Summary
The estrogen receptors ERα and ERβ have been implicated in the progression of a wide variety of cancers. The actions of ER are regulated by ER coregulator proteins, including proline-, glutamic acid-and leucine-rich-protein-1 (PELP1/MNAR). PELP1 has been shown to participate in both genomic and nongenomic functions of ER. The expression and localization of PELP1/MNAR are deregulated in a wide variety of tumors and have been implicated in the development of hormonal resistance in cancer cell lines. Emerging data suggest that PELP1/MNAR interacts with many proteins and activates several oncogenes, including Src kinase, phosphotidyl inositol 3 kinase (PI3K), and signal transducers and activators of transcription 3 (STAT3). These new results suggest that PELP1/MNAR may act as an oncogene as well as cooperating with other oncogenes. Thus, PELP1/MNAR may contribute to the tumorigenic potential of cancer cells by serving as a scaffolding protein that couples various signaling complexes with ER.
Keywords: Estrogen receptors, Coregulators, PELP1, MNAR, Hormonal resistance
Introduction
The estrogen receptors ERα and ERβ are members of the family of nuclear receptors (NR) that mediate the pleotropic actions of the steroid hormone estrogen in a wide variety of tisuues (McDonnell and Norris, 2002). ER signalling has been implicated in the progression of a number of cancers, including those of the breast, endometrium and ovary. ERs have been shown to function as ligand-dependent transcription factors, but accumulating evidence strongly suggests that ERs also require functional interactions with their coregulators for the optimal activation of estrogen responsive genes (Smith and O'Malley, 2004). Several studies have convincingly shown that ER functions are dependent on the formation of large, multi-component complexes in the nucleus (McKenna et al., 1999). ERs have also been found to participate in cytoplasmic and membrane-mediated signaling events (nongenomic signaling), as well as in the stimulation of a number of cytosolic signaling pathways by forming complexes with Src kinase, mitogen-activated protein kinase (MAPK), and phosphatidylinositol-3-kinase (PI3K) (Losel and Wehling, 2003; Bjornstrom and Sjoberg, 2005; Song et al., 2005).
Using genomics/proteomics, several novel genes have been identified as ER-coregulatory proteins (McDonnell and Norris, 2002; Barnes et al., 2004; Smith and O'Malley, 2004). Since ER coregulators modulate ER signaling, they may be important in the progression of ER positive tumors (Hall and McDonnell, 2005). ER coregulators may play a role in hormonal responsiveness and tumor progression and may function as oncogenes (Bagheri-Yarmand et al., 2004; Torres-Arzayus et al., 2004). Deregulation of ER-coregulator protein expression or function has been described in several cancers (Anzick et al., 1997; List et al., 2001; Vadlamudi et al., 2005). However, little is known about the normal functions of these coregulatory proteins and their involvement in cancer progression. Understanding the composition and function(s) of ER regulatory complexes in normal physiology and disease progression may enable the use of these novel pathways for diagnosis and intervention in cancer. In this review, we briefly summarize the emerging data on the novel ER-coregulator proline-, glutamic acid-, and leucine-rich protein-1 (PELP1, also referred to as modulator of nongenomic action of estrogen receptor, MNAR) in various cancers and discuss the clinical significance of PELP1/MNAR deregulation in cancers.
Functions of PELP1/MNAR
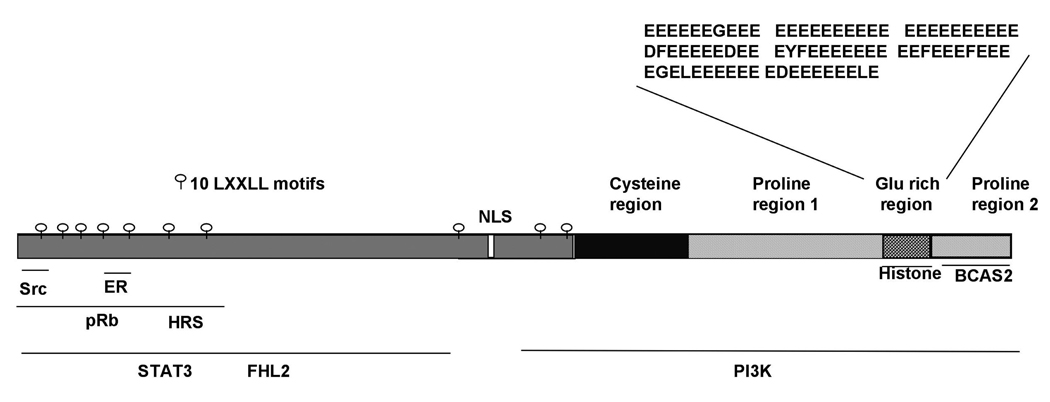
PELP1/MNAR is normally expressed in a wide variety of hormone responsive tissues, including the breast, ovary, and endometrium (Vadlamudi et al., 2001). Initial studies suggested that PELP1/MNAR functions as a coactivator of ER and modulates ER nuclear transactivation functions. In normal mammary epithelial cells, substantial amounts of PELP1 localize in the nuclear compartment. Moreover, hormonal stimulation promotes the colocalization of PELP1 and ER in the nuclear compartment and enhances PELP1/MNAR recruitment to E2-responsive gene promoters (Nair et al., 2004). PELP1/MNAR also interacts with histones (Choi et al., 2004) and participates in chromatin remodeling activity by displacing histone H1 (Nair et al., 2004). In addition, PELP1 has also been found to interact with the general transcriptional activator CBP, and hormonal stimulation promotes PELP1-associated histone acetyl transferase activity (Vadlamudi et al., 2001; Nair et al., 2004). PELP1/MNAR is physiologically associated with the cell cycle switch protein retinoblastoma (pRb) in the nucleus, and these PELP1/MNAR-pRb interactions have been found to play a role in the maximal activation of E2 target genes, such as cyclin D1 (Balasenthil and Vadlamudi, 2003). This, in turn, enhances E2-mediated cell cycle progression by sensitizing cells to G1/S progression (Balasenthil and Vadlamudi, 2003). Emerging evidence also suggests that PELP1/MNAR plays a key role in the ER-mediated generation of non-genomic actions by modulating the interaction of ER with Src kinase, leading to the stimulation of Src enzymatic activity and activation of the mitogen activated protein kinases (MAPK) (Wong et al., 2002; Barletta et al., 2004). PELP1/MNAR also interacts with the p85 subunit of phosphoinositide 3-kinase (PI3K), leading to the activation of the AKT (protein kinase B) pathway (Vadlamudi et al., 2005). Growth factor signals induce the association of PELP1/MNAR with epidermal growth factor receptor (EGFR), resulting in the phosphorylation of PELP1 at tyrosine residues (Vadlamudi et al., 2005). Growth factor signals also promote PELP1/MNAR interactions with STAT3, and these interactions play an important role in the growth factor mediated activation of STAT3 target genes including cyclin D1, fos, and jun (Manavathi and Kumar, 2005). PELP1 is a phosphoprotein, and its phosphorylation status is modulated by growth factor signaling (Nagpal et al., 2006). The ability of PELP1/MNAR to interact with ER, chromatin components, signaling enzymes (e.g. Src and PI3K), and growth factor signaling components (e.g. EGFR, STAT3 and HRS), suggests that PELP1 functions as a scaffolding protein and acts in the assembly of various signaling complexes and in coupling these complexes with steroid receptors such as ER (Fig. 1).
Fig. 1.
Schematic representation of PELP1/MNAR domains and its interacting proteins.
PELP1/MNAR in breast cancer
PELP1/MNAR is widely expressed in breast cancer cells, and its expression is deregulated in breast tumors (Vadlamudi et al., 2001; Greger et al., 2005). PELP1 expression was reported to be 3- to 5-fold higher in breast tumors compared with adjacent normal breast tissue (Vadlamudi et al., 2001). A recent study using tumor arrays (n=252) reported that node positive and metastatic tumors have 2- to 3-fold higher expression of PELP1/MNAR than node negative breast tumors, suggesting that PELP1 may be involved in tumor metastasis (Rajhans et al., 2006). The MCF-7 breast tumor cell line, which overexpresses PELP1, has been shown to promote tumorigenesis in nude mice in the absence of exogenous estrogen, suggesting that PELP1/MNAR deregulation can promote hormonal independence and that this gene can function as an oncogene (Rajhans et al., 2006). PELP1 overexpression has also been found to hypersensitize breast cancer cells to S phase by modulating pRb phosphorylation (Balasenthil and Vadlamudi, 2003). The ability of PELP1 to make MCF-7 cells hypersensitive suggests that the deregulation of PELP1 expression observed in breast tumors may confer an advantage to breast cancer cells by sensitizing them to residual levels of estrogen.
PELP1 has been shown to modulate the expression or function of several oncogenes that have been implicated in breast cancer progression. For example, PELP1 positively upregulates expression of cyclin D (Balasenthil and Vadlamudi, 2003). Because PELP1 expression is deregulated in breast tumors, its ability to modulate cyclin D1 expression may provide a functional advantage to ER-positive tumors and play a role in ER-positive tumor cell proliferation. Similarly, PELP1 has been shown to interact with and activate Src kinase (Wong et al., 2002), another gene commonly overexpressed in breast cancers. Deregulation of PELP1/MNAR may therefore contribute to the constitutive activation of Src kinase in tumors overexpressing PELP1. STAT3 is another oncogene widely expressed in breast cancers, and constitutive phosphorylation at the STAT3 Ser727 residue has been observed in breast tumors, suggesting a role for STAT3 serine phosphorylation in oncogenesis. PELP1 has been observed to interact with STAT3 and to potentiate STAT3 Ser727 phosphorylation (Manavathi et al., 2005), suggesting that deregulation of PELP1 expression in tumors may upregulate STAT3 signaling. Collectively these findings suggest that PELP1 promotes tumorigenesis via its ability to modulate the function of other oncogenes.
PELP1/MNAR is predominantly localized in the nuclei of cells of hormonally responsive tissues. In about 50% of PELP1/MNAR-positive tumors, however, this protein was found to localize in the cytoplasm alone or in both the cytoplasm and nucleus (Vadlamudi et al., 2005). Mechanistic studies have shown that cytoplasmic localization of PELP1 increases its association with Src and PI3K, leading to enhanced MAPK activation and constitutive activation of AKT. Consistent with these results, clones of cells with cytoplasmically localized PELP1, when injected into mammary fat pads, were found to have tumorigenic potential and hormonal independence, suggesting a close relationship between PELP1/MNAR cytoplasmic localization, AKT activation, and tumorigenesis (Vadlamudi et al., 2005). Clones of MCF-7 human breast cancer cells overexpressing PELP1/MNAR in the cytoplasm were found to be much more sensitive to TNF-alpha-induced apoptosis than were wild-type nuclear PELP1- and pcDNA vector-expressing clones (Rayala et al., 2006), suggesting that altered localization of PELP1/MNAR modulates the hormonal sensitivity of breast cancer cells. These findings suggest methods for developing new treatment strategies for tumors with cytoplasmic PELP1 expression.
Emerging results suggest that PELP1/MNAR deregulation may promote hormonal therapy resistance in breast cancer cells. Model cells that mimic PELP1/MNAR cytoplasmic localization in tumors (PELP1-cyto cells) showed hypersensitivity to estrogen but were resistant to tamoxifen (Vadlamudi et al., 2005). Although this agent has been used to treat breast cancer, it is also associated with endometrial thickening, dysfunctional uterine bleeding, endometrial polyps, endometrial hyperplasia, and uterine sarcoma (Bergman et al., 2000). PELP1 enhances the agonist actions of tamoxifen in endometrial cells, suggesting that it may play a role in the tamoxifen-mediated partial agonist action observed in the endometrium (Vadlamudi et al., 2004). Utilizing cDNA microarrays, cell lines with acquired letrozole resistance have been shown to overexpress PELP1/MNAR (Mcpherson et al., 2005). These findings suggest that deregulation of PELP1/MNAR, which can stimulate nongenomic ER functions, may alter the ratio of genomic and nongenomic signaling in breast cancer cells and may therefore promote hormonal independence by modulating ER-transactivating functions and SERM mediated agonist actions.
Growth factor-mediated activation of nongenomic pathways and phosphorylation of ER and ER-coregulatory proteins have been shown to play a role in tamoxifen resistance (Schiff et al., 2005; Gururaj et al., 2006). Signaling by growth factors has been shown to promote the phosphorylation of PELP1/MNAR and to alter its localization (Schiff et al., 2005; Nagpal et al., 2006). Since PELP1/MNAR enhances growth factor mediated ER transactivation activities and promotes tamoxifen resistance, growth factor-mediated posttranslational modification of PELP1 may be important in PELP1-mediated hormonal resistance. Further characterization of cytoplasmic PELP1/MNAR functions showed that it interacts in the cytoplasm with hepatocyte growth factor receptor substrate (HRS), a trafficking molecule that activates MAPK in the presence of EGFR, presumably by sequestering PELP1/MNAR in the cytoplasm. Growth factor signals or signaling components can affect the presence of PELP1/MNAR in the cytoplasm, thus contributing to excessive nongenomic signaling and leading to hormonal resistance (Rayala et al., 2006).
Growth factor signaling may also regulate gene expression through the modulation of RNA splicing efficiency via phosphorylation and modulation of the interactions among the activators, ER and the spliceosome (Masuhiro et al., 2005). PELP1/MNAR interacts with Breast Cancer Amplified Sequence-2 (BCAS2) in the nuclear compartment, and colocalization studies using splicing factor SC35 as a marker revealed that PELP1/MNAR and BCAS2 colocalize at nuclear speckles. PELP1/MNAR can interact with RNA, thus enhancing steroid hormone mediated differential splicing effects. BCAS2-PELP1/MNAR interactions may also be important in ER mediated RNA splicing (Nair et al., 2006).
PELP1/MNAR in endometrial cancer
PELP1/MNAR is normally expressed in various stages of normal endometrium, but it is differently expressed and localized at different stages in benign endometrium. PELP1 expression was shown to be higher in endometrial tumor samples than in postmenopausal specimens (Vadlamudi et al., 2004). In addition, ER is localized in the cytoplasm in postmenopausal endometrium, suggesting that ER nongenomic signaling prevails in postmenopausal endometrium. Downregulation of PELP1 in endometrial cells using siRNA demonstrated that PELP1/MNAR is essential in E2-mediated cell proliferation of endometrial cancer cells (Vadlamudi et al., 2004). Collectively these results suggest that PELP1 mediated genomic and nongenomic signaling is important in the proliferative and secretory phases of endometrium, whereas PELP1 mediated nongenomic functions may be important in postmenopausal endometrium. Thus, deregulation of PELP1 may play important roles in the progression of endometrial cancer.
PELP1/MNAR in ovarian cancer
Female sex hormones have been shown to be involved in the progression of ovarian cancer, with estrogen concentrations in ovarian tissue being at least 100-fold higher than those in the circulation (Lindgren et al., 2002). Both estrogen receptors (ERα and ERβ), the principle targets of estrogen, are present in normal and ovarian epithelial cancer cells (Lau et al., 1999). Analysis of serial analysis of gene expression (SAGE) data in human genome databases suggests that PELP1 expression is altered in ovarian cancers and that ovarian cancer cells express 3- to 4-fold higher levels of PELP1 mRNA than do immortalized ovarian surface epithelial cells, representing the benign stage of ovarian cancer. Analysis of human ovarian cancer tissue arrays revealed that PELP1 is 2- to 3-fold overexpressed in 60% of ovarian tumors. PELP1 has been found to be deregulated in all subtypes of ovarian tumors (including serous, endometrioid, clear cell carcinoma and mucous tumors), whereas PELP1 localization was predominantly cytoplasmic in a subset of ovarian tumors (Vadlamudi et al., unpublished observations). Since cytoplasmic localization of PELP1 promotes the excessive activation of ER-nongenomic functions, the PELP1 deregulation observed in ovarian tumors may have implications in hormonal therapy resistance observed in these tumors.
PELP1/MNAR in salivary tumors
Salivary duct carcinoma (SDC) is a high-grade neoplasm with marked morphologic resemblance to mammary duct carcinoma. Interestingly, immuno-histochemical staining of 70 SDCs revealed strong expression of PELP1/MNAR in 51 (73%) and ERβ in 52 (74%). PELP1/MNAR staining was predominantly cytoplasmic, whereas ERβ staining was predominantly nuclear. The coexpression of ERβ and PELP1/MNAR in the majority of SDCs indicates that these proteins are important in the pathobiology of these tumors through both genomic and nongenomic pathways. These results also suggest that the ERβ pathway may play a role in the biology of SDC and that ERβ agonists and ER nongenomic signaling blockers may have therapeutic value in SDC.
PELP1/MNAR in prostate tumors
Androgen receptor (AR) plays a critical role in prostate carcinogenesis and its progression to the incurable androgen-independent state. PELP1/MNAR has been found to interact with AR and to modulate its transactivation functions (Wong et al., 2002). Recent evidence suggests that PELP1/MNAR may also participate in the nongenomic activities of AR by coupling AR with Src kinase signaling in prostate cancer cells (Unni et al., 2004). PELP1/MNAR is widely expressed in epithelial prostate cancer cell lines, as well as in high-grade prostate tumors (Nair et al., 2005). PELP1 has been shown to interact with FHL2 and to form the trimeric AR-PELP1-FHL2 complex upon ligand stimulation, thus enhancing FHL2-mediated AR transactivation functions (Nair et al., 2005). These results suggest that PELP1/MNAR functions as a molecular adaptor, coupling FHL2 with AR and AR with Src kinase, thus having roles in prostate cancer progression and in AR crosstalk with nongenomic pathways.
Conclusions
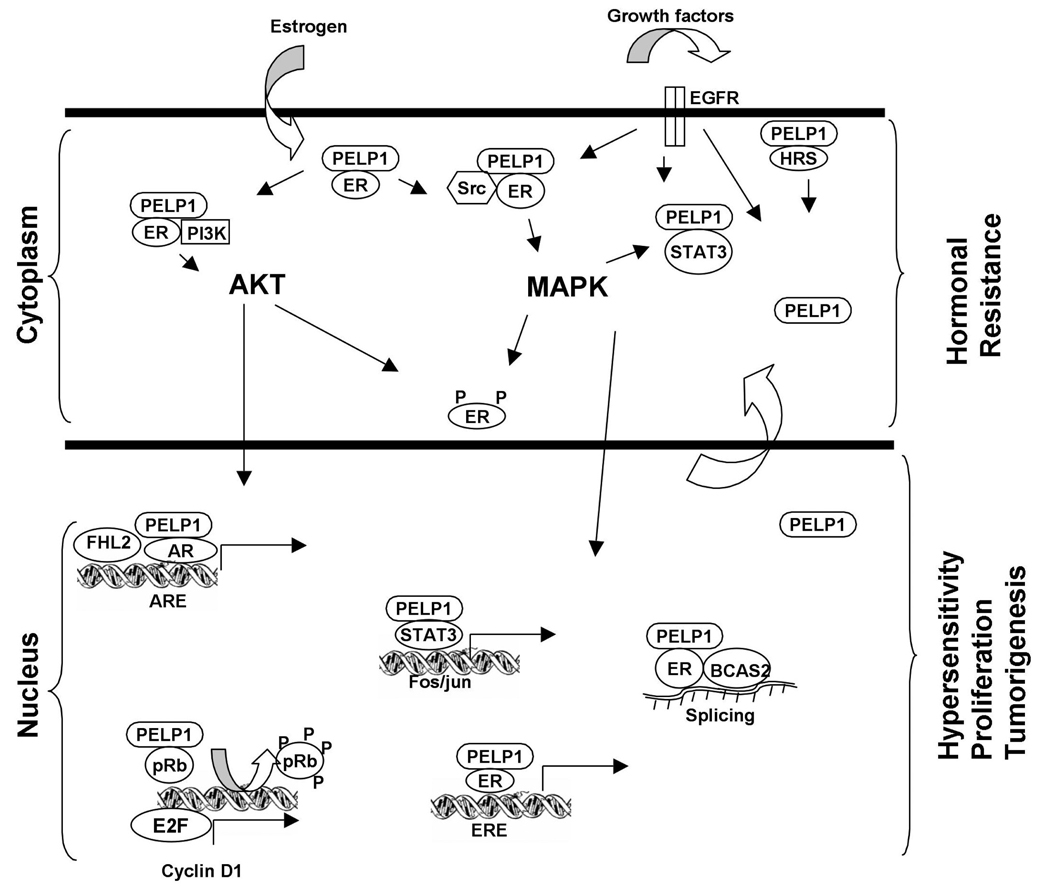
PELP1/MNAR is a novel ER-coactivator that plays an important role in both the genomic and nongenomic actions of ER (Fig. 2). PELP1 enhances the tumorigenic potential of a number of human cancer cell lines in vitro and in vivo. PELP1 expression/localization is altered in a wide variety of tumors. Deregulation of PELP1/MNAR, which can stimulate nongenomic ER functions, may promote hormonal independence by modulating ER-transactivating functions and SERM actions. Collectively, these emerging findings suggest that PELP1/MNAR expression or localization could be used to determine whether a tumor will be resistant or susceptible to hormone therapy. Drugs targeting PELP1/MNAR or its regulatory pathways (Src and AKT) could be beneficial in tumors in which PELP1/MNAR expression/localization is deregulated. Future studies analyzing more tumor samples, validating the role of PELP1/MNAR in animal models of hormone-resistant and -susceptible cancers, and elucidating the mechanisms involved in the deregulation of PELP1/MNAR in tumors will help in the development of ways to target this novel gene for endocrine therapy. The ability of PELP1/MNAR to interact with EGFR, ER, Src and PI3K suggests that it may serve as a novel target for the disruption of the EGFR-ER signaling crosstalk that occurs in hormonal resistant cancer cells.
Fig. 2.
Schematic representation of PELP1/MNAR signaling in cancer cells. PELP1 role in ER genomic and nongenomic functions is depicted by placing PELP1/MNAR in both cytoplasmic and nuclear compartments along with the identified complexes. Deregulation of PELP1/MNAR in cancer cells might contribute to the hypersensitivity of tumor cells by enhancing genomic as well as nongenomic ER actions and thus promote tumorigenesis and hormonal therapy resistance.
Acknowledgements
The work is supported by the NIH grant CA095681 (to R.V).
References
- Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS. AIB1, a steroid receptor coactivator amplified in breast andovarian cancer. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- Bagheri-Yarmand R, Talukder AH, Wang RA, Vadlamudi RK, Kumar R. Metastasis-associated protein 1 deregulation causes inappropriate mammary gland development and tumorigenesis. Development. 2004;131:3469–3479. doi: 10.1242/dev.01213. [DOI] [PubMed] [Google Scholar]
- Balasenthil S, Vadlamudi RK. Functional interactions between the estrogen receptor coactivator PELP1/MNAR and retinoblastoma protein. J. Biol. Chem. 2003;278:22119–22127. doi: 10.1074/jbc.M212822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barletta F, Wong CW, McNally C, Komm BS, Katzenellenbogen B, Cheskis BJ. Characterization of the interactions of estrogen receptor and MNAR in the activation of cSrc. Mol. Endocrinol. 2004;18:1096–1108. doi: 10.1210/me.2003-0335. [DOI] [PubMed] [Google Scholar]
- Barnes CJ, Vadlamudi RK, Kumar R. Novel estrogen receptor coregulators and signaling molecules in human diseases. Cell Mol. Life Sci. 2004;61:281–291. doi: 10.1007/s00018-003-3222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman L, Beelen ML, Gallee MP, Hollema H, Benraadt J, van Leeuwen FE. Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Comprehensive Cancer Centres' ALERT Group. Assessment of Liver and Endometrial cancer Risk following Tamoxifen. Lancet. 2000;356:881–887. doi: 10.1016/s0140-6736(00)02677-5. [DOI] [PubMed] [Google Scholar]
- Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol. Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- Choi YB, Ko JK, Shin J. The transcriptional corepressor, PELP1, recruits HDAC2 and masks histones using two separate domains. J. Biol. Chem. 2004;279:50930–50941. doi: 10.1074/jbc.M406831200. [DOI] [PubMed] [Google Scholar]
- Greger JG, Guo Y, Henderson R, Ross JF, Cheskis BJ. Characterization of MNAR expression. Steroids. 2006;71:317–322. doi: 10.1016/j.steroids.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Gururaj AE, Rayala SK, Vadlamudi RK, Kumar R. Novel mechanisms of resistance to endocrine therapy: genomic and nongenomic considerations. Clin. Cancer Res. 2006;12:1001s–1007s. doi: 10.1158/1078-0432.CCR-05-2110. [DOI] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP. Coregulators in nuclear estrogen receptor action: from concept to therapeutic targeting. Mol. Interv. 2005;5:343–357. doi: 10.1124/mi.5.6.7. [DOI] [PubMed] [Google Scholar]
- Lau KM, Mok SC, Ho SM. Expression of human estrogen receptor-alpha and -beta, progesterone receptor, and androgen receptor mRNA in normal and malignant ovarian epithelial cells. Proc. Natl. Acad. Sci. USA. 1999;96:5722–5727. doi: 10.1073/pnas.96.10.5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren PR, Backstrom T, Cajander S, Damber MG, Mahlck CG, Zhu D, Olofsson JI. The pattern of estradiol and progesterone differs in serum and tissue of benign and malignant ovarian tumors. Int. J. Oncol. 2002;21:583–589. [PubMed] [Google Scholar]
- List HJ, Reiter R, Singh B, Wellstein A, Riegel AT. Expression of the nuclear coactivator AIB1 in normal and malignant breast tissue. Breast Cancer Res. Treat. 2001;68:21–28. doi: 10.1023/a:1017910924390. [DOI] [PubMed] [Google Scholar]
- Losel R, Wehling M. Nongenomic actions of steroid hormones. Nat. Rev. Mol. Cell Biol. 2003;4:46–56. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- Manavathi B, Kumar R. Steering estrogen signals from the plasma membrane to the nucleus: Two sides of the coin. J. Cell Physiol. 2005;207:594–604. doi: 10.1002/jcp.20551. [DOI] [PubMed] [Google Scholar]
- Manavathi B, Nair SS, Wang RA, Kumar R, Vadlamudi RK. Proline-, glutamic acid-, and leucine-rich protein-1 is essential in growth factor regulation of signal transducers and activators of transcription 3 activation. Cancer Res. 2005;65:5571–5577. doi: 10.1158/0008-5472.CAN-04-4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuhiro Y, Mezaki Y, Sakari M, Takeyama K, Yoshida T, Inoue K, Yanagisawa J, Hanazawa S, O'Malley BW, Kato S. Splicing potentiation by growth factor signals via estrogen receptor phosphorylation. Proc. Natl. Acad. Sci. USA. 2005;102:8126–8131. doi: 10.1073/pnas.0503197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell DP, Norris JD. Connections and regulation of the human estrogen receptor. Science. 2002;296:1642–1644. doi: 10.1126/science.1071884. [DOI] [PubMed] [Google Scholar]
- McKenna NJ, Lanz RB, O'Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr. Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- Macpherson NA, Moore S, Brodie A, Thiantanawat A, Jelovac D, Nelson CC. Gene expression changes during acquired resistance to letrozole; a preclinical model of post-menopausal breast cancer. Breast Cancer Res. Treat. 2005;94:4101. [Google Scholar]
- Nagpal J, Nair SS, Pothana S, Tekmal R, Kumar R, Vadlamudi R. Growth factor regulation of PELP1/MNAR functions:Role of PKA-dependent phosphorylation. Proc. Am. Cancer Res. 2006;47:2933. [Google Scholar]
- Nair SS, Mishra SK, Yang Z, Balasenthil S, Kumar R, Vadlamudi RK. Potential role of a novel transcriptional coactivator PELP1 in histone H1 displacement in cancer cells. Cancer Res. 2004;64:6416–6423. doi: 10.1158/0008-5472.CAN-04-1786. [DOI] [PubMed] [Google Scholar]
- Nair SS, Seetharaman B, Muller JM, Schule R, Kumar R, Vadlamudi RK. PELP1/MNAR modulates LIM-only coactivator FHL2 transactivation functions. Proc. Am. Cancer Res. 2005;46:1279. [Google Scholar]
- Nair S, Rajhans R, Nagpal J, Guider J, Kumar R, Vadlamudi R. PELP1/MNAR interacts with BCAS2:Potential role in ERmediated Splicing. Proc. Am. Cancer Res. 2006;47:2930. [Google Scholar]
- Rajhans R, Nair SS, Tekmal R, Kumar R, Vadlamudi RK. Cellular transformation and oncogenesis by PELP1/NMAR, a major broad coactivator in mammalian cells. Proc. Am. Cancer Res. 2006;47:4215. [Google Scholar]
- Rayala SK, Hollander P, Balasenthil S, Molli PR, Bean AJ, Vadlamudi RK, Wang R, Kumar R. Hepatocyte growth factor-regulated tyrosine kinase substrate (HRS) interacts with PELP1 and activates MAPK. J. Biol. Chem. 2005;281:4395–4403. doi: 10.1074/jbc.M510368200. [DOI] [PubMed] [Google Scholar]
- Rayala SK, Mascarenhas J, Vadlamudi RK, Kumar R. Altered localization of a coactivator sensitizes breast cancer cells to tumor necrosis factor-induced apoptosis. Mol. Cancer Ther. 2006;5:230–237. doi: 10.1158/1535-7163.MCT-05-0276. [DOI] [PubMed] [Google Scholar]
- Schiff R, Massarweh SA, Shou J, Bharwani L, Arpino G, Rimawi M, Osborne CK. Advanced concepts in estrogen receptor biology and breast cancer endocrine resistance: implicated role of growth factor signaling and estrogen receptor coregulators. Cancer Chemother. Pharmacol. 2005;56 Suppl. 1:10–20. doi: 10.1007/s00280-005-0108-2. [DOI] [PubMed] [Google Scholar]
- Smith CL, O'Malley BW. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr. Rev. 2004;25:45–71. doi: 10.1210/er.2003-0023. [DOI] [PubMed] [Google Scholar]
- Song RX, Zhang Z, Santen RJ. Estrogen rapid action via protein complex formation involving ERalpha and Src. Trends Endocrinol. Metab. 2005;16:347–353. doi: 10.1016/j.tem.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Torres-Arzayus MI, Font de Mora J, Yuan J, Vazquez F, Bronson R, Rue M, Sellers WR, Brown M. High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell. 2004;6:263–274. doi: 10.1016/j.ccr.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Unni E, Sun S, Nan B, McPhaul MJ, Cheskis B, Mancini MA, Marcelli M. Changes in androgen receptor nongenotropic signaling correlate with transition of LNCaP cells to androgen independence. Cancer Res. 2004;64:7156–7168. doi: 10.1158/0008-5472.CAN-04-1121. [DOI] [PubMed] [Google Scholar]
- Vadlamudi RK, Wang RA, Mazumdar A, Kim Y, Shin J, Sahin A, Kumar R. Molecular cloning and characterization of PELP1, a novel human coregulator of estrogen receptor alpha. J. Biol. Chem. 2001;276:38272–38279. doi: 10.1074/jbc.M103783200. [DOI] [PubMed] [Google Scholar]
- Vadlamudi RK, Balasenthil S, Broaddus RR, Gustafsson JA, Kumar R. Deregulation of estrogen receptor coactivator proline-, glutamic acid-, and leucine-rich protein-1/modulator of nongenomic activity of estrogen receptor in human endometrial tumors. J. Clin. Endocrinol. Metab. 2004;89:6130–6138. doi: 10.1210/jc.2004-0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadlamudi RK, Manavathi B, Balasenthil S, Nair SS, Yang Z, Sahin AA, Kumar R. Functional implications of altered subcellular localization of PELP1 in breast cancer cells. Cancer Res. 2005;65:7724–7732. doi: 10.1158/0008-5472.CAN-05-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CW, McNally C, Nickbarg E, Komm BS, Cheskis BJ. Estrogen receptor-interacting protein that modulates its nongenomic activity-crosstalk with Src/Erk phosphorylation cascade. Proc. Natl. Acad. Sci. USA. 2002;99:14783–14788. doi: 10.1073/pnas.192569699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]