Abstract
Monocytes are central mediators in the advance of atherosclerotic plaque, making them a natural therapeutic target for reducing disease burden. Here, we highlight recent advances in our current understanding of monocyte heterogeneity and its relevance to regulation of monocyte accumulation and function within atherosclerotic plaques. Differences that distinguish monocyte subsets include differential expression of chemokine receptors, especially CCR2 and CX3CR1. Ablation of expression of these two receptors (or their ligands) in mice has an additive inhibition on monocyte recruitment to atherosclerotic plaques. Moreover, simultaneously interfering with three key pathways—CCR2, CX3CR1, and CCR5--essentially abolishes atherosclerosis in mice. Here, we discuss how these chemokine receptors act at multiple points on at least one monocyte subset, regulating their mobilization from bone marrow, survival, and/or recruitment to plaques. Finally, we discuss how this knowledge may be useful clinically, emphasizing that CX3CR1 may in particular be a viable target for therapeutic manipulation of monocyte-derived cell fate in cardiovascular disease.
Introduction
Atherosclerosis is both a metabolic and an immuno-inflammatory disease. Whereas dyslipidemia is required for the initiation and progression of atherosclerotic lesions, the role of inflammatory cells, especially monocytes, is central to plaque development. Indeed, reduction in circulating monocytes limits plaque development in animal models of atherosclerosis 1, 2, blood monocyte counts are an independent risk factor for coronary artery disease in humans 3–5, and alterations in monocyte/macrophage function (i.e., cholesterol efflux) amplify atherosclerosis even under conditions of mild hypercholesterolemia 6. Our understanding of monocyte biology has evolved rapidly in the last several years, as we have learned more about their heterogeneity and homeostasis (mobilization, recruitment, survival). In this review, we will describe the monocyte subsets in mouse and human and consider their functional roles in atherosclerosis. We will also discuss the role chemokine receptors play in mobilizing monocytes into the blood, recruiting them to plaques, and aiding their survival, with an emphasis on the possibility that CX3CR1 (fractalkine receptor) serves as a suitable therapeutic target.
Classical and nonclassical monocyte subsets
There are two major subsets of monocytes that are recognized in humans, mice, and other species (rat, pig) 7, 8 (Fig. 1). They are distinguished by distinct chemokine receptor expression patterns, especially differential expression of CCR2 and CX3CR1 9–11, as well as conservation of other surface markers 7, 8, 12, 13. A recent gene expression analysis comparing human and mouse monocytes reveals further conservation between monocyte subsets, though it also identifies key differences between the species, including patterns of CD36 expression (unpublished observations; MA Ingersoll et al., submitted).
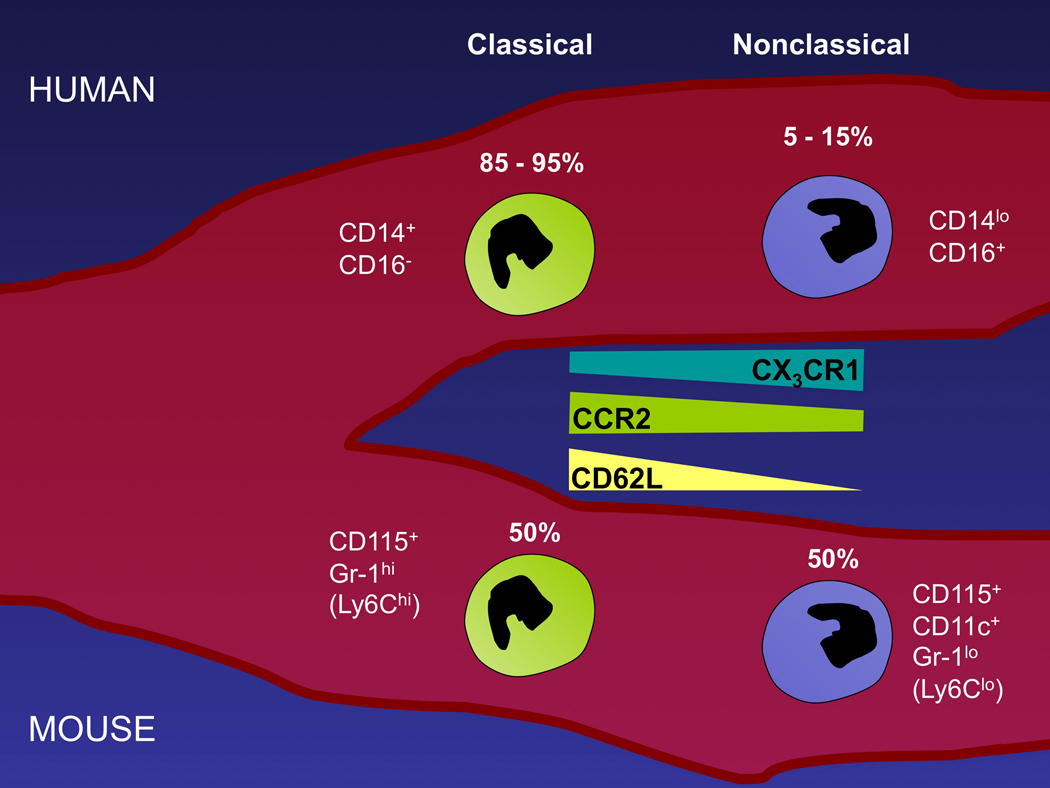
Figure 1.
Cartoon depicting the major markers and frequency (relative %) of the two major monocyte subsets in human (top) and mouse (bottom) blood.
The various terms used in the literature to describe mouse monocyte subsets, and their analogous populations in human, can be confusing (Table 1), with references to different combinations of surface markers for different species, or use of terms like “inflammatory monocytes” that do not refer to the same populations in mice and humans (Table 1). In order to use unifying terms that cross species, we refer to CCR2+ monocytes in all species as “classical monocytes” 14. CCR2+ monocytes comprise the vast majority of human monocytes (≥ 92%), and we regard their ability to respond to the chemokine monocyte chemoattractant protein 1 (MCP-1; CCL2 15) as a classical characteristic. Conversely, CD14loCD16+ human and Ly-6Clo mouse monocytes, low in CCR2 expression 16–18 and relatively rare among monocytes in humans, are “nonclassical monocytes.” In mice, classical and nonclassical monocyte frequency is approximately 1:1 11, 12, compared with the less than 10% of human monocytes that bear the non-classical phenotype. There is evidence that nonclassical monocytes derive from classical monocytes 12, 19, 20, but it remains possible that some nonclassical monocytes arise independently21.
Table 1.
Classification of human and mouse monocyte subsets
| Classical Human | Classical Mouse | Nonclassical Human | Nonclassical Mouse | Refs. | |
|---|---|---|---|---|---|
| Common phenotypic identifiers | CD14++ CD16− | CD115+ Gr1+/Ly-6C+ 7/4hi | CD14+/CD14int CD16+ | CD115+ Gr1-/Ly-6C− 7/4lo | 9, 11, 12, 17, 83 |
| Chemokine receptors differentially expressed | CCR2+ CCR1+ CXCR1+ CXCR2+ CX3CR1int |
CCR2+ CCR1+ CX3CR1int | CCR2− CCR1− CX3CR1hi CCR5 is expressed on CD14+ CD16+ but not CD14int CD16+ monocytes | CCR2− CCR1− CX3CR1hi CCR5+ in inflamed apoE KO mice | 10, 11, 17, 18, 30 |
| Other differentially expressed markers | CD62L+ CD43+ CD11a+ CD11c+ |
CD62L+ CD43+ CD11a+ CD11c− |
CD62L− CD43++ CD11a++ CD11c++ |
CD62L− CD43++ CD11a++ CD11c+ |
7, 11, 12, 22, 23, 30 |
| Functional Assessments | Highly phagocytic relative to nonclassical counterparts; High SR-A and CD36 expression | Not known to differ in phagocytosis relative to nonclassical counterparts | Signaling cascades analyzed by proteomics suggest a phagocytic past for nonclassical monocytes; preference for recognition of oxidized LDL, despite lower scavenger receptor (SR-A, CD36) expression; High TNF secretion; Superior in T cell activation | May be more likely to take up oxidized LDL; high scavenger receptor (SR-A) expression; High TNF secretion; Superior in T cell activation (mixed lymphocyte reaction) | 22–25, 27, 37 , 39, 84–87 |
| Alternative names in the literature |  |
Resident monocytes; Stationary monocytes | 11, 22, 88 | ||
Same coloration of columns indicates human and mouse populations with overlapping features. Red-bordered box reveals terms used to describe monocyte subsets that do not correspond to populations with overlapping features between species.
Functionally, a number of differences between circulating monocyte subsets has been identified, and some of these differences are summarized in Table 1. For example, non-classical monocytes in mouse and man produce more TNF than classical monocytes22, 23 and are more active as antigen-presenting cells in assays measuring T cell stimulation24–27. The role of nonclassical monocytes in regulating T cell responses may be more for the promotion of tolerance than priming, as recently suggested in one study of mouse nonclassical monocytes 28. In humans, nonclassical monocytes include cells that express ILT4, a ligand for HLA-G implicated in fetal tolerance during pregnancy 29.
In line with their differential chemokine receptor profiles, the trafficking patterns of the two monocyte subsets are distinct, at least in mice. Classical mouse monocytes are recruited to sites of inflammation in a “classical” time course (following the typical wave of neutrophils) during acute inflammation 14. The migratory behavior of nonclassical monocytes is far less clear, with different studies on them reaching quite different conclusions, raising the possibility that their behavior is context-dependent. Nonclassical mouse monocytes migrate less abundantly than classical monocytes in acute peritonitis 11, 30. In contrast, in the steady state, they accumulate readily in spleen, liver, brain, and lung, but not in the peritoneum 11. The cell types they differentiate into in these organs and their roles therein remain unclear. In spleen, they likely participate in T-independent antibody production by innate B cells (for review and more extensive discussion on this topic, see 13), and, as mentioned above, participate in cross-tolerance 28. A role for these monocytes in facilitating natural antibody production by innate B cells may be particularly relevant to atherosclerosis, since many natural antibodies react with oxidized LDL 31 and serve atheroprotective roles in mouse models of atherosclerosis 32.
Following injection of Listeria monocytogenes into the peritoneal cavity, nonclassical mouse monocytes appear to accumulate very early 23, earlier than neutrophils. By contrast, after myocardial infarction, nonclassical monocyte entry into the injured heart occurs mainly during later stages of inflammation (day 3 and beyond), where they are thought to coordinate tissue repair, including angiogenesis 33. On the other hand, non-classical monocytes are not obviously recruited to sites of mild 27 or severe 34 skin inflammation, nor at sites of skeletal muscle injury 35. In these sites, however, there is evidence that robustly recruited classical monocytes acquire phenotypical features of non-classical monocytes (upregulation of CX3CR1, induction of CD11c, loss of Gr-1/Ly-6C expression) and these cells in turn promote wound healing 35 and/or egress to lymph nodes 27. Both the myocardial infarction study and the skeletal injury study proposed that classical monocytes were critical to setting the stage for resolution by clearing dead cell debris and that the nonclassical monocyte phenotype mediated the next stages in healing after debridement of the injured tissue 33, 35.
Both classical and nonclassical monocytes enter mouse atherosclerotic lesions 30, but the entry of classical monocytes into plaques occurs in greater magnitude 30, 36, making classical monocytes a natural focal point as major mediators of atherosclerotic disease. It remains to be determined if these populations maintain distinct phenotypes within plaques and/or if one population is more injurious to plaque stability than the other. In general, numbers of nonclassical human monocytes rise in the context of inflammatory disease 14. However, it is not known if this is true in peripheral arterial disease and whether elevations in this typically rare subset clinically correlate with coronary disease as well as or better than total monocyte counts do 4.
Recent studies indicate that nonclassical monocytes, in particular, can become loaded with cholesterol by binding oxidized LDL in the circulation. Nonclassical mouse monocytes express CD11c, in contrast to their classical counterparts 30. Wu et al. recently identified CD11c+ monocytes in apoE knock-out mice as the most prone among circulating monocytes to take up oxidizedLDL 37. This uptake was limited to conditions of high-fat diet feeding but not observed during low-fat chow feeding. This finding is consistent with another recent study in which a significant portion, but not all mouse monocytes, took up minimally modified LDL but not native LDL in the circulation 38. Mosig et al. studied the uptake of oxidatively modified LDL by human monocytes from patients with familial hypercholesterolemia ex vivo 39. In agreement with the mouse studies, human nonclassical monocytes from a highly hypercholesterolemic environment were selectively able to become cholesterol-loaded after incubation with oxidized LDL 39. These data raise the intriguing possibility that nonclassical monocytes, though they enter plaques at a reduced rate compared with classical monocytes, may be especially important as a source of cholesterol that is brought to plaques from the bloodstream under conditions of extreme hypercholesterolemia. Overall, however, much remains to be studied on the fate of monocyte-derived cell subsets in atherosclerosis, and it even remains possible that the two blood subsets do not maintain distinct functional roles or phenotypes (such as dendritic cell versus macrophage) within plaques. Even if they do maintain separate fates within plaque, their proportion relative to the differential degree to which they are recruited 30 might shift, for example, if the subsets have differing half-lives or if they proliferate. More research in this area is required to address these possibilities.
Chemokine receptors in the mobilization, recruitment, and survival of monocyte-derived cells and its relevance to atherosclerosis
Since the central role of monocytes in atherosclerosis was recognized more than a decade ago, major efforts have focused on molecules involved in their recruitment and persistence within plaques. Deficiency or blockade of adhesion molecules including ICAM-1, VCAM-1, CD18/β2 integrin, and P-selectin slow down murine plaque development 40–43. Chemokine or chemokine receptors implicated in monocyte recruitment to plaques, typically studied by genetic deletion in mice, are numerous and include CCR2, its ligand CCL2/MCP-1, CXCR2, its ligand CXCL1/KC/Groα, CCR5, its ligand CCL5/RANTES, CXCL4/platelet factor 4, CX3CR1/fractalkine receptor, and its ligand CX3CL1/fractalkine 44–48,49–51. When three chemokine/chemokine receptor pairs—CCL2, CX3CR1, and CCR5--are simultaneously targeted, murine atherosclerosis is nearly absent 52, fitting with the importance and coordinated roles of these three molecules in the recruitment of monocyte subsets to atherosclerosis plaques 30 (Fig. 2). Reduction of mouse atherosclerosis is in fact markedly reduced even when only two of these pathways (CCL2 and CX3CR1 or CCR2 and CX3CL1) are genetically deleted 52, 53, though additional inhibition is observed by interfering with CCR5 52.
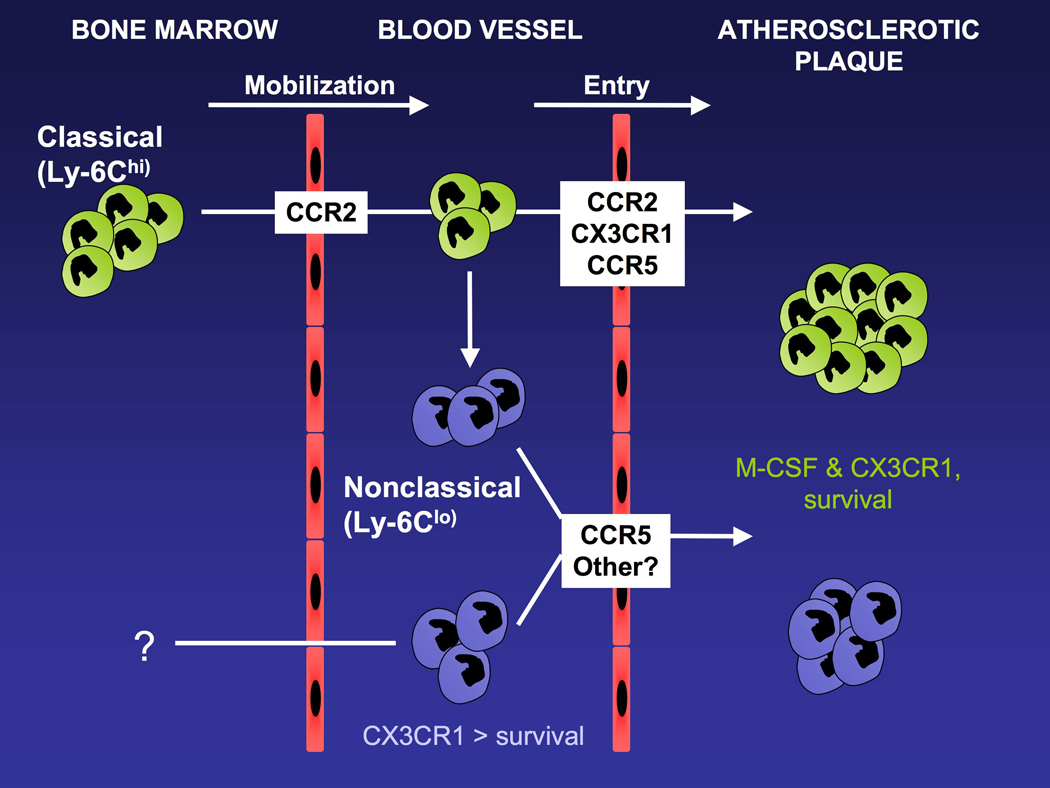
Figure 2.
Cartoon depicts the life cycle of monocyte subsets and their recruitment to atherosclerotic plaques, with an emphasis on the role of chemokine receptors in these processes. Classical monocytes leave the bone marrow in a CCR2-dependent manner 17, 54. It remains unknown whether the development of nonclassical monocytes requires incubation in the bone marrow and mobilization into the bloodstream thereafter. In the bloodstream, the chemokine receptor CX3CR1 regulates the survival of nonclassical monocytes selectively18, 52, 60, whereas the cytokine M-CSF, also a critical factor in atherosclerotic plaque development, controls survival of monocytes generally 89. Chemokine receptors CCR2, CX3CR1, and CCR5 are differentially involved in monocyte subset recruitment into plaques 30. Once in plaques, the persistence of monocyte-derived cells stemming from either subset appears to be regulated by CX3CR1 60 and it may also control their retention 72.
Deficiency in these molecules can alter accumulation of mouse monocyte-derived cells within plaques by means other than controlling their recruitment across the arterial endothelium overlying lesions. For example, deficiency in CCR2 has a profound impact on monocyte homeostasis. Indeed, classical monocyte mobilization from the bone marrow is dramatically impaired in the absence of CCR2 in mice (Fig. 2) 17, 54. As a consequence, CCR2−/− mice have a marked decrease in the number of circulating classical monocytes 17, 27, 54, whereas nonclassical monocyte numbers appear relatively normal 27. Thus, the effect of CCR2 deficiency in ameliorating plaque growth is, at best, likely only partly a consequence of a role for CCR2 is direct recruitment of monocytes into plaques 17, 30, 52. As another example, many chemokines act as scavenger receptors with the capacity to bind oxidized LDL 55. In turn, such binding interferes with their function 55. Interestingly, neither CCL-2 nor CX3CL1, the ligands of CCR2 and CX3CR1, bind oxidized LDL 55, possibly allowing unabated recruitment of monocyte subsets into plaques while the function of other chemokines, such as CCR7 ligands that may mediate migration of monocyte-derived cells out of plaques 56, are impaired 55. The CXCR6 ligand CXCL16 is a rare cleavable, transmembrane chemokine, and was the first chemokine shown to function as a scavenger receptor 55, 57. In mice, CXCL16 deficiency leads to heightened atherosclerosis 58. Its expression on human cultured macrophages allows it to induce cholesterol efflux genes in response to oxidized LDL binding 59. Thus, the concept emerges that CXCL16 on macrophages generates an atheroprotective response after recognition of oxidized LDL.
The multiple roles of CX3CR1 in monocyte biology and atherosclerosis
The homeostasis of nonclassical monocytes, which express approximately two-fold higher levels of surface CX3CR1 than classical monocytes 11, is altered in CX3CR1-deficient mice. Their number in the peripheral blood is mildly decreased in CX3CR1−/− 18, 60, CX3CR1−/− ApoE−/− mice 52 and CX3CR1−/− → Ldl-r−/− chimeric mice 60. In WT mice, the effect of CX3CR1 deficiency is often subtle 52 and can easily be missed. Indeed, while some literature indicates no alterations in nonclassical monocyte numbers in CX3CR1-deficient mice 23, the same authors have later observed reduced nonclassical monocytes in the absence of CX3CR1 61. A more dramatic indication of the role of CX3CR1 in maintaining nonclassical monocyte numbers is evident in mosaic chimeric mice bearing a combination of WT and CX3CR1−/− bone marrow 18. In this context, there is a selective and dramatic loss of nonclassical CX3CR1−/− monocytes in favor of the WT population 18.
In contrast to the role of CCR2 in classical monocyte homeostasis, the role of CX3CR1 in nonclassical monocyte homeostasis is not due to a role for CX3CR1 in nonclassical monocyte retention in the bone marrow 18, 52. Instead, a cause unrelated to a role for this receptor in migration is implicated—control of survival 18, 60 (Fig. 2). Bcl-2 expression is lost in CX3CR1-deficient nonclassical mouse monocytes 18, and overexpression of hBcl-2 restores peripheral blood nonclassical monocyte counts in mice 60.
The role of CX3CR1 in maintaining survival may extend to a role in survival during differentiation of monocytes into macrophages and DCs, possibly including those that derive from classical monocytes whose numbers are normal in blood in the absence of CX3CR1. Mouse CD11b+ pulmonary DCs are deficient in the absence of CX3CR118, and macrophages are scarce in atherosclerotic plaques of CX3CR1-deficient mice, strongly suppressing plaque progression49, 50, 52, 53, 60. This suppression may be related to impaired survival as much as or more than a role for CX3CR1 in monocyte migration to plaques 30, because introduction of a hBcl-2 expression vector regulated by the myeloid-restricted MRP8 promoter corrected the decreased survival stemming from a lack of CX3CR1 and abolished the anti-atherogenic effect of CX3CR1 deficiency 60. However, hBcl-2 over-expression increased plaque development in CX3CR1+/+ control mice as substantially as it did in CX3CR1−/− mice 60. Thus, it remains possible that enhanced survival independently influences plaque development so greatly that other effects of CX3CR1, such as roles in adhesion and migration, are hard to discern. In any case, regulation of monocyte-derived cell survival during or upon recruitment to tissues is generally relevant to atherosclerosis progression 62.
The consideration of an important role for CX3CR1 in monocyte and monocyte-derived cell survival adds one more function of this molecule to its well-established roles 63 in adhesion (its ligand CX3CL1 is the only other known transmembrane chemokine besides CXCL16), chemotaxis, and intercellular interactions between cells within plaques. Indeed, it is likely that classical monocytes, the major subset entering plaques, require CX3CR1 for interacting with or traversing the endothelium 30, 53. Nonetheless, it is often assumed that the dominant role of CX3CR1 is in adhesion or chemotaxis, when in fact its role is not always clear. For example, Nahrendorf et al. argued that absence of Ly6Clo nonclassical monocyte-like cells in infarcted hearts of CX3CR1-deficient mice 7 days after infarction was due to failure of nonclassical monocytes to be recruited to the heart in the absence of CX3CR1 33. Another viable possibility, however, is that these cells did not survive, or that Ly6Chi classical monocytes, recruited earlier to infarcted hearts 33, differentiate into nonclassical monocyte-like cells in situ and need CX3CR1 to survive during this differentiation.
The role of CX3CR1 in affecting the net accumulation of monocyte-derived cells, whether acting at the level of recruitment, retention, or survival, is hard to predict, as it appears to vary depending on the context. Absence of one copy of CX3CR1 is sufficient to impair macrophage accumulation in atherosclerotic plaques 50 and also suppresses the accumulation of some populations in the steady state, such as CD11b+ pulmonary DCs, but leaves many others intact (Jakubzick and Randolph, unpublished observations). Furthermore, net accumulation of monocyte-derived cells in sterile peritonitis is unaffected between 12 h and 3 days 64, even though early recruitment of nonclassical monocytes after instillation of Listeria into the peritoneum appears suppressed 23. Deficiency in CX3CR1 ligand, CX3CL1/fractalkine, also does not lead to major reductions in macrophage accumulation in a variety of inflammatory insults from peritonitis, enterocolitis, and Listeria challenge, even though the role of fractalkine in blood monocyte survival is likely similar to that of its receptor CX3CR1 65.
Targeting chemokines to control monocyte accumulation in human atherosclerosis
Since compound deficiency of two or more distinct chemokine/chemokine receptor pairs produces a stronger impact on plaque development than targeting only one chemokine/chemokine receptor pair 52, 53, targeting multiple chemokine pathways may be ideal in atherosclerosis. One risk, of course, is the enhanced probability of side effects resulting from impaired monocyte recruitment in other organs and during other important inflammatory responses. The fact that lack of CX3CR1 gives rise to subtle changes systemically with more dramatic effects in mouse atherosclerotic plaques, as discussed above, suggests that its antagonism may not be dangerously disruptive to innate or adaptive responses. In contrast, CCR2 deficiency reveals a much greater impact on systemic innate and adaptive responses 66–68. Another risk is the potential for lack of efficacy. It is remarkable that attempts to prevent disease by eliminating monocytes 2 or abrogate pathways like CCR2-mediated mobilization and recruitment 69 once atherosclerosis has reached a highly progressed state are not markedly successful. Since treating advanced disease in humans is more practical at present than treating with compounds to prevent disease, more research effort is needed on how to modify monocyte-derived cell accumulation within advanced plaques. Rather than preventing recruitment of monocytes, therapies that focus on causing monocyte-derived cells to avoid retention in plaque and instead emigrate out of lesions 70, 71 or those that appropriately modulate survival within plaques 62 may be more beneficial. Besides potentially participating in recruitment, CX3CR1 has been implicated in both retention 72 and survival of monocytes and monocyte-derived cells within plaques 18, 60. Moreover, CX3CR1 is one of the few targets already validated in humans, since the M280 polymorphism in the human CX3CR1 gene clearly protects against cardiovascular events 73–75. Polymorphisms in other chemokine receptors, such as CCR5 63, have also been implicated in affecting human atherosclerosis, but the data are strongest for CX3CR1. Inhibition of CX3CR1, therefore, may be a suitable clinical target in humans. A human CX3CR1 antagonist was recently described 76, opening the door to clinical testing. On the other hand, the negative consequences of the CX3CR1 polymorphism, including increased progression of HIV 77–79 and enhanced macular degeneration and retinal vasculitis 80–82 serve as reminders of the considerable challenges in therapeutically targeting chemokines that control human monocyte migration and survival in atherosclerosis. One way around the unwanted systemic side effect of chemokine antagonists may be to target chemokine complexes that may be more uniquely localized to plaques 48 or to develop delivery methods that would restrict therapeutic availability and action to plaques. Altogether, at present, it remains unclear if targeting chemokines to treat atherosclerosis can limit monocyte accumulation in plaques at stages relevant for treatment in humans and do so without impairing immunity during acute inflammatory reactions or infections.
Acknowledgements
The authors’ work is funded by NIH grants AI049653, AI061741 and HL084312, and an Established Investigator Award from the American Heart Association (0740052).
References
- 1.Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony- stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci U S A. 1995;92:8264–8268. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoneman V, Braganza D, Figg N, Mercer J, Lang R, Goddard M, Bennett M. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ Res. 2007;100:884–893. doi: 10.1161/01.RES.0000260802.75766.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman CM, Beilby JP, McQuillan BM, Thompson PL, Hung J. Monocyte count, but not C-reactive protein or interleukin-6, is an independent risk marker for subclinical carotid atherosclerosis. Stroke. 2004;35:1619–1624. doi: 10.1161/01.STR.0000130857.19423.ad. [DOI] [PubMed] [Google Scholar]
- 4.Nasir K, Guallar E, Navas-Acien A, Criqui MH, Lima JA. Relationship of monocyte count and peripheral arterial disease: results from the National Health and Nutrition Examination Survey 1999–2002. Arterioscler Thromb Vasc Biol. 2005;25:1966–1971. doi: 10.1161/01.ATV.0000175296.02550.e4. [DOI] [PubMed] [Google Scholar]
- 5.Johnsen SH, Fosse E, Joakimsen O, Mathiesen EB, Stensland-Bugge E, Njolstad I, Arnesen E. Monocyte count is a predictor of novel plaque formation: a 7-year follow-up study of 2610 persons without carotid plaque at baseline the Tromso Study. Stroke. 2005;36:715–719. doi: 10.1161/01.STR.0000158909.07634.83. [DOI] [PubMed] [Google Scholar]
- 6.Out R, Hoekstra M, Habets K, Meurs I, de Waard V, Hildebrand RB, Wang Y, Chimini G, Kuiper J, Van Berkel TJ, Van Eck M. Combined deletion of macrophage ABCA1 and ABCG1 leads to massive lipid accumulation in tissue macrophages and distinct atherosclerosis at relatively low plasma cholesterol levels. Arterioscler Thromb Vasc Biol. 2008;28:258–264. doi: 10.1161/ATVBAHA.107.156935. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 8.Yrlid U, Jenkins CD, MacPherson GG. Relationships between distinct blood monocyte subsets and migrating intestinal lymph dendritic cells in vivo under steady state conditions. J Immunol. 2006;176:4155–4162. doi: 10.4049/jimmunol.176.7.4155. [DOI] [PubMed] [Google Scholar]
- 9.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–2534. [PubMed] [Google Scholar]
- 10.Ancuta P, Rao R, Moses A, Mehle A, Shaw SK, Luscinskas FW, Gabuzda D. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J Exp Med. 2003;197:1701–1707. doi: 10.1084/jem.20022156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 12.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 13.Randolph GJ, Jakubzick C, Qu C. Antigen presentation by monocytes and monocyte-derived cells. Curr Opin Immunol. 2008;20:52–60. doi: 10.1016/j.coi.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 15.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 16.Palframan RT, Jung S, Cheng G, Weninger W, Luo Y, Dorf M, Littman DR, Rollins BJ, Zweerink H, Rot A, von Andrian UH. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J Exp Med. 2001;194:1361–1373. doi: 10.1084/jem.194.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jakubzick C, Tacke F, Ginhoux F, Wagers AJ, van Rooijen N, Mack M, Merad M, Randolph GJ. Blood Monocyte Subsets Differentially Give Rise to CD103+ and CD103− Pulmonary Dendritic Cell Populations. J Immunol. 2008;180:3019–3027. doi: 10.4049/jimmunol.180.5.3019. [DOI] [PubMed] [Google Scholar]
- 19.Tacke F, Ginhoux F, Jakubzick C, van Rooijen N, Merad M, Randolph GJ. Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J Exp Med. 2006;203:583–597. doi: 10.1084/jem.20052119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, Narni-Mancinelli E, Lauvau G. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol. 2008;86:398–408. doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- 22.Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, Espevik T, Ziegler-Heitbrock L. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–3542. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 23.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 24.Thomas R, Lipsky PE. Human peripheral blood dendritic cell subsets. Isolation and characterization of precursor and mature antigen-presenting cells. J Immunol. 1994;153:4016–4028. [PubMed] [Google Scholar]
- 25.Ancuta P, Weiss L, Haeffner-Cavaillon N. CD14+CD16++ cells derived in vitro from peripheral blood monocytes exhibit phenotypic and functional dendritic cell-like characteristics. Eur J Immunol. 2000;30:1872–1883. doi: 10.1002/1521-4141(200007)30:7<1872::AID-IMMU1872>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 26.Grage-Griebenow E, Zawatzky R, Kahlert H, Brade L, Flad H, Ernst M. Identification of a novel dendritic cell-like subset of CD64(+)/CD16(+) blood monocytes. Eur J Immunol. 2001;31:48–56. doi: 10.1002/1521-4141(200101)31:1<48::aid-immu48>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Qu C, Edwards EW, Tacke F, Angeli V, Llodra J, Sanchez-Schmitz G, Garin A, Haque NS, Peters W, van Rooijen N, Sanchez-Torres C, Bromberg J, Charo IF, Jung S, Lira SA, Randolph GJ. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J Exp Med. 2004;200:1231–1241. doi: 10.1084/jem.20032152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng Y, Latchman Y, Elkon KB. Ly6C(low) monocytes differentiate into dendritic cells and cross-tolerize T cells through PDL-1. J Immunol. 2009;182:2777–2785. doi: 10.4049/jimmunol.0803172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allan DS, Colonna M, Lanier LL, Churakova TD, Abrams JS, Ellis SA, McMichael AJ, Braud VM. Tetrameric complexes of human histocompatibility leukocyte antigen (HLA)-G bind to peripheral blood myelomonocytic cells. J Exp Med. 1999;189:1149–1156. doi: 10.1084/jem.189.7.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Binder CJ, Silverman GJ. Natural antibodies and the autoimmunity of atherosclerosis. Springer Semin Immunopathol. 2005;26:385–404. doi: 10.1007/s00281-004-0185-z. [DOI] [PubMed] [Google Scholar]
- 32.Binder CJ, Horkko S, Dewan A, Chang MK, Kieu EP, Goodyear CS, Shaw PX, Palinski W, Witztum JL, Silverman GJ. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003;9:736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 33.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, Dai XM, Stanley ER, Randolph GJ, Merad M. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7:265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu H, Gower RM, Wang H, Perrard XY, Ma R, Bullard DC, Burns AR, Paul A, Smith CW, Simon SI, Ballantyne CM. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation. 2009;119:2708–2717. doi: 10.1161/CIRCULATIONAHA.108.823740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, Choi J, Perkmann T, Backhed F, Miller YI, Horkko S, Corr M, Witztum JL, Binder CJ. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009;119:1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mosig S, Rennert K, Krause S, Kzhyshkowska J, Neunubel K, Heller R, Funke H. Different functions of monocyte subsets in familial hypercholesterolemia: potential function of CD14+CD16+ monocytes in detoxification of oxidized LDL. Faseb J. 2009;23:866–874. doi: 10.1096/fj.08-118240. [DOI] [PubMed] [Google Scholar]
- 40.Nageh MF, Sandberg ET, Marotti KR, Lin AH, Melchior EP, Bullard DC, Beaudet AL. Deficiency of inflammatory cell adhesion molecules protects against atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1997;17:1517–1520. doi: 10.1161/01.atv.17.8.1517. [DOI] [PubMed] [Google Scholar]
- 41.Collins RG, Velji R, Guevara NV, Hicks MJ, Chan L, Beaudet AL. P-Selectin or intercellular adhesion molecule (ICAM)-1 deficiency substantially protects against atherosclerosis in apolipoprotein E-deficient mice. J Exp Med. 2000;191:189–194. doi: 10.1084/jem.191.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly PW, Milstone DS. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001;107:1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.An G, Wang H, Tang R, Yago T, McDaniel JM, McGee S, Huo Y, Xia L. P-selectin glycoprotein ligand-1 is highly expressed on Ly-6Chi monocytes and a major determinant for Ly-6Chi monocyte recruitment to sites of atherosclerosis in mice. Circulation. 2008;117:3227–3237. doi: 10.1161/CIRCULATIONAHA.108.771048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 45.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 46.Boisvert WA, Santiago R, Curtiss LK, Terkeltaub RA. A leukocyte homologue of the IL-8 receptor CXCR-2 mediates the accumulation of macrophages in atherosclerotic lesions of LDL receptor-deficient mice. J Clin Invest. 1998;101:353–363. doi: 10.1172/JCI1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boisvert WA, Rose DM, Johnson KA, Fuentes ME, Lira SA, Curtiss LK, Terkeltaub RA. Up-regulated expression of the CXCR2 ligand KC/GRO-alpha in atherosclerotic lesions plays a central role in macrophage accumulation and lesion progression. Am J Pathol. 2006;168:1385–1395. doi: 10.2353/ajpath.2006.040748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koenen RR, von Hundelshausen P, Nesmelova IV, Zernecke A, Liehn EA, Sarabi A, Kramp BK, Piccinini AM, Paludan SR, Kowalska MA, Kungl AJ, Hackeng TM, Mayo KH, Weber C. Disrupting functional interactions between platelet chemokines inhibits atherosclerosis in hyperlipidemic mice. Nat Med. 2009;15:97–103. doi: 10.1038/nm.1898. [DOI] [PubMed] [Google Scholar]
- 49.Lesnik P, Haskell CA, Charo IF. Decreased atherosclerosis in CX3CR1−/− mice reveals a role for fractalkine in atherogenesis. J Clin Invest. 2003;111:333–340. doi: 10.1172/JCI15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Combadiere C, Potteaux S, Gao JL, Esposito B, Casanova S, Lee EJ, Debre P, Tedgui A, Murphy PM, Mallat Z. Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation. 2003;107:1009–1016. doi: 10.1161/01.cir.0000057548.68243.42. [DOI] [PubMed] [Google Scholar]
- 51.Teupser D, Pavlides S, Tan M, Gutierrez-Ramos JC, Kolbeck R, Breslow JL. Major reduction of atherosclerosis in fractalkine (CX3CL1)-deficient mice is at the brachiocephalic artery, not the aortic root. Proc Natl Acad Sci U S A. 2004;101:17795–17800. doi: 10.1073/pnas.0408096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 53.Saederup N, Chan L, Lira SA, Charo IF. Fractalkine deficiency markedly reduces macrophage accumulation and atherosclerotic lesion formation in CCR2−/− mice: evidence for independent chemokine functions in atherogenesis. Circulation. 2008;117:1642–1648. doi: 10.1161/CIRCULATIONAHA.107.743872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 55.Shimaoka T, Nakayama T, Hieshima K, Kume N, Fukumoto N, Minami M, Hayashida K, Kita T, Yoshie O, Yonehara S. Chemokines generally exhibit scavenger receptor activity through their receptor-binding domain. J Biol Chem. 2004;279:26807–26810. doi: 10.1074/jbc.C400163200. [DOI] [PubMed] [Google Scholar]
- 56.Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, Randolph GJ, Fisher EA. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc Natl Acad Sci U S A. 2006;103:3781–3786. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shimaoka T, Kume N, Minami M, Hayashida K, Kataoka H, Kita T, Yonehara S. Molecular cloning of a novel scavenger receptor for oxidized low density lipoprotein, SR-PSOX, on macrophages. J Biol Chem. 2000;275:40663–40666. doi: 10.1074/jbc.C000761200. [DOI] [PubMed] [Google Scholar]
- 58.Aslanian AM, Charo IF. Targeted disruption of the scavenger receptor and chemokine CXCL16 accelerates atherosclerosis. Circulation. 2006;114:583–590. doi: 10.1161/CIRCULATIONAHA.105.540583. [DOI] [PubMed] [Google Scholar]
- 59.Barlic J, Zhu W, Murphy PM. Atherogenic lipids induce high-density lipoprotein uptake and cholesterol efflux in human macrophages by up-regulating transmembrane chemokine CXCL16 without engaging CXCL16-dependent cell adhesion. J Immunol. 2009;182:7928–7936. doi: 10.4049/jimmunol.0804112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Landsman L, Bar-On L, Zernecke A, Kim KW, Krauthgamer R, Shagdarsuren E, Lira SA, Weissman IL, Weber C, Jung S. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood. 2009;113:963–972. doi: 10.1182/blood-2008-07-170787. [DOI] [PubMed] [Google Scholar]
- 61.Auffray C, Fogg DK, Narni-Mancinelli E, Senechal B, Trouillet C, Saederup N, Leemput J, Bigot K, Campisi L, Abitbol M, Molina T, Charo I, Hume DA, Cumano A, Lauvau G, Geissmann F. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med. 2009;206:595–606. doi: 10.1084/jem.20081385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gautier EL, Huby T, Witztum JL, Ouzilleau B, Miller ER, Saint-Charles F, Aucouturier P, Chapman MJ, Lesnik P. Macrophage apoptosis exerts divergent effects on atherogenesis as a function of lesion stage. Circulation. 2009;119:1795–1804. doi: 10.1161/CIRCULATIONAHA.108.806158. [DOI] [PubMed] [Google Scholar]
- 63.Barlic J, Murphy PM. Chemokine regulation of atherosclerosis. J Leukoc Biol. 2007;82:226–236. doi: 10.1189/jlb.1206761. [DOI] [PubMed] [Google Scholar]
- 64.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cook DN, Chen SC, Sullivan LM, Manfra DJ, Wiekowski MT, Prosser DM, Vassileva G, Lira SA. Generation and analysis of mice lacking the chemokine fractalkine. Mol Cell Biol. 2001;21:3159–3165. doi: 10.1128/MCB.21.9.3159-3165.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Jr., Broxmeyer HE, Charo IF. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakano H, Lin KL, Yanagita M, Charbonneau C, Cook DN, Kakiuchi T, Gunn MD. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol. 2009;10:394–402. doi: 10.1038/ni.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guo J, de Waard V, Van Eck M, Hildebrand RB, van Wanrooij EJ, Kuiper J, Maeda N, Benson GM, Groot PH, Van Berkel TJ. Repopulation of apolipoprotein E knockout mice with CCR2-deficient bone marrow progenitor cells does not inhibit ongoing atherosclerotic lesion development. Arterioscler Thromb Vasc Biol. 2005;25:1014–1019. doi: 10.1161/01.ATV.0000163181.40896.42. [DOI] [PubMed] [Google Scholar]
- 70.Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Randolph GJ. Emigration of monocyte-derived cells to lymph nodes during resolution of inflammation and its failure in atherosclerosis. Curr Opin Lipidol. 2008;19:462–468. doi: 10.1097/MOL.0b013e32830d5f09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barlic J, Zhang Y, Foley JF, Murphy PM. Oxidized lipid-driven chemokine receptor switch, CCR2 to CX3CR1, mediates adhesion of human macrophages to coronary artery smooth muscle cells through a peroxisome proliferator-activated receptor gamma-dependent pathway. Circulation. 2006;114:807–819. doi: 10.1161/CIRCULATIONAHA.105.602359. [DOI] [PubMed] [Google Scholar]
- 73.McDermott DH, Fong AM, Yang Q, Sechler JM, Cupples LA, Merrell MN, Wilson PW, D'Agostino RB, O'Donnell CJ, Patel DD, Murphy PM. Chemokine receptor mutant CX3CR1-M280 has impaired adhesive function and correlates with protection from cardiovascular disease in humans. J Clin Invest. 2003;111:1241–1250. doi: 10.1172/JCI16790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Apostolakis S, Baritaki S, Kochiadakis GE, Igoumenidis NE, Panutsopulos D, Spandidos DA. Effects of polymorphisms in chemokine ligands and receptors on susceptibility to coronary artery disease. Thromb Res. 2007;119:63–71. doi: 10.1016/j.thromres.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 75.Norata GD, Garlaschelli K, Ongari M, Raselli S, Grigore L, Catapano AL. Effects of fractalkine receptor variants on common carotid artery intima-media thickness. Stroke. 2006;37:1558–1561. doi: 10.1161/01.STR.0000221803.16897.22. [DOI] [PubMed] [Google Scholar]
- 76.Dorgham K, Ghadiri A, Hermand P, Rodero M, Poupel L, Iga M, Hartley O, Gorochov G, Combadiere C, Deterre P. An engineered CX3CR1 antagonist endowed with anti-inflammatory activity. J Leukoc Biol. 2009 doi: 10.1189/jlb.0308158. [DOI] [PubMed] [Google Scholar]
- 77.Faure S, Meyer L, Costagliola D, Vaneensberghe C, Genin E, Autran B, Delfraissy JF, McDermott DH, Murphy PM, Debre P, Theodorou I, Combadiere C. Rapid progression to AIDS in HIV+ individuals with a structural variant of the chemokine receptor CX3CR1. Science. 2000;287:2274–2277. doi: 10.1126/science.287.5461.2274. [DOI] [PubMed] [Google Scholar]
- 78.Faure S, Meyer L, Genin E, Pellet P, Debre P, Theodorou I, Combadiere C. Deleterious genetic influence of CX3CR1 genotypes on HIV-1 disease progression. J Acquir Immune Defic Syndr. 2003;32:335–337. doi: 10.1097/00126334-200303010-00014. [DOI] [PubMed] [Google Scholar]
- 79.Singh KK, Hughes MD, Chen J, Spector SA. Genetic polymorphisms in CX3CR1 predict HIV-1 disease progression in children independently of CD4+ lymphocyte count and HIV-1 RNA load. J Infect Dis. 2005;191:1971–1980. doi: 10.1086/430091. [DOI] [PubMed] [Google Scholar]
- 80.Tuo J, Smith BC, Bojanowski CM, Meleth AD, Gery I, Csaky KG, Chew EY, Chan CC. The involvement of sequence variation and expression of CX3CR1 in the pathogenesis of age-related macular degeneration. Faseb J. 2004;18:1297–1299. doi: 10.1096/fj.04-1862fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wallace GR, Vaughan RW, Kondeatis E, Mathew R, Chen Y, Graham EM, Stanford MR. A CX3CR1 genotype associated with retinal vasculitis in patients in the United Kingdom. Invest Ophthalmol Vis Sci. 2006;47:2966–2970. doi: 10.1167/iovs.05-1631. [DOI] [PubMed] [Google Scholar]
- 82.Combadiere C, Feumi C, Raoul W, Keller N, Rodero M, Pezard A, Lavalette S, Houssier M, Jonet L, Picard E, Debre P, Sirinyan M, Deterre P, Ferroukhi T, Cohen SY, Chauvaud D, Jeanny JC, Chemtob S, Behar-Cohen F, Sennlaub F. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest. 2007;117:2920–2928. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qu C, Moran TM, Randolph GJ. Autocrine Type I IFN and Contact with Endothelium Promote the Presentation of Influenza A Virus by Monocyte-Derived APC. J Immunol. 2003;170:1010–1018. doi: 10.4049/jimmunol.170.2.1010. [DOI] [PubMed] [Google Scholar]
- 84.Wildgruber M, Lee H, Chudnovskiy A, Yoon TJ, Etzrodt M, Pittet MJ, Nahrendorf M, Croce K, Libby P, Weissleder R, Swirski FK. Monocyte subset dynamics in human atherosclerosis can be profiled with magnetic nano-sensors. PLoS One. 2009;4:e5663. doi: 10.1371/journal.pone.0005663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao C, Zhang H, Wong WC, Sem X, Han H, Ong SM, Tan YC, Yeap WH, Gan CS, Ng KQ, Koh MB, Kourilsky P, Sze SK, Wong SC. Identification of Novel Functional Differences in Monocyte Subsets Using Proteomic and Transcriptomic Methods. J Proteome Res. 2009 doi: 10.1021/pr900364p. [DOI] [PubMed] [Google Scholar]
- 86.Draude G, von Hundelshausen P, Frankenberger M, Ziegler-Heitbrock HW, Weber C. Distinct scavenger receptor expression and function in the human CD14(+)/CD16(+) monocyte subset. Am J Physiol. 1999;276:H1144–H1149. doi: 10.1152/ajpheart.1999.276.4.H1144. [DOI] [PubMed] [Google Scholar]
- 87.Randolph GJ, Sanchez-Schmitz G, Liebman RM, Schakel K. The CD16(+) (FcgammaRIII(+)) Subset of Human Monocytes Preferentially Becomes Migratory Dendritic Cells in a Model Tissue Setting. J Exp Med. 2002;196:517–527. doi: 10.1084/jem.20011608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat Immunol. 2007;8:578–583. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- 89.Lagasse E, Weissman IL. Enforced expression of Bcl-2 in monocytes rescues macrophages and partially reverses osteopetrosis in op/op mice. Cell. 1997;89:1021–1031. doi: 10.1016/s0092-8674(00)80290-1. [DOI] [PubMed] [Google Scholar]