Abstract
Twin studies from the Minnesota Twin Family Study (MTFS) suggest negligible genetic effects on eating pathology before puberty, but increased genetic effects during puberty. However, an independent study found no pubertal differences in genetic and environmental effects (Rowe et al., 2002). Discrepant results may be due to methodological differences. The MTFS studies divided twins at mid-puberty, while Rowe et al. (2002) divided twins based on menarche alone. We aimed to reconcile discrepant findings by examining differences in etiologic effects for disordered eating attitudes and behaviors (i.e., levels of weight preoccupation, body dissatisfaction, binge eating, compensatory behaviors) using both classification methods in a new sample of 656 female twins. Using the MTFS method, we observed nominal genetic effects in pre-pubertal twins, but significant genetic effects in pubertal and young adult twins. Conversely, genetic effects were moderate and equal in all groups using the Rowe et al. (2002) method. Findings highlight the potentially important role of puberty in the genetic diathesis of disordered eating attitudes and behaviors and the need to use early indicators of pubertal status in studies of developmental effects.
Keywords: eating disorders, anorexia nervosa, bulimia nervosa, twins, genetic
Twin studies have consistently shown that eating disorders and disordered eating symptoms are heritable (e.g., Bulik, Sullivan, Wade, & Kendler, 2000). However, a series of developmental twin studies from the Minnesota Twin Family Study (MTFS) suggest that the magnitude of genetic and environmental effects on disordered eating attitudes and behaviors vary meaningfully across development. Cross-sectional (Klump, McGue, & Iacono, 2000) and longitudinal (Klump, Burt, McGue, & Iacono, 2007a) studies have indicated nominal genetic influences on disordered eating attitudes and behaviors in pre-adolescent twins, yet substantial genetic effects (>50%) during mid-to-late adolescence. Puberty may account for these age differences, given little-to-no genetic influences on disordered eating attitudes and behaviors in pre-pubertal 11 year-old twins, but significant genetic effects (~50%) in pubertal 11 year-old twins (Klump, McGue, & Iacono, 2003; Klump, Perkins, Burt, McGue, & Iacono, 2007b). Interestingly, the degree of genetic influence in the 11 year-old pubertal twins was equal to that of twins in late adolescence (i.e., ages 16–18). These findings suggest that puberty may account entirely for age differences in heritability and may be critical for the genetic diathesis of disordered eating attitudes and behaviors.
Nevertheless, Rowe et al. (2002) conducted the only independent study to date and found no significant differences in the heritability of disordered eating symptoms based on menarcheal status (i.e., presence/absence). The lack of replication could be due to the use of onset of menses as the only marker of pubertal development. Since menarche occurs during the last stage of puberty (Petersen, Crockett, Richards, & Boxer, 1988), the pre-menarche group would have included twins with advanced pubertal development (e.g., in middle), resulting in increases in genetic effects in the pre-menarche group.
The current study sought to reconcile these discrepant findings. Differences in genetic and environmental influences were examined during puberty using both the Klump et al. (2003) and Rowe et al. (2002) classification methods in a new sample of female twins. Replicating the methods of Klump et al. (2003), we tested whether the magnitude of genetic effects on disordered eating attitudes and behaviors increases from pre-puberty to puberty, but remains constant from puberty into late adolescence and early adulthood. Following the methods of Rowe et al. (2002), we tested whether increased genetic effects are observed if twins are recategorized using menarcheal status as the only marker of pubertal development. Importantly, age and body-mass index (BMI) were controlled for in all analyses to ensure that results were not due to these important developmental and physical variables.
Methods
Participants
Participants were 328 (189 MZ; 139 DZ; see Table 1) same-sex female twin pairs ages 10–28 years from the population-based Michigan State University Twin Registry (MSUTR; Klump & Burt, 2006) and the Minnesota Twin Family Study (MTFS; Iacono, Carlson, Taylor, Elkins, & McGue, 1999). Most twins (89.3%) were from the MSUTR (see Table 1). The remaining (10.7%) comprised a new sample of MTFS twins (described in Iacono, McGue, & Krueger, 2006) who were recruited after the original MTFS sample. Importantly, previous analyses of developmental effects did not include MSUTR twins and were conducted on a different subset of MTFS twins (i.e., Klump et al., 2000; Klump et al., 2003; Klump et al., 2007a; Klump et al., 2007b). Thus, none of the twins were included in previous reports, and therefore represent an independent sample of twins.
Table 1. Descriptive Statistics and Twin Correlations by Pubertal Classifications.
| Pubertal Status | Menarcheal Status | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-pubertal Twins |
Pubertal Twins |
Pre-menarche Twins |
Post-menarche Twins |
Young Adult Twins |
|||||||||||
| Statistics and Correlations | Total | MZ | DZ | Total | MZ | DZ | Total | MZ | DZ | Total | MZ | DZ | Total | MZ | DZ |
| Sample Size (N, twin pairs): | |||||||||||||||
| MSUTR | 62 | 33 | 29 | 61 | 33 | 28 | 72 | 40 | 32 | 49 | 26 | 23 | 162 | 96 | 66 |
| MTFS | 22 | 13 | 9 | 10 | 7 | 3 | 29 | 17 | 12 | 5 | 4 | 1 | 0 | 0 | 0 |
| Total | 84 | 46 | 38 | 71 | 40 | 31 | 101 | 57 | 44 | 54 | 30 | 24 | 162 | 96 | 66 |
| Age: | |||||||||||||||
| Range | 10–13 years | 10–15 years | 10–13 years | 10–15 years | 16–28 years | ||||||||||
| Mean (SD) | 11.35 (0.79) | 13.33 (1.42) | 11.39 (0.83) | 13.65 (1.36) | 20.68 (2.45) | ||||||||||
| MEBS Total Score Descriptives: | |||||||||||||||
| Range (maximum score = 30) | 0–19 | 0–22 | 0–19 | 0–22 | 0–29 | ||||||||||
| Mean (SD) | 5.24 (4.53) | 6.47 (5.46) | 5.61 (4.81) | 6.27 (5.34) | 8.00 (6.16) | ||||||||||
| % above MEBS clinical cut-off (i.e. 15) | 4.82% | 11.27% | 6.44% | 10.19% | 17.08% | ||||||||||
| Twin Intraclass Correlations: | |||||||||||||||
| Twin Correlations | rMZ = .36** | rMZ = .72*** | rMZ = .47*** | rMZ = .69*** | rMZ = .58*** | ||||||||||
| rDZ = .30** | rDZ = .32* | rDZ = .21† | rDZ = .43** | rDZ = .20* | |||||||||||
| Test of Equality (z) | 0.42 | 3.33*** | 2.04* | 1.95* | 4.01*** | ||||||||||
Note: MSUTR = Michigan State University Twin Registry; MTFS = Minnesota Twin Family Study; MEBS = Minnesota Eating Behaviors Survey; MZ = monozygotic; DZ = dizygotic. The significant twin correlations observed in all groups suggest that there was sufficient variability to estimate twin correlations.
p < .06
p<.05
p<.01
p<.001
The methods for the MSUTR and MTFS have been reviewed in detail elsewhere (see Iacono et al., 1999; Klump & Burt, 2006). Briefly, twins from the MSUTR were recruited using a variety of methods (e.g., advertisements, university registrar mailings, birth records). Twins from the MTFS were recruited from birth records through the State of Minnesota. Both MSUTR (see Culbert, Breedlove, Burt, & Klump, 2008) and MTFS (see Iacono et al., 1999; Holdcraft & Iacono, 2004) twins have been shown to be demographically representative of their respective recruitment regions.
Zygosity
Zygosity was established using twin physical similarity questionnaires administered to the twins and/or their mothers that are over 95% accurate (Lykken, Bouchard, McGue, & Tellegen, 1990; Peeters, Van Gestel, Vlietinck, Derom, & Derom, 1998). Physical similarity scores were calculated separately for each reporter (e.g., twin, mother; Klump & Burt, 2006; Iacono et al., 1999). Principal investigators (KLK or SAB) of the MSUTR resolved zygosity discrepancies by reviewing item endorsements and photographs of the twins. MTFS zygosity discrepancies were resolved by examining additional zygosity indices (e.g., the ponderal index, cephalic index, fingerprint ridge counts) and serological examination of 12 blood group antigens and protein polymorphisms (Iacono et al., 1999).
Measures
Disordered Eating
Disordered eating attitudes and behaviors were assessed using the same measure as previous MTFS analyses, namely, the total score from the Minnesota Eating Behavior Survey1 (MEBS; von Ranson, Klump, Iacono, & McGue, 2005). The MEBS total score measures disordered eating attitudes and behaviors in terms of body dissatisfaction (i.e., dissatisfaction with one’s body size and/or shape), weight preoccupation (i.e., preoccupation with dieting, weight, and the pursuit of thinness), binge eating (i.e., engaging in episodes of overeating and attitudes conducive to binge eating), and compensatory behavior (i.e., inappropriate compensatory behaviors to control weight, such as self-induced vomiting). Higher scores indicate greater levels of pathological eating attitudes and behavior. Notably, if 10% or fewer of the MEBS items were missing, the total score was prorated (n = 4 twins).
Previous research supports the reliability and validity of this measure for a range of ages (von Ranson et al., 2005), including children and adolescents as young as 10 years old. Coefficient alphas for the MEBS total score were excellent for all pubertal status and menarcheal status groups (α’s ≥ .85). The MEBS total score also has been shown to successfully discriminate between girls with AN and BN from age-matched, non-eating disorder controls (von Ranson et al., 2005).
Pubertal Development
Pubertal and menarcheal status were determined using the Pubertal Development Scale (PDS; Petersen et al., 1988), which was administered to MSUTR and MTFS twins who were 10–15 years old (n = 155 twin pairs). The PDS assesses changes in secondary sex characteristics (i.e., height spurts, appearance of body hair, skin changes, breast development, onset of menses) using a four-point scale: (1) development has not yet begun, (2) development has barely started, (3) development is definitely underway, and (4) development seems completed. Menstruation was dichotomously rated as present or absent. The PDS has demonstrated excellent reliability and validity (Petersen et al., 1988), including good internal consistency in this study (α ≥ .80).
Two types of pubertal categorizations were used, one based on methods of Klump et al. (2003) (i.e., referred to as “pubertal status”) and the other based on Rowe et al. (2002) (i.e., referred to as “menarcheal status”). Following Klump et al. (2003), PDS responses were summed and averaged across all secondary sex characteristics (including menarche). An average score of ≥ 2.5 (i.e., mid-puberty) was used to categorize twins who had started pubertal development, which resulted in 84 versus 71 pairs in the “pre-pubertal” and “pubertal” groups, respectively.
Following Rowe et al. (2002), we categorized twins based on menarcheal status only. Rowe et al (2002) categorized twins in the “post-menarche” group if they had regular menstrual cycles for at least three months; twins who either had not reached menarche or who had less than three regular menstrual cycles were categorized in the “pre-menarche” group. In the current study, data were available on whether twins had reached menarche (including the approximate date of first menstrual cycle), but direct data attesting to the presence of three regular menstrual cycles were not available. Thus, we estimated three regular menstrual cycles using the twin’s reported date of first menses and the day of her assessment. Extant data indicate that the median length of the first menstrual cycle after menarche is 34 days (Diaz, Laufer, & Breech, 2006). Thus, we estimated three regular menstrual cycles (i.e., 34 days * 3 cycles = 102 days) by subtracting the number of days between onset of menses and the day of the assessment. Twins were then categorized using 102 days as the cut-off, with pre-menarche defined as no menses or menses having occurred <102 days before the assessment (n = 202; range of days = 0–91), and post-menarche was defined as menses having occurred >102 days before the assessment (n = 108; range of days = 142–1,727).
Notably, the PDS was not administered to twins over the age of 15, as variability in pubertal development is very low in this group. Indeed, nationally representative data have shown that over 99% of girls over the age of 15 are post-pubertal (Wu, Mendola, & Buck, 2002). Thus, twins over the age of 15 were categorized into the young adult group (n = 162 twin pairs; see Table 1) for both sets of analyses.
Body Mass Index
Body Mass Index (BMI; Weight (in kilograms)/ Height (in meters) squared) was assessed and used as marker of adiposity. Height was measured with a wall mounted scale, and weight was measured with a digital scale.
Statistical Analyses
Since disordered eating attitudes and behaviors scores were positively skewed, they were log transformed (log10 X + 1) prior to analyses. We then regressed out age and BMI from each twin’s disordered eating attitudes and behaviors score prior to analysis to ensure that age did not account for differences in etiologic effects between the three groups.
Initial indications of genetic and environmental effects on disordered eating attitudes and behaviors between groups were examined with twin intraclass correlations. We then fit the same twin constraint models included in Klump et al. (2003) to determine if we could replicate differences in genetic and environmental effects between pubertal and menarcheal status groups. Notably, these models only allow for the inclusion of pairs who are concordant on pubertal or menarcheal status (i.e., both twins are pre-pubertal or pubertal; both twins are pre-menarche or post-menarche). Although other twin models can examine both concordant and discordant pairs (e.g., moderation models, see Purcell, 2002), we were unable to use these models due to relatively small sample sizes in the groups (see Table 1). Nonetheless, only a small number of pairs (n = 44) were discordant for pubertal status or menarcheal status, and there were no significant differences (pubertal status: t(125.73) = −0.80, p = .43; menarcheal status: t(125.34) = −0.56, p = .57) in disordered eating between concordant and discordant twin pairs. Findings are therefore unlikely to be significantly impacted by the exclusion of discordant pairs.
We initially fit the fully constrained model to the data that allowed additive genetic (A; effects that add across genes), shared environmental (C; environmental factors that are shared by reared-together twins and contribute to their behavioral similarity), and nonshared environmental (E; environmental factors that are unique to twins and contribute to behavioral differences) effects to vary between groups. We then fit three submodels that differentially constrained A, C, and E to be equal in: 1) the pre-pubertal and pubertal groups only; 2) the pubertal and young adulthood groups only; and 3) all three groups (i.e., the fully constrained model). The same models were then tested on menarcheal status groups (i.e., we compared the pre-menarche and post-menarche groups only, the post-menarche and young adulthood groups only, and all three groups).
Models were fit to the raw twin observations using the Mx statistical software program (Neale, 1995). Raw data allowed for the inclusion of all twin pairs even if one twin was missing disordered eating data (Little & Rubin, 1987). Model fit was determined using the difference in minus twice the log-likelihood (−2lnLΔ) between the unconstrained model and nested submodels, resulting in a likelihood ratio χ2 test. A non-significant χ2 indicated that the submodel was preferred, since constraining parameters from the model did not result in a significantly worse fit. Additional model fit was determined by the Akaike’s Information Criteria (AIC). The model with the lowest AIC value was considered best-fitting, as AIC examines model fit against parsimony (Plomin, DeFries, McClearn, & McGuffin, 2001).
Importantly, twin models rest on three key assumptions: 1) genetic effects are additive, with genetic correlations for DZ twins that are half those of MZ twins, 2) there is no assortative mating (i.e., spousal similarity), and 3) equal environmental similarity for MZ and DZ twins (i.e., Equal Environments Assumption). It has been customary to assume additivity of genetic effects in the analysis of twin data (Eaves, Eysenck, & Martin, 1989), as it is not possible to estimate nonadditive genetic, additive genetic, and shared environmental effects simultaneously with reared-together twin data. Additive genetic effects are also supported by our twin intraclass correlations (see Results). While assortative mating for disordered eating is theoretically possible, there has been minimal evidence of this for most psychological characteristics (Price & Vandenberg, 1980). Further, if assortative mating did occur, it would attenuate heritability estimates in all groups.
The validity of the Equal Environments Assumption has been well-established for disordered eating. For example, studies have demonstrated that MZ and DZ twins are equally likely to be influenced by the effects of childhood treatment (Bulik, Sullivan, & Kendler, 1998, Kendler, MacLean, Neale, Kessler, Heath, & Eaves, 1991) and their frequency of contact (Kendler et al., 1991; Tholin, Rasmussen, Tynelius, & Karlsson, 2005) on disordered eating. Evidence also suggests that perceived zygosity (i.e., whether twins believe they are MZ or DZ) does not significantly influence twin resemblance for disordered eating, but actual zygosity does (Kendler, Neale, Kessler, Heath, & Eaves, 1993). Nonetheless, one potentially relevant issue for disordered eating is twin physical similarity, as the physical changes that occur during puberty (e.g., breast development, changes in body weight, growth spurts) could result in greater physical similarity of MZ co-twins, relative to DZ co-twins. Greater physical similarity of MZ twins could, in turn, lead to appearance-based reactions (e.g., weight-based teasing; Suisman, Slane, Burt, & Klump, 2008) from others that cause increased similarity in levels of disordered eating in MZ, relative to DZ, twins. Critically, however, previous research has shown that these processes are unlikely to be the present, as the degree of co-twin physical similarity did not predict twin similarity for disordered eating attitudes and behaviors in early (i.e., age 11) or late (i.e., age 17) adolescence (Klump, Holly, Iacono, McGue, & Willson, 2000). Thus, increased physical similarity of MZ relative to DZ twins is unlikely to account for our findings.
Results
Descriptive Statistics
Ample variability in MEBS scores was present in all age groups (see Tables 1 & 2). A significant proportion of participants scored above the mean MEBS total score (score = 15; Minnesota Twin Family Study, unpublished data, 2007) for women with anorexia nervosa or bulimia nervosa. In addition, the frequency of individual item endorsement also suggested that a range of disordered eating attitudes and behaviors are present in all twin groups (see Table 2).
Table 2. MEBS Item Endorsement Frequencies by Twin Group.
| Item Endorsement Frequency (% of n Twins) |
|||||
|---|---|---|---|---|---|
| Pubertal Status | Menarche Status | ||||
| MEBS Item # and Descriptor | Pre-pubertal (n = 168) |
Pubertal (n = 142) |
Pre-Menarche (n = 202) |
Post-Menarche (n = 108) |
Young Adults (n = 324) |
| Binge Eating | |||||
| 4. Eating in response to emotions | 14.7 | 25.6 | 14.4 | 28.7 | 40.1 |
| 6. Stuffs self with food | 39.5 | 36.1 | 39.6 | 35.2 | 42.3 |
| 15. Eats a large amount of food with a lack of control | 19.2 | 24.1 | 21.3 | 21.3 | 14.8 |
| 19. Frequently thinks about binge eating | 16.4 | 17.3 | 17.3 | 15.7 | 10.8 |
| 23. Restricts food around others, binges when alone | 18.6 | 25.6 | 20.3 | 24.1 | 16.0 |
| 27. Eats alone | 6.2 | 9.8 | 6.4 | 10.2 | 9.6 |
| 28. Afraid will eat when sad | 6.8 | 6.0 | 6.4 | 6.5 | 12.3 |
| Body Dissatisfaction | |||||
| 3. Stomach is too large | 16.4 | 22.6 | 17.3 | 22.2 | 44.4 |
| 8. Thighs are too large | 16.9 | 23.3 | 18.8 | 21.3 | 47.5 |
| 12. Dislikes body shape | 15.3 | 29.3 | 17.8 | 27.8 | 46.9 |
| 16. Buttocks are too large | 5.1 | 18.0 | 8.4 | 14.8 | 32.7 |
| 18. Wishes to be thinner | 31.1 | 41.4 | 34.2 | 38.0 | 45.4 |
| 22. Dislikes hip size | 11.9 | 21.1 | 13.4 | 20.4 | 41.4 |
| Compensatory Behaviors | |||||
| 5. Thinks about trying self-induced vomiting | 1.1 | 8.3 | 2.0 | 8.3 | 21.9 |
| 9. Stops eating for > 1 day | 2.8 | 5.3 | 3.0 | 5.6 | 6.5 |
| 13. Uses laxatives to lose/maintain weight | 1.7 | 1.5 | 2.5 | 0.0 | 1.9 |
| 17. Uses diet pills to lose/maintain weight | 0.6 | 0.0 | 0.5 | 0.0 | 9.9 |
| 26. Uses self-induced vomiting to lose/maintain weight | 0.6 | 3.0 | 1.5 | 1.9 | 3.1 |
| 30. Uses diuretics to lose/maintain weight | 1.7 | 0.8 | 2.0 | 0.0 | 2.2 |
| Weight Preoccupation | |||||
| 2. Frequently diets | 15.3 | 18.0 | 15.8 | 17.6 | 25.3 |
| 7. Frequently thinks about dieting or weight loss | 20.3 | 36.1 | 24.8 | 31.5 | 52.2 |
| 10. Feels guilty about overeating | 22.6 | 30.1 | 25.7 | 25.9 | 33.0 |
| 11. Fears gaining weight | 27.7 | 42.1 | 30.7 | 39.8 | 44.4 |
| 14. Places undue importance on body weight | 55.9 | 62.4 | 56.9 | 62.0 | 55.6 |
| 24. Feels fat after eating a normal amount of food | 26.6 | 21.8 | 28.2 | 17.6 | 18.2 |
| 25. Worries about weight gain | 15.3 | 25.6 | 17.8 | 23.1 | 24.7 |
| 29. Frequently weighs self | 26.0 | 28.6 | 27.2 | 26.9 | 29.0 |
| Additional Items Included in Total Score Only | |||||
| 1. Worries after eating sweets or starches | 14.7 | 11.3 | 14.4 | 11.1 | 33.3 |
| 20. Difficulty perceiving hunger | 44.1 | 51.1 | 46.0 | 49.1 | 32.7 |
| 21. Engages in excessive exercise | 17.0 | 18.0 | 18.3 | 16.7 | 16.4 |
Note: MEBS = Minnesota Eating Behaviors Survey. Only MEBS item descriptors (not the actual items) are presented above. The Minnesota Eating Behavior Survey (previously known as the Minnesota Eating Disorder Inventory) was adapted and reproduced by special permission of Psychological Assessment Resources, Inc., 16204 North Florida Avenue, Lutz, Florida 33549, from the Eating Disorder Inventory (collectively, EDI and EDI-2) by Garner, Olmstead, Polivy, Copyright 1983 by Psychological Assessment Resources, Inc. Further reproduction of the MEBS is prohibited without prior permission from Psychological Assessment Resources, Inc.
Nonetheless, and corroborating previous research (e.g., Garber, Brooks-Gunn, Paikoff, & Warren, 1994; Klump et al., 2003), significant mean differences (see Table 1) in disordered eating attitudes and behaviors were observed between twin groups using both methods of pubertal classification [F(2, 630) = 13.77, p < .001 for pubertal status; F(2, 630) = 8.03, p < .001 for menarcheal status]. Contrast analyses confirmed the presence of linear trends [F(1, 627) = 26.35, p < .001 for pubertal status; F(1, 627) = 15.10, p < .001 for menarcheal status], with increasing levels of disordered eating attitudes and behaviors between the three groups. Not surprisingly, however, these mean differences were no longer significant when age and BMI were regressed out [F(2, 630) = 0.11, p = .90 for pubertal status; F(2, 630) = 0.35, p = .71 for menarcheal status].
Twin Correlations
Twin intraclass correlations, calculated using age and BMI regressed scores, differed depending on classification method. Similar to Klump et al. (2003), the MZ and DZ twin correlations were similar in magnitude in the pre-pubertal group, whereas MZ twin correlations were greater than DZ twin correlations in the pubertal, and young adult groups (see Table 1). In contrast, when menarcheal status was used, findings replicated those of Rowe et al. (2002) as MZ twin correlations were significantly greater than DZ twin correlations in all three groups: pre-menarche, post-menarche, and young adult twins (see Table 1). Importantly, because mean levels of disordered eating attitudes and behaviors increased between groups using both categorization methods (see above and Table 1), mean increases cannot account for our findings either within, or across, methods. This is not surprising, given that twin correlations are based on the degree to which co-twins within a pair show similarity on levels of disordered eating attitudes and behaviors. As long as there is sufficient variability to calculate a correlation (i.e., correlations are non-zero; see Table 1), twin covariation for a trait is largely independent of mean levels (i.e., a high correlation would result if co-twins both score low or high). Taken together, despite similar mean level increases in disordered eating attitudes and behaviors, patterns of genetic and environmental effects differ depending on the pubertal categorization type (i.e., pubertal status vs. menarche status).
Twin Constraint Models
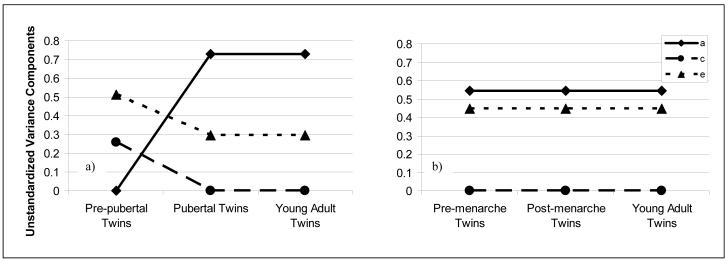
The twin constraint models confirmed impressions of differential etiologic effects by method (see Table 3). Using the methods of Klump et al. (2003), parameter estimates from the fully unconstrained model suggested no genetic influence in pre-pubertal pairs, but the presence of genetic effects in pubertal and young adult twins. Shared and nonshared environmental influences appeared to show the opposite pattern of results, with decreasing influence from prepuberty to puberty. Comparisons between the fully unconstrained model and nested submodels confirmed the presence of significant differences in etiologic effects during puberty. The best fitting model was one in which A, C, and E were allowed to vary in the pre-pubertal group, but were constrained to be equal in the pubertal and young adult groups. This model had the lowest AIC and exhibited a non-significant change in chi-square as compared to the fully unconstrained model (see Table 3). Standardized (or proportional; see Table 3) and unstandardized (or absolute; see Figure 1) parameter estimates from this model showed that genetic influences on disordered eating attitudes and behaviors were negligible (0%) in pre-pubertal twins, where shared and non-shared environmental influences predominated. By contrast, genetic effects on disordered eating attitudes and behaviors were substantial (60%) and equal in pubertal and young adult twins, with the remaining variance accounted for by nonshared environmental effects (40%). Importantly, parameter estimates from this model were nearly identical to those in Klump et al. (2003) who also found that the best fitting model was one which parameters were constrained between pubertal and young adult groups only.
Table 3. Standardized Parameter Estimates and Test Statistics for ACE Constraint Models.
| Categorization Method: Pubertal Status (Klump et al. (2003)method) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Models | Pre-pubertal Twins (N=166; 83 pairs) |
Pubertal Twins (N = 142; 71 pairs) |
Young Adult Twins (N = 324; 162 pairs) |
||||||||||
| A | C | E | A | C | E | A | C | E | −2lnL (df) |
−2lnLΔ (df) |
p | AIC | |
| Fully Unconstrained |
.00 (.00–.52) |
.34 (.00–.51) |
.66 (.47–.87) |
.71 (.17–.82) |
.00 (.00–.48) |
.29 (.18–.47) |
.56 (.21–.68) |
.00 (.00–.30) |
.44 (.33–.58) |
1695.554 (618) |
-- | -- | 459.554 |
| Constrain Pre-pubertal & Pubertal |
.35 (.00–.64) |
.17 (.00–.53) |
.48 (.36–.64) |
-- | -- | -- | .56 (.21–.67) |
.00 (.00–.30) |
.44 (.33–.58) |
1704.036 (621) |
8.482 (3) |
.04 | 462.036 |
|
Constrain Pubertal & Adult |
.00 (.0–.52) |
.34 (.00–.51) |
.66 (.47–.87) |
.60 (.32–.69) |
.00 (.00–.25) |
.40 (.31–.50) |
-- | -- | -- |
1698.988 (621) |
3.434 (3) |
.33 | 456.988 |
| Fully Constrained | .55 (.21–.63) |
.00 (.00–.30) |
.45 (.37–.55) |
-- | -- | -- | -- | -- | -- | 1708.034 (624) |
12.48 (6) |
.05 | 460.034 |
| Categorization Method: Menarcheal Status (Rowe et al. (2002)method) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-menarche Twins (N=202; 101 pairs) |
Post-menarche Twins (N = 108; 54 pairs) |
Young Adult Twins (N = 324; 162 pairs) |
|||||||||||
| A | C | E | A | C | E | A | C | E | −2lnL (df) |
−2lnLΔ (df) |
p | AIC | |
| Fully Unconstrained |
.39 (.00–.60) |
.04 (.00–.50) |
.56 (.40–.78) |
.51 (.00–.81) |
.18 (.00–.67) |
.32 (.18–.55) |
.56 (.21–.68) |
.00 (.00–.30) |
.44 (.32–.58) |
1700.581 (618) |
-- | -- | 464.581 |
| Constrain Pre-menarche & Post-menarche |
.40 (.00–.65) |
.12 (.00–.52) |
.47 (.35–.63) |
-- | -- | -- | .56 (.21–.68) |
.00 (.00–.30) |
.44 (.32–.58) |
1704.275 (621) |
3.694 (3) |
.30 | 462.275 |
| Constrain Post-menarche & Adult |
.39 (.00–.60) |
.04 (.00–.50) |
.56 (.40–.78) |
.59 (.25–.69) |
.00 (.00–.30) |
.41 (.31–.52) |
-- | -- | -- | 1703.281 (621) |
2.70 (3) |
.44 | 461.281 |
|
Fully Constrained |
.55 (.23–.63) |
.00 (.00–.28) |
.45 (.37–.55) |
-- | -- | -- | -- | -- | -- |
1707.800 (624) |
7.219 (6) |
.30 | 459.800 |
Note: A = additive genetic effects; C = shared environmental effects; E = non-shared environmental effects; AIC = Akaike’s Information Criteria; −2lnL = minus 2 times the log likelihood; −2lnLΔ = differences in −2lnL values between model 1 and each of the constrained models. Pre-pubertal = pre-pubertal group; Pubertal = pubertal group; Adult = young adult group; Pre-menarche = pre-menarche group; Post-menarche = post-menarche group. Standardized parameter estimates are listed in columns labeled A, C, E with the 95% confidence intervals within parentheses. Dashed lines for constrained models indicate that parameter estimates were constrained to be equal to those for the preceding group. The best-fitting model is noted in bolded text.
Figure 1.
Unstandardized Estimates of Additive Genetic, Shared Environmental, and Nonshared Environmental Factors from the Best-Fitting Models: a) Pubertal Status, b) Menarcheal Status. a = additive genetic effects; c = shared environment; e = nonshared environment.
In contrast to these results, constraint models suggested similar genetic and environmental influences on disordered eating attitudes and behaviors during pre-menarche, post-menarche, and young adulthood using the Rowe et al. (2002) method (see Table 3). Parameter estimates from the fully unconstrained and best fitting (i.e., the fully constrained model; lowest AIC and non-significant change in chi-square) models suggested substantial and similar genetic influences on disordered eating attitudes and behaviors in pre-menarche, postmenarche, and young-adult twin pairs, with the remaining variance largely accounted for by nonshared environmental influences (see Table 3; Figure 1). Notably, parameter estimates from the best-fitting model (genetic = 55%; nonshared environment = 45%) were strikingly similar to those reported by Rowe et al. (2002) and indicative of no differences in genetic and environmental influences on disordered eating attitudes and behaviors when menarche is used as the sole index of pubertal development. Importantly, identical results were obtained when the presence/absence of menses alone (i.e., no requirement of at least three regular menstrual cycles) was used to categorize twins as “post-menarche” (data not shown). These findings indicate that the use of onset of menses without any requirement of regular cycles would also miss important pubertal differences in genetic effects.
Discussion
The current study sought to reconcile discrepant findings regarding pubertal moderation of genetic effects on disordered eating attitudes and behaviors. Results demonstrated that differences in genetic effects vary as a function of pubertal classification methods. When several secondary sex characteristics are used to categorize twins, findings replicate those of Klump et al. (2003) and show significant increases in genetic effects from pre-puberty to puberty, with no additional increases from puberty through young adulthood. By contrast, when menarche is used as the sole marker of puberty, findings are identical to those of Rowe et al. (2002) and show substantial and equal genetic effects on disordered eating attitudes and behaviors in premenarche, post-menarche, and young adult groups. We conclude that puberty may indeed be a critical time for the genetic diathesis of disordered eating attitudes and behaviors, but that studies examining later stages of puberty may miss these differences in genetic effects.
Moving forward, it will be important to understand possible mechanisms (e.g., psychosocial, neurobiological) underlying these effects. Our findings suggest that factors underlying these effects occur prior to late puberty. Although it is unclear what these factors might be, extant data has shown that early-to-mid-puberty is marked by several changes in psychosocial (e.g., development of autonomy, socially induced awareness of gender or sexuality), psychological (e.g., internalization of the thin ideal), and physical (e.g., body shape and weight) factors, that are associated with disordered eating (Jacobi et al., 2004; Smolak & Levine, 1996) and could be linked to pubertal changes in genetic effects. Importantly, however, while these factors may lead to increases in mean levels of disordered eating attitudes and behaviors, it is unlikely that they operate alone to account for differences in genetic effects. As noted above, estimates of genetic and environmental effects are based on differences in MZ and DZ co-twin similarity for disordered eating attitudes and behaviors. Thus, factors underlying the effects of puberty must differentially affect MZ versus DZ twin resemblance for disordered eating. Given that it is unclear how or why factors such as the internalization of the thin ideal would differentially affect twin resemblance in MZ versus DZ twins, other explanations for these effects may be more plausible.
One possibility is that these psychosocial and psychological factors influence genetic effects during puberty through genotype-environment interactions and/or active genotype-environment correlations. Genotype-environment interactions occur when environmental factors lead to the phenotypic expression of a disorder only in the presence of genetic risk factors (Moffitt, Caspi, & Rutter, 2005). For example, thin-ideal internalization may increase risk for disordered eating pathology, but only for individuals with a genetic susceptibility for eating pathology. Importantly, the presence of gene-environment interactions can increase estimates of genetic effects in twin studies, but only if the environmental factor is shared by co-twins (i.e., they are shared environmental effects). In these cases, the effects of the gene-environment interaction are partitioned into the additive genetic estimate and thus, could account for increases in genetic influences during puberty.
To date, no study has directly investigated gene-shared environment interactions for eating pathology during puberty (although see Racine, Culbert, Larson, & Klump, in press, for a study in adulthood). However, several of the psychological, psychosocial, and physical changes described above could operate in this way to increase genetic estimates during puberty. For example, internalization of the thin-ideal becomes more relevant for disordered eating during puberty given coinciding body and shape changes (Hermes & Keel, 2003). Increasing thin-ideal internalization during puberty may potentiate genetic susceptibility for disordered eating, and consequently, increase genetic effects. In addition, biological changes that are common amongst twins during puberty could contribute. Ovarian hormones are one set of candidates in this regard, as they become prominent in all girls during puberty, they are associated with direct changes in disordered eating symptoms (e.g., binge eating; Blaustein & Wade, 1976; Edler, Lispon, & Keel, 2007; Klump, Keel, Culbert, & Edler, 2008), and they regulate gene transcription within neurotransmitter systems disrupted in eating disorders (e.g., serotonin; Ostlund, Keller, & Hurd, 2003). Thus, it is possible that the activation of ovarian hormones at puberty may result in differential regulation of genetic risk for disordered eating and increased genetic effects (Klump & Culbert, 2007).
In addition to genotype-environment interactions, active genotype-environment correlations may also play a role in the moderating effects of puberty (Klump et al., 2000). Active gene-environment correlations occur when individuals actively create environments that are correlated with their genotype (Scarr & McCartney, 1983). Within twin models, gene-environment correlations are also partitioned into the additive genetic variance, and thus, could account for increases in genetic effects during puberty. In the case of disordered eating, genetic influences on disordered eating may increase during puberty when adolescent girls gain more autonomy. For example, girls with high genetic susceptibility towards body and weight concerns may begin to actively select environments (e.g., weight-focused peers or sports activities) and environmental stimuli (e.g., fashion magazines) consistent with their genetic predispositions (Klump et al., 2000). Such environmental “primers” would enhance genetic predispositions for disordered eating, and consequently increase genetic influences on disordered eating with age and pubertal development. Unfortunately, no studies to date have examined gene-environment correlations for disordered eating, although novel methods have now been developed to do so (Purcell, 2002; Burt, 2008). Clearly, studies directly examining gene-environment interactions and correlations during puberty are needed to better understand the mechanisms underlying the effects of puberty on genetic risk.
Before ending, three key study limitations must be noted. First, our sample was relatively small, resulting in broad confidence intervals for some parameter estimates (e.g., those for shared environmental effects; see Table 3) and potentially lower power to detect differences between models. However, parameter estimates from the reduced models were very similar to the A,C, E estimates from the fully unconstrained models, suggesting that observed effects are not merely the results of constraining parameters. Our findings were also highly similar to those observed in Klump et al. (2003) and Rowe et al. (2002). Nonetheless, future research would benefit from larger samples in order to more fully investigate developmental differences in pubertal effects.
Second, we used a measure of disordered eating attitudes and behaviors rather than eating disorder diagnoses. Given the low prevalence of eating disorders in pre-adolescence, it would have been extremely difficult to examine our developmental hypotheses in a clinical sample. Moreover, given that our aim was to reconcile past discrepant findings, we felt it was important to examine disordered eating continuously in a manner that was similar to Klump et al. (2003) and Rowe et al. (2002). Our measure (i.e., the MEBS) was the same instrument used in previous developmental twin studies from the MTFS (e.g., Klump et al., 2003; Klump et al., 2007a; Klump et al., 2007b), and it shows excellent psychometric properties (von Ranson et al., 2005). Ideally, we would have also examined the same measure as Rowe et al. (2002), but such data were not available. Fortunately, despite different assessment methods, our menarcheal status findings were essentially identical to those of Rowe et al. (2002), suggesting that results are not measure-specific but apply to a range of disordered eating. Replications using other instruments will be important for confirming these impressions. Data thus far are promising, as a recent study (Klump et al., submitted) found similar types of developmental differences in genetic and environmental effects using the Eating Disorders Examination Questionnaire (EDE-Q; Fairburn & Beglin, 1994).
Finally, our study was cross-sectional. We therefore could not examine whether developmental differences actually reflect within-twin pair differences in genetic and environmental influences on disordered eating across puberty. Our findings are consistent with a previous longitudinal study suggesting similar types of developmental differences in genetic and environmental effects (Klump et al., 2007a), yet no previous research has investigated longitudinal changes across puberty or menarche. Longitudinal data will be critical for further characterizing these developmental trajectories.
Despite the limitations of our study, our findings confirm the presence of developmental differences in genetic effects on disordered eating attitudes and behaviors and highlight puberty as a critical stage in the etiology of eating disorders. Future studies should build upon these findings by examining potential mechanisms (e.g., gene-environment interactions and correlations) underlying developmental differences and individual differences in risk for eating pathology. It will be imperative for these studies to use early indicators of pubertal development in order to capture these important effects.
ACKNOWLEDGMENTS
Funding/Support: The research was supported by grants from the National Institute of Mental Health (1F31-MH084470) awarded to Ms. Culbert, the National Institute of Mental Health (1R21 MH070542-01; 1R03 MH63851-01) awarded to Dr. Klump, the Michigan State University Intramural Grants Program (71-IRGP-4831) awarded to Dr. Klump, and the National Institute on Drug Abuse (R01 DA13240) awarded to Drs. McGue and Iacono.
Footnotes
The Minnesota Eating Behavior Survey (previously known as the Minnesota Eating Disorder Inventory) was adapted and reproduced by special permission of Psychological Assessment Resources, Inc., 16204 North Florida Avenue, Lutz, Florida 33549, from the Eating Disorder Inventory (collectively, EDI and EDI-2) by Garner, Olmstead, Polivy, Copyright 1983 by Psychological Assessment Resources, Inc. Further reproduction of the MEBS is prohibited without prior permission from Psychological Assessment Resources, Inc.
Parts of this manuscript were presented at the Behavioral Genetics Association meeting, Louisville, Kentucky, June 25-June 28, 2008.
References
- Blaustein J, Wade G. Ovarian influences on the meal patterns of female rats. Physiology and Behavior. 1976;17:201–208. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- Bulik CM, Sullivan PF, Kendler KS. Heritability of binge-eating and broadly-defined bulimia nervosa. Biological Psychiatry. 1998;44:1210–1218. doi: 10.1016/s0006-3223(98)00280-7. [DOI] [PubMed] [Google Scholar]
- Bulik CM, Sullivan PF, Wade TD, Kendler KS. Twin studies of eating disorders: a review. International Journal of Eating Disorders. 2000;27:1–20. doi: 10.1002/(sici)1098-108x(200001)27:1<1::aid-eat1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Burt SA. Genes and popularity: Evidence of an evocative gene-environment correlation. Psychological Science. 2008;19:112–113. doi: 10.1111/j.1467-9280.2008.02055.x. [DOI] [PubMed] [Google Scholar]
- Culbert KM, Breedlove SM, Burt SA, Klump KL. Prenatal hormone exposure and risk for eating disorders: a comparison of opposite sex and same sex twins. Archives of General Psychiatry. 2008;65:329–336. doi: 10.1001/archgenpsychiatry.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz A, Laufer MR, Breech LL. Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Pediatrics. 2006;118:2245–50. doi: 10.1542/peds.2006-2481. [DOI] [PubMed] [Google Scholar]
- Eaves LJ, Eysenck HJ, Martin NG. Genes, culture, and personality: an empirical approach. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychological Medicine. 2007;37:131–41. doi: 10.1017/S0033291706008956. [DOI] [PubMed] [Google Scholar]
- Garber J, Brooks-Gunn J, Paikoff R, Warren M. Prediction of eating problems: an 8-year study of adolescent girls. Developmental Psychology. 1994;30:823–834. [Google Scholar]
- Hermes SF, Keel PK. The influence of puberty and ethnicity on awareness and internalization of the thin ideal. Internationl Journal of Eating Disorders. 2003;33:465–467. doi: 10.1002/eat.10169. [DOI] [PubMed] [Google Scholar]
- Holdcraft LC, Iacono WG. Cross-generational effects on gender differences in psychoactive drug abuse and dependence. Drug and Alcohol Dependence. 2004;74:147–158. doi: 10.1016/j.drugalcdep.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance use disorders: findings from the Minnesota Twin Family Study. Development and Psychopathology. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Iacono WG, McGue M, Krueger R. Minnesota center for twin and family research. Twin Research and Human Genetics. 2006;9:978–984. doi: 10.1375/183242706779462642. [DOI] [PubMed] [Google Scholar]
- Jacobi C, Hayward C, de Zwaan M, Kraemer HC, Agras WS. Coming to terms with risk factors for eating disorders: application of risk terminology and suggestions for a general taxonomy. Psychological Bulletin. 2004;130:19–65. doi: 10.1037/0033-2909.130.1.19. [DOI] [PubMed] [Google Scholar]
- Kendler KS, MacLean C, Neale MC, Kessler RC, Heath AC, Eaves LJ. The genetic epidemiology of bulimia nervosa. American Journal of Psychiatry. 1991;148:1627–1635. doi: 10.1176/ajp.148.12.1627. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A test of the Equal Environment Assumption in twin studies of psychiatric illness. Behavior Genetics. 1993;23:21–27. doi: 10.1007/BF01067551. [DOI] [PubMed] [Google Scholar]
- Klump KL, Burt SA. The Michigan State University Twin Registry (MSUTR): genetic, environmental, and neurobiological influences on behavior across development. Twin Research and Human Genetics. 2006;9:971–977. doi: 10.1375/183242706779462868. [DOI] [PubMed] [Google Scholar]
- Klump KL, Burt SA, McGue M, Iacono WG. Changes in genetic and environmental influences on disordered eating across adolescence: a longitudinal twin study. Archives of General Psychiatry. 2007a;64:1409–1415. doi: 10.1001/archpsyc.64.12.1409. [DOI] [PubMed] [Google Scholar]
- Klump KL, Burt SA, Spanos A, McGue M, Iacono WG, Wade T. Genetic and environmental influences on key eating disorder symptoms: developmental differences from childhood through middle adulthood for weight and shape concerns. submitted. [Google Scholar]
- Klump KL, Culbert KM. Molecular genetic studies of eating disorders: current status and future directions. Current Directions in Psychological Science. 2007;16:37–41. doi: 10.1111/j.1467-8721.2007.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Holly A, Iacono WG, McGue M, Willson L. Physical similarity and twin resemblance for eating attitudes and behaviors: a test of the Equal Environments Assumption. Behavior Genetics. 2000;30:51–58. doi: 10.1023/a:1002038610763. [DOI] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Culbert KM, Edler C. Ovarian hormones and binge eating: exploring phenotypic associations in community samples. Psychological Medicine. 2008;38:1749–1757. doi: 10.1017/S0033291708002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, McGue M, Iacono WG. Age differences in genetic and environmental influences on eating attitudes and behaviors in preadolescent and adolescent female twins. Journal of Abnormal Psychology. 2000;109:239–251. [PubMed] [Google Scholar]
- Klump KL, McGue M, Iacono WG. Differential heritability of eating pathology in pre-pubertal versus pubertal twins. International Journal of Eating Disorders. 2003;33:287–292. doi: 10.1002/eat.10151. [DOI] [PubMed] [Google Scholar]
- Klump KL, Perkins PS, Burt SA, McGue M, Iacono WG. Puberty moderates genetic influences on disordered eating. Psychological Medicine. 2007b;37:627–634. doi: 10.1017/S0033291707000189. [DOI] [PubMed] [Google Scholar]
- Little RTA, Rubin DB. Statistical Analysis with Missing Data. Wiley; New York: 1987. [Google Scholar]
- Lykken DT, Bouchard TJ, McGue M, Tellegen A. The Minnesota Twin Family Registry: some initial findings. Acta Gemellogicae et Medicae. 1990;39:35–70. doi: 10.1017/s0001566000005572. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Archives of General Psychiatry. 2005;62:473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- Neale MC. Mx: Statistical Modeling. 3rd edition Department of Psychiatry; Richmond, VA 23298: 1995. [Google Scholar]
- Ostlund H, Keller E, Hurd Y. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Annals of the New York Academy of Sciences. 2003;1007:54–63. doi: 10.1196/annals.1286.006. [DOI] [PubMed] [Google Scholar]
- Peeters H, Van Gestel S, Vlietinck R, Derom C, Derom R. Validation of a telephone zygosity questionnaire in twins of known zygosity. Behavior Genetics. 1998;28:159–161. doi: 10.1023/a:1021416112215. [DOI] [PubMed] [Google Scholar]
- Petersen A, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status - reliability, validity, and initial norms. Journal of Youth Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral Genetics. 4th edition Worth Publishers; New York, NY: 2001. [Google Scholar]
- Price RA, Vandenberg SG. Spouse similarity in American and Swedish couples. Behavior Genetics. 1980;10:59–71. doi: 10.1007/BF01067319. [DOI] [PubMed] [Google Scholar]
- Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Research. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Racine SE, Culbert KM, Larson CL, Klump KL. The moderating effects of impulsivity and dietary restraint on associations between serotonin genes and binge eating. Psychiatric Research. doi: 10.1016/j.jpsychires.2009.05.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe R, Pickles A, Simonoff E, Bulik CM, Silberg JL. Bulimic symptoms in the Virginia Twin Study of Adolescent Behavioral Development: correlates, comorbidity, and genetics. Biological Psychiatry. 2002;51:172–182. doi: 10.1016/s0006-3223(01)01257-4. [DOI] [PubMed] [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: a theory of genotype → environment effects. Child Development. 1983;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Smolak L, Levine MP. Adolescent transitions and the development of eating problems. In: Smolak L, Levine MP, Streigel-Moore R, editors. The Developmental Psychopathlogy of Eating Disorders: Implications for Research, Prevention, and Treatment. Erlbaum; Mahwah, NJ: 1996. pp. 207–233. [Google Scholar]
- Suisman JL, Slane JD, Burt SA, Klump KL. Negative affect as a mediator of the relationship between weight-based teasing and binge eating in adolescent girls. Eating Behaviors. 2008;9:493–496. doi: 10.1016/j.eatbeh.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholin S, Rasmussen F, Tynelius P, Karlsson J. Genetic and environmental influences on eating behavior: the Swedish Young Male Twins Study. American Journal of Clinical Nutrition. 2005;81:564–569. doi: 10.1093/ajcn/81.3.564. [DOI] [PubMed] [Google Scholar]
- von Ranson KM, Klump KL, Iacono WG, McGue M. The Minnesota Eating Behavior Survey: a brief measure of disordered eating attitudes and behaviors. Eating Behaviors. 2005;6:373–392. doi: 10.1016/j.eatbeh.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Wu T, Mendola P, Buck G. Ethnic differences in the presence of secondary sex characteristics and menarche among US girls: the third national health and nutrition examination survey, 1988-1994. Pediatrics. 2002;110:752–757. doi: 10.1542/peds.110.4.752. [DOI] [PubMed] [Google Scholar]