Abstract
Objectives
To determine the independent prognostic effect of 7 potential frailty criteria, including 5 from the Fried phenotype, on several adverse outcomes.
Design
Prospective cohort study.
Setting
Greater New Haven, Connecticut.
Participants
Seven hundred fifty-four initially non-disabled, community-living persons aged 70 and older.
Measurements
An assessment of 7 potential frailty criteria (slow gait speed, low physical activity, weight loss, exhaustion, weakness, cognitive impairment and depressive symptoms) was completed at baseline and every 18 months for 72 months. Participants were followed with monthly telephone interviews for up to 96 months to determine the occurrence of chronic disability, long-term nursing home (NH) stays, injurious falls, and death.
Results
In analyses that were adjusted for age, sex, race, education, number of chronic conditions, and the presence of the other potential frailty criteria, 3 of the 5 Fried criteria (slow gait speed, low physical activity, and weight loss) were independently associated with chronic disability, long-term NH stays, and death. Slow gait speed was the strongest predictor of chronic disability (Hazard ratio [HR] 2.97, 95% confidence interval [CI], 2.32–3.80), and long-term NH stays (HR 3.86, 95% CI, 2.23–6.67), and was the only significant predictor of injurious falls (HR 2.19, 95% CI, 1.33–3.60). Cognitive impairment was also associated with chronic disability (HR 1.82, 95% CI, 1.40–2.38), long-term NH stays (HR 2.64, 95% CI, 1.75–3.99), and death (HR 1.54, 95% CI, 1.13–2.10), and the magnitude of these associations was comparable to that of weight loss.
Conclusions
The results of our study provide strong evidence to support the use of slow gait speed, low physical activity, weight loss and cognitive impairment as key indicators of frailty, while raising concerns about the value of self-reported exhaustion and muscle weakness.
Keywords: frailty, disability, cohort study, prognosis
INTRODUCTION
Frailty is increasingly recognized as a geriatric syndrome that is highly prevalent, distinct from disability and co-morbidity, and potentially modifiable, and that increases vulnerability to an array of clinically important outcomes, including functional decline, institutionalization, and falls.(1) Several potential operational definitions of frailty have been proposed,(1–5) but none has become the gold standard for identifying frail older persons in the clinical or research setting. Fried and colleagues (1) have proposed an operational definition, or ‘phenotype,’ of frailty based on five criteria: slow gait speed, low physical activity, unintentional weight loss, self-reported exhaustion and muscle weakness. The presence of three or more of these criteria was independently associated with worsening mobility and disability in activities of daily living (ADLs), incident falls and hospitalizations, and mortality over seven years among a large cohort of community-living older persons.(1)
Although the Fried frailty phenotype has been validated and modified for use in several published reports,(6–9) limitations remain which challenge its generalizability and usefulness in the clinical setting.(10–13) First, cognitive and psychological factors were not included in the frailty phenotype despite their known association with functional decline and disability.(14) Second, persons taking antidepressants and those whose frailty was attributed to a single disease process, such as stroke, Parkinson’s disease, or multiple sclerosis, were excluded from the initial cohort assembled to define the phenotype. Third, assessments of frailty were made only at only a single point in time despite evidence that transitions between frailty states occur frequently.(8) Finally, the independent contribution of the five Fried frailty criteria to subsequent adverse outcomes has not been rigorously evaluated. Thus, while there is strong evidence to support the use of slow gait speed as a criterion for frailty,(13, 15–17) relatively little is known about the prognostic effects of the other four criteria independent of gait speed.
The objective of the current study was to determine the independent prognostic effect of each of the five Fried frailty criteria, as well as two other potential frailty criteria – cognitive impairment and depressive symptoms – on four clinically relevant geriatric outcomes, namely chronic disability, long-term nursing home (NH) stays, injurious falls, and death. Information on the frailty criteria was updated every 18 months and outcomes were assessed over seven and a half years of follow-up.
METHODS
Study Population
The study population included participants of the Precipitating Events Project (PEP), a longitudinal study of 754 initially nondisabled, community-living persons aged 70 years or older. The assembly of the cohort has been described in detail elsewhere.(18) Participants were potentially eligible if they were community-living, English-speaking, and nondisabled (i.e., required no personal assistance) in 4 essential ADLs: bathing, dressing, walking inside the house, and transferring from a chair.(19) Participants were excluded if they had a diagnosis of a terminal illness with a life expectancy less than 12 months, if they planned to move out of the New Haven area during the next 12 months, or if they had significant hearing or cognitive impairment with no available proxy. Persons with slow gait speed were oversampled, as previously described.(18) The participation rate was 75.2%. The study protocol was approved by the Yale Human Investigation Committee; and all participants provided oral informed consent.
Data Collection
Comprehensive home-based assessments were completed by trained research staff at baseline and, subsequently, every 18 months for 72 months. Data were collected on sociodemographic characteristics (age, sex, race, education); 9 self-reported, physician-diagnosed chronic conditions;(20) and seven potential frailty criteria, including those defined previously by Fried et al.(1)
Assessment of Potential Frailty Criteria
As described in an earlier report,(8) our operational definitions differed modestly for three of the five Fried frailty criteria that were originally described in the Cardiovascular Health Study.(1) The slow gait speed criterion was met if the participant took more than 10 seconds to walk back and forth over a ten-foot course as quickly as possible. This cutoff point delineated a threshold response (at the worst quartile) between rapid gait scores and the development of disability in an earlier population-based cohort of older persons.(21, 22) The low physical activity criterion was met for men who scored less than 64 and women who scored less than 52 on the Physical Activity Scale for the Elderly (PASE).(23, 24) These sex-specific cutoff points denote the worse quintile of scores among the first 356 enrolled PEP participants who had been selected randomly from our source population.(18) The weight loss criterion was met if the participant answered “Yes” when asked, “In the past year, have you lost more than 10 pounds?”
Our operational definitions were identical for the remaining two Fried frailty criteria. The exhaustion criterion was met if the participant answered “Much or most of the time” when asked, “How often in the last week did you feel this way” to either of the following two statements from the Center for Epidemiologic Studies Depression (CES-D) Scale: “I felt that everything I did was an effort” and “I could not get going.” The muscle weakness criterion was met when grip strength, assessed as the average of 3 readings by a handheld dynamometer (Chatillon 100; Ametek Inc, Largo, Fla.), was less than or equal to the sex- and body mass index–specific cutoff points provided by Fried et al.(1)
Two additional potential frailty criteria were also evaluated. Cognitive impairment was defined as a score < 24 on the Folstein Mini-Mental Status Exam (MMSE).(25) Depressive symptoms were ascertained using a modified version of the 11-item CES-D scale,(26) which excluded the two questions utilized in the Fried frailty exhaustion criterion, as has been done previously.(1) Scores of this shorter version were transformed to correspond to the standard 20-item scale using the procedure described by Kohout et al,(26, 27) and a cut-off score of 16 or more was used to indicate the presence of depressive symptoms.(26) After exclusion of the two questions, the correlation between the exhaustion criterion and depressive symptoms was reduced from 0.43 to 0.29.
Assessment of Outcomes
Participants were interviewed by telephone every month for up to 7 1/2 years by trained research staff to assess their functional status and ascertain the occurrence of long-term NH stays, injurious falls, and death. Two hundred and eighty three (37.5%) participants died after a median follow-up of 49.0 months, while 28 (3.7%) dropped out of the study after a median follow-up of 26.4 months. Data were otherwise available for 99.1% of the 55,922 telephone interviews.
Participants were assessed for ADL disability using standard questions. For each of the four essential ADLs participants, we asked, “At the present time, do you need help from another person to (complete the task)?” Participants who needed help with a specific task were considered to be disabled in that task. Participants were not asked about eating, toileting, or grooming because the incidence of disability in these three ADLs is low among non-disabled, community-living older persons. Furthermore, it is highly uncommon for disability to develop in these ADLs without concurrent disability in bathing, dressing, walking, or transferring.(21) Chronic disability was defined as a new ADL disability that was present for at least 3 consecutive months.(28) We chose chronic disability as a primary outcome because it is the metric often used to track disability trends among older persons.(29) Furthermore, shorter episodes of disability occur frequently, and a large majority of persons with short-term disability recover within six months.(28)
Nursing home stays that persisted for 4 or more consecutive monthly interviews, corresponding to a minimum length of stay of 90 days, were considered long-term. In an earlier report,(20) the reliability of these data was shown to be high (Kappa=0.96). We chose long-term NH stays as a primary outcome because the vast majority of short-term, post-acute rehabilitation stays in nursing homes result in discharge to the community within 90 days. (30) Those who remain in a nursing home after 90 days are likely to require long-term custodial care.
We defined an injurious fall as a fall leading to a hospital admission for a new fracture, head injury (including subdural hemorrhage, intracranial injury, or open wound), or hematoma or bruise of the face or scalp. To ascertain the occurrence of injurious falls, participants were first asked whether they had stayed at least overnight in a hospital since the last interview (i.e., during the past month). Participants were then asked to provide the primary reason for their admission. Based on an independent review of hospital records among 44 participants, we found that the reliability of this self-reported information was high, with a k value of 0.89 for the presence for the presence or absence of a fall-related injury.
Finally, deaths were ascertained by review of local obituaries and/or from an informant, with a completion rate of 100%.
Statistical Analysis
We used descriptive statistics to define the prevalence of the frailty criteria at baseline and at 18-month intervals. We evaluated the associations between each of the seven potential frailty criteria and chronic disability, long-term NH stays, injurious falls, and death, respectively, using survival analysis. Each outcome was considered independent of the others, and participants who died or were lost to follow-up before developing a specific outcome were censored. The frailty criteria were treated as time-dependent covariates and were updated every 18 months. Two models were developed: Model 1, in which the analyses were adjusted for baseline sociodemographic factors (age, gender, race, education) and the number of chronic conditions, and Model 2, in which the analyses were adjusted for the preceding covariates as well as the presence of the other six potential frailty criteria.
All statistical tests were 2-tailed, and P < 0.05 was considered to indicate statistical significance. All analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
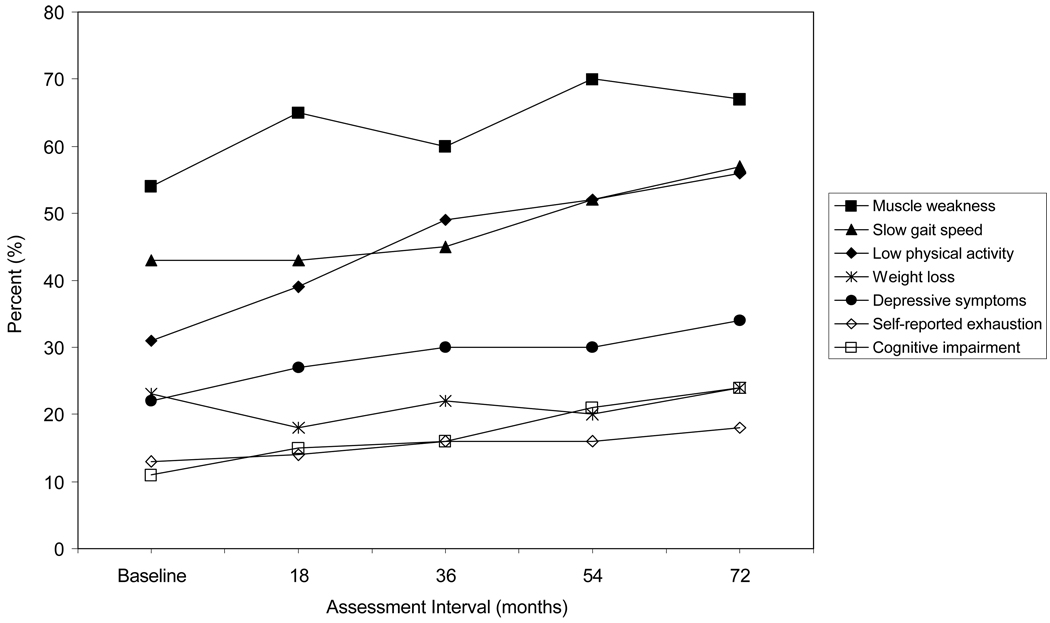
The baseline characteristics of the study participants are shown in Table 1. The majority of participants were female, non-Hispanic white and did not live alone. On average, participants had a high school education and two chronic conditions. Figure 1 shows the prevalence of each potential frailty criterion at 18-month intervals over 72 months. At baseline, the most common potential frailty criteria were slow gait speed and muscle weakness, with prevalence rates of 43% and 54%, respectively. Nearly one-third of participants reported low physical activity at baseline, and this rose to 56% over the course of the study. Among participants with cognitive impairment at baseline, the mean MMSE score was 21.7 (standard deviation 1.45), with a minimum score of 16. Over the 72-month study period, the prevalence of cognitive impairment nearly doubled, while the prevalence of the other 3 potential frailty criteria changed little.
Table 1.
Baseline Characteristics of the 754 Participants
| Age, mean ± SD, y | 78.4 ± 5.3 |
| Sex, No. (%) F | 487 (64.6) |
| Race, No. (%) non-Hispanic white | 682 (90.5) |
| Lives alone, No. (%) | 298 (39.5) |
| Education level, mean ± SD, y | 12.0 ± 2.9 |
| No. of chronic conditions,* mean ± SD | 1.9 ± 1.3 |
| Potential Frailty Criteria,† No. (%) | |
| - Slow Gait Speed | 322 (43) |
| - Low Physical Activity | 232 (31) |
| - Weight Loss | 174 (23) |
| - Self-reported Exhaustion | 96 (13) |
| - Muscle Weakness | 407 (54) |
| - Cognitive Impairment | 86 (11) |
| - Depressive Symptoms | 165 (22) |
The 9 self-reported, physician-diagnosed chronic conditions included hypertension; myocardial infarction; congestive heart failure; stroke; diabetes mellitus; arthritis; hip fracture; chronic lung disease; and cancer (other than minor skin cancers).
As defined in the Methods section.
Figure 1.
Prevalence of Potential Frailty Criteria Over 72 Months
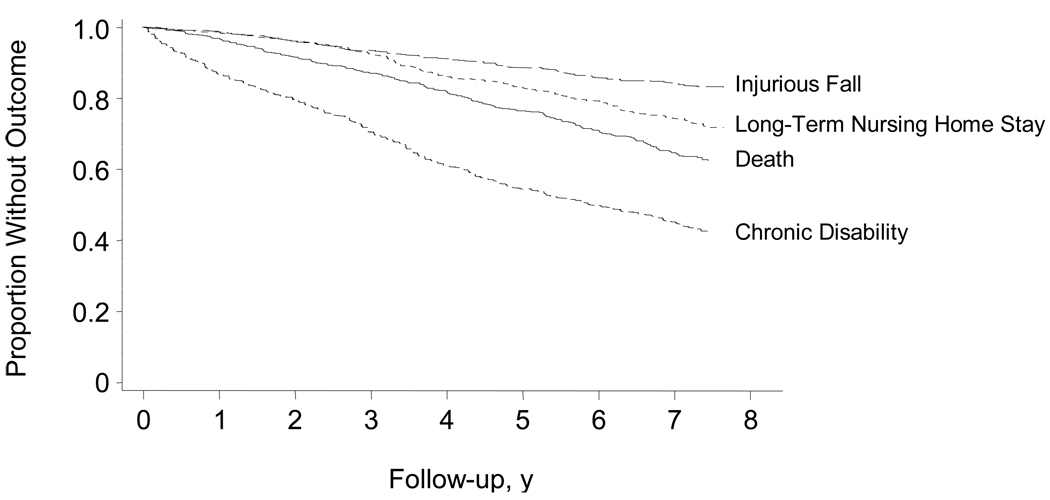
Figure 2 shows the Kaplan-Meier curves for the four adverse outcomes over 7 1/2 years of follow-up. Thirteen percent of participants had an injurious fall, 22% had a long-term NH stay, 38% died, and 52% developed chronic disability.
Figure 2.
Kaplan-Meier Curves for Adverse Outcomes
Results from the Cox proportional hazards analyses are shown in Table 2. In Model 1, slow gait speed was the strongest predictor of chronic disability and long-term NH stays, and was the only criterion associated with injurious falls after adjusting for sociodemographic factors and the presence of chronic conditions. Low physical activity was the strongest predictor of death. The associations between the other five potential frailty criteria – including cognitive impairment and depressive symptoms – and chronic disability, long-term NH stays, and death, respectively, were all of comparable magnitude and were consistently weaker than those for slow gait speed and low physical activity.
Table 2.
Risk of Adverse Outcomes According to Potential Frailty Criteria
| Frailty Criteria* | Chronic Disability |
Long-Term Nursing Home Stay |
Injurious Fall | Death |
|---|---|---|---|---|
| Hazard Ratio (95% Confidence Interval) | ||||
| Slow gait speed | ||||
| Model 1 | 3.8 (3.0 – 4.9) | 5.9 (3.5 – 9.8) | 2.5 (1.6 – 4.0) | 2.7 (2.0 – 3.7) |
| Model 2 | 3.0 (2.3 – 3.8) | 3.9 (2.2 – 6.7) | 2.2 (1.3 – 3.6) | 1.7 (1.2 – 2.3) |
| Low physical activity | ||||
| Model 1 | 2.8 (2.3 – 3.5) | 3.5 (2.3 – 5.4) | 1.5 (0.9 – 2.2) | 3.3 (2.5 – 4.5) |
| Model 2 | 2.1 (1.7 – 2.6) | 2.1 (1.3 – 3.3) | 1.2 (0.8 – 1.9) | 2.2 (1.6 – 3.1) |
| Weight loss | ||||
| Model 1 | 1.9 (1.5 – 2.4) | 2.0 (1.4 – 2.9) | 1.5 (0.9 – 2.3) | 1.7 (1.3 – 2.2) |
| Model 2 | 1.7 (1.4 – 2.1) | 1.7 (1.2 – 2.4) | 1.3 (0.8 – 2.1) | 1.4 (1.1 – 1.8) |
| Self-reported exhaustion | ||||
| Model 1 | 1.6 (1.3 – 2.1) | 2.0 (1.3 – 2.9) | 1.1 (0.6 – 1.9) | 1.7 (1.3 – 2.2) |
| Model 2 | 1.1 (0.8 – 1.4) | 1.1 (0.8 – 1.7) | 0.7 (0.4 – 1.3) | 1.2 (0.8 – 1.6) |
| Weakness | ||||
| Model 1 | 1.5 (1.2 – 1.8) | 1.7 (1.1 – 2.7) | 1.4 (0.8 – 2.2) | 1.8 (1.3 – 2.5) |
| Model 2 | 1.1 (0.9 – 1.4) | 1.0 (0.6 – 1.6) | 1.1 (0.7 – 1.9) | 1.2 (0.9 – 1.7) |
| Cognitive impairment | ||||
| Model 1 | 2.1 (1.7 – 2.8) | 3.7 (2.5 – 5.4) | 1.1 (0.6 – 1.8) | 2.4 (1.8 – 3.1) |
| Model 2 | 1.8 (1.4 – 2.4) | 2.6 (1.7 – 4.0) | 0.9 (0.5 – 1.6) | 1.5 (1.1 – 2.1) |
| Depressive symptoms | ||||
| Model 1 | 1.3 (1.1 – 1.6) | 1.8 (1.3 – 2.6) | 1.5 (1.0 – 2.2) | 1.5 (1.2 – 2.0) |
| Model 2 | 1.1 (0.9 – 1.5) | 1.4 (1.0 – 2.1) | 1.4 (0.9 – 2.2) | 1.2 (0.9 – 1.6) |
Model 1 was adjusted for age, sex, race, education, and chronic conditions. Model 2 was adjusted for age, sex, race, education, chronic conditions, and the presence of the other six frailty criteria.
In Model 2, which also adjusted for the presence of the other frailty criteria, slow gait speed remained the strongest predictor of chronic disability and long-term NH stays and was the only frailty criterion that was independently associated with injurious falls. Of the remaining 6 potential frailty criteria, only low physical activity, weight loss and cognitive impairment were independently associated with chronic disability, long-term NH stays and death in the fully adjusted model. Low physical activity remained the strongest predictor of death.
DISCUSSION
In this prospective cohort study of initially non-disabled community-living older persons, we evaluated the independent prognostic effect of seven potential frailty criteria on four clinically relevant geriatric outcomes. We found that only three of the five original Fried frailty criteria – slow gait speed, low-physical activity, and weight loss – were independently associated with chronic disability, long-term NH stays and mortality. Slow gait speed was the strongest predictor of chronic disability and long-term NH stays, and was the only frailty criterion significantly associated with injurious falls. Cognitive impairment – a potential frailty criterion not included in the Fried phenotype – was associated with three of the four adverse outcomes, and the magnitude of these associations was comparable to or greater than that for weight loss. Neither of the two remaining Fried frailty criteria – self-reported exhaustion or muscle weakness – nor depressive symptoms were independently associated with any of the four adverse outcomes in fully adjusted analyses. These results raise concerns about the validity of the Fried criteria and suggest that the frailty phenotype might be strengthened by the inclusion of alternative criteria such as cognitive impairment.
Frailty is increasingly viewed as a potentially modifiable geriatric syndrome, but a consensus on a standard definition of the syndrome does not yet exist. A recent review of the frailty syndrome described it as a state of increased vulnerability to adverse outcomes, including disability, and several attempts have been made to delineate the physical, cognitive, psychosocial, and environmental components of the frailty syndrome.(13) Despite the demonstrated validity of the Fried frailty phenotype, which focuses exclusively on the physical components of frailty, some have argued that this model omits potentially important clinical features such as cognitive impairment and depressive symptoms.(10, 12, 14) Other definitions of frailty that focus on overall deficit accumulation have also been shown to predict adverse outcomes in older persons.(5, 31, 32) While routine assessment of large numbers of deficits in clinical practice may be difficult at present, the availability of many self-reported patient characteristics will likely increase with the development of electronic medical records.(33) This, in turn, may enhance the feasibility of using deficit accumulation models to assess frailty in clinical practice.(34) Our finding that only three of the five Fried frailty criteria are independently associated with clinically relevant geriatric outcomes suggests that additional research into the epidemiology of frailty is needed before a single goldstandard of the syndrome is accepted or adopted.
In our study, slow gait speed demonstrated the strongest and most consistent associations with the adverse outcomes, and was the only criterion that was independently associated with injurious falls. These findings are consistent with those of prior studies (35–38) Because gait speed is inexpensive to assess and highly reliable, and because it has been shown to predict incident disability, NH stays and mortality almost as well as summary measures of lower extremity performance,(39) it may represent the best single indicator of frailty in the clinical and research setting.(13)
Cognitive impairment is a known risk factor for many geriatric outcomes (40) and the assessment of mental status is a routine part of most geriatric evaluations in the clinical setting. In our study, cognitive impairment (defined as a MMSE score less than 24) was independently associated with three of the four adverse outcomes – chronic disability, long-term NH stays, and death – and the magnitude of these associations was stronger than those for three of the five Fried frailty criteria. This finding highlights the extent to which vulnerability to adverse outcomes is affected by mental status. Including cognitive impairment as an indicator of frailty has high face validity given the high prevalence of cognitive dysfunction in the geriatric population, which is anticipated to rise dramatically over the coming decades.(41)
Self-reported exhaustion and muscle weakness were initially associated with adverse outcomes in analyses that adjusted for sociodemographic factors and the number of chronic conditions, but these associations were attenuated and no longer statistically significant after further adjustment for the presence of the other frailty criteria. These results are consistent with those of an earlier study which found that neither exhaustion nor weakness was independently associated with the onset of ADL disability over 3 years despite a strong association between the composite measure of frailty (any 3 of 5 frailty criteria present) and the onset of disability.(7) Taken together, these results suggest that exhaustion and weakness are not particularly robust indicators of frailty, even though they may strengthen the association with adverse outcomes when aggregated with other frailty indicators.(1) Alternatively, better measures of exhaustion and weakness may be needed. Other studies have shown, for example, that measures of upper body weakness are not as strongly associated with functional decline as measures of lower body weakness.(37)
Depressive symptoms showed a similar pattern of association with the adverse outcomes as self-reported exhaustion and weakness. Although associated with disability, long-term NH stays, and death in the initial set of multivariable models, depressive symptoms was not associated with these outcomes in the fully adjusted models. These results suggest that the presence of depressive symptoms does not add meaningfully to other more robust indicators of frailty such as slow walking speed, low physical activity, weight loss, and cognitive impairment. While it is possible that our assessment of depressive symptoms was weakened by the exclusion of the two items that defined self-reported exhaustion, the associations between the two versions of the depression instrument (i.e. with and without the two self-reported exhaustion items) and the adverse outcomes were comparable in both sets of multivariable analyses (results available upon request).
As per the Fried protocol, we assessed muscle weakness with a handheld dynamometer and exhaustion using identical items from the CES-D questionnaire. Our assessment of frailty, however, included modest modifications to three of the other five criteria that were initially operationalized by Fried et al using data from the Cardiovascular Health Study. While these modifications may have modestly affected the prevalence of these frailty indicators,(8) they should not have had any meaningful affect on the observed associations with adverse outcomes. Similarly, although our stratified sampling strategy yielded a study population with a much higher prevalence of slow gait speed and muscle weakness than in prior studies of frailty, these differences should have had relatively little effect on the longitudinal associations. As is commonly done in clinical practice, we used an MMSE score less than 24 to identify participants with cognitive impairment. While a single cut-point cannot distinguish severe versus mild to moderate cognitive impairment, our study population of community-living older persons, who were initially nondisabled in their essential ADLs, included no participants with an MMSE score less than 16.
Whether our findings can be generalized widely may be questioned. As previously noted, the demographic characteristics of our source population closely mirror those in the United States as a whole, with the exception of race.(42) Despite the absence of a population-based sample, the high participation rate, low rate of attrition for reasons other than death, and nearly complete ascertainment of disability and other outcomes all enhance the generalizability of our findings(43) and at least partially offset the absence of a population-based sample.
The lack of a consensus definition of frailty has been a major obstacle for the development of primary and secondary preventive trials aimed at forestalling or ameliorating this highly prevalent geriatric syndrome. The results of the current study provide strong evidence to support the use of slow gait speed, low physical activity, weight loss and cognitive impairment as key indicators of frailty, while raising concerns about the value of self-reported exhaustion and muscle weakness. Each of the four key indicators of frailty can be easily assessed, thereby enhancing their applicability to clinical practice. Our results also add to the growing body of research showing that slow gait speed may the most useful single indicator of frailty in the clinical setting given its strong and consistent associations with an array of adverse outcomes, low cost, ease of use, and high reliability.(13)
ACKNOWLEDGMENTS
We thank Denise Shepard, BSN, MBA, Shirley Hannan, RN, Andrea Benjamin, BSN, Martha Oravetz, RN, Alice Kossack, Barbara Foster, Shari Lani, Alice Van Wie, and the late Bernice Hebert, RN for assistance with data collection; Evelyne Gahbauer, MD, MPH for data management and programming; Peter Van Ness, PhD, and Lisa Barry, PhD for methodological advice, Wanda Carr and Geraldine Hawthorne for assistance with data entry and management; Peter Charpentier, MPH for development of the participant tracking system; and Joanne McGloin, MDiv, MBA, for leadership and advice as the Project Director.
Funding source: This research was funded by grants from the National Institute on Aging (NIA) (R37AG17560, R01AG022993), and was conducted at Yale's Pepper Center (P30AG21342). Dr. Rothman is a postdoctoral research fellow in geriatrics and clinical epidemiology and is supported by a training grant from the National Institute on Aging (T32AG1934). Dr. Gill is the recipient of a Midcareer Investigator Award in Patient-oriented Research (K24AG021507).
Footnotes
Portions of this work were presented previously at the 2007 Annual Meeting of the American Geriatrics Society.
REFERENCES
- 1.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–389. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 3.Puts MT, Lips P, Deeg DJ. Sex differences in the risk of frailty for mortality independent of disability and chronic diseases. J Am Geriatr Soc. 2005;53:40–47. doi: 10.1111/j.1532-5415.2005.53008.x. [DOI] [PubMed] [Google Scholar]
- 4.Studenski S, Hayes RP, Leibowitz RQ, et al. Clinical Global Impression of Change in Physical Frailty: development of a measure based on clinical judgment. J Am Geriatr Soc. 2004;52:1560–1566. doi: 10.1111/j.1532-5415.2004.52423.x. [DOI] [PubMed] [Google Scholar]
- 5.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women's health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 7.Boyd CM, Xue QL, Simpson CF, et al. Frailty, hospitalization, and progression of disability in a cohort of disabled older women. Am J Med. 2005;118:1225–1231. doi: 10.1016/j.amjmed.2005.01.062. [DOI] [PubMed] [Google Scholar]
- 8.Gill TM, Gahbauer EA, Allore HG, et al. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166:418–423. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- 9.Blaum CS, Xue QL, Michelon E, et al. The association between obesity and the frailty syndrome in older women: the Women's Health and Aging Studies. J Am Geriatr Soc. 2005;53:927–934. doi: 10.1111/j.1532-5415.2005.53300.x. [DOI] [PubMed] [Google Scholar]
- 10.Rockwood K. Frailty and its definition: a worthy challenge. J Am Geriatr Soc. 2005;53:1069–1070. doi: 10.1111/j.1532-5415.2005.53312.x. [DOI] [PubMed] [Google Scholar]
- 11.Gillick M. Pinning down frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M134–M135. doi: 10.1093/gerona/56.3.m134. [DOI] [PubMed] [Google Scholar]
- 12.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 13.Abellan Van Kan G, Rolland Y, Bergman H, et al. Frailty assessment of older people in clinical practice expert opinion of a geriatric advisory panel. J Nutr Health Aging. 2008;12:29–37. doi: 10.1007/BF02982161. [DOI] [PubMed] [Google Scholar]
- 14.Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm--issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62:731–737. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 16.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 17.Buchman AS, Wilson RS, Boyle PA, et al. Change in motor function and risk of mortality in older persons. J Am Geriatr Soc. 2007;55:11–19. doi: 10.1111/j.1532-5415.2006.01032.x. [DOI] [PubMed] [Google Scholar]
- 18.Gill TM, Desai MM, Gahbauer EA, et al. Restricted activity among community-living older persons: incidence, precipitants, and health care utilization. Ann Intern Med. 2001;135:313–321. doi: 10.7326/0003-4819-135-5-200109040-00007. [DOI] [PubMed] [Google Scholar]
- 19.Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 20.Gill TM, Allore HG, Han L. Bathing disability and the risk of long-term admission to a nursing home. J Gerontol A Biol Sci Med Sci. 2006;61:821–825. doi: 10.1093/gerona/61.8.821. [DOI] [PubMed] [Google Scholar]
- 21.Gill TM, Williams CS, Tinetti ME. Assessing risk for the onset of functional dependence among older adults: the role of physical performance. J Am Geriatr Soc. 1995;43:603–609. doi: 10.1111/j.1532-5415.1995.tb07192.x. [DOI] [PubMed] [Google Scholar]
- 22.Gill TM, Richardson ED, Tinetti ME. Evaluating the risk of dependence in activities of daily living among community-living older adults with mild to moderate cognitive impairment. J Gerontol A Biol Sci Med Sci. 1995;50:M235–M241. doi: 10.1093/gerona/50a.5.m235. [DOI] [PubMed] [Google Scholar]
- 23.Pereira MA, FitzerGerald SJ, Gregg EW, et al. A collection of Physical Activity Questionnaires for health-related research. Med Sci Sports Exerc. 1997;29:S1–S205. [PubMed] [Google Scholar]
- 24.Washburn RA, Smith KW, Jette AM, et al. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Kohout FJ, Berkman LF, Evans DA, et al. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 27.Penninx BW, Guralnik JM, Ferrucci L, et al. Depressive symptoms and physical decline in community-dwelling older persons. JAMA. 1998;279:1720–1726. doi: 10.1001/jama.279.21.1720. [DOI] [PubMed] [Google Scholar]
- 28.Hardy SE, Gill TM. Recovery from disability among community-dwelling older persons. JAMA. 2004;291:1596–1602. doi: 10.1001/jama.291.13.1596. [DOI] [PubMed] [Google Scholar]
- 29.Manton KG, Gu X. Changes in the prevalence of chronic disability in the United States black and nonblack population above age 65 from 1982 to 1999. Proc Natl Acad Sci U S A. 2001;98:6354–6359. doi: 10.1073/pnas.111152298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasper J. Using national data on nursing home discharges and long-stay residents to draw implications for nursing home transition programs. Washington, DC: Kaiser Family Foundation; 2005. Who Stays and Who Goes Home. [Google Scholar]
- 31.Rolfson DB, Majumdar SR, Tsuyuki RT, et al. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35:526–529. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kulminski AM, Ukraintseva SV, Akushevich IV, et al. Cumulative index of health deficiencies as a characteristic of long life. J Am Geriatr Soc. 2007;55:935–940. doi: 10.1111/j.1532-5415.2007.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kulminski AM, Ukraintseva SV, Kulminskaya IV, et al. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56:898–903. doi: 10.1111/j.1532-5415.2008.01656.x. PHST-2008/03/21 [aheadofprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62:738–743. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- 35.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardy SE, Perera S, Roumani YF, et al. Improvement in usual gait speed predicts better survival in older adults. J Am Geriatr Soc. 2007;55:1727–1734. doi: 10.1111/j.1532-5415.2007.01413.x. [DOI] [PubMed] [Google Scholar]
- 37.Onder G, Penninx BW, Ferrucci L, et al. Measures of physical performance and risk for progressive and catastrophic disability: results from the Women's Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2005;60:74–79. doi: 10.1093/gerona/60.1.74. [DOI] [PubMed] [Google Scholar]
- 38.Montero-Odasso M, Schapira M, Soriano ER, et al. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2005;60:1304–1309. doi: 10.1093/gerona/60.10.1304. [DOI] [PubMed] [Google Scholar]
- 39.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 40.Inouye SK, Studenski S, Tinetti ME, et al. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007. 2007 May;55(5):780–791. doi: 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 42.Gill TM, Allore HG, Holford TR, et al. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292:2115–2124. doi: 10.1001/jama.292.17.2115. [DOI] [PubMed] [Google Scholar]
- 43.Szklo M. Population-based cohort studies. Epidemiologic reviews. 1998;20:81–90. doi: 10.1093/oxfordjournals.epirev.a017974. [DOI] [PubMed] [Google Scholar]