Abstract
Complete consensus genome sequences were determined for avian paramyxovirus type 8 (APMV-8) strains goose/Delaware/1053/76 (prototype strain) and pintail/Wakuya/20/78. The genome of each strain is 15,342 nucleotides (nt) long, which follows the “rule of six”. The genome consists of six genes in the order of 3′-N-P/V/W-M-F-HN-L-5′. The genes are flanked on either side by conserved transcription start and stop signals, and have intergenic regions ranging from 1 to 30 nt. The genome contains a 55 nt leader region at the 3′-end and a 171 nt trailer region at the 5′-end. Comparison of sequences of strains Delaware and Wakuya showed nucleotide identity of 96.8% at the genome level and amino acid identities of 99.3%, 96.5%, 98.6%, 99.4%, 98.6% and 99.1% for the predicted N, P, M, F, HN and L proteins, respectively. Both strains grew in embryonated chicken eggs and in primary chicken embryo kidney cells, and 293T cells. Both strains contained only a single basic residue at the cleavage activation site of the F protein and their efficiency of replication in vitro depended on and was augmented by, the presence of exogenous protease in most cell lines. Sequence alignment and phylogenic analysis of the predicted amino acid sequence of APMV-8 strain Delaware proteins with the cognate proteins of other available APMV serotypes showed that APMV-8 is more closely related to APMV-2 and -6 than to APMV-1, -3 and -4.
Keywords: APMV-8, Delaware strain, Wakuya strain, Avulavirus, Paramyxoviridae, Complete genome sequence
1. Introduction
Paramyxoviruses are single-stranded, negative-sense, RNA viruses that are large (150–300 nm), enveloped and pleomorphic. Paramyxoviruses have been isolated from and implicated in many disease conditions across ecologically diverse species of animals, fish, birds, and humans (Lamb and Parks, 2007; Nylund et al., 2008). These viruses are grouped under the order Mononegavirales in the family Paramyxoviridae, which includes two subfamilies, Paramyxovirinae and Pneumovirinae. The subfamily Paramyxovirinae is further divided into five genera: Morbillivirus (includes measles [MeV] and canine distemper [CDV] viruses), Rubulavirus (mumps virus and human parainfluenza virus [HPIV-2]), Respirovirus (Sendai virus [SeV] and HPIV-1), Henipavirus (Hendra virus [HeV] and Nipah virus [NiV]) and Avulavirus (Newcastle disease virus (NDV) and other avian paramyxoviruses [APMVs]). The subfamily Pneumovirinae comprises two genera: Pneumovirus (human and bovine respiratory syncytial virus [HRSV and BRSV]), and Metapneumovirus (human and avian metapneumoviruses [HMPV and AMPV]) (Lamb et al., 2005; Mayo and Pringle, 1998).
The genomes of all paramyxoviruses range from 15 to 19 kb and contain 6–10 genes that code for up to 12 different proteins (Lamb and Parks, 2007). Efficient genome replication for the members of subfamily Paramyxovirinae depends on the total genome length being an even multiple of six, known as ‘rule of six’ (Calain and Roux, 1993; Kolakofsky et al., 1998). The genome termini consist of extragenic regions, called 3′-leader and 5′-trailer, which contain conserved promoter sequences involved in RNA replication and genome transcription. Each gene starts with a conserved gene start (GS) sequence and ends with a conserved gene end (GE) sequence. Transcription begins at the 3′-leader region and proceeds in a sequential manner by a start–stop mechanism using GS and GE signals (Lamb and Parks, 2007). Between the gene boundaries, there are non-coding intergenic sequences (IGS).
All members of the family Paramyxoviridae studied to date encode a major nucleocapsid protein (N) that binds the entire length of the genomic and antigenomic RNAs, a nucleocapsid-associated polymerase co-factor called phosphoprotein (P), a major polymerase protein (L) that contains catalytic domains, a matrix protein (M) that lines the inner surface of the virus envelope and is involved in viral morphogenesis, a fusion glycoprotein (F) that is a surface antigen that mediates viral penetration and syncytium formation and whose functional activity depends on host protease cleavage into F1 and F2 subunits, and a major glycoprotein (G) or hemagglutinin-neuraminidase (HN) glycoprotein that is a second surface antigen and mediates attachment (Lamb and Parks, 2007). Most members of subfamily Paramyxovirinae engage in RNA editing, whereby an “editing” motif, located midway along the P gene, directs the non-templated insertion of one or more guanine nucleotides into a proportion of P transcripts during mRNA synthesis. P gene editing shifts the reading frame to access one or more internal open reading frames (ORFs), resulting in mRNAs that encode chimeric proteins in which the N-terminal domain is that of the P protein and the C-terminal is derived from the alternative ORF. For viruses of Respirovirus, Avulavirus, Morbillivirus, and Henipavirus, the addition of one G results in expression of the V protein, which contains a C-terminal domain with a highly conserved cysteine-rich sequence. The addition of two G residues results in expression of the W protein. However, for members of Rubulavirus, it is the unedited mRNA that encodes V, while an mRNA with an insertion of two G residues encodes P (Lamb and Parks, 2007).
All paramyxoviruses that have been isolated from domestic and wild birds are grouped under the genus Avulavirus except for avian metapneumovirus (AMPV), which is classified under genus Metapneumovirus (Lamb and Parks, 2007). APMVs under the genus Avulavirus are divided into nine serotypes (APMV-1–9) based on hemagglutination inhibition (HI) and neuraminidase inhibition (NI) tests (Alexander, 2003). APMV-1 or Newcastle disease virus (NDV) is economically the most important viral disease of poultry and is the most studied member of this group. Very little information is available about the molecular and biological characteristics and pathogenicity of APMV-2 through -9. APMV-2, -3, -6 and -7 have been associated with disease in domestic poultry (Zhang et al., 2006, 2007; Redmann et al., 1991; Alexander and Collins, 1982; Bankowski et al., 1981; Tumova et al., 1979) and APMV-5 has been implicated in a severe pulmonary disease of budgerigars (Nerome et al., 1978). The pathogenicity of the remaining APMV serotypes is not known. Recently, the complete genome sequences of APMV-2 (Subbiah et al., 2008), APMV-3 (Kumar et al., 2008), APMV-4 (Nayak et al., 2008; Jeon et al., 2008) and APMV-6 (Chang et al., 2001) were determined, which has improved our understanding of the members of genus Avulavirus. However, the complete genome sequences of APMV-5, -7, -8 and -9 have not yet been determined.
APMV-8 strain goose/Delaware/1053/76 was first isolated in 1976 (Cloud and Rosenberger, 1980) from a feral Canadian goose (Branta canadensis) in the Atlantic Flyway in the U.S. APMV-8 strain pintail/Wakuya/20/78 was isolated from a feral pintail duck (Anas acuta) in Wakuya, Japan in 1978 (Yamane et al., 1982). Both these strains were later identified as representative strains of a new APMV serotype and designated APMV-8 by HI assay, structural protein profile and double immuno-diffusion tests (Alexander et al., 1983). Strain Delaware is considered to be the prototype strain of APMV-8. Since then, many APMV-8 strains have been isolated from geese and pintail ducks in wild bird population. However, very little is known about the molecular characteristics and pathogenicity of APMV-8.
In this paper, we describe the growth characteristics and complete consensus genome sequences of APMV-8 strains Delaware and Wakuya, and their phylogenetic relationship with the members of family Paramyxoviridae.
2. Materials and methods
2.1. Virus and cells
APMV-8 strains goose/Delaware/1053/76 and pintail/Wakuya/20/78 were received from the National Veterinary Services Laboratory, Ames, Iowa, U.S.A. and Central Veterinary Laboratory, Weybridge, Surrey, U.K., respectively. Delaware and Wakuya strains of APMV-8 were grown in the allantoic cavities of 9-day-old embryonated specific pathogen free (SPF) chicken eggs. Infected allantoic fluids were harvested 3 days post-inoculation. The titer of the virus was determined by hemagglutination (HA) assay using 1% chicken red blood cells (RBCs) at room temperature. Replication of the two strains was evaluated in eight established cell lines, namely, chicken embryo fibroblast (DF-1), quail fibrosarcoma (QT-35), African green monkey kidney (Vero), Madin Darby Bovine Kidney (MDBK), Madin Darby Canine Kidney (MDCK), Pig Kidney (PK-15), Bovine Turbinate (BT) and human embryonic kidney (293T) cells, and in two primary cells, namely, chicken embryo fibroblast (CEF) and chicken embryo kidney (CEK) cells. All cells were grown in Dulbecco’s minimum essential medium (DMEM) containing 10% fetal bovine serum (FBS) and incubated at 37 °C under 5% CO2. Each cell type was grown as a monolayer (70–80% confluency) and infected with 10−3 dilution of 28 HA units of both egg-grown APMV-8 strains, with or without the supplementation of acetyl trypsin (1 μg/ml) (Invitrogen) in serum free medium, as an exogenous source of protease for the cleavage of F protein. The cells were observed daily for 5–7 days for cytopathic effect (CPE) and HA activity of the cell culture supernatant.
2.2. Viral RNA isolation and sequence analysis
The prototype APMV-8 strain Delaware was grown in SPF chicken eggs and the virus from the infectious allantoic fluid was pelleted through 30% sucrose cushion at 25,000 rpm for 2 h in an SW28 rotor and a Beckman XL-80 ultracentrifuge. The pelleted virus was dissolved in 500 μl phosphate buffer saline and used for RNA extraction by Trizol-chloroform method (Invitrogen).
For complete genome sequencing of APMV-8 strain Delaware, RNA was isolated from virions, which were purified on sucrose gradients from allantoic fluid as described above, and was subjected to reverse transcription using random hexamer primers and Superscript II (Invitrogen) reverse transcriptase, using the manufacturer’s protocol. The cDNA served as a template for PCR using degenerate consensus primers that were designed for N, HN and L genes by aligning available gene sequences from members of genera Avulavirus, Morbillivirus, Respirovirus and Rubulavirus. The N gene primer set N230fwd (5′-WWSGKMKWAG CGGAAATACC-3′) (where N = A/C/G/T, S = G/C, M = A/C, W = A/T, K = G/T) and N630rev (5′-AWNCKNARRWCTCTGGTCTCA-3′) (where R = A/G) yielded a 350 bp length amplicon. The HN gene primer set HN540fwd (5′-AGTKGWTGWTTGCTGGAGGTTCT-3′) and HN770rev (5′-CCARTTNARRCGATWAGGACA-3′) generated an amplicon length of around 200 bp. The L gene primer set L4500fwd (5′-GCNGCNGTNGCNAATTATWTACTT-3′) and L4750rev (5′-CANCKNARRTATCTACCNCTGAT-3′) yielded an amplicon size of about 500 bp. The amplicons of the N, HN and L genes corresponded to regions 247–645, 6832–7026 and 12,349–12,842 nucleotides of the genome sequence of strain Delaware, respectively. A new set of primers was designed from these three virus-specific sequences for determining the remaining sequence of strain Delaware.
The leader sequence of the APMV-8 strain Delaware was determined using 3′-rapid amplification of cDNA ends (3′RACE) (Li et al., 2005; Troutt et al., 1992). Briefly, the genomic RNA was ligated to a 3′-blocked RNA oligo-(5′-GGTTTTGCGGTAAAGGTGGAAGAGAAG-3′-blocked) using T4 RNA ligase according to manufacturer’s protocol (Promega). RT-PCR was performed using a DNA complementary oligo-(5′-CCAAAACGCCATTTCCACCTTCTCTTC-3′) with sequence-specific NP190rev primer (5′-TGCTCCTTGCTTCATTGGTGTGG-3′). The resultant PCR product was cloned (see below) and sequenced. The sequence of the trailer region was determined using 5′RACE technique, in which primer L6000fwd (5′-ATG-AGAAAATAATTGCCAGAAAAA-3′) was used to reverse-transcribe the genomic RNA sequence into cDNA, and the cDNA was poly dATP-tailed using T4 terminal deoxynucleotidyl transferase according to the manufacturer’s procedure (Invitrogen). PCR was performed using L6000fwd and oligo-(dT) reverse primer (5′-ACCACGCGTATCGATGTCGACTTTTTTTTTTTTTTTV-3′) and poly-adenylated cDNA as a template. The obtained amplicon was cloned and sequenced.
All PCR reactions were performed in volumes of 50 μl using recombinant Taq polymerase (Invitrogen). The general conditions used for PCR were 95 °C for 5 min, 25 cycles of 95 °C for 1 min (denaturation), 55 °C for 1 min (annealing) and 72 °C for 1.5 min (extension), followed by 72 °C for 10 min (final extension). PCR amplicons were gel-purified and cloned using pCR-4 TOPO TA cloning vector (Invitrogen), or sequenced directly after gel-purification. Sequencing was done using the BigDye terminator v 3.1 cycle sequencing kit (Applied Biosystems) and a 3130xl genetic analyzer, using the manufacturer’s instructions. Sequences were aligned and analyzed using SeqMan and EditSeq programs, and additional primers were designed for genome walking using the PrimerSelect program of DNASTAR Lasergene 7 software package. Fourfold coverage was achieved for each nucleotide of APMV-8 strain Delaware by sequencing three times independently in cloned plasmid DNAs and once directly from PCR amplicons, each using a different lot of viral RNA.
The complete genome sequence of APMV-8 strain Wakuya was determined as described above for strain Delaware except that PCR was performed using specific forward and reverse primers that were designed based on the full-length sequence of strain Delaware. The complete genome sequence was determined three times from cloned PCR products and confirmed once by direct sequencing of PCR products.
2.3. Sequence and phylogenetic tree analysis
For comparative analysis of APMV-8 genome, the sequence of prototype strain Delaware was used. Nucleotide and amino acid sequence similarity searches of the APMV-8 were performed using a basic length alignment search tool (BLAST) from the National Center for Biotechnology Information (NCBI) and blastn or blastp of EditSeq program. The differences between Delaware and Wakuya strains at both nucleotide and amino acid levels were determined using the ClustalW multiple alignment algorithm of MegAlign program. To calculate the phylogenic relatedness of the APMV-8 to other members of the order Mononegavirales, a phylogenetic tree was constructed for each viral protein using the ClustalW multiple alignment algorithm of the MegAlign program of DNASTAR Lasergene 7 software package.
2.4. Database accession numbers
The complete genome sequence of APMV-8 strains Delaware and Wakuya were submitted in GenBank under accession numbers, Delaware strain—FJ215863; Wakuya strain—FJ215864. Various virus sequences used for primer designing and comparative analysis of APMV-8 include NC 002617 for NDV, EU338414 for APMV-2, EU403085 for APMV-3, FJ177514 and EU877976 for APMV-4, NC 003043 for APMV-6, D13990 (F and HN genes) for MrV, NC 003443 for hPIV2, NC 006430 for SV5, NC 004074 for Tioman virus, NC 001552 for Sendai virus, NC 001796 for hPIV3, NC 003461 for hPIV1, AY988601 for Nipah virus, NC 001906 for Hendra virus NC 001498 for Measles virus, NC 001921 for Canine distemper virus and NC 007454 for J virus.
3. Results
3.1. Growth characteristics of APMV-8
Avian paramyxovirus type 8 strains Delaware and Wakuya yielded titers of 27–28 HA units in 9-day-old embryonated SPF chicken eggs 3 days post-inoculation. Eight different cell lines (DF-1, Vero, QT-35, MDCK, MDBK, PK-15, BT and 293T), and two primary cells of chicken origin (CEF and CEK) were evaluated to determine the cell type(s) that can support the growth of APMV-8 strains, as well as whether exogenous protease is required for replication. With the supplementation of acetyl trypsin (1 μg/ml), both the strains of APMV-8 yielded 22, 21 and 21 HA units in Vero, DF-1 and QT-35 cells, respectively, whereas HA was not detectable in MDBK, MDCK, BT and PK-15 cells. The CEK and 293T cells could support the growth of two APMV-8 strains without exogenous protease and yielded 24 HA units for both the strains, but CEF cells required acetyl trypsin, to yield 23 HA units for both APMV-8 strains. The CPE observed were rounding and detachment cells. There was no evidence of syncytium and plaque formation for the strains Delaware and Wakuya of APMV-8 in the cells tested. These results indicated the requirement of acetyl trypsin for APMV-8 to grow in these cell lines and in primary CEF cells excepting CEK and 293T cells.
3.2. Determination of the complete consensus genome sequence of APMV-8
The genomes of APMV-8 strains Delaware and Wakuya each consist of 15,342 nucleotides (nt) (GenBank accession numbers: strain Delaware—FJ215863 and strain Wakuya—FJ215864). The genome length is a multiple of six, conforming to the ‘rule of six’ as is the case for all other members of subfamily Paramyxovirinae (Calain and Roux, 1993; Kolakofsky et al., 1998). The genomes of APMV-8 strains Delaware and Wakuya consist of six tandemly linked genes in the order of 3′-N-P/V/W-M-F-HN-L-5′ (Fig. 1(a)), resembling those of APMV-1, -2, -3 and -4, but lacking the SH protein gene present in APMV-6. The percentage of the genome that encodes proteins is 89.93%, which is similar to the other members of subfamily Paramyxovirinae. The striking feature of the APMV-8 strains Delaware and Wakuya is that both are identical with regard to genome length and to the lengths, positions and spacing of the six genes, the major ORFs and major encoded proteins, and the IGS, which are summarized in Tables 1 and 2 and described in detail below.
Fig. 1.
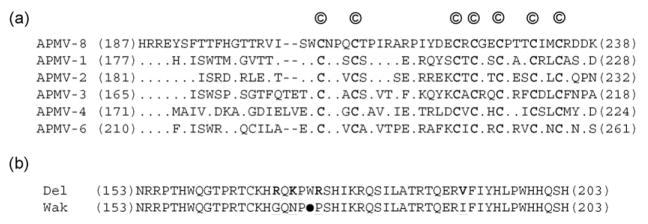
The genome of AMPV-8. (a) Schematic diagram showing the genomic organization of strains Delaware and Wakuya of APMV-8, which are identical with regard to genome length, gene position and length, and the lengths of the IGS, leader and trailer. Numbers over the diagram indicate lengths of the extragenic leader, trailer and IGS sequences, and numbers under the diagram indicate gene length. (b) Alignment and comparison of the 3′-leader regions of available APMVs with that of the Delaware (Del) strain (dots indicate nucleotide identity, underlining indicate a nucleotide difference with Wakuya (Wak) strain and the conserved nucleotides are given in bold). (c) Complementary terminal nucleotides of the 3′-leader and 5′-trailer regions of the Delaware strain (stars indicate complementary base pairs). Sequences are in negative sense.
Table 1.
Molecular features of genes and deduced proteins of APMV-8 strains Delaware and Wakuya.
| Gene | Hexamer phasing position at gene-start | Gene features (nt) |
Intergenic region (nt) | Protein size (aa) | |||
|---|---|---|---|---|---|---|---|
| 5′UTR | ORF | 3′UTR | Total length | ||||
| N | 2 | 75 | 1386 | 88 | 1570 | 2 | 461 |
| P/V (P) | 2 | 55 | 1218 | 80 | 1374 | 30 | 405 |
| P/V (V) | 2 | 55 | 717 | 582 | 1375 | – | 238 |
| M | 2 | 44 | 1110 | 211 | 1385 | 25 | 369 |
| F | 2 | 47 | 1632 | 130 | 1830 | 8 | 543 |
| HN | 4 | 93 | 1734 | 146 | 1994 | 1 | 577 |
| L | 1 | 11 | 6717 | 147 | 6897 | – | 2238 |
Table 2.
Gene-end (GE) motifs, intergenic sequences (IGS), and gene-start (GS) motifs comprising the gene junctions of APMV-8 strains Delaware and Wakuya.a.
| Gene junction | Gene-end | IGS | Gene-start |
|---|---|---|---|
| Leader/N | C5 GCUUC | ||
| N/P | AAUUCU6 | AA | C5 GCUGG |
| P/M | AAUUCU6 | AACUGAUGA(U)UAUUCCUUUCUUGUGGUUGAA | C5 GCUUC |
| M/F | AAUAU6 | AACGAAAUUUCCAAUA(G)ACUGCUCAG | C5 GCUUU |
| F/HN | AAUUAU6 | ACAUUGGA | C5 GCUGG |
| HN/L | AAUUCU6 | A | C2 UC3 GCUGG |
| L/trailer | AAUUCU6 |
Underlined nucleotides indicate assignments in strain Delaware, and nucleotides in parenthesis represent assignments at the same position in strain Wakuya. Sequences are in negative (genome) sense.
The 3′-leader regions of APMV-8 strains Delaware and Wakuya each consist of 55 nt, a length that is conserved among almost all the members of subfamily Paramyxovirinae (Fig. 1(b)). There is a single-nucleotide difference found at position 52, which is residue A in strain Delaware and residue G in strain Wakuya. Comparison of the 55 nt 3′-leader sequence of APMV-8 with APMV-1, -2, -3, -4 and -6 showed that the first 13 nt are identical among APMV-8, -2 and -6. The first 6 nt of the leader sequence (3′-UGGUUU-5′) are same for all the members of genus Avulavirus sequenced to date except for a change in the third position, where the residue G is replaced with residue A in APMV-3 and residue C in APMV-4. Strikingly, the assignments at 42 positions in the leader of strain Delaware and 43 positions in strain Wakuya are identical to the corresponding positions in APMV-2 (Fig. 1(b)).
The length of the 5′-trailer sequences of APMV-8 strains Delaware and Wakuya is 171 nt, and there are 8 nt differences between them. The last 4 nt of the trailer (3′-ACCA-5′) corresponding to 15,339–15,342 nt of the genome is repeated two times in strain Delaware (15,191–15,194 nt and 15,272–15,275 nt) and once in strain Wakuya (15,191–15,194 nt) within their trailer sequences. The sequences of the 3′-leader and 5′-trailer termini showed a high degree of complementarity (Fig. 1(c)). Eleven of the first 14 nt were complementary between the leader and trailer termini of both strains, suggestive of conserved promoter elements at the 3′ termini of the genome and antigenome, respectively.
3.3. Sequences of the GS and GE motifs and the IGS
The GS sequences of APMV-8 strains Delaware and Wakuya are identical: 3′-C5GCUUC-5′ for N and M genes, 3′-C5GCUGG-5′ for P and HN genes, 3′-C5GCUUU-5′ for F gene and 3′-C2UC3GCUGG-5′ for L gene, which are conserved exactly between strains Delaware and Wakuya (Table 2). Similarly, the GE sequences of APMV-8 are 3′-AAUUCU6-5′ for N, P, HN and L genes, 3′-AAUAU6-5′ for M gene and 3′-AAUUAU6-5′ for F gene, which are conserved exactly between the two APMV-8 strains. The IGS of both strains are numerically identical, but there is one nucleotide difference noted in IGS between P and M genes (A–U) and M and F genes (A–G) at positions 3010 and 4432 in the genome, respectively. The lengths of IGS in APMV-8 range from 1 to 30 nt. The IGS between N/P, P/M, M/F, F/HN and HN/L are 2, 30, 25, 8 and 1 nt, respectively. All IGS end with an A residue, as found in many paramyxoviruses (Collins et al., 1986; Crowley et al., 1988; Kawano et al., 1991; Chang et al., 2001), but the M/F IGS in these two APMV-8 strains ends with a G residue. The hexamer phasing positions of the GS sequences of APMV-8 strains Delaware and Wakuya are 2, 2, 2, 2, 4 and 1 (Table 2), which are different from those of other APMVs, namely, APMV-1 (2, 4, 3, 3, 2 and 5), APMV-2 (2, 2, 2, 3, 3 and 3), APMV-3 (2, 5, 5, 2, 2 and 1) and APMV-4 (2, 2, 2, 6, 2 and 2). Thus, the hexamer phasing positions of the GS signals in the APMVs sequenced to date is not conserved.
3.4. The nucleoprotein (N) gene
The N gene of APMV-8 strains Delaware and Wakuya is 1570 nt long, which encodes a N protein of 461 amino acids (aa) with a predicted molecular weight (MW) of 50,956 and 50,971, respectively (Tables 1 and 3). The N protein of the prototype strain Delaware differed from strain Wakuya in three amino acids at positions 409, 441 and 449, where residue H, K and S in strain Delaware were changed to residues R, N and P in strain Wakuya, respectively (Table 3). The N proteins of both APMV-8 strains contained a highly conserved motif, 324-FAPANYSTMYSYAMG-338 (N′-F-X4-Y-X3-Φ-S-Φ-AMG-C′, where X is any amino acid and Φ is any aromatic amino acid), which has been identified in other members of the subfamily Paramyxovirinae and is thought to be responsible for N-N self-assembly during RNA binding (Lamb and Parks, 2007; Yu et al., 1998; Morgan, 1991). Alanine (A), proline (P) and tyrosine (Y) residues at positions 325, 326 and 333, of this motif, are also found conserved in all APMVs characterized to date (Fig. 2). The N protein of strain Delaware has an amino acid sequence identity of 99.3% with strain Wakuya. It shared 72%, 51%, 40.3%, 37.0% and 35.7% amino acid sequence identity with the cognate N proteins of APMV-2, -6, -1, -4 and APMV-3, respectively, and had an amino acid sequence identity of 33–36% with Rubulavirus, 27–28% with Henipavirus, 27% with Morbillivirus, 18–19% with Respirovirus and 25.8% with J virus.
Table 3.
Nucleotide and amino acid assignment differences between strains Delaware and Wakuya.
| Gene | Nucleotide differences in gene | Amino acid differences in protein | Amino acid differences Delaware(position)Wakuya | Strain Delaware |
Strain Wakuya |
||
|---|---|---|---|---|---|---|---|
| MW of protein | pI | MW of protein | pI | ||||
| N | 43/1570 | 3/461 | H(409)R, K(441)N, S(449)P | 50,956 | 5.462 | 50,971 | 5.342 |
| P | 50/1374 | 14/405 | N(18)T, T(59)N, P(62)S, E(75)G, G(79)E, R(91)S, S(97)L, Q(168)R, A(171)P, A(173)T, S(190)N, A(210)V, K(306)R, S(348)A | 43,553 | 5.230 | 43,651 | 5.230 |
| M | 59/1385 | 5/369 | E(35)K, S(70)N, T(71)S, K(227)R, G(315)E | 40,643 | 9.455 | 40,755 | 9.508 |
| F | 61/1830 | 3/543 | Q(3)K, V(6)I, I(28)V | 58,597 | 5.187 | 58,597 | 5.314 |
| HN | 78/1994 | 8/577 | I(42)V, N(98)S, N(108)S, R(246)Q, T(257)I, A(286)T, M(405)I, R(505)G | 63,683 | 5.687 | 63,512 | 5.376 |
| L | 192/6897 | 20/2238 | I(3)V, T(73)A, D(165)N, C(201)R, I(287)T, Q(504)R, L(508)M, E(525)D, A(646)T, H(915)N, H(926)Y, V(1046)M, V(1124)I, E(1419)D, V(1637)I, K(1679)N, R(1705)I, N(1750)S, T(1990)A, R(2189)C | 253,659 | 7.040 | 253,599 | 7.026 |
Fig. 2.

Alignment of a conserved amino acid motif present in the N proteins of all known APMVs (‘X’ refers to any amino acid and ‘Φ’ refers to any aromatic amino acid. Note that the boxed amino acids are conserved among the known APMVs).
3.5. The phosphoprotein (P) gene
The P gene of APMV-8 strains Delaware and Wakuya is 1374 nt long and encodes a P protein of 405 aa with a predicted MW of 43,553 and 43,651, respectively (Tables 1 and 3). There are 14 aa differences noted between the P proteins of strains Delaware and Wakuya at positions N(18)T, T(59)N, P(62)S, E(75)G, G(79)E, R(91)S, S(97)L, Q(168)R, A(171)P, A(173)T, S(190)N, A(210)V, K(306)R and S(348)A (Table 3). It was found that most of the amino acid differences involved the exchange of amino acids containing similar functional groups maintaining an identical isoelectric point (pI) of 5.23 for the P protein in both strains (Table 3), which may be important for interactions with N and L proteins (Hamaguchi et al., 1983; Horikami et al., 1992). The P protein contained 28 potential sites for phosphorylation (21 serine, 6 threonine and 1 tyrosine residues) in strain Delaware and 26 (20, 5 and 1) in strain Wakuya, as predicted by the NetPhos 2.0 program of Expasy proteomics server. The P protein of APMV-8 strain Delaware has 96.5% amino acid sequence identity with strain Wakuya. Similarly, it has 38.4%, 25.8%, 22.9%, 22.4% and 22.3% amino acid sequence identity with the P proteins of APMV-2, -6, -4, -1 and APMV-3, respectively. In addition, it had an amino acid sequence identity of 15–20% with Rubulavirus, 13–14% with Morbillivirus, 11–13% with Respirovirus, 11–12% with Henipavirus and 13% with J virus.
The P gene contains a putative editing site 5′-AAAAAGGG-3′ (mRNA sense) at nucleotide position 471–478 in the P gene (that corresponds to nucleotide position 2098–2105 in the genome sequence) in both strains, which is identical to those of APMV-1, -2, and -6 (Lamb and Parks, 2007; Subbiah et al., 2008; Chang et al., 2001); however, it differed from those of APMV-3 and -4, which contain the nucleotide sequence 5′-UUAAAGGG-3′ (mRNA sense) as the putative P gene editing site (Kumar et al., 2008; Nayak et al., 2008). The addition of a single-G residue at the editing site would produce a V mRNA encoding a V protein of 238 aa with a predicted MW of 26,352 and 26,430 in APMV-8 strains Delaware and Wakuya, respectively (Table 1, and data not shown). The V protein is identical to P for the first 139 N-terminal amino acids followed by a V-specific C-terminal domain of 99 amino acids. This latter domain contains seven conserved invariantly spaced cysteine residues, as reported for other members of subfamily Paramyxovirinae (Fig. 3(a)). There are five amino acid differences between strains Delaware and Wakuya at positions, G(149)S, G(150)S, S(171)T, G(173)D, E(174)Q in the unique C-terminus of V protein. The addition of two G residues at the editing site would form a W mRNA, producing a W protein of 203 aa in strain Delaware that would share the N-terminal 138 aa with the P protein. Interestingly, the putative W protein ORF of strain Wakuya would be identical in length except that it contains a stop (opal or UGA) codon after aa 172 in place of the tryptophan residue (codon UGG) present in strain Delaware (Fig. 3(b)). Apart from this stop codon in strain Wakuya, the unique C-terminal 65 aa of W protein in strain Delaware differed from strain Wakuya in 6 aa at positions, R(149)K, R(150)Q, R(169)G, K(171)N, R(174)P and V(191)I.
Fig. 3.
The V and W proteins. (a) Sequence alignment of the C-terminal end of V proteins of different APMVs (‘©’ denote the conserved cysteine residues that are also given in bold letters; the numbers indicate amino acid positions). (b) Sequence alignment of the C-terminal ends of the putative W protein of APMV-8 strains Delaware (Del) and Wakuya (Wak) (the opal codon (UGA) of W protein in Wakuya strain (●) terminates the reading frame after 172 aa. The amino acid differences between the strains are given in bold and underlined).
3.6. The matrix protein (M) gene
The M gene of APMV-8 strains Delaware and Wakuya is 1385 nt long and encodes a M protein of 369 aa with a predicted MW of 40,643 and 40,755, respectively (Tables 1 and 3). Five amino acid differences were found between strains Delaware and Wakuya at positions E(35)K, S(70)N, T(71)S, K(227)R and G(315)E (Table 3). The M protein of APMV-8 is a highly basic protein with a predicted isoelectric point (pI) of 9.455 and 9.508 in strains Delaware and Wakuya, respectively. The basic property of this protein may be important for ionic interactions with the acidic N protein (Lamb and Parks, 2007). The M protein contained a unique motif manifesting bipartite basic amino acid clustering found at 248–267 (KKTSSKGKPRTLDELKTKVK), which is thought to serve as a nuclear localization signal (NLS) for the protein (Coleman and Peeples, 1993; Peeples et al., 1992) (Fig. 4). The APMV-8 strain Delaware M protein had an amino acid sequence identity of 98.6% with strain Wakuya, and had 52.3%, 38.6%, 29.7%, 29.2% and 28.6% amino acid sequence identity with the M proteins of APMV-2, -6, -3, -1 and APMV-4, respectively. Strain Delaware also had an amino acid sequence identity of 25–28% with Rubulavirus, 20–22% with Morbillivirus, 20–21% with Henipavirus, 18–20% with Respirovirus and 22.9% with J virus.
Fig. 4.

Sequence alignment of the putative bipartite nuclear localization signal (NLS) sequences (boxed) in matrix (M) protein of known APMVs (Coleman and Peeples, 1993; Peeples et al., 1992) (basic residues (R/K) are underlined and their bipartite clustering is boxed).
3.7. The fusion protein (F) gene
The F gene of APMV-8 strains Delaware and Wakuya is 1830 nt long and encodes a F protein of 543 aa in length with a predicted MW of 58,597 (Tables 1 and 3). The F protein is predicted to be a type I transmembrane protein similar to the F proteins of other members of family Paramyxoviridae. It has a predicted N-terminal signal peptide of 22 aa (1–22 aa) and a predicted transmembrane anchor at amino acid positions 487–509. The putative cleavage site of APMV-8 F protein is T-Y-P-Q-T-R↓L, corresponding to amino acid position 98–104 (Table 4). The F protein cleavage site of APMV-8 does not conform to the favored sequence motif for cleavage by the intracellular protease furin, R-X-K/R-R↓F (Hosaka et al., 1991), and instead has a single-basic residue. Strain Delaware differed from strain Wakuya in 3 aa at positions Q(3)T, V(6)I and I(28)V, which all lay within the F2 subunit. The F1 polypeptide of APMV-8 contained the typical heptad repeats designated as HRA and HRB at amino acid positions 129–175 and 448–479, respectively, as predicted by the LearnCoil-VMF program. The F proteins of strains Delaware and Wakuya each contain eight potential N-linked glycosylation sites whose positions are exactly conserved and are located at amino acids 63 and 76 of F2 subunit and 390, 434, 458, 484, 485 and 535 of F1 subunit, as predicted by the NetNGlyc 1.0 program of the Expasy proteomics server. The F protein of strain Delaware has an amino acid identity of 99.4% with that of strain Wakuya and 62.5%, 49%, 41.9%, 36.5% and 31.8% with the F proteins of APMV-2, -6, -1, -4 and APMV-3, respectively. The strain Delaware F protein has an amino acid identity of 28–31% with Rubulavirus, 25–26% with Morbillivirus, 27–28% with Henipavirus, 24–26% with Respirovirus and 26.7% with J virus.
Table 4.
Comparison of fusion (F) protein cleavage sites of various known avian paramyxoviruses and requirement of exogenous protease for cleavage activation.
| APMVS | F protein cleavage sitea | Requirement of exogenous proteaseb |
|---|---|---|
| APMV-8 | TYPQTR ↓ LIGAVIGS | + |
| Virulent-NDV | GRRQKR ↓ FIGAIIGS | − |
| Avirulent-NDV | GGRQGR ↓ LIGAIIGG | + |
| APMV-2 | DKPASR ↓ FVGAIIGS | − |
| APMV-3 | ARPRGR ↓ LFGPIIGS | + |
| APMV-4 | ADIQPR ↓ FIGAIIAT | − |
| APMV-6c | PAPEPR ↓ LIGAIIGT | − |
Polybasic amino acids (R/K) are underlined, the sequence of APMV-8 are given in bold letters, downward arrow indicates the site of cleavage).
Virus replication in cell culture requires the presence of acetyl trypsin (1 μg/ml) in the medium.
APMV-6 does not require exogenous protease for growth in CEK cells (Xiao and Samal, unpublished data).
3.8. The hemagglutinin-neuraminidase protein (HN) gene
The HN gene of APMV-8 strains Delaware and Wakuya is 1994 nt long and encodes a HN protein of 577 aa with a predicted MW of 63,683 and 63,512, respectively (Tables 1 and 3). There are eight aa differences found between strains Delaware and Wakuya at positions I(42)V, N(98)S, N(108)S, R(246)Q, T(257)I, A(286)T, M(405)I and R(505)G. The HN protein is predicted to be a type II integral membrane protein and has a 17 aa long hydrophobic transmembrane domain located between amino acid positions 29 and 45. The HN proteins of strains Delaware and Wakuya each contain 4 potential N-linked glycosylation sites whose positions are exactly conserved and are located at amino acids 121, 280, 392 and 515, as predicted by the NetNGlyc 1.0 program of the Expasy proteomics server. The HN protein also contained the hexapeptide N-R-K-S-C-S between amino acid positions 236 and 241, which is thought to be responsible for sialic acid binding at the cell surface (Varghese et al., 1983). The conserved putative neuraminidase active site residues are found at amino acid positions 176 (R), 401 (E), 416 (R), 506 (R), 534 (Y) and 555 (E), along with eleven conserved cysteine residues at amino acid positions 174, 188, 198, 240, 253, 346, 463, 469, 473, 539 and 550, corresponding to the globular head of HN protein (Langedijk et al., 1997). The strain Delaware HN protein has an amino acid identity of 98.6% with that of strain Wakuya, and 48.3%, 39.3%, 34.6%, 32.7% and 31% with the HN proteins of APMV-2, -6, -1, -4 and APMV-3, respectively. The strain Delaware HN protein has an amino acid identity of 12–13% with the corresponding protein of Morbillivirus, 18–31% with Rubulavirus, 15–16% with Henipavirus, 21–22% with and 23.9% with J virus.
3.9. The large polymerase (L) protein gene
The L gene of APMV-8 strains Delaware and Wakuya is 6897 nt long and encodes a L protein of 2238 aa with a predicted MW of 253,659 and 253,599, respectively (Tables 1 and 3). There are 20 aa observed differences between strains Delaware and Wakuya, found at amino acid positions I(3)V, T(73)A, D(165)N, C(201)R, I(287)T, Q(504)R, L(508)M, E(525)D, A(646)T, H(915)N, H(926)Y, V(1046)M, V(1124)I, E(1419)D, V(1637)I, K(1679)N, R(1705)I, N(1750)S, T(1990)A and R(2189)C. The sequence alignment of the APMV-8 L protein has six linear conserved domains, as found in other members of the family Paramyxoviridae (Poch et al., 1989). The conserved GDNQ sequence motif found within domain III, which is thought to be involved in L protein transcriptional activity (Schnell and Conzelmann, 1995), was also found in the L protein of APMV-8 at amino acid position 774–777 (Fig. 5). The APMV-8 strain Delaware L protein has an amino acid identity of 99.1% with that of strain Wakuya, and 59.3%, 43.7%, 38.6%, 35.2% and 34.2% with the L proteins of APMV-2, -6, -1, -3 and -4, respectively. The strain Delaware L protein has an amino acid identity of 27–28% with the L protein of Morbillivirus, 37–38% with Rubulavirus, 27–28% with Henipavirus, 27–29% with Respirovirus and 28.4% with J virus.
Fig. 5.
Conserved motifs (A–D) in domain III of the L protein of various known paramyxoviruses (bold letters indicate residues responsible for transcriptional activity of the polymerase [Schnell and Conzelmann, 1995] and the numbers indicate amino acid positions).
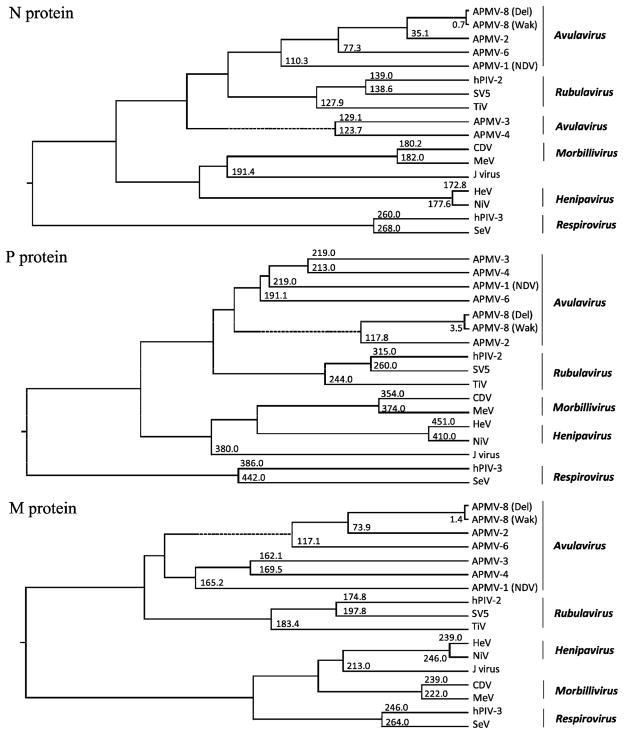
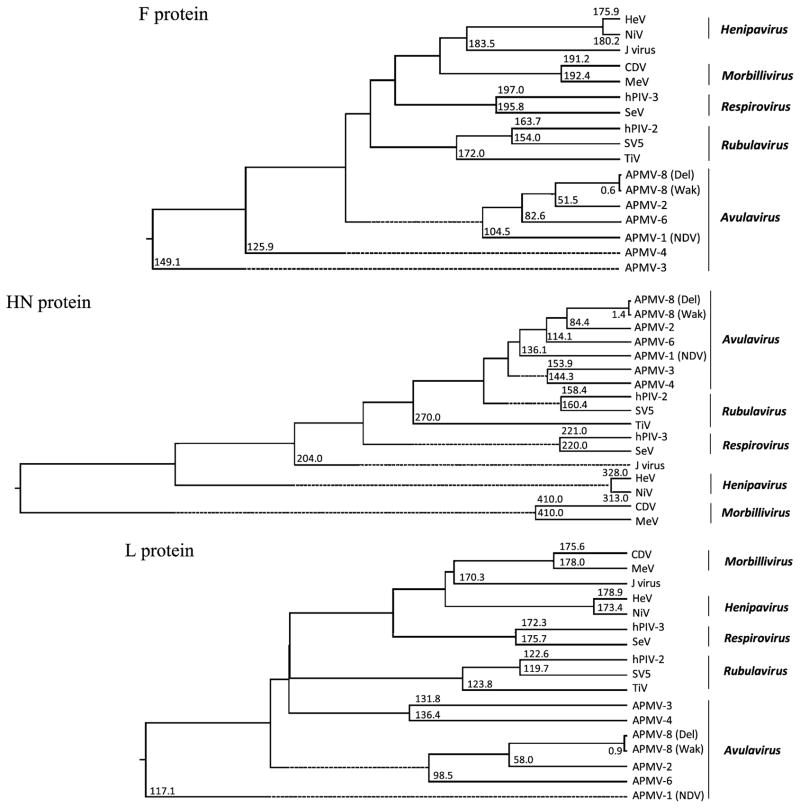
3.10. Phylogenetic analysis
Phylogenetic trees were generated from the amino acid sequence alignments of the N, P, M, F, HN and L proteins of APMV-8 prototype strain Delaware with the closely related strain Wakuya and other cognate proteins of representative viruses of all five genera of subfamily Paramyxovirinae (Fig. 6). The phylogenetic trees clearly indicate the very close genetic relationship of APMV-8 strains Delaware and Wakuya. Moreover, phylogenic analysis confirms the closeness of APMV-8 and APMV-2 when compared to APMV-6. The clustering of APMVs -1, -2, -3, -4 and -6 in the generation of phylogenetic trees strongly supports their classification under the genus Avulavirus. Overall, the rooted phylogenetic trees indicate that the strains Delaware and Wakuya of APMV-8 are closely related to APMV-2 in the genus Avulavirus.
Fig. 6.
Phylogenic analysis of the N, P, M, F, HN and L proteins of AMPV-8 compared to various members of subfamily Paramyxovirinae. The phylogenetic trees and divergence values were obtained using the ClustalW algorithm of the MegAlign program of the DNASTAR Lasergene 7 software package. The values at the branches represent the degree of divergence of APMV-8 strain Delaware with corresponding member viruses.
4. Discussion
APMV-8 strains Delaware and Wakuya grew to high titers (27–28 HA units) in the allantoic cavity of embryonated chicken eggs after 3 days of inoculation. Neither strain caused the death of chicken embryo after 7 days of inoculation with 28 HA units of virus, suggesting that they are apathogenic for chickens. However, the pathogenicity of APMV-8 needs to be evaluated directly in chickens as well as to be determined for other avian species. APMV-8 grew in primary CEK and 293T cells but required the supplementation of acetyl trypsin (1 μg/ml) for growth in Vero, DF-1, QT-35 and in primary CEF cells.
The nucleotide lengths of the genomes of the two strains of APMV-8 were the same, 15,342. This length is longer than those of APMV-1 (15,186 nt) (Krishnamurthy and Samal, 1998), APMV-2 (14,904 nt) (Subbiah et al., 2008), APMV-4 (15,054 nt) (Nayak et al., 2008; Jeon et al., 2008) but is shorter than those of APMV-3 (16,272 nt) (Kumar et al., 2008) and APMV-6 (16,236 nt) (Chang et al., 2001). The nucleotide length of the APMV-8 genome represents the average of the members of the Respirovirus, Rubulavirus, Morbillavirus, and Avulavirus genera, but is less than the characteristically longer genomes of Henipavirus (e.g. Hendra virus, 18,234 nt) and certain unclassified paramyxoviruses (e.g. J virus, 18,954 nt) (Wang et al., 2000; Jack et al., 2005). The genome length of APMV-8 follows the ‘rule of six’, which is a characteristic of subfamily Paramyxovirinae (Calain and Roux, 1993; Kolakofsky et al., 1998), but not of subfamily Pneumovirinae (Samal and Collins, 1996).
The leader region of APMV-8 is 55 nt, a length that is conserved among most members of subfamily Paramyxovirinae (Lamb and Parks, 2007). Generally, the first 12–13 nt of the leader region are more closely related among members of a genus of subfamily Paramyxovirinae and can be used in the classification of new isolates. Comparison of the sequences of leader regions of APMV serotypes sequenced to date showed that the first 13 nt are identical among APMV-8, -2 and -6, indicating a close evolutionary relationship. But, comparison of the leader sequence of APMV-8 with those of APMV-1, -3 and -4 showed 2, 4 and 6 nt changes, respectively, indicating diversity among members of genus Avulavirus. The trailer region of APMV-8 is 171 nt in length, which is longer than the typical length (40–60 nt) for most members of family Paramyxoviridae (Shioda et al., 1986), but is shorter than that of APMV-3 (707 nt) (Kumar et al., 2008). The terminal sequences of the 3′-leader and 5′-trailer regions showed a high degree of complementarity (79% complementarity for the first 14 nt), suggestive of conserved elements in the 3′-promoter region of the genome and antigenome.
APMV-8 genome consists of six genes in the order 3′-N-P/V/W-M-F-HN-L-5′, with IGS that are variable in length from gene junction to gene junction and lack obvious conserved motifs. The same six genes are also found in all members of subfamily Paramyxovirinae, with the exception of the presence of an SH gene in APMV-6 in Avulavirus, SV5 and MuV of Rubulavirus, J virus and BeV, and a U gene in FDLV (Lamb and Parks, 2007; Kurath et al., 2004). The variable, nonconserved nature of the IGS of APMV-8 is similar to those of the members of Rubulavirus and Avulavirus and differ from the typical conserved trinucleotide GAA of Respirovirus, Morbillivirus, and Henipavirus (Nylund et al., 2008).
The genomes of APMV-8 strains Delaware and Wakuya have a nucleotide identity of 96.8%. In addition to being identical in nucleotide length, the two APMV-8 strains also are identical in other genome elements such as the length and spacing of the genes, IGS, and leader and trailer regions. They also shared a very high level of amino acid identity: specifically, 99.3%, 96.5%, 98.6%, 99.4%, 98.6% and 99.1% for the predicted N, P, M, F, HN and L proteins, respectively. The only notable difference was found in the putative W protein of Wakuya strain in which introduction of an opal codon (UGA) would produce a W protein of 172 aa, compared to the 203 aa W protein of strain Delaware. Since the role of W protein in APMV replication and pathogenesis is not known, the functional significance of this difference in the W protein of strain Wakuya remains to be seen. It is noteworthy that strains Delaware and Wakuya are highly related, since they were isolated from two different avian species in two geographically distant countries. Therefore, it will be interesting to compare the pathogenicity of these two strains in different avian species, particularly geese and ducks.
The paramyxovirus F protein is synthesized as an inactive precursor (F0) that is cleaved by host protease into the biologically active form consisting of disulfide-linked F1-F2 subunits (Lamb and Parks, 2007). The F protein cleavage site is a major determinant of NDV pathogenesis in chickens. Virulent NDV strains typically contain a polybasic cleavage site (R-X-K/R-R↓F), which is recognized by furin-like intracellular proteases that are present in most cells, and the cleavage site is followed by an F residue at the beginning of the F1 subunit. In contrast, the cleavage sites of avirulent NDV strains typically contain one or a few basic residues and an L residue at the first position of F1 subunit, and are cleaved by secretary proteases found in the respiratory tract (or, in cell culture, by exogenous protease). However, analysis of recently available sequences of cleavage sites of other APMV serotypes showed that the cleavage sites of some serotypes do not follow the typical cleavage site required for protease activation. For example, it was found that the F protein cleavage sites of APMV-2 (PASR↓F) and APMV-4 (IQPR↓F) contain a single-basic residue (underlined), but do not require exogenous protease for growth in cell culture (Subbiah et al., 2008; Nayak et al., 2008). The putative cleavage site of APMV-8 F protein (PQTR↓L) has a single-basic residue and an L residue at the first position of F1 subunit and requires exogenous protease for growth in cell culture (excepting CEK and 293T cells). This is similar to the F protein cleavage sites of avirulent APMV-1 (RQGR↓L) and APMV-3 (PRGR↓L), irrespective of basic amino acids, require exogenous protease for virus growth in cell culture (Lamb and Parks, 2007; Kumar et al., 2008).
The phylogenetic analysis of APMV-8 with members of family Paramyxoviridae showed that APMV-8 is more closely related to other APMV types than to paramyxoviruses from other genera of Paramyxoviridae, reinforcing the classification of APMVs in the genus Avulavirus. APMV-8 showed a close evolutionary relationship with APMV-2 and -6 by both nucleotide and amino acid analysis. Sequence analysis of additional APMV-8 strains will be needed to understand the full extent of genetic variation within the APMV-8 serotype. Future studies on the epidemiology and pathogenicity of this virus in different avian species will be necessary to understand the role of this virus in the avian population.
Acknowledgments
We thank Daniel Rockemann, Flavia Militino Dias and all our laboratory members for their excellent technical assistance and help. Special thanks to Ireen Dryburgh-Barry for proofreading the manuscript. “This research was supported by NIAID contract no. N01A060009 (85% support) and NIAID, NIH Intramural Research Program (15% support). The views expressed herein do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention of trade names, commercial practices, or organizations imply endorsement by the U.S. Government.”
References
- Alexander DJ, Collins MS. Pathogenecity of PMV-3/Parakeet/Netherland/449/75 for chickens. Avian Pathol. 1982;11:179–185. doi: 10.1080/03079458208436091. [DOI] [PubMed] [Google Scholar]
- Alexander DJ, Hinshaw VS, Collins MS, Yamane N. Characterization of viruses which represent further distinct serotypes (PMV-8 and PMV-9) of avian paramyxoviruses. Arch Virol. 1983;78:29–36. doi: 10.1007/BF01310856. [DOI] [PubMed] [Google Scholar]
- Alexander DJ. Avian paramyxoviruses 2–9. In: Saif YM, editor. Diseases of Poultry. 11. Iowa State University Press; Ames: 2003. pp. 88–92. [Google Scholar]
- Bankowski RA, Almquist J, Dombrucki J. Effect of paramyxovirus yucaipa on fertility, hatchability, and poult yield of turkeys. Avian Dis. 1981;25:517–520. [PubMed] [Google Scholar]
- Calain P, Roux L. The rule of six, a basic feature for efficient replication of Sendai virus defective interfering RNA. J Virol. 1993;67:4822–4830. doi: 10.1128/jvi.67.8.4822-4830.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PC, Hsieh ML, Shien JH, Graham DA, Lee MS, Shieh HK. Complete nucleotide sequence of avian paramyxovirus type 6 isolated from ducks. J Gen Virol. 2001;82:2157–2168. doi: 10.1099/0022-1317-82-9-2157. [DOI] [PubMed] [Google Scholar]
- Cloud SS, Rosenberger JK. Characterization of nine avian paramyxoviruses. Avian Dis. 1980;24:139–152. [Google Scholar]
- Coleman NA, Peeples ME. The matrix protein of Newcastle disease virus localizes to the nucleus via a bipartite nuclear localization signal. Virology. 1993;195 (2):596–607. doi: 10.1006/viro.1993.1411. [DOI] [PubMed] [Google Scholar]
- Collins PL, Dickens LE, Buckler-White A, Olmsted RA, Spriggs MK, Camargo E, Coelingh KV. Nucleotide sequences for the gene junctions of human respiratory syncytial virus reveal distinctive features of intergenic structure and gene order. Proc Natl Acad Sci. 1986;83 (13):4594–4598. doi: 10.1073/pnas.83.13.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley JC, Dowling PC, Menonna J, Silverman JI, Schuback D, Cook SD, Blumberg BM. Sequence variability and function of measles virus 3′ and 5′ ends and intercistronic regions. Virology. 1988;164 (2):498–506. doi: 10.1016/0042-6822(88)90564-8. [DOI] [PubMed] [Google Scholar]
- Hamaguchi M, Yoshida T, Nishikawa K, Naruse H, Nagai Y. Transcriptive complex of Newcastle disease virus1. Both L and P proteins are required to constitute an active complex. Virology. 1983;128:105–117. doi: 10.1016/0042-6822(83)90322-7. [DOI] [PubMed] [Google Scholar]
- Horikami SM, Curran J, Kolakofsky D, Moyer SA. Complexes of Sendai virus NP-P and P-L proteins are required for defective interfering particle genome replication in vitro. J Virol. 1992;66:4901–4908. doi: 10.1128/jvi.66.8.4901-4908.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka M, Nagahama M, Kim WS, Watanabe T, Hatsuzawa K, Ikemizu J, Murakami K, Nakayama K. Arg-X-Lys/Arg-Arg motif as a signal for precursor cleavage catalyzed by furin within the constitutive secretory pathway. J Biol Chem. 1991;266 (19):12127–12130. [PubMed] [Google Scholar]
- Jack PJ, Boyle DB, Eaton BT, Wang LF. The complete genome sequence of J virus reveals a unique genome structure in the family Paramyxoviridae. J Virol. 2005;79:10690–10700. doi: 10.1128/JVI.79.16.10690-10700.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon WJ, Lee EK, Kwon JH, Choi KS. Full-length genome sequence of avian paramyxovirus type 4 isolated from a mallard duck. Virus Genes. 2008;37 (3):342–350. doi: 10.1007/s11262-008-0267-4. [DOI] [PubMed] [Google Scholar]
- Kawano M, Okamoto K, Bando H, Kondo K, Tsurudome M, Komada H, Nishio M, Ito Y. Characterizations of the human parainfluenza type 2 virus gene encoding the L protein and the intergenic sequences. Nucl Acids Res. 1991;19 (10):2739–2746. doi: 10.1093/nar/19.10.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakofsky D, Pelet T, Garcin D, Hausmann S, Curran J, Roux L. Paramyxovirus RNA synthesis and the requirement for hexamer genome length: the rule of six revisited. J Virol. 1998;72:891–899. doi: 10.1128/jvi.72.2.891-899.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy S, Samal SK. Nucleotide sequences of the trailer, nucleo-capsid protein gene and intergenic regions of Newcastle disease virus strain Beaudette C and completion of the entire genome sequence. J Gen Virol. 1998;79 (10):2419–2424. doi: 10.1099/0022-1317-79-10-2419. [DOI] [PubMed] [Google Scholar]
- Kumar S, Nayak B, Collins PL, Samal SK. Complete genome sequence of avian paramyxovirus type 3 reveals an unusually long trailer region. Virus Res. 2008;137 (2):189–197. doi: 10.1016/j.virusres.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurath G, Batts WN, Ahne W, Winton JR. Complete genome sequence of Fer-de-Lance virus reveals a novel gene in reptilian paramyxoviruses. J Virol. 2004;78:2045–2056. doi: 10.1128/JVI.78.4.2045-2056.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RA, Collins PL, Kolakofsky D, Melero JA, Nagai Y, Oldstone MBA, Pringle CR, Rima BK. Family Paramyxoviridae. In: Fauquet CM, editor. Virus Taxonomy: The Classification and Nomenclature of Viruses. The Eighth Report of the International Committee in Taxonomy of Viruses. 2005. [Google Scholar]
- Lamb R, Parks G. In: Paramyxoviridae: The Viruses and Their Replication. Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Lippincott Williams & Wilkins; Philadelphia: 2007. [Google Scholar]
- Langedijk JP, Daus FJ, van Oirschot JT. Sequence and structure alignment of Paramyxoviridae attachment proteins and discovery of enzymatic activity for a morbillivirus hemagglutinin. J Virol. 1997;71:6155–6167. doi: 10.1128/jvi.71.8.6155-6167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Yu M, Zhang H, Wang HY, Wang LF. Improved rapid amplification of cDNA ends (RACE) for mapping both the 5′ and 3′ terminal sequences of paramyxovirus genomes. J Virol Meth. 2005;130:154–156. doi: 10.1016/j.jviromet.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Mayo MA, Pringle CR. Virus taxonomy—1997. J Gen Virol. 1998;79 (4):649–657. doi: 10.1099/0022-1317-79-4-649. [DOI] [PubMed] [Google Scholar]
- Morgan EM. Evolutionary relationships of paramyxovirus nucleocapsid-associated proteins. In: Kingsbury DW, editor. The Paramyxoviruses. Plenum Press; New York: 1991. pp. 163–179. [Google Scholar]
- Nayak B, Kumar S, Collins PL, Samal SK. Molecular characterization and complete genome sequence of avian paramyxovirus type 4 prototype strain duck/Hong Kong/D3/75. Virol J. 2008;5:124. doi: 10.1186/1743-422X-5-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerome K, Nakayama M, Ishida M, Fukumi H. Isolation of a new avian paramyxovirus from budgerigar (Melopsittacus undulatus) J Gen Virol. 1978;38:293–301. doi: 10.1099/0022-1317-38-2-293. [DOI] [PubMed] [Google Scholar]
- Nylund S, Karlsen M, Nylund A. The complete genome sequence of the Atlantic salmon paramyxovirus (ASPV) Virology. 2008;373 (1):137–148. doi: 10.1016/j.virol.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Peeples M, Wang C, Kailash, Gupta KC, Coleman N. Nuclear entry and nucleolar localization of the Newcastle disease virus (NDV) matrix protein occur early in infection and do not require other NDV proteins. J Virol. 1992;66 (5):3263–3269. doi: 10.1128/jvi.66.5.3263-3269.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poch O, Sauvaget I, Delarue M, Tordo N. Identification of four conserved motifs among the RNA-dependent polymerase encoding elements. EMBO J. 1989;8:3867–3874. doi: 10.1002/j.1460-2075.1989.tb08565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmann T, Zeydanli MM, Herbst W, Kaleta EF. Isolation of a paramyxovirus-3 from turkeys with respiratory tract disease in Germany. Dtsch Tierarztl Wochenschr. 1991;98:138–141. [PubMed] [Google Scholar]
- Samal SK, Collins PL. RNA replication by a respiratory syncytial virus RNA analog does not obey the rule of six and retains a nonviral trinucleotide extension at the leader end. J Virol. 1996;70 (8):5075–5082. doi: 10.1128/jvi.70.8.5075-5082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell MJ, Conzelmann KK. Polymerase activity of in vitro mutated rabies virus L protein. Virology. 1995;214:522–530. doi: 10.1006/viro.1995.0063. [DOI] [PubMed] [Google Scholar]
- Shioda T, Iwasaki K, Shibuta H. Determination of the complete nucleotide sequence of the Sendai virus genome RNA and the predicted amino acid sequences of the F, HN and L proteins. Nucl Acids Res. 1986;14:1545–1563. doi: 10.1093/nar/14.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbiah M, Xiao S, Collins PL, Samal SK. Complete sequence of the genome of avian paramyxovirus type 2 (strain Yucaipa) and comparison with other paramyxoviruses. Virus Res. 2008;137 (1):40–48. doi: 10.1016/j.virusres.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troutt AB, McHeyzer-Williams MG, Pulendran B, Nossal GJ. Ligation-anchored PCR: a simple amplification technique with single-sided specificity. Proc Natl Acad Sci. 1992;89:9823–9825. doi: 10.1073/pnas.89.20.9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumova B, Stumpa A, Janout V, Uvizl M, Chmela J. A further member of the Yucaipa group isolated from the common wren (Troglodytes troglodytes) Acta Virol. 1979;23:504–507. [PubMed] [Google Scholar]
- Varghese JN, Laver WG, Colman PM. Structure of the influenza virus glycoprotein antigen neuraminidase at 2.9 Å resolution. Nature. 1983;303:35–40. doi: 10.1038/303035a0. [DOI] [PubMed] [Google Scholar]
- Wang LF, Yu M, Hansson E, Pritchard LI, Shiell B, Michalski WP, Eaton BT. The exceptionally large genome of Hendra virus: support for creation of a new genus within the family Paramyxoviridae. J Virol. 2000;74:9972–9979. doi: 10.1128/jvi.74.21.9972-9979.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane N, Arikawa J, Odagira T, Ishida N. Characterization of avian paramyxoviruses isolated from feral ducks in northern Japan: the presence of three distinct viruses in nature. Microbiol Immunol. 1982;26 (7):557–568. doi: 10.1111/mim.1982.26.7.557. [DOI] [PubMed] [Google Scholar]
- Yu M, Hansson E, Shiell B, Michalski W, Eaton BT, Wang LF. Sequence analysis of the Hendra virus nucleoprotein gene: comparison with other members of the subfamily Paramyxovirinae. J Gen Virol. 1998;79 (7):1775–1780. doi: 10.1099/0022-1317-79-7-1775. [DOI] [PubMed] [Google Scholar]
- Zhang GZ, Zhao JX, Wang M. Serological survey on prevalence of antibodies to avian paramyxovirus serotype 2 in China. Avian Dis. 2007;51:137–139. doi: 10.1637/0005-2086(2007)051[0137:SSOPOA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Zhang GZ, Zhao JX, Wang HW, Yang AM, Bu CY, Wang M. Isolation, identification, and comparison of four isolates of avian paramyxovirus serotype 2 in China. Avian Dis. 2006;50:386–390. doi: 10.1637/7502-010906R1.1. [DOI] [PubMed] [Google Scholar]