Abstract
We have previously demonstrated a function for Neuroglian and Semaphorin1a in Drosophila giant fiber circuit formation. Both molecules are required for guiding the giant fibers out of the brain and have distinct functions during giant synapse formation. In this study we characterized the effects of various combinations of Neuroglian and Semaphorin1a gain and loss of function backgrounds on giant fiber circuitry formation. We found that Neuroglian and Semaphorin1a genetically interact with each other during axon guidance as well as during synapse formation. Our experiments revealed that during pathfinding of the giant fibers out of the brain, Neuroglian function seems to be dependent on Semaphorin1a. In contrast, during giant fiber synapse formation we observed that Semaphorin1a signaling as a receptor can be altered by Neuroglian in the same cell. In summary, our findings suggest that Neuroglian and Semaphorin1a can regulate each other’s function in cis and that the resultant signaling output is possibly different during guidance and synapse formation.
Keywords: Giant fiber, axon pathfinding, synaptogenesis, L1-CAM, Drosophila, cell adhesion
Introduction
It is a special pleasure for us to contribute to this volume celebrating Professor Heisenberg’s achievements in neurogenetics. Early on he saw the significance of a mutational approach to discovering the mechanisms underlying the assembly of the nervous system and described a number of mutants that affected this process. The re-examination of one of the many mutants isolated in the Heisenberg lab has led us to the discovery of a novel role for neuroglian/L1-CAM in forming synaptic connections in the CNS (Godenschwege, Kristiansen, Uthaman, Hortsch, & Murphey, 2006). The present paper is another demonstration that Heisenberg’s work continues to influence the field of neuroscience.
The assembly of neural circuits is a multi-step process involving an array of signaling molecules (Wen & Zheng, 2006). With the exception of pioneer neurons, most axons grow along already established neuronal tracks and receptors on the growth cone integrate signals that are secreted from or exposed on guideposts along the way. During their journey, growth cones usually encounter multiple such choice points which further determine the direction of growth of the axon (Chilton, 2006).
We have shown in two independent studies that Neuroglian and Semaphorin1a (Sema1a) are required for the formation of the giant fiber (GF) circuit in Drosophila, the activation of which mediates the escape response of the fly (Godenschwege, Hu, Shan-Crofts, Goodman, & Murphey, 2002; Godenschwege, Simpson et al., 2002). In wild type specimens, both GFs have their cell bodies in the brain and their axons connect to their respective targets, the TTMn (tergo-trochanteral motorneuron) and the PSI (peripheral synapsing interneuron) in the second thoracic neuromere (Figure 1a) (Allen, Godenschwege, Tanouye, & Phelan, 2006). In Semaphorin1a null mutants (sema1aP1) the GF displays three distinct phenotypes suggesting that Sema1a is involved in decision making at three different steps (Godenschwege, Hu et al., 2002). Approximately half of the GF axons in sema1aP1 mutants grow towards the retina showing that Sema1a has a function in directing the GF towards the connective in order to guide it out of the brain. In all other cases the GFs do reach the target area without any further pathfinding mistakes but most of them either pass by the target or stop at the target without elaborating the large GF presynaptic terminal (Godenschwege, Hu et al., 2002). These phenotypes reveal two additional functions for Sema1a, one in stopping the growth cone at the target as a first step to initiate synaptogenesis and a second in promoting the growth of the GF presynaptic terminal. Additional studies have shown that Sema1a signaling as a receptor in the GF is the “stop signal” for the growth cone at the medial dendrite of the TTMn, while bi-directional signaling of Sema1a on the TTMn with a yet unknown interaction partner on the GF is a “growth signal” for the giant synapse (Godenschwege, Hu et al., 2002; Murphey et al., 2003). In order for synapse formation to occur after the growth cone stopped at the target, the repulsive signaling of Sema1a as a receptor in the GF needs to be tuned off and this process has been shown to involve clathrin-mediated endocytosis (Godenschwege, Hu et al., 2002; Murphey et al., 2003).
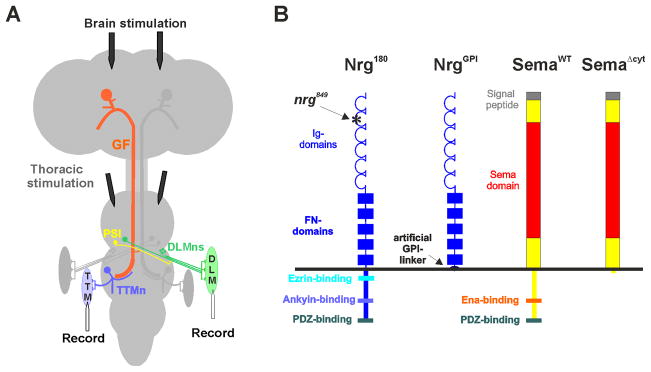
Figure 1. Giant Fiber System and Neuroglian and Semaphorin1a derivatives. A) Giant Fiber System and electrophysiological set up.
The left side of the GF circuit has been highlighted in color. The Giant fiber (GF, orange) soma is in the brain and extends its axon to the second thoracic neuromere. Here it connects to the Peripheral Synapsing Interneuron (PSI, yellow) and the Tergo-Trochanteral Motor neuron (TTMn, blue). The PSI synapses onto the dorsal longitudinal motor neurons (DLMn, green). TTMn and DLMn drive the jump (TTM) and flight muscles (DLM), respectively. Electrodes in the brain were used to activate the GF and the proper function of the GF-TTM and the GF-DLM pathways were tested by recording responses from the jump and flight muscle (Figure 2B). Stimulation electrodes in the thorax were used to test for the proper function of the neuromuscular junction as thoracic stimulation bypasses the GF and excites the TTMn and DLMn motor neurons directly. B) Schematic of Neuroglian and Semaphorin1a derivatives. The asterisk indicates the missense mutation present in the second Ig-domain of the nrg849 allele used in this study.
Neuroglian null mutants are lethal and the complete loss of function of L1-type proteins interferes with neurite outgrowth and axon guidance in early development (Bieber et al., 1989; Lemmon, Farr, & Lagenaur, 1989). However, Neuroglian (Nrg) is important for at least two other steps during GF circuitry formation as revealed by the nrg849 allele (Godenschwege et al., 2006). This allele was originally isolated in the Heisenberg lab as a brain structural mutant (central body deranged 849) with locomotor defects more than two decades ago (Strauss & Heisenberg, 1993). More recently the Callaerts lab found that this allele carries a mis-sense mutation in the neuroglian gene that alters a surface residue in the second Ig domain of the protein (Kang, 2004).
All the GFs in nrg849 flies grow toward the connective but some stall or grow aberrant processes in the subesophageal ganglion (white arrow, Figure 2B). Our studies show that for the axon to leave the brain via the connective Neuroglian function is required in the GF (Godenschwege et al., 2006). In the remaining cases, all other GFs reached and stopped at the TTMn without any further guidance mistakes but failed to grow a fully functional GF presynaptic terminal demonstrating an additional role for Neuroglian in synaptogenesis (Godenschwege et al., 2006).
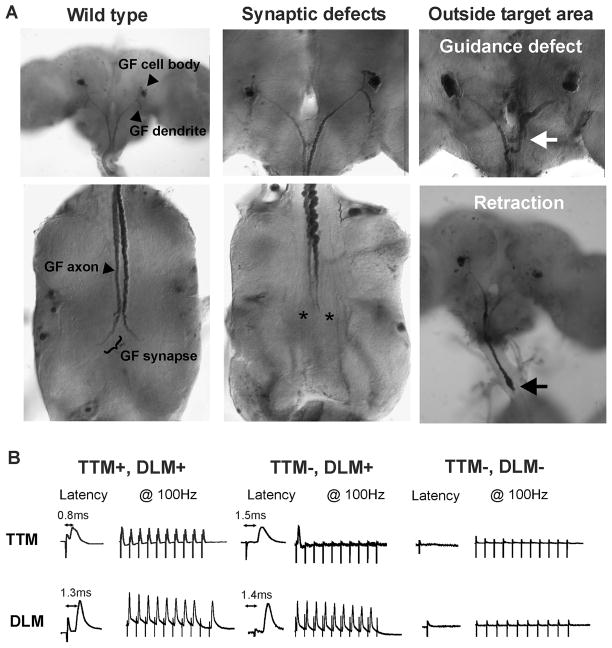
Figure 2. Anatomical and physiological phenotypes of the GF. A) Anatomical phenotypes of adult giant fibers.
Wild type: In this example Nrg overexpression (A307/UAS-nrg180) had no effect on the GF anatomy. Synaptic defects: Overexpression of Sema1a or the extracellular domain of Neuroglian (example of an A307/UAS-nrgGPI specimen is shown) prevented the synaptic terminals of the two GFs from forming (asterisks). Outside target area-Guidance defect: Some GF axons in nrg849 mutants started growing toward the connective but made guidance errors in the subesophageal ganglion (white arrow). The abundance of this type of phenotype was significantly increased in the same mutant background with reduced Sema1a dosage (nrg849/Y, sema1aP1/+, see Table 2). Outside target area-Retraction: Co-expression of Sema1a and NrgGPI in the GF often resulted in GF axons with retraction bulbs (black arrow) seen in the first thoracic neuromere or the connective and sometimes in the brain. B) Wild type and mutant recordings of the GF-TTM and GF-DLM pathway. TTM+, DLM+: In this wild type example the TTM response latency was 0.8 ms and the DLM response latency was 1.3 ms. The TTM and DLM pathways were able to follow stimuli up to 100 Hz. TTM−, DLM+: In mutants with a disrupted GF-TTMn synaptic terminal as seen in A (asterisks) the TTM response latency was increased (1.5 ms) and had defects in following stimuli given at 100Hz. However the responses of the GF-DLM pathway in these mutant flies are usually unaffected in comparison to wild type controls as GF axon makes an en passe synapse with the PSI further anteriorly (see schematic 1A). TTM+, DLM−: In some mutants we could not record any responses from either the TTM or the DLM when stimulated in the brain but thoracic stimulation demonstrated the presence of a NMJ. Such physiological recordings correlate with specimens in which the GF was not present in the target area as seen in A (white and black arrow).
Here, we have studied the genetic interaction between Neuroglian and Semaphorin1a in establishing the GF circuit. We found that Neuroglian and Semaphorin1a in cis can directly or indirectly alter each other’s signaling output with distinct effects during guidance and synapse formation.
Results
In order to determine whether Neuroglian (Nrg) and Semaphorin1a (Sema1a) interact with each other, we generated flies with various combinations of gain and loss of function of each gene and characterized the GF phenotypes anatomically and physiologically using standard methods (Figure 1, 2, Table 1). We used two Gal-4 drivers (c17 and A307) to express the Nrg and Sema1a constructs. The A307 driver expresses strongly in the GF and its postsynaptic targets (Allen, Drummond, & Moffat, 1998). In contrast c17 drives expression in the GF but not in its postsynaptic target, however it’s expression strength is weaker than A307 (Godenschwege, Simpson et al., 2002).
Table 1.
Physiology and Anatomy of the GF circuit under nrg and sema1a single and double mutant conditions
| Physiology in % | Anatomy in% | |||||||
|---|---|---|---|---|---|---|---|---|
| Genotype | n | wild typea | TTM−DLM+b | TTM−DLM−c | n | synaptic terminald | mutant synaptic terminale | outside target areaf |
| +/y or +/+ | 50 | 100 | 0 | 0 | 50 | 100 | 0 | 0 |
| nrg849/+ | 33 | 85 | 15 | 0 | 28 | 94 | 0 | 6* |
| nrg849/y | 52 | 8 | 69 | 23 | 30 | 17 | 60 | 23* |
| semaP1/+ | 20 | 100 | 0 | 0 | 50 | 100 | 0 | 0 |
| nrg849/+;sema1aP1/+ | 29 | 86 | 14 | 0 | 48 | 86 | 4 | 10* |
| nrg849/y;sema1aP1/+ | 30 | 0 | 23 | 77 | 15 | 7 | 13 | 80* |
| UAS-sema1aWT/c17 | 30 | 77 | 23 | 0 | 65 | 88 | 12 | 0 |
| UAS-sema1aWT,A307/+ | 23 | 52 | 48 | 0 | 33 | 55 | 45 | 0 |
| UAS-sema1aΔcyt/c17 | 22 | 95 | 5 | 0 | 48 | 100 | 0 | 0 |
| UAS-sema1aΔcyt,A307/+ | 20 | 95 | 5 | 0 | 50 | 100 | 0 | 0 |
| UAS-nrg180/c17 | 24 | 100 | 0 | 0 | 17 | 100 | 0 | 0 |
| UAS-nrg180/A307 | 16 | 100 | 0 | 0 | 21 | 100 | 0 | 0 |
| UAS-nrgGPI/c17 | 27 | 93 | 7 | 0 | 42 | 95 | 5 | 0 |
| UAS-nrgGPI/A307 | 60 | 0 | 98 | 2 | 41 | 59 | 36 | 5 |
| UAS-nrg180/UAS-sema1aWT,c17 | 21 | 67 | 33 | 0 | 30 | 74 | 23 | 3 |
| UAS-nrg180/UAS-sema1aΔcyt,c17 | 20 | 95 | 0 | 0 | 14 | 100 | 0 | 0 |
| UAS-nrg180/sema1aP1,c17 | 24 | 100 | 0 | 0 | nd | nd | nd | nd |
| UAS-nrg180/UAS-sema1aWT,A307 | 29 | 55 | 45 | 0 | 19 | 15 | 58 | 26 |
| UAS-nrg180/UAS-sema1aΔcyt,A307 | 12 | 92 | 8 | 0 | nd | nd | nd | nd |
| UAS-nrg180/sema1aP1,A307 | 12 | 100 | 0 | 0 | nd | nd | nd | nd |
| UAS-nrgGPI/UAS-sema1aWT,c17 | 15 | 20 | 67 | 13 | 28 | 54 | 32 | 14 |
| UAS-nrgGPI/UAS-sema1aΔcyt,c17 | 12 | 83 | 17 | 0 | 14 | 100 | 0 | 0 |
| UAS-nrgGPI/sema1aP1,c17 | 10 | 100 | 0 | 0 | nd | nd | nd | nd |
| UAS-nrgGPI/UAS-sema1aWT,A307 | 17 | 0 | 0 | 100 | 31 | 0 | 23 | 77 |
| UAS-nrgGPI/UAS-sema1aΔcyt,A307 | 22 | 22 | 69 | 9 | nd | nd | nd | nd |
| UAS-nrgGPI/sema1aP1,A307 | 20 | 30 | 70 | 0 | 71 | 82 | 18 | 0 |
A wild type giant synapse is defined as a TTM with response latency ≤1 ms and ability to follow stimuli one-to-one at 100Hz when stimulated in the brain.
Percent of flies that had a mutant TTM response but the DLM response was present.
Percent of flies that had no TTM and DLM response upon brain stimulation but showed a response upon thoracic stimulation.
Percent of GFs that exhibited a synaptic terminal with normal diameter in the second thoracic neuromere.
Percent of GFs that have reached the target area in the second thoracic neuromere and exhibited abnormally thin, short or no synaptic terminals.
Percent of GFs that did not reach the target area in the second thoracic neuromere. Some GFs (*) terminate in the brain due to guidance error (see Figure 2A white arrow), others display retraction bulbs (see Figure 2A black arrow) often in the first thoracic neuromere and the connective, occasionally in the brain. UAS-lacZ was co-expressed on the X or the second chromosome as a reporter gene in order to reveal the GF anatomy with anti β-galactosidase staining.
nrg849 dependent GF guidance defects are enhanced by reduced Sema1a signaling
In wild type specimens, the GF axon leaves the brain via the connective and forms the large presynaptic terminal onto the medial dendrite of the TTMn in the second thoracic neuromere (Figure 1A, 2A). As previously described, a mutation in the extracellular domain of nrg849 mutants alters the protein’s intracellular output via its ankyrin-binding site (Figure 1A) while other functions of the molecule are likely to be unaffected (Godenschwege et al., 2006). In this mutant approximately 23% of the GFs display guidance defects and terminate in the subesophageal ganglion (Table 1, Figure 2A, white arrow). Correlating with this anatomical phenotype, electrophysiological recordings confirmed that the GF-TTM and GF-DLM pathways are disrupted as no responses were observed in the respective muscles when the GF was activated in the brain (Table 1, Figure 2B). Although heterozygous sema1aP1 mutants are completely wildtype, a reduction in sema1a gene dosage (sema1aP1/+) in the nrg849 mutant background dramatically enhanced both physiological (77% defective TTM−, DLM− synapses) and anatomical (80% defective) nrg849 guidance phenotypes (Table 1). This result suggests that Sema1a can influence Neuroglian function during axon guidance.
Synaptic defects caused by repulsive signaling of Semaphorin1a are enhanced by the NrgGPI
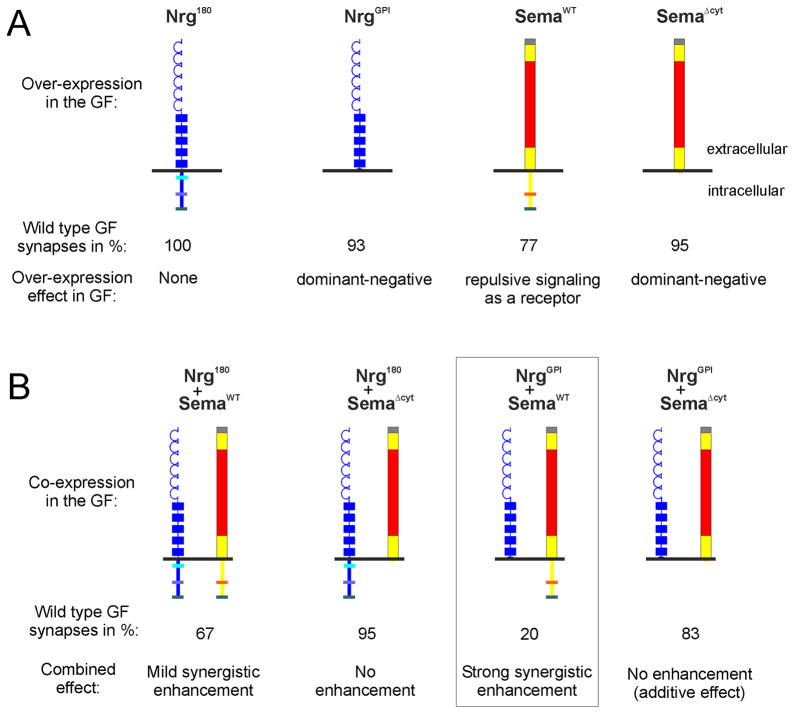
We are especially interested in the role of these two proteins in synapse formation. We therefore focused on the synaptic defects in various genetic interaction experiments. We have previously shown that although Neuroglian is required for giant synapse formation pre- and postynaptically, its over expression (Nrg180) in the GF had no disruptive effect on the function of the giant synapse (Table 1, Figure 2A and 3A) (Godenschwege, Hu et al., 2002; Godenschwege et al., 2006). We and others have also previously demonstrated that while the extracellular domain of Nrg is sufficient for many aspects of axonal guidance, GF synapse formation is completely dependent on the intracellular domain of Nrg (Table 1, Figure 2A) (Godenschwege et al., 2006; Islam, Kristiansen, Romani, Garcia-Alonso, & Hortsch, 2004; Islam, Wei, Chiu, Hortsch, & Hsu, 2003; Kristiansen et al., 2005). Hence, the cell-autonomous expression of the extracellular domain of Nrg tethered to the membrane via an artifical GPI-linker (NrgGPI) in the GF had no effect on GF guidance but disrupts GF synapse formation due to its dominant-negative effect (Table 1, Figure 2A and 3A) (Godenschwege et al., 2006; Kristiansen et al., 2005).
Figure 3. Schematic representation of the Nrg-Sema1A interactions in the GF during synaptogenesis. A) Effects of the overexpression of single Neuroglian and Semaphorin 1a constructs in the GF.
Overexpression (using c17-Gal4 driver) of full length neuronal Nrg (Nrg180) has no effect, while cell-autonomous expression of the extracellular Nrg-domain lacking the intracellular domain (NrgGPI) function as a dominant-negative with respect to giant synapse function (Table 1) (Godenschwege et al., 2006). Excess of Sema1a signaling in the GF as a receptor (SemaWT) disrupts proper giant fiber synapse formation, while the cell-autonomous expression extracellular Sema1a domain (SemaΔcyt) has only mild disruptive effects as a dominant-negative (Table 1) (Godenschwege, Hu et al., 2002). B) Co-expression of various Neuroglian and Semaphorin 1a constructs in the GF. Note that only the combination of NrgGPI and SemaWT (boxed) had very strong synergistically enhanced disruptive effects on the formation of a wild type GF synapse, while all other combinations had no or only mild effects (Table 1).
As described in the introduction Sema1a plays important roles in the assembly of the GF synapse pre- as well as postsynaptically (Godenschwege, Hu et al., 2002; Godenschwege et al., 2006). While initially required in the GF for stopping the growth cone at the target, continued repulsive signaling as a receptor due to expression of full-length Sema1a (Sema1aWT) with the c17 or A307 Gal-4 drivers in GF disrupts the synapse anatomically and physiologically (Table 1, Figure 2A and 3A) (Godenschwege, Hu et al., 2002; Godenschwege et al., 2006). Expression of Sema 1a lacking its intracellular domain (Sema1aΔcyt ) in the GF disrupted the function of the GF synapse mildly, possibly due to dominant-negative effects (Table 1, Figure 2A and 3A) (Godenschwege, Hu et al., 2002; Godenschwege et al., 2006).
In order to test whether Neuroglian and Semaphorin cooperate during GF synapse formation we co-expressed the various Nrg and Sema1a constructs in the GF. Co-expression of Nrg180 and Sema1aΔcyt had little or no effect on the GF anatomy or physiology (Table 1 and Figure 3B). Simultaneous expression of Sema1aΔcyt and NrgGPI exclusively in the GF (using c17) disrupted the GF anatomy and function (83% wild type responses) but was not significantly more than the additive disruptive effect (100%− 5%−7%= 88%) of each construct alone (95% and 93% wild type responses, respectively) (Table 1 and Figure 3). Expression of Nrg180 with Sema1aWT (67% wild type responses) only enhanced the Sema1a gain of function phenotypes (77% wild type responses) mildly if at all (Table 1 and Figure 3). In contrast, the combination of Sema1aWT and NrgGPI had dramatic synergistic effects. When expressed together in the GF (using c17) they led to much more severe phenotypes (20% wild type responses) than the expression of either transgene alone (93% and 77% wild type responses; Table 1, Figure 3). Figure 3 schematizes the critical results showing the interaction in cis between the two proteins. The strong effect produced by cell autonomous expression of Sema1aWT and NrgGPI in the GF (using c17) suggests that the Nrg and Sema1a interact with each other in cis in the GF.
Additional support for the cis interaction was obtained, when the two constructs were expressed with the stronger driver (A307), where in 100% of the cases the GF was functionally completely disconnected and the anatomical studies revealed that most GFs did not terminate in the target area (77%). However, in contrast to the guidance defects seen in the nrg849 mutants in which the GFs were only found to terminate in the subesophageal ganglion, the axons terminals of GFs co-expressing Sema1aWT and NrgGPI terminated anywhere between the target area and the cell bodies in the brain (Table 1, Figure 2A white arrow). In addition, the GFs of these specimens displayed retraction bulbs (Figure 2A black arrow) in most cases, suggesting that the GFs retracted or degenerated in response to excess repulsive signaling after they had reached the target area. Interestingly, such a retraction of the GFs from the target area has also been observed previously when the removal of Sema1a from the surface was prevented by inhibition of clathrin-mediated endocytosis (Murphey et al., 2003). This suggests that Neuroglian may influence the repulsive signaling of Sema1a as a receptor possibly by regulating its trafficking. Another piece of evidence that points to Nrg endogenously regulating Sema1a signaling during GF synapse formation and not vice versa is the finding that reduction of sema1a gene dosage (UAS-nrgGPI/A307 or c17,sema1aP1) or the co-expression of Sema1a lacking its intracellular domain (UAS-nrgGPI/A307 or c17,UAS-sema1aΔcyt ) reduced the disruptive effects of NrgGPI over expression (Table 1).
Discussion
Our results strongly suggest that Neuroglian and transmembrane Sema1a interact in cis during synapse formation and possibly also during guidance in the GF system of Drosophila. This is different from what has been described previously in vertebrates. L1-CAM, the vertebrate homologue of Drosophila Neuroglian, has been shown to be a co-receptor of the Neuropilin/Plexin complex, which mediates the response to secreted Sema3A during axon guidance (Castellani, Chedotal, Schachner, Faivre-Sarrailh, & Rougon, 2000; Castellani, De Angelis, Kenwrick, & Rougon, 2002). Invertebrates don’t have Neuropilins but in Caenorhabditis elegans the L1-CAM/Neuroglian homologue LAD-2 has recently been shown to bind to Plexin PLX-2 as well as to secreted MAB-20/Sema2 (Castellani et al., 2000; Castellani et al., 2002; Wang et al., 2008). The finding that LAD-2 functions as a co-receptor of Plexin in mediating MAB-20/Sema2 response during axon guidance suggests that L1-type proteins in invertebrates may have also assimilated functions of vertebrate Neuropilin (Castellani et al., 2000; Castellani et al., 2002; Wang et al., 2008). The extracellular domain of both, Plexins and Semaphorins, contains a 500 amino acid segment called the “sema domain” that mediates most of their inter-molecular interactions and possibly also the binding to L1-type proteins (Castellani et al., 2002; Fiore & Puschel, 2003; Gherardi, Love, Esnouf, & Jones, 2004; Huber, Kolodkin, Ginty, & Cloutier, 2003; Wang et al., 2008). In addition, LAD-2 has been shown to physically associate with both, MAB-20/Sema2 and PLX2, in c. elegans but the orientation in which secreted MAB-20/Sema2 molecule binds to LAD-2 has not been determined (Castellani et al., 2002; Fiore & Puschel, 2003; Gherardi et al., 2004; Huber et al., 2003; Wang et al., 2008). Hence, it is possible that transmembrane Semaphorin1a physically interacts with Neuroglian/L1-CAM in cis and we propose that this is the case in the GF during synapse formation (Figure 4).
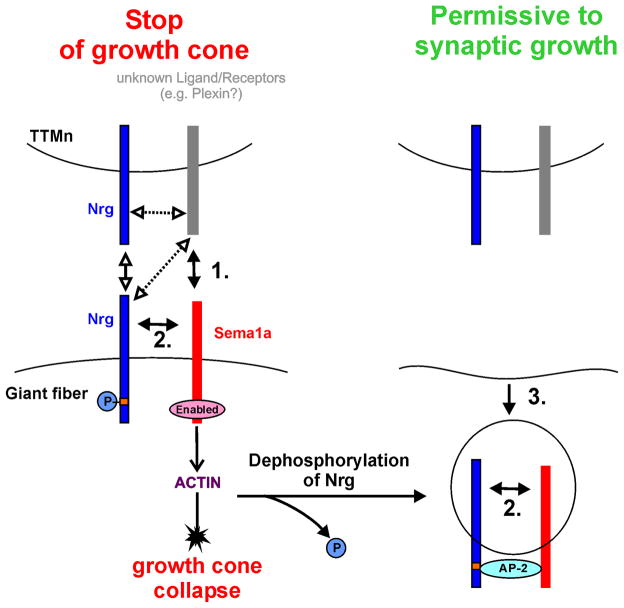
Figure 4. Hypothetical signaling scheme for Neuroglian and Sema1a in GF synapse formation.
Left: Sema1a functions as a repulsive receptor when binding to an unknown interaction partner (arrow no. 1) is hypothesized to stop the giant fiber growth cone at the target (TTMn) (Godenschwege et al., 2006). Right: This repulsive activity must then be switched off to allow for synapse formation to proceed and involves removing Sema1a from the surface (Godenschwege et al., 2006; Murphey et al., 2003). Nrg is involved in switching the GF from axonal growth to synaptic growth by binding in cis to Sema1a (arrows no. 2). De-phosphorylation of Nrg’s intracellular domain may similar to vertebrate L1-CAM target the endocytic apparatus via the clathrin adaptor protein AP-2 to the Nrg-Sema1a complex and facilitates its removal from the surface (arrow no. 3).
In this scenario, Neuroglian could regulate Sema1a signaling as a receptor by co-trafficking or by altering its signaling directly. However, the finding that both, the inhibition of endocytosis (Murphey et al., 2003) as well as the expression of membrane-tethered Nrg lacking its intracellular domain (NrgGPI) in a Sema1a gain-of-function background, had the same effect supports our hypothesis that the regulation of Sema1a by Nrg involves clathrin-mediated endocytosis (Figure 4, Table 1). Hence, similar to vertebrate L1-CAM where de-phosphorylation induces the endocytosis of L1-CAM (Kamiguchi et al., 1998; Schaefer et al., 2002), de-phosphorylation at a yet unidentified motif in invertebrate Nrg may induce the endocytosis of the Sema1a by the binding of the clathrin adaptor protein AP-2 to the Nrg-Sema1a complex. The removal of this repulsive Sema1a signaling important to stop the GF growth cone is essential in order for synapse formation to proceed (Figure 4). Therefore, the phenotypes seen with NrgGPI expression in a wild type background may in part be due to endogenous Sema1a that could not be removed from the surface because of its association with NrgGPI. Therefore the continued repulsive signaling of Sema1a as a receptor could prevent the GF synapse from forming in A307/NrgGPI specimens.
The missense mutation in nrg849 mutants disrupts homophilic binding and reduces the phosphorylation of the intracellular ankyrin binding motif but many of other functions of the molecules remain unaffected (Godenschwege et al., 2006). We have found that the reduction of Sema1a function in the GF dramatically enhanced the guidance defects in nrg849 mutants without having any effect on its own. This strongly suggests that in this situation Sema1a affects Neuroglian output rather than Neuroglian regulating Sema1a signaling.
In conclusion, our results suggest that Semaphorin1a and Neuroglian influence each other’s signaling and the nature of their interaction may be different at the different guideposts an axon encounters during a particular stage of development. We have also shown that the interaction between these two molecules result in distinct responses during the different developmental stages of axon guidance and synapse formation. Our study has provided further insight into the roles that evolutionarily conserved molecules like L1 and Semaphorins play during the formation of functional neuronal circuits.
Material and Methods
Drosophila Stocks
All stocks were grown at 22°C or 25°C on standard medium. The neuroglian and sema1a fly stocks used have been described previously (Bieber et al., 1989; Godenschwege, Hu et al., 2002; Godenschwege et al., 2006; Hall & Bieber, 1997; Islam et al., 2004; Kolodkin, Matthes, & Goodman, 1993; Kristiansen et al., 2005; Strauss & Heisenberg, 1993; Yu, Huang, & Kolodkin, 2000). The two P[GAL]4 lines that drive expression in the GFs were A307, which shows strong expression in the GF and its postsynaptic targets (Allen et al., 1998), and c17, which drives expression weaker than A307 in the GF but does not have expression in its postsynaptic targets (Godenschwege, Simpson et al., 2002).
Immunocytochemistry and Electrophysiology
In order to reveal the lac-Z expression, the CNS of adults and pupae expressing UAS-lacZ were dissected in 100mM Phosphate buffer (PB) and immuncytochemistry with polyclonal rabbit anti β-galactosidase antibody (1:6000, Cappel, Tunhout, Belgium) was performed as previously described (Godenschwege, Hu et al., 2002; Godenschwege, Simpson et al., 2002; Murphey et al., 2003). Details of image capturing and processing have been previously described (Allen et al., 1999; Allen, Shan, & Murphey, 2000; Godenschwege, Hu et al., 2002; Godenschwege, Simpson et al., 2002).
Intracellular recordings from TTM and DLM muscles were obtained from adult flies in a method similar to that described earlier (Tanouye & Wyman, 1980). The modifications of the physiological assay, the analyses of the data have been described elsewhere (Godenschwege, Hu et al., 2002; Godenschwege, Simpson et al., 2002). In brief, the GFs were activated with extracellular stimulation by two tungsten electrodes in the brain by giving pulses of 40–60 mV for 0.03ms. For direct extracellular stimulation of the motorneurons the electrodes were placed into the thoracic ganglion. A tungsten electrode placed in the abdominal cavity served as a ground. Glass electrodes were filled with saline and were driven through the cuticle into the DLM and TTM muscle fibers for intracellular recordings. For each animal the TTM and DLM response latency was measured upon brain or thoracic stimulation. Also the reliability of GF-TTM and GF-PSI-DLM circuit was evaluated by testing its ability to follow 10 pulses given at 100Hz.
Acknowledgments
This work was supported by grants of the National Institute of Health RO1HD050725-01A1 to T.A.G. and RO1NS044609 to R.K.M. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Child Health and Human Development or the National Institutes of Health. We are grateful to the P. Callaerts, C. Goodman, M. Heisenberg, M. Hortsch, A. Kolodkin labs as well as the Bloomington Stock Center for providing fly stocks. We are grateful to Dr S. B. Uthaman for comments on the ms.
References
- Allen MJ, Drummond JA, Moffat KG. Development of the giant fiber neuron of Drosophila melanogaster. J Comp Neurol. 1998;397(4):519–531. doi: 10.1002/(sici)1096-9861(19980810)397:4<519::aid-cne5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Allen MJ, Godenschwege TA, Tanouye MA, Phelan P. Making an escape: development and function of the Drosophila giant fibre system. Semin Cell Dev Biol. 2006;17(1):31–41. doi: 10.1016/j.semcdb.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Allen MJ, Shan X, Caruccio P, Froggett SJ, Moffat KG, Murphey RK. Targeted expression of truncated glued disrupts giant fiber synapse formation in Drosophila. J Neurosci. 1999;19(21):9374–9384. doi: 10.1523/JNEUROSCI.19-21-09374.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen MJ, Shan X, Murphey RK. A role for Drosophila Drac1 in neurite outgrowth and synaptogenesis in the giant fiber system. Mol Cell Neurosci. 2000;16(6):754–765. doi: 10.1006/mcne.2000.0903. [DOI] [PubMed] [Google Scholar]
- Bieber AJ, Snow PM, Hortsch M, Patel NH, Jacobs JR, Traquina ZR, et al. Drosophila neuroglian: a member of the immunoglobulin superfamily with extensive homology to the vertebrate neural adhesion molecule L1. Cell. 1989;59(3):447–460. doi: 10.1016/0092-8674(89)90029-9. [DOI] [PubMed] [Google Scholar]
- Castellani V, Chedotal A, Schachner M, Faivre-Sarrailh C, Rougon G. Analysis of the L1-deficient mouse phenotype reveals cross-talk between Sema3A and L1 signaling pathways in axonal guidance. Neuron. 2000;27(2):237–249. doi: 10.1016/s0896-6273(00)00033-7. [DOI] [PubMed] [Google Scholar]
- Castellani V, De Angelis E, Kenwrick S, Rougon G. Cis and trans interactions of L1 with neuropilin-1 control axonal responses to semaphorin 3A. Embo J. 2002;21(23):6348–6357. doi: 10.1093/emboj/cdf645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton JK. Molecular mechanisms of axon guidance. Dev Biol. 2006;292(1):13–24. doi: 10.1016/j.ydbio.2005.12.048. [DOI] [PubMed] [Google Scholar]
- Fiore R, Puschel AW. The function of semaphorins during nervous system development. Front Biosci. 2003;8:s484–499. doi: 10.2741/1080. [DOI] [PubMed] [Google Scholar]
- Gherardi E, Love CA, Esnouf RM, Jones EY. The sema domain. Curr Opin Struct Biol. 2004;14(6):669–678. doi: 10.1016/j.sbi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Godenschwege TA, Hu H, Shan-Crofts X, Goodman CS, Murphey RK. Bi-directional signaling by Semaphorin 1a during central synapse formation in Drosophila. Nat Neurosci. 2002;5(12):1294–1301. doi: 10.1038/nn976. [DOI] [PubMed] [Google Scholar]
- Godenschwege TA, Kristiansen LV, Uthaman SB, Hortsch M, Murphey RK. A conserved role for Drosophila Neuroglian and human L1-CAM in central-synapse formation. Curr Biol. 2006;16(1):12–23. doi: 10.1016/j.cub.2005.11.062. [DOI] [PubMed] [Google Scholar]
- Godenschwege TA, Simpson JH, Shan X, Bashaw GJ, Goodman CS, Murphey RK. Ectopic expression in the giant fiber system of Drosophila reveals distinct roles for roundabout (Robo), Robo2, and Robo3 in dendritic guidance and synaptic connectivity. J Neurosci. 2002;22(8):3117–3129. doi: 10.1523/JNEUROSCI.22-08-03117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SG, Bieber AJ. Mutations in the Drosophila neuroglian cell adhesion molecule affect motor neuron pathfinding and peripheral nervous system patterning. J Neurobiol. 1997;32(3):325–340. [PubMed] [Google Scholar]
- Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- Islam R, Kristiansen LV, Romani S, Garcia-Alonso L, Hortsch M. Activation of EGF receptor kinase by L1-mediated homophilic cell interactions. Mol Biol Cell. 2004;15(4):2003–2012. doi: 10.1091/mbc.E03-05-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam R, Wei SY, Chiu WH, Hortsch M, Hsu JC. Neuroglian activates Echinoid to antagonize the Drosophila EGF receptor signaling pathway. Development. 2003;130(10):2051–2059. doi: 10.1242/dev.00415. [DOI] [PubMed] [Google Scholar]
- Kamiguchi H, Long KE, Pendergast M, Schaefer AW, Rapoport I, Kirchhausen T, et al. The neural cell adhesion molecule L1 interacts with the AP-2 adaptor and is endocytosed via the clathrin-mediated pathway. J Neurosci. 1998;18(14):5311–5321. doi: 10.1523/JNEUROSCI.18-14-05311.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YY. PhD thesis, Department of Biology and Biochemistry, University of Houston. 2004. The role of the Drosophila L1 CAM Neuroglian in postembryonic brain development: From cell adhesion to signaling. [Google Scholar]
- Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75(7):1389–1399. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- Kristiansen LV, Velasquez E, Romani S, Baars S, Berezin V, Bock E, et al. Genetic analysis of an overlapping functional requirement for L1- and NCAM-type proteins during sensory axon guidance in Drosophila. Mol Cell Neurosci. 2005;28(1):141–152. doi: 10.1016/j.mcn.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Lemmon V, Farr KL, Lagenaur C. L1-mediated axon outgrowth occurs via a homophilic binding mechanism. Neuron. 1989;2(6):1597–1603. doi: 10.1016/0896-6273(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Murphey RK, Froggett SJ, Caruccio P, Shan-Crofts X, Kitamoto T, Godenschwege TA. Targeted expression of shibire ts and semaphorin 1a reveals critical periods for synapse formation in the giant fiber of Drosophila. Development. 2003;130(16):3671–3682. doi: 10.1242/dev.00598. [DOI] [PubMed] [Google Scholar]
- Schaefer AW, Kamei Y, Kamiguchi H, Wong EV, Rapoport I, Kirchhausen T, et al. L1 endocytosis is controlled by a phosphorylation-dephosphorylation cycle stimulated by outside-in signaling by L1. J Cell Biol. 2002;157(7):1223–1232. doi: 10.1083/jcb.200203024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss R, Heisenberg M. A higher control center of locomotor behavior in the Drosophila brain. J Neurosci. 1993;13(5):1852–1861. doi: 10.1523/JNEUROSCI.13-05-01852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanouye MA, Wyman RJ. Motor outputs of giant nerve fiber in Drosophila. J Neurophysiol. 1980;44(2):405–421. doi: 10.1152/jn.1980.44.2.405. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang W, Cheever T, Schwarz V, Opperman K, Hutter H, et al. The C. elegans L1CAM homologue LAD-2 functions as a coreceptor in MAB-20/Sema2 mediated axon guidance. J Cell Biol. 2008;180(1):233–246. doi: 10.1083/jcb.200704178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Zheng JQ. Directional guidance of nerve growth cones. Curr Opin Neurobiol. 2006;16(1):52–58. doi: 10.1016/j.conb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Yu HH, Huang AS, Kolodkin AL. Semaphorin-1a acts in concert with the cell adhesion molecules fasciclin II and connectin to regulate axon fasciculation in Drosophila. Genetics. 2000;156(2):723–731. doi: 10.1093/genetics/156.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]