Abstract
Objectives
We sought to identify a novel gene for dilated cardiomyopathy (DCM).
Background
DCM is a heritable, genetically heterogeneous disorder that remains idiopathic in a majority of patients. Familial cases provide an opportunity to discover unsuspected molecular bases of DCM, enabling preclinical risk detection.
Methods
Two large families with autosomal dominant DCM were studied. Genome-wide linkage analysis was used to identify a disease locus, followed by fine mapping and positional candidate gene sequencing. Mutation scanning was then performed in 278 unrelated subjects with idiopathic DCM, prospectively identified at the Mayo Clinic.
Results
Overlapping loci for DCM were independently mapped to chromosome 10q25-q26. DNA sequencing of affected individuals in each family revealed distinct heterozygous missense mutations in exon 9 of RBM20, encoding RNA binding motif protein 20. Comprehensive coding sequence analyses identified missense mutations clustered within this same exon in six additional DCM families. Mutations segregated with DCM (composite logarithm of the odds score >11.49), were absent in 480 control samples, and altered residues within a highly conserved arginine/serine (RS)-rich region. Expression of RBM20 messenger RNA was confirmed in human heart tissue.
Conclusions
Our findings establish RBM20 as a DCM gene and reveal a mutation hotspot in the RS domain. RBM20 is preferentially expressed in the heart and encodes motifs prototypical of spliceosome proteins that regulate alternative pre-mRNA splicing, thus implicating a functionally distinct gene in human cardiomyopathy. RBM20 mutations are associated with young age at diagnosis, end-stage heart failure, and high mortality.
Keywords: dilated cardiomyopathy, genetics, linkage analysis, mutation, RBM20
INTRODUCTION
Prevention of heart failure has been a major public health focus, founded on knowledge of pathogenic mechanisms and modifiable risk factors for hypertension and coronary artery disease (1). Heart failure remains an idiopathic condition, however, in 50% of adults (2) and 66% of children (3) referred to cardiologists, and end-stage idiopathic dilated cardiomyopathy (DCM) is the most common indication for cardiac transplantation (4,5). Indeed, onset of heart failure symptoms in DCM typically portends advanced myocardial disease and risk for sudden death (6) following years to decades of clinically silent but insidiously progressive myopathy. Even in children, this inherent delay in diagnosis and treatment of DCM accounts for 10-year transplantation-free survival of only 42% (3). Improved prediction, treatment, and prevention of DCM will require discovery of preclinical biomarkers, better tools for risk-stratification, and the molecular and cellular basis of disease to enable mechanism-based therapies (1).
Recognition of DCM as a familial disorder in 20–48% of cases (7–11) has provided a rationale for routine screening echocardiography in at-risk relatives to detect presymptomatic disease (12). Moreover, it has been the impetus for human genetics investigations to uncover the molecular basis of DCM (13–14). Since 1993, pathogenic mutations in over 20 genes encoding cytoskeletal, contractile, nuclear membrane, calcium-regulating, and ion channel proteins have been identified in patients with DCM (15). The majority of studies are hypothesis-based, targeting candidate genes like cardiac actin (16) that encode proteins with established function in the heart. By contrast, unanticipated DCM genes and insights into disease pathobiology have emerged from rare families suitable for whole genome mapping studies (17–20). Here, we used genetic linkage analysis in 2 large families with autosomal dominant DCM to map a disease locus, leading to discovery of a mutation hotspot within an RNA-binding protein gene associated with high morbidity and mortality.
METHODS
Study subjects
Patients with DCM evaluated at the Mayo Clinic in the years 1987–1992 and 1999–2008 and their relatives were recruited and medical records were reviewed. We enrolled 280 unrelated probands; familial DCM was confirmed in 24% (DCM documented in ≥1 first degree relative) and suspected in 27% (based on history alone). Family history of sudden death was present in 18%. The 8 families described in the current study were white and of northern European ancestry by self-reporting. An ethnically-matched group of 480 control subjects with normal echocardiograms was randomly selected from a community-based cohort (21). Subjects provided written informed consent under research protocols approved by the Mayo Clinic institutional review board.
Echocardiograms in relatives were performed for clinical indications or under the auspices of the research study. Diagnostic criteria for DCM were: lack of an identifiable cause for disease, left ventricular diastolic and/or systolic dimensions >95th percentile indexed for body surface area (22), and left ventricular ejection fraction <50%. Subjects with normal echocardiograms were classified as “unaffected” and those with equivocal or insufficient data were classified as “uncertain.” Genomic DNA was isolated from peripheral-blood white cells (Puregene Blood Kit, Gentra/QIAGEN, Valencia, California) or from paraffin-embedded tissue (QIAamp DNA FFPE Tissue Kit, QIAGEN).
Linkage analysis and fine mapping
Genome-wide linkage analysis was performed with the ABI PRISM Linkage Mapping Set MD10, version 2.5 (Applied Biosystems, Foster City, California), consisting of polymerase chain reaction (PCR) primer pairs for 400 short tandem repeat markers. After PCR amplification of DNA samples, fragments were resolved on an ABI PRISM 3130xl and genotypes were scored with GeneMapper Software. Two-point and multipoint linkage analyses were performed with use of the FASTLINK program and specification of the following variables: a phenocopy rate of 0.001, equal marker allele frequencies, and dichotomous liability classes (“affected” and “unaffected”). For mutations, a frequency of 0.001 was specified. Logarithm of the odds (LOD) scores were determined for affected subjects only and for 80% and 100% penetrance models at recombination frequencies of 0.0 to 0.4.
Fine locus mapping was performed with microsatellite markers on physical maps, accessible on the Web site of the National Center for Biotechnology Information (NCBI; www.ncbi.nlm.nih.gov). Genotyping was accomplished by PCR amplification of DNA radiolabeled with [α-32P] deoxycytidine triphosphate, resolution of alleles by polyacrylamide-gel electrophoresis, and visualization by autoradiography. Scored genotypes were assembled as haplotypes to define the critical region.
Mutation detection and haplotype analysis
Expression profiles of candidate genes, derived from Affymetrix GeneChip array data for 12 normal human tissues (accession GDS424) or 61 normal mouse tissues (accession GDS592), were assessed by searching the Gene Expression Omnibus (GEO) link on the NCBI Web site (23). The genomic structure of RBM20 was based on predicted reference mRNA sequence (accession NM_001134363.1), retrieved from NCBI. Primer pairs were designed for genomic DNA PCR-amplification of the coding regions of the 14 predicted exons (Online Table 2), using Oligo Primer Analysis Software, version 6.71 (Molecular Biology Insights, Cascade, Colorado). For sequencing, amplified products were treated with ExoSAP-IT (USB Corp, Cleveland, Ohio) and sequenced by the dye-terminator method with use of an ABI PRISM 3730xl DNA Analyzer (Applied Biosystems). DNA sequences were viewed and analyzed using Sequencher, version 4.5 DNA analysis software (Gene Codes Corp, Ann Arbor, Michigan). The reference mRNA and derived protein sequence (accession NP_001127835.1) were used for annotation of identified mutations.
Denaturing high-performance liquid chromatography (DHPLC) heteroduplex analysis (WAVE DHPLC System, Transgenomic, Omaha, Nebraska) was used to screen for sequence variants in our DCM cohort and control samples. Ideal buffer gradients and column melting temperatures were determined using Transgenomic Navigator™ software version 1.7.0 Build 25 and subsequent optimization (Online Table 2). Chromatographic elution profiles of amplified fragments were compared against the wild-type homoduplex pattern; samples yielding anomalous traces were selected for sequencing. To test for a common founder among families with the same RBM20 mutation, haplotypes for mutant alleles were constructed from an intra-genic tetranucleotide-repeat sequence and single nucleotide polymorphisms, identified by sequencing family members.
Cardiac messenger RNA expression and protein structure analysis
Total RNA was extracted from frozen human heart tissue (RNeasy Fibrous Tissue Midi Kit, QIAGEN) and 1.0 μg was reverse transcribed with an oligo(dT) primer to produce complementary deoxyribonucleic acid (cDNA) from messenger RNA (mRNA) (SMART RACE cDNA Amplification Kit, Clontech, Mountain View, California). Primers cDNA-F (CCTACCCCAGATCATCCAAAATGC) and cDNA-R (AACAAACACTTTGCAGTCAGTTATACA) were designed to PCR amplify and sequence 5'-RACE-Ready cDNA, spanning the RBM20 region containing the identified mutations. A subsequent nested reaction utilizing primers cDNA-2F (GAACCCATTCTCGGTCAGTAACCC) and cDNA-2F/3'UTR-R (TCTCTCTGCCCTTCCTCCATTAGT) was performed to provide optimal sequence quality. To identify conserved structural domains, RBM20 reference protein sequence was subjected to a Conserved Domain Database search performed with BLASTP, accessed on the NCBI Web site. Conservation of amino acids altered by missense RBM20 mutations was investigated by aligning our translated RBM20 cDNA sequence with RBM20 protein sequences of other species.
RESULTS
Phenotype of index families
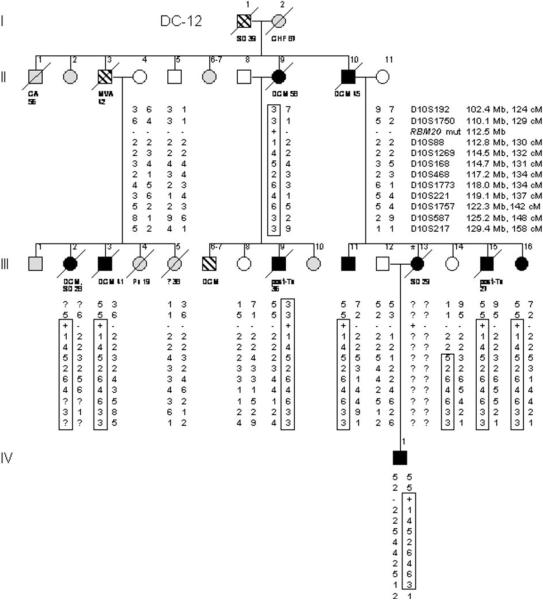
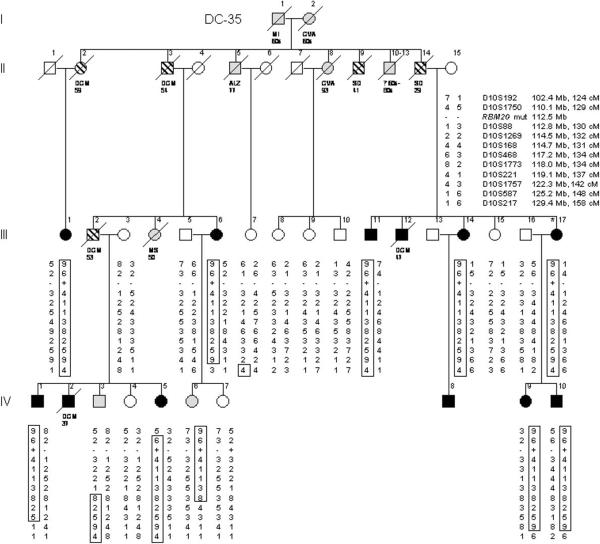
Clinical data and DNA samples were collected from 2 large families in which a clinically aggressive form of DCM segregated as an autosomal dominant trait (Figure 1, Table 1). Kindred DC-12 was recruited for the study in 1991, when an unaffected family member sought medical genetics consultation. The patriarch (Figure 1A: I.1) was of Scottish ancestry and died suddenly at age 39 years. Ten family members developed documented DCM, 2 as young children (mean age at diagnosis = 30.0 years). Two underwent cardiac transplantation as young adults and all but 3 have died of their disease (mean age at death = 37.7 years). Kindred DC–35 was recruited in 2005, following a diagnostic screening echocardiogram in the proband (Figure 1B: III.17) whose father died suddenly at age 29 years. The family was of Norwegian ancestry and comprised of 12 relatives with documented DCM (mean age at diagnosis = 41.3 years) and 5 others with DCM and/or sudden death by history alone. Seven family members with confirmed or suspected DCM died at a mean age of 45.7 years. Five living relatives with DCM had received implantable cardioverter defibrillators (ICDs).
Figure 1. Pedigrees of Index Families with Hereditary Dilated Cardiomyopathy.
Pedigree structures for kindreds DC-12 (A) and DC-35 (B) are shown. Square = male; Circle = female; Black = affected; White = unaffected; Gray = clinical status unknown; Parallel diagonal lines = suspected DCM based on family history; Slash through the symbol = deceased, with cause of/age at death indicated; Asterisk = proband. The gene for RNA binding motif protein 20 (RBM20) is located at chromosome 10q25.2. Markers that were tested for this region of chromosome 10 are listed in order from centromere to q-telomere, with map locations according to the NCBI Web site and given in megabases and centimorgans. The haplotypes for these markers are shown in columns beneath family members who underwent genetic evaluation; the disease-associated haplotypes are boxed. Question marks indicate genotypes that could not be scored from paraffin-embedded samples. Recombination events account for inheritance of portions of the disease haplotype in some affected subjects (DC-12: III.2, III.3, III.11, III.15, III.16, IV.1; DC-35: III.6, IV.1, IV.5, IV.9, IV.10) and enable critical regions to be defined by the minimal region of overlap. RBM20 missense mutations (RBM20 mut), which cosegregate with DCM, are indicated by plus symbols; minus symbols indicate wild-type sequence. ALZ = Alzheimer disease; CA = cancer; CHF = congestive heart failure; CVA = cerebrovascular accident; MI = myocardial infarction; MS = multiple sclerosis; MVA = motor vehicle accident; Pn = pneumonia; SD = sudden death; Tx = cardiac transplantation.
Table 1.
Phenotypic and Genetic Data for Families with Dilated Cardiomyopathy
| Pedigree (Country of origin) |
Age at Diagnosis (y) |
Age at Evaluation (y) (Indication) |
LVID (mm) |
LVEF (%) |
ECG, Arrhythmia |
Other Diagnostic Testing |
Treatment | Outcome | Pathology | Diagnosis | RBM20 Mutation Status |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DC-12 (Scotland) | |||||||||||
| II.5 | - | 58(F) | 55/31 | 68 | Normal | None | Alive 58 y | - | Unaffected | Normal | |
| II.9 | 53 | 53(R) | 64/53 | 39 | LVH, PVC | - | Death 58 y | - | DCM | P638L | |
| II.10 | 44 | 45(S) | Severe LVE | LVSD | AF, PVC | D, F | CHF, Death 45 y | Autopsy: congestive myopathy, fibrosis, myocyte hypertrophy, no CAD | DCM | P638L (inferred) | |
| III.2 | 28 | 28(S) | - | - | - | - | SD 28 y | Autopsy: EFE, congestive myopathy, no CAD | DCM | P638L | |
| III.3 | 37 | 37(F) | 62/57 | 15 | LAD, VT | Neg. angio | D, B | Death 41 y | Autopsy: mild fibrosis | DCM | P638L |
| III.5 | - | 30(R) | 40/24 | 64 | Normal | - | Death 38 y | Autopsy: normal LV and cardiac mass, no CAD | Uncertain (suspected arrhythmia) | Normal | |
| III.8 | - | 36(F) | 43/30 | 51 | Normal | None | Alive 39 y | - | Unaffected | Normal | |
| III.9 | 30's | 36(S) | - | - | LBBB | Transplant 36 y | Death 36 y | - | DCM | P638L | |
| III.11 | 33 | 33(F) | 72/62 | 26 | LVH, IVCD | D, C | Alive 42 y | - | DCM | P638L | |
| III.13 | 29 | 29(S) | - | - | - | - | SD 29 y | Autopsy: CM, mild fibrosis, no CAD | DCM | P638L | |
| III.14 | - | 43(F) | 51/36 | 50 | Normal | None | Alive 46 | - | Unaffected | Normal | |
| III.15 | 24 | 25(F) | 88/79 | 15 | LVH, IVCD | Transplant 26 y | Death 27 y | - | DCM | P638L | |
| III.16 | 14 | 14(F)→22 | 57/41→57/41 | 48→45 | Short PR, SVT | D, So | Alive 24 y | - | DCM | P638L | |
| IV.1 | 3 | 3(F)→12 | 44/30→62/40 | 50→64 | Short PR, LVH | D, L | Alive 12 y | - | DCM | P638L | |
| DC-35 (Norway) | |||||||||||
| III.1 | 55 | 55(F) | 47/39 | 46 | PAC | Neg. stress imaging | - | Alive 58 y | - | DCM | R634Q |
| III.6 | 45 | 45(S)→55 | 70/- →60/50 | 10→30 | LAE, IVCD, ST-T | Neg. angio | D, Cv, L, Sp, W, ICD (EF, FH) | Alive 55 y | - | DCM | R634Q |
| III.7 | - | 60(F) | 44/29 | 60 | Normal | None | Alive 62 y | - | Unaffected | Normal | |
| III.8 | - | 60(F) | 39/29 | 60 | - | None | Alive 60 y | - | Unaffected | Normal | |
| III.9 | - | 52(F) | Normal | 65 | Normal | None | Alive 56 y | - | Unaffected | Normal | |
| III.10 | - | 51(HTN) | 44/28 | 67 | - | None | Alive 52 y | - | Unaffected | Normal | |
| III.11 | 55 | 55(S) | 72/63 | 20 | Short PR, IVCD, ST-T, VT | D, Cv, L, ICD (EF, FH, VT) | Alive 55 y | - | DCM | R634Q | |
| III.12 | 47 | 47(A) | - | - | - | - | Death 47 y | Autopsy: CM and LV dilation | DCM | - | |
| III.14 | 46 | 52(F) | 63/51 | 30 | - | -, ICD (EF, FH) | Alive 52 y | - | DCM | R634Q | |
| III.15 | - | 51 (F) | 54/27 | 55 | - | None | Alive 51 y | - | Unaffected | Normal | |
| III.17 | 48 | 48(F) | 61/44 | 45 | IVCD, ST-T, VT | Neg. angio | M, L, ICD (FH) | Alive 49 y | - | DCM | R634Q |
| IV.1 | 50 | 50(S) | 64/55 | 20 | LAD, LAE, ST-T | Neg. angio | Cv, L | Alive 52 y | - | DCM | R634Q |
| IV.2 | 37 | 37(S) | Severe LVE | 15 | LAE, LAD | Neg. angio, CK 102 U/L | D, E, W | CHF, Death 37 y | Biopsy: Myocyte hypertrophy, mild fibrosis | DCM | - |
| IV.3 | - | 44(F) | 56/36 | 65 | Normal | None | Alive 48 y | - | Uncertain | Normal | |
| IV.4 | - | 44(F) | 51/32 | 52 | Normal | None | Alive 46 y | - | Unaffected | Normal | |
| IV.5 | 40 | 40(F) | 56/43 | 40 | Normal | Neg. stress imaging; CK 70 U/L, cTnI <0.3 ng/ml | M, L | Alive 44 y | - | DCM | R634Q |
| IV.6 | - | 24(F) | 54/35 | 58 | Normal | Neg. stress imaging | None | Alive 27 y | - | Uncertain | R634Q |
| IV.7 | - | 23(F) | 39/27 | 65 | - | None | Alive 23 y | - | Unaffected | Normal | |
| IV.8 | 18 | 18(F) | 61/51 | 37 | IVCD, LVH | Neg. angio | Cv, L, ICD (FH, EF) | Alive 19 y | - | DCM | - |
| IV.9 | 30 | 30(R) | 63/45 | 42 | Normal | - | Alive 30 y | - | DCM | R634Q | |
| IV.10 | 24 | 24(R) | 59/46 | 45 | LVH | - | Alive 24 y | - | DCM | R634Q | |
| DC-50 (Germany) | |||||||||||
| II.3 | 49 | 52(S)→60 | 68/62→71/65 | 17→15 | LVH, ST-T, AF, VT, VF | Neg. angio, CK 43 U/L, cTnI <0.5 ng/ml | D, F, P, C, A, W, ICD (Sy, FH) | CHF, Death 60 y | Autopsy: sev. CM, mild fibrosis | DCM | P638L |
| II.5 | 29 | 29(S) | - | - | ST-T, VT, VF | CK 29 U/L | D, P, PC | CHF, Death 29 y | - | DCM | P638L (inferred) |
| III.3 | 25 | 25(R)→42 | 55/45→51/- | 33→40 | LVH | D, Cv, L | Alive 42 y | - | DCM | P638L | |
| III.4 | 29 | 29(R)→44 | 45/35→52/38 | 40→49 | ST-T | Cv, E | Alive 44 y | - | DCM | P638L | |
| III.5 | 15 | 15(S) | 75/68 | 18 | LVH, ST-T, VT | D, L, W, N, Mx | CHF, SD 18 y | Biopsy: myocyte hypertrophy, mild fibrosis | DCM | - | |
| III.6 | - | 29(R) | 51/28 | 70 | Normal | None | Alive 46 y | - | Unaffected | Normal | |
| III.7 | - | 27(R) | 50/32 | 60 | Normal | None | Alive 36 y | - | Unaffected | Normal | |
| III.8 | 21 | 27(R)→37 | 54/40→56/46 | 51→35 | Short PR, SVT | Cv, L | Alive 38 y | - | DCM | P638L | |
| IV.1 | 17 | 17(S) | 51/40 | 40 | LVH, SVT | M, E | Alive 22 y | - | DCM | P638L | |
| DC-46 (Germany) | |||||||||||
| IV.1 | 26 | 18(F)→26 | 53/36→56/46 | 58→30 | ST-T | cTnT <0.03 ng/ml | Cv, L, ICD (EF, FH) | Alive 27 y | - | DCM | R636S |
| DC-49 (Germany) | |||||||||||
| II.2 | 40 | 40(S)→45 | 71/64→65/52 | 10→20 | LVH, ST-T, IVCD, VT | D, M, L, Sp, F, W, Mx, ICD (CA) | Alive 45 y | - | DCM | R636H | |
| II.3 | 39 | 39(F)→44 | 63/49→52/42 | 43→45 | VT | Neg. angio | Cv, Rm, Cn | Alive 44 y | - | DCM | R636H |
| DC-27 (Norway) | |||||||||||
| II.6 | 70 | 80(S) | 63/- | 25 | SB, AF | D, Cv, L, F, W | CHF, Death 85 y | - | DCM | R636S | |
| III.2 | - | 64(F) | 55/40 | 50 | IRBBB | Cv | Alive 64 y | - | Uncertain | R636S | |
| III.3 | 59 | - | - | - | None | SD 59 y | Autopsy: CM, LVE, CAD but no acute MI, fibrosis | DCM | R636S (inferred) | ||
| III.5 | 55 | 59(F) | 59/44 | 44 | 1° AVB, RBBB, VT | Neg. angio | Cv | Alive 60 y | - | DCM | R636S |
| III.8 | - | 50(F) | 45/- | 60 | Normal | None | Alive 55 y | - | Unaffected | Normal | |
| III.10 | - | 39(F) | 54/38 | 55 | - | None | Alive 47 y | - | Uncertain | R636S | |
| IV.1 | 35 | 35(S) | 68/55 | 38 | LVH, ST-T | Neg. angio | Cv, L | Alive 36 y | - | DCM | R636S |
| IV.5 | 27 | 36(S) | 72/65 | 23 | LAD, IVCD, ST-T | Neg. anglo | Cv, Ln | Alive 37 y | Biopsy: myocyte hypertrophy, mod. fibrosis | DCM | R636S |
| IV.7 | - | 28(R) | 50/33 | 66 | Normal | None | Alive 31 y | - | Unaffected | Normal | |
| IV.9 | - | 15(F) | 52/34 | 57 | Normal | None | Alive 18 y | - | Uncertain | R636S | |
| DC-09 (Norway) | |||||||||||
| III.2 | 57 | 57(R)→68 | 58/46→59/49 | 35→34 | Short PR, PVC | D, E, F,A | Alive 68 y | - | DCM | R636S | |
| III.4 | - | - | - | - | - | Neg. angio | - | Alive 68 y | - | DCM (by history) | R636S |
| IV.2 | 17 | 17(S) | 68/60 | 22 | LVH, ST-T | D, H, N, F, W | CHF, SD 18 y | - | DCM | - | |
| IV.3 | - | 27(R) | 50/32 | 60 | Normal | None | Alive 38 y | - | Unaffected | R636S | |
| IV.4 | - | 24(R) | 53/33 | 61 | Normal | None | Alive 36 y | - | Unaffected | Normal | |
| IV.6 | 19 | 20(S) | - | - | - | Transplant 20 y | Alive 43 y | - | DCM | R636S | |
| DC-22 (England) | |||||||||||
| II.2 | 44 | 45(S) | 53/44 | 25 | ST-T, VT | Neg. angio | D, F, A, Cv, Ln, ICD (EF, FH) | CHF, Alive 54 y | - | DCM | S637G |
| II.3 | 27 | 27(S) | - | - | - | Transplant 32 y | Alive 49y | DCM | S637G | ||
| III.1 | 21 | 21(F) | 53/39 | 35 | ST-T | Cv, L | Alive 28 y | - | DCM | S637G | |
| III.2 | 20 | 20(F) | 48/34 | 46 | Short PR | Neg. stress imaging | Cv | Alive 23 y | - | DCM | S637G |
Indication for evaluation: F, family history; S, symptoms; R, research study; HTN, hypertension; A, autopsy
Echocardiography: LVE, left ventricular enlargement; LVEF, left ventricular ejection fraction (normal ≥50%); LVID, left ventricular internal dimension in diastole/systole (measurements greater than the 95th percentiles, based on body surface area and age, indicated by bold font); LVSD, left ventricular systolic dysfunction
Electrocardiogram, Arrhythmia: 1° AVB, first degree atrioventricular block; AF, atrial fibrillation; IRBBB, incomplete right bundle branch block; IVCD, intraventricular conduction delay; LAD, left axis deviation; LAE, left atrial enlargement; LBBB, left bundle branch block; LVH, left ventricular hypertrophy; PAC, premature atrial contractions; PR, PR interval; PVC, premature ventricular contractions; SB, sinus bradycardia; ST-T, nonspecific ST-T wave changes; SVT, supraventricular tachycardia; VF, ventricular fibrillation; VT, ventricular tachycardia
Other diagnostic testing: CK, creatine kinase; cTnI, cardiac troponin I; cTnT, cardiac troponin T; Neg. angio, no significant coronary artery disease on angiography
Treatment: A, amiodarone; B, benazepril; C, captopril; Cn, candesartan; Cv, carvedilol; D, digoxin; E, enalapril; F, furosemide; H, hydralazine; ICD, implantable cardioverter defibrillator; L, lisinopril; Ln, losartan; M, metoprolol; Mx, mexiletine; N, nitroglycerin; P, propranolol; PC, procainamide; Rm, ramipril; So, sotalol; Sp, spironolactone; Transplant, cardiac transplantation; W, warfarin
Indication for ICD (in parentheses): Sy, syncope; FH, family history; EF, ejection fraction; CA, cardiac arrest; VT, ventricular tachycardia
Outcome: CHF, congestive heart failure; SD, sudden death
Pathology: Sev., severe; CAD, coronary artery disease; CM, cardiomegaly; EFE, endocardial fibroelastosis; MI, myocardial infarction; mod., moderate
-, data not available or applicable
DCM locus mapping
Genome-wide linkage analyses, followed by regional high-density genotyping on chromosome 10, identified a peak two-point LOD score of 3.55 at marker D10S1269 in DC-12 and 4.55 at marker D10S221 in DC-35. Linkage to other regions of the genome with two-point LOD scores >1.0 was excluded by multipoint and/or haplotype analyses with additional markers (data not shown). Fine mapping in DC-12 identified a disease-associated haplotype on chromosome 10q25.1–q26.2 (Figure 1A), a region spanning 19.3 Mb, which was inherited by all affected subjects (peak multipoint LOD score 3.62 for all subjects, assuming 100% mutation penetrance, and 2.67 for affected subjects only). A recombination event within this interval occurred in a 43-year-old female with a normal echocardiogram (III.14). Assuming she did not inherit the disease-associated mutation, the critical region narrowed to 4.6 Mb. Fine mapping in DC-35 identified an overlapping disease-associated haplotype (Figure 1B) spanning 22.8 Mb (peak multipoint LOD score 4.89 for all subjects, assuming 100% mutation penetrance, and 3.58 for affected subjects only). The haplotypes were different for each family suggesting they did not share common ancestry, yet the overlapping disease loci raised the possibility of a shared DCM gene.
Mutation identification
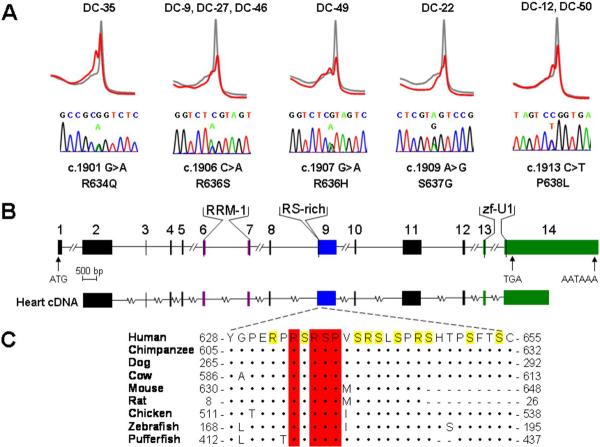
Candidate genes were selected from the 19.3 Mb critical region in DC-12, comprised of more than 150 genes, based on cardiac expression and/or physiologic rationale. Mutations within exons of 25 genes were excluded by DNA sequencing (Online Table 1). RBM20, a gene with unknown function, was included based on its genomic location and expression pattern. Among 12 human tissues, RBM20 is most highly expressed in the heart with 4-fold greater transcript abundance in cardiac than skeletal muscle according to GEO array data. Moreover, it is one of only 19 genes with a mean expression in heart >8-fold higher than the combined mean expression in 11 other tissues. Similarly, among 61 murine tissues it is most highly expressed in heart (5-fold greater than skeletal muscle). Sequencing of the 14 exons of RBM20 identified a distinct heterozygous missense mutation in exon 9 in each family, resulting in a P638L substitution in DC-12 and a R634Q substitution in DC-35 (Figures 1, 3A). Mutations cosegregated with the disease phenotype and were absent in unaffected family members and 480 ethnically-matched control subjects.
Figure 3. RBM20 Mutations Identified in Families with Dilated Cardiomyopathy.
(A) Genomic DNA mutation scans using DHPLC revealed abnormal heteroduplex profiles (red), compared to the control wild-type profile (gray), in exon 9 of RBM20 in 8 DCM families. Abnormal profiles indicated DNA sequence alterations, which were absent in 480 control samples. Below the heteroduplex profiles, DNA sequencing revealed corresponding heterozygous missense mutations. The location of each mutation, and its resultant amino acid substitution, are based on predicted reference RBM20 cDNA (mRNA) and protein sequences and are indicated below each chromatogram. Mutation c.1906 C>A, R636S was shared by three families and c.1913 C>T, P638L was shared by two families. (B) The predicted genomic structure of RBM20, consisting of 14 exons, is depicted to scale. Exons that encode peptides homologous to highly conserved functional domains – RNA-recognition motif 1 (RRM-1, in purple), arginine/serine-rich region (RS-rich, in blue), and U1 zinc finger (zf-U1, in green) – are indicated. Putative start (ATG) and stop (TGA) codons are located in exons 1 and 14, respectively. A polyadenylation signal (AATAAA) is located at the 3' end of exon 14. Directly below the RBM20 genomic structure, cDNA amplification and sequencing confirmed transcription of mRNA from exons 2–14 in human heart tissue, as depicted by parallel alignment. The cDNA transcript contains the complete RS domain and identified RBM20 mutations in the 5' region of exon 9. (C) Alignment of homologous RBM20 protein sequences that flank the amino acid substitutions is shown. The RS domain spans residues 632–654, with arginine (R) and serine (S) residues highlighted in yellow. Residues conserved between human RBM20 and another species are indicated by (•) and amino acid deletions by (-). Amino acids that are altered by the identified RBM20 missense mutations (residues 634, 636, 637, and 638, indicated with red bars) are conserved among all eight species. Accession numbers: XP_291671 for human, XP_508032 for chimpanzee, XP_544017 for dog, XP_603772 for cow, BAE24961 for mouse, NP_001101081 for rat, XP_421755 for chicken, XP_683222 for zebrafish, and CAG01297 for pufferfish.
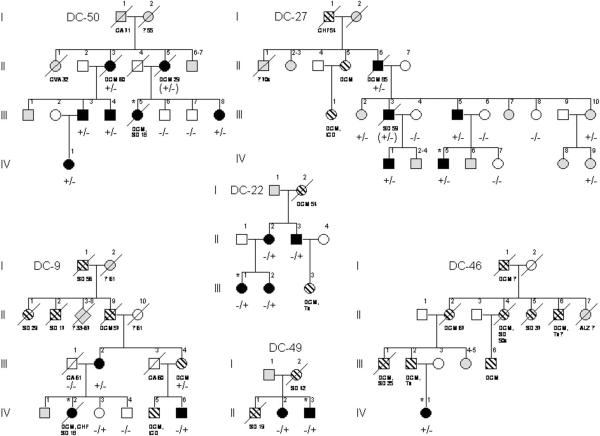
To determine if RBM20 mutations were present in other cases of DCM, we screened the 14 coding exons in our remaining cohort of 278 subjects using DHPLC. Three unique heterozygous missense mutations - R636S, R636H, and S637G - were identified in 6 other families, all clustered within exon 9 (Figures 2, 3A). Among the 8 families with RBM20 mutations, 2 had an identical mutation resulting in P638L substitution and 3 had an identical mutation resulting in R636S substitution. Haplotype analysis (Online Table 3) excluded a common ancestral founder for the P638L substitution. While the disease-associated haplotypes were the same in the three families with an R636S substitution, the majority of individual alleles comprising the haplotype are the most common variants within a white European population. Consequently, a founder effect could not be conclusively established. Mutations were absent in control samples and cosegregated with DCM in the 7 families where DNA samples were available from 2 or more affected subjects. Combined peak two-point LOD scores for mutations versus DCM in the 4 largest families (DC-12, DC-35, DC-27, DC-50) ranged from 8.02 (affected subjects only) to 11.49 (all subjects, assuming 100% mutation penetrance).
Figure 2. Pedigrees of Additional Families with RBM20 Mutations.
Symbols and abbreviations are as for Figure 1. Diamonds = two or more family members of both genders; Parentheses = inferred RBM20 mutation status.
Cardiac mRNA expression and protein structure analysis
Based on the predicted reference cDNA (mRNA), RBM20 is comprised of 14 exons (Figure 3B). Portions of exons 2 and 14 and all of exons 3 through 13 were verified in a single open reading frame cDNA derived from oligo(dT)-primed heart RNA (Figure 3B). This confirmed that these exons are transcribed and spliced into messenger RNA in the heart, including exon 9 which contained the cluster of identified RBM20 mutations. A Conserved Domain Database search of the translated reference RBM20 cDNA indicated homology to an RNA Recognition Motif 1 Superfamily domain spanning exons 6–7 (e-value = 0.005) and a U1 zinc finger domain (e-value = 2e−4) spanning exons 13–14. Additionally, exon 9 encodes an RS-rich domain, which is disrupted by the 5 identified missense mutations. Each resultant amino acid substitution alters a residue in RBM20 conserved among diverse species (Figure 3C).
Genotype-phenotype correlation
RBM20 mutations were associated with clinically aggressive DCM. Collectively, the 39 subjects in our 8 families with a mutation and confirmed DCM were diagnosed 9 years earlier than a comparable series of patients with sporadic and familial DCM who underwent family screening (mean age at diagnosis 35.9 versus 45.2 years) (7). Death occurred in 11 (mean age = 45.2 years) and was deemed sudden in 3; 4 underwent cardiac transplantation (mean age = 28.5 years) and 8 ICD implantation. Subjects who enrolled in our study did not, however, fully represent the malignant nature of their familial disease as revealed by their pedigrees. Among the 32 additional relatives with suspected DCM by family history, for whom medical records were unavailable and/or mutation status could not be determined, 13 died suddenly (mean age = 32.7 years), 3 underwent cardiac transplantation, and 3 had ICD implantation. There were no consistent electrocardiographic features in subjects with an RBM20 mutation; 9 had ventricular tachycardia. Variable degrees of myocyte hypertrophy and interstitial fibrosis were observed on histopathological analysis. Most enrolled subjects with accessible follow up data had advanced disease and exhibited minimal improvement or further deterioration on medical treatment, albeit drug therapy was highly variable. Correlation between RBM20 mutations and phenotype was not without exception, however. There were 5 female subjects who inherited a mutation but did not fulfill diagnostic criteria for DCM: 1 subject in DC-35 (age 24 years) and 3 subjects in DC-27 (ages 15, 39, and 64 years) had left ventricular enlargement with normal ejection fraction; 1 subject in DC-9 (age 27 years) had a normal echocardiogram. No overt non–cardiac phenotypes were evident among subjects with RBM20 mutations.
DISCUSSION
Molecular basis of disease
The majority of known DCM genes encode cytoskeletal or contractile proteins of cardiac myocytes, with direct roles in the generation and/or transmission of contractile force through protein-protein interactions (14). An expanded understanding of the pathobiology of DCM has emerged from identification of mutations that perturb myocardial function via impaired calcium (24), potassium (25), or sodium ion homeostasis (18,19). Collectively, these molecular genetic etiologies for DCM reveal a fundamental defect in excitation-contraction coupling and the heart's capacity to perform under physiologic and stress conditions. Notable exceptions to this paradigm have been revealed through discovery of unsuspected DCM genes, like LMNA and EYA4, in large families suitable for linkage analysis. LMNA encodes lamin A/C, a ubiquitously expressed nuclear membrane protein. By unknown mechanisms, mutations in LMNA cause DCM and conduction system disease (17) or a spectrum of non-cardiac disorders. EYA4 encodes a transcriptional coactivator, which interacts with DNA-binding transcription factors. Mutations in EYA4 are predicted to alter cochlear and cardiac gene expression, causing a syndrome of DCM and sensorineural hearing loss (20). RBM20, here identified as a gene for familial DCM, suggests perturbation of post-transcriptional pre-mRNA processing as a distinct molecular basis for the disorder.
RBM20 encodes RNA binding motif protein 20, with a prototypical RNA-recognition motif followed by an RS domain (26). These structural features are characteristic of a family of RNA-binding SR proteins that assemble in the spliceosome, a large multi-protein complex that orchestrates constitutive and alternative splicing of pre-messenger RNA (27). Indeed, over 70% of human genes express multiple mRNA transcripts via alternative splicing of exons, conferring vast diversity to the proteome (28). Heritable diseases are frequently attributable to cis-acting mutations, which disrupt normal splicing of the gene in which the mutation occurs. However, trans-acting mutations within spliceosome protein genes have been identified in only three human disorders - spinal muscular atrophy, retinitis pigmentosa, and Prader-Willi syndrome (27). Such mutations have the potential to impair normal splicing of multiple genes, as recently demonstrated by exon microarray analysis in a mouse model of spinal muscular atrophy (29). The specific function of RNA binding motif protein 20 in the human heart and the downstream effects of the identified RBM20 mutations that cause DCM remain unknown. However, a pathogenic link between genetic disruption of alternative splicing-regulating SR proteins of the spliceosome and DCM has now been established in mouse models (30).
Clinical implications
Since the first DCM-associated gene was identified by linkage analysis 15 years ago (31,32), clinical application of research findings has proved challenging due to the marked genetic heterogeneity of DCM. While routine genetic testing may be practical in certain heritable cardiac disorders (33), no single gene or mutation for DCM has emerged as common (15). Targeted genetic testing may be practical, however, in clinically defined subgroups. For example, mutations in LMNA and SCN5A have been associated with a cardiac syndrome of DCM, impaired automaticity and conduction, and atrial fibrillation (17–19). By use of genome-wide linkage analysis, the present study further expands the spectrum of DCM genes. Remarkably, the 5 unique RBM20 mutations identified in 8 families are clustered within a single exon that encodes an RS-rich domain. In our cohort, this mutation hotspot accounted for 3% (8/280) of all DCM cases, 5% (8/151) of confirmed or suspected familial cases, and 13% (7/54) of cases with a history of sudden death.
Our study highlights the importance of family screening to detect presymptomatic DCM (7,12). Indeed, 68% (43/63) of the subjects in our 8 families were asymptomatic and first diagnosed with DCM on the basis of a screening echocardiogram. Despite the lack of symptoms, the RBM20 mutations we identified were highly penetrant and only 5 of 44 individuals with a mutation did not fulfill diagnostic criteria for DCM. In fact, 4 of these 5 subjects had left ventricular dilation, a known precursor to overt DCM (7,10). Penetrance of familial DCM is, however, age-dependent and the majority of subjects who enrolled in our study were adults. Discovery of the genetic basis for DCM in these families now enables a preclinical diagnosis in at-risk children and young adults. Given the malignant nature of RBM20 mutations, this knowledge would justify closer clinical follow up, meticulous attention to coexistent modifiable risk factors, and earlier institution of therapies proven to alter the natural history of heart failure (34) and decrease risk of sudden death (6).
Supplementary Material
Acknowledgments
We gratefully acknowledge the patients and families who participated in this study and the physicians who referred them. We thank Jeanne L. Theis, PhD for critical review of the manuscript.
This work was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health (R01 HL071225) and the Marriott Program for Heart Disease Research.
Abbreviations and Acronyms
- cDNA
complementary deoxyribonucleic acid
- DCM
idiopathic dilated cardiomyopathy
- DHPLC
denaturing high-performance liquid chromatography
- GEO
Gene Expression Omnibus
- ICD
implantable cardioverter defibrillator
- LOD
logarithm of the odds
- mRNA
messenger ribonucleic acid
- NCBI
National Center for Biotechnology Information
- PCR
polymerase chain reaction
- RS
arginine/serine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Braunwald E. Cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–9. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 2.Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–84. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 3.Towbin JA, Lowe AM, Colan SD, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296:1867–76. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 4.Taylor DO, Edwards LB, Boucek MM, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult heart transplant report--2007. J Heart Lung Transplant. 2007;26:769–81. doi: 10.1016/j.healun.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Boucek MM, Aurora P, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: tenth official pediatric heart transplantation report--2007. J Heart Lung Transplant. 2007;26:796–807. doi: 10.1016/j.healun.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Desai AS, Fang JC, Maisel WH, Baughman KL. Implantable defibrillators for the prevention of mortality in patients with nonischemic cardiomyopathy: a meta-analysis of randomized controlled trials. JAMA. 2004;292:2874–9. doi: 10.1001/jama.292.23.2874. [DOI] [PubMed] [Google Scholar]
- 7.Michels VV, Moll PP, Miller FA, et al. The frequency of familial dilated cardiomyopathy in a series of patients with idiopathic dilated cardiomyopathy. N Engl J Med. 1992;326:77–82. doi: 10.1056/NEJM199201093260201. [DOI] [PubMed] [Google Scholar]
- 8.Mestroni L, Krajinovic M, Severini GM, et al. Familial dilated cardiomyopathy. Br Heart J. 1994;72:S35–41. doi: 10.1136/hrt.72.6_suppl.s35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keeling PJ, Gang Y, Smith G, et al. Familial dilated cardiomyopathy in the United Kingdom. Br Heart J. 1995;73:417–21. doi: 10.1136/hrt.73.5.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baig MK, Goldman JH, Caforio AL, Coonar AS, Keeling PJ, McKenna WJ. Familial dilated cardiomyopathy: cardiac abnormalities are common in asymptomatic relatives and may represent early disease. J Am Coll Cardiol. 1998;31:195–201. doi: 10.1016/s0735-1097(97)00433-6. [DOI] [PubMed] [Google Scholar]
- 11.Grünig E, Tasman JA, Kücherer H, Franz W, Kübler W, Katus HA. Frequency and phenotypes of familial dilated cardiomyopathy. J Am Coll Cardiol. 1998;31:186–94. doi: 10.1016/s0735-1097(97)00434-8. [DOI] [PubMed] [Google Scholar]
- 12.Burkett EL, Hershberger RE. Clinical and genetic issues in familial dilated cardiomyopathy. J Am Coll Cardiol. 2005;45:969–81. doi: 10.1016/j.jacc.2004.11.066. [DOI] [PubMed] [Google Scholar]
- 13.Collins FS, McKusick VA. Implications of the human genome project for medical science. JAMA. 2001;285:540–4. doi: 10.1001/jama.285.5.540. [DOI] [PubMed] [Google Scholar]
- 14.Olson TM. Monogenic Dilated Cardiomyopathy. In: Walsh RA, editor. Molecular Mechanisms of Cardiac Hypertrophy and Failure. 1st edition Taylor & Francis; Boca Raton: 2005. pp. 525–40. [Google Scholar]
- 15.Hershberger RE, Parks SB, Kushner JD, et al. Coding sequence mutations identified in MYH7, TNNT2, SCN5A, CSRP3, LBD3, and TCAP from 313 patients with familial or idiopathic dilated cardiomyopathy. Clin Translational Science. 2008;1:21–6. doi: 10.1111/j.1752-8062.2008.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson TM, Michels VV, Thibodeau SN, Tai YS, Keating MT. Actin mutations in dilated cardiomyopathy, a heritable form of heart failure. Science. 1998;280:750–2. doi: 10.1126/science.280.5364.750. [DOI] [PubMed] [Google Scholar]
- 17.Fatkin D, MacRae C, Sasaki T, et al. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N Engl J Med. 1999;341:1715–24. doi: 10.1056/NEJM199912023412302. [DOI] [PubMed] [Google Scholar]
- 18.McNair WP, Ku L, Taylor MRG, et al. SCN5A mutation associated with dilated cardiomyopathy, conduction disorder, and arrhythmia. Circulation. 2004;110:2163–7. doi: 10.1161/01.CIR.0000144458.58660.BB. [DOI] [PubMed] [Google Scholar]
- 19.Olson TM, Michels VV, Ballew JD, et al. Sodium channel mutations and susceptibility to heart failure and atrial fibrillation. JAMA. 2005;293:447–54. doi: 10.1001/jama.293.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schönberger J, Wang L, Shin JT, et al. Mutation in the transcriptional coactivator EYA4 causes dilated cardiomyopathy and sensorineural hearing loss. Nat Genet. 2005;37:418–22. doi: 10.1038/ng1527. [DOI] [PubMed] [Google Scholar]
- 21.Redfield MM, Jacobsen SJ, Burnett JC, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community. Appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 22.Henry WL, Gardin JM, Ware JH. Echocardiographic measurements in normal subjects from infancy to old age. Circulation. 1980;62:1054–61. doi: 10.1161/01.cir.62.5.1054. [DOI] [PubMed] [Google Scholar]
- 23.Barrett T, Troup DB, Wilhite SE, et al. NCBI GEO: archive for high-throughput functional genomic data. Nucleic Acids Res. 2009;37(Database issue):D885–90. doi: 10.1093/nar/gkn764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitt JP, Kamisago M, Asahi M, et al. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science. 2003;299:1410–3. doi: 10.1126/science.1081578. [DOI] [PubMed] [Google Scholar]
- 25.Bienengraeber M, Olson TM, Selivanov VA, et al. ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating. Nat Genet. 2004;36:382–7. doi: 10.1038/ng1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long JC, Caceres JF. The SR protein family of splicing factors: master regulators of gene expression. Biochem J. 2009;417:15–27. doi: 10.1042/BJ20081501. [DOI] [PubMed] [Google Scholar]
- 27.Wang GS, Cooper TA. Splicing in disease: disruption of the splicing code and the decoding machinery. Nat Rev Genet. 2007;8:749–61. doi: 10.1038/nrg2164. [DOI] [PubMed] [Google Scholar]
- 28.Johnson JM, Castle J, Garrett-Engele P, et al. Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays. Science. 2003;302:2141–4. doi: 10.1126/science.1090100. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z, Lotti F, Dittmar K, et al. SMN deficiency causes tissue-specific perturbations in the repertoire of snRNAs and widespread defects in splicing. Cell. 2008;133:585–600. doi: 10.1016/j.cell.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding JH, Xu X, Yang D, et al. Dilated cardiomyopathy caused by tissue-specific ablation of SC35 in the heart. EMBO J. 2004;23:885–96. doi: 10.1038/sj.emboj.7600054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Towbin JA, Hejtmancik JF, Brink P, et al. X-linked dilated cardiomyopathy. Molecular genetic evidence of linkage to the Duchenne muscular dystrophy (dystrophin) gene at the Xp21 locus. Circulation. 1993;87:1854–65. doi: 10.1161/01.cir.87.6.1854. [DOI] [PubMed] [Google Scholar]
- 32.Muntoni F, Cau M, Ganau A, et al. Brief report: deletion of the dystrophin muscle-promoter region associated with X-linked dilated cardiomyopathy. N Engl J Med. 1993;329:921–5. doi: 10.1056/NEJM199309233291304. [DOI] [PubMed] [Google Scholar]
- 33.Robin NH, Tabereaux PB, Benza R, Korf BR. Genetic testing in cardiovascular disease. J Am Coll Cardiol. 2007;50:727–37. doi: 10.1016/j.jacc.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Eichhorn EJ, Bristow MR. Medical therapy can improve the biological properties of the chronically failing heart. A new era in the treatment of heart failure. Circulation. 1996;94:2285–96. doi: 10.1161/01.cir.94.9.2285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.