable wavelength detector; column, YWG-C18 (4.6×150mm, 5μm, SHIMADZU); column temperature, 30°C; injection volume, 20 μl; detector wavelength, 283 nm; flow rate, 1 ml/min; and mobile phase, 40:60 (v/v) mixture of methanol and 0.04 M KH2PO4 (pH adjusted to 4.0 using H3PO4). Quantification of hesperidin and hesperetin peaks from all matrices was based on the standard curves. For the analysis of hesperetin and hesperidin, standard solutions containing 0.00, 0.002, 0.005, 0.02, 0.05, 0.2, 0.5 and 1μmol of added hesperetin/L or hesperidin/L were prepared, respectively. The standard curve was obtained by plotting the peak height of standards versus hesperetin and hesperidin concentrations. Day-to-day variation (CV %) for hesperetin and hesperidin from all matrices were 11% and within-day variation for them were 7%. The limits of detection for hesperetin and hesperidin from plasma were 1.5 nmol/L and 3.5 nmol/L, respectively. The linear response ranges of the method for hesperetin and hesperetin were 1.5~1000 nmol/ml and 3.5~1000 nmol/ml, respectively. The equations for the linear responses, described by the relationship between peak height (y) and concentration (x), were y= 0.1676x- 0.0092 (r2= 0.998) for hesperidin and y= 0.1083 x -0.0065 (r2= 0.998) for hesperetin, respectively. Recoveries of these compounds by this methods were >95%.
Animals and Study Design
Male Wistar rats (6 weeks old, 180~220g, Beijing Administration Office of Laboratory Animal, Beijing, China. Certification number: SCXK-Military-2002) were housed in an air-conditioned room (23±2°C) under a 12h light-dark circle for a one-week period of acclimatization. Rats were fasted overnight before the experiments. The animals were handled according to the Guidelines for the Care and Use of Laboratory Animals, and the experiments were conducted at Tianjin Medical University.
Before the experiment started, rats were weighted and divided by weight in ten groups as follows: in test group 1 (test 1), rats were gavaged with purified hesperidin; in test 2, rats were gavaged with purified hesperetin; in test 3, rats were gavaged with the untreated hesperidin extracts; in test 4, were gavaged with hesperidin extract treated with expressed BglA protein; and in test 5 rats, rats were gavaged with hesperidin treated with BglA protein isolated from S. singularis. The other 5 tests (5 rats per test) were carried out using hesperidin or hesperetin administered intravenously with the chemicals in the same order as above. For both oral and IV administration, 18.9mg/kg of hesperidin or 9.4mg/kg of hesperetin was administered as an aqueous isotonic solution at a maximum of 5% (v/v) propylene glycol. The dose of flavonoids given was based on recommended amounts of Chenpi given to people by the Chinese Pharmacopoeia. In cases of oral administration, blood samples were withdrawn from the jugular artery cannula before and at 0, 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 14, and 24 h after flavonoid administration. Before flavonoids solution can be used intravenously, the solution was processed to minimize potential hazards to the rats. First, flavonoid solution was carefully chilled (4°C), filtered and centrifuged (10,000 ×g, 2 min) to remove any particulate matters. After the solution was shown to be non-hemolytic, it was autoclaved at 121°C, cooled, and then used for intravenous administration. Similar to oral administration, the plasma samples were collected before intravenous administration and at 0, 0.5, 1, 2, 4, 6, 8, 12 and 24 h after IV administration. Plasma samples were prepared by centrifugation (10,000 ×g, 2 min), and the plasma was removed and stored at -20°C within 30 min after a blood sample was taken.
The plasma samples after administration were used for the quantification of total hesperetin, after the metabolites are released by hydrolysis with β-glucuronidase. Plasma samples (50 μl) were incubated with appropriate amounts of β-glucuronidase type H-5 (500 unites/ml) in 0.1 mM sodium acetate buffer (pH5.0) at 37°C for 90 min. To stop the reaction of enzyme, 0.01M oxalic acid (100μl) was added into the reaction mixture. Then the mixture was extracted with 500 μl methanol by vortexing the mixture for 2 min and centrifugation (3,000 ×g, 10 min). The supernatant layer was transferred to a microcentrifuge tube and vacuum-dried. The residue was reconstituted with 100 μl methanol, vortexed and then centrifuged (3,000 ×g, 10 min) to produce samples for HPLC analysis.
Calculation of Pharmacokinetic indexes and Statistical Analysis
The pharmacokinetic analysis of the data was performed using WinNonlin (Pharsight, CA), (Version 5.2 Build 200701231637). The pharmacokinetic parameters were derived using the non-compartmental analysis, and linear trapezoidal (Linear Interpolation) method, other pharmacokinetic parameters were determined using standard equalizers. Each rat plasma concentration versus time course was analyzed individually. The statistical analysis of the pharmacokinetic parameters was achieved using one-way ANOVA with pos hoc analysis. Statistically significant difference was set at 5% or P<0.05.
RESULTS
Hydrolysis hesperidin by BglA protein in vitro
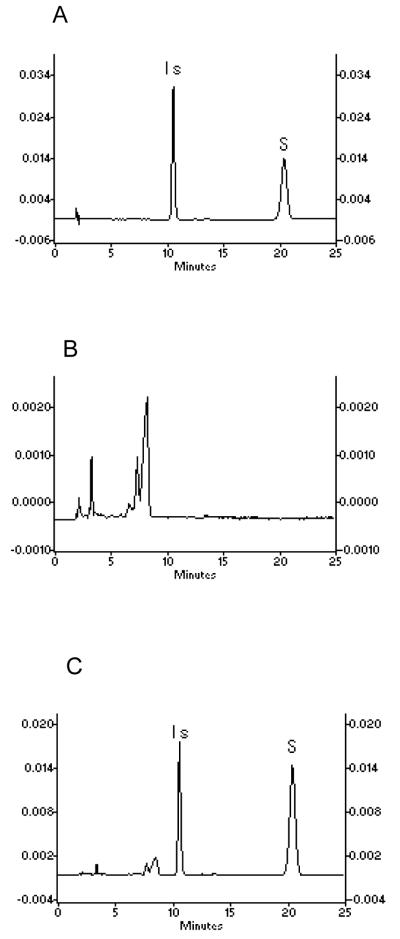
BglA protein, whether isolated from S. Singularis or expressed in E coli BL21 (DE3), was capable of hydrolyzing hesperidin exclusively to hesperetin (Fig. 2), as hesperetin was not detected in a mock mixture free of it. In BglA protein-treated hesperidin extracts, hesperetin concentration was nearly similar to that treated with commercial β-glucosidase (P>0.05) (not shown). In addition, the source of BglA protein did not affect their activities as two different sources of BglA protein had nearly identical activities (not shown). Lastly, the identity of hesperidin was verified by comparing to an authentic standard using HPLC (as shown in Fig. 3), since HPLC clearly separated hesperetin (tR=10.77 min) from hesperidin (tR=20.38 min).
Fig. 2.
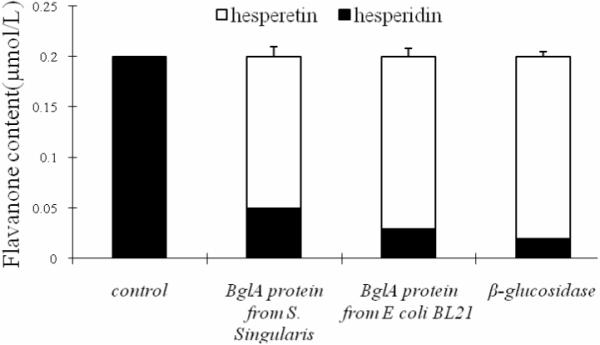
Metabolism of hesperidin by BglA protein and β-glucosidase. The experiments were performed using 0.5 μmol/L hesperidin and 0.01mg of enzyme equivalent prepared from the yeast or expressed in E. Coli (DE3) using procedures described in Materials and Methods.
Fig. 3.
Representative HPLC chromatograms of (A) the standards of hesperetin and hesperidin. (B) blank rat plasma. (C) 0.02 μmol/L hesperetin and 0.02 μmol/L hesperidin in rat plasma.
Identification of BglA expressed in E. Coli
SDS-PAGE analysis of the cell extract of E coli BL21 (DE3) transfected with BglA gene revealed a predominant protein band of approximately 66 KDa (Fig. 4), which was in close agreement with the expected molecular mass of 65.67 KDa deduced from the amino acid sequence of the BglA gene. This predominant protein band was not detected in the cell extract from E. coli BL21 (DE3) not transfected with BglA plasmid. Lastly, the BglA protein activity in transfected E. coli BL21 (DE3) was approximately 100-fold higher than that in cell extracts from S. Singularis (0.3 U mg of protein-1 versus 0.0027 U mg of protein-1).
Fig. 4.
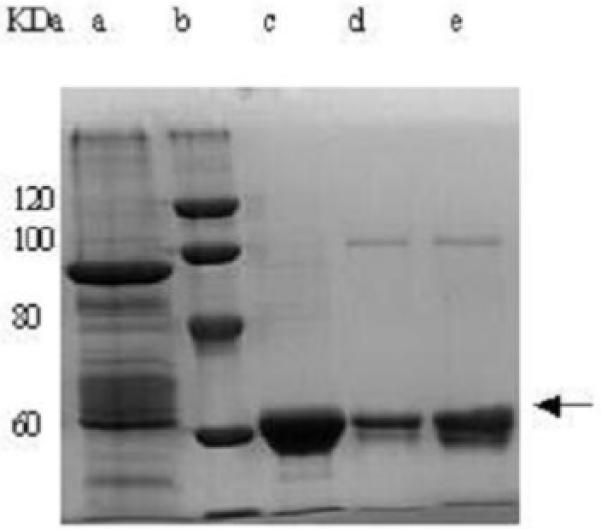
SDS-PAGE of BglA protein extracted from E coli BL21 (DE3) and undergoing various steps of purifications. Lane a: bacteria cell extract; Lane b: molecular mass standards; Lane c: (NH4)2SO4 precipitate (55 to 60% saturation); Lane d: pooled DEAE Sepharose fractions; Lane e: pooled Sephacryl fractions. The narrow band indicates BglA protein.
The BglA protein was enriched as described previously (in Methods) and a summary of the purification procedure of BglA protein from transfected E. coli BL21 (DE3) along with each step’s yield is presented in Table 1. The heterologously expressed BglA protein was purified by ammonium sulfate fractionation and column chromatography with an (overall) 19.3-fold enrichment and the recovery yield was 7.5%.
Table 1.
Purification of BglA protein expressed in E coli BL21(DE3)
| Step | Total activity (U) | Total protein (mg) | Specific activity (U/mg protein)* | Purification (fold) | Recovery (%) |
|---|---|---|---|---|---|
| Crude extract | 216.0 | 772.0 | 0.3 | 1.0 | 100.0 |
| Ammonium sulfate | 49.2 | 19.6 | 2.5 | 8.4 | 22.8 |
| DEAE Sepharose | 25.6 | 8.5 | 3.0 | 10.0 | 11.9 |
| Sephacryl | 16.2 | 2.8 | 5.8 | 19.3 | 7.5 |
One unit of β-glucosidase activity was defined as the amount of enzyme that produced 1μmol of PNP-Glc per min under the assay condition.
Effects of BglA protein treatment on the in vivo pharmacokinetics of total hesperetin in rats after IV administration
We first conducted parallel iv pharmacokinetic studies of hesperetin and hesperidin because it has not been done in the same laboratory. Previously, pharmacokinetic studies of hesperetin or hesperidin have been conducted separately, and the major chemical species found in human plasma are glucuronide conjugates, while the sulfates or sulfate and glucuronide conjugates were only detected in minor quantity 8, 11. This was confirmed by another study in which free hesperetin or hesperidin was not detected in the rat plasma after administration of hesperidin not treated with β-glucuronidase13. Based on these published literatures and the fact that hesperetin glucuronides are not commercially available, plasma samples from the present study were also treated with β-glucuronidase to release aglycone hesperetin. Our experiment could not quantify the hesperetin conjugates due to lack of standards; therefore we termed hesperetin released with the β-glucuronidase from all species of hesperetin conjugates as “total hesperetin.’ And we quantified the plasma concentration of total hesperetin.
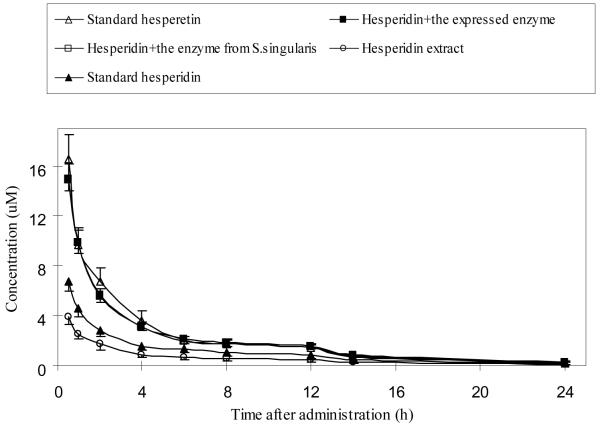
Analysis of plasma concentration-time curves of total hesperetin (shown in Fig. 5) following IV administration indicated that pure hesperetin has a much large AUC value than pure hesperidin, and that the relative bioavailability of hesperetin doubled that of hesperidin (61.5 vs. 30.1 nmol . hr/ml) (Table 2). This large AUC value appears to be consistent with slower clearance of hesperetin when compared to hesperidin (1.17 vs. 0.56 ml/hr). However, we also see that hesperetin has a much smaller volume of distribution, perhaps due to its tight binding to the plasma protein when compared to hesperidin. Several other important pharmacokinetics parameters of pure compounds were shown in Table 2.
Fig. 5.
Changes in the plasma concentration of total hesperetin in rats following IV administration. The rats were divided into five groups, and each rat was administered intravenously 31μmol/kg equivalent amount of hesperidin. Plasma samples were treated with β-glucuronidase and therefore the plasma levels represented total hesperetin equivalent. Values were presented as mean±SEM (n=5).
Table 2.
Pharmacokinetic Parameters of Total Hesperetin After Single IV Administration of Hesperidin or Hesperetin or Hesperidin Treated with Different BlgA Protein Treatment
| Parameters | Pure Hesperidin | Pure Hesperetin | Hesperidin Extract untreated | Hesperidin Extract+BlgA from E. Coli | Hesperidin Extract+BlgA from yeast |
|---|---|---|---|---|---|
| AUCobs AVG (nmol · hr/ml) SD |
30.09 | 61.47 a | 18.01 | 59.40 b | 57.90 b |
| 12.88 | 22.20 | 10.86 | 14.68 | 11.73 | |
| Half-life AVG (hr) SD |
6.28 | 6.52 | 6.25 | 6.05 | 6.15 |
| 0.28 | 0.61 | 0.81 | 1.01 | 0.32 | |
| Vz AVG (ml) SD |
10.73 | 5.40 a | 19.27 | 4.70 b | 4.88 b |
| 4.34 | 2.42 | 7.44 | 0.47 | 0.82 | |
| CL AVG (ml/hr) SD |
1.17 | 0.56 a | 2.10 | 0.54 b | 0.55 b |
| 0.43 | 0.22 | 0.81 | 0.11 | 0.09 |
There are statistically significant differences between AUC0bs for pure hesperidin and pure hesperetin (p<0.05).
There are statistically significant differences between AUC0bs, Vz, CL for hesperidin with different BlgA protein treatment and untreated hesperidin extract (P<0.05).
In addition to pure compounds, we also injected purified hesperidin extracts with or without BlgA protein treatment, which hydrolyzed hesperidin to hesperetin. As expected, the hesperidin extract without BglA treatment behaved just like hesperidin shown earlier, whereas those treated with BglA protein behaved almost like pure hesperetin. The only exception is that the untreated hesperidin extract has even smaller AUC value, faster clearance, and larger volume of distribution than pure hesperidin, suggesting that the herbal mixture contained additional phytochemicals that may interact with hesperidin to moderately change its pharmacokinetic parameters.
Effects of BglA protein treatment on the in vivo pharmacokinetics of total hesperetin in rats after oral administration
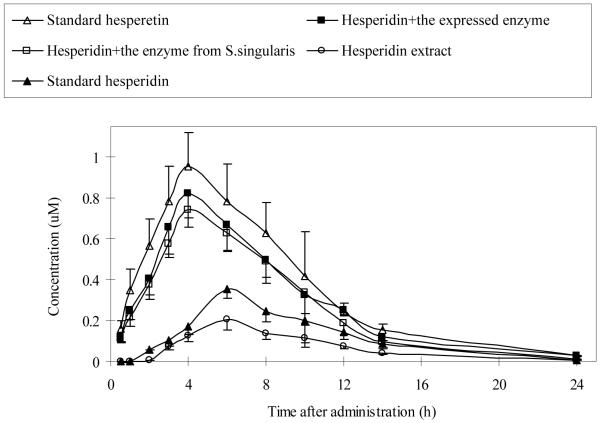
Hesperetin or hesperidin was absorbed by rats from all treatment groups. The corresponding times to reach maximum plasma concentration (Tmax) in rats were significantly shorter after administrations of BglA protein-treated hesperidin and the standard hesperetin than those in the other two groups (Fig. 6). After dosing with the standard hesperidin and hesperidin extract, total hesperetin were detected at 2 h, and reached a maximum (about 0.35 μM and 0.20 μM) at 6 h (Table 3). On the other hand, after the administration of BglA protein-treated hesperidin and the standard hesperetin, total hesperetin were detected as early as 30 min after administration and reached a maximum concentration (about 0.82 μM, 0.74 μM, and 0.95 μM) at 4 h (Table 3). The results showed that plasma Cmax and Tmax levels were similar in hesperetin and BglA treated hesperidin groups since the latter rapidly hydrolyzed hesperidin to hesperetin.
Fig. 6.
Changes in the plasma concentration of total hesperetin in rats following oral administration. The rats were divided into five groups, and each rat was administered orally 31μmol/kg equivalent amount of hesperidin. Plasma samples were treated with β-glucuronidase and therefore the plasma levels represented total hesperetin equivalent. Values were presented as mean±SEM (n=5).
Table 3.
Pharmacokinetic Parameters of Total Hesperetin After Single Oral Administration of Hesperidin or Hesperetin or Hesperidin Treated with Different BlgA Protein Treatment
| Parameters | Pure Hesperidin | Pure Hesperetin | Hesperidin Extract untreated | Hesperidin Extract+BlgA from E. Coli | Hesperidin Extract+BlgA from yeast |
|---|---|---|---|---|---|
| AUCobs AVG (nmol · hr/ml) SD |
2.63 | 7.91 a | 1.50 | 6.82 b | 5.93 b |
| 1.48 | 4.15 | 0.79 | 2.53 | 2.26 | |
| Cmax AVG (nmol/ml) SD |
0.35 | 0.95 | 0.20 | 0.82 | 0.74 |
| 0.10 | 0.37 | 0.10 | 0.27 | 0.19 | |
| Tmax AVG (hr) SD |
6 | 4 | 6 | 4 | 4 |
| 0 | 0 | 0 | 0 | 0 | |
| Vz AVG (ml) SD |
9.86 | 5.65 a | 13.14 | 2.75 b | 4.29 b |
| 5.05 | 5.28 | 1.73 | 1.55 | 2.09 | |
| CL AVG (ml/hr) SD |
56.49 | 19.15 a | 114.06 | 24.48 b | 26.95 b |
| 27.42 | 6.34 | 32.24 | 5.99 | 10.32 | |
| F(%) AVG SD |
8.53 | 13.26 | 10.36 | 11.39 | 10.03 |
| 2.30 | 4.68 | 8.31 | 3.02 | 2.35 |
There are statistically significant differences between AUC0bs for pure hesperidin and pure hesperetin (p<0.05).
There are statistically significant differences between AUC0bs, Vz, CL for hesperidin with different BlgA protein treatment and untreated hesperidin extract (p<0.05).
Whereas the untreated hesperidin not only was absorbed slower, it also had much smaller AUC values following IV administration (2 folds). In oral administration, the difference between pure hesperetin and hesperidin was more than 3 folds, whereas the difference between untreated hesperidin and BglA protein treated hesperidin was even more at 4 folds or higher. Again, this is similar to IV administration where extract had bigger difference since extract may contain additional phytochemicals that may interact with the absorption and/or disposition process of hesperidin.
DISCUSSION
Hesperidin has been reported to have multiple biological activities but has poor bioavailability. Chenpi is the common source of hesperidin, and it has been found to have higher bioactivities after a nine-step fermentation process. Moreover a yeast strain closely related to Sporobolomyces singularis and containing β-glycosidase-like BglA protein was isolated from Chenpi. Therefore, the present hypothesis is that better herbal materials may result from fermentation process because it produces more bioavailable active species.
Our study provided several strong evidences to show that hydrolysis of hesperidin could be enabled in vitro by using yeast enzymes presented in the Chenpi fermentation process. For example, yeast BglA protein extracted from S. singularis or expressed in E coli BL21 (DE3) (Fig.4) are active against hesperidin (Fig. 2). In the presence of this BglA protein (Fig.2), which hydrolyzed hesperidin to hesperetin, the total plasma hesperetin pharmacokinetics was significantly altered following iv and oral administrations (Fig. 5 and Fig.6). For example, the plasma Tmax was significantly shorter and the plasma concentrations of total hesperetin were significantly higher after oral administration of BglA protein-treated hesperidin (Fig. 6). Similarly, AUC values of hesperetin or treated hesperidin were also significantly higher than hesperidin or untreated hesperidin extract (Table 2 and Table 3). Therefore, fermentation process utilizing yeasts capable of secreting BglA protein will likely result in higher bioavailability of the flavonoids presented in Chenpi.
The higher bioavailability is likely the result of increased uptake of hesperetin after administration of BglA protein-treated hesperidin (contained mostly hesperetin) as compared to untreated hesperidin extract (contained mostly hesperidin). Faster absorption of hesperetin as compared to hesperidin was shown previously in the Caco-2 cell culture model and various rat and human pharmacokinetic studies 13, 26. In our own studies, the data appear to suggest that efflux has contributed to slower absorption for hesperidin (glycoside) when comparing to hesperetin (aglycone), since Tmax was longer and Cmax was smaller for the glycoside (Fig. 6).
Another important reason why hesperetin or BglA treated hesperidin extract has higher bioavailability is faster clearance of the glycosides. In fact, clearance values were higher following both oral and iv administrations. Since we have conducted first side-by-side study of this kind, our data provided for the first time strong evidence that hesperidin (a glycoside) was also eliminated much faster than hesperetin (aglycone). This is an important finding since it complements with current theory that glycosides have poor bioavailability because they are poorly permeable, or effluxed by transporters such as MPR2 15, 17, 18, 27. Another reason is extensive metabolism of aglycone (hesperetin) after absorption 8, 13, 26.
Although hesperidin is poorly absorbed and rapidly eliminated, it has reasonable half-life (6 hr). This reasonable half-life came first from prolonged absorption phase, as shown by a longer Tmax (Table 3). The prolonged absorption phase came from its poor absorption, which forced a large portion of glycosides (e.g. hesperidin) to the ileum and the colon, where bacteria β-glucosidases capable of cleaving the flavonoid glycosides are abundantly present and impact bioavailability 28. Because of the action of intestinal microflora, significant absorption of hesperetin would occur after hesperidin is gradually hydrolyzed in the terminal ileum and colon.
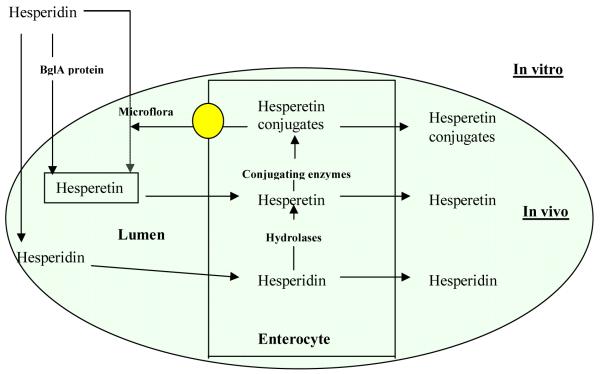
Another reason for hesperetin to have a reasonable plasma half-life, as evidenced by the maintenance of total hesperetin concentration at a reasonable level even at 24 hrs, is that hesperetin participates in enteric and enterohepatic dual recycling 29. This is because hesperetin has been shown to be extensively metabolized into phase II conjugates such as glucuronides and sulfates. These two metabolites are formed and eliminated by the enterocytes and hepatocytes with substantial portion of these metabolites excreted into the intestinal lumen (Fig.7). At lower small intestine and colon, these metabolites may be hydrolyzed by the bacteria β-glucuronidases and sulfatases to release the absorbable aglycones, which may be re-absorbed into the plasma (Fig. 7).
Fig. 7.
Possible pathways for hesperidin and hesperidin disposition in the gut. The figure shows the hydrolysis of hesperidin by BglA protein in vitro, by microflora in lumen in vivo, transport pathways of hesperidin and hesperetin, and disposition pathways of hesperidin and hesperetin.
In conclusion, this is the first comprehensive and comparative study of iv and oral pharmacokinetic of both hesperidin and hesperetin in the same lab. The results indicate for the first time that rapid plasma clearance is partially responsible for poor bioavailability of flavonoid glycosides, adding to our knowledge that rapid clearance together with poor permeation, low solubility, and/or extensive intestinal efflux are responsible for their poor bioavailability. Because hesperetin, the aglycone form of hesperidin, is much better absorbed with slower clearance and therefore higher bioavailability, classical fermentation process utilizing BglA protein-containing yeasts should produce Chenpi that have more bioavailable hesperetin than dried citrus peels, thereby increasing its biological activities.
Acknowledgments
This study was supported by The Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry of P. R. China, Tianjin Municipal Science and Technology Commission (No: 05YFJMJC03900, 08JCZDJC23400), and The National Natural Science Foundation of China (NSFC) (No: 30772483) to all authors except Mng Hu. Ming Hu is supported by a grant from The National Institutes of Health of United States of America, GM070737. We thank Dr. Lin Wen for the assistance with the parameters calculation using the WinNonlin software in the lab of Prof. Tony Kong (Department of Pharmaceutics, Ernest-Mario School of Pharmacy, Rutgers, the State University of New Jersey, Piscataway, NJ).
References
- 1.Mouly P, Gaydou EM, Auffray A. Simultaneous separation of flavanone glycosides and polymethoxylated flavones in citrus juices using liquid chromatography. J Chromatogr A. 1998;800(2):171–9. doi: 10.1016/s0021-9673(97)01131-x. [DOI] [PubMed] [Google Scholar]
- 2.Yeh CC, Kao SJ, Lin CC, Wang SD, Liu CJ, Kao ST. The immunomodulation of endotoxin-induced acute lung injury by hesperidin in vivo and in vitro. Life Sci. 2007;80(20):1821–31. doi: 10.1016/j.lfs.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 3.Kim JY, Jung KJ, Choi JS, Chung HY. Hesperetin: a potent antioxidant against peroxynitrite. Free Radic Res. 2004;38(7):761–9. doi: 10.1080/10715760410001713844. [DOI] [PubMed] [Google Scholar]
- 4.Wilcox LJ, Borradaile NM, de Dreu LE, Huff MW. Secretion of hepatocyte apoB is inhibited by the flavonoids, naringenin and hesperetin, via reduced activity and expression of ACAT2 and MTP. J Lipid Res. 2001;42(5):725–34. [PubMed] [Google Scholar]
- 5.Knekt P, Kumpulainen J, Jarvinen R, Rissanen H, Heliovaara M, Reunanen A, Hakulinen T, Aromaa A. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002;76(3):560–8. doi: 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka T, Kohno H, Mori H. Chemoprevention of Colon Carcinogenesis by Dietary Non-nutritive Compounds. Asian Pac J Cancer Prev. 2001;2(3):165–177. [PubMed] [Google Scholar]
- 7.Yamaguchi M, Hamamoto R, Uchiyama S, Ishiyama K. Effects of flavonoid on calcium content in femoral tissue culture and parathyroid hormone-stimulated osteoclastogenesis in bone marrow culture in vitro. Mol Cell Biochem. 2007;303(12):83–8. doi: 10.1007/s11010-007-9458-x. [DOI] [PubMed] [Google Scholar]
- 8.Erlund I, Meririnne E, Alfthan G, Aro A. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J Nutr. 2001;131(2):235–41. doi: 10.1093/jn/131.2.235. [DOI] [PubMed] [Google Scholar]
- 9.Gil-Izquierdo A, Gil MI, Ferreres F, Tomas-Barberan FA. In vitro availability of flavonoids and other phenolics in orange juice. J Agric Food Chem. 2001;49(2):1035–41. doi: 10.1021/jf0000528. [DOI] [PubMed] [Google Scholar]
- 10.Kanaze FI, Bounartzi MI, Georgarakis M, Niopas I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur J Clin Nutr. 2007;61(4):472–7. doi: 10.1038/sj.ejcn.1602543. [DOI] [PubMed] [Google Scholar]
- 11.Manach C, Morand C, Gil-Izquierdo A, Bouteloup-Demange C, Remesy C. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur J Clin Nutr. 2003;57(2):235–42. doi: 10.1038/sj.ejcn.1601547. [DOI] [PubMed] [Google Scholar]
- 12.Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 13.Yamada M, Tanabe F, Arai N, Mitsuzumi H, Miwa Y, Kubota M, Chaen H, Kibata M. Bioavailability of glucosyl hesperidin in rats. Biosci Biotechnol Biochem. 2006;70(6):1386–94. doi: 10.1271/bbb.50657. [DOI] [PubMed] [Google Scholar]
- 14.Jeong EJ, Liu X, Jia X, Chen J, Hu M. Coupling of conjugating enzymes and efflux transporters: impact on bioavailability and drug interactions. Curr Drug Metab. 2005;6(5):455–68. doi: 10.2174/138920005774330657. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi S, Tanabe S, Sugiyama M, Konishi Y. Transepithelial transport of hesperetin and hesperidin in intestinal Caco-2 cell monolayers. Biochim Biophys Acta. 2007 doi: 10.1016/j.bbamem.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Liu Y, Dai Y, Xun L, Hu M. Enteric disposition and recycling of flavonoids and ginkgo flavonoids. J Altern Complement Med. 2003;9(5):631–40. doi: 10.1089/107555303322524481. [DOI] [PubMed] [Google Scholar]
- 17.Walgren RA, Walle UK, Walle T. Transport of quercetin and its glucosides across human intestinal epithelial Caco-2 cells. Biochem Pharmacol. 1998;55(10):1721–7. doi: 10.1016/s0006-2952(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 18.Walle UK, French KL, Walgren RA, Walle T. Transport of genistein-7-glucoside by human intestinal CACO-2 cells: potential role for MRP2. Res Commun Mol Pathol Pharmacol. 1999;103(1):45–56. [PubMed] [Google Scholar]
- 19.Kim DH, Jung EA, Sohng IS, Han JA, Kim TH, Han MJ. Intestinal bacterial metabolism of flavonoids and its relation to some biological activities. Arch Pharm Res. 1998;21(1):17–23. doi: 10.1007/BF03216747. [DOI] [PubMed] [Google Scholar]
- 20.Lee NK, Choi SH, Park SH, Park EK, Kim DH. Antiallergic activity of hesperidin is activated by intestinal microflora. Pharmacology. 2004;71(4):174–80. doi: 10.1159/000078083. [DOI] [PubMed] [Google Scholar]
- 21.van Rooyen R, Hahn-Hagerdal B, La Grange DC, van Zyl WH. Construction of cellobiose-growing and fermenting Saccharomyces cerevisiae strains. J Biotechnol. 2005;120(3):284–95. doi: 10.1016/j.jbiotec.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Li XL, Ljungdahl LG, Ximenes EA, Chen H, Felix CR, Cotta MA, Dien BS. Properties of a recombinant beta-glucosidase from polycentric anaerobic fungus Orpinomyces PC-2 and its application for cellulose hydrolysis. Appl Biochem Biotechnol. 2004;113-116:233–50. doi: 10.1385/abab:113:1-3:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma YY, Xingqian, Hao Yunbin, Xu Guoneng, Xu Guihua, Liu Donghong. Ultrasound-assisted extraction of hesperidin from Penggan (Citrus reticulata) peel. Ultrasonics Sonochemistry. 2008;15(3):227–232. doi: 10.1016/j.ultsonch.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa E, Sakai T, Ikemura H, Matsumoto K, Abe H. Identification, cloning, and characterization of a Sporobolomyces singularis beta-galactosidase-like enzyme involved in galacto-oligosaccharide production. J Biosci Bioeng. 2005;99(4):331–9. doi: 10.1263/jbb.99.331. [DOI] [PubMed] [Google Scholar]
- 25.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen IL, Chee WS, Poulsen L, Offord-Cavin E, Rasmussen SE, Frederiksen H, Enslen M, Barron D, Horcajada MN, Williamson G. Bioavailability is improved by enzymatic modification of the citrus flavonoid hesperidin in humans: a randomized, double-blind, crossover trial. J Nutr. 2006;136(2):404–8. doi: 10.1093/jn/136.2.404. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Hu M. Absorption and metabolism of flavonoids in the caco-2 cell culture model and a perused rat intestinal model. Drug Metab Dispos. 2002;30(4):370–7. doi: 10.1124/dmd.30.4.370. [DOI] [PubMed] [Google Scholar]
- 28.Silberberg M, Morand C, Mathevon T, Besson C, Manach C, Scalbert A, Remesy C. The bioavailability of polyphenols is highly governed by the capacity of the intestine and of the liver to secrete conjugated metabolites. Eur J Nutr. 2006;45(2):88–96. doi: 10.1007/s00394-005-0568-5. [DOI] [PubMed] [Google Scholar]
- 29.Liu Z, Hu M. Natural polyphenol disposition via coupled metabolic pathways. Expert Opin Drug Metab Toxicol. 2007;3(3):389–406. doi: 10.1517/17425255.3.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]